Abstract
The optimal first‐line palliative systemic treatment strategy for metastatic esophagogastric cancer is not well defined. The aim of our study was to explore real‐world use of first‐line systemic treatment in esophagogastric cancer and assess the effect of treatment strategy on overall survival (OS), time to failure (TTF) of first‐line treatment and toxicity. We selected synchronous metastatic esophagogastric cancer patients treated with systemic therapy (2010–2016) from the nationwide Netherlands Cancer Registry (n = 2,204). Systemic treatment strategies were divided into monotherapy, doublet and triplet chemotherapy, and trastuzumab‐containing regimens. Data on OS were available for all patients, on TTF for patients diagnosed from 2010 to 2015 (n = 1,700), and on toxicity for patients diagnosed from 2010 to 2014 (n = 1,221). OS and TTF were analyzed using multivariable Cox regression, with adjustment for relevant tumor and patient characteristics. Up to 45 different systemic treatment regimens were found to be administered, with a median TTF of 4.6 and OS of 7.5 months. Most patients (45%) were treated with doublet chemotherapy; 34% received triplets, 10% monotherapy and 10% a trastuzumab‐containing regimen. The highest median OS was found in patients receiving a trastuzumab‐containing regimen (11.9 months). Triplet chemotherapy showed equal survival rates compared to doublets (OS: HR 0.92, 95%CI 0.83–1.02; TTF: HR 0.92, 95%CI 0.82–1.04) but significantly more grade 3–5 toxicity than doublets (33% vs. 21%, respectively). In conclusion, heterogeneity of first‐line palliative systemic treatment in metastatic esophagogastric cancer patients is striking. Based on our data, doublet chemotherapy is the preferred treatment strategy because of similar survival and less toxicity compared to triplets.
Keywords: esophageal neoplasms, gastric neoplasms, drug therapy, palliative treatment
Short abstract
What's new?
Metastatic esophagogastric cancer can't be cured, but palliative therapy can improve patients’ quality of life and survival. However, there's no consensus regarding the optimal first‐line palliative systemic therapy for metastatic esophagogastric cancer. Here, the authors evaluated real‐world use of first‐line systemic treatments. The retrospective study included a cohort of 2,204 metastatic patients and found 45 different treatment regimens administered. Patients that received doublet and triplet chemotherapy had similar survival, with triplet patients experiencing higher toxicity. Monotherapy produced significantly worse overall survival and only modest reduction in toxicity. These findings support doublet therapy as the optimal first‐line treatment strategy.
Abbreviations
- 5‐FU
5‐fluorouracil
- CapOx
capecitabine/oxaliplatin
- CI
confidence interval
- CTCAE
Common Terminology Criteria for Adverse Events
- ECC
epirubicin/cisplatin/capecitabine
- EOX
epirubicin/oxaliplatin/capecitabine
- FLOT
docetaxel/oxaliplatin/5‐fluorouracil/leucovorin
- FOLFOX
5‐fluorouracil/oxaliplatin
- HER2
human epidermal growth factor receptor 2
- HR
hazard ratio
- IQR
interquartile range
- NCR
Netherlands Cancer Registry
- NOS
not otherwise specified
- OS
overall survival
- TTF
time to failure
Introduction
Palliative treatment represents an important part of esophagogastric cancer care, since approximately one‐third of esophagogastric cancer patients have metastases at initial diagnoses, and curative treatment options are not available.1, 2 Systemic therapy can improve both survival and quality of life in these patients.3, 4, 5, 6
However, the optimal first‐line palliative systemic therapy regimen for metastatic esophagogastric cancer patients has not yet been identified. Currently, first‐line systemic treatment usually comprises a fluoropyrimidine and a platinum compound with the addition of trastuzumab in the case of human epidermal growth factor receptor 2 (HER2) overexpression, providing a survival benefit up to 9 months compared to no systemic treatment.7, 8, 9, 10, 11 Triplet therapy, in which either an anthracycline or taxane is added to the platinum‐fluoropyrimidine doublet, is suggested in international guidelines for patients in good condition,8, 10, 12, 13 but becomes increasingly controversial because of its toxicity.6, 14, 15 Because of the lack of consensus on optimal palliative systemic treatment, making choices about the best approach for these patients is challenging, which can result in interhospital and interphysician variation in individual systemic treatment. This could eventually affect survival and quality of life, and might be the explanation for stagnating survival rates, despite an increase in the administration of palliative systemic therapy from <10% to 40% of metastatic esophagogastric cancer patients between 1990 and 2011 in the Netherlands.1, 2, 16, 17, 18
Current practice is based on the results of several randomized controlled trials.4, 5, 6 Because of, for example, the underrepresentation of elderly and fragile patients in these trials, the actual patient population may not be adequately reflected. Therefore, more clarity about the administration and effects of palliative systemic therapy in daily clinical practice and evidence for the optimal therapeutic approach are needed. In this nationwide study, we aimed to explore first‐line palliative systemic treatment in patients with metastatic esophagogastric cancer and the effect of treatment strategy on survival and toxicity in a real‐world setting.
Materials and Methods
Data collection
Patients with an adenocarcinoma or squamous cell carcinoma of the esophagus, gastroesophageal junction or stomach (classified as C15 and C16 according to the third edition of the International Classification of Diseases for Oncology19) diagnosed with synchronous metastases (T1–4bNallM1) and treated with systemic therapy were identified from the Netherlands Cancer Registry (NCR). The NCR is a population‐based registry that covers the total Dutch population of more than 17 million people and is directly linked to the pathological archive that comprises all histologically confirmed cancer diagnoses. Data on vital status were obtained by annual linkage to the Dutch Personal Records Database.
All esophagogastric cancer patients with synchronous metastases (metastases diagnosed before or within the first 5 days of the first systemic treatment cycle) treated with systemic therapy were included when diagnosed in a subset of Dutch hospitals between 2010 and 2014, and all hospitals in 2015–2016 (Fig. 1). Due to capacity and financial constraints, we were able to collect additional data of approximately 50% of the patients diagnosed in 2010–2014. For this period, we selected 43 of all 80 hospitals as a representative sample of all hospitals in terms of annual number of patients, type of hospital and location in the Netherlands, and included all patients diagnosed in these hospitals between 2010 and 2014. This sample can therefore be considered as adequately reflecting the nationwide patient population and hospitals (Supporting Information Table S1). Patient characteristics and data on treatment and follow‐up were extracted from the hospital's electronical health record system or medical records by specially trained data managers.
Figure 1.
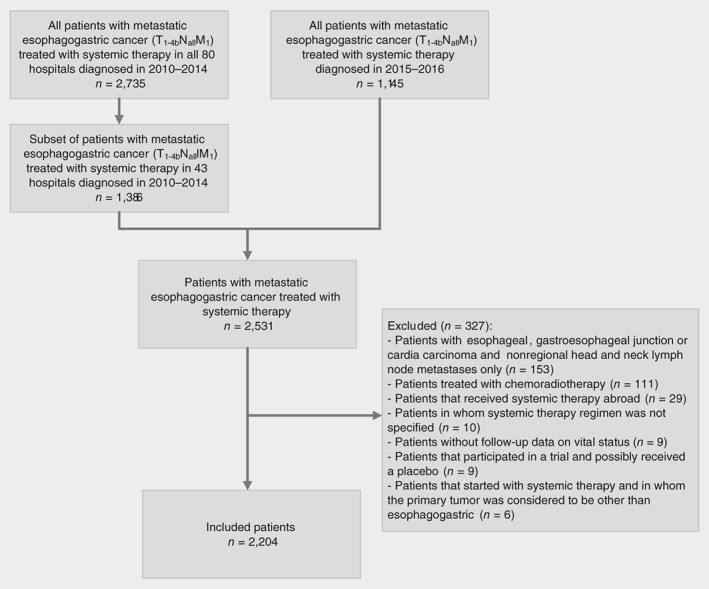
Flowchart of patient selection.
Exclusion
Patients with esophageal, gastroesophageal junction or cardia carcinoma and nonregional lymph node metastases in the head and neck region only (n = 153) were excluded because they could have been eligible for definitive chemoradiotherapy with potential curative intent in cases in which dissemination of metastases was limited to the supraclavicular lymph nodes (Fig. 1).19, 20 Because the exact location of these head and neck lymph node metastases was unknown, we excluded all of these patients. Moreover, patients who received chemoradiotherapy, defined as chemotherapy with concurrent radiotherapy consisting of ≥23 fractions or a total radiation dose of ≥40 Gy, were excluded (n = 111). Patients who received first‐line systemic treatment outside the Netherlands were excluded (n = 29) as were patients without follow‐up data on vital status (n = 9), without information on type of administered systemic therapy regimen (n = 10) or who were included in a trial in which they possibly received a placebo (n = 9). Finally, six patients in whom the primary tumor was first considered to have a different origin than the esophagus or stomach were excluded.
Systemic therapy
First‐line systemic treatment was defined as the first systemic therapy (monotherapy or combination regimen) given until suspension, regardless of reason for discontinuation. A combination regimen was specified as all systemic agents starting within 3 days after the first chemotherapeutic agent started. However, if trastuzumab was added more than 3 days after the start but before the end date of the combination regimen, this was also considered first line (e.g., because of delay in determination of HER2 status). All assumptions regarding first‐line treatment can be found in Supporting Information Table S2.
If the same regimen was restarted after a therapy break, regardless of the duration of this break, this was still considered first line. Continuation of first line was also assumed if one of the agents of the initially started regimen was discontinued and the other agent(s) continued (e.g., capecitabine monotherapy after capecitabine/oxaliplatin [CapOx]), as well as in the case of a switch of a single drug within the same drug group (e.g., 5‐fluorouracil [5‐FU]/oxaliplatin [FOLFOX] to CapOx). If systemic therapy was switched to a regimen containing an agent of a new drug group that was not administered in the first line (e.g., carboplatin/paclitaxel to CapOx) after progression or because of toxicity, or if an agent of a new drug group was added (e.g., oxaliplatin added to 5‐FU), this was considered second‐line treatment.
The systemic therapy strategy was classified into regimens with one, two or three therapeutic agents (monotherapy, doublet therapy and triplet therapy, respectively; all without targeted therapy), trastuzumab‐containing regimens and (nontrastuzumab) targeted therapy‐containing regimens. Subsequently, systemic therapy regimens were subdivided based on the number and type of agents, as described previously6: monotherapy; fluoropyrimidine (F) doublets (with a platinum [but not cisplatin], taxane [T] or irinotecan [I]); cisplatin (C) doublets (with a fluoropyrimidine, taxane or etoposide); gemcitabine (G) doublets (with a platinum/cisplatin); platinum (P; but not cisplatin)/taxane doublets; anthracycline (A) triplets (with a fluoropyrimidine and platinum/cisplatin); taxane triplets (with a fluoropyrimidine and platinum/cisplatin); trastuzumab‐containing regimens; and (nontrastuzumab) targeted therapy‐containing regimens (Supporting Information Fig. S2).
Toxicity
grade 3–5 systemic treatment toxicity according to the Common Terminology Criteria for Adverse Events (CTCAE, version 4.0321) was registered in patients diagnosed between 2010 and 2014. If toxicity was registered but the grade was missing and the patient was not deceased, we considered toxicity as grade 3–4, because grades 1 and 2 were not registered in the NCR.
Overall survival and time to failure of first‐line treatment
Overall survival (OS) was assessed from start of treatment until death or end of follow‐up. Information on vital status was updated until February 1, 2019. Time to failure (TTF) of first‐line treatment was available only in patients with complete follow‐up (i.e., patients diagnosed between 2010 and 2015). TTF was used as a proxy for progression‐free survival and calculated from the start of treatment to the first progression that resulted in termination of the regimen or end of follow‐up. All assumptions regarding TTF are included in Supporting Information Table S1.
Statistical analysis
Patient and tumor characteristics are displayed with counts and percentages, or medians and interquartile ranges (IQRs). Differences between groups were analyzed using chi‐square tests and Fisher's exact tests where appropriate. Kaplan–Meier curves for OS and TTF were compared using the log‐rank test. Multivariable Cox regression analyses were used to identify independently associated treatment strategies with OS and TTF, with adjustment of age, sex, performance status, number of comorbidities, year of diagnosis, tumor location, histology and metastases locations. Values of p < 0.05 were considered statistically significant. Analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC).
Results
Patient characteristics
We included 2,204 patients (Fig. 1), of whom most were male (76%), with a median age of 64 (IQR, 57, 70) years (Table 1). Most patients had a World Health Organisation performance status of 0–1 (55%). Adenocarcinoma was present in 93% of the patients, squamous cell carcinoma in 6% and carcinoma not otherwise specified (NOS) in 1%. Nearly half of the primary tumors were located in the esophagus (46%), followed by noncardia stomach (35%) and gastroesophageal junction or cardia (19%). Most patients had one metastasis location at diagnosis (53%).
Table 1.
Baseline characteristics of all patients subdivided per systemic treatment strategy
| Characteristics | All patients (n = 2,204) | Monotherapy (n = 228) | Doublet (n = 980) | Triplet (n = 758) | Trastuzumab‐containing regimen (n = 215) |
|---|---|---|---|---|---|
| Male, n (%) | 1,670 (75.8%) | 158 (69.3%) | 757 (77.2%) | 564 (74.4%) | 171 (79.5%) |
| Age, years, median (IQR) | 64 (57, 70) | 71 (65, 76) | 64 (57, 70) | 62 (53, 68) | 63 (55, 69) |
| <60 | 741 (33.6%) | 32 (14.0%) | 306 (31.2%) | 311 (41.0%) | 81 (37.7%) |
| 60–69 | 856 (38.8%) | 68 (29.8%) | 404 (41.2%) | 292 (38.5%) | 84 (39.1%) |
| 70–79 | 566 (25.7%) | 110 (48.2%) | 251 (25.6%) | 152 (20.1%) | 49 (22.8%) |
| ≥80 | 41 (1.9%) | 18 (7.9%) | 19 (1.9%) | 3 (0.4%) | 1 (0.5%) |
| BMI, kg/m2, median (IQR) | 24.7 (22.5, 27.7) | 24.2 (21.4, 27.1) | 25.0 (22.5, 27.8) | 24.8 (22.6, 27.7) | 24.4 (22.3, 27.7) |
| <18.5 (underweight) | 57 (2.6%) | 8 (3.5%) | 26 (2.7%) | 20 (2.6%) | 3 (1.4%) |
| 18.5–25 (normal weight) | 829 (37.6%) | 107 (46.9%) | 325 (33.2%) | 311 (41.0%) | 78 (36.3%) |
| >25 (overweight) | 779 (35.3%) | 73 (32.0%) | 337 (34.4%) | 299 (39.4%) | 59 (27.4%) |
| Unknown | 539 (24.5%) | 40 (17.5%) | 292 (29.8%) | 128 (16.9%) | 75 (34.9%) |
| Performance status, n (%) | |||||
| 0 or 1 | 1,220 (55.4%) | 104 (45.6%) | 549 (56.0%) | 406 (53.6%) | 143 (66.5%) |
| ≥2 | 152 (6.9%) | 33 (14.5%) | 75 (7.7%) | 33 (4.4%) | 10 (4.7%) |
| Unknown | 832 (37.7%) | 91 (39.9%) | 356 (36.3%) | 319 (42.1%) | 62 (28.8%) |
| Comorbidities, n (%) | |||||
| 0 | 804 (36.5%) | 61 (26.8%) | 346 (35.3%) | 311 (41.0%) | 77 (35.8%) |
| 1 | 621 (28.2%) | 69 (30.3%) | 271 (27.7%) | 214 (28.2%) | 63 (29.3%) |
| ≥2 | 702 (31.9%) | 94 (41.2%) | 326 (33.3%) | 207 (27.3%) | 65 (30.2%) |
| Unknown | 77 (3.5%) | 4 (1.8%) | 37 (3.8%) | 26 (3.4%) | 10 (4.7%) |
| Tumor location, n (%) | |||||
| Esophagus | 1,014 (46.0%) | 66 (28.9%) | 579 (59.1%) | 241 (31.8%) | 116 (54.0%) |
| Gastroesophageal junction or cardia | 410 (18.6%) | 47 (20.6%) | 148 (15.1%) | 169 (22.3%) | 41 (19.1%) |
| Stomach | 780 (35.4%) | 115 (50.4%) | 253 (25.8%) | 348 (45.9%) | 58 (27.0%) |
| Histology, n (%) | |||||
| Adenocarcinoma | 2,056 (93.3%) | 221 (96.9%) | 858 (87.6%) | 739 (97.5%) | 215 (100.0%) |
| Squamous cell carcinoma | 128 (5.8%) | 6 (2.6%) | 107 (10.9%) | 15 (2.0%) | 0 |
| Carcinoma NOS | 20 (0.9%) | 1 (0.4%) | 15 (1.5%) | 4 (0.5%) | 0 |
| cT stage, n (%) | |||||
| cT1–cT3 | 1,200 (54.4%) | 111 (48.7%) | 543 (55.4%) | 388 (51.2%) | 138 (64.2%) |
| cT4 | 206 (9.3%) | 26 (11.4%) | 79 (8.1%) | 88 (11.6%) | 12 (5.6%) |
| cTx | 798 (36.3%) | 91 (39.9%) | 358 (36.5%) | 282 (37.2%) | 65 (30.2%) |
| cN stage, n (%) | |||||
| cN0 | 342 (15.5%) | 45 (19.7%) | 145 (14.8%) | 128 (16.9%) | 21 (9.8%) |
| cN1–cN2 | 1,474 (66.9%) | 141 (61.8%) | 659 (67.2%) | 500 (66.0%) | 160 (74.4%) |
| cN3 | 192 (8.7%) | 14 (6.1%) | 102 (10.4%) | 53 (7.0%) | 20 (9.3%) |
| cNx | 196 (8.9%) | 28 (12.3%) | 74 (7.6%) | 77 (10.2%) | 14 (6.5%) |
| Histologic grade, n (%) | |||||
| Well differentiated | 34 (1.5%) | 2 (0.9%) | 19 (1.9%) | 7 (0.9%) | 6 (2.8%) |
| Moderately differentiated | 400 (18.1%) | 29 (12.7%) | 179 (18.3%) | 127 (16.8%) | 61 (28.4%) |
| Poorly differentiated | 928 (42.1%) | 86 (37.7%) | 410 (41.8%) | 352 (46.4%) | 68 (31.6%) |
| Unknown | 842 (38.2%) | 111 (48.7%) | 372 (38.0%) | 272 (35.9%) | 80 (37.2%) |
| Metastatic sites, n (%) | |||||
| 1 | 1,172 (53.2%) | 131 (57.5%) | 517 (52.8%) | 423 (55.8%) | 94 (43.7%) |
| ≥2 | 1,032 (46.8%) | 97 (42.5%) | 463 (47.2%) | 335 (44.2%) | 121 (56.3%) |
| Location metastases, n (%)1 | |||||
| Liver | 1,169 (53.0%) | 120 (52.6%) | 519 (53.0%) | 375 (49.5%) | 142 (66.0%) |
| Distant lymph nodes | 890 (40.4%) | 93 (40.8%) | 403 (41.1%) | 287 (37.9%) | 94 (43.7%) |
| Peritoneum | 524 (23.8%) | 62 (27.2%) | 191 (19.5%) | 235 (31.0%) | 30 (14.0%) |
| Lung | 430 (19.5%) | 41 (18.0%) | 195 (19.9%) | 123 (16.2%) | 66 (30.7%) |
| Other | 499 (22.6%) | 39 (17.1%) | 236 (24.1%) | 163 (21.5%) | 51 (23.7%) |
Baseline characteristics of all patients, divided per systemic therapy regimen. Characteristics of patients who received targeted (nontrastuzumab) therapy (n = 23) were not displayed as a subgroup.
More than one location per patient possible; percentages do not add up to 100.
Abbreviations: IQR, interquartile range; BMI, body mass index; NOS, not otherwise specified; cT stage, clinical tumor stage; cN status, clinical lymph node stage.
First‐line systemic treatment regimens and strategies
A total of 45 different first‐line systemic therapy regimens were administered (Supporting Information Fig. S1). The most commonly administered regimen was CapOx (21%), followed by epirubicin, oxaliplatin and capecitabine (EOX; 20%), carboplatin and paclitaxel (13%), epirubicin, cisplatin and capecitabine (ECC; 10%) and capecitabine monotherapy (9%; Supporting Information Table S3). Most patients received doublet chemotherapy (45%), followed by triplet chemotherapy (34%), monotherapy (10%), trastuzumab‐containing regimens (10%) and nontrastuzumab targeted therapy‐containing regimens (1%). The latter group was not displayed as a subgroup in Table 1, and not included in the Kaplan–Meier curves because of the limited number of patients.
All but one patient treated with a trastuzumab‐containing regimen had a HER2‐positive tumor. One patient received trastuzumab monotherapy; all other patients received trastuzumab with chemotherapy. Doublet chemotherapy backbones were used in the majority of the patients (n = 167), of which CapOx (n = 73) and capecitabine/cisplatin (n = 65) were administered most often.
Survival
The median OS was 7.5 (IQR, 3.7, 12.9) months. In 1,700 patients, diagnosed between 2010 and 2015 with complete follow‐up, the median TTF of first‐line systemic treatment was 4.6 (IQR, 2.0, 7.9) months.
Monotherapy resulted in lower survival rates compared to all other treatment strategies in univariable and multivariable analyses (Figs. 2 and 3; Table 2a). The OS and TTF of patients treated with doublet therapy did not differ from patients treated with triplets after adjustment for confounding (OS: adjusted hazard ratio [HR] 0.92, 95% confidence interval [CI] 0.83–1.02; TTF: HR 0.92, 95% CI 0.82–1.04).
Figure 2.
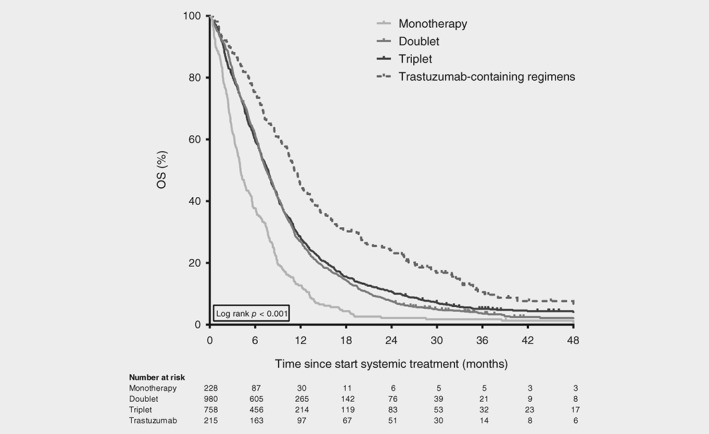
Overall survival of synchronous metastatic esophagogastric cancer patients. Kaplan–Meier curves displaying overall survival in patients treated with one, two or three chemotherapeutic agents (monotherapy, doublet and triplet, respectively) and in patients treated with a trastuzumab‐containing regimen, diagnosed between 2010 and 2016 (n = 1,981). Survival curve of patients treated with a regimen containing (nontrastuzumab) targeted therapy (n = 23) is not displayed.
Figure 3.
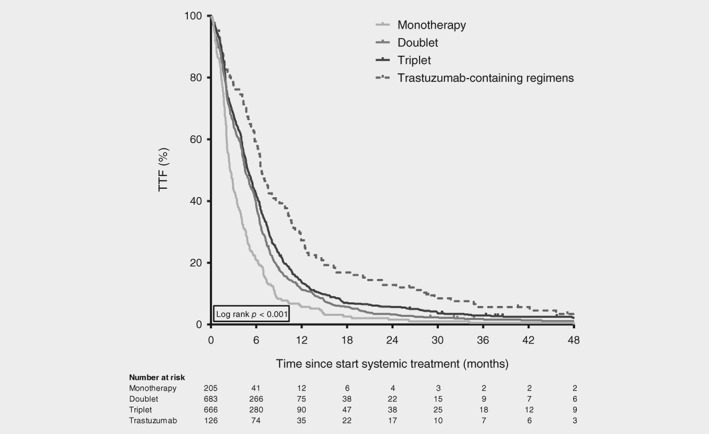
Time to failure of first‐line therapy in synchronous metastatic esophagogastric cancer patients. Kaplan–Meier curves displaying time to failure of first‐line treatment in patients treated with one, two or three chemotherapeutic agents (monotherapy, doublet and triplet, respectively) and in patients treated with a trastuzumab‐containing regimen, diagnosed between 2010 and 2015 (n = 1,680). Survival curve of patients treated with a regimen containing (nontrastuzumab) targeted therapy (n = 20) is not displayed.
Table 2a.
Cox regression analyses for overall survival and time to failure of first‐line treatment per systemic treatment strategy
| Overall survival (n = 2,204) | Time to failure of first‐line treatment (n = 1,700) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients No. | Median OS (months) | Univariable analyses | Multivariable analyses | Patients No. | Median TTF (months) | Univariable analyses | Multivariable analyses | |||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |||||
| Systemic treatment strategy | ||||||||||||||||
| Monotherapy | 228 | 4.1 | 1.71 | 1.48–1.98 | <0.001 | 1.67 | 1.43–1.96 | <0.001 | 205 | 2.5 | 1.51 | 1.29–1.77 | <0.001 | 1.65 | 1.39–1.96 | <0.001 |
| Doublet | 980 | 7.4 | Ref | Ref | 683 | 4.5 | Ref | Ref | ||||||||
| Triplet | 758 | 7.7 | 0.94 | 0.85–1.03 | 0.188 | 0.92 | 0.83–1.02 | 0.110 | 666 | 4.8 | 0.89 | 0.79–0.99 | 0.027 | 0.92 | 0.82–1.04 | 0.179 |
| Trastuzumab‐containing regimen | 215 | 11.2 | 0.62 | 0.53–0.72 | <0.001 | 0.63 | 0.54–0.74 | <0.001 | 126 | 6.7 | 0.62 | 0.51–0.76 | <0.001 | 0.62 | 0.51–0.76 | <0.001 |
| Targeted therapy‐containing regimen (nontrastuzumab) | 23 | 11.9 | 0.73 | 0.48–1.11 | 0.142 | 0.67 | 0.44–1.03 | 0.068 | 20 | 9.2 | 0.54 | 0.35–0.86 | 0.009 | 0.53 | 0.33–0.83 | 0.006 |
| Age, years | ||||||||||||||||
| <60 | 741 | 7.8 | Ref | Ref | 581 | 4.8 | Ref | Ref | ||||||||
| 60–69 | 856 | 7.5 | 1.03 | 0.93–1.14 | 0.542 | 1.01 | 0.91–1.12 | 0.901 | 669 | 4.6 | 0.99 | 0.89–1.11 | 0.904 | 0.95 | 0.85–1.07 | 0.432 |
| 70–79 | 566 | 7.0 | 1.06 | 0.95–1.19 | 0.280 | 1.01 | 0.89–1.14 | 0.893 | 417 | 4.3 | 1.00 | 0.88–1.13 | 0.937 | 0.92 | 0.80–1.06 | 0.227 |
| ≥80 | 41 | 6.5 | 1.26 | 0.92–1.74 | 0.153 | 1.03 | 0.74–1.43 | 0.873 | 33 | 4.1 | 1.19 | 0.84–1.69 | 0.330 | 0.94 | 0.65–1.35 | 0.721 |
| Sex | ||||||||||||||||
| Male | 1,670 | 7.5 | Ref | Ref | 1,290 | 4.6 | Ref | Ref | ||||||||
| Female | 534 | 7.5 | 1.02 | 0.93–1.13 | 0.645 | 0.92 | 0.83–1.03 | 0.135 | 410 | 4.6 | 1.01 | 0.90–1.13 | 0.909 | 0.93 | 0.83–1.05 | 0.251 |
| Performance status | ||||||||||||||||
| 0 or 1 | 1,220 | 8.3 | Ref | Ref | 902 | 4.8 | Ref | Ref | ||||||||
| ≥2 | 152 | 4.7 | 1.73 | 1.46–2.06 | <0.001 | 1.61 | 1.36–1.92 | <0.001 | 114 | 2.9 | 1.53 | 1.26–1.87 | <0.001 | 1.39 | 1.14–1.70 | 0.001 |
| Unknown | 832 | 6.8 | 1.20 | 1.09–1.31 | <0.001 | 1.16 | 1.06–1.27 | 0.002 | 684 | 4.3 | 1.14 | 1.03–1.26 | 0.011 | 1.14 | 1.02–1.26 | 0.016 |
| Comorbidities | ||||||||||||||||
| 0 | 805 | 7.6 | Ref | Ref | 652 | 4.8 | Ref | Ref | ||||||||
| 1 | 621 | 7.0 | 0.94 | 0.84–1.04 | 0.233 | 0.94 | 0.84–1.05 | 0.272 | 475 | 4.1 | 0.95 | 0.85–1.08 | 0.442 | 0.97 | 0.85–1.10 | 0.600 |
| ≥2 | 702 | 7.6 | 1.00 | 0.90–1.11 | 0.975 | 0.96 | 0.86–1.07 | 0.460 | 538 | 4.7 | 0.97 | 0.87–1.09 | 0.654 | 0.95 | 0.84–1.07 | 0.414 |
| Unknown | 76 | 10.5 | 0.69 | 0.54–0.88 | 0.003 | 0.70 | 0.54–0.89 | 0.004 | 35 | 6.2 | 0.77 | 0.55–1.09 | 0.140 | 0.74 | 0.52–1.05 | 0.088 |
| Tumor location | ||||||||||||||||
| Esophagus | 1,014 | 7.8 | Ref | Ref | 772 | 4.6 | Ref | Ref | ||||||||
| Gastroesophageal junction or cardia | 410 | 7.6 | 0.95 | 0.85–1.07 | 0.395 | 0.95 | 0.84–1.08 | 0.417 | 316 | 5.0 | 0.90 | 0.79–1.03 | 0.131 | 0.92 | 0.80–1.06 | 0.268 |
| Stomach | 780 | 6.9 | 1.08 | 0.98–1.18 | 0.132 | 1.02 | 0.91–1.15 | 0.698 | 612 | 4.4 | 0.98 | 0.88–1.09 | 0.691 | 0.98 | 0.85–1.12 | 0.729 |
| Histology | ||||||||||||||||
| Adenocarcinoma | 2,056 | 7.6 | Ref | Ref | 1,580 | 3.7 | Ref | Ref | ||||||||
| Squamous cell carcinoma | 128 | 6.5 | 1.24 | 1.03–1.48 | 0.021 | 1.22 | 1.01–1.48 | 0.040 | 104 | 4.7 | 1.40 | 1.15–1.71 | 0.001 | 1.13 | 1.08–1.67 | 0.008 |
| Carcinoma NOS | 20 | 4.6 | 1.54 | 0.99–2.40 | 0.054 | 1.44 | 0.92–2.25 | 0.112 | 16 | 3.1 | 1.27 | 0.77–2.07 | 0.347 | 1.03 | 0.73–2.00 | 0.452 |
| Liver metastasis | 1,169 | 7.4 | 0.98 | 0.90–1.07 | 0.628 | 1.17 | 1.06–1.29 | 0.002 | 920 | 4.6 | 1.00 | 0.90–1.10 | 0.943 | 1.22 | 1.01–1.26 | 0.041 |
| Distant lymph node metastasis | 890 | 7.2 | 1.06 | 0.97–1.15 | 0.226 | 1.17 | 1.07–1.29 | 0.001 | 684 | 4.5 | 0.97 | 0.88–1.07 | 0.571 | 1.16 | 0.92–1.14 | 0.620 |
| Peritoneal metastasis | 524 | 6.9 | 1.22 | 1.11–1.35 | <0.001 | 1.42 | 1.25–1.61 | <0.001 | 385 | 4.1 | 1.11 | 0.99–1.24 | 0.079 | 1.31 | 1.05–1.41 | 0.009 |
| Lung metastasis | 430 | 7.0 | 1.09 | 0.98–1.21 | 0.122 | 1.16 | 1.04–1.29 | 0.010 | 327 | 4.1 | 1.14 | 1.01–1.29 | 0.032 | 1.13 | 1.02–1.32 | 0.021 |
| Other metastases locations | 499 | 6.6 | 1.25 | 1.13–1.39 | <0.001 | 1.35 | 1.22–1.50 | <0.001 | 379 | 4.1 | 1.23 | 1.09–1.38 | <0.001 | 1.16–1.47 | <0.001 | |
| Year of diagnosis | 0.95 | 0.93–0.97 | <0.001 | 0.97 | 0.95–0.99 | 0.009 | 1.00 | 0.97–1.02 | 0.724 | 1.02 | 0.99–1.05 | 0.230 | ||||
Cox regression analyses in patients diagnosed between 2010 and 2016 for overall survival and patients between 2010 and 2015 for time to failure of first‐line treatment. Both univariable and multivariable analyses are displayed for first‐line systemic therapy subdivided in strategies (Table 2a) as well as regimens (Table 2b). Hazard ratios were adjusted for age, sex, performance status, number of comorbidities, tumor location, histology, metastases locations and year of diagnosis. Systemic treatment strategies were divided in chemotherapy regimens (monotherapy, doublet and triplet), trastuzumab‐containing regimens and nontrastuzumab targeted therapy‐containing regimens. Systemic treatment regimens were dived as follows: monotherapy; fluoropyrimidine doublets (with a platinum [but not cisplatin], taxane or irinotecan); cisplatin doublets (with a fluoropyrimidine, taxane or etoposide); gemcitabine doublets (with a platinum/cisplatin); platinum (but not cisplatin)/taxane doublets; anthracycline triplets (with a fluoropyrimidine and platinum/cisplatin); taxane triplets (with a fluoropyrimidine and platinum/cisplatin); trastuzumab‐containing regimens; and (nontrastuzumab) targeted therapy‐containing regimens.
Abbreviations: A, anthracycline; C, cisplatin; CI, confidence interval; E, etoposide; F, fluoropyrimidine (capecitabine or 5‐FU); G, gemcitabine; HR, hazard ratio; I, irinotecan; NOS, not otherwise specified; OS, overall survival; P, platinum compound (oxaliplatin or carboplatin); T, taxane; TTF, time to failure.
Neither cisplatin, gemcitabine or platinum–taxane doublets nor anthracycline triplets showed survival benefit over fluoropyrimidine doublets in multivariable analyses (Table 2b). OS and TTF of taxane triplets were significantly better than in fluoropyrimidine doublets (HR 0.63, 95% CI 0.46–0.86; HR 0.67, 95% CI 0.45–1.00). Both trastuzumab‐ and targeted therapy‐containing regimens showed significantly better OS and TTF than fluoropyrimidine doublets as well.
Table 2b.
Cox regression analyses for overall survival and time to failure of first‐line treatment per systemic treatment regimen
| Overall survival (n = 2,204) | Time to failure of first‐line treatment (n = 1,700) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients No. | Median OS (months) | Univariable analyses | Multivariable analyses | Patients No. | Median TTF (months) | Univariable analyses | Multivariable analyses | |||||||||
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |||||
| Systemic therapy regimen | ||||||||||||||||
| Monotherapy | 228 | 4.1 | 1.72 | 1.47–2.00 | <0.001 | 1.68 | 1.42–1.98 | <0.001 | 205 | 2.5 | 1.51 | 1.27–1.79 | <0.001 | 1.63 | 1.35–1.96 | <0.001 |
| F‐doublet (FP, FT, FI) | 611 | 7.3 | Ref | Ref | 369 | 4.4 | Ref | Ref | ||||||||
| C‐doublet (CF, CT, CE) | 26 | 6.7 | 0.97 | 0.65–1.47 | 0.901 | 0.80 | 0.52–1.24 | 0.313 | 20 | 4.2 | 1.05 | 0.67–1.65 | 0.833 | 0.86 | 0.52–1.41 | 0.541 |
| G‐doublet (GP, GC) | 50 | 4.7 | 1.84 | 1.38–2.45 | <0.001 | 1.67 | 1.24–2.26 | <0.001 | 46 | 3.0 | 1.71 | 1.26–2.33 | <0.001 | 1.65 | 1.20–2.27 | 0.002 |
| PT‐doublet | 293 | 8.2 | 0.93 | 0.81–1.08 | 0.342 | 0.88 | 0.76–1.03 | 0.115 | 248 | 5.5 | 0.91 | 0.78–1.07 | 0.272 | 0.86 | 0.72–1.03 | 0.093 |
| A‐triplet (ACF, AFOx) | 708 | 7.4 | 0.97 | 0.87–1.09 | 0.620 | 0.94 | 0.83–1.05 | 0.271 | 638 | 4.8 | 0.89 | 0.78–1.02 | 0.085 | 0.91 | 0.80–1.05 | 0.197 |
| T‐triplet (TCF, FOxT) | 50 | 11.8 | 0.61 | 0.45–0.82 | 0.001 | 0.63 | 0.46–0.86 | 0.003 | 28 | 6.0 | 0.67 | 0.45–0.99 | 0.047 | 0.67 | 0.45–1.00 | 0.047 |
| Trastuzumab‐containing regimen | 215 | 11.2 | 0.62 | 0.53–0.73 | <0.001 | 0.62 | 0.52–0.73 | <0.001 | 126 | 6.7 | 0.62 | 0.50–0.76 | <0.001 | 0.60 | 0.49–0.75 | <0.001 |
| Targeted therapy‐containing regimen (nontrastuzumab) | 23 | 11.9 | 0.73 | 0.48–1.12 | 0.145 | 0.66 | 0.43–1.02 | 0.059 | 20 | 9.2 | 0.54 | 0.34–0.86 | 0.009 | 0.51 | 0.32–0.81 | 0.005 |
| Age, years | ||||||||||||||||
| <60 | 741 | 7.8 | Ref | Ref | 581 | 4.8 | Ref | Ref | ||||||||
| 60–69 | 856 | 7.5 | 1.03 | 0.93–1.14 | 0.542 | 1.01 | 0.91–1.12 | 0.842 | 669 | 4.6 | 0.99 | 0.89–1.11 | 0.904 | 0.96 | 0.86–1.09 | 0.551 |
| 70–79 | 566 | 7.0 | 1.06 | 0.95–1.19 | 0.280 | 1.00 | 0.89–1.13 | 0.991 | 417 | 4.3 | 1.00 | 0.88–1.13 | 0.937 | 0.92 | 0.80–1.06 | 0.236 |
| ≥80 | 41 | 6.5 | 1.26 | 0.92–1.74 | 0.153 | 0.99 | 0.71–1.38 | 0.933 | 33 | 4.1 | 1.19 | 0.84–1.69 | 0.330 | 0.92 | 0.64–1.32 | 0.646 |
| Sex | ||||||||||||||||
| Male | 1,670 | 7.5 | Ref | Ref | 1,290 | 4.6 | Ref | Ref | ||||||||
| Female | 534 | 7.5 | 1.02 | 0.93–1.13 | 0.645 | 0.91 | 0.82–1.02 | 0.093 | 410 | 4.6 | 1.01 | 0.90–1.13 | 0.909 | 0.93 | 0.83–1.05 | 0.270 |
| Performance status | ||||||||||||||||
| 0 or 1 | 1,220 | 8.3 | Ref | Ref | 902 | 4.8 | Ref | Ref | ||||||||
| ≥2 | 152 | 4.7 | 1.73 | 1.46–2.06 | <0.001 | 1.61 | 1.35–1.91 | <0.001 | 114 | 2.9 | 1.53 | 1.26–1.87 | <0.001 | 1.37 | 1.12–1.68 | 0.002 |
| Unknown | 832 | 6.8 | 1.20 | 1.09–1.31 | <0.001 | 1.17 | 1.06–1.28 | 0.001 | 684 | 4.3 | 1.14 | 1.03–1.26 | 0.011 | 1.15 | 1.04–1.28 | 0.010 |
| Comorbidities | ||||||||||||||||
| 0 | 805 | 7.6 | Ref | Ref | 652 | 4.8 | Ref | Ref | ||||||||
| 1 | 621 | 7.0 | 0.94 | 0.84–1.04 | 0.233 | 0.94 | 0.84–1.04 | 0.227 | 475 | 4.1 | 0.95 | 0.85–1.08 | 0.442 | 0.96 | 0.85–1.09 | 0.542 |
| ≥2 | 702 | 7.6 | 1.00 | 0.90–1.11 | 0.975 | 0.96 | 0.86–1.07 | 0.442 | 538 | 4.7 | 0.97 | 0.87–1.09 | 0.654 | 0.95 | 0.84–1.07 | 0.371 |
| Unknown | 76 | 10.5 | 0.69 | 0.54–0.88 | 0.003 | 0.68 | 0.53–0.88 | 0.003 | 35 | 6.2 | 0.77 | 0.55–1.09 | 0.140 | 0.74 | 0.52–1.05 | 0.091 |
| Tumor location | ||||||||||||||||
| Esophagus | 1,014 | 7.8 | Ref | Ref | 772 | 4.6 | Ref | Ref | ||||||||
| Gastroesophageal junction or cardia | 410 | 7.6 | 0.95 | 0.85–1.07 | 0.395 | 0.93 | 0.82–1.05 | 0.252 | 316 | 5.0 | 0.90 | 0.79–1.03 | 0.131 | 0.90 | 0.78–1.04 | 0.158 |
| Stomach | 780 | 6.9 | 1.08 | 0.98–1.18 | 0.132 | 1.00 | 0.89–1.13 | 0.995 | 612 | 4.4 | 0.98 | 0.88–1.09 | 0.691 | 0.94 | 0.82–1.08 | 0.410 |
| Histology | ||||||||||||||||
| Adenocarcinoma | 2,056 | 7.6 | Ref | Ref | 1,580 | 3.7 | Ref | Ref | ||||||||
| Squamous cell carcinoma | 128 | 6.5 | 1.24 | 1.03–1.48 | 0.021 | 1.24 | 1.02–1.51 | 0.032 | 104 | 4.7 | 1.40 | 1.15–1.71 | 0.001 | 1.35 | 1.08–1.69 | 0.009 |
| Carcinoma NOS | 20 | 4.6 | 1.54 | 0.99–2.40 | 0.054 | 1.59 | 0.99–2.53 | 0.053 | 16 | 3.1 | 1.27 | 0.77–2.07 | 0.347 | 1.30 | 0.76–2.20 | 0.337 |
| Liver metastasis | 1,169 | 7.4 | 0.98 | 0.90–1.07 | 0.628 | 1.17 | 1.06–1.29 | 0.002 | 920 | 4.6 | 1.00 | 0.90–1.10 | 0.943 | 1.13 | 1.01–1.26 | 0.035 |
| Distant lymph node metastasis | 890 | 7.2 | 1.06 | 0.97–1.15 | 0.226 | 1.16 | 1.06–1.28 | 0.002 | 684 | 4.5 | 0.97 | 0.88–1.07 | 0.571 | 1.02 | 0.92–1.13 | 0.720 |
| Peritoneal metastasis | 524 | 6.9 | 1.22 | 1.11–1.35 | <0.001 | 1.42 | 1.25–1.61 | <0.001 | 385 | 4.1 | 1.11 | 0.99–1.24 | 0.079 | 1.23 | 1.06–1.42 | 0.006 |
| Lung metastasis | 430 | 7.0 | 1.09 | 0.98–1.21 | 0.122 | 1.15 | 1.03–1.29 | 0.015 | 327 | 4.1 | 1.14 | 1.01–1.29 | 0.032 | 1.16 | 1.02–1.31 | 0.024 |
| Other metastases locations | 499 | 6.6 | 1.25 | 1.13–1.39 | <0.001 | 1.35 | 1.21–1.50 | <0.001 | 379 | 4.1 | 1.23 | 1.09–1.38 | <0.001 | 1.30 | 1.15–1.47 | <0.001 |
| Year of diagnosis | 0.95 | 0.93–0.97 | <0.001 | 0.98 | 0.96–1.00 | 0.058 | 1.00 | 0.97–1.02 | 0.724 | 1.02 | 0.99–1.05 | 0.136 | ||||
Of note, if we performed a predictive model and added only add variables with p < 0.1 on univariable analysis, this did not influence statistically significance of the hazard ratios of systemic therapy strategies or regimens in the multivariable models.
Toxicity
Of 1,221 patients diagnosed in 2010–2014, systemic treatment toxicity grade 3–5 was reported in 27% (Table 3). Trastuzumab‐containing regimens induced the highest complication rate (45%), followed by triplets (33%), doublets (21%) and monotherapy (17%). The complication rate differed significantly between the four subgroups (p < 0.001).
Table 3.
Grade 3–5 toxicity in patients treated with monotherapy, doublet chemotherapy and triplet chemotherapy and patients who received a trastuzumab‐containing regimen between 2010 and 2014
| Grade 3–5 toxicity | Patients, n (%) | p value1 | ||||
|---|---|---|---|---|---|---|
| All patients (n = 1,221) | Monotherapy (n = 164) | Doublet (n = 455) | Triplet (n = 511) | Trastuzumab‐containing regimens (n = 71) | ||
| Patients without adverse events | 801 (65.6%) | 115 (70.1%) | 327 (71.9%) | 313 (61.3%) | 36 (50.7%) | <0.001 |
| Patients with adverse events | 332 (27.2%) | 28 (17.1%) | 97 (21.3%) | 166 (32.5%) | 32 (45.1%) | |
| Grade 3–4 | 314 | 26 | 92 | 159 | 29 | |
| Grade 5 | 18 | 2 | 5 | 7 | 3 | |
| Unknown | 88 (7.2%) | 21 (12.8%) | 31 (6.8%) | 32 (6.3%) | 3 (4.2%) | |
| Events, n | ||||||
| Number of adverse events | 486 | 36 | 135 | 245 | 54 | |
Chi‐square test: adverse event rate monotherapy vs. doublet vs. triplet vs. trastuzumab‐containing regimens. Toxicity of patients who received targeted (nontrastuzumab) therapy (n = 20) was not displayed separately.
Of 486 reported adverse events, the most common causes were gastrointestinal complications (43%), followed by blood and lymphatic system disorders, including infections (21%), general disorders (fatigue, pain) and administration site conditions (7%), cardiovascular (6%) and metabolism and nutrition disorders (5%).
Eighteen patients died due to complications of systemic therapy, of whom 7 were treated with a triplet, 5 with a doublet, 2 with monotherapy and 3 with a trastuzumab‐containing regimen. Causes of death were blood and lymphatic system (n = 7), cardiovascular (n = 6) and gastrointestinal (n = 5) disorders.
Discussion
In this nationwide cohort of 2,204 synchronous metastatic esophagogastric cancer patients, we found a strikingly wide variation of 45 different systemic therapy regimens that were administered between 2010 and 2016. This heterogeneity in treatment is undesirable, especially in case of unconventional treatment combinations, since second‐line treatment options are often registered under the assumption that certain compounds have been administered in the first line. The use of an unusual treatment regimen may limit opportunities for second‐line treatment and subsequent OS benefit. Analysis of beyond first‐line treatments is currently ongoing.
Current national and international guidelines recommend a fluoropyrimidine and platinum doublet in metastatic esophagogastric cancer patients, with the addition of an anthracycline or taxane in selected patients.8, 10, 12, 13, 22 Until 2016, Dutch esophageal and gastric cancer guidelines advised systemic therapy only in patients with good performance status, without specifying the type of regimen.23, 24 This could have contributed to the heterogeneity in administered systemic therapy regimens. Another explanation for the variation could be that palliative treatment of esophagogastric cancer is not centralized in specialized hospitals in the Netherlands, in contrast to curative treatment.25, 26
Since the added value of the addition of an anthracycline to a platinum–fluoropyrimidine doublet remains uncertain,15, 27, 28, 29 doublet chemotherapy tends to be the favored choice of first‐line palliative treatment because of its better tolerance.4, 5, 6, 14 In our study, we found less serious (grade 3–5) toxicity in patients receiving doublets (21%) compared to triplets (33%) as well as similar OS and TTF rates, which supports the shift toward doublet therapy as preferred strategy in these patients.
Taxane triplets showed superior OS and TTF compared to fluoropyrimidine doublets. From previous randomized studies, it is known that this increased effectiveness comes at the cost of more toxicity.6 However, because of the limited number of patients who received a taxane triplet, definite conclusions from this real‐world population cannot be drawn. In the curative setting, docetaxel, oxaliplatin and 5‐FU/leucovorin (FLOT) showed longer survival in gastric cancer when used as a perioperative regimen as compared to anthracycline triplets.30 The use of FLOT followed by resection with curative intent in patients with limited metastatic disease is currently being explored in the AIO‐FLOT5 trial.31 However, in the palliative setting, it remains inconclusive whether first‐line taxane triplets or fluoropyrimidine doublets followed by second‐line taxanes should be preferred in view of survival benefit and toxicity.6, 11
Monotherapy showed a significantly worse OS compared to doublets, which is in line with recently published reviews.4, 5, 6 In addition, grade 3–5 toxicity rate was only marginally lower compared to doublets (18% vs. 21%, respectively). This could partly be caused by selection bias, since patients treated with monotherapy are more likely to have a poorer performance status. However, reported HRs were adjusted for both performance status and number of comorbidities. The use of no systemic treatment instead of monotherapy should therefore be considered in patients who potentially do not tolerate doublet therapy, since the median OS is comparable to that of patients who receive best supportive care only.4, 5
A relatively high rate of grade 3–5 toxicity (45%) was seen in patients who received trastuzumab‐containing regimens. In the ToGA trial, trastuzumab did not induce more toxicity compared to chemotherapy only.11 We did not observe the expected increase in cardiovascular toxicity due to trastuzumab. Possibly, the cytotoxic backbone induced the toxicity, since a toxicity rate of 56% was observed in patients who received a triplet backbone, compared to 43% with a doublet backbone. Moreover, lower toxicity rates were found in doublet backbones containing oxaliplatin (33%) compared to cisplatin‐containing doublet backbones (48%), which confirms previously described findings.32
Population‐based data represent a wide variation of patients, including frail patients and patients with comorbidity who are usually not included in conventional clinical trials. Real‐world evidence, if well analyzed and interpreted, is therefore highly potent in efficiently adding information about systemic treatment, alongside the results of these trials.33
We are aware that our study has possible limitations. Although the data have been checked and improved regularly, there could still have been some errors due to misinterpretations by data managers or inadequate reporting by physicians. Because of incomplete medical records, some variables were missing, which may have impaired adjustment for possible confounding. Furthermore, patients with solely head and neck lymph node metastases were excluded, because treatment could have consisted of definitive chemoradiotherapy with curative intent in the case of only positive supraclavicular lymph nodes, as well as patients who had long‐term radiotherapy alongside systemic treatment, since radiotherapy could affect survival rates.20, 34 Nevertheless, the vast majority of the metastatic esophagogastric cancer patient population who received systemic treatment is represented.
Our population‐level findings support doublet chemotherapy as the preferred first‐line treatment strategy in terms of survival rates and toxicity. A trastuzumab‐containing regimen should be considered in patients with HER2 overexpression. Future studies comparing first‐line palliative (doublet) treatment strategies, such as the LyRICX study (NCT03764553), should also focus on quality of life, since this is an important outcome in these patients. Moreover, possible predictive and prognostic characteristics that influence treatment outcomes should be taken into account to improve patient selection and personalize treatment strategies.35
In conclusion, in this nationwide study including real‐world evidence in first‐line systemic treatment of patients with synchronous metastatic esophagogastric cancer, doublet chemotherapy was associated with equal survival rates compared to triplet chemotherapy with a better toxicity profile. Patients treated with a trastuzumab‐containing regimen had the best survival. A remarkable heterogeneity of 45 different systemic therapy regimens was observed, which is undesirable since it may negatively affect outcomes in these patients.
Ethical statement
According to the Central Committee on Research involving Human Subjects, this type of study does not require approval from an ethics committee in the Netherlands. The study was approved by the Privacy Review Board of the NCR and the scientific committee of the Dutch Upper GI Cancer Group.
Supporting information
Table S1 Baseline characteristics of all and selected metastatic esophagogastric cancer patients treated with systemic therapy and diagnosed between 2010 and 2014.
Table S2 List of assumptions regarding definitions of first‐line systemic treatment and time to failure of first‐line treatment.
Table S3 Most frequently administered systemic therapy regimens.
Figure S1 Word cloud of all 45 systemic therapy regimens that were administered. Font size of the word corresponds to the number of patients who received the regimen.
Figure S2 Subdivision of systemic therapy regimens. Systemic treatment regimens were dived as follows: monotherapy; fluoropyrimidine doublets (with a platinum [but not cisplatin], taxane, or irinotecan); cisplatin doublets (with a fluoropyrimidine, taxane or etoposide); gemcitabine doublets (with a platinum/cisplatin); platinum (but not cisplatin)/taxane doublets; anthracycline triplets (with a fluoropyrimidine and platinum/cisplatin); taxane triplets (with a fluoropyrimidine and platinum/cisplatin); trastuzumab‐containing regimens; and (nontrastuzumab) targeted therapy‐containing regimens. The colors of the lines correspond to the different doublet regimens, for example, fluoropyrimidine doublets consist of a fluoropyrimidine with either platinum (but not cisplatin) or taxane, as shown by the blue interconnecting lines.
Acknowledgements
Our study was financially supported by an unrestricted research grant from Lilly Oncology. The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
Conflict of interest: R.H.A.V. has received unrestricted research grants from BMS and Roche. N.H.M. has served as a consultant for BMS, Lilly and MSD. J.d.V. has received nonfinancial support from BTG and Servier, has served as a consultant for Shire and has received unrestricted research grants from Servier. M.G.H.v.O. has received unrestricted research grants from BMS, Merck Serono, Nordic, Roche and Servier. H.W.M.v.L. has served as a consultant for BMS, Celgene, Lilly and Nordic and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips and Roche. The other authors have nothing to disclose.
Part of this study was presented at the ASCO Annual Meeting 2018, Chicago, United States of America; the European Gastric Cancer Congress 2018, Leiden, The Netherlands; and the Scientific Meeting of the European Network of Cancer Registries (ENCR), 2018, Copenhagen, Denmark.
[Correction added on August 31, 2019 after first online publication: reference list updated.]
Data availability
The data that support the findings of our study are available from the Netherlands Cancer Registry. Restrictions apply to the availability of these data, which were used under license for our study.
References
- 1. Haj Mohammad N, Bernards N, van Putten M, et al. Volume‐outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur J Cancer 2017;78:28–36. [DOI] [PubMed] [Google Scholar]
- 2. van Putten M, de Vos‐Geelen J, Nieuwenhuijzen GAP, et al. Long‐term survival improvement in oesophageal cancer in The Netherlands. Eur J Cancer 2018;94:138–47. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Batran SE, Ajani JA. Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer 2010;116:2511–8. [DOI] [PubMed] [Google Scholar]
- 4. Janmaat VT, Steyerberg EW, van der Gaast A, et al. Palliative chemotherapy and targeted therapies for esophageal and gastroesophageal junction cancer. Cochrane Database Syst Rev 2017;11:CD004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;3:CD004064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ter Veer E, Mohammad NH, Van Valkenhoef G, et al. The efficacy and safety of first‐line chemotherapy in advanced Esophagogastric cancer: a network meta‐analysis. J Natl Cancer Inst 2016;108:1–13. [DOI] [PubMed] [Google Scholar]
- 7. Cunningham D, Starling N, Rao S, et al. Capecitabine and Oxaliplatin for advanced Esophagogastric cancer. N Engl J Med 2008;358:36–46. [DOI] [PubMed] [Google Scholar]
- 8. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Eur J Surg Oncol 2016;27:v38–49. [DOI] [PubMed] [Google Scholar]
- 9. Lordick F, Lorenzen S, Yamada Y, et al. Optimal chemotherapy for advanced gastric cancer: is there a global consensus? Gastric Cancer 2014;17:213–25. [DOI] [PubMed] [Google Scholar]
- 10. Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27:v50–7. [DOI] [PubMed] [Google Scholar]
- 11. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet 2010;376:687–97. [DOI] [PubMed] [Google Scholar]
- 12. Ajani JA, D'Amico TA, Almhanna K, et al. Gastric cancer, version 3.2016; clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14:1286–312. [DOI] [PubMed] [Google Scholar]
- 13. Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194–227. [DOI] [PubMed] [Google Scholar]
- 14. Carmona‐Bayonas A, Jiménez‐Fonseca P, Custodio A, et al. Anthracycline‐based triplets do not improve the efficacy of platinum‐fluoropyrimidine doublets in first‐line treatment of advanced gastric cancer: real‐world data from the AGAMEMON national cancer registry. Gastric Cancer 2018;21:96–105. [DOI] [PubMed] [Google Scholar]
- 15. Haj Mohammad N, ter Veer E, Ngai L, et al. Optimal first‐line chemotherapeutic treatment in patients with locally advanced or metastatic esophagogastric carcinoma: triplet versus doublet chemotherapy: a systematic literature review and meta‐analysis. Cancer Metastasis Rev 2015;34:429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nelen SD, van Putten M, Lemmens VEPP, et al. Effect of age on rates of palliative surgery and chemotherapy use in patients with locally advanced or metastatic gastric cancer. Br J Surg 2017;104:1837–46. [DOI] [PubMed] [Google Scholar]
- 17. Dikken JL, Van Sandick JW, Allum WH, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg 2013;100:83–94. [DOI] [PubMed] [Google Scholar]
- 18. Dassen AE, Dikken JL, Bosscha K, et al. Gastric cancer: decreasing incidence but stable survival in The Netherlands. Acta Oncol (Madr) 2014;53:138–42. [DOI] [PubMed] [Google Scholar]
- 19. Fritz A. International classification of diseases for oncology: ICD‐O, 3rd edn. Geneva: World Health Organization, 2000. [Google Scholar]
- 20. Jeene PM, Versteijne E, van Berge Henegouwen MI, et al. Supraclavicular node disease is not an independent prognostic factor for survival of esophageal cancer patients treated with definitive chemoradiation. Acta Oncol (Madr) 2017;56:33–8. [DOI] [PubMed] [Google Scholar]
- 21. National Institute of Cancer . Common Terminology Criteria for Adverse Events (CTCAE). Bethesda, MD: NIH Publication, 2010. [Google Scholar]
- 22.Dutch Clinical Practice Guidelines for Gastric Carcinoma, version 2.2. 2016. Available from: http://www.oncoline.nl [cited December 13, 2018].
- 23.Dutch Clinical Practice Guidelines for Esophageal Carcinoma, version 3.1. 2014. Available from: http://www.oncoline.nl [cited December 13, 2018].
- 24.Dutch Clinical Practice Guidelines for Gastric Carcinoma, version 1.0. 2009. Available from: http://www.oncoline.nl [cited December 13, 2018].
- 25. Van Putten M, Verhoeven RHA, Van Sandick JW, et al. Hospital of diagnosis and probability of having surgical treatment for resectable gastric cancer. Br J Surg 2016;103:233–41. [DOI] [PubMed] [Google Scholar]
- 26. van Putten M, Nelen SD, Lemmens VEPP, et al. Overall survival before and after centralization of gastric cancer surgery in The Netherlands. Br J Surg 2018;105:1807–15. [DOI] [PubMed] [Google Scholar]
- 27. Yun J, Lee J, Park SH, et al. A randomised phase II study of combination chemotherapy with epirubicin, cisplatin and capecitabine (ECX) or cisplatin and capecitabine (CX) in advanced gastric cancer. Eur J Cancer 2010;46:885–91. [DOI] [PubMed] [Google Scholar]
- 28. Kim TW, Choi SJ, Ahn JH, et al. A prospective randomized phase III trial of 5‐fluorouracil and cisplatin (FP) versus epirubicin, cisplatin, and 5‐fu (ECF) in the treatment of patients with previously untreated advanced gastric cancer (AGC). Eur J Cancer 2001;37:S314. [Google Scholar]
- 29. Nio Y. A randomized, comparative study of combination chemotherapies in advanced gastric cancer: 5‐fluorouracil and cisplatin (FP) versus 5‐fluorouracil, cisplatin, and 4′‐epirubicin (FPEPIR). Anticancer Res 1992;12:1983–8. [PubMed] [Google Scholar]
- 30. Al‐Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro‐oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–57. [DOI] [PubMed] [Google Scholar]
- 31. Al‐Batran SE, Goetze TO, Mueller DW, et al. The RENAISSANCE (AIO‐FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited‐metastatic adenocarcinoma of the stomach or esophagogastric junction ‐ a phase III trial of the German AIO/CAO‐V/CAOGI. BMC Cancer 2017;17:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. ter Veer E, Creemers A, de Waal L, et al. Comparing cytotoxic backbones for first‐line trastuzumab‐containing regimens in human epidermal growth factor receptor 2‐positive advanced oesophagogastric cancer: a meta‐analysis. Int J Cancer 2018;143:438–48. [DOI] [PubMed] [Google Scholar]
- 33. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence—what is it and what can it tell us? N Engl J Med 2016;375:2293–7. [DOI] [PubMed] [Google Scholar]
- 34. Jeene PM, van Laarhoven HWM, Hulshof MCCM. The role of definitive chemoradiation in patients with non‐metastatic oesophageal cancer. Best Pract Res Clin Gastroenterol 2018;36–37:53–9. [DOI] [PubMed] [Google Scholar]
- 35. ter Veer E, van Kleef JJ, Schokker S, et al. Prognostic and predictive factors for overall survival in metastatic oesophagogastric cancer: a systematic review and meta‐analysis. Eur J Cancer 2018;103:214–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Baseline characteristics of all and selected metastatic esophagogastric cancer patients treated with systemic therapy and diagnosed between 2010 and 2014.
Table S2 List of assumptions regarding definitions of first‐line systemic treatment and time to failure of first‐line treatment.
Table S3 Most frequently administered systemic therapy regimens.
Figure S1 Word cloud of all 45 systemic therapy regimens that were administered. Font size of the word corresponds to the number of patients who received the regimen.
Figure S2 Subdivision of systemic therapy regimens. Systemic treatment regimens were dived as follows: monotherapy; fluoropyrimidine doublets (with a platinum [but not cisplatin], taxane, or irinotecan); cisplatin doublets (with a fluoropyrimidine, taxane or etoposide); gemcitabine doublets (with a platinum/cisplatin); platinum (but not cisplatin)/taxane doublets; anthracycline triplets (with a fluoropyrimidine and platinum/cisplatin); taxane triplets (with a fluoropyrimidine and platinum/cisplatin); trastuzumab‐containing regimens; and (nontrastuzumab) targeted therapy‐containing regimens. The colors of the lines correspond to the different doublet regimens, for example, fluoropyrimidine doublets consist of a fluoropyrimidine with either platinum (but not cisplatin) or taxane, as shown by the blue interconnecting lines.
Data Availability Statement
The data that support the findings of our study are available from the Netherlands Cancer Registry. Restrictions apply to the availability of these data, which were used under license for our study.


