Abstract
Objective
It was previously determined that group‐based face‐to‐face Mindfulness‐Based Cognitive Therapy (MBCT) and individual internet‐based MBCT (eMBCT) are equally efficacious compared with treatment as usual (TAU) in reducing psychological distress. In this study, the incremental cost‐utility of both interventions compared with TAU was assessed.
Methods
This cost‐utility study included 245 self‐referred heterogeneous cancer patients with psychological distress who were randomized to MBCT, eMBCT or TAU. Healthcare costs and (informal) work‐related productivity losses were assessed by interview. Outcomes were expressed in EuroQol‐5D‐3L utility scores and quality‐adjusted life years (QALY). An economic evaluation with a time‐horizon of 3 months was conducted from the societal perspective in the intention‐to‐treat sample. In addition, secondary explorative analyses of costs and quality of life during the 9‐month follow‐up were conducted based on linear extrapolation of TAU.
Results
Paid work‐related productivity losses and societal costs were lower in both intervention conditions compared with TAU during the 3‐month intervention period. Moreover, quality of life (utility scores) improved in eMBCT versus TAU (Cohen's d: .54) and MBCT versus TAU (.53). At a willingness to pay of €20000 per QALY, the mean incremental net monetary benefit was €1916 (SD=€783) in eMBCT and €2365 (SD=€796) in MBCT versus TAU. Exploration of costs demonstrated an equal pattern of eMBCT and MBCT being superior to TAU. Quality of life at 9‐month follow‐up remained improved in both interventions.
Conclusions
Results indicate that eMBCT and MBCT are cost‐saving treatments whilst simultaneously improving quality of life for distressed cancer patients.
Keywords: cancer, cost and cost analysis, distance counselling, oncology, mindfulness, telemedicine
1. BACKGROUND
Psychological distress is a negative emotional experience which impedes coping with cancer and its treatment.1 Psychological distress is highly prevalent in cancer patients2 and results in serious consequences such as reduced quality of life, decreased compliance with medical care, prolonged duration of hospital stay3, 4 and increased (inadequate) healthcare use.5 Although not all distressed cancer patients subsequently wish for psychological treatment,6 the availability of effective treatment for psychological distress in cancer patients is required.
Psychological treatment in cancer patients yield small to medium effects in reducing psychological distress.7 In addition to cognitive behavioural therapy, mindfulness‐based interventions (MBIs)8, 9 are increasingly offered in oncological settings. Several randomized controlled trials (RCTs) indicate that MBIs result in significant improvements of depressive and anxiety symptoms in cancer patients, e.g.10, 11, 12
MBIs are usually offered as an eight‐week, face‐to‐face group training. However, attending group‐based MBI is not always possible for cancer patients.13 In contrast, Internet‐based interventions are easily accessible, available 24/7 when delivered asynchronously and save travelling time.14, 15 A recent multicentre RCT in 245 self‐referred heterogeneous cancer patients with (mild) psychological distress showed that both group‐based mindfulness‐based cognitive therapy (MBCT) and individual internet‐based MBCT (eMBCT) had a moderate effect in reducing psychological distress in comparison with treatment as usual (TAU).11 The uncontrolled follow‐up period of 9 months demonstrated consolidation of treatment effects in both interventions.16
However, it remains unknown whether (e)MBCT provides value for money.17 Evidence on cost‐effectiveness of MBIs is focused mainly on depression.18A systematic review of economic evaluations of 11 third‐wave cognitive behavioural interventions included 5 studies on MBIs, with two studies on MBIs in recurrent major depression and single studies on MBIs in patients with multiple sclerosis, medically unexplained symptoms or cancer. Evidence on cost‐effectiveness of MBIs in these populations was deemed inconclusive.19 Another review of economic evaluations of acceptance‐ and mindfulness‐based interventions reached a similar conclusions.18
With regard to specific economic evaluations of MBIs in cancer patients, a study in 129 breast cancer patients suffering from persistent pain explored cost‐effectiveness of MBCT compared with wait‐list control with a time horizon of 6 months. When willingness‐to‐pay (WTP) was €0, the MBCT intervention was cost‐effective with a probability of 85%.20 Another study in 104 breast cancer patients compared the cost‐effectiveness of mindfulness‐based stress reduction (MBSR) with wait‐list controls with a time horizon of 12 weeks. MBSR was more costly ($+666) with an incremental QALY gain of +0.03 compared with wait‐list controls, resulting in an ICER of $22,200/QALY.21 Another study in 191 breast cancer patients investigated the cost‐effectiveness of mindfulness‐based art therapy (MBAT) compared with an active support group with a time horizon of 9 weeks. MBAT demonstrated the potential to achieve parity with the support group intervention if some intervention‐related costs were reduced.22
In short, the first studies demonstrate a tentatively positive, but inconclusive view of the economic potential of MBIs. An economic evaluation of an electronically delivered format of MBCT is yet to be conducted. The primary aim of the current study was to evaluate the cost‐effectiveness of both eMBCT and MBCT compared with TAU from the societal perspective in the period from baseline (T0) to post‐treatment (T1). The secondary aim was to explore costs and quality of life during the 9 month‐follow‐up (12‐month time horizon) based on a linear extrapolation of TAU.
2. METHODS
2.1. Trial design, participants, procedure
Study methods have been described in detail elsewhere.11 The present study is an economic evaluation from a societal perspective based on the results of a three‐armed multicentre, parallel group RCT comparing the effectiveness of eMBCT and MBCT with TAU in reducing psychological distress in cancer patients. As patients randomized to TAU received either eMBCT or MBCT after 3 months, the time horizon of the economic evaluation was restricted to 3 months.
Inclusion criteria were: a) any cancer diagnosis, current or past; b) a score of ≥ 11 on the Hospital Anxiety and Depression Scale (HADS);c) ability to attend MBCT both face‐to‐face and online; and d) good command of the Dutch language. Exclusion criteria were: a) severe psychiatric morbidity; b) change in psychotropic medication dosage within a period of three months prior to baseline; c) current or previous participation in ≥ 4 sessions of an MBI. Patients were recruited from April 2014 to December 2015 via self‐referral. The study was approved by the ethical review board of the Radboud University Medical Center (CMO Arnhem‐Nijmegen 2013/542) and all centres provided local ethics approval. The study was registered on http://Clinicaltrials.gov (NCT02138513), reported following CONSORT guidelines.23 A protocol paper was published in advance.24 All participants provided written informed consent prior to enrolment.
2.2. Interventions
2.2.1. Face‐to‐face MBCT
The MBCT protocol9 was followed except for slight tailoring to the cancer patient. MBCT consisted of eight weekly 2.5h group sessions guided by a therapist, a six‐hour one‐day silent retreat and daily home practice assignments of about 45 minutes. All therapists in this study were accredited in concordance with the UK Mindfulness‐Based Teacher Trainer Network Good Practice Guidelines.
2.3. eMBCT
The eMBCT was identical to MBCT in terms of content but was delivered individually and included weekly asynchronous written interaction with a therapist over email. For more information, we refer to our other work.11, 15
2.3.1. Treatment as usual
Treatment as usual (TAU) consisted of all healthcare patients wished to receive. There were no restrictions on healthcare utilization during the study period, except not participating in MBIs.
2.4. Measures
2.4.1. Healthcare costs
The Trimbos/iMTA questionnaire for Costs associated with Psychiatric illness (TiC‐P)25 was used to collect information on direct healthcare use and paid and informal work‐related productivity losses. The TiC‐P is a self‐report instrument, but in the current study the TiC‐P was administered by the researchers in an interview format. The recommended time‐horizon for determining healthcare costs by TiC‐P of three months was used.25
Direct healthcare costs were calculated by multiplying volumes of care by standardized unit prices indexed using Dutch national price indices to the 2016 price level26 (see Table S1 in the Supporting Information). Prescription medication costs were retrieved from the Dutch national tariff list (https://www.medicijnkosten.nl). Societal costs were calculated as the sum of medical and formal and informal productivity loss costs for T1, T1+T2, and T1+T2+(T3*2), reaching a time horizon of 12 months (9 months post‐treatment).
2.4.2. Indirect costs – paid and informal work‐related productivity losses
Indirect costs due to paid work‐related productivity losses included absenteeism and presenteeism costs. Absenteeism costs were calculated by multiplying the number of hours patients were absent from their job by the gross wage per hour according to the Dutch guideline for health economic evaluations.27 Presenteeism costs were calculated by multiplying estimated number of work hours lost by gross wage per hour. Indirect costs related to paid work‐related productivity losses were calculated according to the Friction Cost method.28 Once patients met the friction period criterium of >85 sick days at a specific time point (starting count at baseline) no additional indirect costs due to work were calculated during the rest of the study period. Indirect costs related to productivity loss in informal work were also included.27 The recall period for paid and informal work‐related productivity losses was 4 weeks (as per default), which was proportionately extrapolated from 4 weeks to 3 months to match the recall period of the healthcare use questionnaire. Dutch national price indices were used to index healthcare and productivity costs to the 2016 price level26 and costs were presented in Euros.
2.4.3. Intervention costs
Additionally, intervention costs were €299.00 per person for patients participating in the MBCT and €331.16 per person for patients participating in eMBCT (see Table S2). In MBCT, travel and parking related costs were calculated on an individual basis.27 Intervention development costs were regarded as sunk costs and were therefore disregarded because they would not need to be repeated if the intervention were adopted on a broader scale.29
2.4.4. Quality of life
To measure the health‐related quality of life (QoL) of cancer patients, a validated health‐related QoL instrument was used: the EuroQol‐5D‐3L (EQ‐5D).30, 31 We chose to use a generic QoL measure as opposed to a cancer‐specific measure such as the EORTC QLQ since we were interested in measuring utilities.32 The EQ‐5D index is obtained by applying predetermined weights to the five domains. This index gives a societal‐based global utility score of the participant's health status on a scale between‐.33 (worse than death) and 1 (perfect health). From the utility scores at T0 and T1 QALYs were calculated for each patient using the Area Under the Curve (AUC) method: ((EQ 5D T0 + EQ 5D T1) /2) * (3/12) using the Dutch index tariff.31
2.5. Linear extrapolation
One way to deal with extrapolation of a cost pattern is to assume a linear relationship between costs and volume within some relevant range. Within that relevant range, the total cost varies linearly with volume, at least approximately. In terms of somatic care, patients followed clinical routine with which we did not intervene and which would remain similar after TAU. With regard to psychological care it is known that psychological distress levels are associated with healthcare consumption5 and these did not change in patients receiving TAU only.11 Therefore, the T1 measurement in TAU was linearly extrapolated up to 12 months.
2.6. Analyses
Descriptive analyses of mean differences between conditions were tested by one‐way ANOVAs including treatment (eMBCT, MBCT or TAU) as independent variable and costs/EQ‐5D utility scores/QALYs as dependent variable on the complete‐case intention‐to‐treat (ITT) sample. Analyses of follow‐up costs per category included costs at baseline as a covariate. Post‐hoc tests were conducted by simple contrasts using TAU as reference group with Bonferroni‐corrected (due to two comparisons with TAU) one‐sided P values (considering the positive clinical RCT) rendering P ≤.05 as significant. Because of baseline differences in employment status, we conducted two separate analyses, one including the employed‐at‐baseline subsample only and another including baseline employment status as covariate. Cohen's d effect sizes (ES) were calculated by dividing the difference in means by baseline pooled SDs of the respective conditions33 and were interpreted as small (0.2 to 0.5), medium (0.5 to 0.8), or large (.8).33 Cost‐utility analyses were conducted from the societal perspective on the complete‐case ITT sample including all patients who filled‐out the TiC‐P and EQ‐5D at T1, T2 and T3. The bootstrapped replications (1000 iterations) were graphed on two cost‐utility planes (eMBCT vs. TAU and MBCT vs. TAU). The horizontal axis of these planes represents the incremental effects and the vertical axis represents the incremental costs. The QALY model assumes WTP= willingness to accept compensation.
In addition, the net monetary benefit (NMB) was determined: NMB=(effect E of intervention expressed in QALY * WTP) – costs C for intervention. If the incremental NMB (ΔE * WTP ‐ ΔC) is > 0, the intervention is considered to be cost‐effective compared with an alternative. For the exact WTP is unknown, results of regression analyses with the NMB as dependent variable were subsequently used to obtain a cost‐effectiveness acceptability curve (CEAC) by plotting 1‐P/2 against different levels of WTP (0, 20000, 40000, 60000, 80000) for a QALY where P is the P value from the coefficient on the treatment dummy variable in the regression analyses.34
3. RESULTS
3.1. Sample characteristics
In total, 245 self‐referred heterogeneous cancer patients with psychological distress were randomly assigned to eMBCT (n=90), MBCT (n=77) or TAU (n=78) (See Figure 1). The three conditions did not differ in terms of baseline demographic or clinical characteristics (see Table 1). Intervention dropout was significantly higher in the eMBCT than in the MBCT group: (χ2(1, n=167)=3.92, P = .047). The three conditions did not differ in employment status at baseline, although there were differences at a descriptive level Of the patients who had a job at baseline, relatively more patients met the friction period criterium in both interventions compared with TAU, although this difference was not significant (χ2(2, n=245)=5.25, P = .072) (see Table S3). During the intervention period, a total of n=24 (33%) in eMBCT used a form of mental healthcare compared with n=18 (29%) in MBCT and n=20 (32%) in TAU. This difference was not significant between conditions. Study dropout (number of missing measurements at end of treatment) did not differ between conditions. Study dropouts did not differ from study completers in healthcare costs, informal work costs or EQ‐5D utility scores at baseline. Study dropouts did have marginally significantly lower paid work costs (P = .069) and societal costs (P = .065) at baseline. Study dropouts were relatively more often non‐employed patients compared with employed patients in TAU (P = .025) compared with eMBCT and MBCT (P = ns), which further enhanced the difference in proportion of patients with a job between conditions included in our analyses. Of all patients included in our analyses at end of treatment, 56.9% in eMBCT, 53.1% in MBCT and 71.4% in TAU had a job at baseline. Moreover, study dropouts demonstrated significantly higher psychological distress scores compared with study completers (F(1,244)=5.82, P = .017).
Figure 1.
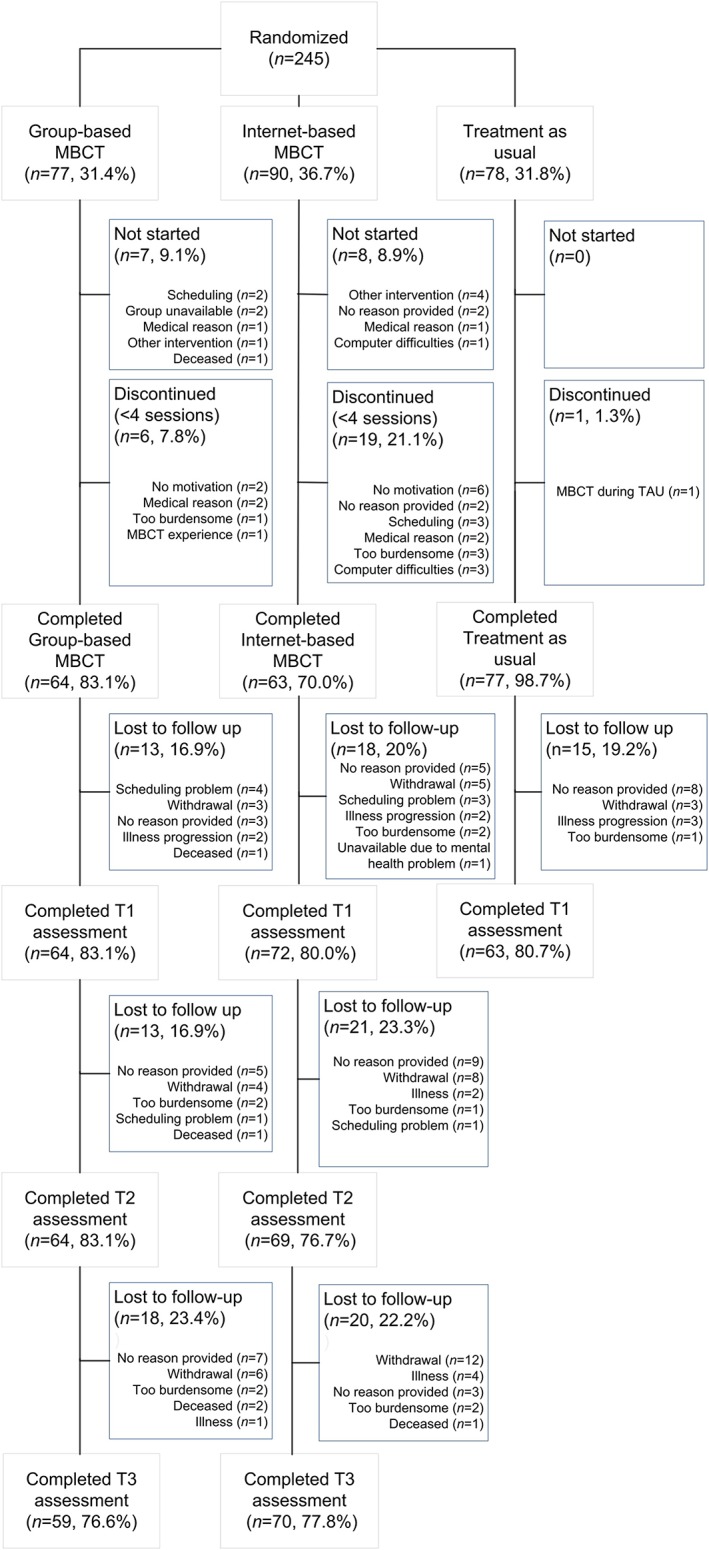
CONSORT flowchart of the cost‐utility trial ran alongside clinical trial
Table 1.
Baseline sociodemographic and clinical characteristics (n=245)
| Characteristic | All | eMBCT | MBCT | TAU | |
|---|---|---|---|---|---|
| n=245 | n=90 | n=77 | n=78 | ||
| n (%) | n (%) | n (%) | n (%) | P | |
| Sex | 0.912 | ||||
| Female | 210 (85.7) | 77 (85.6) | 67 (87.0) | 66 (84.6) | |
| Male | 35 (14.3) | 13 (14.4) | 10 (13.0) | 12 (15.4) | |
| Age, years | 0.464 | ||||
| Mean | 51.7 | 52.4 | 52.1 | 50.4 | |
| SD | 10.7 | 10.7 | 11.4 | 9.9 | |
| Married/in a relationship | 0.491 | ||||
| Yes | 202 (82.4) | 76 (84.4) | 65 (84.4) | 61 (78.2) | |
| No | 43 (17.6) | 14 (15.6) | 12 (15.6) | 17 (21.8) | |
| Children | 0.314 | ||||
| Yes | 169 (69.0) | 65 (72.2) | 48 (62.3) | 56 (71.8) | |
| No | 76 (31.0) | 25 (27.8) | 29 (37.7) | 22 (28.2) | |
| Education | 0.451 | ||||
| High | 166 (67.8) | 56 (62.2) | 54 (70.1) | 56 (71.8) | |
| Middle | 77 (31.4) | 34 (37.8) | 22 (28.6) | 21(26.9) | |
| Low | 2 (0.8) | 0 | 1 (1.3) | 1(1.3) | |
| Diagnosis | 0.724 | ||||
| Breast cancer | 151 (61.6) | 53(58.9) | 53 (68.8) | 45 (57.7) | |
| Gynecological cancer | 18 (7.3) | 9 (10.0) | 2 (2.6) | 7 (9.0) | |
| Prostate cancer | 16 (6.5) | 7 (7.8) | 6 (7.8) | 3 (3.8) | |
| Colon cancer | 12 (4.9) | 4 (4.4) | 4 (5.2) | 4 (5.1) | |
| Non‐Hodgkin's lymphoma | 11 (4.5) | 3 (3.3) | 1 (1.3) | 7 (9.0) | |
| Skin cancer | 5 (2.0) | 3 (3.3) | 1 (1.3) | 1 (1.3) | |
| Thyroid cancer | 4 (1.6) | 1 (1.1) | 1 (1.3) | 2 (2.6) | |
| Bladder cancer | 4 (1.6) | 2 (2.2) | 1 (1.3) | 1(1.3) | |
| Neuroendocrine tumour | 4 (1.6) | 2 (2.2) | 1 (1.3) | 1 (1.3) | |
| Other | 20 (8.2) | 6 (6.7) | 7 (9.1) | 7 (9.0) | |
| Years since diagnosis | 0.616 | ||||
| Mean | 3.5 | 3.3 | 3.9 | 3.2 | |
| SD | 4.7 | 4 | 5.7 | 4.3 | |
| Anticancer treatment intent | 0.472 | ||||
| Curative | 206 (84.1) | 74 (82.2) | 68 (88.3) | 64 (82.1) | |
| Palliative | 39 (15.9) | 16 (17.8) | 9 (11.7) | 14 (17.9) | |
| Current treatment | 0.694 | ||||
| None | 133 (53.1) | 49 (54.4) | 43 (55.8) | 41 (52.6) | |
| Hormone therapy | 79 (32.2) | 28 (31.1) | 22 (28.6) | 29 (37.2) | |
| Combination of treatments | 12 (4.9) | 4 (4.4) | 4 (5.2) | 4 (5.1) | |
| Immunotherapy | 9 (3.7) | 5 (5.6) | 1 (1.3) | 3 (3.8) | |
| Radiotherapy | 8 (3.3) | 3 (3.3) | 5 (6.5) | 0 | |
| Chemotherapy | 4 (1.6) | 1 (1.1) | 2 (2.6) | 1 (1.3) |
3.2. Cost‐utility: 3 month time‐horizon
3.2.1. Costs
Direct healthcare costs did not differ significantly between the two intervention conditions and TAU (see Table 2). Costs associated with paid work‐related productivity losses were lower in both eMBCT and MBCT compared with TAU in post‐hoc comparisons (P = .014 and P = .002, respectively). Costs associated with informal work did not differ significantly between conditions. Societal costs were significantly lower in both eMBCT and MBCT compared with TAU in post‐hoc comparisons (P = .002 and P = .014). Societal costs were significantly lower in eMBCT vs TAU (M = ‐2457, SE = 856, P = .005) and MBCT vs. TAU (M = ‐2998, SE = 904, P = .001) when looking at the employed‐at‐baseline subsample only. Societal costs were significantly lower in eMBCT vs TAU (M = ‐1836, SE = 615, P = .003) and MBCT vs. TAU (M = ‐1394, SE = 594, P = .020) when adjusting for baseline employment status.
Table 2.
(Aggregated) costs per category in Euros and EQ‐5D utility scores per condition and per measurement with TAU costs at T2 and T3 extrapolated from T1. All reported P values concern the P value of the three‐armed comparison
| T0 Baseline | T1 End of treatment | T2 3‐month follow‐up | T3 9‐month follow‐up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eMBCT | MBCT | TAU | P | eMBCT | MBCT | TAU | P | eMBCT | MBCT | TAU | P | eMBCT | MBCT | TAU | P | ||
| n | 90 | 77 | 78 | 72 | 64 | 63 | 63 | 59 | 63 | 59 | 53 | 63 | |||||
| Healthcare | M | 1854.49 | 1858.76 | 1803.16 | .99 | 1475.25 | 1090.34 | 1540.66 | .28 | 2247.32 | 1739.45 | 3081.32 | .05 | 3738.13 | 3869.22 | 6162.65 | .08 |
| SD | 2289.15 | 2226.28 | 2244.21 | 1806.58 | 860.36 | 2437.17 | 2510.26 | 1276.21 | 4874.34 | 3650.48 | 4197.71 | 9748.67 | |||||
| Paid work | M | 3148.11 | 3429.81 | 3782.71 | .69 | 1287.42 | 1223.06 | 2698.39 | .03 | 2467.57 | 1731.85 | 5396.78 | .01 | 3519.78 | 2735.70 | 10793.57 | <.001 |
| SD | 4814.55 | 4835.71 | 4548.91 | 3044.86 | 3011.54 | 3882.26 | 4509.08 | 3862.48 | 7764.52 | 7018.97 | 7229.30 | 15529.04 | |||||
| Informal | M | 831.75 | 680.7 | 708.05 | .57 | 490.18 | 408.43 | 663.53 | .20 | 930.99 | 773.45 | 1327.06 | .08 | 1492.88 | 1514.12 | 2654.12 | .018 |
| SD | 1106.08 | 815.43 | 989.54 | 800.41 | 776.58 | 811.7 | 1352.01 | 1179.54 | 1623.41 | 1881.09 | 2258.14 | 3246.82 | |||||
| Societal | M | 5834.35 | 5969.28 | 6293.91 | .88 | 3252.85 | 2798.80 | 4902.58 | .01 | 5645.88 | 4322.44 | 9805.17 | <.001 | 8750.80 | 8199.31 | 19610.34 | <.001 |
| SD | 6394.91 | 6149.18 | 5540.96 | 3888.31 | 3222.40 | 4895.04 | 5853.85 | 4063.20 | 9790.08 | 9056.53 | 9596.77 | 19580.16 | |||||
| EQ‐5D | n | 90 | 77 | 78 | .92 | 72 | 64 | 63 | <.001 | 69 | 64 | 63 | 70 | 59 | 63 | ||
| M | 0.77 | 0.75 | 0.76 | 0.85 | 0.86 | 0.75 | 0.85 | 0.83 | 0.86 | 0.85 | |||||||
| SD | 0.19 | 0.21 | 0.17 | 0.17 | 0.13 | 0.19 | 0.14 | 0.18 | 0.11 | 0.2 | |||||||
3.2.2. Quality of life
When QoL was expressed in EuroQol‐5D‐3L utility scores, patients in the eMBCT and MBCT conditions reported significantly higher QoL at T1 than patients in TAU (F(2,198)=8.02, P < .001, see Table 2) with moderate effect sizes (eMBCT vs. TAU=.54 and MBCT vs. TAU=.53) . When QoL was expressed in QALYs, there was a non‐significant difference in favour of both interventions compared with TAU (F(2,198)=2.80, P = .063) with small to moderate effect sizes (eMBCT vs. TAU=.37 and MBCT vs. TAU=.34).
3.2.3. Cost‐utility
The cost‐utility planes (Figure 2) revealed that the vast majority cost‐effective pairs are located in the south‐east quadrant where both interventions are more effective and less costly than TAU, i.e., dominate TAU. At a WTP of €20000 the mean incremental net monetary benefit was €1916 (SD=€783) in eMBCT versus TAU and €2365 (SD=€796) in MBCT versus TAU. The cost‐effectiveness Acceptibility Curve (CEAC) indicated that the probability of both interventions being cost‐effective hovers around 99% regardless of the level of WTP per QALY gained (see Figure S1).
Figure 2.
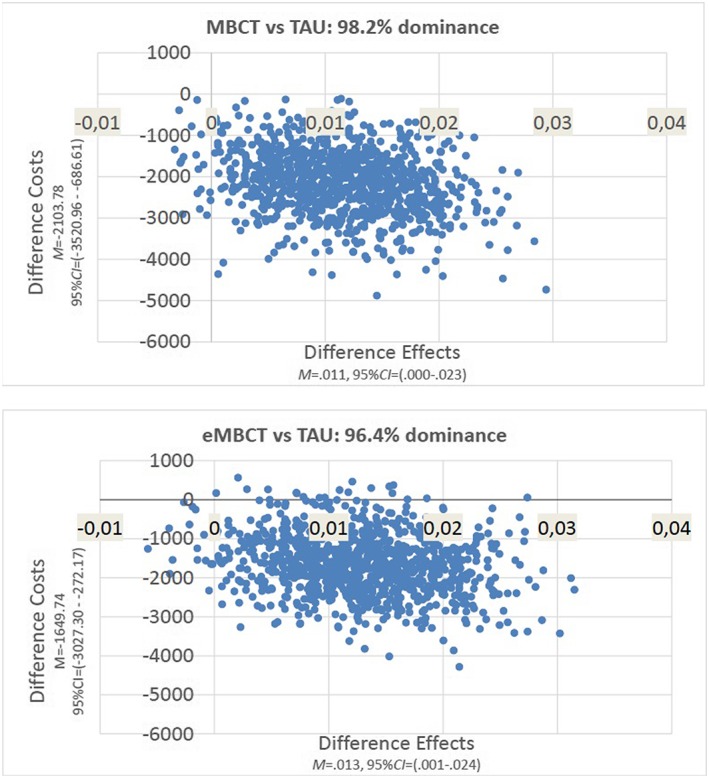
Incremental cost‐effectiveness ratios (societal perspective) for both intervention conditions versus TAU during intervention period (T0‐T1)
3.3. Exploration of costs and quality of life during the 9 month‐follow‐up
3.3.1. Costs
Healthcare costs were significantly lower in eMBCT and MBCT compared with TAU in (P = .035 and P = .048, respectively). Paid work‐related costs were significantly lower in both interventions compared with TAU (both P ≤ .001). Informal work‐related costs were significantly lower in both interventions compared with TAU in post‐hoc comparisons (P = .022 and P = .009). Societal costs were significantly lower in both interventions compared with TAU (both P ≤ .001).
3.3.2. Quality of life
Patients in both interventions maintained the increased QoL over the follow‐up period with no significant differences between eMBCT and MBCT.
4. CONCLUSIONS
The aim of the current study was to evaluate the cost‐utility of both eMBCT and MBCT compared with TAU from the societal perspective in the period from baseline (T0) to post‐treatment (T1) and to explore development of costs and quality of life during the 9 month‐follow‐up results in the period from baseline to 9 month‐follow‐up (T3).
Healthcare costs and informal work‐related productivity losses did not significantly differ between conditions, costs associated with paid work were lower in the interventions compared with TAU. Importantly, the aggregated societal costs were significantly lower in both interventions compared with TAU at all post‐treatment measurements – despite the added intervention costs. Patients in the eMBCT and MBCT conditions reported significantly higher QoL at T1 than patients in TAU with moderate effect sizes, although there were no significant differences between conditions in terms of QALYs. Since the NMB was larger in MBCT than eMBCT, this implies that MBCT provides most value for money compared with TAU. Extrapolated follow‐up results demonstrated comparable favourable effects of both interventions compared with TAU. However, it must be taken into account that selective dropout hinder an unbiased inference of the effect of both interventions in terms of societal costs. In the TAU condition, relatively more patients without a job were lost‐to‐follow up. The results must therefore be interpreted with caution and future studies should preferably stratify for employment status.
Several psychosocial interventions have previously been demonstrated to represent good value for money in cancer care.35 A review of 11 cost‐effectiveness studies of psychosocial interventions in cancer care indicate cost‐effectiveness at different WTP thresholds, but that more research is necessary and that more research should be performed encompassing potential important cost drivers from a societal perspective.36
The current results are partly in line with previous findings on cost‐effectiveness of MBIs for cancer patients20, 21, 22 although it must be noted that there are considerable differences between the studies in terms of population, intervention, measures and analysis perspective. Moreover, the current sample was self‐referred.
4.1. Study limitations
The most important limitation is lack of follow‐up for TAU. As cancer patients might recover spontaneously from psychological distress, it might not be appropriate to assume that costs and QoL remained stable over time. However, our sample consisted of cancer patients on average 3.5 years post diagnosis, rendering them less likely to recover spontaneously.37 Moreover, our period of “watchful waiting” took well over the usual period of “watchful waiting” in other studies, e.g.38 Therefore, we considered it justified to extrapolate TAU from T1.
4.2. Clinical implications
These results imply that offering Internet‐based MBCT in clinical practice improves accessibility of psycho‐oncological care whilst saving societal costs, without compromising intervention efficacy.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Supporting information
Table S1: Dutch reference prices used in determining healthcare costs as mentioned in Dutch manual for conducting economical evaluations in healthcare indexed for 2016 price levels
Table S2: Detailed calculation of eMBCT and MBCT intervention costs
Table S3: Paid work descriptives per measurement (T0 – T3)
ACKNOWLEDGEMENTS
This study was funded by a grant from KWF Kankerbestrijding/Pink Ribbon (KWF 2016‐8185/ PR 2012.WO14.C153). We are grateful to the patients and participating therapists and institutes. We would like to thank Heidi Willemse, Eva Witteveen, Merel Brands, and David Huijts for their assistance in data collection.
Compen F, Adang E, Bisseling E, van der Lee M, Speckens A. Cost‐utility of individual internet‐based and face‐to‐face mindfulness‐based cognitive therapy compared with treatment as usual in reducing psychological distress in cancer patients. Psycho‐Oncology. 2020;29:294‐303. 10.1002/pon.5246
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. National Comprehensive Cancer Network ‐ Clinical Practice Guidelines in Oncology: Distress Management. [cited 2015. November 19th]; Available from: http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf.
- 2. Mehnert A, Hartung TJ, Friedrich M, et al. One in two cancer patients is significantly distressed: Prevalence and indicators of distress. Psychooncology. 2018;27(1):75‐82. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative‐care settings: a meta‐analysis of 94 interview‐based studies. Lancet Oncol. 2011;12(2):160‐174. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell A, Ferguson D, Gill J, Paul J, Symonds P. Depression and anxiety in long‐term cancer survivors compared with spouses and healthy controls: a systematic review and meta‐analysis. Lancet Oncol. 2013;14(8):671‐786. [DOI] [PubMed] [Google Scholar]
- 5. Compen FR, Adang EM, Bisseling EM, et al. Exploring associations between psychiatric disorder, psychological distress, and health care utilization in cancer patients. Psychooncology. 2018;27(6):1671. [DOI] [PubMed] [Google Scholar]
- 6. Van Scheppingen C, Schroevers MJ, Pool G, et al. Is implementing screening for distress an efficient means to recruit patients to a psychological intervention trial? Psychooncology. 2014;23(5):516‐523. [DOI] [PubMed] [Google Scholar]
- 7. Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R. Effects of psycho‐oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta‐analysis. J Clin Oncol. 2013;31(6):782‐793. [DOI] [PubMed] [Google Scholar]
- 8. Kabat‐Zinn J. Full catastrophe living, revised edition: how to cope with stress, pain and illness using mindfulness meditation. UK: Hachette; 2013. [Google Scholar]
- 9. Segal ZV, Williams JMG, Teasdale JD. Mindfulness‐based cognitive therapy for depression: A new approach to relapse prevention. New York: Guilford Press; 2013. [Google Scholar]
- 10. Schellekens MPJ, van den Hurk D, Prins JB, et al. Mindfulness‐Based Stress Reduction added to care as usual for lung cancer patients and/or their partners: A multi‐centre randomized controlled trial. Psychooncology. 2017;26(12):2118‐2126. [DOI] [PubMed] [Google Scholar]
- 11. Compen F, Bisseling E, Schellekens M, et al. Face‐to‐Face and Internet‐Based Mindfulness‐Based Cognitive Therapy Compared With Treatment as Usual in Reducing Psychological Distress in Patients With Cancer: A Multicenter Randomized Controlled Trial. J Clin Oncol. 2018;36(23):2413‐2421. [DOI] [PubMed] [Google Scholar]
- 12. Piet J, Wurtzen H, Zachariae R. The Effect of Mindfulness‐Based Therapy on Symptoms of Anxiety and Depression in Adult Cancer Patients and Survivors: A Systematic Review and Meta‐Analysis. J Consult Clin Psychol. 2012;80(6):1007‐1020. [DOI] [PubMed] [Google Scholar]
- 13. Zernicke KA, Campbell TS, Speca M, McCabe‐Ruff K, Flowers S, Carlson LE. A randomized wait‐list controlled trial of feasibility and efficacy of an online mindfulness–based cancer recovery program: the etherapy for cancer applying mindfulness trial. Psychosom Med. 2014;76(4):257‐267. [DOI] [PubMed] [Google Scholar]
- 14. Spijkerman MPJ, Pots WTM, Bohlmeijer ET. Effectiveness of online mindfulness‐based interventions in improving mental health: A review and meta‐analysis of randomised controlled trials. Clin Psychol Rev. 2016;45:102‐114. [DOI] [PubMed] [Google Scholar]
- 15. Compen FR, Bisseling EM, Schellekens MP, Jansen ET, van der Lee M, Speckens AE. Mindfulness‐Based Cognitive Therapy for Cancer Patients Delivered via Internet: Qualitative Study of Patient and Therapist Barriers and Facilitators. J Med Internet Res. 2017;19(12):e407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cillessen L, Schellekens MPJ, van de Ven M, et al. Consolidation and prediction of long‐term treatment effect of group and online mindfulness‐based cognitive therapy for distressed cancer patients. Acta Oncol. 2018;57(10):1293‐1302. [DOI] [PubMed] [Google Scholar]
- 17. Edwards R, Bryning L, Crane R. Design of Economic Evaluations of Mindfulness‐Based Interventions: Ten Methodological Questions of Which to Be Mindful. Mind. 2015;6(3):490‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duarte R, Lloyd A, Kotas E, Andronis L, White R. Are acceptance and mindfulness‐based interventions ‘value for money’? Evidence from a systematic literature review. Br J Clin Psychol. 2019;58(2):187‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feliu‐Soler A, Cebolla A, McCracken L, et al. Economic Impact of Third‐Wave Cognitive Behavioral Therapies: A Systematic Review and Quality Assessment of Economic Evaluations in Randomized Controlled Trials. Behav Ther. 2018;49(1):124‐147. [DOI] [PubMed] [Google Scholar]
- 20. Johannsen M, Sørensen J, O'Connor M, Jensen AB, Zachariae R. Mindfulness‐based cognitive therapy (MBCT) is cost‐effective compared to a wait‐list control for persistent pain in women treated for primary breast cancerResults from a randomized controlled trial. Psychooncology. 2017;26(12):2208‐2214. [DOI] [PubMed] [Google Scholar]
- 21. Lengacher CA, Kip KE, Reich RR, et al. A Cost‐Effective Mindfulness Stress Reduction Program: A Randomized Control Trial for Breast Cancer Survivors. Nurs Econ. 2015;33(4):210‐232. [PubMed] [Google Scholar]
- 22. Prioli KM, Pizzi LT, Kash KM, et al. Costs and Effectiveness of Mindfulness‐Based Art Therapy versus Standard Breast Cancer Support Group for Women with Cancer. Am Health Drug Benefits. 2017;10(6):288‐294. [PMC free article] [PubMed] [Google Scholar]
- 23. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Compen FR, Bisseling EM, Van der Lee ML, et al. Study protocol of a multicenter randomized controlled trial comparing the effectiveness of group and individual internet‐based Mindfulness‐Based Cognitive Therapy with treatment as usual in reducing psychological distress in cancer patients: the BeMind study. BMC Psychology. 2015;3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hakkaart‐van Roijen L et al. Trimbos/iMTA questionnaire for Costs associated with Psychiatric Illness (TiC‐P). Rotterdam: Institute for Medical Technology Assessment; 2002. [Google Scholar]
- 26. CBS . Consumentenprijzen; prijsindex 2006=100, 1996‐2015. 2016; Available from: http://statline.cbs.nl/Statweb/publication/?DM=SLNL&PA=71311ned&D1=0&D2=0&D3=12,25,38,51,64,77,90,103,116,129,142,155,168,181,194,219,232,245,258,l&HDR=T&STB=G2,G1&VW=T.
- 27. Hakkaart‐van Roijen, L. , et al., Kostenhandleiding. Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. In opdracht van Zorginstituut Nederland. Geactualiseerde versie, 2015.
- 28. Drummond MF et al. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- 29. Tate D, Finkelstein EA, Khavjou O, Gustafson A. Cost Effectiveness of Internet Interventions: Review and Recommendations. Ann Behav Med. 2009;38(1):40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The EuroQol Group . EuroQol‐‐a new facility for the measurement of health‐related quality of life. The EuroQol Group. Health Policy. 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
- 31. Lamers L, Stalmeier PF, McDonnell J, Krabbe PF, van Busschbach J. Kwaliteit van leven meten in economische evaluaties: het Nederlands EQ‐5D‐tarief. Ned Tijdschr Geneeskd. 2005;149(28):1574‐1578. [PubMed] [Google Scholar]
- 32. Fayers P, Bottomley A, EuroQoL Group . Quality of life research within the EORTC—the EORTC QLQ‐C30. Eur J Cancer. 2002;38:125‐133. [DOI] [PubMed] [Google Scholar]
- 33. Cohen J. Statistical power analysis for the behavioral sciences (revised ed.). New York: Academic Press; 1977. [Google Scholar]
- 34. Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost‐effectiveness analysis. Health Econ. 2002;11(5):415‐430. [DOI] [PubMed] [Google Scholar]
- 35. Dieng M, Cust AE, Kasparian NA, Mann GJ, Morton RL. Economic evaluations of psychosocial interventions in cancer: a systematic review. Psychooncology. 2016;25(12):1380‐1392. [DOI] [PubMed] [Google Scholar]
- 36. Jansen F, van Zwieten V, Coupé VM, Leemans CR, Verdonck‐de Leeuw IM. A review on cost‐effectiveness and cost‐utility of psychosocial care in cancer patients. Asia Pac J Oncol Nurs. 2016;3(2):125‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29(2):160‐168. [DOI] [PubMed] [Google Scholar]
- 38. Schuurhuizen C et al. Screening and treatment of psychological distress in patients with metastatic colorectal cancer: study protocol of the TES trial. BMC Cancer. 2015;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Dutch reference prices used in determining healthcare costs as mentioned in Dutch manual for conducting economical evaluations in healthcare indexed for 2016 price levels
Table S2: Detailed calculation of eMBCT and MBCT intervention costs
Table S3: Paid work descriptives per measurement (T0 – T3)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


