Abstract
Background
Troponin T1 (TNNT1) is a subunit of troponin that has been linked to neuromuscular disorder. Recently, it was reported that TNNT1 facilitates the proliferation of breast cancer cells. Interestingly, Cancer Genome Atlas data indicate that its overexpression is associated with an unfavorable prognosis of colorectal cancer (CRC) patients. The present study aimed to explore the expression, function and mechanism of dysregulation of TNNT1 in CRC.
Methods
Immunohistochemical staining and a real‐time polymerase chain reaction were used to compare the expression level of TNNT1 in CRC tissues and adjacent tissues. Western blotting was used to detect the expression of TNNT1 in cell lines. Kaplan–Meier analysis and a chi‐squared test were applied to evaluate the potential of TNNT1 to function as a cancer biomarker. RNA interference was used to inhibit TNNT1 expression in CRC cells, followed by detection of cell proliferation, apoptosis, migration and invasion. A luciferase reporter gene assay was used to determine the regulatory relationship between miR‐873 and TNNT1.
Results
In the present study, we found that TNNT1 was significantly up‐regulated in CRC samples and cell lines. The up‐regulation of TNNT1 was also associated with several clinicopathologic features, and its high expression was correlated with an unfavorable prognosis of the patients. Knockdown of TNNT1 markedly arrested proliferation, migration and invasion, whereas it also promoted apoptosis. TNNT1 was identified as a target gene of miR‐873, and there was a negative correlation among CRC samples.
Conclusions
In conclusion, we have demonstrated that TNNT1, regulated by miR‐873, is an oncogene of CRC associated with patient prognosis.
Keywords: colorectal cancer, invasion, migration, miR‐873, proliferation, TNNT1
1. INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies in the world. According to statistics, CRC causes approximately 390 000 deaths every year, and the relative survival rate is less than 40%.1, 2 Much progress has been made with respect to the diagnostic and treatment technique and curative efficacy of CRC; however, the number of CRC patients is still increasing year by year, especially in some developing countries, including China.3 Hence, an investigation of potential biological mechanisms in the development of CRC is of great significance for the prevention and treatment of such a disease in clinical practice.
Troponin T (TNT), an important protein of about 30–35 kDa, contains approximately 220–300 amino acids and is able to regulate the contraction and relaxation of striated muscle.4 According to previous studies, TNT shows the potential to be a biomarker for some human diseases.5 For example, to evaluate the levels of high‐sensitive TNT before and after treatment, it is important to evaluate cardiac dysfunction risk during the chemotherapy of breast cancer.6 One of the subunits of TNT, troponin T1 (TNNT1), is linked to nemaline myopathy type 5.7 Interestingly, TNNT1 has also been reported to be involved in the progression of breast cancer.8 However, the mechanism of action of TNNT1 in CRC, as well as its clinical significance, still remains to be clarified.
MicroRNAs (miRNAs) are a class of single‐chain non‐coding RNAs containing 18–24 nucleotides. More and more studies indicate that some miRNAs function as tumor‐promoting factors or tumor suppressors in different human tumors.8 For example, miR‐133a‐3p serves as a tumor suppressor in hepatocellular carcinoma9; the dysregulation of miR‐146b is linked to the occurrence and prognosis of papillary thyroid carcinoma10; miR‐26a regulates the proliferation and metastasis of tumor cells by modulating PTEN‐AKT axis11; miR‐873, as a member of microRNAs, plays a crucial role in regulating tumor. As reported previously, miR‐873 can regulate the stemness of breast cancer cells via regulation of PD‐L1.12 In CRC, miR‐873 can suppress the proliferation of CRC cells by targeting TRAF5 and TAB1.13 Nevertheless, the detailed roles of miR‐873 in the occurrence and development of CRC should be explored further.
In the present study, our biological analysis indicated that the prognosis of CRC patients with increased TNNT1 expression was much poorer. An in vitro experiment further showed that TNNT1 expression was up‐regulated in CRC tissues and cells, and also had a cancer‐promoting effect. Further investigation found that miR‐873 could negatively regulate TNNT1 and influence the progression of CRC. In general, the present study was carried out to provide theoretical evidence clarifying the molecular mechanisms responsible for the occurrence and development of CRC, as well as potential therapeutic regimens for CRC patients.
2. MATERIALS AND METHODS
2.1. Collection of pathological tissues
The present study was approved by the ethics review board of Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science. Forty‐five CRC tissue samples and adjacent normal tissues were collected from patients undergoing CRC surgery in Xiangyang Central Hospital. Adjacent normal tissues (at least 3 cm away from the surgical margin) collected from the same patients were used as control specimens, with no tumor cells found in a postoperative pathological examination. All of the specimens were stored in liquid nitrogen at −196°C immediately after removal.
2.2. Immunohistochemistry
Paraffin blocks containing tissues were sliced, xylene dewaxed, dehydrated and rehydrated. Subsequently, they were incubated with primary antibody (anti‐TNNT1 antibody; ab83907; dilution 1:100) overnight and secondary antibody for 30 minutes at room temperature, respectively. Subsequently, the slices were rinsed thoroughly with phosphate‐buffered saline solution. Next, DAB (Beijing Airan Biotechnology Co., Beijing, Ltd) was employed to terminate the reaction before the color was developed. Ultimately, staining was scored by pathologists in our hospital.
2.3. Cell culture
Human CRC cell lines HT29, SW480 and HCT116 and normal colonic cell line CRL1790 were purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Science (Shanghai, China). These cells were respectively cultured in Dulbeccos's modified Eagle's medium medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco) and 100 U/ml penicillin/streptomycin (Invitrogen, Shanghai, China). The cells were placed in a incubator in 5% CO2 and a thermostatic environment at 37°C. The culture solution was changed once every 3 days. Cells in the logarithmic phase were harvested for subsequent experiments.
2.4. Cell transfection
TNNT1 overexpression plasmids were transfected into HT29 cells to build a model of TNNT1 overexpression. TNNT1 targeting small interfering RNA (siRNA) was transfected into SW480 cells to establish a model of TNNT1 knockdown. GenePharma (Shanghai, China) synthesized and provided pcDNA3.1/TNNT1 and pcDNA3.1 plasmid. Ribobio Co., Ltd (Guangzhou, China) supplied siRNA targeting TNNT1, control siRNA, miR‐873 mimics, negative control mimics, miR‐873 inhibitor and control inhibitor, and the sequences were designed as described previously.14, 15 Plasmids were transfected with Lipofectamine 2000 reagent in accordance with the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA, USA). The sequences are listed in Tables 1 and 2.
Table 1.
Sequences of miR mimics and miR inhibitors
| Name | Sequence |
|---|---|
| miR‐873 mimic | 5'‐GCAGGAACUUGUGAGUCUCCUTT‐3' |
| miR‐control mimic | 5'‐UCGCUUGGUGCAGGUCGGGAATT‐3' |
| miR‐873 inhibitor | 5'‐AGGAGACUCACAAGUUCCUGCTT‐3' |
| miR‐control inhibitor | 5'‐UUCUCCGAACGUGUCACGUTT‐3' |
Table 2.
Sequences of control siRNA and TNNT1 siRNAs
| Name | Sequence |
|---|---|
| Si‐TNNT1 | 5'‐CTCTGGA CATTGACTACAT‐3' |
| Si‐control | 5'‐AATTCTCCGAACG TGTCACGT‐3' |
2.5. BrdU staining
The cells transfected were inoculated on the cover glass of a 24‐well plate and cultured overnight. BrdU (10 μg/mL) was added to the medium for 1 hour of culture before these cells were fixed in 4% paraformaldehyde for 10 minutes and stained with anti‐BrdU antibody (Biocompare, South San Francisco, CA, USA) in accordance with the manufacturer's instructions. Then, the cover glass was counterstained with 4',6‐diamidino‐2‐phenylindole to mark nuclei, and images were obtained under a fluorescence microscope (Olympus, Tokyo, Japan).
2.6. Quantitative reverse transcriptase‐polymerase chain reaction (qRT‐PCR)
RNA was extracted from tissues and cells and reversely transcribed into cDNA using a Promega Reverse Transcription System (Promega, Madison, WI, USA) in accordance with the manufacturer's instructions. qRT‐RCR was performed in the ABI 7500 real‐time PCR system (Applied Biosystems, Foster City, CA, USA) with SYBR premix EX TAQ II kit (Takara, Dalian, China) in accordance with the respective manufacturer's instructions. With GAPDH and U6 as internal references, the expression levels of TNNT1 and miR‐873 were statistically analyzed by the 2‐ΔΔCt method. The specific primers used are listed in Table 3.
Table 3.
The sequence of the primers used for qRT‐PCR
| Name | Primer sequence |
|---|---|
| TNNT1 | Forward: 5'‐AACGCGAACGTCAGGCTAAGCT‐3' |
| Reverse: 5'‐CAGGGAGAAACGACCTGGAG‐3' | |
| miR‐873 | Forward: 5'‐CAGAAGGCAAACTGCCTCTGTT‐3' |
| Reverse: 5'‐GTTAAAAGTTGATACCAACAGTG‐3' | |
| U6 | Forward: 5'‐GCTTCGGCAGCACATATACTAA‐3' |
| Reverse: 5'‐AACGCTTCACGAATTTGCGT‐3' | |
| GAPDH | Forward: 5'‐AGCCACATCGCTCAGACAC‐3' |
| Reverse: 5'‐GCCCAATACGACCAAATCC‐3' |
2.7. CCK‐8 assay
A CCK8 assay was carried out using a Cell Counting Kit‐8 (CCK‐8) in accordance with the manufacturer's instructions (MedChem Express, New Jersey, USA). Stably transfected Huh7 cells and Hep3B cells were inoculated to a 96‐well plate (1 × 103 cells/well). A 10 μL CCK‐8 solution (Hubei Biossci Biotechnology Co., Wuhan, Ltd) was added 1, 2, 3 and 4 days later, respectively. Optical density at 450 nm was measured after being cultured further at 37°C for 1 hour.
2.8. Transwell invasion assay
The migration and invasion of cells were detected by a Transwell migration and invasion experiment. Transwell chambers of pore size 8 μm (Corning, Beijing, China), coated with Matrigel (not used for the migration experiment) were used. CRC cells were trypsinized, centrifuged, resuspended in FBS‐free medium, and 5 × 104 cells were placed in the upper compartment, whereas the 10% FBS‐containing medium was added in the lower compartment. After 24 hour of culture at 37°C, cells failing to migrate or invade were removed from the upper compartment. Then, Transwell membrane was fixed in 4% paraformaldehyde for 10 minutes and stained with 0.5% crystal violet. After rinsing with running water, migrated or invaded cells were counted under an inverted microscope (Olympus).
2.9. Luciferase reporter gene assay
HT29 cells were inoculated to a 24‐well plate (5 × 105 cells/well). Wild‐type (WT) or mutant type (Mut) TNNT1 was cloned to pGL 3 Basic vector (Promega) and transfected into HT29 cells. miR‐873 mimics or control miR, respectively, was co‐transfected together with the cells described above. Luciferase activity was determined, respectively, using the dual‐luciferase reporter system (Promega) in accordance with the manufacturer's instructions, 48 hours after transfection.
2.10. Western blotting
Cells were washed with phosphate‐buffered saline and lysed with protease inhibitor‐containing RIAP lysis buffer (Thermo Science, Rockford, IL, USA). Supernatant was collected after centrifugation at a high speed. Protein quantification was performed by bicinchoninic acid method, and supernatant was heated in a water bath kettle for protein denaturation. Proteins were separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred onto a nitrocellulose (NC) membrane (Millipore, MA, USA) before 30 minutes of blocking with skim milk powder at room temperature. After the membrane was washed with Tris‐buffered saline, Tween 20 (TBST), primary antibodies anti‐TNNT1 antibody (ab83907) (dilution 1:1000; Abcam, Cambridge, UK) and anti‐GAPDH antibody (ab181602) (dilution 1:1000; Abcam) were added for culture at 4°C overnight. Then the membrane was rinsed with TBST solution and then incubated together with secondary antibody Goat Anti‐Rabbit IgG H&L (HRP) (ab205718) (dilution 1:2000; Abcam) at room temperature for 1 hour. Subsequently, color rendering was performed using an enhanced chemiluminescence kit (Pierce, Waltham, MA, USA).
2.11. Statistical analysis
SPSS, version 22.0 (IBM Corp., Armonk, NY, USA) was used to perform the statistical analysis. The data obtained are expressed as the mean ± SD. Student's t test was conducted to compare the differences in measurements between two groups. Anslysis of variance was performed to compare differences in measurements among multiple groups. The correlation between TNNT1 and pathological parameters was analyzed by a chi‐squared test. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. TNNT1 is highly expressed in CRC
To explore the characteristics of TNNT1 expression in CRC, the expression of TNTT1 in 45 pairs of CRC tissues/corresponding normal tissues was detected by immunohistochemistry. The results revealed that TNNT1 expression in CRC was significantly higher than that in normal tissues (Figure 1A). Moreover, the survival curve of TNNT1 in CRC patients was obtained from Gepia (http://gepia.cancer-pku.cn/index.html) and it was found that the overall survival of CRC patients highly expressing TNNT1 was significantly poorer (Figure 1B). To further investigate the characteristics of TNNT1 expression in CRC, the expression levels of TNNT1 in CRC tissues and normal tissues were detected by qRT‐PCR. The results suggested that TNNT1 expression in CRC tissues was significantly increased compared to normal tissues (Figure 1C). Next, the characteristics of TNNT1 expression in different cell lines were studied by western blotting, and the results indicated that, compared to the normal cell line CRL1790, the protein expression levels of TNNT1 in CRC cell lines (HT29, SW480 and HCT116) were markedly increased (Figure 1D). Collectively, these data indicated that TNNT1 was up‐regulated in CRC tissues, suggesting its oncogenic role in CRC progression.
Figure 1.
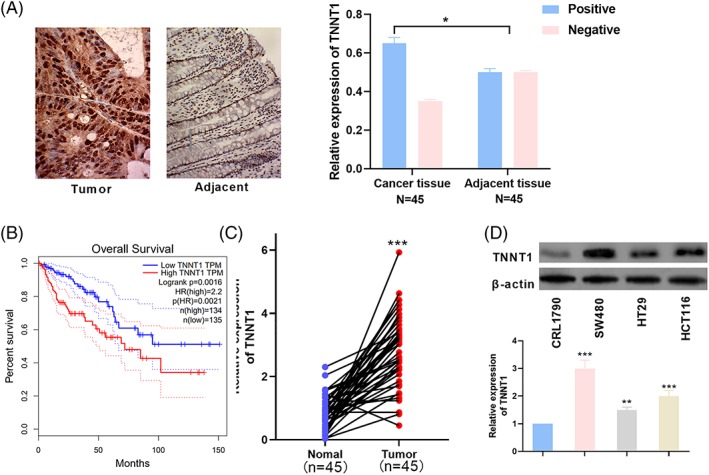
TNNT1 was up‐regulated in CRC. (A) Representative images of immunohistochemical results for TNNT1 in CRC tissues and adjacent normal tissues. (B) The survival curve of TNNT1 in CRC patients was plotted with TCGA data using Gepia. (C) qRT‐PCR was used to detect the expression of TNNT1 in normal tissues and CRC tissues. (D) RT‐PCR was used to determine the expression of TNNT1 in CRL1790, HT29, SW480 and HCT116 cell lines. ***p < 0.001
3.2. Increased TNNT1 expression is associated with pathological indices in CRC
To better understand the clinical significance of TNNT1 expression in CRC, we next investigated the correlation between TNNT1 expression and the clinical characteristics of CRC patients. According to the median mRNA expression level of TNNT1, 45 patients were divided into a high TNNT1 expression group and a low TNNT1 expression group. The data obtained indicated that high TNNT1 expression in CRC tissues was associated with an increase in T stage, degree of differentiation and lymphatic metastasis (p < 0.05), although it was irrelevant to patient's age and gender and tumor size (p > 0.05) (Table 4).
Table 4.
Correlation between TNNT1 and pathological parameters in CRC
| Pathological http://dict.cnki.net/dict_result.aspx?searchword=%e7%97%85%e7%90%86%e6%8c%87%e6%a0%87&tjType=sentence&style=&t=pathological+parameters | Number of patients | TNNT1 | Expression | Chi‐squared | p value |
|---|---|---|---|---|---|
| Low | High | ||||
| All patients | 45 | 22 | 23 | ||
| Age (years) | 0.0178 | 0.89390 | |||
| < 60 | 20 | 10 | 10 | ||
| ≥ 60 | 25 | 12 | 13 | ||
| Gender | 0.6056 | 0.43644 | |||
| Male | 19 | 8 | 11 | ||
| Female | 26 | 14 | 12 | ||
| Tumor size (cm) | 0.2624 | 0.60848 | |||
| < 3 | 29 | 15 | 14 | ||
| ≥ 3 | 16 | 7 | 9 | ||
| T stage | 9.6957 | 0.00784 | |||
| I | 13 | 11 | 2 | ||
| II | 15 | 6 | 9 | ||
| III | 17 | 5 | 12 | ||
| Differentiation | 3.7945 | 0.05142 | |||
| Well and moderate | 18 | 12 | 6 | ||
| Poor | 27 | 10 | 17 | ||
| Lymph node status | 4.1479 | 0.04168 | |||
| Yes | 17 | 5 | 12 | ||
| No | 28 | 17 | 11 |
3.3. TNNT1 can regulate the proliferation, migration and invasion of CRC cells
HT29 and SW480 cell lines were selected to build a model of TNNT1 overexpression and a model of TNNT1 knockdown, respectively (Figure 2A). To explore the influence of TNNT1 on CRC cells, the proliferation of CRC cells was detected by a CCK8 assay and a BrdU assay. The results showed that, compared to the control group, TNNT1 overexpression could promote the proliferation of CRC cells; compared to the control group, TNNT1 knockdown inhibited the proliferation of CRC cells (Figure 2B and C). Migration and invasion of CRC cells were detected by a Transwell assay and the results suggested that TNNT1 overexpression could promote the migration and invasion of CRC cells compared to the control group; TNNT1 knockdown could inhibit the migration and invasion of CRC cells compared to the control group (Figure 2D and E).
Figure 2.
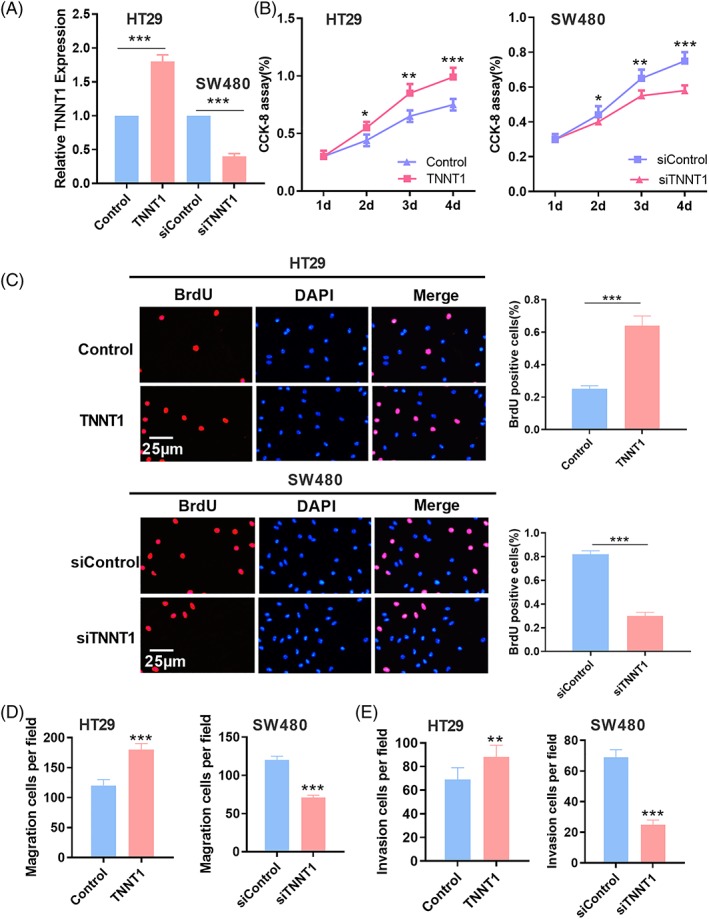
TNNT1 promoted the proliferation, migration and invasion of CRC cells. (A) Overexpression and knockdown models of TNNT1 were established and the expression of TNNT1 was detected by RT‐PCR. (B) The CCK‐8 method was used to detect the proliferation of CRC cells. (C) A BrdU assay was used to detect the proliferation of CRC cells. (D, E) The Transwell method was used to detect the migration and invasion of CRC cells. *p < 0.05, **p < 0.01 and ***p < 0.001
3.4. TNNT1 is targeted by miR‐873
To further explore the mechanism of TNNT1 dysregulation in CRC, we predicted the miRs potentially targeting TNNT1 using the miRanda database (http://www.microrna.org/microrna) and found that miR‐873 had the potential to regulate TNNT1 (Figure 3A). To obtain a deeper understanding of the characteristics of miR‐873 expression in CRC, the expression levels of miR‐873 in normal tissues and CRC tissues and CRC cell lines were detected by RT‐PCR, respectively. The results obtained indicated that, compared to normal tissues, the expression levels of miR‐873 in CRC tissues and CRC cell lines were evidently down‐regulated, which was consistent with a previous study (Figure 3B and C).15 To confirm the target relationship between TNNT1 and miR‐873, HT29 and SW480 cell lines were selected to build a model of miR‐873 overexpression and a model of miR‐873 inhibition (Figure 3D). In addition, a Pearson correlation test was used to analyze the correlation between miR‐873 and TNNT1 in CRC samples. The results confirmed that the level of miR‐873 and TNNT1 mRNA was negatively correlated (Figure 3E). Furthermore, TNNT1 expression following miR‐873 overexpression/inhibition was detected by western blotting, and the results revealed that miR‐873 overexpression decreased TNNT1 expression, whereas miR‐873 inhibition significantly up‐regulated TNNT1 expression (Figure 3F). Furthermore, a dual luciferase reporter assay indicated that miR‐873 mimics could reduce the luciferase activity of the TNNT1‐wt containing luciferase reporter and had no significant influence on the luciferase activity of TNNT1‐mut vector (Figure 3G), further confirming the target relationship between the 3'‐UTR of TNNT1 and miR‐873 in CRC. Based on these results, we concluded that the up‐regulation of TNNT1 in CRC tissues might be a result of the dysregulation of miR‐873.
Figure 3.
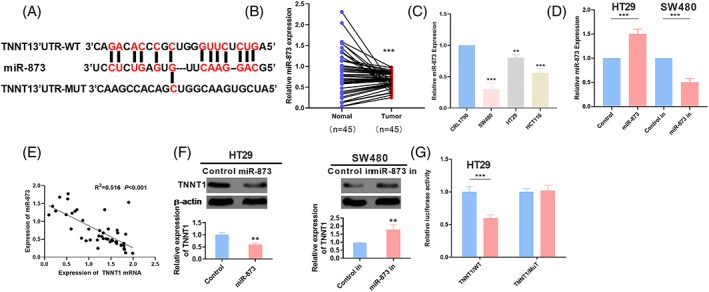
TNNT1 targeted on miR‐873. (A) The miRanda database predicted that TNNT1 was a potential target of miR‐873. (B) The expression of miR‐873 in normal tissues and CRC tissues was detected by qRT‐PCR. (C) qRT‐PCR was used to detect the expression of miR‐873 in CRL1790, HT29, SW480 and HCT116 cell lines. (D) A miR‐873 overexpression and inhibition cell model was constructed. (E) A Pearson correlation test was used to analyze the correlation between mir‐873 and tnnt1 in colorectal cancer. p < 0.001. (F) The expression of TNNT1 was detected by western blot after transfection of miR‐873 mimics or inhibitor. (G) A luciferase assay confirmed the targeted relationship between TNNT1 and miR‐873. *p < 0.05, **p < 0.01 and ***p < 0.001
3.5. miR‐873 can inhibit the proliferation, migration and invasion of CRC cells
To explore the influence of miR‐873 on CRC cells, the proliferation of CRC cells was detected by a CCK8 assay and a BrdU assay. The results indicated that, compared to the NC group, miR‐873 overexpression could significantly restrain the proliferation of CRC cells; conversely, compared to the NC group, miR‐873 inhibitors promoted the proliferation of CRC cells (Figure 4A and B). A Transwell assay was performed to investigate the role of miR‐873 in the migration and invasion of CRC cells. The results suggested that the migration and invasion of CRC cells could be markedly suppressed by the transfection of miR‐873 mimics, whereas the transfection of miR‐873 inhibitors promoted the migration and invasion of CRC cells. These results suggested the role of miR‐873 as a tumor suppressor in CRC.
Figure 4.
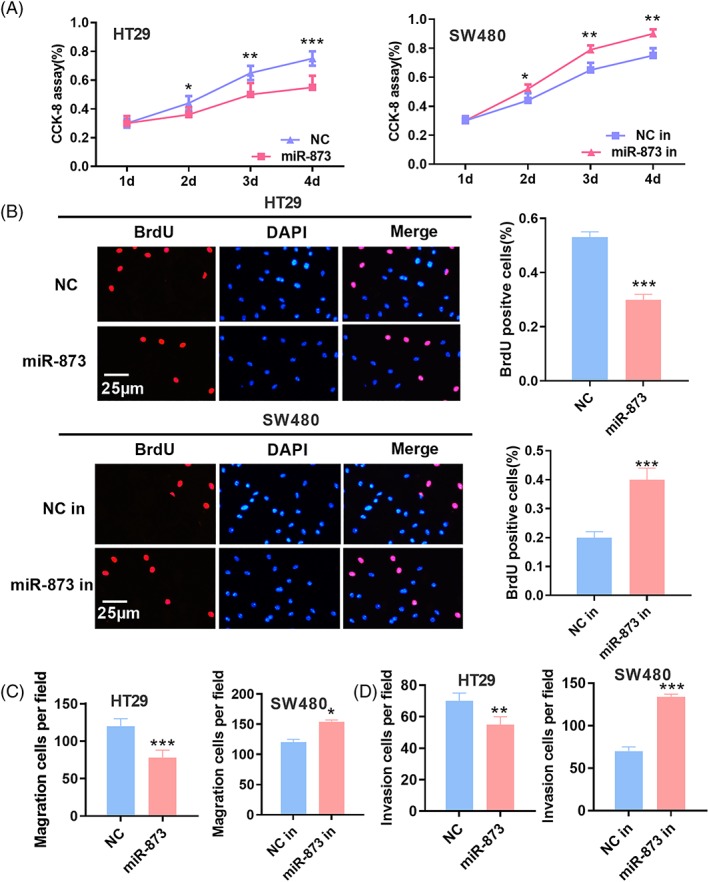
MiR‐873 inhibited the proliferation, migration and invasion of CRC cells. (A) The CCK‐8 method was used to detect the proliferation after transfection of miR‐873 mimics or inhibitors in colon cancer cells. (B) The proliferation of CRC cells was detected by a BrdU assay. (C, D) The Transwell method was used to detect the migration and invasion of CRC cells after transfection of miR‐873 mimics or inhibitor. *p < 0.05, **p < 0.01, ***p < 0.001
3.6. miR‐873/TNNT1 axis can regulate the proliferation, migration and metastasis of CRC cells
To further probe the function of miR‐873/TNNT1 axis in CRC, miR‐873 expression was up‐regulated using miR‐873 mimics on the basis of TNNT1 overexpression. TNNT1 expression and miR‐873 expression were detected by RT‐PCR and western blotting, respectively. The results indicated that, compared to the normal control, miR‐873 overexpression could significantly inhibit TNNT1 protein expression, and the introduction of TNNT1 had no obvious significance on miR‐873 expression (Figure 5A and B). Proliferation, migration and invasion of CRC cells were further detected, and the results suggested that, compared to the control group, proliferation, migration and invasion of CRC cells in miR‐873 mimics group were inhibited, and these effects could be partly reversed by TNNT1 overexpression (Figure 5C–F). These data further validated the important role of the miR‐873/TNNT1 axis with respect to the malignant phenotypes of CRC cells.
Figure 5.
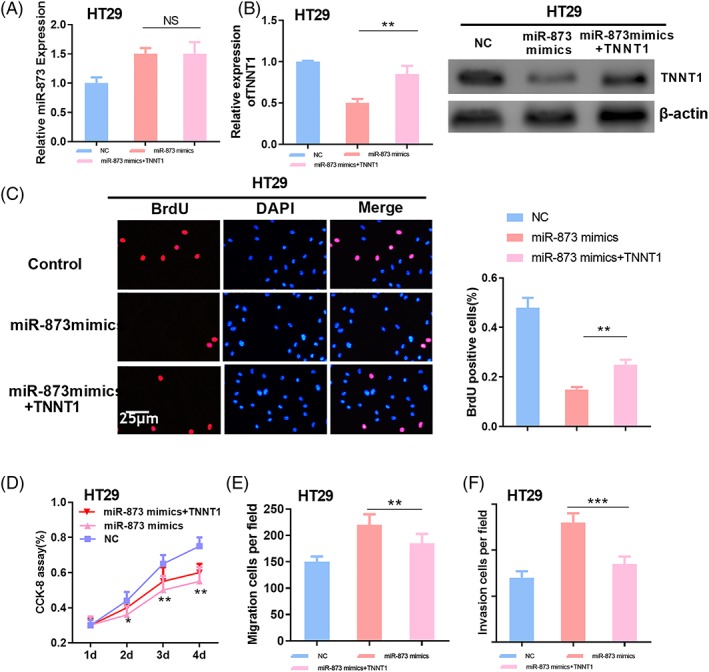
The miR‐873/TNNT1 axis can regulate the proliferation, migration and metastasis of CRC cells. (A, B) The expression of miR‐873 was detected by RT‐PCR. (B) Western blotting was used to detect the expression of TNNT1. (C) A BrdU assay was used to detect the proliferation of CRC cells. (D) The CCK‐8 method was used to detect the proliferation of CRC cells. (E, F) The Transwell method was used to detect the migration and invasion of CRC cells. *p < 0.05, **p < 0.01, ***p < 0.001
4. DISCUSSION
CRC involves a very complicated, multistage pathological process under the control of multiple genes.16 The accurate prediction of the prognosis of a CRC patient and the consequent selection of an effective therapeutic regimen is of great importance for improving patient survival.17 Molecular markers associated with the prognosis of CRC would probably guide individualized therapy to improve the survival rate of CRC patients.18 The present study showed that TNNT1 expression in CRC tissues was up‐regulated, suggesting that TNNT1 might be a biomarker of CRC.
Previous studies have reported that the mutation of TNNT1 gene leads to the complete loss of slow TNT in skeletal muscle, finally resulting in severe nemaline myopathy.19 Surprisingly, studies on the role of TNNT1 in regulating tumors have been increasing in recent years. For example, TNNT1 expression is significantly expressed in breast cancer tissue, and TNNT1 expression is is found to be closely associated with clinical stage and T and N classification, promoting the proliferation of cancer cells by shifting G1/S conversion.20 In the present study, we demonstrated that TNNT1 knockdown significantly suppressed the proliferation, migration and invasion of CRC cells, whereas TNNT1 overexpression promoted these malignant phenotypes, indicating that TNNT1 has a cancer‐promoting effect in the development of CRC, which was similar to that in breast cancer. In the future, in vivo experiments are needed to confirm the role of TNNT1 in CRC, and the downstream mechanism of TNNT1 by which it promotes cancer progression also deserves further investigation.
As an endogenous noncoding RNA, miRNA is also involved in the biological regulation of cells in many tumors, including proliferation, metastasis, multidrug resistance, stemness maintenance, and so on.21, 22 For example, miR‐873 can induce the proliferation and migration of lung adenocarcinoma cells by targeting SRCIN123; miR‐873 mediates the multidrug resistance of ovarian carcinoma cells via targeting ABCB124; miR‐873 can modulate the expression of stemness markers (OCT4 and Nanog) in breast cancer stem cells by regulating PD‐L1.12 Many experiments suggest that miR‐873 has a tumor‐inhibiting effect. The present study also found that miR‐873 expression was down‐regulated in CRC tissues compared to normal tissues. Furthermore, transfection with miR‐873 mimics that inhibit the proliferation, migration and invasion of CRC cells, at the same time as inhibiting miR‐873, could promote the proliferation, migration and invasion of CRC cells, indicating that miR‐873 functions as a tumor suppressor in the development of CRC.
In recent years, numerous studies have shown that 5' terminal (“seed” region) of miRNA can interact with the 3'‐UTR of mRNA by pairing specifically to cause its degradation or inhibit its translation,25, 26 thereby regulating the relevant proteins and its downstream signaling pathways. This process plays a critical role in the progression of tumors. For example, miR‐196b‐5p regulates the migration and metastasis of CRC cells by interacting with HOXB7 and GALNT527; miR‐103 modulates the tumorigenesis of CRC by targeting ZO‐128; and miR‐873‐5p inhibits cell migration, invasion and epithelial‐mesenchymal transition in CRC by targeting ZEB1.29 Intriguingly, our bioinformatics analysis found that there was a targeting relationship between miR‐873 and TNNT1. We designed experiments and found that TNNT1 could promote the proliferation, migration and invasion of CRC cells, and miR‐873 suppressed the proliferation, migration and invasion of CRC cells. Furthermore, our luciferase activity assay confirmed the target relationship between TNNT1 and miR‐873, and we found that miR‐873 probably acts as an upstream target molecule of TNNT1 and has an influence on CRC by regulating TNNT1.
In sum, TNNT1 can promote the proliferation, migration and invasion of CRC cells, suggesting that TNNT1 can be used as a potential marker for the diagnosis and treatment of CRC. In addition, we also found that miR‐873 functions as a tumor suppressor in CRC cells, and TNNT1 negatively regulated by miR‐873 may promote the progression of CRC. In conclusion, the present study explores some new mechanisms in the development of CRC and provides new theoretical evidence for the diagnosis and treatment of CRC.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Chen Y, Wang J, Wang D, et al. TNNT1, negatively regulated by miR‐873, promotes the progression of colorectal cancer. J Gene Med. 2020;22:e3152 10.1002/jgm.3152
Chen Yu and Wang Jinsong contributed equally to this work.
Contributor Information
Zeqiang Yan, Email: greentiger999@hotmail.com.
Manyu Chen, Email: chenmanyu2019@126.com.
DATA AVAILABILITY STATEMENT
The data used to support the findings of the present study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065 10.1038/nrdp.2015.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Issa IA, Noureddine M. Colorectal cancer screening: an updated review of the available options. World J Gastroenterol. 2017;23:5086‐5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zamani M, Hosseini SV, Mokarram P. Epigenetic biomarkers in colorectal cancer: premises and prospects. Biomarkers. 2018;23:105‐114. [DOI] [PubMed] [Google Scholar]
- 4. Jin JP, Zhang Z, Bautista JA. Isoform diversity, regulation, and functional adaptation of troponin and calponin. Crit Rev Eukaryot Gene Expr. 2008;18:93‐124. [DOI] [PubMed] [Google Scholar]
- 5. Prat A, Pineda E, Adamo B, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26‐S35. [DOI] [PubMed] [Google Scholar]
- 6. Advani P, Hoyne J, Moreno‐Aspita A, et al. High‐sensitivity troponin T and NT‐proBNP kinetics in breast cancer chemotherapy. Chemotherapy. 2017;62:334‐338. [DOI] [PubMed] [Google Scholar]
- 7. Kuroda T, Yasuda S, Nakashima H, et al. Identification of a gene encoding slow skeletal muscle troponin T as a novel marker for immortalization of retinal pigment epithelial cells. Sci Rep. 2017;7:8163 10.1038/s41598-017-08014-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tutar Y. miRNA and cancer; computational and experimental approaches. Curr Pharm Biotechnol. 2014;15:429 10.2174/138920101505140828161335 [DOI] [PubMed] [Google Scholar]
- 9. Liang HW, Yang X, Wen DY, et al. Utility of miR133a3p as a diagnostic indicator for hepatocellular carcinoma: an investigation combined with GEO, TCGA, metaanalysis and bioinformatics. Mol Med Rep. 2018;17:1469‐1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chou, C.K. , Liu, R.T. and Kang, H.Y. , MicroRNA‐146b: a novel biomarker and therapeutic target for human papillary thyroid cancer. Int J Mol Sci, 2017; 18: PMC5372649 10.3390/ijms18030636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coronel‐Hernandez J, López‐Urrutia E, Contreras‐Romero C, et al. Cell migration and proliferation are regulated by miR‐26a in colorectal cancer via the PTEN‐AKT axis. Cancer Cell Int. 2019;19:80 10.1186/s12935-019-0802-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao L, Guo Q, Li X, et al. MiR‐873/PD‐L1 axis regulates the stemness of breast cancer cells. EBioMedicine. 2019;41:395‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gong H, Fang L, Li Y, et al. miR873 inhibits colorectal cancer cell proliferation by targeting TRAF5 and TAB1. Oncol Rep. 2018;39:1090‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang G, Dong Y, Liu H, et al. Loss of miR‐873 contributes to gemcitabine resistance in triple‐negative breast cancer via targeting ZEB1. Oncol Lett. 2019;18:3837‐3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hao YH, Yu SY, Tu RS, Cai YQ. TNNT1, a prognostic indicator in colon adenocarcinoma, regulates cell behaviors and mediates EMT process. Biosci Biotechnol Biochem. 2020;84:111‐117. [DOI] [PubMed] [Google Scholar]
- 16. Hamada T, Keum N, Nishihara R, Ogino S. Molecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesis. J Gastroenterol. 2017;52:265‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Binefa G, Rodríguez‐Moranta F, Teule A, Medina‐Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol. 2014;20:6786‐6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston JJ, Kelley RI, Crawford TO, et al. A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet. 2000;67:814‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi Y, Zhao Y, Zhang Y, et al. TNNT1 facilitates proliferation of breast cancer cells by promoting G1/S phase transition. Life Sci. 2018;208:161‐166. [DOI] [PubMed] [Google Scholar]
- 21. Pratap P, Raza ST, Abbas S, Mahdi F. MicroRNA‐associated carcinogenesis in lung carcinoma. J Cancer Res Ther. 2018;14:249‐254. [DOI] [PubMed] [Google Scholar]
- 22. Carter JV, Galbraith NJ, Yang D, Burton JF, Walker SP, Galandiuk S. Blood‐based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta‐analysis. Br J Cancer. 2017;116:762‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao Y, Xue Q, Wang D, du M, Zhang Y, Gao S. miR‐873 induces lung adenocarcinoma cell proliferation and migration by targeting SRCIN1. Am J Transl Res. 2015;7:2519‐2526. [PMC free article] [PubMed] [Google Scholar]
- 24. Wu DD, Li XS, Meng XN, Yan J, Zong ZH. MicroRNA‐873 mediates multidrug resistance in ovarian cancer cells by targeting ABCB1. Tumour Biol. 2016;37:10499‐10506. [DOI] [PubMed] [Google Scholar]
- 25. Guo L, Zhang Q, Ma X, Wang J, Liang T. miRNA and mRNA expression analysis reveals potential sex‐biased miRNA expression. Sci Rep. 2017;7:39812 10.1038/srep39812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Tzeng CM. Integrated analysis of miRNA and mRNA expression profiles to identify miRNA targets. Methods Mol Biol. 2018;1720:141‐148. [DOI] [PubMed] [Google Scholar]
- 27. Stiegelbauer V, Vychytilova‐Faltejskova P, Karbiener M, et al. miR‐196b‐5p regulates colorectal cancer cell migration and metastases through interaction with HOXB7 and GALNT5. Clin Cancer Res. 2017;23:5255‐5266. [DOI] [PubMed] [Google Scholar]
- 28. Ke J, Shao W, Jiang Y, Xu J, Li F, Qin J. MicroRNA103 regulates tumorigenesis in colorectal cancer by targeting ZO1. Mol Med Rep. 2018;17:783‐788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li G, Xu Y, Wang S, Yan W, Zhao Q, Guo J. MiR‐873‐5p inhibits cell migration, invasion and epithelial‐mesenchymal transition in colorectal cancer via targeting ZEB1. Pathol Res Pract. 2019;215:34‐39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of the present study are available from the corresponding author upon reasonable request.


