ABSTRACT
Sclerostin, a protein produced by osteocytes, inhibits bone formation. Administration of sclerostin antibody results in increased bone formation in multiple animal models. Romosozumab, a humanized sclerostin antibody, has a dual effect on bone, transiently increasing serum biochemical markers of bone formation and decreasing serum markers of bone resorption, leading to increased BMD and reduction in fracture risk in humans. We aimed to evaluate the effects of romosozumab on bone tissue. In a subset of 107 postmenopausal women with osteoporosis in the multicenter, international, randomized, double‐blind, placebo‐controlled Fracture Study in Postmenopausal Women with Osteoporosis (FRAME), transiliac bone biopsies were performed either after 2 (n = 34) or 12 (n = 73) months of treatment with 210 mg once monthly of romosozumab or placebo to evaluate histomorphometry and microcomputed tomography‐based microarchitectural endpoints. After 2 months, compared with either baseline values assessed after a quadruple fluorochrome labeling or placebo, significant increases (P < 0.05 to P < 0.001) in dynamic parameters of formation (median MS/BS: romosozumab 1.51% and 5.64%; placebo 1.60% and 2.31% at baseline and month 2, respectively) were associated with a significant decrease compared with placebo in parameters of resorption in cancellous (median ES/BS: placebo 3.4%, romosozumab 1.8%; P = 0.022) and endocortical (median ES/BS: placebo 6.3%, romosozumab 1.6%; P = 0.003) bone. At 12 months, cancellous bone formation was significantly lower (P < 0.05 to P < 0.001) in romosozumab versus placebo and the lower values for resorption endpoints seen at month 2 persisted (P < 0.001), signaling a decrease in bone turnover (P = 0.006). No significant change was observed in periosteal and endocortical bone. This resulted in an increase in bone mass and trabecular thickness with improved trabecular connectivity, without significant modification of cortical porosity at month 12. In conclusion, romosozumab produced an early and transient increase in bone formation, but a persistent decrease in bone resorption. Antiresorptive action eventually resulted in decreased bone turnover. This effect resulted in significant increases in bone mass and improved microarchitecture.© 2019 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals, Inc. on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: BONE HISTOMORPHOMETRY, MICROCOMPUTED TOMOGRAPHY, OSTEOPOROSIS, BONE MODELING, BONE REMODELING
Introduction
Osteoporosis is a skeletal disorder characterized by a deterioration in bone mass, microarchitecture, and strength, with a consequent increase in fracture risk.1 In postmenopausal women, this bone loss results from an imbalance between bone resorption and formation, with resorption exceeding formation. Available antiosteoporotic treatments are divided into two main categories: (1) inhibitors of resorption, such as selective estrogen receptor modulators, bisphosphonates, or denosumab, an antibody against RANKL, which are used for the chronic treatment of osteoporosis; and (2) stimulators of bone formation, such as parathyroid hormone analogs, which act by increasing remodeling, and are used to rapidly improve bone mass and structure. Because resorption and formation are coupled during remodeling, bisphosphonates, the most prescribed antiresorptive drugs, markedly decrease bone turnover, inducing a gradual augmentation of the BMD. BMD is initially increased because of a reduction in the remodeling space, a component of which is the degree of matrix mineralization, at least for the first few years of treatment, with other mechanisms potentially contributing to ongoing gains beyond 5 years, depending on the agent.2, 3 In contrast, parathyroid hormone analogs increase bone formation, resorption, and turnover, as well as increase BMD, mainly by increasing bone formation.4
Sclerostin is a protein produced by the osteocytes that inhibits bone formation by inhibiting canonical Wnt signaling.5, 6 Inherited sclerostin deficiency is characterized by a high bone mass, high BMD, and a reduced fracture risk.7 Romosozumab (Amgen, Thousand Oaks, CA, USA and UCB Pharma, Brussels, Belgium), a bone‐forming agent, is a humanized monoclonal antibody that binds and inhibits sclerostin, thereby promoting osteoblast differentiation and activity. Romosozumab results in increased cortical and cancellous bone formation, mass, and strength, as reported in different animal models.8, 9, 10 Previous studies in humans have shown a transient increase in bone formation markers and a decrease in bone resorption markers.11, 12, 13, 14 In postmenopausal women with moderate osteoporosis, this dual effect of romosozumab led to significant and large increases in BMD and a reduction in fracture risk compared with placebo.15 In postmenopausal women with more severe osteoporosis, romosozumab treatment led to more marked BMD increases than alendronate and superior fracture risk reduction.16
The purpose of the present study was to characterize the effects of romosozumab on bone tissue by bone histomorphometry early in treatment at 2 months and to evaluate in another patient cohort the evolution of these effects after 12 months of romosozumab administration in postmenopausal women with osteoporosis. Bone histomorphometry allows the study of bone at the tissue or cell level to assess the intermediary levels of organization of bone (ie, the osteon or basic structural unit in cortical and cancellous bone). The use of fluorochrome labeling allows the measurement of dynamic parameters and adds a time dimension to the quantitative analysis. Transiliac bone biopsies were obtained at 2 months or 12 months in two different cohorts. For the biopsies obtained at 2 months, the quadruple fluorochrome labeling procedure was used to compare, in a single bone biopsy, the dynamic bone formation parameters at baseline and after 2 months of romosozumab. This technique, when performed over a short period, such as 2 months, allows the evaluation of longitudinal data with only one biopsy, each serving as her own pretreatment control. In addition, the 3D analysis of the bone structure and microarchitecture was performed by microcomputed tomography (µCT).
Materials and Methods
Study design
The Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) was a phase III, multicenter, international, randomized, double‐blind, placebo‐controlled parallel‐group study (ClinicalTrials.gov identifier: NCT01575834) to assess the effects of a monthly subcutaneous administration of 210 mg romosozumab compared with placebo for 12 months; both groups then received an additional 12 months of open‐label denosumab at a dose of 60 mg every 6 months, as previously reported.15 A total of 222 centers in Europe, Central/Latin America, Asia, North America, and Australia/New Zealand participated in this study, and 18 centers in Europe, Central/Latin America, North America, and New Zealand contributed samples to the bone biopsy analysis presented here.
Randomization and masking
In the FRAME trial, patients were randomized 1:1 in a double‐blind manner to either the romosozumab or placebo treatment group using an interactive voice‐response system. Randomization was stratified by age (< 75 years, ≥ 75 years) and prevalent vertebral fracture (yes versus no). The study received ethical review board approval at all sites, and all patients provided written informed consent. The study was conducted in accordance with the principles stated in the Declaration of Helsinki, and was performed according to the rules of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guidelines for Good Clinical Practice.15 Patients enrolled in centers involved in the bone biopsy substudy were invited to participate and have one biopsy at month 2 or 12.
Study population
Ambulatory women with osteoporosis aged 55 to 90 years, were included if a T‐score measured with DXA at the total hip or femoral neck level was ≤ −2.5 SD. Patients were excluded if their T‐score was ≤ −3.5 SD; they had a history of hip fracture; they had any severe or more than two moderate vertebral fractures on lateral spine X‐ray; they had a history of disease affecting bone metabolism other than osteoporosis; they had a history of malignancy during the past 5 years; they had a contraindication for bone biopsy (coagulation abnormality, anticoagulant medication, hip prosthesis, or severe obesity); they were intolerant or contraindicated to demeclocycline or tetracycline or its derivatives; they had current uncontrolled hypo‐ or hyperparathyroidism; they had current hypo‐ or hypercalcemia; they had vitamin D insufficiency defined as 25‐hydoxy vitamin D < 20 ng/mL; they had a history of solid organ or bone marrow transplants; or they had a history of osteonecrosis of the jaw. The use of agents affecting bone metabolism was also exclusionary; however, for selected therapies, permissible off‐treatment periods were allowed before randomization.
A total of 7180 patients were randomized in the FRAME study and received monthly 210 mg romosozumab or placebo. All patients were supplemented with 500 to 1000 mg elemental calcium and 600 to 800 IU vitamin D per day. A separate informed consent was collected on 272 patients to participate in the bone biopsy substudy.
Bone biopsy
One transiliac bone biopsy was performed with a 7.5‐mm inner diameter trephine in 107 patients at either month 2 (n = 34) or at month 12 (n = 73). For the month 2 bone biopsy, patients received oral quadruple labeling as follows: a first set before the initiation of the treatment (baseline labeling) with two 3‐day cycles of 600 mg/day demeclocycline or two 3‐day cycles of 200 mg/day doxycycline with a no‐label 10‐day interval. Treatment was initiated immediately after the completion of the first labeling set. It was followed by a second set before the biopsy (month 2 labeling) with two 2‐day cycles of 1 g/day tetracycline hydrochloride separated by a no‐label 10‐day interval. For the month 12 bone biopsy, patients received oral double labeling as follows: two 3‐day cycles of 1 g/day tetracycline hydrochloride separated by a no‐label 10‐day interval. At month 2 and month 12, biopsies were performed within 5 to 14 days of the last labeling (Fig. 1). The bone biopsy specimens were stored and transported to the central laboratory in 70% ethanol in the dark to prevent the labels from fading (INSERM UMR 1033, Lyon, France) for the µCT analysis, histological processing, reading, and interpretation of the results.
Figure 1.
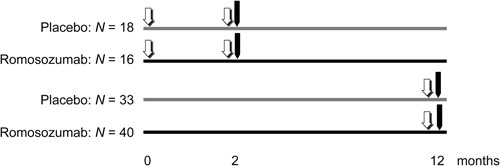
Schema of the bone biopsy substudy. (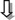 one set of double fluorochrome labeling,
one set of double fluorochrome labeling,  transiliac bone biopsy)
transiliac bone biopsy)
Bone histomorphometry
After fixation in 70% ethanol and dehydration in 100% ethanol, specimens were embedded in methylmethacrylate. Three sets of 8‐µm‐thick sections were cut, separated by 200 µm in the central part of the sample. In each set, sections were stained with modified Goldner's trichrome, solochrome cyanin R, toluidine blue, or May‐Grünwald‐Giemsa. Some sections were left unstained for the measurement of the fluorochrome labels by fluorescent microscopy.17
Sections from each biopsy were evaluated qualitatively for assessment of mineralization, osteomalacia, or any abnormalities of bone marrow (eg, the presence of lymphoid nodules, fibrosis, or metastases), and the type of bone (ie, woven or lamellar bone).
A quantitative analysis was performed on all complete and unbroken samples. The histomorphometry measurements were performed on whole tissue at month 2, including the cancellous (Cn), endocortical (Ec), intracortical (Ct), and periosteal (Ps) envelopes, and on Cn, Ec, and Ct envelopes at month 12 using three sections (one per set), with a total Cn bone tissue area of the three sections ≥ 20 mm2. The Ec surface was defined according to previously published methods.18 For all analyses, the investigators were blinded to treatment allocation, and only the timing of the biopsy (month 2 or 12) was known.
The parameters of bone structure were measured with an automatic image analyzer (Bone V3.5; Explora Nova, La Rochelle, France). The static parameters reflecting resorption and formation, and the dynamic parameters of bone formation and mineralization were measured using a semiautomatic image analyzer (Tablet’Measure V1.54; Explora Nova). The abbreviations of the bone histomorphometric parameters used were those recommended by the American Society for Bone and Mineral Research (ASBMR) Histomorphometric Nomenclature Committee.19 All measured thicknesses (except cortical thickness [Ct.Th]) were multiplied by π/4 for correction of obliquity. Structural parameters included Ct.Th (µm), cortical porosity (Ct.Po, %), and cancellous bone volume (Cn‐BV/TV, %). The parameters of microarchitecture (trabecular thickness [Tb.Th, µm], number [Tb.N, /mm], and separation [Tb.Sp, µm]) were derived from area and perimeter measurements according to Parfitt's formulae.20 Bone resorption was assessed with measurements of eroded surface (ES/BS, %), osteoclast number (Oc.N/BS, /100 mm), and osteoclast surface (Oc.S/BS, %). Static bone formation was reflected by osteoid surface (OS/BS, %), volume (OV/BV, %), and thickness (O.Th, µm). Osteoid seams with a minimum width of 2.5 µm were measured. All of these parameters were measured on Goldner‐stained sections. The mineral apposition rate (MAR, µm/day) and ratio of mineralizing surface to bone surface (MS/BS, % calculated as double plus half of single‐labeled surfaces) were analyzed on unstained sections under ultraviolet light. Mean wall thickness (W.Th, µm) was measured on solochrome cyanin R‐stained sections under polarized light. Bone formation rate (BFR/BS, µm3/µm2/year; = [MS/BS] × MAR × 365), adjusted apposition rate (Aj.AR, µm/day; = BFR/osteoid surface [OS]), formation period (FP, days; = W.Th/Aj.AR), mineralization lag time (Mlt, days; = O.Th/Aj.AR) and activation frequency (Ac.f, per year; = [BFR/BS]/W.Th) were calculated. On month 2 biopsies, all parameters were measured on Cn bone. Dynamic parameters (MAR, MS/BS, and BFR/BS) were also measured on Cn bone on baseline labeling and on Ec, Ct, and Ps bone on the baseline and month 2 labeling. On month 12 biopsies, all parameters were measured on Cn bone; parameters of bone resorption (ES/BS, Oc.S/BS, and Oc.N/BS) were also measured on Ec bone; and dynamic parameters (MAR, MS/BS, and BFR/BS) were measured on Ec and Ct bone.
For biopsy specimens missing double labeling in the analyzed bone, the value of MS/BS was set to 0 and the parameters derived, MAR and BFR/BS, were indicated as missing. When only single labels were present, the dynamic parameters were also derived with MAR value imputed by 0.3 µm/d.21
Microcomputed tomography
Biopsies were scanned before embedding using a Bruker µCT Skyscan 1174 (Bruker, Aarteselaar, Belgium). Scans were performed at 0.6‐degree rotations for 180 degrees (50 kV to 800 µA; 0.5‐mm aluminum filter), with a nominal isotropic voxel size of 19 µm. The 3D analysis was performed using Skyscan CTan software using a bone threshold of 0.3457 g/cm3. Cn and cortical bone parameters included: trabecular bone volume per tissue volume (Tb.BV/TV, %), Tb.Th (mm), Tb.Sp (mm), Tb.N (/mm), structure model index (SMI, #), trabecular bone pattern factor (TBPf, /mm), trabecular connectivity density (Conn. D, #), trabecular BMD (Tb.BMD, mg/cm3), and trabecular tissue BMD (Tb.TMD, mg/cm3), Ct.Th (mm), Ct.Po (%), cortical BMD (Ct.BMD, mg/cm3), and cortical tissue bone mineral density (Ct.TMD, mg/cm3).
Statistical analysis
Continuous variables were expressed as the median (quartiles 1 and 3). Within‐subject paired comparisons between baseline and month 2 in each treatment group were assessed using the Wilcoxon signed rank test. Between‐group comparisons at month 2 and month 12 were based on the Wilcoxon rank sum test. All p values reported were nominal without adjusting for multiplicity. Correlation between Cn‐W.Th and Tb.Th was assessed based on the Pearson correlation coefficient.
Results
Patient baseline characteristics were generally balanced between treatment groups in both the month 2 and month 12 cohorts, with the exception of prior osteoporotic fracture, where a greater proportion of the placebo‐treated patients had prior fractures (Table 1). Overall baseline characteristics were representative of the general study population reported previously.15 A transiliac biopsy was obtained in 34 patients (placebo, n = 18; romosozumab, n = 16) at month 2 and 73 patients (placebo, n = 33; romosozumab, n = 40) at month 12. All bone samples were qualitatively analyzed, but eight biopsies were excluded from the quantitative analysis because of the poor quality of the biopsy (ie, broken or incomplete sample with a Cn bone area lower than 20 mm2).
Table 1.
Baseline Patient Characteristics
| Month 2 cohort | Month 12 cohort | |||
|---|---|---|---|---|
| Romosozumab | Romosozumab | |||
| Placebo | 210 mg QM | Placebo | 210 mg QM | |
| (N = 18) | (N = 16) | (N = 33) | (N = 40) | |
| Age, years | 72.5 | 70.0 | 70.0 | 72.5 |
| (63.0, 75.0) | (65.0, 73.0) | (66.0, 77.0) | (68.0, 76.0) | |
| BMI, kg/m2 | 24.15 | 24.10 | 23.60 | 23.90 |
| (21.80, 27.20) | (22.75, 26.10) | (21.90, 25.70) | (21.75, 26.30) | |
| Prior osteoporotic fracture, n (%) | 5 (27.8) | 0 (0.0) | 14 (42.4) | 11 (27.5) |
| Prevalent vertebral fracture, n (%) | 3 (16.7) | 0 (0.0) | 3 (9.1) | 9 (22.5) |
| BMD T−score: | ||||
| Lumbar L1 to L4 | −3.12 | −2.77 | −3.00 | −2.88 |
| (−3.55, −2.20) | (−3.41, −1.97) | (−3.60, −2.07) | (−3.46, −1.82) | |
| Total hip | −2.39 | −2.47 | −2.56 | −2.52 |
| (−2.72, −2.21) | (−2.61, −2.01) | (−2.90, −2.13) | (−2.78, −2.21) | |
| Femoral neck | −2.67 | −2.72 | −2.81 | −2.74 |
| (−2.88, −2.55) | (−2.87, −2.61) | (−2.94, −2.64) | (−2.93, −2.60) | |
N = number of randomized patients who enrolled in the bone biopsy substudy, received at least one dose of investigational product, and had an evaluable biopsy. Values are median (Q1, Q3) unless otherwise specified.
Q1, Q3 = quartiles 1 and 3; QM = once monthly.
Qualitative analysis
Most patients had a complete biopsy specimen: 14 and 15 patients at month 2 and 31 and 39 patients at month 12 in the placebo and romosozumab groups, respectively. All biopsy specimens had a normal lamellar texture. There was no evidence for osteomalacia, Paget's disease, or bone marrow abnormalities in any of the biopsy specimens. At month 2, labels were present in all biopsy specimens. At month 12, no label was observed in one biopsy in the placebo group and two biopsies in the romosozumab group.
Quantitative bone histomorphometry
Effects of romosozumab at month 2: comparison with baseline
The quadruple labeling allowed the measurements of the dynamic parameters of bone formation at baseline and after 2 months of treatment on the same bone specimen. When compared with baseline, no change was observed in the placebo group (Cn‐MS/BS: 1.60% and 2.31% at baseline and month 2, respectively). In contrast, after 2 months of romosozumab treatment, the labeled surfaces (MS/BS) and BFR/BS were significantly increased in Cn (Cn‐MS/BS: 1.51% and 5.64% at baseline and month 2, respectively, P < 0.001) and Ec bone (Ec‐MS/BS: 6.26% and 24.59% at baseline and month 2, respectively, P < 0.001; Table 2, Fig. 2). The percent change of these parameters between month 2 and baseline was significantly higher in the romosozumab versus the placebo group. MS/BS increased by 325% and 247%, and BFR/BS increased by 328% and 233% in Cn and Ec bone, respectively. In Ct bone, double‐labeled surfaces were significantly increased (P < 0.05) at month 2 when compared with baseline, and no significant change was observed on the Ps bone surface. In the four bone compartments, MAR was not significantly modified by romosozumab.
Table 2.
Dynamic Histomorphometric Parameters of Bone Formation at Baseline and After 2 Months of Romosozumab in Patients With Quadruple Fluorochrome Labeling
| Placebo (N = 18) | Romosozumab 210 mg QM (N = 16) | |||||||
|---|---|---|---|---|---|---|---|---|
| n a | Baseline b | Month 2 | Paired p valuec | n a | Baseline b | Month 2 | Paired p valuec | |
| Cancellous bone | ||||||||
| Cn‐MAR d | 14 | 0.61 | 0.65 | 0.84 | 14 | 0.59 | 0.57 | 0.62 |
| µm/day | (0.56, 0.69) | (0.54, 0.70) | (0.53, 0.65) | (0.51, 0.59) | ||||
| Cn‐MARe | 13 | 0.61 | 0.66 | 0.88 | 13 | 0.59 | 0.57 | 0.29 |
| µm/day | (0.57, 0.69) | (0.61, 0.70) | (0.54, 0.65) | (0.51, 0.58) | ||||
| Cn‐MS/BS | 14 | 1.60 | 2.31 | 0.27 | 15 | 1.51 | 5.64 | <0.001 |
| % | (0.49, 2.19) | (0.72, 3.14) | (0.57, 3.15) | (3.71, 8.42) | ||||
| Cn‐BFR/BS d | 14 | 3.078 | 5.175 | 0.24 | 14 | 3.381 | 12.486 | <0.001 |
| µm3/µm2/year | (1.307, 5.359) | (2.919, 7.165) | (1.647, 6.776) | (7.734, 16.132) | ||||
| Cn‐BFR/BSe | 13 | 3.457 | 5.565 | 0.31 | 13 | 3.584 | 12.898 | <0.001 |
| µm3/µm2/year | (1.648, 5.359) | (4.347, 7.165) | (1.960, 6.776) | (7.788, 16.132) | ||||
| Endocortical bone | ||||||||
| Ec‐MAR d | 12 | 0.62 | 0.61 | 0.65 | 15 | 0.62 | 0.58 | 0.92 |
| µm/day | (0.53, 0.67) | (0.53, 0.72) | (0.51, 0.66) | (0.56, 0.66) | ||||
| Ec‐MARe | 12 | 0.62 | 0.61 | 0.65 | 14 | 0.63 | 0.58 | 0.55 |
| µm/day | (0.53, 0.67) | (0.53, 0.72) | (0.58, 0.66) | (0.56, 0.62) | ||||
| Ec‐MS/BS | 14 | 7.65 | 7.00 | 0.24 | 15 | 6.26 | 24.59 | <0.001 |
| % | (3.49, 12.14) | (3.27, 9.92) | (3.05, 9.52) | (15.98, 31.50) | ||||
| Ec‐BFR/BS d | 12 | 18.715 | 15.191 | 0.34 | 15 | 14.051 | 52.260 | <0.001 |
| µm3/µm2/year | (12.949, 24.711) | (10.985, 21.197) | (8.251, 24.090) | (33.748, 64.875) | ||||
| Ec‐BFR/BSe | 12 | 18.715 | 15.191 | 0.34 | 14 | 14.384 | 52.361 | <0.001 |
| µm3/µm2/year | (12.949, 24.711) | (10.985, 21.197) | (8.506, 24.090) | (35.300, 64.875) | ||||
| Intracortical bone | ||||||||
| Ct‐MAR d | 12 | 0.64 | 0.75 | 0.032 | 15 | 0.60 | 0.67 | 0.31 |
| µm/day | (0.59, 0.70) | (0.65, 0.85) | (0.55, 0.76) | (0.58, 0.75) | ||||
| Ct‐MARe | 11 | 0.64 | 0.76 | 0.032 | 15 | 0.60 | 0.67 | 0.31 |
| µm/day | (0.60, 0.73) | (0.66, 0.85) | (0.55, 0.76) | (0.58, 0.75) | ||||
| Ct‐MS/BS | 14 | 7.46 | 4.59 | 0.95 | 15 | 4.89 | 8.23 | 0.12 |
| % | (2.73, 11.06) | (1.41, 8.47) | (3.62, 6.51) | (4.99, 10.56) | ||||
| Ct‐BFR/BS d | 12 | 20.735 | 17.557 | 0.68 | 15 | 12.515 | 19.202 | 0.064 |
| µm3/µm2/year | (8.914, 27.049) | (5.407, 29.472) | (7.425, 15.411) | (13.113, 32.024) | ||||
| Ct‐BFR/BSe | 11 | 22.302 | 18.383 | 0.70 | 15 | 12.515 | 19.202 | 0.064 |
| µm3/µm2/year | (13.074, 30.450) | (7.103, 32.851) | (7.425, 15.411) | (13.113, 32.024) | ||||
| Periosteal bone | ||||||||
| Ps‐MAR d | 5 | 0.24 | 0.24 | 1.00 | 2 | 0.59 | 0.24 | 0.50 |
| µm/day | (0.24, 0.34) | (0.24, 0.55) | (0.40, 0.78) | (0.24, 0.24) | ||||
| Ps‐MARe | 1 | 0.34 | 0.55 | 1.00 | 0 | ‐ | ‐ | ‐ |
| µm/day | (0.34, 0.34) | (0.55, 0.55) | (−, −) | (−, −) | ||||
| Ps‐MS/BS | 14 | 0.00 | 0.07 | 0.11 | 15 | 0.00 | 0.47 | 0.083 |
| % | (0.00, 0.59) | (0.00, 1.10) | (0.00, 0.00) | (0.00, 1.64) | ||||
| Ps‐BFR/BS d | 5 | 0.507 | 3.195 | 0.063 | 2 | 1.854 | 0.688 | 0.50 |
| µm3/µm2/year | (0.206, 1.199) | (2.273, 5.229) | (1.235, 2.473) | (0.189, 1.187) | ||||
| Ps‐BFR/BSe | 1 | 1.199 | 3.195 | 1.00 | 0 | ‐ | ‐ | ‐ |
| µm3/µm2/year | (1.199, 1.199) | (3.195, 3.195) | (−, −) | (−, −) | ||||
N = number of randomized patients who enrolled in the bone biopsy substudy, received at least one dose of investigational product, and had an evaluable biopsy. All values are median (Q1, Q3) unless otherwise specified.
BFR/BS = bone formation rate per unit of bone surface; Cn = cancellous; Ct = intracortical; Ec = endocortical; MAR = mineral apposition rate; MS/BS = ratio of mineralizing surface to bone surface; Ps = periosteal; Q1, Q3 = quartiles 1 and 3; QM = once monthly.
n = number of biopsies with measurements at both baseline and month 2.
Measurements on the first set of double labeling performed at baseline before treatment. cthe Wilcoxon signed rank test.
With and ewithout imputation when only single labels were identified.
Figure 2.
Effects of romosozumab on bone formation after 2 months. Unstained section of iliac bone biopsy after a quadruple fluorochrome labeling (star: demeclocycline labels at baseline; arrow: tetracycline labels at month 2). Cn = cancellous, Ct = cortical, Ec = endocortical. Original magnification: × 50; box magnification: × 200
Effects of romosozumab: comparison with placebo
At month 2 in Cn bone, the median value of osteoid surfaces was higher in the romosozumab versus the placebo group, but the difference did not reach significance (Cn‐OS/BS: 7.2% and 14.2% in the placebo and romosozumab groups, respectively, P = 0.058; Table 3). Romosozumab induced a significant increase in osteoid volume at month 2 (P = 0.007), but at month 12, osteoid volume was significantly less (P = 0.016) when compared with placebo. At month 2, the dynamic parameters reflecting bone formation at the tissue level, ie, when referred to BS or BV (MS/BS, BFR/BS, and BFR/BV) were significantly augmented (Cn‐MS/BS: 2.3% and 5.6%, P = 0.002; Cn‐BFR/BS: 5.175 and 12.075 µm3/µm2/year, P = 0.004 in the placebo and romosozumab groups, respectively) and the activation frequency appeared to be higher in the romosozumab versus the placebo group; however, at month 12, these parameters were significantly lower in the romosozumab group versus the placebo group. This reduction in bone formation at month 12 was associated with an extension of the formation period (FP), a delay of the onset of mineralization (Mlt), and a reduction of the mineral apposition rate (MAR) versus placebo. The amount of mineralized bone tissue formed at the individual structural unit (W.Th) in Cn bone was significantly higher in the romosozumab versus the placebo group at month 12 (Table 3).
Table 3.
Static and Dynamic Bone Formation Parameters After 2 and 12 Months of Romosozumab
| Month 2 | Month 12 | |||||
|---|---|---|---|---|---|---|
| Romosozumab | Romosozumab | |||||
| Placebo | 210 mg QM | Placebo | 210 mg QM | |||
| N = 14 | N = 15 | p value a | N = 31 | N = 39 | p value a | |
| Cancellous bone | ||||||
| Cn‐W.Th | 31.7 b | 31.6 | 0.91 | 29.5 | 31.8 | 0.014 |
| µm | (30.4, 33.9) | (30.7, 33.6) | (27.8, 32.3) | (30.8, 34.1) | ||
| Cn‐OS/BS | 7.2 | 14.2 | 0.058 | 7.8 | 4.4 | 0.16 |
| % | (1.7, 15.5) | (9.4, 24.3) | (3.7, 15.4) | (2.8, 9.0) | ||
| Cn‐OV/BV | 1.3 | 3.0 | 0.007 | 1.7 | 0.8 | 0.016 |
| % | (0.2, 1.9) | (1.4, 5.4) | (0.8, 4.5) | (0.4, 1.7) | ||
| Cn‐O.Th | 8.6 | 9.7 | 0.029 | 9.9 | 9.7 | 0.57 |
| µm | (6.9, 9.5) | (9.0, 12.6) | (8.5, 12.5) | (8.6, 11.0) | ||
| Cn‐MAR c | 0.65 | 0.57 | 0.097 | 0.54 | 0.48 | 0.015 |
| µm/day | (0.54, 0.70) | (0.50, 0.59) | (0.50, 0.61) | (0.36, 0.55) | ||
| Cn‐MARd | 0.65 | 0.57 | 0.097 | 0.55 | 0.49 | 0.047 |
| µm/day | (0.54, 0.70) | (0.50, 0.59) | (0.50, 0.61) | (0.41, 0.58) | ||
| Cn‐MS/BS | 2.3 | 5.6 | 0.002 | 3.0 | 0.6 | 0.004 |
| % | (0.7, 3.1) | (3.7, 8.4) | (0.9, 5.4) | (0.0, 2.2) | ||
| Cn‐BFR/BS c | 5.175 | 12.075 | 0.004 | 6.755 | 1.577 | 0.014 |
| µm3/µm2/year | (2.919, 7.165) | (7.319, 16.132) | (2.691, 13.213) | (0.928, 6.452) | ||
| Cn‐BFR/BSd | 5.175 | 12.075 | 0.004 | 6.923 | 3.395 | 0.046 |
| µm3/µm2/year | (2.919, 7.165) | (7.319, 16.132) | (2.736, 13.213) | (1.310, 7.332) | ||
| Cn‐BFR/BV | 11.0 | 23.1 | 0.005 | 13.3 | 3.3 | 0.001 |
| %/year | (6.6, 14.8) | (14.2, 31.5) | (4.4, 26.2) | (1.6, 8.9) | ||
| Cn‐Aj.AR | 0.19 | 0.18 | 0.76 | 0.20 | 0.09 | 0.043 |
| µm/day | (0.08, 1.07) | (0.14, 0.36) | (0.13, 0.26) | (0.06, 0.29) | ||
| Cn‐Ac.f | 0.18 | 0.38 | 0.003 | 0.24 | 0.05 | 0.006 |
| /year | (0.10, 0.21) | (0.23, 0.49) | (0.09, 0.46) | (0.03, 0.18) | ||
| Cn‐FP | 166.5 | 176.4 | 0.76 | 150.0 | 369.8 | 0.018 |
| days | (29.7, 379.2) | (84.2, 232.3) | (109.8, 228.7) | (130.3, 524.9) | ||
| Cn‐Mlt | 48.5 | 62.7 | 0.62 | 56.3 | 101.3 | 0.038 |
| days | (6.3, 115.7) | (26.7, 76.2) | (37.2, 90.4) | (44.6, 149.2) | ||
| Endocortical bone | ||||||
| Ec‐MAR c | 0.61 | 0.58 | 0.84 | 0.56 | 0.47 | 0.015 |
| µm/day | (0.53, 0.72) | (0.56, 0.66) | (0.47, 0.67) | (0.24, 0.55) | ||
| Ec‐MARd | 0.61 | 0.58 | 0.84 | 0.59 | 0.48 | 0.060 |
| µm/day | (0.53, 0.72) | (0.56, 0.66) | (0.51, 0.68) | (0.46, 0.61) | ||
| Ec‐MS/BS | 7.0 | 24.6 | <0.001 | 3.6 | 1.9 | 0.25 |
| % | (3.3, 9.9) | (16.0, 31.5) | (1.0, 8.9) | (0.2, 7.6) | ||
| Ec‐BFR/BS c | 15.191 | 52.260 | 0.001 | 10.082 | 6.398 | 0.18 |
| µm3/µm2/year | (10.985, 21.197) | (33.748, 64.875) | (3.902, 19.226) | (1.445, 15.028) | ||
| Ec‐BFR/BSd | 15.191 | 52.260 | 0.001 | 11.164 | 9.195 | 0.67 |
| µm3/µm2/year | (10.985, 21.197) | (33.748, 64.875) | (6.330, 25.020) | (5.894, 24.067) | ||
| Intracortical bone | ||||||
| Ct‐MAR c | 0.76 | 0.67 | 0.13 | 0.64 | 0.61 | 0.099 |
| µm/day | (0.66, 0.85) | (0.58, 0.75) | (0.60, 0.75) | (0.53, 0.69) | ||
| Ct‐MARd | 0.78 | 0.67 | 0.051 | 0.65 | 0.62 | 0.16 |
| µm/day | (0.69, 0.85) | (0.58, 0.75) | (0.60, 0.75) | (0.55, 0.70) | ||
| Ct‐MS/BS | 4.6 | 8.2 | 0.077 | 4.4 | 6.0 | 0.25 |
| % | (1.4, 8.5) | (5.0, 10.6) | (2.2, 8.3) | (3.4, 9.9) | ||
| Ct‐BFR/BS c | 16.731 | 19.202 | 0.41 | 13.448 | 13.866 | 0.38 |
| µm3/µm2/year | (7.103, 26.094) | (13.113, 32.024) | (4.081, 20.648) | (6.344, 28.615) | ||
| Ct‐BFR/BSd | 17.557 | 19.202 | 0.61 | 13.454 | 14.695 | 0.20 |
| µm3/µm2/year | (7.711, 29.472) | (13.113, 32.024) | (4.696, 20.648) | (9.302, 30.097) | ||
| Periosteal bone | ||||||
| Ps‐MAR c | 0.24 | 0.24 | 0.69 | ‐ | ‐ | ‐ |
| µm/day | (0.24, 0.55) | (0.24, 0.50) | (−, −) | (−, −) | ||
| Ps‐MARd | 0.56 | 0.57 | 1.00 | ‐ | ‐ | ‐ |
| µm/day | (0.55, 0.57) | (0.50, 0.83) | (−, −) | (−, −) | ||
| Ps‐MS/BS | 0.07 | 0.47 | 0.48 | ‐ | ‐ | ‐ |
| % | (0.00, 1.10) | (0.00, 1.64) | (−, −) | (−, −) | ||
| Ps‐BFR/BS c | 2.273 | 1.187 | 1.00 | ‐ | ‐ | ‐ |
| µm3/µm2/year | (0.215, 5.229) | (0.404, 7.991) | (−, −) | (−, −) | ||
| Ps‐BFR/BSd | 2.734 | 8.348 | 0.25 | ‐ | ‐ | ‐ |
| µm3/µm2/year | (2.273, 3.195) | (5.484, 14.317) | (−, −) | (−, −) | ||
Ac.f = activation frequency; Aj.AR = adjusted apposition rate; BFR/BS = bone formation rate per unit of bone surface; BFR/BV = bone formation rate per unit of bone volume; Cn = cancellous; Ct = intracortical; Ec = endocortical; FP = formation period; MAR = mineral apposition rate; Mlt = mineralization lag time; MS/BS = ratio of mineralizing surface to bone surface; OS/BS = ratio of osteoid surface to bone surface; O.Th = osteoid thickness; OV/BV = osteoid volume per unit of bone volume; Ps = periosteal; QM = once monthly; W.Th = wall thickness.
the Wilcoxon rank sum test.
All values are median (Q1, Q3) unless otherwise specified.
With and dwithout imputation when single labels were identified.
In Ec bone, the dynamic parameters of bone formation were also significantly higher at month 2 (Ec‐MS/BS: 7.0% and 24.6%, p < 0.001; Ec‐BFR/BS: 15.191 and 52.260 µm3/µm2/year, P = 0.001 in the placebo and romosozumab groups, respectively), but not at month 12. No significant effect was observed on Ct and Ps bone formation (Table 3).
When compared with the placebo, romosozumab induced significant decreases in bone resorption parameters (ES/BS, Oc.S/BS, and Oc.N/BS) at both month 2 and month 12 in Cn bone (Cn‐ES/BS month 2: 3.4% and 1.8%, P = 0.002; month 12: 2.9% and 1.1%, P < 0.001 in the placebo and romosozumab groups, respectively). In Ec bone, ES/BS was significantly lower than placebo at both month 2 and month 12 (Ec‐ES/BS month 2: 6.3% and 1.6%, P = 0.003; month 12: 4.1% and 0.5%, P < 0.001 in the placebo and romosozumab groups, respectively) and Oc.S/BS and Oc.N/BS were decreased at month 12 (Table 4).
Table 4.
Bone Resorption Parameters After 2 and 12 Months of Romosozumab
| Month 2 | Month 12 | |||||
|---|---|---|---|---|---|---|
| Romosozumab | Romosozumab | |||||
| Placebo | 210 mg QM | Placebo | 210 mg QM | |||
| N = 14 | N = 15 | p value a | N = 31 | N = 39 | p value a | |
| Cancellous bone | ||||||
| Cn‐ES/BS | 3.4 | 1.8 | 0.022 | 2.9 | 1.1 | <0.001 |
| % | (1.9, 4.5) | (0.9, 3.2) | (2.0, 4.5) | (0.5, 1.7) | ||
| Cn‐Oc.S/BS | 0.1 | 0.0 | 0.032 | 0.1 | 0.0 | 0.001 |
| % | (0.04, 0.2) | (0.0, 0.1) | (0.0, 0.3) | (0.0, 0.03) | ||
| Cn‐Oc.N/BS | 2.4 | 0.0 | 0.024 | 2.0 | 0.0 | <0.001 |
| /100 mm | (1.0, 5.6) | (0.0, 2.4) | (0.0, 6.5) | (0.0, 1.4) | ||
| Endocortical bone | ||||||
| Ec‐ES/BS | 6.3 | 1.6 | 0.003 | 4.1 | 0.5 | <0.001 |
| % | (3.3, 7.7) | (0.6, 3.5) | (3.0, 6.5) | (0.2, 1.2) | ||
| Ec‐Oc.S/BS | 0.2 | 0.0 | 0.12 | 0.0 b | 0.0 b | <0.001 |
| % | (0.0, 0.3) | (0.0, 0.1) | (0.0, 0.4) | (0.0, 0.0) | ||
| Ec‐Oc.N/BS | 4.4 | 0.0 | 0.14 | 0.0 c | 0.0 c | 0.001 |
| /100 mm | (0.0, 7.7) | (0.0, 4.8) | (0.0, 8.4) | (0.0, 0.0) | ||
Values are median (Q1, Q3) unless otherwise specified.
Cn = cancellous; Ec = endocortical; ES/BS = eroded surface per unit of bone surface; Oc.N/BS = osteoclast number per unit of bone surface; Oc.S/BS = osteoclast surface per unit of bone surface; Q1, Q3 = quartiles 1 and 3; QM = once monthly.
the Wilcoxon rank sum test.
Mean ± SD: 0.3 ± 0.7 in placebo, 0.03 ± 0.09 in romosozumab.
Mean ± SD: 8.1 ± 16.2 in placebo, 1.0 ± 2.9 in romosozumab.
No significant change in bone structure parameters was observed after 2 months of romosozumab when compared with placebo. In contrast, at month 12, romosozumab induced significant increases in Cn‐BV/TV, Tb.Th, and Ct.Th (Table 5). A highly significant correlation was observed between Cn‐W.Th and Tb.Th (r = 0.78, P < 0.001).
Table 5.
Effects of Romosozumab on Structural and Microarchitectural Parameters Assessed by Histomorphometry and µCT
| Month 2 | Month 12 | |||||
|---|---|---|---|---|---|---|
| Romosozumab | Romosozumab | |||||
| Placebo | 210 mg QM | Placebo | 210 mg QM | |||
| N = 18 | N = 16 | p value a | N = 32 | N = 39 | p value a | |
| Histomorphometry | ||||||
| Cancellous bone | ||||||
| Cn‐BV/TV | 12.35 | 15.48 | 0.98 | 11.41 | 15.44 | 0.033 |
| % | (10.88, 16.95) | (8.96, 19.13) | (9.37, 15.54) | (10.97, 20.08) | ||
| Tb.N | 1.25 | 1.35 | 0.84 | 1.21 | 1.17 | 0.97 |
| /mm | (1.00, 1.59) | (0.98, 1.59) | (1.00, 1.34) | (0.97, 1.46) | ||
| Tb.Th | 99.5 | 105.9 | 0.35 | 100.2 | 132.0 | 0.006 |
| µm | (85.0, 133.4) | (95.8, 125.4) | (86.1, 125.2) | (101.9, 158.4) | ||
| Tb.Sp | 717.4 | 617.6 | 0.78 | 734.0 | 703.9 | 0.62 |
| µm | (513.6, 828.4) | (524.8, 914.9) | (638.4, 887.2) | (532.6, 899.1) | ||
| Cortical bone | ||||||
| Ct.Th | 714.5 | 611.0 | 0.68 | 627.0 | 741.0 | 0.014 |
| µm | (402.0, 907.0) | (484.0, 767.0) | (494.0, 692.0) | (633.0, 861.0) | ||
| Ct.Po | 4.40 | 4.61 | 0.81 | 4.36 | 3.83 | 0.47 |
| % | (3.58, 7.17) | (2.64, 8.04) | (2.70, 7.69) | (2.67, 5.56) | ||
| µCT | ||||||
| Cancellous bone | ||||||
| Tb.BMD | 185 | 225 | 0.36 | 170 | 230 | 0.026 |
| mg/cm3 | (150, 210) | (140, 250) | (140, 235) | (170, 300) | ||
| Tb.TMD | 701 | 716 | 0.75 | 704 | 727 | 0.013 |
| mg/cm3 | (689, 723) | (667, 749) | (678, 722) | (699, 744) | ||
| Tb.BV/TV | 17.10 | 20.42 | 0.42 | 15.97 | 22.04 | 0.006 |
| % | (15.17, 18.66) | (12.91, 23.54) | (13.92, 21.13) | (17.92, 28.61) | ||
| Tb.N | 0.79 | 0.87 | 0.70 | 0.85 | 0.89 | 0.21 |
| /mm | (0.70, 0.99) | (0.74, 0.98) | (0.69, 0.95) | (0.77, 0.98) | ||
| Tb.Th | 0.191 | 0.222 | 0.35 | 0.204 | 0.241 | 0.001 |
| mm | (0.184, 0.233) | (0.198, 0.231) | (0.180, 0.232) | (0.215, 0.293) | ||
| Tb.Sp | 0.858 | 0.762 | 0.046 | 0.788 | 0.793 | 0.86 |
| mm | (0.807, 0.894) | (0.710, 0.809) | (0.717, 0.838) | (0.732, 0.860) | ||
| SMI | 1.32 | 1.42 | 0.42 | 1.49 | 1.36 | 0.22 |
| # | (1.15, 1.53) | (1.16, 1.65) | (1.33, 1.80) | (1.20, 1.69) | ||
| TBPf | 3.51 | 3.68 | 0.84 | 3.99 | 3.24 | 0.030 |
| /mm | (2.93, 4.58) | (2.74, 5.07) | (3.25, 5.61) | (2.11, 4.34) | ||
| Conn.D | 3.41 | 4.17 | 0.07 | 3.45 | 3.11 | 0.26 |
| #/mm3 | (2.14, 4.02) | (3.78, 5.14) | (2.86, 4.05) | (2.49, 4.32) | ||
| Cortical bone | ||||||
| Ct.BMD | 774 | 780 | 0.98 | 789 | 770 | 0.15 |
| mg/cm3 | (733, 803) | (736, 795) | (754, 812) | (748, 794) | ||
| Ct.TMD | 813 | 804 | 0.78 | 805 | 790 | 0.054 |
| mg/cm3 | (775, 829) | (788, 821) | (784, 840) | (775, 814) | ||
| Ct.Th | 0.854 | 0.719 | 0.42 | 0.661 | 0.786 | 0.056 |
| mm | (0.585, 1.016) | (0.565, 0.810) | (0.535, 0.837) | (0.621, 0.977) | ||
| Ct.Po | 5.07 | 3.74 | 0.73 | 3.87 | 3.39 | 0.47 |
| % | (3.31, 7.12) | (3.40, 6.82) | (2.28, 5.74) | (2.34, 4.93) | ||
Values are median (Q1, Q3).
Cn‐BV/TV = cancellous bone volume; Conn.D = connectivity density; Ct.BMD = cortical bone mineral density; Ct.Po = cortical porosity; Ct.Th = cortical thickness; Ct.TMD = cortical tissue bone mineral density; µCT = microcomputed tomography; Q1, Q3 = quartiles 1 and 3; QM = once monthly; SMI = structure model index; Tb.N = trabecular number; Tb.Sp = trabecular separation; Tb.Th = trabecular thickness; Tb.BMD = trabecular bone mineral density; Tb.TMD = trabecular tissue bone mineral density; Tb.BV/TV = trabecular bone volume per tissue volume; TBPf = trabecular bone pattern factor.
the Wilcoxon rank sum test.
Microcomputed tomography analysis
The mean volume of interest of the biopsies analyzed by µCT was 122 ± 63 mm3 and ranged from 14 to 311 mm3. The 3D assessment by µCT of structural parameters was consistent with the histomorphometric findings. At month 2, in the romosozumab group, Tb.Sp was already significantly lower than in the placebo group. At month 12, parameters reflecting the mineral density (Tb.BMD) and amount of bone (Tb.BV/TV and Tb.Th) were significantly higher (Fig. 3), and at the cortical level, Ct.Th tended to increase (P = 0.056). At month 12, improved microarchitecture, as shown by a significant decrease in TBPf and a trend in improved connectivity as reflected by Conn.D, was observed in the romosozumab group versus the placebo group (Table 5).
Figure 3.
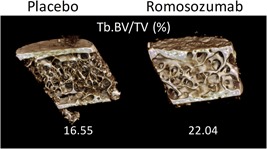
Effects of romosozumab at month 12 on bone mass and microarchitecture assessed by µCT. Tb.BV/TV = trabecular bone volume per tissue volume
Discussion
Early positive effect on bone formation
We evaluated the effects of romosozumab at the bone tissue level with quadruple fluorochrome labeling performed at baseline and after 2 months of treatment to allow for within‐subject comparisons of dynamic parameters of formation. This technique, which can be reliably used only over a short period like 2 months, presents two advantages over paired biopsies: only one sample is collected in each patient, and each serves as her own pretreatment control, eliminating the problems caused by the interindividual variability in histomorphometric variables.22 With the quadruple labeling technique, the extent of BS at baseline was unknown. Over 2 months, the extent of BS changes was expected to be very small and the calculation error considered minor. Thus, for the calculation it was assumed that BS at baseline and BS measured at month 2 were identical and the baseline labels were expressed using BS measured at month 2 as referent. When compared with baseline, marked increases in some dynamic parameters of bone formation—MS/BS and BFR/BS—were observed on Cn and Ec bone surfaces at month 2 in the romosozumab group. These early effects on bone formation were also significant when compared with the placebo group. Accompanying the positive effects on dynamic bone formation parameters were significant increases in static bone formation parameters—OV/BV and O.Th—and a trend for OS/BS, with no effect on the mineralization rate when compared with placebo. These observations reflected a rapid increase in bone formation at the tissue level reflected by MS/BS and BFR/BS, rather than at the individual cell level, supported by the absence of significant change of Aj.AR and MAR. Our results showed increased surface‐based bone formation, reflecting an increased osteoblast number, but MAR and Aj.AR and the total FP did not change at month 2. In contrast, a previous study using kinetic reconstruction, reported transient increases in osteoblast vigor (MAR) and sustained increases in osteoblast efficiency (Aj.AR) in sclerostin antibody‐ (Scl‐Ab‐) treated cynomolgus monkeys.23 These differences may reflect the different time points of analysis between the studies. At month 2, the higher amount of bone deposited during the same FP with an unchanged MAR suggests that the percentage of time osteoblast activity increases, as previously observed after kinetic reconstruction in cynomolgus monkeys.23
No significant effect on the Ps and Ct iliac bone was observed in the present study, in contrast to those previously reported in long bones in aged ovariectomized cynomolgus monkeys at clinically relevant systemic exposures of romosozumab.10 These apparent differences in response of the Ps surface may be related to the different mechanical function between the long bones, which are mechanically loaded, and the iliac crest.
A possible modeling process
Notably, at month 2 when compared with placebo, indices of bone formation were higher on both Cn and Ec bone surfaces, whereas indices of bone resorption were lower. Essentially, when bone formation doubled, the bone resorption (ES/BS, Oc.S/BS, and Oc.N/BS) was halved. Our observations on formation and resorption parameters are in agreement with the increase in serum bone formation markers, and a decrease in resorption markers, reported one week after the first injection of romosozumab in healthy and osteoporotic women.11, 12, 13, 15 These data suggest that at month 2, increased bone formation occurred independent of resorption, consistent with modeling‐based bone formation, with formation occurring without previous resorption. Evidence of the exact contribution of modeling‐based formation to the total increase in formation observed will require further investigation. Transient activation of modeling‐based formation in Cn and Ec bone in response to Scl‐Ab early in the course of treatment has been demonstrated in rats and monkeys.23, 24 Activation of modeling‐based bone formation appears to involve the reactivation of quiescent bone‐lining cells,25 with subsequent recruitment of osteoprogenitors to maintain bone formation.26 Because modeling‐based formation is independent of resorption, the interpretation of the increased Ac.f, which assumes a coupling between bone resorption and formation, at month 2 does not reflect the true Ac.f of remodeling units; hence, it cannot be interpreted.
Remodeling process at month 12
The increase in bone formation early in the initiation of the treatment was followed by decreases in all static and dynamic parameters of formation at month 12. MAR was lower and FP and Mlt were higher than placebo. Although these changes suggest reduced osteoblast function, this may simply be a consequence of the reduction in Ac.f as observed after long‐term antiresorptive therapy, which results in reduced Ac.f, increased FP and Mlt, and decreased MAR. Kinetic reconstruction reveals that FP prolongation occurs largely at the terminal phase of the FP, where forming packets normally have reduced indices of osteoblast function,27, 28 resulting in a greater contribution of these sites to mean values. Kinetic reconstruction is currently ongoing to further characterize these effects at month 12.
These effects on bone formation were associated with a sustained decrease of bone resorption in both Cn and Ec bone in the romosozumab group compared with the placebo group. The antiresorptive effect of romosozumab may be explained by the Wnt‐mediated increase in osteoprotegerin (OPG) expression, a known Wnt target gene. However, in experimental studies, modifications in OPG and RANKL expressions are not always observed after Scl‐Ab administration.29 In addition to OPG/RANKL, other pathways are probably involved in the antiresorptive effect of Scl‐Ab.9, 30 These findings suggest that after an initial bone‐forming effect, which seems to involve both modeling and remodeling formation, romosozumab had an antiresorptive action through a classic remodeling process, resulting in decreased bone turnover (Ac.f), with a coupled reduction in bone resorption and bone formation. A very similar biphasic pattern has been reported in animals.23 This complex pattern of bone formation responses, with an early increase followed by an attenuation, has been suggested by the kinetics of serum bone turnover markers.11, 13, 15 In patients receiving the same dose, procollagen type 1 N‐terminal propeptide (P1NP), a marker of formation, reaches a peak on day 14, then returns to baseline by month 9, whereas serum C‐telopeptide of type 1 collagen (sCTX), a marker of resorption, remains decreased up to month 12.15 In animal studies, the effects of Scl‐Ab are similar, with an initial increase in the biochemical and histological parameters of formation, which returns to baseline values with long‐term administration.9, 10
Self‐regulatory mechanism of bone formation
A so‐called self‐regulatory mechanism with long‐term administration of Scl‐Ab has been suggested.31 The self‐regulatory mechanism invoked to limit bone formation in response to Scl‐Ab is not fully understood. Recent studies in rats suggest that following the initial activation of canonical Wnt signaling, numerous downstream pathways are upregulated, notably pathways that would inhibit cell‐cycle progression and mitogenesis. These effects ensue simultaneously with a reduction in osteoprogenitor number and proliferation, but occur before the diminution of osteoblast number and bone formation,24, 26, 30 suggesting that osteoprogenitor response may contribute to self‐regulation. In addition, DKK‐1 expression in bone tissue increases in SOST knockout mice and in Scl‐Ab treated rats32 similar to serum DKK‐1 levels in patients with sclerosteosis.7 A compensatory increase in expression of other Wnt antagonists suggests possible Wnt feedback regulation through multiple pathway components.32
Bone structure and microarchitecture
Despite a major increase in bone formation, W.Th did not differ in romosozumab versus placebo at month 2, likely because most bone packets formed under the effects of romosozumab were not yet completed. W.Th estimates would, therefore, largely reflect bone packets completed before romosozumab treatment. Assessment of the effects of romosozumab on W.Th at this point would require kinetic reconstruction to project the completed W.Th from the currently forming sites.
The initial bone‐forming effect, followed by an antiresorptive mechanism, resulted in an increased amount of bone at month 12, as shown by the increased W.Th, BV/TV, and Tb.Th; the thickening of the trabeculae was related to the increased W.Th. These findings suggest that a positive bone‐balance at the individual, basic multicellular unit may contribute to bone mass gain. In addition to increasing BV/TV, Ct.Th, and Tb.Th, µCT analysis—the reference method to assess the 3D microarchitecture—indicated that romosozumab improved trabecular connectivity as evidenced by a significant decrease in TBPf. In addition, the low bone turnover observed at month 12 contributed to the increase in the Tb.TMD estimated by the µCT analysis, as previously shown with other antiresorptive agents such as bisphosphonates.3 Improvement in bone mass, trabecular microarchitecture, and degree of mineralization may all contribute to the increased BMD observed in romosozumab‐treated patients and the associated reduction in fracture risk after 12 months.15 This newly formed bone had normal lamellar texture, with no evidence of mineralization defects.
Romosozumab appeared to be well‐tolerated; the adverse events previously reported were balanced between the romosozumab and placebo groups in the FRAME trial.15 Recently, in the ARCH trial conducted in more than 4000 postmenopausal women at high risk for fracture, cardiovascular adverse events were observed more often with romosozumab (2.5%) than with alendronate (1.9%).16
Most currently used therapeutic agents, such as bisphosphonates, inhibit bone resorption and bone turnover, increasing BMD but not stimulating new bone formation.2 The anabolic bone agents teriparatide and abaloparatide stimulate both bone formation and resorption. In contrast to romosozumab, previous histomorphometric studies with all those therapies did not show evidence of an effect on BV/TV after 18 months.4, 33
This study presents some limitations, including a relatively small sample size at month 2, a higher number of patients with a prior osteoporotic fracture at baseline in the placebo group, and the absence of baseline biopsies. The use of quadruple labeling in the subgroup of month 2 biopsies allowed the assessment of the within‐subject variation of some dynamic parameters of formation, but could not provide information about the changes of bone resorption between baseline and month 2.
In conclusion, the administration of romosozumab to postmenopausal women with osteoporosis produced an early, transient, and significant bone‐forming effect associated with a concomitant and sustained decrease in bone resorption. This dual but opposite effect suggests a transient absence of coupling between resorption and formation early in the initiation of treatment, with bone formation potentially occurring without prior resorption, consistent with a modeling process. Later in treatment, the sustained decrease in bone resorption resulted in reduced bone turnover. These changes in bone formation and resorption were associated with increased bone mass and improved microarchitecture that would contribute to the reduced fracture risk previously reported in postmenopausal women with osteoporosis treated with romosozumab.
Disclosures
PC: Travel grant from Amgen Inc and UCB. RC: Grants/research support from Amgen, Chugai‐Roche, and Merck; consultant for Amgen, Janssen, Radius, Sandoz, and UCB; and speaking fees from AbbVie, Amgen, Bristol‐Myers Squibb, Eli Lilly, MSD, and Pfizer. NP‐M has no conflict of interest. J‐PR: Travel grant from Amgen Inc. PG: Grants/research support from Amgen, Eli Lilly, and Pfizer. JPB: Grants/research support from Amgen and Eli Lilly; consultant for Amgen, Eli Lilly, and Merck; and speakers’ bureau for Amgen and Eli Lilly. CL: Employee and shareholder of UCB Pharma. RB: Former employee of Amgen Inc. AW and AG: Employees and shareholders of Amgen Inc.
Acknowledgments
Amgen Inc., Astellas, and UCB Pharma sponsored this study (NCT01575834). Amgen Inc. and UCB Pharma provided assistance and funded all costs associated with development of this manuscript. James Ziobro (funded by Amgen Inc.) and Lisa Humphries (Amgen Inc.) provided medical writing assistance in the preparation of this manuscript. AW performed the analyses according to prespecified statistical analysis plan. The authors are grateful to Stéphane Horlait (Amgen Inc) for his helpful contributions in the study conduct, data interpretation, and draft preparation. The authors thank the investigators who collected bone biopsy specimen samples in the FRAME study.
Authors’ roles: PC and RC had full access to the data and developed the initial and subsequent drafts of the manuscript. PC and RC conducted the study. PC, NP‐M, and J‐PR collected the data. AW performed statistical analyses. PC, RC, NP‐M, J‐PR, RB, CL, and AG interpreted the data. AG designed and led the underlying clinical study. PG and JPB were investigators. All authors revised and approved the final version of the manuscript and had final responsibility for the decision to submit the manuscript for publication.
References
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. Osteoporosis prevention, diagnosis and therapy. JAMA. 2001;285:785–95. [DOI] [PubMed] [Google Scholar]
- 2. Chavassieux P, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long‐term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–94. [DOI] [PubMed] [Google Scholar]
- 4. Arlot M, Meunier PJ, Boivin G, et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res. 2005;20:1244–53. [DOI] [PubMed] [Google Scholar]
- 5. Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–4. [DOI] [PubMed] [Google Scholar]
- 6. Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280:19883–7. [DOI] [PubMed] [Google Scholar]
- 7. Van Lierop AH, Appelman‐Dijkstra N, Papapoulos SE. Sclerostin deficiency in humans. Bone. 2017;96:51–62. [DOI] [PubMed] [Google Scholar]
- 8. Ominsky MS, Danielle L, Brown DL, et al. Differential temporal effects of sclerostin antibody and parathyroid hormone on cancellous and cortical bone and quantitative differences in effects on the osteoblast lineage in young intact rats. Bone. 2015;81:380–91. [DOI] [PubMed] [Google Scholar]
- 9. Ominsky MS, Boyce RW, Li X, Ke HZ. Effects of sclerostin antibodies in animal models of osteoporosis. Bone. 2017;96:63–75. [DOI] [PubMed] [Google Scholar]
- 10. Ominsky MS, Boyd SK, Varela A, et al. Romosozumab improves bone mass and strength while maintaining bone quality in ovariectomized cynomolgus monkeys. J Bone Miner Res. 2017;32:788–801. [DOI] [PubMed] [Google Scholar]
- 11. McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–20. [DOI] [PubMed] [Google Scholar]
- 12. Padhi D, Jang G, Stouch B, Fang L, Posvar E. Single‐dose, placebo‐controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26:19–26. [DOI] [PubMed] [Google Scholar]
- 13. Padhi D, Allison M, Kivitz AJ, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: a randomized, double‐blind, placebo‐controlled study. J Clin Pharmacol. 2014;54:168–78. [DOI] [PubMed] [Google Scholar]
- 14. Natasha M, Appelman‐Dijkstra N, Papapoulos SE. Sclerostin inhibition in the management of osteoporosis. Calcif Tissue Int. 2016;98:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43. [DOI] [PubMed] [Google Scholar]
- 16. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27. [DOI] [PubMed] [Google Scholar]
- 17. Chavassieux P, Arlot M, Meunier PJ. Clinical use of bone biopsy In: Marcus R, Feldman D, & Kelsey J, editors, Osteoporosis (Vol2, 2nd Ed San Diego, CA: Academic Press, 2001) pp. 501–9. [Google Scholar]
- 18. Arlot ME, Delmas PD, Chappard D, Meunier PJ. Trabecular and endocortical bone remodeling in postmenopausal osteoporosis: comparison with normal postmenopausal women. Osteoporos Int. 1990;1:41–9. [DOI] [PubMed] [Google Scholar]
- 19. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols and units for bone histomorphometry. A 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 2013;28:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parfitt AM, Mathews CH, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983;72:1396–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Recker RR, Kimmel DB, Dempster D, Weinstein RS, Wronski TJ, Burr DB. Issues in modern bone histomorphometry. Bone. 2011;49:955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lindsay R, Cosman F, Zhou H, et al. A novel tetracycline labeling schedule for longitudinal evaluation of the short‐term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2005;21:366–73. [DOI] [PubMed] [Google Scholar]
- 23. Boyce RW, Niu QT, Ominsky MS. Kinetic reconstruction reveals time‐dependent effects of romosozumab on bone formation and osteoblast function in vertebral cancellous and cortical bone in cynomolgus monkeys. Bone. 2017;101:77–87. [DOI] [PubMed] [Google Scholar]
- 24. Nioi P, Taylor S, Hu R, et al. Transcriptional profiling of laser capture microdissected subpopulations of the osteoblast lineage provides insight into the early response to sclerostin antibody in rats. J Bone Miner Res. 2015;30:1457–67. [DOI] [PubMed] [Google Scholar]
- 25. Kim SW, Lu Y, Williams EA, et al. Sclerostin antibody administration converts bone lining cells into active osteoblasts. J Bone Miner Res. 2017;32:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boyce RW, Brown D, Felx M, et al. Decreased osteoprogenitor proliferation precedes attenuation of cancellous bone formation in ovariectomized rats treated with sclerostin antibody. Bone Rep. 2018;8:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eriksen EF, Gundersen HJ, Melsen F, Mosekilde L. Reconstruction of the formative site in iliac trabecular bone in 20 normal individuals employing a kinetic model for matrix and mineral apposition. Metab Bone Dis Relat Res. 1984;5:243–52. [DOI] [PubMed] [Google Scholar]
- 28. Eriksen EF, Melsen F, Sod E, Barton I, Chines A. Effects of long‐term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone. 2002;31:620–5. [DOI] [PubMed] [Google Scholar]
- 29. Stolina M, Dwyer D, Niu QT, et al. Temporal changes in systemic and local expression of bone turnover markers during six months of sclerostin antibody administration to ovariectomized rats. Bone. 2014;67:305–13. [DOI] [PubMed] [Google Scholar]
- 30. Taylor S, Ominsky MS, Hu R, et al. Time‐dependent cellular and transcriptional changes in osteoblast lineage associated with sclerostin antibody treatment in ovariectomized rats. Bone. 2016;84:148–59. [DOI] [PubMed] [Google Scholar]
- 31. Delgado‐Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Florio M, Gunasekaran K, Stolina M, et al. A bispecific antibody targeting sclerostin and DKK‐1 promotes bone mass accrual and fracture repair. Nature Com. 2016;7:11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moreira CA, Fitzpatrick LA, Wang Y, Recker RR. Effects of abaloparatide‐SC (BA058) on bone histology and histomorphometry: the ACTIVE phase 3 trial. Bone. 2017;97:314–9. [DOI] [PubMed] [Google Scholar]


