Abstract
Background
Atrial fibrillation (AF) is a frequent finding in HFpEF. However, its association with invasive haemodynamics, imaging parameters and outcome in HFpEF is not well established. Furthermore, the relevance of AF subtype with regard to outcome is unclear. This study sought to investigate the prognostic impact of paroxysmal and persistent AF in a well‐defined heart failure with preserved ejection fraction (HFpEF) population.
Materials and methods
Between 2010 and 2016, 254 HFpEF patients were prospectively enrolled. All patients underwent echocardiography as well as left and right heart catheterization. Patients without contraindications underwent CMR including T1 mapping. Follow‐up and outcome data were collected. Patients with significant coronary artery disease were excluded.
Results
A total of 153 patients (60%) suffered from AF, 119 (47%) had persistent and 34 (13%) had paroxysmal AF. By multiple logistic regression analysis, persistent AF was independently associated with NT‐proBNP (P = .003), NYHA functional class (P = .040), left and right atrial size (P = .022 and <.001, respectively), cardiac output (P = .002) and COPD (P = .034). After a median follow‐up of 23 months (interquartile range 5‐48), 92 patients (36%) reached the primary end point defined as hospitalization for heart failure or cardiovascular death. By multivariate Cox regression analysis, only persistent AF (P = .005) and six‐minute walk distance (P = .011) were independently associated with the primary end point.
Conclusions
Sixty percent of our HFpEF patients suffered from AF. Persistent but not paroxysmal AF was strongly associated with event‐free survival and was independently related to NYHA functional class, serum NT‐proBNP, atrial size, cardiac ouput and presence of COPD.
Keywords: atrial fibrillation, heart failure with preserved ejection fraction
1. INTRODUCTION
Heart failure is a global epidemic, affecting millions of adults worldwide. The same holds true for atrial fibrillation (AF), which may occur independently from heart failure, but is frequently linked to it.1 Almost half of heart failure patients present as heart failure with preserved ejection fraction (HFpEF).2 AF is highly prevalent in the HFpEF population, affecting up to 65% of HFpEF patients.3 HFpEF and AF share similar risk factors such as arterial hypertension, overweight/obesity, chronic obstructive pulmonary disease (COPD), diabetes and advanced age.4 Thus, a close link of HFpEF and AF has already been assumed.5, 6 However, the confounding factors of AF in HFpEF are largely unknown, and whether AF subtype (persistent versus paroxysmal) is important in terms of prognosis has so far not been investigated.
Increasing left ventricular stiffness is accompanied by LA remodelling, which corresponds to the development of LA fibrosis.7 Kosiuk et al have developed and prospectively validated a probability score for the presence of LA arrhythmogenic substrate, based mainly on clinical comorbidities.8 Interestingly, factors associated with LA arrhythmogenic substrate were identical to known risk factors for HFpEF such as diabetes, renal dysfunction, advanced age, female gender and hypertension. These findings underline the close association of HFpEF and LA remodelling. LA remodelling and atrial fibrosis are closely related and well‐known risk factor for the perpetuation of AF, that is persistent AF.8, 9
The present study was designed to investigate the prevalence of paroxysmal and persistent AF in a well‐defined prospective HFpEF cohort and relate it with clinical, imaging and invasive haemodynamic parameters, and with event‐free survival.
2. MATERIALS AND METHODS
2.1. Study design
This was a prospective observational study performed at the Medical University of Vienna. Between December 2011 and November 2016, consecutive patients with suspected HFpEF were invited to participate. The local Ethics Committee approved the study protocol (EK No. 796/2010). All participants gave written informed consent.
2.2. Clinical definitions
HFpEF was diagnosed according to the Guidelines of the European Society of Cardiology2 and the American Heart Association.10 The following criteria had to be fulfilled: (a) signs or symptoms of heart failure, (b) preserved left ventricular systolic function (ejection fraction ≥50%), (c) N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) 220 pg/mL and (d) evidence of left ventricular diastolic dysfunction or structural changes (LA enlargement and left ventricular hypertrophy) by transthoracic echocardiography. The haemodynamic diagnosis of HFpEF was confirmed whether the pulmonary artery wedge pressure exceeded 12 mm Hg by right heart catheterization.
Atrial fibrillation lasting <7 days was defined as paroxysmal AF and AF ≥7 days as persistent AF. AF lasting longer than one year was defined as longstanding persistent AF.4 Incident AF was diagnosed on follow‐up electrocardiograms.
Reasons for exclusion were invasively confirmed significant coronary artery disease, moderate to severe and severe aortic and mitral valve heart disease irrespective of aetiology as evaluated by transthoracic echocardiography.11, 12, 13 As severe tricuspid regurgitation is a frequent finding in HFpEF,14 these patients were not excluded. Other reasons for exclusion were congenital heart disease and cardiac amyloidosis. Screening for amyloidosis was performed according to current recommendations15 including transthoracic echocardiography, cardiac magnetic resonance imaging (CMR), 99mTc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy and, if necessary, endomyocardial biopsy.
2.3. Outcome measures
Patients were prospectively followed by ambulatory visits including electrocardiograms and telephone calls at 6‐month intervals. Additionally, electrocardiograms and/or Holter monitoring was performed in case of palpitations. The main outcome measure was a combined end point consisting of hospitalization for heart failure or death from cardiovascular causes. End points were ascertained by follow‐up visits and phone calls and adjudicated by our internal adjudication committee consisting of DB and JM, who were blinded to patient characteristics as well as imaging and haemodynamic data.
2.4. Assessment techniques
2.4.1. Transthoracic echocardiography with tissue Doppler analysis
All transthoracic echocardiography studies were performed by board‐certified physicians using scanners such as GE Vivid 7 and Vivid S70 (GE Healthcare). Left ventricular ejection fraction was assessed by biplane Simpson technique. Transmitral inflow was measured by pulsed wave Doppler, septal and lateral e' by pulsed wave tissue Doppler. Signs for severe mitral regurgitation were a vena contracta jet width ≥0.7 cm with a large central regurgitant jet (area >40% of left atrium) or a wall impinging jet of any size accompanied by corresponding quantitative measurements (eg effective regurgitant orifice area ≥40 cm2, regurgitant volume ≥60 mL/beat).11, 12, 13
2.4.2. Exercise capacity
For assessment of submaximal exercise capacity, six‐minute walk distance (6MWD) on a 50 metre indoor track was used. For statistical analysis, the percentage of the predicted 6MWD ([6MWD/predicted 6MWD] ×100) was calculated.16
2.4.3. Right and left heart catheterization
For right heart catheterization, a 7F Swan‐Ganz catheter (Baxter) was inserted via a jugular or femoral access. Pressures were documented as a digitized mean over the whole respiratory cycle including at least eight consecutive heart cycles using CathCorLX (Siemens AG). In addition to mean pulmonary artery wedge pressure, the systolic, diastolic and mean pulmonary artery pressures were documented. Left ventricular end‐diastolic pressure was manually checked in each patient.
Cardiac output was measured by thermodilution. Furthermore, the transpulmonary gradient was calculated by subtracting wedge pressure from mean pulmonary artery pressure. Diastolic pulmonary vascular pressure gradient was defined as the difference between diastolic pulmonary artery pressure and pulmonary artery wedge pressure during a pullback. Pulmonary vascular resistance was calculated by dividing transpulmonary gradient by cardiac output. Following right heart catheterization, coronary angiography was performed in the same procedure.
2.4.4. Cardiac magnetic resonance imaging
cardiac magnetic resonance imaging examinations were performed on a 1.5 Tesla scanner (MAGNETOM Avanto, Siemens Healthcare GmbH). Patients with an estimated glomerular filtration rate of <30 mL/min/1.73 m2 were excluded. CMR was only performed when the heart rate was below 90/min. CMR examinations were performed according to standard protocols17, 18 including late gadolinium enhancement imaging (0.1 mmoL/kg gadobutrol, Gadovist, Bayer Vital GmbH) and T1 mapping using the modified Look‐Locker inversion (MOLLI) sequence. For pre‐contrast T1 mapping, electrocardiographically triggered MOLLI was applied using a 5(3)3 prototype (5 acquisition heartbeats followed by 3 recovery heartbeats and further 3 acquisition heartbeats). For post‐contrast T1 mapping, a 4 (1) 3 (1) 2 prototype was used. T1 values from a mid‐cavity two‐ and four‐chamber view were averaged. Regions of interest for T1 blood pool values were derived with sufficient distance to papillary muscles and the endomyocardial border.
MOLLI‐extracellular volume (ECV) was calculated according to the following formula18:
T1 myo pre/T1 blood pre indicates myocardial/blood native T1 times, and T1 myo post/T1 blood post indicates T1 times of myocardium/blood 15 minutes after gadobutrol application.
2.4.5. Statistical analysis
IBM SPSS version 21 (SPSS Inc) was used for statistical analyses. For all tests, the significance level was set to P < .05. Continuous variables are expressed as mean ± standard deviation or as median and interquartile ranges. Categorical variables are presented as numbers and per cent. Continuous variables were compared using the Mann‐Whitney U test, for dichotomous variables the chi‐square test was applied. Parameters were divided into clinical, echocardiographic, CMR and invasive haemodynamic categories. To define factors associated with persistent AF, univariate logistic regression analysis was calculated for each parameter. Significant parameters then entered the multivariate analysis in the respective parameter category. Significance limit to enter this model was P < .05.
To assess associations with event‐free survival, separate univariate Cox regression models were performed for all baseline parameters, followed by a multivariate Cox regression model for each parameter category with stepwise forward selection. The significance level for a predictor to enter the model was 0.05, and the limit to stay in the model was 0.1. In a final step, all parameters associated with outcome within the respective group were entered into an additional combined pooled multivariate model using forced entry. Kaplan‐Meier plots (log‐rank test) were applied to verify the time‐dependent discriminative power of paroxysmal and persistent AF on cardiovascular outcome.
3. RESULTS
3.1. Study population
Between December 2010 and November 2016, 296 HFpEF patients were screened for enrollment. After exclusion of 42 patients [significant coronary artery disease (n = 15), NT‐proBNP <220 pg/mL (n = 14), cardiac amyloidosis (n = 13)], 254 patients were prospectively enrolled. All patients with paroxysmal AF were in sinus rhythm at the time of CMR scan and invasive haemodynamic assessment. On the other hand, all patients with persistent AF were in AF at the time of CMR and invasive haemodynamic assessment.
Baseline characteristics are summarized in Table 1. Mean age was 71 ± 8 years, 70% of study participants were female, and mean body mass index was 30 ± 7. No significant differences were found between the paroxysmal AF and the sinus rhythm cohort. Patients with persistent AF, however, were more frequently male (P = .040), presented with worse exercise capacity (P = .026), more frequent COPD (P = .006), worse functional status (P = .003) and higher levels of NT‐pro BNP and gamma‐glutamyl‐transferase (each P < .001, respectively). Regarding medical therapy, persistent AF patients were less often on 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A reductase inhibitors (P = .023) and compared to patients with paroxysmal AF they were also less often on specific antiarrhythmic drugs (P < .001). Echocardiography as well as CMR revealed more pronounced LA, right atrial and right ventricular dilatation in persistent AF patients (each P < .001, respectively). Furthermore, by CMR these patients had worse right ventricular ejection fractions (P < .001) and higher levels of ECV (P = .007).
Table 1.
Baseline characteristics
| Variable | Sinus rhythm (n = 101) | Paroxysmal AF (n = 34) | Persistent AF (n = 119) | a P‐value |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, y | 70 ± 9 | 72 ± 10 | 72 ± 7 | .536 |
| Female sex, n (%) | 79 (79) | 23 (68) | 75 (63) | .040 |
| Body mass index, kg/m2 | 31 ± 8 | 31 ± 7 | 30 ± 6 | .299 |
| Diabetes mellitus type II, n (%) | 40 (40) | 6 (26) | 42 (35) | .896 |
| Hyperlipidaemia, n (%) | 60 (60) | 17 (50) | 61 (51) | .379 |
| Arterial hypertension, n (%) | 96 (95) | 33 (97) | 114 (96) | 1.000 |
| Heart rate, (beats/min) | 71 ± 16 | 68 ± 10 | 73 ± 14 | .093 |
| % of predicted 6MWD, % | 75 ± 26 | 79 ± 22 | 67 ± 25 | .026 |
| Sleep apnoea, n (%) | 11 (11) | 3 (9) | 12 (10) | 1.000 |
| COPD, n (%) | 28 (28) | 6 (18) | 48 (40) | .006 |
| NYHA functional class, n (%) | ||||
| II | 45 (45) | 14 (41) | 29 (24) | .003 |
| III | 48 (47) | 19 (56) | 80 (67) | |
| IV | 8 (8) | 1 (3) | 10 (8) | |
| NT‐proBNP, pg/mL | 560 (360 to 1160) | 680 (370 to 1690) | 1630 (1050 to 2450) | <.001 |
| Glycated haemoglobin, % | 6.2 ± 1.1 | 5.5 ± 1.4 | 6.2 ± 0.9 | .120 |
| eGFR, mL/min/1.73 m2 | 62 (50 to 74) | 53 (43 to 72) | 58 (43 to 71) | .265 |
| Gamma‐glutamyl‐transferase, U/l | 29 (19 to 49) | 28 (20 to 50) | 52 (33 to 95) | <.001 |
| HMG‐CoA reductase inhibitor, n (%) | 58 (58) | 15 (44) | 47 (39) | .023 |
| Betablocker, n (%) | 74 (73) | 26 (76) | 91 (76) | .883 |
| Diuretics, n (%) | 71 (70) | 26 (76) | 100 (84) | .046 |
| ACE inhibitor, n (%) | 27 (27) | 9 (26) | 41 (34) | .220 |
| AT II rezeptor antagonist, n (%) | 42 (42) | 11 (32) | 41 (34) | .435 |
| Specific antiarrhythmic drugs, n (%) | 0 (0) | 8 (24) | 12 (10) | <.001 b |
| Echocardiographic parameters | ||||
| LA diameter, mm | 59 ± 6 | 62 ± 9 | 66 ± 8 | <.001 |
| LV diameter, mm | 43 ± 5 | 45 ± 6 | 44 ± 5 | .380 |
| RA diameter, mm | 58 ± 7 | 62 ± 9 | 67 ± 8 | <.001 |
| RV diameter, mm | 35 ± 6 | 36 ± 7 | 39 ± 8 | <.001 |
| Interventricular septum, mm | 13 ± 3 | 13 ± 2 | 13 ± 3 | .116 |
| E/E' ratio | 15 (10 to 21) | 15 (9 to 20) | 13 (10 to 18) | .314 |
| LV ejection fraction, % | 60 ± 6 | 60 ± 6 | 59 ± 7 | .438 |
| Systolic PAP, mm Hg | 56 (43 to 71) | 51 (44 to 74) | 59 (49 to 74) | .117 |
| Cardiac magnetic resonance imaging parameters | ||||
| LV end‐diastolic diameter, mm | 46 ± 5 | 51 ± 9 | 48 ± 5 | .136 |
| RV end‐diastolic diameter, mm | 38 ± 7 | 39 ± 7 | 43 ± 8 | <.001 |
| Interventricular septum, mm | 11 ± 2 | 11 ± 2 | 11 ± 2 | .179 |
| LA diameter, mm | 61 ± 8 | 63 ± 9 | 69 ± 9 | <.001 |
| LA area, cm2 | 27 (23 to 33) | 28 (25 to 36) | 32 (28 to 37) | <.001 |
| RA diameter, mm | 61 ± 8 | 65 ± 8 | 70 ± 9 | <.001 |
| RA area, cm2 | 25 (21 to 28) | 24 (22 to 28) | 33 (27 to 39) | <.001 |
| LV ejection fraction, % | 67 ± 12 | 63 ± 10 | 60 ± 10 | <.001 |
| LV end‐diastolic volume, mL | 118 (102 to 136) | 122 (95 to 183) | 118 (103 to 142) | .796 |
| Cardiac output, l/min | 6.0 ± 3.4 | 5.7 ± 2.6 | 5.0 ± 1.5 | .078 |
| RV ejection fraction, % | 56 ± 12 | 54 ± 14 | 48 ± 9 | <.001 |
| RV end‐diastolic volume, mL | 138 (115 to 166) | 128 (115 to 179) | 148 (116 to 199) | .077 |
| Native T1 time myocardium, ms | 426 (371 to 476) | 428 (382 to 475) | 406 (351 to 460) | .216 |
| MOLLI‐ECV | 28.8 ± 3.6 | 29.0 ± 3.8 | 30.9 ± 5.0 | .007 |
| Invasive haemodynamics | ||||
| Systolic PAP, mm Hg | 52 (39 to 64) | 51 (44 to 59) | 51 (43 to 64) | .946 |
| Diastolic PAP, mm Hg | 21 (17 to 28) | 20 (16 to 25) | 23 (18 to 27) | .122 |
| Mean PAP, mm Hg | 33 (26 to 41) | 32 (25 to 38) | 34 (29 to 38) | .415 |
| PAWP, mm Hg | 19 ± 6 | 21 ± 6 | 21 ± 6 | .133 |
| LV end‐diastolic pressure, mm Hg | 21 ± 7 | 19 ± 6 | 20 ± 6 | .423 |
| TPG, mm Hg | 14 (10 to 19) | 12 (9 to 15) | 13 (10 to 18) | .779 |
| Diastolic pressure gradient, mm Hg | 3.0 (0.0 to 6.0) | 0.5 (−2.8 to 3.8) | 1.5 (−1.0 to 5.0) | .607 |
| CO thermodilution, l/min | 5.5 ± 1.4 | 5.3 ± 1.3 | 5.1 ± 1.3 | .050 |
| PVR, dyn‐s‐cm−5 | 212 (161 to 278) | 198 (119 to 235) | 204 (142 to 281) | .884 |
Variables with a significance level of p < .05 are displayed with bold letters.
Values are given as mean ± SD or median and interquartile range or total numbers and per cent.
Abbreviations: 6MWD, 6 min walk distance; ACE, angiotensine converting enzyme; AF, atrial fibrillation; AT II, angiotensin II; CO, cardiac output; COPD, chronic obstructive pulmonary disease; E, early mitral inflow velocity; E', early diastolic mitral annular velocity; eGFR, estimated glomerular filtrationrate; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; LA, left atrial; LV, left ventricular; MOLLI‐ECV, modified Look‐Locker inversion recovery sequence‐derived extracellular volume; NT‐proBNP, N‐terminalprohormone of brain natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; TPG, transpulmonary pressure gradient.
P‐value indicates the difference between the persistent AF cohort and the rest.
P‐value indicates the difference between the persistent and the paroxysmal AF cohort.
At study enrollment 153 (60%) patients presented with AF. A total of 119 patients (47%) had persistent AF. Of these, 116 (97%) had been diagnosed with AF at least one year prior to enrollment and thus were in longstanding persistent AF. A total of 34 patients (13%) had paroxysmal AF. During the course of the study, four patients developed a new onset of paroxysmal AF. None of these patients experienced a cardiovascular event. Eight patients developed new (direct) onset of persistent AF. Two of them patients experienced a cardiovascular event. However, no close temporal correlation of persistent AF onset and cardiovascular event was observed. In one case, the event of left sided heart failure was 24 months before persistent AF onset, in the other case it was vice versa. Only one conversion from paroxysmal to persistent AF was observed. This patient suffered an episode of left‐sided heart failure 14 months prior the conversion from paroxysmal to persistent AF. Thus, the cumulative incidence of AF over the whole study duration was 165 (65%) with 127 patients (50%) in persistent AF and 38 patients (15%) in paroxysmal AF. Only 16 patients of the AF cohort were on specific antiarrhythmic drugs, namely 13 on amiodarone, 1 on dronedarone and 1 on flecainide. One patient was switched from flecainide to amiodarone during the course of the study.
3.2. Factors associated with atrial fibrillation
The association of clinical, imaging and invasive haemodynamic parameters with persistent AF was tested by uni‐ and multivariate logistic regression analyses (Table 2). By multivariate analysis, persistent AF was significantly associated with COPD (P = .034), New York Heart Association (NYHA) functional class (P = .040), serum NT‐proBNP (P = .003), LA (P = .022) and right atrial size (P < .001) and cardiac output by CMR (P = .002).
Table 2.
Univariable und multivariable logistic regression analysis investigating the association of clinical, imaging and haemodynamic parameters with persistent atrial fibrillation
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P‐value | Hazard Ratio (95% CI) | P‐value | |
| Clinical parameters | ||||
| Age | 1.02 (0.99‐1.05) | .252 | ||
| Female sex | 0.55 (0.32‐0.95) | .031 | ||
| Body mass index | 0.97 (0.94‐1.01) | .233 | ||
| Diabetes mellitus type II | 0.96 (0.57‐1.60) | .868 | ||
| Hyperlipidaemia | 0.79 (0.48‐1.30) | .357 | ||
| Arterial hypertension | 1.06 (0.32‐3.57) | .924 | ||
| % of predicted 6MWD | 0.98 (0.97‐0.99) | .013 | ||
| Sleep apnoea, n | 0.97 (0.43‐2.19) | .940 | ||
| COPD | 2.20 (1.27‐3.82) | .005 | 2.06 (1.06‐4.01) | .034 |
| NYHA class ≥ III | 2.29 (1.33‐3.95) | .003 | 2.08 (1.04‐4.18) | .040 |
| NT‐proBNP | 1.23 (1.06‐1.42) | .006 | 1.51 (1.15‐1.99) | .003 |
| Glycated haemoglobin | 1.15 (0.90‐1.48) | .258 | ||
| eGFR | 1.00 (0.98‐1.01) | .436 | ||
| Gamma‐glutamyl‐transferase | 1.01 (1.00‐1.01) | .006 | ||
| Echocardiographic parameters | ||||
| LA diameter | 1.12 (1.07‐1.17) | <.001 | ||
| LV diameter | 1.02 (0.98‐1.07) | .354 | ||
| RA diameter | 1.13 (1.09‐1.18) | <.001 | 1.13 (1.09‐1.18) | <.001 |
| RV diameter | 1.08 (1.04‐1.13) | <.001 | ||
| Interventricular septum | 0.93 (0.84‐1.03) | .175 | ||
| E/E' ratio | 0.95 (0.88‐1.02) | .172 | ||
| LV ejection fraction | 0.98 (0.93‐1.03) | .420 | ||
| Systolic PAP | 1.01 (0.99‐1.03) | .249 | ||
| Cardiac magnetic resonance imaging parameter | ||||
| LV end‐diastolic diameter | 1.02 (0.97‐1.08) | .498 | ||
| RV end‐diastolic diameter | 1.09 (1.04‐1.15) | .001 | ||
| Interventricular septum | 0.92 (0.78‐1.08) | .257 | ||
| LA diameter | 1.11 (1.06‐1.16) | <.001 | ||
| LA area | 1.09 (1.04‐1.15) | <.001 | 1.12 (1.02‐1.23) | .022 |
| RA diameter | 1.13 (1.08‐1.19) | <.001 | ||
| RA area | 1.17 (1.11‐1.24) | <.001 | 1.21 (1.10‐1.33) | <.001 |
| LV ejection fraction | 0.94 (0.91‐0.97) | <.001 | ||
| LV end‐diastolic volume | 1.00 (0.99‐1.00) | .431 | ||
| Cardiac output | 0.79 (0.65‐0.97) | .021 | 0.54 (0.36‐0.79) | .002 |
| RV ejection fraction | 0.94 (0.91‐0.97) | <.001 | ||
| RV end‐diastolic volume | 1.01 (0.99‐1.01) | .055 | ||
| Native T1 time myocardium | 1.00 (0.99‐1.00) | .409 | ||
| MOLLI‐ECV | 1.12 (1.02‐1.23) | .017 | ||
| Invasive haemodynamics | ||||
| Systolic PAP | 1.00 (0.99‐1.02) | .976 | ||
| Diastolic PAP | 1.03 (0.99‐1.06) | .193 | ||
| Mean PAP | 1.01 (0.98‐1.04) | .483 | ||
| PAWP | 1.04 (0.99‐1.09) | .101 | ||
| LV end‐diastolic pressure | 0.98 (0.93‐1.03) | .347 | ||
| TPG | 0.99 (0.96‐1.03) | .667 | ||
| Diastolic pressure gradient | 0.99 (0.95‐1.04) | .793 | ||
| CO thermodilution | 0.82 (0.67‐1.01) | .066 | ||
| PVR | 1.00 (1.00‐1.00) | .764 | ||
Variables with a significance level of p < .05 are displayed with bold letters.
Abbreviations: 6MWD, 6 min walk distance; ACE, angiotensine converting enzyme; AF, atrial fibrillation; AT II, angiotensin II; CO, cardiac output; COPD, chronic obstructive pulmonary disease; E, early mitral inflow velocity; E', early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; LA, left atrial; LV, left ventricular; MOLLI‐ECV, modified Look‐Locker inversion recovery sequence‐derived extracellular volume; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; TPG, transpulmonary pressure gradient.
3.3. Association of atrial fibrillation with cardiovascular outcome
After a median follow‐up of 23 months (interquartile range 5‐48), 92 patients (36%) reached the combined end point. In nine patients, cardiovascular death was the first event and 83 were hospitalized for acute heart failure.
Kaplan‐Meier plots showed significantly reduced event‐free survival in patients suffering from persistent AF (log‐rank test P < .001). This was not the case for patients with paroxysmal AF (Figure 1).
Figure 1.
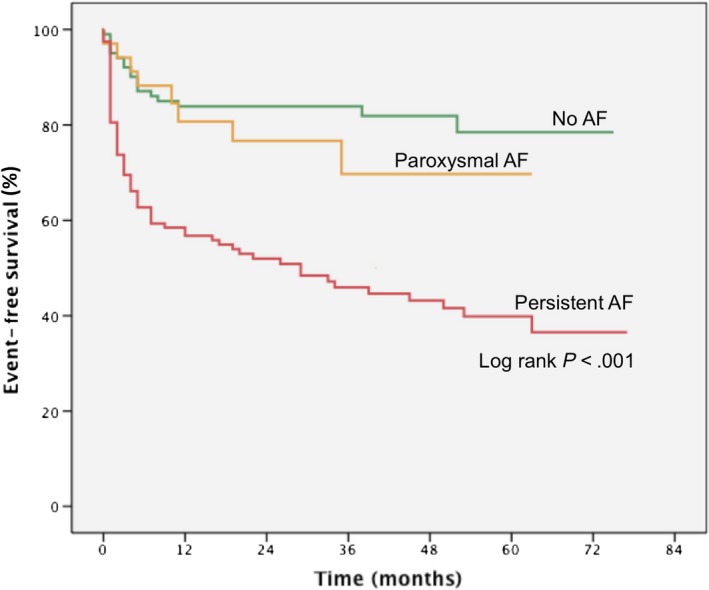
Kaplan‐Meier plot according to atrial fibrillation subtype. Patients with persistent atrial fibrillation had significantly worse event‐free survival rates than those with paroxysmal atrial fibrillation or sinus rhythm. AF indicates atrial fibrillation
Table 3 shows the results of the uni‐ and multivariate Cox regression analysis, clustered for clinical, imaging and haemodynamic parameters. Among clinical parameters, persistent AF (P < .001), 6MWD (P < .001) and reduced estimated glomerular filtration rate (P = .030) were significantly associated with outcome in the multivariate analysis. Among invasive haemodynamic parameters, only diastolic pulmonary arterial pressure (P = .001) and, among imaging variables, right ventricular size (P < .001) and function (P = .017) were independently related to event‐free survival.
Table 3.
Univariable and multivariable Cox regression analyses
| Variable | no event (n = 162) | event (n = 92) | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P‐value | Hazard Ratio (95% CI) | P‐value | |||
| Clinical parameters | ||||||
| Persistent AF, n (%) | 53 (33) | 66 (72) | 3.43 (2.18‐5.41) | <.001 | 3.04 (1.77‐5.24) | <.001 |
| Paroxysmal AF, n (%) | 26 (16) | 8 (9) | 1.39 (0.59‐3.24) | .452 | ||
| Age, y | 71 ± 9 | 72 ± 8 | 1.01 (0.99‐1.04) | .389 | ||
| Female sex, n (%) | 119 (73) | 58 (63) | 1.48 (0.97‐2.27) | .070 | ||
| Body mass index, kg/m2 | 30.2 ± 6.5 | 31.0 ± 7.5 | 1.02 (0.99‐1.05) | .233 | ||
| Diabetes mellitus type II, n (%) | 46 (28) | 45 (49) | 1.96 (1.30‐2.96) | .001 | ||
| Hyperlipidaemia, n (%) | 88 (54) | 50 (54) | 0.96 (0.63‐1.44) | .827 | ||
| Arterial hypertension, n (%) | 155 (96) | 88 (96) | 1.02 (0.37‐2.77) | .974 | ||
| Heart rate, (beats/min) | 70 ± 13 | 74 ± 16 | 1.01 (1.00‐1.03) | .058 | ||
| % of predicted 6MWD, % | 78 ± 23 | 60 ± 24 | 0.98 (0.97‐0.98) | <.001 | 0.98 (0.97‐0.99) | <.001 |
| Sleep apnoea, n (%) | 13 (8) | 13 (14) | 1.51 (0.84‐2.71) | .170 | ||
| COPD, n (%) | 44 (27) | 38 (41) | 1.71 (0.98‐2.97) | .032 | ||
| NYHA class ≥ III, n (%) | 86 (53) | 78 (85) | 3.41 (1.93‐6.02) | <.001 | ||
| NT‐proBNP, pg/mL | 780 (390 to 1440) | 1940 (1150 to 2860) | 1.11 (1.06‐1.16) | <.001 | ||
| Glycated haemoglobin, % | 6.4 ± 4.4 | 6.2 ± 1.2 | 0.98 (0.90‐1.08) | .695 | ||
| eGFR, mL/min/1.73 m2 | 64 (50 to 80) | 53 (40 to 65) | 0.98 (0.97‐0.99) | <.001 | 0.98 (0.97‐0.99) | .030 |
| Gamma‐glutamyl‐transferase, U/l | 33 (22 to 55) | 49 (29 to 104) | 1.00 (1.00‐1.00) | <.001 | ||
| Echocardiographic parameters | ||||||
| LA diameter, mm | 62 ± 8 | 64 ± 7 | 1.03 (1.00‐1.05) | .042 | ||
| LV diameter, mm | 44 ± 5 | 44 ± 6 | 0.99 (0.96‐1.03) | .708 | ||
| RA diameter, mm | 61 ± 9 | 65 ± 8 | 1.03 (1.01‐1.05) | .012 | ||
| RV diameter, mm | 36 ± 7 | 39 ± 8 | 1.06 (1.03‐1.08) | <.001 | 1.05 (1.02‐1.08) | <.001 |
| Interventricular septum, mm | 13 ± 3 | 13 ± 2 | 0.98 (0.91‐1.07) | .697 | ||
| E/E' ratio | 13.8 (10.6 to 18.9) | 15.0 (10.2 to 18.0) | 1.03 (0.97‐1.09) | .365 | ||
| LV ejection fraction, % | 59 ± 7 | 59 ± 7 | 1.00 (0.96‐1.05) | .885 | ||
| Systolic PAP, mm Hg | 45 (54 to 69) | 61 (51 to 75) | 1.02 (1.01‐1.03) | .002 | ||
| Cardiac magnetic resonance imaging parameters | ||||||
| LV end‐diastolic diameter, mm | 47 ± 6 | 47 ± 6 | 1.00 (0.96‐1.05) | .906 | ||
| RV end‐diastolic diameter, mm | 40 ± 7 | 42 ± 8 | 1.04 (1.01‐1.08) | .009 | ||
| Interventricular septum, mm | 11 ± 2 | 11 ± 2 | 1.03 (0.91‐1.15) | .681 | ||
| LA diameter, mm | 64 ± 9 | 69 ± 9 | 1.05 (1.02‐1.08) | .001 | ||
| LA area, cm2 | 28 (24 to 34) | 31 (28 to 36) | 1.03 (1.01‐1.05) | .018 | ||
| RA diameter, mm | 64 ± 9 | 68 ± 10 | 1.04 (1.01‐1.07) | .010 | ||
| RA area, cm2 | 27 (22 to 34) | 30 (25 to 35) | 1.03 (1.00‐1.05) | .025 | ||
| LV ejection fraction, % | 63 ± 11 | 62 ± 12 | 1.00 (0.98‐1.02) | .888 | ||
| LV end‐diastolic volume, mL | 117 (103 to 142) | 124 (99 to 150) | 1.00 (1.00‐1.01) | .667 | ||
| Cardiac output, l/min | 5.6 ± 2.9 | 5.3 ± 1.9 | 0.96 (0.85‐1.09) | .561 | ||
| RV ejection fraction, % | 54 ± 11 | 49 ± 11 | 0.97 (0.95‐0.99) | .005 | 0.97 (0.95‐0.99) | .017 |
| RV end‐diastolic volume, mL | 136 (113 to 169) | 155 (121 to 205) | 1.00 (1.00‐1.00) | .577 | ||
| Native T1 time myocardium, ms | 419 (371 to 461) | 407 (354 to 471) | 1.00 (1.00‐1.00) | .788 | ||
| MOLLI‐ECV | 29.1 ± 3.4 | 31.3 ± 5.8 | 1.09 (1.02‐1.18) | .016 | ||
| Invasive haemodynamics | ||||||
| Systolic PAP, mm Hg | 48 (39 to 59) | 55 (47 to 69) | 1.02 (1.01‐1.03) | <.001 | ||
| Diastolic PAP, mm Hg | 21 (17 to 25) | 24 (19 to 30) | 1.05 (1.02‐1.08) | <.001 | 1.05 (1.02‐1.08) | .001 |
| Mean PAP, mm Hg | 32 (26 to 37) | 36 (31 to 43) | 1.04 (1.02‐1.06) | <.001 | ||
| PAWP, mm Hg | 19 ± 6 | 22 ± 6 | 1.06 (1.02‐1.10) | .002 | ||
| LV end‐diastolic pressure, mm Hg | 20 ± 6 | 21 ± 6 | 1.01 (0.97‐1.06) | .517 | ||
| TPG, mm Hg | 12 (9 to 17) | 14 (10 to 20) | 1.03 (1.01‐1.06) | .022 | ||
| Diastolic pressure gradient, mm Hg | 1 (−1 to 5) | 2 (−1 to 5) | 1.02 (0.98‐1.07) | .232 | ||
| CO thermodilution, l/min | 5.3 ± 1.3 | 5.2 ± 1.4 | 0.97 (0.82‐1.14) | .671 | ||
| PVR, dyn‐s‐cm−5 | 196 (143 to 252) | 223 (155 to 334) | 1.00 (1.00‐1.00) | .008 | ||
| Pooled multivariate analysis | ||||||
| Persistent AF | 2.44 (1.31‐4.54) | .005 | ||||
| % of predicted 6 MWD | 0.98 (0.97‐0.99) | .011 | ||||
Abbreviations: 6MWD, 6‐minute walk distance; ACE, angiotensine converting enzyme; AF, atrial fibrillation; AT II, angiotensin II; CO, cardiac output; COPD, chronic obstructive pulmonary disease; E, early mitral inflow velocity; E', early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; LA, left atrial; LV, left ventricular; MOLLI‐ECV, modified Look‐Locker inversion recovery sequence‐derived extracellular volume; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial; RV, right ventricular; TPG, transpulmonary pressure gradient.
However, after pooled multivariate Cox regression analysis, only persistent AF (P = .005) and 6MWD (P = .011) remained independently associated with cardiovascular outcome.
4. DISCUSSION
In the present study, persistent AF was found in nearly 50% of patients. Persistent but not paroxysmal AF was independently associated with event‐free survival. Furthermore, persistent AF was closely related to important markers of disease severity such as NYHA functional class, serum NT‐proBNP, atrial dilatation, cardiac output and presence of COPD.
Several prior studies have addressed the prognostic influence of AF on HFpEF.3, 6, 19, 20 All of these studies were retrospective analyses or sub‐studies of multicentre trials. In contrast to these, we prospectively enrolled our patients, HFpEF was confirmed by invasive haemodynamic assessment, and coronary artery disease was ruled out by coronary angiography.
4.1. Factors associated with atrial fibrillation
We identified several parameters independently associated with persistent AF. By multivariate analysis, NYHA functional status, serum levels of NT‐proBNP, atrial dilatation, low cardiac output and COPD were associated with persistent AF (Table 2). Impaired pulmonary function and COPD have previously been linked with the development of AF.21 Worse NYHA status and elevated NT‐proBNP reflect advanced stages of disease, previously related to outcome in HFpEF.22 There are two main differences when comparing AF to SR: loss of atrial contraction, also called the booster pump function, and irregular heart rate. While the relative importance of the atrial booster pump function remains controversial,23 the negative influence of irregular heart rate on cardiac output has previously been described.24
Compared with published reference values,25 atrial dimensions in general were markedly increased in our study population. Furthermore, the extent of atrial enlargement was independently associated with persistent AF. In a recent sheep model of induced persistent AF, the degree of atrial dilatation and atrial fibrosis were significantly related to persistent AF.9 More pronounced atrial enlargement may also be linked to more advanced stages of HFpEF, indicated by significantly higher ECV values by CMR T1 mapping in the persistent AF cohort. Extracellular volume expansion plays a key role in the pathogenesis and prognosis of HFpEF.26, 27 It enhances left ventricular stiffness, which causes atrial enlargement due to increased atrial afterload. Of note, elevated ECV values did not remain significantly associated with persistent AF in the multivariate regression analysis. Patients suffering from persistent AF presented with markedly dilated right ventricles and decreased right ventricular ejection fractions, which also not remained significantly associated with persistent AF after multivariate analysis. However, impaired right ventricular ejection fraction is a known predictor for worse cardiovascular outcome28, 29, 30 and an association between AF and right heart dysfunction has previously been described.29 Interestingly, obesity, a well‐known risk factor for AF development,4 was not associated with AF in the present cohort.
4.2. Association of atrial fibrillation with outcome
Our data indicate a strong and independent association of persistent AF with cardiovascular outcome in HFpEF. Most studies that investigated AF and heart failure included both HFpEF and heart failure due to reduced ejection fraction populations and thus compared AF in HFpEF with AF in heart failure in reduced ejection fraction rather than with HFpEF in sinus rhythm.6, 20 Thus, previous data regarding the association of AF with all cause mortality or mortality and hospitalization for heart failure in HFpEF patients are limited and, moreover, report conflicting results.3, 19, 31
Our findings are in line with a previous large, population‐based study that showed AF to be associated with increased mortality after adjustment for age, sex, body size, kidney function, hypertension, COPD, angiotensin receptor‐, ß‐blocker and statin use.3 On the contrary, in I‐PRESERVE AF was associated with outcome only in the univariate analysis but was not among those demographic, clinical and biological variables that provided consistent independent prognostic information.31 In the present study, no differences in outcome were observed between patients in SR and patients presenting with paroxysmal AF. Thus, failure to show an association of AF with event‐free survival in the aforementioned study may also be related to the fact that paroxysmal and persistent AF patients were not analysed separately.31
4.3. Limitations
This was a single‐centre study, thus a centre‐specific bias cannot be ruled out although single‐centre studies have several advantages such as homogenous patient selection, continuous workflow and constant follow‐up. As continuous electrocardiogram monitoring by implantable loop recorders was not provided, asymptomatic AF episodes may have been missed and real‐life burden of AF episodes might be higher. In incident AF, we observed a high rate of direct onset of persistent AF. This rate may be overestimated as persistent AF might be preceded by asymptomatic and not documented short episodes of paroxysmal AF. The fact that no differences with regard to baseline characteristics and outcome were observed between patients in SR and with paroxysmal AF may be due to the low rate of paroxysmal AF in the present study. Functional imaging parameters such as ejection fraction and cardiac output on CMR may be influenced by heart rate and irregular heart rhythm. However, baseline heart rate was 73/min in persistent AF patients and did not differ significantly from patients with paroxysmal AF or SR. The cross‐sectional observational study design limits conclusions about cause—effect relationships.
5. CONCLUSIONS AND CLINICAL PERSPECTIVE
AF is a frequent finding in HFpEF and may affect more than 60% of patients in the course of their disease. In the present study, persistent AF was the predominant AF subtype. It was closely and independently related to event‐free survival and related to NYHA class, NT‐proBNP, atrial size, cardiac function and COPD. Up to now, no specific treatment has been approved for HFpEF patients.2 Similarly, effective antiarrhythmic treatment options are limited for patients with persistent AF and no survival benefit has been documented for treatments targeting rhythm control.32 Only the modulation of associated risk factors such as obesity has so far shown significant improvement for both HFpEF and AF patients.33, 34 Besides pharmacologic trials, future representative studies investigating dedicated antiarrhythmic treatment of HFpEF patients are highly warranted.
CONFLICT OF INTEREST
None.
AUTHORS' CONTRIBUTIONS
RS and JM were responsible for concept and drafting of the manuscript; FD, AAK, SA, CB, CZ‐T, MK, LF FXR, and DB performed data collection, interpretation and approved the final version.
ACKNOWLEDGEMENTS
All authors approved the final version of the article, including the authorship list.
Schönbauer R, Duca F, Kammerlander AA, et al. Persistent atrial fibrillation in heart failure with preserved ejection fraction: Prognostic relevance and association with clinical, imaging and invasive haemodynamic parameters. Eur J Clin Invest. 2020;50:e13184 10.1111/eci.13184
Funding information
This study received support from the Austrian Society of Cardiology (to JM and FD), the Österreichischer Herzfonds (to JM) and the Austrian fellowship grants KLI 246 (to DB), and KLI 245 (to JM).
REFERENCES
- 1. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: Treatment considerations for a dual epidemic. Circulation. 2009;119:2516‐2525. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 3. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community‐based study. Circulation. 2013;128:1085‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609‐1678. [DOI] [PubMed] [Google Scholar]
- 5. Zotter‐Tufaro C, Mascherbauer J, Duca F, et al. Prognostic significance and determinants of the 6‐min walk test in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2015;3:459‐466. [DOI] [PubMed] [Google Scholar]
- 6. Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GYH. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: A systematic review and meta‐analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660‐666. [DOI] [PubMed] [Google Scholar]
- 7. Melenovsky V, Hwang S‐J, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295‐303. [DOI] [PubMed] [Google Scholar]
- 8. Kosiuk J, Dinov B, Kornej J, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR‐FLASH score. Heart Rhythm. 2015;12:2207‐2212. [DOI] [PubMed] [Google Scholar]
- 9. Martins RP, Kaur K, Hwang E, et al. Dominant frequency increase rate predicts transition from paroxysmal to long‐term persistent atrial fibrillation. Circulation. 2014;129:1472‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147‐e239. [DOI] [PubMed] [Google Scholar]
- 11. Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease. Eur Heart J. 2007;28:230‐268. [DOI] [PubMed] [Google Scholar]
- 12. Lancelotti P, Tribouilloy C, Hagendorff A, et al. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14(7):611‐644. [DOI] [PubMed] [Google Scholar]
- 13. Baumgartner H, Hung J, Bermejo J, et al. Recommendations for the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18(3):254‐275. [DOI] [PubMed] [Google Scholar]
- 14. Mascherbauer J, Kammerlander AA, Zotter‐Tufaro C, et al. Presence of ‘isolated’ tricuspid regurgitation should prompt the suspicion of heart failure with preserved ejection fraction. PLoS ONE. 2017;12(2):e0171542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonderman D, Agis H, Kain R, Mascherbauer J. Amyloid in the heart: an under‐recognized threat at the interface of cardiology, haematology, and pathology. Eur Heart J Cardiovasc Imaging. 2016;17:978‐980. [DOI] [PubMed] [Google Scholar]
- 16. Enright PL, Sherrill DL. Reference equations for the six‐minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384‐1387. [DOI] [PubMed] [Google Scholar]
- 17. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E, Society for Cardiovascular Magnetic Resonance and Board of Trustees Task Force on Standardized Protocols . Standardized cardiovascular magnetic resonance protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. 2017;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Granger CB, Michelson EL, Mcmurray JJV, Puu M, Yusuf S, Hil DP. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction of reduction in mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47:1997‐2004. [DOI] [PubMed] [Google Scholar]
- 20. Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J, Agarwal SK, Alonso A, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the atherosclerosis risk in communities (ARIC) study. Circulation. 2014;129:971‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dalos D, Mascherbauer J, Zotter‐Tufaro C, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:189‐199. [DOI] [PubMed] [Google Scholar]
- 23. Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039‐1045. [DOI] [PubMed] [Google Scholar]
- 25. Pfaffenberger S, Bartko P, Graf A, et al. Size matters! Impact of age, sex, height, and weight on the normal heart size. Circ Cardiovasc Imaging. 2013;6:1073‐1079. [DOI] [PubMed] [Google Scholar]
- 26. Duca F, Kammerlander AA, Zotter‐Tufaro C, et al. Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2016;9:e005277. [DOI] [PubMed] [Google Scholar]
- 27. Mascherbauer J, Marzluf BA, Tufaro C, et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013;6:1056‐1065. [DOI] [PubMed] [Google Scholar]
- 28. Kammerlander AA, Marzluf BA, Graf A, et al. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol. 2014;64:2633‐2642. [DOI] [PubMed] [Google Scholar]
- 29. Aschauer S, Kammerlander AA, Zotter‐tufaro C, et al. The right heart in heart failure with preserved ejection fraction: insights from cardiac magnetic resonance imaging and invasive haemodynamics. Eur J HeartFail. 2016;18:71‐80. [DOI] [PubMed] [Google Scholar]
- 30. Aschauer S, Zotter‐Tufaro C, Duca F, et al. Modes of death in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2017;228:422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Komajda M, Carson PE, Hetzel S, et al. Factors associated with outcome in heart failure with preserved ejection fraction. Circ Hear Fail. 2011;4:27‐35. [DOI] [PubMed] [Google Scholar]
- 32. Chatterjee S, Sardar P, Lichstein E, Mukherjee D, Aikat S. Pharmacologic rate versus rhythm‐control strategies in atrial fibrillation: An updated comprehensive review and meta‐analysis. PACE ‐ Pacing Clin Electrophysiol. 2013;36:122‐133. [DOI] [PubMed] [Google Scholar]
- 33. Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype‐specific treatment of heart failure with preserved ejection fraction. Circulation. 2016;134:73‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation. JAMA. 2013;310:2050. [DOI] [PubMed] [Google Scholar]


