Abstract
Purpose
Run‐in periods are used to identify placebo‐responders and washout. Our aim was to assess the association of run‐in periods with clinical outcomes of antipsychotics in dementia.
Methods
We searched randomized placebo‐controlled trials of conventional and atypical antipsychotics for neuropsychiatric symptoms (NPS) in dementia in electronic sources and references of selected articles. We extracted (a) the presence of a run‐in period, use of placebo/investigated drug during run‐in (versus washout only), and run‐in duration (1 week or more) and (b) the reduction in NPS, number of participants with somnolence, extrapyramidal symptoms (EPS), and deaths per treatment group. We pooled clinical outcomes comparing antipsychotic and placebo groups in trials with and without run‐in.
Results
We identified 35 trials. Twenty‐nine trials used run‐in. The pooled standardized mean difference in the reduction of NPS was −0.170 (95% CI, −0.227 to −0.112) in trials with run‐in and −0.142 (95% CI, −0.331 to 0.047) in trials without run‐in. The pooled odds ratio for somnolence was 2.8 (95% CI, 2.3‐3.5) in trials with run‐in and 3.5 (95% CI, 1.2‐10.7) in trials without run‐in; for EPS, these ORs were 1.8 (95% CI, 1.4‐2.2) and 2.0 (95% CI, 1.3‐3.1) respectively, and for mortality 1.4 (95% CI, 1.0‐2.0) and 1.6 (95% CI, 0.7‐3.4). The use of placebo/investigated drug during run‐in and run‐in duration did not affect the estimates in a consistent way.
Conclusions
The use of run‐in in trials might have led to overestimated efficacy and especially underestimated risks of side effects of antipsychotics compared with placebo for NPS in dementia.
Keywords: antipsychotics, dementia, efficacy, meta‐analysis, pharmacoepidemiology, run‐in, side effects
Key Points.
Run‐in periods have been used in the majority of trials that tested conventional and atypical antipsychotics in dementia.
The use of run‐in periods increased the estimated efficacy of antipsychotics in dementia.
The use of run‐in periods decreased the risk of somnolence, extrapyramidal symptoms, and mortality of antipsychotics in dementia.
The use of run‐in periods did not affect drop‐out substantially.
The effect of run‐in on clinical outcomes of trials needs to be addressed as part of reviews.
1. INTRODUCTION
Results of randomized controlled trials are important for regulatory and clinical decisions. Researchers have therefore sought to optimize treatment effects and identify patients that will benefit most from treatment. One way of enhancing trial design is using a run‐in period between screening for eligibility and before randomization.1, 2 During this period of usually 1 to 2 weeks, drugs that the eligible patients already used are washed out. In some trials, the drugs are replaced by placebo to blind the participants for the change in treatment. Drug‐naïve patients can also be given placebo, or the active drug of interest. Patients with high placebo response, poor compliance, low treatment response, or intolerance for the drug can thus be identified.3, 4 At the end of the run‐in phase, the researchers select the participants that are definitively included in the study. It is assumed that a run‐in period will decrease placebo response and dropout during the trial and consequently increase the effect size and the power of a trial.5, 6
A small number of reviews have studied the effect that a run‐in period can have on trial outcomes of psychopharmacological drugs. Antidepressants in children were 15% more effective in trials with a run‐in period than in trials without a run‐in period and above the threshold for a small effect size (standardized mean difference 0.26 vs 0.17, respectively; cut‐off for small effect is 0.20).7 Another meta‐analysis of antidepressant trials in depressed outpatients showed that a placebo run‐in period was associated with higher efficacy and more power.8 On the other hand, run‐in periods were not associated with greater efficacy in trials of antidepressants for major depression, benzodiazepines for anxiety, and naltrexon for alcohol addiction.9, 10, 11, 12, 13
To our knowledge, the effect of a run‐in period on efficacy and side effects of antipsychotics has not been investigated before. This is notable, because high placebo response rates, high dropout rates, and decreasing effectiveness over the years are the major problems in antipsychotic trials.14 An association between use of a run‐in period and drug safety is not unlikely, because run‐in periods can lead to exclusion of persons not tolerating the drugs and of noncompliant subjects.15 Moreover, atypical antipsychotics have been marketed with the claim of a more favorable side effects profile compared with conventional antipsychotics, ie, lower rates of somnolence and extrapyramidal symptoms (EPS).16
Antipsychotics are often prescribed for neuropsychiatric symptoms in dementia. Trials that tested the efficacy of antipsychotics for this indication commonly used run‐in periods. The aim of this study was to assess the association of run‐in periods in trials of conventional and atypical antipsychotics in dementia with clinical outcomes and also dropout.
2. METHODS
We performed a meta‐epidemiological study. We wrote a research proposal for the sponsor in advance, and it can be requested from the corresponding author.
2.1. Search strategy
Four sources were used to identify trials. Two reviewers (T.A.H. and H.J.L.) first searched the electronic databases Cinahl, Embase, Pubmed, and Cochrane library with the strings “generic name atypical/conventional antipsychotic” AND trial AND dementia (see the Appendix S1). We composed a list of all conventional and atypical antipsychotics from the websites of the World Health Organization, Food and Drug Administration, and Wikipedia to enable this search.17, 18, 19 Secondly, we hand‐searched the references of published systematic reviews, which were identified with the same electronic databases. Titles and abstracts of potentially eligible studies were retrieved in Pubmed. Thirdly, we sought RCTs in trial registration websites with the same keywords where possible. Finally, we searched the databases of the Dutch Medicines Evaluation Board and the FDA for unpublished trials of atypical antipsychotics.
2.2. Study selection
Randomized placebo‐controlled trials that tested the efficacy of orally administered conventional or atypical antipsychotics for neuropsychiatric symptoms in dementia were included. If studies seemed potentially eligible given title and abstract, full articles were retrieved as well as online protocols of unpublished studies. Two reviewers (T.A.H. and H.J.L.) reviewed these articles for definitive eligibility. Studies with no information on the use of a run‐in period and with multiple drugs in one intervention arm were excluded. There were no restrictions with respect to publication date, language, flexible or fixed dosing of the active treatment, and duration of the study. The search was last rerun in June 2019.
2.3. Data extraction
Two reviewers (T.A.H. and H.J.L.) independently extracted data from the included trials. First, we extracted general study characteristics: publication year, type of antipsychotic groups, setting, type of neuropsychiatric symptoms for which the antipsychotics were tested, and total number of randomized patients. Next, we extracted characteristics of the run‐in period: presence/absence, replacement drug (no drug, placebo, or active treatment), and duration, as well as the percentage of patients excluded at the end of the run‐in phase.
We then extracted the clinical outcomes for the drug and placebo groups. Efficacy of antipsychotics in dementia can be measured with a generic instrument that covers various neuropsychiatric symptoms (eg, NPI and BEHAVE‐AD) or an instrument for specific symptoms such as agitation (eg, CMAI). We used the results measured with the instrument that matched the symptoms at enrollment, eg, if patients had to have agitation to enter a trial, we used the result reported with an agitation scale. We extracted the mean change from baseline to end point. When multiple dosages or multiple drug groups were included in a trial, an average change was calculated. We also extracted the standard deviation (SD) of the difference between the groups in mean change. If the SD was not reported, it was calculated with the P value, range, or confidence interval reported for the difference in mean change. Otherwise, the SD was imputed with the average of the reported SD of all trials with the same indication and instrument. For two trials that did not report the data we needed, we obtained the IPD and calculated the mean changes and SDs.20, 21 In addition, we abstracted the number of patients with somnolence (sedation and drowsiness), and with EPS, and the number that died.
Finally, we extracted the total number of patients that dropped out (total drop‐out) and the drop‐out in the groups (selective drop‐out). Run‐in is often used to decrease drop‐out and enhance power. Drop‐out is also considered to represent the balance between efficacy and side effects.20
The published main results' article of a trial was our primary source of information. When the article did not report the data that we needed, secondary publications, trial reports, and meta‐analysespublished online by industry were our secondary source. We contacted the authors of eligible trials to provide missing data, or individual patient data, and received such data of four studies.20, 21, 22, 23 The reviewers discussed differences in the extracted data until consensus was reached.
2.4. Data analyses
First, we assessed the relationship between the presence of a run‐in period with the four clinical outcomes and selective dropout. We performed meta‐analyses to pool efficacy, risk of somnolence, EPS, and mortality of the antipsychotic versus placebo groups in trials with and without run‐in periods. For efficacy in terms of reduction in NPS, we calculated standardized mean differences (SMDs) to take into account the use of different instruments in the trials. SMDs were calculated with a 95% confidence interval (CI). For risk of somnolence, EPS, mortality, and selective dropout, we calculated odds ratios (ORs) with 95% CIs. Heterogeneity, presented as I 2, was calculated for all meta‐analyses. A fixed‐effects model was applied when I 2 was below 40%, otherwise a random‐effects model.24
We then investigated the relationship of the characteristics of the run‐in periods with the clinical outcomes and selective dropout. The outcomes of trials that had run‐in periods with and without a placebo were pooled (we did not find trials with active drugs in the run‐in period). We also pooled outcomes of trials with a run‐in period up to 1 week versus those with a longer run‐in period (8 to 14 days). Finally, we tested the association between run‐in characteristics and total drop‐out with meta‐regression.
We ran the above analyses for all antipsychotics combined first and then for the conventional antipsychotics and atypical antipsychotics separately. We wanted to perform an a priori sensitivity‐analysis for atypical antipsychotics without quetiapine, because its efficacy and side effect profile are considered to differ. A post hoc analysis was run in which trials that had tested both an atypical drug and haloperidol were excluded from the analysis. All analyses were performed with STATA statistical software version 15.0.25
3. RESULTS
Our search yielded 2768 potentially relevant RCTs (Figure 1). We obtained the reports of 65 RCTs for full text review. We identified 45 eligible RCTs, but four did not report whether a run‐in period was used, four did not report any of the outcomes of interest, and two were ongoing. We used the other 35 studies in the current study.20, 21, 22, 23, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56
Figure 1.
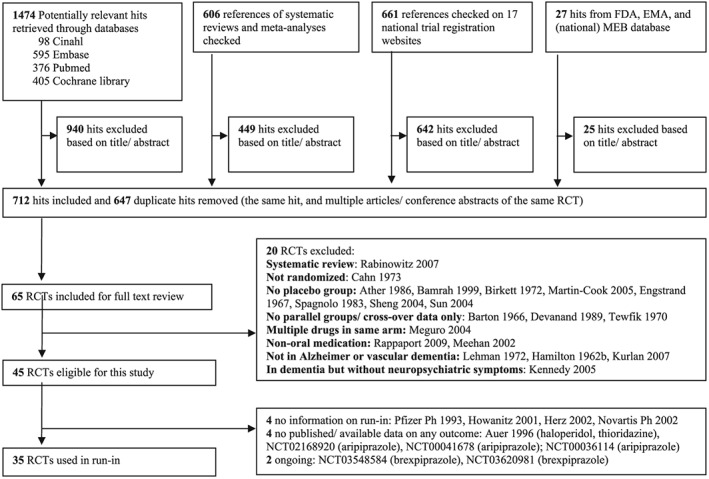
Flow diagram of literature search and study selection
Table 1 presents the general study characteristics. Twenty‐nine of the 35 studies had a run‐in period: nine of 11 conventional antipsychotic trials, 18 of 21 atypical antipsychotic trials, and two of three trials with both antipsychotics. Fourteen studies used placebo during the run‐in period, two trials the investigated drug, and 19 studies no placebo or active treatment (washout only). The duration of the run‐in periods varied between 2 days and 6 weeks. The percentage of patients excluded at the end of the run‐in period varied from 0% to 29%. In five of seven relatively old conventional antipsychotic trials, the percentage was 0%, while in the atypical trials, it was at least 7%.
Table 1.
Randomized placebo‐controlled trials of antipsychotics in dementia with neuropsychiatric symptoms related to dementia
| Author, Year | Antipsychotic | Setting | Neuropsychiatric Symptom | Run‐in Period (Duration in Weeks) | Patients Excluded After Run‐in, n (%) | Randomized Patients, n |
|---|---|---|---|---|---|---|
| Conventional antipsychotic trials (11) | ||||||
| Hamilton, 1962 | Trifluoperazine | Hospital | Diverse | No | NA | 27 |
| Sugerman, 1964 | Haloperidol | Hospital | Diverse | No | NA | 18 |
| Rada, 1976 | Thiothixene | Hospital | Diverse | Yes, with placebo (1) | 0 (0) | 42 |
| Barnes, 1982 | Thioridazine Loxapine | Nursing home | Diverse | Yes, with placebo (2) | 7 (11.7) | 53 |
| Petrie, 1982 | Haloperidol Loxapine | Hospital | Diverse | Yes, with placebo (2) | 0 (0) | 61 |
| Stotsky, 1984 | Thioridazine | Nursing home and hospital | Diverse | Yes, washout only (2) | NR | 358 |
| Finkel, 1995 | Thiothixene | Nursing home | Agitation | Yes, washout only (1) | 0 (0) | 33 |
| Auchus, 1997 | Haloperidol | Outpatients | Agitation | Yes, washout only (2) | 0 (0) | 12 |
| Devanand, 1998 | Haloperidol | Outpatients | Diverse | Yes, with placebo (1) | 5 (7.0) | 66 |
| Teri, 2000 | Haloperidol | Hospital | Agitation | Yes, washout only (2) | 0 (0) | 70 |
| Pollock, 2002 | Perphenazine | Nursing home | Diverse | Yes, washout only (<1) | NR | 54 |
| Atypical antipsychotic trials (21) | ||||||
| Satterlee, 1995 | Olanzapine | Nursing home | Psychosisa | Yes, washout only | 51 (17.7) | 238 |
| Janssen Ph, 1997 | Risperidone | NR | Diverse | Yes, with placebo (1) | NR | 39 |
| Katz, 1999 | Risperidone | Nursing home | Diverse | Yes, with placebo (1) | 104 (14.3) | 625 |
| Street, 2000 | Olanzapine | Nursing home | Diverse | Yes, with placebo (2) | 82 (28.5) | 206 |
| Brodaty, 2003 | Risperidone | Nursing home | Aggression | Yes, with placebo (1) | 39 (10.2) | 345 |
| Janssen Ph, 2003 | Risperidone | Nursing home | Psychosis | Yes, with placebo (1) | NR | 18 |
| De Deyn, 2004 | Olanzapine | Nursing home | Psychosis | Yes, with placebo (2) | NR | 652 |
| Ballard, 2005 | Quetiapine | Nursing home | Agitation | No | NA | 62 |
| De Deyn, 2005 | Aripiprazole | Outpatients | Psychosis | Yes, washout only (1) | NR | 208 |
| Deberdt, 2005 | Risperidone Olanzapine | Nursing home and outpatients | Psychosis | Yes, with placebo (2) | NR | 494 |
| Janssen Ph, 2005 | Risperidone | NR | Psychosis | Yes, with risperidone (1) | NR | 33 |
| Mintzer, 2006 | Risperidone | Nursing home | Psychosis | Yes, with placebo (1) | 87 (15.5) | 473 |
| Schneider, 2006 |
Risperidone Olanzapine Quetiapine |
Outpatients | Diverse | No | NA | 421 |
| Mintzer, 2007 | Aripiprazole | Nursing home | Psychosis | Yes, washout only (1) | NR | 487 |
| Zhong, 2007 | Quetiapine | Nursing home | Agitation | No | NA | 333 |
| Paleacu, 2008 | Quetiapine | Not reported | Diverse | Yes, with quetiapine (2) | NR | 40 |
| Streim, 2008 | Aripiprazole | Nursing home | Psychosis | Yes, washout only (1) | NR | 256 |
| Otsuka Ph, 2017a | Brexpiprazole | Nursing home | Agitation | Yes, washout only (6) | NR | 413 |
| Otsuka Ph, 2017b | Brexpiprazole | Nursing home and outpatients | Agitation | Yes, washout only (6) | NR | 270 |
| ACADIA, 2018 | Pimavanserin | Nursing home and outpatients | Agitation | Yes, washout only (4) | NR | 111 |
| Ballard, 2018 | Pimavanserin | Nursing home | Psychosis | Yes, washout only (3) | 25 (12.1) | 181 |
| Trials with conventional and atypical antipsychotic drug group (3) | ||||||
| Allain, 2000 |
Tiapride Haloperidol |
Nursing home and hospital | Agitation | No | NA | 306 |
| De Deyn, 1999 |
Risperidone Haloperidol |
Nursing home | Diverse | Yes, with placebo (1) | 27 (7.3) | 344 |
| Tariot, 2006 |
Quetiapine Haloperidol |
Nursing home | Psychosis | Yes, washout only (1)b | 123 (24.6) | 284 |
Abbreviations: NA not applicable; NR, not reported; Ph, pharmaceuticals.
Reduction measured with generic instrument (in all other studies indication and outcome scale were congruent).
At least 2 days.
3.1. Run‐in and clinical outcome
The analysis of efficacy encompassed 29 of 35 studies (Table 2). The reduction in NPS in the drug versus placebo groups was somewhat higher in trials with a run‐in period (SMD −0.170; 95% CI, −0.227 to −0.112) than in trials without a run‐in period (SMD −0.142; 95% CI, −0.331 to 0.047). Efficacy was somewhat higher when placebo or active drug was used (SMD −190; 95% CI, −0.267 to −0.112), and when run‐in lasted 1 week at most (SMD −0.214; 95% CI, −0.289 to −0.138).
Table 2.
Use of run‐in periods and clinical outcomes of antipsychotics versus placebo in randomized trials
| Efficacy | Somnolence | EPS | Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| SMD (95% CI) | N | OR (95% CI) | N | OR (95% CI) | N | OR (95% CI) | N | |
| Conventional and atypical antipsychotics | ||||||||
| No run‐in | −0.142 (−0.331 to 0.047)a | 4 | 3.5 (1.2‐10.7)a | 4 | 2.0 (1.3‐3.1) | 5 | 1.6 (0.7‐3.4) | 6 |
| With run‐in | −0.170 (−0.227 to −0.112) | 25 | 2.8 (2.3‐3.5) | 16 | 1.8 (1.4‐2.2) | 14 | 1.4 (1.0‐2.0) | 28 |
| ‐washout only | −0.146 (−0.267 to −0.024)a | 12 | 2.6 (1.4‐4.9)a | 8 | 1.7 (1.1‐2.7) | 5 | 1.2 (0.7‐2.1) | 14 |
| ‐with placebo/drug | −0.190 (−0.267 to −0.112) | 13 | 2.7 (2.1‐3.6) | 8 | 1.8 (1.4‐2.4) | 9 | 1.5 (1.0‐2.4) | 14 |
| ‐duration ≤ 1 week | −0.214 (−0.289 to −0.138) | 13 | 3.0 (2.1‐4.5)a | 9 | 1.8 (1.4‐2.4) | 10 | 1.4 (0.9‐2.0) | 14 |
| ‐duration > 1 week | −0.108 (−0.197 to −0.019) | 12 | 2.5 (1.7‐3.9) | 7 | 1.8 (0.8‐4.1)a | 4 | 1.5 (0.7‐3.0) | 13 |
| Conventional antipsychotics | ||||||||
| No run‐in | −0.389 (−0.669 to −0.110) | 1 | 4.3 (0.2‐110.2)a | 2 | 2.7 (1.4‐5.1) | 2 | 1.4 (0.3‐6.9) | 3 |
| With run‐in | −0.345 (−0.492 to −0.199) | 9 | 5.4 (3.2‐9.3) | 4 | 3.0 (1.9‐4.8) | 4 | 1.2 (0.6‐2.3) | 11 |
| Atypical antipsychotics | ||||||||
| No run‐in | −0.133 (−0.266 to 0.001) | 4 | 2.8 (0.9‐8.3)a | 3 | 1.6 (0.7‐3.6)a | 4 | 1.6 (0.7‐3.8) | 4 |
| With run‐in | −0.141 (−0.202 to −0.081) | 18 | 2.6 (2.1‐3.3) | 14 | 1.6 (1.2‐2.0) | 12 | 1.4 (1.0‐2.1) | 19 |
Abbreviations: EPS, extrapyramidal symptoms; OR, odds ratio; SMD, standardized mean difference.
A random effect model was used.
The number of participants with somnolence during the study period was reported in 20 of 35 studies. The pooled risk for somnolence was lower when a run‐in period was present (OR 2.8; 95% CI, 2.3‐3.5) versus when it was absent (OR 3.5; 95% CI, 1.2‐10.7). The use of placebo or active drug did not seem to affect the risk of somnolence further. The risk was lower for trials with a run‐in period of 1 week at most (OR 2.5; 95% CI, 1.7‐3.9).
Nineteen of 35 studies reported the number of participants with EPS in the treatment groups. The risk of EPS in trials with a run‐in period was slightly lower (OR 1.8; 95% CI, 1.4‐2.2) than in trials without a run‐in period (OR 2.0; 95% CI, 1.3‐3.1). Use of placebo or duration of run‐in did not seem to reduce the risk of EPS further.
The data of all but one trial could be used for the analysis of mortality risk. The risk of mortality was 1.6 (95% CI, 0.7‐3.4) and 1.4 (95% CI, 1.0‐2.0) in trials with and without run‐in, respectively. The risk was slightly lower when no placebo was used, but run‐in duration did not seem to affect it.
The sub‐analyses in trials of atypical antipsychotics only yielded similar results for use of run‐in versus no run‐in on all outcomes (Table 2). In trials of conventional antipsychotics, however, efficacy seemed lower and risk of somnolence and EPS higher in trials with run‐in compared to trials without run‐in, but the number of trials without run‐in was 3 at most and confidence intervals were large.
3.2. Run‐in and drop‐out
Thirty‐one of 35 studies reported dropout rates for the antipsychotic and placebo groups (Table 3). The number of participants that dropped out was 1519 of 4963 in the antipsychotic groups (30.6%) and 783 of 2747 in the placebo groups (28.5%). The odds ratio of selective dropout was 1.0 (95% CI, 0.9‐1.2) for trials with a run‐in period and 1.0 (95% CI, 0.7‐1.3) for those without.
Table 3.
Use of run‐in periods and dropout in antipsychotic trials in dementia
| Selective Dropouta | Total Dropout | |||
|---|---|---|---|---|
| OR (95% CI) | N | Beta (95% CI) | N | |
| Conventional and atypical antipsychotics | ||||
| No run‐in | 1.0 (0.7‐1.3) | 5 | ref | |
| With run‐in | 1.0 (0.9‐1.2) | 26 | 0.3 (−15.1 to 15.7) | 33 |
| washout only | 0.9 (0.7‐1.1) | 13 | ref | |
| with placebo/drug | 1.2 (1.0‐1.4) | 13 | −4.7 (−14.9 to 5.5) | 27 |
| duration ≤ 1 week | 0.9 (0.8‐1.1) | 14 | ref | |
| duration > 1 week | 1.2 (1.0‐1.5) | 12 | −5.0 (−14.7 to 4.6) | 26 |
| Conventional antipsychotics | ||||
| No run‐in | 1.4 (0.7‐2.8) | 2 | ref | |
| With run‐in | 1.0 (0.7‐1.3) | 9 | 16.0 (−7.5 to 39.6) | 13 |
| Atypical antipsychotics | ||||
| No run‐in | 0.9 (0.5‐1.7)b | 4 | ref | |
| With run‐in | 1.0 (0.9‐1.2) | 19 | −7.7 (−25.1 to 9.7) | 23 |
Abbreviation: OR, odds ratio.
Drug group versus placebo.
A random effects model was used.
Total dropout in the studies varied between 0% and 81.7%. The use of a run‐in period was not associated with a decreased total dropout (beta 0.3%; 95% CI, −15.1 to 15.7) (Table 3).
3.3. Sensitivity analyses
There were not enough trials with and without run‐in periods for the sensitivity‐analysis of atypical antipsychotics without quetiapine.57 The results of the analysis without trials that tested both haloperidol and an atypical drug confirmed the pattern of higher efficacy and lower risk of side effects for the conventional and atypical antipsychotic drugs in trials with versus without run‐in(Table S1).
4. DISCUSSION
We assessed the association between use of a run‐in period and clinical outcomes of 35 antipsychotic trials in dementia. The reduction in neuropsychiatric symptoms of antipsychotics versus placebo was somewhat higher in trials with a run‐in period than in trials without a run‐in period. The risk of somnolence, EPS, and mortality was lower in trials with than without run‐in. Accordingly, the risk of dropout in the antipsychotic compared with the placebo groups, which represents the balance between beneficial and harmful effects, was not affected when run‐in was used. Use of run‐in periods did not influence total dropout either.
Several reviews have reported an association between the use of run‐in periods and the efficacy of psychotropics. Two meta‐analyses of antidepressant trials showed that a placebo run‐in period was associated with higher effectiveness and more power.7, 8 We found that the use of run‐in periods was associated with a small increase in efficacy of antipsychotics in dementia. Additionally, we found an association between run‐in periods and a decreased risk of side effects of antipsychotics in dementia. The exclusion of placebo‐responders and drug‐intolerant patients after the run‐in period might have led to increased efficacy and a more favorable side effect profile. The effect of run‐in periods on outcomes of trials has not been investigated often and remains an under‐investigated and likely underestimated source of bias.
A common argument for use of run‐in periods is the reduction of noncompliance and dropout. Our findings showed that a placebo run‐in was not associated with lower between‐group or total dropout rate.
4.1. Bias due to run‐in periods
In the studies that we identified, patients that met the inclusion criteria could have been excluded from trial participation as a result of the outcomes during the run‐in period. It is therefore not surprising that the use of run‐in period yielded higher efficacy estimates and lower risks of side effects.3, 58 In observational studies, bias due to (de)selection of patients based on prior treatment and its outcomes, whether before or after the start of the study, is generally called selection bias.59
Bias due to run‐in periods in trials is not commonly discussed in the literature. Most tools for risk of bias assessment in trials do not require consideration of run‐in periods either. In eight of 11 meta‐analyses of antipsychotic trials in dementia, more than 80% of the included trials used a run‐in period (Table S2).60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70 Risperidone and olanzapine, currently the two most popular antipsychotic drugs for use in dementia, have been tested in ten trials of which nine included a run‐in period. The general assumption is that selection of patients before randomization is said to reduce only generalizability of study results, not the internal validity of the trial results.1
We propose a different view. The screening and selection of patients before randomization should be based on (contra‐)indications that are applied in daily medical practice. The selected patients will then represent the patients of interest to doctors and the population of interest as defined in the PICOs of reviews. This selection needs to be distinguished from deselection of eligible patients based on observed treatment effects during run‐in (between screening and randomization).2 The remaining randomized group does not represent the population of interest any more. Estimates of efficacy and risk of side effects will be biased for the population of interest. Therefore, run‐in needs to be considered as a source of bias in trials and reviews of trials.
Another issue to consider is the ethics of entering patients who are doing (relatively) well on a certain antipsychotic drug into a trial of another or the same antipsychotic. During washout, symptoms could return, and it is questionable whether the patient will respond as favorably to a new drug. Especially when it is difficult to convince patients to use antipsychotics and find an antipsychotic that has the desired effect, which is often the case in schizophrenia, switching to another drug for the sake of a trial is even more questionable. Including new instead of prevalent users in trials would be preferable, as is the recognized practice in observational epidemiology.
4.2. Strengths and limitations
To our knowledge, this is the first study that investigated the relationship of run‐in periods with clinical outcomes of antipsychotic trials including side effects. Our pooled estimates of efficacy seemed low but are corroborated by previous reviews reporting SMDs between 0.12 and 0.21.61, 66, 70 SMDs above the threshold of 0.200 for a small treatment effect were mainly found in meta‐analyses that focused on aggression or agitation.63, 64, 68, 70
A limitation of our research was that only six studies did not use a run‐in phase. Most of these studies were performed in outpatients and with atypical antipsychotics, in particular quetiapine. As a result of this distribution, the higher efficacy in trials with run‐in might be partly attributable to a higher efficacy of conventional antipsychotics. Nevertheless, our sensitivity analysis in atypical antipsychotic trials showed a higher efficacy for trials with a run‐in period as well. Additionally, one would expect the risk of side effects to increase with run‐in as well, but it did not.
5. CONCLUSION
The use of a run‐in period is very common in antipsychotic trials for dementia. In these trials, efficacy was higher compared with trials without run‐in, while the risk of side effects was lower. Therefore, the use of a run‐in period in trials might have led to overestimated efficacy and especially underestimated side effects of antipsychotics for neuropsychiatric symptoms in dementia. Meta‐analyses should include sensitivity‐analyses of trials with and without run‐in periods.
AUTHOR CONTRIBUTIONS
T.A. Hulshof and H.J. Luijendijk searched and selected the trials, extracted the data, and drafted the manuscript. T.A. Hulshof performed the data‐analysis. H.J. Luijendijk designed the study. S.U. Zuidema and C.C. Gispen‐de Wied critically commented on the design and results of the study. All authors reviewed the manuscript and suggested revisions.
Supporting information
Table S1. Use of run‐in periods and the clinical outcomes of antipsychotics in placebo‐controlled trials with conventional OR/AND atypical group
Table S2. Trials with run‐in in meta‐analyses of antipsychotics in dementia
ACKNOWLEDGEMENT
The authors received a grant from the Dutch Medicines Evaluation Board (College ter Beoordeling van Geneesmiddelen).
Hulshof TA, Zuidema SU, Gispen‐de Wied CC, Luijendijk HJ. Run‐in periods and clinical outcomes of antipsychotics in dementia: A meta‐epidemiological study of placebo‐controlled trials. Pharmacoepidemiol Drug Saf. 2020;29:125–133. 10.1002/pds.4903
REFERENCES
- 1. Rothwell P. External validity of randomised controlled trials: to whom do the results of this trial apply? Lancet. 2005;365(1):82‐93. [DOI] [PubMed] [Google Scholar]
- 2. Leber P, Davis C. Threats to the validity of clinical trials sample selection. Control Clin Trials. 1998;19(2):178‐187. [DOI] [PubMed] [Google Scholar]
- 3. Pablos‐Méndez A, Barr R, Shea S. Run‐in periods in randomized trials: implications for the application of results in clinical practice. JAMA. 1998;279(3):222‐225. [DOI] [PubMed] [Google Scholar]
- 4. Cipriani A, Gedds J. What is a run‐in phase? Epidemiol Psichiatr Soc. 2010;19(1):21‐22. [PubMed] [Google Scholar]
- 5. Ulmer M, Robinaugh D, Friedberg J, Lipsitz S, Natarajan S. Usefulness of a run‐in period to reduce drop‐outs in a randomized controlled trial of a behavioral intervention. Contemp Clin Trials. 2008;29(5):705‐710. [DOI] [PubMed] [Google Scholar]
- 6. Brittain E, Wittes J. The run‐in period in clinical trials. The effect of misclassification on efficiency. Control Clin Trials. 1990;11(5):327‐338. [DOI] [PubMed] [Google Scholar]
- 7. Bridge JA, Iyengar S, Salary CB. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta‐analysis of randomized controlled trials. JAMA. 2007;297(15):1683‐1696. [DOI] [PubMed] [Google Scholar]
- 8. Khan A, Cohen S, Dager S, Avery D, Dunner D. Onset of response in relation to outcome in depressed outpatients with placebo and imipramine. J Affect Disord. 1989;17(1):33‐38. [DOI] [PubMed] [Google Scholar]
- 9. Trivedi M, Rush H. Does a placebo run‐in or a placebo treatment cell affect the efficacy of antidepressant medications? Neuropsychopharmacology. 1994;11(1):33‐43. [DOI] [PubMed] [Google Scholar]
- 10. Lee S, Walker J, Jakul L, Sexton K. Does elimination of placebo responders in a placebo run‐in increase the treatment effect in randomized clinical trials? A meta‐analytic evaluation. Depress Anxiety. 2004;19(1):10‐19. [DOI] [PubMed] [Google Scholar]
- 11. Greenberg R, Fisher S, Riter J. Placebo washout is not a meaningful part of antidepressant drug trials. Percept Mot Skills. 1995;81(2):688‐690. [DOI] [PubMed] [Google Scholar]
- 12. Mitte K, Noack P, Steil R, Hautzinger M. A meta‐analytic review of the efficacy of drug treatment in generalized anxiety disorder. J Clin Psychopharmacol. 2005;25(2):141‐150. [DOI] [PubMed] [Google Scholar]
- 13. Del Re A, Maisel N, Blodgett J, Finney J. The declining efficacy of naltrexone pharmacotherapy for alcohol use disorders over time: a multivariate meta‐analysis. Alcohol Clin Exp Res. 2013;37(6):1064‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agid O, Siu C, Potkin S, Kapur S, Watsky E, Vanderburg D. Meta‐regression analysis of placebo response in antipsychotic trials 1970‐2010. Am J Psychiatry. 2013;170(11):1335‐1344. [DOI] [PubMed] [Google Scholar]
- 15. Schechtman K, Gordon M. A comprehensive algorithm for determining whether a run‐in strategy will be a cost‐effective design modification in a randomized clinical trial. Stat Med. 1993;12(2):111‐128. [DOI] [PubMed] [Google Scholar]
- 16. Arnt J, Skarsfeldt T. Do Novel Antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18(2):63‐101. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . WHO Collaborating Centre for Drug Statistics Methodology. http://www.whocc.no/atc_ddd_index/?code = N05A. Published 2013.
- 18. US Food and Drug Administration . Atypical Antipsychotic Drugs Information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm094303.htm. Published 2013.
- 19.Wikipedia. Typical antipsychotic. Wikipedia, the free encyclopedia. https://en.wikipedia.org/wiki/Typical_antipsychotic. Published 2015. Accessed January 8, 2015.
- 20. Schneider L, Tariot P, Dagerman K, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355(15):1525‐1538. [DOI] [PubMed] [Google Scholar]
- 21. Paleacu D, Barak Y, Mirecky I, Mazeh D. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer's disease patients: a 6‐week,double‐blind,placebo‐controlled study. Int J Geriatr Psychiatry. 2008;23(September 2007):393‐400. [DOI] [PubMed] [Google Scholar]
- 22. De Deyn P, Rabheru K, Rasmussen A. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:945‐956. [DOI] [PubMed] [Google Scholar]
- 23. De Deyn P, Jeste D, Swanink R, Kostic D, Breder C. Aripiprazole for the treatment of psychosis in patients with Alzheimer's disease. J Clin Psychopharmacol. 2005;25(5):463‐467. http://content.wkhealth.com/linkback/openurl?sid = WKPTLP:landingpage&an = 00004714‐200510000‐00010. Accessed April 17, 2014 [DOI] [PubMed] [Google Scholar]
- 24. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.handbook.cochrane.org [Google Scholar]
- 25.StataCorp. Stata Statistical Software: Release 13. 2013.
- 26. Auchus A, Bissey‐Black C. Pilot study of haloperidol, and placebo agitation in Alzheimer's disease. Neuropsychiatry Clin Neurosci. 1997;9:591‐593. [DOI] [PubMed] [Google Scholar]
- 27.RIS‐BEL‐14. Janssen Pharmaceutical. 1997.
- 28. Devanand D, Sackeim HA, Marder K, et al. A randomized, placebo‐controlleddose‐comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer's disease. Am J Psychiatry. 1998;155(November):1512‐1520. [DOI] [PubMed] [Google Scholar]
- 29. Katz I, Jeste D, Mintzer J, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double‐blind trial. J Clin Psychiatry. 1999;60(February):107‐115. [DOI] [PubMed] [Google Scholar]
- 30. Allain H, Dautzenberg P, Maurer K, Schuck S, Bonhomme D, Gerard D. Double blind study of tiapride versus haloperidol and placebo in agitation and aggressiveness in elderly patients with cognitive impairment. Psychopharmacology (Berl). 2000;148(4):361‐366. [DOI] [PubMed] [Google Scholar]
- 31. Street J, Clark S, Gannon K, Cummings J, Bymaster F. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities. Arch Gen Psychiatry. 2000;57(10):968‐976. [DOI] [PubMed] [Google Scholar]
- 32. Teri L, Logsdon RG, Peskind E. Treatment of agitation in AD: a randomized, placebo‐controlled clinical trial. Neurology. 2000;55(November):1271‐1277. [DOI] [PubMed] [Google Scholar]
- 33. Pollock B, Mulsant B, Rosen J, et al. Comparison of citalopram, perphenazine, and placebo for the acute treatment of psychosis and behavioral disturbances in hospitalized, demented patients. Am J Psychiatry. 2002;159(March):460‐465. [DOI] [PubMed] [Google Scholar]
- 34. Brodaty H, Ames D, Snowdon J, et al. A Randomized placebo‐controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;(February);64(2):134‐143. [DOI] [PubMed] [Google Scholar]
- 35. Hamilton L, Bennett J. The use of trifluoperazine in geriatric patients with chronic brain syndrome. J Am Geriatr Soc. 1962; (February); 10:140‐147. [DOI] [PubMed] [Google Scholar]
- 36. De Deyn P, Carrasco M, Deberdt W, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19(2):115‐126. [DOI] [PubMed] [Google Scholar]
- 37. Ballard C, Margallo‐Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer's disease: randomised double blind placebo controlled trial. BMJ. 2005;330(7496):874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deberdt W, Dysken M, Rappaport S, Feldman P, Young C, Hay D. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. Am J Geriatr Psychiatry. 2005;13(August):722‐730. [DOI] [PubMed] [Google Scholar]
- 39. Mintzer J, Greenspan A, Caers I, et al. Risperidone in the treatment of psychosis of Alzheimer disease: results from a prospective clinical trial. Am J Geriatr Psychiatry. 2006;(March;14(3):280‐291. [DOI] [PubMed] [Google Scholar]
- 40. Tariot P, Schneider L, Katz I, et al. Quetiapine treatment of psychosis associated with dementia: a double‐blind, randomized, placebo‐controlled clinical trial. Am J Geriatr Psychiatry. 2006;14(9):767‐776. [DOI] [PubMed] [Google Scholar]
- 41. Mintzer J, Tune L, Breder C, Swanink M, Marcus R. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, of three fixed doses. Am J Geriatr Psychiatry. 2007;(November);15(11):918‐931. [DOI] [PubMed] [Google Scholar]
- 42. Zhong K, Tariot P, Mintzer J, Minkwitz M, Devine N. Quetiapine to treat agitation in dementia: a randomized, double‐blind,placebo‐controlled study. Curr Alzheimer Res. 2007;4(1):81‐93. [DOI] [PubMed] [Google Scholar]
- 43. Streim J, Porsteinsson A, Breder C, Swanink R, Marcus R. Placebo‐controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. Am J Geriatr Psychiatry. 2008;(July);16(7):537‐550. [DOI] [PubMed] [Google Scholar]
- 44.RIS‐INT‐83. Janssen Pharmaceutical. 2003.
- 45. Sugerman A, Williams B, Alderstein A. Haloperidol in the psychiatric disorders of old age. Clin notes. 1964; (June); 120:1190‐1192. [DOI] [PubMed] [Google Scholar]
- 46. Janssen Pharmaceutical . Clinical study report synopsis. 2005;9–12.
- 47. Otsuka Pharmaceutical . 2017a. www.clinicaltrailsregister.eu. (EudraCT Number 2013‐000504‐41).
- 48. Otsuka Pharmaceutical . 2017b. www.clinicaltrailsregister.eu. (EudraCT Number 2013‐000503‐17).
- 49. Ballard C, Banister C, Khan Z, Cummings J, Demos GCB. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer's disease psychosis: a phase 2, randomised, placebo‐controlled,double‐blind study. Lancet Neurol. 2018;17(3):213‐222. [DOI] [PubMed] [Google Scholar]
- 50. ACADIA Pharmaceutical . 2018. http://www.clinicaltrails.gov. (NCT02992132).
- 51. Rada R, Kellner R. Thiothixene in the treatment of geriatric patients with chronic organic brain syndrome. J Am Geriatr Soc. 1976;24(3):105‐107. [DOI] [PubMed] [Google Scholar]
- 52. Barnes R, Veith R, Okimoto J, Raskind M, Gumbrecht G. Efficacy of antipsychotic medications in behaviorally disturbed dementia patients. Am J Psychiatry. 1982;139(9):1170‐1174. [DOI] [PubMed] [Google Scholar]
- 53. Petrie W, Ban T, Berney S, et al. Loxapine in psychogeriatrics: a placebo‐ and standard‐controlled clinical investigation. J Clin Psychopharmacol. 1982;2(2):122‐126. [PubMed] [Google Scholar]
- 54. Stotsky B. Multicenter study comparing thioridazine with diazepam and placebo in elderly, nonpsychotic patients with emotional and behavioral disorders. Clin Ther. 1984;6(4):546‐559. [PubMed] [Google Scholar]
- 55. Finkel S, Lyons J, Anderson R, Sherrell K. A randomized, placebo‐controlled trial of thiothixene in agitated, demented nursing home patients. Int J Geriatr Psychiatry. 1995;10(June 1994):129‐136. [Google Scholar]
- 56. Satterlee W. Olanzapine versus placebo in the treatment of patients with psychosis associated with dementia (F1D‐MC‐HGAO). 1995.
- 57. Borenstein M, Hedges L, Higgins J, Rothstein H. Subgroup analyses In: Introduction to Meta‐Analysis. Chichester, UK: John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 58. Berger V, Rezvani A, Makarewicz V. Direct effect on validity of response run‐in selection in clinical trials. Control Clin Trials. 2003;24(2):156‐166. [DOI] [PubMed] [Google Scholar]
- 59. Hernán M, Hernández‐Díaz S, Robins J. A structural approach to selection bias. Epidemiology. 2004;15(5):615‐625. 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 60. Farlow MR, Shamliyan TA. Benefits and harms of atypical antipsychotics for agitation in adults with dementia. Eur Neuropsychopharmacol. 2017;27(3):217‐231. 10.1016/j.euroneuro.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 61. Wang J, Yu J, Wang H, et al. Pharmacological treatment of neuropsychiatric symptoms in Alzheimer's disease: a systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry. 2015;(Jan);86(1):101‐109. [DOI] [PubMed] [Google Scholar]
- 62. Lee P, Gill S, Freedman M, Bronskill S, Hillmer M, Rochon P. Atypical antipsychotic drugs in the treatment of behavioural and psychological symptoms of dementia: systematic review. BMJ. 2004;329(June):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tan L, Tan L, Wang H, et al. Efficacy and safety of atypical antipsychotic drug treatment for dementia: a systematic review and meta‐analysis. Alzheimers Res Ther. 2015;7(20):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Ma H, Huang Y, Cong Z, et al. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta‐analysis of randomized placebo‐controlled trials. J Alzheimers Dis. 2014;42(3):915‐937. [DOI] [PubMed] [Google Scholar]
- 65. Seitz D, Gill S, Herrmann N, et al. Pharmacological treatments for neuropsychiatric symptoms of dementia in long‐term care: a systematic review. Int Psychogeriatr. 2013;25(2):185‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maher A, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off‐label uses in adults. JAMA. 2011;306(12):1359‐1370. [DOI] [PubMed] [Google Scholar]
- 67. Cheung G, Stapelberg J. Quetiapine for the treatment of behavioural and psychological symptoms of dementia (BPSD): a meta‐analysis of randomised placebo‐controlled trials. N Z Med J. 2011;124(1336):39‐50. [PubMed] [Google Scholar]
- 68. Lonergan E, Luxenberg J, Colford J, Birks J. Haloperidol for agitation in dementia (Review). Cochrane Database Syst Rev.. 2002;(2):CD002852. [DOI] [PubMed] [Google Scholar]
- 69. Carson S, McDonagh M, Peterson K. A systematic review of the efficacy and safety of atypical antipsychotics in patients with psychological and behavioral symptoms of dementia. J Am Geriatr Soc. 2006;54(2):354‐361. [DOI] [PubMed] [Google Scholar]
- 70. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer's disease. Cochrane Database Syst Rev. 2006. Jan 25;(1):CD003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Use of run‐in periods and the clinical outcomes of antipsychotics in placebo‐controlled trials with conventional OR/AND atypical group
Table S2. Trials with run‐in in meta‐analyses of antipsychotics in dementia


