Abstract
This review assesses harmful algal bloom (HAB) modeling in the context of climate change, examining modeling methodologies that are currently being used, approaches for representing climate processes, and time scales of HAB model projections. Statistical models are most commonly used for near-term HAB forecasting and resource management, but statistical models are not well suited for longer-term projections as forcing conditions diverge from past observations. Process-based models are more complex, difficult to parameterize, and require extensive calibration, but can mechanistically project HAB response under changing forcing conditions. Nevertheless, process-based models remain prone to failure if key processes emerge with climate change that were not identified in model development based on historical observations. We review recent studies on modeling HABs and their response to climate change, and the various statistical and process-based approaches used to link global climate model projections and potential HAB response. We also make several recommendations for how the field can move forward: 1) use process-based models to explicitly represent key physical and biological factors in HAB development, including evaluating HAB response to climate change in the context of the broader ecosystem; 2) quantify and convey model uncertainty using ensemble approaches and scenario planning; 3) use robust approaches to downscale global climate model results to the coastal regions that are most impacted by HABs; and 4) evaluate HAB models with long-term observations, which are critical for assessing long-term trends associated with climate change and far too limited in extent.
Keywords: harmful algal blooms, climate change, numerical modeling
1. Motivation and background
Climate change is expected to affect the frequency, magnitude, biogeography, phenology, and toxicity of harmful algal blooms (HABs) (Moore et al. 2008; Hallegraeff 2010; Anderson et al. 2015; Wells et al. 2015). Projecting likely responses of HABs to climate change is critical for informing the development of societal response strategies to mitigate their impacts and requires development and application of various types of models. Models used to project HAB response range from simple conceptual exercises to complex, highly resolved dynamical systems (Anderson et al., 2015). Regardless of model complexity, their efficacy depends on how well fundamental physical, biological, and biogeochemical processes are represented, as well as the ability to prescribe accurate initial conditions (i.e., model starting conditions) and model forcing at boundaries (i.e., time series of external variables essential to run the model). The challenges associated with representing physical and biological processes important for HAB development and prescribing accurate forcing vary greatly by region, HAB species, and time horizon, and inevitably introduce some level of uncertainty in model output. HAB scientists have struggled with how to address this uncertainty, as the complexity and multitude of processes that influence HAB response can be overwhelming (e.g., Wells et al. 2015). This difficult conundrum of anticipating climate change effects but struggling with how to evaluate potential HAB response has been described as a “formidable predictive challenge” (Hallegraeff 2010), and has inhibited the development of actionable projections to increase resilience to future HABs.
The term “harmful algal bloom” applies to a diverse subset of algae that cause a variety of negative impacts when they bloom, including human illness from eating contaminated food, drinking contaminated water, or breathing harmful aerosols, fish kills, and environmental degradation due to high biomass (Erdner et al. 2008). Major types of HABs include toxin-producing pelagic diatoms (e.g., Pseudo-nitzchia), dinoflagellates (e.g., Alexandrium, Pyrodinium, Gymnodinium, Dinophysis, Karenia), and cyanobacteria (e.g., Microcystis, Nodularia); toxin-producing benthic dinoflagellates (e.g., Gambierdiscus); fish-killing raphidophytes (e.g., Heterosigma); and high-biomass events (e.g., Phaeocystis, Ulva). Consistent with this diversity in HAB organisms, the expected HAB response to climate change is also diverse. The sensitivity and even the sign of the response of HABs to climate change may vary depending on the organism and the setting. For example, increased temperature may increase growth rates of organisms that are currently at the poleward limit of their thermal habitat at a particular location, but may also result in some locations becoming too hot to support growth (e.g., Kibler et al. 2015).
A number of in-depth reviews of climate change impacts on HABs identify a range of potential responses to environmental factors including warming temperature, increased stratification, altered nutrient availability and composition, light intensity, and ocean acidity (Moore et al. 2008; Hallegraeff 2010; Anderson et al. 2015; Wells et al. 2015). HAB response may also depend on how climate change will affect zooplankton grazers or microbial pathogens that limit their growth, which is particularly difficult to characterize since grazer activity may also respond to the same changes in environmental factors that determine HAB response and are also likely to be regionally specific (Wells et al. 2015). Many of the projected responses of HABs to changing environmental factors rely primarily on theory or laboratory studies that isolate particular organisms or processes. The derived rates and responses from these culture studies do not always correspond with those observed in the field, potentially reflecting variation among isolates, effects of competition, and/or interactions among factors that occur in the environment (Fu et al. 2012; Wells et al. 2015). Consequently, these interactions are typically not well parameterized in HAB models, if they are included at all. This may lead to greater uncertainty in model projections if interactions emerge or become more important to HAB formation in the future as a result of changing climate conditions.
Directly linking changes in observed HAB distribution, frequency, or intensity to shifts in climatic forcing remains difficult (Moore et al. 2008; Wells et al. 2015), but examples are emerging as time series of observations accumulate. Identifying HAB responses (or lack thereof) to anomalous climate events or natural climate cycles provide the best opportunities for formulating hypotheses as to how HABs might respond to climate change (Trainer et al., 2019 this special issue). For example, anomalously warm water associated with the 2014–16 northeast Pacific marine heatwave was associated with an intense, widespread Pseudo-nitzschia bloom along the U.S. West Coast beginning in spring 2015 that may have been fueled by the combination of higher growth rates at warmer temperatures and nutrients supplied by upwelling (McCabe et al. 2016). Increased closures of shellfish harvesting due to domoic acid from Pseudo-nitzschia and saxotoxin from Alexandrium were linked with anomalously warm sea surface temperatures off the coast of Oregon during a positive phase of the Pacific Decadal Oscillation (PDO) and strong El Niño event (McKibben et al. 2015). In the Rias Baixas along the Northwest Iberian Peninsula, a decrease in upwelling intensity over the past 40 years was linked to increased time scales for flushing, which corresponded with increased Dinophysis occurrence and shellfish harvest closures (Álvarez-Salgado et al. 2008). The frequency and magnitude of Pseudo-nitzschia blooms off the coast of Southern California was linked to the PDO and more directly with the North Pacific Gyre Oscillation (NPGO), but the correlations were weak and exact mechanisms unclear (Sekula-Wood et al. 2011). Long time series also reveal systems that are not responsive to climate regimes. For example, warm water anomalies in Puget Sound (Washington State) generated during El Niño winters do not persist into the seasonal window (summer and fall) when blooms of the dinoflagellate Alexandrium typically occur. Because of this mismatch in timing, no robust relationship exists between levels of paralytic shellfish toxins in Puget Sound shellfish and an index of the El Niño-Southern Oscillation (ENSO) (Moore et al. 2010). The use of models prognostically to represent mechanistic links between climate and HABs enables some hypotheses of HAB response to future climate change to be tested and remains a research priority.
Most models used to project HAB response at climate time scales (i.e., decades to a century) were initially developed and applied over shorter time scales (i.e., several days to a season) to provide hindcasts or forecasts of present conditions. Other reviews have richly detailed the current state of HAB modeling over shorter time scales (Glibert et al. 2010; McGillicuddy 2010; Flynn and McGillicuddy 2018; Franks 2018), so modeling applications of present conditions will be addressed here only in the context of how such models might be applied to understand future conditions. As a simplification, most HAB models can be characterized as being primarily statistical or process-based. Statistical models are developed from relationships between input and response variables in observations. While they have proven effective for hindcasts and near-term forecasts, the statistical relationships become less predictable as forcing conditions shift outside the range of past observations (Flynn and McGillicuddy 2018). Process-based models may be more robust for projecting HAB response under novel environmental conditions, but this assumes that the dominant processes remain unchanged under a different set of forcing conditions. Additionally, models of response to climate change are dependent on the ability to predict forcing conditions such as water temperature, wind strength, or river discharge at spatial and temporal scales relevant to the processes represented in the HAB model. The uncertainty in the environmental conditions increases greatly with the time scale of forecast, in part because of greater uncertainty in the global circulation models (GCM) at longer time scales but also because the unpredictability of human behavior becomes a greater factor. For example, representing the source of nutrients that might fuel a bloom or affect toxicity could depend on resolving shifts in upwelling wind intensity or hydrologic response to precipitation events from local watersheds, but changes in land use or direct anthropogenic nutrient inputs may have even greater effects on regional nutrient concentrations (Glibert et al. 2010). The paucity of successful HAB models at even interannual time scales and the uncertainties in predicting future environmental conditions make extending meaningful projections to climate time scales challenging.
This review examines the state of HAB modeling in the context of climate change. We assess the key components of modeling HAB response to climate change, starting with an overview of the HAB modeling methodologies currently in use, reviewing studies that have examined HAB response to climate change, and offering recommendations on how to move forward by incorporating approaches used in the broader climate and ecosystem modeling communities. Considerations include the spatial resolution, time horizon, and forecast accuracy of HAB models developed in the present climate, representation of future forcing conditions that govern bloom development and transport, and an assessment of whether the models developed and calibrated under present forcing conditions can adequately represent future response, or if additional factors might emerge to dominate bloom dynamics.
2. Modeling HABs in the present climate
Most HAB models currently in use for present climate conditions focus on either hindcasts in process studies or near-term (a few days to seasonal) forecasts for operational and management uses. These existing HAB models are the most likely bases for projecting future response to climate change. They use a wide range of methodologies, in part reflecting the diversity of HAB species, the availability of data for model forcing or calibration, and differences in motivation for model development. Here we broadly classify HAB models as those that apply statistical (or empirical) techniques, process-based formulations, or merge multiple approaches (i.e., hybrid models). The categorizations are not meant to be rigid. Other key model attributes could instead be used to distinguish methodologies, such the level of complexity from a single organism to full ecosystem, the degree of spatial and temporal resolution, the time scales of simulation (event, seasonal, interannual, or longer), and whether models are diagnostic hindcasts or prognostic forecasts. Nevertheless, we find our categorization of the current modeling approaches facilitates thinking about how each of the methodologies might be adapted to assess HAB response to climate change. A brief summary of the modeling studies reviewed here is given in Table 1, including this categorization, HAB organism, geographic region, and model type and time scales.
Table 1.
Table summarizing the HAB modeling studies reviewed here. Models are categorized based on whether they focus on present-day (hindcasts, event-based, near-term forecasts) or future climate conditions (using climate model projections) and the modeling approach (statistical, process-based, or a hybrid). Information on the HAB organism being modeled, geographic region, and a brief description of the model formulation and timescale are listed.
| Present vs future climate | Model type | HAB organism | Region | Brief description | |
|---|---|---|---|---|---|
| Allen et al., 2008 | Present | Process-based | High biomass species | NW European shelf | Ecosystem + circulation; seasonal |
| Anderson et al., 2009 | Present | Statistical | Pseudo-nitzschia | California | Regression; spatial + temporal; seasonal |
| Anderson et al., 2010 | Present | Statistical | Pseudo-nitzschia | Chesapeake Bay | Generalized linear regression; spatial + temporal; interannual |
| Brown et al., 2013 | Present | Statistical | Karlodinium, Prorcentrum, Microcystis | Chesapeake Bay | Neural network and logistic regression; spatial + temporal; interannual |
| Cusack et al., 2015 | Present | Statistical | Pseudo-nitzschia | SW Ireland | Zero-inflated negative binomial regression; interannual |
| Cusack et al., 2016 | Present | Hybrid | Pseudo-nitzschia | SW Ireland | Observations + particle tracking; near-term |
| Diaz et al, 2016 | Present | Statistical | Dinophysis | Portugal | General additive model; interannual |
| Giddings et al., 2014 | Present | Hybrid | Pseudo-nitzschia | Pacific NW | Particle tracking + ecosystem; seasonal to interannual |
| Gillibrand et al., 2016 | Present | Process-based | Karenia mikimotoi | Scotland | IBM with growth; event |
| Gonzalez Vilas et al., 2014 | Present | Statistical | Pseudo-nitzschia | NW Spain | Machine learning; spatial + temporal; interannual |
| Henrichs et al., 2015 | Present | Process-based | Karenia brevis | Gulf of Mexico | IBM with behavior; interannual |
| Lane et al., 2009 | Present | Statistical | Pseudo-nitzschia | California | Logistic regression; interannual |
| Stock et al., 2005; Li et al., 2009 | Present | Process-based | Alexandrium | Gulf of Maine | Ecosystem + circulation; seasonal to interannual |
| Moore et al., 2009 | Present | Statistical | Alexandrium | Puget Sound | Regression + trend analysis; interannual |
| Raine et al., 2010 | Present | Statistical | Dinophysis | SW Ireland | Based on wind index, near-term forecast |
| Ralston et al., 2014 | Present | Process-based | Alexandrium | Cape Cod | Local growth; seasonal to interannual |
| Ralston et al., 2015 | Present | Process-based | Alexandrium | Cape Cod | Ecosystem + circulation; seasonal to interannual |
| Ruiz-Villarreal etal., 2016 | Present | Process-based | Dinophysis | NW Spain | Particle tracking; seasonal to interannual |
| Stumpf et al., 2008; Stumpf et al., 2009 | Present | Hybrid | Karenia | Gulf of Mexico | Observations + particle tracking; near-term forecast |
| Stumpf et al., 2012 | Present | Statistical | Cyanobacteria | Lake Erie | Regression; spatial + temporal; seasonal |
| Velo-Suarez et al., 2010 | Present | Process-based | Dinophysis | NW Spain | Particle tracking; event |
| Wynne et al., 2011 | Present | Process-based | Microcystis | Lake Erie | Particle tracking; event |
| Gobler et al., 2017 | Future | Process-based | Alexandrium, Dinophysis | NEandNW Atlantic, NE Pacific, Alaska | Growth rates (temperature) |
| Jacobs et al., 2015 | Future | Process-based | Alexandrium, Vibrio | Chesapeake, Puget Sound, Alaska | Growth rates/windows (temperature) |
| Kibler et al., 2015 | Future | Process-based | Gambierdiscus | Caribbean | Growth rates (temperature) |
| Lin et al., 2018 | Future | Process-based | Karlodinium | Chesapeake Bay | Growth rates (temperature, nutrients) |
| Moe et al., 2016 | Future | Statistical | Cyanobacteria | Norway lake | Bayesian network linking ecosystem and watershed models |
| Moore et al., 2011 | Future | Statistical | Alexandrium | Puget Sound | Regression + trend analysis; growth window |
| Moore et al., 2015 | Future | Process-based | Alexandrium | Puget Sound | Regional physical models; growth windows (temperature, salinity) |
| Glibert et al., 2014 | Future | Process-based | Prorocentrum, Karenia | NW European shelf, NE Asia, SE Asia | Regional physical + ecosystem models; habitat suitability (temperature, salinity, nutrients) |
| Townhill et al., 2018 | Future | Statistical | Pseudo-nitzschia, Alexandrium, Dinophysis | NW European shelf | Habitat suitability (temperature, salinity, bathymetry); maximum entropy approach |
2.1. Statistical models
Statistical models use observations to relate key forcing variables (e.g., a nutrient concentration, temperature, upwelling wind index, or time of year) to relevant measures of HABs (e.g., the timing of HAB events or the abundance, toxicity, and spatial distributions of HAB species). A wide range of forcing variables are typically considered during model development, some of which may be interrelated (e.g., temperature and time of year, salinity and river discharge). While the choice of forcing variables is often guided by our understanding (theoretical or empirical) of the underlying physical and biological processes, statistical models do not attempt to represent those processes directly, only the cumulative effects of them. Statistical models require extensive observations to develop robust relationships between forcing variables and HAB response. As such, some of the most compelling examples come from regions with long records of HAB monitoring and investigation. Examples include Pseudo-nitzschia and Dinophysis blooms off the Iberian Peninsula and Ireland (Raine et al. 2010; Cusack et al. 2015; Díaz et al. 2016), Pseudo-nitzschia off the U.S. West Coast (Anderson et al. 2009; Lane et al. 2009), Alexandrium in Puget Sound and the U.S. Northeast (Moore et al. 2009; Ralston et al. 2014), Karenia in the Gulf of Mexico (Stumpf et al. 2009), and multiple HABs on the Northwest European Shelf and in Chesapeake Bay (Anderson et al. 2010; Brown et al. 2013). Statistical models are typically used in hindcasting, but may provide nowcasts if real-time observations of forcing variables are available or limited forecasts if lags are built in to the model. Alternatively, output from operational physical models can be used in place of observations to provide input for statistical models, enabling near-term forecasts of HABs. A wide variety of statistical approaches have been used to model HABs in the present climate, ranging from simple linear regressions to more complex analyses using artificial neural networks, fuzzy logic, or Bayesian inference. Here, we highlight a few approaches that have been used to predict the timing and distribution of HABs.
Statistical analysis of observational data sets that record HAB response to changes in environmental forcing at climate-relevant time scales can be informative for identifying forcing variables that are climate sensitive. Past performance is no guarantee of future results, but multi-decadal observations provide evidence at time scales relevant to climate change of HAB variation with forcing conditions. For example, in Puget Sound (Washington State), optimal conditions for Alexandrium catenella blooms – warm air and water temperatures in combination with low river discharge and wind speed – have become more common over the past 30 years, as have the frequency and duration of toxic blooms (Moore et al. 2009). In many cases, identification of a “window of opportunity” with increased risk for bloom development and toxin accumulation, and potential alterations to that window of opportunity with climate change, is a primary goal of HAB modeling rather than representing specific events or the phytoplankton community. Another example is a study of a 30-year record of Dinophysis acuta in the rias of northwest Spain that used a general additive model (GAM) based on upwelling intensity, thermocline depth, tidal range, and inoculum strength to predict cell abundances. The analysis did not find evidence for increasing trends in bloom frequency or intensity, nor clear relationships to long-term climate indices like the North Atlantic Oscillation (NAO) (Díaz et al. 2016). The study did, however, find that an exceptional bloom in 1989–1990 appeared to be associated with high positive anomalies in sea surface temperature (SST) and the NAO index. That analysis did not extend their GAM to climate time scales. To do so effectively, a GCM would need to represent the combination of upwelling and solar heating that are ideal for HAB development. These ideal physical conditions occur relatively briefly and infrequently, and remain challenging to reproduce in finer scale regional models that would be needed to adequately represent the blooms (Ruiz-Villarreal et al. 2016).
Forcing variables that represent dominant physical and biogeochemical processes can serve as the basis for forecasting the timing of HABs. For example, in southwestern Ireland, stratified, wind-driven circulation during summer months can bring harmful Dinophysis spp. from the continental shelf into coastal embayments where they can cause toxic events (Raine et al. 2010). A simple model based on the 5-day weather forecast for cross-shore wind and time of year was used to predict Dinophysis import events and Diarrheic Shellfish Poisoning (DSP) toxicity, and these model results were used to guide near-term shellfish resource management. In Monterey Bay (California), a logistic regression model incorporating multiple forcing factors including time of year, chlorophyll, silicic acid, water temperature, upwelling index, river discharge, and nitrate was developed from 8 years of observations and used to predict the probability of Pseudo-nitzschia blooms (Lane et al. 2009). Similarly, Pseudo-nitzschia blooms off the coast of Ireland were linked to upwelling, and a statistical model using a wind index, water temperature, and recent cell densities helped predict the timing, but not intensity, of bloom events (Cusack et al. 2015).
Statistical models that spatially resolve forcing variables can provide information on HAB distribution based on habitat suitability for the causative organism. For example, a regression model using satellite ocean color and sea surface temperature (SST) detected 98% of toxic Pseudo-nitzschia blooms in Santa Barbara Channel (California) with less than 30% false positive cases (Anderson et al. 2009). In Lake Erie, satellite imagery of Microcystis spp. bloom extent was correlated with river discharge and nutrient loading, and could be used to generate a seasonal forecast because of the several month lag between input variables and bloom response (Stumpf et al. 2012). In northwest Spain, the presence or absence of Pseudo-nitzschia blooms in several coastal embayments was linked to location, day of year, temperature, salinity, upwelling index, and, most importantly, recent bloom occurrence using a support vector machine, which is a common machine-learning algorithm (González Vilas et al. 2014). In Chesapeake Bay, a Generalized Linear Model (regression-based approach allowing for both Gaussian and non-Gaussian distributions) was developed with 22 years of cell abundance data and used to make hindcast maps of Pseudo-nitzschia bloom probability based on factors including time of year, temperature, salinity, nutrients (phosphate, nitrate, silicic acid), river discharge, dissolved organic carbon, and Secchi depth (Anderson et al. 2010). Another approach in Chesapeake Bay used output from a physical model as input for empirical habitat suitability models to make near-term forecasts of HAB occurrence (Brown et al. 2013). The methodologies (neural network or logistic regression) and input variables (time of year, temperature, salinity, chlorophyll, nutrients, Secchi depth, total suspended solids, dissolved oxygen) for the habitat models varied for the three HAB species (Karlodinium veneficum, Prorocentrum minimum, and Microcystis aeruginosa) modeled. This approach relied on both physical model results and extensive HAB observations for development of the empirical model.
2.2. Process-based models
Process-based (or mechanistic) models use mathematical equations to explicitly simulate key physical and biological processes that govern HABs and HAB outcomes. Their development requires detailed knowledge of critical life history characteristics and the factors that modulate them as well as transport pathways. As such, they require large amounts data to represent the many processes in the system and can be limited by their parameterizations of rates of growth, mortality, mobility, toxin production, and other key processes that are typically derived from simplified laboratory studies of isolated strains. In situations where observational or laboratory data are limited, process-based models instead may be informed by data on similar organisms or may be limited to focusing on a subset of processes that are particularly important to bloom dynamics. Because process-based models are more comprehensive than statistical models, they take more time and effort to develop and are more computationally expensive to run. Process-based models can be difficult to constrain given the nonlinearity and intermittency of HABs, but they are usually more transferable across regions because of their explicit representation of physical and biological processes.
In systems where transport processes are negligible, models based only on biological processes have utility. For example, in Nauset Estuary on Cape Cod (Massachusetts), a small embayment with limited exchange and long residence times, interannual variability in timing of A. catenella blooms was reproduced with a simple model based temperature-dependent growth rates (Ralston et al. 2014). In contrast, for many HABs physical transport provides the dominant control on bloom distribution. For these cases a common approach is to use velocity fields from a circulation model to advect particles that are representative of the HAB. For example, the accumulation of Dinophysis acuminata in the Bay of Biscay at temperature and salinity gradients associated with river plumes, and subsequent dispersion of the bloom by winds and tides, was well represented by passive particle tracking and circulation model hindcasts (Velo-Suárez et al. 2010). A passive particle tracking approach was also used in a forecast system for Dinophysis for the rias (drowned river valleys) of the northwestern Iberian coast (Ruiz-Villarreal et al. 2016). Particle tracking similar to that used for oil spills was used for a Microcystis aeruginosa bloom in western Lake Erie by linking satellite ocean color observations and a hydrodynamic model, and importantly the study included quantitative skill assessment of the predictions relative to persistence, or no influence of transport on the bloom (Wynne et al. 2011).
More commonly, both physical and biological processes play important roles in HAB development and they cannot be treated independently. Individual-based models (IBMs), like passive particle tracking, can be run within a circulation model or offline using model output to represent advection by currents, but IBMs also can incorporate biological processes specific to the organism of interest. For example, an IBM with growth dependent on temperature, mortality dependent on shear and population density, and phototaxic vertical migration was used to hindcast Karenia mikimotoi blooms along coastal Scotland (Gillibrand et al. 2016). Results showed a strong dependence on bloom source region and uncertainty in the biological rate parameters, making the model less practical for forecasts. In the Gulf of Mexico, an IBM of Karenia brevis that included vertical migration based on internal nutrient ratios was used to identify potential source regions by running simulations backwards in time (Henrichs et al. 2015).
Rather than IBMs, HAB growth, mortality, and redistribution can also be represented as cell concentrations within circulation or biogeochemical models. For example, a model of A. catenella that represents cyst germination, growth dependent on temperature, salinity, nutrients, and light, and mortality has been used in diagnostic hindcasts and operational forecasts in the Gulf of Maine (Stock et al. 2005; Li et al. 2009), and a related model that also imposed diel vertical migration was used to simulate A. catenella in an estuary (Ralston et al. 2015). Those models treated the HAB as independent of the broader plankton community by simulating only the species of interest and prescribing the nutrient field based on observations rather than having it evolve dynamically. A more complete ecosystem, biogeochemical, and circulation model of the northwest European shelf incorporated multiple phytoplankton, zooplankton, and bacteria functional groups and benthic-pelagic coupling to simulate high biomass events, providing predictions after calibration to satellite ocean color (Allen et al., 2008).
In general, the many biological processes that contribute to HAB development remain poorly defined and present major sources of uncertainty in process-based models. Passive particle tracking models ignore this and IBM or Eulerian-based hindcasts typically calibrate model parameters within acceptable ranges that optimally correspond to observed blooms. However, models used to generate forecasts that have operational utility cannot rely on retrospective calibration, and so many adopt hybrid approaches that use physical models to predict transport processes along with empirical models to integrate biological response. For example, near-term forecasts for Pseudo-nitzschia in Bantry Bay in southwest Ireland were based on the combination of a passive particle tracking model to represent cross-shore advection by upwelling, a circulation model, satellite observations, and in-situ sensors to characterize local water properties, and recent toxicity reports (Cusack et al. 2016). Similarly, transport of Pseudo-nitzschia from formation regions offshore to the coast depending on upwelling or relaxation along the Pacific Northwest coast of the U.S. was simulated with particle tracking, and the rate of false positives for toxicity events was reduced by incorporating thresholds for overall phytoplankton abundance from an ecosystem model (Giddings et al. 2014). A hybrid approach using satellite SST and ocean color along with particle tracking was used to explain accumulations of Karenia spp. in the eastern Gulf of Mexico (Stumpf et al. 2008), although bloom forecasts are based primarily on satellite data (Stumpf et al. 2009). Satellite algorithms for bloom identification are important components of many hybrid systems for early warning, using either overall levels of chlorophyll-a (Stumpf et al. 2008; Cusack et al. 2016) or specific spectral response like for Microcystis in Lake Erie (Stumpf et al. 2012). The utility of satellite data in hybrid models depends on the HAB, as for example in Europe it was found to be useful for early warning of Karenia mikimotoi and Lepidodinium chlorophorum but not Dinophysis (Maguire et al. 2016).
3. Modeling HABs in a changing climate – what has been done?
Projecting HAB response to climate change involves extending the simulation period of existing HAB models to decades, centuries, or potentially paleo time scales for retrospective climate analyses. Data describing future forcing conditions can be obtained from GCM simulations and used as input variables to HAB models. GCMs forecast ocean circulation and water properties under future climate scenarios informed by various greenhouse gas concentration trajectories. These scenarios describe a range of possible futures based on greenhouse gas emissions, economic development, population growth, and other factors. The output generated by GCMs quantify changes in physical and biogeochemical conditions and can be combined with statistical relationships from past observations to project changes in HABs. Additional model layers to represent climate change effects outside of the ocean, such as watershed hydrology or land use, can also be integrated. This offers a relatively simple approach for examining climate impacts on HABs, but statistical models become increasingly error-prone when projecting into conditions different from the training data set (Flynn and McGillicuddy 2018). This is because the statistical relationships may represent the cumulative effect of multiple processes or interactions that cannot be extrapolated, and also because thresholds or tipping points that were not identified or characterized by prior observations may be exceeded in the projections. Process-based models are less prone to these potential issues, but they represent only a portion of the physical and biological complexity due to computational constraints and data limitations, and so even process-based models validated under present conditions may not simulate many of the hypothesized responses to climate change. Here we discuss some of the approaches for using statistical and process-based HAB models to project HAB response to climate change. The different approaches vary in complexity in terms of how many forcing variables are considered and how they are derived.
3.1. Statistical models
A statistical modeling approach was used to link HAB observations in Puget Sound (Washington State) with physical observations and climate model forecasts to evaluate long-term shifts in environmental conditions favorable for blooms (Moore et al., 2011). Based on a 15-year record of paralytic shellfish poisoning toxins in shellfish tissues, A. catenella blooms were associated with warm air and water temperatures, low streamflow, weak winds, and small tidal height variability. The relationship was extrapolated back in time using observations of the forcing variables, and the annual window of favorable environmental conditions for A. catenella was found to have increased from 1967 to 2006, with two step-like increases occurring in 1978 and 1992 when higher annual values were attained compared to previous years. The 1978 step change may have been related to the reversal of the Pacific Decadal Oscillation (PDO) from cool to warm phase in 1977. The 1992 shift did not directly correspond with regional climate indices, and a lagged response to a regime shift to warmer summer SST off the Washington coast in 1989 could not be distinguished from natural variability. Projections of the statistical relationship using output from a GCM indicated that by the end of the 21st century, the duration of favorable environmental conditions for A. catenella would increase by about 2 weeks annually on average (Moore et al., 2011).
Another statistical approach to climate response defined habitat zones for the shelf sea of northwest Europe based on temperature, salinity, depth, and stratification from regional climate projections, finding a general northward shift in HAB species composition (Townhill et al. 2018). Species distribution modeling based on current distributions was projected forward using a maximum entropy approach for multiple HAB species. On the shelf, Dinophysis acuta and Gymnodinium catanatum had the greatest northward shift of 200–500 km by 2055, while optimal habitat suitability for three species (A. ostenfeldii, A. minutum, and P. australis) shifted southward. The southward shift was attributed to factors in addition to temperature change, including how the regional bathymetry affects habitat suitability.
Models of HAB response have also been coupled to models of future changes in freshwater or nutrient delivery from rivers, which are often not resolved in global models. For example, a Bayesian network model was used to link GCM results with process-based models of watershed hydrology and a lake ecosystem model to project climate impacts on cyanobacteria biomass in Lake Vansjø (Norway) (Moe et al. 2016). The Bayesian approach allowed assessment of multiple land use scenarios and incorporation of monitoring data and expert knowledge in the probabilistic links between nodes. Results suggest that the benefits of better land-use management were partly counteracted by future warming.
3.2. Process-based models
Temperature is a keystone parameter of climate change, and warming of the sea surface is apparent in many regions in observational records from satellites and in-situ measurements. Because temperature is a strong determinant of growth, changes in temperature can be used to approximate changes in potential growth rates of HAB organisms. Warmer waters may already be affecting bloom dynamics. For example, sea surface temperature records from 1982 to 2016 were combined with laboratory-based growth rates for A. catenella (fundyense) and D. acuminata (Gobler et al. 2017). In the North Atlantic, calculated mean growth rates increased by about 0.01 d−1 over the study period and the duration of favorable growth conditions increased by 2 to 3 weeks. In the North Pacific trends were less clear, but some regions (the Salish Sea and coastal Alaska) were identified as having increasingly favorable growth conditions and HAB prevalence.
Temperature is an important forcing variable in nearly every HAB model of climate response reviewed here. A number of studies use projected changes in sea surface temperature at certain locations to approximate changes in growth rates and identify expansions (or contractions) of optimal growth windows for HAB organisms. The windows are defined as the number of days each year when temperatures are projected to be within thresholds that support optimal growth (e.g., Moore et al. 2008). For example, an ensemble of GCM projections were used to quantify changes in temperature-dependent growth rates of Gambierdiscus and Fukuyoa species, dinoflagellates associated with ciguatera fish poisoning (CFP), at six sites in the Gulf of Mexico through the end of the 21st century (Kibler et al. 2015). The results suggest increased abundance and diversity of Gambierdiscus spp. and greater CFP risk in the Gulf of Mexico, but a shift in the species composition at higher temperatures suggests lower overall risk in the Caribbean. A similar ensemble approach was used to calculate shifts in the timing of temperature growth windows for A. catenella and Vibrio spp. bacteria in Puget Sound and Chesapeake Bay, with the A. catenella bloom period predicted to start 1 month earlier and end 1 month later (Jacobs et al. 2015). In addition to changes in bloom timing, the study identified geographic shifts in optimal temperature zones along coastal Alaska for Vibrio, which while not a HAB, presents a methodology that could be applied in HAB studies to examine potential latitudinal shifts in species distribution without directly simulating HAB dynamics.
Potential shifts in the timing of optimal growth windows as well as the spatial distributions of HABs can be evaluated by utilizing spatially resolved information on future forcing conditions from GCMs or regional models of climate change rather than projections at a single location. For example, in Puget Sound, regional scale atmospheric, ocean, and hydrologic models were combined to represent multiple potential influences on optimal temperature (and salinity) windows for growth of A. catenella (Moore et al. 2015). Comparing model results for circa-1990 and circa-2050, atmospheric heating was projected to increase the duration of favorable growth conditions by 30 days per year with the biggest increases in HAB-favorable conditions occurring in the North Basin and Strait of Juan de Fuca. Changes in the timing and magnitude of river discharge and upwelling on temperature and salinity were found to have less effect on calculated growth rates. The study did not address potential changes in nutrient loading due to upwelling or anthropogenic sources.
In addition to HAB growth rates, warming temperature may also be expected to increase growth rates of some grazers that prey on HAB species, including zooplankton, benthic invertebrates, and fishes. Moreover, predator-prey interactions and the response to changing environmental conditions are more complex than species growth rates, as changes in the distribution, abundance, community composition, toxicity, and nutritional quality of HAB species may all depend on temperature and can affect the relative balance of growth rates and loss from predation, and thus bloom development (Wells et al. 2015). Representing quantitatively the many factors contributing to effects of predation on HAB growth and decline, including temperature, remains a major challenge for process-based models in both current and climate change scenarios. To this point, most of the modeling of temperature impacts has focused on HAB growth rates alone rather than assessing the potentially differential responses of grazers and prey.
The above examples directly link changes in temperature to temperature-dependent growth rates of HAB organisms to examine changes in bloom timing and spatial distribution. Some other examples also consider salinity, but the relatively small changes in salinity projected in the study regions meant that the growth responses were primarily driven by changes in temperature. Nutrients are another forcing variable that strongly determine growth rates and toxicity of HAB organisms and are projected to be altered by climate change. For example, a model of the mixotrophic dinoflagellate Karlodinium veneficum and its algal prey, Rhodomonas salina, was used to simulate growth under various temperature and nutrient stoichiometry scenarios (Lin et al. 2018). While these scenarios were not directly linked to GCM output of future climate change scenarios, they were informative of future HAB response and suggest that warmer, wetter springs combined with increased nitrogen inputs to Chesapeake Bay may be more favorable to HAB development. In contrast, GCM output was used as boundary conditions for a coupled oceanographic and biogeochemical model with four classes of phytoplankton, three for zooplankton, one for bacteria, nitrogen and phosphorous in different forms, and benthic mineralization on three regional grids at 1/10-degree resolution to assess conditions for Prorocentrum and Karenia spp. around 2100 (Glibert et al. 2014). The study defined regions of suitable habitat or propensity for toxicity based on temperature, salinity, and nutrients for two time slices: the period 1980–1990 for the present day and 2090–2100 for the future climate scenario. Model results showed expansion both spatially and temporally of both species on the northwest European shelf and northeast Asia, and relatively little change in southeast Asia.
4. Modeling HABs in a changing climate – what should be done?
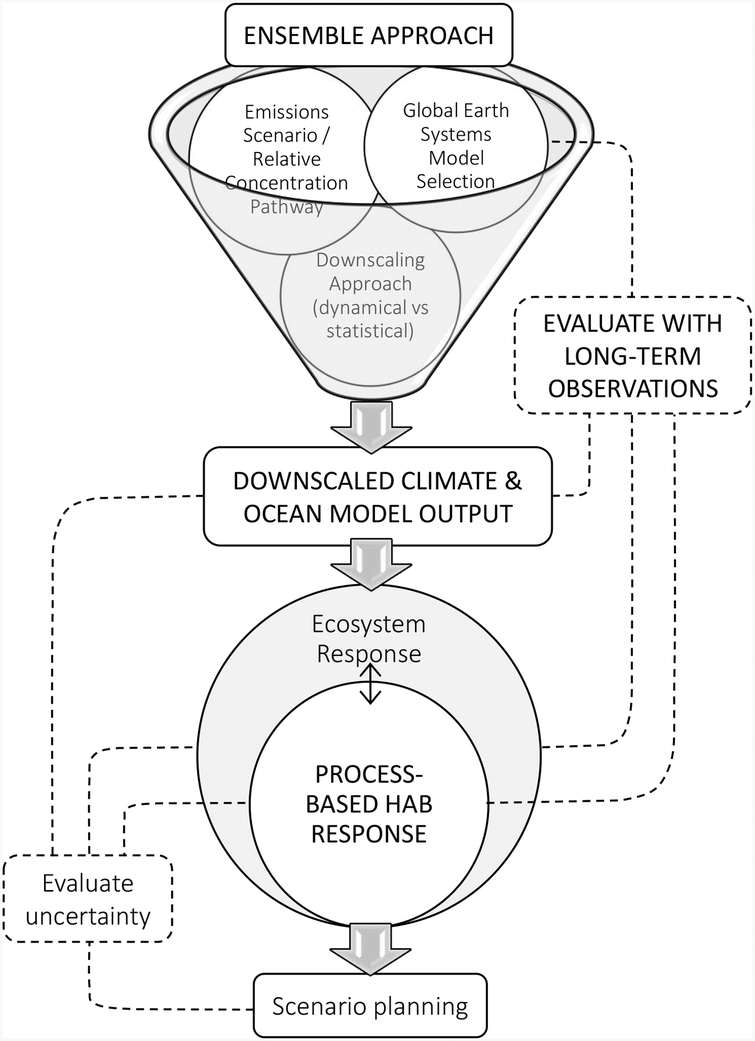
The fact that relatively few modeling studies quantitatively project how climate change may affect the distribution and abundance of HAB populations or toxicity is symptomatic of the challenges associated with this important task. Challenges associated with understanding the biological response of HABs to climate change, as well as suggestions for best practices that should be employed to address them, are discussed in Wells et al. (2015); however, little attention was given to the modeling infrastructure needed to project HAB response to climate change. Generating useful projections of HAB response to climate change will require engagement with other communities that can help refine the representation of future conditions in HAB models, including climate scientists, marine ecologists, watershed hydrologists, invasive species biologists, and environmental managers and policy makers (Glibert et al. 2010). Here we offer several suggestions to improve modeling of HABs in a changing climate, schematically summarized in Figure 1.
Figure 1.
Schematic diagram summarizing considerations for improving modeling of HAB response to climate change. Multiple global earth systems models, emissions scenarios/relative concentration pathways, and downscaling approaches should be considered in an ensemble approach to generate downscaled climate and ocean model output. Downscaling is necessary to resolve critical physical and biogeochemical processes for HAB development at coastal scales. These downscaled data should be used to force process-based models of HAB response with the results considered in an ecosystem context. Models should be evaluated with long-term observations. This step can be informative for selecting global models, identifying biases in downscaled model projections, and validating models of HAB and ecosystem response. An important final step is to identify components of the model system that are key sources of uncertainty in the long-term HAB response (i.e., evaluate uncertainty) and to develop scenarios (i.e., scenario planning) around those sources of uncertainty in the development of societal response strategies.
4.1. Use process-based models
Even though there are challenges associated with uncertainty in model parameterizations, nonlinear feedbacks, and computing power, process-based models have distinct advantages over statistical approaches for projecting impacts of climate change on HABs. In many cases, data limitations initially hinder development of process-based models for emergent HABs or regions without many observations, and so statistical models can be extremely important in the diagnosis of bloom mechanisms and development of process-based models. Statistical models are often well suited for shorter-term projections and management applications, particularly when the models incorporate a dominant influence of periodic forcing like from ENSO or PDO. Importantly for climate change response, process-based models explicitly represent physical and biological mechanisms involved in HAB development, and so they are less likely to lose validity when forcing variables are applied that extend outside of periods of historical observation. Incorporating multiplicative effects of changes in temperature, nutrient availability, or stratification (among other factors) into process-based HAB models requires focused, process-oriented field or laboratory studies that record organism response beyond just abundance, ideally in the context of the ecosystem response rather than just for individual strains (Flynn and McGillicuddy 2018). Changes in HAB severity will depend on the cumulative effects of factors including differential responses of predators and prey, changing nutrient availability, and shifts in transport patterns rather than a simple parameter dependence from on lab studies. Circulation models can be directly coupled with ecosystem models to simulate projected physical and biogeochemical changes at climate time scales. This approach is intrinsic to many earth system models that have been used to examine changes in ecosystem and nutrient dynamics globally and regionally using various downscaling methods. For HAB models, the limited understanding of complex predator-prey interactions and competition among classes within the ecosystem limit our ability to parameterize process-based models (Wells et al. 2015), and should be a focus of future research.
Process-based models are typically more complex than statistical models. The introduction of additional processes and parameters may improve model fit, but can also reduce predictive skill if not based on a robust representation of the underlying processes (Bell and Schlaepfer 2016). Regime shifts, in which the dominant processes or forcing variables controlling bloom development change in large, abrupt, and persistent ways, are particularly challenging to model, and additional complexity may increase variability in the results without incorporating the relevant combination of stressors leading to the regime shift, particularly if the model is not validated with data independent from the training region and forcing conditions. HAB models used to assess climate impacts should be rigorously evaluated to identify model parameters that most sensitively determine model outcomes, and this should guide efforts to simplify complex models and to focus laboratory and field studies to refine the uncertainty in those key parameters (Flynn and McGillicuddy 2018). The development of process-based models requires parallel efforts of laboratory and observational studies to refine key rate parameters and process dependencies, including the effects of changes to multiple forcing factors changing simultaneously. The applicability of process-based models is predicated on validation across a broad set of forcing conditions, and so data collection is particularly critical for in developing models for HABs in regions that have a sparse history of monitoring and research. Statistical approaches should continue to play an important role in HAB modeling, particularly for resource management and public health protection over event to seasonal time scales, but extending statistical models to predict climate change response has limited merit.
4.2. Use an ensemble approach
An ensemble approach can be used to address the uncertainty that is introduced to long-term projections of HAB response from a wide range of sources, including HAB or ecosystem model parameterizations, variability in the climate model forcing (GCM selection, emissions scenario, downscaling approach), and the stochastic response of non-linear physical-biological interactions within the model system. An ensemble approach considers multiple model scenarios to quantify how different choices of key input factors, and potentially within the model formulation as well, affects the uncertainty in model projections. The selection of scenarios to use in an ensemble approach depends on the particular application and available resources, but sensitivity testing based on a subset of potential cases can be used to identify components of the model system that are particularly important sources of uncertainty in the long-term response. The central tendency (or “most likely” scenario) of the ensemble might be the focus of analysis and reporting on the modeling, but it may also be informative to select scenarios that encompass the full range of possible future outcomes. The process used to develop the scenarios and the sensitivity to various model aspects within the ensemble provide critical context for interpreting the results and for guiding future research efforts to minimize or mitigate model uncertainty.
HAB models constitute a small subset of the broader array of ocean biogeochemical models, so models representing similar processes can provide context for assessing climate change response. A common approach is to couple global or regional circulation models with biogeochemistry models of varying complexity to project ecosystem response under future climate forcing. The ecosystem response depends both on the circulation model and the biogeochemical formulation, so generally an ensemble approach evaluating multiple, independent models with the same set of forcing conditions provides critical context for evaluating model results. For example, a study using six climate model simulations along with an empirical model for predicting chlorophyll from physical model fields projected a global increase in primary productivity of 0.7–8% in response to warming over the 21st century (Sarmiento et al. 2004). In contrast, analysis of four coupled climate-carbon cycle models projected a global decrease in primary productivity of 2–20% (Steinacher et al. 2010). The differences between the results were attributed to differences in the biological model formulations, in that nutrient availability was incorporated in the coupled model but not directly in the empirical approach. Both studies found large regional variability in the response to climate change, as well as regional differences in the agreement among the ensemble members. Model skill varied regionally depending on the model, so appropriately weighting the ensemble members based on their skill regionally can provide a better solution than a simple average of ensemble members, and quantifying the inter-model variation provides a valuable measure of the uncertainty in the region of interest (Steinacher et al. 2010; Stock et al. 2011). Evaluation of model skill for ecosystem response requires long-term observations, as discussed in greater detail below. For chlorophyll, identifying observational declines at both regional and global scales required using Secchi depth measurements spanning more than 100 years because fluctuations in chlorophyll at the interannual to decadal time scales were sufficiently large that long-term trends were not robust over the ~30 years of satellite data (Boyce et al. 2010).
Modeling studies of climate impacts on HABs have typically examined responses at time scales of 50 to 100 years (e.g., Moore et al. 2008; Glibert et al. 2014; Townhill et al. 2018), as this is when greenhouse gas concentration trajectories associated with the different potential futures diverge and high emission scenarios become distinguishable from natural variability. Yet for management and public policy decisions, characterizing changes in HAB risks at shorter time scales (i.e., decadal) may be more critical. For physical models, projection of climate response at decadal time scales remains a major challenge (Zhang and Kirtman 2019). At decadal time scales, both external forcing and internal ocean response can be dominated by noise, making model response unpredictable. Internal climate variations like ENSO, AMO, or PDO may dominate responses of key climate variables like upwelling strength or river discharge, particularly at decadal time scales, swamping trends at century time scales that are more robustly represented across the suite of climate models. Climate predictability at decadal time scales varies regionally with the local modes of internal variability, such that some regions have greater predictability (North Pacific, North Atlantic, Southern Ocean) than others (tropical Pacific) (Zhang and Kirtman 2019). An understanding of the regional predictability of climate model, including variation among models, is particularly important for HAB models that are typically only simulating regional scales at decadal time scales.
Using validation and sensitivity testing to understand uncertainty in HAB models, in addition to the uncertainty in projections of the physical and biogeochemical conditions, is a critical step prior to projecting HAB response to climate change. HAB models of present conditions need to include more thorough assessments of model uncertainty, with ensemble sensitivity studies or more formal means like Bayesian models that incorporate uncertainty estimates in the results (Anderson et al. 2015), as the uncertainty compounds when run in climate forecast scenarios. HAB model failures also are instructive particularly in the context of potential regime shifts with climate change when major shifts in forcing conditions are not adequately represented in the model setup, as with anomalous conditions that affected Alexandrium in the Gulf of Maine (McGillicuddy et al. 2011).
Scenario planning is becoming a popular approach for decision-makers to address uncertainty in future projections and help prepare for conditions that may be substantially different from current conditions (Star et al. 2016). Scenario planning involves crafting stories about how the world might turn out in the future, it is not about predicting what will happen. Scenarios are developed around major uncertainties, or what ifs, in how key parameters m ight change in the future. Scenario planning can combine both quantitative and qualitative components, and involve input from researchers as well as stakeholders. Working through scenarios not only informs the development of societal response strategies to deal with future HABs, but also helps to understand how socioecological systems work and respond to HABs under current climate conditions. Benefits from scenario planning include increased flexibility to react quickly to a changing world, more thoughtful strategic planning and decisions, innovative ideas, early and broad risk assessment, and increased ability to achieve a common vision (Star et al. 2016). The use of scenario planning for evaluating HAB response to climate change offers a path forward for addressing some of the major uncertainties in biological responses identified in Wells et al. (2015) while still providing actionable projections.
4.3. Use downscaled climate models
Global earth system models typically have spatial resolution too coarse (nominally 1° for CMIP5 generation of climate models) to represent regional variability like tides, river inflows, coastal topography, or water column structure in detail. Even high resolution global models at 1/12° can’t resolve features at the scale of the baroclinic Rossby radius (ci/f, where ci is the internal wave speed and f the Coriolis parameter), which is relevant to coastal upwelling, frontal jets, and buoyant plumes, in more than 90% of the coastal ocean. To get to 70% coverage, 6 times higher resolution would be required (Holt et al. 2017). Higher resolution regional circulation models provide better model skill for resolving stratification and variability at seasonal time scales, but linking regional scale models to forcing from GCMs requires accounting for the coarse resolution and regional biases through downscaling, bias corrections, and multi-model ensembles (Stock et al. 2011). Resolving physical and biogeochemical processes at coastal scales is critical for HAB modeling, as the HABs that have the greatest impacts on fisheries, aquaculture, or through direct exposure typically occur near the coast.
Downscaling from global models can be statistical or dynamical. Dynamical downscaling provides physically consistent representations of the dynamical system at higher resolution, but it is comparatively expensive to setup and run the models and remains subject to regional biases in the global models (Stock et al. 2011). For example, dynamical downscaling was used to model the North Sea at 3 km resolution to project changes in bottom temperature and salinity, and these physical model fields were used to project changes in distributions of 75 benthic species (Weinert et al. 2016). The results indicated northward shifts for about 2/3 of species and southward shifts for the rest, and the downscaled model illustrated the strong influence of bottom topography on habitat gains and losses. An ensemble of dynamically downscaled regional models of the Baltic Sea with different nutrient loading scenarios was used to assess hypoxic and anoxic extent and potential influences of changes in river discharge, air-sea fluxes, and intensified nutrient cycling (Meier et al. 2011). The variance in biogeochemical response with forcing from three physical models with different structures but similar forcing provided a metric of the robustness of the results relative to model variability.
Statistical downscaling can take various forms, including linear regression, general additive models, and neural networks, and can link global climate model output variables to variables of interest in a particular region. Approaches for selecting appropriate downscaling approaches are reviewed elsewhere (e.g., Wilby et al. 2004; Haylock et al. 2006). The robustness of the downscaling depends in part on the data available to develop statistical relationships between predictor and response variables, and it requires keeping a subset of the observations separate from the training data for validation. Statistical downscaling also faces limitations when extrapolating into climate conditions that are outside the bounds of the observational record, as model failures may not be apparent even when using independent validation data from the same parameter space as the training data (Bell and Schlaepfer 2016).
Various statistical downscaling approaches have been used to link climate model outputs to biogeochemical models at regional, coastal, or estuarine scales. A constructed analogues approach that represents sharp geographical gradients and daily variability through linear regressions of model output to observations (Hidalgo et al. 2008) was used to relate air temperatures from GCMs to water temperature in the San Francisco Estuary, and thus project climate impacts on an endangered fish species (Brown et al. 2016). Four different downscaling methods were trained on 20 years of observations to downscale air temperature and precipitation fields from four GCMs to the Susquehanna River watershed to generate inputs to a water balance model and predict changes in surface salinity and temperature in Chesapeake Bay (Muhling et al. 2018). Those downscaled salinity and temperature projections were combined with habitat models for three Vibrio species to predict future increases in the seasonal duration and spatial extent of the pathogens (Muhling et al. 2017). Several examples using statistical downscaling, bias correction, and ensemble approaches to model climate change impacts on regional fisheries are examined in Stock et al. (2011), which details many of the considerations in using downscaled climate models to drive ecosystem forecasts that are relevant to HAB models.
4.4. Evaluate models with long-term observations
Global climate models are known to have biases and skill that vary regionally, and these can be assessed by comparison with observation records during GCM model hindcast periods. Observations to evaluate physical parameters like air temperature or wind speed, and to lesser extent water temperature and salinity, are far more prevalent than long-term observations of biogeochemical parameters like nutrient or chlorophyll concentrations. Extended time series of HAB abundance or toxicity that are needed to evaluate HAB model hindcasts at climate time scales are even rarer. Long-term observations of biologically relevant data are critical to identify trends in what are often sparse, patchy distributions (Ducklow et al. 2009), and they also need to be incorporated into assessments of climate forecasts. Fisheries surveys are an example of a rich data type that has been used to identify decadal scale variability associated with the PDO or NAO as well as seasonal to interannual variability with ENSO (Lehodey et al. 2006). Models of climate impacts on fisheries incorporate these long-term records into statistical relationships between physical fields and the response of the variable of interest, and those relationships can be continually updated as additional data are collected (Hollowed et al. 2009; Hare et al. 2010). The Continuous Plankton Recorder (CPR) survey is another observational record that goes back more than half a century, and it has been used to document shifts in community composition with decreased abundance of dinoflagellates and increases of some diatoms, including Pseudo-nitzschia, which were attributed to increased sea surface temperatures and stronger stratification (Hinder et al. 2012). CPR data were used to identify increases in warm-water phytoplankton and zooplankton species and decreases in cold-water species that were correlated with sea surface temperature in the northeastern Atlantic, air temperature in the Northern Hemisphere, and the NAO (Beaugrand and Reid 2003). Northward shifts in community composition in a coupled physical and biogeochemical model that were consistent with CPR observations were used to diagnose the processes leading to the changes, and showed that in addition to warmer temperatures that changes in circulation and stratification contributed to the patterns in the model (Barton et al. 2016).
To be useful for assessing climate impacts on biological systems, models must be able to distinguish the response to climate variability from internal biological dynamics (Lehodey et al. 2006), and ideally HAB models of climate response should help in identifying similar responses among different regions. Successful modeling approaches can be transferred to new regions, but requires accounting for similarities and differences in the physical environment, ecosystem characteristics, and HAB population, all of which are multi-dimensional and difficult to quantify without observations. Identifying climate effects in observations requires at least several decades of consistent HAB monitoring, and yet few regions have such high-quality time series data, nor is there monitoring in regions where future outbreaks may occur (Anderson et al. 2015; Wells et al. 2015). In addition to climate change, anthropogenic stressors such as fishing pressure, nutrient inputs, and invasive species introduction increase the challenges of identifying trends in observations of HAB abundance and distribution. Nutrient inputs have increased more than ten-fold in some coastal regions over the past few decades with usage of synthetic nitrogen fertilizer usage and urbanization, but the impacts vary widely (Howarth 2008). Projecting future nutrient conditions may require accounting for regional increases or decreases in nutrient loading with watershed land-use changes (Bouwman et al. 2009; Glibert et al. 2010) in addition to physical changes in the nutrient delivery by river discharge or coastal upwelling that are incorporated in models of HAB dynamics presently. Shifts in nutrient inputs by eutrophication or climate change may also affect nutrient limitation and require incorporating currencies in addition to nitrogen into HAB models (Flynn and McGillicuddy 2018).
While it is generally accepted that HABs are globally increasing in severity and extent, the role of climate change in the observed trends has been challenging to isolate mechanistically among the many other contributing factors (Moore et al. 2008). HAB models applied retrospectively at climate time scales may provide a useful means of hypothesis testing as opposed to focusing on predictions of future impacts. As has been done with observations (Moore et al. 2011), weather events, anomalous seasonal conditions, or sharp changes in forcing can be simulated retrospectively with HAB models as analogues for climate change impacts. Such scenarios can more realistically incorporate multiple stressors, and allow for quantitative assessment of model performance and uncertainty using observations that are independent from the model calibration. For example, laboratory studies have found that growth rates for Alexandrium spp. increase up to 20–24 °C (Watras et al. 1982; Etheridge and Roesler 2005; Bill et al. 2016), suggesting that warmer water will lead to faster growth and greater bloom intensity. Observations of A. catenella in an estuary in the northeastern U.S. found that the blooms in warmer years occurred earlier but did not have longer duration or greater maximum cell abundance, and instead the blooms terminated before water temperatures reached the values corresponding with maximum growth rates from the laboratory (Ralston et al. 2014). A process-based, single-species model that used the laboratory growth rates could effectively reproduce the growth phase across multiple years with widely varying temperature conditions, but an empirical formulation for mortality that was not strictly temperature-dependent was needed to represent bloom termination across the years, and could only be calibrated based on comparison with the multi-year observations (Ralston et al. 2015). Bloom dynamics in that system remained similar enough over several years that the empirical formulation for mortality had predictive skill, but climate change can potentially induce more fundamental shifts in ecosystem dynamics, for example changing from bottom-up (nutrient availability regulating growth) to top-down (grazing control) control (Wells et al. 2015). Developing robust models of the interactions between HAB growth rates and grazer response under changing forcing conditions, particularly when the relationships may be strongly non-linear, remains a central challenge for HAB modeling across all simulation time scales (Flynn and McGillicuddy 2018).
5. Conclusions
Modeling HAB response to future climate change is still an emerging field, as evidenced by the limited number of studies (fewer than 10) and diversity of approaches reviewed here. Extending HAB models to decadal time scales or longer, extrapolating into forcing regimes that are outside historical observations, representing potential regime shifts in the dominant processes controlling HAB development, and incorporating uncertainty and variability in physical climate model projections are challenging but feasible tasks. Based on this review, we offer several recommendations for how to best move forward with modeling HAB response to climate change. Statistical models have predominantly been used for near-term and operational HAB forecasts, but the uncertainty in model output increases as forcing conditions diverge from the historical observations that were used to develop them. Process-based models more directly represent key physical and biological factors in bloom development, and thus are better suited to extrapolation into future climate forcing conditions. HAB models should be developed in the context of the ecosystem response to climate change, recognizing that the response of many key processes and the potential for regime shifts are common to the broader ecosystem. Uncertainty in HAB model projections associated with process formulations or climate model forcing should be quantified and conveyed using ensemble approaches and scenario planning. Downscaling of global (and potentially regional) climate models to coastal scales should be done robustly in collaboration with physical climate modelers to preserve features of the forcing that are key to HAB development. Finally, long-term observations of HABs and forcing conditions are essential to identify trends associated with climate change and for rigorously assessing HAB model results. Long-term observations are critically lacking in many HAB impacted regions, and this may represent the biggest impediment to the development of models that can effectively assess HAB response to climate change. Multiple decades of HAB monitoring are often necessary to distinguish long-term trends from the response to cyclic climate forcing, so any model-based assessment of HAB response to climate change needs to be closely coupled to high quality observations. Modeling studies of HAB response to climate change will likely expand as resource managers and policy makers increasingly demand projections of HAB impacts at both near-term and longer time scales. As such, HAB models will be crucial for informing the development of strategies to reduce socioeconomic and public health impacts as well as to increase resilience of socioecological systems to future HABs.
Highlights:
Process-based models preferred to statistical for projecting climate change impacts
Long-term observations are critical for model development and evaluation
Evaluate model uncertainty with ensemble approaches and scenario planning
Use robust downscaling of climate model projections
Acknowledgements
Support for DKR was provided through the Woods Hole Center for Oceans and Human Health with grants from the National Science Foundation (OCE-1314642 and OCE-1840381) and the National Institute of Environmental Health Sciences (P01ES021923 and P01ES028938). SKM was supported by the Northwest Fisheries Science Center, National Marine Fisheries Service. The authors thank Dennis McGillicuddy for valuable input, and Greg Williams for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
David K. Ralston, Applied Ocean Physics and Engineering, Woods Hole Oceanographic Institution, Woods Hole, MA, USA.
Stephanie K. Moore, Environmental and Fisheries Sciences Division, Northwest Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Seattle, WA, USA
References
- Álvarez-Salgado XA, Labarta U, Fernández-Reiriz MJ, Figueiras FG, Rosón G, Piedracoba S, Filgueira R, and Cabanas JM. 2008. Renewal time and the impact of harmful algal blooms on the extensive mussel raft culture of the Iberian coastal upwelling system (SW Europe). Harmful Algae 7: 849–855. doi: 10.1016/j.hal.2008.04.007. [DOI] [Google Scholar]
- Anderson Clarissa R., Moore Stephanie K., Tomlinson Michelle C., Silke Joe, and Cusack Caroline K.. 2015. Chapter 17 - Living with Harmful Algal Blooms in a Changing World: Strategies for Modeling and Mitigating Their Effects in Coastal Marine Ecosystems In Coastal and Marine Hazards, Risks, and Disasters, ed. Shroder John F., Ellis Jean T., and Sherman Douglas J., 495–561. Boston: Elsevier. doi: 10.1016/B978-0-12-396483-0.00017-0. [DOI] [Google Scholar]
- Anderson Clarissa R., Sapiano Mathew R. P., Krishna Prasad M. Bala, Long Wen, Tango Peter J., Brown Christopher W., and Murtugudde Raghu. 2010. Predicting potentially toxigenic Pseudo-nitzschia blooms in the Chesapeake Bay. Journal of Marine Systems 83 GEOHAB Modeling: 127–140. doi: 10.1016/j.jmarsys.2010.04.003. [DOI] [Google Scholar]
- Anderson Clarissa R., Siegel David A., Kudela Raphael M., and Brzezinski Mark A.. 2009. Empirical models of toxigenic Pseudo-nitzschia blooms: Potential use as a remote detection tool in the Santa Barbara Channel. Harmful Algae 8: 478–492. doi: 10.1016/j.hal.2008.10.005. [DOI] [Google Scholar]
- Barton Andrew D., Irwin Andrew J., Finkel Zoe V., and Stock Charles A.. 2016. Anthropogenic climate change drives shift and shuffle in North Atlantic phytoplankton communities. Proceedings of the National Academy of Sciences 113: 2964–2969. doi: 10.1073/pnas.1519080113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugrand Grégory, and Reid Philip C.. 2003. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Global Change Biology 9: 801–817. doi: 10.1046/j.1365-2486.2003.00632.x. [DOI] [Google Scholar]
- Bell David M., and Schlaepfer Daniel R.. 2016. On the dangers of model complexity without ecological justification in species distribution modeling. Ecological Modelling 330: 50–59. doi: 10.1016/j.ecolmodel.2016.03.012. [DOI] [Google Scholar]
- Bill Brian D., Moore Stephanie K., Hay Levi R., Anderson Donald M., and Trainer Vera L.. 2016. Effects of temperature and salinity on the growth of Alexandrium (Dinophyceae) isolates from the Salish Sea. Journal of Phycology 52: 230–238. doi: 10.1111/jpy.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman AF, Beusen AHW, and Billen G. 2009. Human alteration of the global nitrogen and phosphorus soil balances for the period 1970–2050. Global Biogeochemical Cycles 23. doi: 10.1029/2009GB003576. [DOI] [Google Scholar]
- Boyce Daniel G., Lewis Marlon R., and Worm Boris. 2010. Global phytoplankton decline over the past century. Nature 466: 591–596. doi: 10.1038/nature09268. [DOI] [PubMed] [Google Scholar]
- Brown CW, Hood RR, Long W, Jacobs J, Ramers DL, Wazniak C, Wiggert JD, Wood R, and Xu J. 2013. Ecological forecasting in Chesapeake Bay: Using a mechanistic–empirical modeling approach. Journal of Marine Systems 125 Advances in Marine Ecosystem Modelling Research III: 113–125. doi: 10.1016/j.jmarsys.2012.12.007. [DOI] [Google Scholar]
- Brown Larry R., Komoroske Lisa M., Wagner R. Wayne, Morgan-King Tara, May Jason T., Connon Richard E., and Fangue Nann A.. 2016. Coupled Downscaled Climate Models and Ecophysiological Metrics Forecast Habitat Compression for an Endangered Estuarine Fish. PLOS ONE 11: e0146724. doi: 10.1371/journal.pone.0146724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack Caroline, Dabrowski Tomasz, Lyons Kieran, Berry Alan, Westbrook Guy, Salas Rafael, Duffy Conor, Nolan Glenn, and Silke Joe. 2016. Harmful algal bloom forecast system for SW Ireland. Part II: Are operational oceanographic models useful in a HAB warning system. Harmful Algae 53 Applied Simulations and Integrated Modelling for the Understanding of Toxic and Harmful Algal Blooms (ASIMUTH): 86–101. doi: 10.1016/j.hal.2015.11.013. [DOI] [PubMed] [Google Scholar]
- Cusack Caroline, Mouriño Helena, Teresa Moita Maria, and Silke Joe. 2015. Modelling Pseudo-nitzschia events off southwest Ireland. Journal of Sea Research 105: 30–41. doi: 10.1016/j.seares.2015.06.012. [DOI] [Google Scholar]
- Díaz Patricio A., Manuel Ruiz-Villarreal Yolanda Pazos, Moita Teresa, and Reguera Beatriz. 2016. Climate variability and Dinophysis acuta blooms in an upwelling system. Harmful Algae 53 Applied Simulations and Integrated Modelling for the Understanding of Toxic and Harmful Algal Blooms (ASIMUTH): 145–159. doi: 10.1016/j.hal.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Ducklow Hugh W., Doney Scott C., and Steinberg Deborah K.. 2009. Contributions of Long-Term Research and Time-Series Observations to Marine Ecology and Biogeochemistry. Annual Review of Marine Science 1: 279–302. doi: 10.1146/annurev.marine.010908.163801. [DOI] [PubMed] [Google Scholar]
- Erdner Deana L., Dyble Julianne, Parsons Michael L., Stevens Richard C., Hubbard Katherine A., Wrabel Michele L., Moore Stephanie K., Lefebvre Kathi A., Anderson Donald M., and Bienfang Paul. 2008. Centers for Oceans and Human Health: a unified approach to the challenge of harmful algal blooms In Environmental Health, 7:S2 BioMed Central. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge Stacey M., and Roesler Collin S.. 2005. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep Sea Research II 52: 2491–2500. doi: 10.1016/j.dsr2.2005.06.026. [DOI] [Google Scholar]
- Flynn Kevin J., and McGillicuddy Dennis J.. 2018. Modeling Marine Harmful Algal Blooms: Current Status and Future Prospects In Harmful Algal Blooms, 115–134. John Wiley & Sons, Ltd. doi: 10.1002/9781118994672.ch3. [DOI] [Google Scholar]
- Franks Peter J. S. 2018. Recent Advances in Modelling of Harmful Algal Blooms In Global Ecology and Oceanography of Harmful Algal Blooms, ed. Glibert Patricia M., Berdalet Elisa, Burford Michele A., Pitcher Grant C., and Zhou Mingjiang, 359–377. Ecological Studies. Cham: Springer International Publishing. doi: 10.1007/978-3-319-70069-4_19. [DOI] [Google Scholar]
- Fu Fx, Tatters Ao, and Hutchins Da. 2012. Global change and the future of harmful algal blooms in the ocean. Marine Ecology Progress Series 470: 207–233. doi: 10.3354/meps10047. [DOI] [Google Scholar]
- Giddings SN, MacCready P, Hickey BM, Banas NS, Davis KA, Siedlecki SA, Trainer VL, Kudela RM, Pelland NA, and Connolly TP. 2014. Hindcasts of potential harmful algal bloom transport pathways on the Pacific Northwest coast. Journal of Geophysical Research: Oceans: n/a-n/a. doi: 10.1002/2013JC009622. [DOI] [Google Scholar]
- Gillibrand PA, Siemering B, Miller PI, and Davidson K. 2016. Individual-based modelling of the development and transport of a Karenia mikimotoi bloom on the North-west European continental shelf. Harmful Algae 53 Applied Simulations and Integrated Modelling for the Understanding of Toxic and Harmful Algal Blooms (ASIMUTH): 118–134. doi: 10.1016/j.hal.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Glibert Patricia M., Allen J. Icarus, Artioli Yuri, Beusen Arthur, Bouwman Lex, Harle James, Holmes Robert, and Holt Jason. 2014. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Global Change Biology 20: 3845–3858. doi: 10.1111/gcb.12662. [DOI] [PubMed] [Google Scholar]
- Glibert Patricia M., Allen J. Icarus, Bouwman AF, Brown Christopher W., Flynn Kevin J., Lewitus Alan J., and Madden Christopher J.. 2010. Modeling of HABs and eutrophication: Status, advances, challenges. Journal of Marine Systems 83 GEOHAB Modeling: 262–275. doi: 10.1016/j.jmarsys.2010.05.004. [DOI] [Google Scholar]
- Vilas Luis González, Spyrakos Evangelos, Torres Palenzuela Jesus M., and Pazos Yolanda. 2014. Support Vector Machine-based method for predicting Pseudo-nitzschia spp. blooms in coastal waters (Galician rias, NW Spain). Progress in Oceanography 124: 66–77. doi: 10.1016/j.pocean.2014.03.003. [DOI] [Google Scholar]
- Hallegraeff Gustaaf M. 2010. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. Journal of Phycology 46: 220–235. doi: 10.1111/j.1529-8817.2010.00815.x. [DOI] [Google Scholar]
- Hare Jonathan A., Alexander Michael A., Fogarty Michael J., Williams Erik H., and Scott James D.. 2010. Forecasting the dynamics of a coastal fishery species using a coupled climate–population model. Ecological Applications 20: 452–464. [DOI] [PubMed] [Google Scholar]
- Haylock Malcolm R., Cawley Gavin C., Harpham Colin, Wilby Rob L., and Goodess Clare M.. 2006. Downscaling heavy precipitation over the United Kingdom: a comparison of dynamical and statistical methods and their future scenarios. International Journal of Climatology: A Journal of the Royal Meteorological Society 26: 1397–1415. [Google Scholar]
- Henrichs Darren W., Hetland Robert D., and Campbell Lisa. 2015. Identifying bloom origins of the toxic dinoflagellate Karenia brevis in the western Gulf of Mexico using a spatially explicit individual-based model. Ecological Modelling 313: 251–258. doi: 10.1016/j.ecolmodel.2015.06.038. [DOI] [Google Scholar]
- Hidalgo Hugo G., Dettinger Michael D., and Cayan Daniel R.. 2008. Downscaling with constructed analogues: Daily precipitation and temperature fields over the United States. California Energy Commission PIER Final Project Report CEC-500-2007-123. [Google Scholar]
- Hinder Stephanie L., Hays Graeme C., Edwards Martin, Roberts Emily C., Walne Anthony W., and Gravenor Mike B.. 2012. Changes in marine dinoflagellate and diatom abundance under climate change. Nature Climate Change 2: 271–275. doi: 10.1038/nclimate1388. [DOI] [Google Scholar]
- Hollowed Anne Babcock, Bond Nicholas A., Wilderbuer Thomas K., Stockhausen William T., A’mar Z. Teresa, Beamish Richard J., Overland James E., and Schirripa Michael J.. 2009. A framework for modelling fish and shellfish responses to future climate change. ICES Journal of Marine Science 66: 1584–1594. [Google Scholar]
- Holt Jason, Hyder Patrick, Ashworth Mike, Harle James, Hewitt Helene T., Liu Hedong, New Adrian L., et al. 2017. Prospects for improving the representation of coastal and shelf seas in global ocean models. Geoscientific Model Development 10: 499–523. doi: 10.5194/gmd-10-499-2017. [DOI] [Google Scholar]
- Howarth Robert W. 2008. Coastal nitrogen pollution: A review of sources and trends globally and regionally. Harmful Algae 8 HABs and Eutrophication: 14–20. doi: 10.1016/j.hal.2008.08.015. [DOI] [Google Scholar]
- Jacobs John, Moore Stephanie K., Kunkel Kenneth E., and Sun Liqiang. 2015. A framework for examining climate-driven changes to the seasonality and geographical range of coastal pathogens and harmful algae. Climate Risk Management 8: 16–27. doi: 10.1016/j.crm.2015.03.002. [DOI] [Google Scholar]
- Kibler Steven R., Tester Patricia A., Kunkel Kenneth E., Moore Stephanie K., and Litaker R. Wayne. 2015. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecological Modelling 316: 194–210. doi: 10.1016/j.ecolmodel.2015.08.020. [DOI] [Google Scholar]
- Lane Jenny Q., Raimondi Peter T., and Kudela Raphael M.. 2009. Development of a logistic regression model for the prediction of toxigenic Pseudo-nitzschia blooms in Monterey Bay, California. Marine Ecology Progress Series 383: 37–51. doi: 10.3354/meps07999. [DOI] [Google Scholar]
- Lehodey P, Alheit J, Barange M, Baumgartner T, Beaugrand G, Drinkwater K, Fromentin J-M, et al. 2006. Climate Variability, Fish, and Fisheries. Journal of Climate 19: 5009–5030. doi: 10.1175/JCLI3898.1. [DOI] [Google Scholar]
- Li Yizhen, He Ruoying, McGillicuddy Dennis J. Jr., Anderson Donald M., and Keafer Bruce A.. 2009. Investigation of the 2006 Alexandrium fundyense bloom in the Gulf of Maine: In-situ observations and numerical modeling. Continental Shelf Research 29: 2069–2082. doi: 10.1016/j.csr.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire Julie, Cusack Caroline, Manuel Ruiz-Villarreal Joe Silke, McElligott Deirdre, and Davidson Keith. 2016. Applied simulations and integrated modelling for the understanding of toxic and harmful algal blooms (ASIMUTH): Integrated HAB forecast systems for Europe’s Atlantic Arc. Harmful Algae 53 Applied Simulations and Integrated Modelling for the Understanding of Toxic and Harmful Algal Blooms (ASIMUTH): 160–166. doi: 10.1016/j.hal.2015.11.006. [DOI] [PubMed] [Google Scholar]
- McCabe Ryan M., Hickey Barbara M., Kudela Raphael M., Lefebvre Kathi A., Adams Nicolaus G., Bill Brian D., Gulland Frances M. D., Thomson Richard E., Cochlan William P., and Trainer Vera L.. 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophysical Research Letters 43: 10,366–10,376. doi: 10.1002/2016GL070023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ 2010. Models of harmful algal blooms: Conceptual, empirical, and numerical approaches. Journal of Marine Systems 83 GEOHAB Modeling: 105–107. doi: 10.1016/j.jmarsys.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ, Townsend DW, He R, Keafer BA, Kleindinst JL, Li Y, Manning JP, Mountain DG, Thomas MA, and Anderson Donald M.. 2011. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnology and Oceanography 56: 2411–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgaine McKibben, S., Watkins-Brandt Katie S., Wood A. Michelle, Hunter Matthew, Forster Zach, Hopkins Alyssa, Du Xiuning, Eberhart Bich-Thuy, Peterson William T., and White Angelicque E.. 2015. Monitoring Oregon Coastal Harmful Algae: Observations and implications of a harmful algal bloom-monitoring project. Harmful Algae 50: 32–44. doi: 10.1016/j.hal.2015.10.004. [DOI] [Google Scholar]
- Meier HEM, Andersson HC, Eilola K, Gustafsson BG, Kuznetsov I, Müller‐Karulis B, Neumann T, and Savchuk OP. 2011. Hypoxia in future climates: A model ensemble study for the Baltic Sea. Geophysical Research Letters 38. doi: 10.1029/2011GL049929. [DOI] [Google Scholar]
- Jannicke Moe, S., Haande Sigrid, and Couture Raoul-Marie. 2016. Climate change, cyanobacteria blooms and ecological status of lakes: A Bayesian network approach. Ecological Modelling 337: 330–347. doi: 10.1016/j.ecolmodel.2016.07.004. [DOI] [Google Scholar]
- Moore Stephanie K., Johnstone James A., Banas Neil S., and Salathé Eric P.. 2015. Present-day and future climate pathways affecting Alexandrium blooms in Puget Sound, WA, USA. Harmful Algae 48: 1–11. doi: 10.1016/j.hal.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Moore Stephanie K., Mantua Nathan J., Hickey Barbara M., and Trainer Vera L.. 2009. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae 8: 463–477. doi: 10.1016/j.hal.2008.10.003. [DOI] [Google Scholar]
- Moore Stephanie K., Mantua Nathan J., Hickey Barbara M., and Trainer Vera L.. 2010. The relative influences of El Niño‐Southern Oscillation and Pacific Decadal Oscillation on paralytic shellfish toxin accumulation in northwest Pacific shellfish. Limnology and Oceanography 55: 2262–2274. [Google Scholar]
- Moore Stephanie K., Mantua Nathan J., and Salathé Eric P.. 2011. Past trends and future scenarios for environmental conditions favoring the accumulation of paralytic shellfish toxins in Puget Sound shellfish. Harmful Algae 10: 521–529. doi: 10.1016/j.hal.2011.04.004. [DOI] [Google Scholar]
- Moore Stephanie K., Trainer Vera L., Mantua Nathan J., Parker Micaela S., Laws Edward A., Backer Lorraine C., and Fleming Lora E.. 2008. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environmental Health 7: S4. doi: 10.1186/1476-069X-7-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhling Barbara A., Gaitán Carlos F., Stock Charles A., Saba Vincent S., Tommasi Desiree, and Dixon Keith W.. 2018. Potential Salinity and Temperature Futures for the Chesapeake Bay Using a Statistical Downscaling Spatial Disaggregation Framework. Estuaries and Coasts 41: 349–372. doi: 10.1007/s12237-017-0280-8. [DOI] [Google Scholar]
- Muhling Barbara A., Jacobs John, Stock Charles A., Gaitan Carlos F., and Saba Vincent S.. 2017. Projections of the future occurrence, distribution, and seasonality of three Vibrio species in the Chesapeake Bay under a high-emission climate change scenario. GeoHealth 1: 278–296. doi: 10.1002/2017GH000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine Robin, Georgina McDermott Joe Silke, Lyons Kieran, Nolan Glenn, and Cusack Caroline. 2010. A simple short range model for the prediction of harmful algal events in the bays of southwestern Ireland. Journal of Marine Systems 83 GEOHAB Modeling: 150–157. doi: 10.1016/j.jmarsys.2010.05.001. [DOI] [Google Scholar]
- Ralston David K., Brosnahan Michael L., Fox Sophia E., Lee Krista D., and Anderson Donald M.. 2015. Temperature and Residence Time Controls on an Estuarine Harmful Algal Bloom: Modeling Hydrodynamics and Alexandrium fundyense in Nauset Estuary. Estuaries and Coasts: 1–19. doi: 10.1007/s12237-015-9949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston David K., Keafer Bruce A., Brosnahan Michael L., and Anderson Donald M.. 2014. Temperature dependence of an estuarine harmful algal bloom: Resolving interannual variability in bloom dynamics using a degree-day approach. Limnology and Oceanography 59: 1112–1126. doi: 10.4319/lo.2014.59.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Villarreal Manuel, García-García Luz M., Cobas Marcos, Díaz Patricio A., and Reguera Beatriz. 2016. Modelling the hydrodynamic conditions associated with Dinophysis blooms in Galicia (NW Spain). Harmful Algae 53 Applied Simulations and Integrated Modelling for the Understanding of Toxic and Harmful Algal Blooms (ASIMUTH): 40–52. doi: 10.1016/j.hal.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Sarmiento JL, Slater R, Barber R, Bopp L, Doney SC, Hirst AC, Kleypas J, et al. 2004. Response of ocean ecosystems to climate warming. Global Biogeochemical Cycles 18. doi: 10.1029/2003GB002134. [DOI] [Google Scholar]
- Sekula-Wood Emily, Claudia Benitez-Nelson Steve Morton, Anderson Clarissa, Burrell Christopher, and Thunell Robert. 2011. Pseudo-nitzschia and domoic acid fluxes in Santa Barbara Basin (CA) from 1993 to 2008. Harmful Algae 10: 567–575. doi: 10.1016/j.hal.2011.04.009. [DOI] [Google Scholar]
- Star Jonathan, Rowland Erika L., Black Mary E., Enquist Carolyn A. F., Garfin Gregg, Catherine Hawkins Hoffman Holly Hartmann, Jacobs Katharine L., Moss Richard H., and Waple Anne M.. 2016. Supporting adaptation decisions through scenario planning: Enabling the effective use of multiple methods. Climate Risk Management 13: 88–94. doi: 10.1016/j.crm.2016.08.001. [DOI] [Google Scholar]
- Steinacher M, Joos F, Frölicher TL, Bopp L, Cadule P, Cocco V, Doney SC, et al. 2010. Projected 21st century decrease in marine productivity: a multi-model analysis. Biogeosciences 7: 979–1005. doi: 10.5194/bg-7-979-2010. [DOI] [Google Scholar]
- Stock Charles A., Alexander Michael A., Bond Nicholas A., Brander Keith M., Cheung William W. L., Curchitser Enrique N., Delworth Thomas L., et al. 2011. On the use of IPCC-class models to assess the impact of climate on Living Marine Resources. Progress in Oceanography 88: 1–27. doi: 10.1016/j.pocean.2010.09.001. [DOI] [Google Scholar]
- Stock Charles A., McGillicuddy Dennis J. Jr., Solow Andrew R., and Anderson Donald M.. 2005. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical–biological model. Deep Sea Research Part II: Topical Studies in Oceanography 52: 2715–2744. doi: 10.1016/j.dsr2.2005.06.022. [DOI] [Google Scholar]
- Stumpf Richard P., Litaker R. Wayne, Lanerolle Lyon, and Tester Patricia A.. 2008. Hydrodynamic accumulation of Karenia off the west coast of Florida. Continental Shelf Research 28 Ecology and Oceanography of Harmful Algal Blooms in Florida: 189–213. doi: 10.1016/j.csr.2007.04.017. [DOI] [Google Scholar]
- Stumpf Richard P., Tomlinson Michelle C., Calkins Julie A., Kirkpatrick Barbara, Fisher Kathleen, Nierenberg Kate, Currier Robert, and Wynne Timothy T.. 2009. Skill assessment for an operational algal bloom forecast system. Journal of Marine Systems 76: 151–161. doi: 10.1016/j.jmarsys.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf Richard P., Wynne Timothy T., Baker David B., and Fahnenstiel Gary L.. 2012. Interannual Variability of Cyanobacterial Blooms in Lake Erie. PLOS ONE 7: e42444. doi: 10.1371/journal.pone.0042444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townhill Bryony L., Tinker Jonathan, Jones Miranda, Pitois Sophie, Creach Veronique, Simpson Stephen D., Dye Stephen, Bear Elizabeth, and Pinnegar John K.. 2018. Harmful algal blooms and climate change: exploring future distribution changes. ICES Journal of Marine Science 75: 1882–1893. doi: 10.1093/icesjms/fsy113. [DOI] [Google Scholar]
- Velo-Suárez L, Reguera B, González-Gil S, Lunven M, Lazure P, Nézan E, and Gentien P. 2010. Application of a 3D Lagrangian model to explain the decline of a Dinophysis acuminata bloom in the Bay of Biscay. Journal of Marine Systems 83 GEOHAB Modeling: 242–252. doi: 10.1016/j.jmarsys.2010.05.011. [DOI] [Google Scholar]
- Watras Carl J., Chisholm Sallie W., and Anderson Donald M.. 1982. Regulation of growth in an estuarine clone of Gonyaulax tamarensis Lebour: Salinity-dependent temperature responses. Journal of Experimental Marine Biology and Ecology 62: 25–37. doi: 10.1016/0022-0981(82)90214-3. [DOI] [Google Scholar]
- Weinert Michael, Mathis Moritz, Kröncke Ingrid, Neumann Hermann, Pohlmann Thomas, and Reiss Henning. 2016. Modelling climate change effects on benthos: Distributional shifts in the North Sea from 2001 to 2099. Estuarine, Coastal and Shelf Science 175: 157–168. doi: 10.1016/j.ecss.2016.03.024. [DOI] [Google Scholar]
- Wells Mark L., Trainer Vera L., Smayda Theodore J., Karlson Bengt S. O., Trick Charles G., Kudela Raphael M., Ishikawa Akira, et al. 2015. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 49: 68–93. doi: 10.1016/j.hal.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilby Robert L., Charles SP, Zorita Eduardo, Timbal Bertrand, Whetton Penny, and Mearns LO. 2004. Guidelines for use of climate scenarios developed from statistical downscaling methods. Supporting material of the Intergovernmental Panel on Climate Change, available from the DDC of IPCC TGCIA 27. [Google Scholar]
- Wynne Timothy T., Stumpf Richard P., Tomlinson Michelle C., Schwab David J., Watabayashi Glen Y., and Christensen John D.. 2011. Estimating cyanobacterial bloom transport by coupling remotely sensed imagery and a hydrodynamic model. Ecological Applications 21: 2709–2721. doi: 10.1890/10-1454.1. [DOI] [PubMed] [Google Scholar]
- Zhang Wei, and Kirtman Ben. 2019. Estimates of Decadal Climate Predictability From an Interactive Ensemble Model. Geophysical Research Letters 46: 3387–3397. doi: 10.1029/2018GL081307. [DOI] [Google Scholar]