Abstract
The U.S. Preventive Services Task Force has recommended that primary care clinicians screen all adults for obesity and provide those affected intensive multicomponent behavioral interventions. Approximately 95 million U.S. adults qualify for such care, also referred to as lifestyle modification. Using the Guidelines (2013) for Managing Overweight and Obesity in Adults as a framework, this article reviews the principal components of comprehensive lifestyle modification, which include diet, physical activity, and behavior therapy. To lose weight, the Guidelines recommend participation for 6 months in high-intensity programs that provide ≥14 counseling sessions with a trained interventionist. When provided face-to-face, individual or group treatment, participants lose up to 8 kg (8% of weight) in 6 months and experience improvements in cardiovascular disease risk factors and quality of life. To prevent weight regain, the Guidelines recommend participation for 1 year in weight-loss-maintenance programs that provide at least monthly counseling. High levels of physical activity, frequent monitoring of body weight, and consumption of a reduced calorie diet are associated with long-term weight loss. Investigators currently are seeking to increase the availability of lifestyle modification by delivering it in community-based programs, as well as on digital platforms (e.g., Internet and Smartphone). Digitally-delivered programs lower costs and expand treatment reach; their efficacy is likely to improve further with the addition of new technologies for monitoring food intake, activity, and weight. Ultimately, to improve long-term weight management, individual lifestyle counseling must be joined with collective and institutional efforts to improve our nation’s eating and activity environments.
Keywords: obesity, weight loss, intensive behavioral therapy, diet, physical activity
In 2018, the United States Preventive Services Task Force (USPSTF) reaffirmed its recommendation that primary care health professionals screen all adults for obesity and offer those affected “intensive, multicomponent behavioral interventions” (p. 374) (USPSTF et al., 2018). This recommendation is likely gratifying to psychologists and other behavioral scientists who helped to develop these interventions; it explicitly recognizes the substantial evidence base that supports the behavioral management of obesity. At the same time, the recommendation is daunting in its scope and charge to primary care physicians and other health professionals. How can they possibly provide intensive behavioral counseling, either directly or by referral, to the approximately 95 million U.S. adults who suffer from obesity and its associated health complications (Hales, Carroll, Fryar, & Ogden, 2017)? Moreover, who will pay for this care, given that many insurers offer limited coverage of weight management (Downey & Kyle, 2018)?
This article describes lifestyle modification for obesity in adults, its short- and long-term benefits, and efforts to increase its availability through the use of digital delivery and an ever-expanding assortment of apps. The examination of these topics is guided by recent systematic reviews and meta-analyses, as well as by analysis of selected studies. Obesity is defined by a body mass index (BMI) ≥30 kg/m2, but many of the studies reviewed included persons with overweight (i.e., BMI of 25–29.9 kg/m2) and one or more weight-related co-morbidities (e.g., type 2 diabetes). Numerous treatment algorithms recommend lifestyle modification as the cornerstone of treatment for all individuals with overweight or obesity (Garvey et al., 2016; Jensen et al., 2014; World Health Organization, 1998). (We note that the terms lifestyle modification, lifestyle intervention, behavioral weight control, intensive behavioral therapy, and behavioral treatment are used interchangeably by researchers, as they are in this paper.)
Context of Lifestyle Modification for Obesity
Appetite and body weight are regulated by a highly integrated gut-to-brain, neuroendocrine system that tracks both short- and long-term changes in energy intake and expenditure, with the goal of ensuring energy and body weight homeostasis (Schwartz et al. 2017). This system evolved principally to defend against food scarcity. The capacity to store excess energy as body fat is critical to survival. So is the body’s capacity, during food scarcity, to reduce, by 20% or more, both resting metabolic rate (i.e., the energy required to maintain basic biological functions such as body temperature) and energy expended during physical activity (as required to obtain food) (Rosenbaum & Leibel, 2016).
Today in America and other developed nations, the neuroendocrine regulation of body weight has been largely overwhelmed by what Brownell and Horgen (2004) have labeled a toxic food environment. For most Americans, food is now cheap, plentiful, and highly processed and palatable, with unending combinations of sugar, fat, and salt that are served up in ever-increasing portion sizes (Hall et al., 2019). Eating is the new national pastime, fueled by aggressive marketing and by dramatic changes in how individuals and families prepare and consume meals (Roberto et al., 2015); they are increasingly eaten out or ordered-in, with a marked increase in calories as compared with home cooking (Brownell & Horgen, 2004. Increases in mean daily energy intake have occurred in tandem with reductions in energy expenditure at work, with our nation’s transition to an information (digital) economy. For many, a hard day’s work may now be measured in key strokes and conference calls. The neuroendocrine system that defends against the perils of food scarcity and weight loss it is not equally adept in responding to food abundance or an environment in which energy expenditure is no longer prerequisite to eating. Regrettably, it also cannot distinguish intentional efforts to lose weight from starvation. The body’s hypometabolic responses to intentional weight loss frustrate weight control efforts.
This sketch does not do justice to the complexity of behavioral, biological, cultural, economic, environmental, ethnic, psychological, and social factors that influence an individual’s lifestyle behavior and body weight and which lifestyle modification is expected to address (Tronieri & Wadden, 2018). Individuals must increasingly fortify the neuroendocrine control of body weight with cognitive and behavioral efforts to regulate food intake and physical activity. Lifestyle modification provides principles and techniques to do so.
Lifestyle Modification Recommended for Weight Loss
Converging recommendations for the provision of lifestyle modification have come from numerous expert panels, including the USPSTF (2018), the Centers for Medicare and Medicaid Services (2011), and the Guidelines (2013) for Managing Overweight and Obesity in Adults, hereafter referred to as the Obesity Guidelines (Jensen et al., 2014). Recommendations from the Obesity Guidelines were based on a systematic review of studies published through 2011 that sought to identify the components of lifestyle modification programs needed to achieve clinically meaningful weight loss, typically defined as a reduction ≥5% of initial weight (Jensen et al., 2014). These guidelines recommend that individuals participate in a comprehensive intervention for at least 6 months. Such programs are delivered by a trained interventionist and provide instruction in behavioral principles and techniques that are designed to modify dietary intake and physical activity. Interventionists typically are health professionals, including registered dietitians (R.D.), psychologists, or health counselors, as well as trained lay persons (Jensen et al., 2014).
Table 1 summarizes the schedule and content of treatment recommended by the Obesity Guidelines for achieving and maintaining weight loss (Jensen et al., 2014; Heymsfield & Wadden, 2017). These guidelines preferentially recommend in-person, individual or group treatment, as well as telephone-delivered interventions. These recommendations are based on the large evidence base supporting these traditional interventions. These approaches, however, are quickly being complemented or supplanted by comprehensive digitally-delivered interventions, for which there was not as large an evidence base when the Obesity Guidelines were developed.
Table 1.
Recommended components of a high-intensity comprehensive lifestyle intervention to achieve and maintain a 5–10% reduction in body weight.
| Component | Weight Loss | Weight Loss Maintenance |
|---|---|---|
| Frequency and duration of treatment | 14 or more in-person counseling sessions in 6 months with a trained interventionist (individual or group contact). | Monthly or more frequent in-person or telephone sessions for ≥1 year with a trained interventionist. |
| contact | Similarly structured, comprehensive web-based interventions, as well as evidence-based commercial programs may be recommended. | |
| Diet | Low-calorie diet (typically 1200–1500 kcal/d for women, 1500–1800 kcal/d for men), with macronutrient composition based on patient’s preferences and health status. † | Reduced-calorie diet, consistent with reduced body weight, with macronutrient composition based on patient’s preferences and health status. |
| Physical activity‡ | ≥150 min/wk of aerobic physical activity (e.g., brisk walking). | 200–300 min/wk of aerobic activity (e.g., brisk walking). |
| Behavior therapy | Daily monitoring of food intake and physical activity, facilitated by paper diaries or apps. | Occasional to frequent monitoring of food intake and physical activity, as needed. |
| Weekly monitoring of weight. | Weekly to daily monitoring of weight. | |
| Structured curriculum of behavior change (e.g., DPP), including goal setting, problem solving, and stimulus control. | Curriculum of behavior change, including problem solving, cognitive restructuring, and relapse prevention. | |
| Regular feedback and support from a trained interventionist. | Regular feedback from trained interventionist. |
Adapted from the Guidelines (2013) for Managing Overweight and Obesity in Adults, Jensen et al. (2014). DPP = Diabetes Prevention Program.
The Guidelines concluded that a variety of dietary approaches, which differ widely in macronutrient composition, can produce weight loss, provided they induce an adequate energy deficit. This includes ad libitum approaches in which a lower calorie intake is achieved by restriction or elimination of particular food groups or by the provision of prescribed foods.
The Guidelines did not address the possible benefits of strength training, in addition to aerobic activity.
Table reprinted with permission from Heymsfield & Wadden, 2017.
During the first 6 months of lifestyle modification, individuals should receive at least 14 individual or group treatment sessions, the frequency defined as high intensity by the Obesity Guidelines. Frequent contact is critical for inducing clinically meaningful weight loss, as affirmed by a randomized controlled trial (RCT) by Perri et al. (2014). However, increasing intensity further (e.g., 24 rather than 16 sessions in 6 months) does not appear to increase weight loss appreciably, while certainly increasing costs. Cost considerations also generally favor the use of group rather than individual treatment, given the approximately equivalent weight losses produced by the two approaches, with some studies reporting greater efficacy with group treatment (Renjilian, Perri, Nezu, McKelvey, Shermer, & Anton, 2001).
Principal Components of Lifestyle Modification
The principal components of comprehensive lifestyle intervention are briefly described below. More detailed descriptions can be obtained by reviewing the Obesity Guidelines or the treatment protocols for the Diabetes Prevention Program (DPP; Knowler et al., 2002), or the Look AHEAD (Action for Health in Diabetes) study (Look AHEAD Research Group, 2013).
Diet
Calorie restriction is essential to inducing clinically meaningful weight loss. The Obesity Guidelines recommend consumption of a diet designed to achieve a deficit of 500–750 kcal/day, with a resulting mean loss of 0.5–0.75 kg (1.0–1.5 lb) per week. Accordingly, women often are prescribed 1200–1500 kcal/day and men 1500–1800 kcal/day, with higher targets within the ranges for individuals with higher body weights (or physical activity levels) (Jensen et al., 2014). Another option is to prescribe 1200–1500 kcal/d for individuals who weigh <113 kg (250 lb) and 1500–1800 kcal/d for those >113 kg (Look AHEAD Research Group et al., 2006). The U.S. Dietary Guidelines (2015) recommend that approximately 15–20% of daily calories be derived from protein, 20–35% from fat (with no more than 10% from saturated fat) and the remainder from carbohydrates, particularly fresh fruits, vegetables, and grains. Reducing portion sizes, as well as excess sugar and fat, are convenient ways to achieve desired calorie deficits (Rolls, 2018).
Lifestyle modification programs have traditionally prescribed low-fat, high carbohydrate diets (Knowler et al., 2002). However, extensive research over the past 15 years has shown that a variety of dietary approaches including low-carbohydrate/high-protein, Mediterranean-style, and low-glycemic-load diets can induce weight loss, if they facilitate achievement of desired calorie deficits (Jensen et al., 2014). Some RCTs have revealed larger 3- or 6-month weight losses with low-carbohydrate/high protein diets, as compared with other approaches, but long-term (>1 year) weight losses are generally comparable (Makris & Foster, 2011). There is little evidence that macronutrient composition per se (i.e., amount of fat, protein, etc.) affects weight loss, when different diets are matched for calorie level (Sacks et al., 2009). Macronutrient composition, however, may affect improvement in cardiometabolic risk factors (Jensen et al., 2014). Fabricatore et al. (2011), for example, found that a low glycemic index diet produced significantly larger reductions in hemoglobin A1c in patients with type 2 diabetes than did a low-fat diet, although the diets produced similar weight losses. Thus, an individual’s cardiometabolic risk factors and dietary preferences should be considered when choosing a diet. The use of portion-controlled diets -- including liquid shakes, meal bars, and prepared entrees – also facilitates achievement of weight loss goals (Heymsfield et al., 2003).
Physical Activity
Lifestyle modification programs typically prescribe 150–180 minutes per week of moderately vigorous aerobic activity, such as brisk walking or cycling (Butryn, Webb, & Wadden, 2011). Regular aerobic activity is associated with numerous benefits including improvements in physical (e.g., lipids, blood pressure) and mental health (e.g., anxiety, depression) (Powell et al., 2018). Physical activity also is associated with improved fitness, which may attenuate the risk of mortality associated with obesity (Barry et al., 2014). Persons who report lack of time to exercise are encouraged to engage in multiple brief bouts (e.g., 10 minutes) of activity throughout the day and to increase their lifestyle activity (e.g., by using stairs rather than elevators) (Jakicic, Rogers, Sherman, & Kovacs, 2018). A new generation of standing desks, including those with treadmills, potentially provides convenient means of increasing daily energy expenditure. Decreasing screen time and other sedentary behaviors is another important consideration for potentially increasing physical activity (and weight loss) (Otten, Jones, Littenberg, & Harvey-Berino, 2009). The addition of twice-weekly strength training to an activity program may protect against the loss of muscle that accompanies aging (Powell et al., 2018).
Physical activity alone produces minimal short-term weight loss if not combined with calorie restriction. Jakicic et al. (2011), for example, found that groups of non-dieting individuals who were instructed to engage in either 150 or 300 minutes of weekly activity both lost less than 2% of initial weight at 6 months. Thus, in the short term, lifestyle modification participants are encouraged to exercise primarily for its important health benefits. However, high levels of activity are critical for maintaining weight loss, as discussed later.
Behavior Therapy
Behavior therapy provides tools to facilitate changing eating and physical activity patterns (Butryn et al., 2011). Behavioral principles are applied in an interrelated manner consistent with Kanfer’s (1975) theory of self-regulation. First, the interventionist and patient collaboratively set specific, attainable goals for behavior change, which clearly identify the behavior to be modified and how it will be achieved. Self-monitoring (i.e., recording) of food and calorie intake, physical activity, and body weight is the cornerstone of treatment because it provides critical information about progress towards goals. More frequent self-monitoring of food intake is associated with greater weight loss (Wadden et al., 2005). Record keeping helps individuals identify behavioral patterns and target areas for change. Regularly reviewing patients’ self-monitoring records to evaluate progress toward goals provides accountability. This also creates an opportunity for the interventionist to provide reinforcement when goals are achieved (Butryn et al., 2011). If progress towards a goal is suboptimal, the interventionist instructs patients in problem solving, which involves analyzing barriers to behavior change and developing solutions.
Participants practice stimulus control by altering eating- and activity-related cues in their environment and use cognitive techniques to address maladaptive thinking. Behavioral treatment also may incorporate motivational interviewing to support an individual’s commitment to change, as well as acceptance-based approaches to improve long-term adherence (Butryn, Schumacher, & Forman, 2018). One of the greatest challenges to behavioral treatment is identifying the specific eating, activity, cognitive, and emotional factors that contribute to an individual’s obesity and then focusing specifically on these targets. Too often, particularly with group treatment, all individuals receive the same manualized approach to lifestyle modification.
Efficacy of Lifestyle Modification: Short Term Results (6–12 Months)
The Obesity Guideline’s systematic review of high-intensity lifestyle interventions revealed mean weight losses of up to 8 kg (about 8% of weight) at 6 months, with similar weight losses at 1 year (Jensen et al., 2014). These losses were significantly greater than those observed for usual care, a treatment arm required for studies to be included in the review. Numerous trials have reported mean 1-year losses of 8–12% of initial weight (e.g., Foster et al., 2010). Many of these studies, however, were not included in the Obesity Guidelines’ review because they did not include a usual care group, and instead compared two or more diet or physical activity regimens.
The Diabetes Prevention Program (DPP) provides an excellent example of a high-intensity intervention and is perhaps the most important weight management study conducted to date (Knowler et al., 2002). The trial enrolled 3234 individuals with overweight/obesity who also had impaired glucose control (i.e., prediabetes). Participants in the intensive lifestyle intervention (ILI) received 16 individual, in-person counseling sessions (approximately 30 minutes each) with an R.D. in the first 6 months, with at least every-other-month sessions through month 12. ILI participants lost a mean of 7.1 kg (about 7%) at month 6 and maintained this loss at month 12, at which time 62% of participants achieved a loss ≥5% of initial weight. By contrast, participants randomized to metformin (a diabetes medication) lost 2.8 kg, with no loss in the placebo group.
The DPP was followed by the Look AHEAD study, which enrolled 5,145 participants with overweight/obesity and type 2 diabetes (Look AHEAD Research Group, 2013). In year 1, participants in the ILI condition had two to three weekly group treatment sessions (60–90 minutes each) per month, with one individual monthly counseling visit to tailor treatment to individuals’ specific needs. At month 12, ILI participants lost a mean of 8.6% of initial weight, and 68.0% lost ≥5% of initial weight. Participants in the usual care group, referred to as diabetes support and education (DSE), lost 0.7%, with only 13.6% losing ≥5% (Wadden et al., 2009).
New Technology Supporting Traditional Lifestyle Modification
Individuals in the DPP and Look AHEAD interventions used paper records to monitor their food intake and physical activity, as in most studies reviewed by the Obesity Guidelines. The past decade, however, has witnessed a dramatic proliferation of technology-based tools for recording food intake, measuring physical activity, and tracking weight. Table 2 describes several of these tools and their potential advantages over traditional paper records, including lower numeracy demands and greater convenience and accuracy.
Table 2.
Technology to support self-monitoring in traditional lifestyle modification programs, as well as in digitally-delivered interventions.
| Description | Accuracy | Considerations | |
|---|---|---|---|
| Food Intake | |||
| Smartphone applications (apps) | Users add individual foods to their record by searching a database containing verified and user-submitted entries, selecting a matching item, and entering the portion consumed. The app summarizes total calorie intake (by food, meal, and day), calories remaining relative to a target goal, and additional nutrition information. Most apps include barcode scanners that automatically identify and add packaged items to the food record. Users can tag commonly eaten foods and save recipes and for future use. Popular apps include MyFitnessPal, Lose It!, and MyPlate. | Accuracy of calorie estimates using apps is similar to that obtained with traditional calorie-counting books and paper records.1,2 |
|
| Bite counter | A device with a microelectromechanical gyroscope is worn on the wrist to count bites of food by detecting “wrist roll” motion when the hand is lifted from the table to the mouth. Bite count and demographic information are used to estimate caloric intake; information about the specific foods consumed is not needed. Wearable sensors that detect chewing and swallowing are also under development.3 | In one study, the bite counter captured a mean of 81.2% of bites taken.4 In a free living situation, correlation with calorie intake estimated via self-administered dietary recall was moderate (r=.53).5 |
|
| Digital food photography | Smartphones are used to capture images of food servings and food waste. A standardized object, such as a card, is sometimes included as a reference. Images are wirelessly transmitted to a server and compared to standardized portions to produce a calorie estimate. Bitesnap is an early commercial version (that requires some user input). | When calculated using rater-assisted estimates, portion size and energy estimates correlate highly (rs>.90) with weighed portions.6 Fully automated methods are still in development. |
|
| Physical Activity | |||
| Wearable sensors | These devices are worn on the wrist or upper arm (or clipped to the torso) and use tri-axial or multisensor accelerometers to measure body movement. They provide estimates of daily step counts, total energy expenditure, and time spent engaging in moderate to vigorous physical activity. Many devices also monitor sleep quality and duration. Popular brands include Fitbit, Garmin, and Samsung (as well as most smart phones and smart watches). | A meta-analysis found that most sensors provide reliable estimates of step counts (CCs > .80). The accuracy of energy expenditure estimates varied widely (r=.32 to .96); most devices underestimated expenditure.7 |
|
| Weight | |||
| Smart scales | Smart scales wirelessly transmit weight measurements to a server. Coordinated online or app-based platforms provide a visual summary of weight change over time. Some scales provide measures of body composition and resting metabolic rate. Popular brands include Fitbit, RENPHO, Withings, and GreaterGoods. | Weight measurements are presumed to have similar accuracy to other home scales. |
|
| Situational Factors | |||
| Ecological momentary assessment (EMA) or intervention (EMI) | EMA can be used to capture other features of the internal or external environment that may predict lapses from health behavior goals. An app or web link is used to record momentary information about thoughts, emotions, and other situational factors, as well as about the target behavior (e.g., overeating). Participants can be prompted by the EMA to enter data or enter information unprompted to report a target episode or situation. Machine learning can be used to identify individual factors that predict the target behavior. | Several studies have used EMA to evaluate behavior patterns.8 Further research is needed to assess the efficacy of EMIs that employ this approach to prompt an individual to modify their behavior to reduce lapse risk. |
|
Researchers have hypothesized that these advantages would improve adherence to self-monitoring that, in turn, would enhance weight loss. In RCTs, participants assigned to monitor food intake with smartphone apps completed significantly more food records than those instructed to keep paper diaries (Burke et al., 2012; Spring et al., 2017). None of these studies, however, found significant differences in weight loss with these different approaches. Another study compared the accuracy of calorie intake recorded with paper-, computer-, and smartphone-based methods (Hutchesson et al., 2015). The three approaches resulted in similar levels of underreporting (by 456 to 510 kcal/day), relative to participants’ total daily energy expenditure, as determined by indirect calorimetry. However, 89% of participants rated the paper records as the least preferred tracking method. Taken together, these findings suggest that, at present, greater acceptability, convenience, and adherence are the principal benefits of using apps.
Web and smartphone apps are beneficial but still require participants to input information. By contrast, wearable devices, such as watches and armbands that track physical activity, fully automate the recording process. An early pilot study found that participants who received an armband tracker lost significantly more weight than those assigned to conventional activity recording (Shuger et al., 2011). However, a larger, subsequent RCT found that participants provided a wearable tracker lost significantly less weight at 24 months than those instructed to track their physical activity manually on a website (3.5 and 5.9 kg, respectively) (Jakicic et al., 2016). The groups did not differ significantly in their recorded physical activity or dietary intake.
Wearable bite counters, calorie-tracking utensils, and related devices are being developed that automatically track food intake. Most have yet to be adequately tested. One study randomly assigned participants to monitor food intake with either a mobile app or a wearable bite counter (Turner-McGrievy et al., 2017). The app produced significantly greater weight loss than the bite counter (6.8 vs 3.0 kg, respectively). Thus, while current wearable devices clearly reduce the effort of self-monitoring, further research is needed to improve the accuracy of some instruments and to determine the most effective means of using devices to support behavior change.
Weight Loss Maintenance: Recommendations and Long-term Results
Without further intervention, lifestyle modification participants typically regain one-third of their lost weight in the year following treatment, with continued weight gain thereafter (Butryn et al., 2011). Numerous factors contribute to weight regain, including individuals’ renewed vulnerability, in the absence of support, to an environment that discourages adherence to eating and activity habits learned in treatment. Moreover, participants have to work just as hard to maintain their weight loss as they did to achieve it; most report that maintaining a weight loss is far less rewarding than losing weight (Butryn et al., 2011). Previously described reductions in resting metabolic rate and energy expenditure during physical activity decrease patients’ allowable calorie intake more than would be expected based on their reduced body weight alone (Rosenbaum & Leibel, 2016). In addition, changes in appetite-related hormones, including ghrelin and leptin, may result in increased hunger and cravings that undermine dietary adherence (Schwartz et al, 2017).
Behavioral Weight-Loss-Maintenance Interventions
The Obesity Guidelines advise individuals who have lost weight to participate in a long-term -- at least 1 year -- weight loss maintenance program (Table 1). This intervention should provide monthly or more frequent contact with an interventionist. Perri and colleagues (2008) have repeatedly demonstrated the benefits of such contact. In addition to consuming a reduced-calorie diet, appropriate for a reduced body weight, individuals should engage in high levels of physical activity (200–300 minutes/week). Multiple trials have shown that higher levels of physical activity are associated with larger long-term weight losses (Jakicic et al., 2018; Jeffery, Wing, Sherwood, & Tate, 2003). Increased physical activity is likely necessary to compensate for the increased energy efficiency that accompanies weight loss (Rosenbaum & Leibel 2016). Regular self-monitoring of body weight is also critical. The National Weight Control Registry – which follows individuals who have maintained a loss ≥13.6 kg (30 lb) for at least 1 year – found that frequent (weekly or more) weighing was associated with better maintenance of lost weight (Wing & Phelan, 2005).
The Obesity Guidelines’ review found that comprehensive maintenance programs significantly reduced weight regain compared to usual care. Approximately 35–60% of persons who received weight-loss maintenance sustained a loss ≥5% of initial weight for more than 2 years of follow-up (Jensen et al., 2014). The Look AHEAD study provides an excellent example of a weight maintenance intervention. ILI participants received twice-monthly counseling contacts (one in-person meeting and one phone call/email per month) during years 2–4. In addition, they had optional monthly group sessions and periodic refresher courses, designed to reverse weight gain. At the end of year 4, ILI participants lost 4.7% of initial weight, compared with 1.0% for the DSE group; 45% and 26% of participants, respectively, maintained a loss ≥5% (Wadden et al., 2011). Black and Hispanic participants achieved long-term weight losses comparable to their white counterparts, replicating findings from the DPP (Knowler et al., 2002).
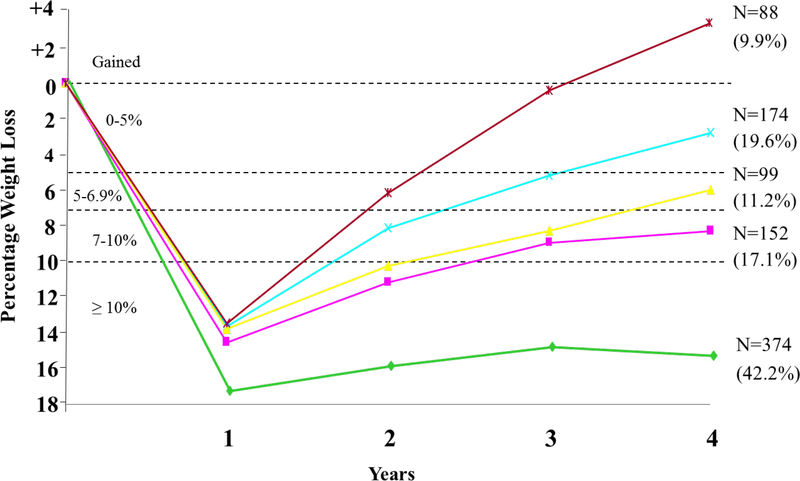
The large sample of ILI participants at year 4 (N = 2419, 94.1% retention) facilitated the analysis of subsets of individuals with different degrees of long-term weight loss success. Figure 1 presents findings for 877 participants (36.3% of the original sample) who lost ≥10% of initial weight at the end the first year. At year 4, 42% of this sub-sample (of 877 participants) maintained a weight loss ≥10%; another 28.5% of these participants sustained a loss of 5–9.9%. These findings, with those of the National Weight Control Registry, underscore that a significant minority of persons can achieve long-term, clinically meaningful weight loss, despite the environmental and metabolic barriers that confront them. A pressing challenge is to identify the biological and behavioral characteristics that make these participants successful, while also developing new therapies for the significant percentage of persons who regain their lost weight.
Figure 1.
The figure presents weight loss trajectories over 4 years in the 887 participants in the intensive lifestyle intervention (ILI) who, at year 1, lost ≥ 10% of initial weight. The figure shows the number of participants who, at year 4, maintained a loss of 10% or more of initial weight (N = 374), of 5.0–9.9% (N = 251), or of 0–4.9% (N = 174) or who gained above their baseline weight (N = 88). The percentages shown in parentheses are based on the sample size for the subgroup. Thus, the 374 of 887 participants who maintained a 10% loss at year 4 comprised 42.2% of this subgroup of participants. The figure is reprinted with permission from Wadden et al., 2011.
Combining Behavioral Treatment with Pharmacotherapy for Obesity
Pharmacotherapy offers another option for facilitating the maintenance of lost weight. Five medications currently are approved by the Food and Drug Administration (FDA) for chronic weight management when prescribed to appropriate individuals (i.e., BMI ≥30 kg/m2 or ≥27 kg/m2 with an obesity co-morbidity). They include orlistat, lorcaserin, and liraglutide, as well as two combination therapies, phentermine plus topiramate and naltrexone plus bupropion (Apovian et al, 2015; Heymsfield & Wadden, 2017). The medications increase mean 1-year weight losses, as compared with placebo, by approximately 3 to 8 kg; liraglutide and the combination therapies have the largest effects. Adding weight loss medication to high-intensity lifestyle modification substantially increases initial weight reduction, compared with lifestyle counseling alone, satisfying many patients’ desires for larger losses (Wadden et al., 2005; Wadden et al., 2019). Medications, however, could play an even more important role in obesity management by facilitating the maintenance of lost weight, potentially by counteracting previously described unfavorable changes in appetite-related hormones and energy expenditure that occur with weight loss (Apovian et al., 2015; Rosenbaum & Leibel, 2016). From this perspective, patients would take anti-obesity medications chronically (i.e., indefinitely), as approved by the FDA, to maintain their reduced body weight, just as medications to control hypertension, diabetes, and hyperlipidemia also are taken chronically. Current medications increase long-term weight loss (>1 year) by approximately 3 to 6 kg, compared to lifestyle modification alone (Apovian et al. 2015).
Health Benefits of Lifestyle Modification
Physical Health
The most impressive evidence of the health benefits of moderate (5–10%) weight loss comes from lifestyle interventions designed to prevent the progression from impaired glucose tolerance (i.e., prediabetes) to type 2 diabetes. In the previously described DPP, at 2.8 years, lifestyle intervention participants lost a mean of 5.6 kg, which was associated with a 58% reduction in the risk of developing type 2 diabetes compared with placebo, and a 31% reduction compared with metformin (Knowler et al., 2002). Multiple studies have replicated these findings, with several showing benefit with weight losses as little as 2–3 kg at 3–4 years (Gregg & Flores, 2018). A 10-year follow-up of the DPP found that lifestyle participants, despite regaining to within 2 kg of their baseline weight, continued to have a 34% reduced risk of developing type 2 diabetes compared with placebo (Knowler et al., 2009). These are critically important findings, which indicate that weight loss, even when followed by partial or full weight regain, improves long-term glycemic control and potentially other health outcomes (Gregg & Flores, 2018; Wing et al., 2016). These results may allay discouragement or shame about weight loss and regain.
Moderate weight loss induces clinically meaningful improvements in other cardiovascular disease (CVD) risk factors, including blood pressure, triglycerides, and sleep apnea (Gregg & Flores, 2018). Improvements are generally dose dependent, with greater losses (>10%) producing greater benefits (Wing et al., 2011). Thus, patients should not be discouraged from losing large amounts of weight if they can. Moderate weight loss also may improve nonalcoholic fatty liver disease, osteoarthritis, asthma/reactive airway disease, and conditions in women that include polycystic ovary syndrome, urinary stress incontinence, and infertility (Gregg & Flores, 2018). There also is increasing interest in the relationship between obesity and weight-related cancers, including breast, colon, rectal, and esophageal. Randomized trials currently are evaluating whether weight reduction reduces the risk of breast cancer reoccurrence (Ligibel et al., 2017).
At present, there is no evidence (including one negative finding) from RCTs that intentional weight loss with lifestyle modification reduces CVD mortality, despite improving individual CVD risk factors (Look AHEAD Research Group, 2013). By contrast, numerous medications for hypertension, hyperlipidemia, and type 2 diabetes significantly reduce CVD mortality (Wadden, Bakizada, Wadden, & Alamuddin, 2018). Thus, with moderate or greater levels of disease severity, weight reduction is recommended in combination with well-established medical therapies for CVD and other conditions, not in lieu of them. Medications potentially can be tapered as weight loss and improvements in health occur.
Quality of Life and Mental Health
When quality of life is measured by obesity-specific inventories, such as the Impact of Weight on Quality of Life (IWQOL) Lite scale (Kolotkin et al., 2009), intentional weight loss is associated with marked improvements in physical function and self-esteem, and smaller but still potentially clinically meaningful improvements in sexual life, work, and public distress about weight. Greater weight loss is generally associated with greater improvement in psychosocial function (Kolotkin et al., 2009). Lifestyle modification also is associated with small to medium-sized improvements in body image dissatisfaction (Chao, 2015; Sarwer, Thompson, & Cash, 2005). These improvements appear to be more strongly associated with perceived physical changes than with the amount of weight lost.
Moderate weight loss also is associated with reduced symptoms of depression. In most studies, participants were not depressed at baseline and, thus, mean scores on screening inventories declined by only a few points, with larger, potentially clinically meaningful improvements in participants who had moderate or greater depression at the start of treatment (Faulconbridge et al., 2018). Caution suggests treating clinically significant depression in individuals with obesity in the same manner that it would be addressed in persons of average weight – with cognitive behavior therapy for depression (and possibly anti-depressant medication) (Tronieri & Wadden, 2018). Similarly, evidence-based psychological therapies are recommended to address anxiety disorders, severe body image concerns, eating disorders, and other psychiatric conditions encountered in patients with obesity. Weight reduction may ameliorate these conditions, but it generally has not been established as a first-line intervention for them.
Evolving Methods of Delivering Lifestyle Modification
The Challenge of Dissemination
The majority of the findings described above are from large, funded trials in which treatment was delivered in person by experienced providers working in academic medical centers. Providers in other settings often lack the time and training to deliver intensive lifestyle interventions. This treatment also can be time-consuming and costly for participants, and many individuals, including those in rural communities and economically disadvantaged urban areas, do not have access to care. Identifying methods to disseminate effective lifestyle modification to the millions of people who would benefit from it is thus a key priority. These efforts have evaluated delivering treatment in different settings and using new modalities.
Primary Care Practice
Primary care clinicians have been encouraged by the USPSTF and the Centers for Medicare and Medicaid Services (CMS) to offer intensive behavioral treatment (IBT) to all patients with obesity, either directly or by referral. In 2011, CMS began reimbursing IBT for beneficiaries treated in primary care settings (Centers for Medicare & Medicaid Services, 2011). Treatment provides 14–15 brief (15 minute) counseling visits over 6 months; individuals who lose 3 kg are eligible for six additional monthly visits. This was a landmark coverage decision by CMS, which may well stimulate other insurers to increase their provision of behavioral weight loss interventions. A major limitation, however, of CMS’ IBT benefit is the requirement that counseling be delivered by a physician, nurse practitioner, nurse specialist, or physician assistant, few of whom have the time or training to provide such care. The very low utilization of the CMS benefit (<1%) likely reflects these barriers. We recommend that IBT be delivered in primary care settings by auxiliary health professionals, including R.D.s and trained health counselors, who have more experience in providing such care and can do so at a lower cost than physicians and nurses. Psychologists and social workers also potentially could deliver IBT in this setting; we advise all interventionists to use a structured behavioral approach (Wadden, Tsai, & Tronieri, in press). Primary care physicians can play a critical role in weight management by evaluating and treating weight-related health conditions, by non-judgmentally discussing weight and weigh management with patients, and by helping them find effective weight loss programs.
Community Programs and Lay Interventionists
Researchers have adapted the DPP protocol for delivery in community settings by using group rather than individual treatment sessions and making other changes to reduce costs (West, Krukowski, & Larsen, 2018). Ackermann et al. (2008), for example, studied a 16-session DPP-based group intervention delivered by YMCA staff. Intervention participants lost 6.0% of initial weight at 6 and 12 months, compared with 2.0% for a control group that received brief advice. The cost of delivering the YMCA intervention was estimated to be $275–325 per person, compared to $1400 in the original DPP study. Based on these and other positive results, this adapted DPP program is now offered at over 200 YMCAs nationwide to individuals with overweight/obesity who have prediabetes (West, Krubowski, & Larsen, 2018). DPP-based interventions also have been delivered effectively by laypersons in other community settings, as described by West et al. (2018). A meta-analysis of community-delivered DPP programs showed a mean loss of 4% of initial weight at 12 months (Ali, Echouffo-Tcheugui, & Williamson, 2012). To improve efficacy, lay interventionists, as well as community weight loss professionals (registered dietitians, nurses), may need additional training in behavior therapy. Without this training, it is common to use a psychoeducational approach -- telling patients what to do -- without instructing them in how to change eating and activity habits.
Commercial Programs
The Obesity Guidelines indicate that commercial programs that have published peer-reviewed evidence of their safety and efficacy are another option for providing high-intensity lifestyle modification (Jensen et al., 2014). The two best studied programs are Weight Watchers (now named WW) and Jenny Craig. WW provides a points-based dietary plan of conventional foods with support from in-person meetings or online sessions. A 2015 review identified nine studies that supported the program’s efficacy, with mean weight losses ranging from 3.1 to 7.3% of initial weight at 6 to 12 months (Gudzune & Clark, 2018). Jenny Craig provides prepackaged meals along with individual in-person or phone-based counseling sessions. In three studies, program participants achieved mean weight losses of 7.1 to10.9% of initial weight at 12 months (Gudzune & Clark, 2018). Costs per month are approximately $40 for WW and $570 for Jenny Craig (including the cost of food for the latter program). Several additional commercial meal replacement programs, such as Optifast, have demonstrated their safety and efficacy, although program costs can exceed $650 per month due to charges for food, medical monitoring, and counseling (Gudzune & Clark, 2018). Thus, prospective consumers should consider both the potential benefits and financial costs of enrolling in a particular commercial program.
Telephone-Delivered Weight Management
Telephone-delivered programs can be used to extend the reach of lifestyle interventions to persons in rural areas, and they are likely to be more convenient and less expensive for all individuals. Several studies have shown that their efficacy is comparable to in-person treatment. For example, Donnelly et al. (2007) provided participants with a 26-week lifestyle modification program (which included a 12-week, 1200–1500 kcal/day portion-controlled diet) delivered via in-person group visits or group conference calls. Median weight losses were similar for the two delivery methods at week 26 (12.5 and 12.8 kg, respectively). Perri et al. (2008) showed that telephone counseling is also effective for facilitating weight loss maintenance. Women from a rural community who had lost an average of 10.0 kg in an initial 6-month intervention were assigned to a newsletter control group or to either telephone or in-person delivery of twice monthly maintenance sessions. One year after randomization, both the phone and in-person groups had regained an average of 1.2 kg, compared with 3.7 kg for the control group.
Digitally-Delivered Weight Management Interventions
Technology-based delivery methods, often referred to as eHealth (electronic health) or mHealth (mobile health), have the greatest potential to provide convenient, affordable weight management. Most comprehensive digitally-delivered interventions include features that facilitate goal setting and self-monitoring, as well as provide feedback on weight, diet, and/or physical activity. Specific intervention components may vary considerably (Schippers, Adam, Smolenski, Wong, & de Wit, 2017). Systematic reviews have identified the provision of personalized feedback as a key feature of effective digital interventions (Jensen et al., 2014; Schippers et al., 2017). Increasingly, these programs rely on automated, personalized feedback, generated from algorithms that analyze participants’ self-monitoring data (e.g., Tate, Jackvony, & Wing, 2006).
Internet-based Interventions
Early digital studies sought to determine whether the internet could be used to increase the reach of comprehensive behavioral interventions. Harvey-Berino et al (2010) were among the first to test the efficacy of the same group lifestyle intervention delivered: 1) in-person; 2) by internet (including online content, self-monitoring tools, and weekly chat groups); or 3) by a hybrid format (the internet program combined with monthly in-person meetings). All participants received 24 weekly sessions, at the end of which time weight losses for the three groups were 8.0, 5.5, and 6.0 kg, respectively (with in-person treatment superior to the other two groups). These findings, with others, suggest that digitally-delivered interventions typically produce mean short-term losses that are at least 20–35% smaller than those achieved with in-person counseling (Schippers et al., 2017). The smaller losses may be attributable to reduced feedback and accountability (e.g., no weekly weigh-in at clinic). However, differences in weight loss between delivery methods decrease over time (with weight regain), and internet programs are cheaper and have greater potential reach than in-person care.
Mobile and Smartphone Interventions
The steady increase in cellphone ownership over the past several decades, with the more recent proliferation of smartphones, has facilitated the development of increasingly sophisticated mobile interventions. Text messaging can be used to disseminate mobile interventions to nearly any cellphone user. Most studies that have evaluated text-based lifestyle interventions have achieved modest weight losses, consistent with their delivery of abbreviated program content. For example, Steinberg et al (2013a) had participants report, using text, on their progress in meeting behavioral goals. They received fully-automated brief feedback messages, in addition to texts describing weight loss strategies. The intervention group lost 1.3 kg, compared to a 1.1 kg mean gain in the education control group.
Smartphone apps have the capacity to deliver more comprehensive intervention content, including features similar to those of internet programs. However, the majority of commercially available weight loss apps include only a small percentage of the behavioral strategies featured in most lifestyle programs (Pagoto, Schneider, Jojic, DeBiasse, & Mann, 2013), and most apps do not provide tailored feedback. Such programs are not likely to induce significant weight loss for most individuals. For example, the highly popular app, MyFitnessPal, which helps users set a calorie goal and track food intake, produced a mean loss of only 0.03 kg in 6 months in primary care patients, compared with a gain of 0.3 kg in controls (Laing et al., 2014). The frequency of logins declined sharply after the first month (to close to 0), underscoring the problem of maintaining user engagement with digitally-delivered interventions.
Svetkey et al. (2015) developed a comprehensive app that provided individualized behavioral goals, self-monitoring tools, challenge games, and social support. Participants were assigned to: 1) the app intervention; 2) an education control group; or 3) 6 weekly, in-person group sessions, followed by monthly phone contacts with a coach. Weight loss in the app group did not differ from that of controls at month 12 (1.5 vs. 2.3 kg) or month 24 (1.0 vs. 1.4 kg). The coaching group had significantly larger losses than the app group at month 12 (3.6 kg) but not at month 24 (2.5 kg). The authors hypothesized that, despite continued twice weekly use over 2 years, engagement with the app was not sufficiently frequent to produce meaningful weight loss.
Enhanced Digital Interventions
More recent efforts have sought to improve weight loss with digital delivery by adding enhancements to increase user engagement. One such approach has been to increase the interactive quality of the digital program. Thomas, Leahey, and Wing (2015) tested the efficacy of a 12-week online program that provided interactive lessons, rather than static materials, as used in most digital programs. The enhanced lessons incorporated videos, quizzes, and practical exercises. The program also provided self-monitoring tools and fully automated weekly feedback based on participants’ recorded data. At 6 months, intervention participants lost 5.4 kg, compared to 1.3 kg loss for control participants who received static lessons about the benefits of weight loss (without behavioral strategies). Other efforts to increase interactivity have incorporated lifestyle programs into social media platforms, virtual reality, and online games.
Another approach to improving digital interventions has been to pair them with automated self-monitoring devices and enhanced user feedback (Table 2). Steinberg et al. (2013b) evaluated the efficacy of a 6-month, e-mail-delivered intervention based on the DPP that encouraged daily self-weighing. Participants were provided with a smart scale and feedback (based on an algorithm) on their weighing frequency and achievement of weight loss goals. The intervention group lost 6.6% of weight at 6 months, compared with 0.4% for waitlist participants who received a smart scale but were instructed not to change their self-weighing habits. A smartphone app developed by Martin et al. (2015) provided participants with weight loss targets and highly personalized feedback, in addition to app-based lesson materials. Participants also were given activity monitors and smart scales, and the app delivered graphic feedback depicting individuals’ physical activity and weight loss, compared to expected targets (calculated based on their starting weight and calorie prescription). If participants’ weight losses fell outside of the expected range, they were prompted to select a behavioral strategy (e.g., use portion-controlled foods) to get back on track. They also received at least weekly feedback on their progress, delivered by a trained interventionist, by phone, email, or app. Intervention participants in this12-week pilot study lost 9.4 kg compared to 0.6 kg in the control group.
Other researchers have combined enhanced digital interventions with low-intensity provider contact and tested whether these combined interventions benefit socioeconomically disadvantaged patients, who have suboptimal weight loss in most programs. In a recent study, primary care patients were provided with monthly calls, a smart scale, and an app integrated with their electronic health record that offered behavior change goals, tools for self-monitoring, and personalized feedback (Bennett et al., 2018). Engagement rates were high, and intervention participants lost 4.0 kg at 1 year, compared to 0.1 kg in the usual care group.
Taken together, these studies demonstrate that it is feasible to produce clinically meaningful weight loss with a digital format. However, we note that we have highlighted some of the strongest results achieved with digitally-delivered lifestyle interventions. The average losses produced with these interventions are only 2–3 kg greater than those of attention or wait-list control groups (Schippers et al., 2017). Further research is needed to determine which behavioral strategies and enhanced features are essential for effective digitally-delivered interventions. The optimal balance of “touch” versus “tech” also must be studied. Innovative methods are needed for providing supportive accountability in the digital sphere. The presence of an interventionist facilitates self-evaluation and self-regulation, and positive feedback from a provider further enhances motivation (Mohr, Cuijpers, & Lehman, 2011; Wing, Tate, Gorin, Raynor, & Fava, 2006).
Additional research at present is investigating the potential for just-in-time adaptive interventions (JITAIs) that use machine learning to identify individual risk factors for behavioral lapses and provide intervention strategies at the times when an individual is most at risk. As technology continues to advance, new opportunities will emerge to improve individuals’ awareness of their eating and activity behaviors and to provide novel interventions that facilitate short- and long-term behavioral control (and weight loss).
Understanding Obesity-Related Phenotypes to Improve Lifestyle Modification
Individual response to behavioral treatment can vary markedly (as it does with pharmacologic and surgical interventions for obesity). A substantial minority (35–54%) of participants in intensive behavioral programs do not achieve a clinically meaningful loss ≥5% of initial weight (Jensen et al., 2014; Wadden et al., 2009). Obesity-related phenotypes, consisting of clusters of behavioral, psychosocial, and physiological characteristics, may facilitate or limit response to lifestyle modification. Numerous studies have attempted to examine whether specific characteristics predict weight loss. The strongest predictors are adherence-related factors and early weight loss (Stubbs et al., 2011). These process-oriented variables can inform decision-making once an individual has enrolled in an intervention, but they are not useful for initial treatment selection and do not explain why some individuals have early success.
The Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) initiative was established to coordinate the evaluation of weight loss predictors, with the goal of better understanding weight loss variability in order to inform the development of tailored interventions (MacLean et al., 2018). Expert panels identified a set of core constructs in each of four domains: 1) behavioral (e.g., physical activity, self-weighing, sleep); 2) biological (e.g., genetics, metabolic function, energy homeostasis); 3) environmental (e.g., food availability, characteristics of social network); and 4) psychosocial (e.g., binge eating, affect, executive function). The panels also provided suggestions for how to measure each construct, and a list is available online (https://www.gem-measures.org/workspaces/ADOPT). The goal of ADOPT is to promote the inclusion of uniform measures of potential weight loss predictors in all clinical trials, facilitating the accumulation of large data sets to identify the multiple pathways that affect weight loss and maintenance. Additional studies that use neuroimaging or assess the role of other factors (such as the gut microbiome) in predicting weight loss also are likely to make important contributions.
Efforts to explain the heterogeneity in weight loss constitute an important first step towards the development of precision-medicine approaches to improve treatment outcomes. An essential complement to this research is the identification of treatment techniques that address specific barriers to short- and long-term success. For example, techniques from acceptance and commitment therapy (ACT), when combined with behavioral treatment, may improve weight loss for individuals with higher levels of depression and disinhibited eating (Forman et al., 2013). In addition to better targeting individual behavioral and psychosocial factors, multifactorial interventions likely will be needed, including behavioral and pharmacologic therapies that address the biological adaptations precipitated by weight loss.
Conclusion
High-intensity lifestyle modification programs can help individuals with overweight and obesity lose 5–10% of initial weight to achieve clinically meaningful improvements in multiple health outcomes. The two most pressing needs in this field remain increasing individuals’ access to lifestyle modification, most likely through digital platforms, and improving the long-term maintenance of lost weight. These goals can only be met with accompanying improvements in the reimbursement of lifestyle modification for obesity, which is supposed to be a covered benefit under the Affordable Care Act but has not been widely embraced (Downey & Kyle, 2018).
This review also has revealed that intensive lifestyle interventions for obesity focus principally on the individual, whom it exhorts to adopt exemplary eating and physical activity behaviors. Such changes are difficult to enact and particularly to sustain in the face of daily interactions with an environment that often encourages exactly the opposite behaviors. This is not a fair fight for persons with obesity, particularly for individuals who live in economically disadvantaged neighborhoods that offer neither affordable fresh foods nor safe settings for physical activity. For lifestyle modification approaches to be successful long term, individual responsibility for behavior change must be joined with collective and institutional responsibility to create a food and activity environment that supports healthier eating and activity choices for all persons (Brownell & Horgen, 2004; Roberto et al., 2015). To this end, bold policy initiatives are needed at the community, city, state, and federal level, as well as from industry. This too is a daunting charge but one that must be addressed to prevent the development of obesity in future generations and to improve the lives of individuals already affected by this disease.
Public Significance Statement:
This review describes lifestyle modification approaches for overweight and obesity. Such programs provide instruction in cognitive-behavioral strategies designed to facilitate the individual’s consumption of a satisfying, reduced-calorie diet and to increase physical activity. Treatment that offers 14 or more counseling contacts in 6 months helps individuals lose an average of 5–8% of initial weight (for example, 10–16 lb), which is associated with improvements in health and quality of life.
Acknowledgments
Completion of this paper was supported, in part, by Mentored Patient Oriented Research Award (K23DK116935) to Jena S. Tronieri, Ph.D., from the National Institutes of Health/National Institute of Diabetes, Digestive, and Kidney Disease.
Biography



Footnotes
Disclosures: Thomas Wadden reports serving on scientific advisory boards for Novo Nordisk and WW (formerly Weight Watchers). Jena S. Tronieri serves as a consultant to Novo Nordisk. Meghan Butryn reports no conflicts of interest.
Contributor Information
Thomas A. Wadden, Perelman School of Medicine at the University of Pennsylvania, and Haverford College
Jena S. Tronieri, Perelman School of Medicine at the University of Pennsylvania
Meghan L. Butryn, Drexel University
References
- Ackermann RT, Finch EA, Brizendine E, Zhou H, & Marrero DG (2008). Translating the diabetes prevention program into the community. The DEPLOY pilot study. American Journal of Preventive Medicine, 35(4), 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali MK, Echouffo-Tcheugui J, & Williamson DF (2012). How effective were lifestyle interventions in real-world settings that were modeled on the diabetes prevention program? Health Affairs (Project Hope), 31(1), 67–75. [DOI] [PubMed] [Google Scholar]
- Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, … Endocrine Society. (2015). Pharmacological management of obesity: an endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology & Metabolism, 100(2), 342–362. [DOI] [PubMed] [Google Scholar]
- Barry VW, Baruth M, Beets MW, Durstine JL, Liu J, & Blair SN (2014). Fitness vs. fatness on all-cause mortality: A meta-analysis. Progress in Cardiovascular Diseases, 56(4), 382–390. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Steinberg D, Askew S, Levine E, Foley P, Batch BC, … DeVries A (2018). Effectiveness of an app and provider counseling for obesity treatment in primary care. American Journal of Preventive Medicine, 55(6), 777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD, & Horgen KB (2004). Food fight: The inside story of the food industry, America’s obesity crisis, and what we can do about it. New York: McGraw-Hill. [Google Scholar]
- Burke LE, Styn MA, Sereika SM, Conroy MB, Ye L, Glanz K, … Ewing LJ (2012). Using mHealth technology to enhance self-monitoring for weight loss: A randomized trial. American Journal of Preventive Medicine, 43(1), 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butryn ML, Schumacher LM, & Forman EM (2018). Alternative behavioral weight loss approaches: Acceptance and commitment therapy and motivational interviewing In Wadden TA & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 508–521). New York: Guilford Press. [Google Scholar]
- Butryn ML, Webb V, & Wadden TA (2011). Behavioral treatment of obesity. Psychiatric Clinics of North America, 34(4), 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services. Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N). November 29, 2011. [Google Scholar]
- Chao HL (2015). Body image change in obese and overweight persons enrolled in weight loss intervention programs: a systematic review and meta-analysis. PloS one, 10(5), e0124036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desendorf J, Bassett DR, Raynor HA, & Coe DP (2014). Validity of the bite counter device in a controlled laboratory setting. Eating Behaviors, 15(3), 502–504. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Smith BK, Dunn L, Mayo MM, Jacobsen DJ, Stewart EE, … Sullivan DK (2007). Comparison of a phone vs clinic approach to achieve 10% weight loss. International Journal of Obesity, 31(8), 1270–1276. [DOI] [PubMed] [Google Scholar]
- Downey M, & Kyle TK (2018). Coverage of obesity treatment: costs and benefits In Wadden TA & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 425–436). New York: Guilford Press. [Google Scholar]
- Evenson KR, Goto MM, & Furberg RD (2015). Systematic review of the validity and reliability of consumer-wearable activity trackers. International Journal of Behavioral Nutrition and Physical Activity, 12(1), 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, & Ludwig DS (2011). Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: A randomized controlled trial. Diabetes Research and Clinical Practice, 92(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulconbridge LF, Driscoll CFB, Hopkins CM, Bailer Benforado B, Bishop-Gilyard C, Carvajal R, … Wadden TA (2018). Combined treatment for obesity and depression: A pilot study. Obesity, 26(7), 1144–1152. [DOI] [PubMed] [Google Scholar]
- Fontana JM, Higgins JA, Schuckers SC, Bellisle F, Pan Z, Melanson EL, … Sazonov E (2015). Energy intake estimation from counts of chews and swallows. Appetite, 85, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman E, Butryn M, Juarascio A, Bradley L, Lowe M, Herbert J, & Shaw J (2013). The mind your health project: A randomized controlled trial of an innovative behavioral treatment for obesity. Obesity, 21(6), 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EM, Schumacher LM, Crosby R, Manasse SM, Goldstein SP, Butryn ML, … Graham Thomas J (2017). Ecological momentary assessment of dietary lapses across behavioral weight loss treatment: Characteristics, predictors, and relationships with weight change. Annals of Behavioral Medicine, 51(5), 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, … Klein S (2010). Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Annals of Internal Medicine, 153(3), 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, … Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. (2016). American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. EndocrinePractice, 22(Supplement 3), 1–203. [DOI] [PubMed] [Google Scholar]
- Gregg EW, & Flores MR (2018). The impact of intentional weight loss on major morbidity and mortality In Wadden TA & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 185–207). New York: Guilford Press. [Google Scholar]
- Gudzune KA, & Clark JM (2018). Commercial weight-loss programs In Wadden TA, & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 480–491). New York: Guilford Press. [Google Scholar]
- Hall KD, Ayuketah A, Brychta R, Cai H, Cassimatis T, Chen KY, … Zhou M (2019). Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Cell Metabolism (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, & Ogden CL (2017). Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief, (288), 1–8. [PubMed] [Google Scholar]
- Harvey-Berino J, West D, Krukowski R, Prewitt E, VanBiervliet A, Ashikaga T, & Skelly J (2010). Internet delivered behavioral obesity treatment. Preventive Medicine, 51(2), 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, van Mierlo C. a. J., van der Knaap HCM, Heo M, & Frier HI (2003). Weight management using a meal replacement strategy: Meta and pooling analysis from six studies. International Journal of Obesity and Related Metabolic Disorders, 27(5), 537–549. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, & Wadden TA (2017). Mechanisms, pathophysiology, and management of obesity. New England Journal of Medicine, 376(15), 1492. [DOI] [PubMed] [Google Scholar]
- Hutchesson MJ, Rollo ME, Callister R, & Collins CE (2015). Self-monitoring of dietary intake by young women: Online food records completed on computer or smartphone are as accurate as paper-based food records but more acceptable. Journal of the Academy of Nutrition and Dietetics, 115(1), 87–94. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, … Belle SH (2016). Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. Journal of the American Medical Association, 316(11), 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakicic JM, Otto AD, Lang W, Semler L, Winters C, Polzien K, & Mohr KI (2011). The effect of physical activity on 18-month weight change in overweight adults. Obesity,19(1),100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakicic JM, Rogers RJ, Sherman SA, & Kovacs SJ (2018). Physical activity and weight management In Wadden TA & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 322–335). New York: Guilford Press. [Google Scholar]
- Jeffery RW, Wing RR, Sherwood NE, & Tate DF (2003). Physical activity and weight loss: Does prescribing higher physical activity goals improve outcome? American Journal of Clinical Nutrition, 78(4), 684–689. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, … Yanovski SZ (2014). Guidelines (2013) for managing overweight and obesity inadults. Obesity, 22(S2), S1–S410. [Google Scholar]
- Kanfer FH (1975). Self-management methods In Kanfer FH, & Goldstein AP (Eds), Helping people change (pp. 309–355). New York: Pergamon Press. [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, … Diabetes Prevention Program Research Group. (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine, 346(6), 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, … Diabetes Prevention Program Research Group. (2009). 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet, 374(9702), 1677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotkin RL, Norquist JM, Crosby RD, Suryawanshi S, Teixeira PJ, Heymsfield SB, … Nguyen AM (2009). One-year health-related quality of life outcomes in weight loss trial participants: Comparison of three measures. Health and Quality of Life Outcomes, 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing B, Mangione C, Tseng C, Leng M, Vaisberg E, Mahida M, … Bell D (2014). Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: A randomized, controlled trial. Annals of Internal Medicine, 161(10), S5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligibel JA, Barry WT, Alfano C, Hershman DL, Irwin M, Neuhouser M, … Goodwin PJ (2017). Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (alliance A011401): Study design. NPJ Breast Cancer, 3, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AHEAD Research Group, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, … Kumanyika S (2006). The Look AHEAD study: A description of the lifestyle intervention andthe evidence supporting it. Obesity, 14(5), 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look AHEAD Research Group (2013). Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New England Journal of Medicine, 369(2), 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PS, Rothman AJ, Nicastro HL, Czajkowski SM, Agurs-Collins T, Rice EL, … Loria CM (2018). The accumulating data to optimally predict obesity treatment (ADOPT) core measures project: Rationale and approach. Obesity, 26, S6–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris A, & Foster GD (2011). Dietary approaches to the treatment of obesity. Psychiatric Clinics of North America, 34(4), 813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Miller AC, Thomas DM, Champagne CM, Han H, & Church T (2015). Efficacy of SmartLoss, a smartphone-based weight loss intervention: Results from a randomized controlled trial. Obesity, 23(5), 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, & Champagne C (2014). Measuring food intake with digital photography. Journal of Human Nutrition and Dietetics, 27 Suppl 1, 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr D, Cuijpers P, & Lehman K (2011). Supportive accountability: a model for providing human support to enhance adherence to eHealth interventions. Journal of Medical Internet Research, 13(1), e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten JJ, Jones KE, Littenberg B, & Harvey-Berino J (2009). Effects of television viewing reduction on energy intake and expenditure in overweight and obese adults: a randomized controlled trial. Archives of Internal Medicine, 169(22), 2109–2115. [DOI] [PubMed] [Google Scholar]
- Pagoto SL, Schneider KL, Jojic M, DeBiasse MA, & Mann DM (2013). Weight loss using evidence-based strategies in mobile apps. American Journal of Preventive Medicine, 45(5), 576–82. [DOI] [PubMed] [Google Scholar]
- Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, … Martin AD (2008). Extended-care programs for weight management in rural communities: The treatment of obesity in underserved rural settings (TOURS) randomized trial. Archives of Internal Medicine, 168(21), 2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri MG, Limacher MC, von Castel-Roberts K, Daniels MJ, Durning PE, Janicke DM, … Martin AD (2014). Comparative effectiveness of three doses of weight-loss counseling: Two-year findings from the rural LITE trial. Obesity, 22(11), 2293–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell KE, King AC, Buchner DM, Campbell WW, DiPietro L, Erickson KI, … Whitt-Glover MC (2018). The scientific foundation for the physical activity guidelines for Americans, 2nd edition Journal of Physical Activity & Health, 17, 1–11. [DOI] [PubMed] [Google Scholar]
- Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, & Anton SD (2001). Individual versus group therapy for obesity: Effects of matching participants to their treatment preferences. Journal of Consulting and Clinical Psychology, 69(4), 717–721. [PubMed] [Google Scholar]
- Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, … Brownell KD (2015). Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. The Lancet, 385(9985), 2400–2409. [DOI] [PubMed] [Google Scholar]
- Rolls BJ (2018). The role of portion size, energy density, and variety in obesity and weight management In Wadden TA & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 93–104). New York: Guilford Press. [Google Scholar]
- Rosenbaum M, & Leibel RL (2016). Models of energy homeostasis in response to maintenance of reduced body weight. Obesity, 24(8), 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, … Williamson DA (2009). Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. New England Journal of Medicine, 360(9), 859–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwer DB, Thompson JK, & Cash TF (2005). Body image and obesity in childhood. Psychiatric Clinics, 28(1), 69–87. [DOI] [PubMed] [Google Scholar]
- Schippers M, Adam PCG, Smolenski DJ, Wong HTH, & de Wit JBF (2017). A meta-analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration: Mobile phone obesity interventions meta-analysis. Obesity Reviews, 18(4), 450–459. [DOI] [PubMed] [Google Scholar]
- Scisco JL, Muth ER, & Hoover AW (2014). Examining the utility of a bite-count–based measure of eating activity in free-living human beings. Journal of the Academy of Nutrition and Dietetics, 114(3), 464–469. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, & Leibel RL (2017). Obesity pathogenesis: An Endocrine Society scientific statement. Endocrine Reviews, 38(4), 267–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuger SL, Barry VW, Sui X, McClain A, Hand GA, Wilcox S, … Blair SN (2011). Electronic feedback in a diet- and physical activity-based lifestyle intervention for weight loss: A randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity, 8, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pellegrini CA, Pfammatter A, Duncan JM, Pictor A, McFadden HG, … Hedeker D (2017). Effects of an abbreviated obesity intervention supported by mobile technology: The ENGAGED randomized clinical trial. Obesity, 25(7), 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg DM, Levine EL, Askew S, Foley P, & Bennett GG (2013a). Daily text messaging for weight control among racial and ethnic minority women: Randomized controlled pilot study. Journal of Medical Internet Research, 15(11), e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, & Ward DS (2013b). The efficacy of a daily self-weighing weight loss intervention using smart scales and e-mail. Obesity, 21(9), 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J, Whybrow S, Teixeira P, Blundell J, Lawton C, Westenhoefer J, … Raats M (2011). Problems in identifying predictors and correlates of weight loss and maintenance: Implications for weight control therapies based on behaviour change. Obesity Reviews, 12(9), 688–708. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Batch BC, Lin P, Intille SS, Corsino L, Tyson CC, … Bennett GB (2015). Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity, 23(11), 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate DF, Jackvony EH, & Wing RR (2006). A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an internet weight loss program. Archives of Internal Medicine, 166(15), 1620–1625. [DOI] [PubMed] [Google Scholar]
- Thomas G, Leahey TM, & Wing RR (2015). An automated internet behavioral weight-loss program by physician referral: A randomized controlled trial. Diabetes Care, 38(1), 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronieri JS, & Wadden TA (2018). Behavioral assessment of patients with obesity In Wadden TA, & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 253–282). New York: Guilford Press. [Google Scholar]
- Turner-McGrievy GM, Wilcox S, Boutté A, Hutto BE, Singletary C, Muth ER, & Hoover AW (2017). The dietary intervention to enhance tracking with mobile devices (DIET mobile) study: A 6-month randomized weight loss trial. Obesity, 25(8), 1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services and U. S. Department of Agriculture. (2015). 2015–2020 Dietary Guidelines for Americans: 8th Edition. December 2015 Available at http://health.gov/dietaryguidelines/2015/guidelines/ 2015–2020.
- U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, … Wong JB (2018). Behavioral weight loss interventions to prevent obesity-related morbidity and mortality in adults: US preventive services task force recommendation statement. Journal of the American Medical Association, 320(11), 1163–1171. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Bakizada ZM, Wadden SZ, & Alamuddin N (2018). An overview of the treatment of obesity in adults In Wadden TA & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 283–308). New York: Guilford Press. [Google Scholar]
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, … Stunkard AJ (2005). Randomized trial of lifestyle modification and pharmacotherapy for obesity. New England Journal of Medicine, 353(20), 2111–2120. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, … Look AHEAD Research Group. (2011). Four-year weight losses in the look AHEAD study: Factors associated with long-term success. Obesity, 19(10), 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Tsai AG, & Tronieri JS (in press). A protocol to deliver intensive behavioral therapy (IBT) for obesity in primary care settings: The MODEL-IBT Program. Obesity, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Walsh OA, Berkowitz RI, Chao AM, Alamuddin N, Gruber K, … Tronieri JS (2019). Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity, 27(1), 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, West DS, Neiberg RH, Wing RR, Ryan DH, Johnson KC, … Vitolins MZ (2009). One-year weight losses in the look AHEAD study: Factors associated with success. Obesity, 17(4), 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DS, Krukowski RA, & Larsen CA (2018). Treatment of obesity in community settings In Wadden TA, & Bray GA (Eds.), Handbook of obesity treatment (2nd ed., pp. 492–507). New York: The Guilford Press. [Google Scholar]
- Wing RR, Espeland MA, Clark JM, Hazuda HP, Knowler WC, Pownall HJ, … Look AHEAD Research Group. (2016). Association of weight loss maintenance and weight regain on 4-year changes in CVD risk factors: The action for health in diabetes (Look AHEAD) clinical trial. Diabetes Care, 39(8), 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, … Look AHEAD Research Group. (2011). Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care, 34(7), 1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, & Phelan S (2005). Long-term weight loss maintenance. American Journal of Clinical Nutrition, 82(1), 222–225S. [DOI] [PubMed] [Google Scholar]
- Wing RR, Tate DF, Gorin AA, Raynor HA, & Fava JL (2006). A self-regulation program for maintenance of weight loss. New England Journal of Medicine, 355(15), 1563–1571. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1998). Obesity: Preventing and managing the global epidemic (Publication No. WHO/NUT/NCD/98.1). Geneva: Author. [PubMed] [Google Scholar]