Abstract
Background
Wheeze in infancy and early childhood is common and appears to be increasing though the magnitude of any increase is unclear. Most wheezing episodes in infancy are precipitated by respiratory viral infections. Treatment of very young children with wheeze remains controversial. Anti‐cholinergics are often prescribed but practice varies widely and the efficacy of this form of therapy remains the subject for debate.
Objectives
Wheeze in infancy and early childhood is common and appears to be increasing. Most wheezing episodes in infancy are a result of viral infection. Bronchodilator medications such as beta2‐agonists and anti‐cholinergic agents are often used to relieve symptoms, but patterns of use vary. The objective of this review was to assess the effects of anti‐cholinergic therapy in the treatment of wheezing infants. This is a second update of this review.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials and the reference lists of articles. We contacted researchers in the field and industry sources. Searches were current as of June 2008.
Selection criteria
Randomised trials that compared anti‐cholinergic therapy with placebo or beta2‐agonists in wheezing children under two years of age. Children with acute bronchiolitis and chronic lung disease were excluded.
Data collection and analysis
Eligibility for inclusion and quality of trials were assessed independently by two reviewers.
Main results
Six trials involving 321 infants in three different settings were included. Compared with beta2‐agonist alone, the combination of ipratropium bromide and beta2‐agonist was associated with a reduced need for additional treatment, but no difference was seen in treatment response, respiratory rate or oxygen saturation improvement in the emergency department. There was no significant difference in length of hospital stay between ipratropium bromide and placebo; or between ipratropium bromide and beta2‐agonist combined compared with beta2‐agonist alone. However, combined ipratropium bromide and beta2‐agonist compared to placebo showed significantly improved clinical scores at 24 hours. Parents preferred ipratropium bromide over nebulised water or placebo for relief of their children's symptoms at home. A further updated search conducted in June 2005 did not yield any new studies.
Authors' conclusions
There is not enough evidence to support the uncritical use of anti‐cholinergic therapy for wheezing infants, although parents using it at home were able to identify benefits.
Plain language summary
Anticholinergic drugs for wheeze in children under the age of two years
Anti‐cholinergic drugs are widely used to treat infants and young toddlers with acute and recurrent wheeze though the role of these agents remain controversial. Six trials involving 321 infants in three different settings were reviewed. The review was unable to identify clear benefits in outcomes such as duration of hospitalisation or improvement in oxygenation though there were suggestions that some patients may benefit particularly in recurrently wheezy infants treated at home. Well designed studies are required to clarify the role of these agents in young children with wheeze.
Background
Wheeze in infancy and early childhood is common and appears to be increasing. It is now clear that there are a number of causes for recurrent wheeze in this age group with less than half of affected individuals continuing to wheeze beyond the age of five. In many, factors such as pre‐natal maternal smoking, appear to contribute significantly to the likelihood of recurrent wheeze while atopy can only be identified as a risk factor in a minority of patients. Furthermore it appears that these infants, unlike older children with atopic asthma, do not improve dramatically after bronchodilator therapy. The spectrum of sub‐groups of wheezing infants is only slowly being defined but includes conditions such as 'viral associated wheeze', 'atopic asthma', 'bronchiolitis' and chronic lung disease of prematurity. There is currently no agreement as to how to classify recurrent wheeze in these young children and there are no diagnostic clinical or laboratory investigations on which to base a diagnosis other than history in those with chronic lung disease of prematurity and the presence of widespread crepitation on auscultation in acute bronchiolitis.
A number of possibilities have been suggested for the generally poor response to bronchodilator therapy in young children and these include: ‐failure to deliver drugs to their site of action ‐immature receptors that are unable to respond to the drugs ‐lack of effective muscle development in the airways
A number of studies including assessment of lung function before and after bronchodilator therapy and radio labelled deposition studies have shown clearly that aerosolised drugs can be delivered to their site of action in the infant lungs, at least when the infants are relatively well and that the airway receptors and smooth muscle in the airways of the very young children are able to respond to drugs delivered in this way.
This suggests that other factors such as excessive airways secretions or fluid, mucosal oedema and airway geometry rather than bronchoconstriction maybe responsible for much of the airways obstruction in these young wheezing patients. The relative contribution made by bronchoconstriction may vary depending upon the particular sub‐group of wheezing illness.
Most wheezing episodes in infancy are induced by viral infections. They are generally self limiting and resolve with the resolution of the viral respiratory tract infection. Bronchodilators such as beta2‐agonists and anticholinergic agents are frequently administered to infants and young children with viral induced wheeze with the aim of providing symptomatic relief. It has been suggested that anti‐cholinergic drugs are useful in the treatment of some infants with wheeze in this age group and that infants generally respond better to this form of therapy than they do to beta2‐agonists. However there is relatively little evidence on which to base such assertions. Clinical practice is largely determined by local preference.
Objectives
To determine whether there is evidence to support the use of anti‐cholinergic therapy in the treatment of wheezing infants. Effects of anticholinergic therapy on severity symptoms and duration of symptoms will be assessed.
Methods
Criteria for considering studies for this review
Types of studies
To be considered for inclusion, clinical studies had to be randomised controlled clinical trials involving infants and young children under the age of two years treated with anticholinergic therapy for wheeze.
Due to the low numbers of trials and patients, studies that did not contain a placebo arm were included.
Types of participants
To be included, the participants had to be less than two years of age and experience wheeze due to reversible airways obstruction. Studies recruiting subjects with chronic lung disease or infants born prematurely were excluded.
Studies undertaken in three clinical settings have been included:‐ 1. Treatment of participants with recurrent wheeze at home 2. Treatment of acute respiratory distress associated with wheezing in the emergency department 3. Treatment of participants hospitalised for acute respiratory distress and associated wheezing.
To avoid difficulties with defining terms such as wheezy bronchitis, bronchiolitis and asthma, the term wheeze has been chosen as the unifying clinical sign defining the group of patients included in this study.
Studies in which participants had widespread crepitations on auscultation of the chest, with or without wheeze, have been excluded. Such subjects would be classified as having acute bronchiolitis by many clinicians though others would reserve this term for infants experiencing their first episode of wheeze and would exclude infants with crepitations from this category.
For this reason, airways obstruction with wheeze but without crepitations has been chosen to characterise the subjects to be included. Diagnostic labels were not used to select appropriate studies.
Types of interventions
Participants were included in the review if the studies had included patients randomised to receive anti‐cholinergic therapy or placebo or a beta2‐agonist. Treatment with anti‐cholinergic therapy was administered as mono therapy or combined with additional therapy such as a beta2‐agonist.
Types of outcome measures
1. At home ‐ effect on symptom scores ‐ parental perception
2. Accident and emergency department ‐ requirement for additional inhaled therapy ‐ effect on respiratory rates ‐ effect on oxygenation ‐ observed response
3. Hospitalised patients ‐ effects on symptom scores ‐ effect on oxygenation ‐ effect on duration of hospital stay
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and hand‐searching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(Anticholinergic* or anti‐cholinergic* or "anti cholinerg*" or cholinergic* or antagonist* or atropin* or atrovent* or oxitropium or ipratropium* or muscarinic* or Sch1000) and (child* or paediat* or pediat* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat*)
The most recent serach was conducted in June 2008.
Searching other resources
The bibliographies of trial reports were checked for additional references. Personal contact with colleagues and trialists working in the field of paediatric respiratory disease was made in order to identify other potentially relevant studies. Boehringer Ingelheim and authors of identified trials were also contacted.
Data collection and analysis
Selection of studies
All titles and abstracts identified by the search that appeared relevant were selected for full text review by ME, MK and AB. Agreement was reached by consensus. There were no disagreements.
Data extraction and management
Data was extracted from trials by two reviewers (ME, AB) and entered into Review Manager.
Assessment of risk of bias in included studies
Using the Cochrane approach to assessment of allocation concealment quality of the selected papers was assessed using the following principals:
Grade A: Adequate concealment Grade B: Uncertain Grade C: Clearly inadequate concealment
Further quality assessments were carried out at the update stage of this review by TE using the Jadad five point assessment for quality of reports of randomised clinical trials as follows.
Was the study described as randomised? (1=yes; 0=no) Was the study described as double‐blind? (1=yes; 0=no) Was there a description of the withdrawals and dropouts? (1=yes; 0=no Was the method of randomisation well described and appropriate? (1=yes; 0=no) Was the method of double‐blinding well described and appropriate? (1=yes; 0=no) Points are deducted for either inappropriate randomisation or blinding.
Unit of analysis issues
One study (Henry 1984) included in the review is a crossover trial and outcome measured from this study have been regarded separately from the other parallel studies.
Data synthesis
For the continuous variables; respiratory rate, oxygen saturation, days to discharge and symptom score a fixed effects weighted mean difference (WMD) and 95% confidence interval (95%CI) have been calculated for each study. For the dichotomous variables; improvement in symptoms, parental assessment, requirement for further inhaled therapy, excellent responders and clinical score at 24 hours a fixed effects odds ratio (OR) with 95%CI have been calculated for individual studies.
Results
Description of studies
Six studies met the criteria for inclusion in this meta analysis. The patients included in these studies were under two years of age and were treated for airways obstruction with associated wheeze. These studies included patients recruited from three settings: the emergency room (two studies), hospitalised patients (three studies) and outpatients (one study). An update search conducted in June 2008 did not identify any further studies for inclusion. For full details of the search history, please see Table 6.
1. Search history.
| Date | Search detail |
| All years (June 2005) | References identified: 354 References retrieved: 15 Studies failing to meet inlcusion criteria: 9 Studies included (total): 6 |
| June 2005‐2006 | References identified: 17 References retrieved: 1 Studies failing to meet inlcusion criteria: 1 Studies included (total): 6 |
| June 2006 ‐ June 2007 | References identified: 25 References retrieved: 0 |
The studies utilised a variety of delivery systems (nebulisers and pressurised metered dose inhalers with holding chambers and masks), doses and dosing regimes. Outcomes varied in all studies. The only data that could be aggregated was the effect of ipratropium with a beta2‐agonist vs beta2‐agonist alone on the number of days to discharge.
The two studies undertaken in an emergency department setting included a total of 130 patients. Three studies involving hospitalised patients included 168 patients. Seventy‐nine of these were from one study in which all patients were aged less than one year. One study followed outpatients treated regularly with nebulised therapy for a period of two months. A total of 23 patients were included in a three way cross over study.
Outcomes assessed in the emergency department were requirement for repeat therapy, subjective assessment of 'poor' vs 'excellent' response, change in respiratory rate and change in oxygen saturation. Comparisons were made between ipratropium plus beta2‐agonist vs beta2‐agonist plus placebo. Outcomes from the two studies could not be aggregated.
Outcomes assessed in the hospitalised patients were duration of hospitalisation in two studies and change in clinical score at 24 hours in one. Clinical score in this case includes four clinical parameters: respiratory rate, wheezing, cyanosis and accessory muscle utilization where the maximum score possible is 12, indicating severe illness. This outcome is reported in terms of the number of patients who failed to decrease their clinical score in the first 24 hours of admission.
Outcomes in the maintenance treatment study included parental preference and symptoms experienced.
Risk of bias in included studies
Henry 1984 Jadad score NA Allocation concealment B ‐ Unclear This study is described as a double blind crossover trial although the method of double blinding is not explicit. Withdrawals are reported and accounted for.
Mallol 1987b Jadad score 2 Allocation concealment B ‐ Unclear This study is described as randomised but gives no description of the randomisation method. Double blinding methods are not used. withdrawals were made at 24 hours if patients did not meet a certain criteria. This is described in the text.
Schuh 1992 Jadad score 5 Allocation concealment A ‐ Adequate This study is described as randomised and double blind. Both the method of randomisation and double blinding are described in the text and are appropriate methods. Withdrawals and dropouts are well described.
Wang 1992 Jadad score 5 Allocation concealment A ‐ Adequate This study is described as randomised and double blind. Both the method of randomisation and double blinding are described in the text and are appropriate methods. Withdrawals and dropouts are well described.
Naspitz 1992 Jadad score 3 Allocation concealment A ‐ Adequate The patients in this study are described as being 'divided at random' although no indication is given of randomisation method. Double blinding methods are adequate but withdrawals and dropouts are not indicated in the text.
Effects of interventions
HOME In one study [Henry 1984], maintenance treatment was perceived by parents to be preferable to nebulised water overall (OR 0.15; 95% CI 0.04 to 0.64) and better than placebo for immediate response to treatment (OR 0.11; 95% CI 0.02, 0.58) although there was no significant difference in the relief of symptoms as defined by diary cards (OR 0.60; 95% CI 0.19 to 1.88).
EMERGENCY DEPARTMENT The two studies undertaken in this setting did not include a placebo arm [Naspitz 1992; Schuh 1992]. The use of ipratropium bromide in the emergency department in addition to beta2‐agonist resulted in significantly fewer patients requiring further therapy 45 minutes after initial therapy compared with beta2‐agonist alone in one study (Odds Ratio 0.22; 95% Confidence Interval 0.08 to 0.61). However in the other study in this setting, there was no difference in the frequency of a perceived 'excellent' response, change in respiratory rate or improvement in oxygen saturation.
HOSPITAL A single study compared ipratropium alone vs placebo [Wang 1992]. No significant reduction in duration of hospitalisation was identified in the 31 patients (Weighted Mean Difference ‐0.4 days; 95% CI ‐1.4 to 0.61 days). The addition of ipratropium to beta2‐agonist therapy had no effect on duration of hospitalisation when compared with beta agonist alone (WMD ‐0.4 days; 95% CI ‐1.41 to 0.61 days). However, ipratropium plus beta2‐agonist therapy produced a significant decrease in the number of patients who failed to improve their clinical score at 24 hours when compared to nebulised saline used as a placebo [Mallol 1987b], OR 0.06; 95% CI 0.01 to 0.23. The quality of this study is very low (Jadad=2) and the study design does not permit any conclusions to be drawn as to the cause of this improvement which may have been due to the combination of drugs or to one or other alone. The data that are available from the third study (Mallol 1987] could not be included and further data from the authors have been requested.
Discussion
Anticholinergic agents, in particular ipratropium bromide, are widely used for the treatment of infants with airways obstruction with associated wheeze. We have identified only six randomised clinical trials that have attempted determine whether ipratropium bromide is of benefit when used to treat such patients. These trials have been undertaken in three different settings.
The protocol sought specifically to exclude trials which include patients with chronic lung disease of prematurity and those in whom a diagnosis of 'bronchiolitis' was made on the basis of crepitations within the chest. The results of this analysis apply specifically to otherwise healthy infants with recurrent wheeze.
This review indicates that the use of ipratropium bromide alone to treat infants hospitalised with wheeze does not result in a reduction in the duration of hospitalisation when compared to placebo. Similarly its use with a beta2‐agonist did not shorten hospitalisation compared with using a beta2‐agonist alone but the combination did produce a more rapid improvement in clinical score at 24 hours than placebo alone.
The addition of ipratropium bromide to a single dose of beta2‐agonist therapy resulted in a reduction in the requirement for further therapy 45 minutes later when compared to beta2‐agonist alone in one emergency room based study but no benefit was observed in a second study comparing the same two interventions. No study in this setting has compared the use of ipratropium bromide with or without beta2‐agonists with placebo in a randomised trial. These results do not suggest that the use of ipratropium in the emergency room setting confers significant benefit when treating wheezing infants. Further studies comparing its use alone and in combination with beta2‐agonists vs placebo are indicated before firm conclusions can be drawn. Such studies will need to ensure that the maximum reasonable dose of beta2‐agonist has been given first, if they are to test for an additive effect between the two agents.
In the home setting, regular ipratropium therapy for a period of two months was preferred by parents to nebulised water in a cross over study. They were able to identify an immediate improvement following nebulised ipratropium significantly more frequently than when nebulised water was administered. However, there was no significant reduction in the frequency of reported symptoms during the period of regular ipratropium therapy. The study used distilled water as the placebo and hence it is possible that the nebulised distilled water caused bronchoconstriction in the placebo group. However, a jet nebuliser was used and hence the rate of delivery of the nebulised water is likely to be low and whilst it is unlikely that induced bronchoconstriction would account for these results, this possibility cannot be discounted.
It is now recognised that there are sub‐groups of infants with wheeze though identifying these sub‐groups is currently not practical. We have excluded infants with 'bronchiolitis' as defined by the presence of crepitations on auscultation even though a number of these infants may have had audible wheeze at some point in their illness. Further clarification of the sub‐groups of wheezing infants may identify infants who do respond consistently to an intervention such as ipratropium bromide. If such sub‐group(s) represent a small proportion of the total wheezing illness in infancy, any benefit derived from anticholinergic therapy will be obscured by the lack of response in the majority of infants. This may explain why 'excellent' response to therapy is observed in some individuals despite the overall conclusion that anticholinergics do not confer significant benefit when used to treat all infants with wheeze.
These studies did not consider the role of steroid therapy in the treatment of these young patients with or without anticholinergic therapy.
The results presented here do not support the uncritical use of anticholinergic for the treatment of wheeze in infancy. Further work is required to clarify its exact role if any.
Authors' conclusions
Implications for practice.
The results of this review do not support the widespread, indiscriminate use of anticholinergic agents in the treatment of children under the age of two years with airways obstruction and wheeze. In the emergency room setting one study was able to identify a reduction in the need for additional therapy 45 minutes after ipratropium and beta agonist vs beta agonist alone but no benefits were observed in a second study in the same setting. The results from the included studies do not identify any major impact on the severity of symptoms or clinical course of the acute illness.
It is possible that infants did obtain symptomatic relief but that this was not identified by the outcomes chosen. Interestingly, parents were able to express a preference for anti‐cholinergic therapy in the home setting and this may reflect their ability to identify changes in their infant that were not identified in any of the outcome criteria chosen in the other studies.
Implications for research.
A large placebo controlled study with carefully chosen outcome criteria, may be able to identify benefits in terms of a reduction in symptom severity not identified in these studies. Alternatively it is possible that some infants do obtain benefit from such therapy but that such benefits in a minority of subjects is obscured by the lack of benefit in the majority of subjects. This issue can only be addressed when the spectrum of wheeze and airways obstruction in infancy is defined in more detail.
Feedback
Ezzo and Alderson 1999
Summary
1. Description of methods is very sparse, for example there is no discussion about methods of data analysis such as approach to heterogeneity.
2. The method of quality assessment seems to confuse blinding with allocation concealment.
3. Losses to follow up and intention‐to‐treat issues are not discussed.
4. Outcomes: it would be helpful to have some discussion/justification that oxygenation is a predictor of symptom score.
5. Conclusions: it would be more useful to the reader to say something like there is not enough evidence rather than the conclusions in the text which say "The results of this review do not support the widespread, indiscriminate use of anticholinergic agents in the treatment of children under the age of two years with airways obstruction".
Reply
1. Only one comparison ' (Ipatropium bromide + beta agonist) vs (beta agonist) in hospitalised patients ' was suitable for data pooling in this review. Two studies investigated this comparison using the outcome measure ' days to discharge'. Chi‐squared testing for heterogeneity did yield a significant result (p=0.04). However, the overall effect of the intervention on this outcome is non‐significant WMD = 0.29 95%CI (‐0.38, 0.95) as are the individual effects of the studies; Mallol 1987b WMD = 0.8 95%CI (‐0.02, 1.62): Wang 1992 WMD = ‐0.7 95%CI (‐1.84, 0.44).
2. Our intended approach to heterogeneity if it were to become an issue and an expansion of the methodology section is proposed for inclusion in the update of this review.
3. In retrospect this statement does appear to be true and although at the time when the review was carried out we were using what was established quality criteria, we do accept that our interpretation of these was slightly confused. It is intended to remedy this situation in the review update by a re‐assessment of the quality of all the papers included in the review using the Jadad blind assessment of the quality of trial reports.
4. We realise that it has become increasingly important within the context of a systematic review to discuss withdrawals and dropouts of trials in terms of how well they were described in the report. This issue too will be addressed in the review update.
5. Nowhere in the review does it state that oxygenation is a predictor of symptom score nor would we support this statement.
6. The use of anticholinergic agents is widespread. We feel that to state "there is not enough evidence to support this" would indicate that this is known to be a useful and worthwhile practice and that the supporting evidence for this has not yet emerged. Our statement that "The results of this review do not support this practice" is based solely on the available evidence and is a logical conclusion to draw from the results of the review.
Contributors
Jeanette Ezzo and Phil Alderson
What's new
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | New search has been performed | Literature search re‐run; no new studies identified |
| 30 June 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 1 April 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank Anna Bara and Steve Milan for all their support during the (over long) gestation of this review.
Data and analyses
Comparison 1. HOME SETTING: Ipratropium bromide vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in symptoms | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Parental assessment ‐ overall | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Parental assessment ‐ immediate | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
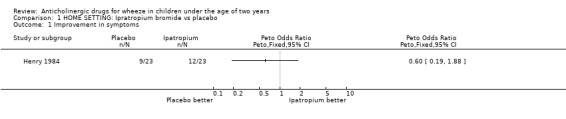
Comparison 1 HOME SETTING: Ipratropium bromide vs placebo, Outcome 1 Improvement in symptoms.
1.2. Analysis.
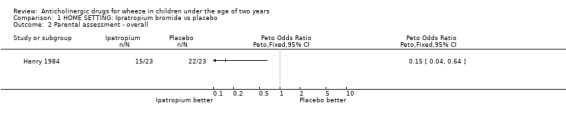
Comparison 1 HOME SETTING: Ipratropium bromide vs placebo, Outcome 2 Parental assessment ‐ overall.
1.3. Analysis.
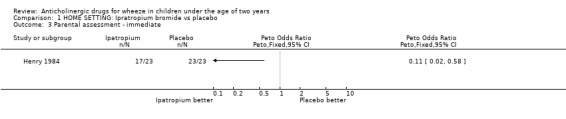
Comparison 1 HOME SETTING: Ipratropium bromide vs placebo, Outcome 3 Parental assessment ‐ immediate.
Comparison 2. EMERGENCY DEPT: Ipratropium bromide + beta agonist vs beta agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Requirement for further inhaled therapy at 45 mins | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Respiratory rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Oxygen saturation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 'Excellent' responders | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2 EMERGENCY DEPT: Ipratropium bromide + beta agonist vs beta agonist, Outcome 1 Requirement for further inhaled therapy at 45 mins.
2.2. Analysis.
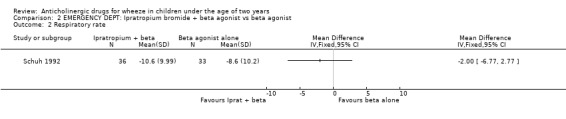
Comparison 2 EMERGENCY DEPT: Ipratropium bromide + beta agonist vs beta agonist, Outcome 2 Respiratory rate.
2.3. Analysis.

Comparison 2 EMERGENCY DEPT: Ipratropium bromide + beta agonist vs beta agonist, Outcome 3 Oxygen saturation.
2.4. Analysis.
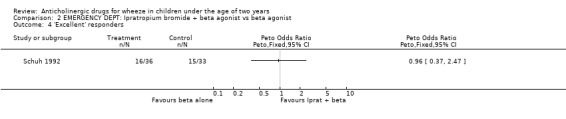
Comparison 2 EMERGENCY DEPT: Ipratropium bromide + beta agonist vs beta agonist, Outcome 4 'Excellent' responders.
Comparison 3. HOSPITALISED: Ipratropium bromide vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days to discharge | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in oxygen saturation at discharge or day 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in symptom scores at discharge or day 3 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
3.1. Analysis.
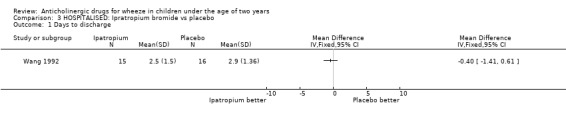
Comparison 3 HOSPITALISED: Ipratropium bromide vs placebo, Outcome 1 Days to discharge.
3.2. Analysis.
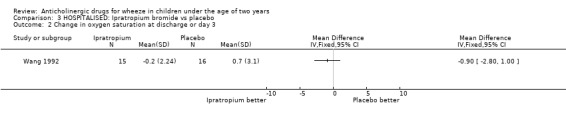
Comparison 3 HOSPITALISED: Ipratropium bromide vs placebo, Outcome 2 Change in oxygen saturation at discharge or day 3.
3.3. Analysis.
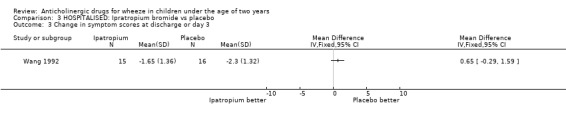
Comparison 3 HOSPITALISED: Ipratropium bromide vs placebo, Outcome 3 Change in symptom scores at discharge or day 3.
Comparison 4. HOSPITALISED: Ipratropium bromide + beta agonist vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure to decrease clinical score at 24 hours | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Days to discharge | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
4.1. Analysis.
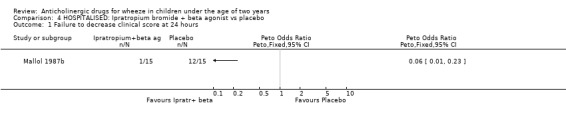
Comparison 4 HOSPITALISED: Ipratropium bromide + beta agonist vs placebo, Outcome 1 Failure to decrease clinical score at 24 hours.
4.2. Analysis.
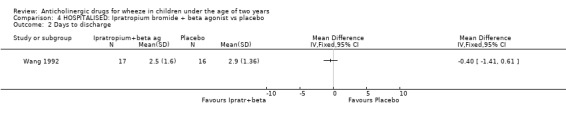
Comparison 4 HOSPITALISED: Ipratropium bromide + beta agonist vs placebo, Outcome 2 Days to discharge.
Comparison 5. HOSPITALISED: Ipratropium bromide + beta agonist vs beta agonist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days to discharge | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.38, 0.95] |
5.1. Analysis.
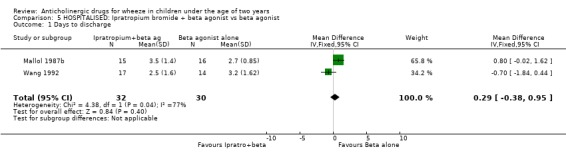
Comparison 5 HOSPITALISED: Ipratropium bromide + beta agonist vs beta agonist, Outcome 1 Days to discharge.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Henry 1984.
| Methods | Double blind controlled trial. Three way crossover study. | |
| Participants | 23 infants (19 males 4 females), aged 4 ‐ 23 months. Recurrent wheeze managed at home. 10 previously admitted to hospital with acute bronchiolitis. No premature infants | |
| Interventions | Nebulised therapy three times per day for two months 1) 20mgs sodium cromoglycate; 2) 250 mcg ipratropium bromide; 3) 2mls of water | |
| Outcomes | Clinical score (in‐house scoring system) derived from last 50 days of treatment. Parental preference. Symptom free days. Parental perception of immediate benefit Additional bronchodilator use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Mallol 1987.
| Methods | Single blinded randomised controlled trial. Parallel group study. Randomisation allocated by table of random numbers. | |
| Participants | 28 infants (average age 10 months). Hospiltalised. | |
| Interventions | Three groups of patients given: ipratropium bromide (60 micrograms), beta‐agonist (fenoterol) or placebo, delivered by an open spacer. Treatment given hourly for four hours. | |
| Outcomes | Clinical severity score (TAL), measured hourly for four hours just before treatment was given. | |
| Notes | Additional data to be obtained from author. Trial did not excude participants with bronchiolitis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mallol 1987b.
| Methods | Randomised controlled trial. Parallel group study. Random allocation of five treatments. No evidence that investigators or parents blinded to treatment arm. | |
| Participants | 79 infants, (53 males 26 females), mean age 5.9 months, range 1‐11 months. Admitted to hospital with "moderately severe" acute wheezing illness. Infants with clinical scores <6 or >10 (TAL) excluded. . | |
| Interventions | Five treatments given: 1) Nebulized fenoterol FNT (0.04ml/kg/dose, six hourly ‐ 0.5%solution) and nebulised ipratropium bromide IB (250microgram/dose, six hourly) (n=15) 2) Nebulised FNT (n=16) 3) Oral FNT + oral (prdnisolone 2mg/kg/day, 8 hourly) or IV/IM (dexamethasone 0.3mg/kg/day, 8 hourly) steroids (n=16) 4) oral FNT+ oral or IV aminophylline + oral or IV steroids (n=11) 5) nebulised 0.9% saline (n=15) | |
| Outcomes | Improvement in clinical scores (TAL) at 24 hours. Days of hospitalisation. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Naspitz 1992.
| Methods | Randomised double blind clinical controlled trial | |
| Participants | 61children (33 males 28 females), ages 8‐24 months
Patients with acute wheezing seen in the emergency department. excl. febrile, cyanosis. Wheeze alone on auscultaion |
|
| Interventions | Fenoterol (0.5% aqueous soln. 1 drop/ 3kg) + 4 drops of 0.9% saline vs fenoterol (0.5% aqueous soln. 1 drop/ 3kg) + 4 drops ipratropium bromide 0.025% soln. Single treatment using ultrasonic nebuliser (CEL‐SP). | |
| Outcomes | Clinical score at 15, 30 & 45 minutes (Ben‐Zvi). Requirement for further treatment at 45 mins. | |
| Notes | No placebo arm, none with crepitations on auscultat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schuh 1992.
| Methods | Randomised double blind controlled trial. Parallel group study. | |
| Participants | 69 infants (49 male, 20 females), aged 6 weeks to 24 months.
First episode of wheeze presenting to an emergency department. Excl. cyanosis, resp rate > 90/min prsentation between 24.00 and 08.00am., previous wheeze, mechanical ventilation after birth. recurrent aspiratiopn, concurrent cardiopulmonarty disease, previous bronchodilator therapy |
|
| Interventions | 1) Albuterol 0.15mg/kg + ipratropium bromide 250 mcg 2) Albuterol 0.15mg/kg + 0.9% saline placebo Administered via jet nebulised 1 hour apart. | |
| Outcomes | Decline in respiratory rate from baseline at 120 minutes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Wang 1992.
| Methods | Ranndomised placebo controlled double blind trial. | |
| Participants | 62 patients, aged 2 months to 24 months. Hospitalised for first time with acute wheeze. | |
| Interventions | Nebulised therapy 4 hourly. 1) Salbutamol (0.15mg/kg/dose) (sal)+ placebo (0.9% saline) 2) Placebo + ipratropium bromide (125mcgs if<6/12 ‐ 250mcg if>6/12) (IpBr) 3) Sal + IpBr 4) placebo = placebo | |
| Outcomes | Change in oxygen saturation from baseline at discharge or day 3. Change in clinical score from baseline at discharge or day 3. Duration of hospitalisation. | |
| Notes | Over 90% were treated with inhaled salbutamol prior to admission with an 'inadequate' response. Responders were discharged from the emergency room and not eligible for the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chowdhury 1995 | Participants had widespread crepitations on auscultation; i.e, they suffered from bronchiolitis. |
| Henry 1983 | Patients included had a diagnosis of 'acute bronchiolitis' ‐ clinical diagnosis included fine crepitations |
| Huls 1990 | Acute obstructive bronchitis |
| Mallol 1987c | Not a clinical trial |
| Mikawa 1997 | Study recruited children up to 16 years of age |
| Riedler 1990 | Confirmed acute airway obstruction |
| Stokes 1983 | Not a clinical trial |
| Stokes 1983b | Acute bronchiolitis with crepitations ‐ assessment of work of breathing |
| Wesley 1990 | Comparison of two methods of administration of ipratropium bromide, no placebo group. |
| Yuksel 2001 | Study aim is to assess the potential arrhythmogenic risk of albuterol and ipratropium |
Contributions of authors
Mark Everard was involved in writing the protocol, assessing studies for eligibility and quality, data extraction, imputing data into ReVMan, interpreting the analysis and writing the review. Anna Barr was invalauable in extracting data from papers entering data into ReVMan and making a major contribution to the analysis Matthew Kurrian was involved in assessment of eligibility and quality of papers. Tracy Elliott was involved in the update of the review and further quality assessment of the papers Varaidzo Mayowe was involved in the latest update of the review and further quality assessment of the papers
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
Francine Ducharme has received some travel support from Boehringer Ingelheim to attend national and international conferences. No other potential conflicts of interest known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Henry 1984 {published data only}
- Henry RL, Hiller EJ, Milner AD, Hodges IG, Stokes GM. Nebulised ipratropium bromide and sodium cromoglycate in the first two years of life. Archives of Disease in Childhood 1984;59(1):54‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mallol 1987 {published data only}
- Mallol J, Barrueto L, Girardi G, Toro O. Bronchodilator effect of fenoterol and ipratropium bromide in infants with acute wheezing: use of MDI with a spacer device. Pediatric Pulmonology 1987;3(5):352‐6. [DOI] [PubMed] [Google Scholar]
Mallol 1987b {published data only}
- Mallol J, Barrueto L, Girardi G, Munoz R, Puppo H, Ulloa V, et al. Use of nebulized bronchodilators in infants under 1 year of age: analysis of four forms of therapy. Pediatric Pulmonology 1987;3(5):298‐303. [DOI] [PubMed] [Google Scholar]
Naspitz 1992 {published data only}
- Naspitz CK, Sole D. Treatment of acute wheezing and dyspnea attacks in children under 2 years old: inhalation of fenoterol plus ipratropium bromide versus fenoterol. Journal of Asthma 1992;29(4):253‐8. [DOI] [PubMed] [Google Scholar]
Schuh 1992 {published data only}
- Schuh S, Johnson D, Canny G, Reisman J, Shields M, Kovesi T, et al. Efficacy of adding nebulized ipratropium bromide to nebulized albuterol therapy in acute bronchiolitis. Pediatrics 1992;90(6):920‐3. [PubMed] [Google Scholar]
Wang 1992 {published data only}
- Wang EE, Milner R, Allen U, Maj H. Bronchodilators for treatment of mild bronchiolitis: a factorial randomised trial. Archives of Disease in Childhood 1992;67(3):289‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chowdhury 1995 {published data only}
- Chowdhury D, Al‐Howasi M, Khalil M, Al‐Frayh AS, Chowdury S, Ramia S. The role of bronchodilators in the management of bronchiolitis: a clinical trial. Annals of Tropical Paediatrics 1995;15:77‐84. [DOI] [PubMed] [Google Scholar]
Henry 1983 {published data only}
- Henry RL, Milner AD, Stokes GM. Ineffectiveness of ipratropium bromide in acute bronchiolitis. Archives of Disease in Childhood 1983;58(11):925‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huls 1990 {published data only}
- Hüls G, Bultmann C, Scheunemann C, Lindermann H. Effects of bronchodilator agents in acute obstructive bronchitis in early childhood [Zur Wirkung bronchodilatierender Substanzen bei der akuten obstruktiven Bonchitis im frühen Kindesalter]. Pneumologie 1990;44:1188‐9. [PubMed] [Google Scholar]
Mallol 1987c {published data only}
- Mallol J, Munoz R, Puppo H, Ulloa V, Toro O, Girardi G, Barrueto L. Effects of nebulized fenoterol, associated with ipratropium or steroids, on the heart rate of infants under one year of age with acute wheezing. Pediatric Pulmonology 1987;3(2):83‐5. [DOI] [PubMed] [Google Scholar]
Mikawa 1997 {published data only}
- Mikawa H, Baba M, Nakashima M. Clinical usefulness of a leukotriene antagonist; pranlukast dry syrup on pediatric bronchial asthma in multicenter comparative double‐blind clinical study with oxatomide dry syrup. Journal of Clinical Therapeutics and Medicines 1997;13(2):423‐56. [Google Scholar]
Riedler 1990 {published data only}
- Riedler J. Inhalation bronchodilators in infancy‐‐comparative study between salbutamol and ipratropium bromide. Pneumologie 1990;44(5):777‐80. [PubMed] [Google Scholar]
Stokes 1983 {published data only}
- Stokes GM, Milner AD, Hodges IG, Henry RL. Nebulised ipratropium bromide in wheezy infants and young children. European Journal of Respiratory Diseases ‐ Supplement 1983;128(2):494‐8. [PubMed] [Google Scholar]
Stokes 1983b {published data only}
- Stokes GM, Milner AD, Hodges IG, Henry RL, Elphic MC. Nebulised therapy in acute severe bronchiolitis in infancy. Archives of Disease in Childhood 1983;58(4):279‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wesley 1990 {published data only}
- Wesley AG, Paruk F, Broughton MH, Gouws E. Ipratropium bromide delivered by metered‐dose aerosol to infant wheezers. South African Medical Journal 1991;79:536‐8. [PubMed] [Google Scholar]
Yuksel 2001 {published data only}
- Yuksel H, Coskun S, Polat M, Onag A. Lower arrythmogenic risk of low dose albuterol plus ipratropium. Indian Journal of Pediatrics 2001;68(10):945‐9. [DOI] [PubMed] [Google Scholar]


