Abstract
Background
There are at least three reasons to believe antidepressants might help in smoking cessation. Firstly, nicotine withdrawal may produce depressive symptoms or precipitate a major depressive episode and antidepressants may relieve these. Secondly, nicotine may have antidepressant effects that maintain smoking, and antidepressants may substitute for this effect. Finally, some antidepressants may have a specific effect on neural pathways (e.g. inhibiting monoamine oxidase) or receptors (e.g. blockade of nicotinic‐cholinergic receptors) underlying nicotine addiction.
Objectives
The aim of this review is to assess the effect and safety of antidepressant medications to aid long‐term smoking cessation. The medications include bupropion; doxepin; fluoxetine; imipramine; lazabemide; moclobemide; nortriptyline; paroxetine; S‐Adenosyl‐L‐Methionine (SAMe); selegiline; sertraline; St. John's wort; tryptophan; venlafaxine; and zimeledine.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register which includes reports of trials indexed in the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, and PsycINFO, and other reviews and meeting abstracts, in July 2013.
Selection criteria
We considered randomized trials comparing antidepressant medications to placebo or an alternative pharmacotherapy for smoking cessation. We also included trials comparing different doses, using pharmacotherapy to prevent relapse or re‐initiate smoking cessation or to help smokers reduce cigarette consumption. We excluded trials with less than six months follow‐up.
Data collection and analysis
We extracted data and assessed risk of bias using standard methodological procedures expected by the Cochrane Collaboration.
The main outcome measure was abstinence from smoking after at least six months follow‐up in patients smoking at baseline, expressed as a risk ratio (RR). We used the most rigorous definition of abstinence available in each trial, and biochemically validated rates if available. Where appropriate, we performed meta‐analysis using a fixed‐effect model.
Main results
Twenty‐four new trials were identified since the 2009 update, bringing the total number of included trials to 90. There were 65 trials of bupropion and ten trials of nortriptyline, with the majority at low or unclear risk of bias. There was high quality evidence that, when used as the sole pharmacotherapy, bupropion significantly increased long‐term cessation (44 trials, N = 13,728, risk ratio [RR] 1.62, 95% confidence interval [CI] 1.49 to 1.76). There was moderate quality evidence, limited by a relatively small number of trials and participants, that nortriptyline also significantly increased long‐term cessation when used as the sole pharmacotherapy (six trials, N = 975, RR 2.03, 95% CI 1.48 to 2.78). There is insufficient evidence that adding bupropion (12 trials, N = 3487, RR 1.19, 95% CI 0.94 to 1.51) or nortriptyline (4 trials, N = 1644, RR 1.21, 95% CI 0.94 to 1.55) to nicotine replacement therapy (NRT) provides an additional long‐term benefit. Based on a limited amount of data from direct comparisons, bupropion and nortriptyline appear to be equally effective and of similar efficacy to NRT (bupropion versus nortriptyline 3 trials, N = 417, RR 1.30, 95% CI 0.93 to 1.82; bupropion versus NRT 8 trials, N = 4096, RR 0.96, 95% CI 0.85 to 1.09; no direct comparisons between nortriptyline and NRT). Pooled results from four trials comparing bupropion to varenicline showed significantly lower quitting with bupropion than with varenicline (N = 1810, RR 0.68, 95% CI 0.56 to 0.83). Meta‐analyses did not detect a significant increase in the rate of serious adverse events amongst participants taking bupropion, though the confidence interval only narrowly missed statistical significance (33 trials, N = 9631, RR 1.30, 95% CI 1.00 to 1.69). There is a risk of about 1 in 1000 of seizures associated with bupropion use. Bupropion has been associated with suicide risk, but whether this is causal is unclear. Nortriptyline has the potential for serious side‐effects, but none have been seen in the few small trials for smoking cessation.
There was no evidence of a significant effect for selective serotonin reuptake inhibitors on their own (RR 0.93, 95% CI 0.71 to 1.22, N = 1594; 2 trials fluoxetine, 1 paroxetine, 1 sertraline) or as an adjunct to NRT (3 trials of fluoxetine, N = 466, RR 0.70, 95% CI 0.64 to 1.82). Significant effects were also not detected for monoamine oxidase inhibitors (RR 1.29, 95% CI 0.93 to 1.79, N = 827; 1 trial moclobemide, 5 selegiline), the atypical antidepressant venlafaxine (1 trial, N = 147, RR 1.22, 95% CI 0.64 to 2.32), the herbal therapy St John's wort (hypericum) (2 trials, N = 261, RR 0.81, 95% CI 0.26 to 2.53), or the dietary supplement SAMe (1 trial, N = 120, RR 0.70, 95% CI 0.24 to 2.07).
Authors' conclusions
The antidepressants bupropion and nortriptyline aid long‐term smoking cessation. Adverse events with either medication appear to rarely be serious or lead to stopping medication. Evidence suggests that the mode of action of bupropion and nortriptyline is independent of their antidepressant effect and that they are of similar efficacy to nicotine replacement. Evidence also suggests that bupropion is less effective than varenicline, but further research is needed to confirm this finding. Evidence suggests that neither selective serotonin reuptake inhibitors (e.g. fluoxetine) nor monoamine oxidase inhibitors aid cessation.
Plain language summary
Do medications used to treat depression help smokers who are trying to quit
Background and review questions
Some medications and supplements that have been used to treat depression (antidepressants) have been tested to see whether they also help people who are trying to stop smoking. Two antidepressants, bupropion (Zyban) and nortriptyline, are sometimes prescribed to help with quitting smoking. This review set out to determine if using antidepressants increased people's likelihood of successfully quitting smoking at six months or longer and to determine the safety of using these medications to help quit smoking.
Study characteristics
The evidence is current to July 2013. This update includes 24 new studies, and this review includes 90 studies overall. The studies included people who smoked and people who had recently quit smoking. There were 65 trials of bupropion, which is licensed for use as a smoking cessation medication under the trade name 'Zyban'. There were ten trials of nortriptyline which is a tricyclic antidepressant which is not licensed specifically for smoking cessation. We only included studies which measured long term quitting (whether or not people had quit smoking at six months or longer from the start of the study).
Key results and quality of evidence
Trials of bupropion (Zyban) for smoking cessation show high quality evidence that it increases the likelihood of a quit attempt being successful after at least six months (44 trials, over 13,000 participants). The side effects of bupropion include insomnia, dry mouth and nausea and rarely (1:1000) seizures and perhaps psychiatric problems, but the last is unclear. There is also moderate quality evidence, limited by a relatively small number of included studies and participants, that the antidepressant nortriptyline increases quit rates (six trials, 975 participants). The side effects of this medication include dry mouth, constipation, nausea, and sedation, and it can be dangerous in overdose. Selective serotonin reuptake inhibitor antidepressants (for example, fluoxetine), monoamine oxidase inhibitor antidepressants (for example, selegiline), and the antidepressant venlaxafine have not been shown to help smoking cessation, nor has the herbal therapy St John's wort, or S‐Adenosyl‐L‐Methionine (SAMe), a dietary supplement that is thought to have antidepressant properties.
Discussion and considerations
The way in which bupropion and nortriptyline might work is not fully understood. Both appear to help people quit smoking whether or not they have a history of depression, or have depressive symptoms when they stop smoking. The likelihood of quitting using bupropion or nortriptyline appears to be similar to that for nicotine replacement therapy, but the likelihood of quitting using bupropion appears to be lower than the likelihood of quitting using varenicline.
Summary of findings
Background
Whilst nicotine replacement is the most widely used pharmacotherapy for smoking cessation, some people prefer a treatment that does not use nicotine. Others require alternative treatments having failed to quit with nicotine replacement. Observations that a history of depression is found more frequently amongst smokers than nonsmokers, that cessation may precipitate depression, that nicotine may have antidepressant effects, and that antidepressants influence the neurotransmitters and receptors involved in nicotine addiction provided a rationale for the study of antidepressant medications for smoking cessation (Benowitz 2000; Kotlyar 2001).
Description of the intervention
The following medications and substances regarded as having antidepressant properties have been investigated for their effect on smoking behaviour in at least one study:
the tricyclic antidepressants (TCAs) doxepin, imipramine and nortriptyline
the monoamine oxidase inhibitors (MAOI) moclobemide, selegiline, lazabemide, and EVT302
the selective serotonin reuptake inhibitors (SSRIs) fluoxetine, paroxetine, sertraline, citalopram, and zimeledine
the atypical antidepressants bupropion, tryptophan, venlafaxine, imipramine, and doxepin
extracts of St. John's wort (Hypericum perforatum L.)
the dietary supplement S‐Adenosyl‐L‐Methionine (SAMe)
Of the antidepressants tested for smoking cessation, the most commonly used medication is bupropion. This antidepressant has both dopaminergic and adrenergic actions, and appears to be an antagonist at the nicotinic acetylcholinergic receptor (Fryer 1999). It may work by blocking nicotine effects, relieving withdrawal (Cryan 2003; West 2008), or reducing depressed mood (Lerman 2002a). It has been licensed as a prescription aid to smoking cessation in many countries. The usual dose for smoking cessation is 150 mg once a day for three days increasing to 150 mg twice a day continued for 7 to 12 weeks, and the quit attempt is generally initiated a week after starting pharmacotherapy.
Following bupropion, the second most commonly tested medication for smoking cessation is the tricyclic antidepressant nortriptyline. Its presumed mechanism of action is increased noradrenergic activity. It is sometimes prescribed when first‐line treatments have been unsuccessful, and is licensed for smoking cessation in New Zealand. The recommended regimen is 10 to 28 days of titration before the quit attempt, followed by a 12‐week dose of 75 to 100 mg daily (Cahill 2013).
No other antidepressants are currently licensed for use as smoking cessation aids, though others have been tested for possible use. It has been hypothesized that MOAIs may aid smoking cessation because they could substitute for the ability of smoking to act as a monoamine oxidase‐A (MOA) inhibitor. Inhibiting MOA increases dopamine and noradrenaline and hence it has been hypothesized that it should decrease negative affect and make quitting smoking easier. It has been hypothesised that SSRIs might be helpful because they increase serotonin which is also associated with improving negative affect. Other antidepressants including doxepin, tryptophan, and venlafaxine, and alternative therapies for depression such as St. John's wort and SAMe, may help with smoking cessation through similar biological mechanisms. Whether antidepressants work mostly due to reducing negative affect, reducing urges to smoke or withdrawal symptoms, or by acting as nicotine blockers is unclear.
The focus of this review and meta‐analysis is on trials that provide evidence for an effect of antidepressants on long‐term smoking cessation. We describe these in the Effects of interventions section. For pharmacotherapies for which there is still a lack of long‐term data, we briefly describe results from excluded short‐term trials in the Description of studies section.
Objectives
To assess the evidence for the efficacy and safety of medications with antidepressant properties in assisting long‐term smoking cessation, including: bupropion; citalopram; doxepin; fluoxetine; imipramine; lazabemide; moclobemide; nortriptyline; paroxetine; tryptophan; SAMe; selegiline; sertraline; St John's wort; venlafaxine; and zimeledine.
For each medication identified as having been used in a smoking cessation trial we evaluated whether it was more effective than placebo or an alternative treatment in achieving long‐term smoking cessation.
Methods
Criteria for considering studies for this review
Types of studies
For efficacy, we examined randomized trials comparing antidepressant with placebo or with an alternative therapeutic control, or comparing different dosages of an antidepressant, that reported six‐month or longer follow‐ups. For safety, we examined data from randomized controlled trials comparing antidepressant with placebo or no pharmacotherapy controls, and also considered observational data. Studies were included irrespective of their publication status and language of publication.
Types of participants
Current cigarette smokers, or recent quitters (for trials of relapse prevention).
Types of interventions
Treatment with any medication with antidepressant properties to aid a smoking cessation attempt or to prevent relapse, or to reduce the number of cigarettes smoked and aid subsequent cessation. Trials in which all participants received the same pharmacotherapy regimen but different behavioural support were not included.
Types of outcome measures
Efficacy was measured via a) abstinence from smoking or b) incidence of reducing cigarette consumption to 50% or less of baseline, both assessed at follow‐up at least six months from start of treatment. Safety was assessed by incidence of serious and other adverse events, and drop‐outs due to adverse events.
Search methods for identification of studies
We identified studies from the Cochrane Tobacco Addiction Group's Specialised Register. At the time of the updated search in July 2013, the Register included the results of searches of the Cochrane Central Register of Controlled trials (CENTRAL), issue 5, 2013; MEDLINE (via OVID) to update 20130607; EMBASE (via OVID) to week 201324; and PsycINFO (via OVID) to update 20130610. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and a list of other resources searched. We searched the Register for reports of studies evaluating bupropion, nortriptyline or any other pharmacotherapy generally classified as having an antidepressant effect. Search terms included relevant individual drug names or antidepressant* or antidepressive*. We checked the citation lists of these studies, recent reviews of non‐nicotine pharmacotherapy, and abstracts from the meetings of the Society for Research on Nicotine and Tobacco. See Appendix 1 for the register search strategy.
Data collection and analysis
One author (LS) conducted the searches, screened titles and abstracts for relevance, and obtained full text of reports of eligible or possibly eligible studies. For conference abstracts reporting potentially eligible studies, attempts were made to contact study investigators to obtain additional data. Papers that reported secondary analyses from eligible studies were listed as additional references under the main study identifier. Two authors (LS and TL or JHB) independently extracted study data and compared the findings. Any discrepancies were resolved by mutual consent. Where available, the following information is recorded in the Characteristics of included studies table:
Type of antidepressant
Country and setting
Recruitment method
Definition of smoker used
Participant demographics (i.e. average age, gender, average cigarettes per day)
Intervention and control description (including dose, schedule, and behavioural support common to all arms)
Outcome(s) used in meta‐analysis, including length of follow‐up, definition of abstinence, and biochemical validation of smoking cessation
Sources of funding
Assessment of risk of bias in included studies
We assessed included studies for risks of selection bias (method of random sequence generation and allocation concealment), performance and detection bias (the presence or absence of blinding), attrition bias (levels and reporting of loss to follow‐up), and any other threats to study validity.
Studies were considered at high risk of performance and detection bias where there was no blinding of participants or personnel or where there was evidence of unblinding, at unclear risk if insufficient information was available with which to judge, and at low risk if the study reported blinding of participants and personnel in detail and there was no evidence of unblinding. Studies were considered to be at low risk of attrition bias where over half of the participants were followed up at the longest follow‐up and where numbers followed up were similar across arms (difference < 20%).
Measures of treatment effect
In each study, we used the strictest available criteria to define cessation, so we used the longest reported follow‐up and extracted figures for sustained abstinence in preference to point prevalence where both were presented. In studies that used biochemical validation of cessation, only those subjects meeting the criteria for biochemically confirmed abstinence were regarded as having stopped smoking. As far as possible, we used an intention‐to‐treat analysis with people who dropped out or were lost to follow‐up treated as continuing smokers. Where subjects appeared to have been randomized but were not included in the data presented by the author we noted this in the study description (see Characteristics of included studies). Assuming that people lost to follow‐up were smokers will ensure that actual quit rates are conservative, but may not necessarily lead to conservative relative treatment effects (e.g. risk ratios) if loss to follow‐up is higher in the control group than in the intervention group (Hall 2001). Some studies now use alternative methods to model effects of missing data (Hall 2001; Niaura 2002). Where differential results using alternative models were reported we considered whether the results of the meta‐analysis were sensitive to the use of different denominators.
Assessment of heterogeneity
To investigate statistical heterogeneity we use the I² statistic, given by the formula [(Q ‐ df)/Q] x 100%, where Q is the Chi² statistic and df is its degrees of freedom (Higgins 2003). This describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance). We used threshold values of 25% and 50% as suggesting moderate and substantial heterogeneity respectively. Although we give a summary statistic, the conclusions that can be drawn from it must be cautious. Where trials are small and few in number the confidence intervals will be wide.
Assessment of reporting biases
The derivation of the summary statistic implicitly assumes that data from all randomized trials are available without any bias due to non‐publication of unpromising results or to exclusion of randomized individuals. There is evidence that publication bias occurs in the field of smoking cessation research (Egger 1997), and this issue is discussed further in the Cochrane review of nicotine replacement therapy (NRT) (Stead 2012). Thus, we included unpublished studies or studies found only as abstracts where sufficient detail was available. We contacted authors for further data if necessary. Where sufficient data were available (ten or more studies in a comparison), we used funnel plots to investigate publication bias.
Data synthesis
We summarized individual study results as a risk ratio (RR), calculated as: (number of quitters in intervention group/ number randomized to intervention group) / (number of quitters in control group/ number randomized to control group). A risk ratio greater than 1.0 indicates a higher rate of quitting in the treatment group than in the control group. For each type of medication where more than one eligible trial was identified, we performed meta‐analysis using a Mantel‐Haenszel fixed‐effect method to estimate a pooled risk ratio with 95% confidence intervals (Mantel 1959). Where studies contributed more than one intervention arm to a pooled analysis, we split the control arm to avoid double counting. We created summary of findings tables for the efficacy of bupropion and nortriptyline, using standard Cochrane methodology.
Throughout, when we discuss effect we are referring to risk ratios, and not to absolute quit rates.
Subgroup analysis and investigation of heterogeneity
We distinguished between trials testing an antidepressant as a single pharmacotherapy and those testing an antidepressant as an adjunct to NRT for initial cessation. We also distinguished between cessation trials and those where the intervention addressed relapse prevention or reduction in number of cigarettes smoked. For trials of bupropion, we have included subgroup analyses by length of follow‐up, recruitment method (clinical/community), and level of behavioural support. For the subgroup analysis based on level of additional support, we used the same criteria applied in the Cochrane NRT review (Stead 2012); low intensity support was regarded as part of the provision of routine care, so the duration of time spent with the smoker (including assessment for the trial) had to be less than 30 minutes at the initial consultation, with no more than two further assessment and reinforcement visits.
Where reported, we also extracted data from analyses evaluating a potential interaction between current depression or past history of depression and quit rates. We relied upon the definition of depression used by study authors, which included both formal diagnoses and scores on validated depression scales.
None of the trials located were specifically designed to directly compare antidepressant pharmacotherapy with non‐pharmacological therapies.
Adverse events
Tables in the results section summarize the adverse events reported in clinical trials for smoking cessation for medications which have shown evidence of efficacy (bupropion and nortriptyline). These tables include all studies of bupropion and nortriptyline in which one arm received the pharmacotherapy and the other arm did not.
In addition, for this update we have created meta‐analyses for serious adverse events (SAEs) for bupropion and nortriptyline. As per the definition provided by the U.S. Food and Drug Adminstration, SAEs were defined as any event that was life‐threatening, resulted in hospitalization, death, disability, or permanent damage, or required intervention to prevent one of the above outcomes (FDA definition). Studies had to meet three criteria to be included in SAE analyses:
the active treatment was compared to a placebo or no pharmacotherapy control;
neither arm received additional pharmacotherapy above the antidepressant being tested; and
SAEs were reported during or within 30 days of active drug treatment.
We differentiate between studies which stated that no SAEs occurred (included in the analyses) and those that did not report on SAEs, in which it is possible that no events occurred, but not explicitly made clear (excluded from analyses and recorded in AE tables). In addition to screening included studies, we also screened excluded studies of nortriptyline and bupropion where the reason for exclusion was short‐term follow‐up. Where these studies met the above three criteria, they were included in the SAE meta‐analysis.
As with smoking cessation meta‐analyses, we used number randomized as the denominator and calculated risk ratios for each comparison, calculated as (number of events in the treatment group/number randomized to the treatment group)/(number of events in the control group/number randomized to the control group). For nortriptyline and bupropion, we used any SAE as the outcome of interest. Due to concerns about specific adverse effects of bupropion, we also analysed psychiatric and cardiovascular SAEs separately for bupropion studies.
Results
Description of studies
We identified twenty‐four additional trials for this update, yielding a total of 90 included trials. The new trials were of bupropion (Cinciripini 2013; Cox 2012; Eisenberg 2013; Gariti 2009; Hall 2011; Hays 2009; Kalman 2011; Levine 2010; Piper 2009; Planer 2011; Rose 2013; Rovina 2009; Schnoll 2010 (previously in 'awaiting classification' as Schnoll 2005) Siddiqi 2013; Smith 2009; Stapleton 2013; Wittchen 2011), fluoxetine (Brown 2013), nortriptyline (Richmond 2013), SAMe (Sood 2012, not previously covered in this review), selegiline ( Kahn 2012; Killen 2010), and St John's wort (Parsons 2009; Sood 2010, not previously covered in this review). One study previously included based on unpublished data is now published (Weinberger 2010). There were sixty‐six trials of bupropion, including seven testing the medication for relapse prevention (Covey 2007; Croghan 2007; Hall 2011; Hays 2001; Hays 2009; Hurt 2003; Killen 2006) and one for reduction (Hatsukami 2004). Eight of the bupropion trials allowed a direct comparison with nicotine replacement therapy (Gariti 2009; Górecka 2003; Jorenby 1999; Piper 2009; Smith 2009; Stapleton 2013; Uyar 2007; Wittchen 2011), and four a direct comparison with the nicotine receptor partial agonist varenicline (Cinciripini 2013; Gonzales 2006; Jorenby 2006; Nides 2006). Ten trials used nortriptyline including three which also used bupropion (Haggsträm 2006; Hall 2002; Wagena 2005). There were five trials of fluoxetine (Blondal 1999; Brown 2013; Niaura 2002; Saules 2004; Spring 2007), five of selegiline (Biberman 2003; George 2003; Kahn 2012; Killen 2010; Weinberger 2010), two of St John's wort (Parsons 2009; Sood 2010), one of paroxetine (Killen 2000), one of sertraline (Covey 2002), one of venlafaxine (Cinciripini 2005), and one of SAMe (Sood 2012). One study included only patients with current depression (Brown 2013). All other studies but two (Ahluwalia 2002; Schnoll 2010) excluded smokers with current depression but almost all included smokers with a past history of depression. Further details of the study designs are given in the table 'Characteristics of included studies'.
We list 80 excluded studies. Most of these were short‐term or laboratory‐based studies. For medications where there is little or no evidence from long‐term studies we briefly describe the results of the excluded short‐term trials. The reasons for exclusion are given in the table 'Characteristics of excluded studies'. Two placebo‐controlled trials studied the use of bupropion for smokeless tobacco cessation (Dale 2002; Glover 2002). These trials are excluded from the present review but are covered in the Cochrane review of interventions for smokeless tobacco cessation (Ebbert 2011). Papers reporting additional outcomes or subgroup analyses from included studies are listed as references under the study identifier. One further study is potentially relevant but did not have sufficient data to assess for inclusion at the time of publication (Rose 2013a).
Bupropion
Sixty‐six studies of bupropion with long‐term follow‐up are included. Outcomes for four studies are based on conference abstracts or pharmaceutical company data (Ferry 1992; Ferry 1994; Selby 2003; SMK20001).
The majority of trials were conducted in North America but studies are also included from Europe (Aubin 2004; Dalsgarð 2004; Fossati 2007; Górecka 2003; Rovina 2009; Stapleton 2013; Wagena 2005; Wittchen 2011; Zellweger 2005 ); Brazil (Haggsträm 2006); Australia (Myles 2004); Israel (Planer 2011); New Zealand (Holt 2005); Pakistan (Siddiqi 2013); Turkey (Uyar 2007); and two multi‐continent studies (Tonnesen 2003; Tonstad 2003). Special populations recruited include smokers with the following conditions: chronic obstructive pulmonary disease (Górecka 2003; Tashkin 2001; Wagena 2005); schizophrenia (Evins 2001; Evins 2005; Evins 2007; George 2002; George 2008); post traumatic stress disorder (Hertzberg 2001); cancer (Schnoll 2010); suspected tuberculosis (Siddiqi 2013); alcoholism (Grant 2007; Hays 2009); and cardiovascular disease (Eisenberg 2013; Planer 2011; Rigotti 2006; Tonstad 2003). Three of the studies in patients with cardiovascular disease, and one other, enrolled hospital inpatients (Eisenberg 2013; Planer 2011; Rigotti 2006;Simon 2009). Other populations included adolescents (Killen 2004; Muramoto 2007); smokers awaiting surgery (Myles 2004); hospital staff (Dalsgarð 2004); healthcare workers (Zellweger 2005); African‐Americans (Ahluwalia 2002; Cox 2012); and Maori (Holt 2005). Two studies recruited smokers who had previously failed to quit smoking using bupropion (Gonzales 2001; Selby 2003), and two included smokers who had just failed to quit using NRT (Hurt 2003; Rose 2013).
More than half the bupropion studies followed participants for at least 12 months from the start of treatment or the target quit day. Twenty‐six studies (40%) had only six months‐follow‐up (Ahluwalia 2002; Aubin 2004; Cinciripini 2013; Collins 2004; Cox 2012; Dalsgarð 2004; Evins 2001; Evins 2005; George 2002; George 2008; Grant 2007; Haggsträm 2006; Hatsukami 2004; Hertzberg 2001; Kalman 2011; Killen 2004; Muramoto 2007; Myles 2004; Rose 2013; Schnoll 2010; Siddiqi 2013; Simon 2009; Smith 2009; Stapleton 2013; Uyar 2007; Wagena 2005). The majority of studies reported an outcome of sustained abstinence. In 18 (28%) only point prevalence rates were given, or the definition of abstinence was unclear (Cox 2012; Evins 2005; Gariti 2009; George 2002; George 2008; Grant 2007; Hall 2011; Killen 2004; Muramoto 2007; Myles 2004; Piper 2007; Piper 2009; Schmitz 2007; Schnoll 2010; Selby 2003; Smith 2009; Swan 2003; Uyar 2007).
Forty‐four trials evaluated bupropion as a single pharmacotherapy to assist initial cessation. Twelve trials that tested bupropion as an adjunct to nicotine replacement therapy are pooled separately (Evins 2007; Kalman 2011; Killen 2004; Jorenby 1999 (part); George 2008; Grant 2007; Piper 2009; Rose 2013; Schnoll 2010; Simon 2004; Smith 2009; Stapleton 2013), as are the eight trials making direct comparisons between bupropion and nicotine replacement therapy (Gariti 2009; Górecka 2003; Jorenby 1999; Piper 2009; Smith 2009; Stapleton 2013; Uyar 2007; Wittchen 2011), three comparing bupropion and nortriptyline (Haggsträm 2006; Hall 2002; Wagena 2005), and four comparing bupropion and varenicline (Cinciripini 2013; Gonzales 2006, Jorenby 2006; Nides 2006). Trials testing the extended use of bupropion for relapse prevention (Covey 2007; Croghan 2007; Hall 2011; Hays 2001; Hays 2009; Hurt 2003; Killen 2006) and its use for assisting in reducing the amount smoked (Hatsukami 2004) are pooled separately.
The seven studies that evaluated bupropion SR for relapse prevention each had slightly different designs. These studies also contribute to a separate Cochrane review on interventions for relapse prevention (Hajek 2013).
One study evaluated bupropion for reducing smoking in people not wanting to make a quit attempt but interested in reducing smoking (Hatsukami 2004). During treatment, if participants decided they wanted to try to quit, they were enrolled in a cessation programme during which they continued to use bupropion and were then followed up for 19 weeks.
Most of the bupropion trials excluded participants with current depression but not those with a history of depression. Two studies did include participants with current depression (Ahluwalia 2002; Schnoll 2010). Two studies explicitly excluded participants with a past history of major depression (Dalsgarð 2004) or any psychiatric disorder (Collins 2004). Amongst the studies recording the prevalence of a past history of depression at baseline, the proportion ranged from 6% (Hatsukami 2004) to 44% (Swan 2003), but was typically 20 to 30%.
Nortriptyline
Ten published studies of the tricyclic antidepressant nortriptyline are included. Richmond 2013 is new since the last review, and was conducted in prisoners. Hall and colleagues conducted three trials, and Prochazka and colleagues two, both in the USA. Two studies were conducted in Brazil (Da Costa 2002; Haggsträm 2006), one in the Netherlands (Wagena 2005), one in the UK (Aveyard 2008), and one in Australia (Richmond 2013). Eight studies excluded participants with current depression but most of these included people with a history of depression. All studies were placebo controlled and used doses of 75 to 100 mg/day or titrated doses to serum levels recommended for depression during the week prior to the quit date. Treatment duration ranged from 12 to 14 weeks. All studies used a definition of cessation based on a sustained period of abstinence. The three studies by Hall and colleagues, Aveyard 2008, and Richmond 2013 reported outcomes after 12 months of follow‐up and the other five studies had six months of follow‐up.
The three studies by Hall and colleagues (Hall 1998; Hall 2002; Hall 2004) used factorial designs to test nortriptyline versus placebo crossed with different intensities of behavioural support, whereas the remaining studies provided a set amount of behavioural support to all participants, ranging from brief behavioural counselling to repeated group and individual sessions. Four studies tested nortriptyline as an adjunct to NRT (Aveyard 2008; Hall 2004; Prochazka 2004; Richmond 2013) and six tested nortriptyline on its own (Da Costa 2002; Haggsträm 2006; Hall 1998; Hall 2002; Prochazka 1998; Wagena 2005).
Selective Serotonin Reuptake Inhibitors (SSRIs)
One new study of an SSRI has been added since the last update of this review (Brown 2013, evaluating fluoxetine).
Fluoxetine
Five trials with long‐term follow‐up are included. Two studies used fluoxetine as the only pharmacotherapy and had six months follow‐up: a multicentre trial compared 30 mg daily, 60 mg daily, or placebo for 10 weeks (Niaura 2002) and a single‐site study used 60 mg or placebo for 12 weeks (Spring 2007). Three trials provided NRT to all participants and evaluated the addition of fluoxetine over 12 months follow‐up: Blondal 1999 used 20 mg/day or placebo for three months as an adjunct to nicotine inhaler; Saules 2004 used 20 or 40 mg/day or placebo for 10 weeks as an adjunct to nicotine patch; and Brown 2013 compared 10 weeks of fluoxetine, 16 weeks of fluoxetine, or no additional treatment in participants using nicotine patch for eight weeks. Brown 2013 was conducted in smokers with elevated depressive symptoms. Participants in all other trials were not currently depressed but may have had a past history of depression. Spring 2007 stratified by history of depression.
We list as excluded other short‐term studies, one with three month abstinence outcomes (Spring 1995) and others which reported outcomes other than abstinence (Cornelius 1997; Cornelius 1997; Dalack 1995; Naranjo 1990; Pomerleau 1991; Stein 1993). Another pharmaceutical company‐sponsored multicentre trial was completed but its results were never presented or published (personal communication).
Paroxetine
One trial with six‐month follow‐up assessed paroxetine (40 mg, 20 mg, or placebo) for nine weeks as an adjunct to nicotine patch (Killen 2000).
Sertraline
One trial with six‐month follow‐up assessed sertraline (200 mg/day) for 11 weeks versus placebo in conjunction with six individual counselling sessions. There were 134 participants, all current smokers with a past history of major depression (Covey 2002). One trial that combined sertraline with buspirone was excluded because the specific effect of sertraline could not be evaluated (Carrão 2007).
Citalopram/zimelidine
There were no long‐term studies of citalopram or zimelidine. One short‐term study used a crossover design to investigate the effect of the SSRIs citalopram or zimelidine on the smoking behaviour of heavy drinkers who were not attempting to stop smoking. Their cigarette use did not change significantly between active medication and placebo periods (Sellers 1987).
Monoamine oxidase inhibitors
Moclobemide
Moclobemide has been tested for smoking cessation in one long‐term placebo‐controlled trial in France (Berlin 1995). Treatment with 400 mg/day began one week before quit day and continued for two months, reducing to 200 mg/day for a further month. No behavioural counselling was provided. Final follow‐up was at 12 months.
Selegiline
Five long‐term trials have been published. Three used 10 mg/day oral treatment (Biberman 2003; George 2003; Weinberger 2010) and two used 6 mg/day patch treatment (Kahn 2012; Killen 2010). Three had treatment durations of nine weeks (George 2003; Kahn 2012; Weinberger 2010), one had a treatment duration of eight weeks (Killen 2010), and one continued therapy for 26 weeks (Biberman 2003). One excluded study was terminated early due to lack of efficacy and reported results to nine weeks only (Le Foll 2009). One other short‐term study (Houtsmuller 2002) and one reporting only preliminary short‐term data (Brauer 2000) are also excluded.
Lazabemide
There were no long‐term studies of this selective MAO‐B inhibitor. It was evaluated in an eight‐week, dose finding, exploratory study (Berlin 2002). The trial was halted early due to liver toxicity observed in trials of the medications for other indications, and lazabemide is not being developed further. Continuous four‐week quit rates at the end of treatment, including all drop‐outs as treatment failures, were 17% (18/108) for 200 mg/day, 11% (12/108) for 100 mg/day, and 9% (10/114) for placebo.
EVT302
There are no long‐term studies of this selective reversible MAO‐B inhibitor. It has been evaluated in an eight‐week placebo controlled study (Berlin 2012), excluded due to short follow‐up. There was no evidence of clinical benefit and no significant difference between active and placebo groups. Continuous four‐week quit rates at the end of treatment, including all drop‐outs as treatment failures were 17.2% (25/145) for EVT302, 15.2 % (22/145) for placebo, 32.8 % (22/61) for EVT302 plus nicotine patch, and 26.8 % (17/61) for placebo plus nicotine patch.
Other antidepressants
Three studies of other antidepressants have been included since the last review: two studies of St John's wort (Parsons 2009; Sood 2010) and one trial of the dietary supplement SAMe (Sood 2012).
Venlafaxine
One long‐term trial with 147 participants compared venlafaxine at a dose of up to 225 mg/day with placebo. All participants also received nicotine patches and nine brief individual counselling sessions; follow‐up was for 12 months (Cinciripini 2005). An unpublished short‐term study (Frederick 1997) reported no difference in abstinence rates at eight weeks, and frequent side effects in the treatment group.
Hypericum (St John's wort)
Two studies with long‐term follow‐up are included (Parsons 2009; Sood 2010). Both reported prolonged abstinence at six months. Parsons 2009 compared 14 weeks of 900 mg/day St John's wort to placebo and Sood 2010 compared 900 mg/day, 1800 mg/day, and placebo for 12 weeks.
Two studies are excluded. One unpublished study of an oral spray and placebo control with 45 participants detected no difference in abstinence at one month follow‐up (Becker 2003). Barnes 2006 compared two doses for smoking cessation in an open randomized study with no placebo control. Quit rates were low and did not differ between dose levels. No participants maintained abstinence for 12 months.
S‐Adenosyl‐L‐Methionine (SAMe)
One long‐term trial with 120 participants compared 1600 mg/day or 800 mg/day SAMe to placebo for 8 weeks with follow‐up at six months.
Doxepin
There are no long‐term studies of this serotonergic tricyclic antidepressant. It has been evaluated in a single small trial with short‐term follow‐up (Edwards 1989). Treatment was with 150 mg doxepin daily for three weeks prior to quit day and four weeks afterwards. Subjects forfeited a US$135 deposit if they failed to stop smoking for seven days. Two months after cessation, 78% (7/9) of the doxepin group and 10% (1/10) of the placebo group reported abstinence, a statistically significant difference (P < 0.02). However one week post‐cessation abstinence rates using stringent validated abstinence criteria failed to show a statistically significant difference. Among withdrawal symptoms, there was a significant group difference only for craving.
Imipramine
There are no long‐term studies of this noradrenergic tricyclic antidepressant. One trial (Jacobs 1971) compared imipramine (25 mg 3 times/day) with lobeline, dextroamphetamine, placebo and a no‐medication control. Some participants attended group support sessions. After three months, success rates, which included a reduction in smoking to less than 10% of baseline, were 56% (10/18) for imipramine, 40% (6/15) for placebo, and 69% (27/39) for the no‐medications control. These differences were not statistically significant.
Tryptophan
There have been no long‐term studies reported. Bowen and colleagues postulated that this serotonin‐enhancing action in conjunction with a high carbohydrate (CHO) diet might relieve the negative affect of cigarette withdrawal. Oral l‐tryptophan (50mg/kg/day) and instructions to follow a high CHO, low‐protein diet were compared with placebo pills and instructions for a low‐carbohydrate diet (Bowen 1991). Participants in both groups also received four two‐hour weekly multi‐component group therapy sessions. Two weeks following the target cessation date 75% (12/16) of the tryptophan and high CHO diet group were abstinent versus 47% (7/15) of the placebo and low CHO diet group. This difference was not statistically significant.
Risk of bias in included studies
The majority of studies were judged to be at unclear risk for selection, performance and detection bias and at low risk for attrition bias. Figure 1 displays risk of bias judgements across each domain.
1.
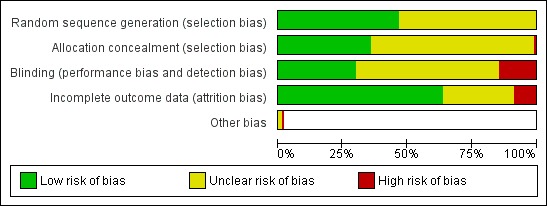
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Risk of bias in each area is summarized below. Additional details about the methodology of individual trials are given in the table Characteristics of included studies. Consistent with Cochrane methods, we included some trials that have only been published as abstracts, which have limited information on methodological issues (Higgins 2008). For some studies we have obtained additional information from authors, or from the pharmaceutical company funding the study. Use of unpublished data in the meta‐analysis is noted in the Characteristics of included studies table.
Allocation
All of the trials were described as randomized, but most failed to report randomization and concealment methods in detail. Twenty‐nine studies (32%) reported a method of sequence generation and allocation concealment judged to place the results at low risk of selection bias. One study was judged to be at high risk of selection bias due to a lack of allocation concealment (Wittchen 2011). All other trials were judged to be at unclear risk of selection bias because the method of sequence generation or allocation was not described in sufficient detail.
Blinding
Twenty‐seven trials (30%) provided sufficient information on blinding to be judged to be at low risk of performance and detection bias. These trials were placebo controlled, with both participants and personnel blinded to treatment allocation. Thirteen studies either did not blind participants or personnel or reported evidence suggesting unblinding, and were hence judged to be at high risk of performance and detection bias. The remaining studies did not provide sufficient information on blinding with which to judge risk of bias. For the most part, these trials reported that they were "double‐blind" but provided no additional detail on the nature of the blinding used.
Incomplete outcome data
The majority of studies (63%, 57 trials) reported low losses to follow‐up and were judged to be at low risk of attrition bias. Eight studies reported high (> 50%) or differential (> 20% difference between arms) loss to follow‐up and were judged to be at high risk of bias for this domain. The remaining 25 studies did not report the number or percentage of participants lost to follow‐up in each arm, and were hence judged to be at unclear risk for attrition bias.
Other potential sources of bias
One cluster‐randomized trial (Siddiqi 2013) was judged to be at high risk of other bias. In this trial, despite adequate reported methods of sequence generation and allocation concealment, the authors found substantial heterogeneity of program effects across clusters and a baseline imbalance in cigarette smoking behaviour (20% of participants in the control arm smoked only waterpipes (no cigarettes) compared to 4% in the intervention arm).
Definition of abstinence
The definition of abstinence was not always explicit and biochemical validation of self‐reported smoking status was not always used. However, all but four of the bupropion studies (Planer 2011; Smith 2009; Swan 2003; Wittchen 2011) and all but one of the nortriptyline studies (Da Costa 2002) used biochemical verification for most self‐reported quitters at some assessment points. In a small number of studies we were able to obtain a sustained outcome that was not given in the published report. Most of the sustained abstinence rates are based on self‐reported slip‐free abstinence from the start of the third week after the target quit date (TQD) onward and validated at intermediate and final follow‐ups.
Effects of interventions
Summary of findings for the main comparison. Bupropion for smoking cessation.
| Bupropion for smoking cessation | ||||||
| Patient or population: people who smoke Intervention: bupropion | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Bupropion | |||||
| Bupropion versus placebo/control. Abstinence Follow‐up: 6+ months | 115 per 10001 | 187 per 1000 (172 to 203) | RR 1.62 (1.49 to 1.76) | 13728 (44 studies) | ⊕⊕⊕⊕ high2,3 | |
| Bupropion and NRT versus NRT alone. Abstinence Follow‐up: 6+ months | 186 per 10001 | 221 per 1000 (175 to 281) | RR 1.19 (0.94 to 1.51) | 3487 (12 studies) | ⊕⊕⊝⊝ low3,4,5 | |
| Bupropion versus NRT. Abstinence Follow‐up: 6+ months | 254 per 10001 | 244 per 1000 (216 to 277) | RR 0.96 (0.85 to 1.09) | 4086 (8 studies) | ⊕⊕⊕⊝ moderate4 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control risk calculated as mean across included studies 2 Sensitivity analyses including only those studies judged to be at low risk of bias did not impact the pooled results 3 Funnel plot did not show evidence of asymmetry 4 All but one study at unclear or high risk for selection bias 5 Inconsistency across pooled results (I squared = 52%)
Summary of findings 2. Nortriptyline for smoking cessation.
| Nortriptyline for smoking cessation | ||||||
| Patient or population: people who smoke Intervention: nortriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nortriptyline | |||||
| Nortriptyline versus placebo. Abstinence Follow‐up: 6+ months | 99 per 10001 | 201 per 1000 (147 to 275) | RR 2.03 (1.48 to 2.78) | 975 (6 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| Nortriptyline and NRT versus NRT alone. Abstinence Follow‐up: 6+ months | 116 per 10001 | 141 per 1000 (109 to 180) | RR 1.21 (0.94 to 1.55) | 1644 (5 studies) | ⊕⊕⊕⊝ moderate2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Control risk based on average across all control groups 2 Though majority of studies at unclear risk of bias, sensitivity analyses suggests this is unlikely to affect the point estimate 3 Total number of events less than 300
(Selected analyses are displayed as Figures in the text. Other analyses are shown in the Data and analyses section online and full PDF versions of the review.)
Smoking cessation
Bupropion
We distinguish between the subgroup of trials where bupropion was tested as the only pharmacotherapy, and those trials that assessed the effect of bupropion when added to NRT. Two trials contributed arms to both subgroups (Jorenby 1999; Piper 2009).
Compared to placebo or no pharmacotherapy control, no other pharmacotherapy
There were 44 trials in which bupropion was the sole pharmacotherapy, with over 13,000 participants. The pooled risk ratio [RR] was 1.62 (95% confidence interval [CI] 1.49 to 1.76) with little evidence of heterogeneity (I² = 18%), see Figure 2, Analysis 1.1. The control group quit rates ranged from 0% to 33%, with a weighted average of 9%. Intervention group quit rates ranged from 4% to 43% with a weighted average of 18%. A sensitivity analysis did not suggest that results were sensitive to the exclusion of studies at high or unclear risk of bias across any domains. One cluster randomized trial of bupropion versus no pharmacotherapy was not included in the meta‐analysis due to substantial heterogeneity of program effects across clusters. It detected no significant difference between groups at any follow‐up point (adjusted RR 1.1, 95% CI 0.5 to 2.3) (Siddiqi 2013). A funnel plot (not shown) did not suggest the presence of publication bias.
2.
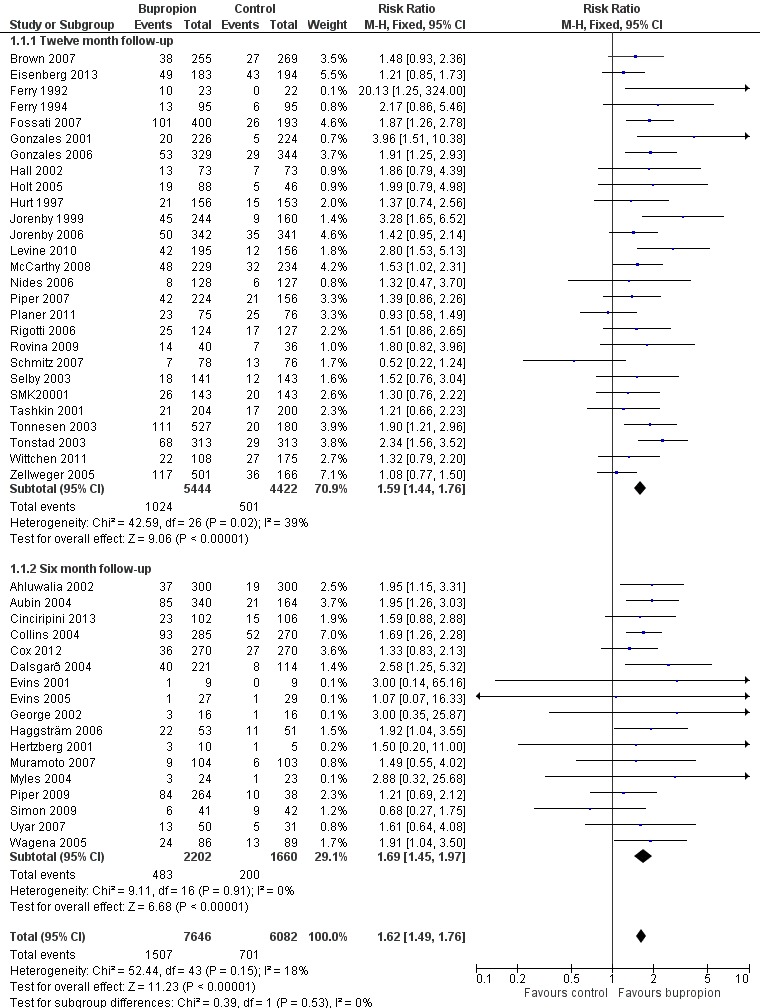
Forest plot of comparison: 1 Bupropion. Abstinence at 6m or greater follow‐up, outcome: 1.1 Bupropion versus placebo/control. Subgroups by length of follow‐up.
1.1. Analysis.
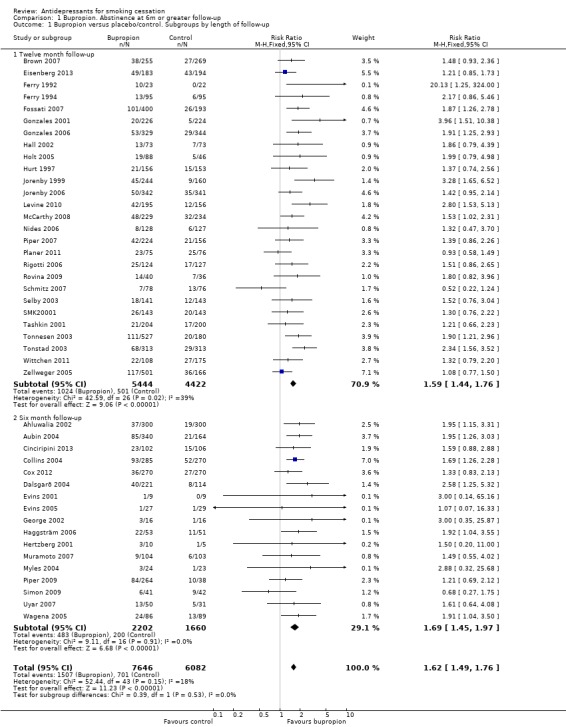
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 1 Bupropion versus placebo/control. Subgroups by length of follow‐up.
Sensitivity to length of follow‐up
Of the 44 trials of bupropion included in the main analysis, 27 had 12‐month follow‐up and 17 had six months. The estimated RR for the 12‐month follow‐up group was 1.59 (95% CI 1.44 to 1.76, I² = 39%, Analysis 1.1.1) which was not substantially different than that for trials with only six months (RR 1.69, 95% CI 1.45 to 1.97, I² = 0%, Analysis 1.1.2).
Sensitivity to clinical setting
In a post hoc subgroup analysis we distinguished between trials that recruited community volunteers and trials that recruited patients in healthcare settings or with specific diagnoses. Whilst the estimated effect was marginally smaller amongst trials that recruited community volunteers than those recruiting in health care settings, the confidence intervals overlapped and effects were significant in both groups (Analysis 1.2).
1.2. Analysis.
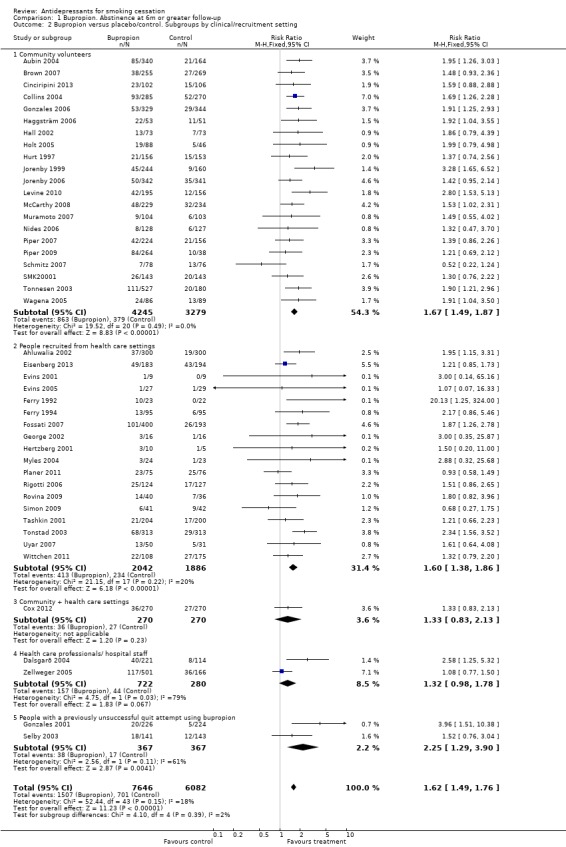
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 2 Bupropion versus placebo/control. Subgroups by clinical/recruitment setting.
Effect of level of behavioural support
Three trials compared bupropion and placebo in factorial designs varying the behavioural support. There was no evidence from any that the efficacy of bupropion differed between lower and higher levels of behavioural support (Hall 2002; McCarthy 2008) or by type of counselling approach used (Schmitz 2007). Other studies have compared different levels of behavioural support for people prescribed bupropion. These did not include placebo arms so do not provide evidence about within‐study interactions between behavioural interventions and pharmacotherapy. We also explored a between‐study subgroup analysis of the possible interaction with behavioural support using the classification into low and high intensity used in the Cochrane NRT review (Stead 2012). Low intensity was less than 30 minutes at the initial consultation, with no more than two further assessment and reinforcement visits. Only one of the included trials had such low intensity support (Myles 2004) and it was too small to draw conclusions from. Fossati 2007 (in a primary care setting) and part of McCarthy 2008 had limited behavioural support but in both cases there were more than three visits. We also examined, within the more intensive therapy trials, evidence of a different effect of bupropion versus placebo in ten trials that provided group‐based behavioural interventions compared to the majority (30) where individual therapy was provided. We found no evidence of a difference between subgroups (Analysis 1.3). (This subgroup analysis was based on the trials contributing to Analysis 1.1 but excludes four trials where the level of support could not be classified and one factorial trial where data were not provided in a usable manner.)
1.3. Analysis.
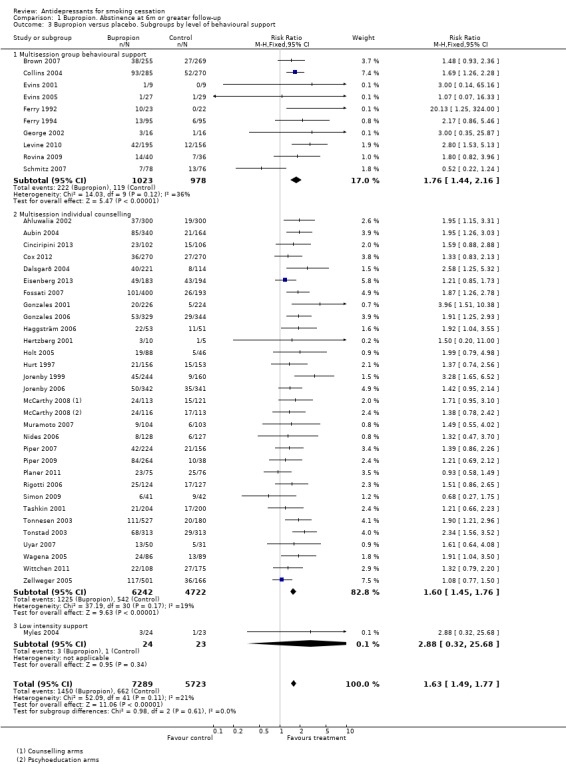
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 3 Bupropion versus placebo. Subgroups by level of behavioural support.
Effect of dose
In the first multi‐dose study (Hurt 1997), cessation rate was linearly related to dose (100 mg versus 150 mg versus 300 mg) through the end of treatment, consistent with pharmacological efficacy, although the difference between 300 mg and 150 mg doses was not significant at long‐term follow‐up. A larger study compared 150 mg and 300 mg daily doses, without a placebo group, and reported similar 12‐month point prevalence quit rates for both doses (Swan 2003). A study in adolescents also included 150 mg and 300 mg doses (Muramoto 2007), with higher quit rates in the larger does group. Doses above 300 mg have not been tested. Pooling the three studies and comparing 300 mg versus 150 mg shows no evidence of a significant difference in abstinence (RR 1.08, 95% CI 0.93 to 1.26, Analysis 1.4), with moderate statistical heterogeneity (I2 = 49%).
1.4. Analysis.
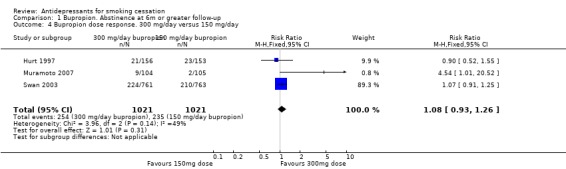
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 4 Bupropion dose response. 300 mg/day versus 150 mg/day.
Combined bupropion and nicotine replacement therapy compared to NRT alone
There was substantial statistical heterogeneity in the results of twelve studies adding bupropion to NRT (I² = 52%). Although two studies detected a clinically and statistically significant benefit of adding bupropion, the two studies contributing the largest weights to the analysis had point estimates very close to 1. Pooling using a fixed effect model does suggest a small benefit (RR 1.19, 95% CI 1.05 to 1.36, analysis not shown), but the level of heterogeneity makes a more conservative random‐effects model more appropriate. This does not alter the point estimate but the wider confidence intervals no longer exclude no effect (RR 1.19, 95% CI 0.94 to 1.51, Analysis 1.5), although neither do they exclude a benefit that would be clinically useful. A funnel plot (not shown) did not suggest the presence of publication bias. Of the twelve trials, five recruited people who were potentially hard to treat: adolescents (Killen 2004); smokers with schizophrenia (Evins 2007; George 2008); smokers in treatment for alcohol dependence (Grant 2007); and smokers who had failed to quit using NRT alone (Schnoll 2010). George 2008 was a small study with no quitters at all in the control group. The significant benefit seen in one trial (Jorenby 1999) may be due in part to the unusually poor results from nicotine patch alone in this study. Most studies used nicotine patch but two studies provided nicotine lozenge (Piper 2009; Smith 2009) and one offered a choice of NRT (Stapleton 2013); this does not explain the heterogeneity, nor does the exclusion of studies that did not use a bupropion placebo in the control arm.
1.5. Analysis.
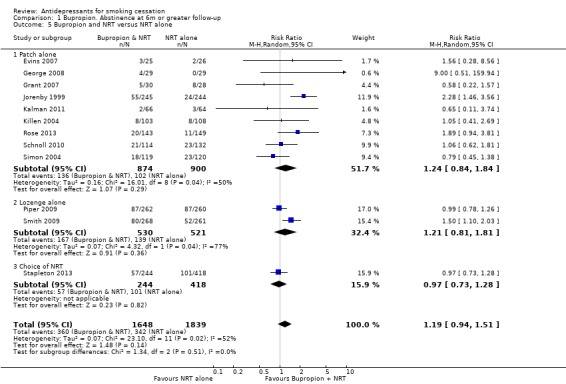
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 5 Bupropion and NRT versus NRT alone.
One relapse prevention study (Croghan 2007) compared nicotine inhaler, bupropion or both combined as initial therapy for cessation. In this open label phase, the combination had higher quit rates than either single therapy. After 12 weeks there was a second phase of randomization, so long‐term effects cannot be compared.
Bupropion for relapse prevention
Seven trials have evaluated extended use of bupropion for preventing relapse in people who have already stopped smoking. Pooling studies suggests the possibility of a small benefit but confidence intervals just include 1 (RR 1.15, 95% CI 1.00 to 1.33, Analysis 1.6). The studies were heterogeneous with respect to the length of initial abstinence, the period of pharmacotherapy and the length of post treatment follow‐up. The results of these studies are discussed in more detail in a Cochrane review of relapse prevention interventions (Hajek 2013).
1.6. Analysis.
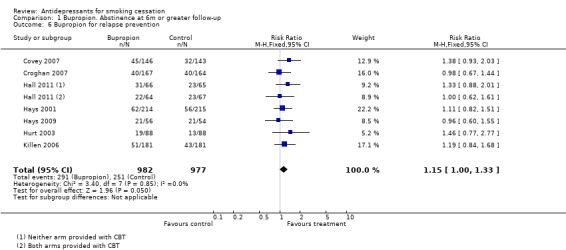
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 6 Bupropion for relapse prevention.
Bupropion and depression
Most studies did not report outcomes separately for participants with and without a history of depression, current depression, or depressive symptoms, even if any were enrolled. A separate Cochrane review on smoking cessation interventions for smokers with current or past depression has identified a small number of studies with data for these subgroups (van der Meer 2013). They identified data from five trials that included a total of 410 participants with current depressive symptoms (Brown 2007; Ahluwalia 2002 (reported in Catley 2005); Levine 2010; Rigotti 2006 (reported in Thorndike 2008); Schnoll 2010). When pooled the effect was non‐significant but the confidence intervals did not rule out a benefit in this population (RR 1.37, 95% CI 0.83 to 2.27). In five trials with a total of 404 participants with a past history of depression (Brown 2007; Hurt 1997 (reported in Hayford 1999); Piper 2009; Jorenby 1999 (reported in Smith 2003)), there was evidence of benefit (RR 2.04, 95% CI 1.31 to 3.18) although the review authors note the small number of participants and the post hoc nature of the subgroup analyses. Some study reports have noted a lack of evidence for any interaction between depression and treatment effect (Hurt 2002 & Cox 2004 subgroup analyses of Hays 2001; Kalman 2011). However, in a within trial analysis of a recent study comparing bupropion with NRT, a significant interaction was detected between participants with a past history of depression and treatment with bupropion, which suggested that bupropion was more effective than NRT for those with a past history of depression (Stapleton 2013).
Bupropion may alleviate some subclinical symptoms of depression during treatment (Ahluwalia 2002; Catley 2005; Lerman 2002a), but although this may facilitate smoking cessation, other mechanisms are probably more important (Catley 2005). In one trial (Collins 2004 reported in Lerman 2004), there was an interaction between nicotine dependence and treatment on post‐cessation depression symptoms. Most smokers showed a reduction in depression symptoms during the treatment phase, whether they received bupropion or placebo. The reduction was maintained during follow‐up. However highly dependent smokers showed a greater reduction in depression scores whilst receiving bupropion than whilst receiving placebo, and an increase when treatment ended.
Gender/age differences with bupropion
Too few of the studies have published data on long‐term quit rates by gender for it to be possible to conduct a definitive subgroup meta‐analysis. A meta‐analysis of mainly short‐term outcomes and including 12 trials with 4421 participants showed no evidence of a treatment‐gender interaction (Scharf 2004). In these trials, women were less successful at quitting than men overall, but bupropion was equally beneficial in men and women. A subgroup analysis of long‐term data from one study (Jorenby 1999, reported in Smith 2003) did report an interaction such that women appeared to benefit relatively more from medication. A more recent study reported a significant gender by smoking rate by treatment group interaction, such that bupropion seemed to benefit male heavy smokers and female light smokers but not others (Collins 2004). This study also showed an interaction among treatment effect, gender, and genotype (Lerman 2002b). At the end of treatment, women with a variant CYP2B6 gene had significantly higher quit rates when treated with bupropion than on placebo. The bupropion treatment effect was not significant for the other three gender/genotype subgroups. A study in smokers with chronic obstructive pulmonary disease (COPD) noted a larger treatment effect for women (ORs 2.7 versus 1.7), although the statistical significance of this interaction was not tested (Tashkin 2001). One study has reported a larger treatment effect for four‐ to seven‐week abstinence in males (Gonzales 2001). This was a study re‐treating smokers who had already failed to quit with bupropion. In the Hays 2001 relapse prevention study, there were no significant gender effects (Gonzales 2002a) and in a recent analysis of data from two included studies comparing bupropion with NRT and with placebo (Piper 2009; Smith 2009), there were also no significant gender effects (Piper 2010). In summary, gender does not appear to consistently influence the efficacy of bupropion. Whilst most reports have not indicated any difference in treatment effects between older and younger smokers, subgroup analyses of two trials, Hays 2001 (reported in Hurt 2002) and Hurt 1997 (reported in Dale 2001), found evidence of an interaction, with a larger treatment effect for older smokers. One study in adolescents did not show evidence of an effect for bupropion over nicotine patch alone (Killen 2004).
Bupropion as second treatment
One trial, included in the analysis of combined bupropion and NRT versus NRT alone, randomized participants immediately after failing to quit using NRT alone or lapsing within one week of quitting using NRT. More participants successfully quit in the combined arm than in the NRT alone arm, although the result was not statistically significant (Rose 2013, RR 1.89, 95% CI 0.94 to 3.81). In addition, one relapse prevention trial described above (Hurt 2003) also randomized 194 smokers who had not quit successfully using nicotine patch therapy to bupropion or placebo as a second line treatment. Only one person, in the bupropion group, quit and sustained abstinence at six months. This is consistent with the results of some other studies, which find low overall success rates in smokers offered further pharmacotherapy soon after treatment failure (e.g. Gourlay 1995; Tonnesen 1993), though Rose 2013 detected somewhat higher rates (six month continuous abstinence: 14% combined arm; 11% NRT only). In addition, a subgroup analysis of Jorenby 1999 (reported in Durcan 2002) suggested that bupropion was equally effective in smokers with and without a past history of failure with NRT. In this trial, the gap between the previous failed attempt and the second attempt at cessation would have been longer.
Bupropion versus NRT
Eight studies provided direct comparisons between bupropion and NRT, some comparing more than one type. Six allowed a comparison with the patch. Pooled results for all forms of NRT did not detect a significant difference between the two types of pharmacotherapy (RR 0.96, 95% CI 0.85 to 1.09, I2 = 27%, Analysis 1.7). No single type of NRT (patch, lozenge or a choice) showed a significant difference with bupropion, but two studies included a comparison with combination nicotine patch and lozenge and when these are pooled the combination is more effective than bupropion (RR 0.74, 95% CI 0.55 to 0.98). This is consistent with evidence that using two types of NRT is more effective than using the patch alone (Fiore 2008; Stead 2012). Statistical heterogeneity was low within all subgroups, with the exception of patch (I2 = 48%). All of the statistical heterogeneity in this subgroup can be attributed to Jorenby 1999, which, unlike the other studies, detected a significant effect in favour of bupropion. However, nicotine patch itself was not efficacious in this particular study.
1.7. Analysis.
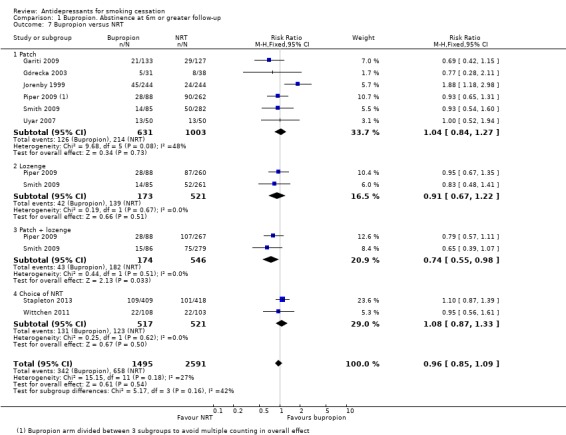
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 7 Bupropion versus NRT.
Bupropion versus varenicline
Four studies there directly compared bupropion and varenicline (Cinciripini 2013; Gonzales 2006; Jorenby 2006; Nides 2006). The pooled estimate showed a significantly lower rate of quitting with bupropion than varenicline (N = 1810, RR 0.68, 95% CI 0.56 to 0.83, Analysis 1.8), with no evidence of heterogeneity.
1.8. Analysis.
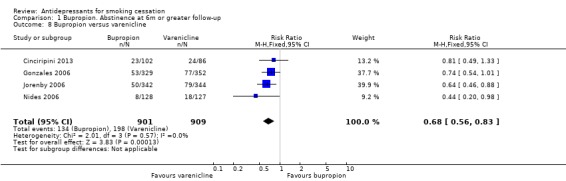
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 8 Bupropion versus varenicline.
Bupropion for smoking reduction
One study offered bupropion to smokers not wishing to quit (Hatsukami 2004). There were no significant differences in reduced cigarettes/day, cotinine, or cessation (Analysis 1.9).
1.9. Analysis.
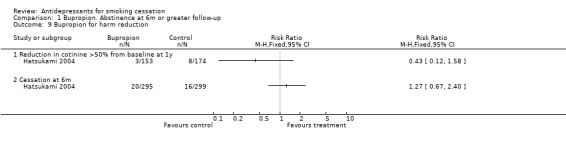
Comparison 1 Bupropion. Abstinence at 6m or greater follow‐up, Outcome 9 Bupropion for harm reduction.
Nortriptyline
Compared to placebo control, no other pharmacotherapy
Pooling six trials using nortriptyline as the only pharmacotherapy shows evidence of a significant benefit of nortriptyline over placebo (N = 975, RR 2.03, 95% CI 1.48 to 2.78, Figure 3, Analysis 2.1) without evidence of statistical heterogeneity (I² = 7%).
3.
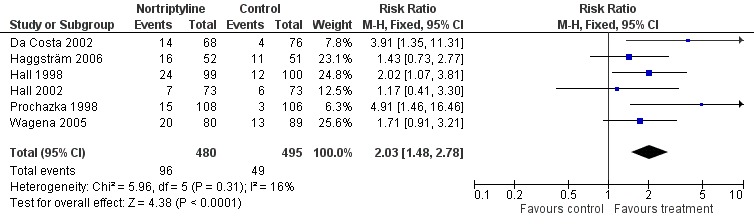
Nortriptyline versus placebo, long‐term abstinence
2.1. Analysis.
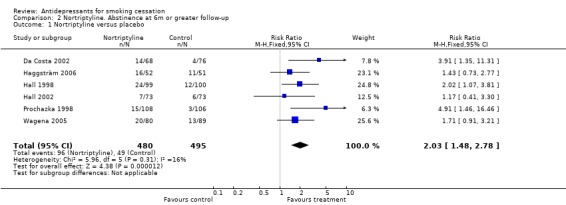
Comparison 2 Nortriptyline. Abstinence at 6m or greater follow‐up, Outcome 1 Nortriptyline versus placebo.
Combined nortriptyline and nicotine replacement therapy compared to NRT alone
Pooling four trials (one with a factorial design entered in two parts) using nortriptyline as an adjunct to nicotine patch therapy does not show evidence of an additional benefit from nortriptyline (N = 1644, RR 1.21, 95% CI 0.94 to 1.55, Analysis 2.2), with moderate heterogeneity (I² = 26%).
2.2. Analysis.
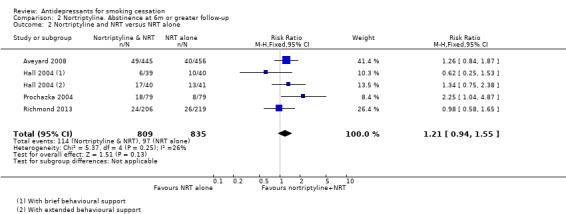
Comparison 2 Nortriptyline. Abstinence at 6m or greater follow‐up, Outcome 2 Nortriptyline and NRT versus NRT alone.
Subgroup and sensitivity analyses
There were too few trials of nortriptyline to examine effect of duration of follow‐up, past depression, or amount of behavioural therapy between subgroups of trials. In one within‐study comparison, a past history of depression did not appear to moderate the efficacy of nortriptyline, but subgroup numbers were small (Hall 1998). In two within‐study comparisons, the intensity of adjunctive behaviour therapy did not influence the active versus placebo effect (Hall 1998; Hall 2002). In the study by Hall and colleagues of extended treatment (longer duration of both nortriptyline and behaviour therapy) versus brief treatment (similar to other nortriptyline trials), the confidence intervals for nortriptyline versus placebo included 1.0 (i.e. no evidence of an effect) for each treatment. The extended treatment increased absolute rates of abstinence and the relative effect for nortriptyline (RR 1.34 versus 0.62) but this was not statistically significant. Dose‐response studies with nortriptyline have not been reported.
Bupropion versus nortriptyline
Three trials included a direct comparison between bupropion and nortriptyline (Haggsträm 2006; Hall 2002; Wagena 2005). In each study, the comparison favoured bupropion but none showed significant differences. There was no evidence of heterogeneity. When pooled the difference remained non‐significant, but does not exclude a clinically useful difference in favour of bupropion (N = 417, RR 1.30, 95% CI 0.93 to 1.82, Analysis 3.1).
3.1. Analysis.
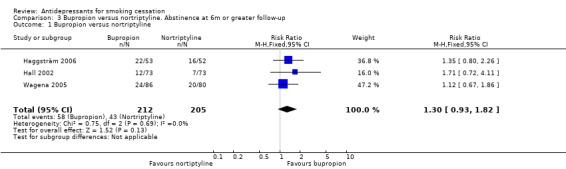
Comparison 3 Bupropion versus nortriptyline. Abstinence at 6m or greater follow‐up, Outcome 1 Bupropion versus nortriptyline.
Selective serotonin reuptake inhibitors (SSRIs)
Seven trials of SSRIs were included. Four compared an SSRI to control (two fluoxetine: Niaura 2002; Spring 2007; one paroxetine: Killen 2000; one sertraline: Covey 2002). Pooled, they did not detect a significant effect (N = 1546, RR 0.93, 95% CI 0.71 to 1.22, I2 = 0%, Analysis 5.1), nor did any of the individual therapies. Three trials which evaluated fluoxetine as an adjunct to NRT (Blondal 1999; Brown 2013; Saules 2004) also did not detect a significant effect (N = 466, RR 0.70, 95% CI 0.48 to 1.03, I2 = 0%, Analysis 5.2).
5.1. Analysis.

Comparison 5 Selective Serotonin Reuptake Inhibitors (SSRIs) versus placebo. Abstinence at 6m or greater follow‐up., Outcome 1 SSRI versus placebo/control.
5.2. Analysis.
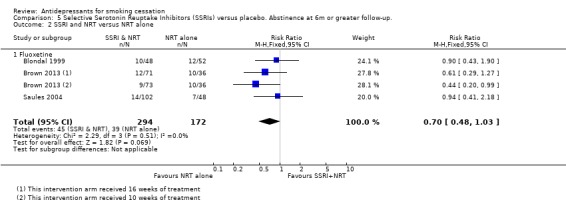
Comparison 5 Selective Serotonin Reuptake Inhibitors (SSRIs) versus placebo. Abstinence at 6m or greater follow‐up., Outcome 2 SSRI and NRT versus NRT alone.
Monoamine oxidase inhibitors (MAOIs)
One trial of moclobemide (Berlin 1995) and five of selegiline (Biberman 2003; George 2003; Kahn 2012; Killen 2010; Weinberger 2010) were included. When pooled, there was no evidence of benefit (N = 827, RR 1.29, 95% CI 0.93 to 1.79, I² = 9%, Analysis 6.1) and the two contributing the highest weights reported higher quit rates in the placebo group (Killen 2010; Weinberger 2010).
6.1. Analysis.
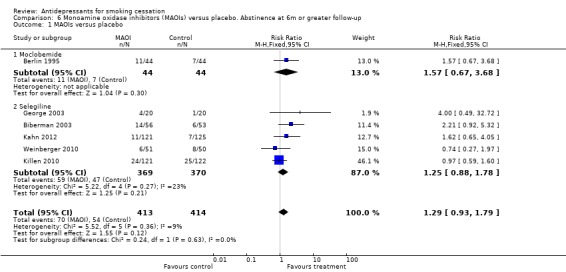
Comparison 6 Monoamine oxidase inhibitors (MAOIs) versus placebo. Abstinence at 6m or greater follow‐up, Outcome 1 MAOIs versus placebo.
One trial of befloxatone showed no effect on cessation but data are unpublished (Berlin 2005).
Venlafaxine
One trial of venlafaxine (Cinciripini 2005) failed to detect a significant increase in 12‐month quit rates compared to nicotine patch and counselling alone, but confidence intervals do not exclude a clinically useful effect (RR 1.22, 95% CI 0.64 to 2.32, Analysis 7.1). Post hoc subgroup analyses suggested that there might be greater evidence for an effect amongst lighter smokers.
7.1. Analysis.
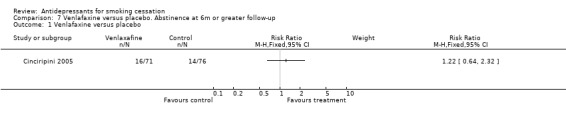
Comparison 7 Venlafaxine versus placebo. Abstinence at 6m or greater follow‐up, Outcome 1 Venlafaxine versus placebo.
St John's wort
Neither of the two small trials of St John's wort detected a significant effect (Parsons 2009; Sood 2010). In one, more participants quit in the placebo than in the intervention arm. The pooled result was not significant, with some evidence of heterogeneity (N = 261, RR 0.81, 95% CI 0.26 to 2.53, I2 = 29%).
S‐Adenosyl‐L‐Methionine (SAMe)
One trial of SAMe (Sood 2012) had lower quit rates in the active than control groups. The trial tested two doses which had similar outcomes and are pooled in the analysis (RR 0.70, 95% CI 0.24, 2.07, Analysis 9.1). The authors concluded that further clinical trials were not justified.
9.1. Analysis.
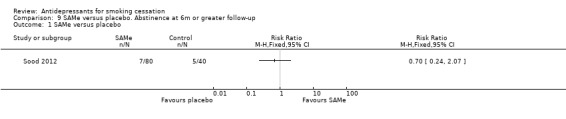
Comparison 9 SAMe versus placebo. Abstinence at 6m or greater follow‐up, Outcome 1 SAMe versus placebo.
Adverse Events
We summarize all adverse events (AEs) reported in trials of bupropion (Analysis 4.1) and nortriptyline (Analysis 4.2). In this update, we also conducted meta‐analyses of serious adverse events (SAEs) that occurred during treatment for studies in which bupropion or nortriptyline were compared with placebo or no pharmacotherapy controls. We also consider findings from recent large observational studies. A summary of historic data on bupropion from national surveillance schemes in the United Kingdom (UK), Australia and Canada is given in Appendix 2.
4.1. Analysis.
Comparison 4 Bupropion and nortriptyline. Adverse events, Outcome 1 Nortriptyline vs placebo. 'No report' = no information, 'None occurred' = explicit statement.
| Nortriptyline vs placebo. 'No report' = no information, 'None occurred' = explicit statement | |||
|---|---|---|---|
| Study | Serious events | Other adverse events | Withdrawal due to AE |
| Aveyard 2008 | 2 admissions to hospital (1 intervention, 1 control) with collapse or palpitations judged possibly caused by treatment | Dry mouth (80% vs 'more than half, OR 6.67, 5.12 to 8.69), Constipation (OR 2.06, 1.66 to 2.56) and Sweating (OR 1.37, 1.11 to 1.68) significantly more common | No report |
| Da Costa 2002 | None occurred | No significant differences between groups. Dry mouth 44% vs 24%, constipation 29% vs 12%, irritation 18% vs 24%, anxiety 18% vs 28%. | No report |
| Haggsträm 2006 | None occurred | Dry mouth (67.3% vs 31.4%) and drowsiness (19.2% vs 11.8%) significantly more common with nortriptyline Constipation (15.4% vs 9.8%) NS | No report |
| Hall 1998 | None occurred | Dry mouth (78% vs 33%), lightheadedness (49% vs 22%), shaky hands (23% vs 11%) and blurry vision (16% vs 6%) were significantly more common. | 4 (4%) dropouts due to side effects in drug and 1 (1%) in placebo group. Total dropout rates 30% in drug and 17% in placebo groups |
| Hall 2002 | None occurred | Dry mouth (72% vs 33%) and constipation ( 32% vs 14%) significantly more common with nortriptyline | No report |
| Hall 2004 | None occurred | Dry mouth (85% vs 40%), lightheadedness (44% vs 22%), shaky hands (30% vs 14%) constipation (38% vs 15%), blurry vision (23% vs 7%), difficulty urinating (13% vs 2%), all p<0.01. Sexual difficulties (19% vs 2% p <0.02) | 4 (10%) in active brief treatment, 10 (25%) in active extended treatment, 9 (11%) in placebo stopped medication due to adverse effects (active vs placebo p=0.18, Fisher's exact test) |
| Prochazka 1998 | None occurred | Dry mouth (64% vs 23%), dysgeusia (20% vs 8%), GI upset (41% vs 24%), drowsiness (24% vs 8%) significantly more common | 10 (9%) treatment withdrawal due to adverse events in nortriptyline group, vs 3 (3%) in placebo group. |
| Prochazka 2004 | None occurred | Dry mouth (38% vs 8%) & sedation (20% vs 3%) significantly more common. | 10 (13%) discontinued nortriptyline including 1 subject with a normal baseline ECG who developed asymptomatic prolongation of QT interval, vs 1 placebo |
| Wagena 2005 | One death in placebo group, previously hospitalised with dermatological reactions | Dry mouth (61% vs 20%), diarrhoea or constipation (48% vs 26%) and fatigue (20% vs 9%) significantly more common in nortriptyline group | 24% discontinued nortriptyline vs 9% placebo |
4.2. Analysis.
Comparison 4 Bupropion and nortriptyline. Adverse events, Outcome 2 Bupropion versus control. 'No report' = no information, 'None occurred' = explicit statement.
| Bupropion versus control. 'No report' = no information, 'None occurred' = explicit statement | |||
|---|---|---|---|
| Study | Serious events | Other adverse events | Withdrawal due to AE |
| Ahluwalia 2002 | No seizures occurred No serious adverse events reported. | Insomnia (29.3 vs 20.7%) more common with bupropion. Dry mouth (28% vs 24%) | No information |
| Aubin 2004 | No seizures occurred 5 bupropion and 1 placebo serious AE during treatment, 2 bupropion during f‐up. 1 chest pain, tremor & sweating & 1 depressive syndrome after end of treatment considered possibly due to bupropion. | 61% on bupropion and 45% on placebo experienced at least one AE Sleep disorder 33% bupropion vs 19% placebo | 10% bupropion & 5% placebo withdrew due to AEs |
| Cinciripini 2013 | No seizures occurred 3 SAEs (within 30 days of treatment) in bupropion group: bilateral mammoplasty, facial paralysis and syncope. 2 SAEs in placebo group: diabetes mellitus and chest pain. |
Significantly higher rates of influenza (7.8% vs 1.9%), nausea (16.7% vs 7.5%) and insomnia (31.4% vs 19.8%) in bupropion than placebo group. Significantly less diarrhoea in intervention than control group (11.3 vs 3.9%). No other significant differences detected. | 2% bupropion and 1% placebo withdrew due to AEs |
| Collins 2004 | Not reported in paper | Not reported in paper | Not reported in paper |
| Covey 2007 | One seizure during open label phase (before randomization to relapse prevention) | 'The number of reported side effects (e.g. nervousness, constipation, insomnia, stomach‐ache, depressed mood) was low (mean = 0.43, SD = 0.91), and did not vary by treatment group (P = 0.69)' | None reported |
| Cox 2012 | None occurred in first 3 weeks of treatment (not reported beyond that) | Type of AE not reported. All AEs in first week of treatment 8.9% bupropion and 28.5% placebo group. | Not reported in paper |
| Dalsgarð 2004 | No seizures occurred No serious adverse event during treatment phase. 3 events during follow‐up not considered to be drug related including 1 death in bupropion group, | Insomnia (28% vs 18%), dizziness (8% vs 1%) and skin problems (15% vs 7%) significantly more common with bupropion. Major depression more common in placebo (1% vs 5%), | 12% bupropion & 8% placebo withdrew due to adverse event. |
| Eisenberg 2013 | No seizures occurred. Over the course of 12 months, 17.7% bupropion and 18.5% placebo experienced SAE. No SAEs considered related to treatment. | Over course of study treatment, no significant difference in AEs. Most common were insomnia (22.3% bupropion vs 18.2% placebo), dry mouth (13.3% bupropion vs 9.1% placebo), and dizziness (8.0% bupropion vs 8.6% placebo). | Not reported |
| Evins 2001 | No seizures occurred | No information (only 19 participants) | No information |
| Evins 2005 | Not reported in abstract | Not reported in abstract | Not reported in abstract |
| Ferry 1992 | No seizures occurred (data from FDA submission) | No information from abstract | No information from abstract |
| Ferry 1994 | No seizures occurred (data from FDA submission) | No information from abstract | 3% bupropion & 3% placebo withdrew due to adverse experience (data from FDA submission) |
| Fossati 2007 | No seizures occurred 8 serious adverse events in bupropion group, of which 1 thought to be medication related (suspected cholangitis) | Dry mouth (6.3% vs 2.1%), Insomnia (17.3% vs 6.2%), and constipation (11.0% vs 3.6%) significantly more common on bupropion | ˜14% bupropion & 7% placebo withdrew due to AEs |
| George 2002 | No seizures occurred | Dry mouth (62.5% vs 25.0%) , headache (56.3% vs 37.5%), insomnia (43.8% vs 27.8%), memory problems (50.0% vs 31.3%), blurred vision (50.0% vs 25.0%, irregular heartbeat (37.5% vs 12.5%), nausea/vomiting (43.8% vs 18.8%) diarrhoea (50.0% vs 25.0%), anxiety/agitation (50.0% vs 25.0%), tremor (31.3% vs 12.5%) | 2 bupropion & 5 placebo withdrew during treatment, no reasons given |
| George 2008 | No seizures occurred. Three serious adverse events (SAEs) involved psychotic decompensation, 2 placebo, 1 bupropion. All deemed unrelated to study medications | There were significant (p <.05) group differences on concentration, jitteriness, lightheadedness, muscle stiffness, and frequent nocturnal awakening | No information on AE related withdrawals |
| Gonzales 2001 | No seizures occurred. One serious adverse event (rash with pruritus and edema) in the bupropion group was assessed as being due to study medication | No significant differences between bupropion & placebo. 72% on bupropion reported adverse event vs 58% placebo. Most common adverse events insomnia (24% vs 11%), viral infections (13% vs 19%) dry mouth (13% vs 9%), headache (8% vs 13%), | 30 people discontinued medication due to adverse event, 11 (5%) placebo, 19 (8%) bupropion. For patients on bupropion most common events were anxiety (4), dry mouth (3) and rash (3) |
| Gonzales 2006 | 1 seizure after 20 days of bupropion. No other serious events assessed as due to medication | Dry mouth (8.8% vs 5.5%, NS), nausea (12.5% vs 8.4%, NS), insomnia (21.9% vs 12.8%) | 9.0% placebo, 15.2% bupropion discontinued medication |
| Grant 2007 | No seizures occurred | Insomnia (37% vs 7%) | 10 (33%) discontinued bupropion vs 3 (11%) placebo |
| Haggsträm 2006 | No seizures or other serious adverse events occurred | Insomnia (50.9% vs 17.6%), dry mouth (50.9% vs 31.4%), diarrhoea (11.0% vs 3.9%) | No report |
| Hall 2002 | No seizures occurred | No significant differences between bupropion & placebo | No report |
| Hatsukami 2004 | No seizures occurred. 8 serious AEs in bupropion, 3 placebo. One case of chest pain thought to be treatment related. | No details | 60 people discontinued medication due to adverse events, 22 (7%) placebo, 38 (13%) bupropion. |
| Hays 2001 | No seizures occurred. No serious adverse events assessed as being caused by study medication | No significant differences between bupropion and placebo. Most common adverse events during 45 week double blind medication phase insomnia (10% vs 7%) and headache (24% vs 17%) also rhinitis, influenza URI and accidental injury. | 41 people discontinued medication due to adverse events, 17 (8%) placebo, 24 (11%) bupropion. |
| Hertzberg 2001 | No seizures occurred. One patient receiving bupropion suffered ataxia, headache and jitteriness. | No details | One bupropion (ataxia, headache and jitteriness) |
| Holt 2005 | No seizures or serious adverse events | Insomnia 26% vs 9% | Three discontinued bupropion due to a rash. |
| Hurt 1997 | No seizures occurred. One of three serious adverse events could have been associated with bupropion; extreme irritability restlessness, anger, anxiety and craving in a man who stopped smoking. | Bupropion 300mg was associated with significantly more insomnia (34.6% vs 20.9%) and dry mouth (12.8% vs 4.6%) than placebo. | 37 people discontinued medication due to adverse events; 6 (5%) placebo; 9 (6%) 100mg; 7 (5%) 150mg; 13 (8%) 300mg. Tremor, headaches, rash and urticaria were the most common reasons for stopping treatment. |
| Hurt 2003 | No seizures occurred | No significant differences. Anxiety (16% vs 9%) and nervousness (13% vs 6%) more common in bupropion group. Insomnia less common (10% vs 17%). | Not stated. |
| Jorenby 1999 | No seizures occurred. Three serious adverse events were attributed to bupropion, all consisted of rash and pruritus, one with shortness of breath and chest tightness. All had full resolution of symptoms | Bupropion was associated with more insomnia (42.4% vs 19.5%) and dry mouth (10.7% vs 4.4%) than placebo. | 79 people discontinued medication due to adverse events; 6 (3.8%) placebo; 16 (6.6%) patch; 29 (11.9%) bupropion and 28 (11.4%) combined treatment group. 20% dropped out of study, and 35% were lost to follow‐up at 12 months. |
| Jorenby 2006 | No seizures occurred 1 serious adverse event attributed to bupropion; angioedema. | Bupropion was associated with more insomnia (21.2% vs 12.4%), sleep disorder (6.8% vs 2.6%), constipation (6.5% vs 1.5%), dry mouth (7.6% vs 3.2%). | 16 (4.7%) bupropion vs 13 (3.8%) placebo discontinued study due to AEs. 12.6% vs 7.3% discontinued medication. |
| Killen 2004 | No seizures occurred. No adverse effects judged to be severe by study physician | 22 (21%) reported severe AEs in bupropion & patch group vs 25 (23%) in placebo & patch. 24 (23%) vs 35 (32%) reported moderate AEs | None reported |
| Killen 2006 | 1 seizure during open‐label phase 2 other serious adverse events during open label phase (oedema, depression) and 2 during extended treatment (diagnosis of hyperthyroidism in bupropion group, onset of immune thrombocytopenia purpura in placebo group). | During open‐label phase 53% reported insomnia, 47% dry mouth, 44% vivid dreams, 23% nausea, 22% headache, 17% racing heart rate, 12% skin rash and 7% irregular heart rate. | 30 (8%) discontinued medication during open‐label phase. 1 bupropion and 2 placebo discontinued during extended treatment phase. |
| Levine 2010 | "No serious events were associated with medication use." No further information reported. | "Overall, bupropion was well tolerated." No further detail provided. | "Reasons for medication discontinuation did not differ between bupropion and placebo, with the exception of allergic reaction (5.64% vs 0.65% in bupropion vs placebo, p = 0.02). No further detail provided. |
| Muramoto 2007 | No seizures occurred. 1 hospitalisation (150 mg/d group) for deliberate ingestion of Datura innoxia for recreational purposes. 1 hospitalisation (150 mg/d group) for intentional overdose of study medication, other drugs & alcohol. | Headache and cough were commonest reported AEs. No others significantly different. | Eight subjects discontinued medication early because of the following adverse events: feeling depressed, irritable, or angry; sleep disturbance; headache; urticaria; anxiety; heart palpitations; suicide attempt; anticholinergic crisis related to recreational drug use; and pregnancy. |
| Nides 2006 | Two seizures, 2 other serious AEs in bupropion (persistent intermittent bloody diarrhoea, syncope) all considered to be possibly related to bupropion | 90% of bupropion and 88% on placebo experienced at least one AE. Insomnia 45% bupropion vs 22% placebo, constipation 14% bupropion vs 4% placebo, dry mouth 12% bupropion vs 6% placebo. | 16% bupropion & 10% placebo discontinued medication |
| Piper 2007 | No report of seizures | 4.7% of adverse events was insomnia, not reported by condition | Not reported |
| Piper 2009 | 1 seizure in bupropion group, but other SAEs not reported | In bupropion vs placebo, more diarrhea (1.5% vs 1.1%), vomiting (1.9% vs 1.1%), dry mouth (3.8% vs 1.0%), and sleep disturbance and abnormal dreams (16.8% vs 5.6%). In all other AEs, same or more occurred in placebo arm. | 2 in bupropion group withdrew because of events related to medication (1 interaction related to other antidepressants; 1 heartburn). 1 placebo withdrew because of "negative experience" whilst on medication. |
| Planer 2011 | Over course of one year, 3% bupropion and 1% placebo MI, 3% bupropion and 7% placebo ACS. | No significant differences in any AEs over one year except for increased rate of dizziness in bupropion users (14% bupropion vs 1.4% placebo) | Not reported |
| Rigotti 2006 | No report of seizures. Two deaths in placebo group. Non cardiac serious adverse events; 37% bupropion vs 31% placebo, ns Rate of new cardiovascular events did not differ significantly at 3 months or 1 year. After 30 days off drug more bupropion group sustained a cardiovascular event, borderline significance after adjustment for cardiac risk factors. | Not reported | Withdrawals not reported |
| Rovina 2009 | No seizures in either two arms included in this analysis. 0.9% tachycardia in bupropion arm vs 0 in control arm during 19 weeks of treatment. | Higher rates in bupropion than in control arm of: insomnia (15.8% vs 3.2%), dry mouth (12.9% vs 0), dizziness (8.8% vs 0), constipation (5.5% vs 1.2%), headache (5.8% vs 4.5%), anxiety (4.8% vs 3.7%), nausea (5% vs 0), concentration issues (4.2% vs 1.2%), allergic reaction (5.8% vs 0), sadness (2.6% vs 0.4%), and sleepiness (1.4% vs 0). | Not reported |
| Simon 2004 | No report of seizures or other serious AE | Frequency of insomnia and abnormal dreams similar in both groups. Dry mouth 22% bupropion vs 8% placebo, gastrointestinal upset 9% bupropion vs 1% placebo | Withdrawals not reported |
| Simon 2009 | No seizures occurred. 1 death in each group, causes not given (hospital population) | 11 (26%) bupropion vs 4 (9%) in the placebo reported any AEs. Hyperactivity and insomnia reported solely in bupropion group | Not reported |
| SMK20001 | No seizures or deaths occurred. 7 patients (bupropion 4, placebo 3) experienced a serious AE, none considered related to medication. | Overall rate of reporting of adverse events 90% vs 83%. Sleep disorders 46% vs 27% | 7% vs 1% withdrew due to AEs |
| Stapleton 2013 | No seizures occurred. 4 patients receiving bupropion had an SAE whilst on treatment (2 allergic reactions resulting in anaphylaxis, 1 transient suicidal thoughts, 1 severe chest pain). No SAEs occured in group receiving NRT only. | Higher rates of some adverse events in participants receiving bupropion compared to those receiving NRT only: disturbed sleep, dry mouth, headache, nausea, dizziness, low mood/depression, anxiety/panic, chest pain, disorientation, loss of appetite. | 20% of abstinent participants allocated to bupropion only had switched to NRT. No further detail reported on withdrawal due to AEs. |
| Swan 2003 | No seizures or deaths occurred. No serious AEs reported | Higher dose associated with more difficulty sleeping (48% vs 41%), difficulty concentrating (35% vs 28%), gastrointestinal problems (27% vs 20%) and shakiness/tremor (24% vs 17%) than lower dose. | 26% discontinued medication in 150 mg group and 31% in 300 mg group |
| Tashkin 2001 | No seizures occurred. 6 patients (placebo 5, bupropion 1) experienced a serious adverse event. One event (transient ischaemic attack) in placebo group thought to be related to study treatment. | Bupropion associated with more insomnia (24% vs 12%). Rates for headache (6% vs 6%) and dry mouth (6% vs 5%) similar in 2 groups. | 27 people discontinued medication, bupropion 14 (7%), placebo 13 (6.5%). Commonest reasons in bupropion group were anxiety (5), insomnia (4) |
| Tonnesen 2003 | No seizures occurred 7 patients (bupropion 6, placebo 1) experienced serious AEs within a week of ending treatment. A reasonable possibility that SAEs in 3 bupropion patients related to study medication (fainting due to insomnia, urticaria/angioedema (2). In addition one bupropion patient delivered twin girls 4 weeks after treatment termination, one still born. | Bupropion associated with more insomnia (24% vs 15%), dry mouth (13% vs 5%) headache (13% vs 10%) sleep disorder (10% vs 7%), constipation (6% vs 1%) and dizziness (7% vs 4%) | 8% on bupropion and 6% on placebo withdrew due to adverse events. |
| Tonstad 2003 | No seizures occurred. Five serious adverse events during treatment, all on bupropion. Only 1 (lupus disseminatus) considered related to medication. None led to medication discontinuation. Three SAEs within a week of treatment, none related to bupropion use. 36 patients (Bupropion 24, placebo 14) reported cardiovascular adverse events. 4 deaths (2, 2) during follow‐up phase, none related to study medication. | Bupropion associated with more insomnia (24% vs 12%), dry mouth (18% vs 10%), nausea (13% vs 6%), dizziness (8% vs 5%). 11% in each group reported headache. No evidence of any effect on vital signs in CVD patients. | 5% on bupropion and 6% on placebo withdrew due to adverse events. |
| Uyar 2007 | No seizures occurred | 56% on bupropion reported dry mouth, 44% headache, 40% insomnia. Sleep disturbance rates significantly higher than control (38% vs 9.6%). | 4 (8%) discontinued bupropion due to adverse effects |
| Wagena 2005 | No seizures occurred One death in placebo group, previously hospitalised with dermatological reactions | No significant differences between bupropion & placebo. Insomnia 34% vs 24%, Dry mouth 28% vs 20%, diarrhoea or constipation 34% vs 26% | 15% on bupropion and 9% on placebo discontinued medication |
| Wittchen 2011 | None occurred | Insomnia (7%) and dry mouth (7%) most frequently reported in bupropion group, rates not given for control group | 7 on bupropion discontinued due to adverse effects (11 in NRT group, 0 in control group) |
| Zellweger 2005 | Two seizures occurred in bupropion group. One patient had a possible familial predisposition and the other was sleep deprived. 1 patient on placebo suffered a transient ischemic attack and 1 a pulmonary sequestration | Bupropion associated with more insomnia (39% vs 22%). Similar rates of dry mouth (12% vs 10%), agitation (10% vs 11%), nausea (10% vs 7%). | 9% on bupropion and 5% on placebo withdrew due to adverse events, most commonly due to nervous system events in both groups. |
Assessing AEs in smoking cessation medications is difficult because any AEs may be due, not to the medication, but to nicotine withdrawal (i.e., physical dependence). In addition, given smokers are more likely to have several medical and psychiatric illnesses, some "new" AEs may be exacerbations of pre‐existing illnesses (Hughes 2008).
Adverse affects of bupropion
The most common side effects of bupropion are insomnia, occurring in 30% to 40% of patients, dry mouth (10%), and nausea (GlaxoSmithKline; Goldstein 1998). Typical drop‐out rates due to adverse events range from 7% to 12%, but in one study 31% of those on 300 mg and 26% on 150 mg discontinued medication (Swan 2003) and in a second small study testing the 300 mg dose in alcoholic smokers, 33% of those receiving active medication withdrew due to adverse events (compared to 11% on placebo) (Grant 2007). In a pragmatic, non‐blinded effectiveness trial, 20% of abstinent participants who had been allocated to bupropion at baseline switched to NRT due to adverse events (Stapleton 2013).
Allergic reactions have also been reported with bupropion. These include pruritus, hives, angioedema and dyspnoea. Symptoms of this type requiring medical treatment have been reported at a rate of about 1 to 3 per thousand in clinical trials (GlaxoSmithKline), and this is approximately the level at which they are being reported in the national surveillance schemes. Hypersensitivity reactions are listed as possible rare (occurring at rates less than 1 per 1000) adverse effects in the product data.
Bupropion is also an inhibitor of CYP2D6 so care is needed when starting or stopping bupropion use in patients taking other medication metabolized by this route (Kotlyar 2005).
Serious adverse events
For this update, we conducted meta‐analyses of SAEs reported in the studies of bupropion versus placebo or a no pharmacotherapy control (including studies that were excluded from efficacy analyses due to short length of follow‐up). Meta‐analyses of the 33 trials in which SAEs whilst on treatment were measured and reported found a marginal and statistically non‐significant excess of any SAE in the bupropion groups compared with the control groups (RR 1.30, 95% CI 1.00 to 1.69, 9631 participants, Figure 4), with event rates of 2.1% for bupropion and 1.9% for placebo users. Of the 33 trials, 11 reported no SAEs in either arm during the period of interest. Subgroup analysis of psychiatric SAEs detected no difference between the bupropion and placebo arms, with an RR of 0.60 (95% CI 0.28 to 1.28, 19 trials, analysis not shown). The event rates were 0.4% and 0.7%, respectively. Subgroup analysis of cardiovascular events also detected no difference between the two groups, with an RR of 1.16 (95% CI 0.65 to 2.06, 25 trials, analysis not shown) and event rates of 0.5% for bupropion and 0.4% for placebo.
4.
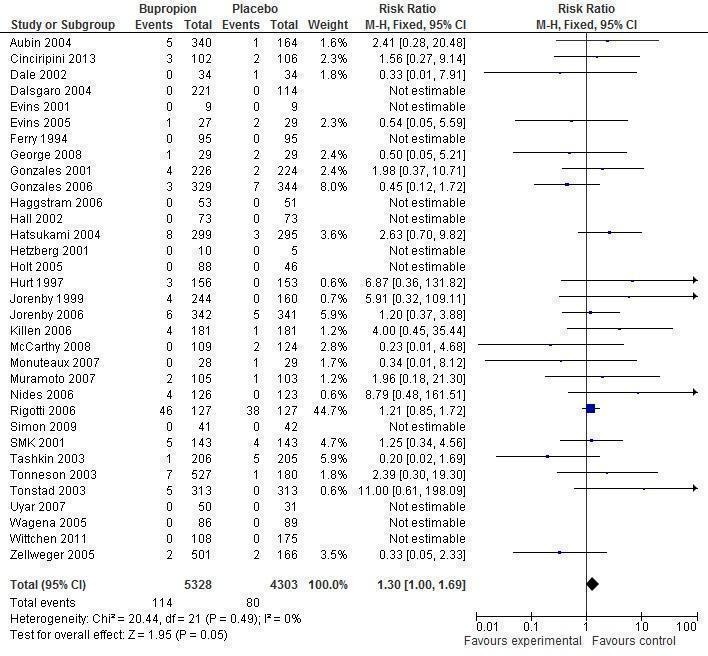
Seizures
Early trials of bupropion as a treatment for depression using the immediate release formulation and often doses greater than 300 mg/day suggested it increased the risk of seizures in those with a prior history of alcohol withdrawal, anorexia, or head trauma. This led to the development of the slow release (SR) preparation licensed for smoking cessation (and used in all but the earliest of the included studies of bupropion). Using this preparation in doses of 300 mg/day or less, and excluding those at risk of seizures, no seizures had been reported in any of the smoking cessation trials until the study in physicians and nurses in Europe (Zellweger 2005). In this study there were two seizures amongst 502 people randomized to bupropion, one of whom had a familial history (data from GlaxoSmithKline). Since then two seizures have been reported in a study in which 126 participants received bupropion (Nides 2006), one in a study with 329 treated (Gonzales 2006), one in a study with 289 treated (Covey 2007), one in a study with 362 treated (Killen 2006), and one in a study with 264 treated (Piper 2009). Two seizures were also reported in an unpublished study with 100 participants prescribed bupropion (Strayer 2004, personal communication). This gives a total of 10 seizures amongst over 13,000 people exposed in clinical trials, so despite these reports the overall seizure rate remains similar to the rate of 1:1000 given in product safety data. The figure of 1:1000 derives from a large, open, uncontrolled observational safety surveillance study conducted by the manufacturers (Dunner 1998) which examined 3100 adult patients using slow release bupropion for eight weeks for treatment of depression (not smoking cessation). Treatment was extended if necessary to a year, at a maximum dose of 150 mg twice daily. Patients with a history of eating disorder, or a personal or family history of epilepsy were excluded. Three participants (i.e. 1:1000) had a seizure considered to be related to the therapeutic use of bupropion.
The evidence on seizure risk from trials is consistent with findings from large observational studies of use of bupropion SR for smoking cessation. Boshier 2003 reported on a cohort of 11,753 English patients who had been dispensed bupropion. Eleven seizures were reported for a rate of 1 in 1000; four of these were associated with a past history of seizure. Hubbard 2005 used a UK general practice database to estimate the relative incidence of death or seizure in 9329 individuals over a mean (SD) follow‐up of 1.9 (0.9) years comparing each individual during a 'high risk' period following prescription of bupropion with him/herself outside this period. High risk periods of either 28 or 63 days were evaluated. The reported seizure rates were non‐significantly higher during the high risk periods (28 days: 3.62, 95% CI 0.87 to 15.09; 63 days: 2.38, 95% CI 0.72 to 7.93). A total of 45 seizures in 28 people were reported but only two occurred in the first 28 days of treatment, in one individual with no previous history of epilepsy. Of note in this study was that 12 people had been prescribed bupropion despite previous diagnoses of seizure.
Psychiatric adverse events
In 2009, the US Food and Drug Administration (FDA) added new warnings about the risk of serious mental health events including changes in behaviour, depressed mood, hostility, and suicidal thoughts in patients using bupropion for smoking cessation (US FDA 2009a; US FDA 2009b). The added warnings were based on the continued review of postmarketing adverse event reports for varenicline (a more recently licensed smoking cessation treatment, see Cahill 2012) and bupropion received by the FDA. There were 46 reports of suicidal ideation and 29 of suicidal behaviour for bupropion to November 27 2007 (US FDA 2009a). Prior to this, a review of the safety of bupropion had been undertaken by the European Agency for the Evaluation of Medicines for Human Use (EMEA 2002) which had stated that there was neither a pharmacological nor a clinical reason for suspecting bupropion to be causally associated with depression or suicide. Suicidal ideation had been observed in six out of a total of 4067 participants in clinical trials for smoking cessation, a rate of 1: 677. The rate of suicidal ideation with bupropion was stated to be low compared with the rates found in the general population but no data were presented. The EMEA review committee concluded that the benefit/risk balance remained favourable, but made recommendations to strengthen warnings on hypersensitivity and depression by advising clinicians to be aware of the possible emergence of significant depressive symptoms in patients undergoing a smoking cessation attempt.
Since the last update, three new observational analyses of psychiatric events in people using bupropion for smoking cessation have been published. All three compared bupropion with varenicline and two also included comparisons with NRT. A registry‐based cohort study in Denmark evaluated risk of psychiatric adverse events in people prescribed bupropion or varenicline over a period of three years. In propensity score‐matched analyses, there was no significant difference in psychiatric adverse events between participants receiving varenicline and participants receiving bupropion (hazard ratio 0.85, 95% CI 0.55 to 1.30) (Pasternak 2013). Similarly, in an analysis of five years of data from general practices in the UK, no differences in rates of depression, suicide and non‐fatal self‐harm were detected between people prescribed varenicline, bupropion or NRT for smoking cessation (bupropion versus NRT hazard ratio for fatal and non‐fatal self‐harm 0.83, 95% CI 0.30 to 2.31; for treated depression 0.63, 95% CI 0.46 to 0.87) (Thomas 2013). Conversely, an analysis based on US data comparing suicidal behaviour and depression in people prescribed bupropion,varenicline or NRT for smoking cessation did detect significant between group differences. Using NRT as a reference group, bupropion showed a statistically significant increased risk of suicidal behaviour and depression (OR 2.9, 95% CI 2.3 to 3.7), and varenicline showed statistically significant increased risk substantially higher than that of bupropion (OR 8.4, 95% CI 6.8 to 10.4) (Moore 2011).
Overdoses and deaths
Although no patient is reported to have died while taking bupropion in trials for smoking cessation, some have died while taking bupropion prescribed for smoking cessation in routine practice. There has been no formal epidemiological analysis of these deaths, but no national reporting scheme has concluded that bupropion caused these deaths. In a self‐controlled case series analysis (Hubbard 2005), death rates were non‐significantly lower during the high risk period (28 days after being prescribed bupropion) compared to the period before taking bupropion and after 28 days of being prescribed bupropion (0.5, 95% CI 0.12 to 2.05; 63 days: 0.47, 95% CI 0.18 to 1.19).
Bupropion may cause adverse effects in overdose. A review of bupropion‐only non‐therapeutic exposures reported to the US Toxic Exposure Surveillance System for 1998‐1999 identified 3755 exposures to Wellbutrin SR, 2184 to Wellbutrin, and 1409 to Zyban (Belson 2002). Non‐therapeutic exposures included intentional overdose and unintentional ingestion as well as reports of adverse reactions. Of those exposed to Zyban who showed any symptoms,13% developed a seizure. There were no deaths associated with Zyban.
Bupropion in pregnancy
A follow‐up of 136 women exposed to bupropion prescribed for smoking cessation or depression during the first trimester of pregnancy suggested that bupropion does not increase the rates of major malformations, but there were significantly more spontaneous abortions (Chun‐Fai‐Chan 2005). An assessment of potential infant exposure to bupropion and active metabolites in breast milk suggests that the exposure of an infant whose mother was taking a therapeutic dose would be small (Haas 2004).
Adverse effects of nortriptyline
Five studies comparing nortriptyline to placebo or to a no pharmacotherapy control measured and reported SAEs occurring during treatment only. However, no SAEs occurred whilst on treatment in any arms of these trials, meaning we were unable to calculate an RR for SAEs whilst on nortriptyline. The only serious adverse event in someone treated with nortriptyline was collapse/palpitations thought possibly caused by treatment (Aveyard 2008). The other adverse events reported included the well known tricyclic effects of dry mouth, drowsiness, light‐headedness and constipation observed in studies treating depression in which doses were often > 150 mg (Khawam 2006). When used at 75 to 150 mg doses in smokers, drop‐out rates in the trials reporting this outcome have ranged from 4% to 12%, with one exception (Wagena 2005). This rate is similar to that for bupropion and NRT. Nortriptyline can be lethal in overdoses. Studies of nortriptyline used for depression suggest it may have the potential for more serious adverse events than those reported in trials of nortriptyline for smoking cessation. Since nortriptyline is not approved for smoking cessation in any country, we are unaware of any observational data.
Discussion
Bupropion and nortriptyline
Forty‐four trials now provide a large, high quality evidence base confirming the benefit from bupropion used as single pharmacotherapy for smoking cessation (Table 1). There is no substantial statistical heterogeneity evident and the pooled estimate suggests that bupropion increased long‐term quitting success by relative factor of 1.5 to 1.8. Treatment effects appear to be comparable in a range of populations, settings and types of behavioural support and in smokers with and without a past history of depression. Clear evidence of an additional benefit from adding bupropion to NRT was not demonstrated.
Meta‐analysis of the three bupropion trials that compared the recommended dose of 300 mg/day (150 mg twice daily) with a dose of only 150 mg failed to show a significant long‐term benefit of the higher dose. Whilst the power of the comparison is not sufficient to establish equivalence, for people with troubling side effects such as insomnia, a reduction in dose to 150 mg in the morning would be an alternative to discontinuing pharmacotherapy altogether.
Evidence from eight head‐to‐head trials did not detect a significant difference between bupropion and NRT. This is consistent with a recent network meta‐analysis which found that, in indirect, across‐study comparison, the efficacy was similar, suggesting no advantage for one treatment over the other (Cahill 2013). The choice between these two therapies will depend on patient preferences, including a consideration of the risks of adverse events.
Pooled results from four trials found that participants treated with bupropion were significantly less likely to quit than those treated with varenicline, a partial nicotinic agonist. This is also consistent with results from the recent network meta‐analysis (Cahill 2013). Although this suggests varenicline should be preferred over bupropion as a first line therapy, further study is warranted for several reasons. First, the number of studies is still small. Second, the trials used very similar optimal samples, settings and procedures. Whether the same degree of superiority for varenicline would occur in a more real‐world setting is unclear. Finally, given that both bupropion and varenicline block nicotine receptors and increase dopamine, a biological explanation for superior efficacy for varenicline has not been proposed. The evidence for efficacy of varenicline is covered by another Cochrane review (Cahill 2012).
Two further trials of extended therapy with bupropion for individuals who have recently quit bring the number included to seven, and the pooled estimate narrowly misses statistical significance (RR 1.15, 95% CI 1.00 to 1.33) but the clinical importance of any effect seems likely to be small. Preventing relapse remains a major challenge.
Nortriptyline has also been shown to assist cessation; there is a moderate evidence base, limited by a relatively small number of trials and total number of participants (Table 2). As with bupropion, there is no evidence that the combination of nortriptyline and NRT is more effective than NRT alone.
There are no direct comparisons of nortriptyline with NRT or varenicline. Head‐to‐head comparison with bupropion in three trials favour bupropion but do not show a significant difference. The pooled risk ratios of efficacy of nortriptyline and bupropion appear broadly similar. One argument for considering nortriptyline as a first line therapy is its lower cost when compared to licensed therapies (Wagena 2005a). The main argument against this is based on the potential for serious adverse effects (Hughes 2005).
Data from two included studies new to this update suggest there may be a significant interaction between bupropion and current or past history of depression, with bupropion appearing more effective in participants with a history of depression (Schnoll 2010) or with current depression (Stapleton 2013). This contrasts with previous versions of this review which found that, although not widely tested, the efficacy of bupropion and nortriptyline appeared to be independent of a past history of depression (Hall 1998; Hayford 1999; Hurt 2002) and post‐cessation depression (Catley 2005, reporting an analysis of Ahluwalia 2002). These data suggested that their efficacy is not due to a traditional antidepressant effect and that they benefit those with no history of depression. Although the pharmacological mechanism of action of bupropion is still unclear, animal studies suggest that it may act as an antagonist at the nicotine receptor (Cryan 2003; Wiley 2002, Young 2002). How nortriptyline increases cessation is unclear. Regardless of relative efficacy, both treatments have been shown to be effective in people with no history of depression.
Although there is considerable research interest in genetic differences that could help predict response to pharmacotherapy (Uhl 2008), there is currently no genetic test that can be used for treatment matching in a clinical setting, and a recent analysis concluded that a single gene test to aid choice of treatment was not the most cost effective approach (Welton 2008). There is preliminary evidence that smokers with normal dopamine receptor availability and function might respond better to bupropion than to NRT (David 2005; Lerman 2006) whilst genotypes that are associated with impaired dopaminergic systems could have relatively better outcomes with NRT (Johnstone 2004). It is also possible that gender may moderate the interaction between bupropion and genotype (Swan 2005). The rate of metabolism of nicotine has also been suggested as a moderator of treatment effect (Collins 2004, reported in Patterson 2008).
Adverse effects
No seizures were reported in the first large studies of bupropion for smoking cessation but more recently seven studies (Covey 2007; Gonzales 2006; Killen 2006; Nides 2006; Piper 2009; Strayer 2004; Zellweger 2005) report a total of ten seizures. Since about 13,000 people have been exposed to the slow release formulation of bupropion in the cessation studies included in this review, the averaged rate is still less than the 1:1000 estimated risk used in product safety information, although the clustering of seizures in a few small studies is unexpected. Some suicides and deaths while taking bupropion have been reported. Currently, like many other antidepressants and varenicline, bupropion has a warning about the possibility of serious mood and behavioural changes. However, it remains unclear whether these outcomes were caused by bupropion effects. Meta‐analyses did not detect any difference between bupropion and placebo or no pharmacotherapy controls in rate of psychiatric or cardiovascular SAEs, and detected a marginal and non‐statistically significant increased rate of any SAEs in people randomized to bupropion.
In studies in depressed patients, nortriptyline sometimes caused sedation, constipation, urinary retention and cardiac problems, and when taken as an overdose could be fatal. Based on the rate of significant adverse events when used to treat depression, nortriptyline would be expected to have higher rate of drop‐outs in smoking cessation studies. This has not been the case in the relatively small number of subjects receiving nortriptyline in the existing studies (about 1300), perhaps because the dose of nortriptyline used (75 to 150 mg) is generally smaller than that used for depression and smokers are not acutely ill. No SAEs were reported in any trials comparing nortriptyline to placebo or a no pharmacotherapy control. However, given the small sample size, the safety of these doses of nortriptyline for smoking cessation is still unclear.
Other antidepressants
The seven long‐term trials of selective serotonin reuptake inhibitors (SSRIs) (fluoxetine, paroxetine and sertraline) and other short‐term trials have, somewhat surprisingly, failed to show that this class of antidepressants helps smoking cessation. Some studies have found SSRIs effective in post hoc‐determined subgroups (Borrelli 2004; Swan 1999) but this requires verification. The most recent trial of fluoxetine alone (Spring 2007) found that although fluoxetine initially increased cessation amongst smokers with a history of depressive disorder, by the end of the study it impaired cessation regardless of depressive history. A recent study of fluoxetine as an adjunct to NRT in people with elevated symptoms of depression (Brown 2013) found it significantly impaired cessation in the group receiving 10 weeks of treatment, and did not have a significant effect on the group receiving 16 weeks of treatment, though again less people quit in the group receiving fluoxetine.
There is no evidence of long‐term efficacy for monoamine oxidase inhibitors. Two early trials of selegiline suggested a possible benefit but the more recent trials do not support this.
This update saw the inclusion of studies of alternative therapies for depression. Two studies of St John's wort did not detect a significant effect, with conflicting results and low quit rates across all arms. The one study of the dietary supplement SAMe also did not detect a significant effect.
Mechanism of action of antidepressants
Whether the efficacy of bupropion and nortriptyline is specific to the unique pharmacology of these medications or whether it would occur in all antidepressants has not been completely resolved. The SSRI antidepressants appear not to be efficacious. This suggests serotonin modulation is not important, leaving the dopaminergic or noradrenergic effects of nortriptyline and bupropion to account for their efficacy. Although the efficacy of bupropion was initially thought to be due to its dopaminergic actions, nortriptyline, which is also effective, has relatively weak dopaminergic activity. In addition, bupropion has as much noradrenergic activity as dopaminergic activity. Another possibility, at least for bupropion, is that it acts as a nicotinic receptor blocker (Warner 2005). Whether the same is true for nortriptyline is not clear (Gambassi 1999). If noradrenergic effects are important in treatments for smoking, then monoamine oxidase inhibitors and other tricyclic antidepressants should be effective; however, the six small trials of these present conflicting results.
Agreements and disagreements with other studies or reviews
The findings of this review are in agreement with the conclusions of other reviews and guidelines (Aubin 2002; Haustein 2003; Hughes 2005; Jorenby 2002; Martinez‐Raga 2003; McRobbie 2005; RCP 2000; Tonstad 2002; Tracey 2002; West 2000; West 2003). US smoking cessation guidelines (Fiore 2008) continue to recommend bupropion as a first line therapy and nortriptyline as a second‐line therapy due to possible adverse events. Open uncontrolled trials and observational studies of bupropion have shown real‐life quit rates comparable to those found in clinical trials (Holmes 2004; Paluck 2006; Wilkes 2005). Studies of cost‐effectiveness also support the utility of bupropion (Bolin 2006; Javitz 2004; Welton 2008) and nortriptyline (Hall 2005). Findings regarding the efficacy of bupropion in smokers with current or past depression are consistent with those from a separate Cochrane review evaluating smoking cessation treatments exclusively in these populations (van der Meer 2013).
However, our findings on the effectiveness of bupropion as an adjunct to NRT differ from the results of the USPHS clinical practice guideline. Whereas we did not detect a significant difference, the US guideline reported an odds ratio of 1.3 (95% CI 1.0 to 1.8) for a combination versus nicotine patch alone (Fiore 2008 table 6.28). The difference in meta‐analytic outcomes may be because the current analysis included several studies of hard‐to‐treat populations not included in the USPHS analysis. Also, it could be because the Cochrane analysis was a collation of 12 within‐study randomized comparisons whereas the USPS was an across‐study comparison of the results from the combination arm in three trials and the results from the patch alone arm in 32 studies.
Similar to our findings, other systematic reviews looking at the serious adverse events profile of bupropion remain inconclusive. A meta‐analysis of suicidality in placebo‐controlled trials of bupropion detected no significant difference between bupropion and placebo groups (Wightman 2010) and a review of trials in which bupropion was prescribed to hospitalised patients with cardiovascular disease highlighted the need for larger randomized controlled trials to determine the long‐term safety of bupropion in this population (Grandi 2011a).
Authors' conclusions
Implications for practice.
The existing evidence supports a role for bupropion and nortriptyline in clinical practice. Nicotine replacement therapy has proven efficacy in over 100 studies (Stead 2012) and has a very benign side‐effect profile. There is insufficient published evidence to conclude either bupropion or nortriptyline has superior efficacy to NRT or vice versa. The confidence intervals around the efficacy estimates for bupropion, nortriptyline and NRT overlap. Nortriptyline appears equally effective in smokers with and without a history of depression. Data are conflicting on whether there is an interaction between bupropion and current or past depression, though efficacy has been established for all groups. The efficacy of bupropion and nortriptyline does not appear to be mediated by improving post‐cessation depression. Whether smokers with a previous history of depression or mild current depression would do better with bupropion or nortriptyline than NRT has not been tested. Whether bupropion prevents depressive symptoms or relapse to depression better than NRT has also not been studied. Patient preferences, cost, availability, and side‐effect profile will all need to be taken into account in choosing among medications. Bupropion and nortriptyline may be helpful in those who fail on NRT. Studies comparing bupropion with varenicline have shown higher quit rates with varenicline.
All smoking cessation medications can produce clinically significant adverse effects. When people are initially screened for potential adverse effects, however, fewer than 10% of those on antidepressants for smoking cessation stop taking the medications due to adverse effects. Although bupropion use has been associated with deaths in lay public reports, currently there is insufficient evidence to state that bupropion caused these deaths. There has also been concern about antidepressants such as bupropion being associated with psychiatric disorders including suicidal ideation and suicide attempts. Again, it is not clear that there is a causal relationship. Smoking cessation may also precipitate depression (Hughes 2007). Also, although nortriptyline is associated with more side effects when used for depression, in the doses used for smoking cessation this may not be true.
Slow release bupropion, under the name Zyban, is licensed for smoking cessation in many parts of the world, including North America, Australia, and Europe, but is not available in many other countries. Often, bupropion is available in these countries under the name Wellbutrin SR as a treatment for depression. Nortriptyline is marketed as an antidepressant in many countries but is not currently marketed as a smoking cessation aid in any country. In almost all countries, bupropion and nortriptyline are available only as prescription medications.
Implications for research.
More research is needed with different antidepressants to determine which antidepressants or classes of antidepressant are effective in smoking cessation. Determining this could provide insight not only into the mechanism of action of antidepressant efficacy but also into the biological factors controlling nicotine dependence and smoking. Antidepressants of the SSRI category are not effective, which suggests serotonin may not be an important factor. However, it is unclear whether dopaminergic, noradrenergic, or nicotinic‐cholinergic monoaminergic activity or blockage of nicotine receptors is most important for cessation efficacy. Similarly, the suggestive findings that genotype might moderate antidepressant treatment efficacy deserve follow‐up. Research on the biological and behavioural mediators of the efficacy of bupropion and nortriptyline is needed; e.g. how much of their efficacy is due to craving or withdrawal relief, blocking nicotine reinforcement, or preventing lapses from becoming relapses. Knowledge of whether NRT or antidepressants have more efficacy in decreasing depression post‐cessation would help decide whether smokers with a past history of depression should prefer antidepressants over NRT.
Given the concern by some about deaths and psychiatric disorders from antidepressants used for smoking cessation, continued monitoring is indicated.
What's new
| Date | Event | Description |
|---|---|---|
| 14 June 2016 | Amended | Corrected typographical error in Abstract results. Risk Ratio for buproprion + NRT (12 trials) changed from 1.9 to 1.19. Now matches meta‐analysis 1.5 |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 3, 1997
| Date | Event | Description |
|---|---|---|
| 8 October 2013 | New search has been performed | Updated with 24 new included studies. Studies of S‐Adenosyl‐L‐Methionine and St John's wort included for the first time. Meta‐analyses of serious adverse events added. |
| 8 October 2013 | New citation required but conclusions have not changed | Conclusions largely unchanged. Efficacy findings unchanged. |
| 22 June 2011 | Amended | Additional table converted to appendix to correct pdf format |
| 5 October 2009 | Amended | Correction to excluded studies table, detail for Carrão 2007 |
| 30 July 2009 | New search has been performed | Updated with 13 new included trials including 3 of selegiline, not previously covered. No substantial change to effects, main conclusions not altered |
| 17 June 2008 | Amended | Converted to new review format. |
| 11 October 2006 | New citation required but conclusions have not changed | Seventeen new trials were added to the review for issue 1, 2007. There were no major changes to the reviewers' conclusions. |
| 16 July 2004 | New citation required but conclusions have not changed | New trials of bupropion, nortriptyline and fluoxetine were added for issue 4, 2004, and additional information on adverse effects was included. There were no major changes to the reviewers' conclusions. |
| 8 January 2003 | New citation required but conclusions have not changed | New trials of bupropion and nortriptyline were added to the review in Issue 2 2003. There were no major changes to the reviewers' conclusions |
| 19 September 2001 | New citation required but conclusions have not changed | Four new studies on bupropion, and one each on nortriptyline and paroxetine were added to the review in Issue 1 2002. In press data from a trial of fluoxetine are included which differ from unpublished data previously used. The reviewers' conclusions about the efficacy of bupropion and nortriptyline were not changed substantively. |
| 28 August 2000 | New citation required and conclusions have changed | Updates the earlier Cochrane review 'Anxiolytics and antidepressants for smoking cessation'. Anxiolytics are evaluated in a separate review. |
Notes
This review was first published as part of the review 'Anxiolytics and antidepressants for smoking cessation'. From Issue 4 2000 the classes of drugs are reviewed separately.
Acknowledgements
Our thanks to Drs Niaura, Borrelli, Spring, Fiore, Hurt, Mizes, Ferry, Schuh, Cinciripini, Hays, Prochazka, Ahluwalia, Mayo, Collins, Novotny, Brown, David, Evins, Le Foll, Glover & Piper for assistance with additional information or data on studies.
Appendices
Appendix 1. Specialised Register search strategy
Searched using CRS (Cochrane Register of Studies) software
#1 (bupropion or zyban):TI,AB,MH,EMT,KY,XKY
#2 nortriptyline:TI,AB,MH,EMT,KY,XKY
#3 (monoamine oxidase inhib*):TI,AB,MH,EMT,KY,XKY
#4 (moclobemide or selegiline or lazabemide):TI,AB,MH,EMT,KY,XKY
#5 (SSRI* or (selective serotonin re?uptake inhibitor*)):TI,AB,MH,EMT,KY,XKY
#6 (fluoxetine or sertraline or paroxetine or zimelidine):TI,AB,MH,EMT,KY,XKY
#7 (doxepin or imipramine or tryptophan or venlafaxine):TI,AB,MH,EMT,KY,XKY
#8 ((john?s wort) or hypericum):TI,AB,MH,EMT,KY,XKY
#9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
(MH, EMT, KY and XKY are keyword fields)
Appendix 2. Suspected adverse events from national reporting schemes
| Country | No. of reports | No. of users | Rate of reports | Events reported | Source/ date |
| UK (safety notice 2002) | 7,630 24 July 2002 | 540,000 patients (31 March 2002) | 14/1000 | Commonest: Urticaria/rashes/pruritus (2357, 31% of total reports) Insomnia (994, 13%) Headache (779, 10%) Dizziness (747, 10%) Seizures: 184 (2.4% of reports, est rate 0.3/1000) Deaths: 60 | Medicines Control Agency 26 July 2002 http://www.mca.gov.uk/ourwork/ monitorsafequalmed/ safetymessages/zyban26702.pdf |
| UK (MHRA routine data on suspected adverse drug reactions). Includes reports summarised in UK safety notice 2002 | 8,452 (18,319 reactions) to May 2004 | Estimated approximately 1,000,000 community prescriptions dispensed in UK 2000‐April 2004, based on England data of 762,200 for 2000‐2003. Average prescription was a 4 week course. | approx 8/1000 prescriptions | By organ class: ‐General disorders: 4127 Includes insomnia (1030), dizziness (805), chest pain (384) ‐Skin & subcutaneous tissue disorders: 3728 Includes pruritus (421), rashes (1094), urticaria (1104), angioedema (481) ‐Psychiatric disorders: 2417 Includes affective disorders (627), suicidal ideation/ suicide/parasuicide (87) ‐Neurological disorders: 2338 Includes convulsions & epilepsy (212) ‐Gastrointestinal disturbances: 2176 Includes nausea / vomiting (775) ‐Deaths: 70, includes 4 suicides | Medicine and Healthcare products Regulatory Agency (MHRA) Adverse Drug Reactions Information Service. Data provided April 2004. Prescription data from DoH Prescription Cost Analysis (www.publications.doh.gov.uk/prescriptionstatistics/index.htm) |
| Canada (safety notice 2001) | 1,127 28 May 2001 | 1,245,000 Zyban, 699,000 Wellbutrin (April 30 2001) | 0.6/1000 | Full list not given Serious: 682 Seizures: 172 (Zyban 120, Wellbutrin 46 bupropion 6) (15% of reports, est rate 0.1/1000 for Zyban Deaths: 19 Serum sickness: 37 | Canadian Adverse Reaction Monitoring Programme (CADRMP)GSK/ Health Canada 'Dear Doctor' letter 3 July 2001 http://www.hc‐sc.gc.ca/hpfb‐dgpsa/tpd‐dpt/zyban_e.html |
| Australia (safety alert 2001) | 1,237 August 2001 | Not given | not available | Full list not given skin reactions (499 reports), psychiatric disturbances (427), nervous system (406, includes convulsions/twitching 74), gastrointestinal tract (258), facial/angioedema (89), serum sickness (63) Deaths: 18 | Therapeutic Goods Administration (TGA) alert 31 August 2001 http://www.health.gov.au/tga/ docs/html/zyban.htm |
| Australia (routine data) | 1,672 (4,390 reactions) to March 2004 | Approx 534,000 prescriptions 2000‐March 2004, mainly 30 pill/2 week | approx 3/1,000 prescriptions | Deaths: 31 Skin & subcutaneous tissue: 930. Includes urticaria (366), pruritus (90), rash (164), oedema (160) Nervous system: 792. Includes convulsions (105), Psychiatric disorders: 992. Includes suicide/self‐injurious ideation (32), depression (13) Gastointestinal disorders: 440. Includes nausea/ vomiting (221) | TGA data supplied 31 March 2004 |
| France (analysis of pharmacovigilance database & GSK reports) | 1682 of which 475 classified as serious, September 2001 to September 2004 | 698,000 patients treated | approx 2.4/1,0000 people | Deaths: 21 (including 3 suicides)
Neuropsychiatric: 62, includes suicide attempts (21), suicidal ideation (19), seizures: (75, incidence 0.01%).
Skin & subcutaneous tissue: 148, includes angiodema (50), serum sickness like reaction (40), urticaria (27). 11 intentional overdose including 2 deaths |
Beyens 2008 |
Data and analyses
Comparison 1. Bupropion. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bupropion versus placebo/control. Subgroups by length of follow‐up | 44 | 13728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.49, 1.76] |
| 1.1 Twelve month follow‐up | 27 | 9866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.44, 1.76] |
| 1.2 Six month follow‐up | 17 | 3862 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.45, 1.97] |
| 2 Bupropion versus placebo/control. Subgroups by clinical/recruitment setting | 44 | 13728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.49, 1.76] |
| 2.1 Community volunteers | 21 | 7524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.49, 1.87] |
| 2.2 People recruited from health care settings | 18 | 3928 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.38, 1.86] |
| 2.3 Community + health care settings | 1 | 540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.83, 2.13] |
| 2.4 Health care professionals/ hospital staff | 2 | 1002 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.98, 1.78] |
| 2.5 People with a previously unsuccessful quit attempt using bupropion | 2 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.29, 3.90] |
| 3 Bupropion versus placebo. Subgroups by level of behavioural support | 41 | 13012 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.49, 1.77] |
| 3.1 Multisession group behavioural support | 10 | 2001 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [1.44, 2.16] |
| 3.2 Multisession individual counselling | 30 | 10964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.45, 1.76] |
| 3.3 Low intensity support | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.32, 25.68] |
| 4 Bupropion dose response. 300 mg/day versus 150 mg/day | 3 | 2042 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.26] |
| 5 Bupropion and NRT versus NRT alone | 12 | 3487 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.94, 1.51] |
| 5.1 Patch alone | 9 | 1774 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.84, 1.84] |
| 5.2 Lozenge alone | 2 | 1051 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.81, 1.81] |
| 5.3 Choice of NRT | 1 | 662 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.28] |
| 6 Bupropion for relapse prevention | 7 | 1959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.00, 1.33] |
| 7 Bupropion versus NRT | 8 | 4086 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.09] |
| 7.1 Patch | 6 | 1634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.27] |
| 7.2 Lozenge | 2 | 694 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.22] |
| 7.3 Patch + lozenge | 2 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 0.98] |
| 7.4 Choice of NRT | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.87, 1.33] |
| 8 Bupropion versus varenicline | 4 | 1810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.83] |
| 9 Bupropion for harm reduction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Reduction in cotinine >50% from baseline at 1y | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Cessation at 6m | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Nortriptyline. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nortriptyline versus placebo | 6 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.48, 2.78] |
| 2 Nortriptyline and NRT versus NRT alone | 4 | 1644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.55] |
Comparison 3. Bupropion versus nortriptyline. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bupropion versus nortriptyline | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.93, 1.82] |
Comparison 4. Bupropion and nortriptyline. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nortriptyline vs placebo. 'No report' = no information, 'None occurred' = explicit statement | Other data | No numeric data | ||
| 2 Bupropion versus control. 'No report' = no information, 'None occurred' = explicit statement | Other data | No numeric data |
Comparison 5. Selective Serotonin Reuptake Inhibitors (SSRIs) versus placebo. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SSRI versus placebo/control | 4 | 1594 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.71, 1.22] |
| 1.1 Fluoxetine | 2 | 1236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.30] |
| 1.2 Paroxetine | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.64, 1.82] |
| 1.3 Sertraline | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.30, 1.64] |
| 2 SSRI and NRT versus NRT alone | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.03] |
| 2.1 Fluoxetine | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.03] |
Comparison 6. Monoamine oxidase inhibitors (MAOIs) versus placebo. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 MAOIs versus placebo | 6 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.79] |
| 1.1 Moclobemide | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.67, 3.68] |
| 1.2 Selegiline | 5 | 739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.88, 1.78] |
Comparison 7. Venlafaxine versus placebo. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Venlafaxine versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 8. St John's wort versus placebo. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 St John's wort versus placebo | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.26, 2.53] |
8.1. Analysis.
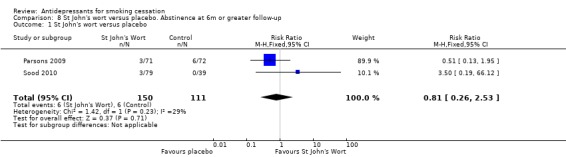
Comparison 8 St John's wort versus placebo. Abstinence at 6m or greater follow‐up, Outcome 1 St John's wort versus placebo.
Comparison 9. SAMe versus placebo. Abstinence at 6m or greater follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SAMe versus placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahluwalia 2002.
| Methods | BUPROPION
Randomized controlled trial Setting: community‐based health care centre, USA Recruitment: community volunteers |
|
| Participants | 600 African American smokers, > 10 CPD; 70% F, av. age 44, av. CPD 17, 27% had possible clinical depression (CES‐D > 16) | |
| Interventions | 1. Bupropion 300 mg/day for 7 weeks 2. Placebo Both arms: 8 sessions of in‐person or telephone counselling & S‐H guide | |
| Outcomes | Abstinence at 26w (prolonged) Validation: CO <= 10 ppm, discrepancies resolved with cotinine <= 20 mg | |
| Notes | Continuous abstinence rates shown in Figure 3 of paper. Figures obtained from authors. Funding: National Cancer Institute. GlaxoSmithKline provided study medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization codes were generated in blocks of 50 and sent to the pharmaceutical company..." |
| Allocation concealment (selection bias) | Low risk | Blinded drugs provided to investigator; " ... [the pharmaceutical company]... packaged the treatment and then shipped the blinded drug to the investigator." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. "Blinding was successful. At the end of treatment, 58% (150/259) of participants correctly guessed that they received bupropion SR, and 41% (104/253) correctly guessed they received placebo." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Approximately 32% lost to follow‐up in each group; included as smokers. |
Aubin 2004.
| Methods | BUPROPION Randomized controlled trial Setting: 74 cessation outpatient clinics, France Recruitment: volunteers Randomization: computer‐generated, blind |
|
| Participants | 504 smokers, >= 10 CPD; 56% F, av age 41, av CPD NS, 16% history of MDD | |
| Interventions | 1. Bupropion 300 mg for 7 weeks 2. Placebo Both arms: motivational support at clinic visits at baseline, w3, w7, w12 & 3 phone calls TQD, 2‐3 days later, w5, w18 | |
| Outcomes | Abstinence at 26w (continuous from w4) Validation: CO < 10 ppm | |
| Notes | First included as Lebargy 2003 based on abstract. Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The computerized randomization schedule, prepared by the sponsor, was inaccessible to the investigator who was provided with a specific set of sequential treatment numbers." |
| Allocation concealment (selection bias) | Low risk | "The computerized randomization schedule, prepared by the sponsor, was inaccessible to the investigator who was provided with a specific set of sequential treatment numbers." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" "Blinding was assured by matching the placebo to the bupropion tablets..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26% of the placebo and 27% of the bupropion groups lost; included as smokers. |
Aveyard 2008.
| Methods | NORTRIPTYLINE Randomized controlled trial Setting: National Health Service stop smoking clinics, UK Recruitment: People attending clinics |
|
| Participants | 901 smokers, ≥10/day; 46% F, av. age 43, av. CPD 21 | |
| Interventions | 1. Nortriptyline 75 mg/day, for 8 w including tapering (max dose for 6w) 2. Placebo capsules All participants received free NRT and had behavioural support, the majority attending group sessions run by cessation specialists | |
| Outcomes | Abstinence at 12 months (prolonged from day 15 post quit) Validation: CO at 4w, saliva cotinine (collected by post) at 6m & 12m | |
| Notes | Funding: Cancer Research UK and National Institute for Health Research. Medication provided by King Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent statistician generated the randomisation schedule in Stata. We used block randomisation, with randomly ordered block sizes of two, four, and six, stratified by stop smoking adviser." |
| Allocation concealment (selection bias) | Low risk | Study nurses recruited participants, and the study administrator (who had not met the participants) allocated participants in sequence against the list for each adviser. Only the administrator and the pharmacist knew the allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Advisers, participants, and study staff...were blind to allocation... tablets were encapsulated, and identical powder filled capsules provided the placebos." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% I, 17% C lost at 12m, included as smokers. Authors note that majority of losses were already smoking. |
Berlin 1995.
| Methods | MOCLOBEMIDE Randomized controlled trial Setting: clinic, France Recruitment: By adverts in general practices or from occupational medicine depts |
|
| Participants | 88 smokers, >20/day and FTQ>=6. No current major depression or anxiety disorders. 57% had history of MDD | |
| Interventions | 1. Moclobemide 400 mg/day for 1w pre‐ and 2m post‐TQD, 200 mg for 3rd month 2. Placebo (P) No behavioural intervention or counselling | |
| Outcomes | Abstinence at 1 year (prolonged) Abstinence verified at all visits up to 6m by plasma cotinine <= 20 ng/ml. 1 year abstinence based on telephone self report by 6m quitters. | |
| Notes | There were no serious adverse reactions. Insomnia was more common in drug (36%) than P (7%) groups. There were 4 drop‐outs for adverse effects/relapse in drug and 2 in P. Funding: Roche |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind, but blinding at allocation not explicit. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not reported. "Relapses and subjects lost from follow‐up were considered treatment failures." |
Biberman 2003.
| Methods | SELEGILINE Randomized controlled trial Setting: 3 community‐based clinic, Israel Recruitment: mailing to members of public health service provider |
|
| Participants | 109 smokers (15+ CPD); 38% F, av. age 42, av. CPD 27‐30 | |
| Interventions | 1. Selegiline 10 mg/day for 26 weeks, nicotine patch 21 mg for 8 weeks incl tapering 2. Placebo & nicotine patch Both arms: Behavioural support from trained family physician; weekly then fortnightly visits for 12w | |
| Outcomes | Abstinence at 52 w, continuous with validation at each visit Validation: negative for urine nicotine, cotinine, 3‐hydroxycotinine (Niccheck) | |
| Notes | No serious AEs, no significant differences in AEs, 2 selegiline discontinuations. Funding not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Four hundred dice‐throwing generated a randomized sequence code; 199 containers prepacked with selegiline and 201 containers prepacked with placebo were numbered accordingly." Judged adequate. |
| Allocation concealment (selection bias) | Low risk | "The code was sealed, kept secretly and was revealed for the first time when the last participant finished the 12 months of follow‐up. The first participant who joined the trial after the initial visit run‐in phase received the first bottle from the container set number 001, the second participant from set number 002 and so on. The trial coordinator arranged participant’s allocation." Judged adequate. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" (see above) "No discontinuation difference for selegiline or placebo was observed among the groups, which implies masking success." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 lost to follow‐up, included as smokers in MA. |
Blondal 1999.
| Methods | FLUOXETINE Randomized controlled trial Setting: cessation clinic, Iceland Recruitment: community volunteers |
|
| Participants | 100 smokers (excl 5 early withdrawals), > 10 CPD; 62% F, av age 41, av CPD 28, 38% fluoxetine/56% placebo had history of depression | |
| Interventions | 1. Nicotine inhaler and fluoxetine for 3m, option of continuing for 3m more. Fluoxetine 10 mg/day initiated 16 days before TQD, increased to 20 mg/day on day 6. 2. Nicotine inhaler and placebo Both arms: 5 x 1 hr group behaviour therapy. Advised to use 6‐12 inhalers/day for up to 6m. | |
| Outcomes | Abstinence at 1 year (sustained from quit day) Validation: CO < 10 ppm at all assessments (6w, 3,6, 12 m) | |
| Notes | Funding: Oddur Olafsson Fund, Pharmacia & Upjohn Consumer Health Care. Delta Pharmaceutical Company provided fluoxetine and placebo and fluoxetine analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization; part of the randomization procedure was performed by the manufacturer at another location where the code was also kept until it was broken in May 1997. |
| Allocation concealment (selection bias) | Low risk | Randomization codes applied to pill boxes which were then allocated sequentially. "This part of the randomization procedure was performed by the manufacturer at another location where the code was also kept." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind." "...pill boxes, with either fluoxetine or an identical appearing placebo containing the same ingredients except fluoxetine, were labelled with numbers ranging from 100 to 210.." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low numbers lost to follow up but reported results exclude 5 withdrawals; 3 from fluoxetine group due to adverse effects ‐ nervousness and anxiety, 1 from fluoxetine due to pregnancy, 1 from placebo who had purchased fluoxetine. |
Brown 2007.
| Methods | BUPROPION
Randomized controlled trial, 2x2 factorial design Setting: 2 clinical sites (Butler Hospital, Miriam Hospital) Rhode Island, USA Recruitment: community volunteers |
|
| Participants | 524 smokers >= 10 CPD; 48% F, av. age 44, av. CPD 25, 17.6% with history of MDD single episode, 3.1% recurrent MDD | |
| Interventions | 2 x 2 factorial design. Alternative psychosocial treatments were standard cessation therapy or plus CBT for depression. Both had 12 x 90 min groups twice weekly/ weekly/ monthly for 12w. TQD 5th session. Collapsed in this analysis 1. Bupropion 300 mg/day for 12 weeks 2. Placebo | |
| Outcomes | Abstinence at 12m (sustained at 4 visits) Validation: CO <= 10 ppm, saliva cotinine <= 15 ng/ml | |
| Notes | First included as Brown 2006, part unpublished data. Some genotyping studies combine these participants with those reported in Collins 2004 Funding: National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned to one of two treatment sites, where they were to receive one of two manualized group treatments ... Participants were then randomly assigned to receive one of two medication conditions, bupropion or placebo, using the urn randomization technique." |
| Allocation concealment (selection bias) | Unclear risk | "Whereas we were able to balance the drug and placebo conditions on an individual basis, behavioral treatments were randomized by group and thus were more susceptible to fluctuations in recruitment and to the availability at both sites of pairings of a senior and a junior therapist trained in CBTD". Knowledge of behavioural assignment was probably not concealed but seems unlikely to have lead to individual selection bias. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind." Psychological condition unlikely to be blinded but unlikely to affect comparisons included in this review. "All participants and study staff were blind to medication condition." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81% provided complete outcome data at all follow ups, not related to treatment condition. All participants included in ITT analyses |
Brown 2013.
| Methods | FLUOXETINE Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers |
|
| Participants | 216 smokers with elevated depressive symptoms (CES‐D score ≥ 6) smoking ≥ 10 cpd. 38.4% F, av age 46, av cpd 21, mean FTND 5.6, mean CES‐D 11.4 | |
| Interventions | 1. 10 weeks of 20 mg fluoxetine (beginning 2 weeks prior to TQD) 2. 16 weeks of 20 mg fluoxetine (beginning 8 weeks prior to TQD) 3. Control (no placebo) All arms: nicotine patch for 8 weeks starting on TQD (21 mg/day for 4 weeks, 14mg/day for 2 weeks, 7 mg/day for last 2 weeks), 5 sessions of brief behavioural smoking cessation treatment (in person and phone over 4 weeks, 20‐ 30 mins each) |
|
| Outcomes | Continuous abstinence at 12m Validation: salivary cotinine < 10 ng/ml |
|
| Notes | New for 2013. Significantly higher abstinence in 16 week arm than in 10 week arm, results presented separately in meta‐analysis with control divided. N abstinent not reported, extrapolated from percentages provided. Funding: American Cancer Society |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Urn randomization, no further detail provided |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% followed up at 12m. Similar rates across arms. |
Cinciripini 2005.
| Methods | VENLAFAXINE Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers |
|
| Participants | 135 smokers, >= 10 CPD; 50% F, av age 46, av CPD 27 | |
| Interventions | 1. Venlafaxine titrated to max of 225 mg/day from 3w before quit day for 21w, including 2w tapering. 2. Placebo Both arms: 6w 22 mg nicotine patch, and 9x 15 min behavioural counselling. | |
| Outcomes | Abstinence at 12m (PP) Validation: CO <= 10 ppm and/or saliva cotinine < 15 ng/ul Adverse events/withdrawals: not reported | |
| Notes | First included as Cinciripini 1999 based on abstract. Funding: National Institutes for Health and National Institute for Drug Abuse. Medication provided free of charge by Wyeth Ayerst Laboratories. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. Stratification by depression history. |
| Allocation concealment (selection bias) | Low risk | Randomization by pharmacy, all study personnel with direct patient contact blind. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind... Blinding of the study staff to the medication was maintained using prenumbered pill containers, assigned to each participant at randomization by the pharmacy. All study personnel with direct patient contact were blind to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Majority of participants followed‐up (65 intervention; 63 control), participants lost to follow‐up counted as smokers |
Cinciripini 2013.
| Methods | BUPROPION Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers |
|
| Participants | 294 smokers of ≥ 5 cpd 61% M, av age 44, av cpd 20, mean FTND 4.5 |
|
| Interventions | 1. 12 weeks bupropion started 12‐19 days before TQD (150mg/d days 1‐3, 300mg/d thereafter) 2. 12 weeks varenicline on same schedule (0.5mg/day days 1‐3, 1.0mg/day days 4‐7, 2.0mg/day thereafter) 3. Placebo on same schedule All arms: 10 individual counselling sessions (6 in person, 4 via phone, 240 mins total) |
|
| Outcomes | Continuous abstinence after 2 week grace period at 6m (Other prolonged abstinence outcomes also reported) Validation: CO < 10 ppm or salivary cotinine < 15 ng/mL |
|
| Notes | New for 2013 In less than 1% of the total cases, participants who did not attend a follow‐up were coded as abstinent because they were abstinent at the following data point. All other losses to follow‐up counted as smokers. Author provided further detail on AE measurements via e‐mail. Funding: National Institute on Drug Abuse, National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Adaptive randomization,” no further detail provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “Blinded” but no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73% followed up at 6m, similar rates across arms, all lost to follow‐up known to be smokers |
Collins 2004.
| Methods | BUPROPION Randomized controlled trial Setting: 2 clinical research sites (Georgetown University Medical Center & State University of New York), USA Recruitment: community volunteers |
|
| Participants | 555 smokers, >= 10 CPD, excluding history of psychiatric disorder including MDD; 57% F, av. age 46, av. CPD 21 | |
| Interventions | 1. Bupropion 300 mg/day for 10 w begun 2 w before TQD 2. Placebo Both arms: 7 sessions group behavioural counselling | |
| Outcomes | Abstinence at 6m (prolonged from w2, 7 consecutive days of smoking defined as relapse) Validation: saliva cotinine <= 15 ng/ml | |
| Notes | Replaces Lerman 2002 which reported subset of data. Denominators supplied by 1st author, excludes 114 who withdrew before intervention. Some study details from Lerman 2006. Some genotyping studies combine these participants with those reported in Brown 2007. Funding: National Cancer Institute, National Institute on Drug Abuse, National Center for Research Resources. Treatment provided free of charge by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was determined by a computer‐generated randomization scheme operated by a senior data manager; stratification was carried out by study site" (Lerman 2006). |
| Allocation concealment (selection bias) | Low risk | Centrally generated & allocation concealed from counsellors & assessors. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo used but blinding procedure not described in detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% lost to follow‐up at 6 month follow‐up; included as smokers. |
Covey 2002.
| Methods | SERTRALINE Randomized controlled trial Setting: clinic, USA Recruitment: volunteers |
|
| Participants | 134 smokers with a history of past MDD; 65% F, av age 44.5, 47% had history of recurrent MDD | |
| Interventions | 1. Sertraline starting dose 50 mg/day, 200 mg/day by week 4 quit day. 9 day taper. Total duration 10w + 9 day taper, including 1w placebo washout prior to randomization 2. Placebo Both arms: 9 x 45 min individual counselling sessions at clinic visits | |
| Outcomes | Abstinence 6m after end of treatment (7 day PP) Validation: serum cotinine < 25 ng/ml | |
| Notes | Funding: Pfizer, Inc and National Institute on Drug Abuse | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double‐blind" "Medications were provided in prepared bottles that were numbered according to the randomization schedule and dispensed at each visit. All study staff at the clinic site were blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Total participants lost to follow‐up at 6m not reported. "The subjects lost to follow‐up after random assignment were considered treatment failures." |
Covey 2007.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: Cessation clinic, USA Recruitment: community volunteers quit after 8w bupropion & nicotine patch |
|
| Participants | 289 abstainers (excludes 5 withdrawing consent before starting medication); 45% F, av. age 43, av. cpd 21 | |
| Interventions | Relapse prevention study. All participants received 8 w open‐label bupropion & nicotine patch (21mg with weaning) for 7w from TQD. Transition procedures preserved blinding for RP phase but allowed weaning from bupropion. Individual counselling including CBT techniques, 15 min x6 during open label, x4 during RP, x2 during follow up. 1. Bupropion (300 mg) & nicotine gum (2 mg, use as needed to manage craving) for 16 w 2. Bupropion & placebo gum 3. Nicotine gum & placebo pill (150 mg bupropion for first week) 4. Double placebo (150 mg bupropion for first week) | |
| Outcomes | Abstinence (no relapse to 7 days of smoking) for 12m (10m after randomization, 6m after EOT) (Primary outcome for study was time to relapse) Validation: CO ≤8ppm at each visit | |
| Notes | Quit rate after open‐label treatment was 52% so the final quit rate of 30% for combination therapy is equivalent to ˜16% of people starting treatment Funding: National Institute on Drug Abuse. GlaxoSmithKline provided medications. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A statistician who did not participate in the clinical phases of the study provided computer‐generated randomization lists that were not accessible to the clinical staff", stratified by gender & depression history. |
| Allocation concealment (selection bias) | Low risk | A research nurse who did not have direct contact with participants prepared individual medication kits based on the randomization schedule. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind" "Participants and clinical researchers with direct participant contact were blinded to the randomization" At end, subjects asked to guess treatment assignment; half guessed correctly and guess was not associated with treatment outcome. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 randomized participants withdrew before double blind phase. Less than 50% followed up in each group. Greater loss to follow up in double placebo, losses included in ITT analysis. |
Cox 2012.
| Methods | BUPROPION Randomized controlled trial Setting: urban community‐based clinic, USA Recruitment: volunteers, via healthcare settings and via community |
|
| Participants | 540 African American light smokers (≤ 10 cpd for ≥ 2 years, smoked on ≥25 days in past month). 66% F, av. age 47, av. cpd 8, av. FTND 3.2 | |
| Interventions | 1. 300 mg bupropion for 7 weeks (150 mg 1xd for 3d, then 150 mg 2xd for remainder) 2. Placebo on same schedule Both arms: up to 6 one‐to‐one 15‐20 minute individual counselling sessions, self‐help guide at start |
|
| Outcomes | 7d PP at 6 months Validation: salivary cotinine <15 ng/mL |
|
| Notes | New for 2013 update SAEs only reported at week 3 (none reported), not included in SAE analysis. Funding: National Cancer Institute, National Institutes of Health, National Institute for Minority Health and Disparities |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Both participants and investigators were blinded to the pharmacotherapy condition." No further information provided, unclear if counsellors blinded to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30% lost to follow‐up at 6m, no difference between groups |
Croghan 2007.
| Methods | BUPROPION Randomized controlled trial Setting: clinics, USA Recruitment: community volunteers for pharmacotherapy cessation & relapse prevention trial |
|
| Participants | 405 abstainers after 3m pharmacotherapy, 74 from inhaler, 141 bupropion, 190 combination. Participant characteristics not presented at start of RP phase | |
| Interventions | Relapse prevention study. In cessation phase participants had been randomized to bupropion (300mg), nicotine inhaler (up to 16 cartridges/day) or combination. Physician advice at entry, brief (<10 min) counselling at monthly study visits (total 12‐18 including RP phase) & S‐H. Abstainers (7 day PP after 3m therapy) eligible for RP phase. RP intervention randomized single therapy abstainers to continue cessation therapy or placebo for 9m. Combined therapy abstainers randomized to 4 groups: combination, placebo & single therapy, or double placebo | |
| Outcomes | Abstinence at 15m (from TQD, 12m from RP start, 3m from EOT) (PP) Validation: CO ≤8ppm | |
| Notes | All arms with bupropion combined, compared to the respective placebo arms.
Cessation rates at end of induction phase were 14% for inhaler, 26% for bupropion and 34% for combination. Funding: Public Health Service |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a dynamic allocation procedure balancing stratification factors. |
| Allocation concealment (selection bias) | Low risk | Randomization procedure makes prior knowledge of allocation unlikely. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo used, but insufficient information provided re: blinding to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow up post‐medication were high and not enumerated by group, but all included in ITT analysis. |
Da Costa 2002.
| Methods | NORTRIPTYLINE Randomized controlled trial Setting: cessation clinic, Brazil Recruitment: volunteers to a smokers' support group |
|
| Participants | 144 smokers, >= 15 CPD; 'predominantly female' , age, CPD not described, 48% had a history of depression | |
| Interventions | 1. Nortriptyline max 75 mg/day for 6w incl titration period, begun 1w before start of behaviour therapy 2. Placebo Both arms: 6 weekly group cognitive behavioural therapy | |
| Outcomes | Abstinence 6m after end of treatment (prolonged) Validation: none | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each patient chose a blind number from a box ...' Probably adequate. |
| Allocation concealment (selection bias) | Unclear risk | "... with each number corresponding to a “medication kit” that was externally undistinguishable. Patients and professionals participating in this study were blindfolded for this distribution." Potentially adequate but difference in numbers in each group not accounted for. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but insufficient detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost in each group not clear. |
Dalsgarð 2004.
| Methods | BUPROPION Randomized controlled trial Setting: 5 hospitals, Denmark Recruitment: hospital staff |
|
| Participants | 335 smokers incl physicians, nurses, other hospital service and admin staff, >= 10 CPD, no history of MDD; 75% F, av. age 43, av. CPD 19 | |
| Interventions | 1. Bupropion 300 mg/day for 7 weeks 2. Placebo Both arms: motivational support around TQD, at w3 & 7, and at 12w follow up | |
| Outcomes | Abstinence at 6m (prolonged from w4) Validation: CO < 10 ppm | |
| Notes | Funding: GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was computer generated and blinded. |
| Allocation concealment (selection bias) | Low risk | Allocation was double‐blinded and bupropion and placebo tablets were identical in form and number. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" Clear that participants were blinded but unclear if all staff were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32% of the bupropion group and 43% the placebo group discontinued treatment, included in analysis. |
Eisenberg 2013.
| Methods | BUPROPION Randomized controlled trial Setting: 38 hospitals, Canada Recruitment: hospital patients with acute myocardial infarction (AMI) |
|
| Participants | 392 smokers of at least 10 cpd, hospitalized with enzyme positive AMI. 84% M, av. age 54, av. cpd 23, av. FTND NR. | |
| Interventions | 1. Bupropion 300 mg/day for 9 weeks (150 mg for 3d, then 150 mg 2xd for remainder) 2. Placebo on same schedule Both arms: 7 one‐to‐one counselling sessions by research nurses at baseline and all follow‐ups of < 20 mins (avg. 5) – mix of phone and in‐person |
|
| Outcomes | 12m continuous abstinence (7d PP also reported) Validation: CO ≤ 10 ppm |
|
| Notes | New for 2013 update Patients not allowed to smoke whilst hospitalized. SAEs reported over 12m so not included in analysis. n quit extracted from percentages provided; denominators do not include 9 deaths in bupropion and 6 deaths in placebo group, all deemed not to be related to study medication. Adherence to treatment: 72.3% bupropion 82% placebo took at least 1 pill per day Funding: Canadian Institutes of Health Research and Heart and Stroke Foundation of Quebec |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done via an internet website using random blocks of 2 and 4 and was stratified by center to ensure that similar numbers of patients were randomized to the 2 arms of the study at each study center" |
| Allocation concealment (selection bias) | Low risk | Allocation performed centrally, see above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind." "All clinical end points were adjudicated by members of the Endpoints Evaluation Committee who were blinded to treatment assignment." No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 77% followed up at 12m, similar across arms |
Evins 2001.
| Methods | BUPROPION Randomized controlled trial Setting: outpatient clinic, USA Recruitment: volunteers Randomization: no details |
|
| Participants | 18 smokers with stable schizophrenia (excl 1 drop‐out prior to medication) 39% F, av age 45.5/42.7, av CPD 38/30 | |
| Interventions | 1. Bupropion 300 mg/day for 3m. TQD after w3 2. Placebo Both arms: 9x 1 hr weekly group CBT | |
| Outcomes | Abstinence at 6m, (prolonged) Validation: CO < 9 ppm or serum cotinine < 14 ng/mL | |
| Notes | 2 year follow up also reported (Evins, et al 2004). 3 additional quitters, not used in meta‐analysis since additional therapy used Funding: National Association for Research on Schizophrenia and Affective Disorders. Medication provided by Glaxo Wellcome Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Subjects were randomly assigned to 12 weeks of double‐blind bupropion SR, 150 mg/day, or an identical appearing placebo tablet added to their usual medication regimen." Unclear if all staff members were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Nineteen subjects were enrolled and 18 subjects completed the 6‐month smoking cessation trial." |
Evins 2005.
| Methods | BUPROPION Randomized controlled trial Setting: clinic, USA Recruitment: volunteers |
|
| Participants | 56 smokers with schizophrenia (>=10 CPD) (excl 6 drop‐outs prior to medication); 27% F, av age 45, av CPD 37/26 | |
| Interventions | 1. Bupropion 300 mg/day for 3m. 2. Placebo Both arms: 12 session group CBT | |
| Outcomes | Abstinence at 6m (7 day PP) Validation: CO < 9 ppm | |
| Notes | There was a significant treatment effect at EOT.
Originally included as Evins 2003 based on abstracts Funding: National Association for Research on Schizophrenia and Affective Disorders. Medication provided by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" with "identical placebo tablets." No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only people taking at least one dose of study medication included in analyses in paper. 5 in each group lost to follow‐up and included as smokers. |
Evins 2007.
| Methods | BUPROPION Randomized controlled trial Setting: community mental health centre, USA Recruitment: outpatients |
|
| Participants | 51 smokers (>=10 CPD) with schizophrenia; av. age 44, av. CPD 28/25 | |
| Interventions | 1. Bupropion 300 mg/day for 3m, nicotine patch, 21 mg for 8w incl tapering, 2 mg nicotine gum 2. Placebo + NRT as 1. Both arms: 12 session group CBT, TQD week 4 | |
| Outcomes | Abstinence at 12m (from TQD) Validation: CO <= 8 ppm | |
| Notes | First included as Evins 2006 based on unpublished data
Used in bupropion+NRT vs NRT comparison. Funding: Massachusetts Department of Mental Health. Medication provided by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and investigators remained blind to the treatment condition (bupropion or placebo) throughout the follow‐up period." "Assessment of treatment assignment was at the level of chance for both participants and staff at Weeks 4 and 12 for both treatment assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% of the bupropion group and 18% of the placebo group were lost to follow‐up at week 12; included as smokers. All other participants followed up at 12m. |
Ferry 1992.
| Methods | BUPROPION Randomized controlled trial Setting: clinic, USA Recruitment: NS |
|
| Participants | 42 male smokers | |
| Interventions | 1. Bupropion 300 mg/day for 3m 2. Placebo Both arms: group smoking cessation and relapse prevention counselling | |
| Outcomes | Abstinence at 6m from end of treatment (sustained) Validation: saliva cotinine | |
| Notes | Abstract with no further details Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further detail provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given. |
Ferry 1994.
| Methods | BUPROPION Randomized controlled trial Setting: Veterans Medical Centre, USA Recruitment: NS |
|
| Participants | 190 smokers | |
| Interventions | 1. Bupropion 100 mg x 3/day for 12w 2. Placebo Both arms: group smoking cessation and relapse prevention counselling; TQD within first 4w | |
| Outcomes | Abstinence at 12m (prolonged from day 29) Validation: saliva cotinine <= 15 ng/ml at 6m and 12m | |
| Notes | Abstract with long‐term abstinence data supplied by author. Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further detail provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 72% followed‐up intervention, 61% followed up control. "The most conservative approach to analysis would reclassify all of these individuals as smokers due to protocol violation." |
Fossati 2007.
| Methods | BUPROPION Randomized controlled trial Setting: primary care clinics, Italy Recruitment: patients of 71 general practitioners |
|
| Participants | 593 smokers, ≥ 10 CPD; 40% F, av. age 49, av. CPD 22 | |
| Interventions | 1. Bupropion 300 mg/day for 7 weeks 2. Placebo Both arms: GP visits at enrolment & 4, 7, 26 & 52w, phone calls 1 day pre TQD, 3 days post TQD, 10w post enrolment. Classified as low intensity | |
| Outcomes | Abstinence at 12m (continuous from week 4) Validation: CO ≤10ppm at each visit | |
| Notes | Funding: Mario Negri Institute and GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Stated to be double‐blind, but not explicit that GPs blind to randomization code. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind", further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15% Bupropion & 17% Placebo did not attend 12m follow‐up, included as smokers. |
Gariti 2009.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: university, USA Recruitment: self‐referral from community |
|
| Participants | 260 light smokers (6‐15 cpd) motivated to quit 57% F, av.age 54, av.cpd 11, av.FTND 4 |
|
| Interventions | 1. Placebo patch for 8 wks + 9 wks bupropion SR + 10 wks individualized counselling sessions 2. Placebo patch for 8 wks + 9 wks bupropion SR + 4x5‐10min counselling sessions 3. Nicotine patch + 9 wks placebo bupropion + 10 wks individualized counselling sessions 4. Nicotine patch for 8 wks + 9 wks placebo bupropion + four 5‐10min counselling sessions |
|
| Outcomes | 7d PP at 12m. Validation: CO<10ppm; urinary cotinine <200ng/ml |
|
| Notes | New for 2013 update. Used in direct comparison of bupropion and NRT only, pooling 1+2 versus 3+4. Funding: National Institute on Drug Abuse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized ‘urn randomization’ |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‘double‐blind, double‐dummy’ for medication component. 'Neither the nurses nor the participants knew which of the two formulations contained the active formulation.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data included as smokers. Similar losses to follow‐up across both groups. |
George 2002.
| Methods | BUPROPION Randomized controlled trial Setting: mental health clinic, USA Recruitment: outpatients |
|
| Participants | 32 smokers with schizophrenia motivated to quit; 44% F, av. age 41/45, av. CPD 24 | |
| Interventions | 1. Bupropion 300 mg/day for 9 weeks. TQD w3 2. Placebo Both arms: 10x 60 min weekly group therapy | |
| Outcomes | Abstinence at 6m (7‐day PP) Validation: CO < 10 ppm | |
| Notes | Funding: National institute on Drug Abuse, U.S. Department of Veterans Affairs, National Alliance for Research on Schizophrenia and Depression. Medication provided by GlaxoSmithKline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Both subjects and research staff were blinded to study medication assignment. Study medications were prepared by research pharmacists at CMHC, using encapsulation of SR bupropion tablets with blue 00 opaque capsules; placebo capsules contained only a dextrose matrix." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number followed‐up at 6m not reported."Subjects who were lost during the trial or at 6‐month follow‐up were counted as smokers." |
George 2003.
| Methods | SELEGILINE Randomized controlled trial Setting: outpatient smoking research clinic, USA Recruitment: community volunteers |
|
| Participants | 40 smokers, CO ≥10 ppm; 63% F, av. age 49, av. CPD 23, 25% MDD history positive | |
| Interventions | 1. Selegiline 10 mg/day for 9 weeks (5 mg/day in w1 & w9) 2. Placebo | |
| Outcomes | Abstinence at 6m (7 day PP) Validation: CO < 10ppm | |
| Notes | "The main side effects of SEL were anorexia, gastrointestinal symptoms, and insomnia. None of the differences in adverse event ratings were significant in the SEL compared with the PLA group, and the drug was well tolerated compared with the placebo group. Reports of anxiety/agitation in both the SEL and PLA groups during the trial were high." Funding: National Institute on Drug Abuse, U.S. Department of Veteran Affairs, National Alliance for Research on Schizophrenia and Depression |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, adequacy of blinding tested in research staff; results suggested blinding was adequate. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29/40 not assessed at 6m. Greater loss to follow‐up in placebo, exact data not reported. |
George 2008.
| Methods | BUPROPION Randomized controlled trial Setting: Mental Health CentreUSA Recruitment: Outpatients |
|
| Participants | 58 smokers with schizophrenia or schizoaffective disorder (excludes 1 receiving no study medication); 40% F, av. age 40, av. CPD ˜23 | |
| Interventions | 1. Bupropion 300 mg/day for 9w, begun 7 days pre‐TQD 2. Placebo Both arms: Nicotine patch (21mg/24hrs) for 8w from TQD & group behaviour therapy 10 weekly sessions | |
| Outcomes | Abstinence at 6m, PP Validation: CO <10 ppm | |
| Notes | Bupropion as adjunct to NRT Funding: National Institute on Drug Abuse, National Alliance for Research on Schizophrenia and Depression |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double blind" but no additional details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/29 intervention & 10/29 control did not complete trial, included as smokers. |
Gonzales 2001.
| Methods | BUPROPION Randomized controlled trial Setting: 16 clinical trial centres, USA Recruitment: volunteers who had previously failed to quit using bupropion |
|
| Participants | 450 smokers, >= 15 CPD, who had previously used bupropion for at least 2w without adverse effects; 55% F (Placebo), 48% F (Bup); av. age 45, av. CPD not specified, no details of depression history | |
| Interventions | 1. Bupropion 300 mg/day for 12w, begun 7 days pre‐TQD. 2. Placebo Both arms: brief individual counselling at visits w1‐7, 9, 12, + telephone counselling at 4 and 5m | |
| Outcomes | Abstinence at 12m, prolonged from w4 Validation: CO <= 10 ppm at each visit | |
| Notes | 6m data published. 12m data presented in a poster used since 2003 update Funding: GlaxoWellcome Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants who satisfied the inclusion criteria were randomized to the treatment phase and received either bupropion SR ... or matching placebo. Eligible participants were assigned a protocol‐specific treatment number on the basis of a randomization code provided by GlaxoWellcome." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Even though participants and the site staff were blinded to the drug assignments and the site staff did not encourage participants to speculate on their assignments, the lower placebo abstinence rates in the current study may be attributable to the previous experiences of participants with bupropion in their previous cessation attempts." However, little difference in completion between two arms, suggesting blinding may have been successful. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants followed‐up at 12m unclear. "...all participants who stopped participating in the study during the treatment phase were considered to be smokers." |
Gonzales 2006.
| Methods | BUPROPION Randomized controlled trial Setting: 19 clinical trial centres, USA Recruitment: community volunteers |
|
| Participants | 1025 smokers of >= 10 CPD (673 in relevant arms), recent MDD excluded, prior exposure to bupropion excluded; 46% F, av. age 42, av. CPD 21, no details of depression history | |
| Interventions | 1. Bupropion 300 mg/day for 12w, begun 7 days pre‐TQD 2. Varenicline 2mg/day 3. Placebo All arms: Brief (<10 min) standardized individual counselling at 12 weekly visits during drug phase and 11 clinic/phone visits during follow up, problem solving and relapse prevention | |
| Outcomes | Abstinence at 1 year (sustained from w4) Validation: CO <= 10 ppm at each visit | |
| Notes | Bupropion was an active control for varenicline.
Bupropion vs placebo and bupropion vs varenicline comparisons contribute to review. Funding: Pfizer, Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'predefined ... computer‐generated randomization sequence', 1:1:1, using block size of 6, stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Participants and investigators were blinded to drug treatment assignments[, and] ... were not encouraged to guess their treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up similar across conditions; 44% bupropion, 39.5% varenicline, 46% placebo, all included in analyses. |
Grant 2007.
| Methods | BUPROPION Randomized controlled trial Setting: 2 substance use disorder clinics, USA Recruitment: Alcoholics in residential or outpatient treatment programmes |
|
| Participants | 58 alcoholic smokers (20+ CPD); 84% M, av. age 40, av. CPD 25 | |
| Interventions | 1. Bupropion 300 mg for 60 days + nicotine patch 21 mg for 8 weeks incl tapering 2. Placebo & nicotine patch Both arms: 1 hour cessation group (& 4 weekly assessment visits) | |
| Outcomes | Abstinence at 6m, 7 day PP Validation: no biochemical val, collaterals contacted, inconsistent, adjusted rates not reported. | |
| Notes | Funding: National Institute on Alcohol Abuse and Alocholism | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but unclear who was blinded, no further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher loss in bupropion (40%) than placebo (21%) but still within 20% range of each other. ITT analysis. |
Górecka 2003.
| Methods | BUPROPION Randomized controlled trial Setting: Smokers' clinic, Poland Recruitment: smokers with a diagnosis of COPD and failure to stop smoking with advice alone |
|
| Participants | 70 smokers with COPD 43% F, av age 56, av CPD 24 | |
| Interventions | 1. Bupropion 300 mg/day for 7w 2. Nicotine patch (15mg) for 8w Common components: support at clinic visits at baseline, 2w, EOT | |
| Outcomes | Abstinence at 1 year (sustained) Validation: CO < 10ppm | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described but presumably no blinding, as participants will have known assignment based on patch versus pill |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described. |
Haggsträm 2006.
| Methods | BUPROPION & NORTRIPTYLINE Randomized controlled trial Setting: Smoking cessation clinic, Brazil Recruitment: community volunteers. |
|
| Participants | 156 smokers, FTND at least 4; 70% F placebo & nortriptyline, 59% Bup, av. age 44, av. CPD NS | |
| Interventions | 1. Bupropion 300 mg/day for 60 days, placebo nortriptyline, TQD during week 2 2. Nortripytyline 75 mg/day for 60 days, placebo bupropion 3. Double placebo All arms: 6x 15‐min individual CBT, weekly then bi‐weekly. | |
| Outcomes | Abstinence at 6m (continuous from TQD) Validation: CO <= 10 ppm at 3 & 6m | |
| Notes | Funding not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, double dummy. "Both investigators and patients were blind to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers lost to follow‐up not reported, all included as smokers. |
Hall 1998.
| Methods | NORTRIPTYLINE Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers. Exclusion criteria included MDD within 3m of baseline |
|
| Participants | 199 smokers of >= 10 CPD, 33% had history of MDD 55% F, av age 40, av CPD 21‐25 | |
| Interventions | 2 x 2 factorial design. Alternative psychological Rxs were 10 sessions of CBT or 5 sessions of health education control. Collapsed in this analysis 1. Nortriptyline titrated to therapeutic levels ‐ usually 75‐100 mg/day, 12w 2. Placebo | |
| Outcomes | Abstinence at 1 year post‐EOT, prolonged. PP rates also reported. Validation: CO at weeks 12, 24, 39 and 64 | |
| Notes | There were no significant main or intervention effects for MDD category so these are pooled. Funding: National Instutute on Drug Abuse and Veterans Administration |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomization, after stratification on history of MDD and number of cigarettes smoked. |
| Allocation concealment (selection bias) | Low risk | Allocation generated at enrolment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Medication was placebo controlled and double blind. Placebo and active drug were identical in appearance." However, no detail on who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30% did not complete treatment in placebo and 17% in active groups. Analyses with missing =smoking given. |
Hall 2002.
| Methods | BUPROPION & NORTRIPTYLINE Randomized controlled trial, 3x2 factorial Setting: cessation research centre, USA Recruitment: community volunteers |
|
| Participants | 220 smokers, >= 10 CPD; 40‐47% F, av. age 37‐43, av. CPD 20‐23, 33% had history of MDD | |
| Interventions | 3 x 2 factorial design. Alternative psychological interventions were Medical Management (MM, physician advice, S‐H, 10‐20 min 1st visit, 5 minds at 2,6,11 weeks) or Psychosocial Intervention (PI, as MM plus 5x 90 min group sessions at 4,5,7,11w) Pharmacotherapy: 1. Bupropion 300 mg/day, 12w 2. Nortriptyline titrated to therapeutic levels, 12w 3. Placebo | |
| Outcomes | Abstinence at 1 year (47w post quit date), prolonged. PP also reported Validation: CO <= 10 ppm, urine cotinine <= 60 ng/mL | |
| Notes | No significant interaction between pharmacotherapy and behaviour therapy, so BT arms collapsed in main analysis. Bupropion & nortriptyline compared to placebo and head‐to‐head. Levels of support compared for bupropion only, PP rates used. Not included in behavioural support subgroup. Funding: National Institute on Drug Abuse, National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were stratified by number of cigarettes smoked, sex and history of depression vs no history, and randomly assigned to 1 of the 6 experimental cells." |
| Allocation concealment (selection bias) | Low risk | "We encapsulated both drugs to maintain the patency of the bupropion formulation and to provide a blinded drug. All participants received capsules that were identical in number and appearance" but blinding of allocation not explicit. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Double blind but participants informed about adverse effects of each drug and 87% of participants taking active drug guessed that they were (compared to 67% placebo group). Bupropion participants no more likely than nortriptyline participants to correctly identify which drug they had received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% lost to follow‐up at 52 w. No significant difference across conditions. Included as smokers in analyses. |
Hall 2004.
| Methods | NORTRIPTYLINE Randomized controlled trial, 2x2 factorial Setting: clinic, USA Recruitment: community volunteers. |
|
| Participants | 160 smokers of ≥ 10 CPD 41% F, av age ˜38, av CPD ˜19, 21% MDD history positive | |
| Interventions | 2 x 2 factorial design. Nortriptyline vs placebo and brief vs extended treatment. Brief treatment: Nicotine patch for 8w from quit date, & 5 group counselling sessions, total 7.5 hrs Extended treatment: First 12w as for Brief, then same dose continued to week 52 then tapered. Individual counselling every 4w, total 3‐4.5 hrs. Phone counselling, total 40‐80 mins. 1. Nortriptyline titrated to 50‐150 ng/ml (˜75‐100 mg) for 12w, quit date week 5 2. Placebo |
|
| Outcomes | Abstinence at 52w, repeated PP at 24, 36, 52w. Validation: CO ≤ 10 ppm and urine cotinine ≤ 50 ng/ml at each point. | |
| Notes | Factorial design, Brief and extended treatment entered in meta‐analysis separately. In the active extended treatment arm participants were still receiving nortriptyline at the time of final follow up. Funding: National Institute on Drug Abuse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization stratified on CPD, prior NRT use, MDD history; method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Double blind, but "participants given active drug were more likely to guess that they had received active drug (63%) than the placebo participants were to believe they were taking active drug (37%)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% lost at week 52, included as smokers. |
Hall 2011.
| Methods | BUPROPION Randomized controlled trial Setting: Clinic, USA Recruitment: community volunteers |
|
| Participants | 406 smokers of ≥ 10 cpd 39% F, av age 41, av cpd 19, mean FTND 4.9 |
|
| Interventions | Relapse prevention study. All arms received same cessation intervention (5 sessions group counselling, 10wks NRT and 12wks bupropion at 150 mg/d for first 3 days and 300 mg/d for remainder). After cessation treatment ended: 1. Bupropion for 40wks (300mg/d) 2. As per 1, but placebo 3. As per 1, plus 11 sessions of individual CBT over 40 wks 4. As per 3, but placebo 5. No further treatment |
|
| Outcomes | 7d PP at 2y CO ≤ 10 ppm and urinary cotinine ≤ 60 ng/ml |
|
| Notes | New for 2013 update. Study report does not present absolute values for N abstinent, only adjusted ORs. N quit extrapolated from graph. Group 5 does not contribute to any analyses. Funding: National Institute on Drug Abuse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomly assigned,” methods not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded successfully but blinding of staff not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 83% followed up at 2y, similar rates across groups |
Hatsukami 2004.
| Methods | BUPROPION Randomized controlled trial Setting: 12 clinical trial sites, USA Recruitment: community volunteers |
|
| Participants | 594 smoker of >= 20 CPD wanting to reduce amount smoked. Not quit for > 3m in previous year, at least 2 failed quit attempts including 1 with NRT, not currently depressed, 6% had history of MDD. Excludes 15 who took no study medication. | |
| Interventions | Not a cessation trial 1. Bupropion 300 mg/day, 26w 2. Placebo Both arms: written materials suggesting reduction techniques, monthly brief individual counselling, telephone contact 2 days, 12 days, 5w after target reduction date. Participants indicating a willingness to quit at any time were enrolled in a 7w cessation programme with weekly visits followed by 19w of follow up | |
| Outcomes | Abstinence 6m after quit date (denominator 594; 214 entered cessation phase Validation: urine cotinine | |
| Notes | Not used in main analysis
38% of bupropion and 34% of placebo group entered cessation phase. Median time to attempting cessation shorter in bupropion group Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were assigned randomly using a computer‐generated schedule..." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," unclear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Very high levels of attrition (at 6 months, 43% placebo and 39% control followed up). For cessation analyses, subjects who dropped out were considered to have resumed smoking [after withdrawal date]. |
Hays 2001.
| Methods | BUPROPION Randomized controlled trial Setting: 5 clinical trial centres, USA Recruitment: 784 community volunteers |
|
| Participants | 429 smokers of >= 15 CPD who quit after 7 weeks open label bupropion; 51% F, av age 46, av CPD 26, 19% history of MDD | |
| Interventions | Relapse prevention study 1. Bupropion 300 mg/day for 45 weeks 2. Placebo Both arms: physician advice, S‐H materials and brief individual counselling at follow‐up visits. |
|
| Outcomes | Abstinence at 2 years (1 year after end of pharmacotherapy), prolonged Validation: CO <= 10 ppm | |
| Notes | Relapse prevention trial Funding: Glaxo Wellcome Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization to the placebo or bupropion groups was computer generated at a central location;..." |
| Allocation concealment (selection bias) | Low risk | Centralized (see above) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. "...the investigators did not know the patient assignments. All bupropion and placebo pills were identical in shape, size, and color." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Participants who dropped out were considered to have relapsed to smoking, but information on other important factors, such as weight gain, was not collected and therefore could not be included in the analysis." Approximately 26% of the bupropion group and 27% of the placebo group did not complete the study. |
Hays 2009.
| Methods | BUPROPION Randomized controlled trial Setting: Clinic, USA Recruitment: community volunteers |
|
| Participants | 110 recovering alcoholic abstainers with at least 1 y continuous abstinence from alcohol and drugs, 18+ years old, smoking ≥ 20 cpd for previous year. Quit for at least last week of 8w patch therapy 78% M; av age 44; av cpd 29.9 (in initial population of 195 volunteers) |
|
| Interventions | Relapse prevention study. All participants first received brief weekly counselling sessions and nicotine patch for 8 w. Patch tailored on the basis of baseline serum cotinine concentration 1. Bupropion: 150 mg/day first 3 d, then 300 mg/d until w 52 2. Placebo on same schedule Brief individual counselling (≤ 10 min) at each clinic visit (weekly for w 9‐12, monthly for w 13‐24, then at 52, 53, 64 and 76 w) |
|
| Outcomes | Abstinence at 76 w (continuous and 7 d PP) Validation: CO < 8 ppm |
|
| Notes | New for 2013 update Study does not report number of participants allocated to each group or number of successful abstainers in each group; numbers obtained through extrapolation Funding: National Institute on Alcohol Abuse and Alocholism |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized", method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as "double‐blind", placebo used, but no further information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At w 76, similar rate of dropout in both groups (34% intervention; 37% control). Participants lost to follow‐up counted as relapsed smokers |
| Other bias | Unclear risk | Discrepancy in data: at 76 w, 7 d PP less than continuous abstinence |
Hertzberg 2001.
| Methods | BUPROPION Randomized controlled trial Setting: Veterans Affairs Medical Centre (VAMC), USA Recruitment: VAMC outpatient volunteers |
|
| Participants | 15 male veterans with Post Traumatic Stress Disorder, av age 50, av CPD 33 | |
| Interventions | 1. Bupropion 300 mg/day, 12w begun at least 1w before TQD. 2. Placebo Both arms: individual counselling pre‐quit, weeks 1,2,4,8,12. | |
| Outcomes | Abstinence at 6m, prolonged, validated at weeks 2, 8, 12. Validation: CO <= 10ppm Paper includes as abstinent one person with a slip at week 12 | |
| Notes | 2 of the successful quitters were taking bupropion at 6m, prescribed after end of study. Funding: Glaxo Wellcome Inc, National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind, no further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Uneven attrition between arms; very high percentage lost to follow‐up in placebo group. 30% of the participants receiving bupropion SR did not complete the full 12‐week trial; 80% of the placebo group failed to complete the trial and were considered to have resumed smoking. |
Holt 2005.
| Methods | BUPROPION Randomized controlled trial Setting: Cessation clinic, New Zealand Recruitment: Maori community volunteers aged 16‐70 |
|
| Participants | 134 smokers, >=10 CPD; 72% F, av age 42/38 | |
| Interventions | 1. Bupropion 300mg/day for 7w 2. Placebo Both arms: counselling at 3 clinic visits during medication & 3 monthly follow ups, motivational phone call 1 day before & 2 days after TQD | |
| Outcomes | Abstinence at 12m (continuous, undefined) Validation: CO at each visit | |
| Notes | Funding: GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using a computer generated code. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, "Neither the study team nor the participant was aware of which treatment had been allocated until the end of the 12 month study period." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High and uneven loss to follow‐up, with less than half of placebo group followed up at 12 months. 36% lost in bupropion group and 52% in placebo at 12 months. "Participants who were lost to follow up were categorised as smokers ... often this was confirmed by family members or friends." |
Hurt 1997.
| Methods | BUPROPION Randomized controlled trial Setting: multi‐centre, USA Recruitment: community volunteers |
|
| Participants | 615 smokers, > 15 CPD, without current depression; 55% F, av. age 44, av. CPD 27, 3% had a history of major depression and alcoholism, 15% depression alone, 7% alcoholism alone. | |
| Interventions | 1. Bupropion 100 mg/day for 7 weeks 2. Bupropion 150 mg/day 3. Bupropion 300 mg/day 4. Placebo All arms: physician advice, S‐H materials, and brief individual counselling by study assistant at each visit | |
| Outcomes | Abstinence at 12m (prolonged from day 22, data provided by Glaxo Wellcome) (continuous abstinence to week 6 and 7 day PP abstinence at 12m reported in paper) Validation: expired CO <= 10ppm | |
| Notes | 300 mg compared with placebo in main analysis
There was no evidence that history of major depression or alcoholism interacted with treatment condition or was associated with poorer outcomes. Prolonged abstinence rates at 12m as supplied by Glaxo Wellcome: 300 mg 21; 150 mg 23; Placebo 15 Funding: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, stratified by site, method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but no detail given on who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Subjects who missed a follow‐up visit were considered to be smoking.... The rate of completion of the study increased with the dose and was 57 percent, 65 percent, 64 percent, and 71 percent for the placebo, 100‐mg, 150‐mg, and 300‐mg groups, respectively..." |
Hurt 2003.
| Methods | BUPROPION Randomized controlled trial Setting: multi‐centre 14 North Central Cancer Treatment Group sites, USA Recruitment: community volunteers. |
|
| Participants | 578 smokers recruited to first stage of study: >= 15 CPD; 57% F, av age 42, 21% history of MDD 176 smokers abstinent after 8w nicotine patch treatment randomized to relapse prevention intervention 194 non‐abstinent smokers randomized to bupropion as second line therapy | |
| Interventions | (All participants first received nicotine patch for 8w, dose based on cig consumption) Relapse prevention arm: 1. Bupropion for 26w 2. Placebo Second line therapy arm: 1. Bupropion for 8w 2. Placebo | |
| Outcomes | Relapse prevention arm: Abstinence at 12m, (PP, 6m after end of therapy). Second line therapy arm: Abstinence at 6m (4m after end of therapy) Validation: CO < 8 ppm | |
| Notes | Does not contribute to primary analysis.
Long‐term follow up for 2nd line Rx arm from authors. Funding: National Cancer Institute, Public Health Service. Medication provided by Glaxo Wellcome and Elan Pharmaceutical. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using 'dynamic allocation'. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants lost to follow‐up not given. Patients lost to follow‐up considered to be smoking. |
Jorenby 1999.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: multi‐centre clinical trial units, USA Recruitment: community volunteers |
|
| Participants | 893 smokers, > 15 CPD, no current major depressive episode, 15‐20% had history of MDD 52% F, av age 43 av CPD 25 | |
| Interventions | 1. Nicotine patch (24 hr, 21 mg for 6w, tapered for 2w) and sustained release bupropion 300 mg for 9w from 1w before quit day 2. Bupropion 300 mg and placebo patch 3. Nicotine patch and placebo tablets 4. Placebo patch and placebo tablets All arms: Brief (< 15 min) individual counselling session at each weekly assessment. One telephone call 3 days after quit day | |
| Outcomes | Abstinence at 12m, (continuous) Validation: Expired CO < 10ppm at each clinic visit | |
| Notes | Primary outcome for study was PP abstinence; this analysis uses continuous abstinence since quit day. Funding: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were randomly assigned to one of four treatments with use of an unequal‐cell design...[but] Randomization was not balanced within sites." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All subjects who discontinued treatment early or who were lost to follow‐up were classified as smokers." Approximately 20% left the study and provided no additional information. 15% stopped taking medication but participated in follow‐up assessments. |
Jorenby 2006.
| Methods | BUPROPION Randomized controlled trial Setting: multi‐centre clinical trial units, USA Recruitment: community volunteers |
|
| Participants | 683 smokers (in relevant arms) >=10 CPD, no recent treatment for MDD, prior exposure to bupropion excluded; 41% F, av. age 42, av. CPD 22 | |
| Interventions | 1. Bupropion 300mg for 12 w +placebo varenicline 2. Varenicline 2mg for 12 w +placebo bupropion 3. Placebo bupropion + placebo varenicline All arms: Brief (< 10 min) individual counselling at each weekly assessment for 12w & 5 follow‐up visits. One telephone call 3 days after quit day | |
| Outcomes | Abstinence at 12m, (sustained from week 9) Validation: Expired CO < 10 ppm at each clinic visit | |
| Notes | Bupropion was an active control for varenicline.
Bupropion vs placebo and bupropion vs varenicline comparisons contribute to review. Funding: Pfizer Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was completed centrally by using a computer‐generated list and sites used an electronic system to assign participants to treatment." |
| Allocation concealment (selection bias) | Low risk | "Folders [containing medication or placebo] for all participants (regardless of treatment assignment) were identical throughout the treatment phase including a period of dose titration (week 1) and treatment at the target dose (weeks 2‐12)." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "in a double‐blind manner," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over the period of treatment and follow‐up 14% of those receiving varenicline were lost to follow‐up; 14% randomized to bupropion lost to follow‐up; 16% of the placebo group were lost to follow‐up. "Participants whose smoking status was unknown or whose carbon monoxide level was higher than 10 ppm were classified as smoking during both the treatment phase and follow‐up." |
Kahn 2012.
| Methods | SELEGILINE Randomized controlled trial Setting: clinics, USA Recruitment: community |
|
| Participants | 246 smokers of ≥ 15 cpd in 30d prior to enrolment, smoked for past 5 years and expired CO ≥ 9 ppm, motivated to quit. 51% M, av. age 46, av. cpd 22. | |
| Interventions | 1. Selegiline patch (6mg/24hr) for 9 weeks, starting 7 days before TQD 2. Placebo patch on same schedule Both arms: 9 weekly individual counselling sessions of approx. 10mins each |
|
| Outcomes | Prolonged abstinence at 6m (continuous from week 6 onwards) Validation: CO < 9 ppm |
|
| Notes | New for 2013 update. Some additional information on study characteristics provided by author. Mean compliance rates 91.6% and 91.3% for the STS and placebo groups Funding: National Institutes of Health, National Institute on Drug Abuse |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Adaptive randomization," method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70% placebo and 74% STS followed up at 12 months |
Kalman 2011.
| Methods | BUPROPION Randomized controlled trial Setting: not specified (but presumably clinic‐based), USA Recruitment: Veterans Administration Medical Center |
|
| Participants | 143 smokers with 2 to 12 months alcohol abstinence, smoking at least 10 cpd with history of alcohol abuse or dependence. Mean age 49, 83% M, avg. cpd 20.8, mean FTND 5.9. | |
| Interventions | 1. Bupropion (8 weeks) (started 1 week before TQD, first 3 days 150mg/day, rest of period 2 x 150 mg/day) 2. Placebo as above Both arms: nicotine patch (7 weeks starting on TQD; 21mg weeks 1‐4, 14mg weeks 5‐6, 7mg week 7) and 8 weekly counselling sessions starting 1 week before TQD (one‐to‐one sessions based on CBT and MI) |
|
| Outcomes | Prolonged abstinence at 24 weeks (no smoking after first 2 weeks after TQD) Validation: salivary cotinine ≤ 15ng/ml |
|
| Notes | New for 2013 update. N quit calculated from percentages provided. Funding: National Institute of Drug Abuse, National Institute on Alcohol Abuse and Alocholism |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Urn randomization," no further details provided |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but no detail on who was blinded in terms of study staff, including counsellors. "Both medication groups performed at the chance level in judging medication assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13 participants who dropped out prior to receiving medication, not included in denominators. Further 18% intervention and 14% control lost at 24 weeks,counted as smoking in analyses. |
Killen 2000.
| Methods | PAROXETINE Randomized controlled trial Setting: clinic, USA Recruitment: Advertisements |
|
| Participants | 224 smokers, > 10 CPD, no current major depression. 12‐25% had history of MDD; 46% F, av age 46, av CPD 26 | |
| Interventions | 1. Nicotine patch (24 hr, 21 mg, 8w) + 40 mg paroxetine (9w incl tapering) 2. Patch as 1 + 20 mg paroxetine 3. Patch as 1 + placebo paroxetine All arms: S‐H manual and 15 min behavioural counselling at weeks 1 & 4. | |
| Outcomes | Abstinence at 6m (7 day PP at 10 & 26w) Validation: CO < 9 ppm and saliva cotinine < 20 ng/ml at each visit. | |
| Notes | 40 mg & 20 mg dose pooled in MA from 2009. 20/75 quit on 40mg, 15/75 on 20mg Funding: University of California Tobacco‐Related Disease Research Program, SmithKline Beecham |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but unclear who exactly was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not reported. "Those failing to provide confirmation [of smoking status] were reclassified as smokers." |
Killen 2004.
| Methods | BUPROPION Randomized controlled trial Setting: continuation high schools, USA Recruitment: adolescents at schools |
|
| Participants | 211 adolescent smokers, >= 10 CPD, at least 1 failed quit attempt; 31% F, av. age 17, av. CPD 15 | |
| Interventions | 1. Bupropion 150mg for 9w from 1w before TQD, Nicotine patch for 8w 2. Placebo & nicotine patch Both arms: Weekly 45 min group sessions, skills training | |
| Outcomes | Abstinence at 6m (7 day PP) Validation: Saliva cotinine < 20 ng/ml at 6m (CO at EOT) | |
| Notes | Low compliance with both bupropion & patch therapy Funding: National Cancer Institute. GlaxoSmithKline provided medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double‐blind." Though further details not provided, assessment of blind suggests it was successful (30% placebo and 31% bupropion correctly guessed assignment) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 38% bupropion & 35% placebo lost at 6 months, included in analysis. |
Killen 2006.
| Methods | BUPROPION Randomized controlled trial Setting: clinics, USA Recruitment: community volunteers |
|
| Participants | 362 smokers >=10 CPD, no current major depression; 46% F, av age 45, av CPD 20, 25% previous bupropion use | |
| Interventions | Extended treatment for relapse prevention after successful quitting. All received open label combination pharmacotherapy of bupropion 300 mg for 11w, nicotine patch for 10w. TQD day 7, 30 min individual relapse prevention skills training at 6 clinic visits. 1. Bupropion 150 mg for 14w 2. 2w tapering bupropion then placebo. Both arms had 4 further clinic visits during extended therapy | |
| Outcomes | Abstinence at 12m (continuous). PP and 7day relapse‐free outcomes also reported. Validation: CO (10 people not required to provide samples) | |
| Notes | Relapse prevention, does not contribute to main analysis.
PP outcomes favour placebo but no outcomes showed significant effects Funding: National Cancer Institute. Medication provided by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally generated pre‐assigned random sequence stratified by gender, prior to open label phase. |
| Allocation concealment (selection bias) | Low risk | Centrally assigned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Blinded drugs provided to investigator; " ... [the pharmaceutical company]... packaged the treatment and then shipped the blinded drug to the investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8% bupropion & 13% placebo lost at 12 months, included in analysis. |
Killen 2010.
| Methods | SELEGILINE Randomized controlled trial Setting: community, USA Recruitment: radio, newspapers, community website and notices distributed via local organizations |
|
| Participants | 243 smokers of ≥ 10 cpd, 18‐65 years old. 70% M, av age 45, av cpd 19. | |
| Interventions | 1. Selegiline patch for 8 weeks, 6mg/24hr, starting on TQD 2. Identical placebo on same schedule Both groups: 9 sessions of individual counselling to develop cognitive and behavioural skills to resist urges to smoke. |
|
| Outcomes | 7d PP at 12m Validation: CO < 10ppm |
|
| Notes | New for 2013 update Funding: National Institute on Drug Abuse. Medication and matching placebo provided by Somerset Pharmaceuticals, Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Participant assigned sequential ID numbers corresponding with drug “pre‐packaged and labelled by ID only at an off‐site location by an individual who had no association with the participants.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Treatment assignment was concealed from staff and both research staff and participants were blind to week 52.” Assessment of blinding in participants and study staff suggests it was successful |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87% followed up at 12m, same in both arms. Missing counted as smokers. |
Levine 2010.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: not specified, USA Recruitment: community volunteers |
|
| Participants | 349 weight‐concerned women smokers of ≥ 10 cpd, motivated to quit Av age 42, av cpd 21, mean FTND 5.2 |
|
| Interventions | Factorial trial 1. Bupropion SR for 26 weeks. 150mg/d for first 2 days and 300mg/d for remainder of treatment. 2. Placebo on same schedule Counselling conditions: 1. Standard cessation counselling 2. Standard cessation counselling + material on weight concerns All arms received 12, 90 minute group counselling sessions delivered over 3 months |
|
| Outcomes | Prolonged abstinence at 12m (7d PP at 3, 6 and 12m) Validation: CO ≤ 8 ppm and salivary cotinine ≤ 15 ug |
|
| Notes | New for 2013 update. Counselling arms collapsed in analyses (same intensity, just differed in content). N abstinent calculated from percentages given. Funding: National Institute on Drug Abuse. Medication supplied by GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked randomization, method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over half lost to follow‐up at 12m. 48% followed up overall, similar rates between groups. |
McCarthy 2008.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: Cessation clinic, USA Recruitment: community volunteers |
|
| Participants | 463 smokers; 50% F, av. age 36‐41 across arms, av. CPD 22 | |
| Interventions | Factorial trial 1. Bupropion SR 300mg for 8 weeks 2. Placebo Counselling conditions: 1. Counselling; 8 x10min session, 2 prequit, TQD, 5 over 4 wks 2. Psychoeducation about medication, support & encouragement. Same no. of sessions, 80mins less contact time | |
| Outcomes | Abstinence at 12m (7 day PP). Prolonged self‐reported abstinence also assessed Validation: CO ≤10ppm | |
| Notes | Counselling conditions collapsed in main analysis, entered separately in subgroup analysis by intensity. Psychoeducation arms placed in multisession individual counselling subgroup due to high level of contact received, though not classified as counselling in paper. Funding: National Cancer Institute, National Instutute on Drug Abuse. GlaxoSmithKline provided placebo medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Staff who screened and enrolled participants were unaware of the experimental condition to be assigned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind (for medication). "Research staff who interacted with participants were blind to participants’ medication condition assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 171 (37%) failed to attend quit date visit or lost to follow up, similar across groups, included in ITT analysis. |
Muramoto 2007.
| Methods | BUPROPION Randomized controlled trial Setting: research clinic, USA Recruitment: adolescent community volunteers |
|
| Participants | 312 adolescents (14 to 17) smoking ≥6 CPD; 46% F, median age 16, median CPD 11 | |
| Interventions | 1. Bupropion 300 mg for 7w 2. Bupropion 150 mg 3. Placebo All arms: Brief (10‐20 min) individual counselling session pre quit and at each weekly assessment. | |
| Outcomes | Abstinence at 6m (7 day PP; 30 day PP abstinence assessed but not reported) Validation: CO <10ppm (cotinine at weeks 2 & 6 only) | |
| Notes | 300 mg arm contributes to main analysis. 2/105 quit in 150mg group Funding: National Cancer Institute, The Robert Wood Johnson Foundation, GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Active study medication and identical‐appearing placebo were prepackaged into 3 sets of identical‐appearing blister cards in accordance with a computer‐generated randomization list." |
| Allocation concealment (selection bias) | Low risk | "... a research assistant assigned the subject the next treatment number (and associated blister cards) in sequence." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Study subjects and researchers remained blind to treatment group assignment throughout the study." "9.6% in the 300 mg group accurately guessed their treatment assignment. Across all treatment groups, there were no significant differences in the proportion of subjects who accurately guessed their treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Slightly higher lost to follow‐up/ declined further participation in placebo group (30%) than active arms (18%). ITT analysis. |
Myles 2004.
| Methods | BUPROPION Randomized controlled trial Setting: preoperative clinic, Australia Recruitment: Smokers awaiting surgery |
|
| Participants | 47 smokers expected to undergo surgery within 8‐14w 34% F, av age 45/40, 49% smoked 21‐30 CPD | |
| Interventions | 1. Bupropion 300 mg for 7w 2. Placebo Both arms: Advice at baseline, 1 phone call 2‐4 days after TQD. Low intensity | |
| Outcomes | Abstinence at 6m (28 day PP ‐ classified as sustained) Validation CO <= 10 ppm | |
| Notes | More drop‐outs in placebo group. Only 20 had surgery. Funding: Alfred Hospital Research Trust, Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated from a table of random numbers into one of two groups: active (bupropion) or placebo (identical appearance). |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further detail provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17% lost to follow‐up in the bupropion group; 9% lost to follow‐up in the placebo group. "Patients lost to follow‐up were assumed to still be smoking." |
Niaura 2002.
| Methods | FLUOXETINE Randomized controlled trial Setting: 16 clinical trial centres, USA Recruitment: Community volunteers |
|
| Participants | 989 non‐depressed smokers, no history of bipolar or current psychiatric disorder 61% F, av age 42 av CPD 28 | |
| Interventions | 1. Fluoxetine 30 mg for 10w, starting 2w before TQD 2. Fluoxetine 60 mg for 10w, starting 2w before TQD 3. Placebo All arms: 9 sessions (60‐90 mins) individual CBT. Included coping skills, stimulus control techniques and relapse prevention. | |
| Outcomes | Abstinence at 32w from TQD, multiple PP Validation: saliva cotinine < 20 ng/mL at each visit | |
| Notes | Originally based on abstract and data from authors. From 2002 based on full report. Numbers quit derived from rounded quit rates (10% quit in each group). Funding: Eli Lilly and Company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data in treatment phase addressed, but unclear whether missing data in follow‐up phase addressed. At 12m, 42% missing data, similar across all arms; missing data counted as smokers in our analyses. |
Nides 2006.
| Methods | BUPROPION Randomized controlled trial Setting: 5 clinical sites, USA Recruitment: Volunteers (phase II study) |
|
| Participants | 638 smokers (255 in relevant arms, incl 2 bupropion & 4 placebo who did not start medication). No major depression in past year 51% F, av age 41, av CPD 20. 13‐20% had used bupropion | |
| Interventions | 1. Bupropion 300 mg for 7w 2. Varenicline 2 mg for 7w (other dose regimens not used in review 3. Placebo All arms: Up to 10 mins counselling at 7 weekly clinic visits, 12 & 24w. | |
| Outcomes | Abstinence at 12m (continuous from week 4) Validation: CO | |
| Notes | Bupropion was an active control for varenicline.
Bupropion vs placebo and bupropion vs 2mg varenicline comparisons contribute to review.
Inclusion of 6 pretreatment drop‐outs has minimal effect on RR. Funding: Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a randomization list was computer generated using a method of randomly permuted blocks and a pseudorandom number generator." |
| Allocation concealment (selection bias) | Low risk | "Investigators assigned medication to subjects in numerical order of acceptance into the study." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "double‐blind", "to preserve treatment blinding," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Subjects who dropped out for any reason were considered to be smokers at all subsequent time points." 9.5% of varenicline tartrate 0.3 mg, once daily; 7% of varenicline tartrate 1.0 mg, once daily; 11 % of varenicline tartrate 1.0 mg, twice daily; 6% of bupropion hydrochloride 150 mg, twice daily and 13% of the placebo group were lost to follow‐up. |
Parsons 2009.
| Methods | ST JOHN'S WORT Randomized controlled trial, 2x2 factorial Setting: Smoking cessation clinic, UK Recruitment: direct mail from GP, stop smoking service, newspaper advertisements |
|
| Participants | 143 adult smokers of at least 10 cpd, motivated to quit 38% M, av age 46, av cpd 21, mean FTND 5.5 |
|
| Interventions | 1. St John's wort 900 mg/day (300mg x 3/day) for 14 w, started 2 w prior to TWD 2. Placebo on same schedule Both arms: 7 weekly individual behavioural support sessions in clinic |
|
| Outcomes | Prolonged abstinence at 6m Validation: CO ≤ 10 ppm |
|
| Notes | New for 2013. Factorial trial ‐ also tested the use of chromium versus placebo for weight loss. Arms collapsed for analysis; no difference detected. Funding: Cancer Research UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Via computer program |
| Allocation concealment (selection bias) | Low risk | Independent statistician sent randomization codes to medication packing company, medication allocated in sequence. Researchers blind to allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Participants, therapists, and outcome assessors were blind to the treatment allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Over 90% followed up at 6m, similar between groups |
Piper 2007.
| Methods | BUPROPION Randomized controlled trial Setting: USA Recruitment: volunteers Randomization: method not stated |
|
| Participants | 608 smokers of ≥ 10 CPD; 58% F, av. age 42, av CPD 22, no details of depression history | |
| Interventions | 1. Nicotine gum (4 mg) and bupropion (300 mg) (not used in this review) 2. Placebo gum and bupropion 3. Double placebo All arms: 3x 10 min counselling over 3 weeks | |
| Outcomes | Abstinence at 12m (PP) Validation: CO or blood cotinine | |
| Notes | First included with 6m data as Piper 2004 based on abstract Funding: National Institutes for Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was conducted in double‐blind fashion using blocked randomization within each of the 10 [orientation session] cohorts." No further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind, but no further information. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32% of bupropion & 36% of placebo groups lost at 12 months. "Participants who could not be reached at follow‐up were considered to be smoking for the purposes of follow‐up analyses." |
Piper 2009.
| Methods | BUPROPION Randomized controlled trial Setting: community, USA Recruitment: volunteers |
|
| Participants | 1504 smokers motivated to quit. 58% F, av.age 45, av.cpd 21.4 |
|
| Interventions | 1. Bupropion SR (150 mg bid, 1 wk pre‐quit, 8 wks postquit) 2. Bupropion + NRT (lozenge) (duration and dosage as below) 3. Nicotine lozenge 2 or 4 mg for 12 wks (based on dose‐for‐dependence level as per instructions) 4. Nicotine patch (24hr, 21, 14, and 7 mg titrated down over 8 wk period postquit) 5. Lozenge + patch (duration and dosage as above) 6. Placebo bupropion 7. Placebo bupropion + placebo lozenge 8. Placebo lozenge 9. Placebo patch 10. Placebo lozenge + placebo patch All arms: 7 one‐to‐one 10‐20min counselling sessions |
|
| Outcomes | 7d PP abstinence at 6m; initial cessation. Validation: CO<10ppm |
|
| Notes | New for 2013 update. Placebo outcomes reported as a whole in published report, author provided data for individual groups. 1 versus 6 in Analyses 1.1, 1.2 and 1.3. 2 versus 3 included in Analysis 1.5. 1 versus 4 in Analysis 1.7.1, 1 versus 3 in Analysis 1.7.2 and 1 versus 5 in Analysis 1.7.3 (intervention arm split in three to avoid triple counting). Majority of funding from National Institute on Drug Abuse and National Center for Research Resources. Medication provided to participants at no extra cost by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not specified. "Randomization was double‐blind and used a block randomization scheme with sex and self‐reported race as the blocking variables." |
| Allocation concealment (selection bias) | Low risk | "Staff did not know to which type(s) of medication a participant would be assigned until the moment of randomization, and study staff were blinded to whether the medication was active or placebo." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double blind" but no further detail provided. "Study staff were blinded to whether the medication was active or placebo" (Type of medication (i.e. patch, gum, pill) would have been apparent to both groups) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 drop‐outs (out of 1504). Analyses conducted using ITT. Individuals with missing data considered to be smoking. |
Planer 2011.
| Methods | BUPROPION Randomized controlled trial Setting: hospitals, Jersulem, Israel Recruitment: patients hospitalised for acute coronary syndrome in 2 separate campuses in Jerusalem |
|
| Participants | 151 smokers of > 10 cpd with diagnosis of acute coronary syndrome, motivated to quit. av. age: 51.9 yrs, 79.9% M, av. cpd 31 |
|
| Interventions | 1. Bupropion 150 mg 1xday for 3 days, then 2x day for 2m 2. Placebo, same schedule Both arms: counselling (at least 15 min of motivational support) during hospitalisation and continued after discharge (at least 2 visits with physician and nurse at 1 and 2m and weekly telephone call by nurse during first and second month, then monthly telephone calls during rest of the year) |
|
| Outcomes | Self‐reported continuous abstinence at 12m Validation: none |
|
| Notes | New for 2013 update. Study stopped early after interim analysis indicated no benefit OR adjusted for age, sex, invasive procedure, risk factors, Fagerstrom score, cpd: 0.90 (95% CI 0.39‐2.09) Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not specified |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and staff blind to treatment assignment, "Numbered study bottles were supplied by the study coordinator and remained concealed from the patients and medical staff." No biochemical validation but participants blind to condition so differential misreport unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 lost to follow‐up in each group |
Prochazka 1998.
| Methods | NORTRIPTYLINE Randomized controlled trial Setting: VAMC & Army Medical Centre, USA Recruitment: outpatient clinics and campus advertisements |
|
| Participants | 214 smokers, >10 CPD (Excludes 29 early drop‐outs); 38% F, av age 47, av CPD 21,12% had a history of depression | |
| Interventions | 1. Nortriptyline max 75 mg/day from 10 days pre‐quit date to 8w after, tapered for 2w. 2. Placebo capsules. Both arms: 2 behavioural group sessions prior to drug therapy. During treatment individual support was provided by the study nurse. | |
| Outcomes | Abstinence at 6m (prolonged) Validation: CO =< 9 ppm at each visit and urine cotinine < 50 ng/mL at 6m. | |
| Notes | Funding: Department of Veterans Affairs, US Department of Defense | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | "An unblinded research pharmacist recommended dosage reductions for those above the therapeutic range and dosage increases for those who were subtherapeutic. To maintain blinding, dose reductions and increases on an equal number of randomly selected placebo‐treated subjects were also recommended...our blinding was only partially effective. Because of the high frequency of dry mouth, the nurse and subjects were often able to identify the active drug." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 75% drop‐out rate in placebo, 61% in drug group, majority classified as ineffective therapy. |
Prochazka 2004.
| Methods | NORTRIPTYLINE Randomized controlled trial Setting: clinic, USA Recruitment: outpatient clinic & community volunteers |
|
| Participants | 158 smokers, > 10 CPD, excluding current depression; 54% F, av. CPD 22, 6% history of depression | |
| Interventions | 1. Nortriptyline max 75 mg/day for 14w, from 2w before TQD tapered for 2w + nicotine patch 8w from TQD 2. Placebo capsules + active nicotine patch. All arms: brief counselling from nurse at weekly visits | |
| Outcomes | Abstinence at 6m (prolonged) Validation: CO ≤ 9 ppm at each visit, cotinine < 50 ng/ml at 6 months | |
| Notes | First included based on unpublished data, Prochazka 2001. One fewer nortriptyline quitter in published paper Funding: Department of Veterans Affairs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were stratified by history of previous major depression and randomized by means of a computer‐generated random number list that was held by the Research Pharmacy Service of the Denver Veterans Affairs Medical Center." |
| Allocation concealment (selection bias) | Low risk | "Once a patient was enrolled, the Research Pharmacy Service randomized the subject according to the randomization list." Judged adequate. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Authors caution "...our blinding was only partially effective. Because of the high frequency of dry mouth, the study nurse was often able to identify the active drug." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Subjects who dropped out were counted as smokers." Number of dropouts not given. |
Richmond 2013.
| Methods | NORTRIPTYLINE Randomized controlled trial Setting: 18 prisons, Australia Recruitment: referral from clinic staff, flyers and posters in prisons |
|
| Participants | 425 male prisoners aged >18, incarcerated for ≥ 1m with ≥ 6m of current sentence remaining, FTND ≥ 5. av age 34, av cpd 23, 83% FTND ≥ 6 | |
| Interventions | 1. Nortriptyline in tablet form for 13 weeks (TQD week 3. Week 1: 25 mg/day for 3 days, 50 mg/day for 4 days. Weeks 2 to 12 75 mg/day. Week 13 50 mg/day for 4 days, then 25 mg/day for 3 days) 2. Placebo on same schedule Both groups: Two 30 minute counselling sessions with CBT. Self‐help materials, access to quitline. 10 weeks NRT patch started on TQD; 21 mg weeks 1‐6, 14 mg/day weeks 7‐8, 7 mg/day weeks 9‐10. |
|
| Outcomes | Continuous abstinence at 12m Validation: CO < 10 ppm |
|
| Notes | New for 2013 update N quit extrapolated from percentages provided Funding: National Health and Medical Research Council, NSW Department of Health, Queensland Department of Health. NRT provided free of charge by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Randomization algorithm,” no further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Identical placebo. “Follow‐up assessments were conducted… by a prison nurse research assistant who was blind to group allocation.” No further information on blinding provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80% followed up at 12m, similar in both groups |
Rigotti 2006.
| Methods | BUPROPION Randomized controlled trial Setting: hospitals, USA Recruitment: volunteers |
|
| Participants | 248 smokers hospitalised with cardiovascular disease (excludes 3/3 dropped prior to treatment & 2 placebo deaths during follow up) 31% F, av age 56, av CPD 23/21. 30%/20% had prior use of bupropion, 54%/56% prior use of NRT | |
| Interventions | 1. Bupropion 300 mg for 12w 2. Placebo Both arms: Multicomponent CBT cessation & relapse prevention programme, motivational interviewing approach, Begun in hospital, 30‐45 mins, 5 X10 min post‐discharge contacts (2 days,1,3,8, 12w), S‐H, chart prompt for physician. Total time 80‐95 mins | |
| Outcomes | Abstinence at 12m (sustained at multiple follow ups) Validation: saliva cotinine at 12 & 52w, CO at 2 & 4w | |
| Notes | Funding: National Heart, Lung and Blood Institute, National Institutes of Health General Clinical Research Centers Program, GlaxoSmithKline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a computer program, the study statistician generated a sequence of randomly‐permuted blocks of 4 within strata formed by study site and daily cigarette consumption (10 vs 10)." |
| Allocation concealment (selection bias) | Low risk | "The study pharmacist used this sequence, concealed from enrolment staff, to assign participants to study arm. Subjects and study personnel, except the statistician and pharmacist, were blind to treatment assignment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Subjects and study personnel, except the statistician and pharmacist, were blind to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Subjects were considered smokers if they were lost to follow‐up..."; 23% lost to follow up in the bupropion group and 23% in the placebo group. |
Rose 2013.
| Methods | BUPROPION Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers |
|
| Participants | 440 smokers of ≥ 10cpd who did not respond successfully to cessation treatment with NRT (phase 1 = 335 participants whose smoking did not decrease by >50% after 1 week NRT (prior to TQD); phase 2 = 105 participants who lapsed within one week after TQD) 50% M, av age 43, av cpd 22, mean FTND 5.8 |
|
| Interventions | 1. 12 weeks bupropion (150 mg/day for 3 days, 300 mg/d for remainder) and nicotine patch (patch dose based on expired CO, 21 mg/day for CO ≤ 30 ppm, 42 mg/day for CO > 30 ppm) 2. Varenicline alone (not included in any analyses as bupropion comparison would be confounded by addition of NRT) 3. Nicotine patch only (dosing as above) Both arms: Cessation programme with nicotine patch (discontinued after 1w in Phase 1 varenicline arm) and 4 to 6 brief (<15min) counselling sessions |
|
| Outcomes | Continuous abstinence at 6m Validation: CO ≤ 10 ppm |
|
| Notes | New for 2013 update Phase 1 and Phase 2 combined in meta‐analysis. Sensitivity analyses including both separately did not detect any significant effect on the pooled result. Funding: Supported by grant to Duke University from Philip Morris USA. NRT donated by GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | < 50% followed up at 6m in both phases, similar rates of dropout across all arms. 27 participants censored from reported analyses, mainly for protocol violations, included a smoking here. |
Rovina 2009.
| Methods | BUPROPION Randomized controlled trial Setting: cessation clinic, Greece Recruitment: Clinic attenders invited to participate |
|
| Participants | 205 smokers of average >15 cpd daily 40% F, av. age 45, av. cpd 37 |
|
| Interventions | 1. Bupropion 300 mg/day for 19 wks + 15 mins physician counselling 2. Bupropion 300 mg/day for 19 wks + nonspecific group therapy (NSGT), 1 hour weekly for 1 m, then every 3 wks until 19 wks 3. Bupropion 300 mg/day for 19 wks + cognitive behavioral group therapy (CBGT), same schedule 4. CBGT without bupropion |
|
| Outcomes | Abstinence at 12m after end of treatment (continuous) Validation: CO ≤ 10 ppm |
|
| Notes | New for 2013 update. 3 versus 4 used analyses, 1 and 2 not included in any analyses (effect of different counselling would confound effect of bupropion) Authors do not report n abstinent, numbers included in MA extrapolated from applying percentage to overall n randomized Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not stated, 3:1:1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label, participants and staff aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 90% followed up at 12m, but authors do not specify percentage per group and do not specify how participants lost to follow‐up were treated. Authors only provide percentages abstinent, so n abstinent in this review may be inflated. |
Saules 2004.
| Methods | FLUOXETINE Randomized controlled trial Setting: cessation clinic, USA Recruitment: volunteers |
|
| Participants | 150 smokers, 20% history of MDD; 55% F, av. age 40 | |
| Interventions | 1. Fluoxetine 40 mg for 14w, nicotine patch for 10w 2. Fluoxetine 20 mg for 14w, nicotine patch for 10w 3. Placebo & nicotine patch All arms: TQD end of w4, CBT 6 sessions starting 2w before TQD, 11 clinic visits | |
| Outcomes | Abstinence at 12m (not defined) Validation: CO < 10 ppm | |
| Notes | Authors provided quit numbers by treatment group Funding: National Institute on Drug Abuse, State of Michigan. Nicotine patch provided by McNeil Consumer Healthcare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers lost to follow up not provided by study arm but high: at six months, only 58 of 150 subjects followed‐up. Subjects who dropped out of the study or lost to follow‐up were considered to be smoking again. |
Schmitz 2007.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: Research clinic, USA Recruitment: Community volunteers |
|
| Participants | 154 women smokers >20 CPD; av. age 48, av. CPD 21 | |
| Interventions | Factorial trial of bupropion and 2 group therapies 1. Bupropion 300 mg/day for 7 weeks 2. Placebo Both arms: either CBT based on relapse prevention model, or group support therapy, both 7 weekly 60 min meetings, TQD morning of 1st session, 10 days after start of medications | |
| Outcomes | Abstinence at 12m (7 day PP) Validation: CO ≤ 10ppm, saliva cotinine < 15ng/ml | |
| Notes | Group therapy variants collapsed in main analysis Funding: National Institute on Drug Abuse. Bupropion provided by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn procedure, balancing on a range of outcome‐related variables. |
| Allocation concealment (selection bias) | Low risk | "Investigators and research staff were blind to the randomization codes, which were kept by a faculty member independent of the research and treatment team." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," further information not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 'enrolment failures' who did not receive any treatment are excluded from analyses. Other non‐completers and losses to follow up included in ITT analysis. |
Schnoll 2010.
| Methods | BUPROPION Randomized controlled trial Setting: not specified (presumably clinic), USA Recruitment: Patient lists from physicians treating people with cancer |
|
| Participants | 246 cancer patients smoking ≥ 2 cpd 48% F, av age 54.8, av cpd 17.5, mean FTND 3.2. 32% had tobacco related tumours. |
|
| Interventions | 1. Bupropion for 9 weeks, started 2 weeks before TQD (150mg/d first week, 300mg/d remaining 8 weeks) 2. Placebo on same schedule Both arms: 8 weeks nicotine patches and 5 sessions of behavioural counselling (3 in person, 2 over phone) |
|
| Outcomes | 7d PP at 6m Validation: CO ≤ 10ppm |
|
| Notes | New for 2013. Previously listed as Scholl 2005 in 'Studies awaiting classification.' Funding: National Cancer Institute. NRT provided free of charge from GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by depression status. Method of sequence generation not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind," no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 65% intervention and 72% control followed up at 6m |
Selby 2003.
| Methods | BUPROPION Randomized controlled trial Setting: 15 clinical centres, Canada Recruitment: community volunteers |
|
| Participants | 284 smokers previously exposed to bupropion for at least 2w, not quit for more than 24 hours in previous month | |
| Interventions | 1. Bupropion 300mg for 12w 2. Placebo Behavioural support not described | |
| Outcomes | Abstinence at 12m (PP) Validation: CO <= 10 ppm at treatment visits | |
| Notes | Based on abstract Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given, unclear how participants lost to follow‐up treated in outcome data. 70% intervention group and 50% control group completed study. |
Siddiqi 2013.
| Methods | BUPROPION Cluster randomized trial Setting: health centres, Pakistan Recruitment: patients from participating health centres with suspected pulmonary tuberculosis |
|
| Participants | 33 health centres covering 1955 adult smokers with suspected tuberculosis (1299 included in arms relevant to this review), smoking ≥ 1cpd or smoking hookah on a daily basis 95%M, av age 41, av cpd 19 (where one hookah counts as 2 cigarettes) |
|
| Interventions | 1. 7 weeks bupropion (75mg/d first week, 150mg/d thereafter) 2. No pharmacotherapy Both arms: 2 sessions of brief, in‐person behavioural support (Note, third arm received usual care only, not included in this review) |
|
| Outcomes | Continuous abstinence at 6m Validation: CO ≤ 9 ppm |
|
| Notes | New for 2013 Reported narratively only due to substantial heterogeneity of program effects across clusters. 275/659 quit intervention vs 254/640 control, adjusted RR 1.1 (0.5–2.3). Funding: International Development Research Centre |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Cluster randomized trial. “A researcher who was blinded to center identity” allocated conditions |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clinics dropped out post‐randomization. Over 90% of participants followed up at 6m |
| Other bias | High risk | Substantial heterogeneity of program effects across clusters. 20% of participants in control arm smoked only hookah (no cigarettes) compared to 4% in intervention arm. |
Simon 2004.
| Methods | BUPROPION Randomized controlled trial Setting: VAMC outpatient units, USA Recruitment: outpatients |
|
| Participants | 244 smokers, 79% veterans; 5% F, av. age 50, av. CPD 24, 17% history of depression. | |
| Interventions | 1. Bupropion 300 mg for 7w, nicotine patch for 2m 2. Placebo bupropion, nicotine patch for 2m Both arms: 3m CBT counselling, S‐H materials and telephone follow‐up counselling | |
| Outcomes | Abstinence at 12m (sustained at multiple follow ups) Validation: saliva cotinine | |
| Notes | Used in bupropion+NRT vs NRT comparison.
2 placebo & 3 bupropion deaths excluded from denominators
Originally based on abstract, now uses published data and sustained quitting outcome. Funding: California Tobacco‐Related Disease Research Program |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We assigned participants to the 2 study arms by using a computer algorithm to generate a random list of treatment assignments." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "All study personnel engaged in providing interventions to participants were blinded to treatment assignment." "Blinding appeared to be effective in our study; an approximately equal number of participants were able to guess what their treatment had been at the end of the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 244 participants enrolled, 3 (1%) were lost to follow‐up (all randomized to the placebo arm)...Participants lost to follow‐up were considered smokers." |
Simon 2009.
| Methods | BUPROPION Randomized controlled trial Setting: VAMC hospital, USA Recruitment: hospitalised volunteers |
|
| Participants | 83 inpatients smoking at least 5 CPD in previous year, smoking in week before admission, in contemplation or preparation stage of change; | |
| Interventions | 1. Bupropion 300 mg for 7w 2. Placebo Both arms: Individual cognitive behavioural 30‐60 min during hospital stay + 5 phone calls at w1, w3, w5, w8, w12, recycling encouraged. | |
| Outcomes | Abstinence at 6m, continuous at each assessment Validation: saliva cotinine <15 ng/ml | |
| Notes | 1 death in bupropion, 1 in placebo excluded from analyses Funding: California Tobacco‐Related Disease Research Program |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer algorithm to generate a random list of treatment assignments." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All study personnel engaged in providing interventions to participants were blinded to treatment assignment." "A significant percentage of participants were able to guess correctly whether they were taking active bupropion or placebo" but as results did not favour intervention group, authors suggest this unblinding did not bias the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 withdrawals, 1 lost to follow‐up, 1 death in placebo, 2 withdrawals, 1 lost, 1 death in bupropion. All except deaths included in MA |
Smith 2009.
| Methods | BUPROPION Randomized controlled trial Setting: 12 primary care clinics, USA Recruitment: volunteers from primary care clinics |
|
| Participants | 1346 smokers of >10 cpd for past 6m. 56% F, av.age 44, av.cpd 20.3, motivated to quit | |
| Interventions | 1. Bupropion only (up‐titrated during wk pre‐quitting, 150 mg bid for 8 wks postquit) 2. Nicotine lozenge only (4 mg lozenge if first cig of day smoked >30 min after waking, 2 mg otherwise. 1 lozenge every 1‐2hrs postquit wk 1‐6; 1 lozenge every 2‐4hrs wk 7‐9; 1 lozenge every 4‐8hrs wk 10‐12) 3. Nicotine patch only (21 mg post‐quit wk 1‐4; 14 mg wk 5‐6; 7 mg wk 7‐8) 4. Bupropion and lozenge (dosage as above) 5. Patch and lozenge (dosage as above) Both arms: Quitline counselling (state provided). All participants received initial session, then could elect to receive up to 4 additional calls + could call for additional support if required. |
|
| Outcomes | 7d PP at 6m and number of days to relapse. Validation: none |
|
| Notes | New for 2013 update. No control so does not contribute to primary analysis. 4 vs 2 used in Analysis 1.5. 1 vs 3 used in Analysis 1.7.1, 1 vs 2 used in Analysis 1.7.2, and 1 vs 5 used in Analysis 1.7.3 (n in 1 divided equally between subgroups to avoid triple counting). Majority of funding from National Institutes of Health, National Institute on Drug Abuse, and National Cancer Institute. Medication provided to participants at no cost by GlaxoSmithKline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient detail with which to judge. "Smokers were randomized to the 5 treatment conditions within each clinic with blocking on sex and self‐identified race." |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 158 individuals who did not pick up study medication at first point not included in analyses; 122 withdrawals & 9 deaths considered to be smoking. |
SMK20001.
| Methods | BUPROPION Randomized controlled trial Setting: 6 clinical trial centres, USA Recruitment: volunteers for phase II trial |
|
| Participants | 286 smokers >=15 CPD, no prior use of bupropion 48% F, av age 42, av CPD NS | |
| Interventions | 1. Bupropion 300 mg for 7w & placebo novel therapy 2. Double placebo No information about behavioural support | |
| Outcomes | Abstinence at 12m (continuous) Validation: CO <= 10 ppm | |
| Notes | Identified from GSK trials website. Also included a novel cessation aid Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34% lost in bupropion, 29% placebo, included as smokers. |
Sood 2010.
| Methods | ST JOHN'S WORT Randomized controlled trial Setting: Community, USA Recruitment: press releases and local advertising |
|
| Participants | 118 adult smokers of ≥ 10 cpd, motivated to quit. 18% M, av age 38, av cpd 20, mean FTND 5.0. |
|
| Interventions | 1. St John's wort 900 mg/day (300 mg tablet 3xday for 12 weeks) 2. St John's wort 1800 mg/day (3 x 300 mg/day tablet first week, 3 x 600 mg/day tablet weeks 2‐12) 3. Matched placebo on same schedule Both arms:12 week behavioural intervention using Mayo Clinic ‘Smoke Free and Living It’ manual (type and number of sessions not stated) |
|
| Outcomes | Prolonged abstinence at 24 weeks (2 week grace period following quit date) (7d PP also reported) Validation: CO ≤ 8ppm |
|
| Notes | New for 2013 update Groups 1 and 2 combined in meta‐analysis; no significant difference between the two (at 24 weeks, 1/39 abstinent intervention 1, 2/40 abstinent intervention 2). Funding: National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated centrally by Mayo Clinic Division of Biostatistics |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “Blinded” with matched placebo, no further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 43% dropped out within first 12 weeks, unclear how many dropped out by 24 weeks. Not given by arm. |
Sood 2012.
| Methods | S‐ADENOSYL‐L‐METHIONINE (SAMe) Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers |
|
| Participants | 120 smokers of ≥ 10 cpd motivated to quit 53% M, av age 40, av cpd 20, mean FTND 5.2 |
|
| Interventions | 1. SAMe 1600 mg/day (via mouth) for 8w 2. SAMe 800 mg/day same schedule 3. Placebo same schedule All arms: Behavioural counselling using “Smoke Free and Living It” manual at every clinic visit (approx. 7) |
|
| Outcomes | 7d PP at 6m (prolonged abstinence measured but not reported) Validation: CO ≤ 8 ppm |
|
| Notes | New for 2013 update. SAMe is a dietary supplement used to treat depression. No difference between arms 1 and 2, hence combined in meta‐analysis. Funding: National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Blinded," no further detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57% followed up overall, similar rates between groups |
Spring 2007.
| Methods | FLUOXETINE Randomized controlled trial Setting: clinic, USA Recruitment: community volunteers |
|
| Participants | 247 smokers, >= 10 CPD; 54% F, av. age 44, av. CPD 23, 44% history of MDD | |
| Interventions | 1. Fluoxetine 60 mg (titrated up over 2 w) for 12 weeks 2. Placebo Both arms: group behavioural counselling, 9 meetings over 12 weeks | |
| Outcomes | Abstinence at 6m (prolonged from 2 w after quit date) Validation: CO < 10 ppm, urine cotinine < 20 ng/ml | |
| Notes | First included as Spring 2004 with unpublished data. Full publication reports sustained abstinence Funding: National Institutes of Health, Veterans Affairs. Medication provided by Eli Lilly and Company. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study pharmacist stratified participants by depression history and used computer‐ generated random numbers to assign them to drug or placebo." |
| Allocation concealment (selection bias) | Unclear risk | Allocated by unblinded pharmacist, method not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double blind, "Research staff and participants were blinded to medication status." "Drug assignment was guessed correctly by 59.8% of placebo and 64.6% of fluoxetine participants. Facilitators guessed correctly for 65.3% of placebo and 55.6% of fluoxetine participants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals/lost to follow‐up 40% for fluoxetine, 48% placebo. Authors report similar results from missing assumed smoking and GEE analyses. All participants included in MA. |
Stapleton 2013.
| Methods | BUPROPION Randomized controlled trial Setting: Smoking cessation clinics, UK Recruitment: People attending smoking cessation clinics |
|
| Participants | 1071 daily smokers; 47% M, av age 41, av CPD 20, >25% history of depression | |
| Interventions | 1. Bupropion for 8 weeks, started prior to TQD (exact period NS), 150 mg/d for first 6d, then 300 mg for remainder 2. Bupropion (as above) + NRT (choice of single product, 12 weeks started on TQD, dosage determined on individual basis) 3. NRT only (as above) All groups: 7 weekly behavioural support sessions as per standard service protocol. Mainly group, 60‐90 mins each |
|
| Outcomes | Prolongued abstinence at 6m Validation: CO < 10 ppm |
|
| Notes | Funding: Department of Health for England. Study medication provided free of charge by Pfizer UK, GSK UK and Novartis UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized. “Randomization and packaging was organized by an independent statistician at the host site.” |
| Allocation concealment (selection bias) | Low risk | “On enrolment, participants selected their envelope from a large batch and signed it before breaking the seal to reveal their allocation.” |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label, no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61.5% followed up at both 1 and 6m, no significant difference between groups. Prolonged abstinence only imputed for 16% of total. |
Swan 2003.
| Methods | BUPROPION Randomized controlled trial, 2x2 factorial Setting: HMO, USA Recruitment: volunteers from Group Health Co‐op membership |
|
| Participants | 1524 smokers >= 10 CPD; 57% F, av age 45, av CPD 23, 44% history of depression | |
| Interventions | Factorial design crossing 2 drug doses with 2 intensities of behavioural counselling: Bupropion 300 mg/day versus 150 mg/day Free & Clear proactive telephone counselling (4 brief calls), access to quitline and S‐H materials vs Zyban Advantage Program (ZAP) tailored S‐H materials, single telephone call after TQD, access to Zyban support line Prescription was mailed. No face‐to‐face contact during enrolment or Rx | |
| Outcomes | Abstinence at 12m (7‐day PP) Validation: none | |
| Notes | Based on published data from 2004
No dose/behavioural treatment interaction at 12m so arms collapsed to compare 300 vs 150
Effects differed at 3 and 12m. Effect of higher dose disappeared and additional support aided recycling. Funding: National Cancer Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Open‐label randomized trial...The computer code for the procedure calculated probabilities of group assignment that were dynamically modified based on the number of members in each group so that final group sizes were equal. No restrictions such as stratification or blocking were used as part of the randomization process." |
| Allocation concealment (selection bias) | Low risk | Procedure built into study database. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentage lost to follow‐up across all groups (approx 15%). Nonresponders treated as smoking. |
Tashkin 2001.
| Methods | BUPROPION Randomized controlled trial Setting: multi‐centre, USA Recruitment: advertisements for volunteers |
|
| Participants | 404 smokers with mild to moderate COPD. (Excludes 7 early drop‐outs who did not take any study medication); 45% F, av. age 53‐54, av. CPD 28, 18% in Bupropion group and 23% in Placebo had a history of depression. | |
| Interventions | 1. Bupropion SR 300 mg/day for 12w from 1w before TQD 2. Placebo All participants had brief face‐to‐face counselling at each clinic visit (weeks 1‐7, 10, 12), telephone counselling 3 days after TQD | |
| Outcomes | Abstinence at 52w, sustained from w4 (unpublished data from GSK, Lancet paper reports 6m data) Validation: CO =< 10 ppm at each visit | |
| Notes | 12m unpublished data used from 2003/2.
ITT population defined as those taking at least one dose of study medication. Funding: Glaxo Wellcome Inc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised as per code provided by Glaxo Wellcome, using block sizes of four stratified by centre. Within each block of four, two participants were assigned placebo and two bupropion SR. The randomisation codes were kept at the study sites during the trial and we instructed investigators to break the code only for a medical emergency." |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind study, but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64% intervention and 73% control followed up at 6m. "All participants who withdrew from the study were taken to be smokers thereafter." |
Tonnesen 2003.
| Methods | BUPROPION Randomized controlled trial Setting: 28 clinical trial centres in 8 European countries, Australia, NZ Recruitment: community volunteers |
|
| Participants | 710 smokers >= 10 CPD; 51% F, av. age 42, median CPD 20, no details of depression history | |
| Interventions | 1. Bupropion SR 300 mg/day for 7w 2. Placebo Both arms: brief motivational support at weekly clinic visits and telephone support during follow up. 11 clinic visits and 10 phone calls scheduled. | |
| Outcomes | Abstinence at 52w (prolonged from w4) Validation: CO <= 10 ppm | |
| Notes | First included 2003 as Tonstad 2001.
ITT population defined as those taking at least one dose of study medication excludes 3 randomized participants Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "GlaxoSmithKline created a randomization schedule in a 3 : 1 bupropion: placebo ratio. Each centre received a list with treatment numbers and subjects were consecutively assigned a treatment number at the baseline visit." |
| Allocation concealment (selection bias) | Low risk | As per above. "GlaxoSmithKline supplied bupropion SR 150 mg and placebo‐to‐match tablets for oral administration as white, film‐coated tablets." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double blind but methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of bupropion SR and 12% placebo were lost to follow‐up. |
Tonstad 2003.
| Methods | BUPROPION Randomized controlled trial Setting: 28 clinical trial centres in 10 countries incl Europe, Australia, NZ Recruitment: volunteers with CVD |
|
| Participants | 629 smokers with stable cardiovascular disease (CVD), >= 10 CPD; 23% F, av. age 55, av. CPD 25, 49% had history of MI, no details of depression history | |
| Interventions | 1. Bupropion SR 300 mg/day for 7w, begun 1‐2w before TQD 2. Placebo Both arms: brief motivational support at weekly clinic visits and telephone support during follow up. 9 clinic visits and 10 phone calls scheduled. | |
| Outcomes | Abstinence at 12m (prolonged from w4) Validation: CO <= 10 ppm | |
| Notes | First included 2003 as McRobbie 2003. ITT population = 626 defined as those taking at least one dose of study medication. Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but no further detail provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number missing follow‐up in each group not provided. At 12m, 38% bupropion and 50% placebo had prematurely discontinued treatment. "Subjects with missing investigator assessments were assumed to be smokers at that visit." |
Uyar 2007.
| Methods | BUPROPION Randomized controlled trial Setting: cessation clinic, Turkey Recruitment: cessation clinic patients |
|
| Participants | 131 smokers; 81% M, av. age 36 | |
| Interventions | 1. Bupropion 300mg for 7 weeks 2. Nicotine patch 21mg for 6 weeks incl tapering 3. Advice and follow up only All arms: Brief counselling on consequences of smoking with follow up for 24 weeks‐ more than low intensity | |
| Outcomes | Abstinence at 24w (not defined) Validation: CO < 10 ppm | |
| Notes | First included based on abstract. Contributes to bupropion vs control and bupropion vs nicotine patch Funding not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly allocated', method not described, unclear why fewer in control condition. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of any losses to follow‐up. |
Wagena 2005.
| Methods | BUPROPION & NORTRIPTYLINE Randomized controlled trial Setting: university medical centre, Netherlands Recruitment: community volunteers |
|
| Participants | 255 smokers (>= 10 CPD) with or at risk of COPD; 51% F, av. age 51, av. CPD 23, 20% had possible depression, 7% previous use of bupropion | |
| Interventions | 1. Bupropion SR 300 mg/day for 12w 2. Nortriptyline 75 mg/day for 12w 3. Placebo bupropion or placebo nortriptyline All arms: Individual counselling 10‐20 mins at baseline, 1w & 3w post TQD (TQD typically day 11). Telephone support TQD, 2, 4, 6, 8, 11w. | |
| Outcomes | Abstinence at 26w (prolonged puff‐free from w4) Validation: Urine cotinine <= 60 ng/ml at 4, 12 & 26w | |
| Notes | Funding: Netherlands Asthma Foundation, Netherlands Organization for Health Research and Development. Lundbeck BV provided nortriptyline free of charge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated by pharmacist, stratified by COPD severity, block size 33. |
| Allocation concealment (selection bias) | Low risk | Research staff blinded throughout study. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double blind but "at both time points, participants receiving active drug compared with those receiving placebo were more likely to guess that they had received bupropion SR and nortriptyline treatment (72% vs 43% , P.01; and 62% vs 37%; P=.001; respectively)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 (12%) bupropion, 13 (16%) nortriptyline, 12 (13%) lost or withdrawn. All included in ITT analysis. |
Weinberger 2010.
| Methods | SELEGILINE Randomized controlled trial Setting: clinics, USA Recruitment: community volunteers |
|
| Participants | 101 smokers (excludes 2 taking no medication), 50% F, av. age 47, av. CPD 22, 28% had history of MDD | |
| Interventions | 1. Selegiline 10 mg/day for 9 weeks (5 mg/day in w1 & w9) 2. Placebo Both arms; brief weekly counselling | |
| Outcomes | Abstinence at 6m (7‐day PP) Validation: CO & cotinine | |
| Notes | Previously included as Weinberger 2009 based on unpublished data. Minor change to data based on published report in 2013 update. Funding: National Institute of Drug Abuse, Veteran's Administration, Women's Health Research at Yale, NIH, University of Toronto. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Both participants and research staff were blinded to study medication assignment," assessments of staff and participants suggest blinding was adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27.5% selegiline, 42% placebo lost at 6 months. Including all participants is less conservative. |
Wittchen 2011.
| Methods | BUPROPION Randomized controlled trial Setting: 167 primary care clinics, Germany Recruitment: patients at participating primary care clinics |
|
| Participants | 467 'current regular smokers' attending primary care clinic for any reason and willing to consider treatment in next 7d. 48% M, av.age 43, av.cpd 20 | |
| Interventions | 1. Minimal intervention (not used in review) 2. CBT (4‐5 one on one counselling sessions for 20‐30min) 3. CBT (as above) + bupropion SR (9‐12 wks, 150mg;1/d for first 6d; 2/d thereafter) 4. CBT (as above) + NRT for 9‐12 wks, patient's choice of patch (7mg‐52.5 mg), gum (2 or 4 mg) or spray (10mg/ml) |
|
| Outcomes | Abstinence at 12m (from EoT) Validation: none |
|
| Notes | New for 2013 update 3 vs 2 included in primary analyses. 2 vs 4 included in Analysis 1.7 comparison of NRT with bupropion. 1 not used as results vs. bupropion would be confounded with CBT. Patients covered all costs for pharmaceutical treatments. Sponsored by the Federal Ministry of Education and Research; additional support provided by GlaxoSmithKline GmbH & Co and Pharmacia GmbH. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Generated by the study center"; used to put 4 different coloured questionnaires in random order |
| Allocation concealment (selection bias) | High risk | No concealment: "questionnaires were distributed consecutively to all attending patients on the target days by nurses. Thus, the assignment of patients was entirely dependent on the consecutive attendance of patients and the random assignment of a color. Doctors were not allowed to interfere with this study procedure." But numbers allocated to groups very uneven and discussion states: "Random checks of this procedure [randomization] and quality assurance tests by study monitors revealed that in some cases in the latter part of the study treatment was based on patient and physician preferences." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Neither participants nor providers were blind. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar number of drop‐outs between groups; participants lost to follow‐up considered smokers for MA |
Zellweger 2005.
| Methods | BUPROPION Randomized controlled trial Setting: 26 clinical trial centres in 12 European countries Recruitment: volunteers, healthcare professionals (qualified practising physician or nurse) |
|
| Participants | 667 smokers (>= 10 CPD) (excludes 1 centre enrolling 20 people, and 3 people who took no medication) 64% F, Av age 40, av CPD 23. 32% doctor, 68% nurse, no details of depression history | |
| Interventions | 1. Bupropion SR 300 mg/day for 7w 2. Placebo Both arms: Brief (10‐15 min) motivational support at weekly clinic visits and telephone support one day before TQD, 3 days after TQD, monthly during follow up | |
| Outcomes | Abstinence at 52w (prolonged from w4) Validation: CO <= 10 ppm | |
| Notes | Continuous abstinence rates and information on adverse events from GlaxoSmithKline data. One centre excluded Funding: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind but further detail not provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number lost to follow‐up not stated. Participants with missing assessments or drop‐outs considered to be smoking. |
av: average
AE: adverse event CBT: cognitive behavioural therapy CES‐D: Center for Epidemiologic Studies Depression Scale CO: carbon monoxide (in exhaled breath) COPD: chronic obstructive pulmonary disease CPD: cigarettes per day CVD: cardiovascular disease EOT: end of treatment F: female FTND: Fagerstrom Test for Nicotine Dependence FTQ: Fagerstrom Tolerance Questionnaire ITT: intention to treat m: month/s MA: meta‐analysis MDD: major depressive disorder MI: myocardial infarction mins: minutes
NRT: nicotine replacement therapy NS: not stated P: placebo PP: point prevalence abstinence RP: relapse prevention Rx: treatment S‐H: self‐help TQD: target quit date VAMC: Veterans Affairs Medical Center w: week/s
y: year/s
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akbarpour 2010 | Bupropion ‐ short follow‐up |
| Banham 2010 | Not RCT ‐ review of smoking cessation treatment for people with severe mental illness |
| Barnes 2006 | St John's wort ‐ pilot study comparing two doses of St John's wort, no quitters at 12 months. |
| Becker 2003 | St John's wort ‐ short follow‐up (1 month) |
| Berlin 2002 | Lazabemide (monoamine oxidase‐B inhibitor) ‐ short follow up |
| Berlin 2005 | Befloxatone (reversible monoamine oxidase‐B inhibitor) ‐ data not published, treatment reported to have had no effect on abstinence rates. |
| Berlin 2012 | EVT302 (MAO‐B inhibitor) ‐ short follow‐up. (No evidence of short‐term benefit; 412 participants, RR 1.21, 95% CI 0.83 to 1.76) |
| Bloch 2010 | Bupropion ‐ trial in people with schizophrenia, short follow‐up and cessation not reported |
| Bowen 1991 | Tryptophan ‐ short follow up Tryptophan 50 mg/kg/day, with high carbohydrate low protein diet (7/1 ratio), vs placebo and low carbohydrate high protein diet (1/1 ratio) for two weeks. |
| Brauer 2000 | Selegiline ‐ only preliminary short‐term results available. Six month follow‐up planned |
| Breitling 2008 | Trial of practitioner education and financial incentives, or cessation drug costs reimbursement |
| Carrão 2007 | Sertraline ‐ combined with buspirone so effect of sertraline could not be isolated |
| Chan 2005 | Bupropion ‐ case control study in pregnant women |
| Cornelius 1997 | Fluoxetine ‐ cessation not an outcome. Fluoxetine reduced the amount smoked by depressed alcoholic smokers. |
| Cornelius 1999 | Fluoxetine ‐ short‐term outcome in a study of depressed alcoholic patients not attempting to quit. |
| Dalack 1995 | Fluoxetine ‐ refers to but does not report on a cessation study. |
| Dale 2002 | Bupropion ‐ used for smokeless tobacco cessation, not smoking cessation. |
| Dale 2007 | Bupropion ‐ for smokeless tobacco cessation, see Ebbert 2011 |
| Daniela 2008 | Sertraline and buspirone ‐ effect of antidepressant confounded with that of anxiolytic |
| Edwards 1989 | Doxepin ‐ short follow‐up (2 months) |
| Elsasser 2002 | Bupropion ‐ only 12 week follow‐up reported to date. 17 teenage (14‐19) smokers treated. |
| Evins 2008 | Bupropion ‐ long‐term results not presented due to high loss to follow‐up |
| Fatemi 2005 | Bupropion ‐ short‐term crossover trial |
| Frederick 1997 | Venlafaxine ‐ short follow‐up (8 weeks) |
| Gawin 1989 | Buspirone ‐ open trial |
| Gifford 2011 | Bupropion ‐ test of behavioural therapy, all participants received bupropion |
| Glover 2002 | Bupropion ‐ used for smokeless tobacco cessation, not smoking cessation |
| Gold 2002 | Bupropion ‐ non random assignment, patient preference |
| Grandi 2011 | Bupropion ‐ not RCT, review of bupropion use in patients with CVD |
| Grassi 2009 | Not an RCT, pre‐post study of influence of smoking ban on people's selection of smoking cessation treatment |
| Gray 2011 | Bupropion ‐ short follow‐up |
| Hall 2009 | Bupropion ‐ all participants received bupropion for quitting, test of extended CBT or NRT |
| Hawk 2008 | Bupropion ‐ short follow‐up (12 weeks). Compares 1 week to 4 week prequit use. |
| Hilberink 2005 | Bupropion ‐ test of NRT + counselling, one cluster received bupropion but is not a test of bupropion |
| Hitsman 1999 | Fluoxetine ‐ the majority of patients in this study were also part of the multi‐centre trial reported in Niaura 2002. |
| Houtsmuller 2002 | Selegiline ‐ short‐term laboratory study |
| Hussain 2010 | Bupropion ‐ short follow‐up, trial in unmotivated smokers |
| Jacobs 1971 | Imipramine ‐ short follow‐up. Outcome was reduction in smoking to less than 10% of baseline. |
| Kalman 2004 | Bupropion ‐ short follow‐up (12 weeks) |
| Karam‐Hage 2011 | Bupropion ‐ short follow‐up (to end of medication phase). Pilot study, 11 participants |
| Kotz 2009 | Nortriptyline ‐ pharmacotherapy was confounded with additional counselling from nurse (control group 1), compared to usual care |
| Kras 2010 | St John's wort ‐ short follow‐up |
| Lawvere 2006 | St John's wort ‐ uncontrolled study |
| Le Foll 2009 | Selegiline ‐ study terminated early due to lack of efficacy, results available at 9 weeks only |
| Li 2009 | Bupropion ‐ short follow‐up |
| Miller 2003 | Bupropion ‐ short follow‐up (8 weeks) |
| Monuteaux 2007 | Bupropion ‐ participants were adolescent non‐smokers, not for cessation |
| Mooney 2008 | Bupropion ‐ short follow‐up, bupropion for opioid and tobacco dependence |
| Naranjo 1990 | Fluoxetine ‐ study of short‐term smoking behaviour. |
| Neumann 2000 | Bupropion ‐ smokers randomized to 1 or 2 months of medication (300 mg/day). 91/165 randomized were not included in the analysis, including some 1‐month group participants who requested further medication. |
| Neumann 2002 | Bupropion ‐ short‐term follow‐up. Comparison of 300 mg and 150 mg doses |
| Niederhofer 2004 | Bupropion ‐ short‐term. 22 adolescents followed up during 90 days of treatment |
| Olmstead 1999 | Bupropion ‐ all participants received bupropion. Short‐term follow‐up. |
| Paluck 2006 | Bupropion ‐ uncontrolled prospective observational study |
| Pomerleau 1991 | Fluoxetine ‐ no cessation data reported |
| Raynor 2005 | Bupropion ‐ short (90 day) follow‐up. Sub‐study within a larger trial with long‐term follow up, not yet published |
| Robinson 1991 | Buspirone ‐ case series |
| Rovina 2003 | Bupropion ‐ abstract only, trial report not available. Insufficient information to determine inclusion |
| Schepis 2006 | Bupropion ‐ abstract only, trial report not available. Insufficient information to determine inclusion |
| Sellers 1987 | Zimelidine or citalopram (SSRIs) ‐ placebo‐controlled crossover design study of smoking behaviour and alcohol use in non‐depressed heavy drinkers |
| Sheng 2013 | Bupropion ‐ follow‐up less than 6 months |
| Sherman 2008 | Bupropion ‐ trial of NRT as adjunct to bupropion |
| Shiffman 2000 | Bupropion ‐ placebo‐controlled short‐term study of effects on craving and withdrawal in patients not wanting to quit smoking permanently |
| Shoptaw 2008 | Bupropion ‐ tested for methamphetamine dependence. Reduction in smoking was a secondary outcome. Only 48/73 participants smoked, quitting not reported. |
| Singh 2010 | Bupropion ‐ short‐term follow‐up |
| Sittipunt 2007 | Nortriptyline ‐ only 3‐month follow‐up |
| Sonntag 2003 | Bupropion ‐ abstract only, trial report not available. Insufficient information to determine inclusion |
| Spring 1995 | Fluoxetine ‐ 6‐month cessation not reported. Primarily a study of post‐cessation weight gain. |
| Stein 1993 | Fluoxetine ‐ does not report outcomes from a double‐blind study |
| Steinberg 2009 | Bupropion ‐ confounded with nicotine inhaler and treatment duration in comparison with nicotine patch alone |
| Strayer 2004 | Bupropion ‐ all participants prescribed bupropion. Test of behavioural interventions, not bupropion. Adverse event data from author used. |
| Swanson 2003 | Bupropion +/‐ nicotine patch. Unable to confirm correct denominators. |
| Tidey 2009 | Bupropion ‐ laboratory study, outcomes included urge to smoke, not cessation |
| Toll 2007 | Bupropion ‐ all participants had same pharmacotherapy |
| Weinberger 2008 | Bupropion for people with bipolar disorder. Short follow‐up (8 weeks). Only 5 participants. |
| Weiner 2001 | Bupropion ‐ no control group |
| Weiner 2012 | Bupropion ‐ short follow‐up |
| White 2005 | Bupropion versus gabapentin ‐ Short follow‐up (6 weeks) |
| Zernig 2008 | Bupropion ‐ used as an active control to a psychosocial intervention, cannot estimate pharmacotherapy effect |
| ZYB30011 2002 | Bupropion ‐ only follow‐up to end of treatment (7 weeks) |
Characteristics of ongoing studies [ordered by study ID]
Rose 2013a.
| Trial name or title | Combination varenicline/bupropion treatment for NRT‐nonresponders |
| Methods | Double‐blind randomized controlled trial |
| Participants | 222 NRT non‐responders |
| Interventions | 1. Varenicline and bupropion 2. Varenicline alone |
| Outcomes | Abstinence at 8‐11 weeks postquit and at 6 months |
| Starting date | March 2011 |
| Contact information | Jed Rose, jed.rose@duke.edu |
| Notes | Results at 8 to 11 weeks found significant benefit of adding bupropion for male participants, but not for female participants |
Contributions of authors
All authors contribute to the text of the review. LS and TL extracted study data, and JHB also extracted data for the 2013 update.
Sources of support
Internal sources
-
Department of Primary Health Care, Oxford University, UK.
Editorial base for the Cochrane Tobacco Addiction Group
-
National Institute for Health Research School for Primary Care Research, UK.
Support for the Department of Primary Health Care, Oxford University
External sources
National Institute on Drug Abuse (NIDA), USA.
NHS Research and Development Programme, UK.
Declarations of interest
JR Hughes has received consultancy fees from many pharmaceutical companies that provide tobacco related services or products or are developing new products, including Pfizer (the maker of NRTs and varenicline) and GlaxoSmithKline (the makers of bupropion and NRTs).
Edited (no change to conclusions)
References
References to studies included in this review
Ahluwalia 2002 {published data only}
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained‐release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002;288:468‐74. [DOI] [PubMed] [Google Scholar]
- Boardman T, Catley D, Mayo MS, Ahluwalia JS. Self‐efficacy and motivation to quit during participation in a smoking cessation program. International Journal of Behavioral Medicine 2005;12:266‐72. [DOI] [PubMed] [Google Scholar]
- Catley D, Harris KJ, Okuyemi KS, Mayo MS, Pankey E, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine & Tobacco Research 2005;7:859‐70. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Ahluwalia JS, Catley D, Okuyemi KS, Mayo MS, Resnicow K. Successful recruitment of minorities into clinical trials: the Kick It at Swope project. Nicotine & Tobacco Research 2003;5:575‐84. [DOI] [PubMed] [Google Scholar]
- Harris KJ, Okuyemi KS, Catley D, Mayo MS, Ge B, Ahluwalia JS. Predictors of smoking cessation among African‐Americans enrolled in a randomized controlled trial of bupropion. Preventive Medicine 2004;38:498‐502. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers?. Addiction 2003;98:1387‐93. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Guo H, Lynam IM, Powell JN, Okuyemi KS, Bronars CA, et al. The impact of perceived treatment assignment on smoking cessation outcomes among African‐American smokers. Journal of General Internal Medicine 2008;23(9):1361‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aubin 2004 {published data only}
- Aubin HJ, Lebargy F, Berlin I, Bidaut‐Mazel C, Chemali‐Hudry J, Lagrue G. Efficacy of bupropion and predictors of successful outcome in a sample of French smokers: a randomized placebo‐controlled trial. Addiction 2004;99:1206‐18. [DOI] [PubMed] [Google Scholar]
- Lebargy F, Aubin HJ, Lagrue G, Bidaut‐Mazel C, Chemali‐Hudry J, Poulain L. A placebo‐controlled, double‐blind study of Zyban LP: An effective and well‐tolerated aid to smoking cessation ‐ preliminary results (POS4‐69). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 New Orleans, Louisiana. 2003.
Aveyard 2008 {published data only}
- Aveyard P, Johnson C, Fillingham S, Parsons A, Murphy M. Nortriptyline plus nicotine replacement versus placebo plus nicotine replacement for smoking cessation: pragmatic randomised controlled trial. BMJ 2008;336(7655):1223‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aveyard P, Johnson C, Murphy M, Johnstone E, Walton R, Fillingham S, et al. A pragmatic randomised controlled trial to test the efficacy of nortriptyline plus nicotine replacement therapy (NRT) versus a placebo plus NRT in helping smokers to stop and testing the role of noradrenergic and dopaminergic genetic variants in smoking cessation [PI‐TS‐02]. Society for Research on Nicotine and Tobacco 8th European Meeting, September 2006; Kusadasi, Turkey. 2006.
Berlin 1995 {published data only}
- Berlin I, Said S, Spreux Varoquaux O, Launay JM, Olivares R, Millet V, et al. A reversible monoamine oxidase A inhibitor (moclobemide) facilitates smoking cessation and abstinence in heavy, dependent smokers. Clinical Pharmacology and Therapeutics 1995;58:444‐52. [DOI] [PubMed] [Google Scholar]
- Berlin I, Spreux Varoquaux O, Said S, Launay JM. Effects of past history of major depression on smoking characteristics, monoamine oxidase‐A and ‐B activities and withdrawal symptoms in dependent smokers. Drug and Alcohol Dependence 1997;45:31‐7. [DOI] [PubMed] [Google Scholar]
Biberman 2003 {published data only}
- Biberman R, Neumann R, Katzir I, Gerber Y. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo plus nicotine skin patch for smoking cessation. Addiction 2003;98:1403‐07. [DOI] [PubMed] [Google Scholar]
Blondal 1999 {published data only}
- Blondal T, Gudmundsson LJ, Tomasson K, Jonsdottir D, Hilmarsdottir H, Kristjansson F, et al. The effects of fluoxetine combined with nicotine inhalers in smoking cessation ‐ a randomized trial. Addiction 1999;94:1007‐15. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Abrantes AM, Strong DR, Lloyd‐Richardson EE, Niaura R, Kahler CW, Brown RA. Regular exercise as a protective factor in relapse following smoking cessation treatment. American Journal on Addictions 2009;18(1):100‐1. [DOI] [PubMed] [Google Scholar]
- Brown RA, Niaura R, Lloyd‐Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive‐behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research 2007;9:721‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd‐Richardson EE, Munafo MR, et al. Pharmacogenetic clinical trial of sustained‐release bupropion for smoking cessation. Nicotine & Tobacco Research 2007;9:821‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Niaura R, Papandonatos GD, Shadel WG, Burkholder GJ, Britt DM, et al. Does the DRD2‐Taq1 A polymorphism influence treatment response to bupropion hydrochloride for reduction of the nicotine withdrawal syndrome?. Nicotine & Tobacco Research 2003;5:935‐42. [DOI] [PubMed] [Google Scholar]
- David SP, Strong DR, Munafo MR, Brown RA, Lloyd‐Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: Analysis of pooled data from two clinical trials. Nicotine & Tobacco Research 2007;9(12):1251‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, David SP, Brightman M, Strong D, McGeary JE, Brown RA, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics Journal 2012;12(1):86‐92. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wileyto EP, Heitjan DF. Modeling smoking cessation data with alternating states and a cure fraction using frailty models. Statistics in Medicine 2010; Vol. 29, issue 6:627‐38. [] [DOI] [PMC free article] [PubMed]
- Okun ML, Levine MD, Houck P, Perkins KA, Marcus MD. Subjective sleep disturbance during a smoking cessation program: associations with relapse. Addictive Behaviors 2011; Vol. 36, issue 8:861‐4. [; 9400123000011571] [DOI] [PMC free article] [PubMed]
- Strong DR, Kahler CW, Leventhal AM, Abrantes AM, Lloyd‐Richardson E, Niaura R, et al. Impact of bupropion and cognitive‐behavioral treatment for depression on positive affect, negative affect, and urges to smoke during cessation treatment. Nicotine & Tobacco Research 2009; Vol. 11, issue 10:1142‐53. [] [DOI] [PMC free article] [PubMed]
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome‐wide association study results. Archives of General Psychiatry 2008;65(6):683‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2013 {published data only (unpublished sought but not used)}
- Brown RA, Abrantes AM, Strong DR, Niaura R, Kahler CW, Miller IW, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Nicotine & Tobacco Research 2013;epub Sep 20. [DOI] [PubMed] [Google Scholar]
- Brown RA, Strong DR, Abrantes AM, Miller IW, Kahler CW, Niaura R, et al. Efficacy of sequential use of fluoxetine for smoking cessation in elevated depressive symptom smokers. Society for Research on Nicotine & Tobacco 17th Annual Meeting, February 2011, Toronto 2011:28S. [] [DOI] [PubMed]
- Brown RA, Strong DR, Miller IW, Kahler CW, Niaura R, Price LH. Efficacy of sequential vs. concurrent use of fluoxetine in smoking cessation for elevated depressive symptom smokers (SYM8D). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
Cinciripini 2005 {published data only}
- Cinciripini PM, Tsoh JY, Friedman K, Wetter D, Cinciripini LG, Skaar KL. A placebo controlled evaluation of venlafaxine for smoking cessation: preliminary findings [Abstract A18]. Society for Research on Nicotine and Tobacco Annual Meeting; Mar 27‐29 1998; New Orleans, Louisiana. 1998.
- Cinciripini PM, Tsoh JY, Wetter DW, Lam C, Moor C, Cinciripini L, et al. Combined effects of venlafaxine, nicotine replacement, and brief counseling on smoking cessation. Experimental and Clinical Psychopharmacology 2005;13:282‐92. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter D, Minna J, et al. The effects of brief counseling, transdermal nicotine replacement and antidepressant therapy on smoking cessation among smokers carrying the DRD2 A1 allele (PA3A). Society for Research on Nicotine and Tobacco Annual Meeting; Mar 5‐7 1999; San Diego, California. 1999.
- Cinciripini PM, Wetter DW, Tomlinson GE, Tsoh JY, Moor CA, Cinciripini LG, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: Evidence for a pharmacogenetic effect on mood. Nicotine & Tobacco Research 2004;6:229‐39. [DOI] [PubMed] [Google Scholar]
Cinciripini 2013 {published data only}
- Cinciripini PM, Robinson JD, Karam‐Hage M, Minnix JA, Lam C, Versace F, et al. Effects of varenicline and bupropion sustained‐release use plus intensive smoking cessation counseling on prolonged abstinence from smoking and on depression, negative affect, and other symptoms of nicotine withdrawal. JAMA Psychiatry 2013;70(5):522‐33. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2004 {published data only}
- Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, et al. Catechol‐O‐methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological Psychiatry 2007;61(1):111‐8. [DOI] [PubMed] [Google Scholar]
- Collins B, Wileyto P, Patterson F, Rukstalis M, Audrain‐McGovern J, Kaufmann V, et al. Gender differences in smoking cessation in a placebo‐controlled trial of bupropion with behavioral counseling. Nicotine & Tobacco Research 2004;6:27‐37. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, BD, Thomas PD, et al. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems‐based candidate gene study of smoking cessation. Human Molecular Genetics 2008;17(18):2834‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Munafo MR, Brown RA, Lloyd‐Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: Analysis of pooled data from two clinical trials. Nicotine & Tobacco Research 2007;9(12):1251‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AB, Wileyto EP, Jepson C, Lori A, Cubells JF, Lerman C. Galanin receptor 1 (GALR1) SNP is associated with craving and smoking relapse. Neuropsychopharmacology 2011; Vol. 36:S378. [; 9400123000012457]
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology 2012;37(7):1683‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Redden DT, Berrettini WH, Shields PG, Restine SL, Pinto A, et al. No evidence for a major role of polymorphisms during bupropion treatment. Obesity 2006;14(11):1863‐7. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Lerman C. The dynamics of the urge‐to‐smoke following smoking cessation via pharmacotherapy. Addiction 2011; Vol. 106, issue 10:1835‐45. [] [DOI] [PubMed]
- Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biological Psychiatry 2007;62(6):635‐41. [DOI] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 2006;31:231‐42. [DOI] [PubMed] [Google Scholar]
- Lerman C, Niaura R, Collins BN, Wileyto P, Audrain MJ, Pinto A, et al. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychology of Addictive Behaviors 2004;18:362‐6. [DOI] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu AY, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence 2002;67:219‐23. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology 2003;22:541‐8. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Pinto A, Hawk L, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics 2002;12:627‐34. [DOI] [PubMed] [Google Scholar]
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo‐controlled trial of bupropion. Clinical Pharmacology & Therapeutics 2008;84:320‐5. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Epstein L, Audrain J, Niaura R, Hawk L, Shields PG, et al. Can the blind see? Participant guess about treatment arm assignment may influence outcome in a clinical trial of bupropion for smoking cessation. Journal of Substance Abuse Treatment 2008;34(2):234‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome‐wide association study results. Archives of General Psychiatry 2008;65(6):683‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain‐McGovern J, et al. Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research 2005;7:257‐68. [DOI] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain‐McGovern J, et al. Do small lapses predict relapse to smoking behavior under bupropion treatment?. Nicotine & Tobacco Research 2004;6:357‐66. [DOI] [PubMed] [Google Scholar]
Covey 2002 {published data only}
- Berlin I, Chen H, Covey LS. Depressive mood, suicide ideation and anxiety in smokers who do and smokers who do not manage to stop smoking after a target quit date. Addiction 2010; Vol. 105, issue 12:2209‐16. [] [DOI] [PubMed]
- Covey LS, Glassman AH, Stetner F, Rivelli S. A trial of sertraline for smokers with past major depression. Society for Research on Nicotine and Tobacco Meeting. Arlington, VA (http://www.srnt.org/events/abstracts99/index.htm) 2000.
- Covey LS, Glassman AH, Stetner F, Rivelli S, Stage K. A randomized trial of sertraline as a cessation aid for smokers with a history of major depression. American Journal of Psychiatry 2002;159:1731‐7. [DOI] [PubMed] [Google Scholar]
Covey 2007 {published data only}
- Covey LS, Botello‐Harbaum M, Glassman AH, Masmela J, LoDuca C, Salzman V, et al. Smokers' response to combination bupropion, nicotine patch, and counseling treatment by race/ethnicity. Ethnicity & Disease 2008;18:59‐64. [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Jiang H, Fried J, Masmela J, LoDuca C, et al. A randomized trial of bupropion and/or nicotine gum as maintenance treatment for preventing smoking relapse. Addiction 2007;102:1292‐302. [DOI] [PubMed] [Google Scholar]
Cox 2012 {published data only}
- Buchanan TS, Cox LS, Nollen NL, Thomas JL, Berg CJ, Mayo MS, et al. Perceived treatment assignment and smoking cessation in a clinical trial of bupropion. Cancer Epidemiology, Biomarkers & Prevention 2011;20(4):721. [] [Google Scholar]
- Cox LS, Faseru B, Mayo MS, Krebill R, Snow TS, Bronars CA, et al. Design, baseline characteristics, and retention of African American light smokers into a randomized trial involving biological data. Trials 2011; Vol. 12:22. [] [DOI] [PMC free article] [PubMed]
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. Journal of the National Cancer Institute 2012;104(4):290‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faseru B, Choi WS, Krebill R, Mayo MS, Nollen NL, Okuyemi KS, et al. Factors associated with smoking menthol cigarettes among treatment‐seeking African American light smokers. Addictive Behaviors. England, 2011; Vol. 36, issue 12:1321‐4. [] [DOI] [PMC free article] [PubMed]
- Faseru B, Nollen NL, Mayo MS, Krebill R, Choi WS, Benowitz NL, et al. Predictors of cessation in African American light smokers enrolled in a bupropion clinical trial. Addictive Behaviors 2013;38(3):1796‐803. [CRS: 9400107000000930] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, et al. CYP2B6 and bupropion's smoking‐cessation pharmacology: the role of hydroxybupropion. Clinical Pharmacology and Therapeutics 2012;92(6):771‐7. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Croghan 2007 {published data only}
- Clark MM, Hurt RD, Croghan IT, Patten CA, Novotny P, Sloan JA, et al. The prevalence of weight concerns in a smoking abstinence clinical trial. Addictive Behaviors 2006;31:1144‐52. [DOI] [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Croghan GA, Sloan JA. Comparing nicotine inhaler, bupropion and nicotine inhaler plus bupropion in treating tobacco dependence [abstract]. Nicotine & Tobacco Research 2005;7:680‐1. [Google Scholar]
- Croghan IT, Hurt RD, Dakhil SR, Croghan GA, Sloan JA, Novotny PJ, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clinic Proceedings 2007;82:186‐95. [DOI] [PubMed] [Google Scholar]
- Croghan IT, Hurt RD, Ebbert JO, Croghan GA, Polk Jr OD, Stella PJ, et al. Racial differences in smoking abstinence rates in a multicenter, randomized, open‐label trial in the United States. Journal of Public Health 2010; Vol. 18, issue 1:59‐68. [] [DOI] [PMC free article] [PubMed]
Da Costa 2002 {published data only}
- Costa C, Younes R, Lourenco M. Smoking cessation: A randomized double‐blind study comparing nortriptyline to placebo [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A354. [Google Scholar]
- Costa CL, Younes RN, Lourenco MT‐C. Stopping smoking: a prospective, randomized, double‐blind study comparing nortriptyline to placebo. Chest 2002;122:403‐8. [DOI] [PubMed] [Google Scholar]
Dalsgarð 2004 {published data only}
- Dalsgarð OJ, Hansen NC, Søes‐Petersen U, Evald T, Høegholm A, Barber J, et al. A multicenter, randomized, double‐blind, placebo‐controlled, 6‐month trial of bupropion hydrochloride sustained‐release tablets as an aid to smoking cessation in hospital employees. Nicotine & Tobacco Research 2004;6:55‐61. [DOI] [PubMed] [Google Scholar]
- Dalsgarð OJ, Vestbo J. A multicenter, randomised, double‐blind, placebo‐controlled 6 month trial to evaluate efficacy and tolerability of bupropion hydrochloride sustained release (SR) tablets as treatment for nicotine dependency in healthcare workers and as an aid to smoking cessation (ZYB30009). Poster and oral presentation. European Congress on Tobacco or Health, Warsaw, Poland, 20‐22 June 2002. 2002.
Eisenberg 2013 {published data only}
- Benowitz NL, Prochaska JJ. Smoking cessation after acute myocardial infarction. Journal of the American College of Cardiology 2013;61(5):533‐5. [] [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Grandi SM, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: A randomized, placebo‐controlled trial. Canadian Journal of Cardiology 2011;27(5 SUPPL 1):S344. [] [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Grandi SM, Gervais A, O'Loughlin J, Paradis G, Rinfret S, et al. Bupropion for smoking cessation in patients hospitalized with acute myocardial infarction: A randomized, placebo‐controlled trial. Journal of the American College of Cardiology 2013;61(5):524‐32. [] [DOI] [PubMed] [Google Scholar]
- Grandi SM, Filion KB, Gervais A, Joseph L, O'Loughlin J, Paradis G, et al. The effect of smoking cessation on weight at 12 months in patients post‐myocardial infarction [abstract]. Circulation. 2012; Vol. 10 Suppl:P090. []
Evins 2001 {published data only}
- Evins AE, Cather C, Rigotti NA, Freudenreich O, Henderson DC, Olm Shipman CM, et al. Two‐year follow‐up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. Journal of Clinical Psychiatry 2004;65:307‐11. [DOI] [PubMed] [Google Scholar]
- Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine & Tobacco Research 2001;3:397‐403. [DOI] [PubMed] [Google Scholar]
Evins 2005 {published data only}
- Evins AE, Cather C, Culhane M, Freudenreich O, Rigotti NA, Goff DC. Smoking cessation in schizophrenia: A double blind placebo controlled trial of bupropion SR added to cognitive behavioral therapy. Biological Psychiatry 2004;55:806. [Google Scholar]
- Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm‐Shipman CM, et al. A double‐blind placebo‐controlled trial of bupropion sustained‐release for smoking cessation in schizophrenia. Journal of Clinical Psychopharmacology 2005;25:218‐25. [DOI] [PubMed] [Google Scholar]
- Evins AE, Deckersbach T, Cather C, Freudenreich O, Culhane MA, Henderson DC, et al. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. Journal of Clinical Psychiatry 2005;66:1184‐90. [DOI] [PubMed] [Google Scholar]
Evins 2007 {published data only}
- Evins AE, Cather C, Culhane M, Birnbaum AS, Horowitz J, Hsieh E, et al. A placebo‐controlled study of bupropion SR added to high dose nicotine replacement therapy for smoking cessation or reduction in schizophrenia (POS2‐104). Society for Research on Nicotine and Tobacco 12th Annual Meeting; February 15‐18, Orlando, Florida. 2006.
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12‐week double‐blind, placebo‐controlled study of bupropion SR added to high‐dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. Journal of Clinical Psychopharmacology 2007;27:380‐6. [DOI] [PubMed] [Google Scholar]
Ferry 1992 {published and unpublished data}
- Ferry LH, Robbins AS, Scariati PD, et al. Enhancement of smoking cessation using the antidepressant bupropion hydrochloride [abstract 2670]. Circulation 1992;86(4 Suppl 1):I‐671. [Google Scholar]
Ferry 1994 {published and unpublished data}
- Ferry LH, Burchette RJ. Efficacy of bupropion for smoking cessation in non depressed smokers [Abstract]. Journal of Addictive Diseases 1994;13(4):249. [Google Scholar]
Fossati 2007 {published data only}
- Ferketich AK, Fossati R, Apolone G. An evaluation of the Italian version of the Fagerstrom Test for Nicotine Dependence. Psychological Reports 2008;102:687‐94. [DOI] [PubMed] [Google Scholar]
- Fossati R, Apolone G, Negri E, Compagnoni A, Vecchia C, Mangano S, et al. A double‐blind, placebo‐controlled, randomized trial of bupropion for smoking cessation in primary care. Archives of Internal Medicine 2007;167:1791‐7. [DOI] [PubMed] [Google Scholar]
Gariti 2009 {published data only}
- Gariti P, Lynch K, Alterman A, Kampman K, Xie H, Varillo K. Comparing smoking treatment programs for lighter smokers with and without a history of heavier smoking. Journal of Substance Abuse Treatment 2009; Vol. 37, issue 3:247‐55. [] [DOI] [PMC free article] [PubMed]
George 2002 {published data only}
- George TP, Vessicchio JC, Termine A, Bregartner TA, Feingold A, Rounsaville BJ, et al. A placebo controlled trial of bupropion for smoking cessation in schizophrenia. Biological Psychiatry 2002;52:53‐61. [9400123000002832] [DOI] [PubMed] [Google Scholar]
George 2003 {published data only}
- George TP, Vessicchio JC, Termine A, Jatlow PI, Kosten TR, O'Malley SS. A preliminary placebo‐controlled trial of selegiline hydrochloride for smoking cessation. Biological Psychiatry 2003;53(2):136‐43. [DOI] [PubMed] [Google Scholar]
- Lara XD, Vessicchio JC, Termine A, Kosten TR, O'Malley SS, George TP. Selegiline versus placebo for smoking cessation in nicotine dependent refractory smokers (PO2 02). Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 Seattle Washington. 2001:53. []
George 2008 {published data only}
- George TP, Vessicchio JC, Sacco KA, Weinberger AH, Dudas MM, Allen TM, et al. A placebo‐controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biological Psychiatry 2008;63(11):1092‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Weinberger AH, Sacco KA. Sustained‐release bupropion combined with transdermal nicotine patch for smoking cessation in schizophrenia (SYM11C). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
- Moss TG, Sacco KA, Allen TM, Weinberger AH, Vessicchio JC, George TP. Prefrontal cognitive dysfunction is associated with tobacco dependence treatment failure in smokers with schizophrenia. Drug and Alcohol Dependence 2009; Vol. 104, issue 1‐2:94‐9. [] [DOI] [PMC free article] [PubMed]
Gonzales 2001 {published data only}
- Gonzales D, Nides M, Ferry LH, Segall N, Herrero L, Modell J, et al. Retreatment with bupropion SR: results from 12‐month follow‐up (RP‐83). Rapid Communication Poster Abstracts. Society for Research on Nicotine and Tobacco 8th Annual Meeting, February 20‐23 Savannah, Georgia. 2002.
- Gonzales DH, Nides MA, Ferry LH, Kustra RP, Jamerson BD, Segall N, et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: A randomized placebo‐controlled study. Clinical Pharmacology and Therapeutics 2001;69:438‐44. [DOI] [PubMed] [Google Scholar]
Gonzales 2006 {published data only}
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained‐release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296:47‐55. [DOI] [PubMed] [Google Scholar]
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010; Vol. 12, issue 6:574‐81. [] [DOI] [PubMed]
- Jackson KC, Nahoopii R, Said Q, Dirani R, Brixner D. An employer‐based cost‐benefit analysis of a novel pharmacotherapy agent for smoking cessation. Journal of Occupational & Environmental Medicine 2007;49(4):453‐60. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo‐controlled clinical trials. Neuropsychopharmacology 2012;37(3):641‐50. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prignot J. Care for adherence to treatment for tobacco dependence. Breathe 2011; Vol. 7, issue 3:291. []
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197(3):371‐7. [DOI] [PubMed] [Google Scholar]
Górecka 2003 {published data only}
- Górecka D, Bednarek M, Nowinski A, Puscinska E, Goljan‐Geremek A, Zielinski J. Effect of treatment for nicotine dependence in patients with COPD [Wyniki leczenia uzaleznienia od nikotyny chorych na przewlekla obturacyjna chorobe pluc]. Pneumonologia i Alergologia Polska 2003;71:411‐7. [PubMed] [Google Scholar]
Grant 2007 {published data only}
- Grant KM, Kelley SS, Smith LM, Agrawal S, Meyer JR, Romberger DJ. Bupropion and nicotine patch as smoking cessation aids in alcoholics. Alcohol 2007;41(5):381‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haggsträm 2006 {published data only}
- Haggsträm FM, Chatkin JM, Sussenbach‐Vaz E, Cesari DH, Fam CF, Fritscher CC. A controlled trial of nortriptyline, sustained‐release bupropion and placebo for smoking cessation: preliminary results. Pulmonary Pharmacology & Therapeutics 2006;19:205‐9. [DOI] [PubMed] [Google Scholar]
Hall 1998 {published data only}
- Haas AL, Munoz RF, Humfleet GL, Reus VI, Hall SM. Influences of mood, depression history, and treatment modality on outcomes in smoking cessation. Journal of Consulting & Clinical Psychology 2004;72:563‐70. [DOI] [PubMed] [Google Scholar]
- Hall SM, Gorecki JA, Reus VI, Humfleet GL, Munoz RF. Belief about drug assignment and abstinence in treatment of cigarette smoking using nortriptyline. Nicotine & Tobacco Research 2007;9(4):467‐71. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive‐behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry 1998;55:683‐90. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Reus VI, Gorecki J, Hall SM, Humfleet GL, Munoz RF, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clinical Pharmacology & Therapeutics 2008;83:436‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2002 {published data only}
- Hall SM, Gorecki JA, Reus VI, Humfleet GL, Munoz RF. Belief about drug assignment and abstinence in treatment of cigarette smoking using nortriptyline. Nicotine & Tobacco Research 2007;9(4):467‐71. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet G, Maude‐Griffin R, Reus VI, Munoz R, Hartz DT. Nortriptyline versus bupropion and medical management versus psychological intervention in smoking treatment (PA 5A). Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 Seattle, Washington. 2001:31.
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude‐Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Archives of General Psychiatry 2002;59:930‐36. [DOI] [PubMed] [Google Scholar]
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Munoz R. Cost‐effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32:381‐92. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Reus VI, Gorecki J, Hall SM, Humfleet GL, Munoz RF, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clinical Pharmacology & Therapeutics 2008;83:436‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2004 {published data only}
- Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry 2004;161:2100‐7. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Reus VI, Gorecki J, Hall SM, Humfleet GL, Munoz RF, et al. Therapeutic drug monitoring of nortriptyline in smoking cessation: a multistudy analysis. Clinical Pharmacology & Therapeutics 2008;83:436‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2011 {published data only}
- Hall SM, Humfleet GL, Munoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. American Journal of Public Health 2011; Vol. 101, issue 12:2349‐56. [] [DOI] [PMC free article] [PubMed]
- Prochaska JJ, Hall SM, Humfleet G, Munoz RF, Reus V, Gorecki J, et al. Physical activity as a strategy for maintaining tobacco abstinence: a randomized trial. Preventive Medicine 2008; Vol. 47, issue 2:215‐20. [] [DOI] [PMC free article] [PubMed]
Hatsukami 2004 {published data only}
- Hatsukami DK, Rennard S, Patel MK, Kotlyar M, Malcolm R, Nides MA, et al. Effects of sustained‐release bupropion among persons interested in reducing but not quitting smoking. American Journal of Medicine 2004;116:151‐7. [DOI] [PubMed] [Google Scholar]
- Rennard S, Hatsukami D, Malcolm R E, Patel MK, Jamerson BD, Dozier G. Zyban (bupropion HCL SR) vs placebo as an aid to smoking reduction among smokers unwilling and unable to quit smoking (PO4 77). Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 Seattle, Washington. 2001:117.
Hays 2001 {published data only}
- Abel GA, Hays JT, Decker PA, Croghan GA, Kuter DJ, Rigotti NA. Effects of biochemically confirmed smoking cessation on white blood cell count. Mayo Clin Proceedings 2005;80:1022‐28. [DOI] [PubMed] [Google Scholar]
- Cox LS, Patten CA, Niaura RS, Decker PA, Rigotti N, Sachs DPL, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. Journal of General Internal Medicine 2004;19:828‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, et al. The effect of bupropion sustained‐release on cigarette craving after smoking cessation. Clinical Therapeutics 2002;24:540‐51. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Johnston JA, White J, Gonzales D, Sachs DP, Rigotti N, Niaura R. Bupropion SR for relapse prevention: a "slips‐allowed" analysis. American Journal of Health Behavior 2004;28(5):456‐63. [PubMed] [Google Scholar]
- Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, et al. Effects of gender on relapse prevention in smokers treated with bupropion SR. American Journal of Preventive Medicine 2002;22:234‐39. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained‐release bupropion for pharmacologic relapse prevention after smoking cessation. A randomized, controlled trial. Annals of Internal Medicine 2001;135:423‐33. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Wolter TD, Rigotti N, Hays JT, Niaura R, Durcan MJ, et al. Bupropion for pharmacologic relapse prevention to smoking ‐ Predictors of outcome. Addictive Behaviors 2002;27(4):493‐507. [DOI] [PubMed] [Google Scholar]
- Rigotti N, Thorndike AN, Durcan MJ, White JD, Johnston AJ, Niaura R, et al. Attenuation of post‐cessation weight gain in smokers taking bupropion: The effect of gender. Abstract Book. Society for Research on Nicotine and Tobacco 6th Annual Meeting; Feb 18‐20 2000; Arlington VA. 2000.
Hays 2009 {published data only}
- Hays JT, Hurt RD, Decker PA, Croghan IT, Offord KP, Patten CA. A randomized, controlled trial of bupropion sustained‐release for preventing tobacco relapse in recovering alcoholics. Nicotine & Tobacco Research 2009; Vol. 11, issue 7:859‐67. [] [DOI] [PMC free article] [PubMed]
Hertzberg 2001 {published data only}
- Hertzberg MA, Moore SD, Feldman ME, Beckham JC. A preliminary study of bupropion sustained‐release for smoking cessation in patients with chronic posttraumatic stress disorder. Journal of Clinical Psychopharmacology 2001;21:94‐8. [DOI] [PubMed] [Google Scholar]
Holt 2005 {published data only}
- Holt S, Timu‐Parata C, Ryder‐Lewis S, Weatherall M, Beasley R. Efficacy of bupropion in the indigenous Maori population in New Zealand. Thorax 2005;60:120‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurt 1997 {published and unpublished data}
- Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, et al. Bupropion for smoking cessation: predictors of successful outcome. Chest 2001;119:1357‐1364. [DOI] [PubMed] [Google Scholar]
- Glaxo Wellcome. Presentation for FDA approval of Bupropion sustained release for smoking cessation. Dr J. Andrew Johnston December 10 1996.
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. British Journal of Psychiatry 1999;174:173‐8. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Glover ED, Sachs DPL, et al. Bupropion for smoking cessation: A double‐blind, placebo‐controlled dose response trial [Abstract]. Journal of Addictive Diseases 1996;15:137. [Google Scholar]
- Hurt RD, Sachs DPL, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained‐release bupropion and placebo for smoking cessation. New England Journal of Medicine 1997;337:1195‐202. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Fiedler‐Kelly J, Glover ED, Sachs DP, Grasela TH, DeVeaugh‐Geiss J. Relationship between drug exposure and the efficacy and safety of bupropion sustained release for smoking cessation. Nicotine & Tobacco Research 2001;3:131‐40. [DOI] [PubMed] [Google Scholar]
Hurt 2003 {published and unpublished data}
- Hurt RD, Croghan GA, Sloan JA, Krook JE, Silberstein PT. Bupropion for relapse prevention after nicotine patch therapy [PA 5B abstract ]. Society for Research on Nicotine and Tobacco 7th Annual Meeting, March 23‐23 2001, Seattle, Washington. 2001:32.
- Hurt RD, Krook JE, Croghan IT, Loprinzi CL, Sloan JA, Novotny PJ, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. Journal of Clinical Oncology 2003;21:914‐20. [DOI] [PubMed] [Google Scholar]
Jorenby 1999 {published data only}
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behavior 2002;26:213‐20. [DOI] [PubMed] [Google Scholar]
- Jamerson BD, Nides M, Jorenby DE, Donahue R, Garrett P, Johnston JA, et al. Late‐term smoking cessation despite initial failure: an evaluation of bupropion sustained release, nicotine patch, combination therapy, and placebo. Clinical Therapeutics 2001;23:744‐52. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained‐release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine 1999;340:685‐91. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Fiore MC. Cost‐benefit analysis of sustained‐release bupropion, nicotine patch, or both for smoking cessation. Preventive Medicine 2000;30:209‐216. [DOI] [PubMed] [Google Scholar]
- Smith SS, Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston AJ, et al. Targeting smokers at increased risk for relapse: treating women and those with a history of depression. Nicotine & Tobacco Research 2003;5:99‐109. [DOI] [PubMed] [Google Scholar]
Jorenby 2006 {published data only}
- Hays JT, Leischow SJ, Lawrence D, Lee TC. Adherence to treatment for tobacco dependence: association with smoking abstinence and predictors of adherence. Nicotine & Tobacco Research 2010; Vol. 12, issue 6:574‐81. [] [DOI] [PubMed]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained‐release bupropion for smoking cessation: a randomized controlled trial. JAMA 2006;296:56‐63. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo‐controlled clinical trials. Neuropsychopharmacology 2012;37(3):641‐50. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197(3):371‐7. [DOI] [PubMed] [Google Scholar]
Kahn 2012 {published data only}
- Kahn R, Gorgon L, Jones K, McSherry F, Glover ED, Anthenelli RM, et al. Selegiline transdermal system (STS) as an aid for smoking cessation. Nicotine & Tobacco Research. United Kingdom: Oxford University Press (Great Clarendon Street, Oxford OX2 6DP, United Kingdom), 2012; Vol. 14, issue 3:377‐82. [] [DOI] [PMC free article] [PubMed]
Kalman 2011 {published data only}
- Kalman D, Herz L, Monti P, Kahler CW, Mooney M, Rodrigues S, et al. Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: results from a randomized, double‐blind, placebo‐controlled study. Drug and Alcohol Dependence 2011;118(2‐3):111‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Kalman D. Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine & Tobacco Research 2010; Vol. 12, issue 4:416‐22. [] [DOI] [PMC free article] [PubMed]
- McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently‐abstinent alcoholics: Preliminary results using an aggregate genetic risk score. Substance Abuse : Research and Treatment 2012;6(1):107‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Killen 2000 {published data only}
- Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A. Onset of major depression during treatment for nicotine dependence. Addictive Behaviors 2003;28:461‐70. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, Hayward C, Sussman L, Rothman M, et al. Nicotine patch and paroxetine for smoking cessation. Journal of Consulting and Clinical Psychology 2000;68:883‐9. [PubMed] [Google Scholar]
Killen 2004 {published data only}
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Samuels D. Major depression among adolescent smokers undergoing treatment for nicotine dependence. Addictive Behaviors 2004;29:1517‐26. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology 2004;72:729‐35. [DOI] [PubMed] [Google Scholar]
Killen 2006 {published data only}
- Killen JD, Fortmann SP, Murphy GM Jr, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with Bupropion SR for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 2006;74(2):286‐94. [DOI] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5‐A3‐B4) predict severity of nicotine addiction and response to smoking cessation therapy. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics 2011; Vol. 156, issue 3:275‐84. [] [DOI] [PubMed]
Killen 2010 {published data only}
- Killen JD, Fortmann SP, Murphy GMJ, Hayward C, Fong D, Lowenthal K, et al. Failure to improve cigarette smoking abstinence with transdermal selegiline + cognitive behavior therapy. Addiction 2010; Vol. 105, issue 9:1660‐8. [] [DOI] [PMC free article] [PubMed]
Levine 2010 {published data only}
- Levine MD, Perkins KA, Kalarchian MA, Cheng Y, Houck PR, Slane JD, et al. Bupropion and cognitive behavioural therapy for weight‐concerned women smokers. Archives of Internal Medicine 2010; Vol. 170, issue 6:543‐50. [] [DOI] [PMC free article] [PubMed]
- Okun ML, Levine MD, Houck P, Perkins KA, Marcus MD. Subjective sleep disturbance during a smoking cessation program: associations with relapse. Addictive Behaviors 2011; Vol. 36, issue 8:861‐4. [] [DOI] [PMC free article] [PubMed]
McCarthy 2008 {published data only}
- McCarthy DE. Mechanisms of tobacco cessation treatment: Self‐report mediators of counseling and bupropion sustained release treatment. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9‐B):5414. [Google Scholar]
- McCarthy DE, Piasecki TM, Jorenby DE, Lawrence DL, Shiffman S, Baker TB. A multi‐level analysis of non‐significant counseling effects in a randomized smoking cessation trial. Addiction 2010; Vol. 105, issue 12:2195‐208. [] [DOI] [PMC free article] [PubMed]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Baker TB. Psychological mediators of bupropion sustained‐release treatment for smoking cessation. Addiction 2008;103(9):1521‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research 2008;10:717‐29. [DOI] [PubMed] [Google Scholar]
- McCarthy DE. Piasecki TM, Lawrence DL, Fiore MC, Baker TB. Efficacy of bupropion SR and individual counseling among adults attempting to quit smoking (POS1‐041). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
Muramoto 2007 {published data only}
- Best D. Bupropion assists with tobacco cessation in adolescents but relapse is high. Journal of Pediatrics 2008; Vol. 152, issue 5:738‐9. [] [DOI] [PubMed]
- Muramoto ML, Leischow SJ, Sherrill D, Matthews E, Strayer LJ. Randomized, double‐blind, placebo‐controlled trial of 2 dosages of sustained‐release bupropion for adolescent smoking cessation. Archives of Pediatrics & Adolescent Medicine 2007;161:1068‐74. [DOI] [PubMed] [Google Scholar]
- Taren D, Fankem S, Muramoto M. Weight loss in adolescents who quit smoking with bupropion [RP‐071]. Society for Research on Nicotine and Tobacco 11th annual meeting, March 2005; Prague, Czech Republic. Rapid Communications posters. 2005.
Myles 2004 {published data only}
- Myles PS, Leslie K, Angliss M, Mezzavia P, Lee L. Effectiveness of bupropion as an aid to stopping smoking before elective surgery: a randomised controlled trial. Anaesthesia 2004;59:1053‐8. [DOI] [PubMed] [Google Scholar]
Niaura 2002 {published data only}
- Borrelli B, Niaura R, Keuthen NJ, Goldstein MG, Depue JD, Murphy C, et al. Development of major depressive disorder during smoking‐cessation treatment. Journal of Clinical Psychiatry 1996;57:534‐8. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter‐defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction 2004;99:378‐85. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short‐term relapse to smoking in a cessation trial. Journal of Consulting and Clinical Psychology 2001;69:511‐15. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Kristeller J, Ockene JK, Keuthen NJ. Weight suppression and weight rebound in ex‐smokers treated with fluoxetine. Journal of Consulting and Clinical Psychiatry 1999;67:124‐31. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue DE, Borrelli B, Hitsman B, Niaura R, et al. Influence of fluoxetine on positive and negative affect in a clinic‐based smoking cessation trial. Psychopharmacology 2004;173:153‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran N, Spring B, Borrelli B, McChargue D, Hitsman B, Niaura R, Hedeker D. Elevated positive mood: a mixed blessing for abstinence. Psychology of Addictive Behaviors 2006;20:36‐43. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Borrelli B, Niaura R, Papandonatos GD. Influence of antidepressant pharmacotherapy on behavioral treatment adherence and smoking cessation outcome in a combined treatment involving fluoxetine. Experimental & Clinical Psychopharmacology 2001;9:355‐62. [DOI] [PubMed] [Google Scholar]
- Mizes JS, Sloan DM, Segraves K, Spring B, Pingatore R, Kristeller J. Fluoxetine and weight‐gain in smoking cessation ‐ examination of actual weight‐gain and fear of weight‐gain [abstract]. Psychopharmacology Bulletin 1996;32:491. [Google Scholar]
- Niaura R, Goldstein M, Spring B, Keuthen N, Kristeller J, DePue J, et al. Fluoxetine for smoking cessation: A multicenter randomized double blind dose response study. Society for Behavioral Medicine Annual Meeting; April 18 1997; San Francisco, CA.
- Niaura R, Spring B, Borrelli B, Hedeker D, Goldstein MG, Keuthen N, et al. Multicenter trial of fluoxetine as an adjunct to behavioral smoking cessation treatment. Journal of Consulting and Clinical Psychology 2002;70:887‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Niaura R, Borrelli B, Spring B. Subgroups of smokers with different success rates after treatment with fluoxetine for smoking cessation [abstract]. Nicotine & Tobacco Research 1999;1:281. [Google Scholar]
Nides 2006 {published data only}
- Nides M, Oncken C, Gonzales D, Rennard S, Watsky EJ, Anziano R, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: Results from a 7‐week, randomized, placebo‐ and bupropion‐controlled trial with 1‐year follow‐up. Archives of Internal Medicine 2006;166:1561‐8. [DOI] [PubMed] [Google Scholar]
- Oncken C, Watsky E, Reeves K, Anziano R. Varenicline is efficacious and well tolerated in promoting smoking cessation: results from a 7‐week, randomized, placebo‐ and bupropion‐controlled trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005, Prague, Czech Republic. 2005.
Parsons 2009 {published data only}
- Parsons A, Ingram J, Inglis J, Aveyard P, Johnstone E, Brown K, et al. A proof of concept randomised placebo controlled factorial trial to examine the efficacy of St John's wort for smoking cessation and chromium to prevent weight gain on smoking cessation. Drug and Alcohol Dependence 2009; Vol. 102, issue 1‐3:116‐22. [] [DOI] [PubMed]
Piper 2007 {published data only}
- Piper ME. Bupropion alone and in combination with nicotine gum: Efficacy, mediation and moderation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;67(9‐B):5418. [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine & Tobacco Research 2007;9:947‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Mediators of bupropion treatment effects (SYM 2C). Society for Research on Nicotine and Tobacco 14th Annual Meeting February 26‐March 1, Portland, Oregon 2008:31. [; 9400123000005187]
- Piper ME, Federman EB, Smith SS, Fiore MC, Baker TB. Efficacy of bupropion SR alone and combined with 4‐mg gum (PA2‐2). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004:18.
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology 2008;117:94‐105. [DOI] [PubMed] [Google Scholar]
Piper 2009 {published and unpublished data}
- Asthana A, Johnson HM, Piper ME, Fiore MC, Baker TB, Stein JH. Effects of smoking intensity and cessation on inflammatory markers in a large cohort of active smokers. American Heart Journal 2010; Vol. 160, issue 3:458‐63. [] [DOI] [PMC free article] [PubMed]
- Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. American Heart Journal 2011; Vol. 161, issue 1:145‐51. [] [DOI] [PMC free article] [PubMed]
- Japuntich SJ, Leventhal AM, Piper ME, Bolt DM, Roberts LJ, Fiore MC, et al. Smoker characteristics and smoking‐cessation milestones. American Journal of Preventive Medicine 2011; Vol. 40, issue 3:286‐94. [] [DOI] [PMC free article] [PubMed]
- Japuntich SJ, Piper ME, Leventhal AM, Bolt DM, Baker TB. The effect of five smoking cessation pharmacotherapies on smoking cessation milestones. Journal of Consulting and Clinical Psychology 2011; Vol. 79, issue 1:34‐42. [] [DOI] [PMC free article] [PubMed]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction 2011; Vol. 106, issue 2:418‐27. [] [DOI] [PMC free article] [PubMed]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research 2010;12(6):647‐57. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Kenford S, Fiore MC, Baker TB. Smoking cessation and quality of life: changes in life satisfaction over 3 years following a quit attempt. Annals of Behavioral Medicine 2012; Vol. 43, issue 2:262‐70. [] [DOI] [PMC free article] [PubMed]
- Piper ME, Schlam TR, Cook JW, Sheffer MA, Smith SS, Loh WY, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology 2011;216(4):569‐78. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo‐controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry 2009; Vol. 66, issue 11:1253‐62. [] [DOI] [PMC free article] [PubMed]
Planer 2011 {published data only}
Prochazka 1998 {published data only}
- Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Archives of Internal Medicine 1998;158:2035‐9. [DOI] [PubMed] [Google Scholar]
Prochazka 2004 {published data only}
- Prochazka AV, Kick S, Steinbrunn C, Miyoshi T, Fryer GE. A randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation. Archives of Internal Medicine 2004;164:2229‐33. [DOI] [PubMed] [Google Scholar]
- Prochazka AV, Reyes R, Steinbrunn C, Miyoshi T. Randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation (PO3 26). Society for Research on Nicotine and Tobacco 7th Annual Meeting, March 23‐23 2001, Seattle, Washington. 2001:73.
Richmond 2013 {published data only}
- Richmond R, Indig D, Butler T, Wilhelm K, Archer V, Wodak A. A randomized controlled trial of a smoking cessation intervention conducted among prisoners. Addiction 2013;108(5):966‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2006 {published data only}
- Rigotti N, Thorndike A, Regan S, Pasternak R, Chang Y, McKool K, et al. Safety and efficacy of bupropion for smokers hospitalized with acute cardiovascular disease [abstract]. Nicotine & Tobacco Research 2005;7:682. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Thorndike AN, Regan S, McKool K, Pasternak RC, Chang Y, et al. Bupropion for smokers hospitalized with acute cardiovascular disease. American Journal of Medicine 2006;119:1080‐7. [DOI] [PubMed] [Google Scholar]
- Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres‐Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Archives of Internal Medicine 2008;168(2):186‐91. [DOI] [PubMed] [Google Scholar]
Rose 2013 {published data only}
- Rose JE, Behm FM. Adapting smoking cessation treatment according to initial response to precessation nicotine patch. American Journal of Psychiatry 2013;170:860‐7. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovina 2009 {published data only}
Saules 2004 {published and unpublished data}
- Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double‐blind placebo‐controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive‐behavioral group therapy. American Journal on Addictions 2004;14:438‐46. [DOI] [PubMed] [Google Scholar]
- Schuh LM, Downey KK, Hopper JA, Tancer M, Schuster CR. Fluoxetine in smoking cessation treatment. College on Problems of Drug Dependence Annual Meeting, San Juan, Puerto Rico 2000.
Schmitz 2007 {published data only}
- Schmitz JM, Stotts AL, Mooney ME, Delaune KA, Moeller GF. Bupropion and cognitive‐behavioral therapy for smoking cessation in women.[erratum appears in Nicotine Tob Res. 2007 Jul;9(7):785]. Nicotine & Tobacco Research 2007;9(6):699‐709. [DOI] [PubMed] [Google Scholar]
Schnoll 2010 {published data only}
- Martinez E, Tatum KL, Weber DM, Kuzla N, Pendley A, Campbell K, et al. Issues related to implementing a smoking cessation clinical trial for cancer patients. Cancer Causes & Control 2009; Vol. 20, issue 1:97‐104. [] [DOI] [PMC free article] [PubMed]
- Schnoll R, Lazev A, Sobel M, Tatum K, Butler D, Lerman C. Preliminary results from a randomized trial of bupropion for smoking cessation among cancer patients. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March, Prague, Czech Republic. 2005.
- Schnoll RA, Martinez E, Langer C, Miyamoto C, Leone F. Predictors of smoking cessation among cancer patients enrolled in a smoking cessation program. Acta Oncologica 2011; Vol. 50, issue 5:678‐84. [] [DOI] [PubMed]
- Schnoll RA, Martinez E, Tatum KL, Weber DM, Kuzla N, Glass M, et al. A bupropion smoking cessation clinical trial for cancer patients. Cancer Causes & Control 2010; Vol. 21, issue 6:811‐20. [] [DOI] [PubMed]
Selby 2003 {published and unpublished data}
- GlaxoSmithKline Clinical Trials Register. Study No: ZYB40001. A randomized, double‐blind, placebo‐controlled, 12‐week smoking cessation trial of Zyban (150 mg bid) in adult smokers previously treated with Zyban. http‐‐ctr.glaxowellcome.co.uk‐Summary‐bupropion‐IV_ZYB40001.pdf (accessed 23rd August 2006).
- Selby P, Ainslie M, Stepner N, Roberts J. Sustained‐release bupropion (Zybanr) is effective in the re‐treatment of relapsed adult smokers. Am J Respir Crit Care Med 2003;167(7):A47. [Google Scholar]
- Selby P, Brands B, Stepner N. Retreatment with ZYban SR: 52 week follow‐up of a Canadian Multicentre trial (POS3‐63). Society for Research on Nicotine and Tobacco 9th Annual Meeting, February 19‐22, New Orleans. 2003.
- Selby P, Brosky G, Baker R, Lertzman M, Dakin P, Roberts J. Zyban is effective in the retreatment of relapsed adult smokers (PO4 68). Society for Research on Nicotine and Tobacco 7th Annual Meeting March 23‐23 Seattle Washington. 2001:114.
Siddiqi 2013 {published data only}
- Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, et al. Action to stop smoking in suspected tuberculosis (assist) in Pakistan: A cluster randomized, controlled trial. Annals of Internal Medicine 2013;158(9):667‐75. [] [DOI] [PubMed] [Google Scholar]
- Siddiqi K, Khan A, Ahmad M, Shafiq‐ur‐Rehman. An intervention to stop smoking among patients suspected of TB‐‐evaluation of an integrated approach. BMC Public Health 2010;10:160. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2004 {published data only}
- Caplan BJ. The "bupropion for smoking cessation" trial from a family practice perspective. Archives of Internal Medicine 2005;165:470. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion for smoking cessation: a randomized trial. Archives of Internal Medicine 2004;164:1797‐803. [DOI] [PubMed] [Google Scholar]
- Simon JA, Duncan C, Carmody TP, Hudes ES. Bupropion plus nicotine replacement no better than replacement alone. Journal of Family Practice 2004;53:953‐4. [Google Scholar]
Simon 2009 {published data only}
- Simon JA, Duncan C, Huggins J, Solkowitz S, Carmody TP. Sustained‐release bupropion for hospital‐based smoking cessation: a randomized trial. Nicotine & Tobacco Research 2009;11(6):663‐9. [DOI] [PubMed] [Google Scholar]
Smith 2009 {published data only}
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research 2010;12(6):647‐57. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, McCarthy DE, Japuntich SJ, Christiansen B, Piper ME, Jorenby DE, et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Archives of Internal Medicine 2009; Vol. 169, issue 22:2148‐55. [] [DOI] [PMC free article] [PubMed]
SMK20001 {unpublished data only}
- GlaxoSmithKline Clinical Trials Register. Study No: SMK 20001. A Multi‐Center, Double‐Blind, Double‐Dummy, Placebo‐Controlled, Randomized, Parallel Group, Dose Response Evaluation of a New Chemical Entity (NCE) and ZYBAN (bupropion hydrochloride) Sustained Release (300mg/day) versus Placebo As Aids to Smoking Cessation. http://www.gsk‐clinicalstudyregister.com/files/pdf/812.pdf (accessed 4th August 2009).
Sood 2010 {published data only}
- Sood A, Ebbert JO, Prasad K, Croghan IT, Bauer B, Schroeder DR. A randomized clinical trial of St. John's wort for smoking cessation. Journal of Alternative and Complementary Medicine 2010; Vol. 16, issue 7:761‐7. [] [DOI] [PMC free article] [PubMed]
Sood 2012 {published data only}
- Sood A, Prasad K, Croghan IT, Schroeder DR, Ehlers SL, Ebbert JO. S‐Adenosyl‐L‐methionine (SAMe) for smoking abstinence: A randomized clinical trial. Journal of Alternative and Complementary Medicine 2012;18(9):854‐9. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spring 2007 {published data only}
- Carrington A, Doran N, Spring B. Fluoxetine moderates the association between trait‐anxiety and smoking status following behavioral treatment for smoking cessation (POS4‐81). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 New Orleans, Louisiana. 2003.
- Spring B, Doran N, Pagoto S, McChargue D, Cook JW, Bailey K, et al. Fluoxetine, smoking, and history of major depression: A randomized controlled trial. Journal of Consulting & Clinical Psychology 2007;75:85‐94. [DOI] [PubMed] [Google Scholar]
- Spring B, Doran N, Pagoto S, McChargue DE, Cook JW, Bailey K, et al. Reduced abstinence for smokers previously treated with fluoxetine (PA1‐1). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
Stapleton 2013 {published data only}
- Stapleton J, West R, Hajek P, Wheeler J, Vangeli E, Abdi Z, et al. Randomized trial of NRT, bupropion, and NRT plus bupropion for smoking cessation: effectiveness in clinical practice. Addiction 2013;108(12):2193‐201. [DOI: 10.1111/add.12304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swan 2003 {published data only}
- Catz S, Jack LM, Swan GE, McClure J. Adherence to bupropion SR in a smoking cessation effectiveness trial (POS2‐77). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Jack LM, Swan GE, Thompson E, Curry SJ, McAfee T, Dacey S, Bergman K. Bupropion SR and smoking cessation in actual practice: methods for recruitment, screening, and exclusion for a field trial in a managed‐care setting. Preventive Medicine 2003;36:585‐93. [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost‐effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10:217‐26. [PubMed] [Google Scholar]
- McAfee T, Zbikowski SM, Bush T, McClure J, Swan G, Jack LM, Curry S. The effectiveness of bupropion SR and phone counseling for light and heavy smokers (PA2‐1). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
- Swan GE, Jack LM, Curry S, Chorost M, Javitz H, McAfee T, Dacey S. Bupropion SR and counseling for smoking cessation in actual practice: Predictors of outcome. Nicotine & Tobacco Research 2003;5:911‐21. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Javitz HS, McAfee T, McClure JB. Predictors of 12‐month outcome in smokers who received bupropion sustained‐release for smoking cessation. Central Nervous System Drugs 2008;22(3):239‐56. [DOI] [PubMed] [Google Scholar]
- Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychology 2007;26:361‐8. [DOI] [PubMed] [Google Scholar]
- Swan GE, Javitz HS, Jack LM, Curry SJ, McAfee T. Heterogeneity in 12‐month outcome among female and male smokers. Addiction 2004;99:237‐50. [DOI] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, Bergman K. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Archives of Internal Medicine 2003;163:2337‐44. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5:21‐9. [DOI] [PubMed] [Google Scholar]
Tashkin 2001 {published data only}
- Patel MK, Tashkin DP, Kanner RE, Bailey WC, Buist A, Anderson PJ, et al. A multicenter evaluation of the effects of bupropion hydrochloride sustained release tablets (Bup SR) versus placebo in a population of smokers with chronic obstructive pulmonary disease (PO130). 11th World Conference on Tobacco or Health; Aug 6‐11 2000; Chicago, ILL. 2000; Vol. 1:118.
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double‐blind, placebo‐controlled, randomised trial. Lancet 2001;357:1571‐75. [DOI] [PubMed] [Google Scholar]
Tonnesen 2003 {published data only}
- Bolliger CT, Gilljam H, Lebargy F, Spiegel PI, Edwards J, Hider A, et al. Bupropion hydrochloride (Zyban) is effective and well tolerated as an aid to smoking cessation ‐ a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22‐26 2001. European Respiratory Journal 2001;18 (Suppl 33):12s. [Google Scholar]
- Tonnesen P, Tonstad S, Hjalmarson A, Lebargy F, Spiegel PI, Hider A, et al. A multicentre, randomized, double‐blind, placebo‐controlled, 1‐year study of bupropion SR for smoking cessation. Journal of Internal Medicine 2003;254:184‐92. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Aaserud E, Hjalmarson A, Peiffer G, Molen T, Hider A, et al. Zyban is an effective and well tolerated aid to smoking cessation in a general smoking population ‐ a multi‐country study. Society for Research on Nicotine and Tobacco 3rd Europe Conference, September 19‐22 2001, Paris, France 2001:46.
Tonstad 2003 {published data only}
- McRobbie H, Brath H, Astbury C, Hider A, Sweet R. Bupropion hydrochloride sustained release (SR) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease 12 month follow‐up phase data (ZYB40014). Abstract and presentation at European Respiratory Society meeting, 14‐18 September 2002, Stockholm, Sweden. 2002.
- Tonstad S, Farsang C, Klaene G, Lewis K, Manolis A, Perruchoud AP, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. European Heart Journal 2003;24:946‐55. [DOI] [PubMed] [Google Scholar]
- Spiegel PI, Lewis K, Seinost G, Astbury C, Hider A, Sweet R. Bupropion hydrochloride (Zyban) is an effective and well tolerated aid to smoking cessation in smokers with cardiovascular disease ‐ a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22‐26. European Respiratory Journal 2001;18(Suppl 33):13s. [Google Scholar]
Uyar 2007 {published data only}
- Uyar M, Bayram N, Filiz A, Elbek O, Topçu A, Dikensoy O, et al. Comparison of nicotine patch and bupropion in treating tobacco dependence. European Respiratory Journal 2005;26(Suppl 49):388s. [Google Scholar]
- Uyar M, Filiz A, Bayram N, Elbek O, Herken H, Topcu A, et al. A randomized trial of smoking cessation. Medication versus motivation. Saudi Medical Journal 2007;28(6):922‐6. [PubMed] [Google Scholar]
Wagena 2005 {published data only}
- Quaak M, Schayck Constant P, Postma Dirkje S, Wagena Edwin J, Schooten Frederik J. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction 2012; Vol. 107, issue 1:178‐87. [] [DOI] [PubMed]
- Wagena EJ, Knipschild PG, Huibers MJH, Wouters EFM, Schayck CPR. Efficacy of bupropion and nortriptyline for smoking cessation among people who are at risk for or have chronic obstructive pulmonary disease: Results from a randomized, placebo‐controlled trial. Nicotine & Tobacco Research 2005;7(4):683‐4. [] [Google Scholar]
- Schayck CP, Kaper J, Wagena EJ, Wouters EF, Severens JL. The cost‐effectiveness of antidepressants for smoking cessation in chronic obstructive pulmonary disease (COPD) patients. Addiction 2009; Vol. 104, issue 12:2110‐7. [] [DOI] [PubMed]
Weinberger 2010 {published and unpublished data}
- Weinberger AH, George TP, Mckee SA. Differences in smoking expectancies in smokers with and without a history of major depression. Addictive Behaviors 2011; Vol. 36, issue 4:434‐7. [] [DOI] [PMC free article] [PubMed]
- Weinberger AH, Mckee SA, George TP. Changes in smoking expectancies in abstinent, reducing, and non‐abstinent participants during a pharmacological trial for smoking cessation. Nicotine & Tobacco Research 2010; Vol. 12, issue 9:937‐43. [] [DOI] [PMC free article] [PubMed]
- Weinberger AH, Reutenauer EL, Jatlow PI, O'Malley SS, Potenza MN, George TP. A double‐blind, placebo‐controlled, randomized clinical trial of oral selegiline hydrochloride for smoking cessation in nicotine‐dependent cigarette smokers. Drug and Alcohol Dependence 2010; Vol. 107, issue 2‐3:188‐95. [] [DOI] [PMC free article] [PubMed]
- Weinberger AH, Reutenauer EL, O'Malley SS, Potenza MN, George TP. A randomized placebo‐controlled clinical trial of selegiline for smoking cessation: Preliminary results (POS5‐29). Society for Research on Nicotine and Tobacco 15th Annual Meeting April 27‐30, 2009, Dublin, Ireland.
- Weinberger AH, Reutenauer EL, Solorzano M, O'Malley SS, Potenza MN, George TP. A randomized placebo controlled clinical trial of selegiline for smoking cessation (abstract 653). CPDD 71st Annual Meeting, June 20‐25 2009, Reno Nevada.
Wittchen 2011 {published data only}
- Wittchen HU, Hoch E, Klotsche J, Muehlig S. Smoking cessation in primary care ‐ a randomized controlled trial of bupropion, nicotine replacements, CBT and a minimal intervention. International Journal of Methods in Psychiatric Research 2011;20(1):28‐39. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zellweger 2005 {published data only}
- Puska P, Brath H, Astbury C, Hider AE. Zyban is an effective and well tolerated aid to smoking cessation in a healthcare professionals population ‐ a multi‐country study. Society for Research on Nicotine and Tobacco 3rd European Conference, September 2001, Paris, France. 2001:45.
- Zellweger JP, Blaziene A, Astbury C, Hider A, Hogue S. Bupropion hydrochloride sustained release is an effective and well tolerated aid to smoking cessation in a healthcare professionals population ‐ a multicountry study. Abstract and presentation at 11th Annual meeting of European Respiratory Society, Berlin, September 22‐26 2001. European Respiratory Journal 2001;18 (Suppl 33):166s. [Google Scholar]
- Zellweger JP, Boelcskei PL, Carrozzi L, Sepper R, Sweet R, Hider AZ. Bupropion SR vs placebo for smoking cessation in health care professionals. American Journal of Health Behavior 2005;29:240‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akbarpour 2010 {published data only}
- Akbarpour F, Rezaei O, Khodaie‐Ardakani MR, Sheikhvatan M, Goodarzi H, Dolatshahi B. A double‐blind placebo‐controlled trial of bupropion for smoking abstinence and cognition improvement in schizophrenia. Minerva Psichiatrica 2010; Vol. 51, issue 4:263‐9. []
Banham 2010 {published data only}
Barnes 2006 {published data only}
- Barnes J, Barber N, Wheatley D, Williamson EM. A pilot randomised, open, uncontrolled, clinical study of two dosages of St John's wort (Hypericum perforatum) herb extract (LI‐160) as an aid to motivational/behavioural support in smoking cessation. Planta Medica 2006;72(4):378‐82. [DOI] [PubMed] [Google Scholar]
Becker 2003 {unpublished data only}
- Becker B, Bock B, Carmona‐Barros R. St. John's Wort oral spray reduces withdrawal symptoms during quitting smoking (POS4‐82). Society for Research on Nicotine and Tobacco 9th Annual Meeting February 19‐22 New Orleans, Louisiana. 2003.
Berlin 2002 {published data only}
- Berlin I, Aubin HJ, Pedarriosse AM, Rames A, Lancrenon S, Lagrue G. Lazabemide, a selective, reversible monoamine oxidase B inhibitor, as an aid to smoking cessation. Addiction 2002;97:1347‐54. [DOI] [PubMed] [Google Scholar]
Berlin 2005 {published data only}
- Berlin I, Covey LS, Jiang HP, Hamer D. Lack of effect of D2 dopamine receptor TaqI A polymorphism on smoking cessation. Nicotine & Tobacco Research 2005;7:725‐8. [DOI] [PubMed] [Google Scholar]
Berlin 2012 {published data only}
- Berlin I, Hunneyball IM, Greiling D, Jones SP, Fuder H, Stahl HD. A selective reversible monoamine oxidase B inhibitor in smoking cessation: effects on its own and in association with transdermal nicotine patch. Psychopharmacology 2012;223(1):89‐98. [] [DOI] [PubMed] [Google Scholar]
Bloch 2010 {published data only}
Bowen 1991 {published data only}
- Bowen DJ, Spring B, Fox E. Tryptophan and high‐carbohydrate diets as adjuncts to smoking cessation therapy. Journal of Behavioral Medicine 1991;14(2):97‐110. [DOI] [PubMed] [Google Scholar]
Brauer 2000 {unpublished data only}
- Brauer LH, Paxton DA, Stock CT, Rose JE. Selegiline and transdermal nicotine for smoking cessation. Society for Research on Nicotine and Tobacco Annual Conference; 2000 Feb 18‐20; Arlington VA (http://www.srnt.org/events/abstracts99/index.htm) 2000.
Breitling 2008 {published data only}
- Breitling LP, Twardella D, Brenner H. High effectiveness of short treatment with bupropion for smoking cessation in general care. Thorax 2008;63:476‐7. [DOI] [PubMed] [Google Scholar]
Carrão 2007 {published data only}
- Carrao JL, Moreira LB, Fuchs FD. The efficacy of the combination of sertraline with buspirone for smoking cessation. A randomized clinical trial in nondepressed smokers. European Archives of Psychiatry & Clinical Neuroscience 2007;257:383‐8. [DOI] [PubMed] [Google Scholar]
Chan 2005 {published data only}
- Chan B, Einarson A, Koren G. Effectiveness of bupropion for smoking cessation during pregnancy. Journal of Addictive Diseases 2005;24(2):19‐23. [DOI] [PubMed] [Google Scholar]
Cornelius 1997 {published data only}
- Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, Black A, et al. Double‐blind fluoxetine in depressed alcoholic smokers. Psychopharmacology Bulletin 1997;33:165‐70. [PubMed] [Google Scholar]
Cornelius 1999 {published data only}
- Cornelius JR, Perkins KA, Salloum IM, Thase ME, Moss HB. Fluoxetine versus placebo to decrease the smoking of depressed alcoholic patients [letter]. Journal of Clinical Psychopharmacology 1999;19:183‐4. [DOI] [PubMed] [Google Scholar]
Dalack 1995 {published data only}
- Dalack GW, Glassman AH, Rivelli S, Covey LS, Stetner F. Mood, major depression, and fluoxetine response in cigarette smokers. American Journal of Psychiatry 1995;152:398‐403. [DOI] [PubMed] [Google Scholar]
Dale 2002 {published data only}
- Dale LC, Ebbert JO, Schroeder DR, Croghan IT, Rasmussen DF, Trautman JA, et al. Bupropion for the treatment of nicotine dependence in spit tobacco users: a pilot study. Nicotine Tobacco Research 2002;4(3):267‐74. [DOI] [PubMed] [Google Scholar]
Dale 2007 {published data only}
- Dale LC, Ebbert JO, Glover ED, Croghan IT, Schroeder DR, Severson HH, et al. Bupropion SR for the treatment of smokeless tobacco use. Drug & Alcohol Dependence 2007;90(1):56‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Ebbert JO, Patten CA, Dale LC, Bronars CA, Schroeder DR. Measuring nicotine dependence among smokeless tobacco users. Addictive Behaviors 2006;31(9):1511‐21. [DOI] [PubMed] [Google Scholar]
Daniela 2008 {published data only}
- Daniela I, Carmen C. The combination of sertraline with buspirone for smoking cessation process ‐ The effectiveness and adverse events. Toxicology Letters 2008;180(Suppl 1):S130. [Google Scholar]
Edwards 1989 {published data only}
- Edwards NB, Murphy JK, Downs AD, Ackerman BJ, Rosenthal TL. Doxepin as an adjunct to smoking cessation: a double‐blind pilot study. American Journal of Psychiatry 1989;146(3):373‐6. [DOI] [PubMed] [Google Scholar]
- Murphy JK, Edwards NB, Downs AD, Ackerman BJ, Rosenthal TL. Effects of doxepin on withdrawal symptoms in smoking cessation. American Journal of Psychiatry 1990;147(10):1353‐7. [DOI] [PubMed] [Google Scholar]
Elsasser 2002 {published data only}
- Elsasser GN, Guck TP, Destache CJ, Daher PM, Frey DR, Jones J, et al. Sustained release bupropion in the treatment of nicotine addiction among teenage smokers (RP‐32). Rapid Communication Poster Abstracts. Society for Research on Nicotine and Tobacco 8th Annual Meeting, February 20‐23 Savannah, Georgia. 2002.
Evins 2008 {published data only}
- Evins AE, Alpert JE, Pava J, Petersen TJ, Farabaugh AH, Fava M. A double blind placebo controlled trial of bupropion added to nicotine patch and cognitive behavioral therapy in smokers with current or past unipolar depressive disorder. Neuropsychopharmacology 2005;30 Suppl 1:S91. [Google Scholar]
- Evins AE, Culhane MA, Alpert JE, Pava J, Liese BS, Farabaugh, A, et al. A controlled trial of bupropion added to nicotine patch and behavioral therapy for smoking cessation in adults with unipolar depressive disorders. Journal of Clinical Psychopharmacology 2008;28:660‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fatemi 2005 {published data only}
- Fatemi SH, Stary JM, Hatsukami DK, Murphy SE. A double‐blind placebo‐controlled cross over trial of bupropion in smoking reduction in schizophrenia. Schizophrenia Research 2005;76(2‐3):353‐6. [DOI] [PubMed] [Google Scholar]
Frederick 1997 {published data only}
- Frederick SL, Hall SM, Sees KL. The effect of venlafaxine on smoking cessation in subjects with and without a history of depression. NIDA Research Monograph 1997;174:208. [Google Scholar]
Gawin 1989 {published data only}
- Gawin F, Compton M, Byck R. Buspirone reduces smoking [letter]. Archives of General Psychiatry 1989;46(3):288‐9. [DOI] [PubMed] [Google Scholar]
Gifford 2011 {published data only}
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, et al. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy 2011; Vol. 42, issue 4:700‐15. [] [DOI] [PubMed]
Glover 2002 {published data only}
- Glover ED, Glover PN, Sullivan CR, Cerullo CL, Hobbs G. A comparison of sustained‐release bupropion and placebo for smokeless tobacco cessation. American Journal of Health Behavior 2002;26(5):386‐93. [DOI] [PubMed] [Google Scholar]
Gold 2002 {published data only}
- Gold PB, Rubey RN, Harvey RT. Naturalistic, self‐assignment comparative trial of bupropion SR, a nicotine patch, or both for smoking cessation treatment in primary care. American Journal of Addiction 2002;11(4):315‐31. [DOI] [PubMed] [Google Scholar]
Grandi 2011 {published data only}
- Grandi S, Shimony A, Eisenberg MJ. The efficacy and safety of bupropion started in‐hospital for smoking cessation in patients with cardiovascular disease: A systematic review and meta‐analysis. Circulation 2011; Vol. 124, issue 21 SUPPL 1. [; 9400123000012459]
Grassi 2009 {published data only}
Gray 2011 {published data only}
- Carpenter MJ, Baker NL, Gray KM, Upadhyaya HP. Assessment of nicotine dependence among adolescent and young adult smokers: A comparison of measures. Addictive Behaviors 2010; Vol. 35, issue 11:977‐82. [] [DOI] [PMC free article] [PubMed]
- Gray KM, Carpenter MJ, Baker NL, Hartwell KJ, Lewis AL, Hiott DW, et al. Bupropion SR and contingency management for adolescent smoking cessation. Journal of Substance Abuse Treatment 2011; Vol. 40, issue 1:77‐86. [] [DOI] [PMC free article] [PubMed]
- Gray KM, Carpenter MJ, Baker NL, Klintworth EM, Leinbach AS, Upadhyaya HP, et al. Bupropion SR and contingency management in adolescent smokers: main findings. College on Problems of Drug Dependence 71st Annual Meeting. Reno/Sparks, Nevada, 2009:74.
Hall 2009 {published data only}
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction 2009; Vol. 104, issue 6:1043‐52. [] [DOI] [PMC free article] [PubMed]
Hawk 2008 {unpublished data only}
- Hawk LW, Mahoney MC, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, et al. Preliminary evidence of extinction of smoking behavior with bupropion (PA9‐4). Society for Research on Nicotine and Tobacco 14th Annual Meeting February 26‐March 1, Portland, Oregon. 2008.
Hilberink 2005 {published data only}
Hitsman 1999 {published data only}
- Hitsman B, Pingitore R, Spring B, Mahableshwarkar A, Mizes JS, Segraves KA, et al. Antidepressant pharmacotherapy helps some cigarette smokers more than others. Journal of Consulting and Clinical Psychiatry 1999;67:547‐54. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Spring B, Borrelli B, Niaura R, Papandonatos G. Adherence to medication versus behavioral therapy as predictors of smoking cessation in combined treatment involving fluoxetine [abstract]. Society for Research on Nicotine and Tobacco Annual Conference; 2000 Feb 18‐20; Arlington VA 2000.
Houtsmuller 2002 {published data only}
- Houtsmuller EJ, Stitzer ML. Selegiline effects on smoking and abstinence [abstract]. CPDD Annual Meeting; 1998; Scottsdale, AZ 1998.
- Houtsmuller EJ, Thornton JA, Stitzer ML. Effects of selegiline (l‐deprenyl) during smoking and short‐term abstinence. Psychopharmacology (Berl) 2002;163:213‐20. [DOI] [PubMed] [Google Scholar]
Hussain 2010 {published data only}
Jacobs 1971 {published data only}
- Jacobs MA, Spilken AZ, Norman MM, Wohlberg GW, Knapp PH. Interaction of personality and treatment conditions associated with success in a smoking control program. Psychosomatic Medicine 1971;33(6):545‐56. [DOI] [PubMed] [Google Scholar]
Kalman 2004 {unpublished data only}
- Kalman D, Engler P, Monti P. Preliminary findings from a pilot treatment study of smokers in early alcohol recovery (POS1‐072). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
Karam‐Hage 2011 {published data only}
- Karam‐Hage M, Strobbe S, Robinson JD, Brower KJ. Bupropion‐SR for smoking cessation in early recovery from alcohol dependence: a placebo‐controlled, double‐blind pilot study. American Journal of Drug and Alcohol Abuse 2011;37(6):487‐90. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotz 2009 {published data only}
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confrontational counselling for smoking cessation in smokers with previously undiagnosed mild to moderate airflow limitation: study protocol of a randomized controlled trial. BMC Public Health 2007;7:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJH, Schayck OCP. Efficacy of confronting smokers with airflow limitation for smoking cessation. European Respiratory Journal 2009;33:754‐62. [DOI] [PubMed] [Google Scholar]
Kras 2010 {published data only}
- Kras M, Stough C, Scholey A, Kure C, Camfield D. Hypericum perforatum, nicotine patches and combination hypericum perforatum/nicotine patches for smoking cessation. European Neuropsychopharmacology 2010; Vol. 20:S608‐9. []
Lawvere 2006 {published data only}
- Lawvere S, Mahoney MC, Cummings KM, Hyland AJ. St John's Wort for smoking cessation: twelve months post cessation. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Lawvere S, Mahoney MC, Cummings KM, Kepner JL, Hyland A, Lawrence DD, et al. A Phase II study of St. John's Wort for smoking cessation. Complementary Therapies in Medicine 2006;14(3):175‐84. [DOI] [PubMed] [Google Scholar]
Le Foll 2009 {published data only (unpublished sought but not used)}
- Le Foll, B. Testing a full substitution therapy approach as treatment of tobacco dependence (NCT00390923). Clinicaltrials.gov 2009:http://clinicaltrials.gov/show/ NCT00390923, accessed on 25 June 2013. [Google Scholar]
Li 2009 {published data only}
- Li J, Zhang T, Wang B, Li X. An efficacy analysis of bupropion for smoking cessation in schizophrenia. Zhongguo Xinyao Yu Linchuang Zazhi (Chinese Journal of New Drugs and Clinical Remedies) 2009; Vol. 28, issue 3:231‐4. []
Miller 2003 {published data only}
- Miller H, Ranger‐Moore J, Hingten M. Bupropion SR for smoking cessation in pregnancy: a pilot study [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6):S133. [Google Scholar]
Monuteaux 2007 {published data only}
- Monuteaux MC, Spencer TJ, Faraone SV, Wilson AM, Biederman J. A randomized, placebo‐controlled clinical trial of bupropion for the prevention of smoking in children and adolescents with attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2007;68(7):1094‐101. [DOI] [PubMed] [Google Scholar]
Mooney 2008 {published data only}
- Mooney ME, Poling J, Gonzalez G, Gonsai K, Kosten T, Sofuoglu M. Preliminary study of buprenorphine and bupropion for opioid‐dependent smokers. American Journal on Addictions 2008;17(4):287‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M, Gonzalez G, Gonsai K, Poling J, Kosten T. Buprenorphine and bupropion combination for opioid‐dependent smokers. 68th Annual Scientific Meeting of the College on Problems of Drug Dependence. 2006.
Naranjo 1990 {published data only}
- Naranjo CA, Kadlec KE, Sanhueza P, Woodley Remus D, Sellers EM. Fluoxetine differentially alters alcohol intake and other consummatory behaviors in problem drinkers. Clinical Pharmacology and Therapeutics 1990;47:490‐8. [DOI] [PubMed] [Google Scholar]
Neumann 2000 {published and unpublished data}
- Neumann JK, Peeples B, East J, Ellis AR. Nicotine reduction: effectiveness of bupropion. British Journal of Psychiatry 2000;177:87‐88. [DOI] [PubMed] [Google Scholar]
Neumann 2002 {published data only}
- Neumann JK, Peeples B, Seneker A. Nicotine reduction and bupropion. Chest 2002;121:1378. [DOI] [PubMed] [Google Scholar]
Niederhofer 2004 {published data only}
- Niederhofer H, Huber M. Bupropion may support psychosocial treatment of nicotine‐ dependent adolescents: Preliminary results. Pharmacotherapy 2004;24(11):1524‐8. [DOI] [PubMed] [Google Scholar]
Olmstead 1999 {published data only}
- Olmstead R, Kelly J, Chin C, Iwamoto‐Schaap PN, Madsen DC, Huerta L, et al. Combined bupropion and mecamylamine treatment for smoking cessation: a pilot trial. Society for Research on Nicotine and Tobacco Fifth Annual Meeting March 5‐7 San Diego CA. 1999.
Paluck 2006 {published data only}
- Paluck EC, McCormack JP, Ensom MH, Levine M, Soon JA, Fielding DW. Outcomes of bupropion therapy for smoking cessation during routine clinical use. Annals of Pharmacotherapy 2996;40(2):185‐90. [DOI] [PubMed] [Google Scholar]
Pomerleau 1991 {published data only}
- Pomerleau OF, Pomerleau CS, Morrell EM, Lowenbergh JM. Effects of fluoxetine on weight gain and food intake in smokers who reduce nicotine intake. Psychoneuroendocrinology 1991;16:433‐40. [DOI] [PubMed] [Google Scholar]
Raynor 2005 {published data only}
- Raynor DA. Adherence to pharmacological smoking cessation treatment among weight‐concerned women. Dissertation Abstracts International: Section B: The Sciences and Engineering 2005;65(8‐B):4301. [Google Scholar]
Robinson 1991 {published data only}
- Robinson MD, Smith WA, Cederstrom EA, Sutherland DE. Buspirone effect on tobacco withdrawal symptoms: a pilot study. Journal of the American Board of Family Practice 1991;4(2):89‐94. [PubMed] [Google Scholar]
Rovina 2003 {unpublished data only}
- Gratziou C, Rovina N, Athanassa Z, Francis K, Evangelou E, Chiotis D, et al. Evaluation of prolonged bupropion treatment as an aid in smoking cessation [abstract]. European Respiratory Journal 2002;20 Suppl 38:611s. [Google Scholar]
- Rovina N, Gratziou C, Nikoloutsou I, Athanassa Z, Francis K, Roussos C. Ideal duration of therapy with bupropion HCL: Comparison between short and long treatment. Society for Research on Nicotine and Tobacco 5th European Meeting November 20‐22 2003 Padua: Abstract book. 2003.
- Rovina N, Gratziou C, Nikoloutsou I, Athanassa Z, Francis K, Roussos C. Short or prolonged treatment with bupropion HCL in smoking cessation therapy. European Respiratory Journal 2003;22 Suppl 45:165s. [Google Scholar]
Schepis 2006 {unpublished data only}
- Schepis TS, Warren KA, Rao U. Evaluation of a cognitive‐behavioral smoking cessation treatment for adolescents and young adults (POS2‐53). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
Sellers 1987 {published data only}
- Sellers EM, Naranjo CA, Kadlec K. Do serotonin uptake inhibitors decrease smoking? Observations in a group of heavy drinkers. Journal of Clinical Psychopharmacology 1987;7:417‐20. [PubMed] [Google Scholar]
Sheng 2013 {published data only}
- Sheng LX, Tang YL, Jiang ZN, Yao CH, Gao JY, Xu GZ, et al. Sustained‐release bupropion for smoking cessation in a Chinese sample: a double‐blind, placebo‐controlled, randomized trial. Nicotine & Tobacco Research 2013;15(2):320‐5. [] [DOI] [PubMed] [Google Scholar]
Sherman 2008 {published data only}
- Sherman SE, Aldana I, Estrada M, York L. Comparing the tolerability and effectiveness of two treatment regimens in a smoking clinic. Military Medicine 2008;173(6):550‐4. [DOI] [PubMed] [Google Scholar]
Shiffman 2000 {published data only}
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology 2000;148:33‐40. [DOI] [PubMed] [Google Scholar]
Shoptaw 2008 {published data only}
- Shoptaw S, Heinzerling KG, Rotheram‐Fuller E, Steward T, Wang J, Swanson AN, et al. Randomized, placebo‐controlled trial of bupropion for the treatment of methamphetamine dependence. Drug and Alcohol Dependence 2008;96(3):222‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2010 {published data only}
- Singh P, Kumar R. Assessment of the effectiveness of sustained release Bupropion and intensive physician advice in smoking cessation. Lung India 2010; Vol. 27, issue 1:11‐8. [] [DOI] [PMC free article] [PubMed]
Sittipunt 2007 {published data only}
- Sittipunt C, Kawkitinarong K, Wongtim S, Udompanich V. The effectiveness of nortriptyline plus brief motivation counseling for the treatment of smoking cessation in Thai active smokers [Abstract]. Respirology 2007;12(Suppl 4):A223. [Google Scholar]
Sonntag 2003 {published data only}
- Hoch E, Wittchen HU. Population health perspective on smoking cessation: A randomized controlled trial of different methods in primary health care (RPOS 3‐71). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Sonntag H, Hoch E, Jahn B, Spiegel B, Pfister H, Wittchen HU. Smoking cessation in primary care: implementation effectiveness and optimized allocation. Suchtmedizin in Forschung und Praxis 2003;5:137‐41. [Google Scholar]
Spring 1995 {published data only}
- Spring B, Wurtman J, Wurtman R, Khoury A, Goldberg H, McDermott J, et al. Efficacies of dexfenfluramine and fluoxetine in preventing weight gain after smoking cessation. American Journal of Clinical Nutrition 1995;62(6):1181‐7. [DOI] [PubMed] [Google Scholar]
Stein 1993 {published data only}
- Stein RA, Jarvik ME, Gorelick DA. Impairment of memory by fluoxetine in smokers. Experimental and Clinical Psychopharmacology 1993;1:188‐93. [Google Scholar]
Steinberg 2009 {published data only}
- Steinberg MB, Greenhaus S, Schmelzer AC, Bover MT, Foulds J, Hoover DR, et al. Triple‐combination pharmacotherapy for medically ill smokers: a randomized trial. Annals of Internal Medicine 2009;150(7):447‐54. [DOI] [PubMed] [Google Scholar]
Strayer 2004 {unpublished data only}
- Strayer SM, Flusche A, Hodge J, Martindale JR. Effectiveness trial of Zyban for smoking cessation in the outpatient setting (POS1‐044). Abstract Book. Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004:45.
Swanson 2003 {published data only}
- Swanson NA, Burroughs CC, Long MA, Lee RW. Controlled trial for smoking cessation in a Navy shipboard population using nicotine patch, sustained‐release bupropion, or both. Military Medicine 2003;168:830‐4. [PubMed] [Google Scholar]
Tidey 2009 {published data only}
- Tidey JW, Rohsenow DJ. Intention to quit moderates the effect of bupropion on smoking urge. Nicotine & Tobacco Research 2009;11(3):308‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toll 2007 {published data only}
- Jatlow P, Toll BA, Leary V, Krishnan‐Sarin S, O'Malley SS. Comparison of expired carbon monoxide and plasma cotinine as markers of cigarette abstinence. Drug and Alcohol Dependence 2008;98(3):203‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Mckee SA, Toll BA, Krishnan‐Sarin S, Cooney JL, Makuch RW, et al. Risk factors for treatment failure in smokers: Relationship to alcohol use and to lifetime history of an alcohol use disorder. Nicotine & Tobacco Research 2008;10(12):1793‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, O'Malley SS, Katulak NA, Wu R, Dubin JA, Latimer A, et al. Comparing gain‐ and loss‐framed messages for smoking cessation with sustained‐release bupropion: a randomized controlled trial. Psychology of Addictive Behaviors 2007;21(4):534‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll BA, Salovey P, O'Malley SS, Mazure CM, Latimer A, Mckee SA. Message framing for smoking cessation: the interaction of risk perceptions and gender. Nicotine & Tobacco Research 2008;10(1):195‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2008 {published data only}
- Weinberger AH, Vessicchio JC, Sacco KA, Creeden CL, Chengappa KN, George TP. A preliminary study of sustained‐release bupropion for smoking cessation in bipolar disorder. Journal of Clinical Psychopharmacology 2008;28(5):584‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiner 2001 {published data only}
- Weiner E, Ball MP, Summerfelt A, Gold J, Buchanan RW. Effects of sustained‐release bupropion and supportive group therapy on cigarette consumption in patients with schizophrenia. American Journal of Psychiatry 2001;158(4):635‐7. [DOI] [PubMed] [Google Scholar]
Weiner 2012 {published data only}
- Mann‐Wrobel MC, Bennett ME, Weiner EE, Buchanan RW, Ball MP. Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophrenia Research 2011; Vol. 126, issue 1‐3:277‐83. [] [DOI] [PubMed]
- Weiner E, Ball MP, Buchholz AS, Gold JM, Evins AE, McMahon RP, et al. Bupropion sustained release added to group support for smoking cessation in schizophrenia: a new randomized trial and a meta‐analysis. Journal of Clinical Psychiatry. United States, 2012; Vol. 73, issue 1:95‐102. [] [DOI] [PubMed]
White 2005 {published data only}
- White WD, Crockford D, Patten S, Guebaly N. A randomized, open‐label pilot comparison of gabapentin and bupropion SR for smoking cessation. Nicotine & Tobacco Research 2005;7(5):809‐13. [DOI] [PubMed] [Google Scholar]
Zernig 2008 {published data only}
- Zernig G, Wallner R, Grohs U, Kriechbaum N, Kemmler G, Saria A. A randomized trial of short psychotherapy versus sustained‐release bupropion for smoking cessation. Addiction 2008;103(12):2024‐31. [DOI] [PubMed] [Google Scholar]
ZYB30011 2002 {unpublished data only}
- GlaxoSmithKline Clinical Trials Register. A multicentre, randomised, double‐ blind, placebo controlled study to evaluate the efficacy and tolerability of bupropion hydrochloride (SR) sustained release (2 x 150mg per day) versus placebo as an aid to smoking cessation in smokers with at least one cardiovascular (CV) risk factor. http://www.gsk‐clinicalstudyregister.com/files/pdf/730.pdf (accessed 4th August 2009).
References to ongoing studies
Rose 2013a {unpublished data only}
- Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits male NRT‐Nonresponders. Society for Research on Nicotine and Tobacco 19th Annual Meeting March 13‐16 Boston MA 2013:261. [] [Google Scholar]
Additional references
Aubin 2002
- Aubin HJ. Tolerability and safety of sustained‐release bupropion in the management of smoking cessation. Drugs 2002;62 Suppl 2:45‐52. [DOI] [PubMed] [Google Scholar]
Belson 2002
- Belson MG, Kelley TR. Bupropion exposures: clinical manifestations and medical outcome. Journal of Emergency Medicine 2002;23:223‐30. [DOI] [PubMed] [Google Scholar]
Benowitz 2000
- Benowitz NL, Peng MW. Non‐nicotine pharmacotherapy for smoking cessation. CNS Drugs 2000;13:265‐85. [Google Scholar]
Beyens 2008
- Beyens MN, Guy C, Mounier G, Laporte S, Ollagnier M. Serious adverse reactions of bupropion for smoking cessation: analysis of the French Pharmacovigilance Database from 2001 to 2004. Drug Safety 2008;31:1017‐26. [DOI] [PubMed] [Google Scholar]
Bolin 2006
- Bolin K, Lindgren B, Willers S. The cost utility of bupropion in smoking cessation health programs: simulation model results for Sweden. Chest 2006;129:651‐60. [DOI] [PubMed] [Google Scholar]
Borrelli 2004
- Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter‐defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction 2004;99:378‐85. [DOI] [PubMed] [Google Scholar]
Boshier 2003
- Boshier A, Wilton LV, Shakir SA. Evaluation of the safety of bupropion (Zyban) for smoking cessation from experience gained in general practice use in England in 2000. European Journal of Clinical Pharmacology 2003;59(10):767‐73. [DOI] [PubMed] [Google Scholar]
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6] [DOI] [PubMed] [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD009329.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Catley 2005
- Catley D, Harris KJ, Okuyemi KS, Mayo MS, Pankey E, Ahluwalia JS. The influence of depressive symptoms on smoking cessation among African Americans in a randomized trial of bupropion. Nicotine & Tobacco Research 2005;7(6):859‐870. [DOI] [PubMed] [Google Scholar]
Chun‐Fai‐Chan 2005
- Chun‐Fai‐Chan B, Koren G, Fayez I, Kalra S, Voyer‐Lavigne S, Boshier A, et al. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. American Journal of Obstetrics and Gynecology 2005;192(3):932‐936. [DOI] [PubMed] [Google Scholar]
Cox 2004
- Cox LS, Patten CA, Niaura RS, Decker PA, Rigotti N, Sachs DPL, et al. Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. Journal of General Internal Medicine 2004;19:828‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cryan 2003
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 2003;168:347‐58. [DOI] [PubMed] [Google Scholar]
Dale 2001
- Dale LC, Glover ED, Sachs DP, Schroeder DR, Offord KP, Croghan IT, et al. Bupropion for smoking cessation: predictors of successful outcome. Chest 2001;119:1357‐64. [DOI] [PubMed] [Google Scholar]
David 2005
- David SP, Papandonatos GD, Munafo MR, McCaffery JM, Lerman C, Lloyd‐Richardson EE, et al. DRD2‐TAQ1A genotypic moderation of bupropion treatment efficacy for smoking cessation at 6‐month follow‐up [abstract]. Nicotine & Tobacco Research 2005;7:704. [Google Scholar]
Dunner 1998
- Dunner DL, Zisook S, Billow AA, Batey SR, Johnston JA, Ascher JA. A prospective safety surveillance study for bupropion sustained‐release in the treatment of depression. Journal of Clinical Psychiatry 1998;59:366‐73. [DOI] [PubMed] [Google Scholar]
Durcan 2002
- Durcan MJ, White J, Jorenby DE, Fiore MC, Rennard SI, Leischow SJ, et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. American Journal of Health Behavior 2002;26(3):213‐20. [DOI] [PubMed] [Google Scholar]
Ebbert 2011
- Ebbert J, Montori VM, Erwin PJ, Stead LF. Interventions for smokeless tobacco use cessation. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD004306.pub4] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMEA 2002
- European Agency for the Evaluation of Medicines for Human Use. Committee for Proprietary Medicinal Products. Opinion following an article 36 referral. Bupropion hydrochloride. http://www.emea.eu.int/pdfs/human/referral/2761002en.pdf Accessed 29 April 2004.
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. AHRQ publication No. 00‐0032. Rockville, MD: US Dept of Health and Human Services. Public Health Services, May 2008. [Google Scholar]
Fryer 1999
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. Journal of Pharmacology and Experimental Therapeutics 1999;288:88‐92. [PubMed] [Google Scholar]
Gambassi 1999
- Gambassi G, Bernabei R. Antidepressants and smoking cessation [Comment]. Archives of Internal Medicine 1999;159:1257‐8. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline
- GlaxoSmithKline. Zyban(R) bupropion hydrochloride Sustained‐Release Tablets. Product Information. www.gsk.com/products/assets/us_zyban.pdf (accessed 15/11/2006).
Goldstein 1998
- Goldstein MG. Bupropion sustained release and smoking cessation. Journal of Clinical Psychiatry 1998;59 suppl 4:66‐72. [PubMed] [Google Scholar]
Gonzales 2002a
- Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, et al. Effects of gender on relapse prevention in smokers treated with bupropion SR. American Journal of Preventive Medicine 2002;22(4):234‐39. [DOI] [PubMed] [Google Scholar]
Gourlay 1995
- Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ 1995;311:363‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grandi 2011a
- Grandi S, Shimony A, Eisenberg MJ. The efficacy and safety of bupropion started in‐hospital for smoking cessation in patients with cardiovascular disease: A systematic review and meta‐analysis. Circulation 2011;124(21):Suppl 1. [Google Scholar]
Haas 2004
- Haas JS, Kaplan CP, Barenboim D, Jacob P, Benowitz NL. Bupropion in breast milk: an exposure assessment for potential treatment to prevent post‐partum tobacco use. Tobacco Control 2004;13:52‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2013
- Hajek P, Stead LF, West R, Jarvis M, Hartmann‐Boyce J, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD003999.pub4] [DOI] [PubMed] [Google Scholar]
Hall 2001
- Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger‐Moore J, Hedeker D, et al. Statistical analysis of randomized trials in tobacco treatment: longitudinal designs with dichotomous outcome. Nicotine & Tobacco Research 2001;3:193‐202. [DOI] [PubMed] [Google Scholar]
Hall 2005
- Hall SM, Lightwood JM, Humfleet GL, Bostrom A, Reus VI, Munoz R. Cost‐effectiveness of bupropion, nortriptyline, and psychological intervention in smoking cessation. Journal of Behavioral Health Services & Research 2005;32:381‐92. [DOI] [PubMed] [Google Scholar]
Haustein 2003
- Haustein KO. Bupropion: pharmacological and clinical profile in smoking cessation. International Journal of Clinical Pharmacology and Therapeutics 2003;41:56‐66. [DOI] [PubMed] [Google Scholar]
Hayford 1999
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. British Journal of Psychiatry 1999;174:173‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Holmes 2004
- Holmes S, Zwar N, Jimenez‐Ruiz CA, Ryan PJ, Browning D, Bergmann L, et al. Bupropion as an aid to smoking cessation: a review of real‐life effectiveness. International Journal of Clinical Practice 2004;58(3):285‐291. [DOI] [PubMed] [Google Scholar]
Hubbard 2005
- Hubbard R, Lewis S, West J, Smith C, Godfrey C, Smeeth L, et al. Bupropion and the risk of sudden death: a self‐controlled case‐series analysis using The Health Improvement Network. Thorax 2005;60(10):848‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2005
- Hughes JR, Stead LF, Lancaster T. Nortriptyline for smoking cessation: A review. Nicotine & Tobacco Research 2005;7:491‐9. [DOI] [PubMed] [Google Scholar]
Hughes 2007
- Hughes JR. Depression during tobacco abstinence. Nicotine & Tobacco Research 2007;9:443‐6. [DOI] [PubMed] [Google Scholar]
Hughes 2008
- Hughes JR. Smoking and suicide: A brief overview. Drug and Alcohol Dependence 2008;198:169‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurt 2002
- Hurt RD, Wolter TD, Rigotti N, Hays JT, Niaura R, Durcan MJ, et al. Bupropion for pharmacologic relapse prevention to smoking ‐ Predictors of outcome. Addictive Behaviors 2002;27(4):493‐507. [DOI] [PubMed] [Google Scholar]
Javitz 2004
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost‐effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10(3):217‐26. [PubMed] [Google Scholar]
Johnstone 2004
- Johnstone EC, Yudkin Pl, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short‐term effectiveness of the nicotine patch. Pharmacogenetics 2004;14:83‐90. [DOI] [PubMed] [Google Scholar]
Jorenby 2002
- Joreby D. Clinical efficacy of bupropion in the management of smoking cessation. Drugs 2002;62 Suppl 2:25‐35. [DOI] [PubMed] [Google Scholar]
Khawam 2006
- Khawam EA, Laurencic G, Malone DA. Side effects of antidepressants: An overview. Cleveland Clinic Journal of Medicine 2006;73:351‐61. [DOI] [PubMed] [Google Scholar]
Kotlyar 2001
- Kotlyar M, Golding M, Hatsukami DK, Jamerson BD. Effect of nonnicotine pharmacotherapy on smoking behavior. Pharmacotherapy 2001;21:1530‐48. [DOI] [PubMed] [Google Scholar]
Kotlyar 2005
- Kotlyar M, Brauer LH, Tracy TS, Hatsukami DK, Harris J, Bronars CA, et al. Inhibition of CYP2D6 activity by bupropion. Journal of Clinical Psychopharmacology 2005;25:226‐9. [DOI] [PubMed] [Google Scholar]
Lerman 2002a
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu AY, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence 2002;67:219‐23. [DOI] [PubMed] [Google Scholar]
Lerman 2002b
- Lerman C, Shields PG, Wileyto EP, Audrain J, Pinto A, Hawk L, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics 2002;12:627‐34. [DOI] [PubMed] [Google Scholar]
Lerman 2004
- Lerman C, Niaura R, Collins BN, Wileyto P, Audrain MJ, Pinto A, et al. Effect of bupropion on depression symptoms in a smoking cessation clinical trial. Psychology of Addictive Behaviors 2004;18:362‐6. [DOI] [PubMed] [Google Scholar]
Lerman 2006
- Lerman C, Jepson C, Wileyto EP, Epstein LH, Rukstalis M, Patterson F, et al. Role of functional genetic variation in the dopamine D2 receptor (DRD2) in response to bupropion and nicotine replacement therapy for tobacco dependence: results of two randomized clinical trials. Neuropsychopharmacology 2006;31:231‐42. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
Martinez‐Raga 2003
- Martinez‐Raga J, Keaney F, Sutherland G, Perez Galvez B, Strang J. Treatment of nicotine dependence with bupropion SR: review of its efficacy, safety and pharmacological profile. Addiction Biology 2003;8:13‐21. [DOI] [PubMed] [Google Scholar]
McRobbie 2005
- McRobbie H, Lee M, Juniper Z. Non‐nicotine pharmacotherapies for smoking cessation. Respiratory Medicine 2005;99:1202‐12. [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore TJ, Furberg CD, GJ, Maltsberger JT, Singh S. Suicidal behavior and depression in smoking cessation treatments. PloS One 2011;6(11):e27016. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasternak 2013
- Pasternak B, Svanström H, Hviid A. Use of varenicline versus bupropion and risk of psychiatric adverse events. Addiction 2013;108(7):1336‐43. [DOI] [PubMed] [Google Scholar]
Patterson 2008
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo‐controlled trial of bupropion. Clinical Pharmacology & Therapeutics 2008;84:320‐5. [DOI] [PubMed] [Google Scholar]
Piper 2010
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, et al. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research 2010;12(6):647‐57. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
RCP 2000
- Working party of the Royal College of Physicians of London. Non‐nicotine medications for treating nicotine addiction. Nicotine Addiction in Britain. London: Royal College of Physicians, 2000:147‐151. [Google Scholar]
Scharf 2004
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled‐ and meta‐analyses of clinical trials of Bupropion SR. Addiction 2004;99:1462‐69. [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith SS, Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston AJ, et al. Targeting smokers at increased risk for relapse: treating women and those with a history of depression. Nicotine & Tobacco Research 2003;5:99‐109. [DOI] [PubMed] [Google Scholar]
Stead 2012
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
Swan 1999
- Swan GE, Jack LM, Niaura R, Borrelli B, Spring B. Subgroups of smokers with different success rates after treatment with fluoxetine for smoking cessation [abstract]. Nicotine & Tobacco Research 1999;1(3):281. [Google Scholar]
Swan 2005
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5:21‐9. [DOI] [PubMed] [Google Scholar]
Thomas 2013
- Thomas KH, Martin RM, Davies NM, Metcalfe C, Windmeijer F, Gunnell D. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ 2013;347:f5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorndike 2008
- Thorndike AN, Regan S, McKool K, Pasternak RC, Swartz S, Torres‐Finnerty N, et al. Depressive symptoms and smoking cessation after hospitalization for cardiovascular disease. Archives of Internal Medicine 2008;168(2):186‐91. [DOI] [PubMed] [Google Scholar]
Tonnesen 1993
- Tonnesen P, Norregaard J, Sawe U, Simonsen K. Recycling with nicotine patches in smoking cessation. Addiction 1993;88:533‐9. [DOI] [PubMed] [Google Scholar]
Tonstad 2002
- Tonstad S. Use of sustained‐release bupropion in specific patient populations for smoking cessation. Drugs 2002;2 Suppl 2:37‐43. [DOI] [PubMed] [Google Scholar]
Tracey 2002
- Tracey JA. Zyban ‐ is there a cause for concern?. Expert Opinion on Drug Safety 2002;1:303‐5. [DOI] [PubMed] [Google Scholar]
Uhl 2008
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome‐wide association study results. Archives of General Psychiatry 2008;65(6):683‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
US FDA 2009a
- Anon. Varenicline and bupropion. Reports of suicidality associated with used of varenicline (marketed as CHANTIX) and bupropion (marketed as Zyban and generics). FDA Drug Safety Newsletter 2009;2(1):1‐4. [Google Scholar]
US FDA 2009b
- Public Health Advisory: FDA Requires New Boxed Warnings for the Smoking Cessation Drugs Chantix and Zyban. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm169988.htm accessed 20th November 2013.
van der Meer 2013
- Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD006102.pub2] [DOI] [PubMed] [Google Scholar]
Wagena 2005a
- Wagena EJ, Knipschild P, Zeegers MP. Should nortriptyline be used as a first‐line aid to help smokers quit? Results from a systematic review and meta‐analysis. Addiction 2005;100:317‐26. [DOI] [PubMed] [Google Scholar]
Warner 2005
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid?. Addiction Biology 2005;10(3):219‐31. [DOI] [PubMed] [Google Scholar]
Welton 2008
- Welton NJ, Johnstone EC, David SP, Munafò MR. A cost‐effectiveness analysis of genetic testing of the DRD2 Taq1A polymorphism to aid treatment choice for smoking cessation. Nicotine & Tobacco Research 2008;10(1):231‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2000
- West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Thorax 2000;55(12):987‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2003
- West R. Bupropion SR for smoking cessation. Expert Opinion on Pharamcotherapy 2003;4:533‐40. [DOI] [PubMed] [Google Scholar]
West 2008
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 2008;197(3):371‐7. [DOI] [PubMed] [Google Scholar]
Wightman 2010
- Wightman DS, Foster VJ, Krishen A, Richard NE, Modell JG. Meta‐analysis of suicidality in placebo‐controlled clinical trials of adults taking bupropion. Primary Care Companion to the Journal of Clinical Psychiatry 2010;12(5):e1‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiley 2002
- Wiley JL, Lavecchia KL, Martin BR, Damaj MI. Nicotine‐like discriminative stimulus effects of bupropion in rats. Experimental and Clinical Psychopharmacology 2002;10:129‐135. [DOI] [PubMed] [Google Scholar]
Wilkes 2005
- Wilkes S, Evans A, Henderson M, Gibson J. Pragmatic, observational study of bupropion treatment for smoking cessation in general practice. Postgraduate Medical Journal 2005;81:719‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2002
- Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. European Journal of Pharmacology 2002;443:113‐118. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hughes 1994
- Hughes JR. Non‐nicotine pharmacotherapies for smoking cessation. Journal of Drug Development 1994;6:197‐203. [Google Scholar]
Hughes 2000
- Hughes JR, Stead LF, Lancaster T. Anxiolytics and antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI] [PubMed] [Google Scholar]
Hughes 2002
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI] [PubMed] [Google Scholar]
Hughes 2003
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000031] [DOI] [PubMed] [Google Scholar]
Hughes 2004
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000031.pub2] [DOI] [PubMed] [Google Scholar]
Hughes 2007a
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD000031.pub3] [DOI] [PubMed] [Google Scholar]


