Abstract
Objective:
This RCT tested effectiveness of an adaptive “SMART” stepped-care approach to “standard” behavioral weight loss (BWL (Standard)) for patients with binge-eating disorder (BED) and obesity.
Method:
191 patients with BED/obesity were randomly assigned to six-months BWL (Standard) (N=39) or Stepped-care (N=152). Within Stepped-care, patients started with BWL for one month; treatment-responders continued BWL while non-responders switched to CBT. All patients receiving Stepped-care were additionally randomized to weight-loss medication or placebo (double-blind) for the remaining five months. Independent assessments were performed reliably at baseline, throughout-treatment, and post-treatment.
Results:
ITT analyses of remission rates (zero binges/month) revealed BWL (Standard) and Stepped-care did not differ significantly overall (74.4% vs 66.5%); within Stepped-care, remission rates ranged 40.0%−83.3% with medication significantly superior to placebo (overall) and among non-responders switched to CBT. Mixed-models analyses of binge-eating frequency revealed significant time-effects but BWL (Standard) and Stepped-care did not differ overall; within Stepped-care, medication was significantly superior to placebo (overall) and among non-responders switched to CBT. Mixed models revealed significant weight loss but BWL (Standard) (5.1% weight-loss) and Stepped-care (5.8% weight-loss) did not differ overall; within Stepped-care (range 0.4%−8.8% BMI loss), medication was significantly superior to placebo (overall) and among both responders continued on BWL and non-responders switched to CBT.
Conclusions:
Overall, BWL (Standard) and adaptive Stepped-care treatments produced robust improvements in binge-eating (high remission-rates and binge-eating reductions) and weight-loss in patients with BED/obesity but differed little. Within adaptive Stepped-care, weight-loss medication enhanced outcomes for BED/obesity. Implications for clinical practice and future adaptive designs are offered.
TRIAL REGISTRATION:
Clinicaltrials.gov Identifier: NCT blinded number to be inserted
Clinicaltrials.gov registration number:
Keywords: obesity, eating disorders, cognitive-behavioral therapy, binge eating, weight loss
Binge-eating disorder (BED), the most prevalent eating disorder among adults (Udo & Grilo, 2018), is characterized by recurrent binge eating (i.e., eating unusually large quantities of food accompanied by feelings of loss of control), marked distress about binge eating, and absence of extreme weight-compensatory behaviors (e.g., purging) that characterize bulimia nervosa. BED is associated with elevated rates of medical and psychiatric comorbidities (Udo & Grilo, 2019) and is associated particularly strongly with obesity (Kessler et al., 2013; Udo & Grilo, 2018). BED is characterized by greater eating-disorder and general psychopathology than obesity (Grilo, Hrabosky, White, Allison, Stunkard, & Masheb, 2008) and appears to heighten risk for developing future metabolic problems associated with obesity (Hudson et al., 2010); unfortunately, weight losses in treatment studies for BED seem dampened relative to those for obesity without BED (Blaine & Rodman, 2007).
Although randomized clinical trials (RCT) for BED have produced general empirical support for certain psychological (Grilo, 2017; Wilson, Grilo, & Vitousek, 2007), pharmacological (McElroy, 2017; Reas & Grilo, 2015), and combination or “multi-modal” (Grilo, Reas, & Mitchell, 2016) treatments, even with the “best-available” interventions, many patients do not benefit sufficiently or fail to cease binge eating or lose weight (Hilbert et al., 2019). Treatment research for BED has utilized primarily “traditional” parallel-group RCT designs. RCTs “combining” pharmacological and psychological approaches, a typical clinical-practice approach, have nearly all failed to improve outcomes for BED (Grilo, Reas, & Mitchell, 2016; Hilbert et al., 2019). The very few treatment studies for BED that have tested longer and sequenced methods (Agras et al., 1995; Agras et al., 1994; Grilo, Masheb, Wilson et al., 2011) have also not reported better outcomes. For example, Grilo et al (2012) reported that a sequential approach of providing behavioral weight loss (BWL) following cognitive-behavioral therapy (CBT) did not offer any advantage over either CBT or BWL despite the longer intervention time.
To date, RCTs bear little resemblance to treatment approaches offered in clinical settings (Kazdin & Blasé, 2001; Shafran et al., 2009) or naturally sought-out by persons with eating/weight disorders in the U.S. (Coffino, Udo, & Grilo, in press; Hart et al., 2011). Most research with BED (and with obesity and other eating disorders) has addressed the important question of which treatments can help the most patients (Hilbert et al., 2019). Emerging stepped-care models have evaluated testing scalable versions of effective treatments such as guided-self-help versions of CBT (e.g. Peterson et al., 2009; see: Wilson & Zandberg, 2012) with the view of increasing dissemination in cost-effective ways. Typical stepped-care treatment models propose starting with scalable methods and only if non-response moving on to more intensive or “specialist” treatments (Wilson & Zandberg, 2012). Very little research, however, has examined how or whether to alter or combine treatments in unresponsive patients (Hilbert et al., 2019).
Expert reviews have highlighted the pressing need for more complex models of care (Hilbert et al., 2019) to find ways to improve outcomes. Adaptive treatment strategies have been proposed for chronic diseases (Lavori & Dawson, 2007) such as obesity (Almirall, Nahum-Shani, Sherwood & Murphy, 2014) but these remain conceptual models still in development (Almirall, Compton, Gunlicks-Stoessel, Duan, & Murphy, 2012) and in need of empirical evaluation. Adaptive approaches to stepped-care propose that initial responses to treatments, usually less intensive or more widely available interventions, inform subsequent treatments. Two studies, one with bulimia nervosa (BN) (Mitchell et al., 2011) and one with BN and BED (Chen et al., 2017) found that approaches starting with guided self-help CBT (CBTgsh) and shifting to more intensive treatments resulted in outcomes that did not differ significantly at post-treatment from more intensive treatments administered in full (Mitchell et al., 2011) or from those who showed early response to CBTgsh (Chen et al., 20127). Both studies, however, suggested that early non-response to CBTgsh can perhaps be overcome and that continued research is needed to test innovative adaptive approaches to enhance treatment outcomes.
The current study aimed to perform an adaptive RCT (Lavori & Dawson, 2007) using a “sequential multiple assignment randomized trial” (SMART) design (Nahum-Shani et al., 2012) to evaluate a proposed treatment model for patients with BED with co-existing obesity. The proposed adaptive treatment aims to benefit from reliable findings from RCTs showing that rapid response to treatments for eating disorders has robust prognostic significance (Linardon, Brennan, & de la Piedad Garcia, 2016). A series of studies found that rapid response to treatment, defined empirically (using receiver operating characteristic (ROC) curves) as 65%−70% reductions in binge eating after one month of treatment predicted favorable outcomes for diverse treatments for BED (Grilo & Masheb, 2007; Grilo, Masheb, & Wilson, 2006; Grilo, White, Masheb, & Gueorguieva, 2015; Grilo, White, Wilson, Gueorguieva, & Masheb, 2012; Masheb & Grilo, 2017). Importantly, rapid response specifically to BWL was associated with superior reductions in both weight and binge eating (Grilo, White et al., 2017; Masheb & Grilo, 2007) whereas non-rapid-response signaled poor outcomes. The adaptive SMART RCT design for BED focused on these rapid-response findings for BWL, rather than with different forms of CBT, for two important reasons. First, rigorous RCTs for BED found that BWL was roughly comparable to CBT but had the added advantage of producing weight losses (Grilo, Masheb, Wilson, Gueorguieva, & White, 2011; Wilson, Wilfley, Agras, & Bryson, 2010), which do not occur with CBT (Hilbert et al., 2019). The strong association between BED and obesity dictates the need to find ways to address both issues. Second, although CBTgsh was developed as a scalable version to address the dissemination challenge for CBT (Shafran et al., 2009; Wilson & Zandberg, 2012), BWL, a “generalist” treatment is a clearly much more widely available treatment option (Wilson et al., 2010).
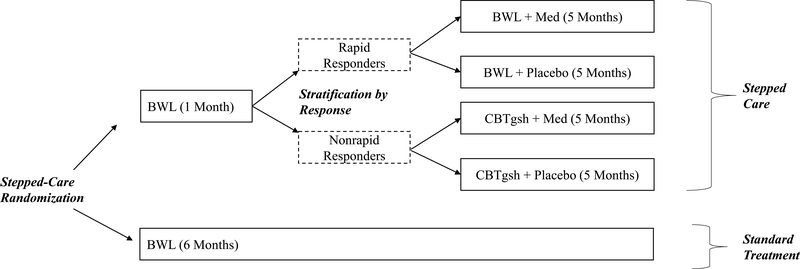
Figure 1 shows the adaptive SMART design. Participants were randomized to either Stepped-care (top of Figure) or to BWL (Standard) (bottom of Figure) for 6 months (“Stepped-care Randomization”). In Stepped-care, following one month of BWL, patients were then stratified by whether or not they had a “rapid response” to BWL (“Stratification by Response”). Rapid response was defined as a 65% reduction in binge-eating from baseline to the month one assessment; this definition, informed by signal detection methods (ROC curves) has reliabily shown prognostic significance in previous RCTs for BED (Grilo et al., 2006; Grilo 2015; Masheb & Grilo, 2007). Rapid responders were then randomized to receive, in double-blind fashion, either weight-loss medication (sibutramine or orlistat) or placebo in additton to continuing to receive BWL for another 5 months (“Randomization for Rapid Responders”). Participants who did not show a rapid response to BWL were switched to a “specialist” CBTgsh and additionally randomized to also receive, in double-blind fashion, either weight-loss medication or placebo (“Randomization for Non-Rapid Responders”). The BWL in both treatment arms followed the same manualized protocol.
Figure 1.
Study design.
BWL = behavioral weight loss; Med = medication (weight-loss medication); CBTgsh = cognitive-behavioral therapy via guided self-help
The primary aim was to test whether Stepped-care, which began with BWL and then followed an adaptive approach for additional interventions based on rapid treatment response is superior to BWL (Standard) treatment. Secondary aims were to explore whether, within Stepped-care, groups with rapid response continuing BWL and without rapid response switched to CBTgsh, the addition of weight-loss medications is superior to placebo for enhancing outcomes.
Methods
Participants
Participants were a series of 191 consecutively assessed patients recruited (December 17, 2008 to July 10, 2012) via media advertisement who met DSM-IV research criteria for BED. Additional eligibility criteria included age between 18 and 65 years and body mass index (BMI; weight (kg) divided height (m2)) between 30 and 50 kg/ m2. Consistent with principles for conducting controlled effectiveness trials (Kraemer, 2003), exclusion criteria were kept to a minimum and reflected primarily clinical issues that, regardless of setting, would indicate need for different treatment or represent a contraindication to the study medications. Exclusionary criteria included: concurrent treatment for eating/weight problems; taking contraindicated medications (e.g., MAOIs, SSRIs); uncontrolled medical conditions (e.g., diabetes or thyroid problems) that influence eating/weight or representing contraindications to study medications (e.g., cardiovascular disease, systolic blood pressure greater than 160 mmHg, diastolic blood pressure greater than 95 mmHg, or heart rate > 100 beats/minute); and pregnancy or unable to comply with birth control. The study received full review and approval by the Yale IRB. After complete description of the study to participants, written informed consent was obtained.
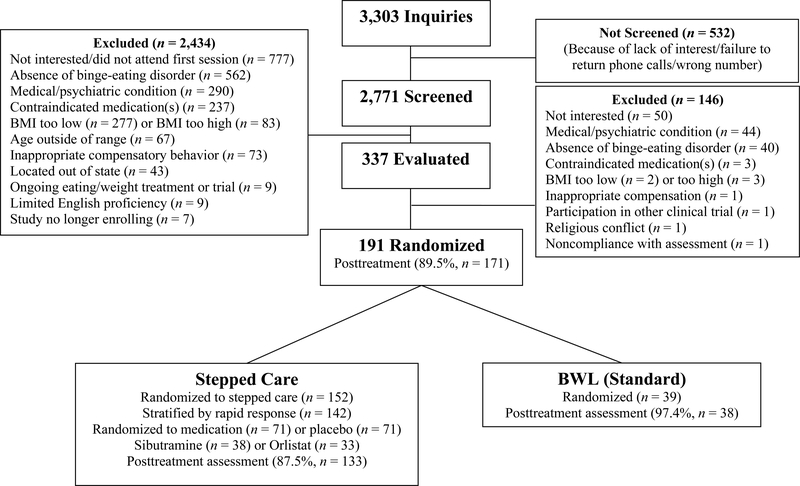
Figure 2 summarizes the flow of participants throughout the study. Of the 2771 individuals screened by telephone, 337 were scheduled for in-person assessments to determine eligibility. Of these, 191 individuals were interested in participating, met eligibility requirements, completed baseline assessments, and were randomized to treatment. The 191 participants had a mean age of 48.4 (SD=9.5) years and mean BMI of 39.0 (SD=6.0) kg/m2. Seventy-one percent (N=136) of participants were female, 84% (N=160) attended/finished college, and 79% (N=150) were Caucasian, 15% (N=28) were African-American, 4% (N=8) were Hispanic, and 2% (N=5) were “other” ethnicity/race.
Figure 2.
Participant flow throughout the study.
Diagnostic Assessments and Repeated Measures
Diagnostic and assessment procedures were performed by trained doctoral-level research-clinicians monitored throughout the study. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P; First, Spitzer, Gibbon, & Williams, 1996) was used to establish the BED diagnosis and to determine co-existing (current and lifetime) DSM-IV psychiatric disorders.
Eating Disorder Examination Interview (EDE-16th edition; Fairburn & Cooper, 2008), a semi-structured, investigator-based interview, was administered to assess eating-disorder psychopathology and to confirm the BED diagnosis at baseline and was re-administered at post-treatment. The EDE focuses on the previous 28 days except for diagnostic items, which are rated for DSM-based duration and frequency stipulations. The EDE assesses the frequency of objective binge-eating episodes (OBE; corresponds to DSM-based definition of binge-eating) and also comprises four subscales of eating-disorder psychopathology which are averaged to a total global score reflecting severity. The EDE, empirically-supported and used widely in eating-disorder treatment research (Berg, Peterson, Frazier, & Crow, 2012), has demonstrated good validity (Grilo, Masheb, & Wilson, 2001) and inter-rater and test-retest reliability in BED (Grilo, Masheb, Lozano-Blanco, & Barry, 2004). In the present RCT, inter-rater (N=37 ratings of taped interviews) reliability of the EDE was excellent; inter-rater intra-class correlation coefficients (ICC) were 0.86 for binge-eating frequency and 0.94 for EDE global score.
Beck Depression Inventory (BDI; Beck & Steer, 1987) 21-item version is a well-established self-report (Beck, Steer, & Garbin, 1998) measure of symptoms of depression (higher scores reflect higher levels). The BDI was given at baseline, monthly, and at post-treatment. In the present study, coefficient alpha was 0.90 for the BDI, indicating good internal consistency.
Physical measures were also obtained. Weight and height (used to calculate BMI) were measured at baseline and weight was measured monthly throughout treatment and at post-treatment using a large-capacity digital scale. Waist circumference, measured following NHBLI (2000) protocols, offers clinical advantages (Pouliot et al., 1994) to classifying excess abdominal fat and predicting cardiovascular risk, Cholesterol was obtained as part of standard lipid-panel following established protocols (morning draw,12-hour overnight fast, no exercise for 48 hours).
Treatment Randomization Protocol
Three different randomization schedules were created for this adaptive SMART study design (Figure 1). Stepped-care randomization followed a ratio of 4:1 (Stepped-care to BWL (Standard) ratio) for all eligible consented participants. In Stepped-care, rapid-response status – defined as 65% reduction in binge-eating frequency - was determined after one month to influence the next (adaptive stage) in treatment delivery labelled as “stratification by response.” Rapid responders continued with BWL and were block-randomized to also receive, in double-blind fashion, either weight-loss medication or placebo (1:1 ratio). Non-rapid responders were all switched to start CBTgsh and were block-randomized to also receive, in double-blind fashion, either weight-loss medication or placebo (1:1 ratio). Block randomization at each of the randomization points was intended to achieve balance among treatment conditions. Block sizes, used to protect the blind, varied between 10 and 15 for the initial “Stepped-care randomization” and between 4 and 6 for the subsequent “Stratification by response” randomizations.
Treatment Blind and Independent Assessments
Randomization assignment was kept blinded from participants until they started treatment. Study medications were delivered in double-blind fashion. To ensure concealment of the double-blind randomization, study medications were provided in identical-appearing capsules (in matching “dosing”) prepared by the Yale Investigational Drug Service. Assessors performing the independent evaluations at post-treatment and follow up points were additionally kept blinded with respect to whether participants received Stepped-care (including whether BWL was switched to CBTgsh and whether any (double-blind) medication was received) or BWL (Standard) treatment. Participants were reminded before each assessment meeting to refrain from disclosing any details about which treatments they received during meetings with evaluators.
Treatments
Treatments were delivered by trained and monitored research-clinicians, all experienced with eating/weight disorders. We followed the approach by Wilson and colleagues (2010) and had masters-level clinicians deliver “generalist” BWL and doctoral-level clinicians deliver “specialist” CBT; we additionally had MD-level psychiatrist (P.M.M.) deliver pharmacotherapy with minimal clinical management. Research-clinicians received intensive training in the psychological treatments (by C.M.G.) and were then supervised weekly (BWL by M.A.W. and CBT by R.M.M.). In addition, clinicians were monitored using audiotapes of sessions throughout the study to prevent drift following methods used in previous RCTs of CBT and BWL for BED (Grilo et al., 2011). Session tapes were reviewed for adherence to the manualized treatments and included assessment of structure, process, and content elements used to augment supervision.
The design involved careful matching of total sessions and time to reduce some potential confounds due to “differences in attention” between the 6-month treatment conditions (described below). The BWL (Standard) condition comprised 16 sessions. The Stepped-care condition comprised a total of 15 sessions of either BWL or 15 sessions of BWL followed by CBTgsh with the added time for the pharmacotherapy (minimal clinical management) intended to result in roughly comparable overall total time as the 16-session BWL (Standard).
Behavioral Weight Loss (BWL).
BWL was delivered following the same protocols in both the BWL (Standard) and the BWL conditions within Stepped-care. BWL followed the protocol developed initially as the LEARN Program for Weight Management (Brownell, 2000) and since refined for RCTs testing BWL for BED (e.g., Devlin, et al., 2007; Grilo et al., 2012) and obesity (e.g., Foster et al., 2003; Wadden et al., 2011). BWL was delivered in individual sessions ranging 50–60 minutes following the manualized protocol and keyed to specific patient readings to reinforce the materials. Participants were provided with a copy of the patient self-care version of the LEARN program. BWL focuses on making gradual behavioral lifestyle changes including moderate caloric decreases and improved nutrition quality (goals of 1200–1500 kcal/day with less than 30% fat) and moderate increases in physical activity (goals of 30 minutes physical activity five times weekly). Behavioral strategies included goal setting, recording food intake and physical activity, and problem-solving to address challenges. The steps are presented in an additive fashion yet with some redundancy to facilitate learning and consolidation.
Cognitive-behavioral Therapy via Guided-Self-Help (CBTgsh).
CBTgsh was delivered following manualized protocols used previously (Grilo & Masheb, 2005; Grilo et al., 2005b; Wilson et al., 2010; see Wilson & Zandberg, 2012) in RCTs testing CBTgsh for BED. CBTgsh followed manualized sessions (11 individual sessions ranging 25–30 minutes) keyed to specific patient reading and monitoring materials. Participants were provided with a copy of Overcoming Binge Eating (Fairburn, 1995), the patient self-care step-by-step version of CBT. CBTgsh consists of six steps that address how to assess and change eating behaviors and associated cognitive features. The six steps are additive and designed to be followed sequentially, such that some progress on one step is required prior to moving on. Clinicians focus on (a) maintaining and enhancing motivation; (b) correcting any misunderstanding of the CBT information; (c) addressing difficulties with relevant behavioral experiments and cognitive-restructuring exercises in the manuals; and (d) reinforcing the necessity for self-monitoring and record keeping.
Medication (Weight-loss Medication or Placebo).
The SMART design was developed with the concept that “medication” would be examined, in a randomized placebo-controlled fashion, for whether it might enhance outcomes within the Stepped care condition. When the study was designed, there was no FDA-approved medication for BED and clinically it seemed that the most pressing need was to enhance weight loss outcomes. Of the FDA-approved weight-loss medications at the time, sibutramine was selected because it had demonstrated efficacy for both reducing binge-eating and weight in two RCTs with BED (Appolinario et al., 2003; Wilfley et al., 2008) and for enhancing BWL outcomes in obesity (Wadden et al., 2005). Sibutramine, a serotonin and norepinephrine reuptake inhibitor, was given using a 15 mg per day fixed-dose (Appolinario et al., 2003; Wilfley et al., 2008) throughout the last five months of Stepped-care.
In October 2010, when sibutramine was withdrawn from the market by the manufacturer, we continued with the study as designed by using the FDA-approved weight-loss medication orlistat which had received some empirical support for BED (Golay et al., 2005; Grilo, Masheb, & Salant, 2005) and for producing weight loss in obesity (Davidson et al., 1999). Orlistat, a lipase inhibitor, is a non-centrally-acting medication that produces a dose-dependent reduction in dietary-fat absorption, with a maximum 30% reduction accomplished with dosing of 120 mg three times daily. Orlistat dosing was 120mg three times daily used in previous RCTs (Davidson et al., 1999; Grilo, Masheb, & Salant, 2005).
The same randomization, double-blind, and minimal clinical management procedures were followed for both study medications. Randomization involved 50/50 assignment to medication or placebo, in matching capsules that were visually identical and dosing frequencies.
Statistical Analyses
Sample size was based on power calculations for primary aim (Stepped-care vs BWL (Standard)) and secondary aims (within Stepped-care) based on data from RCTS for BED testing BWL, CBTgsh, and sibutramine cited above, considering clinically-meaningful effect sizes, and performing sensitivity analyses for different outcomes. A target sample of N=175 allocated in a 4:1 ratio for Stepped-care versus BWL (Standard) yielded 80–85% power for medium effect sizes (Cohen’s d=0.57) for main outcomes at two-sided alpha level of 0.05.
Analyses designed to compare treatments were all “intent-to-treat” (ITT) and were performed for all randomized patients. Baseline characteristics (demographic, psychiatric, and clinical variables) for the treatment groups were compared using chi-square analyses for categorical variables and ANOVAs for continuous measures.
The two primary treatment outcome variables were binge eating and weight loss. Binge eating was analyzed using complementary approaches (remission and frequency) and weight loss was calculated as percent weight loss from baseline. “Remission” from binge eating was defined as zero binges (OBEs) during the previous 28 days (assessed by the EDE); for “remission,” any instances of treatment dropouts or missing data, pre-treatment baseline data were carried forward (i.e., failure imputed). Secondary treatment outcomes were eating-disorder psychopathology (global EDE), depression (BDI), and physical variables (waist circumference, total cholesterol).
For analyses of continuous measures, ITT approach was followed with all available data used in mixed models analyses without imputation. All treatment outcomes (except remission) were analyzed using mixed effects models (Gueorguieva & Krystal, 2004; Gueorguieva & Sanacora, 2006) with group (Stepped-care vs. BWL (Standard)) as a between-subject factor, time as a within-subject factor and the interaction between group and time. Treatment groups were compared on remission rates (yes/no) using chi-square tests at post-treatment.
To examine secondary goals, additional analyses examined whether the different treatments differed, whether the averages for the weight-loss medication conditions were superior to those for the averages for the placebo, and whether each medication condition differed from each respective control (i.e., BWL+Med vs. BWL+Pla, and CBTgsh+Med vs. CBTgsh+Pla). Lastly, because the weight-loss medication used changed during the course of the study, analyses compared subitramine, orlistat, and placebo within Stepped-care.
Variables not conforming to normality were log-transformed prior to analysis. The best-fitting variance-covariance structure (among unstructured, autoregressive, auto-regressive heterogeneous, compound symmetry, compound symmetry heterogeneous) was selected using Schwartz-Bayesian criterion. Significant main/interaction effects were explained by tests of simple effects or pairwise comparisons. Overall tests were considered significant at 0.05 level.
Results
Randomization, Stratification, and Participant Characteristics
Figure 2 shows the flow of participants throughout the study. Of the 191 randomized patients, N=152 received Stepped-care and N=39 received BWL (Standard). Within Stepped-care, N=142 were stratified by rapid response status following one month of BWL. Within Stepped-care, N=93 were categorized with rapid response (this represents 65% of the N=142 who were stratified and 61% of the overall N=152); for context, 28 (72%) of the N=39 BWL (Standard) would have been categorized with rapid response at month one (these proportions did not differ significantly with p-values >0.3). Of the 142, N=71 were randomized to placebo and N=71 to active medication (the first consecutive N=38 to sibutramine and the next consecutive N=33 to orlistat). Within Stepped-care, these assignments resulted in the following four treatment conditions: BWL+Placebo (N=46), BWL+Medication (N=47; of these, N=22 were with sibutramine and N=25 were with orlistat), CBTgsh+Placebo (N=25), and CBTgsh+Medication (N=24; of these, N=16 were with sibutramine and N=8 were with orlistat).
Treatment groups did not differ significantly in age, sex, race/ethnicity, education, psychiatric co-morbidity, or age of BED onset (Table 1) or on pretreatment levels of any outcome variables (Table 2). Stepped-care and BWL (Standard) treatment conditions did not differ significantly in the number of sessions attended (mean = 13.1, 13.3, respectively) nor did the number of sessions attended within Stepped-care groups (means range 12.2 to 14.7) differ significantly (all p-values > .11). Post-treatment assessments were obtained for 90% of all participants; assessment completion rates did not differ significantly for Stepped-care and BWL (Standard) (88%, 97%, respectively; Figure 2).
Table 1.
Demographic and Clinical Characteristics of 191 Randomized Patients and Separately for BWL (Standard) (N=39) and Stepped-care (N=152)
| Variable | OVERALL (N=191) |
BWL (Standard) (N=39) |
Stepped-care (N=152) |
Test statistic | p value |
|---|---|---|---|---|---|
| Age, mean (SD) | 48.4 (9.5) | 50.0 (9.2) | 48.0 (9.6) | F(1,189)=1.29 | .26 |
| Female, No (%) | 136 (71.2) | 32 (82.1) | 104 (68.4) | X2(1)=2.81 | .09 |
| Race/Ethnicity, No (%)1 | X2(1)=0.07 | .79 | |||
| White | 150 (78.5) | 30 (76.9) | 120 (78.9) | ||
| Black | 28 (14.7) | 5 (12.8) | 23 (15.1) | ||
| Hispanic | 8 (4.2) | 3 (7.7) | 5 (3.3) | ||
| Asian | 2 (1.0) | 1 (2.6) | 1 (0.7) | ||
| Other | 3 (1.6) | 0 (0) | 3 (2.0) | ||
| Education, No (%)1 | X2(1)=2.27 | .13 | |||
| College | 94 (49.2) | 15 (38.5) | 79 (52.0) | ||
| Some college | 66 (34.6) | 18 (46.2) | 48 (31.6) | ||
| High school | 29 (15.2) | 6 (15.4) | 23 (15.1) | ||
| Some high school | 2 (1.0) | 0 (0) | 2 (1.3) | ||
| DSM-IV co-morbidity lifetime, No (%) | |||||
| Mood disorders | 99 (51.8) | 22 (56.4) | 77 (50.7) | X2(1)=0.41 | .52 |
| Major depressive disorder | 93 (48.7) | 21(53.8) | 72 (47.4) | X2(1)=0.52 | .47 |
| Anxiety disorders | 73 (38.2) | 15 (38.5) | 58 (38.2) | X2(1)=0.001 | .97 |
| Substance use disorders | 58 (30.4) | 10 (25.6) | 48 (31.6) | X2(1)=0.52 | .47 |
| Age onset BED, mean (SD) | 28.7 (14.6) | 30.2 (14.5) | 28.3 (14.7) | F(1,189)=0.48 | .49 |
Note: Test statistic = chi-square for categorical variables and ANOVAs for dimensional variables.
P values are for two-tailed tests. BWL = behavioral weight loss; SD = standard deviation. No = number. BED = binge-eating disorder.
denotes that chi-square was performed for two collapsed categories given low frequencies of some variables (i.e., white versus non-white and college graduate versus less than college degree).
Table 2.
Clinical Variables for BWL (Standard) (N=39) and Stepped-care (N=152) at Pre-treatment and Post-treatment.
| Pre-treatment | Post-treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| BWL (Standard) | Stepped-care | BWL (Standard) | Stepped -care | |||||
| Variable | M | SD | M | SD | M | SD | M | SD |
| Binge-eating/month (EDE) | 17.8 | (12.9) | 20.2 | (15.1) | 1.7 | (2.3) | 2.7 | (8.1) |
| Global EDE Score | 2.6 | (0.9) | 2.7 | (0.9) | 1.8 | (0.9) | 1.8 | (0.9) |
| Depression (BDI) | 14.0 | (8.7) | 15.3 | (8.7) | 10.0 | (9.3) | 8.9 | (7.8) |
| Body mass index | 37.5 | (5.7) | 39.4 | (6.0) | 35.7 | (6.1) | 36.9 | (5.8) |
| Weight (kg) | 103.5 | (21.4) | 111.4 | (22.7) | 97.9 | (20.0) | 104.5 | (21.2) |
| Percent weight loss | -- | -- | -- | -- | 5.1 | (5.4) | 5.8 | (7.1) |
| Waist Circumference | 45.3 | (5.9) | 46.9 | (5.9) | 41.6 | (5.5) | 43.6 | (5.8) |
| Cholesterol | 192.6 | (30.2) | 201.5 | (36.4) | 187.8 | (32.6) | 187.0 | (34.8) |
Note: BWL = behavioral weight loss; M = mean; SD = standard deviation; EDE = Eating Disorder Examination interview; BDI = Beck Depression Inventory.
Binge-Eating Outcomes
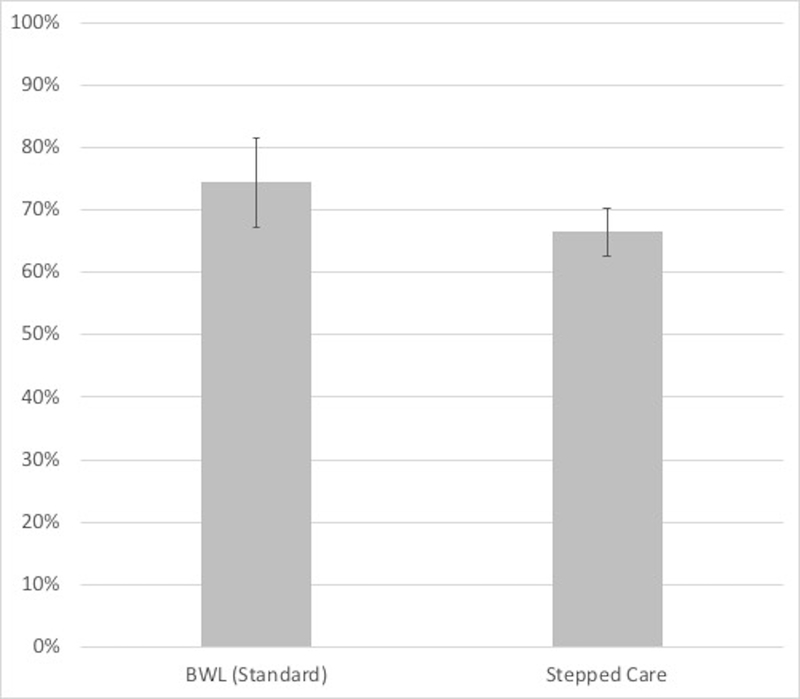
Figure 3 summarizes findings for binge-eating remission at post-treatment across the different treatment conditions. Binge-eating remission for Stepped-care (66.5%) and BWL (Standard) (74.4%) did not differ significantly (chi-square (1)=0.89, p=0.34). Analysis of remission rates within the Stepped-care conditions of the design (which ranged 40.0% – 83.3%) revealed significant differences (chi-square (4)=15.00, p=0.005). Overall, the medication groups had a significantly higher remission rate (80.3%) than the placebo groups (57.8%) (chi square (1)=8.43, p=0.004). Within rapid-responders continuing BWL, adding medication did not significantly improve remission rates relative to placebo (chi-square (1)=1.52, p=0.22) but within non-rapid responders to BWL switched to CBTgsh, adding medication was associated with significantly higher remission rates than adding placebo (chi-square (1)=9.69, p=0.002).
Figure 3. Binge-eating remission at post-treatment across treatment conditions.
Left panel shows overall remission rates for the BWL (Standard) and Stepped-care conditions (non-significant, p=0.34). Right panel shows overall remission rates for the BWL (Standard) condition and for the four arms within the Stepped-care condition; overall, medication superior to placebo (p=0.004), within rapid-responders continuing BWL, medication and placebo did not differ (p=0.22), within non-rapid responders switched to CBTgsh, medication superior to placebo (p=0.002).
Note: BWL = behavioral weight loss; CBTgsh = cognitive-behavioral therapy via guided self-help; Med = weight-loss medication
Mixed models analyses of binge-eating frequency (Table 2) revealed overall significant improvement from baseline to post-treatment across conditions (F(1,172)=556.75, p<.0001) but no significant differences between Stepped-care and BWL (Standard) (F(1,172)=0.86, p=0.36). Analyses of binge-eating frequency across different conditions of the design (Tables 2 and 3) revealed a significant treatment-by-time interaction (F(4,168)=3.62, p=0.01) and significant time (F(1,170)=794.22, p<.0001) and significant treatment (F(4,170)=4.12, p=0.003) main effects. Within Stepped-care, adding medication was associated with significantly greater reduction in binge-eating frequency than adding placebo (F(1,172)=7.00, p=0.01). Within rapid responders continuing BWL, adding medication was not significantly different from adding placebo (F(1,164)=0.13, p=0.72) but within non-rapid responder to BWL switched to CBTgsh, adding medication was associated with significantly greater reductions in binge-eating than adding placebo (F(1,175)=8.65, p=0.004). There were significant between group differences at post-treatment (F(4,157)=4.71, p=0.001). At post-treatment, within Stepped-care, the medication groups had on average lower binge-eating frequency than the placebo groups (F(1,157)=14.93, p=0.0002) and adding medication to CBTgsh was associated with lower binge-eating frequency than adding placebo (F(1,157)=16.53, p<.0001). Analysis of medication type (sibutramine or orlistat) vs placebo, there was a significant medication effect (F(2,131)=4.21, p=0.02) and a significant time effect (F(1,128)=708.26, p<.0001) but no significant interaction between medication and time (F(2,129)=1.72, p=0.18). Sibutramine (p<.01), but not orlistat (p=.08), had significantly better response than placebo; sibutramine and orlistat did not differ (p=0.44).
Table 3.
Clinical Variables Across the Four Treatments Within Stepped-care at Pre-treatment and Post-treatment.
| Pre-treatment | Post-treatment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BWL+Pla | BWL+Med | CBTgsh+Pla | CBTgsh+Med | BWL+Pla | BWL+Med | CBTgsh+Pla | CBTgsh+Med | |||||||||
| Variable | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD |
| Binge-eating/ month (EDE) | 23.7 | 19.4 | 19.7 | 11.9 | 18.6 | 14.0 | 14.8 | 10.4 | 1.7 | 3.7 | 1.0 | 2.5 | 6.2 | 9.6 | 0.2 | 0.4 |
| Global EDE Score | 2.8 | 0.8 | 2.7 | 0.9 | 2.9 | 0.8 | 2.5 | 0.9 | 1.8 | 0.9 | 1.7 | 0.6 | 1.8 | 1.0 | 1.6 | 0.9 |
| Depression (BDI) | 14.2 | 8.0 | 15.0 | 8.6 | 19.4 | 10.2 | 12.4 | 7.3 | 7.9 | 6.2 | 7.5 | 5.7 | 15.1 | 13.4 | 7.4 | 4.9 |
| Body mass index | 40.0 | 6.3 | 38.0 | 4.8 | 40.8 | 6.5 | 38.4 | 5.5 | 37.1 | 5.5 | 34.9 | 5.5 | 40.6 | 5.6 | 36.3 | 5.0 |
| Weight (kg) | 112.1 | 24.0 | 107.9 | 19.0 | 118.1 | 24.0 | 109.2 | 23.0 | 104.8 | 22.7 | 99.0 | 17.6 | 116.9 | 22.0 | 104.2 | 19.8 |
| Percent weight loss | -- | -- | -- | -- | -- | -- | -- | -- | 6.1 | 5.9 | 8.6 | 7.0 | 0.5 | 5.7 | 4.6 | 8.4 |
| Waist Circumference | 47.1 | 6.1 | 45.9 | 4.6 | 47.6 | 6.8 | 47.3 | 6.1 | 43.6 | 5.9 | 41.9 | 4.8 | 47.4 | 6.5 | 43.4 | 5.4 |
| Cholesterol | 203.7 | 37.4 | 202.5 | 33.3 | 199.4 | 33.4 | 200.0 | 45.3 | 194.4 | 32.2 | 183.4 | 33.9 | 187.9 | 37.9 | 181.8 | 37.4 |
Note: BWL = behavioral weight loss; CBTgsh = cognitive-behavioral therapy via guided self-help; Pla = placebo; Med = medication (weight-loss medication); M = mean; SD = standard deviation; EDE = Eating Disorder Examination interview; BDI = Beck Depression Inventory.
Within Stepped-care, stratification by rapid response status following one month of BWL resulted in the following four treatment conditions: BWL+Pla (N=46), BWL+Med (N=47; of these, N=22 were with sibutramine and N=25 were with orlistat), CBTgsh+Pla (N=25), and CBTgsh+Med (N=24; of these, N=16 were with sibutramine and N=8 were with orlistat).
Percent Weight Loss
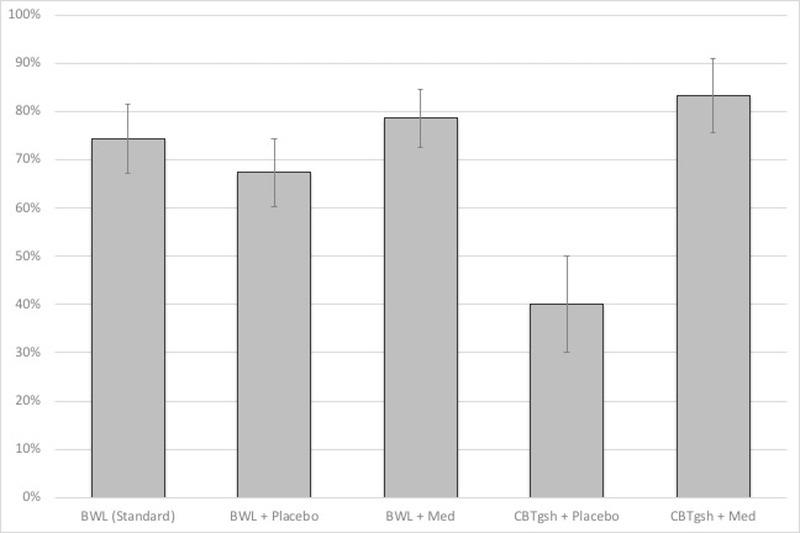
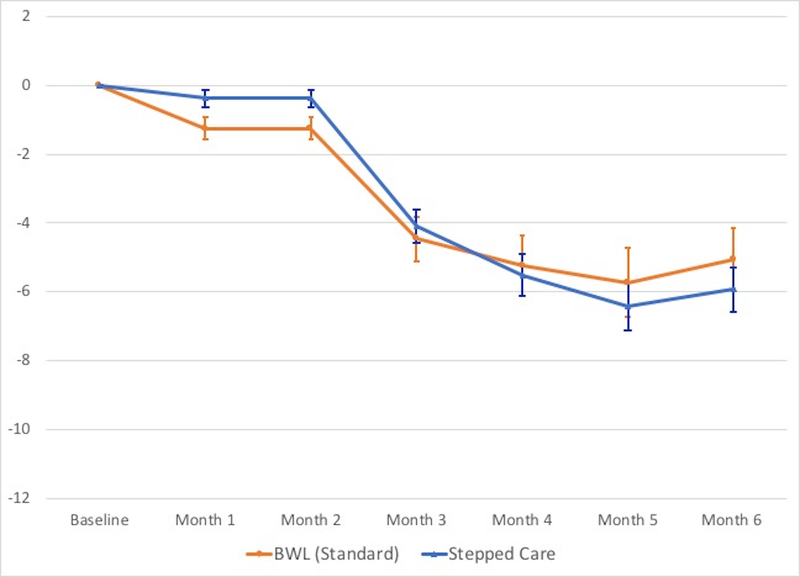
Figure 4 summarizes findings for percent weight loss throughout treatment across the different treatment conditions (see Tables 2 and 3 for weight (kg) and BMI values). Mixed models analyses revealed overall significant weight losses (F(5,139)=22.98, p<.0001) but no significant differences between Stepped-care (5.8% weight loss) and BWL (5.1% weight loss) (F(1,183)=0.05, p=0.83).
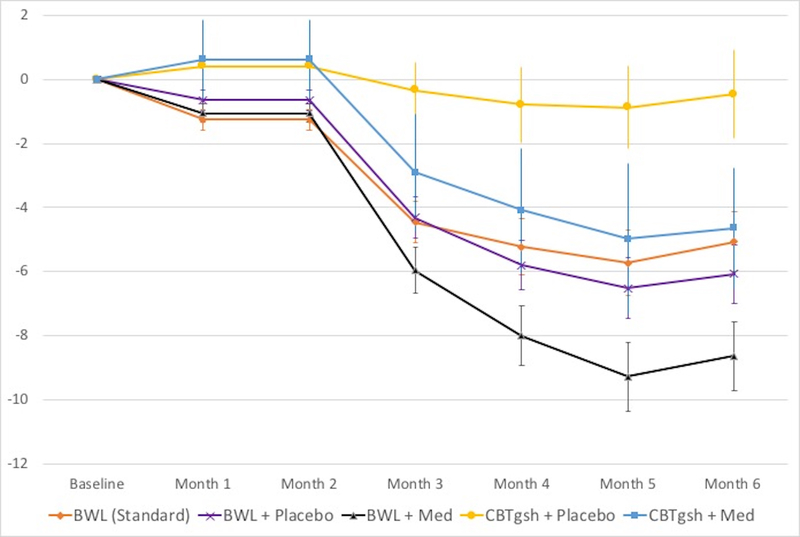
Figure 4. Percent weight loss throughout the course of the treatments.
Left panel shows overall percent weight loss for the BWL (Standard) and Stepped-care conditions (p-levels non-significant).Right panel shows overall percent weight loss for the BWL (Standard) condition and for the four arms within the Stepped-care condition; medication was associated with significantly greater percent weight loss than placebo throughout treatment (p<.04 at month 1, p<.002 at month 2, and p<.0001 at months 3, 4, and 5) and at post-treatment (p<.0001). Among rapid responders continuing BWL, medication was associated with significantly greater percent weight loss than placebo from month 2 onward (all p-values < 0.04) and among non-responders to BWL switched to CBTgsh, medication was significantly superior than placebo from month 3 onward (p<.03 at month 3, p<.01 at months 4 and 5, and p<.008 at post-treatment).
Note: BWL = behavioral weight loss; CBTgsh = cognitive-behavioral therapy via guided self-help; Med = weight-loss medication.
Analyses of percent weight loss throughout the course of the different conditions (Figure 4, Table 3) revealed significant treatment-by-time interaction (F(20,152)=2.51, p<0.0009), significant time (F(5,136)=36.12, p<.0001), and significant treatment (F(4,178)=7.37, p<.0001) main effects. Within Stepped-care, overall adding medication was associated with significantly greater percent weight loss than placebo observed throughout the course of treatment (p<.04 at month 1, p<.002 at month 2, and p<.0001 at month 3 onward) and at post-treatment (p<.0001). Within rapid responders continuing BWL, adding medication was associated with greater percent weight loss than placebo from month 2 onward (all p-values < 0.04) and within non-rapid responder to BWL switched to CBTgsh, adding medication was significantly superior than placebo from month 3 onward (p < .03 at month 3, p < .01 at months 4 and 5, and p < .008 at post-treatment). Analyses of medication type (sibutramine or orlistat) versus placebo revealed significant medication-by-time interaction (F(10,107)=3.47, p=0.0005), time effect (F(5,105)=41.71, p<.0001), and medication effect (F(2,139)=5.04, p<0.01). Sibutramine and placebo differed significantly from month 2 onward (p < .03 at month 2, p < .003 at month 3, p < .0005 at months 4 and 5 and at post-treatment).
Secondary Outcomes: Eating-Disorder Psychopathology, Depression, and Physical Measures
Tables 2 and 3 show descriptive analyses for secondary outcome continuous measures of eating-disorder psychopathology (EDE global score), depression (BDI), and physical variables (waist circumference, total cholesterol) across treatments. Mixed models revealed significant reductions in EDE global scores (F(1,171)=133.42, p<.0001), BDI scores (F(4,522)=11.83, p<.0001), waist circumference (F(1,162)=106.03, p<.0001), and in total cholesterol (F(1,168)=15.52, p=0.0001), but no significant differences between Stepped-care and BWL (Standard) in any of these outcomes (all p-values >0.05).
Discussion
This RCT, the largest single-site trial to date for BED, tested the effectiveness of adaptive “SMART” stepped-care treatment versus BWL (Standard) treatment. The adaptive arm, which began with BWL, a viable alternative to CBT (Grilo et al., 2011; Wilson et al., 2010), made use of whether participants had a rapid response or not. Previous research found that failure to respond quickly to BWL for BED predicted with poor binge-eating and weight-loss outcomes (Grilo et al., 2012; Masheb & Grilo, 2007). Thus, this adaptive trial tested a “common-sense” clinical strategy (if treatment is working, then continue and try to enhance it, but if treatment is not working try a different (evidence-supported) treatment plus try to enhance that using a sequential multiple allocation randomized trial (SMART) approach. Within the adaptive stepped-care arm, the SMART design examined, using a randomized double-blind approach, the potential utility of weight-loss medications that had previously also received some support for BED (Grilo et al., 2005; Wilfley et al., 2008) in an attempt to enhance outcomes among both rapid and non-rapid responders. In the case of rapid responders to initial BWL, the BWL was continued and in the case of non-rapid responders, participants were switched to a “specialist” CBTgsh as advocated in traditional Stepped-care approaches (Wilson & Zandberg, 2012).
Stepped-care and BWL (Standard) were associated with robust improvements in binge-eating and in associated secondary measures of eating-disorder psychopathology and depression that did not differ significantly from each other. Remission rates (i.e., abstinence from binge eating) for Stepped-care and BWL (Standard) were 66.5% and 74.4%, respectively, exceeding highest rates previously reported for both BWL and for specialist therapies (Wilson et al., 2010), but did not differ significantly from each other. Both conditions had significant reductions in frequency of binge eating throughout the course of treatment and at post-treatment, but the reductions did not differ significantly from each other. Additionally, both Stepped-care and BWL (Standard) treatments produced significant reductions in weight-loss (over 5% mean BMI losses) but these did not differ significantly between treatments. Within Stepped-care, relative to placebo, weight-loss medication enhanced the primary outcomes of both binge eating and weight loss. Because of the design and non-randomized allocation of patients to continuing BWL or switching to CBTgsh, we could not specifically compare them to each other.
Although this study did not find support for the specific adaptive Stepped-care condition relative to BWL (Standard) treatment, the findings suggest the potential utility of using certain weight-loss medications to enhance outcomes in BED. Within Stepped-care, adding weight-loss medication resulted in significantly greater reductions than adding placebo for reducing both binge eating (i.e., higher remission rates and lower frequency) and weight. For example, overall, the addition of medication was associated with an 80% remission rate versus 58% for the addition of placebo. Whereas the addition of medication did not significantly enhance outcomes further among the initial responders to BWL, significant benefits were observed for the non-responders in terms of better binge-eating and weight-loss outcomes. The overall benefits of the weight-loss medication condition for enhancing both binge-eating and weight-loss outcomes were due to the significant effects of sibutramine, not orlistat. These findings echo the greater effects reported for sibutramine (Wilfley et al., 2008) than orlistat (Grilo et al., 2005) for BED.
The observed weight-loss outcomes are encouraging given the well-known challenge in achieving weight loss in this specific patient group of persons with BED and co-existing obesity (Blaine & Rodman, 2007). Among initial responders to BWL, the addition of weight-loss medication was associated with a mean 8.8% weight loss, whereas among initial non-responders to BWL switched to CBTgsh (which is well-known to not produce any weight loss; Grilo & Masheb, 2005; Wilson et al., 2010), the addition of weight-loss medication was associated with a mean 4.8% weight loss. The overall weight-loss outcomes for both Stepped-care and BWL (Standard) conditions exceeded findings reported by Grilo and colleagues (2011) for BWL and approximated findings for BWL (Wilson et al., 2010) and sibutramine (Wilfley et al., 2008) for BED; the weight losses, however, were less than those reported by Wadden and colleagues (2005) in their study of BWL and sibutramine, alone and in combination, for patients with obesity without BED. Finding ways to enhance weight losses further in this specific patient group remains a research need although the present findings challenge concerns that weight loss is contraindicated for persons with BED.
Several strengths and potential limitations are noted as context for interpreting the findings for clinical practice and for informing future adaptive SMART designs. The manualized therapy protocols (BWL and CBTgsh) and pharmacotherapy were delivered by highly trained and monitored doctoral research-clinicians and psychiatrists. Generalizability of the robust improvements to other clinical settings and practitioners is therefore uncertain. Research has, for example, documented relatively low rates of naturalist treatment utilization by persons with BED (Coffino et al., in press) and studies of the delivery of treatments in the community suggest that they likely depart considerably from the manualized treatments delivered in this and other treatment studies (Shafran et al., 2009). This was a major motivation for the selection of BWL, a “generalist” treatment considered to be widely available Wilson et al., 2010) and across clinical settings (Wadden et al., 2005). CBTgsh was used as the second-line approach for initial non-responders as advocated by some models (see Wilson & Zandberg, 2012); CBT, however, is unlikely to produce any weight loss (Grilo et al., 2011) though it might stabilize or prevent further weight gain (Blomquist, Barnes, White, Masheb, Morgan, & Grilo, 2011). Future adaptive designs might consider integrating more intensive forms of CBT to address continued binge eating although adjunctive interventions would be needed to address excess weight. Importantly, when this study was designed, there were few relevant pharmacotherapy options. One of the weight-loss medications tested was sibutramine, which was subsequently withdrawn from the market. The findings, however, have heuristic value for planning adaptive SMART designs. Presently, there are several weight-loss medications that might be relevant given their putative mechanisms of action, such as the combination of naltrexone and bupropion, in addition to their weight-loss effects (__Greenway et al., 2010). Additionally, there now exists a FDA-approved medication for BED (lisdexamfetamine) (McElroy et al., 2016) that has yet to be tested in combination with behavioral or psychological methods. Additionally, the findings pertain only for acute outcomes (i.e., following 6-months of treatment) and longer-term follow-up analyses will address questions regarding durability or maintenance of effects and relapse. In one of the very few follow-up studies of any medication for BED, weight regain quickly followed discontinuation of sibutramine (Grilo, Masheb, White, et al., 2015). Each of these issues merit consideration in the design of longer-term adaptive SMART designs for BED.
Public Health Significance:
This study shows that behavioral weight loss treatment can provide substantial benefits to patients with binge-eating disorder who also have excess weight. Behavioral weight loss treatment resulted in 74.4% of patients achieving abstinence from binge eating and an average of 5.1% weight loss. This study also shows that weight-loss medications have potential utility for enhancing outcomes particularly amongst those patients who do not respond initially to behavioral weight loss treatment.
Acknowledgments
This research was supported by National Institutes of Health grant R01 DK49587. Dr. Grilo was also supported by NIH grant K24 DK070052.
Biography






Footnotes
Dr. Grilo reports no conflicts of interest but wishes to report several broader interests which did not influence this research or paper. Dr. Grilo’s broader interests include: Consultant to Sunovion and Weight Watchers International; Honoraria for lectures, CME activities, and presentations at scientific conferences; and Royalties from Guilford Press and Taylor & Francis Publishers for academic books.
Contributor Information
Carlos M. Grilo, Department of Psychiatry, Yale University School of Medicine
Marney A. White, Department of Biostatistics, Yale University School of Public Health
Robin M. Masheb, Department of Psychiatry, Yale University School of Medicine
Valentina Ivezaj, Department of Psychiatry, Yale University School of Medicine.
Peter Morgan, Department of Psychiatry, Yale University School of Medicine.
Ralitza Gueorguieva, Department of Biostatistics, Yale University School of Public Health.
References
- Agras WS, Telch C, Arnow B, Eldredge K, Detzer M, Henderson J, Marnell M (1995). Does interpersonal therapy help patients with binge eating disorder who fail to respond to cognitive-behavioral therapy? Journal of Consulting & ClinicalPsychology, 63, 356–360. [DOI] [PubMed] [Google Scholar]
- Agras WS, Telch CF., Arnow B, Eldredge K, Wilfley DE Raeburn SD, et al. (1994) Weight loss, cognitive-behavioral, and disipramine treatments in binge eating disorder: An additive design. Behavior Therapy, 25, 225–238. [Google Scholar]
- Almirall D, Nahum-Shani I, Sherwood NE, & Murphy SA (2014). Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Translational Behavioral Medicine, 4, 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders, Fifth edition (DSM-5). Washington, DC: American Psychiatric Association. [Google Scholar]
- Appolinario JC, Bacaltchuk J, Sichieri R, Claudino AM, Godoy-Matos A, Morgan C, Zanella MT, & Coutinho W (2003). A randomized, double-blind, placebo-controlled study of sibutramine in the treatment of binge-eating disorder. Archives of General Psychiatry, 60, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Beck AT, & Steer R (1987). Manual for revised Beck Depression Inventory. New York: Psychological Corporation. [Google Scholar]
- Beck AT, Steer R, & Garbin M (1998). Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clinical Psychology Review, 8, 77–100. [Google Scholar]
- Berg KC, Peterson CB, Frazier P, & Crow SJ (2012). Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: a systematic review of the literature. International Journal of Eating Disorders, 45, 428–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine B, & Rodman J (2007). Responses to weight loss treatment among obese individuals with and without BED: matched-study meta-analysis. Eating Weight Disorders,12,54–60. [DOI] [PubMed] [Google Scholar]
- Blomquist KK, Barnes RD, White MA, Masheb RM, Morgan PT, & Grilo CM (2011). Exploring weight gain in year before treatment for binge eating disorder: a different context for interpreting limited weight losses in treatment studies. International Journal of Eating Disorders, 44, 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD (2000). LEARN program for weight management. Dallas: American Health Pub. [Google Scholar]
- Chen EY, Cacioppo J, Fettich K, Gallop R, McClosky MS, Olino T, & Zeffino TA (2017). An adaptive randomized trial of dialectical behavior therapy and cognitive behavior therapy for binge-eating. Psychological Medicine, 47, 703–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino JA, Udo T, & Grilo CM (in press). Rates of help-seeking in U.S. adults with lifetime DSM-5 eating disorders: Prevalence across diagnoses and sex and ethnic/racial differences. Mayo Clinic Proceedings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, et al. (1999). Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. Journal of the American Medical Association, 281, 235–242. [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Goldfein JA, Petkova E, Jiang H, Raizman PS, Wolk S, Mayer L, Carino J, Bellace D, Kamenetz C, Dobrow I, & Walsh BT (2005). Cognitive behavioral therapy and fluoxetine as adjuncts to group behavioral therapy for binge eating disorder. Obesity Research, 13, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, O’Connor M (2008). The Eating Disorder Examination (16.0D) In Fairburn CG. Cognitive Behavior Therapy and Eating Disorders. NY: Guilford Press. [Google Scholar]
- Fairburn CG, Marcus MD, & Wilson GT (1993). Cognitive-behavioral therapy for binge eating and bulimia nervosa: comprehensive treatment manual In Fairburn CG & Wilson GT (eds.), Binge eating: nature, assessment, and treatment. NY: Guilford Press. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (1996). Structured clinical interview for DSM-IV Axis I disorders-patient Edition (SCID-I/P, Ver 2.0). NY: NYSPI. [Google Scholar]
- Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, & Klein S (2003). A randomized trial of a low-carbohydrate diet for obesity. New England Journal of Medicine, 348, 2082–2090. [DOI] [PubMed] [Google Scholar]
- Golay A, Laurent-Jaccard A, Habicht F, et al. (2005). Effect of orlistat in obese patients with binge eating disorder. Obesity Research, 13, 1077–1088. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E; COR-I Study Group. (2010). Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multi-centre randomized double-blind placebo-controlled phase 3 trial. Lancet, 376, 595–605. [DOI] [PubMed] [Google Scholar]
- Grilo CM (2017). Psychological and behavioral treatments for binge-eating disorder. Journal of Clinical Psychiatry, 78(S1), 20–24. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Hrabosky JI, White MA, Allison KC, Stunkard AJ, & Masheb RM (2008). Overvaluation of shape and weight in binge-eating disorder and overweight controls: refinement of BED diagnostic construct. Journal of Abnormal Psychology, 117, 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, & Masheb RM (2005). A randomized controlled comparison of guided self-help cognitive behavioral therapy and behavioral weight loss for binge eating disorder. Behaviour Research and Therapy, 43, 1509–1525. [DOI] [PubMed] [Google Scholar]
- Grilo CM, & Masheb RM (2007). Rapid response predicts binge eating and weight loss in binge eating disorder: findings from a controlled trial of orlistat with guided self-help cognitive behavioral therapy. Behaviour Research and Therapy, 45, 2537–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Lozano-Blanco C, & Barry DT (2004). Reliability of the Eating Disorder Examination in patients with binge eating disorder. International Journal of Eating Disorders, 35, 80–85. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, & Salant SL (2005). Cognitive behavioral therapy guided self-help and orlistat for the treatment of binge eating disorder: a randomized, double-blind, placebo-controlled trial. Biological Psychiatry, 57, 1193–1201. [DOI] [PubMed] [Google Scholar]
- Grilo CM Masheb R., White MA, Gueorguieva R, Barnes R, Walsh BT, McKenzie K, Genao I, & Garcia R (2014). Treatment of binge eating disorder in racially and ethnically diverse obese patients in primary care: randomized placebo-controlled clinical trial of self-help and medication. Behaviour Research and Therapy, 58, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, & Wilson GT (2001). A comparison of different methods for assessing the features of eating disorders in patients with binge eating disorder. Journal of Consulting and Clinical Psychology, 69, 317–322. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, & Wilson GT (2006). Rapid response to treatment for binge eating disorder. Journal of Consulting and Clinical Psychology, 74, 602–613. [DOI] [PubMed] [Google Scholar]
- Grilo CM, Masheb RM, Wilson GT, Gueorguieva R, & White MA (2011). Cognitive-behavioral therapy, behavioral weight loss, and sequential treatment for obese patients with binge-eating disorder: a randomized controlled trial. Journal of Consulting and Clinical Psychology, 79, 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, Reas DL, & Mitchell JE (2016). Combining pharmacological and psychological treatments for binge eating disorder: current status, limitations, and future directions. Current Psychiatry Reports, 18(6), 55. [DOI] [PubMed] [Google Scholar]
- Grilo CM, White MA, Masheb RM, & Gueorguieva R (2015). Predicting meaningful outcomes to medications and self-help treatments for binge-eating disorder in primary care: The significance of early rapid response. Journal of Consulting and Clinical Psychology, 83, 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo CM, White MA, Wilson GT, Gueorguieva R, & Masheb RM (2012). Rapid response predicts 12-month post-treatment outcomes in binge eating disorder: theoretical and clinical implications. Psychological Medicine, 42, 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueorguieva RV, & Krystal J (2004). Move over ANOVA: Progress in analyzing repeated measures data and its reflection in papers published in the Archives of General Psychiatry. Archives of General Psychiatry, 61, 310–317. [DOI] [PubMed] [Google Scholar]
- Gueorguieva RV, & Sanacora G (2006). Joint analysis of repeatedly observed continuous and ordinal measures of disease severity. Statistics in Medicine, 25, 1307–1322. [DOI] [PubMed] [Google Scholar]
- Hart LM, Granillo MT, Jorm AF, & Paxton SJ (2011). Unmet need for treatment in the eating disorders: a systematic review of eating disorder specific treatment seeking among community cases. Clinical Psychology Review, 31, 727–735. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Petroff D, Herpertz S, Pietrowsky R, Tuschen-Caffier B, Vocks S, & Schmidt R (2019). Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. Journal of Consulting and Clinical Psychology, 87, 91–105. [DOI] [PubMed] [Google Scholar]
- Kazdin AE, & Blase SL (2011). Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspectives on Psychological Science, 6, 21–37. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, Deitz AC, Hudson JI, Shahly V, . . . Xavier M (2013). The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biological Psychiatry, 73(9), 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC (2003). “Rules” of evidence in assessing the efficacy and effectiveness of treatments. Developmental Neuropsychology, 24, 705–718. [DOI] [PubMed] [Google Scholar]
- Lavori PW, & Dawson R (2008). Adaptive treatment strategies in chronic disease. Annual Review Medicine, 59, 443p–453p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardon J, Brennan L, & de la Piedad Garcia X (2016). Rapid response to eating disorder treatment: a systematic review and meta-analysis. International Journal of Eating Disorders, 49, 905–919. [DOI] [PubMed] [Google Scholar]
- Masheb RM & Grilo CM (2007). Rapid response predicts treatment outcomes in binge-eating disorder: implications for stepped care. Journal of Consulting and Clinical Psychology, 75, 39–644. [DOI] [PubMed] [Google Scholar]
- McElroy SL (2017). Pharmacologic treatments for binge-eating disorder. Journal of Clinical Psychiatry, 78 Suppl 1, 14–19. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson J, Ferreira-Cornwell M, Radewonuk J, Whitaker T, & Gasior M (2016). Lisdexamfetamine dimesylate for adults with moderate to severe binge-eating disorder: Results of two pivotal phase 3 randomized controlled trials. Neuropsychopharmacology, 41, 1251–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Agras S, Crow S, Halmi K, Fairburn CG, Bryson S, & Kraemer H (2011). Step care and cognitive-behavioural therapy for bulimia nervosa: randomized trial. British Journal of Psychiatry, 198, 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Qian M, Almirall D, Pelham WE, Gnagy B, Fabiano GA, Waxmonsky JG, & Yu J (2012). Experimental design and primary data analysis methods for comparing adaptive interventions. Psychological Methods, 17, 457–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI (2000). Practical guide: identification, evaluation, and treatment of overweight and obesity in adults. NIH Publication Number 00–4084. [Google Scholar]
- Peterson CB, Mitchell JE, Crow SJ, Crosby RD, & Wonderlich SA (2009). The efficacy of self-help group treatment and therapist-led group treatment for binge eating disorder. American Journal of Psychiatry, 166, 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ (1994). Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. American Journal of Cardiology, 73, 460–468. [DOI] [PubMed] [Google Scholar]
- Reas DL, & Grilo CM (2015). Pharmacological treatment of binge eating disorder: update review and synthesis. Expert Opinion in Pharmacotherapy, 16, 1463–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafran R, Clark DM, Fairburn CG, et al. (2009). Mind the gap: improving the dissemination and implementation of CBT. Behaviour Research and Therapy, 47, 902–909. [DOI] [PubMed] [Google Scholar]
- Udo T, & Grilo CM (2018). Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of US adults. Biological Psychiatry, 84, 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udo T, & Grilo CM (2019). Psychiatric and medical correlates of DSM-5 eating disorders in a nationally representative sample of adults in the United States. International Journal of Eating Disorders, 52, 42–50. [DOI] [PubMed] [Google Scholar]
- Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, Hesson LA, Osei SY, Kaplan R, & Stunkard AJ (2005). Randomized trial of lifestyle modification and pharmacotherapy for obesity. New England Journal of Medicine, 353, 2111–2120. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Crow SJ, Hudson JI, Mitchell JE, Berkowitz RI, Blakesley V, Walsh BT, & Sibutramine Binge Eating Disorder Research Group. (2008). Efficacy of sibutramine for the treatment of binge eating disorder: A randomized multi-center placebo-controlled double-blind study. American Journal of Psychiatry, 165, 51–58. [DOI] [PubMed] [Google Scholar]
- Wilson GT, Grilo CM, & Vitousek KM (2007). Psychological treatments of eating disorders. American Psychologist, 62, 199–216. [DOI] [PubMed] [Google Scholar]
- Wilson GT, & Zandberg LJ (2012). Cognitive-behavioral guided self-help for eating disorders: effectiveness and scalability. Clinical Psychology Review, 32, 343–357. [DOI] [PubMed] [Google Scholar]