Abstract
During adaptation to an increase in environmental luminance, retinal signaling adjustments are mediated by the neuromodulator dopamine. Retinal dopamine is released with light and can affect center-surround receptive fields, the coupling state between neurons, and inhibitory pathways through inhibitory receptors and neurotransmitter release. While the inhibitory receptive field surround of bipolar cells becomes narrower and weaker during light adaptation, it is unknown how dopamine affects bipolar cell surrounds. If dopamine and light have similar effects, it would suggest that dopamine could be a mechanism for light-adapted changes. We tested the hypothesis that dopamine D1 receptor activation is sufficient to elicit the magnitude of light-adapted reductions in inhibitory bipolar cell surrounds. Surrounds were measured from OFF bipolar cells in dark-adapted mouse retinas while stimulating D1 receptors, which are located on bipolar, horizontal, and inhibitory amacrine cells. The D1 agonist SKF-38393 narrowed and weakened OFF bipolar cell inhibitory receptive fields, but not to the same extent as with light adaptation. However, the receptive field surround reductions differed between the glycinergic and GABAergic components of the receptive field. GABAergic inhibitory strength was reduced only at the edges of the surround while glycinergic inhibitory strength was reduced across the whole receptive field. These results expand the role of retinal dopamine to include modulation of bipolar cell receptive field surrounds. Additionally, our results suggest that D1 receptor pathways may be a mechanism for the light-adapted weakening of glycinergic surround inputs and the largest wide-field GABAergic inputs to bipolar cells. However, remaining differences between light-adapted and D1 receptor-activated inhibition demonstrate that non-D1 receptor mechanisms are necessary to elicit the full effect of light adaptation on inhibitory surrounds.
Keywords: bipolar cell, GABA, glycine, amacrine cell, luminance
Introduction
To enable the visual system to respond in a wide range of light intensities the retina adapts to background luminance at many stages including at the photoreceptors (Tamura et al., 1991; Woodruff et al., 2008), the horizontal cells (Xin & Bloomfield, 1999b), the excitatory bipolar and inhibitory amacrine cells (Green et al., 1975; Naka et al., 1979; Green & Powers, 1982; Shapley & Enroth-Cugell, 1984; Page-McCaw et al., 2004; Dunn et al., 2006; Dunn et al., 2007; Mazade & Eggers, 2016), and the ganglion cells. As luminance increases, ganglion cell receptive field responses, which are modulated by inhibitory input through horizontal cells and inner retinal amacrine cells (Dedek et al., 2008; Stroh et al., 2018), increase signal sensitivity and strength (Barlow et al., 1957; Merwine et al., 1995; Troy et al., 1999; Dedek et al., 2008; Farrow et al., 2013; Borghuis et al., 2018). Ganglion cell response changes with light adaptation could in part be a consequence of changes in pre-synaptic bipolar cell inhibitory receptive field surround strength and extent, which are mediated largely by amacrine cells. Bipolar cell receptive field surrounds are reduced during light adaptation, which could lead to stronger ganglion cell input from small stimuli and may play a role in increased retinal acuity (Mazade & Eggers, 2016). It is unclear what may be mediating these receptive field changes however the neuromodulator dopamine is compelling as a major candidate.
Dopamine is released from specialized amacrine cells (Bauer et al., 1980; Godley & Wurtman, 1988; Witkovsky, 2004) and has been shown to be important for visual processing and light adaptation by affecting many retinal circuits (Godley & Wurtman, 1988; Boatright et al., 1989; Doyle et al., 2002). Mice lacking dopamine are impaired in many functions associated with light adaptation including reduced light responses, contrast sensitivity, circadian rhythms, and visual acuity thresholds (Jackson et al., 2012). Since dopamine dysfunction reduces visual properties that rely on excitatory-inhibitory interactions, and inhibitory pathways shape center-surround structure (Flores-Herr et al., 2001; Russell & Werblin, 2010; Buldyrev & Taylor, 2013; Protti et al., 2014), dopaminergic pathways likely play a prominent role in the weakening of inner retinal receptive field surrounds with light adaptation.
Dopamine receptors are located on many retinal neurons in all layers (Veruki & Wassle, 1996; Nguyen-Legros et al., 1997; Farshi et al., 2016). Dopamine signaling directly shapes ganglion cell receptive fields (Jensen & Daw, 1984; Maguire & Smith, 1985; Jensen & Daw, 1986; Jensen, 1989, 1991, 1992; Maguire & Hamasaki, 1994) and heavily regulates amacrine cell coupling (Hampson et al., 1992; Xia & Mills, 2004; Kothmann et al., 2009). Dopamine regulation of cell coupling is similar to the regulation by luminance and likely one mechanism for receptive field changes with light adaptation (Bloomfield et al., 1997). Additionally, dopamine D1 receptors modulate GABAA (Feigenspan & Bormann, 1994a) and GABAC (Feigenspan & Bormann, 1994b) receptor currents as well as GABAergic and glycinergic amacrine cell release (Pycock & Smith, 1983; Kato et al., 1985; O’Brien & Dowling, 1985; Calaza et al., 2001; Mazade et al., 2019b). A recent study demonstrated that D1 receptor activation decreased local inhibition onto mouse bipolar cells to the same extent as light adaptation (Mazade et al., 2019b). However, the similarity between dopamine and luminance regulation of inner retinal receptive fields is unknown.
We hypothesized that activation of D1 receptors would be sufficient to elicit light-adapted inhibitory changes in bipolar cell receptive field surrounds. We focused on OFF bipolar cells, which respond to the offset of light, because they receive inhibition from both dark- and light-adapted pathways and show robust changes in receptive field surrounds with light (Mazade & Eggers, 2013, 2016; Mazade et al., 2019b). OFF bipolar cell surrounds are composed of glycinergic and GABAergic sources (Eggers et al., 2007), that may be differentially modulated by dopamine (Veruki & Wassle, 1996; Nguyen-Legros et al., 1997; Farshi et al., 2016). Our previous results suggested that D1 receptors are primarily modulating glycinergic, and not GABAergic, local amacrine cell inputs to OFF bipolar cells (Mazade et al., 2019b). Here, we show that this is mostly replicated across the whole OFF bipolar cell receptive field surround. These results expand the role of dopamine to likely modulating light-adapted changes in bipolar cell receptive fields but indicate non-D1 receptor pathways are also required for adaptation.
Materials and Methods
Preparation of mouse retinal slices
All animal protocols for this study were approved by The University of Arizona Institutional Animal Care and Use Committee (IACUC). As described previously (Eggers & Lukasiewicz, 2006; Eggers et al., 2013; Mazade & Eggers, 2013), male mice (n = 27), fed ad libitum, (C57BL/6J strain, Jackson Laboratories, Bar Harbor, ME, USA) 35–60 days of age were euthanized using carbon dioxide and their eyes enucleated. The retina was removed and a large rectangle of the central retina was cropped from the periphery. A nitrocellulose membrane filter paper (0.45 μm pore size, Millipore, Ireland) was placed on the retina and an average of six 250 μm slices were cut with a hand chopper, rotated 90 degrees, and mounted onto glass cover slips using vacuum grease. Cells used from these slices were selected using previous criteria (Mazade & Eggers, 2016). The tissue was maintained in oxygenated extracellular solution at room temperature. All dissection and storage procedures were performed under infrared illumination to preserve retinal light sensitivity.
Solutions and drugs
The extracellular recording solution used for dissection and for measuring light-evoked currents contained (in mM) 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 20 glucose, and 26 NaHCO3. For voltage clamp recordings, the intracellular solution contained (in mM) 120 CsOH, 120 gluconic acid, 1 MgCl2, 10 HEPES, 10 TEA-Cl, 10 phosphocreatine-Na2, 4 Mg-ATP, 0.5 Na-GTP, 10 EGTA and 50 μM Alexa Fluor 488 (Invitrogen, Carlsbad, California, USA) and was adjusted to pH 7.2 with CsOH. Extracellular solutions were bubbled with 95% O2–5% CO2. To isolate the inhibitory receptor inputs, SR-95531 (SR, 20 μM) to block GABAA receptors, (1,2,5,6-tetrahydropyridine-4yl) methyphosphinic acid (TPMPA, 50 μM) to block GABAC receptors, and strychnine (1 μM,) to block glycine receptors were used (Mazade & Eggers, 2013, 2016). To test dopamine D1 receptors, the D1 receptor agonist SKF-38393 (SKF, 20 μM, Tocris) was used with a concentration similar to previous studies (Ichinose & Lukasiewicz, 2007; Liu et al., 2016; Mazade et al., 2019b). All drug solutions were applied to the slice for five minutes before recordings began using a gravity-driven superfusion system (Cell Microcontrols, Norfolk, VA), with continuous perfusion throughout the experiment (~1–2 mL/minute). Unless otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Whole cell recordings
Light-evoked inhibitory post synaptic currents (L-IPSCs) were recorded from OFF bipolar cells in retinal slices using whole cell patch-clamp sampled at 10 kHz and voltage clamped to 0 mV, the reversal potential of nonselective cation channels. Liquid junction potentials (20 mV) were calculated with Clampex software (Molecular Devices, Sunnyvale, California, USA) and corrected for at the start of the recording. Electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) on a P97 Flaming/Brown puller (Sutter Instruments, Novato, California, USA) and had resistances of 5–7 MΩ. Mice were dark-adapted overnight, and all recordings were performed in the dark under infrared illumination to preserve retinal light sensitivity. Recording extracellular solution was heated to 32°C using thin stage and inline heaters (Cell Microcontrols, Norfolk, VA). Responses were filtered at 6 kHz with the four-pole Bessel filter on a Multi-clamp 700B patch-clamp amplifier (Molecular Devices, Sunnyvale, California, USA) and digitized with a Digidata 1140 data acquisition system (Molecular Devices, Sunnyvale, California, USA).
Morphological identification of cells
The intracellular solution contained Alexa 488 to label OFF bipolar cells. Cells were classified as OFF bipolar cells using axonal morphology, inner plexiform layer terminal stratification, and the soma position in the inner nuclear layer (Ghosh et al., 2004). Cells were imaged with a Nikon Digital Sight camera with Elements software using a Nikon Intensilight C-HGFIE Fluorescent lamp (Nikon Instruments, Tokyo, Japan). Axon terminal morphology and response properties (Mazade & Eggers, 2013) were used to identify OFF bipolar cells. A total of 29 bipolar cells were included in this study (12 OFF type 1/2/4 and 17 OFF type 3). In previous studies we found no difference between subtype spatial inhibitory response profiles or center receptive field responses when comparing between OFF types 1,2 and 4 and OFF type 3 (Mazade & Eggers, 2016; Mazade et al., 2019b). Therefore, we combined all OFF bipolar cell subtypes for averaging their spatial response profiles in each condition.
Visual stimulus
Bar stimuli (25 μm wide, flashed every 100 μm) were presented using a white organic light-emitting diode (OLED Microdisplay, eMagin EMA-100503 SXGA Monochrome White XL, Bellevue, WA) projected through the camera port of the microscope, which elicited strong, stable, and reproducible responses (Mazade & Eggers, 2016). The stimulus was presented through the 4x objective and the stimulus field was limited to the size of the OLED screen that was 3 mm long x 2.1 mm tall. The length of the retinal slice varied but was always < 3 mm long (average of 1.5 mm long). The OLED screen (e.g. stimulus field) was always centered over the recorded cell so that the bar stimulated the retina up to 600 μm to either side of the cell and the bar extended well over the height of the retinal slice (~0.25 mm). Therefore, the stimulus presentation fully activated retinal neurons at each stimulus location. Recordings were made in the central retinal region of mixed green/UV cone opsins (Applebury et al., 2000; Haverkamp et al., 2005). The stimulus was presented and controlled with custom MATLAB (Mathworks, Natick, MA, 1984) code with Psychtoolbox-3 (Brainard, 1997) extensions by controlling the intensity (7.83 × 104 photons/μm2/sec), size, location, and duration of the stimulus. The background luminance used to light adapt the retina was 1150 photons/μm2/sec (Mazade & Eggers, 2016). A one-second stimulus was used to elicit a robust response and determine the type of inhibition for identification (Mazade & Eggers, 2013) with an inter-stimulus interval of 29 seconds.
Data analysis and statistics
Clampfit software (Molecular Devices, Sunnyvale, California, USA) was used to measure the peak amplitude and charge transfer of L-IPSC traces averaged from at least two stimulus repeats. The peak amplitude of L-IPSCs was estimated by reducing the sampling rate of averaged traces (50 fold) with each point replaced with the average of those data points, after which the maximum value was measured. This was performed to reduce variations from spontaneous activity. A peak amplitude measurement was included if the L-IPSC was larger than one standard deviation (SD) above the baseline current, leaving a positive value after the baseline + SD was subtracted from the response. If the measurement after subtraction was negative, indicating no light response, the value was set at zero for including a “no response” value in the quantification for that stimulus position and condition. For current charge transfer, the area under the response was measured in Clampfit over the length of the response (1–2 seconds) using the same time window in each condition for the same cell. As for peak amplitude, an evoked light-response was included if it was larger than one standard deviation above baseline noise. The mean + SD of the baseline current was subtracted from all evoked responses for analysis to limit baseline or spontaneous noise. All example light response traces in the figures were filtered with a low pass Gaussian filter (1000 Hz) to limit noise for display purposes. Example L-IPSCs show responses to the central bar stimulus directly over the recorded cell and 400 μm away from the cell in each direction. The light gray bar under the example trace notes the start and end of the light stimulus. A disconnected bar indicates that the light stimulus occurred before or proceeded after the example trace shown.
By mapping the receptive field with a series of bars, we could quantify the extent (size) and strength of the surround by measuring the strength of the light-evoked response at each location. Spatial light-evoked response charge transfer, normalized to the dark-adapted response at zero μm (bar stimulus over the recorded cell), and peak amplitude measurements were used. Responses to SKF or light-adapted conditions in the figure panels comparing the two, were normalized to their respective dark-adapted responses. Normalized responses were used to control for variability between bipolar cell L-IPSCs and standardize response magnitude so that the widths of the receptive field could be better compared without influence of any initial amplitude change. Peak amplitude was used as a reliable maximum response current. 2-way Analysis of Variance (ANOVA) with Student-Newman-Keuls (SNK) posthoc test was used to compare spatial profiles before and after light adaptation or D1 receptor activation as well as between response characteristics at each stimulus distance. All data are mean ± SEM, showing only the outer error bars in the figure plots for visualization. Significance is exact p values in text (p value less than 0.001 noted as p<0.001) and as p<0.05 (*), p<0.01 (**), and p<0.001 (***) in figures.
Results:
D1 receptor activation partially replicates OFF bipolar cell surround reductions with light adaptation
D1 receptors are a likely candidate for mediating light-adapted changes to OFF pathway surrounds, since some populations of OFF bipolar cells and the glycinergic and GABAergic amacrine cells providing inhibitory input express D1 receptors (Figure 1A). Horizontal cells are also important for shaping receptive field surrounds, however a vast majority of OFF bipolar cell input in the dark is glycinergic (Eggers et al., 2007; Mazade & Eggers, 2013, 2016; Mazade et al., 2019b) so inner retinal amacrine cells likely play a larger role in OFF bipolar cell surrounds. While D1 receptor activation modulates local light-evoked and spontaneous inhibition to OFF bipolar cells in a similar manner as with light-adaptation (Mazade et al., 2019b), it is unknown whether D1 receptor activation and light adaptation similarly affect the spatial inhibitory input to OFF bipolar cells (Figure 1B). Previously we found that light adaptation narrows and weakens OFF bipolar cell light-evoked inhibitory surrounds (Mazade & Eggers, 2016) and we predicted that activation of D1 receptors with SKF would be sufficient to replicate this result. To test this prediction, we mapped the spatial extent of light-evoked OFF bipolar cell inhibition using a narrow light bar flashed across the retinal slice (bar width: 25 μm, inter-stimulus distance: 100 μm, flash duration: 1 s, inter-stimulus interval: 29 s). All OFF bipolar cell subtypes were grouped together for the following analyses (see Methods). We found that OFF bipolar cell inhibition became significantly narrower and smaller after SKF application (Figure 2). OFF bipolar cell L-IPSCs in the dark were robust, with inhibitory responses at distances more than 400 μm away from the recorded cell (Figure 2A). After SKF application, most OFF bipolar cells did not have measurable inhibition if stimuli were presented more than 400 μm away from the cell (Figure 2B). The spatial profile of response charge transfer normalized to the center bar in each condition became significantly narrower with SKF (n=7, p<0.001, ANOVA, Figure 2C) as the average responses in the periphery became significantly smaller compared to dark-adapted responses. Similarly, the L-IPSC peak amplitude spatial profile became significantly smaller after SKF application (n=7, p<0.001, ANOVA, Figure 2D).
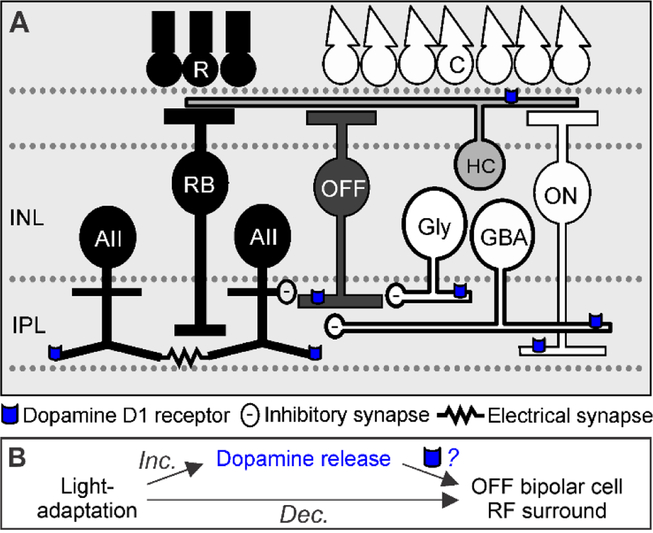
Figure 1.
Schematic of dopamine D1 receptor locations on major inhibitory inputs to OFF bipolar cells. A. In dark-adapted conditions, AII amacrine cells (AII) are activated by the rod pathway (dark pathway, R = rod photoreceptors, RB = rod bipolar cells) and release glycine onto OFF cone bipolar cells (OFF). AII amacrine cells are coupled to other AII amacrine cells through electrical gap junctions. In light-adapted conditions, other narrow-field glycinergic amacrine cells (Gly) and wide-field GABAergic amacrine cells (GBA) are activated by cone pathways (white pathways, C = cone photoreceptor, ON = ON cone bipolar cell), which provide inhibitory input to OFF bipolar cells. In the inner retina, dopamine is released onto D1 receptors (blue) located on cone bipolar and amacrine terminals. INL = inner nuclear layer, IPL = inner plexiform layer. B. Light adaptation increases dopamine release and decreases OFF bipolar cell receptive field (RF) surround size and strength. However, it is unknown to what extent dopamine, through dopamine D1 receptor activation, replicates the light-adapted effects on OFF bipolar cell surrounds.
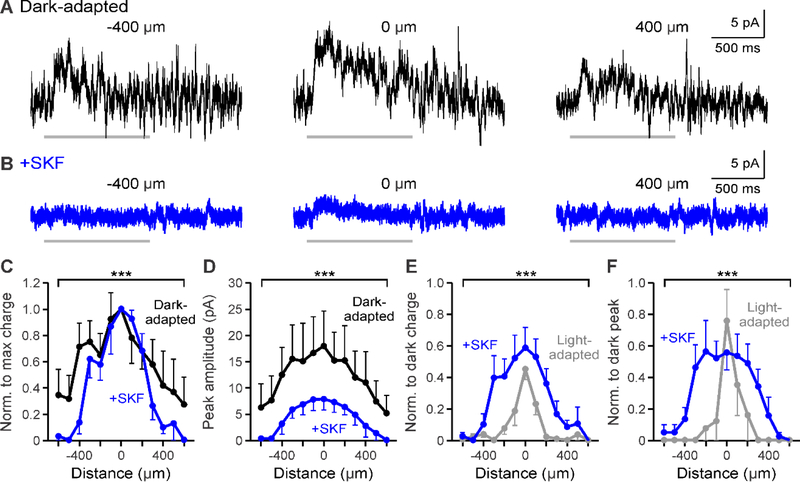
Figure 2.
D1 receptor activation weakens the spatial inhibitory input to OFF bipolar cells. A-B. Example L-IPSCs recorded in dark-adapted (black, A) and dark-adapted + SKF (+SKF, blue, B) conditions in response to a light stimulus (1 second, 25 μm bar) presented at −400, 0, and 400 μm from the cell. SKF application reduced L-IPSC strength at all stimulus distances. Light stimulus = light gray bar under example OFF type 1/2 traces. C. Spatial inhibition profiles of response charge transfer normalized to the center bar stimulus in dark-adapted and SKF conditions (n=7). The spatial profile became significantly narrower with SKF. D. Same as in C but for response peak amplitude. The peak amplitude profile was significantly narrower and smaller with light adaptation. E. Spatial inhibition profiles of response charge transfer normalized to the respective dark-adapted center bar stimulus in SKF and light-adapted (n=5, gray) conditions. Light-adapted spatial input was significantly narrower and smaller than with SKF application. F. Same as in E but for normalized response peak amplitude. The peak amplitude profile was significantly smaller in the periphery with light adaptation. Cells included in averages: SKF = 3 OFF type 1/2/4 and 4 OFF type 3; light-adapted = 1 OFF type 1/2/4 and 4 OFF type 3. Light-adapted data was adapted from Mazade and Eggers, 2016 Fig. 2C,F for comparison. Error bars are ±SEM and only show the outer bar for each data point. (*** p<0.001)
To determine if activation of D1 receptors replicated light-adapted changes in spatial input, we compared the response charge transfer and peak amplitude spatial profiles between light-adapted (data adapted from Mazade & Eggers, 2016) and SKF conditions. The spatial responses for SKF and light-adapted conditions were normalized to their respective dark-adapted center responses to determine whether activating D1 receptor signaling is sufficient to produce the magnitude of inhibitory changes caused with increased background luminance. Although there was a significant decrease in spatial charge transfer to OFF bipolar cells with SKF application (Figure 2C), the spatial response profile was still significantly larger and wider than under light-adapted conditions (light-adapted n=5, SKF vs. light-adapted p<0.001, ANOVA, Figure 2E). The normalized inhibitory peak amplitude profile was also significantly larger under SKF than light-adapted conditions (light-adapted n=5, SKF vs. light-adapted p<0.001, ANOVA, Figure 2F). The main differences between SKF and light-adapted spatial changes were localized to the central surround area (<400 μm). Therefore, D1 receptor activation replicated the reduction in spatial surrounds but not the magnitude of change evoked with light adaptation. This suggests that D1 receptors are only part of the mechanism for light-adapted inner retinal OFF pathway receptive field adaptation.
D1 receptors reduced GABAergic input at the edges of OFF bipolar cell surrounds
Inhibition to OFF bipolar cells is mediated by inputs from GABAergic and glycinergic amacrine cells onto GABAA, GABAC, and glycine receptors, and each of these components may be independently modulated by D1 receptors. Previous work found that the light-adapted narrowing of OFF bipolar surrounds was due in part to narrowing and weakening of the GABAergic component of the receptive field (Mazade & Eggers, 2016). Since activation of D1 receptors caused significant narrowing of total spatial inhibitory input (Figure 2) and decreases in spontaneous GABA release to OFF bipolar cells (Mazade et al., 2019b), we wanted to determine whether D1 receptor activation reduces the strength of the GABAergic component of the spatial surround. Pharmacologically isolated GABAergic spatial profiles (in strychnine to block glycine receptors) were measured in the dark before and after SKF application. Bar stimuli elicited small L-IPSCs at both near and far distances from the cell (Figure 3A). Small GABAergic responses in OFF bipolar cells in the dark are expected due to the relatively minor GABAergic input compared to glycinergic input. However, GABAergic responses at 400 μm away still remained after SKF application (Figure 3B). The GABAergic spatial profile was not different in the SKF condition (p=0.085, ANOVA) however there were significant reductions in response strength localized only to the farthest stimulus distances (n=4, −600 μm: p=0.006, −500 μm: p<0.001, 500 μm: p=0.031, 600 μm: p=0.028, ANOVA SNK-Posthoc, Figure 3C). Likewise, the changes in the peak amplitude of GABAergic responses were only reduced at the farthest stimulus distances (−600 μm: p=0.022, 500 μm: p=0.007, ANOVA SNK-Posthoc, Figure 3D). Weak responses at the edges but not the center of the receptive field surround suggests D1 receptors affect long-distance connections from wide-field GABAergic amacrine cells or at least the distal processes responsible for long range inputs.
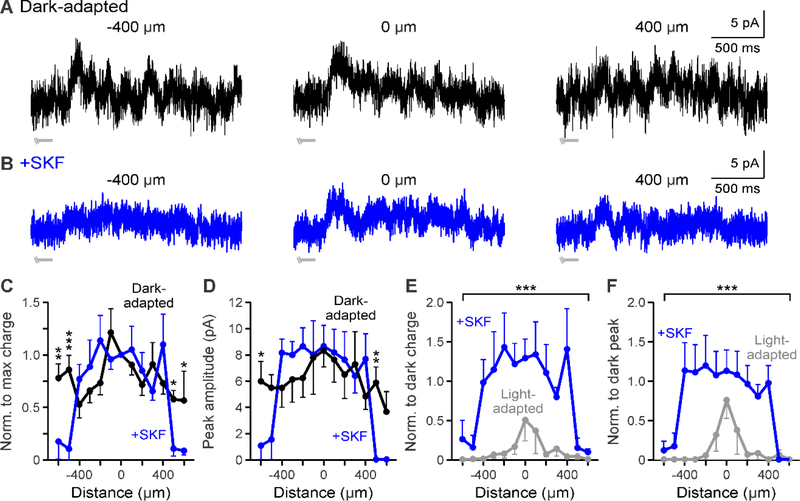
Figure 3.
D1 receptor activation affects the edges of GABAergic spatial input to OFF bipolar cells. A-B. Example GABAergic L-IPSCs recorded in dark-adapted (black, A) and dark-adapted + SKF (+SKF, blue, B) conditions. SKF application did not reduce overall response strength 400 μm away from the cell. Light stimulus = light gray disconnected bar under example traces; this OFF type 3 cell responded to the offset of the stimulus so only the end of the light stimulus is shown. C. Spatial inhibition profiles of GABAergic response charge transfer normalized to the center bar stimulus in dark-adapted and SKF conditions (n=4). Inhibition was significantly reduced only at far stimulus distances. D. Same as in C but for GABAergic response peak amplitude. Peak amplitude was only reduced at far stimulus distances. The peak amplitude spatial profile was significantly narrower and smaller with light adaptation. E. Spatial inhibition curves of GABAergic response charge transfer normalized to the respective dark-adapted center bar stimulus in SKF and light-adapted (n=5, gray) conditions. Spatial inhibitory strength was reduced in the near surround more with light adaptation than with SKF. F. Same as in E but for normalized response peak amplitude. The peak amplitude spatial profile was significantly smaller with light adaptation only in the local surround. Cells included in averages: SKF = 1 OFF type 1/2/4 and 3 OFF type 3; light-adapted = 4 OFF type 3. Light-adapted data was adapted from Mazade and Eggers, 2016 Fig. 3C,F for comparison. Error bars are ± SEM and only show the outer bar for each data point. (* p<0.05, ** p<0.01, and *** p<0.001)
To determine the extent that D1 receptor activation is similar to light-adapted GABAergic spatial changes, we compared the spatial response and peak amplitude profiles between light-adapted conditions (data adapted from a previous report, Fig. 3C,D; Mazade & Eggers, 2016) and SKF, when both were normalized to their respective dark-adapted center responses. We found that GABAergic spatial charge transfer was significantly wider and larger after SKF application than under light-adapted conditions (SKF n=4, light-adapted n=4, p<0.001, ANOVA, Figure 3E). All but the farthest distances (<500 μm, all comparisons p<0.05, ANOVA SNK-Posthoc) were significantly larger after SKF. Likewise, the normalized spatial peak amplitude profile was significantly larger with D1 receptor activation than with light adaptation (SKF n=4, light-adapted n=4, p<0.001, ANOVA, Figure 3F). While these results indicate that D1 receptors affect the edges of receptive field surrounds, other factors must modulate the central GABAergic surround changes seen with increased luminance.
D1 receptor stimulation reduces glycinergic surround extent and strength to light-adapted levels
The results from Figure 3 show that dopaminergic signaling can affect GABA receptor-mediated currents but the effects of dopamine on glycinergic surround inhibition is unknown. Total light-evoked glycinergic input to OFF bipolar cells decreases with light adaptation (Mazade & Eggers, 2013) and the glycinergic component of the receptive field surround becomes narrower and smaller (Mazade & Eggers, 2016). Additionally, D1 receptor activation suppresses local glycinergic light-evoked and spontaneous currents by reducing glycine release (Mazade et al., 2019b). Since activation of D1 receptors causes significant narrowing and weakening of total spatial inhibition to OFF bipolar cells (Figure 2) and glycine is the dominant inhibitory signal (Eggers et al., 2007), we determined whether dopamine modulation of light-evoked glycinergic spatial input is similar to that with light adaptation.
Using the same spatial mapping stimulus in Figures 2 and 3, the spatial extent of pharmacologically isolated glycinergic inhibitory input (in SR and TPMPA to block all GABA receptors) was measured in dark-adapted conditions before and after SKF application. Center bar stimuli elicited strong L-IPSCs with smaller L-IPSCs at 400 μm away (Figure 4A). However, SKF application reduced all L-IPSCs at each mapping location (Figure 4B). The spatial profile of normalized charge transfer became significantly narrower in the SKF condition (n=5, p<0.001, ANOVA, Figure 4C), similar to total inhibition with SKF. Likewise, the peak amplitude spatial profile became significantly smaller in the SKF treated retina (n=5, p=0.003, ANOVA, Figure 4D). Unlike the GABAergic dark-adapted response profiles (Figure 3), glycinergic dark-adapted spatial profiles are similar in shape and magnitude to the total inhibition dark-adapted spatial profiles (compare Figure 2 and Figure 4) likely due to the glycine-dominated input to OFF bipolar cells in the dark. These results suggest that D1 receptor modulation is contributing to the observed reduction of glycinergic spatial inhibition to OFF bipolar cells (Mazade & Eggers, 2016), which is likely the primary driver of weakening the total inhibitory spatial surround. To determine similarity to changes with light adaptation, we compared the response charge transfer and peak amplitude spatial profiles between glycinergic light-adapted (data adapted from Mazade & Eggers, 2016, Fig. 5C,F) and SKF conditions normalized to their respective dark-adapted center responses. We found that there was no difference between SKF (n=5) and light-adapted conditions (n=4) in the response charge transfer (p=0.892, ANOVA, Figure 4E) or peak amplitude (p=0.190, ANOVA, Figure 4F) profiles. These results indicate that D1 receptor activation is sufficient to replicate light-adapted levels of change in glycinergic surround size and magnitude.
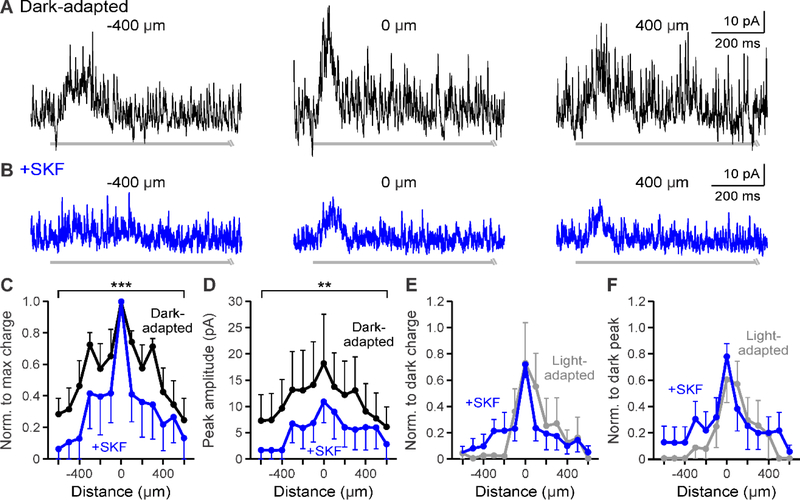
Figure 4.
D1 receptor stimulation reduces glycinergic spatial inhibitory strength to light-adapted levels. A-B. Example glycinergic L-IPSCs recorded in dark-adapted (black, A) and dark-adapted + SKF (+SKF, blue, B) conditions. SKF application reduced overall response 400 μm away from the OFF bipolar cell. Light stimulus = light gray disconnected bar under example OFF type 4 traces. The response ended before the end of the light stimulus, so only the initial portion of the light stimulus is shown. C. Spatial inhibition profiles of glycinergic response charge transfer normalized to the center bar stimulus in dark-adapted and SKF conditions (n=5). The spatial profile of inhibitory responses was reduced with D1 receptor activation. D. Same as in C but for glycinergic response peak amplitude. The peak amplitude spatial profile was significantly smaller with SKF activation. E. Spatial inhibition curves of glycinergic response charge transfer normalized to the respective dark-adapted center bar stimulus in SKF and light-adapted (n=4, gray) conditions. Light-adapted spatial input was not different from that with SKF application. F. Same as in E but for normalized glycinergic response peak amplitude. The peak amplitude spatial profile was not significantly different from that with light adaptation. Cells included in averages: SKF = 4 OFF type 1/2/4 and 1 OFF type 3; light-adapted = 3 OFF type 1/2/4 and 1 OFF type 3. Light-adapted data was adapted from Mazade and Eggers, 2016 Fig. 5C,F for comparison. Error bars are ± SEM and only show the outer bar for each data point. (** p<0.01 and *** p<0.001)
Discussion:
We found that D1 receptor activation can replicate many but not all light-adapted changes in OFF bipolar cell surround inhibition by reducing the size and extent of spatial input. This was likely primarily due to modulation of glycinergic inputs, as the effects of activating D1 receptors were comparable to light-adapted glycinergic decreases, and less so from GABAergic sources as D1 receptor activation reduced responses only at the edges of the surround. Interestingly, while these results suggest that dopamine (through D1 receptors) is one factor in light-adapted changes in inner retinal inhibition our findings show that other mechanisms are required to reach the full magnitude of adaptation effects for OFF bipolar receptive fields.
D1 receptor regulation of OFF bipolar cell surrounds may modulate OFF ganglion cell responses
Our results showing that D1 receptor activation replicates some bipolar cell light-adapted surround changes add context to several studies investigating the role of dopamine in modulating ganglion cell light-evoked signals. Work in vitro and in vivo in rabbit OFF ganglion cells found that dopamine reduced their sensitivity to light stimuli (Jensen & Daw, 1984, 1986) and blocking D1 receptors reduced surround responses (Jensen & Daw, 1984). Similar effects were seen in vivo in the cat where blocking D1 receptors reduced light-evoked activity of OFF ganglion cells, suggesting that D1 receptors normally increase light responses (Maguire & Hamasaki, 1994). The authors hypothesized that the site of action could include the outer retina and inner retinal amacrine cells, which is supported by changes we find here. Additionally, blocking D1 receptors in the mouse decreased OFF ganglion cell responses in light-adapted retinas where the suggested site of action was through OFF bipolar cell glutamate release (Yang et al., 2013). A common theme in many of these studies includes the inhibition modulating upstream bipolar cell release. The D1 receptor-mediated reduction in spatial inhibition to OFF bipolar cells that we show here could help explain increased OFF ganglion cell responses reported previously and modeled in a previous study (Mazade & Eggers, 2016). For example, light adaptation acting through D1 receptor activation would reduce the inhibitory input to OFF bipolar cells in response to a small spot of light leading to increased bipolar cell output to ganglion cells. This could be one way of strengthening ganglion cell responses to smaller stimuli to increase acuity in bright light conditions. This predicts that blocking dopamine would attenuate this inhibitory reduction, leading to a more inhibited bipolar cell output and reduced signaling to ganglion cells, which may explain the previous results using D1 receptor antagonists. The data here suggest that the modulation observed in ganglion cell spiking responses in bright light conditions are likely to arise from dopamine-mediated mechanisms at the bipolar cell level. However, this remains to be demonstrated directly.
The weakening in size and strength of OFF bipolar cell surrounds with dopamine and light adaptation contrasts recent evidence suggesting the opposite for ON bipolar cells. Interestingly, it was found that D1 receptor activation increased GABAergic activity in ON bipolar cell dendrites, likely mediated through horizontal cells, suggesting a strengthening of their receptive field surrounds (Chaffiol et al., 2017). Furthermore, following a period of darkness, when D1 receptors are presumably the least active, GABAergic function was weak. Differences between ON and OFF bipolar cell pathways are not unexpected given the unique rod-mediated AII amacrine cell inhibitory connections to OFF but not ON bipolar cells in addition to many other retinal ON and OFF pathway asymmetries (Chichilnisky & Kalmar, 2002; Ravi et al., 2018). Previously, we predicted that weaker OFF bipolar cell surrounds may increase OFF ganglion cell preference for small stimuli. It could be that stronger ON bipolar cell surrounds with D1 receptors/light-adaptation produces the opposite change: ON ganglion cells become less sensitive to smaller stimuli. This matches the larger receptive field sizes for ON than OFF ganglion cells (Ravi et al., 2018 ) and parallels recent work showing cortical ON pathways prefer larger stimuli than cortical OFF pathways in the cat (Mazade et al., 2019a). However, whether ON bipolar cells show changes in receptive field surrounds coming from amacrine cells, not horizontal cell inputs to dendrites, is less clear.
D1 receptors mediate wide-field GABAergic and narrow-field glycinergic amacrine cell inhibitory changes to OFF bipolar cells
We report that D1 receptor modulation of GABAergic pathways could be partially responsible for the light-induced reduction in the GABAergic component of the receptive field surround (Mazade & Eggers, 2016). Previous results suggest that D1 receptors do not modulate the post-synaptic GABA receptors on OFF bipolar cells as the peak amplitude of GABAergic spontaneous events did not change (Mazade et al., 2019b). Instead, the effects on GABAergic currents were likely due to decreased GABA release from distal amacrine cells, since D1 receptors decrease the frequency of GABAergic spontaneous events (Mazade et al., 2019b) and light-evoked reductions only occurred at the edges of the OFF bipolar cell surround (Figure 3). Given that the steep decline in GABAergic response at the edges of the surround occurred only in D1-receptor activated conditions, and not light adaptation, it is likely that D1-receptors are strongly linked to long-range GABAergic input to OFF bipolar cells. Although we observe a steep decline, there may be a gradual weakening of the response within the 100 μm distance between our stimuli. However, in general the GABAergic component of OFF bipolar cell inhibition is very small, especially in the dark-adapted condition as can be seen by comparing with glycinergic and total inhibition here and in previous studies (Eggers et al., 2007; Mazade & Eggers, 2013, 2016; Mazade et al., 2019b). Therefore, it is unlikely that GABAergic inhibition is playing a major role in the adaptation of OFF bipolar cell receptive fields. Together with previous findings, our results suggest that the main effect of D1 receptors on GABAergic inhibition is on wide-field GABAergic amacrine cells but that additional mechanisms are required to mediate GABAergic changes in the central or local surround.
In contrast to the GABAergic component of the OFF bipolar cell surround, D1 receptor activation reduced glycinergic inputs at both the central and peripheral surround (Figure 4). While there have been relatively few studies investigating how dopaminergic signaling affects retinal glycinergic pathways, work in the rabbit retina showed that the effects of D1 antagonists on the surround response of OFF ganglion cells (Falch et al., 1986; Jensen, 1992) was blocked by inhibiting glycine receptors (Jensen, 1989). This suggests dopamine may be acting through glycinergic pathways to increase ganglion cell responses, which is supported by our data here and implied by previous work (Mazade & Eggers, 2016). A reduction in the glycinergic surround strength of OFF bipolar cells would greatly alter the inhibitory input to the OFF bipolar cell and would subsequently increase signal transmission to downstream ganglion cells. Our current findings suggest that D1 receptors are primarily modulating glycinergic amacrine cell inputs to OFF bipolar cells which is consistent with our previous work on D1 receptor-mediated presynaptic effects on local glycinergic inputs (Mazade et al., 2019b).
The conclusion that dopamine-mediated effects mainly occur presynaptically on amacrine cells and are correlated with light-adapted changes in receptive fields, adds further support for amacrine cell importance in adaptation. Additionally, we propose that glycinergic spatial inhibition to OFF bipolar cells is more important than previously thought for contributing to lateral, and not only vertical or cross-over, inhibition. We find here and in previous studies (Mazade & Eggers, 2016), that the glycinergic component of OFF pathway receptive fields can extend to ~800 μm away from the recorded cell (Figure 3). This suggests that they are playing a role in lateral inhibition that may be mediated heavily by gap junction connects between narrow-field glycinergic amacrine cells. We previously proposed (Mazade & Eggers, 2016) that the reduced extent of glycinergic inputs with light adaptation could be explained by uncoupling of retinal gap junctions between glycinergic amacrine cells. Dopamine is well documented to modulate coupling state and synchronized activity throughout the retina (Lasater & Dowling, 1985; Dong & McReynolds, 1991; Hampson et al., 1992; Ribelayga et al., 2008; Kothmann et al., 2009; Hu et al., 2010; Bu et al., 2014). Both D1 receptors, (Hampson et al., 1992; Xia & Mills, 2004; Kothmann et al., 2009) and increased background illumination (Bloomfield et al., 1997; Xin & Bloomfield, 1997, 1999a) uncouple glycinergic AII amacrine cells that provide large inhibitory input to OFF bipolar cells (Figure 1).
Our data is consistent with the idea that D1 receptors cause uncoupling and thus reduce the spatial spread of glycinergic signals (Veruki et al., 2010; Mazade & Eggers, 2016) in a similar manner to light adaptation. However, uncoupling of other glycinergic amacrine cells are also likely involved since not all OFF bipolar cell subtypes receive AII amacrine cell input (Mazade & Eggers, 2013; Tsukamoto & Omi, 2017). In fact, AII amacrine cells appear to preferentially contact OFF types 1/2 (Graydon et al., 2018) but all OFF bipolar cells receive a majority of glycinergic input (Eggers et al., 2007). Therefore, other glycinergic amacrine cells, such as the A8, which are coupled to each other and also provide glycinergic inhibition to OFF bipolar cells, may be involved (Lee et al., 2015; Yadav et al., 2019). However, A8 amacrine cell coupling was not modulated by D1 receptors and it is unknown how coupling state is affected by luminance (Yadav et al., 2019). Regardless, OFF bipolar cells not receiving AII input are likely receiving input from other glycinergic amacrine cells that perform similar functions as the AII.
We propose that D1 receptor activation has two main effects on OFF bipolar cell inhibition. The first is uncoupling of AII or other glycinergic amacrine cells to reduce surround extent and magnitude while also reducing distal GABAergic inputs in the far surround. The second is a decrease in spontaneous noise leading to more excitable OFF bipolar cells in bright environments (Mazade & Eggers, 2016; Mazade et al., 2019b). Overall, the results of this study suggest that while dopamine signaling through D1 receptors is a prominent component in mediating OFF pathway light-adapted changes, other factors must also contribute to achieve the complete light-adapted reduction in receptive field surround. Light adaptation causes a shift from rod-dominant to cone-dominant pathways, can activate additional dopamine receptors (Cohen et al., 1992; Nguyen-Legros et al., 1999; Li et al., 2013) and can cause the release of other retinal neuromodulators such as nitric oxide (Neal et al., 1998). Therefore, activation of D1 receptors may be only one part of increasing visual acuity through light (Wilcox, 1932), at least at the level of inner retinal signal processing.
Acknowledgements:
We thank the members of the Eggers laboratory for helpful comments.
Grants: This work was supported by NSF-1552184 (EDE), Army Research Office W911NF-15-1-0613 (EDE), the University of Arizona Office of Research and Discovery, Faculty Seed Grant (EDE), National Eye Institute Grant R01-EY-026027 (EDE), the ARCS Foundation (REM), and the University of Arizona Graduate and Professional Student Council Research and Project Grant (REM).
Footnotes
Disclosures: The authors declare no conflicts of interest regarding the present study.
References Cited
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA & Robbins JT. (2000). The murine cone photoreceptor: a single cone type expresses both S and M opsins with retinal spatial patterning. Neuron 27, 513–523. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R & Kuffler SW. (1957). Change of organization in the receptive fields of the cat’s retina during dark adaptation. J Physiol 137, 338–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B, Ehinger B & Aberg L. (1980). [3H]-dopamine release from the rabbit retina. Albrecht Von Graefes Arch Klin Exp Ophthalmol 215, 71–78. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D & Osborne T. (1997). Light-induced modulation of coupling between AII amacrine cells in the rabbit retina. Vis Neurosci 14, 565–576. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Hoel MJ & Iuvone PM. (1989). Stimulation of endogenous dopamine release and metabolism in amphibian retina by light- and K+-evoked depolarization. Brain Res 482, 164–168. [DOI] [PubMed] [Google Scholar]
- Borghuis BG, Ratliff CP & Smith RG. (2018). Impact of light-adaptive mechanisms on mammalian retinal visual encoding at high light levels. J Neurophysiol 119, 1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. (1997). The Psychophysics Toolbox. Spatial vision 10, 433–436. [PubMed] [Google Scholar]
- Bu JY, Li H, Gong HQ, Liang PJ & Zhang PM. (2014). Gap junction permeability modulated by dopamine exerts effects on spatial and temporal correlation of retinal ganglion cells’ firing activities. J Comput Neurosci 36, 67–79. [DOI] [PubMed] [Google Scholar]
- Buldyrev I & Taylor WR. (2013). Inhibitory mechanisms that generate centre and surround properties in ON and OFF brisk-sustained ganglion cells in the rabbit retina. J Physiol 591, 303–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaza KC, de Mello FG & Gardino PF. (2001). GABA release induced by aspartate-mediated activation of NMDA receptors is modulated by dopamine in a selective subpopulation of amacrine cells. J Neurocytol 30, 181–193. [DOI] [PubMed] [Google Scholar]
- Chaffiol A, Ishii M, Cao Y & Mangel SC. (2017). Dopamine Regulation of GABAA Receptors Contributes to Light/Dark Modulation of the ON-Cone Bipolar Cell Receptive Field Surround in the Retina. Curr Biol 27, 2600–2609 e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ & Kalmar RS. (2002). Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci 22, 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AI, Todd RD, Harmon S & O’Malley KL. (1992). Photoreceptors of mouse retinas possess D4 receptors coupled to adenylate cyclase. Proc Natl Acad Sci U S A 89, 12093–12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedek K, Pandarinath C, Alam NM, Wellershaus K, Schubert T, Willecke K, Prusky GT, Weiler R & Nirenberg S. (2008). Ganglion cell adaptability: does the coupling of horizontal cells play a role? PLoS One 3, e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CJ & McReynolds JS. (1991). The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. J Physiol 440, 291–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W & Menaker M. (2002). Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci 19, 593–601. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP & Rieke F. (2006). Controlling the gain of rod-mediated signals in the Mammalian retina. J Neurosci 26, 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Lankheet MJ & Rieke F. (2007). Light adaptation in cone vision involves switching between receptor and post-receptor sites. Nature 449, 603–606. [DOI] [PubMed] [Google Scholar]
- Eggers ED & Lukasiewicz PD. (2006). GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, Mazade RE & Klein JS. (2013). Inhibition to retinal rod bipolar cells is regulated by light levels. J Neurophysiol 110, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers ED, McCall MA & Lukasiewicz PD. (2007). Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina. J Physiol 582, 569–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falch E, Hedegaard A, Nielsen L, Jensen BR, Hjeds H & Krogsgaard-Larsen P. (1986). Comparative stereostructure-activity studies on GABAA and GABAB receptor sites and GABA uptake using rat brain membrane preparations. J Neurochem 47, 898–903. [DOI] [PubMed] [Google Scholar]
- Farrow K, Teixeira M, Szikra T, Viney TJ, Balint K, Yonehara K & Roska B. (2013). Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron 78, 325–338. [DOI] [PubMed] [Google Scholar]
- Farshi P, Fyk-Kolodziej B, Krolewski DM, Walker PD & Ichinose T. (2016). Dopamine D1 receptor expression is bipolar cell type-specific in the mouse retina. J Comp Neurol 524, 2059–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A & Bormann J. (1994a). Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Natl Acad Sci U S A 91, 10893–10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A & Bormann J. (1994b). Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol 481 ( Pt 2), 325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA & Wassle H. (2001). Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21, 4852–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A & Wassle H. (2004). Types of bipolar cells in the mouse retina. J Comp Neurol 469, 70–82. [DOI] [PubMed] [Google Scholar]
- Godley BF & Wurtman RJ. (1988). Release of endogenous dopamine from the superfused rabbit retina in vitro: effect of light stimulation. Brain Res 452, 393–395. [DOI] [PubMed] [Google Scholar]
- Graydon CW, Lieberman EE, Rho N, Briggman KL, Singer JH & Diamond JS. (2018). Synaptic Transfer between Rod and Cone Pathways Mediated by AII Amacrine Cells in the Mouse Retina. Curr Biol 28, 2739–2751 e2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DG, Dowling JE, Siegel IM & Ripps H. (1975). Retinal mechanisms of visual adaptation in the skate. J Gen Physiol 65, 483–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DG & Powers MK. (1982). Mechanisms of light adaptation in rat retina. Vision Res 22, 209–216. [DOI] [PubMed] [Google Scholar]
- Hampson EC, Vaney DI & Weiler R. (1992). Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci 12, 4911–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G & Euler T. (2005). The primordial, blue-cone color system of the mouse retina. J Neurosci 25, 5438–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu EH, Pan F, Volgyi B & Bloomfield SA. (2010). Light increases the gap junctional coupling of retinal ganglion cells. J Physiol 588, 4145–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T & Lukasiewicz PD. (2007). Ambient light regulates sodium channel activity to dynamically control retinal signaling. J Neurosci 27, 4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CR, Ruan GX, Aseem F, Abey J, Gamble K, Stanwood G, Palmiter RD, Iuvone PM & McMahon DG. (2012). Retinal dopamine mediates multiple dimensions of light-adapted vision. J Neurosci 32, 9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RJ. (1989). Mechanism and site of action of a dopamine D1 antagonist in the rabbit retina. Vis Neurosci 3, 573–585. [PubMed] [Google Scholar]
- Jensen RJ. (1991). Involvement of glycinergic neurons in the diminished surround activity of ganglion cells in the dark-adapted rabbit retina. Vis Neurosci 6, 43–53. [DOI] [PubMed] [Google Scholar]
- Jensen RJ. (1992). Effects of the dopamine antagonist (+)-SCH 23390 on intracellularly recorded responses of ganglion cells in the rabbit retina. Vis Neurosci 8, 463–467. [DOI] [PubMed] [Google Scholar]
- Jensen RJ & Daw NW. (1984). Effects of dopamine antagonists on receptive fields of brisk cells and directionally selective cells in the rabbit retina. J Neurosci 4, 2972–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RJ & Daw NW. (1986). Effects of dopamine and its agonists and antagonists on the receptive field properties of ganglion cells in the rabbit retina. Neuroscience 17, 837–855. [DOI] [PubMed] [Google Scholar]
- Kato S, Negishi K & Teranishi T. (1985). Dopamine inhibits calcium-independent gamma-[3H]aminobutyric acid release induced by kainate and high K+ in the fish retina. J Neurochem 44, 893–899. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC & O’Brien J. (2009). Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci 29, 14903–14911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater EM & Dowling JE. (1985). Electrical Coupling Between Pairs of Isolated Fish Horizontal Cells Is Modulated by Dopamine and cAMP. Gap Junctions, 393–404. [Google Scholar]
- Lee SC, Meyer A, Schubert T, Huser L, Dedek K & Haverkamp S. (2015). Morphology and connectivity of the small bistratified A8 amacrine cell in the mouse retina. J Comp Neurol 523, 1529–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP & O’Brien J. (2013). Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci 33, 3135–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grove JC, Hirano AA, Brecha NC & Barnes S. (2016). Dopamine D1 receptor modulation of calcium channel currents in horizontal cells of mouse retina. J Neurophysiol 116, 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G & Hamasaki DI. (1994). The retinal dopamine network alters the adaptational properties of retinal ganglion cells in the cat. J Neurophysiol 72, 730–741. [DOI] [PubMed] [Google Scholar]
- Maguire GW & Smith EL 3rd. (1985). Cat retinal ganglion cell receptive-field alterations after 6-hydroxydopamine induced dopaminergic amacrine cell lesions. J Neurophysiol 53, 1431–1443. [DOI] [PubMed] [Google Scholar]
- Mazade R, Jin J, Pons C & Alonso JM. (2019a). Functional Specialization of ON and OFF Cortical Pathways for Global-Slow and Local-Fast Vision. Cell Rep 27, 2881–2894 e2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazade RE & Eggers ED. (2013). Light adaptation alters the source of inhibition to the mouse retinal OFF pathway. J Neurophysiol 110, 2113–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazade RE & Eggers ED. (2016). Light adaptation alters inner retinal inhibition to shape OFF retinal pathway signaling. J Neurophysiol 115, 2761–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazade RE, Flood MD & Eggers ED. (2019b). Dopamine D1 receptor activation reduces local inner retinal inhibition to light-adapted levels. J Neurophysiol 121, 1232–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwine DK, Amthor FR & Grzywacz NM. (1995). Interaction between center and surround in rabbit retinal ganglion cells. J Neurophysiol 73, 1547–1567. [DOI] [PubMed] [Google Scholar]
- Naka KI, Chan RY & Yasui S. (1979). Adaptation in catfish retina. J Neurophysiol 42, 441–454. [DOI] [PubMed] [Google Scholar]
- Neal M, Cunningham J & Matthews K. (1998). Selective release of nitric oxide from retinal amacrine and bipolar cells. Invest Ophthalmol Vis Sci 39, 850–853. [PubMed] [Google Scholar]
- Nguyen-Legros J, Simon A, Caille I & Bloch B. (1997). Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Vis Neurosci 14, 545–551. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C & Vernier P. (1999). Dopamine receptor localization in the mammalian retina. Mol Neurobiol 19, 181–204. [DOI] [PubMed] [Google Scholar]
- O’Brien DR & Dowling JE. (1985). Dopaminergic regulation of GABA release from the intact goldfish retina. Brain Res 360, 41–50. [DOI] [PubMed] [Google Scholar]
- Page-McCaw PS, Chung SC, Muto A, Roeser T, Staub W, Finger-Baier KC, Korenbrot JI & Baier H. (2004). Retinal network adaptation to bright light requires tyrosinase. Nat Neurosci 7, 1329–1336. [DOI] [PubMed] [Google Scholar]
- Protti DA, Di Marco S, Huang JY, Vonhoff CR, Nguyen V & Solomon SG. (2014). Inner retinal inhibition shapes the receptive field of retinal ganglion cells in primate. J Physiol 592, 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ & Smith LF. (1983). Interactions of dopamine and the release of [3H]-taurine and [3H]-glycine from the isolated retina of the rat. Br J Pharmacol 78, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi S, Ahn D, Greschner M, Chichilnisky EJ & Field GD. (2018). Pathway-Specific Asymmetries between ON and OFF Visual Signals. J Neurosci 38, 9728–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribelayga C, Cao Y & Mangel SC. (2008). The circadian clock in the retina controls rod-cone coupling. Neuron 59, 790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell TL & Werblin FS. (2010). Retinal synaptic pathways underlying the response of the rabbit local edge detector. J Neurophysiol 103, 2757–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley RM & Enroth-Cugell C. (1984). Visual adaptation and retinal gain controls. Prog Retin Eye Res 3, 263–346. [Google Scholar]
- Stroh S, Puller C, Swirski S, Holzel MB, van der Linde LIS, Segelken J, Schultz K, Block C, Monyer H, Willecke K, Weiler R, Greschner M, Janssen-Bienhold U & Dedek K. (2018). Eliminating Glutamatergic Input onto Horizontal Cells Changes the Dynamic Range and Receptive Field Organization of Mouse Retinal Ganglion Cells. J Neurosci 38, 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Nakatani K & Yau KW. (1991). Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol 98, 95–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy JB, Bohnsack DL & Diller LC. (1999). Spatial properties of the cat X-cell receptive field as a function of mean light level. Vis Neurosci 16, 1089–1104. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y & Omi N. (2017). Classification of Mouse Retinal Bipolar Cells: Type-Specific Connectivity with Special Reference to Rod-Driven AII Amacrine Pathways. Frontiers in neuroanatomy 11, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Oltedal L & Hartveit E. (2010). Electrical coupling and passive membrane properties of AII amacrine cells. J Neurophysiol 103, 1456–1466. [DOI] [PubMed] [Google Scholar]
- Veruki ML & Wassle H. (1996). Immunohistochemical localization of dopamine D1 receptors in rat retina. Eur J Neurosci 8, 2286–2297. [DOI] [PubMed] [Google Scholar]
- Wilcox WW. (1932). The Basis of the Dependence of Visual Acuity on Illumination. Proc Natl Acad Sci U S A 18, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P. (2004). Dopamine and retinal function. Doc Ophthalmol 108, 17–40. [DOI] [PubMed] [Google Scholar]
- Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH & Fain GL. (2008). Modulation of phosphodiesterase6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci 28, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XB & Mills SL. (2004). Gap junctional regulatory mechanisms in the AII amacrine cell of the rabbit retina. Vis Neurosci 21, 791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin D & Bloomfield SA. (1997). Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol 383, 512–528. [DOI] [PubMed] [Google Scholar]
- Xin D & Bloomfield SA. (1999a). Comparison of the responses of AII amacrine cells in the dark- and light-adapted rabbit retina. Vis Neurosci 16, 653–665. [DOI] [PubMed] [Google Scholar]
- Xin D & Bloomfield SA. (1999b). Dark- and light-induced changes in coupling between horizontal cells in mammalian retina. J Comp Neurol 405, 75–87. [DOI] [PubMed] [Google Scholar]
- Yadav SC, Tetenborg S & Dedek K. (2019). Gap Junctions in A8 Amacrine Cells Are Made of Connexin36 but Are Differently Regulated Than Gap Junctions in AII Amacrine Cells. Front Mol Neurosci 12, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pahng J & Wang GY. (2013). Dopamine modulates the off pathway in light-adapted mouse retina. J Neurosci Res 91, 138–150. [DOI] [PubMed] [Google Scholar]