Abstract
Background and Objective
As the interface between the oral cavity and the teeth, the junctional epithelial barrier is critical for gingival defense. The junctional epithelium is subject to mechanical stresses from biting force or external insults such as bacterial attacks, but little is known about the effects of mechanical stimuli on epithelial functions. Transient receptor potential vanilloid 4 (TRPV4) functions as a mechanosensitive nonselective cation channel. In the present study, based on marked expression of TRPV4 in the mouse junctional epithelium, we aimed to clarify the putative links between TRPV4 and junctional complexes in the junctional epithelium.
Methods and Results
Histological observations revealed that the junctional epithelium in TRPV4‐deficient (TRPV4−/−) mice had wider intercellular spaces than that in wild‐type (TRPV4+/+) mice. Exogenous tracer penetration in the junctional epithelium was greater in TRPV4−/− mice than in TRPV4+/+ mice, and immunoreactivity for adherens junction proteins was suppressed in TRPV4−/− mice compared with TRPV4+/+ mice. Analysis of a mouse periodontitis model showed greater bone volume loss in TRPV4−/− mice compared with TRPV4+/+ mice, indicating that an epithelial barrier deficiency in TRPV4−/− mice may be associated with periodontal complications.
Conclusion
The present findings identify a crucial role for TRPV4 in the formation of adherens junctions in the junctional epithelium, which could regulate its permeability. TRPV4 may be a candidate pharmacological target to combat periodontal diseases.
Keywords: adherens junction, gingiva, periodontal diseases, transient receptor potential channels
1. INTRODUCTION
Periodontal disease is characterized by chronic inflammation in tissues surrounding teeth after attacks by microorganisms followed by host immune responses. The initial site of disease onset is considered to be the junctional epithelium, which forms a unique epithelial attachment to teeth. The junctional epithelium has a higher turnover rate and wider intercellular spaces than other oral epithelia with continuous gingival fluid flow and neutrophil infiltrates.1 Cell‐cell attachment in gingival epithelia is important for maintenance of physical and functional integrity and as a barrier to protect the mucosa against harmful stimuli in the oral cavity. Groeger and Meyle2 demonstrated that Porphyromonas gingivalis exposure to human gingival keratinocytes caused downregulation of claudin expression with a reduction in transepithelial electrical resistance. Meanwhile, Damek‐Poprawa et al3 showed that Aggregatibacter actinomycetemcomitans toxin exposure to human gingival epithelia altered the distribution of the adherens junction proteins E‐cadherin, β‐catenin, and filamentous actin. Based on this evidence, an understanding of cell‐cell contacts and associated cell‐cell junction complexes in the junctional epithelium is critical for the treatment of periodontal diseases.
Extracellular calcium is involved in the regulation of human keratinocyte differentiation and stratification.4 The Ca2+ gradient plays an important role in epidermal differentiation5 to organize the stratified epithelium and withstand various stimuli in the oral cavity. Concurrently, extracellular Ca2+ is required to stiffen the extracellular domain of E‐cadherin and to form homophilic interactions that stabilize cell‐cell junctions.6
We previously reported that oral epithelia express thermosensitive transient receptor potential (TRP) channels.7, 8, 9, 10 Activation of TRP channels allows cation influx into cells, leading to a variety of physiological or pathological consequences mediated by Ca2+‐dependent processes. Among the thermosensitive TRP channels, TRP vanilloid 4 (TRPV4) is highly expressed in epithelia and activated by moderately warm temperature (<27‐35°C), hypo‐osmolality, shear stress, mechanical stretching, or anandamide metabolites including arachidonic acid.11, 12 Sokabe et al13 demonstrated that TRPV4 contributes to cell‐cell junctions in skin keratinocytes by interacting with β‐catenin and E‐cadherin.
Recently, we showed that oral epithelia have thermosensitive properties mediated by TRPV3 and TRPV4 and wound‐healing processes regulated by TRPV3.10 In the present study, we reveal marked TRPV4 expression in the junctional epithelium in addition to oral epithelia. We hypothesize that TRPV4 may provide a molecular basis for the observed characteristics of the junctional epithelium. We further explore whether TRPV4 contributes to periodontal disease in a mouse model. Finally, we propose a potential role for TRPV4 in the gingival epithelial barrier.
2. MATERIAL AND METHODS
2.1. Animals
All animal protocols were reviewed and approved by the Animal Care and Use Committee of Kyushu University. We used 9‐11‐week‐old male C57BL/6N mice (TRPV4+/+; CLEA Japan) and TRPV4‐deficient mice (TRPV4−/−)14 backcrossed with C57BL/6N mice for more than 10 generations. All animals were housed in a temperature‐controlled room with a 12‐h/12‐h light/dark cycle and free access to food (CE‐2; CLEA Japan) and water. Mice were anesthetized with a combination of medetomidine, butorphanol, and midazolam.
2.2. Primary culture of mouse oral epithelial keratinocytes
Primary cultures of oral epithelial cells were performed as previously described.10 Briefly, the palatal mucosa of neonatal mice (n = 8 per group) was incubated in 400 PU/mL dispase I (Godoshusei Ltd.) at 4°C overnight. After subsequent incubation of the epithelial sheet with 0.25% trypsin (Invitrogen) at 37°C for 10 minutes, the cells were resuspended in CnT‐Prime medium (CELLnTEC) containing 1% fetal bovine serum (FBS; Thermo Fisher Scientific). The incubation medium was replaced with FBS‐free CnT‐Prime medium after 12‐18 hours. At 3‐5 days of culture, the cells were fixed with 4% paraformaldehyde in phosphate buffer for 5 minutes, washed three times with phosphate‐buffered saline (PBS), and permeabilized with 0.1% Triton X‐100 for 5 minutes. To explore the effect of TRPV4 on cell‐cell contacts, the cell culture medium was changed to differentiation medium by adding 2 mmol/L CaCl2 for 24 hours.
To compare the proliferative activity in the primary cultures from TRPV4+/+ and TRPV4−/− mice, we performed immunofluorescence staining of proliferation marker Ki67 with an anti‐Ki67 antibody (Abcam) together with DAPI staining of all nuclei. Photomicrographs of eight fields were analyzed using ImageJ software (National Institutes of Health) to quantify the rate of Ki67‐positive cells (%) among all cells detected by DAPI staining of the nuclei.
2.3. Antibody generation, histology, immunohistochemistry, and immunocytochemistry
Guinea pigs were immunized with a peptide corresponding to the mouse TRPV4 C‐terminus (CDGHQQGYAPKWRTDDAPL) coupled with hemocyanin (Sucram). Antibody reactivity and specificity were confirmed by immunoblotting, in which bands of the correct size were observed in protein extracts of TRPV4‐transfected HEK293 cells, but not pcDNA3.1‐transfected cells. Immunohistochemistry was performed as described.8 Animals (n = 3 per group) were perfused transcardially with heparinized PBS followed by 4% paraformaldehyde in phosphate buffer. The maxilla was dissected out and decalcified with 10% EDTA for 7 days. For histology, paraffin sections were cut and stained with hematoxylin‐eosin. For immunohistochemistry, 10‐µm‐thick frozen sections were incubated with anti‐TRPV4 (2 µg/mL), rat anti‐E‐cadherin (1:200; Takara Bio Inc, Shiga, Japan), and rabbit anti‐β‐catenin (1:400; Cell Signaling Technology) antibodies at 4°C overnight. The antibodies were visualized with fluorochrome‐conjugated secondary antibodies (Alexa Fluor® 488 or 594 donkey anti‐guinea pig/rabbit or rat IgG; 1:400; Jackson ImmunoResearch). F‐actin was visualized with Alexa Fluor® 546 phalloidin (Thermo Fisher Scientific). All sections were observed and analyzed with an Axio Imager 2 microscope with ApoTome or an LSM700 confocal microscope (Carl Zeiss).
2.4. In situ hybridization
In situ hybridization was performed with an ISH Reagent Kit (Genostaff Co. Ltd.) according to the manufacturer's instructions. The maxilla sections were deparaffinized with G‐Nox (Genostaff Co. Ltd.), rehydrated through an ethanol series and PBS, fixed with 10% formalin in PBS for 15 minutes at room temperature, washed in PBS, and treated with 4 µg/mL Proteinase K (Wako) for 10 minutes at 37°C. Next, the sections were washed in PBS, refixed with 10% formalin in PBS for 15 minutes at room temperature, washed in PBS, placed in 0.2 N HCl for 10 minutes at room temperature, and washed in PBS. The sections were heat‐treated for 5 minutes at 80°C, cooled immediately, and placed in a Coplin jar containing G‐Wash (Genostaff Co. Ltd.). Hybridization was performed with specific probes (Table S1) at a concentration of 300 ng/mL in G‐Hybo (Genostaff Co. Ltd.) for 16 hours at 60°C. After hybridization, the sections were washed in G‐Wash for 10 minutes at 60°C, 50% formamide in G‐Wash for 10 minutes at 60°C, twice in G‐Wash for 10 minutes at 60°C, and twice in Tris‐buffered saline containing 0.1% Tween‐20 (TBST) at room temperature. After treatment with G‐Block (Genostaff Co. Ltd.) for 15 minutes at room temperature, the sections were incubated with anti‐DIG AP conjugate (Roche) diluted 1:2000 with G‐Block in TBST for 1 hour at room temperature. The sections were washed twice in TBST and incubated in 100 mmol/L Tris‐HCl pH 9.5 containing 100 mmol/L NaCl, 50 mmol/L MgCl2, and 0.1% Tween‐20. Color reactions were performed with NBT/BCIP solution (Sigma‐Aldrich) overnight, following by washing in PBS. The sections were counterstained with Kernechtrot stain solution (Muto Pure Chemicals) and mounted with G‐Mount (Genostaff Co. Ltd.).
2.5. Scanning electron microscopy
Maxillae from TRPV4+/+ and TRPV4−/− mice (n = 3 per group) were fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer overnight and decalcified with 10% EDTA. The gingival tissues were carefully separated from the teeth and postfixed with 1% osmium tetroxide for 1 hour at 4°C. The tissues were dehydrated in a graded ethanol series, immersed in t‐butyl alcohol, freeze‐dried, sputter‐coated with platinum using an ion coater (JEC‐550; JEOL) and examined under a scanning electron microscope (JSM‐5400; JEOL).
2.6. Western blot analysis
Palatal mucosae from TRPV4+/+ and TRPV4−/− mice (n = 3 per group) were lysed in 50 mmol/L Tris‐HCl (pH 7.4) containing 150 mmol/L NaCl, 1% Triton X‐100, 0.5% NP‐40, Protease Inhibitor Cocktail (Nacalai Tesque), and PhosSTOP phosphatase inhibitor cocktail (Roche). The protein samples were separated by electrophoresis in TGX FastCast Gels (Bio‐Rad) and transferred onto PVDF membranes (GE Healthcare). The membranes were blocked with 5% bovine serum albumin for 1 hour at room temperature and incubated with an anti‐TRPV4 antibody overnight at 4°C. Antibody‐bound bands were visualized with ECL Prime Western Blotting Detection Reagent (GE Healthcare).
2.7. Proliferation assay
Mice (n = 4 per group) were injected with 100 µg of 5‐ethynyl‐2'‐deoxyuridine (EdU). After 19.5 hours, the mice were fixed as described for the immunohistochemistry analysis. EdU was visualized with Click‐iT® reaction cocktail (C35002; Thermo Fisher Scientific) according to the manufacturer's instructions. EdU‐positive cells in every fifth gingival section around the first molar in all four animals from each group were analyzed morphometrically in a 190 × 250‐µm square under a 20 × objective lens.
2.8. Permeability assay
Dextran‐Texas Red (1 mg/mL; MW: 3000; lysine fixable; Thermo Fisher Scientific) was diluted with distilled water and used as a tracer. Under stereomicroscopic observation, 2 µL of tracer was applied by pipette into the mouth of anesthetized mice toward the gingival sulcus of the first molar every 5 minutes as described previously.15 After six applications of the tracer, the maxilla was dissected out, immersed in 4% paraformaldehyde in phosphate buffer, and decalcified with 10% EDTA for 3 days. The palatal mucosa was carefully removed from the maxilla, cut into 10‐µm frontal sections, and observed under an Axio Imager 2 microscope (Carl Zeiss). The fluorescence intensity and area with fluorescence in the junctional epithelium at the middle area of the first molar were quantified using analysis software (ZEN2012, blue edition; Carl Zeiss).
2.9. Experimental periodontitis model and micro‐CT analysis
An experimental periodontitis model was induced as described by Abe and Hajishengallis.16 Under anesthesia, a sterilized 5‐0 silk ligature was tied around the maxillary left second molar and the right side was left unligated as a control. At 10 days after suturing, the animals were anesthetized, euthanized, and fixed. Their maxillae were dissected and scanned by micro‐CT (SkyScan1076; Bruker). The bone volume change (Δbone volume [ΔBV]/tissue volume [TV]) was analyzed with CTAn software (Bruker). A standardized three‐dimensional region of interest on the alveolar bone was defined according to the definition in Trombetta‐Esilva et al17: from the palatal root apex to the most cervical portion of the furcation of the first molar; in the coronal plane from the mesial aspect of the palatal root of the first molar to the distal aspect of the second molar distal root; and from the palatal end of the palatal root to the buccal end of the distal root of the first molar. The bone volume was calculated with exclusion of the dental root volume.
2.10. Statistical analysis
Statistical comparisons were performed by Student's t test or ANOVA followed by a Bonferroni‐type multiple t test. Values of P < .05 were considered statistically significant. Data are presented as mean ± SE.
3. RESULTS
3.1. Marked TRPV4 expression in the junctional epithelium
We generated specific antibodies against TRPV4 using a previously confirmed epitope peptide.18 We selected an antibody that recognized TRPV4 with low binding in TRPV4−/− mice. TRPV4‐specific immunofluorescence was present in the palatal and buccal gingival epithelia, and the staining was stronger in the junctional epithelium compared with the oral sulcular and oral epithelia (Figure 1A,B). Immunofluorescence was heterogeneous in the epithelia, and greater TRPV4 immunoreactivity was observed in the basal cell layer of gingival epithelia compared with other layers. The immunoreactivity delineated the plasma membrane of basal epithelial cells. Primary cultured TRPV4+/+ neonatal oral epithelial cells had clear TRPV4 immunoreactivity compared with low levels observed in TRPV4−/− cells (Figure 1C). In situ hybridization revealed gingival epithelial labeling with the TRPV4 riboprobe (Figure 1D). Immunoblotting confirmed TRPV4 expression (100 kDa) in the gingival mucosal crude extract from TRPV4+/+ mice, but not that from TRPV4−/− mice (Figure 1E).
Figure 1.
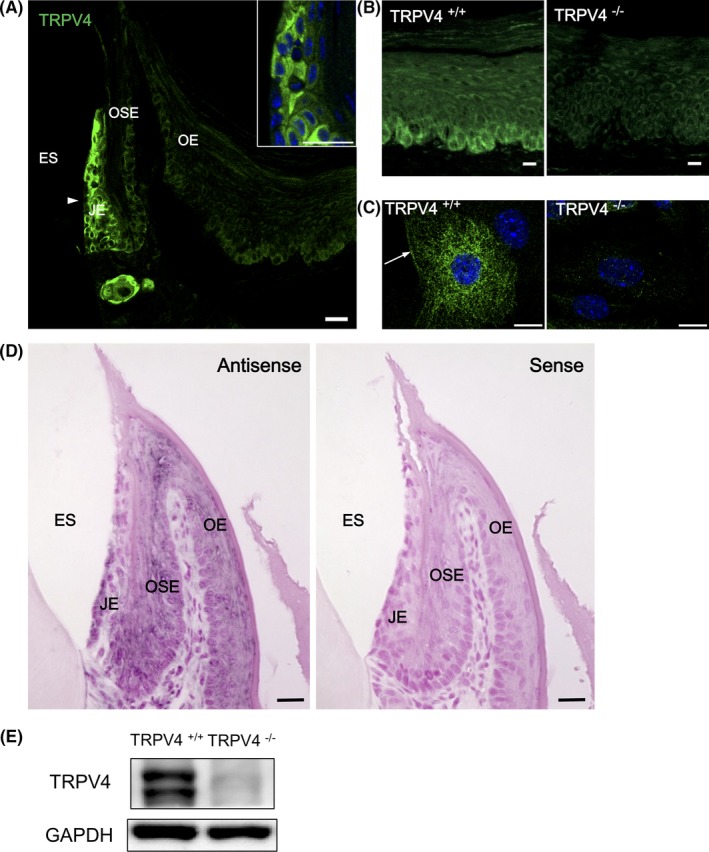
TRPV4 expression in oral epithelia. A, Immunofluorescence photomicrograph of the frontal section of a mouse palatal gingiva. Marked TRPV4 immunoreactivity is present in the gingival epithelium in TRPV4+/+ mice especially in the JE (arrowhead) and basal layers of the OSE and OE. The inset shows a higher magnification image of the JE. Heterogeneous TRPV4 immunoreactivity is observed in the JE. B, Higher magnification of TRPV4 immunohistochemistry of the palatal epithelium in TRPV4+/+ and TRPV4−/− mice. Basal cell immunoreactivity is present in the TRPV4+/+ mice, but low in TRPV4−/− mice. C, Immunocytochemistry of primary oral epithelial cells. TRPV4+/+ cells show cytoplasmic filamentous and plasma membrane (arrow) immunoreactivity for TRPV4. Low immunoreactivity is present in TRPV4−/− cells. D, In situ hybridization of mouse gingiva in the maxilla. Trpv4 mRNA is present in the JE, OSE, and OE. No staining is observed for the sense probe. E, Immunoblotting analysis. Detection of TRPV4 protein in the palatal mucosa from TRPV4+/+ mice. No bands are observed in the tissues from TRPV4−/− mice. ES, enamel space; JE, junctional epithelium; OE, oral epithelium; OSE, oral sulcular epithelium. Scale bars: 20 µm (A and D) and 10 µm (B and C)
3.2. Structural differences and enhanced permeability of exogenous substances in TRPV4 −/− gingiva
No apparent gingival structural differences were found between TRPV4+/+ and TRPV4−/− mice by light microscopy. The thickness of the epithelial layer in the junctional epithelium appeared slightly thicker in TRPV4−/− mice compared with TRPV4+/+ mice, but the difference was not significant. Wider intercellular spaces in the junctional epithelium were observed in TRPV4−/− mice compared with TRPV4+/+ mice (Figure 2A, Figure S2A). The cell sizes in the junctional epithelium in TRPV4−/− mice were smaller than those in TRPV4+/+ mice (Figure S2B). The surface structure of the junctional epithelium facing the enamel in TRPV4+/+ mice was smooth, continuous, and flat with occasional irregular gaps among polygonal cell shapes, while it was covered by numerous individual polygonal cells with larger gaps in TRPV4−/− mice (Figure 2B). To clarify the differences in the junctional epithelium properties between TRPV4+/+ and TRPV4−/− mice, fluorescent dextran was topically applied to the gingival sulcus of the first molars. The tracer was observed in the top of the junctional epithelium, bottom of the gingival sulcus, and coronal portion of the junctional epithelium (Figure 2C). The amount of tracer and the area with fluorescence were significantly greater in TRPV4−/− tissues than in TRPV4+/+ tissues (Figure 2D). Intercellular and intracellular dextran labeling in the junctional epithelium was present in TRPV4−/− tissues. Little labeling was observed in the oral sulcular epithelium. These results showed that TRPV4 deficiency enhanced permeability in the junctional epithelium.
Figure 2.
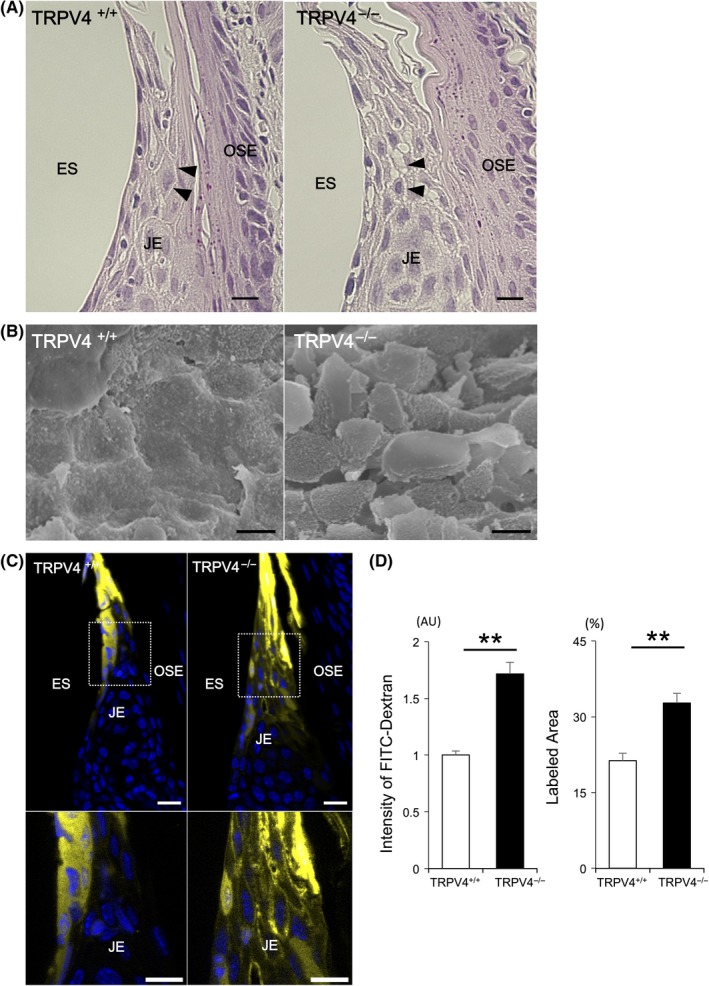
Morphological characteristics and permeability of the JE. A, Light micrographs of hematoxylin and eosin staining of the JE. No apparent structural differences in the JE are observed between TRPV4+/+ and TRPV4−/− mice. Note the wider intercellular spaces (arrowheads) in TRPV4−/− mice compared with TRPV4+/+ mice. B, Scanning electron micrographs of the junctional epithelial surface in TRPV4+/+ and TRPV4−/− mice. Rough epithelial arrangement of the junctional epithelial surface is observed in TRPV4−/− mice compared with TRPV4+/+ mice. An apparent polygonal cell shape is seen in TRPV4−/− mice. C, Permeability assay of the JE. The coronal JE was labeled with fluorescent dextran. Dextran is observed at higher levels in the intercellular spaces of the coronal JE in TRPV4−/− mice compared with TRPV4+/+ mice. D, The intensity in the JE and ratio of labeled area are significantly higher in TRPV4−/− mice than in TRPV4+/+ mice. All data are expressed as mean ± SE (n = 4). **P < .01. ES, enamel space; JE, junctional epithelium; OE, oral epithelium; OSE, oral sulcular epithelium. Scale bars: 10 µm
3.3. TRPV4 deficiency alters adherens junction proteins in the epithelium
Epithelial permeability is dependent on intercellular junctions, such as adherens junctions. We performed immunohistochemistry to observe the expression of the adherens junction proteins actin, β‐catenin, and E‐cadherin. E‐cadherin immunoreactivity delineated the cell membranous structures as a mesh‐like arrangement in the oral epithelia, oral sulcular epithelium, and junctional epithelium (Figure 3A). In the oral epithelia, the mesh‐like arrangement of E‐cadherin was intense from the spinous layer to the granular layer, while the basal layer showed rather weak labeling (Figure S3). The labeling in the granular layer appeared as a thin straight line depicting a polygonal cell shape, while the labeling in the spinous layer appeared as a bold line with notches.
Figure 3.
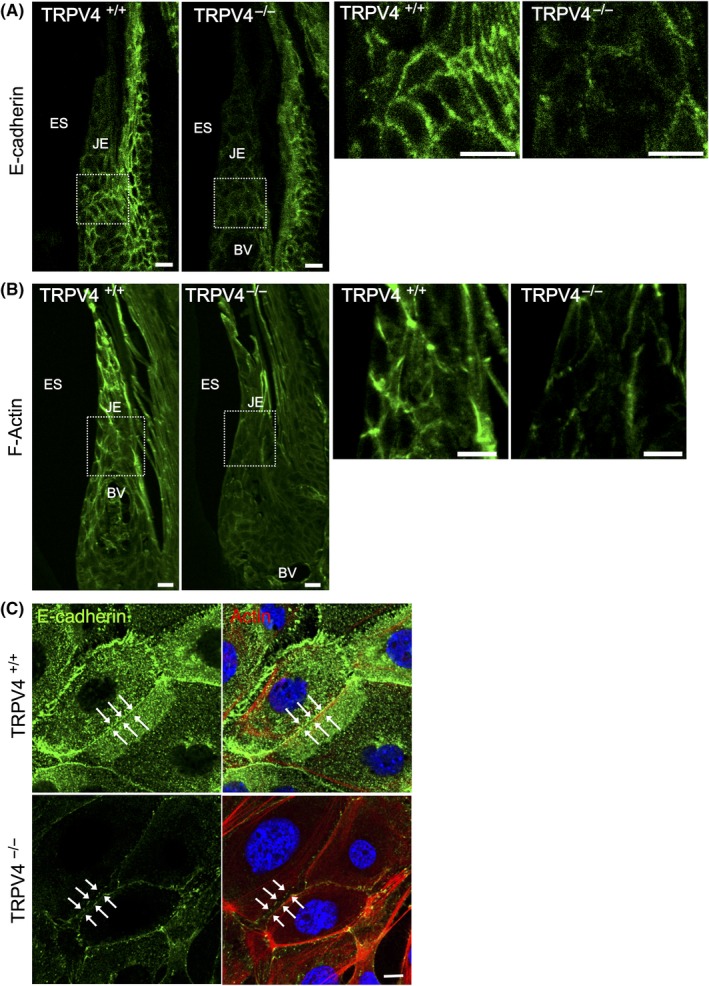
Effects of TRPV4 on the distribution of adherens junction proteins. A, E‐cadherin immunostaining in the JE has a mesh‐like pattern depicting an intercellular arrangement. TRPV4−/− mice show weaker E‐cadherin immunoreactivity in the JE compared with TRPV4+/+ mice. B, Rhodamine‐phalloidin staining of the JE in TRPV4+/+ and TRPV4−/− mice. Peripheral actin delineates the cell surface in TRPV4+/+ mice, but is rough and weak in TRPV4−/− mice. C, Immunofluorescence photomicrographs of primary cultured oral epithelial cells from TRPV4+/+ and TRPV4−/− mice after high‐calcium medium treatment for 24 h. Representative data from one of five experiments are shown. Cells from TRPV4+/+ mice form cell‐cell contacts showing E‐cadherin immunoreactivity (green) with a linear appearance (arrows). Cells from TRPV4−/− mice have intercellular gaps with weaker wavy lines (arrows) of E‐cadherin immunoreactivity compared with cells from TRPV4+/+ mice. Punctate E‐cadherin and F‐actin staining is present in the cells from TRPV4−/− mice. Rhodamine‐phalloidin: red; DAPI: blue. BV, blood vessels; ES, enamel space; JE, junctional epithelium. Scale bars: 10 µm
In the junctional epithelium, weak E‐cadherin immunofluorescence was observed at the bottom adjacent to the cement‐enamel junction and the coronal portion of the junctional epithelium, and strong labeling was observed in the middle portion. Gingival E‐cadherin epithelial labeling was more intense in TRPV4+/+ mice compared with TRPV4−/− mice (Figure 3A), although no significant differences in the amounts of E‐cadherin proteins in mucosal crude extracts were observed between TRPV4+/+ and TRPV4−/− mice by immunoblotting (Figure S4A). Meanwhile, the amounts of β‐catenin in TRPV4−/− mice were significantly lower than those in TRPV4+/+ mice (Figure S4B,C).
The formation of cadherin‐mediated cell‐cell junctions was accompanied by intense reorganization of the actin cytoskeleton, which controls cell mobility during differentiation and stratification.19, 20 In TRPV4+/+ gingiva, F‐actin was present in the coronal portion of the junctional epithelial cell periphery, while in TRPV4−/− gingiva, F‐actin labeling was weak and faint (Figure 3B). To investigate the function of TRPV4 in cell‐cell contacts, we performed immunocytochemistry of primary cultured oral epithelial cells after high‐calcium medium treatment. The cells from TRPV4+/+ mice showed intimate cell‐cell junctions depicted as a line of E‐cadherin staining, while gaps were present between the cells from TRPV4−/− mice (Figure 3C).
3.4. Elevated junctional epithelial proliferation in TRPV4 −/− mice
The junctional epithelium has a high turnover rate and E‐cadherin can inhibit cell cycle progression.21 To determine whether TRPV4 deficiency affects gingival proliferation, we compared the numbers of EdU‐labeled cells in TRPV4+/+ and TRPV4−/− mice. EdU‐positive cells were found in the basal layers of the gingiva, especially in the oral sulcular and junctional epithelia. In TRPV4+/+ mice, EdU‐labeled cells in the basal layer of the oral epithelia were singular or had a scattered distribution, while in TRPV4−/− mice, a larger number of labeled cells lined the basal layers and some were present in and above the basal cell layers (Figure 4A). The numbers of EdU‐labeled cells were significantly greater in TRPV4−/− gingival epithelia compared with TRPV4+/+ gingival epithelia (Figure 4B). Enhancement of proliferation was confirmed in primary cultures of oral epithelial cells. The rate of Ki67‐positive cells among all cells with DAPI‐stained nuclei was significantly higher in primary cultures of oral epithelial cells from TRPV4−/− mice than in those from TRPV4+/+ mice (Figure 4C,D).
Figure 4.
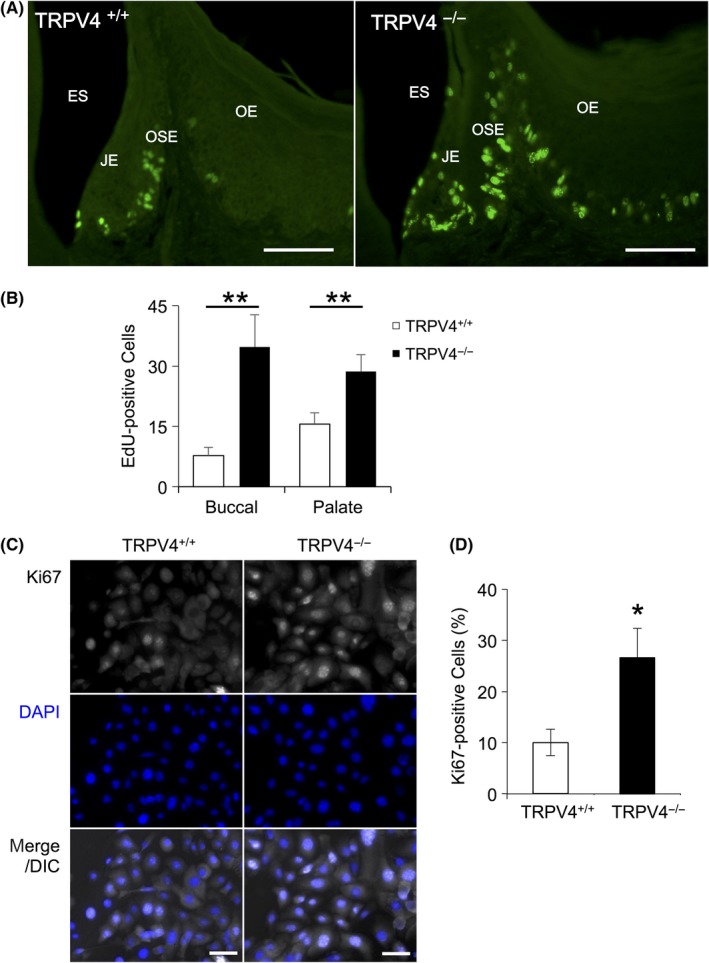
Effect of TRPV4 on gingival proliferation. A, Representative images of EdU staining of the gingiva from TRPV4+/+ and TRPV4−/− mice. EdU‐labeled nuclei are observed in the basal layer in TRPV4+/+ mice and in and above the basal cell layer in TRPV4−/− mice. B, Summary of the numbers of EdU‐positive cells in the gingival epithelia. The cell numbers are significantly higher in TRPV4−/− mice compared with TRPV4+/+ mice. Data are expressed as mean ± SE (n = 4). **P < .01. C, Representative images of immunofluorescence staining for Ki67 in primary cultures of oral epithelial cells from TRPV4+/+ and TRPV4−/− mice. D, Summary of Ki67‐positive cell rates in oral epithelial cell cultures. Data are expressed as mean ± SE (n = 8). *P < .05. ES, enamel space; JE, junctional epithelium; OE, oral epithelium; OSE, oral sulcular epithelium. Scale bars: 50 µm
3.5. Effect of TRPV4 deficiency on a ligature‐induced periodontitis model
It is widely believed that disruption of the junctional epithelial barrier leads to the onset of periodontal diseases. To evaluate whether TRPV4 is involved in periodontal destruction, we tied a silk ligature around the second maxillary molars of TRPV4+/+ and TRPV4−/− mice (Figure 5A). Of note, it was easier to insert silk ligatures around the molars of TRPV4−/− mice compared with TRPV4+/+ mice. There was no apparent difference in the health status between non‐ligatured and ligatured animals. Consistent with a previous study,16 placement of ligatures decreased the bone volume in both groups. At 10 days after the ligature placement, the alveolar ΔBV/TV was markedly larger in TRPV4−/− mice compared with TRPV4+/+ mice (Figure 5B,C).
Figure 5.
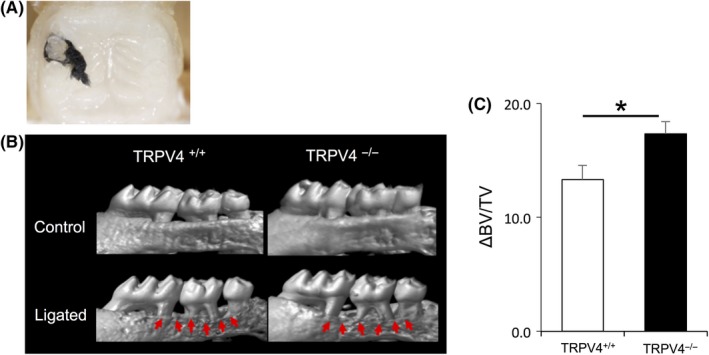
TRPV4 effect on a mouse periodontitis model. A, Representative intraoral image demonstrating a ligature placement around the second maxillary molar. B, Reconstructed micro‐CT images of the maxilla. The upper panels show no suture placement and the lower panels show suture placement. Arrows indicate areas of bone loss with increased root structure visible above the alveolar bone crest. C, Summary of the changes in bone volume per tissue volume (ΔBV/TV) at 10 d after ligature placement. Significantly larger changes are observed in TRPV4−/− mice compared with TRPV4+/+ mice. All data are expressed as mean ± SE (n = 10). *P < .05
4. DISCUSSION
The junctional epithelium forms a seal around teeth to retain mechanical strength between the tooth and the connecting mucosa and protect against compression or stretching.22 Here, we found that oral epithelia, especially the junctional epithelium, had intense TRPV4 expression. TRPV4 is activated by a variety of physical and chemical stimuli, including heat, mechanical force, hypo‐osmolarity, and endogenous substances such as arachidonic acid and endocannabinoids (for a review, see Everaerts et al23). We previously reported that the junctional epithelium shows extensive expression of TRPV2,8 a mechanosensitive24 and thermosensitive25 channel. As the junctional epithelium has a prominent nerve supply from the trigeminal sensory ganglion26 compared with the oral sulcular and oral epithelia, we propose that the junctional epithelium may play a role in sensing environmental changes around teeth via TRPV4 and TRPV2, and relaying messages to nerves to maintain the epithelial integrity and periodontal tissues.
In the present study, we confirmed TRPV4 protein expression by in situ hybridization, Western blotting, immunohistochemistry, and immunocytochemistry, in accord with our previous reports on TRPV4 mRNA expression in rats9 and mice.10 Antibody specificity against TRP channels has challenged many researchers, including us. We generated an antibody against the C‐terminal peptide of TRPV4 that had negligible reactivity with TRPV4−/− tissues. Our data are consistent with previous reports on the skin27 and urinary bladder18, 28 using an antibody against the same epitope peptide. In addition to its oral epithelial expression, TRPV4 was predominantly expressed in the junctional epithelium. In the oral and oral sulcular epithelia, TRPV4 immunoreactivity was stronger in the basal cell layers than in other layers. In the junctional epithelium, the staining intensity and distribution were heterogeneous and noticeably stronger toward the coronal side. Subcellular TRPV4 immunoreactivity was observed at the plasma membrane in oral epithelia, suggesting a role as a receptor for external stimuli. To the best of our knowledge, no detailed morphological analyses of the TRPV4 spatial localization in the oral mucosa in vivo have been reported. Further experiments are needed to clarify the TRPV4 subcellular localization in response to stimuli or functional adaptations such as migration or proliferation of oral epithelia.
Gross structural differences in the junctional epithelium between TRPV4+/+ and TRPV4−/− mice were not apparent under microscopic observation. The junctional epithelium is a non‐keratinized squamous epithelium characterized by wide intercellular spaces and thin epithelial cytoplasmic processes. Ordinary light microscopic observation revealed wider intercellular spaces in the TRPV4−/− junctional epithelium. By electron microscopy, the surface of the junctional epithelium facing the enamel in TRPV4−/− mice was rough and individual cells were distinct compared with those in TRPV4+/+ mice. Because TRPV4 is activated by hypo‐osmolarity11, 12 and plays a role in regulatory volume decreases,29 the smaller cell sizes and wider intercellular spaces may arise from impaired function of TRPV4. Constant renewal and repair of the epithelium are critically important to maintain the epithelial barrier. At the same time, as epithelial cells differentiate and stratify, they may form specific intercellular junctions via TRPV4 to maintain the internal environment.
As light microscopic observation indicated wider intercellular spaces in the junctional epithelium, we expected the exogenous tracer dextran to penetrate easily into the TRPV4−/− gingiva. However, the tracer was restricted to the coronal portion of the junctional epithelium, and rarely found in the oral sulcular or oral epithelia. Labeling in the junctional epithelium was in accord with a previous study involving topical application of horseradish peroxidase to the rat gingiva to label the coronal portion of the junctional epithelium.30 The dextran‐labeled area was wider in the junctional epithelium of TRPV4−/− mice compared with TRPV4+/+ mice, suggesting that TRPV4 deficiency affects the permeability in the junctional epithelium. Dextran fluorescence clearly labeled the intercellular spaces in both TRPV4+/+ and TRPV4−/− mice. Furthermore, dextran was present in epithelial cells, suggesting that TRPV4 regulates paracellular and transcellular transport in the epithelium. It remains controversial whether activation or inactivation of TRPV4 strengthens the epithelial barrier, based on cell culture experiments using TRPV4 agonists or antagonists.13, 31 Our in vivo study demonstrated that TRPV4 deficiency enhanced junctional epithelial permeability.
Although epithelial permeability is dependent on cell‐cell junctional complexes, the detailed mechanisms of cell‐cell junctions in the junctional epithelium remain to be elucidated. Infection of immortalized human gingival keratinocytes with the periodontitis pathogen Porphyromonas gingivalis reduced transepithelial electrical resistance,2 suggesting a crucial role of the epithelial barrier in periodontal destruction. We found disorganization and weak labeling of E‐cadherin, β‐catenin, and F‐actin in TRPV4−/− mice. Furthermore, there was greater dextran invasion in TRPV4−/− mice compared with TRPV4+/+ mice, possibly because of the impaired epithelial adherens junctions in TRPV4−/− mice. We found that high‐calcium treatment induced cell‐cell contacts in primary cultures of oral epithelial cells from TRPV4+/+ mice, while intercellular gaps were apparent between the oral epithelial cells from TRPV4−/− mice. These findings are reasonable because TRPV4 directly interacts with the adherens junction molecules actin,29, 32 β‐catenin, and E‐cadherin.13
The junctional epithelium is a unique free‐end epithelium facing hard tissue enamel and has a continuous high turnover rate compared with other oral epithelia. In the present study, TRPV4−/− mice exhibited a larger number of proliferating cells and wider intercellular spaces than TRPV4+/+ mice. Expression of E‐cadherin has been implicated in cell growth arrest, termed “contact inhibition of proliferation,” in epithelial cells.33 Several constituent molecules of adherens junctions are known to arrest proliferation signals when they form junctional complexes at contact sites. Thus, we speculate that impaired junctions with E‐cadherin may attenuate contact inhibition, resulting in the higher turnover rate in TRPV4−/− mice. Furthermore, Stockinger et al21 demonstrated that β‐catenin transcriptional activity affected cell proliferation by interacting with E‐cadherin in the cytoplasm of epithelial cells. In the junctional epithelium, suppressed interactions between TRPV4 and β‐catenin or E‐cadherin may enhance proliferation followed by reduced cell‐cell adhesion. Upregulation of epithelial proliferation in TRPV4−/− mice would be implicated in pathological growth of oral epithelial cells in periodontal diseases.
We hypothesized that impaired barrier function via cell‐cell contacts at the junctional epithelium may contribute to periodontal dysfunction. In our mouse periodontitis model, there was a markedly larger bone volume decrease in TRPV4−/− mice compared with TRPV4+/+ mice. Junctional complexes including E‐cadherin, actin fibers, and TRPV4 in the junctional epithelium are suggested to play a role against exogenous insults from the gingival sulcus. Recently, epithelial barrier dysfunction in the skin or mucosa was found to be associated with pathogenesis in gut or pulmonary diseases.34 As TRPV4 agonists or antagonists can regulate inflammation in pulmonary or bowel disease models, TRPV4 has been implicated as a potential target for the management of lung injury35 or inflammatory bowel diseases.36 In addition, we recently revealed an impairment of the epithelial cell‐cell contact structure with an irregular pattern of adherens junctional proteins and TRPV4 in the labial mucosal epithelium of Sjögren syndrome patients.37 Impaired epithelia with weaker TRPV4 expression than normal epithelia were observed together with a larger number of inflammatory cells in Sjögren syndrome patients. Thus, TRPV4‐associated epithelial cell‐cell junctions appear to play an important role in human oral mucosal epithelia. It is well known that bacteria and their components can intrude into the junctional epithelium, which increase neutrophil penetration and enhance periodontal inflammation.38, 39 Impaired epithelial cell‐cell contact via TRPV4 may lead to more serious vulnerability of periodontal tissues to bacteria or mechanical stresses and aggravate periodontal inflammation. Taking the present findings together, we suggest that TRPV4 is a potential therapeutic target for restoration of the junctional epithelium barrier. Further studies on TRPV4 functions in periodontal tissues are needed, because this protein is considered to be ubiquitously expressed in endothelial cells, bone cells, and immune cells.
Supporting information
ACKNOWLEDGEMENTS
We appreciate the technical support from the Research Support Center, Graduate School of Medical Sciences, Kyushu University. We deeply appreciate the kind gift of TRPV4‐deficient mice from Drs. Atsuko Mizuno and Makoto Suzuki, Jichi Medical School. We also appreciate the kind gift of protein extracts from pcDNA3.1‐transfected HEK293 cells with or without TRPV4 from Drs. Yasunori Takayama and Makoto Tominaga, Okazaki Institute for Integrative Bioscience. We thank Alison Sherwin, PhD, from Edanz Editing (http://www.edanzediting.com/ac) for editing a draft of this manuscript. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16H05558 and JP16K15825 to MAK. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article. This study is submitted in partial fulfillment of the requirements for Dr Kitsuki's thesis.
Kitsuki T, Yoshimoto RU, Aijima R, et al. Enhanced junctional epithelial permeability in TRPV4‐deficient mice. J Periodont Res. 2020;55:51–60. 10.1111/jre.12685
Tomoko Kitsuki and Reiko U. Yoshimoto have contributed equally to the work.
REFERENCES
- 1. Schroeder HE. The periodontium. Schroeder HE Handbook of microscopic anatomy. Berlin: Springer‐Verlag; 1986:233‐323. [Google Scholar]
- 2. Groeger SE, Meyle J. Epithelial barrier and oral bacterial infection. Periodontol 2000. 2015;69(1):46‐67. [DOI] [PubMed] [Google Scholar]
- 3. Damek‐Poprawa M, Korostoff J, Gill R, DiRienzo JM. Cell junction remodeling in gingival tissue exposed to a microbial toxin. J Dent Res. 2013;92:518‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharpe GR, Gillespie JI, Greenwell JR. An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Lett. 1989;254:25‐28. [DOI] [PubMed] [Google Scholar]
- 5. Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245‐254. [DOI] [PubMed] [Google Scholar]
- 6. Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of e‐cadherin upon calcium binding. FEBS J. 1994;223:1019‐1026. [DOI] [PubMed] [Google Scholar]
- 7. Kido MA, Muroya H, Yamaza T, Terada Y, Tanaka T. Vanilloid receptor expression in the rat tongue and palate. J Dent Res. 2003;82:393‐397. [DOI] [PubMed] [Google Scholar]
- 8. Shimohira D, Kido MA, Danjo A, et al. TRPV2 expression in rat oral mucosa. Histochem Cell Biol. 2009;132:423‐433. [DOI] [PubMed] [Google Scholar]
- 9. Wang B, Danjo A, Kajiya H, Okabe K, Kido MA. Oral epithelial cells are activated via TRP channels. J Dent Res. 2011;90:163‐167. [DOI] [PubMed] [Google Scholar]
- 10. Aijima R, Wang B, Takao T, et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015;29:182‐192. [DOI] [PubMed] [Google Scholar]
- 11. Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695‐702. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434‐438. [DOI] [PubMed] [Google Scholar]
- 13. Sokabe T, Fukumi‐Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285:18749‐18758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96‐101. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda H, Shiraiwa M, Yamaza T, et al. Difference in penetration of horseradish peroxidase tracer as a foreign substance into the peri‐implant or junctional epithelium of rat gingivae. Clin Oral Implants Res. 2002;13:243‐251. [DOI] [PubMed] [Google Scholar]
- 16. Abe T, Hajishengallis G. Optimization of the ligature‐induced periodontitis model in mice. J Immunol Methods. 2013;394:49‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trombetta‐Esilva J, Yu H, Arias DN, Rossa C Jr, Kirkwood KL, Bradshaw AD. LPS induces greater bone and PDL loss in SPARC‐null mice. J Dent Res. 2011;90:477‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gevaert T, Vriens J, Segal A, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453‐3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bershadsky A. Magic touch: how does cell‐cell adhesion trigger actin assembly? Trends Cell Biol. 2004;14:589‐593. [DOI] [PubMed] [Google Scholar]
- 20. Mege RM, Gavard J, Lambert M. Regulation of cell‐cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541‐548. [DOI] [PubMed] [Google Scholar]
- 21. Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E‐cadherin regulates cell growth by modulating proliferation‐dependent beta‐catenin transcriptional activity. J Cell Biol. 2001;154:1185‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schroeder HE, Listgarten MA. Fine structure of the developing epithelial attachment of human teeth. Monogr Dev Biol. 1971;2:1‐134. [PubMed] [Google Scholar]
- 23. Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog Biophys Mol Biol. 2010;103:2‐17. [DOI] [PubMed] [Google Scholar]
- 24. Muraki K, Iwata Y, Katanosaka Y, et al. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829‐838. [DOI] [PubMed] [Google Scholar]
- 25. Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin‐receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436‐441. [DOI] [PubMed] [Google Scholar]
- 26. Kondo T, Ayasaka N, Nagata E, Tanaka T. A light and electron microscopic anterograde WGA‐HRP tracing study on the sensory innervation of junctional and sulcular epithelium in the rat molar. J Dent Res. 1992;71:60‐65. [DOI] [PubMed] [Google Scholar]
- 27. Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat‐evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408‐6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Everaerts W, Vriens J, Owsianik G, et al. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298:F692‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Becker D, Bereiter‐Hahn J, Jendrach M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur J Cell Biol. 2009;88:141‐152. [DOI] [PubMed] [Google Scholar]
- 30. Yamaza T, Kido MA, Kiyoshima T, Nishimura Y, Himeno M, Tanaka T. A fluid‐phase endocytotic capacity and intracellular degradation of a foreign protein (horseradish peroxidase) by lysosomal cysteine proteinases in the rat junctional epithelium. J Periodontal Res. 1997;32:651‐660. [DOI] [PubMed] [Google Scholar]
- 31. Reiter B, Kraft R, Günzel D, et al. TRPV4‐mediated regulation of epithelial permeability. FASEB J. 2006;20:1802‐1812. [DOI] [PubMed] [Google Scholar]
- 32. Shin SH, Lee EJ, Hyun S, Chun J, Kim Y, Kang SS. Phosphorylation on the Ser 824 residue of TRPV4 prefers to bind with F‐actin than with microtubules to expand the cell surface area. Cell Signal. 2012;24:641‐651. [DOI] [PubMed] [Google Scholar]
- 33. St Croix B, Sheehan C, Rak JW, Flørenes VA, Slingerland JM, Kerbel RS. E‐Cadherin‐dependent growth suppression is mediated by the cyclin‐dependent kinase inhibitor p27(KIP1). J Cell Biol. 1998;142:557‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799‐809. [DOI] [PubMed] [Google Scholar]
- 35. Morty RE, Kuebler WM. TRPV4: An exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol. 2014;307:L817‐821. [DOI] [PubMed] [Google Scholar]
- 36. Vergnolle N. TRPV4: New therapeutic target for inflammatory bowel diseases. Biochem Pharmacol. 2014;89:157‐161. [DOI] [PubMed] [Google Scholar]
- 37. Yoshimoto RU, Aijima R, Ohyama Y, et al. Impaired junctions and invaded macrophages in oral epithelia with oral pain. J Histochem Cytochem. 2019;67:245‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005;84:9‐20. [DOI] [PubMed] [Google Scholar]
- 39. Ji S, Choi YS, Choi YN. Bacterial invasion and persistence: critical events in the pathogenesis of periodontitis? J Periodont Res. 2015;50:570‐585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


