Abstract
The influence of arousal on visual attention was examined in 6.5‐month‐old infants (N = 42) in the context of a visual search task. Phasic increases in arousal were induced with brief sounds and measured with pupil dilation. Evidence was found for an inverted U‐shaped relation between pupil dilation amplitude and visual orienting, with highest likelihood of a target fixation at intermediate levels of arousal. Effects were similar for facial stimuli and simple objects. Together, these results contribute to our understanding of the relation between arousal and attention in infancy. The study also demonstrates that infants have a bias to orient to human eyes, even when presented in isolation.
Young infants orient quickly to visual stimuli that are socially meaningful (such as faces) or that differ from their environment in terms of low‐level visual characteristics (e.g., Adler & Orprecio, 2006; Catherwood, Skoien, & Holt, 1996; Kwon, Setoodehnia, Baek, Luck, & Oakes, 2016). In humans and other animals, visual selection is affected by general arousal, and particularly by activity in the brains locus coeruleus‐norephinephrine (LC‐NE) system (Aston‐Jones & Cohen, 2005; Mather, Clewett, Sakaki, & Harley, 2016). Typically, increases in LC‐NE arousal enhance the tendency to orient to targets that are salient or related to current goals. However, very high levels of arousal may instead lead to increased distractibility and unselective responses (Aston‐Jones & Cohen, 2005; Mather et al., 2016), which may be linked to an inhibition of prefrontal functions (Arnsten, 2009). Most human studies of arousal‐attention interactions have been conducted with adults and children beyond toddlerhood, but a growing literature has also linked arousal to visual attention in infants (Colombo, 2002; de Barbaro, Chiba, & Deák, 2011). Recently, de Barbaro et al. (2017) reported that infants made shorter and more frequent gaze shifts during periods of high arousal. This suggests that the increase in arousal may have enhanced the infants’ attention to novel stimuli. Previous studies have also suggested that infants who react with higher arousal to stress and sensory input may also be more attentive to faces, as well as to other salient stimuli (de Barbaro, Clackson, & Wass, 2016; Jones, Dawson, & Webb, 2018). This study sought to extend knowledge about this relation by experimentally manipulating short‐term arousal.
Arousal was induced by auditory cues and measured with pupil dilation (an index of LC‐NE activity). We examined arousal effects on orienting to both facial (whole faces or isolated eyes) targets and visually simple objects (color deviants). Infants were seen at around 6.5 months. During this age period, infants are capable of both stimulus‐driven and endogenous visual orienting (Posner, Rothbart, Sheese, & Voelker, 2014).
Before we turn to the specific hypotheses of our study, we will introduce the key constructs in some more detail.
Phasic Alerting and Arousal
A change in behavior caused by a temporary increase in general arousal after sensory input is referred to as phasic alerting (Posner & Fernandez‐Duque, 2006; Sturm & Willmes, 2001). In behavioral studies, phasic alerting is typically manipulated by presenting auditory or visual cues (henceforth alerting cues) simultaneously or shortly before the visual stimuli. The vast majority of the human studies about phasic alerting effects have been conducted in adults. These studies have shown that phasic alerting influences early perceptual processing and spatial attention (Kleberg, Thorup, & Falck‐Ytter, 2017a; Kusnir, Chica, Mitsumasu, & Bartolomeo, 2011; Petersen, Hilkjaer Petersen, Bundesen, Vangkilde, & Habekost, 2017). In visual search tasks, phasic alerting typically increases processing speed and decreases the threshold for target detection and conscious perception (Asutay & Västfjäll, 2017; Kusnir et al., 2011). However, phasic alerting can have the opposite effect in highly complex visual environments or when several responses compete (Callejas, Lupiàñez, Funes, & Tudela, 2005; Fan et al., 2009; Weinbach & Henik, 2014).
On a physiological level, phasic alerting is linked to tonic and phasic firing rates of the LC‐NE system (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005). Pupil dilation is an index for LC‐NE functioning in both humans (Murphy, O'connell, O'sullivan, Robertson, & Balsters, 2014; Wang & Munoz, 2015) and animals (Reimer et al., 2016) that is increased by cognitive load and emotional arousal (Laeng, Sirois, & Gredebäck, 2012) as well as unexpected sensory input (Wetzel, Buttelmann, Schieler, & Widmann, 2016).
The Pop‐Out Effect
One ecologically valid task that requires selective attention and that infants master from early on is the pop out paradigm. In this task, visual stimuli that differ from the surrounding on a unique stimulus dimension such as orientation, color, or social relevance (henceforth targets) are presented among distractors. When a target stimulus captures attention in a bottom‐up fashion, it is said to “pop‐out” of the visual scene. The pop‐out effect can be operationalized as a tendency to orient attention to a target with a higher likelihood than would be expected by chance (e.g., Gliga, Elsabbagh, Andravizou, & Johnson, 2009; Gluckman & Johnson, 2013). Another operationalization is that a pop‐out effect is found if the time to locate the targets does not depend on the number of distractors (e.g., Treisman & Gelade, 1980).
Pop‐out effects have previously been described in infants and adults for both facial stimuli and simple visual features such as colors (Adler & Orprecio, 2006; Catherwood et al., 1996; Kwon et al., 2016). Previous studies have linked the pop‐out effect to responses in early visual cortical areas (e.g., Bogler, Bode, & Haynes, 2013), but it is also clear that the effect is modulated by task goals and motivational states (Hsieh, Colas, & Kanwisher, 2011). The first aim of this study was to examine the effects of phasic alerting on visual orienting in the context of a pop‐out task. The effects of phasic alerting are likely to be broad and affect attention to multiple classes of stimuli (e.g., Mather et al., 2016). Therefore, we studied orienting to both simple visual objects and facial stimuli. Simple objects were geometrical shapes differing from surrounding objects in terms of color (color deviants). In addition to the primary aim, we sought to outline some of the visual characteristics of stimuli that elicit preferential orienting to social information by comparing infants tendency to orient to isolated eyes and whole faces. This topic is introduced in the following section.
Social Orienting in Infants
Infant orienting toward faces is driven by a largely subcortical brain network including the amygdala (Johnson, Senju, & Tomalski, 2015). A number of studies have found that faces and face‐like configurations trigger orienting in infants more reliably than control stimuli such as inverted faces and nonfacial patterns (Cassia, Simion, Umilta, & Macchi Cassia, 2001; Farroni et al., 2005; Shah, Happé, Sowden, Cook, & Bird, 2015; Simion, Valenza, Umiltà, & Barba, 1998), even when presented among visually salient distractors (Di Giorgio, Turati, Altoè, & Simion, 2012; Elsabbagh et al., 2013; Gliga et al., 2009; Gluckman & Johnson, 2013). However, the literature is not entirely consistent, as some studies have not found evidence for preferential orienting to faces (Di Giorgio et al., 2012; Kwon et al., 2016). Infant orienting to faces is believed to be highly important for sociocommunicative and socioemotional development (e.g., Johnson et al., 2015), and a better understanding of the necessary and sufficient properties of stimuli that trigger this preference is therefore needed. Previous research has identified a number of unique visual characteristics associated with facial stimuli that seem important for triggering social orienting, including low spatial frequency information from faces (Johnson et al., 2015), contrast polarity (Farroni et al., 2005), and the typical top‐heavy configuration of faces with two eyes at the top and the mouth on the bottom. One currently unanswered question is whether eyes alone are sufficient to elicit an attentional bias. Human infants are highly attentive to the eye region of whole faces (Batki, Baron‐Cohen, Wheelwright, Connellan, & Ahluwalia, 2000; Dupierrix et al., 2014; Oakes & Ellis, 2013), and neurophysiological studies in adults and children have suggested the existence of neural mechanisms that are maximally activated by eyes rather than by whole faces or facial features other than eyes (Gliga & Dehaene‐Lambertz, 2007; Hoehl, 2015; Itier & Batty, 2009). Gluckman and Johnson (2013) found that 6‐month‐old infants had a tendency to orient to body parts, including eyes, hands, mouths, and feet. Orienting was more consistent to whole faces than to objects in the combined “body parts” category and in turn more consistent to face parts than to other body parts. However, eyes were not compared to faces or other body parts. In other words, no study has directly compared orienting to whole faces versus only eyes in infants. In the following, we use the term social orienting to refer to preferential orienting to faces and other forms of social stimuli.
Hypotheses
We expected that phasic alerting cues would increase the likelihood and speed of orienting to the visual targets and that this effect would be linked to an increase in pupil dilation. In line with previous studies (Catherwood et al., 1996; Gliga et al., 2009; Gluckman & Johnson, 2013), we expected pop‐out effects for faces and color deviants. On the basis of previous literature about the importance of eyes for face processing in infancy, we hypothesized that a pop‐out effect would also be found for eyes alone. We did not expect that the effects of phasic alerting cues would differ depending on the type of visual target.
Method
Participants
An initial sample of 44 infants was recruited from a register of families who had expressed interest to participate in developmental research. Based on descriptive statistics from previous studies of social orienting in infants (e.g., Gliga et al., 2009), this sample size can be assumed to be adequate for detecting a true effect of Cohens d = .6 with a power of ~.8. The vast majority of infants were born in Sweden and came from middle‐income families with an academic background.
Two male infants were excluded from all analyses because they contributed too little valid data (see criteria for rejection of trials under Statistics and Data Reduction). The remaining 42 infants (15 male) had a mean age of 6.64 months (SD = 0.66; range = 5.7–8). Infants were included in all analyzes where they contributed at least four valid trials in the relevant conditions (see Recording and Analysis of Eye Tracking Data). As a consequence, the number of included infants varied between 39 and 42 (see Tables 1 and 2). The study was approved by the regional research ethics committee of Stockholm, Sweden, and the research was conducted in accordance with the 1964 declaration of Helsinki.
Table 1.
Average Number of Trials per Participant and Number of Included Participants in Analyses Related to the Effect of Alerting Cue
| Condition | Silent | Simple alerting cue | Vocal alerting cue | Number of participants |
|---|---|---|---|---|
| First fixation analysis | ||||
| Trials per participant | 18.62 (3.38) [11–24] | 9.21 (1.39) [6–11] | 10.12 (2.67) [2–13] | 40 |
| Latency analysis | ||||
| Trials per participant | 10.85 (3.26) 5–19 | 6.03 (1.55) 3–10 | 5.77 (2.30) 2–10 | 39 |
Table 2.
Average Number of Trials Per Participant and Number of Included Participants in Analyses Related to the Effect of Visual Stimulus Type
| Condition | Trials per participant | Number of participants |
|---|---|---|
| Whole faces | 42 | |
| Trials per participant | 12.93 (2.15) 9–16 | |
| Isolated eyes | 42 | |
| Trials per participant | 12.83 (2.66) 8–16 | |
| Color deviants | 42 | |
| Trials per participant | 11.83 (3.14) 4–16 |
Stimulus Presentation
The eye‐tracking paradigm was a modified version of the one used in previous studies (Kleberg, Högström, et al., 2017; Kleberg, Thorup, & Falck‐Ytter, 2017b). Stimuli consisted of four images in a circular display (see Figure 1), of which one was the target. Targets were either a full face (full face condition), a pair of isolated eyes (eye condition), or a colored geometrical shape in red, yellow, or green (color deviant condition). Sixteen trials of each condition were presented. In the eye and whole face conditions, two of the three distractor images were nonsocial objects sharing a common configuration (cell phones, trees, cars or houses). The third distractor was either a blurred face turned 180 degrees (in the whole face trials) or a pair of black circles (in the eye condition). These distractors were included to resemble low‐level visual features of eyes and faces, such as hue and contrast polarity. In the color deviant condition, the target had a different color than the three distractors, but the stimuli were otherwise identical.
Figure 1.
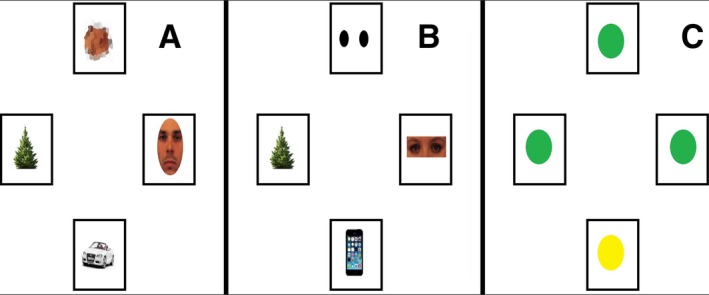
Example of stimuli in the whole face condition (A), eyes (B), and color deviant (C), conditions. Areas of interest are marked as black rectangles.
Auditory alerting cues were presented on 50% of the trials. To examine the generalizability of the putative phasic alerting effects, we included alerting cues that were either simple (a brief beep, 25% of the total trials) or vocal (a single vowel, 25% of the total trials). Auditory cues had a sound pressure level (SPL) of approximately 70–75 decibel and were presented at a variable interval between 400 and 80 ms before the onset of the visual stimuli and varied in duration between 200–300 ms. The simple and vocal alerting cues did not differ on SPL (db SPL; independent samples t‐test, p > .25) but were not matched on other parameters. Due to a technical error, an unbalanced number of vocal (N = 5) and simple (N = 3) alerting cues were presented in the eye condition. Therefore, trial number was included as random factor in the analyses. We also computed all analyses after removing the two last presented vocal trials in the eye condition from analysis. This did not change any of the significant effects. All trials were preceded by a small animated attention‐grabbing figure at the center of the screen during a variable time interval ranging between 1,000–1,500 ms. The attention grabber was extinguished 400 ms before the onset of the visual stimuli. The visual stimuli remained on screen for 3,000 ms, even if the infant successfully oriented to the target earlier.
Recording and Analysis of Eye Tracking Data
A five‐point calibration was completed before the experiment. Data were recorded using a Tobii T120 corneal reflection eye tracker (Tobii Inc. Danderyd, Sweden) at a sampling rate of 60 Hz. Calibration accuracy was checked manually and repeated if it was not judged to be successful by the experimenter. Raw gaze coordinates were extracted using the Tobii‐studio software, and further analyses were conducted in MATLAB (The MathWorks, Inc., Natick, MA, US) using scripts written by the first author. The gaze coordinates were averaged for the right and left eye. Samples with validity codes > 2 assigned by the Tobii software (indicating a poor estimate of the pupil position) were discarded. Trials were rejected if gaze was not in the center of the screen for at least 50% of the 200 ms before stimulus onset (11% of the total number of trials). We interpolated linearly over gaps in the data shorter than 150 ms. Rectangular areas of interest (AOIs) covering approximately 7.3 × 5.9° of the visual field were defined around the four stimulus positions (see Figure 1). A gaze entry into one of the AOIs was detected by the scripts if (a) the coordinates of one sample was within the AOI; and (b) at least three of the subsequent four samples (~60 ms) were within the same AOI. The latency to enter an AOI was defined from the first accepted sample.
Gaze shifts occurring quicker than 150 ms after target onset were considered anticipatory (Kenward et al., 2017). If an anticipatory gaze shift was detected, the whole trial was discarded (1.47% of trials). Initial plots showed that the distribution of latencies to first fixations at the targets was positively skewed, due to a small proportion of very long latencies (see Supporting Information). We therefore excluded latencies above the 97.5th percentile (> 2130 ms),
Pupil diameter was averaged for the left and right eye, and the signal was filtered using a moving average filter with a window size corresponding to five samples (~150 ms). Samples with unlikely pupil diameters (< 2 mm or > 7 mm) were removed from analysis. A linear interpolation was used over gaps in the data shorter than 150 ms. For each individual, we computed a measure of baseline pupil size by taking the mean pupil size during the 250 ms preceding the visual stimuli during all valid silent trials. The average pupil waveform is shown in Figure 2. Pupil response amplitude was defined as the peak amplitude during the 0–1,500 ms time window after the onset of the visual stimulus, subtracted from the baseline pupil size, expressed as percent change from baseline. The peak was calculated as the median of the three highest values during the response interval. Trials with pupil response amplitudes higher than 25% or smaller than −6% (corresponding to the 97.5th and 2.5th percentile, respectively) were removed from further analysis. Post hoc analyses indicated that pupil size did not vary with gaze point on the screen or the number of eye movements during the trial interval (see Supporting Information).
Figure 2.
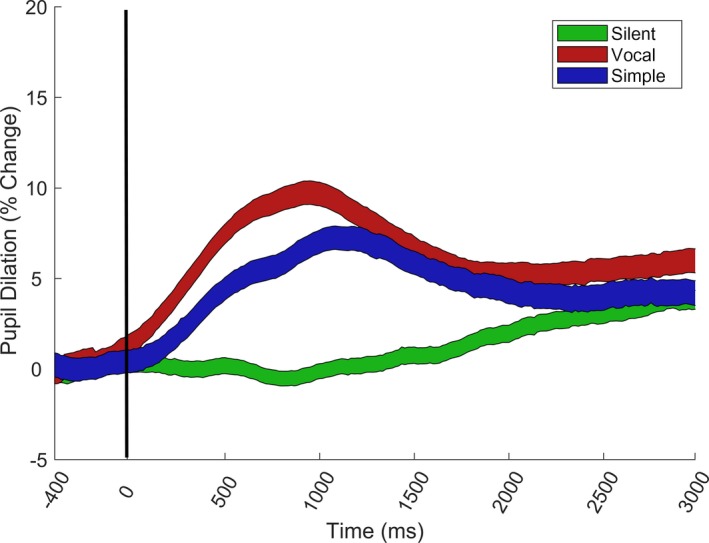
Pupil dilation as a function of time in the vocal, simple and silent conditions. The onset of the visual stimuli is marked as 0 on the X axis. Alerting cues were presented at variable intervals during the −400 to 80 ms interval before the onset of the visual stimuli (see Method). The colored areas cover mean pupil size ± 95% confidence intervals. Pupil dilation is expressed as percentual change from individual baseline.
Analysis Plan
In the analyses related to the effects of alerting cues on visual orienting, data from the three types of visual stimuli (full face, eyes, color deviants) were combined. In the analyses related to the effect of visual stimulus type, data from the three alerting cue conditions (silent, vocal, simple) were combined. To test the validity of this approach, we tested interaction effects between alerting cue (silent, vocal, simple) and visual stimulus type No significant interactions were found (all p > .25).
Statistics and Data Reduction
Data were analyzed using generalized linear models with random intercepts for participants (equivalent to treating multiple observations from one participant as repeated measures). We used three dependent variables: First gaze shifts was a binary variable (coded as a “hit” if the first fixation was at the visual target, and as “miss” if the first fixation was at a distractor). Target latency was defined as the time in milliseconds to the first gaze shift to the target. Our third dependent variable was pupil dilation amplitude (see Recording and Analysis of Eye Tracking Data).
In analyses related to first gaze shifts, the data were analyzed using models with a binominal distribution and a logit link function. In analyses where the dependent variables were continuous (target latency and pupil dilation amplitude), the models were fitted with a Gaussian distribution and an identity link function.
We tested the significance of main and interaction effects by comparing models with and without the effects using likelihood ratio tests. In other words, this analysis tests whether the effect of interest is beneficial for explaining the dependent variable. Outlier observations, defined as standardized residuals outside the ± 3 SD range were removed from analysis. All analyses were conducted in MATLAB. To compare the likelihood of orienting to the visual stimuli to chance level, we computed bootstrapped 95% confidence intervals around the means with 1,000 iterations and compared this value to chance level (0.25).
Results
Effects of Phasic Alerting Cues
We analyzed the likelihood of a first gaze shift at the target as a function of auditory alerting cues using a mixed effects model with alerting cue (silent, simple, vocal) as predictor. Forty infants contributed valid data to this analysis (see Table 1). A significant main effect of alerting cue was found χ2(2) = 6.91, p = .032. Planned follow‐up comparisons demonstrated that first gaze shifts to the target were more likely after simple alerting cues compared to silent trials, χ2(1) = 4.20, p = .040, b = −0.28, SE = 0.14, OR = 0.75. First gaze shifts to targets were also more likely in the simple cue condition compared to the vocal condition χ2(1) = 6.69, p = .010, b = −0.41, SE = 0.16, OR = 0.66. The vocal condition and the silent condition did not differ, χ2(1) = 0.81, p = .367, b = 0.12, SE = 0.14, OR = 1.13. These results are shown in Figure 3. No effect of alerting cue was found on target latency, χ2(1) = 3.52, p = .172, b = −97.97, SE = 62.45.
Figure 3.
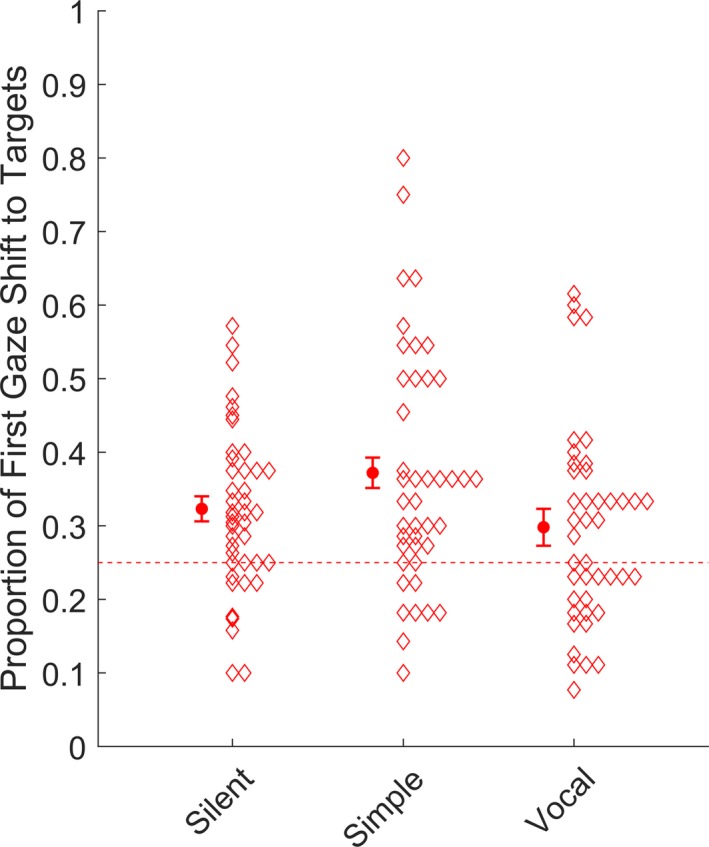
Proportion of first gaze shifts to target silent, simple, and vocal alerting cues. The dashed line represents chance level (0.25). Error bars cover ± 1 SEM. Diamonds represent means for individual participants.
Additionally, we explored whether alerting cues affected the latency to a first fixation inside any AOI, after moving ones gaze from the central attention getter. Thirty‐nine infants contributed valid data to this analysis. We found a main effect of alerting cue, χ2(2) = 19.54, p < .001. Follow‐up comparisons demonstrated that vocal alerting cues reduced the latency to a first gaze shift to any AOI compared to silent trials, χ2(1) = 16.28, p < .001, b = 23.85, SE = 5.89, and compared to simple alerting cues, χ2(1) = 15.49, p < .001, b = 28.65, SE = 7.24. Simple alerting cues did not reduce the latency to a first gaze shift compared to silent trials, χ2(1) = 0.36, p = .548, b = 3.80, SE = 6.32.
Pupil Dilation
Average pupil dilation amplitude differed significantly between the three alerting conditions, as shown in Figure 2.The main effect of condition was highly significant, χ2(1) = 224.52, p < .001. Vocal sounds resulted in larger pupil dilation amplitude than simple cues, χ2(1) = 16.55, p = < .001, b = −1.56, SE = 0.38, and simple cues in turn resulted in larger pupil dilation amplitude than silent trials, χ2(1) = 87.12, p = < .001, b = 3.43, SE = 0.36.
Relation Between Pupil Dilation and Visual Orienting
The above findings demonstrate that simple alerting cues increased the likelihood of a first gaze shifts to targets and were also associated with an increase in pupil dilation amplitude. This suggests that the effect on visual attention may have been mediated by an increase in arousal. Intriguingly, however, only the simple auditory cues produced a facilitating effect. The vocal sounds did not affect visual selection, even if this condition resulted in the strongest effect on pupil dilation. This suggests that the relation between pupil dilation and target selection may follow an inverted U‐shaped pattern, with optimal detection of targets at intermediate levels of arousal. To test this hypothesis, we compared two logistic generalized regression models with first gaze shift to targets as dependent variable and pupil response as a continuous predictor to the data. In the first model, we included a linear term only. This model was significant, χ2(1) = 6.38, p = .012, b = 0.02, SE = 0.01, OR = 1.02. In the second model, we included a quadratic term. This model was significant and indicated an inverted U‐shaped response, χ2(1) = 9.29, p = .010, b = < −0.01, SE = < 0.01 (Figure 4). A comparison between the two models showed that the quadratic model was superior to the linear, χ2(1) = 3.96, p = .047. To sum up, the relation between pupil dilation amplitude and first gaze shifts to targets was best explained by an inverted U‐shaped function. These results are shown in Figure 4. The relation between pupil dilation amplitude and likelihood of a first fixation at the targets was not qualified by any interaction effects involving sound or visual stimulus type (p > .25).
Figure 4.
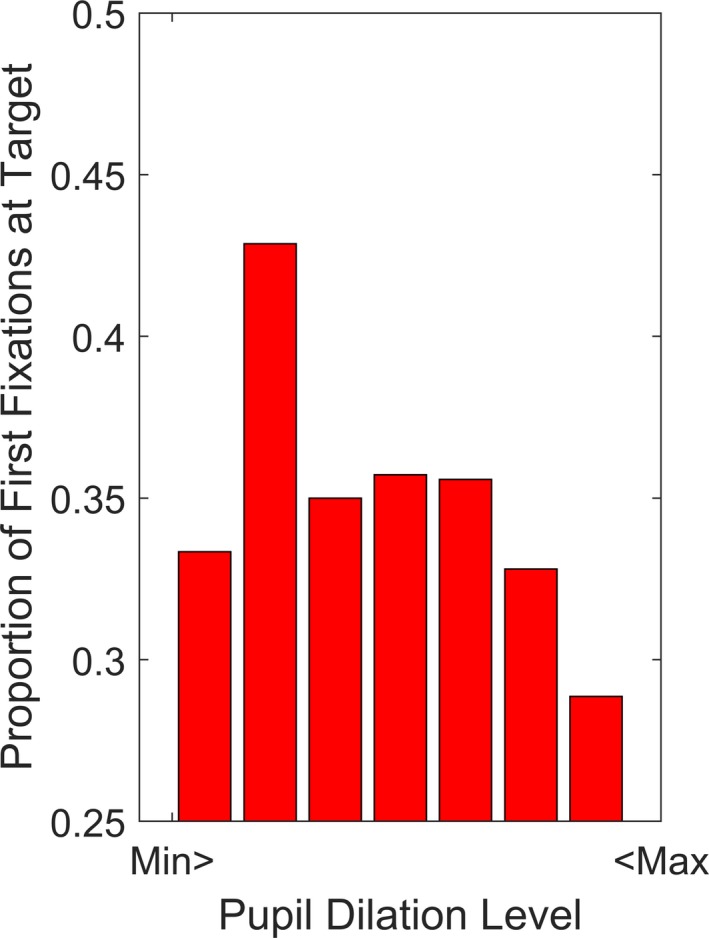
Proportion of first fixations at the target at different levels of pupil dilation amplitude (see Method). Each bar covers 50% of the interquartile range. The Y axis starts at chance level (0.25).
In a second step, we examined the relation between pupil dilation amplitude and the latency to a first gaze shift inside any AOI (target or distractor). This analysis showed a significant negative linear response, χ2(1) = 36.84, p < .001, b = 5.46, SE = 0.89, A quadratic model did not fit the data better than the linear model, χ2(1) = 0.06, p = .810, b = 0.03, SE = 0.10. To sum up, this suggests that on the level of individual trials, larger pupil response amplitude was linked to quicker gaze shifts to any AOI, and that this relation was linear. The relation was not qualified by any interaction effects involving sound or visual stimulus type (p > .25).
Effects of Visual Stimulus Type
In total, 42 infants contributed data to the analysis of first gaze shifts as a function of visual stimulus type (see Table 2). A main effect of condition was found, χ2(2) = 53.29, p < .001. Follow‐up comparisons showed that first gaze shifts to targets were more likely in the whole face than in the eyes condition, χ2(1) = 25.98, p = < .001, b = 0.66, SE = 0.13, OR = 1.93, and in turn higher for eyes than for color deviants, χ2(1) = 4.17, p = .041, b = −0.29, SE = 0.14, OR = 0.75. These data are shown in Figure 5.
Figure 5.
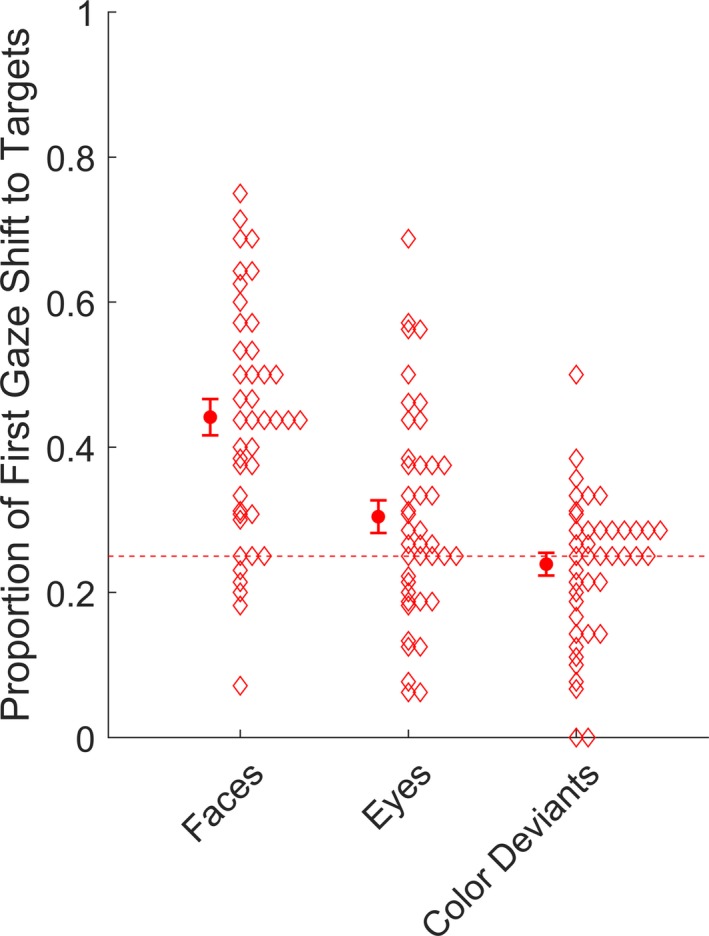
Proportion of first gaze shifts to whole faces, eyes, and color deviants. The dashed line represents chance level (0.25). Error bars cover ± 1 SEM. Diamonds represent means for individual participants.
We compared the proportion of gaze shifts to targets against chance level (i.e., a proportion of 0.25) by bootstrapping 95% studentized confidence intervals (CI) around the means of each of the variables and comparing these values to chance level. The bootstrapped CIs did not include chance level for whole faces, M = .45, 95% CI [.41, .50], or eyes M = .30, 95% CI [.26, .34]. In contrast, the mean proportion of first fixation at the color deviants had a confidence interval that included chance level, M = .24, 95% CI [.20, .28]. We found no evidence for differences in target latency between the three visual stimulus types, χ2(2) = 2.89, p = .24.
Discussion
The first aim of this study was to examine the effect of phasic alerting on visual orienting. We hypothesized that alerting cues would increase orienting to visual targets. This hypothesis was supported but only for auditory simple and not for more complex (vocal) phasic alerting cues. That is, a brief sound presented just before the onset of the visual stimulus increased the likelihood that the first fixated object was the visual target rather than a distractor, irrespective of whether the target was a facial stimulus or a color deviant. Despite the lack of a clear effect of vocal sounds on the likelihood of a first fixation at the targets, vocal sound increased overall speed of visual orienting.
As noted in the introduction, phasic alerting affects are believed to be related to transient increase in arousal (Fernandez‐Duque & Posner, 1997; Sturm & Willmes, 2001; but see Weinbach & Henik, 2014). Monoaminergic neurotransmitters, and particularly the LC‐NE system, have been implicated phasic alerting (Aston‐Jones & Cohen, 2005). In line with our hypothesis, we demonstrated a relation between pupil dilation, a peripheral marker of LC‐NE activity, and phasic alerting that has previously not been described in infancy. First, both simple and vocal alerting reliably caused larger pupil dilations than silent trials. Second, we found evidence for an inverted U‐shaped relation between pupil dilation and the likelihood of a first fixation at the targets, with highest likelihood of a first fixation at the targets at intermediate levels of arousal. Higher pupil dilation amplitude was also linked to faster gaze shifts to any object (target or distractor), again suggesting that the nonspecific increase in speed of visual orienting observed after vocal alerting cues was linked to an effect on arousal.
Current models of LC‐NE functioning in attention describe an inverted U‐shaped relation between attention and tonic LC‐NE arousal. Intermediate levels of arousal increase attention to salient and task‐relevant targets, whereas high levels of arousal lead to quick but unselective responses that ultimately impair performance (Aston‐Jones & Cohen, 2005; Bouret & Sara, 2004). Our results suggest a similar relation between phasic alerting and visual orienting. We also found fast, but nonselective responses after vocal sounds, which together with the observed high pupil dilation level, is consistent with a hyper‐aroused state. It is notable that in previous research, an inverted U‐shaped relation between LC‐NE arousal and attention has mainly been described in relation to tonic activity (e.g., Aston‐Jones & Cohen, 2005), and it is less clear whether this pattern would also be expected for phasic activity—as the current data suggest is the case in infancy. A second explanation is that the vocal sounds failed to increase the likelihood of orienting to targets not only because they led to high levels of arousal but also because their social or speech‐like character triggered additional processing that interfered with an otherwise facilitating alerting effect. A recent study in 12‐month‐old infants (de Barbaro, Clackson, & Wass, 2017) reported that indices of slow phasic changes in autonomic arousal (heart rate and skin conductance) occurring on a time scale of approximately 20 s predicted a more vigilant visual scanning.
In a previous study (Kleberg, Högström, et al., 2017), we found a facilitating effect of phasic alerting on social orienting in young children with autism, a disorder of social communication. In these children, both simple and vocal sounds led to faster social orienting. In contrast, a group of typically developing children showed the opposite effect, with slower social orienting after alerting cues. Taken together, this suggests that the role of phasic arousal in social orienting may change with development and could be different in autism.
A recent study in adults (Asutay & Västfjäll, 2017) demonstrated that phasic alerting facilitates visual search (i.e., by enhancing the pop‐out effect) in adults. Asutay and Västfjäll (2017) used nonsocial visual targets and a range of both social and nonsocial auditory phasic alerting cues. The authors reported that the strength of the facilitating effect was predicted by participants’ arousal ratings of the auditory cues. Together with this study, this suggests that the auditory alerting cues affect visual orienting through an effect on arousal level and that these effects may be found from infancy to adulthood.
A surprising finding of this study was that no pop‐out effect was found for the color deviant stimuli. Color pop out is a robust phenomenon in adults (e.g., Treisman, 1980) and has previously been reported in one infant study (Catherwood et al., 1996). Although the pop‐out effect is typically interpreted as a bottom‐up driven phenomenon, studies in adults as well as in animals have documented that the motivational value of the stimuli affects the pop‐out effect via top‐down modulation (Hsieh et al., 2011). It is possible that the color deviant stimuli used in this study were not sufficiently motivationally salient to elicit a pop‐out effect. It is also possible that the inclusion of many different types of stimuli in a between subjects design may have influenced the infants’ responses to these, presumably least salient, visual targets.
In addition to the results related to phasic alerting and pupil dilation reported above, we found that, as predicted, both eyes and whole faces were more likely to be the target of a first gaze shift than would be expected by chance. This demonstrates that, even in isolation, human eyes trigger a pop‐out effect in infants. However, the pop‐out effect was stronger for whole faces than for eyes, demonstrating that other sources of information than eyes alone contribute to orienting to faces. It should also be noted that the pop‐out effect was stronger for facial information than for color deviants. There is an ongoing debate about the visual characteristics of stimuli that elicit preferential orienting to social stimuli in infants (e.g., Farroni et al., 2005; Johnson, Senju & Tomalski, 2015; Viola Macchi, Turati, & Simion, 2004; Wilkinson, Paikan, Gredebäck, Rea, & Metta, 2014). Our results contribute to this literature by showing that eyes can elicit preferential visual orienting even when presented in isolation. This supports the theory that the brain has perceptual mechanisms that respond to eyes rather than to whole face configurations (Batki et al., 2000). Our results are also in line with a previous study (Gluckman & Johnson, 2013) that reported pop‐out effects in infants for other social stimuli than faces.
Future Directions
We used two types of alerting cues, one simple and one vocal. As noted in the introduction, these two cues differ on multiple dimensions, including their social significance. Because this was the first study using the current paradigm in infancy, we contrasted to disparate types of alerting cues. Future studies should manipulate several dimensions of the alerting cues such as perceived arousal, emotional valence and linguistic content to get a more detailed understanding of how these dimensions relate to the phasic alerting effect. As can be seen in Figures 3 and 5, there was considerable individual variation in the strength of the experimental effects. Future studies should examine whether this variability is related to other areas of infant development, both cross‐sectionally and longitudinally. It could also be interesting to manipulate additional aspects of the visual distractors, including their top heaviness.
Supporting information
Figure S1. Mean Pupil Size as a Function of X Coordinate (Left) and Y Coordinate (Right) Plotted as Black Lines (‐‐)
Figure S2. Proportion of All Valid Trials With One, Two, and Three Areas of Interest Visited During the First 1,500 ms
Figure S3. Mean Pupil Dilation Response on Trials With One (N = 854), Two (N = 725), and Three (N = 34) Areas of Interest Visited
Figure S4. Distribution of All Latencies to a First Gaze Shift at the Target (Social or Nonsocial)
This research was supported by grants to Terje Falck‐Ytter from the Swedish Research Council (2015‐03670), Stiftelsen Riksbankens Jubileumsfond (NHS14‐1802:1) and the Strategic Research Area Neuroscience at Karolinska Institutet (StratNeuro). The authors declare that they have no conflicts of interest.
We thank Gustaf Gredebäck for advice on pupil dilation analyses and Marcus Nyström for statistical advice.
References
- Adler, S. A. , & Orprecio, J. (2006). The eyes have it: Visual pop‐out in infants and adults. Developmental Science, 9, 189–206. 10.1111/j.1467-7687.2006.00479.x [DOI] [PubMed] [Google Scholar]
- Arnsten, A. F. T. (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Neuroscience, 10, 410–422. 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston‐Jones, G. , & Cohen, J. D. (2005). An integrative theory of locus coeruleus‐norephinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Asutay, E. , & Västfjäll, D. (2017). Exposure to arousal‐inducing sounds facilitates visual search. Scientific Reports, 7, 10363 10.1038/s41598-017-09975-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batki, A. , Baron‐Cohen, S. , Wheelwright, S. , Connellan, J. , & Ahluwalia, J. (2000). Is there an innate gaze module? Evidence from human neonates. Infant Behaviour and Development, 23, 223–229. [Google Scholar]
- Bogler, C. , Bode, S. , & Haynes, J.‐D. (2013). Orientation pop‐out processing in human visual cortex. NeuroImage, 81, 73–80. 10.1016/j.neuroimage.2013.05.040 [DOI] [PubMed] [Google Scholar]
- Bouret, S. , & Sara, S. J. (2004). Reward expectation, orientation of attention and locus coeruleus‐medial frontal cortex interplay during learning. European Journal of Neuroscience, 20, 791–802. 10.1111/j.1460-9568.2004.03526.x [DOI] [PubMed] [Google Scholar]
- Callejas, A. , Lupiàñez, J. , Funes, M. J. , & Tudela, P. (2005). Modulations among the alerting, orienting and executive control networks. Experimental Brain Research, 167, 27–37. 10.1007/s00221-005-2365-z [DOI] [PubMed] [Google Scholar]
- Cassia, V. M. , Simion, F. , Umilta, C. , & Macchi Cassia, V. (2001). Face preference at birth: The role of an orienting mechanism. Developmental Science, 4, 101–108. 10.1111/1467-7687.00154 [DOI] [Google Scholar]
- Catherwood, D. , Skoien, P. , & Holt, C. (1996). Colour pop‐out in infant response to visual arrays. British Journal of Developmental Psychology, 14, 315–326. 10.1111/j.2044-835X.1996.tb00708.x [DOI] [Google Scholar]
- Colombo, J. (2002). Infant attention grows up: The emergence of a developmental cognitive neuroscience perspective. Current Directions in Psychological Science, 11, 196–200. 10.1111/1467-8721.00199 [DOI] [Google Scholar]
- de Barbaro, K. , Chiba, A. , & Deák, G. O. (2011). Micro‐analysis of infant looking in a naturalistic social setting: Insights from biologically based models of attention. Developmental Science, 14, 1150–1160. 10.1111/j.1467-7687.2011.01066.x [DOI] [PubMed] [Google Scholar]
- de Barbaro, K. , Clackson, K. , & Wass, S. (2016). Stress reactivity speeds basic encoding processes in infants. Developmental Psychobiology, 58, 546–555. 10.1002/dev.21399 [DOI] [PubMed] [Google Scholar]
- de Barbaro, K. , Clackson, K. , & Wass, S. V. (2017). Infant attention is dynamically modulated with changing arousal levels. Child Development, 88(2), 629–639. [DOI] [PubMed] [Google Scholar]
- Di Giorgio, E. , Turati, C. , Altoè, G. , & Simion, F. (2012). Face detection in complex visual displays: An eye‐tracking study with 3‐ and 6‐month‐old infants and adults. Journal of Experimental Child Psychology, 113, 66–77. 10.1016/j.jecp.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Dupierrix, E. , Hillairet De Boisferon, A. , Méary, D. , Lee, K. , Quinn, P. C. , Di Giorgio, E. , … Pascalis, O. (2014). Preference for human eyes in human infants. Journal of Experimental Child Psychology, 123, 138–146. 10.1016/j.jecp.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh, M. , Gliga, T. , Pickles, A. , Hudry, K. , Charman, T. , & Johnson, M. H. (2013). The development of face orienting mechanisms in infants at‐risk for autism. Behavioural Brain Research, 251, 147–154. 10.1016/j.bbr.2012.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , Gu, X. , Guise, K. G. , Liu, X. , Fossella, J. , Wang, H. , & Posner, M. I. (2009). Testing the behavioral interaction and integration of attentional networks. Brain and Cognition, 70, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, J. , McCandliss, B. D. , Fossella, J. , Flombaum, J. I. , & Posner, M. I. (2005). The activation of attentional networks. NeuroImage, 26, 471–479. [DOI] [PubMed] [Google Scholar]
- Farroni, T. , Johnson, M. H. , Menon, E. , Zulian, L. , Faraguna, D. , & Csibra, G. (2005). Newborns’ preference for face‐relevant stimuli: Effects of contrast polarity. Proceedings of the National Academy of Sciences of the United States of America, 102, 17245–17250. 10.1073/pnas.0502205102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Duque, D. , & Posner, M. I. (1997). Relating the mechanisms of orienting and alerting. Neuropsychologia, 35, 477–486. 10.1016/S0028-3932(96)00103-0 [DOI] [PubMed] [Google Scholar]
- Gliga, T. , & Dehaene‐Lambertz, G. (2007). Development of a view‐invariant representation of the human head. Cognition, 102, 261–288. 10.1016/j.cognition.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Gliga, T. , Elsabbagh, M. , Andravizou, A. , & Johnson, M. H. (2009). Faces attract infants’ attention in complex displays. Infancy, 14, 550–562. 10.1080/15250000903144199 [DOI] [PubMed] [Google Scholar]
- Gluckman, M. , & Johnson, S. P. (2013). Attentional capture by social stimuli in young infants. Frontiers in Psychology, 4, 1–7. 10.3389/fpsyg.2013.00527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl, S. (2015). How do neural responses to eyes contribute to face—sensitive ERP components in young infants? A rapid repetition study. Brain and Cognition, 95, 1–6. 10.1016/j.bandc.2015.01.010 [DOI] [PubMed] [Google Scholar]
- Hsieh, P.‐J. , Colas, J. T. , & Kanwisher, N. (2011). Pop‐out without awareness: Unseen feature singletons capture attention only when top‐down attention is available. Psychological Science, 22, 1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier, R. J. , & Batty, M. (2009). Neural bases of eye and gaze processing: The core of social cognition. Neuroscience & Biobehavioral Reviews, 33, 843–863. 10.1016/j.neubiorev.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. , Senju, A. , & Tomalski, P. (2015). The two‐process theory of face processing: Modifications based on two decades of data from infants and adults. Neuroscience and Biobehavioral Reviews, 50, 169–179. 10.1016/j.neubiorev.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Jones, E. J. H. , Dawson, G. , & Webb, S. J. (2018). Sensory hypersensitivity predicts enhanced attention capture by faces in the early development of ASD. Developmental Cognitive Neuroscience, 29, 11–20. 10.1016/j.dcn.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenward, B. , Koch, F.‐S. , Forssman, L. , Brehm, J. , Tidemann, I. , Sundqvist, A. , … Gredebäck, G. (2017). Saccadic reaction times in infants and adults: Spatiotemporal factors, gender, and interlaboratory variation. Developmental Psychology, 53, 1750–1764. 10.1037/dev0000338 [DOI] [PubMed] [Google Scholar]
- Kleberg, J. L. , Högström, J. , Nord, M. , Bölte, S. , Serlachius, E. , & Falck‐Ytter, T. (2017). Autistic traits and symptoms of social anxiety are differentially related to attention to others’ eyes in social anxiety disorder. Journal of Autism and Developmental Disorders, 47, 3814–3821. 10.1007/s10803-016-2978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleberg, J. L. , Thorup, E. , & Falck‐Ytter, T. (2017a). Reduced visual disengagement but intact phasic alerting in young children with autism. Autism Research, 10, 539–545. 10.1002/aur.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleberg, J. L. , Thorup, E. , & Falck‐Ytter, T. (2017b). Visual orienting in children with autism: Hyper‐responsiveness to human eyes presented after a brief alerting audio‐signal, but hyporesponsiveness to eyes. Autism Research, 10, 246–250. 10.1002/aur.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusnir, F. , Chica, A. B. , Mitsumasu, M. A. , & Bartolomeo, P. (2011). Phasic auditory alerting improves visual conscious perception. Consciousness and Cognition, 20, 1201–1210. [DOI] [PubMed] [Google Scholar]
- Kwon, M. K. , Setoodehnia, M. , Baek, J. , Luck, S. J. , & Oakes, L. M. (2016). The development of visual search in infancy: Attention to faces versus salience. Developmental Psychology, 52, 537–555. 10.1037/dev0000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng, B. , Sirois, S. , & Gredebäck, G. (2012). Pupillometry. A Window to the Preconscious?. Perspectives on Psychological Science, 7, 18–27. 10.1177/1745691611427305 [DOI] [PubMed] [Google Scholar]
- Mather, M. , Clewett, D. , Sakaki, M. , & Harley, C. W. (2016). Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences, 39, 1–75. 10.1017/S0140525X15000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, P. R. , O'Connell, R. G. , O'Sullivan, M. , Robertson, I. H. , & Balsters, J. H. (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping, 35, 4140–4154. 10.1002/hbm.22466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes, L. M. , & Ellis, A. E. (2013). An eye‐tracking investigation of developmental changes in infants’ exploration of upright and inverted human faces. Infancy, 18, 134–148. 10.1111/j.1532-7078.2011.00107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, A. , Hilkjaer Petersen, A. , Bundesen, C. , Vangkilde, S. , & Habekost, T. (2017). The effect of phasic auditory alerting on visual perception. Cognition, 165, 73–81. 10.1016/j.cognition.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Posner, M. I. , Rothbart, M. K. , Sheese, B. E. , & Voelker, P. (2014). Developing attention: Behavioral and brain mechanisms. Advances in Neuroscience, 2014, 1–9. 10.1155/2014/405094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer, J. , McGinley, M. J. , Liu, Y. , Rodenkirch, C. , Wang, Q. , McCormick, D. A. , & Tolias, A. S. (2016). Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nature Communications, 7, 13289 10.1038/ncomms13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, P. , Happé, F. , Sowden, S. , Cook, R. , & Bird, G. (2015). Orienting toward face‐like stimuli in early childhood. Child Development, 86, 1693–1700. 10.1111/cdev.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simion, F. , Valenza, E. , Umiltà, C. , & Barba, B. D. (1998). Preferential orienting to faces in newborns: A temporal–nasal asymmetry. Journal of Experimental Psychology: Human Perception and Performance, 24, 1399–1405. 10.1037/0096-1523.24.5.1399 [DOI] [PubMed] [Google Scholar]
- Sturm, W. , & Willmes, K. (2001). On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage, 14(1), S76–S84. 10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- Treisman, A. M. , & Gelade, G. (1980). A feature‐integration theory of attention. Cognitive Psychology, 12(1), 97–136. [DOI] [PubMed] [Google Scholar]
- Viola Macchi, C. , Turati, C. , & Simion, F. (2004). Can a nonspecific bias toward top‐heavy patterns explain newborns’ face preference? Psychological Science, 15, 379–383. 10.1111/j.0956-7976.2004.00688.x [DOI] [PubMed] [Google Scholar]
- Wang, C.‐A. , & Munoz, D. P. (2015). A circuit for pupil orienting responses: Implications for cognitive modulation of pupil size. Current Opinion in Neurobiology, 33, 134–140. 10.1016/j.conb.2015.03.018 [DOI] [PubMed] [Google Scholar]
- Weinbach, N. , & Henik, A. (2012). Temporal orienting and alerting ‐the same or different?. Frontiers in Psychology, 3, 236, 10.3389/fpsyg.2012.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbach, N. , & Henik, A. (2014). Alerting enhances attentional bias for salient stimuli: Evidence from a global/local processing task. Cognition, 133, 414–419. [DOI] [PubMed] [Google Scholar]
- Wetzel, N. , Buttelmann, D. , Schieler, A. , & Widmann, A. (2016). Infant and adult pupil dilation in response to unexpected sounds. Developmental Psychobiology, 58, 382–392. 10.1002/dev.21377 [DOI] [PubMed] [Google Scholar]
- Wilkinson, N. , Paikan, A. , Gredebäck, G. , Rea, F. , & Metta, G. (2014). Staring us in the face? An embodied theory of innate face preference. Developmental Science, 17, 809–825. 10.1111/desc.12159 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean Pupil Size as a Function of X Coordinate (Left) and Y Coordinate (Right) Plotted as Black Lines (‐‐)
Figure S2. Proportion of All Valid Trials With One, Two, and Three Areas of Interest Visited During the First 1,500 ms
Figure S3. Mean Pupil Dilation Response on Trials With One (N = 854), Two (N = 725), and Three (N = 34) Areas of Interest Visited
Figure S4. Distribution of All Latencies to a First Gaze Shift at the Target (Social or Nonsocial)


