Abstract
Naldemedine is a peripherally acting μ‐opioid‐receptor antagonist for the treatment of opioid‐induced constipation. Two phase 1 single‐dose studies investigated the pharmacokinetics and safety of a 0.2‐mg oral dose of naldemedine in subjects with renal impairment (mild, n = 9; moderate, n = 9; severe, n = 6; and end‐stage renal disease, n = 8) or hepatic impairment (mild or moderate, n = 8 each) and demographically matched healthy subjects with normal renal and hepatic function (n = 8, both studies). Pharmacokinetic assessments indicate that dose adjustments for naldemedine are not necessary for subjects with any degree of renal impairment or for subjects with mild or moderate hepatic impairment. In subjects with renal impairment compared with healthy subjects with normal renal function, the geometric mean ratios of naldemedine area under the concentration‐time curve (AUC0‐inf) ranged from 82.8% (90%CI 69.5% to 98.6%) to 137.8% (90%CI 114.0% to 166.5%). Renal clearance decreased with reduced renal function (normal function 1.3 L/h; mild impairment 1.1 L/h; moderate impairment 1.0 L/h; severe impairment 0.5 L/h), and only 2.7% of naldemedine was removed by hemodialysis. In subjects with hepatic impairment compared with healthy subjects with normal hepatic function, the geometric mean ratio of AUC0‐inf ranged from 82.8% (90%CI 65.7% to 104.5%) to 105.2% (90%CI 83.4% to 132.6%). Naldemedine was well tolerated in both healthy subjects and subjects with renal or hepatic impairment, and reported adverse events were generally consistent with the known safety profile.
Keywords: hepatic impairment, naldemedine, opioid‐induced constipation, peripherally‐acting μ‐opioid‐receptor antagonist, renal impairment
Opioids are commonly used to manage moderate to severe pain as evidenced by nearly 300 million opioid prescriptions in the United States in 2012 alone.1, 2 Despite their effectiveness, treatment with opioids can lead to adverse effects, such as opioid‐induced constipation.1, 3 Opioid‐induced constipation results from the binding of opioids to peripheral μ‐opioid receptors in the gastrointestinal tract and is one of the most common adverse opioid reactions for patients receiving opioid analgesics.1, 4 Laxatives are considered a first‐line treatment in patients with opioid‐induced constipation; however, their mechanism of action does not treat the underlying cause of opioid‐induced constipation, and they are ineffective in >50% of individuals.5, 6, 7 Moreover, laxatives are often associated with side effects such as gas and bloating in patients with opioid‐induced constipation, which further diminishes patients’ quality of life.6
Naldemedine is an oral peripherally acting μ‐opioid‐receptor antagonist approved for the treatment of opioid‐induced constipation in adults with chronic noncancer pain (United States and Japan) and adults with cancer (Japan).8 Naldemedine minimally crosses the blood‐brain barrier and, therefore, effectively treats opioid‐induced constipation without negating the analgesic effects of opioid therapy.9 In 2 randomized, placebo‐controlled, phase 1 studies in healthy subjects, naldemedine was rapidly absorbed; the median time to the maximum plasma concentration (Tmax) of naldemedine ranged from 0.5 to 0.75 hours with either single ascending doses (0.1 to 100 mg) or multiple ascending doses (3 to 30 mg) over the course of 10 days.10 Moreover, systemic exposure to naldemedine was nearly dose proportional for single ascending doses, and only slight increases in peak plasma concentration (Cmax) and area under the concentration‐time curve (AUC0‐t) (1‐ to 1.3‐fold) were observed following multiple daily doses of naldemedine.10 No major safety or tolerability issues were observed in either study.10
Naldemedine is primarily metabolized via cytochrome P450 (CYP)3A4 to nor‐naldemedine.10, 11 Another phase 1 study, in which healthy subjects were administered 2 mg of [oxadiazole‐14C]‐ or [carbonyl‐14C]‐naldemedine, showed that nor‐naldemedine was the primary metabolite observed in the plasma and accounted for 9% to 13% of the systemic exposure of unchanged naldemedine.11 Following administration of [oxadiazole‐14C]‐naldemedine, 57% of the total radioactivity was recovered in the urine, and 35% was recovered in the feces.11 Additionally, 16% to 18% of the administered dose was excreted unchanged in the urine.11
Although it has been demonstrated that naldemedine is metabolized and excreted by the kidney and the liver,10, 11 the pharmacokinetics and safety of naldemedine in subjects with renal or hepatic impairment have yet to be determined. Here, we report results from 2 phase 1 studies evaluating the pharmacokinetics and safety of oral naldemedine in subjects with renal impairment (renal impairment study; study number 1401V921B) or hepatic impairment (hepatic impairment study; study number 1402V921C) compared with healthy, demographically matched subjects without renal or hepatic impairment, respectively.
Methods
Study Design
Both nonrandomized open‐label parallel‐cohort phase 1 studies were approved by an Institutional Review Board (IntegReview IRB, Austin, Texas) and conducted in accordance with the International Council for Harmonisation Good Clinical Practice guideline and the Declaration of Helsinki. All subjects provided written informed consent. Both studies were conducted across clinical research units located in the United States (DaVita Clinical Research, Lakewood, Colorado [renal and hepatic impairment studies]; DaVita Clinical Research, Minneapolis, Minnesota [renal and hepatic impairment studies]; Orlando Clinical Research Center, Orlando, Florida [renal impairment study]; Clinical Pharmacology of Miami, Inc, Hialeah, Florida [hepatic impairment study]).
The renal and hepatic impairment studies were designed in accordance with US FDA Guidance for Industry (Pharmacokinetics in Patients With Impaired Renal Function and Pharmacokinetics in Patients With Impaired Hepatic Function, respectively) as described below.12, 13 A 0.2‐mg dose of naldemedine was selected for these studies based on results from prior phase 2 and phase 3 studies, which demonstrated that 0.2 mg naldemedine administered once daily was a well‐tolerated dose with efficacy for the treatment of opioid‐induced constipation.14, 15, 16 Additionally, 0.2 mg naldemedine is the approved therapeutic dose in the United States.8
The renal impairment study consisted of 5 cohorts categorized as healthy subjects with normal renal function and subjects with mild renal impairment, moderate renal impairment, severe renal impairment, or end‐stage renal disease (ESRD) requiring hemodialysis. The coprimary objectives of the renal impairment study were to assess the pharmacokinetics of naldemedine after administration of a single, oral, 0.2‐mg dose of naldemedine in subjects with renal impairment compared with healthy subjects with normal renal formal function and to assess the effect of hemodialysis on removal of naldemedine from blood (in the cohort of subjects with ESRD requiring hemodialysis).
The hepatic impairment study consisted of 3 cohorts categorized as healthy subjects with normal hepatic function or subjects with mild or moderate hepatic impairment. Subjects with severe hepatic impairment were not enrolled in this study because opioids are known to precipitate or aggravate hepatic encephalopathy.17, 18 The primary objective of the hepatic impairment study was to evaluate the pharmacokinetics of naldemedine after administration of a single oral 0.2‐mg dose of naldemedine in subjects with hepatic impairment compared with healthy subjects with normal hepatic function. The secondary objectives in both studies were to evaluate both the safety and tolerability of naldemedine in subjects with renal or hepatic impairment compared with healthy subjects with normal hepatic function.
All subjects were screened between day –28 and day –2 to assess study eligibility. Qualified subjects were admitted to the clinical research units 1 day before administration of naldemedine and were discharged 3 days postdose. Except for the cohort of subjects with ESRD requiring hemodialysis, all subjects received a single dose of naldemedine after an overnight fast of ≥8 hours, which continued for 4 hours postdose. Subjects returned to the clinical research unit at 15 days (±2 days) postdose for a follow‐up visit. Subjects with ESRD requiring hemodialysis received the first dose of naldemedine 1 to 2 hours after completion of hemodialysis, following a fast of ≥4 hours (defined as treatment period 1). Subjects continued fasting for 2 hours postdose, followed by a snack and then a regular meal at 4 hours postdose. On day 15, subjects in this cohort received a second dose of 0.2 mg naldemedine 2 hours before hemodialysis, following an overnight fast of ≥8 hours (defined as treatment period 2). Subjects continued fasting for 4 hours postdose. Subjects in the ESRD cohort returned to the clinical research unit 29 days (±2 days) postdose for a follow‐up visit.
Study Population
In both studies eligible subjects were male or female, with a body weight >50 kg, a body mass index of 18.5 to 38.0 kg/m2, and age 20 to 75 years (renal impairment study) or 20 to 70 years (hepatic impairment study) at the time of informed consent.
In the renal impairment study healthy subjects with normal renal function had an estimated creatinine clearance ≥90 mL/min as calculated by the Cockcroft‐Gault equation.19 Each of these subjects was matched demographically to a subject with moderate renal impairment with respect to sex, age (±10 years), and body mass index (±20%). The degree of renal impairment was determined by estimated glomerular filtration rate (eGFR) calculated according to the Modification of Diet in Renal Disease criteria.20 Mild renal impairment was defined as eGFR ≥60 to <90 mL/(min·1.73 m2), moderate renal impairment was defined as eGFR ≥30 to <60 mL/(min·1.73 m2), and severe renal impairment was defined as eGFR <30 mL/(min·1.73 m2) (including subjects with ESRD not receiving hemodialysis). Further, all subjects with mild, moderate, or severe renal impairment had stable renal function (<30% difference between eGFR values at screening and the day before administration of naldemedine). Subjects requiring hemodialysis were eligible if they required hemodialysis ≥3 times per week for ≥6 months before screening, were considered clinically stable with respect to underlying renal impairment, and had not started any new drug or changed dosages from 14 days before the administration of naldemedine until the study completion. Subjects with any degree of renal impairment (including ESRD with hemodialysis) and hypertension were eligible if they had satisfactory control of blood pressure.
In the hepatic impairment study healthy subjects with normal hepatic function (Child‐Pugh score <5) were each matched demographically to a subject with moderate hepatic impairment with respect to sex, age (±10 years), and body mass index (±20%). Degrees of hepatic impairment were determined by Child‐Pugh score.21 Mild hepatic impairment was defined as a Child‐Pugh score of 5 or 6, and moderate hepatic impairment was defined as a Child‐Pugh score between 7 and 9. Subjects with hepatic impairment were included if they had stable hepatic function for ≥1 month before screening and did not start a new drug or change dosages within 14 days before naldemedine administration until the study completion.
Subjects were excluded from either study if they had a life expectancy <3 months, a clinically significant medical history that would introduce additional safety risk to the subject by participation or would interfere with study results, or a history of a gastrointestinal surgery that would potentially interfere with absorption of naldemedine. In the original protocol for the renal impairment study, a history of cholecystectomy was not indicated as an exclusion criterion. When subjects with a history of cholecystectomy were enrolled, they were included in the safety evaluation; pharmacokinetic evaluations were not conducted, thereby excluding any potential influence from prior cholecystectomy. Following a protocol amendment, additional subjects with a history of cholecystectomy were excluded from the renal impairment study.
Naldemedine is a known substrate of the P‐glycoprotein efflux transporter (P‐gp) and is primarily metabolized by CYP3A4.10, 11, 22 Therefore, prior and concomitant medications were recorded, and P‐gp receptor and CYP3A enzyme inhibitors were prohibited from 2 weeks before admission to the clinical research units until study completion. P‐gp receptor and CYP3A enzyme inducers were prohibited from 4 weeks before admission to the clinical research units until the study completion.
Sample Collection and Analyses of Blood, Urine, and Dialysate
In both studies venous blood samples for determination of plasma naldemedine concentrations were collected predose (−0.25 hours) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 12, 24, 36, 48, 60, and 72 hours postdose. Blood samples for determination of serum protein binding were collected 0.75 and 24 hours postdose. Urine samples were collected predose (−12 to 0 hours) and at 0 to 12, 12 to 24, 24 to 36, 36 to 48, 48 to 60, and 60 to 72 hours postdose. For subjects in the renal impairment study with ESRD requiring hemodialysis, blood samples (collected on the arm opposite the dialyzer during treatment period 2) and urine samples were collected during each treatment period. For hemodialysis the same dialyzer equipment with a new membrane was used for each treatment period. During treatment period 2, aliquots of dialysate solution were collected 3, 4, 5, and 6 hours postdose or at the end of hemodialysis.
Determination of plasma, urinary, or dialysate naldemedine concentrations in both studies was performed by Shin Nippon Biomedical Laboratories, Ltd (Kainan, Wakayama, Japan), using fully validated liquid chromatography/tandem mass spectrometry methods. Naldemedine and its internal standard (naldemedine‐15Nd 5) were extracted from plasma and dialysate using a solid‐phase extraction cartridge (Sep‐Pak Vac tC18 1 cc [100 mg]; Waters Corporation, Milford, Massachusetts; for plasma) or a solid‐phase extraction plate (Sep‐Pak Vac tC18 [100 mg]; Waters Corporation; for dialysate). Urine samples were extracted by protein precipitation using methanol (with dissolved naldemedine‐15Nd 5) and then diluted. Plasma and dialysate extracts and processed urine samples were chromatographed on a Shimadzu 10A (renal impairment samples) or Shimadzu 20A (renal and hepatic impairment samples) HPLC (Shimadzu, Kyoto Prefecture, Japan) using a binary gradient system. For plasma and urine samples, mobile phase A was ultrapure water/1 mol/L ammonium formate/formic acid (98.0%) (990:10:1, v/v/v), and mobile phase B was acetonitrile/methanol/2‐propanol/ultrapure water/1 mol/L ammonium formate/formic acid (98.0%) (400:400:100:90:10:1, v/v/v/v/v/v). For dialysate samples, mobile phase A was ultrapure water/1 mol/L ammonium formate/formic acid (98.0%) (980:20:1, v/v/v), and mobile phase B was acetonitrile/formic acid (98.0%) (1000:1, v/v). A Capcell Pak CR 1:20 (5 μm, 2.0 mm × 150 mm) column (Shiseido, Ltd, Tokyo, Japan), with a polytetrafluoroethylene filter (#2227) and filter box, large (#2226; Shiseido Irica Technology, Inc, Kyoto Prefecture, Japan), were used for plasma and urine samples; and an Atlantis HILIC (3 μm, 2.1 mm × 50 mm) column (Waters Corporation) was used for dialysate samples.
Naldemedine was detected and quantified by tandem mass spectrometry in positive ion mode with electrospray ionization on an API 5000 mass spectrometer (AB Sciex, Framingham, Massachusetts) using multiple reaction monitoring with m/z transitions of 571 → 368 for naldemedine and 577 → 368 for naldemedine‐15Nd 5. Calibration curves of naldemedine ranged from 0.01 to 10 ng/mL in plasma, 0.1 to 100 ng/mL in urine, and 0.005 to 5 ng/mL in dialysate. The lower limit of quantification for naldemedine was 0.01 ng/mL, 0.1 ng/mL, and 0.005 ng/mL in plasma, urine, and dialysate samples, respectively. The precision and accuracy of each analytical method were within ±15%. Specifically, precision was 4.2% to 9.7% for plasma samples, 6.1% to 7.1% for urine samples, and 3.4% to 9.5% for dialysate samples; accuracy was −5.3% to 4.8% for plasma samples, 2.0% to 7.6% for urine samples, and −5.3% to 7.2% for dialysate samples.
The protein‐binding analysis for naldemedine was performed at Huntingdon Life Sciences Inc (now Envigo, East Millstone, New Jersey). Concentrations of naldemedine in serum and serum ultrafiltrate were determined by fully validated liquid chromatography/tandem mass spectrometry methods using an Atlantis HILIC (3 μm, 2.1 mm × 50 mm) column (Waters Corporation) after solid‐phase extraction with an internal standard (naldemedine‐15Nd 5), similar to dialysate. Naldemedine was separated using a binary gradient system: mobile phase A was 0.1% formic acid in 20 mmol/L ammonium formate; and mobile phase B was 0.1% formic acid in acetonitrile. Naldemedine was detected and quantified by tandem mass spectrometry on an API 5000 mass spectrometer (AB Sciex) using selected reaction monitoring. Calibration curves of naldemedine ranged from 0.05 to 20 ng/mL in serum and 0.00586 to 1.17 ng/mL in serum ultrafiltrate. The lower limit of quantification for naldemedine was 0.05 ng/mL and 0.00586 ng/mL for serum and ultrafiltrate samples, respectively. The precision and accuracy of each analytical method were within ±15%. Specifically, precision was 2.4% to 9.1% for serum samples and 3.9% to 10.9% for ultrafiltrate samples; accuracy was 3.2% to 6.3% for serum samples and −5.7% to 6.0% for ultrafiltrate samples.
Pharmacokinetic Assessments
The following pharmacokinetic parameters (in plasma or urine) were calculated: cumulative amount of the drug excreted in urine from time 0 to 72 hours postdose (Aeu0‐72), calculated as the product of the urinary volume and the urinary concentration; area under the plasma concentration‐time curve from time 0 to the time of the last quantifiable concentration after dosing (AUC0‐t), calculated by linear trapezoidal method when concentrations are increasing and by logarithmic trapezoidal method when concentrations are decreasing (linear up/log down trapezoidal method); area under the plasma concentration‐time curve extrapolated from time 0 to infinity (AUC0‐inf), defined as AUC0‐t + the last measurable concentration/λz, and λz, the apparent terminal elimination rate constant, is the magnitude of the slope of the linear regression of the log concentration–versus‐time profile in the terminal phase; apparent total clearance, estimated as dose/AUC0‐inf; renal clearance (CLR), calculated as Aeu0‐72/AUC0‐72; Cmax; fraction excreted in urine from time 0 to 72 hours postdose (Feu0‐72), calculated as Aeu0‐72/dose × 100; serum protein unbound fraction, estimated as the ratio of unbound to total concentration of naldemedine in serum; Tmax; and terminal elimination half‐life (t½,z), calculated as t½,z = (ln 2)/λz. The following pharmacokinetic parameters (in dialysate) were calculated only for the renal impairment study: amount of naldemedine recovered in dialysate over the hemodialysis period (Ad); fraction of naldemedine recovered in dialysate, calculated as Ad/dose × 100; and hemodialysis clearance, calculated as Ad/AUC over the hemodialysis period.
Safety and Tolerability
In both studies the safety and tolerability of naldemedine was assessed by monitoring adverse events (AEs), physical examinations, vital sign measurements (blood pressure, pulse rate, respiratory rate, and body temperature), 12‐lead electrocardiograms, and clinical laboratory tests (hematology, serum chemistry tests, and urinalysis). Abnormal laboratory values that were considered clinically significant by the investigator were reported as AEs. All AEs were coded using the Medical Dictionary for Regulatory Activities, version 17.0.
Statistical Analyses
Approximately 36 to 40 subjects were planned for enrollment in the renal impairment study (8 healthy subjects with normal renal function, 8 subjects each with mild or moderate renal impairment, 4 to 8 subjects with severe renal impairment, and 8 subjects with ESRD requiring hemodialysis), and 24 subjects were planned for enrollment in the hepatic impairment study (8 subjects in each cohort). Statistical analysis and programming were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina). The pharmacokinetic parameters for naldemedine were estimated based on their plasma and urinary concentrations by noncompartmental methods using Phoenix WinNonlin version 6.4 (Certara, St. Louis, Missouri). An analysis of variance model was used to analyze log‐transformed pharmacokinetic parameters Cmax, AUC0‐t, AUC0‐inf, and t½,z as response variables, with fixed‐effect terms for renal function (healthy subjects with normal renal function and subjects with mild, moderate, or severe renal impairment, and ESRD requiring hemodialysis [treatment period 1]) or for hepatic function (healthy subjects with normal hepatic function and subjects with mild or moderate hepatic impairment). Pharmacokinetic parameters, unless otherwise specified, are reported as geometric means.
Point estimates of the geometric mean ratio (GMR) and associated 90%CIs were calculated for treatment differences in subjects with mild, moderate, or severe renal impairment and subjects with ESRD requiring hemodialysis (treatment period 1) compared with healthy subjects with normal renal function. Similarly, point estimates of the GMR and 90%CIs were calculated for treatment differences in subjects with mild or moderate hepatic impairment compared with healthy subjects with normal hepatic function. The point estimates of the GMR for the log‐transformed pharmacokinetic parameters and 90%CIs were then back‐transformed to give point estimates and 90%CIs for the GMR of the parameters for each treatment comparison.
Results
Subjects
Between February 17, 2015 and July 17, 2015, 41 subjects entered the renal impairment study and received 0.2 mg oral naldemedine (mild impairment, n = 9; moderate impairment, n = 9; severe impairment, n = 6; ESRD requiring hemodialysis, n = 8; and normal function, n = 9). All subjects completed the study. Of note, only 4 of 8 subjects with ESRD produced enough urine to evaluate the CLR and Feu0‐72 of naldemedine. Additionally, pharmacokinetic assessments and plasma concentrations were not measured in 3 subjects: 2 subjects had a prior medical history of cholecystectomy (mild renal impairment, n = 1; moderate renal impairment, n = 1); and 1 healthy subject with normal renal function (who was demographically matched to the subject with moderate renal impairment).
Between March 16, 2015 and July 31, 2015, 24 subjects entered the hepatic impairment study and received 0.2 mg oral naldemedine (mild impairment, n = 8; moderate impairment, n = 8; and normal function, n = 8). All subjects completed the study.
Baseline characteristics for subjects in the renal impairment and the hepatic impairment study are summarized in Table 1. In the renal impairment study, subjects had a mean eGFR ranging from 97.9 mL/(min·1.73 m2) (healthy subjects with normal renal function) to 9.9 mL/(min·1.73 m2) (subjects with ESRD requiring hemodialysis).
Table 1.
Baseline Demographics and Subject Characteristics in the Renal Impairment Study and the Hepatic Impairment Study
| Renal Impairment Study | |||||
|---|---|---|---|---|---|
| Parameter | Mild (n = 9) | Moderate (n = 9) | Severe (n = 6) | ESRD (n = 8) | Normal Function (n = 9) |
| Age, y | 61.2 (10.6) | 66.6 (5.9) | 61.2 (3.3) | 51.8 (10.7) | 62.8 (4.1) |
| Weight, kg | 80.9 (19.0) | 80.0 (14.4) | 94.4 (6.9) | 75.6 (16.0) | 87.3 (13.2) |
| BMI, kg/m2 | 27.8 (5.0) | 28.6 (4.0) | 34.1 (2.9) | 25.9 (4.3) | 29.1 (3.3) |
| Sex, n (%) | |||||
| Male | 7 (77.8) | 4 (44.4) | 4 (66.7) | 4 (50.0) | 5 (55.6) |
| Female | 2 (22.2) | 5 (55.6) | 2 (33.3) | 4 (50.0) | 4 (44.4) |
| Race, n (%) | |||||
| White | 8 (88.9) | 7 (77.8) | 5 (83.3) | 2 (25.0) | 6 (66.7) |
| Black | 1 (11.1) | 1 (11.1) | 1 (16.7) | 5 (62.5) | 2 (22.2) |
| American Indian or Alaska Native | 0 (0.0) | 1 (11.1) | 0 (0.0) | 1 (12.5) | 1 (11.1) |
| eGFR, mL/(min·1.73 m2) | 72.6 (7.3) | 50.3 (5.6) | 20.0 (3.4) | 9.9 (5.0) | 97.9 (26.0) |
| CLcr, mL/min | 82.0 (17.0) | 58.8 (11.4) | 31.8 (4.1) | 14.3 (8.5) | 114.4 (16.9) |
| Hepatic Impairment Study | |||
|---|---|---|---|
| Parameter | Mild (n = 8) | Moderate (n = 8) | Normal Function (n = 8) |
| Age, y | 57.5 (6.4) | 57.0 (5.8) | 51.4 (5.9) |
| Weight, kg | 90.3 (16.6) | 79.1 (14.0) | 82.9 (16.7) |
| BMI, kg/m2 | 30.6 (4.5) | 28.0 (4.6) | 29.0 (4.0) |
| Sex, n (%) | |||
| Male | 5 (62.5) | 5 (62.5) | 5 (62.5) |
| Female | 3 (37.5) | 3 (37.5) | 3 (37.5) |
| Race, n (%) | |||
| White | 7 (87.5) | 6 (75.0) | 7 (87.5) |
| Black | 1 (12.5) | 1 (12.5) | 1 (12.5) |
| American Indian or Alaska Native | 0 (0.0) | 1 (12.5) | 0 (0.0) |
| Child‐Pugh score, n (%) | |||
| 5 or 6 | 8 (100.0) | 0 | 0 |
| 7 to 9 | 0 | 8 (100.0) | 0 |
Data are mean (SD) unless otherwise specified. BMI indicates body mass index; CLcr, creatinine clearance; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease.
Pharmacokinetics
Renal Impairment Study
The mean plasma concentrations of naldemedine over time for subjects with renal impairment are shown in Figure 1, and pharmacokinetic parameters of naldemedine in plasma, dialysate, urine, and serum are summarized in Table 2. In plasma the geometric mean t½,z of naldemedine ranged from 14.2 to 18.7 hours in subjects with renal impairment and was 13.8 hours in healthy subjects with normal renal function. The geometric mean Cmax of naldemedine ranged from 2.23 to 3.01 ng/mL in subjects with renal impairment and was 3.39 ng/mL in healthy subjects with normal renal function. Naldemedine was rapidly absorbed with a median Tmax of 0.50 to 0.88 hours regardless of renal function. The geometric mean AUC0‐inf for naldemedine ranged from 18.6 to 32.4 ng·h/mL in subjects with renal impairment and was 23.6 ng·h/mL in healthy subjects with normal renal function.
Figure 1.
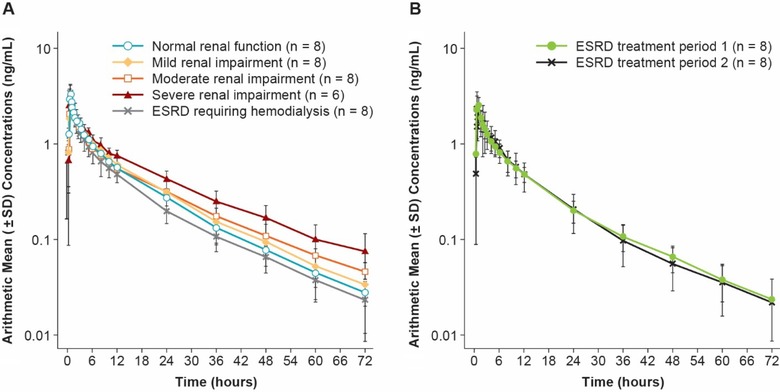
Mean (±SD) plasma concentrations of naldemedine in (A) subjects with mild (n = 8), moderate (n = 8), or severe (n = 6) renal impairment, ESRD requiring hemodialysis (treatment period 1) (n = 8), and healthy subjects with normal renal function (n = 8), and (B) mean plasma concentrations of naldemedine before and after hemodialysis in the cohort of subjects with ESRD during treatment periods 1 and 2 (n = 8 each). Figures are presented in a semilogarithmic scale. ESRD indicates end‐stage renal disease.
Table 2.
Pharmacokinetics of Naldemedine in the Renal Impairment Study
| Mild (n = 8) | Moderate (n = 8) | Severe (n = 6) | ESRD: Treatment Period 1 (n = 8) | ESRD: Treatment Period 2 (n = 8) | Normal Function (n = 8) | |
|---|---|---|---|---|---|---|
| Plasma | ||||||
| AUC0‐t (ng·h/mL) | ||||||
| Geometric mean (CV%) | 24.6 (23.5) | 23.8 (22.4) | 30.4 (16.1) | 18.9 (17.3) | 18.1 (25.9) | 22.9 (18.3) |
| Arithmetic mean (SD) | 25.2 (5.8) | 24.3 (5.6) | 30.7 (4.9) | 19.1 (3.1) | 18.7 (5.5) | 23.3 (4.2) |
| AUC0‐inf (ng·h/mL) | ||||||
| Geometric mean (CV%) | 25.4 (24.6) | 25.0 (23.6) | 32.4 (18.1) | 19.5 (17.9) | 18.6 (26.1) | 23.6 (18.9) |
| Arithmetic mean (SD) | 26.0 (6.3) | 25.6 (6.2) | 32.9 (6.1) | 19.8 (3.4) | 19.2 (5.6) | 23.9 (4.4) |
| Cmax (ng/mL) | ||||||
| Geometric mean (CV%) | 3.01 (23.7) | 2.56 (25.5) | 2.76 (13.4) | 2.81 (24.8) | 2.23 (26.5) | 3.39 (20.7) |
| Arithmetic mean (SD) | 3.08 (0.70) | 2.63 (0.63) | 2.78 (0.38) | 2.89 (0.68) | 2.30 (0.68) | 3.46 (0.74) |
| Tmax (h)a | 0.50 (0.25, 0.75) | 0.63 (0.50, 1.50) | 0.75 (0.50, 0.75) | 0.79 (0.50, 1.00) | 0.88 (0.25, 2.00) | 0.75 (0.50, 0.75) |
| t½,z (h) | ||||||
| Geometric mean (CV%) | 14.2 (25.4) | 17.2 (23.1) | 18.7 (15.7) | 15.2 (28.1) | 15.0 (24.1) | 13.8 (17.7) |
| Arithmetic mean (SD) | 14.6 (3.2) | 17.5 (3.5) | 18.9 (2.9) | 15.7 (4.4) | 15.4 (3.6) | 13.9 (2.5) |
| CL/F (L/h) | ||||||
| Geometric mean (CV%) | 7.9 (24.6) | 8.0 (23.6) | 6.2 (18.1) | 10.3 (17.9) | 10.7 (26.1) | 8.5 (18.9) |
| Arithmetic mean (SD) | 8.1 (2.0) | 8.2 (1.8) | 6.2 (1.1) | 10.4 (2.0) | 11.0 (2.5) | 8.6 (1.6) |
| Dialysate | ||||||
| CLhd (L/h) | ||||||
| Geometric mean (CV%) | … | … | … | … | 1.7 (14.4) | … |
| Arithmetic mean (SD) | … | … | … | … | 1.7 (0.2) | … |
| Fd (%) | ||||||
| Geometric mean (CV%) | … | … | … | … | 2.7 (32.8) | … |
| Arithmetic mean (SD) | … | … | … | … | 2.8 (1.1) | … |
| Urine | ||||||
| Feu0‐72 (%) | ||||||
| Geometric mean (CV%) | 14.0 (28.2) | 11.6 (35.2) | 6.9 (40.1) | 0.7 (103.3)b | 0.9 (110.4)b | 14.7 (20.7) |
| Arithmetic mean (SD) | 14.4 (3.8) | 12.2 (4.3) | 7.3 (3.0) | 0.5 (0.8) | 0.7 (1.2) | 15.0 (2.9) |
| CLR (L/h) | ||||||
| Geometric mean (CV%) | 1.1 (16.5) | 1.0 (26.3) | 0.5 (29.3) | 0.1 (95.5)b | 0.1 (111.0)b | 1.3 (25.5) |
| Arithmetic mean (SD) | 1.1 (0.2) | 1.0 (0.3) | 0.5 (0.1) | < 0.1 (0.1) | 0.1 (0.1) | 1.3 (0.3) |
| Serum | ||||||
| At 0.75 h | ||||||
| Cunbound (ng/mL) | ||||||
| Geometric mean (CV%) | 0.217 (23.6) | 0.190 (28.6) | 0.230 (17.1) | 0.159 (23.1) | 0.182 (32.9) | 0.219 (20.7) |
| Arithmetic mean (SD) | 0.222 (0.049) | 0.198 (0.063) | 0.233 (0.041) | 0.162 (0.037) | 0.190 (0.063) | 0.223 (0.047) |
| Ctotal (ng/mL) | ||||||
| Geometric mean (CV%) | 3.03 (22.5) | 2.59 (28.2) | 2.69 (10.5) | 2.67 (23.6) | 1.97 (23.0) | 3.11 (21.2) |
| Arithmetic mean (SD) | 3.09 (0.72) | 2.67 (0.74) | 2.70 (0.28) | 2.73 (0.62) | 2.02 (0.46) | 3.17 (0.67) |
| Fu (%) | ||||||
| Geometric mean (CV%) | 7.2 (12.8) | 7.4 (15.2) | 8.6 (13.6) | 6.0 (13.2) | 9.2 (14.3) | 7.1 (12.7) |
| Arithmetic mean (SD) | 7.2 (0.9) | 7.4 (1.2) | 8.6 (1.2) | 6.0 (0.8) | 9.3 (1.4) | 7.1 (0.9) |
| At 24 h | ||||||
| Cunbound (ng/mL) | ||||||
| Geometric mean (CV%) | 0.0226 (41.3) | 0.0268 (37.7) | 0.0348 (33.6) | 0.0168 (44.1) | 0.0170 (40.8) | 0.0186 (26.7) |
| Arithmetic mean (SD) | 0.0243 (0.0102) | 0.0282 (0.0089) | 0.0365 (0.0122) | 0.0183 (0.0085) | 0.0182 (0.0070) | 0.0191 (0.0048) |
| Ctotal (ng/mL) | ||||||
| Geometric mean (CV%) | 0.340 (39.2) | 0.326 (34.8) | 0.455 (20.1) | 0.216 (29.2) | 0.210 (37.6) | 0.289 (25.6) |
| Arithmetic mean (SD) | 0.361 (0.128) | 0.342 (0.114) | 0.463 (0.091) | 0.224 (0.067) | 0.222 (0.084) | 0.298 (0.076) |
| Fu (%) | ||||||
| Geometric mean (CV%) | 6.6 (16.4) | 8.2 (24.1) | 7.7 (23.0) | 7.8 (31.2) | 8.1 (22.0) | 6.4 (12.8) |
| Arithmetic mean (SD) | 6.7 (1.1) | 8.4 (1.9) | 7.8 (1.7) | 8.1 (2.2) | 8.3 (1.7) | 6.5 (0.8) |
AUC0‐t indicates area under the plasma concentration‐time curve from time 0 to the time of the last quantifiable concentration after dosing; AUC0‐inf, area under the plasma concentration‐time curve extrapolated from time 0 to infinity; CL/F, apparent total clearance; CLhd, hemodialysis clearance; CLR, renal clearance; Cmax, maximum observed plasma concentration; Ctotal, total concentration; Cunbound, unbound concentration; CV%, coefficient of variation; ESRD, end‐stage renal disease; Fd, fraction of naldemedine recovered in dialysate; Feu0‐72, fraction excreted in urine from time 0 to 72 hours postdose; Fu, serum protein unbound fraction; Tmax, time to maximum plasma concentration; t½,z, terminal elimination half‐life.
Median (minimum, maximum).
n = 4.
In subjects with ESRD, administration of naldemedine before hemodialysis (treatment period 2) resulted in a geometric mean hemodialysis clearance of 1.7 L/h, and the fraction of naldemedine recovered in the dialysate was 2.7% during the 3‐ to 4‐hour hemodialysis period (Table 2).
Pharmacokinetics in urine demonstrated that the geometric mean CLR of naldemedine decreased with reduced renal function (normal function 1.3 L/h; mild impairment 1.1 L/h; moderate impairment 1.0 L/h; severe impairment 0.5 L/h; ESRD [treatment period 1] 0.1 L/h; ESRD [treatment period 2] 0.1 L/h; Table 2). Similarly, the geometric mean Feu0‐72 of naldemedine decreased with reduced renal function (normal function 14.7%; mild impairment 14.0%; moderate impairment 11.6%; severe impairment 6.9%; ESRD [treatment period 1] 0.7%; ESRD [treatment period 2] 0.9%). The geometric mean unbound fraction of naldemedine in serum was similar across cohorts at 0.75 and 24 hours, ranging from 6.0% to 9.2% (Table 2).
The GMRs (90%CI) for the AUC0‐inf of naldemedine in subjects with mild, moderate, or severe renal impairment and with ESRD requiring hemodialysis compared with healthy subjects with normal renal function were 107.7% (90.4%, 128.3%), 106.0% (89.0%, 126.4%), 137.8% (114.0%, 166.5%), and 82.8% (69.5%, 98.6%), respectively (Table 3).
Table 3.
Geometric Mean Ratioa (and 90%CI) of Pharmacokinetic Parameters for Subjects With Renal Impairment Versus Healthy Subjects With Normal Renal Function
| Renal Impairment Study | ||||
|---|---|---|---|---|
| Mild | Moderate | Severe | ESRDb | |
| AUC0‐t | 107.4 | 103.8 | 132.6 | 82.3 |
| (90.8, 126.9) | (87.8, 122.7) | (110.7, 158.8) | (69.7, 97.3) | |
| AUC0‐inf | 107.7 | 106.0 | 137.8 | 82.8 |
| (90.4, 128.3) | (89.0, 126.4) | (114.0, 166.5) | (69.5, 98.6) | |
| Cmax | 88.7 | 75.5 | 81.3 | 82.9 |
| (73.5, 107.0) | (62.5, 91.0) | (66.3, 99.5) | (68.7, 100.0) | |
| t½,z | 103.3 | 124.7 | 136.1 | 110.3 |
| (85.4, 125.0) | (103.1, 150.9) | (110.8, 167.1) | (91.2, 133.4) | |
AUC0‐t indicates area under the plasma concentration‐time curve from time 0 to the time of the last quantifiable concentration after dosing; AUC0‐inf, area under the plasma concentration‐time curve extrapolated from time 0 to infinity; Cmax, maximum observed plasma concentration; ESRD, end‐stage renal disease; t½,z, terminal elimination half‐life.
The ratio of the various renal impairment groups compared with healthy subjects with normal renal function (ie, renal impairment/normal function × 100).
Treatment period 1.
Hepatic Impairment Study
Following administration of a single 0.2‐mg dose of naldemedine, the mean plasma concentrations of naldemedine over time in subjects with hepatic impairment, compared with healthy subjects with normal hepatic function, are shown in Figure 2. Pharmacokinetic parameters of naldemedine in plasma, urine, and serum are summarized in Table 4.
Figure 2.
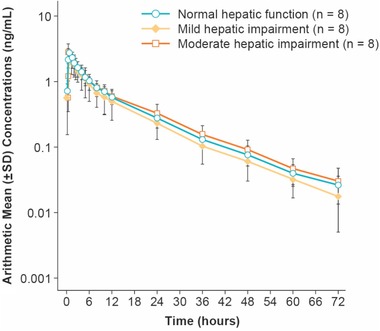
Mean (±SD) plasma concentrations of naldemedine in subjects with mild (n = 8) or moderate (n = 8) hepatic impairment and healthy subjects with normal hepatic function (n = 8). Figure is presented in a semilogarithmic scale.
Table 4.
Pharmacokinetics of Naldemedine in the Hepatic Impairment Study
| Mild (n = 8) | Moderate (n = 8) | Normal Function (n = 8) | |
|---|---|---|---|
| Plasma | |||
| AUC0‐t (ng·h/mL) | |||
| Geometric mean (CV%) | 19.1 (36.4) | 24.2 (21.7) | 23.1 (22.8) |
| Arithmetic mean (SD) | 20.2 (7.8) | 24.7 (5.1) | 23.6 (5.5) |
| AUC0‐inf (ng·h/mL) | |||
| Geometric mean (CV%) | 19.6 (35.9) | 24.8 (21.8) | 23.6 (22.8) |
| Arithmetic mean (SD) | 20.7 (7.8) | 25.3 (5.3) | 24.1 (5.6) |
| Cmax (ng/mL) | |||
| Geometric mean (CV%) | 2.44 (47.4) | 2.93 (16.8) | 2.71 (26.3) |
| Arithmetic mean (SD) | 2.67 (1.27) | 2.96 (0.50) | 2.80 (0.77) |
| Tmax (h)a | 0.75 (0.50, 2.00) | 0.63 (0.50, 0.75) | 0.75 (0.50, 1.00) |
| t½,z (h) | |||
| Geometric mean (CV%) | 14.0 (15.1) | 13.3 (21.5) | 13.5 (9.3) |
| Arithmetic mean (SD) | 14.1 (2.1) | 13.6 (2.9) | 13.6 (1.3) |
| CL/F (L/h) | |||
| Geometric mean (CV%) | 10.2 (35.9) | 8.1 (21.8) | 8.5 (22.8) |
| Arithmetic mean (SD) | 10.7 (3.4) | 8.2 (1.8) | 8.7 (1.8) |
| Urine | |||
| Feu0‐72 (%) | |||
| Geometric mean (CV%) | 11.5 (20.9) | 17.2 (25.5) | 18.7 (15.9) |
| Arithmetic mean (SD) | 11.7 (2.4) | 17.7 (4.2) | 18.9 (3.0) |
| CLR (L/h) | |||
| Geometric mean (CV%) | 1.2 (23.0) | 1.4 (26.7) | 1.6 (19.2) |
| Arithmetic mean (SD) | 1.2 (0.3) | 1.5 (0.3) | 1.6 (0.3) |
| Serum | |||
| At 0.75 h | |||
| Cunbound (ng/mL) | |||
| Geometric mean (CV%) | 0.188 (52.8) | 0.244 (34.1) | 0.210 (27.9) |
| Arithmetic mean (SD) | 0.207 (0.089) | 0.257 (0.098) | 0.217 (0.057) |
| Ctotal (ng/mL) | |||
| Geometric mean (CV%) | 2.21 (57.5) | 2.88 (22.3) | 2.81 (32.4) |
| Arithmetic mean (SD) | 2.47 (1.15) | 2.94 (0.61) | 2.94 (0.98) |
| Fu (%) | |||
| Geometric mean (CV%) | 8.5 (15.8) | 8.5 (24.4) | 7.5 (10.1) |
| Arithmetic mean (SD) | 8.6 (1.4) | 8.7 (2.4) | 7.5 (0.8) |
| At 24 h | |||
| Cunbound (ng/mL) | |||
| Geometric mean (CV%) | 0.0227 (36.8) | 0.0283 (52.2) | 0.0214 (27.9) |
| Arithmetic mean (SD) | 0.0241 (0.0089) | 0.0313 (0.0142) | 0.0221 (0.0061) |
| Ctotal (ng/mL) | |||
| Geometric mean (CV%) | 0.239 (45.6) | 0.324 (36.7) | 0.287 (29.3) |
| Arithmetic mean (SD) | 0.260 (0.124) | 0.340 (0.103) | 0.298 (0.087) |
| Fu (%) | |||
| Geometric mean (CV%) | 9.5 (25.1) | 8.8 (20.0) | 7.5 (10.4) |
| Arithmetic mean (SD) | 9.8 (2.4) | 8.9 (1.8) | 7.5 (0.8) |
AUC0‐t indicates area under the plasma concentration‐time curve from time 0 to the time of the last quantifiable concentration after dosing; AUC0‐inf, area under the plasma concentration‐time curve extrapolated from time 0 to infinity; CL/F, apparent total clearance; CLR, renal clearance; Cmax, maximum observed plasma concentration; Ctotal, total concentration; Cunbound, unbound concentration; CV%, coefficient of variation; Feu0‐72, fraction excreted in urine from time 0 to 72 hours postdose; Fu, serum protein unbound fraction; Tmax, time to maximum plasma concentration; t½,z, terminal elimination half‐life.
Median (minimum, maximum).
In plasma the geometric mean t½,z of naldemedine ranged from 13.3 to 14.0 hours in subjects with hepatic impairment and was 13.5 hours for healthy subjects with normal hepatic function (Table 4). The geometric mean Cmax of naldemedine ranged from 2.44 to 2.93 ng/mL in subjects with hepatic impairment and was 2.71 ng/mL in healthy subjects with normal hepatic function. Naldemedine was absorbed rapidly with median Tmax ranging from 0.63 to 0.75 hour across all cohorts. The geometric mean AUC0‐inf for naldemedine ranged from 19.6 to 24.8 ng·h/mL in subjects with hepatic impairment and was 23.6 ng·h/mL in healthy subjects with normal hepatic function.
Pharmacokinetic analyses in urine demonstrated that the geometric mean CLR and Feu0‐72 of naldemedine were similar across cohorts, ranging from 1.2 to 1.6 L/h and 11.5% to 18.7%, respectively (Table 4). The geometric mean unbound fraction of naldemedine in serum ranged from 7.5% to 9.5% across cohorts at 0.75 and 24 hours (Table 4).
The GMRs (90%CI) for the AUC0‐inf of naldemedine in subjects with mild or moderate hepatic impairment compared with healthy subjects with normal hepatic function were 82.8% (65.7%, 104.5%) and 105.2% (83.4%, 132.6%), respectively (Table 5).
Table 5.
Geometric Mean Ratioa (and 90%CI) of Pharmacokinetic Parameters for Subjects With Hepatic Impairment Versus Healthy Subjects With Normal Hepatic Function
| Hepatic Impairment Study | ||
|---|---|---|
| Mild | Moderate | |
| AUC0‐t | 82.7 (65.5, 104.5) | 104.7 (82.9, 132.3) |
| AUC0‐inf | 82.8 (65.7, 104.5) | 105.2 (83.4, 132.6) |
| Cmax | 90.0 (68.6, 118.0) | 107.8 (82.3, 141.4) |
| t½,z | 103.7 (90.4, 119.0) | 98.5 (85.9, 113.0) |
AUC0‐t indicates area under the plasma concentration‐time curve from time 0 to the time of the last quantifiable concentration after dosing; AUC0‐inf, area under the plasma concentration‐time curve extrapolated from time 0 to infinity; Cmax, maximum observed plasma concentration; t½,z, terminal elimination half‐life.
The ratio for mild or moderate hepatic impairment compared with healthy subjects with normal hepatic function (ie, hepatic impairment/normal function × 100).
Safety
In the renal impairment study 43.9% (18/41) of subjects reported treatment‐emergent AEs (TEAEs). The most frequent TEAEs were headache (12.2% [5/41]), nausea (9.8% [4/41]), and diarrhea (7.3% [3/41]). There were no notable differences in the incidences of TEAEs across cohorts. In the hepatic impairment study 33.3% (8/24) of subjects reported TEAEs. The most frequent TEAEs were diarrhea (12.5% [3/24]), abdominal pain (8.3% [2/24]), flatulence (8.3% [2/24]), and somnolence (8.3% [2/24]). The incidence of TEAEs was numerically higher in subjects with mild (37.5% [3/8]) or moderate (50.0% [4/8]) hepatic impairment compared with healthy subjects with normal hepatic function (12.5% [1/8]). There were no deaths or TEAEs leading to withdrawal of naldemedine in either study.
Discussion
The pharmacokinetic parameters in both the renal and hepatic impairment studies were generally similar to the findings in a previous phase 1 study of naldemedine in healthy subjects.10
In subjects with moderate or severe renal impairment, mean plasma pharmacokinetic profiles suggest a slightly lower clearance of naldemedine compared with healthy subjects with normal renal function, subjects with mild renal impairment, and subjects administered naldemedine 1 to 2 hours after hemodialysis (ESRD requiring hemodialysis, treatment period 1). The GMRs for the AUC0‐inf of naldemedine in subjects with moderate or severe renal impairment compared with healthy subjects with normal renal function were 106.0% and 137.8%, respectively. Similarly, the GMRs for the Cmax of naldemedine in subjects with moderate or severe renal impairment compared with healthy subjects with normal renal function were 75.5% and 81.3%, respectively, suggesting that the exposure to naldemedine did not increase substantially with renal impairment. In contrast, naloxegol, an approved peripherally acting μ‐opioid‐receptor antagonist for opioid‐induced constipation, was found to have higher mean exposures in subjects with moderate and severe renal impairment compared with healthy subjects.23
The geometric mean AUC0‐inf and Cmax of naldemedine were lower in subjects with ESRD requiring hemodialysis than in healthy subjects with normal renal function or subjects with mild, moderate, or severe renal impairment; however, the geometric mean t½,z was longer in subjects with ESRD requiring hemodialysis than in healthy subjects with normal renal function. Although the reason for the observed lower geometric mean AUC0‐inf and Cmax of naldemedine in subjects with ESRD requiring hemodialysis is unclear, we speculate that gut‐wall edema induced by ESRD may have reduced the absorption and absolute bioavailability of naldemedine.24
The mean exposure to naldemedine was not affected by hepatic impairment as demonstrated by the GMRs for the AUC0‐inf (≤105.2%) and Cmax (≤107.8%) of naldemedine in subjects with mild or moderate hepatic impairment compared with healthy subjects with normal hepatic function. Subjects with severe hepatic impairment (Child‐Pugh score between 10 and 15) were not enrolled in this study because opioids are known to precipitate or aggravate hepatic encephalopathy in these subjects.17, 18
The unbound fraction of naldemedine was similar across cohorts in the renal impairment study (6.0% to 9.2%) and the hepatic impairment study (7.5% to 9.5%), suggesting that renal or hepatic impairment did not affect the degree of naldemedine protein binding. The quantities of unbound naldemedine at 0.75 and 24 hours postdose were similar between cohorts in each study, suggesting that protein binding was independent of naldemedine concentration over the range of concentrations evaluated. In the renal impairment study only a small amount of naldemedine (2.7%) was removed from plasma by a 3‐ to 4‐hour hemodialysis treatment, demonstrating that hemodialysis did not affect the pharmacokinetics of naldemedine and was not an effective means for removing naldemedine in instances of excessive dosing.
Urinary pharmacokinetics of naldemedine varied slightly between the 2 studies. In the renal impairment study the fraction of naldemedine excreted in urine decreased with decreasing renal function, although the fraction of naldemedine excreted in urine was consistent across cohorts in the hepatic impairment study. Reported TEAEs in each study were generally consistent with the known safety profile of naldemedine.10, 11
A limitation of the hepatic impairment study was that it did not evaluate subjects with severe hepatic impairment. Additionally, both renal and hepatic impairment studies were small‐scale phase 1 studies; it would therefore be valuable to study the efficacy and safety of naldemedine in subjects with opioid‐induced constipation and renal or hepatic impairment in large‐scale studies. However, in these phase 1 studies, exposure to naldemedine did not increase substantially with either renal or hepatic impairment. Moreover, naldemedine was generally well‐tolerated in both healthy subjects and subjects with renal or hepatic impairment. Together, these results along with results of a population pharmacokinetic analysis and exposure‐response analysis of naldemedine25 suggest that no dose adjustment is warranted in subjects with any degree of renal impairment (with or without dialysis) or in subjects with mild or moderate hepatic impairment.
Conclusions
The AUC0‐inf of naldemedine did not increase substantially (GMRs <138%) in subjects with renal or hepatic impairment. Naldemedine was well tolerated in subjects with renal impairment, hepatic impairment, and healthy subjects with normal renal or hepatic function.
Acknowledgments
All authors had complete access to the data that support this publication. The authors would like to thank the Principal Investigator of both studies, Jolene Kay Berg, MD. Medical writing support was provided by Jeffrey Bratz, PhD, of Oxford PharmaGenesis, Newtown, PA, USA, and this was funded by Shionogi & Co, Ltd.
Data Sharing
The data used in this manuscript are commercially confidential and not accessible to the public.
Disclosures
All authors are employees of Shionogi. This study was funded by Shionogi. Editorial assistance was provided by Oxford PharmaGenesis Inc, Newtown, Pennsylvania and was funded by Shionogi.
There will not be reprints offered for this article.
References
- 1. Camilleri M, Drossman DA, Becker G, Webster LR, Davies AN, Mawe GM. Emerging treatments in neurogastroenterology: a multidisciplinary working group consensus statement on opioid‐induced constipation. Neurogastroenterol Motil. 2014;26:1386‐1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levy B, Paulozzi L, Mack KA, Jones CM. Trends in opioid analgesic‐prescribing rates by specialty, U.S., 2007‐2012. Am J Prev Med. 2015;49:409‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swegle JM, Logemann C. Management of common opioid‐induced adverse effects. Am Fam Physician. 2006;74:1347‐1354. [PubMed] [Google Scholar]
- 4. Lembo AJ. Introduction: opioid‐induced constipation. Am J Gastroenterol Suppl. 2014;2:2. [DOI] [PubMed] [Google Scholar]
- 5. Nelson AD, Camilleri M. Opioid‐induced constipation: advances and clinical guidance. Ther Adv Chronic Dis. 2016;7:121‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emmanuel A, Johnson M, McSkimming P, Dickerson S. Laxatives do not improve symptoms of opioid‐induced constipation: results of a patient survey. Pain Med. 2017;18:1932‐1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brenner DM, Chey WD. An evidence‐based review of novel and emerging therapies for constipation in patients taking opioid analgesics. Am J Gastroenterol Suppl. 2014;2:38‐46. [Google Scholar]
- 8. Markham A. Naldemedine: first global approval. Drugs. 2017;77:923‐927. [DOI] [PubMed] [Google Scholar]
- 9. Inagaki M, Kume M, Tamura Y, et al. Discovery of naldemedine: a potent and orally available opioid receptor antagonist for treatment of opioid‐induced adverse effects. Bioorg Med Chem Lett. 2019;29:73‐77. [DOI] [PubMed] [Google Scholar]
- 10. Fukumura K, Yokota T, Baba Y, Arjona Ferreira JC. Phase 1, randomized, double‐blind, placebo‐controlled studies on the safety, tolerability, and pharmacokinetics of naldemedine in healthy volunteers. Clin Pharmacol Drug Dev. 2018;7:474‐483. [DOI] [PubMed] [Google Scholar]
- 11. Ohnishi S, Fukumura K, Kubota R, Wajima T. Absorption, distribution, metabolism, and excretion of radiolabeled naldemedine in healthy subjects. Xenobiotica. 2018:1‐36. [DOI] [PubMed] [Google Scholar]
- 12. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) . Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. March 2010. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf. Accessed December 17, 2018.
- 13. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) . Guidance for industry: pharmacokinetics in patients with impaired hepatic function—study design, data analysis, and impact on dosing and labeling. May 2003. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072123.pdf. Accessed December 17, 2018.
- 14. Webster LR, Yamada T, Arjona Ferreira JC. A phase 2b, randomized, double‐blind placebo‐controlled study to evaluate the efficacy and safety of naldemedine for the treatment of opioid‐induced constipation in patients with chronic noncancer pain. Pain Med. 2017;18:2350‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Webster LR, Nalamachu S, Morlion B, et al. Long‐term use of naldemedine in the treatment of opioid‐induced constipation in patients with chronic noncancer pain: a randomized, double‐blind, placebo‐controlled phase 3 study. Pain. 2018;159:987‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hale M, Wild J, Reddy J, Yamada T, Arjona Ferreira JC. Naldemedine versus placebo for opioid‐induced constipation (COMPOSE‐1 and COMPOSE‐2): two multicentre, phase 3, double‐blind, randomised, parallel‐group trials. Lancet Gastroenterol Hepatol. 2017;2:555‐564. [DOI] [PubMed] [Google Scholar]
- 17. Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J. Analgesics in patients with hepatic impairment: pharmacology and clinical implications. Drugs. 2012;72:1645‐1669. [DOI] [PubMed] [Google Scholar]
- 18. Soleimanpour H, Safari S, Shahsavari Nia K, Sanaie S, Alavian SM. Opioid drugs in patients with liver disease: a systematic review. Hepat Mon. 2016;16:e32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31‐41. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247‐254. [DOI] [PubMed] [Google Scholar]
- 21. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646‐649. [DOI] [PubMed] [Google Scholar]
- 22. Watari R, Matsuda A, Ohnishi S, Hasegawa H. Minimal contribution of P‐gp on the low brain distribution of naldemedine, a peripherally acting μ‐opioid receptor antagonist [published online ahead of print 2018]. Drug Metab Pharmacokinet. [DOI] [PubMed] [Google Scholar]
- 23. Bui K, She F, Sostek M. The effects of renal impairment on the pharmacokinetics, safety, and tolerability of naloxegol. J Clin Pharmacol. 2014;54:1375‐1382. [DOI] [PubMed] [Google Scholar]
- 24. Roberts DM, Sevastos J, Carland JE, Stocker SL, Lea‐Henry TN. Clinical pharmacokinetics in kidney disease: application to rational design of dosing regimens. Clin J Am Soc Nephrol. 2018;13:1254‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kubota R, Fukumura K, Wajima T. Population pharmacokinetics and exposure‐response relationships of naldemedine. Pharm Res. 2018;35:225. [DOI] [PMC free article] [PubMed] [Google Scholar]


