Abstract
Introduction
For adequate pain treatment in patients with cancer, it is important to monitor and evaluate pain regularly. Although the numeric rating scale (NRS) is implemented in hospitals in the Netherlands, pain is still not systematically registered during outpatient consultations. The aim of this study was to assess whether home telemonitoring increases pain registration in medical records of outpatients with cancer.
Methods
Patients with cancer were included in the intervention group (IG) when they visited the outpatient clinic. They received a short message service and an interactive voice response on their mobile phones 3 times a week, asking them to provide their pain score (NRS). When the reported NRS pain score was ≥5, a specialized oncology nurse adapted the pain treatment when necessary. Outcomes were compared to a control group (CG) without home telemonitoring. In both groups, medical records were analyzed and data on pain and analgesics were collected.
Results
In each group, the medical records of 54 patients were analyzed on 3 consecutive outpatient visits. In the CG, pain registration or its absence was described in 60 visits (37.0%). In the IG, pain registration or its absence was reported in 83 visits (51.2%). Patients in the IG received a prescription for analgesics significantly more often (36/54 patients [66.6%]) than did patients in the CG (18/54 patients [33.3%]), P < 0.01).
Conclusion
Home telemonitoring for patients with cancer significantly increases registration of pain and prescriptions of analgesics in outpatient medical records. Home telemonitoring helps to increase the awareness of pain and its management.
Keywords: telemonitoring, cancer pain, registration of pain, numeric rating scale, pain assessment
Introduction
Pain is one of the most common and feared symptoms in patients with cancer. Pain prevalence rates are 39% after curative treatment; 55% during anticancer treatment; and 66% in advanced, metastatic, or terminal disease.1 Cancer pain management is frequently suboptimal, despite effective treatments being available.2 Undertreatment appears to be common and has been ascribed to some combination of professional‐related and patient‐related factors and system issues, such as fear of opioids and poor assessment of pain.2, 3, 4, 5, 6 Other barriers included physicians’ reluctance to prescribe opioids and limitations of oncologists’ knowledge.3, 7
To improve the quality of pain treatment in the Netherlands, a revised multidisciplinary, evidence‐based guideline on the diagnosis and treatment of pain in patients with cancer was published in 2016.8 A validated pain assessment tool using the numeric rating scale (NRS) or VAS was recommended each time the patient visited the outpatient clinic. Although the NRS and VAS are implemented in hospitals in the Netherlands, pain is not yet systematically registered in the outpatients’ medical records because oncologists and nurses do not register pain regularly during consultations.6
The use of modern communication tools can be useful for early detection and management of moderate to severe pain, without the need for face‐to‐face contact. Other benefits include improved self‐management skills for patients, fewer hospital visits, and increased patient satisfaction and compliance with care agreements.9 Patients who used telemonitoring felt closer contact with doctors and felt better cared for. Monitoring their own health data gave patients more self‐awareness about their pain.10
Home telemonitoring by means of interactive voice response (IVR) is such a tool. IVR with or without short message service (SMS) has been effectively used in health care in the treatment of asthma11, diabetes mellitus,12, 13 and anticoagulant management.14
Pain management is part of the daily work at a pain center, but not for an oncological outpatient clinic. In our hospital, the pain center and the outpatient oncology department are two separate departments, but there is good mutual contact. From our pain center, we wanted to investigate whether home monitoring (managed by the pain center) could increase the registration of pain in the oncologist's patient medical record.
We used home telemonitoring with an external computer database and communicated with the patient by automatic telephone call. A human computer voice automatically calls the patient periodically. In case of pain management, the voice invites the patient to give an actual NRS pain score. The patient answers by entering a number between 0 and 10 on his or her mobile phone. This number is sent back and stored in an external computer database. In this way, a large group of patients can be called at the same time without active human intervention (and time). The effect of home telemonitoring can be twofold: early detection of moderate to severe pain and increased adequacy of patients’ pain treatment.
The aim of this study was to assess whether home telemonitoring increased registration of pain in medical records of patients visiting a Dutch teaching hospital.
Methods
Study Population
An intervention study was performed in a before‐and‐after design: (1) before: control group not using home telemonitoring; (2) after: intervention group using home telemonitoring. Both patient groups visited the outpatient clinic of hematology and pulmonary oncology of the Reinier de Graaf Hospital (RdGH), and data regarding pain in their medical records were analyzed. It was hypothesized that the use of home telemonitoring would increase registration of pain in the medical records.
The inclusion criteria for both groups were diagnosis of cancer, 18 years of age or older, and living at home.
The patients in the intervention group also had to have access to a mobile phone. Exclusion criteria for the intervention group were patients not speaking Dutch, patients who could not handle a mobile phone, and those with cognitive disorders. The patients in the control group received usual care.
The Medical Ethical Committee (METC) of Zuid west Holland (METC protocol number 2017‐013) approved this study. This study was also approved by the science office of the RdGH. Anonymity of every patient was guaranteed. Informed consent was not needed for the control group. The intervention group gave informed consent with oral and written permission.
Study Procedure
After the first visit with the oncologist or specialized oncological nurse, the patient received an information leaflet. After 2 weeks, the nurse practitioner of the pain center called the patients to invite them to participate. The patients were informed about what home telemonitoring is and how it works. Participation in home telemonitoring was voluntarily.
Intervention Home Telemonitoring
Home telemonitoring is an automatic computer‐based monitoring system that communicates with the patient by SMS and IVR. Our hospital contracted a company specializing in SMS/IVR messaging (EasyCareSolutions B.V.).
Each patient received an SMS at 9:25 a.m. to announce the IVR (Figure 1). The text of the SMS was: “Good day, Ms./Mr. (family name of the patient). In a couple of minutes, you will receive a phone call from our computer asking you to give a pain score.” At 9:30 a.m. the IVR call was made by a male voice: “Good day, this is an automatic call from the pain center. Please press hash tag (#) to continue. After the beep, on a scale from 0 to 10, enter a score for the pain you are experiencing at this moment. 0 means no pain at all, 10 means the worst pain you can imagine.” After providing the score, the voice said: “We thank you for your cooperation. We will hang up the phone.”
Figure 1.
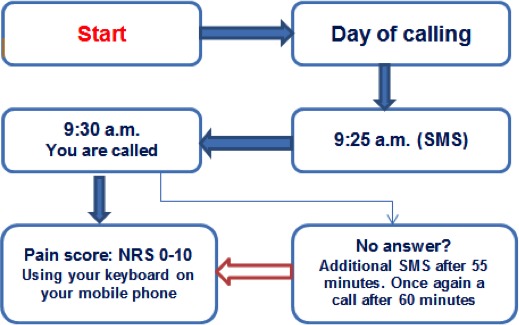
Home telemonitoring. NRS, numeric rating scale; SMS, short message service.
The time of the phone call at 9:30 a.m. was chosen to allow the specialized oncology nurses time to discuss management of the pain. At 11:30 a.m., an appointment was set for consultation with the oncologist or nurse practitioner specializing in pain and palliative care.
During the first month, each patient received an SMS 3 times a week (Monday, Wednesday, and Friday). If the NRS pain score did not exceed 3 (out of 10) after 2 weeks, the patient was called only once a week, on Wednesday.
Data Collection
For both groups, patient medical records were examined from the electronic patient file, and the names were selected from the consultation list of the oncologist. Patients were chosen in order of first consultation. Sociodemographic data (eg, date of birth, gender), medical data (eg, type of cancer, presence of metastasis), and pain‐related data (eg, pain registration or its absence, prescription of analgesics, general information about pain, vague descriptions of symptoms related to pain) were derived from the medical record.
In the intervention group, the NRS pain scores were registered in the database (EasyCareSolutions B.V.), including responses and nonresponses. The database (managed by the pain center) was a stand‐alone database and was not linked to the electronic medical records of patients. Home telemonitoring is a secure system that guarantees patients’ privacy.
When the NRS pain score was 5 or higher, the computer automatically generated an e‐mail to the pain center. The nurse practitioner of the pain center then contacted the specialized oncology nurse, supplying the names of the patients with a high NRS pain score. The specialized oncology nurse called the patients the same day to (re)‐assess and manage the pain. The effect of pain management was evaluated during the next SMS/IVR.
Data Analysis
Data from the control group and intervention group were extracted from the medical records and imported and analyzed using SPSS version 21 (IBM Corp., Armonk, NY, U.S.A.). Descriptive statistics were assessed on the pain registration or its absence; NRS pain scores and prescriptions of analgesics were derived from the medical records. Mean scores were calculated and compared with paired t‐tests. Differences in proportions were tested with the chi‐squared test and Fisher's exact test. A P value of <0.05 (2‐sided) was considered statistically significant.
Results
Between September 2016 and December 2016, we assessed 64 patient records and included 54 patients in the control group. Between February 2017 and May 2017 we approached 80 patients, of whom 54 were included in the intervention group (Figure 2).
Figure 2.
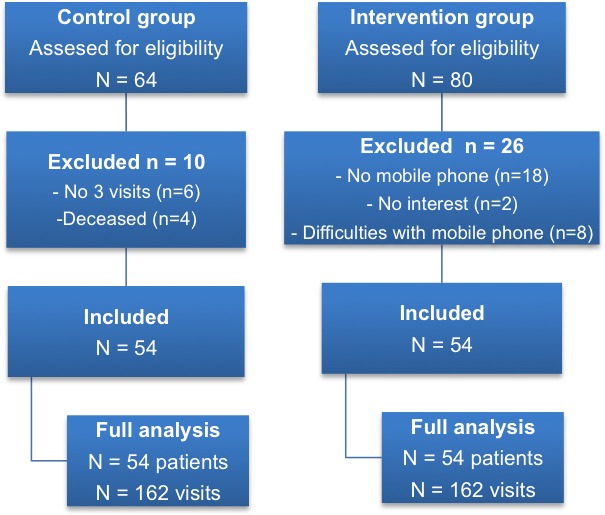
Flow chart of the study.
The sociodemographic data are shown in Table 1. In the intervention group, significantly more men and more patients with a pulmonary type of cancer participated than in the control group. Most patients had breast cancer or urologic/gynecologic cancer. Patient characteristics, except for gender and pulmonary type of cancer, were not significantly different between the groups.
Table 1.
Patient Characteristics
| Characteristics | Control Group (n = 54) | Intervention Group (n = 54) | |
|---|---|---|---|
| n (%) | n (%) | P value | |
| Gender, male | 18 (33.3) | 30 (55.6) | 0.0327 |
| Age group, years | |||
| <45 | 1 (1.9) | 4 (7.4) | |
| 45 to 60 | 22 (40.7) | 22 (40.7) | |
| 60 to 75 | 21 (38.8) | 18 (33.3) | |
| ≥75 | 10 (18.5) | 10 (18.5) | |
| Type of cancer | |||
| Colorectal | 11 (20.4) | 6 (11.1) | 0.1916 |
| Breast | 16 (29.6) | 11 (20.4) | 0.3743 |
| Urologic/gynecologic | 14 (25.9) | 11 (20.4) | 0.6488 |
| Upper abdomen | 4 (7.4) | 9 (16.7) | 0.2362 |
| Pulmonary | 0 | 9 (16.7) | 0.0027 |
| Hematologic | 9 (16.6) | 8 (14.7) | 1 |
| Presence of metastasis | |||
| Yes | 24 (44.4) | 30 (55.6) | 0.336 |
P values ≤ 0.05 are considered significant.
Some patients visited the doctor or specialized oncology nurse less frequently or did not want to participate in home telemonitoring. In both groups, we analyzed medical records from the first 3 visits (total of 162 visits). All patients in the intervention group received an SMS/IVR from the pain center 3 times a week during the first month. In the beginning, 2 of 54 patients in the intervention group experienced some technical problems in reporting their pain scores. The nurse practitioner called the patients to identify the problems. They did not have enough time to enter an NRS score on the phone. After lengthening the time to respond from 4 to 8 seconds, no further difficulties were reported. After this episode, the rate of response of all patients was 100% on each SMS/IVR, and all NRS pain scores were registered in the stand‐alone database. Only NRS scores of 5 or higher were automatically sent to the pain center and then passed on to the specialized oncology nurse.
Pain Registration
In the control group, nothing was described about pain in the medical records in 63% of the visits (Table 2), including no pain scores. In 37% of the visits, pain and location were described, but nothing about the intensity or type of pain. “No pain” or “nonspecific pain” also was reported. The description in the medical records of “no pain” could mean that pain management was adequate or that patients had no pain. The “nonspecific pain” description varied between “it is bearable” (n = 11), “less pain” (n = 18), “neuropathic pain” (n = 10), and “joint pain” (n = 14). All results between the visits were not significant.
Table 2.
Pain Registration in the Medical Records
| No. of Visits | Pain Registration | No Pain Registration | ||||||
|---|---|---|---|---|---|---|---|---|
| Registration of Pain or Its Absence | NRS/VAS Registration | |||||||
| CG | IG | CG | IG | CG | IG | CG | IG | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||
| First visit | 54 | 54 | 21 (38.9) | 27 (50) | 0 | 5 (9.2) | 33 (61.1) | 22 (40.8) |
| Second visit | 54 | 54 | 22 (40.8) | 21 (38.9) | 0 | 7 (12.9) | 32 (59.2) | 27 (50.0) |
| Third visit | 54 | 54 | 17 (31.5) | 18 (33.3) | 0 | 5 (9.2) | 37 (68.5) | 30 (55.6) |
| Total | 162 | 162 | 60 (37.0) | 66 (40.7) | 0 | 17 (10.5) | 102 (63.0) | 79 (48.8) |
CG, control group; IG, intervention group; NRS, numeric rating scale.
In the intervention group, in 48.8% of the visits there was nothing described about pain in the medical records (see Table 2). In 51.2% of the visits, pain or absence of pain was documented, among which 10.5% included a description of the intensity of pain using the NRS/VAS, compared to 0% in the control group. “No pain” or “nonspecific pain” also was reported. The “nonspecific pain” description varied between “it is bearable” (n = 16), “less pain” (n = 24), “neuropathic pain” (n = 8), and “joint pain” (n = 10).
The total number of “pain registrations” in the medical records was higher in the intervention group (51.2%) compared to the control group (37%; P = 0.034; see Table 2).
Registration of Analgesics
Registration of analgesics was analyzed per patient (Table 3). In the intervention group, more patients had a prescription for analgesics than in the control group (36 vs. 18; P = 0.005). In the control group, 36 of 54 patients did not use pain medication, and for 20 of these patients pain was not described in the medical record. In the intervention group, 18 patients did not use any analgesics, and for 4 of these patients pain was not described in the medical record. Most patients with a prescription for analgesics used slow‐release opioids.
Table 3.
Registration of Analgesics
| Registration | Control (n = 54 patients) | Intervention (n = 54 patients) |
|---|---|---|
| Registration of analgesics | 18 | 36 |
| No registration of analgesics | 36 | 18 |
Discussion
The aim of this intervention study was to evaluate whether the introduction of home telemonitoring of NRS pain scores increases the registration of pain and NRS pain scores in the medical records of oncology outpatients.
Because we only studied the registration of pain by the oncologists in the medical records, we did not include analysis of the NRS pain scores in the stand‐alone database in this article. This study showed that the rate of registration of pain improved from 37.0% in the control group to 51.2% in the intervention group. Documentation of the NRS pain scores increased by 0% in the control group and by 10.5% in the intervention group. Registration of analgesics was higher in the intervention group.
Almost all patients with a prescription for analgesics used a form of slow‐release opioids, with stable plasma levels that did not affect the background cancer pain. We assumed that the 9:30 a.m. time of calling did not affect the time the medication was taken.
In 2015, te Boveldt et al.15 analyzed pain registration in the medical records of oncology outpatients in 6 Dutch hospitals and found that pain was not systematically registered. Our analysis of the control group confirmed this conclusion. Our result of 63.0% of “no pain registration” in the control group corresponded with their results of nonacademic hospitals. Despite the implementation of the first Dutch evidence‐based guideline “Diagnoses and Pain Management in Cancer Patients” in 2008, this proportion is still remarkably high.
In 2015, Besse et al.16 found that home telemonitoring yielded a reliable assessment of pain intensity and facilitated immediate intervention if patients with cancer‐related pain needed urgent treatment in the palliative phase of their disease. They concluded that there appeared to be no barrier to using home telemonitoring. Our study also showed that telemonitoring does not have any barriers at home, despite the 2 patients who had easily soluble technical problems in the beginning.
In 2012, Kim et al.17 found in a randomized controlled trial with outpatients diagnosed with stage IV advanced solid tumors that standardized pain education and telemonitoring used by nurse practitioners is an efficient way to improve pain management in the outpatient clinic. Although Kim et al. used telemonitoring to measure pain for only 1 week without continuation, their findings were consistent with our results. Unlike Kim et al., we have continued home telemonitoring.
After introducing home telemonitoring, the use of pain medication increased, probably related to increased recognition of pain. Our intervention group of 54 patients is small, but with consecutive patients, the expectation is that pain registration and prescription of pain medication will increase even more when NRS scores are linked in the medical records.
An explanation for the increased rate of registration of NRS pain scores in the medical files in the intervention group (from 0% to 10.5%) may be that home telemonitoring helps to increase the awareness of pain and its management, which has a positive effect on the treatment of pain, resulting in better communication about pain between doctors, nurses, and patients. If NRS pain scores in the database are directly linked to the medical records, the rate of registration of the scores will increase to 100%, giving doctors and specialized oncology nurses insight into the pain itself and the process of pain treatment. This will direct more attention to the pain and will immediately lead to an improvement in pain management.
Home telemonitoring can be used as a tool to identify pain at an early stage, to register pain, and to treat it more effectively. However, home telemonitoring for registration of cancer pain is relatively new. The RdGH is one of the first hospitals in the Netherlands that offers a home telemonitoring service to this patient group.9 Home telemonitoring facilitates the guidance of patients with pain and prevents them from having additional pain in the home situation, consequently improving their quality of life. It also can be a tool that creates more contact with the patient, which improves the quality of care. To the patient it is an additional service in the treatment of pain.
This is the first intervention study to assess the use of home telemonitoring with SMS/IVR among patients with cancer pain. Home telemonitoring helped to increase the awareness of pain and its management in patients, nurses, and physicians. Our study confirmed the findings of the study of te Boveldt et al.15 and the feasibility study by Besse et al.16 However, this study had limitations. First, it was restricted to the hematology and pulmonary oncology department of a Dutch nonacademic teaching hospital, making it less easy to extrapolate our results to other departments or hospitals (eg, university hospitals). Second, we could not make a distinction between cancer‐related pain and non‐cancer‐related pain in all medical records because the information was not available. Last, in the control group, patients were consecutively included in the order of registration at the oncology outpatient clinic. The differences between both groups were based on coincidence.
Recommendation
Home telemonitoring of pain creates awareness in patients and healthcare providers. Ideally NRS pain scores should be automatically registered in an electronic medical record. When both systems are linked, the rate of NRS pain score registration will increase to 100%. This allows oncologists and specialized oncology nurses to see the actual scores directly in the medical records and consequently improve communication and assessment of pain. In order to assess the optimal frequency of SMS/IVR calls, it is important to check the scores of all patients regularly.
For further implementation of home telemonitoring, a financial investment for the acquisition of a modern communication system is necessary. Further research is required to determine the generalizability and (cost) effectiveness of the implementation of home telemonitoring for this specific patient group.
Conclusion
Home telemonitoring for patients with cancer significantly increased registrations of pain and prescriptions of analgesics in the outpatient medical records of a Dutch teaching hospital. Home telemonitoring helped to increase the awareness of pain and its management among patients, nurses, and physicians.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgements
The authors thank the specialized oncology nurses who instructed and monitored the patients.
References
- 1. van den Beuken‐van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan‐Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta‐analysis. J Pain Symptom Manag. 2016;51:1070.e9–1090.e9. [DOI] [PubMed] [Google Scholar]
- 2. Breivik H, Cherny N, Collett B, et al. Cancer‐related pain: a pan‐European survey of prevalence, treatment, and patient attitudes. Ann Oncol. 2009;20:1420–1433. [DOI] [PubMed] [Google Scholar]
- 3. Oldenmenger WH, Sillevis Smitt PA, van Dooren S, Stoter G, van der Rijt CC. A systematic review on barriers hindering adequate cancer pain management and interventions to reduce them: a critical appraisal. Eur J Cancer. 2009;45:1370–1380. [DOI] [PubMed] [Google Scholar]
- 4. Antón A, Montalar J, Carulla J, et al. Pain in clinical oncology: patient satisfaction with management of cancer pain. Eur J Pain. 2012;16:381–389. [DOI] [PubMed] [Google Scholar]
- 5. Jacobsen R, Liubarskiene Z, Møldrup C, Christrup L, Sjøgren P, Samsanaviciene J. Barriers to cancer pain management: a review of empirical research. Medicinal (Kaunas). 2009;45:427–433. [PubMed] [Google Scholar]
- 6. te Boveldt N, Vernooij‐Dassen M, Burger N, Ijsseldijk M, Vissers K, Engels Y. Pain and its interference with daily activities in medical oncology outpatients. Pain Physician. 2013;16:379–389. [PubMed] [Google Scholar]
- 7. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29:4769–4775. [DOI] [PubMed] [Google Scholar]
- 8. Vissers KCP, Van den Beuken‐van Everdingen MHJ, Dijkstra D, et al. Richtlijn diagnostiek en behandeling van pijn bij patiënten met kanker modulaire herziening, 2015, Nederlansde vereniging voor Anesthesiologie. https://www.anesthesiologie.nl/uploads/files/KD_RL_Pijn_bij_kanker.pdf (accessed June 20, 2019)
- 9. Oldenmenger WH, Baan MAG, van der Rijt CCD. Development and feasibility of a web application to monitor patients’ cancer‐related pain. Support Care Cancer. 2018;26:635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morton K, Dennison L, May C, et al. Using digital interventions for self‐management of chronic physical health conditions: a meta‐ethnographic review of published studies. Patient Educ Couns. 2017;100:616–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaana M, Paré G, Sicotte C. Home telemonitoring for respiratory conditions: a systematic review. Am J Managed Care. 2009;15:313–320. [PubMed] [Google Scholar]
- 12. Schiaffini R, Tagliente I, Carducci C, et al. Impact of long‐term use of eHealth systems in adolescents with type 1 diabetes treated with sensor‐augmented pump therapy. J Telemed Telecare. 2016;22:277–281. [DOI] [PubMed] [Google Scholar]
- 13. Jaana M, Paré G. Home telemonitoring of patients with diabetes: a systematic assessment of observed effects. J Eval Clin Pract. 2007;13:242–253. [DOI] [PubMed] [Google Scholar]
- 14. Oake N, van Walraven C, Rodger MA, Forster AJ. Effect of an interactive voice response system on oral anticoagulant management. CMAJ. 2009;180:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. te Boveldt N, Vernooij‐Dassen MJFJ, Jansen A, Vissers KPC, Engels Y. Pain is not systematically registered in Dutch medical oncology outpatients. Pain Pract. 2015;15:364–370. [DOI] [PubMed] [Google Scholar]
- 16. Besse KT, Faber‐te Boveldt ND, Janssen GH, Vernooij‐Dassen M, Vissers KC, Engels Y. Pain assessment with short message service and interactive voice response in outpatients with cancer and pain: a feasibility study. Pain Pract. 2016;16:320–326. [DOI] [PubMed] [Google Scholar]
- 17. Kim HS, Shin SJ, Kim SC, et al. Randomized controlled trial of standardized education and telemonitoring for pain in outpatients with advanced solid tumors. Support Care Cancer. 2013;21:1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]


