Abstract
Background
In recent years, bacterial cellulose (BC), or biocellulose, a natural polymer synthesized by certain bacteria, has attracted great interest in dermatology and cosmetic applications.
Several bioactive ingredients are currently loaded into BC masks. However, only a few studies have reported the effectiveness of such delivery systems.
Aim
The aim of this study was to evaluate the effect on skin parameters of three biocellulose masks formulated to have different cosmetic effects (anti‐aging, lifting, and cell renewal). In particular, skin moisturizing, skin color, skin viscoelastic properties, skin surface smoothness, wrinkle reduction, dermal homogeneity, and stratum corneum renewal were evaluated.
Materials and methods
The study involved 69 healthy Caucasian female volunteers between 25 and 64 years, who were divided into three different studies. Biocellulose facial masks were applied using the split‐face method three times a week for 4‐8 weeks depending on the study.
Results
The results obtained from this work highlight that biocellulose masks are very well tolerated. A significant decrease in skin roughness and wrinkle breadth, and an improvement in dermal homogeneity and firmness, was observed after 2 months of treatment with “anti‐aging” masks. A significant improvement in skin firmness and elasticity was observed after 1 month of treatment with “lifting” masks. Furthermore, a 1‐month treatment with “cell renewal” masks promoted the production of new skin cells through a mild exfoliating action.
Conclusions
This study highlights that biocellulose masks are effective delivery systems to successfully release into the skin several types of active compounds exerting many beneficial effects.
Keywords: bacterial cellulose, cosmetic facial mask, efficacy evaluation, in vivo study, tolerability evaluation
1. INTRODUCTION
Facial masks are traditional cosmetic treatments and have recently undergone modifications, with new aspects, ingredients, and vehicles offering new perspectives for skincare. Facial masks are very promising cosmetic formulations as they are effective and easy and quick to apply. Furthermore, they are accessible, being formulated as a gel, cream, sheet, or paste, and show instant effects on the skin.1
Among several types of face‐mask formulations, sheet masks have some distinctive traits. They are face‐shaped fabric sheets soaked in active solution, are generally used once, and are individually packaged, making them fast, convenient, and easy to use. Furthermore, they can prevent quick evaporation of the water phase, thus allowing the ingredients to penetrate deep into the skin.
Although a large number of synthetic materials have been developed for use in face masks, there has been growing interest in the development of materials based on natural polymers, mainly due to their natural biocompatibility and biodegradability, but also to their renewable character and specific properties. Among natural polymers, cellulose fibers have found extensive applications in the fields of biomedicine and cosmetics.2, 3, 4, 5, 6, 7, 8
Bacterial cellulose (BC) is produced by several species of bacteria, such as those belonging to the genera Gluconacetobacter (formerly Acetobacter), Aerobacter, Agrobacterium, Azotobacter, Achromobacter, Alcaligenes, Rhizobium, Pseudomonas, Salmonella, and Sarcina.9, 10 Among these species, Gluconacetobacter xylinus can produce a relatively high level of BC and has been widely studied as a model BC‐producing microorganism.
The material properties of BC can be tailored by using various in situ techniques, such as the addition of various substances, the change of culturing conditions, and the use of genetically modified strains, as well as ex situ strategies, such as physical and chemical modification, specialized drying conditions, and exposure to electromagnetic irradiation.11, 12
Bacterial cellulose is an alternative cellulosic substrate for facial masks, mainly due to its ultrafine network structure. Bacterial cellulose displays unique physicochemical properties, including a high Young's modulus, water‐holding capability, crystallinity, porosity, transparency, water‐retention capability, tensile strength, and biocompatibility, as well as an ultrafine fiber network.13
Like all celluloses, BC is a polymer of glucose, in which the pyranose rings are joined together by b‐1,4 bonds, giving rise to macromolecules. Bacterial cellulose is a natural and totally biocompatible polymer.14, 15 Although BC and plant cellulose possess a similar chemical structure, the self‐assembling nanofibrous structure of BC is different to that of plant cellulose. Despite the many positive effects of biocellulose, it is essential to extensively check all steps of face‐mask production in order to meet the legal requirements of quality and safety specified for cosmetic products.16
Bioactive ingredients, including those found in moisturizers, exfoliants, and skin‐lightening cosmetics, as well as herbal ingredients, vitamins, proteins, minerals, and more unusual ingredients such as pearl, snail extract, and seaweed, are currently loaded into BC masks. However, there are very few studies to support the effectiveness of these ingredients.17, 18, 19, 20
The main aim of this work is to present a scientific protocol in order to evaluate the efficacy of topical treatments based on an in vivo use of biocellulose masks (BM) with different claimed effects, namely anti‐aging BM (ABM), lifting BM (LBM), and purifying and regenerating BM (PRBM). The objective of these experimental studies was to test the effects of cellulose sheet masks loaded with different active ingredients on skin moisturization, skin color, skin viscoelastic properties, skin surface smoothness, the presence of wrinkles, dermal homogeneity, and cell renewal.
2. MATERIALS AND METHODS
2.1. Materials
Bacterial cellulose was obtained by the incubation of G xylinus in a sterile broth at 30°C for a period of up to 120 hours at a pH of 6.5‐7.0 in static conditions. After partially de‐watering the gel, the BC sheets were die‐cut to obtain a mask shape with holes for the eyes, nose, and mouth. Then, BC sheets (BCS) were supplied in stacks soaked with a preserved water solution (phenoxyethanol—0.075%, iodopropynyl butyl carbamate—0.0015%).
2.2. Preparation of face masks
Active biocellulose masks (ABM, LBM, PRBM) and placebo biocellulose masks (PLBM) were prepared, starting from BCS that were folded and embedded with 20 mL of active solution and placebo solution, respectively. The folded masks were inserted into polyethylene terephthalate (PET)/aluminum/polyethylene (PE) sachets and then thermosealed.
The placebo solution contained 95.7% water, 3% butylene glycol, 0.9% phenoxyethanol, 0.1% ethylhexylglycerin, and 0.3% xanthan gum.
The solutions for the active masks contained the following active ingredients:
Anti‐aging mask: Adansonia digitata fruit extract, Hibiscus sabdariffa flower extract, acetyl decapeptide‐3, Coffea arabica seedcake extract, hydrolyzed hyaluronic acid, palmitoyl tripeptide‐38, Kigelia africana fruit extract, Acacia senegal gum, dimethylaminoethanol tartrate, and Crocus chrysanthus bulb extract;
Lifting mask: hydrolyzed Chenopodium quinoa seed, hydrolyzed hyaluronic acid, and acetyl decapeptide‐3;
Purifying and regenerative mask: Portulaca oleracea flower/leaf/stem extract, sodium hyaluronate cross‐polymer, Chlorella vulgaris extract, kaolin, and magnesium sulfate.
In particular, fruit extract of A digitata and H sabdariffa flower extract were chosen because of the high concentration of compounds with antioxidant efficacy that are present in both of them, hydrolyzed hyaluronic acid acts improving hydration and skin elasticity. Furthermore, peptides were chosen because of their biomimetic action, contributing to the activation of regeneration processes and initiation of mechanisms that prevent aging.21, 22, 23, 24, 25
All masks were produced following Good Manufacturing Practices (GMP) guidelines and were checked for quality, microbial content, and the effectiveness of the preservative system through the Challenge test, and for all masks, a confirmation of tolerability was performed through an in vivo test (Patch Test) in order to ensure a safe and high‐quality product suitable to be applied to volunteers.16, 26
2.3. In vivo study design
The study was carried out according to the Helsinki declaration (Ethical Principles for Medical Research Involving Human Subjects).27 A total of 69 healthy female volunteers between 25 and 64 years old were recruited according to the following general inclusion and exclusion criteria.
General inclusion criteria:
Good general health.
Absence of cutaneous diseases.
Absence of cutaneous lesions or other lesions in the area of interest that could interfere with the study evaluation.
No history of hypersensitivity to the common components of the cosmetic formulations.
Not pregnant or breastfeeding.
Subjects agreed not to undergo other treatments in the treated area for the entire duration of the test.
Subjects signed the informed consent form.
General exclusion criteria:
Subjects that did not meet the inclusion criteria above.
Subjects undergoing pharmacological therapy.
Subjects that had participated in a similar study less than 60 days prior to the start of the present study.
Subjects with known allergies.
Volunteers selected according to the specific inclusion criteria were divided into three different studies:
ABM: a total of 24 subjects between 42 and 64 years old with facial skin aging (lines, wrinkles, thin skin, and loss of skin tone) were enrolled in a 2‐month study of the continuous use of ABM and PLBM;
LBM: A total of 20 subjects between 38 and 57 years old with facial skin sagging were enrolled in a 1‐month study of the continuous use of LBM and PLBM;
PRBM: A total of 25 subjects between 25 and 40 years old with dull facial skin were enrolled in a 1‐month study of the continuous use of PRBM and PLBM.
In each study, subjects were trained to apply, by the split‐face method in a self‐controlled study, the placebo mask on one side of the face, and the active mask (ABM, LBM, or PRBM) on the other side. In this way, a comparison was made between the active and placebo masks on the same subject so as to remove or reduce as much as possible the effect of unintentional factors other than treatment on the subject response. Each subject therefore served as her own control, thereby giving greater statistical power to the study.
Masks were applied at specified intervals three times a week for 4 or 8 weeks depending on the study design. The two half masks were placed on the face after mild cleansing and left on for up to 20 minutes. The specific face sides to which the active and placebo masks were applied were randomized among volunteers.
2.4. Instrumental efficacy evaluation
Assessments were performed at the beginning of each study (T0) and at predetermined subsequent times (T1 or T2) depending on the aim of the study. The investigated areas were the forehead on both sides of the face, the forehead fold for wrinkle investigation, and the cheekbone for the investigation of the skin renewal effect.
All measurements were made in an air‐conditioned room with controlled temperature and humidity (T = 22°C, relative humidity [RH] = 70 ± 5%). Subjects were preconditioned in the room for at least 15 minutes before the measurements were made.
The instruments used in the evaluation of skin parameters involve contact between the skin and a series of probes that do not cause discomfort, pain, or damage the skin.
2.5. Evaluation of tolerability and moisturizing effect
Assessments of the tolerability and moisturizing effects of the facial masks were performed at T0 and at T1 (after 12 applications). The investigated area was the forehead on both sides of the face.
The tolerability of the masks was assessed by measurement of the erythema index (EI) using a reflectance spectrophotometer (Mexameter MX 18, Courage & Khazaka), which measures levels of melanin and hemoglobin (erythema), the two components that are mainly responsible for the color of the skin. To make the measurement, a probe with a diameter of 5 mm emits three wavelengths of light (568, 660, and 870 nm) in order to quantify the amount of the selected biochromophores (melanin, hemoglobin), thus providing an arbitrary melanin index (MI; range = 0‐999) and EI (range = 0‐999).
The stratum corneum water content (SCWC), which reflects the hydrating effect of the face mask, was evaluated by a CM 825 corneometer (cutometer MPA580, Courage & Khazaka). Corneometry is a technique used to assess the hydration of the outer layer of the epidermis, which is known as the stratum corneum.28 Since skin is a dielectric medium, all variations in hydration result in a corresponding change in the skin's electrical capacity.29 The device used in our trial was equipped with a 49 mm2 surface probe that allows precise measurement in 1 second within a depth range of 10‐20 μm in the stratum corneum. The parameters were expressed using an arbitrary score scale (0‐100 AU).
The assessment of skin barrier integrity and the possible occlusive or irritant effect of the facial masks used in this study were performed by the measurement of transepidermal water loss (TEWL) using a TM 300 Tewameter (cutometer MPA580, Courage & Khazaka).30 TEWL was quantified in g/m2 h by a skin evaporimeter made of a small cylindrical open chamber (1 cm in diameter, 2 cm in height) with two hygrometric sensors connected to a microprocessor plugged into a computer workstation. The device allows the recording of TEWL values (ranging from 0 to 90 g/m2 h) as well as the relative humidity (ranging from 0% to 100%) and probe temperature.
2.6. Biocellulose mask specific activity
2.6.1. Anti‐aging effect (ABM)
Assessments were performed at T0, after 12 applications (T1), and at the end of treatment after 24 applications (T2). The investigated areas were the forehead on both sides of the face and the forehead fold for wrinkle investigation.
Skin surface roughness
Skin surface roughness was evaluated using a PRIMOSpico instrument (GFM), which allows the acquisition of a 3D image of the skin surface by an optical analysis system and its transformation using dedicated software. The 3D image acquired with the instrument can be transformed using specific software into a color image in which each shade of color (from blue to yellow) is related to a height; thus, the presence of a groove will be indicated by a color gradient that can transition from blue to green according to its depth. Parameters characterizing skin micro‐reliefs which can be measured at a glance thanks to this 3D topographic representation of the skin are the following:
Ra represents the average skin roughness. It is the integral of the function describing the skin roughness profile curve; the higher the value of Ra, the higher the skin roughness.
Rmax represents the maximal skin roughness. Single roughness depths are first determined by measuring the peak‐to‐valley distance within each evaluated sampling length. Rmax is the greatest peak‐to‐valley distance within any single sampling length, which is defined as the vertical distance between the top of the highest peak and the bottom of the lowest valley.
Dermis homogeneity
Ultrasound images were produced and further processed by a 22 MHz DUB system (tpmTaberna pro medicum GmbH), which allows the acquisition of an ultrasound image of the skin and the measurement of skin tissue thickness in μm (A‐scan and B‐scan mode, respectively), thus permitting the visualization of structures up to 8 mm in depth. The dermis is echogenic, with echoes originating from the fiber network comprised of collagen and elastic fibers; fat and edema are echo‐poor areas.
2.6.2. Lifting effect (LBM)
Assessments were performed at T0 and at the end of treatment after 12 applications (T1). The investigated area was the forehead on both sides of the face.
Viscoelastic properties of the skin
The effect of the products on skin mechanical properties was evaluated using an MPA580 cutometer (Courage & Khazaka). In this technique, the skin is evaluated through the measurement of the vertical deformation of the skin induced by vacuum aspiration with a negative pressure of 450 mbar, which is applied to the skin for between 1 and 3 seconds through a probe with a diameter of 2 mm. Each aspiration is followed by a release time, allowing the skin to return to its resting conditions. The considered parameters were:
R0: Skin firmness. This parameter measures the maximum amplitude that the skin reaches following the application of suction by the cutometer and represents the passive response of the skin to applied force. R0 represents the ratio between skin looseness and skin tightness; the lower R0 is, the higher the firmness of the skin.
R2: An index representing the capability of the skin to return to its rest condition after being exposed to mechanical stress, that is, it is an index of the total elasticity of the skin. The closer R2 is to 1, the more elastic the skin is.
R5: The ratio between the skin's immediate recovery and its immediate deformation. Therefore, it is a measure of the net elasticity.
R7: The portion of the skin elasticity compared to the complete skin deformation curve. The closer R7 is to 1, the more elastic the skin is.
2.6.3. Purifying and renewal effect (PRBM)
Assessments were performed at T0 and at the end of treatment after 12 applications (T1). To study the stratum corneum renewal effect, the investigated area was the cheekbone.
Stratum corneum renewal effect
The stratum corneum renewal effect was investigated by the use of tape stripping, which is one of the most frequently used approaches to sample the stratum corneum.31 This method involves the application of adhesive tape with a diameter of 14 mm (D101 D‐Squame Stripping Discs, Cuderm) to the skin, and its subsequent removal to strip off a layer of stratum corneum. A constant pressure of 225 g/cm2 is impressed on the disk surface. The infrared densitometry (IRD) technique was used to quantify the absolute amount of stratum corneum removed by each tape strip.
In particular, a SquameScan™ device (CuDerm) was used to measure the protein content (PC) of the stratum corneum samples removed by tape stripping. This apparatus has a diode which emits light at a peak wavelength of 850 nm that shines through the tape strip onto a device which measures the amount of transmitted light. The results are expressed as PC in µg/cm2.
2.7. Statistical analysis
Data were processed both for descriptive statistical analysis and statistical analysis with comparison tests for parametric data. In particular, in the self‐controlled study methodology used in this work, a paired t test was applied. For each parameter analyzed in the clinical studies, a comparison was made between the values measured at time Ti (4 or 8 weeks) with respect to the initial value at time T0 and an evaluation of the net active‐placebo effect, for both active and placebo masks. For each data series compared using the t test, normal distribution was verified by the Kolmogorov‐Smirnov test. A significance level of 5% was chosen, so changes were considered statistically significant for P < 0.05.
3. RESULTS
All volunteers used an active mask on one half of the face and a placebo mask on the other half of the face for the entire duration of the test. A randomized application procedure was applied.
3.1. Tolerability and moisturizing effect of biocellulose masks
The instrumental evaluation of the EI can give information about mask tolerability.
Figure 1 reports the main results from the erythema analysis at T0 and after 12 applications in the facial area for treatment with active masks (ABM, LBM, PRBM) and PLBM.
Figure 1.
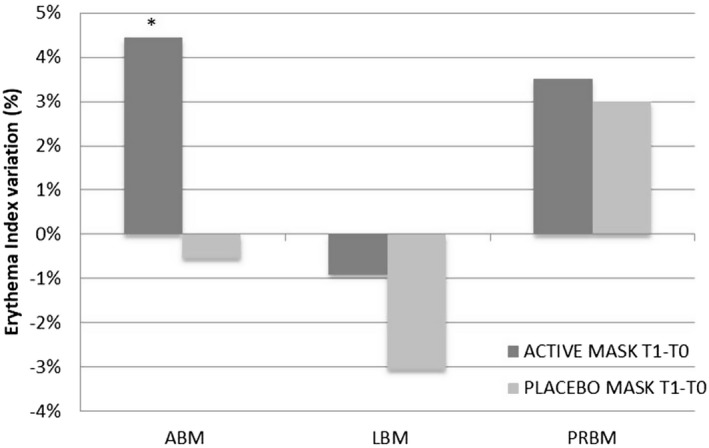
Comparison of the variation of the erythema index in the areas treated with anti‐aging biocellulose masks (ABM), lifting biocellulose masks (LBM), purifying and regenerating biocellulose masks (PRBM), and placebo biocellulose masks (PLBM). *, P < 0.05 is significant; **, P < 0.01 is strongly significant; ***, P < 0.001 is very strongly significant
The variation of the EI (erythema index) after 4 weeks of application was found to be statistically significant only in the ABM test, in which an increase of 4.45% was observed. In the ABM study, the final observation was made at T2 (after 8 weeks). At this time, the variation in erythema with respect to T0 was 3.71% and was not statistically significant (P = 0.1677). These results highlight that the tested products were very well tolerated.
SCWC and TEWL were investigated since they are relevant parameters for the evaluation of skin‐moisturizing effectiveness. Furthermore, a significant reduction in the TEWL may be symptomatic of an occlusive effect of the formulation in use, while an increase can highlight a specific alteration reaction of the skin barrier caused by the use of the product. Therefore, TEWL is considered to be an important measure of epidermal barrier function.
Figure 2 reports the main results for SCWC and TEWL after 12 applications in the areas treated with active masks (ABM, LBM, PRBM) and in the area treated with a PLBM.
Figure 2.
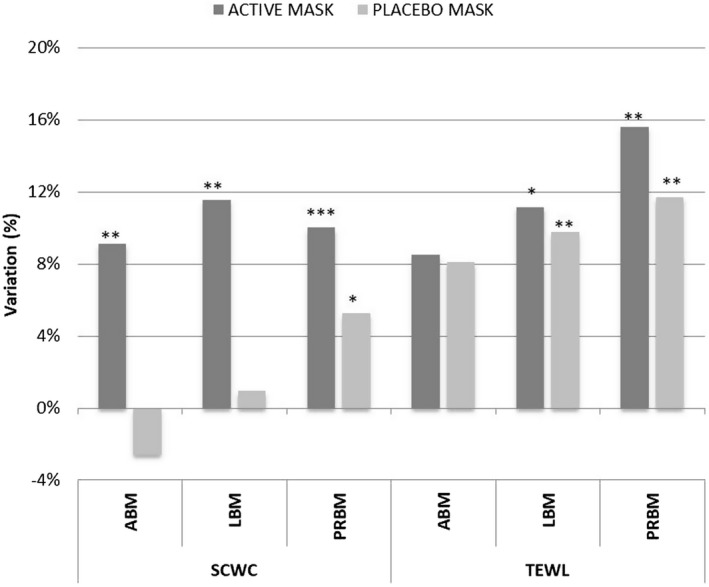
Comparison of the variation in stratum corneum water content (SCWC) and transepidermal water loss (TEWL) in the areas treated with ABM, LBM, PRBM, and PLBM. *, P < 0.05 is significant; **, P < 0.01 is strongly significant; ***, P < 0.001 is very strongly significant
The results show that, after 12 applications, all active masks, namely ABM, LBM, and PRBM, led to statistically significant increases of the SCWC, of 9.1% (P = 0.006), 11.54% (P = 0.0045), and 10% (P = 0.0001), respectively. The increment of the SCWC parameter in the placebo area was statistically significant (P = 0.0324) only for PRBM, with an increase of 5.24%. Variations in TEWL values were statistically significant both in the LBM and in the PRBM tests for both active and placebo masks (Figure 2). In any case, TEWL values remained within the range of healthy skin, confirming the high tolerability of the products.
The net active‐placebo effect for all studies is shown in Figure 3. The net active‐placebo effect for the SCWC parameter was always statistically significant and was more than 10% for the ABM and LBM and less than 5% for the PRBM. No statistically significant differences in the net active‐placebo effect were found for TEWL or EI.
Figure 3.
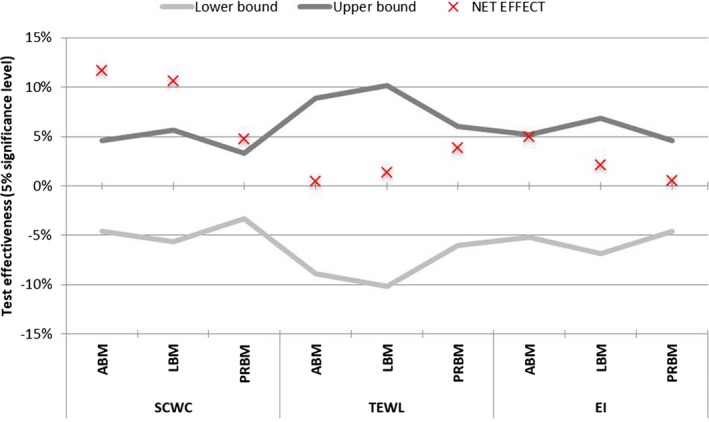
Confidence intervals of the net active‐placebo effect. The area between the lower and upper bounds corresponds to the region of the acceptance of the null hypothesis, that is, that there is no difference between the active and placebo masks
3.2. Specific activity of biocellulose masks
3.2.1. Anti‐aging effect
In order to evaluate the anti‐aging effect of ABM, 24 subjects between 42 and 64 years old with signs of facial skin aging were enrolled to use the active mask and a placebo mask three times a week for 2 months using the split‐face method.
Skin texture improvement and wrinkle‐reduction effect
The in vivo evaluation of the surface topography of human skin is of great interest for dermatological research, as such topography represents the 3D organization of the dermis and the subcutaneous tissue. The surface topography can be considered to reflect the functional status of the skin; it is an expression of the ability of the skin to respond to mechanical stimuli and threats. Various treatments exist to reverse or at least decrease changes in skin texture. In order to assess the success of an anti‐aging treatment, wrinkles and the variation in micro‐relief depth need to be measured before and after the application of the cosmetic product.
The 3D system permitted the evaluation, by matching, of the same skin area in the three subsequent analyses. The parameters Ra (the average skin roughness correlated to sensory perception) and Rmax (the greatest peak‐to‐valley distance in a single wrinkle) were measured. The greater the values of Ra and Rmax, the greater the skin roughness caused by skin texture, lines, and wrinkles.
The results obtained are reported in Table 1.
Table 1.
Mean values, variation, net effect, and statistical significance of average skin roughness (Ra; µm) and maximal skin roughness (Rmax; µm) obtained for all subjects in the areas treated with anti‐aging biocellulose masks (ABM) and placebo biocellulose masks (PLBM) after 4 wk (T1) and 8 wk (T2) of treatment
| Parameter | Study | Mean | T1‐T0 | T2‐T0 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | Variation | Variation (%) | P‐value | Variation | Variation (%) | P‐value | ||
| Ra | ABM | 31.13 | 29.46 | 27.63 | −1.667 | −5.13 | 0.0101 | −3.500 | −10.99 | 0.0000 |
| PLBM | 29.79 | 29.25 | 29.08 | −0.542 | −1.29 | 0.2965 | −0.708 | −1.92 | 0.5461 | |
| Net Effect | 1.33 | 0.21 | −1.46 | −1.125 | −3.84 | 0.1180 | −2.792 | −9.07 | 0.0000 | |
| Rmax | ABM | 217.88 | 209.04 | 194.46 | −8.833 | −3.62 | 0.1253 | −23.417 | −10.33 | 0.0000 |
| PLBM | 201.33 | 200.46 | 201.25 | −0.875 | 0.75 | 0.8678 | −0.083 | 0.72 | 0.9891 | |
| Net Effect | 16.54 | 8.58 | −6.79 | −7.958 | −4.37 | 0.1846 | −23.333 | −11.04 | 0.0007 | |
The results show that the application of the ABM led to a significant decrease in the skin roughness of 5.13% (P = 0.0101) after 12 applications, and a significant decrease of 10.99% (P = 0.0000) after 2 months of treatment. The variation of this parameter in the placebo area was not significant at T1 or at T2 (Figure 4). The net ABM‐PLBM effect showed a trend of decreasing skin roughness that was not statistically significant at T1 (P = 0.1180) but was statistically significant at T2, when a decrease of about 9% (P = 0.0000) was observed, as shown in Figure 5.
Figure 4.
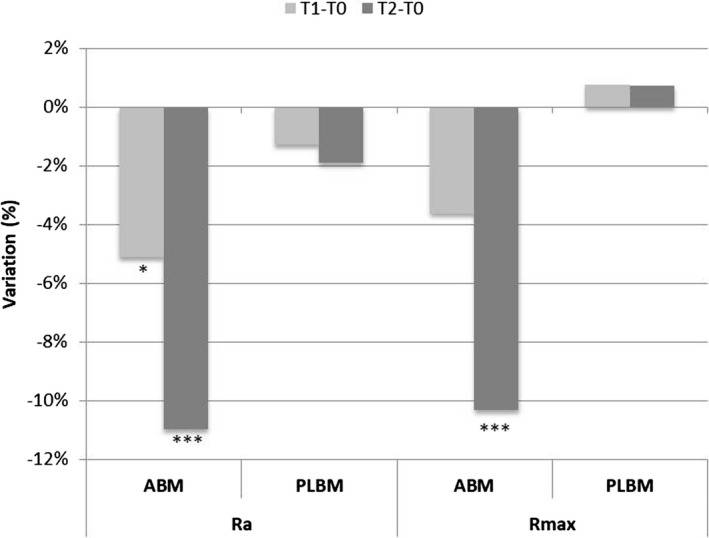
Variation and statistical significance of average skin roughness (Ra) and maximal skin roughness (Rmax) in the areas treated with ABM and PLBM. *, P < 0.05 is significant; **, P < 0.01 is strongly significant; ***, P < 0.001 is very strongly significant
Figure 5.
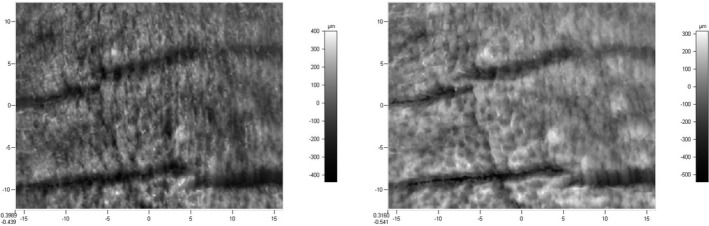
3D skin images of a forehead before treatment (left) and after 2 mo of treatment with ABM (right)
At the same time, a significant decrease in the breadth of wrinkles of 10.33% (P = 0.0000) was detected after 2 months of treatment. The variation of this parameter in the PLBM area was not statistically significant at T1 or T2 (Figure 4).
These effects could be due to the presence of various active ingredients in the ABM. Fruit extract of A digitata, also known as the baobab tree, contains a high concentration of vitamin C—seven times more than orange fruit—and flavonoid compounds showing a high antioxidant efficacy.21 Hibiscus sabdariffa flower extract is rich in phenolic acids, flavonoids, and anthocyanins, which exert protective effects against oxidative stress.22 Furthermore, acetyl decapeptide‐3 and palmitoyl tripeptide‐38 are biomimetic peptides identical to amino acid sequences synthesized by humans and so could interact with growth factor receptors and provide clinical anti‐aging effects.23
The 3D images shown in Figure 5, which were created using the software, clearly show the reduction in wrinkle breadth following 2 months of treatment with ABM.
Improvement in dermis homogeneity and thickness
The evaluations carried out by ultrasound permitted the total thickness of the epidermis and dermis to be visualized and measured. Measurements of skin thickness were performed using the A‐scan mode on all subjects at baseline and after 12 and 24 applications.
Figure 6 shows examples of ultrasound images of skin at baseline and after 12 and 24 applications of ABM treatment.
Figure 6.
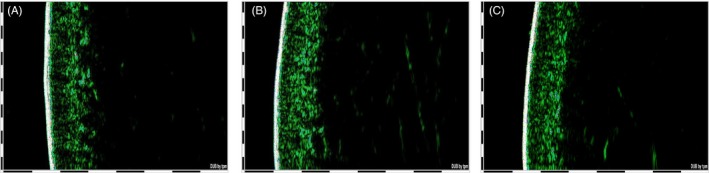
B‐mode ultrasound images of skin at T0 (A), after 12 applications of ABM (B), and at the end of the study after 24 applications of ABM (C). The epidermis and dermis are visible as echo‐rich (green) areas
The comparison of ultrasound images before and after treatment clearly shows a difference in the organization of the tissue below the epidermis resulting in an improvement of dermis homogeneity and firmness. These results were confirmed by measurements of dermis thickness.
The ABM led to an average reduction in dermis thickness of more than 3% (P = 0.0001) after 12 applications and of more than 4% (P = 0.0000) after 24 applications. In the areas treated with the placebo, no significant differences were observed at T1, while a significant reduction of dermis thickness of more than 1% (P = 0.0117) was observed at T2 (Figure 7).
Figure 7.
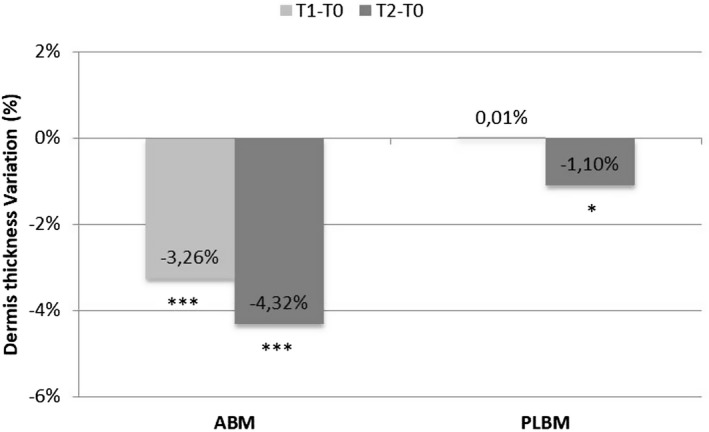
Variation and statistical significance of dermis thickness obtained for all subjects in the areas treated with ABM and PLBM after 4 wk (T1) and 8 wk of treatment (T2). *, P < 0.05 is significant; **, P < 0.01 is strongly significant; ***, P < 0.001 is very strongly significant
The net ABM‐PLBM effects for Ra, Rmax, and dermis thickness are shown in Figure 8. The trend of decrease in wrinkle breadth was not statistically significant at T1; however, it was statistically significant at T2, when a decrease of about 11% (P = 0.0007) was observed. The net effect ABM‐PLBM showed a statistically significant reduction in the dermis thickness at T1 of about 3.25% (P = 0.0000).
Figure 8.
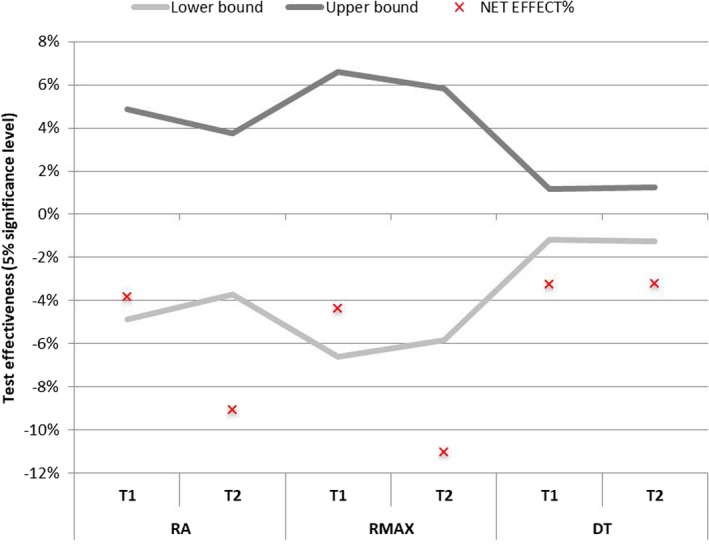
Confidence intervals of the active‐placebo net effect. The area between the lower and upper bounds corresponds to the region of acceptance of the null hypothesis, that is, that there is no difference between the active and placebo masks
3.2.2. Lifting effect
In order to evaluate the lifting effect of LBM, 20 subjects between 38 and 57 years old with facial skin sagging were enrolled to use this mask and a placebo mask three times a week for 1 month using a split‐face method.
Several elasticity parameters were evaluated. Table 2 shows the mean values, variation, net effect, and statistical significance of skin elasticity obtained for all subjects in the areas treated with LBM and PLBM after 4 weeks of treatment (T1).
Table 2.
Values, variation, net effect, and statistical significance of skin elasticity obtained for all subjects treated with LBM and PLBM
| Parameter | Study | Mean | T1‐T0 | |||
|---|---|---|---|---|---|---|
| T0 | T1 | Variation | Variation (%) | P‐value | ||
| R0 | LBM | 0.262 | 0.198 | −0.0634 | −24.22 | 0.0000 |
| PLBM | 0.247 | 0.222 | −0.0252 | −10.63 | 0.0064 | |
| Net effect | 0.014 | −0.024 | −0.0381 | −13.59 | 0.0002 | |
| R2 | LBM | 0.561 | 0.621 | 0.0594 | 10.78 | 0.0004 |
| PLBM | 0.593 | 0.633 | 0.0399 | 7.29 | 0.0553 | |
| Net effect | −0.031 | −0.012 | 0.0195 | 3.49 | 0.4905 | |
| R5 | LBM | 0.426 | 0.498 | 0.0724 | 18.21 | 0.0103 |
| PLBM | 0.473 | 0.476 | 0.0034 | 4.68 | 0.9072 | |
| Net effect | −0.047 | 0.022 | 0.0691 | 13.53 | 0.0963 | |
| R7 | LBM | 0.252 | 0.280 | 0.0279 | 11.42 | 0.0050 |
| PLBM | 0.275 | 0.278 | 0.0029 | 2.67 | 0.8302 | |
| Net effect | −0.023 | 0.002 | 0.0250 | 8.75 | 0.1509 | |
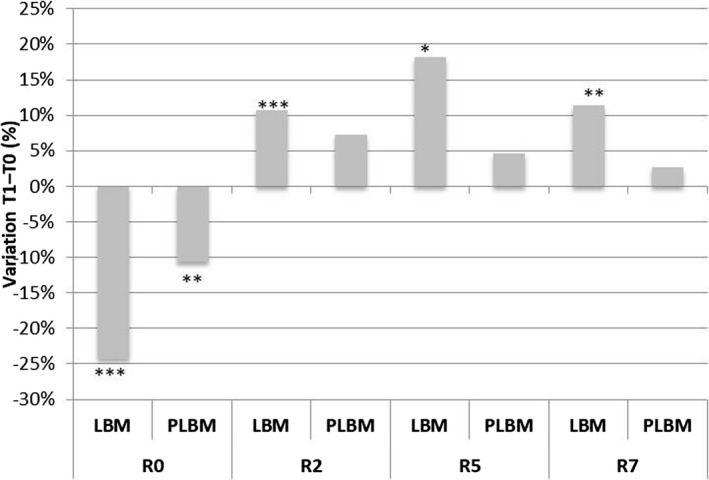
R0 = skin firmness; R2 = gross skin elasticity index; R5 = net elasticity; R7 = viscoelasticity index.
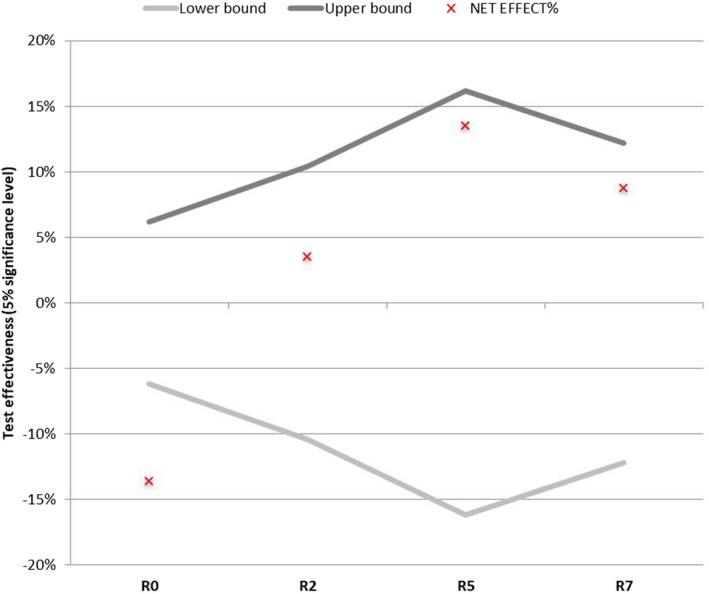
The results show that the application of LBM led to an improvement in skin firmness, with a significant decrease in the R0 parameter of 24.22% (P = 0.0000) observed after 1 month of treatment (Figure 9). The variation of this parameter in the placebo area was significant (P = 0.0064), and the net active‐placebo effect showed a statistically significant decrease of about 13.59% (P = 0.0002; Figure 10).
Figure 9.
Variation and statistical significance of skin elasticity in the areas treated with LBM and PLBM. *, P < 0.05 is significant; **, P < 0.01 is strongly significant; ***, P < 0.001 is very strongly significant
Figure 10.
Confidence intervals of the net active‐placebo effect. The area between the lower and upper bounds corresponds to the region of acceptance of the null hypothesis, that is, that there is no difference between the active and placebo masks
The application of LBM led to a significant increase in the R2 parameter of about 10.78% (P = 0.0004) after 1 month of treatment. The variation of this parameter in the placebo area was not significant.
The results show that the application of LBM led to a significant increase in the R5 parameter of about 18.21% (P = 0.0103) after 1 month of treatment. The variation of this parameter in the placebo area was not significant.
The results show that the application of LBM led to a significant increase in the R7 parameter of about 11.42% (P = 0.0050) after 1 month of treatment. The variation of this parameter in the placebo area was not significant.
The net active‐placebo effect was not statistically significant after 12 applications of LBM for the parameters R2, R5, and R7, as shown in Figure 10.
Among several ingredients contained in the LBM, hydrolyzed hyaluronic acid could be responsible for the improvement of skin elasticity. Furthermore, the presence of this ingredient together with acetyl decapeptide‐3 could lead to clinical effects such as the slowing of aging, due to the action of acetyl decapeptide‐3 on the formation of wrinkles.24, 25 Skin roughness decreased significantly after 1 month of treatment with LBM (by 6.7%). The variation of this parameter in the placebo area was not significant. Unlike for ABM, no significant difference was detected in wrinkle breadth following the use of LBM.
3.2.3. Skin purifying and renewal effect
In order to evaluate the skin purifying and renewal effect of PRBM, subjects between 25 and 40 years old with dull facial skin were enrolled in a 1‐month study involving the continuous use of PRBM and PLBM.
At the beginning (T0) and the end of the study (T1), four different tape strippings (S1, S2, S3, S4) were performed sequentially on the same area of the cheekbone treated with PRBM and PLBM. The tape stripping procedure is able to remove the first layers of the stratum corneum.
Table 3 reports total and single tape stripping PC data in µg/cm2.
Table 3.
Total and single tape stripping protein content (PC) data showing variation, net effect, and statistical significance
| Sample | Study | Mean (µg/cm2) | T1‐T0 | |||
|---|---|---|---|---|---|---|
| T0 | T1 | Variation | Variation (%) | P‐value | ||
| PCtot | PRBM | 80.59 | 51.99 | −28.60 | −33.02 | 0.0000 |
| PLBM | 79.62 | 76.08 | −3.54 | −0.92 | 0.4294 | |
| Net effect | 0.97 | −24.09 | −25.06 | −32.09 | 0.0000 | |
| PCS1 | PRBM | 23.59 | 17.50 | −6.09 | −20.00 | 0.0008 |
| PLBM | 23.57 | 23.98 | 0.42 | 9.41 | 0.7950 | |
| Net effect | 0.03 | −6.48 | −6.51 | −29.41 | 0.0003 | |
| PCS2 | PRBM | 21.74 | 13.44 | −8.30 | −35.64 | 0.0000 |
| PLBM | 21.00 | 20.00 | −1.00 | 0.74 | 0.4974 | |
| Net effect | 0.74 | −6.56 | −7.30 | −36.38 | 0.0002 | |
| PCS3 | PRBM | 18.76 | 11.32 | −7.44 | −36.53 | 0.0000 |
| PLBM | 18.52 | 16.65 | −1.87 | −7.63 | 0.0954 | |
| Net effect | 0.24 | −5.33 | −5.57 | −28.89 | 0.0015 | |
| PCS4 | PRBM | 16.50 | 9.73 | −6.77 | −38.41 | 0.0000 |
| PLBM | 16.53 | 15.45 | −1.08 | 0.46 | 0.3714 | |
| Net effect | −0.03 | −5.72 | −5.69 | −38.87 | 0.0004 | |
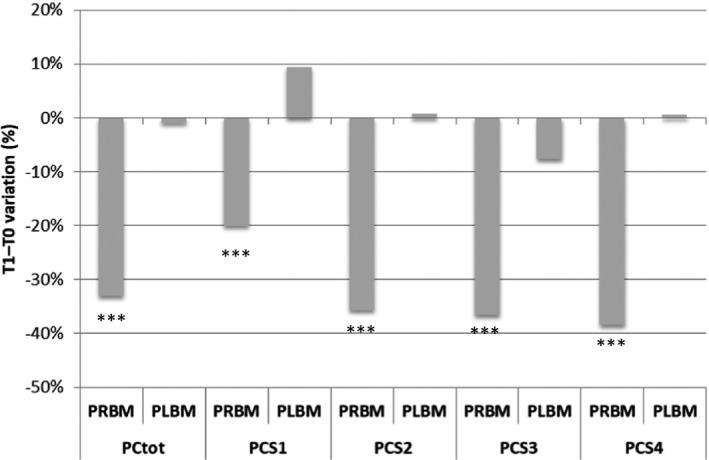
The results show that PRBM led to a significant decrease of the average PC and then of the stratum corneum cohesion of 33.02% (P = 0.0000) after 1 month of treatment. The variation of the average PC in the placebo area was not statistically significant (P = 0.4294) and was equal to −0.92% (Figure 11).
Figure 11.
Variation and statistical significance of the protein content (PC) of total and single tape‐strips (Si) in the areas treated with PRBM and PLBM. *, P < 0.05 is significant; **, P < 0.01 is strongly significant; ***, P < 0.001 is very strongly significant
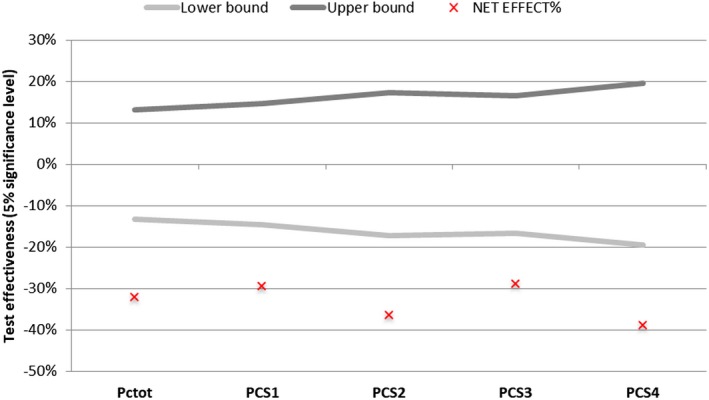
The net active–placebo effect on the average PC was statistically significant and corresponded to a variation of about −32.09% (P = 0.0000; Figure 12).
Figure 12.
Confidence intervals of the net active‐placebo effect for protein content (PC). The area between the lower and upper bounds corresponds to the region of acceptance of the null hypothesis, that is, that there is no difference between the active and placebo masks
The stratum corneum appears to be more cohesive with a reduction of the removed PC, which results in an improvement in the smoothness of the skin surface. The PRBM seems to exert a renewal effect on the epidermis through a mild exfoliating action.
By analyzing the PC of each single tape, it is clear that, at the beginning of the study, the amount of proteins was the same for both sides of the face. After 1 month of treatment with PRBM, a lower amount of protein was removed by each tape strip with a strong statistical significance, as highlighted in Figure 11, and the net active‐placebo effect was always statistically significant for each strip (Figure 12).
The smoothing effect could be due to an exfoliating mechanism. Additionally, if this effect is present, no erythema is highlighted in the PRBM‐treated area, probably due to the presence of P oleracea extract, which alleviates skin irritation and allergic responses and has pharmacological effects such as antibacterial and anti‐inflammatory effects. Thus, this extract is used mainly in acne care products, cosmetics for sensitive skin, etc.31
4. CONCLUSIONS
Bacterial cellulose is an innovative substrate for facial masks, mainly due to its favorable properties such as very high water‐holding capability, tolerability, and biocompatibility.
Even though there is a growing skincare market for cosmetic sheet masks, there are very few studies addressing their effectiveness in the literature. This work mainly focused on the in vivo evaluation of the effectiveness of three different face masks based on biocellulose sheets that were formulated to have specific cosmetic effects (anti‐aging, lifting, and cell renewal).
Scientific protocols using noninvasive skin bioengineering techniques and adequate statistical methods were employed in order to discriminate the efficacy of the different mask formulations tested with respect to placebo comparison.
In particular, the self‐controlled study methodology used in this work revealed to be the best approach to investigate both the effectiveness of active masks and the evaluation of the net active‐placebo effect.
The results obtained in this study highlight the possibility to develop cosmetic masks with very high quality, tolerability, and efficacy based on biocellulose sheet embedded with several bioactive ingredients, such as peptides, natural extracts, and biopolymers. A statistically significant decrease in skin roughness and wrinkle breadth, and a statistically significant improvement in dermis homogeneity and firmness, was observed after 2 months of treatment with “anti‐aging” biocellulose masks. Furthermore, a statistically significant improvement of skin firmness and elasticity was observed after 1 month of treatment with “lifting” biocellulose masks. Moreover, 1‐month treatments with “cell renewal” “purifying and regenerating biocellulose mask, which promote the production of new skin cells through a mild exfoliating action, significantly improved stratum corneum cohesion.
In conclusion, this study highlights that biocellulose masks represent an effective tailored system to exert statistically significant beneficial effects to the skin.
Perugini P, Bleve M, Redondi R, Cortinovis F, Colpani A. In vivo evaluation of the effectiveness of biocellulose facial masks as active delivery systems to skin. J Cosmet Dermatol. 2020;19:725–735. 10.1111/jocd.13051
REFERENCES
- 1. Nilforoushzadeh MA, Amirkhani MA, Zarrintaj P, et al. Skin care and rejuvenation by cosmeceutical facial mask. J Cosmet Dermatol. 2018;17(5):693‐702. [DOI] [PubMed] [Google Scholar]
- 2. Sulaeva I, Henniges U, Rosenau T, Potthast A. Bacterial cellulose as a material for wound treatment: properties and modifications. A review. Biotechnol Adv. 2015;33(8):1547‐1571. [DOI] [PubMed] [Google Scholar]
- 3. Lina F, Yue Z, Jin Z, Guang Y. Bacterial cellulose for skin repair materials, biomedical engineering In: Fazel‐Rezai R, ed. Frontiers and Challenges. Rijeka, Croatia: InTech Publisher; 2011:249‐274. [Google Scholar]
- 4. Ullah H, Santos HA, Khan T. Applications of bacterial cellulose bin food, cosmetics and drug delivery. Cellulose. 2016;23(4):2291‐2314. [Google Scholar]
- 5. Czaja WK, Young DJ, Kawecki M, Brown RM Jr. The future prospects of microbial cellulose in biomedical applications. Biomacromol. 2007;8(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 6. Iguchi M, Yamanaka S, Budhiono A. Review bacterial cellulose—a masterpiece of nature's arts. J Mater Sci. 2000;35:261‐270. [Google Scholar]
- 7. Du R, Zhao F, Peng Q, Zhou Z, Han Y. Production and characterization of bacterial cellulose produced by Gluconacetobacter xylinus isolated from Chinese persimmon vinegar. Carbohydr Polym. 2018;15(194):200‐207. [DOI] [PubMed] [Google Scholar]
- 8. Petersen N, Gatenholm P. Bacterial cellulose‐based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol. 2011;91:1277‐1286. [DOI] [PubMed] [Google Scholar]
- 9. Chawla PR, Bajaj IB, Survase SA, Singhal RS. Fermentative production of microbial cellulose. Food Technol Biotechnol. 2009;47(2):107‐124. [Google Scholar]
- 10. Shao W, Wu J, Liu H, Ye S, Jiang L, Liu X. Novel bioactive surface functionalization of bacterial cellulose membrane. Carbohydr Polym. 2017;15(178):270‐276. [DOI] [PubMed] [Google Scholar]
- 11. Stumpf TR, Yang X, Zhang J, Cao X. In situ and ex situ modifications of bacterial cellulose for applications in tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;1(82):372‐383. [DOI] [PubMed] [Google Scholar]
- 12. Hasan N, Biak A, Kamarudin S. Application of bacterial cellulose (BC) in natural facial scrub. IJASEIT. 2012;2(4):1‐4. [Google Scholar]
- 13. Gallegos A, Carrera SH, Parra R, Keshavarz T, Iqbal H. Bacterial cellulose: a sustainable source to develop value‐added products ‐ a review. BioResources. 2016;11(2).5641-5655. [Google Scholar]
- 14. Torres FG, Commeaux S, Troncoso OP. Biocompatibility of bacterial cellulose based biomaterials. J Funct Biomater. 2012;3:864‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almeida IF, Pereira T, Silva N, et al. Bacterial cellulose membranes as drug delivery systems: an in vivo skin compatibility study. Eur J Pharm Biopharm. 2014;86:332‐336. [DOI] [PubMed] [Google Scholar]
- 16. Perugini P, Bleve M, Cortinovis F, Colpani A. Biocellulose masks as delivery systems: a novel methodological approach to assure quality and safety. Cosmetics. 2018;5:66. [Google Scholar]
- 17. Velasco MV, Vieira RP, Fernandes AR, et al. Short‐term clinical of peel‐off facial mask moisturizers. Int J Cosmet Sci. 2014;36(4):355‐360. [DOI] [PubMed] [Google Scholar]
- 18. Amnuaikit T, Chusuit T, Raknam P, Boonme P. Effects of a cellulose mask synthesized by a bacterium on facial skin characteristics and user satisfaction. Med Devices (Auckl). 2011;4:77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pacheco G, de Mello CV, Chiari‐Andréo BG, et al. Bacterial cellulose skin masks‐properties and sensory tests. J Cosmet Dermatol. 2017;17:840‐847. [DOI] [PubMed] [Google Scholar]
- 20. Silva N, Drumond I, Almeida IF, et al. Topical caffeine delivery using biocellulose membranes: a potential innovative system for cellulite treatment. Cellulose. 2014;21:665‐674. [Google Scholar]
- 21. Kamatou G, Vermaak I, Viljoen AM. An updated review of Adansonia digitata: a commercially important African tree. S Afr J Bot. 2011;77:908‐919. [Google Scholar]
- 22. Mahadevan N, Kamboj S, Kamboj P. Hibiscus sabdariffa Linn. An overview. Nat Prod Radiance. 2009;8(1):77‐83. [Google Scholar]
- 23. Gazitaeva ZI, Drobintseva AO, Chung Y, Polyakova VO, Kvetnoy IM. Cosmeceutical product consisting of biomimetic peptides: antiaging effects in vivo and in vitro. Clin Cosmet Investig Dermatol. 2017;10:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lima TN, Pedriali Moraes CA. Bioactive peptides: applications and relevance for cosmeceuticals. Cosmetics. 2018;5:21. [Google Scholar]
- 25. Saxena SJ, Marini JL.Skin firming cream, Applicant: Jan Marini Skin Research, US 9,089,505 B1, Jul. 28; 2015.
- 26. SCCS Opinion concerning “Guidelines on the use of human volunteers in compatibility testing of finished cosmetic products”, adopted by The Scientific Committee on Cosmetics and Non‐food Products Intended for consumers, 23 June 1999.
- 27. World Medical Association . World Medical Association declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310:20. [DOI] [PubMed] [Google Scholar]
- 28. Blichman CW, Serup J. Assessment of skin moisture. Measurement of electrical conductance, capacitance and transepidermal water loss. Acta Derm Venereol (Stockh). 1988;68:284. [PubMed] [Google Scholar]
- 29. Lee CM, Maibach HI Bioengineering analysis of water hydration: an overview. Exog Dermatol. 2002;1:269‐275. [Google Scholar]
- 30. Pinnagoda J, Tupkek R, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermatitis. 1990;22(3):164‐178. [DOI] [PubMed] [Google Scholar]
- 31. Escobar‐Chávez JJ, Merino V, López‐Cervantes M, et al. The tape‐stripping technique as a method for drug quantification in skin. J Pharm Pharmaceut Sci. 2008;11(1):104‐130. [DOI] [PubMed] [Google Scholar]


