Abstract
Objectives
To assess the performance of the commercially available Magmaris sirolimus‐eluting bioresorbable scaffold (BRS) with invasive imaging at different time points.
Background
Coronary BRS with a magnesium backbone have been recently studied as an alternative to polymeric scaffolds, providing enhanced vessel support and a faster resorption rate. We aimed to assess the performance of the commercially available Magmaris sirolimus‐eluting BRS at different time points.
Methods
A prospective, single‐center, nonrandomized study was performed at the Thoraxcenter, Erasmus Medical Center, Rotterdam, The Netherlands. Six patients with stable de novo coronary artery lesions underwent single‐vessel revascularization with the Magmaris sirolimus‐eluting BRS. Invasive follow‐up including intravascular imaging using optical coherence tomography (OCT) was performed at different time points.
Results
At a median of 8 months (range 4–12 months) target lesion failure occurred in one patient. Angiography revealed a late lumen loss of 0.59 ± 0.39 mm, a percentage diameter stenosis of 39.65 ± 15.81%, and a binary restenosis rate of 33.3%. OCT showed a significant reduction in both minimal lumen area (MLA) and scaffold area at the site of the MLA by 43.44 ± 28.62 and 38.20 ± 25.74%, respectively. A fast and heterogeneous scaffold degradation process was found with a significant reduction of patent struts at 4–5 months.
Conclusions
Our findings show that the latest iteration of magnesium BRS suffers from premature dismantling, resulting in a higher than expected decrease in MLA.
Keywords: bioresorbable scaffolds, constrictive remodeling, Magmaris sirolimus‐eluting bioresorbable scaffold, scaffold bioresorption, scaffold collapse, scaffold recoil
Abbreviations
- AMS
absorbable magnesium scaffolds
- BRS
bioresorbable scaffold
- DAPT
dual antiplatelet therapy
- DES
drug‐eluting stent
- DS
diameter stenosis
- LA
lumen area
- LLL
late lumen loss
- MLA
minimal lumen area
- MSA
minimal scaffold area
- NC
noncompliant
- OCT
optical coherence tomography
- post‐PCI
post‐percutaneous coronary intervention
- pre‐PCI
pre‐percutaneous coronary intervention
- QCA
quantitative coronary analysis
- RVD
reference vessel diameter
- SA
scaffold area
- SE‐MEA
scaffold expansion according to manufacturer's expected area
- SE‐RVA
scaffold expansion according to reference vessel area
1. INTRODUCTION
Bioresorbable scaffolds (BRS) provide short‐term vessel scaffolding while avoiding long‐term consequences of metallic drug‐eluting stents (DES). Recent studies demonstrated higher rates of clinical events with polymeric BRS as compared to contemporary metallic DES.1 In order to improve BRS performance, alternative backbone materials such as magnesium are currently under investigation. First generations of absorbable magnesium scaffolds (AMS‐1 and AMS‐2; Biotronik, Berlin, Germany) failed to maintain vessel support due to rapid degradation process.2 Later iterations of the device (DREAMS and DREAMS 2G; Biotronik AG, Bülach, Switzerland) demonstrated safety and efficacy in the BIOSOLVE I and BIOSOLVE II trials.3, 4 The Magmaris sirolimus‐eluting BRS (Biotronik AG) represents the latest generation and is currently being tested in the BIOSOLVE III and IV studies.
We present the findings of clinical and intravascular imaging at different time points following the commercial use of the Magmaris sirolimus‐eluting BRS.
2. MATERIALS AND METHODS
All commercial cases treated with the Magmaris BRS (six cases) in the Thoraxcenter of the Erasmus Medical Center, Rotterdam, The Netherlands, from September 2016 to April 2017 were included in the analysis. Angiographic follow‐up was available for all patients at a median of 8 months (range 4–12 months). Quantitative coronary analysis (QCA) and optical coherence tomography (OCT) imaging analysis were performed offline. In‐device measurements are reported and presented as mean ± 1 standard deviation (SD) or percentages. Wilcoxon's signed rank test was used to analyze paired comparisons between continuous values. The coefficient of correlation of Pearson (r) was used to determine the linear relationship between quantitative variables. A value of p < .05 was considered statistically significant. Statistical analysis was conducted using SPSS for Windows version 21 (SPSS Inc., Chicago, IL) (see Supporting Information, Methods).
3. RESULTS
All patients presented with stable angina and noncomplex single‐vessel coronary artery disease. Mean age was 57.2 years and 50% were male. One scaffold per patient was implanted; mean BRS diameter and length were 3.08 ± 0.20 and 20.00 ± 4.47 mm, respectively. High‐pressure predilatation and post‐dilatation was implemented in all cases using noncompliant (NC) balloons. (see Tables S1 and S2). OCT post‐procedure was performed in five cases. Procedural and device success was 100%. Patients were discharged on dual antiplatelet therapy (DAPT) for at least 12 months along with high‐intensity statin treatment.
Offline pre‐percutaneous coronary intervention (pre‐PCI) QCA revealed a lesion length of 16.50 ± 4.61 mm, percentage diameter stenosis (%DS) of 61.95 ± 5.30%, and a reference vessel diameter (RVD) of 2.75 ± 0.25. Pre‐dilatation balloon diameter/RVD ratio was 1.10 ± 0.10. BRS diameter/RVD ratio was 1.13 ± 0.10, and post‐dilatation balloon diameter/BRS diameter ratio was 1.14 ± 0.10. Residual %DS was 22.41 ± 8.13%. Acute recoil was 5.34 ± 3.99%. Offline post‐percutaneous coronary intervention (post‐PCI) OCT showed a minimal lumen area (MLA) of 5.64 ± 1.47 mm2 and a minimal scaffold area (MSA) of 5.62 ± 1.60 mm2. Scaffold expansion (SE) according to reference vessel area (SE‐RVA) was 91.04 ± 18.13% and scaffold expansion according to manufacturer's expected area (SE‐MEA) was 73.84 ± 16.33%. Eccentricity index and symmetry index were 0.88 ± 0.01 and 0.32 ± 0.08, respectively. Incomplete strut apposition was 3.16 ± 4.22% (Table 1). No edge dissections were found.
Table 1.
QCA and OCT measurements
| Post‐PCI | Follow‐up | Absolute difference | Relative difference (%) | p value | |
|---|---|---|---|---|---|
| QCA measurementsa | |||||
| Lesion length (mm) | 19.79 ± 4.48 | 20.12 ± 4.64 | −0.33 ± 0.41 | −1.59 ± 1.97 | .116 |
| Reference vessel diameter (mm) | 2.92 ± 0.26 | 2.74 ± 0.39 | 0.18 ± 0.21 | 6.47 ± 7.05 | .080 |
| Minimal lumen diameter (mm) | 2.25 ± 0.20 | 1.66 ± 0.48 | 0.59 ± 0.39 | 26.33 ± 19.52 | .028 |
| Mean lumen diameter (mm) | 2.67 ± 0.23 | 2.30 ± 0.35 | 0.37 ± 0.22 | 14.00 ± 8.79 | .028 |
| In device diameter stenosis (%) | 22.41 ± 8.13 | 39.65 ± 15.81 | −17.24 ± 16.48 | −99.88 ± 103.13 | .046 |
| Acute recoil (%) | 5.34 ± 3.99 | NA | NA | NA | NA |
| OCT measurements | |||||
| MLA (mm2)b | 5.64 ± 1.47 | 3.24 ± 1.85 | 2.41 ± 1.51 | 43.44 ± 28.62 | .042 |
| Mean lumen area (mm2)b | 7.03 ± 1.91 | 6.82 ± 3.79 | 0.22 ± 2.64 | 5.49 ± 36.04 | .686 |
| MSA (mm2) | 5.62 ± 1. 60 | NA | NA | NA | NA |
| Mean SA (mm2) | 6.87 ± 1.73 | NA | NA | NA | NA |
| SA at MLA site (mm2)c | 6.06 ± 1.70 | 3.76 ± 1.77 | 2.30 ± 1.48 | 38.20 ± 25.74 | .043 |
| ISA (%)d | 3.16 ± 4.22 | 0.44 ± 0.88 | 2.72 ± 3.37 | 70.25 ± 47.68 | .109 |
| SE‐RVA (%) | 91.04 ± 18.13 | NA | NA | NA | NA |
| SE‐MEA (%) | 73.84 ± 16.36 | NA | NA | NA | NA |
| Eccentricity index | 0.88 ± 0.01 | NA | NA | NA | NA |
| Symmetry index | 0.32 ± 0.08 | NA | NA | NA | NA |
| Maximum attenuation valuesb | 16.4 ± 2.63 | 8.71 ± 5.38 | 7.70 ± 5.74 | 46.62 ± 30.7 | .080 |
| Maximum backscattering valuesb | 8.97 ± 0.33 | 8.13 ± 0.59 | 0.84 ± 0.68 | 9.23 ± 7.22 | .080 |
Note: Paired comparison of the pooled data measurements at baseline post‐intervention (post‐PCI) and at a median follow‐up of 8 months by QCA and OCT. Data area expressed as mean ± SD.
Abbreviations: ISA, incomplete strut apposition; MLA, minimal lumen area; MSA, minimal scaffold area; OCT, optical coherence tomography; post‐PCI, post‐percutaneous coronary intervention; QCA, quantitative coronary analysis; SA, scaffold area; SE‐MEA, scaffold expansion according to manufacturer's expected area; SE‐RVA, scaffold expansion according to reference vessel area.
Comparison made for six patients.
Comparison made for five patients.
Comparison made for five patients with the SA at baseline and follow‐up matching the same cross‐sectional area.
Comparison made for four patients.
At a median of 8 months (minimum 4 months, maximum 12 months), all patients were under DAPT and target lesion failure occurred in one patient (patient #5, see Figure S1) based on severe constrictive remodeling. All other patients remained asymptomatic.
Offline QCA of the invasive follow‐up procedure revealed a late lumen loss (LLL) of 0.59 ± 0.39 mm, %DS of 39.65 ± 15.81%, and a binary restenosis rate of 33.3%. Offline OCT at follow‐up demonstrated a decrease in MLA by 43.44 ± 28.62% (p = .042), along with a significant decrease in scaffold area (SA) at the site of the MLA by 38.20 ± 25.74% (p = .043) (Figure 1 and Table 1). Percentage lumen area (%LA) stenosis was 56.64% with a binary restenosis rate of 83.3%.
Figure 1.
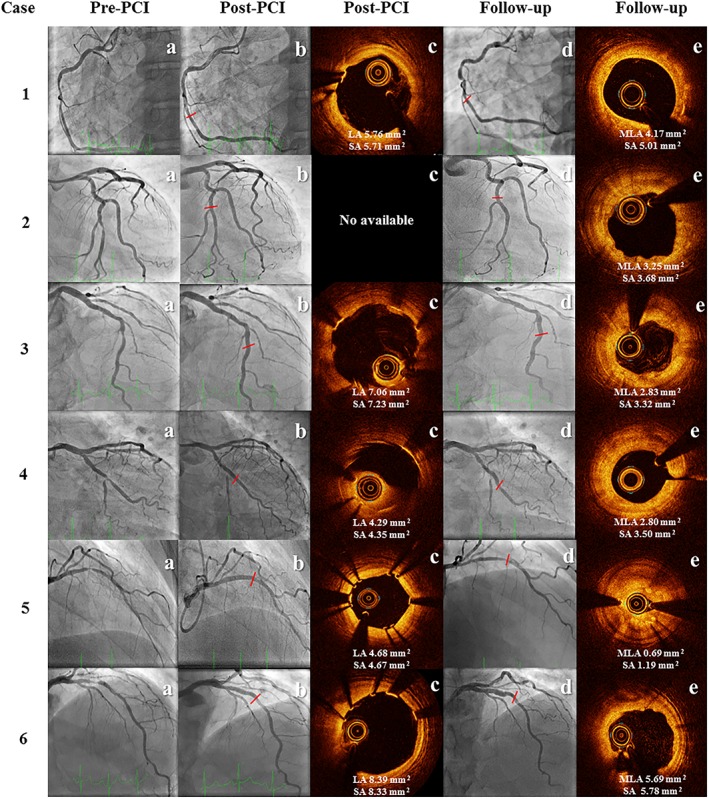
Angiography and OCT imaging at baseline and follow‐up. Angiography imaging available for six patients showing target vessel at baseline before intervention (pre‐PCI), post‐intervention (post‐PCI) and at invasive control (follow‐up). OCT imaging available for five patients at post‐intervention (post‐PCI) and for six patients at invasive control (follow‐up). Comparison of cross‐sectional image at follow‐up with the MLA matched with the same cross‐sectional image at baseline. LA, lumen area; MLA, minimal lumen area; OCT, optical coherence tomography; post‐PCI, post‐percutaneous coronary intervention; pre‐PCI, pre‐percutaneous coronary intervention; SA, scaffold area [Color figure can be viewed at http://wileyonlinelibrary.com]
In a per‐scaffold subsegment analysis, a strong linear correlation between SA at baseline and %LA reduction at follow‐up was found (r = −.87, p = .001). Furthermore, a similar correlation was present between SE at baseline and %LA reduction at follow‐up according to both RVA and MEA (r = −.86, p = .001 and r = − .85, p = .002, respectively) (see Figure S2a–c). Attenuation and backscattering analysis demonstrated a numerical reduction of maximum indices that correspond with strut degradation. Yet, both high and low values were within the same scaffolded segment at follow‐up illustrating a heterogeneous strut degradation process (see Figures S3 and S4). No evidence of edge dissection or thrombosis was found.
4. DISCUSSION
Sufficient radial force to overcome elastic recoil and plaque resistance is an essential feature of contemporary stents. The mechanical properties of coronary stents are influenced by backbone/polymer material, geometry, and strut thickness5; recently, with the advent of BRS, the resorption time was also added to this equation. Driven by the presentation of case #5 with severe early scaffold collapse at 4 months, and the recent data on adverse events related to Absorb Bioresorbable vascular scaffold (BVS), our hospital institutional board mandated us to call back all previous cases treated with the commercially available Magmaris BRS and to perform invasive control in order to assess the performance of this device. This resulted in imaging assessment at different follow‐up time points.
The Magmaris BRS starts its degradation process as soon as 3 months and completes at 12 months.6 Our results revealed a significant decrease in patent struts with a heterogeneous resorption pattern as soon as 4–5 months postimplantation; the latest was demonstrated by an important reduction of attenuation and backscattering indices; this is in line with a serial OCT imaging analysis performed in the BIOSOLVE II trial, showing a significant decrease in MLA of 28.3% at 6 months, and reduction of attenuation and backscattering values with fewer struts remnants visible.3, 7 A rapid bioresorption rate might induce nonuniform vessel support and loss of radial strength, which has been corroborated with the first generation of Absorb BVS and magnesium BRS.2, 8 The present report suggests that the most recent version of magnesium BRS also suffers from premature dismantling.
Whether the high LA reduction as found in our study could be attributed to a lower radial strength secondary to rapid bioresorption, an excessive neointimal formation, or both, is yet to be proven. Complete SA and neointimal volumes throughout the scaffolded segment could not be determined due to the difficulty in recognizing patent struts; however, when the SA was analyzable, a significant reduction of SA was found compared to the postimplantation result at the same location. These findings are in line with seven recently published case reports on severe lumen reduction and scaffold collapse after Magmaris BRS implantation.9, 10, 11, 12, 13, 14, 15 Furthermore, acute recoil was only 5.3%, in line with current generation metallic DES and BVS.16, 17 Preclinical data have suggested that increased local inflammation is responsible for the higher LLL obtained during the Magmaris degradation process, and a switch to a progressive positive vessel remodeling once the bioresorption is completed might be expected.18 The latest warrants for serial invasive evaluation of the vessel response beyond 12 months. Nevertheless, vessel constrictive remodeling occurs between 1 and 6 months after PCI; therefore, assuring optimal radial support for at least 6 months after device implantation seems to be crucial.19
Although careful lesion preparation and systematic high‐pressure post‐dilatation with NC balloons were routinely performed in all cases, a trend toward enhanced lumen loss in the distal scaffold edges was noticed. The latter appeared to be strongly linked to the post‐procedural SA and lower SE at the most distal scaffold subsegments, compared to the middle and proximal subsegments. This discrepancy could be explained by the presence of smaller vessel diameter in the distal subsegments leading to scaffold oversizing, and potentially less aggressive post‐dilatation due to the challenge in visualizing the tantalum markers. OCT assessment of magnesium BRS has shown an increase of lumen volume loss when expansion index is >1,20 and previous data on Absorb BVS have correlated implantation in small vessel diameter with a higher rate of in‐device restenosis,21 eccentricity, and asymmetry.22 Performing pre‐procedural intracoronary imaging for vessel sizing and lesion characterization might help to further improve lesion/device selection.23 On the other hand, increasing device visibility under fluoroscopy for future BRS generations could boost expansion optimization.
4.1. Study limitations
This is a nonrandomized single‐center study with small sample size. Patient and lesion selection was at the operator's discretion. Pre‐PCI intracoronary imaging was not systematically performed. The heterogeneity of the invasive follow‐up time points might have resulted in high SD caused by extreme values, and the clack of serial invasive imaging after 12 months prohibited statements on potential long‐term lumen enlargement. The difficulty of identifying patent struts at follow‐up did not allow us to perform methodical analysis of the entire scaffold and precluded any detailed analyses on neointimal hyperplasia volumes.
5. CONCLUSIONS
The latest magnesium BRS iteration suffers from premature dismantling with subsequent loss of vessel support; together with incomplete distal device expansion, could have contributed to a higher than expected lumen loss. While our findings need to be confirmed in a randomized fashion, it seems imperative to follow the European working group recommendations on BRS and limiting their use to clinical trials or registries with adequate follow‐up.24 Finally, our results suggest that clinical and angiographic follow‐up alone might not be sufficient to establish the safety and long‐term efficacy of new BRS, and warrants the use of serial invasive coronary imaging at baseline and follow‐up.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Supporting information
Table S1 Baseline clinical and angiographic characteristics
Table S2 Baseline procedural characteristics
Figure S1 Severe scaffold collapse at 4 months post‐implantation of Magmaris BRS
Optical coherence tomography imaging at baseline and follow‐up at the same cross‐section sites illustrating proximal and distal constrictive remodeling and scaffold severe recoil.
Lumen Area (LA); Minimal Lumen Area (MLA); Minimal Scaffold Area (MSA); Scaffold Area (SA).
Figure S2 Per scaffold 10‐subsegment serial changes in OCT measurements
Per scaffold subsegment correlation between SA at baseline and LA reduction at follow‐up (a). Per scaffold subsegment correlation between SE‐RVA at baseline and LA reduction at follow‐up (b). Per scaffold subsegment correlation between SE‐MEA at baseline and LA reduction at follow‐up. Most distal subsegments (light color). Most proximal subsegments (dark color). Scaffold Area (SA); Lumen Area (LA); Scaffold Expansion according to Manufacturer Expected Area (SE‐MEA); Scaffold Expansion according to Reference Vessel Area (SE‐RVA).
Figure S3. OCT of Magmaris degradation from baseline to 12 months.
Upper row shows OCT representation of the degradation process of different scaffolds visualized at different follow‐up time points. The bottom row shows attenuation values of the scaffolded segment. Attenuation values reduce from very high to low with struts bioresorption; higher attenuation values correspond to patent struts, middle attenuation values correspond to struts undergoing degradation. Lower attenuation values correspond to absence of struts remains. Optical Coherence Tomography (OCT).
Figure S4 In‐scaffold heterogeneous strut degradation
Optical coherence tomography longitudinal view (first row) and cross‐section images of one Magmaris BRS illustrating different stages of strut degradation at 5‐month follow‐up in patient #6.
Tovar Forero MN, van Zandvoort L, Masdjedi K, et al. Serial invasive imaging follow‐up of the first clinical experience with the Magmaris magnesium bioresorbable scaffold. Catheter Cardiovasc Interv. 2020;95:226–231. 10.1002/ccd.28304
REFERENCES
- 1. Sorrentino S, Giustino G, Mehran R, et al. Everolimus‐eluting bioresorbable scaffolds versus everolimus‐eluting metallic stents. J Am Coll Cardiol. 2017;69(25):3055‐3066. [DOI] [PubMed] [Google Scholar]
- 2. Erbel R, Di Mario C, Bartunek J, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non‐randomised multicentre trial. Lancet. 2007;369(9576):1869‐1875. [DOI] [PubMed] [Google Scholar]
- 3. Haude M, Ince H, Abizaid A, et al. Sustained safety and performance of the second‐generation drug‐eluting absorbable metal scaffold in patients with de novo coronary lesions: 12‐month clinical results and angiographic findings of the BIOSOLVE‐II first‐in‐man trial. Eur Heart J. 2016;37(35):2701‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haude M, Erbel R, Erne P, et al. Safety and performance of the drug‐eluting absorbable metal scaffold (DREAMS) in patients with de‐novo coronary lesions: 12 month results of the prospective, multicentre, first‐in‐man BIOSOLVE‐I trial. Lancet. 2013;381(9869):836‐844. [DOI] [PubMed] [Google Scholar]
- 5. Chichareon P, Katagiri Y, Asano T, et al. Mechanical properties and performances of contemporary drug‐eluting stent: focus on the metallic backbone. Expert Rev Med Devices. 2019;16(3):211‐228. [DOI] [PubMed] [Google Scholar]
- 6. Sotomi Y, Onuma Y, Collet C, et al. Bioresorbable scaffold: the emerging reality and future directions. Circ Res. 2017;120(8):1341‐1352. [DOI] [PubMed] [Google Scholar]
- 7. Garcia‐Garcia HM, Haude M, Kuku K, et al. In vivo serial invasive imaging of the second‐generation drug‐eluting absorbable metal scaffold (Magmaris—DREAMS 2G) in de novo coronary lesions: insights from the BIOSOLVE‐II first‐in‐man trial. Int J Cardiol. 2018;255:22‐28. [DOI] [PubMed] [Google Scholar]
- 8. Ormiston JA, Serruys PW, Regar E, et al. A bioabsorbable everolimus‐eluting coronary stent system for patients with single de‐novo coronary artery lesions (ABSORB): a prospective open‐label trial. Lancet. 2008;371(9616):899‐907. [DOI] [PubMed] [Google Scholar]
- 9. Cubero‐Callego H. Early collapse of a magnesium Bioresorbable scaffold. J Am Coll Cardiol Intv. 2017;10(18):e171‐e172. [DOI] [PubMed] [Google Scholar]
- 10. Barkholt TO, Neghabat O, Terkelsen CJ, Christiansen EH, Holm NR. Restenosis in a collapsed magnesium bioresorbable scaffold. Circ Cardiovasc Interv. 2017;10:e005677. [DOI] [PubMed] [Google Scholar]
- 11. Garcia‐Blas S, Minana G, Sanchis J. Optical coherence tomography of magnesium bioresorbable scaffold restenosis. Rev Esp Cardiol (Engl Ed). 2018;71:1069. [DOI] [PubMed] [Google Scholar]
- 12. Yang H, Zhang F, Qian J, Chen J, Ge J. Restenosis in Magmaris stents due to significant collapse. JACC Cardiovasc Interv. 2018;11(10):e77‐e78. [DOI] [PubMed] [Google Scholar]
- 13. Roa‐Garrido J, Cardenal‐Piris RM, El Amrawy AM, Gomez‐Menchero A, Camacho Freire S, Diaz‐Fernandez JF. Optical coherence tomographic image pattern of metallic bioresorbable vascular scaffold restenosis. JACC Cardiovasc Interv. 2018;11(7):707‐708. [DOI] [PubMed] [Google Scholar]
- 14. Marynissen T, McCutcheon K, Bennett J. Early collapse causing stenosis in a resorbable magnesium scaffold. Catheter Cardiovasc Interv. 2018;92(2):310‐312. [DOI] [PubMed] [Google Scholar]
- 15. Mitomo S, Demir OM, Giannini F, Latib A, Colombo A. Magmaris Bioresorbable scaffold—possible dismantling 2 months after implantation on intravascular ultrasound. Circ J. 2019;83(6):1418. [DOI] [PubMed] [Google Scholar]
- 16. Onuma Y, Serruys PW, Gomez J, et al. Comparison of in vivo acute stent recoil between the bioresorbable everolimus‐eluting coronary scaffolds (revision 1.0 and 1.1) and the metallic everolimus‐eluting stent. Catheter Cardiovasc Interv. 2011;78(1):3‐12. [DOI] [PubMed] [Google Scholar]
- 17. van Bommel RJ, Lemmert ME, van Mieghem NM, van Geuns RJ, van Domburg RT, Daemen J. Occurrence and predictors of acute stent recoil—a comparison between the xience prime cobalt chromium stent and the promus premier platinum chromium stent. Catheter Cardiovasc Interv. 2018;91:E21‐E28. [DOI] [PubMed] [Google Scholar]
- 18. Waksman R, Zumstein P, Pritsch M, et al. Second‐generation magnesium scaffold Magmaris: device design and preclinical evaluation in a porcine coronary artery model. EuroIntervention. 2017;13(4):440‐449. [DOI] [PubMed] [Google Scholar]
- 19. Kimura T, Kaburagi S, Tamura T, et al. Remodeling of human coronary arteries undergoing coronary angioplasty or atherectomy. Circulation. 1997;96(2):475‐483. [DOI] [PubMed] [Google Scholar]
- 20. Ozaki Y, Garcia‐Garcia HM, Hideo‐Kajita A, et al. Impact of procedural characteristics on coronary vessel wall healing following implantation of second‐generation drug‐eluting absorbable metal scaffold in patients with de novo coronary artery lesions: an optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging. 2019;20(8):916‐924. [DOI] [PubMed] [Google Scholar]
- 21. Baquet M, Nef H, Gori T, et al. Restenosis patterns after bioresorbable vascular scaffold implantation: angiographic substudy of the GHOST‐EU registry. Catheter Cardiovasc Interv. 2018;92(2):276‐282. [DOI] [PubMed] [Google Scholar]
- 22. Suwannasom P, Sotomi Y, Ishibashi Y, et al. The impact of post‐procedural asymmetry, expansion, and eccentricity of bioresorbable Everolimus‐eluting scaffold and metallic Everolimus‐eluting stent on clinical outcomes in the ABSORB II trial. JACC Cardiovasc Interv. 2016;9(12):1231‐1242. [DOI] [PubMed] [Google Scholar]
- 23. Ali ZA, Karimi Galougahi K, Shlofmitz R, et al. Imaging‐guided pre‐dilatation, stenting, post‐dilatation: a protocolized approach highlighting the importance of intravascular imaging for implantation of bioresorbable scaffolds. Expert Rev Cardiovasc Ther. 2018;16(6):431‐440. [DOI] [PubMed] [Google Scholar]
- 24. Byrne RA, Stefanini GG, Capodanno D, et al. Report of an ESC‐EAPCI task force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. Eur Heart J. 2017;39:1591‐1601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Table S1 Baseline clinical and angiographic characteristics
Table S2 Baseline procedural characteristics
Figure S1 Severe scaffold collapse at 4 months post‐implantation of Magmaris BRS
Optical coherence tomography imaging at baseline and follow‐up at the same cross‐section sites illustrating proximal and distal constrictive remodeling and scaffold severe recoil.
Lumen Area (LA); Minimal Lumen Area (MLA); Minimal Scaffold Area (MSA); Scaffold Area (SA).
Figure S2 Per scaffold 10‐subsegment serial changes in OCT measurements
Per scaffold subsegment correlation between SA at baseline and LA reduction at follow‐up (a). Per scaffold subsegment correlation between SE‐RVA at baseline and LA reduction at follow‐up (b). Per scaffold subsegment correlation between SE‐MEA at baseline and LA reduction at follow‐up. Most distal subsegments (light color). Most proximal subsegments (dark color). Scaffold Area (SA); Lumen Area (LA); Scaffold Expansion according to Manufacturer Expected Area (SE‐MEA); Scaffold Expansion according to Reference Vessel Area (SE‐RVA).
Figure S3. OCT of Magmaris degradation from baseline to 12 months.
Upper row shows OCT representation of the degradation process of different scaffolds visualized at different follow‐up time points. The bottom row shows attenuation values of the scaffolded segment. Attenuation values reduce from very high to low with struts bioresorption; higher attenuation values correspond to patent struts, middle attenuation values correspond to struts undergoing degradation. Lower attenuation values correspond to absence of struts remains. Optical Coherence Tomography (OCT).
Figure S4 In‐scaffold heterogeneous strut degradation
Optical coherence tomography longitudinal view (first row) and cross‐section images of one Magmaris BRS illustrating different stages of strut degradation at 5‐month follow‐up in patient #6.


