Abstract
Objectives
Painful diabetic peripheral neuropathy (PDPN) is a long‐term complication of diabetes mellitus (DM). Dorsal Root Ganglion Stimulation (DRGS) has recently emerged as a neuromodulation modality in the treatment of chronic neuropathic pain. The objective of this study was to compare the effect of burst DRGS (Burst‐DRGS) and conventional DRGS (Con‐DRGS) in an experimental model of PDPN.
Materials and Methods
DM was induced in female Sprague–Dawley rats by intraperitoneal injection of streptozotocin (STZ, n = 48). Animals were tested for mechanical hypersensitivity (50% hind paw withdrawal threshold on Von Frey test) before, and 4 weeks after STZ injection. PDPN rats were then implanted with a unilateral bipolar lead at the L5 DRG (n = 22) and were stimulated for 30 min at days 2 and 3 postimplantation. Animals received Con‐DRGS and Burst‐DRGS in a randomized crossover design (n = 10), or received Sham‐DRGS (n = 7) for 30 min, and were tested for mechanical hypersensitivity at baseline, 15 and 30 min during DRGS, and 15 and 30 min following DRGS. Five animals were withdrawn from the study due to electrode‐related technical problems.
Results
Con‐DRGS and Burst‐DRGS normalized STZ‐induced mechanical hypersensitivity at 15 and 30 min during stimulation. A significant difference in terms of mechanical hypersensitivity was observed between both of the stimulated groups and the Sham‐DRGS group at 15 and 30 min during stimulation. Interestingly, Burst‐DRGS showed signs of a residual effect at 15 min after cessation of stimulation, while this was not the case for Con‐DRGS.
Conclusions
Under the conditions tested, Con‐DRGS and Burst‐DRGS are equally effective in attenuating STZ‐induced mechanical hypersensitivity in an animal model of PDPN. Burst‐DRGS showed signs of a residual effect at 15 min after cessation of stimulation, which requires further investigation.
Keywords: Burst stimulation, dorsal root ganglion stimulation, mechanical hypersensitivity, neuropathic pain, painful diabetic peripheral neuropathy
INTRODUCTION
Painful diabetic peripheral neuropathy (PDPN) is a debilitating consequence of DM, with a prevalence ranging from 10 to 26% 1, 2, 3. PDPN typically presents as burning, electric, stabbing, or tingling neuropathic pain that starts in the lower limbs, and is characterized by diffuse damage to small nerve fibers, specifically to those of the Aδ and C type 4. Numerous pharmacological drugs for neuropathic pain have been introduced over the years 5. As the efficacy of pharmacological drugs in PDPN is limited, there is an urgent need for the development of novel treatment options.
Spinal cord stimulation of the dorsal columns (SCS) is a recommended last resort therapy for PDPN patients who do not respond to conventional pharmacological medication. The effectiveness of SCS in PDPN has been demonstrated in two randomized clinical trials (RCTs) 6, 7, 8. Despite considerable improvements, there are limitations to the efficacy of SCS. First, approximately 60% of patients with PDPN achieve pain reductions of ≥50% 6, 7, 8. Second, SCS is often unable to satisfactorily and specifically stimulate difficult‐to‐reach areas, such as the extremities in PDPN. Third, placement of the leads on top of the dorsal columns makes SCS with conventional settings susceptible to postural variations due to changes in distance between stimulation lead and stimulation target, leading to unpleasant paresthesias and/or overstimulation 9. Last, the energy consumption of SCS is relatively high, as there is significant energy loss to surroundings, such as the cerebrospinal fluid, before stimulation reaches the spinal cord dorsal columns.
In the field of neuromodulation, new developments aimed to change not only the anatomical target but also to introduce new stimulation waveforms have been suggested to overcome most of the disadvantages of SCS. Change of anatomical target from the spinal cord to the dorsal root ganglion (dorsal root ganglion stimulation, DRGS) not only increased treatment success in chronic intractable neuropathic pain but also resulted in less postural variation in paresthesia intensity and less battery consumption as compared to SCS 10. Additionally, DRGS was found to be a safe and effective neuromodulation modality that improves painful symptoms in PDPN patients 11. The introduction of new stimulation waveforms including use of burst paradigms 12 have been shown to result in clinically relevant pain reductions, without eliciting paresthesias. Studies have shown that burst SCS (Burst‐SCS) decreases pain intensity to a greater degree than conventional SCS (Con‐SCS) 13, 14. This superior effect might be attributed to the underlying mechanism of action of Burst‐SCS, as it is hypothesized that Burst‐SCS, besides targeting brain areas related to the location and intensity of pain (lateral pain pathway), also targets areas related to the emotional and affective components of pain (medial pain pathway) 13.
Over the years, preclinical research has provided valuable information with regard to the therapeutic effects of neuromodulation for PDPN. The effectiveness of SCS in experimental PDPN has been demonstrated in both the short 15, 16 and long term 17. Interestingly, the first in vivo study to test the effectiveness of DRGS in an animal model of peripheral nerve injury was recently published 18. The authors showed that DRGS attenuated both reflex‐based pain behavior as well as affective pain behavior, with no signs of histological damage to the DRG.
In line with the recent changes in the field of neuromodulation, a combination of novel anatomical targeting and the use of novel stimulation waveforms might provide a platform to further improve neuromodulatory therapies for chronic neuropathic pain. To date, no study has explored the effect of novel DRGS modalities, like Burst‐DRGS, in experimental or clinical PDPN. The present study, therefore, aimed to assess the effect of Burst‐DRGS vs. Con‐DRGS in an animal model of PDPN. To this end, we used an animal model for DRGS, which was demonstrated to relieve neuropathic pain in rats 18. Based on the aforementioned evidence on Burst stimulation in the field of SCS, we hypothesized that Burst‐DRGS leads to significantly higher pain relief and higher responder rates compared to Con‐DRGS.
METHODS
Ethical Statement
All experiments were conducted in a humane manner in accordance with the European Directive for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (86/609/EU). The study was approved by the Animal Research Committee of Maastricht University (DEC‐protocol 2013‐079).
Animals
All experiments were performed using young‐adult, female Sprague–Dawley rats (6 weeks at study onset, 180–220 g, n = 48). Animals were housed per 2 in filter‐top polycarbonate cages in a climate controlled room (temperature 21 ± 1°C, humidity 55 ± 15%) with constant background music (approximately 45 decibel) and under artificial lightning (12:12 reversed light/dark cycle). Distilled water and food was at all times available to the animals ad libitum. Animals were allowed to acclimatize to the housing facility without experimenter contact for 1 week after arrival, and were handled properly before the onset of the experiments.
Induction of Diabetes Mellitus
DM was induced by a single intraperitoneal injection of 65 mg/kg Streptozotocin (STZ; Sigma‐Aldrich, Schnelldorf, Germany; n = 48). Prior to STZ injection, animals were weighed and fasted overnight. STZ was then freshly dissolved in sterile 0.9% NaCl to a solution of 65 mg/mL. In the first week after STZ injection, blood glucose levels were assessed in blood derived from the saphenous vein using a blood glucose meter (Accu‐Chek Aviva®, Roche Diagnostics GmbH, Mannheim, Germany). Rats with a glucose level of ≥15 mmol/L were considered diabetic 19 and were included in the study. When glucose levels exceeded 31.4 mmol/L, one‐third of a slow releasing insulin pellet (LinShin Canada, Inc.) was placed subcutaneously in the trunk of the animal.
Assessment of Mechanical Hypersensitivity (Von Frey Assay)
Mechanical hypersensitivity was assessed by measuring the response of the hind paws to Von Frey filaments using the “up‐down” method 20. In short, rats were placed in a transparent box on an elevated mesh floor. Animals were allowed to acclimate to the behavioral set‐up for 15 min before testing. Subsequently, a series of Von Frey filaments with incrementing stiffness (bending forces 0.6, 1.2, 2.0, 3.6, 5.5, 8.5, 15.1, and 28.84 g) were applied to the plantar surface of the hind paws of the animals for 5 sec. In case of a negative response (no withdrawal of hind paw), the next filament with higher bending force was applied. In case of a positive response (withdrawal of hind paw), the previous filament with lower bending force was applied. The 50% withdrawal threshold (WT) was calculated after completion of a sequence of six consecutive responses. A cut‐off value of 28.84 g was defined to prevent tissue damage. Last, the 50% WT was multiplied by 10,000 and logarithmically transformed to account for Weber's law 21 and obtain a linear scale.
Development of Mechanical Hypersensitivity
Only animals showing mechanical hypersensitivity on the Von Frey assay at 4 weeks after STZ injection were implanted and treated with DRGS. Animals without mechanical hypersensitivity were excluded from the study. The presence of mechanical hypersensitivity was defined as a decrease of ≥0.2 unit in log10 (10,000 x 50% WT) when compared to pre‐STZ values 15, 16, 17.
Dorsal Root Ganglion Stimulation Lead
Preparation of the DRGS lead was performed as previously described 18. Briefly, the lead was manufactured out of two platinum–iridium wires with different diameters (0.010 and 0.005 in). The insulation at the termini of both wires was removed, and the terminal of the large wire (0.010 in) was bent back upon itself to produce an atraumatic tip. The smaller wire (0.005 in) was then wrapped around the insulated part of the larger wire. A few spots of dental cement were added to strengthen the lead. Last, the lead was tested with an Ohmmeter to confirm proper functioning of the lead (Fig. 1).
Figure 1.

Bipolar DRGS lead.
Implantation of the DRGS Lead
Implantation of the DRGS lead at the L5 DRG was performed as previously described 18. Briefly, the intervertebral foramen at the level of the fifth lumbar (L5) spinal nerve was exposed via a paravertebral incision under general anesthesia. Subsequently, the foramen was gently opened by probing with a small, blunt nerve hook to provide a passage for the lead to enter the foramen on the dorsolateral aspect of the L5 DRG. The lead was secured onto the transverse process caudal to the foramen using a stainless steel ligature and a small screw (diameter 0.86 mm, length 3.2 mm). This produced a device capable of providing bipolar contact in apposition to the L5 DRG. Last, the lead was tunneled subcutaneously through the neck of the animals and the wounds were closed in layers. After implantation of the lead, the rats were allowed to recover for 2 days before the start of DRGS.
DRG Stimulation
For stimulation of the L5 DRG, an A‐M systems stimulator (MultiStim: Programmable 8‐Channel Stimulator (Model 3800) 220 V/50 Hz) fitted with an additional stimulus Isolator (Model 3820 for A‐M Systems MultiStim) was used. After connecting the implanted lead to the stimulator, the motor threshold (MT) was determined using a frequency of 2 Hz and pulse width of 200 μsec for Con‐DRGS, and a pulse width of 1000 μsec, five pulses (500 Hz intraburst frequency) administered at an interburst frequency of 2 Hz for Burst‐DRGS. MT was defined as the current inducing contractions of the lower trunk or hind limb(s). For Con‐DRGS, the stimulation settings were as follows: biphasic stimulation with frequency = 50 Hz, pulse width = 200 μsec, amplitude = 67% of motor threshold (Fig. 2a). For Burst‐DRGS, the stimulation settings were as follows: monophasic stimulation with interburst frequency = 40 Hz, intraburst frequency = 500 Hz, pulse width = 1000 μsec, interpulse interval = 1000 μsec, burst pulse count = 5, amplitude = 67% MT 12, 13, 14 (Fig. 2b). Animals with an MT of ≥1 mA at stimulation days were excluded from analysis. For sham stimulated animals, the amplitude was set at zero. Animals were unrestrained during DRGS.
Figure 2.
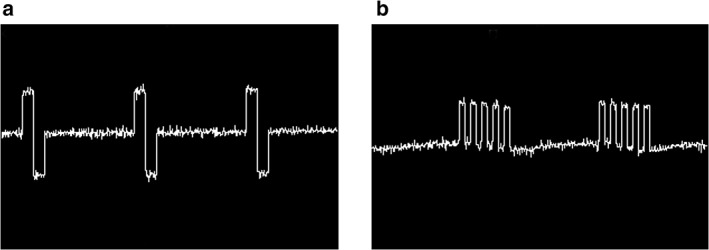
Oscilloscope output of the Con‐DRGS (a) and Burst‐DRGS (b) waveform. Con‐DRGS: biphasic mode with frequency = 50 Hz, pulse width = 200 μsec. Burst‐DRGS: monophasic mode with interburst frequency = 40 Hz, intraburst frequency = 500 Hz, pulse width = 1000 μsec, interpulse interval = 1000 μsec, burst pulse count = 5.
Timeline of Experiments
Following baseline measurements for mechanical hypersensitivity (Von Frey; week −1), animals were injected with STZ (week 0). In the first week after STZ injection (week 1), blood glucose of the animals was measured to confirm DM (DM defined as blood glucose level ≥15 mmol/L 19). Four weeks after STZ injection (week 4), animals were again tested for mechanical hypersensitivity, to select animals that developed PDPN (≥0.2 decrease in log10 (10,000 x 50% WT) on Von Frey when compared to the pre‐STZ baseline 15, 16, 17) for DRGS implantation (week 5). PDPN animals received either Con‐DRGS and Burst‐DRGS in a randomized cross‐over design on days 2 and 3 postimplantation, or were assigned to a Sham‐DRGS group (50% WT measured on day 2). The experimenter was blinded for the DRGS paradigm used. On stimulation days, animals were first tested for MT, after which the amplitude was set accordingly. Animals were then tested for mechanical hypersensitivity on Von Frey just before DRGS onset (baseline), 15 and 30 min during DRGS (or sham‐DRGS), and 15 and 30 min after DRGS (45 and 60 min; Fig. 3).
Figure 3.
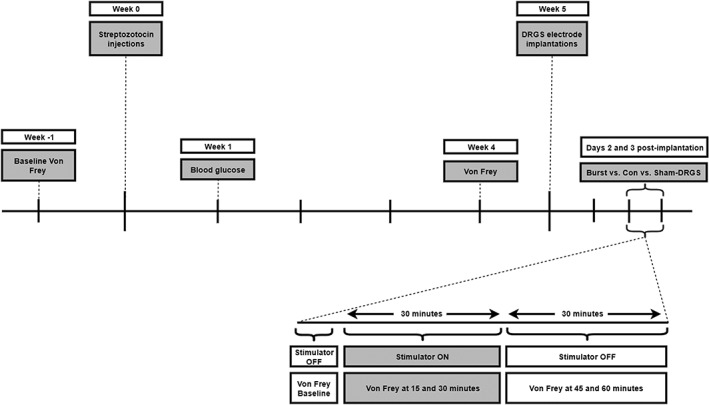
Timeline of experiments.
Statistical Analysis
The WTs to Von Frey filaments are presented as mean ± standard error of the mean. For statistical analysis, Von Frey data were logarithmically transformed to account for Weber's Law 21 and obtain a linear scale. Data were tested for a normal distribution using the Shapiro–Wilk normality test and were confirmed to be normally distributed. For analysis of intragroup changes in WTs over time, one‐way repeated measures analysis of variance (ANOVA) was performed, followed by Tukey's multiple comparison test. For between‐groups analysis (Con‐DRGS vs. Burst‐DRGS vs. Sham‐DRGS), a two‐way ANOVA followed by Tukey's multiple comparisons test was used. For comparisons between pre‐STZ WTs and preimplant WTs, and comparisons of MTs between the Con‐DRGS and Burst‐DRGS group, a paired‐samples t‐test was used.
RESULTS
Flowchart of Animals
Out of the 48 animals that were injected with STZ, 43 developed DM (90%; blood glucose level ≥15 mmol/L). One animal died as a result from STZ‐related health deterioration. Thirteen animals required insulin treatment (blood glucose level ≥31.4 mmol/L). Twenty‐five out of the 43 diabetic animals developed subsequent PDPN 4 weeks post‐STZ injection (58%; ≥0.2 decrease in log10 (10,000 x 50% WT) on Von Frey when compared to the pre‐STZ baseline 15, 16, 17), of which 22 were successfully implanted with a DRGS device. Two out of the 22 implanted PDPN animals were withdrawn from the study due to connector breakage before the first stimulation day, one animal was withdrawn from the study due to not being able to finish the complete study period (no motor threshold observed on second stimulation day), and two animals were excluded from the study because of high MT (MT ≥1 mA). Consequently, 17 animals were left for analysis. Of these 17 animals, 10 animals received Con‐DRGS and Burst‐DRGS in a randomized cross‐over design, and 7 animals received Sham‐DRGS.
Development of STZ‐Induced Mechanical Hypersensitivity
The mean log10 (10,000 x 50% WT) value of the 17 stimulated animals (animals that underwent Con‐DRGS, Burst‐DRGS, or Sham‐DRGS) dropped from 5.06 ± 0.04 before STZ injection to 4.47 ± 0.04 preimplantation (4 weeks following STZ injection; p < 0.0001; Fig. 4).
Figure 4.
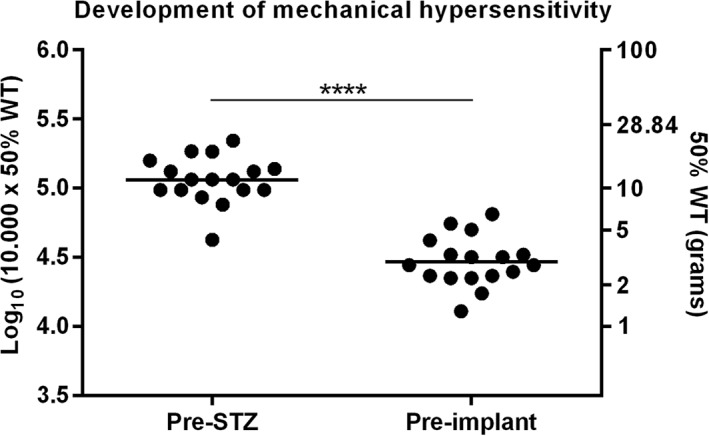
Development of mechanical hypersensitivity after STZ injection of all stimulated rats (animals that underwent Con‐DRGS, Burst‐DRGS, or Sham‐DRGS; n = 17). **** p < 0.0001 compared to pre‐STZ baseline.
Effect of Con‐DRGS on STZ‐Induced Mechanical Hypersensitivity
For animals receiving Con‐DRGS, the average baseline log10 (10,000 x 50% WT) score (before start of Con‐DRGS) was 4.38 ± 0.07. Con‐DRGS resulted in a significant reduction of mechanical hypersensitivity at 15 min (4.81 ± 0.12, p < 0.05) and 30 min (5.01 ± 0.12, p < 0.01), when compared to baseline. Log10 (10,000 x 50% WT) scores returned to baseline values after cessation of Con‐DRGS at 45 min (4.41 ± 0.06, p > 0.99) and 60 min (4.42 ± 0.08, p > 0.99). No residual effect was observed for Con‐DRGS, as both the 45 and 60 min time point were significantly different compared to the 30 min time point (p < 0.01), while no significant difference was observed between the 45 and 60 min time points and baseline (p > 0.99; Fig. 5a).
Figure 5.
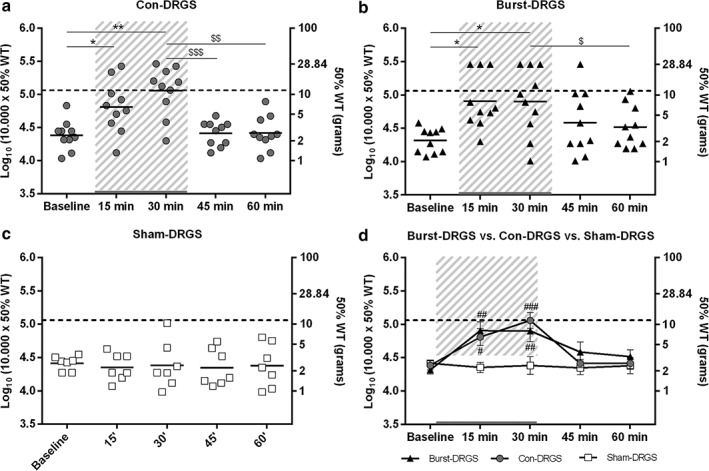
Scatter plot of the effect of Con‐DRGS (n = 10) (a), Burst‐DRGS (n = 10) (b), and Sham‐DRGS (n = 7) (c) on STZ‐induced mechanical hypersensitivity. A combined presentation of the effect of Con‐DRGS, Burst‐DRGS, and Sham‐DRGS is presented in (d). Dotted line = the mean pre‐STZ baseline of all stimulated animals. Gray area = period of DRGS. *, **p < 0.05, p < 0.01 compared to prestimulation baseline; $, $$, $$$p < 0.05, p < 0.01, p < 0.001 compared to T = 30 min; #, ##, ###p < 0.05, p < 0.01, p < 0.001 compared to the Sham‐DRGS group at the same time point.
Effect of Burst‐DRGS on STZ‐Induced Mechanical Hypersensitivity
For animals receiving Burst‐DRGS, the average baseline log10 (10,000 x 50% WT) score (before start of Burst‐DRGS) was 4.31 ± 0.06. Burst‐DRGS resulted in a significant reduction of mechanical hypersensitivity at 15 min (4.91 ± 0.13, p = 0.01) and 30 min (4.90 ± 0.16, p = 0.02), when compared to baseline. Log10 (10,000 x 50% WT) scores returned to baseline values after cessation of Burst‐DRGS at 45 min (4.58 ± 0.15, p = 0.40) and 60 min (4.52 ± 0.10, p = 0.15). Importantly, Burst‐DRGS showed signs of a residual effect at 45 min (15 min after stimulation), as there was no significant difference in the efficacy of Burst‐DRGS at the 45 min time point when compared to the 30 min time point (p > 0.05) and baseline (p = 0.40; Fig. 5b).
Effect of Sham‐DRGS on STZ‐Induced Mechanical Hypersensitivity
For animals receiving Sham‐DRGS, the average baseline log10 (10,000 x 50% WT) score (before start of Sham‐DRGS) was 4.42 ± 0.04. No significant differences in mechanical hypersensitivity were found at 15 min (4.35 ± 0.08, p = 0.88), 30 min (4.38 ± 0.13, p > 0.99), 45 min (4.35 ± 0.10, p = 0.90), and 60 min (4.38 ± 0.12, p > 0.99), when compared to baseline. Furthermore, no significant difference was observed between any of the tested time points (p > 0.88; Fig. 5c).
Effect of Con‐DRGS vs. Burst‐DRGS vs. Sham‐DRGS on STZ‐Induced Mechanical Hypersensitivity
A significant difference between Con‐DRGS and Sham‐DRGS log10 (10,000 x 50% WT) values was observed at 15 min of stimulation (4.81 ± 0.12 vs. 4.35 ± 0.08, p = 0.02) and 30 min of stimulation (5.01 ± 0.12 vs. 4.38 ± 0.13, p < 0.001). No significant differences in terms of log10 (10,000 x 50% WT) values were observed between Con‐DRGS and Sham‐DRGS at baseline (4.38 ± 0.07 vs. 4.42 ± 0.04, p = 0.98), 45 min (4.41 ± 0.06 vs. 4.35 ± 0.10, p = 0.92), and 60 min (4.42 ± 0.08 vs. 4.38 ± 0.12, p = 0.97).
A significant difference was also observed between Burst‐DRGS and Sham‐DRGS log10 (10,000 x 50% WT) values at 15 min of stimulation (4.91 ± 0.13 vs. 4.35 ± 0.08, p < 0.01) and 30 min of stimulation (4.90 ± 0.16 vs. 4.38 ± 0.13, p < 0.01). No significant differences in terms of log10 (10,000 x 50% WT) values were observed between Burst‐DRGS and Sham‐DRGS at baseline (4.31 ± 0.06 vs. 4.42 ± 0.04, p = 0.81), 45 min (4.58 ± 0.15 vs. 4.35 ± 0.10, p = 0.32), and 60 min (4.52 ± 0.10 vs. 4.38 ± 0.12, p = 0.68).
No significant differences were observed between Con‐DRGS and Burst‐DRGS at any time point: Baseline: 4.38 ± 0.07 vs. 4.31 ± 0.06, p = 0.88; 15 min: 4.81 ± 0.12 vs. 4.91 ± 0.13, p = 0.80; 30 min: 5.01 ± 0.12 vs. 4.90 ± 0.16, p = 0.55; 45 min: 4.41 ± 0.06 vs. 4.58 ± 0.15, p = 0.48; 60 min: 4.42 ± 0.08 vs. 4.52 ± 0.10, p = 0.78 (Fig. 5d). Additionally, observed MTs were lower in the Burst‐DRGS group compared to the Con‐DRGS group, albeit not significant (p = 0.12; Fig. 6).
Figure 6.
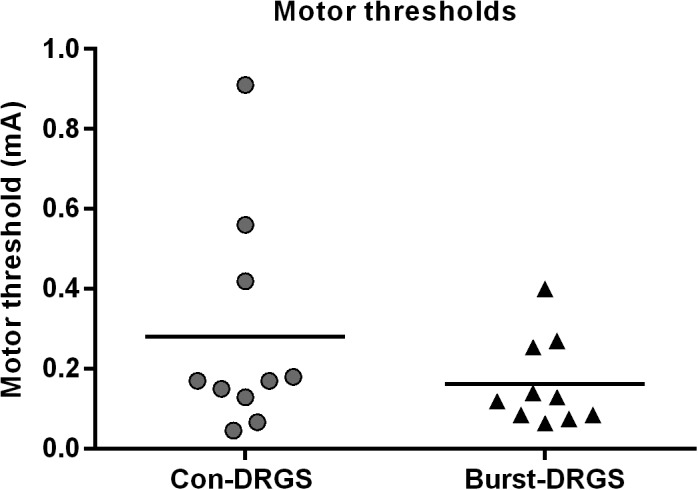
Motor thresholds (MT) assessed by means of Con‐DRGS and Burst‐DRGS. The MT of Con‐DRGS was assessed at 2 Hz and pulse width of 200 μsec. The MT of Burst‐DRGS was assessed using a pulse width of 1000 μsec, and five pulses (500 Hz intraburst frequency) administered at an interburst frequency of 2 Hz.
Percentage Responders: Con‐DRGS vs. Burst‐DRGS
The percentage of responders to Con‐DRGS was 70% (seven out of ten) at 15 min, 90% (nine out of ten) at 30 min of stimulation, 20% (two out of ten) at 45 min, and 33% (three out of ten) at 60 min. In the Burst‐DRGS group, the percentage of responders was 80% (eight out of ten) at 15 min, 70% (seven out of ten) at 30 min of stimulation, 50% (five out of ten) at 45 min, and 40% (four out of ten) at 60 min. A responder was defined as an animal with an increase of ≥0.2 unit in log10 (10,000 x 50% WT) at the 15, 30, 45, or 60 min marks when compared to baseline before stimulation onset (Table 1).
Table 1.
Percentage Responders to Con‐DRGS and Burst‐DRGS at 15, 30, 45, and 60 min.
| Group | T = 15 min | T = 30 min | T = 45 min | T = 60 min |
|---|---|---|---|---|
| Con‐DRGS | 7/10 (70%) | 9/10 (90%) | 2/10 (20%) | 3/10 (33%) |
| Burst‐DRGS | 8/10 (80%) | 7/10 (70%) | 5/10 (50%) | 4/10 (40%) |
A responder to stimulation was defined as an animal with an increase of the 10log (10,000 x 50%WT) ≥0.2 during stimulation compared to the prestimulation baseline.
DISCUSSION
This is the first study to compare Burst‐DRGS with Con‐DRGS in PDPN. Our findings showed that both Con‐DRGS and Burst‐DRGS are equally effective in reversing STZ‐induced mechanical hypersensitivity to pre‐STZ baseline values. Since Con‐DRGS was already very effective in bringing log10 (10,000 x 50% WT) values back to the healthy baseline level after 30 min of stimulation, it might have been challenging to show statistically significant improvements with Burst‐DRGS over this effective Con‐DRGS therapy. Nevertheless, Burst‐DRGS showed signs of a residual effect (not significant) at 15 min after cessation of stimulation, while this was not the case for Con‐DRGS‐treated animals. Also the responder rates were considerably higher in the Burst‐DRGS (5/10; 50%) group when compared to the Con‐DRGS group (2/10; 20%) 15 min after cessation of stimulation. Our results are in line with the work of Pan et al., who were the first to perform in vivo Con‐DRGS in a unilateral peripheral nerve injury model of neuropathic pain 18. The authors concluded that Con‐DRGS attenuates both reflex‐based as well as affective pain behavior. Also the observed motor thresholds in the study of Pan et al. 18 were comparable to those observed in our study. Importantly, the study of Pan and colleagues also showed that DRGS produces no signs of histological or behavioral injury to the DRG.
Also clinically, the effectiveness of Con‐DRGS and the superiority of Con‐DRGS over Con‐SCS for the treatment of chronic intractable pain of the lower limbs attributed to complex regional pain syndrome and causalgia has been published in a RCT 10. Additionally, the first retrospective study to assess the effect of Con‐DRGS for refractory PDPN patients was recently published by Eldabe et al. 11. Despite the retrospective nature and small sample size (ten patients) of this study, the authors concluded that Con‐DRGS is a safe and effective neuromodulation modality to improve painful symptoms in PDPN patients 11.
In the field of Burst stimulation, contradictory findings regarding the superiority of Burst‐SCS over Con‐SCS have been reported. While some studies show a clear advantage of Burst‐SCS over Con‐SCS 13, 14, 22, other studies show no difference between the two stimulation modalities in terms of their pain relieving effect 23, which may have resulted from the different disease indications assessed in these studies. The superiority of Burst‐SCS observed in some studies might be attributed to the mechanism of action of Burst‐SCS, as it is hypothesized that Burst‐SCS, besides targeting brain areas related to the location and intensity of pain (lateral pain pathway), also target areas related to the emotional and affective components of pain (medial pain pathway) 13. Furthermore, a study by Tang et al. found that Burst‐SCS at 60% MT reduced neural activity significantly more than Con‐SCS at the same amplitude, which might explain the superiority of Burst‐SCS over Con‐SCS 24. Last, the total charge per second is higher with Burst‐SCS when compared to Con‐SCS, something that is hypothesized to correlate with stimulation efficacy 25. In our study, no significant difference was found between Con‐DRGS and Burst‐DRGS on STZ‐induced mechanical hypersensitivity. Nevertheless, the inclusion of only reflex‐based tests in the present study might limit our window for detecting differences related to motivational affective aspects of pain 13. Also, the Burst waveform used in the present study, albeit monophasic, varies slightly from the clinically‐used BurstDR waveform, which is monophasic with a passive recharge balance.
The present preclinical DRGS model mimics features that are typical of clinical DRGS. As is the case in clinical DRGS, pain relief occurred very promptly (after 15 min of stimulation) in our animal model for both Con‐DRGS and Burst‐DRGS, and was maintained for the full 30 min stimulation period. Interestingly, a recent study by Meuwissen et al., which compared Con‐SCS with Burst‐SCS in an animal model of peripheral nerve injury, showed Burst‐SCS to have a delayed onset and a delayed carry‐over of analgesic effect when compared to Con‐SCS 26. While no differences in terms of a delayed onset of analgesic effect was observed between Con‐DRGS and Burst‐DRGS in the present study, Burst‐DRGS appeared to show signs of a residual effect at 45 min when compared to Con‐DRGS. Differences in the delayed wash‐in of stimulation might be attributed to differences in the experimental model used, the location of stimulation (dorsal column vs. DRG), the type of stimulation (quadripolar vs. bipolar), and/or the Burst paradigm used (biphasic vs. monophasic). The motor thresholds necessary to evoke contractions of the hind paws of the animals were lower for Burst‐DRGS than Con‐DRGS in our study, albeit not significant. This is in line with preclinical findings that Burst‐SCS requires significantly lower amplitudes to obtain a motor response when compared to Con‐SCS 24, 26, 27, 28, 29, 30. The latter has important consequences for the stimulation amplitude, which is generally lower with Burst‐SCS when compared to Con‐SCS 12.
To date, few studies have been conducted to elucidate the mechanism underlying DRGS. DRGS was found to inhibit neuronal excitability, by reducing the amplitude and/or the amount of action potentials arising from the DRG 31. The unique pseudo‐unipolar design and the T‐junction of the DRG may act as a low‐pass filter for electrical stimuli traveling from the periphery to the spinal cord 32. Furthermore, it was shown by fMRI that DRGS is capable of attenuating BOLD signals in brain regions that are considered part of the pain matrix, like the contralateral thalamic VPL/VPM nuclei, and cortical S1 and S2 33. However, the involvement of the spinal pain gate should not be overlooked, as modulating firing rates of DRG neurons by DRGS may also affect interneurons and GABAergic systems in the dorsal horn as is the case in traditional SCS 34, 35, 36. Interestingly, it was shown that Burst‐SCS does not rely on GABAergic mechanisms as is the case for Con‐SCS, as it was shown that the effect of Con‐SCS, but not Burst‐SCS, is blocked by administration of a GABA‐B receptor antagonist 28, suggesting that different mechanisms of action underlie different stimulation waveforms. Recently, a study by Du et al. found an extensive GABAergic communication network between sensory neuron somata inside the DRG 37. The authors showed that sensory neurons in the DRG express major proteins required for GABA synthesis and release, and are capable of releasing GABA upon depolarization. Furthermore, it was found that local infusion of GABA reuptake inhibitors into the DRG alleviated neuropathic pain, whereas focal application of GABA receptor antagonists triggered neuropathic pain. The authors proposed the idea that this GABAergic system in the DRG acts as a second gate, in addition to the Gate Control Theory 38, and that neuromodulation of the DRG might exert its analgesic action by engaging this second gate 37. More research into the underlying mechanisms of DRGS and its relation to specific stimulation waveforms is necessary to fine‐tune DRGS for chronic neuropathic pain diseases.
Limitations of this study include the use of only a short‐term stimulation protocol. Long‐term stimulation protocols, as being used in clinical practice, require further investigation. Second, only female Sprague–Dawley rats were included in our study as female Sprague–Dawley rats reach their maximal body weight and nerve conduction values faster and at a lower weight when compared to male Sprague–Dawley rats or either sex of other strains 19. As there is strong evidence for sex differences in pain and analgesia, one should be cautious when extrapolating these data to the male sex 39. As mentioned earlier, also the inclusion of only reflex‐based tests has its limitations and the Burst waveform used in the present study varies slightly from the clinically used BurstDR waveform.
We conclude that under the conditions tested, Con‐DRGS and Burst‐DRGS are equally effective in attenuating streptozotocin‐induced mechanical hypersensitivity in an animal model of PDPN. Importantly, Burst‐DRGS showed signs of a residual effect at 15 min after cessation of stimulation, which was not the case with Con‐DRGS. Further work needs to be done to confirm this residual effect of Burst‐DRGS. The present study provides a first insight into the pain relieving effect of Burst‐DRGS. Further optimization and analysis of DRGS driven by insights into the underlying mechanisms of the various stimulation paradigms is necessary.
Authorship Statements
Glenn Franken performed the experiments, analyzed the data, and wrote the manuscript. Elbert A.J. Joosten and Glenn Franken conceived and designed the experiment. Jacques Debets performed the DRGS implantations. All authors have approved the final version of the manuscript.
COMMENTS
I especially appreciate the discussion in which the authors adequately combine the knowledge of basic and clinical research.
Frank Huygen, MD, PhD
Rotterdam, The Netherlands
***
The importance of painful diabetic peripheral neuropathy as a potential application for neuromodulation cannot be overstated. The commonness of this condition means that it has the potential to become the biggest single indication for stimulation. It is therefore critical that we develop a robust evidence base for treatment, incorporating laboratory studies such as this as well as clinical trials.
James Fitzgerald, MA, BM BCh, PhD
Oxford, UK
Comments not included in the Early View version of this paper.
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: This work was supported by a sponsored research contract titled ‘Dorsal Root Ganglion Stimulation in neuropathic pain: Effect of stimulation settings and pain relief in an experimental model of painful diabetic polyneuropathy’ from Abbott, Inc., granted to Elbert A.J. Joosten.
Conflict of Interest: Elbert A.J. Joosten is a consultant for Abbott, Inc. Glenn Franken and Jacques Debets have no conflicts of interest to disclose.
REFERENCES
- 1. Abbott CA, Malik RA, van Ross ER, Kulkarni J, Boulton AJ. Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care 2011;34:2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daousi C, MacFarlane IA, Woodward A, Nurmikko TJ, Bundred PE, Benbow SJ. Chronic painful peripheral neuropathy in an urban community: A controlled comparison of people with and without diabetes. Diabet Med 2004;21:976–982. [DOI] [PubMed] [Google Scholar]
- 3. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–1522. [DOI] [PubMed] [Google Scholar]
- 4. Kaur S, Pandhi P, Dutta P. Painful diabetic neuropathy: an update. Ann Neurosci 2011;18:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finnerup NB, Otto M, Jensen TS, Sindrup SH. An evidence‐based algorithm for the treatment of neuropathic pain. MedGenMed 2007;9:36. [PMC free article] [PubMed] [Google Scholar]
- 6. Slangen R, Schaper NC, Faber CG et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two‐center randomized controlled trial. Diabetes Care 2014;37:3016–3024. [DOI] [PubMed] [Google Scholar]
- 7. van Beek M, Slangen R, Schaper NC et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24‐month follow‐up of a prospective two‐center randomized controlled trial. Diabetes Care 2015;38:e132–e134. [DOI] [PubMed] [Google Scholar]
- 8. de Vos CC, Meier K, Zaalberg PB et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain 2014;155:2426–2431. [DOI] [PubMed] [Google Scholar]
- 9. Kramer J, Liem L, Russo M, Smet I, Van Buyten JP, Huygen F. Lack of body positional effects on paresthesias when stimulating the dorsal root ganglion (DRG) in the treatment of chronic pain. Neuromodulation 2015;18:50–57. discussion 57. [DOI] [PubMed] [Google Scholar]
- 10. Deer TR, Levy RM, Kramer J et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain 2017;158:669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eldabe S, Espinet A, Wahlstedt A et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation 2018; e‐pub ahead of print. doi:10.1111/ner.12767. [DOI] [PubMed] [Google Scholar]
- 12. De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T. Burst spinal cord stimulation: toward paresthesia‐free pain suppression. Neurosurgery 2010;66:986–990. [DOI] [PubMed] [Google Scholar]
- 13. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;80:642–649. [DOI] [PubMed] [Google Scholar]
- 14. De Ridder D, Lenders MW, De Vos CC et al. A 2‐center comparative study on tonic versus burst spinal cord stimulation: amount of responders and amount of pain suppression. Clin J Pain 2015;31:433–437. [DOI] [PubMed] [Google Scholar]
- 15. Pluijms WA, van Kleef M, Honig WM, Janssen SP, Joosten EA. The effect of spinal cord stimulation frequency in experimental painful diabetic polyneuropathy. Eur J Pain 2013;17:1338–1346. [DOI] [PubMed] [Google Scholar]
- 16. van Beek M, van Kleef M, Linderoth B, van Kuijk SM, Honig WM, Joosten EA. Spinal cord stimulation in experimental chronic painful diabetic polyneuropathy: delayed effect of high‐frequency stimulation. Eur J Pain 2017;21:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Beek M, Hermes D, Honig WM et al. Long‐term spinal cord stimulation alleviates mechanical hypersensitivity and increases peripheral cutaneous blood perfusion in experimental painful diabetic polyneuropathy. Neuromodulation 2018;21:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan B, Yu H, Fischer GJ, Kramer JM, Hogan QH. Dorsal root ganglionic field stimulation relieves spontaneous and induced neuropathic pain in rats. J Pain 2016;17:1349–1358. [DOI] [PubMed] [Google Scholar]
- 19. Calcutt NA. Modeling diabetic sensory neuropathy in rats. Methods Mol Med 2004;99:55–65. [DOI] [PubMed] [Google Scholar]
- 20. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. [DOI] [PubMed] [Google Scholar]
- 21. Mills C, Leblond D, Joshi S et al. Estimating efficacy and drug ED50's using von Frey thresholds: impact of weber's law and log transformation. J Pain 2012;13:519–523. [DOI] [PubMed] [Google Scholar]
- 22. de Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation 2014;17:152–159. [DOI] [PubMed] [Google Scholar]
- 23. Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double‐blind, randomized and placebo‐controlled crossover trial. Eur J Pain 2017;21:507–519. [DOI] [PubMed] [Google Scholar]
- 24. Tang R, Martinez M, Goodman‐Keiser M, Farber JP, Qin C, Foreman RD. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation 2014;17:143–151. [DOI] [PubMed] [Google Scholar]
- 25. Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation 2016;19:373–384. [DOI] [PubMed] [Google Scholar]
- 26. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Burst spinal cord stimulation in peripherally injured chronic neuropathic rats: a delayed effect. Pain Pract 2018;18:988–996. [DOI] [PubMed] [Google Scholar]
- 27. Gong WY, Johanek LM, Sluka KA. A comparison of the effects of burst and tonic spinal cord stimulation on hyperalgesia and physical activity in an animal model of neuropathic pain. Anesth Analg 2016;122:1178–1185. [DOI] [PubMed] [Google Scholar]
- 28. Crosby ND, Weisshaar CL, Smith JR, Zeeman ME, Goodman‐Keiser MD, Winkelstein BA. Burst and tonic spinal cord stimulation differentially activate GABAergic mechanisms to attenuate pain in a rat model of cervical radiculopathy. IEEE Trans Biomed Eng 2015;62:1604–1613. [DOI] [PubMed] [Google Scholar]
- 29. Crosby ND, Goodman Keiser MD, Smith JR, Zeeman ME, Winkelstein BA. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation. 2015;18:1–8. discussion 8. [DOI] [PubMed] [Google Scholar]
- 30. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional‐SCS vs. burst‐SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: effect of amplitude. Neuromodulation 2018;21:19–30. [DOI] [PubMed] [Google Scholar]
- 31. Koopmeiners AS, Mueller S, Kramer J, Hogan QH. Effect of electrical field stimulation on dorsal root ganglion neuronal function. Neuromodulation 2013;16:304–311. discussion 310‐301. [DOI] [PubMed] [Google Scholar]
- 32. Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. 2015;18:24–32. discussion 32. [DOI] [PubMed] [Google Scholar]
- 33. Pawela CP, Kramer JM, Hogan QH. Dorsal root ganglion stimulation attenuates the BOLD signal response to noxious sensory input in specific brain regions: insights into a possible mechanism for analgesia. Neuroimage 2017;147:10–18. [DOI] [PubMed] [Google Scholar]
- 34. Stiller CO, Cui JG, O'Connor WT, Brodin E, Meyerson BA, Linderoth B. Release of gamma‐aminobutyric acid in the dorsal horn and suppression of tactile allodynia by spinal cord stimulation in mononeuropathic rats. Neurosurgery 1996;39:367–374. discussion 374‐365. [DOI] [PubMed] [Google Scholar]
- 35. Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain 1997;73:87–95. [DOI] [PubMed] [Google Scholar]
- 36. Janssen SP, Gerard S, Raijmakers ME, Truin M, Van Kleef M, Joosten EA. Decreased intracellular GABA levels contribute to spinal cord stimulation‐induced analgesia in rats suffering from painful peripheral neuropathy: the role of KCC2 and GABA(A) receptor‐mediated inhibition. Neurochem Int 2012;60:21–30. [DOI] [PubMed] [Google Scholar]
- 37. Du X, Hao H, Yang Y et al. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J Clin Invest 2017;127:1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–979. [DOI] [PubMed] [Google Scholar]
- 39. Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain 2004;8:397–411. [DOI] [PubMed] [Google Scholar]


