Abstract
This registry‐linkage study evaluates familial aggregation of cancer among relatives of a population‐based series of early‐onset (≤40 years) cancer patients in Finland. A cohort of 376,762 relatives of early‐onset cancer patients diagnosed between 1970 and 2012 in 40,538 families was identified. Familial aggregation of early‐onset breast, colorectal, brain and other central nervous system (CNS) cancer and melanoma was explored by standardized incidence ratios (SIR), stratified by relatedness. Gender‐, age‐ and period‐specific population cancer incidences were used as reference. Cumulative risks for siblings and offspring of the proband up to age ≤40 years were also estimated. Almost all early‐onset cancers were sporadic (98% or more). Among first‐degree relatives, SIR was largest in colorectal cancer (14, 95% confidence interval 9.72–18), and lowest in melanoma (1.93, 1.05–3.23). Highest relative‐specific SIRs were observed for siblings in families, where also parent had concordant cancer, 90 (43–165) for colorectal cancer and 29 (11–64) for CNS cancer. In spouses, all SIRs were at population level. Cumulative risk of colorectal cancer by age 41 was 0.98% in siblings and 0.10% in population, while in breast cancer the corresponding risks were 2.05% and 0.56%. In conclusion, early‐onset cancers are mainly sporadic. Findings support high familial aggregation in early‐onset colorectal and CNS cancers. Familial aggregation in multiplex families with CNS cancers was mainly attributed to neurofibromatosis and in colorectal cancer to FAP‐ and HNPCC‐syndromes. The pattern of familial aggregation of early‐onset breast cancer could be seen to support very early exposure to environmental factors and/or rare genetic factors.
Keywords: early‐onset cancer, familial aggregation
Short abstract
What's new?
The tendency for certain cancer types to cluster in families generally is explained by shared environmental exposures or inherited mutations. In particular, early‐onset cancer, diagnosed between ages 0 and 40, is considered indicative of familial factors. Here, investigation of cancer risk among more than 376,760 relatives of probands, or individuals with early‐onset cancer, shows that the likelihood of early‐onset cancer affecting even just one other relative in addition to the proband is exceedingly rare. Nearly all early‐onset cancers in the study population were sporadic. Estimated cumulative risks observed for specific cancers may prove useful in the context of genetic counseling.
Abbreviations
- CI
confidence interval
- CNS
central nervous system
- FAP
familial adenomatous polyposis
- FCR
Finnish Cancer Registry
- HNPCC
hereditary nonpolyposis colorectal cancer
- NF
neurofibromatosis
- RR
relative risk
- SIR
standardized incidence ratio
Background
The role of genetic and environmental factors in the development of cancers remains an intriguing question and familial aggregation of cancers provides possibilities to evaluate their contribution. Familial clustering of cancer can be explained either by inherited germline mutations or by shared exposure to environmental factors and lifestyle, resulting in somatic changes predisposing to cancer.
While major epidemiological evidence supports a significant causal role of environmental exposure in the risk of a wide range of cancers,1, 2 inherited mutations are more likely to contribute in early age‐at‐onset cancers and respective family members.3 Despite the fact that more than 50 hereditary cancer syndromes have been recognized,3 the known inherited mutations are evaluated to contribute only about 4% of all childhood cancer cases4 and 5–10% of adult cancers.5, 6
In order to evaluate familial risk, it is useful to assess the risk using pedigree information by age‐at‐onset of cancers.7 Familial aggregation of cancers using population‐based data has been studied previously.8, 9, 10 In the analysis of the Swedish Family Cancer Database,10 several familial cancer associations were established, including cancers of the colon, breast, skin and nervous system, when multiple same primary cancers were diagnosed in the same family. In a study investigating familial risks of the concordant cancers in parents and offspring by age at diagnosis, the highest cancer risks were observed in offspring, whose parents were diagnosed of a concordant cancer at earlier ages.11 In a large Nordic collaborative study on familial melanoma, no difference in risk by morphological type was observed.12
The primary aim of the present study was to evaluate relative and cumulative risks of the concordant cancer in family members of early‐onset index cases called probands. We also studied sibling risk stratified by parental cancer status and compared early‐ vs. late age‐at‐onset of concordant cancers. By exploring the pattern of familial aggregation in brain and other central nervous system (CNS) cancers, colorectal and breast cancer and melanoma of the skin, we aim to evaluate the contribution of environmental and genetic components in the etiology of these early‐onset cancers.
Methods
Study design
Our study utilizes data of a prospective observational cohort of 376,762 relatives in 40,538 families of early‐onset cancer patients diagnosed at age ≤40 years in Finland between 1970 and 2012 (Table 1). The cohort was originally built to assess late effects in the offspring of early‐onset cancer survivors.13, 14 The Finnish Cancer Registry (FCR) and Population Information System maintained by the Population Registry Centre, served as the data sources. The FCR contains information on all diagnosed cancer cases in Finland since 1953, including the unique personal ID number and diagnostic details, such as tumor morphology. The registry covers 96% of solid and 86% of nonsolid tumors.15 The Population Information System is a registry of all permanent Finnish residents and holds data on, for example, personal ID number, family relations and date of birth and death, and allows reliable identification of family members. Links to siblings are reliably available for individuals born after 1955, and alive in 1967. Links to offspring, including legal children of males, are nonsystematically available for children born after 1940, and systematically for children born after 1955, and alive in 1967.
Table 1.
Numbers of family members of probands and their person‐years, number of families by number of cancer cases among family members and number of familial cancers and of families with familial cancers by primary site, including follow‐up and cancer cases of family members at 0–40 years of age
| Number of families | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family members of probands | Number of cancer cases among family members | Familial cancers | ||||||||||
| Primary site | ICD‐10 | Number | Person‐years | 01 | 1 | 2 | 3 | 4 | >4 | Total1 | Number of cancers | Number of families (proportion of families with familial cancers) |
| Brain and other CNS | C70–72, D32–33, D42–43 | 49,712 | 935,387 | 5,346 | 76 | 0 | 1 | 0 | 12 | 5,424 | 85 | 78 (1.4) |
| Colorectum | C18–20 | 20,536 | 383,189 | 1,922 | 33 | 5 | 1 | 0 | 0 | 1,961 | 46 | 39 (2.0) |
| Breast | C50 | 54,766 | 992,156 | 5,465 | 96 | 2 | 0 | 0 | 0 | 5,563 | 100 | 98 (1.8) |
| Melanoma | C43 | 29,894 | 536,409 | 3,070 | 26 | 0 | 0 | 0 | 0 | 3,096 | 26 | 26 (0.8) |
| Other primary | C00–17, C21–42, C44–49, C51–69, C73–96 D09.0‐1, D41, D45–47, D76 | 226,870 | 2,650,641 | 24,231 | 253 | 8 | 1 | 1 | 0 | 24,494 | 276 | 263 (1.1) |
| Total | 376,7623 | 40,034 | 484 | 15 | 3 | 1 | 1 | 40,538 | ||||
Excludes families where proband has no family members.
Six cancers in one family.
The number of unique relatives.
Families were identified by a proband, who was the first cancer case in the family diagnosed at or under the age of 40 years between January 1, 1970 and December 31, 2012, in Finland. The family members (probands’ offspring, mother, father, siblings, spouse and siblings’ offspring and siblings’ spouse) were linked to the probands from the Population Information System. We were not able to comprehensively and systematically retrieve information on grandparents or other older second degree relatives from the Population Information System.
Cancers were classified according to the International statistical classification of diseases and related health problems (ICD‐10) as follows: CNS (ICD10 codes C70–72, D32–33 and D42–43), colorectum (C18–20), breast (C50), melanoma (C43) and all other malignancies. The study was approved by the National Institute for Health and Welfare (Permit no. THL/1006/5.05.00/2017).
Follow‐up and outcomes
The ascertainment of probands and family members, as well as follow‐up of cancer outcomes, is illustrated by a Lexis diagram (Supporting Information Fig. S1). For all family members of the proband, the follow‐up begins either at the date of birth or January 1, 1953. In order to avoid immortal time bias16 (period of time when by study design cancer could not be diagnosed due to the ascertainment), we did not consider family members of the proband to be at risk of cancer between January 1, 1970, and the date of diagnosis of the proband at 0–40 years of age. The cancer‐specific follow‐up ended either at date of the cancer diagnosis, date of death or emigration, or December 31, 2016, whichever came first. This leads to inclusion of certain time periods at risk of family members prior to the date of diagnosis of the proband: all ages from 1953 to 1970, and age ≥41 years from 1970 onwards. The SIR was estimated using all follow‐up outside the immortal periods.
Characteristics of cancer patients
The proportions of male and female probands, by primary site and median age at diagnosis, are given in Supporting Information Table S1. There were 17,852 (42%) males and 24,166 (58%) females among the probands. The median age at diagnosis for probands was 28 years for CNS cancer, 37 years for breast cancer, 34 years for colorectal cancer and 33 years for melanoma of the skin. The most prevalent early‐onset cancer diagnoses among probands were lymphomas and leukemias (21%, 8,791 families). Even if hematological malignancies are common in children, their distribution differs from that of young adults (20–40 years) or all adults. In addition, the most recent classification of hematological malignancies into groups not following the traditional leukemia and lymphoma‐division has to be taken into account. Additionally, transformation of one malignancy into something else, for example, myelodysplasias into acute myeloid leukemias found in many adult cases needs to be studied in more detail. We felt this complex group of different malignancies should be reported separately and are not within the scope of the current study.
The next most prevalent diagnostic groups were breast cancer (14%), CNS cancer (13%), melanoma (8%) and colorectal cancer (5%). These cancer types were selected as examples of some of the common early‐onset cancer types, where familial aggregation would be expected due to known predisposing genetic mutations (such as hereditary nonpolyposis colorectal cancer [HNPCC], familial adenomatous polyposis [FAP], BRCA1/2 gene mutations and neurofibromatosis [NF]).
More focused analysis was performed for siblings of the proband, who had, in addition to the proband, at least one parent diagnosed with a concordant cancer (later referred to as multiplex families). For cancers in the multiplex families, we checked the original clinical and pathology notifications from the FCR and extracted any information concerning selected cancer syndromes, FAP and HNPCC for multiplex families with colorectal cancer, and NF for CNS cancers. Also, any notes indicating BRCA1/2 gene mutation were extracted from multiplex breast cancer families.
Statistical methods
Standardized incidence ratios (SIR) were used as measures of familial aggregation as they compare sex‐, age‐ and period‐specific cancer incidence among family members to that in the population of Finland. SIRs were estimated for all first‐degree relatives of the proband combined and for family members separately by relatedness to the proband. Siblings of the proband were further divided by parental cancer status: siblings who had at least one parent diagnosed with a concordant cancer, and siblings whose parents had not been diagnosed with the cancer in question. We also report SIRs for cancers of the probands’ relatives diagnosed at ≤40 years (early‐onset) and at ≥41 years (late‐onset) separately by relatedness. The estimates of SIRs were corrected for nonrandom selection of families through the proband (ascertainment bias) by excluding the proband from the analysis.7, 17 Poisson regression was used in the estimation of SIRs. In addition, we estimated cumulative risks and expected cumulative risks from 0 to 41 years of age in offspring and siblings. In the estimation of the cumulative risks, we considered only follow‐up after the diagnosis date of the proband. Details of the estimation of SIRs and cumulative risks are given in the Supporting Information Statistical Methods. All reported p values are two‐sided, and to control for overall type‐1 error, we used Benjamini–Hochberg procedure.
Our data had at least 80% power of finding a SIR of 1.7 among first‐degree relatives diagnosed at ≤40 years for cancers of CNS, SIR 3.0 for colorectal cancer, SIR 1.7 for breast cancer and SIR 2.2 for melanoma of the skin. Data on cancers are of high coverage15 and are also complete for date of the diagnosis and family members’ relatedness to the proband. However, we are missing some parents of the probands. Information on both parents was missing for 17.0% of the probands, whereas 6.4% had information on one parent only (see chapter Study design on registry linkages).
Analysis was performed using the R software, version 3.5.0 (R project for Statistical Analysis) and packages Epi, version 2.30 and popEpi, version 0.4.5.
The data that support the findings of our study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Results
The numbers and proportions of families, subjects and familial cancers diagnosed at ≤40 years of age by primary site are presented in Table 1. Almost all, 98% or more, of the early‐onset cancers of the studied primary sites were sporadic. There were 78 families with at least one early‐onset family member diagnosed with CNS cancer (1.4% of the families). Accordingly, there were 39 families with at least one family member diagnosed with early‐onset colorectal cancer (2.0%), 98 (1.8%) with early‐onset breast cancer and 26 (0.8%) cases of early‐onset melanoma of the skin. Additional tables for the numbers and proportions of families, subjects, and familial cancers by primary site for familial cancers at any age are shown in Supporting Information Table S2, and for cancers diagnosed at ≥41 years in Supporting Information Table S3. Age distributions of family members at diagnoses and at the end of follow‐up are presented in Supporting Information Tables S4 and S5.
Relative risks of early‐onset cancer among relatives of early‐onset proband
SIRs for early‐onset cancers in family members diagnosed at ≤40 years of age are shown in Table 2. When considering all first‐degree relatives of the proband combined, the SIRs for early‐onset familial cancers were elevated in all studied cancer sites, ranging from 1.93 (95% CI 1.05–3.23) for melanoma of the skin to 14 (9.72–18) for colorectal cancer.
Table 2.
Numbers of family members of the proband, number of observed cancer cases and standardized incidence ratios (SIR) for concordant cancers in family members by relatedness to the proband, when also the family member was diagnosed at ≤40 years
| Relatedness to the proband | Number of family members of the proband1 | Number of cancers2 | Person‐years | SIR for family member for concordant cancer3 | (95% CI) |
|---|---|---|---|---|---|
| Brain and other CNS cancers4 | |||||
| First‐degree relatives5 | 25,861 | 54 | 465,429 | 2.50 | (1.88–3.27) |
| Offspring | 5,829 | 23 | 126,523 | 3.66 | (2.32–5.49) |
| Father | 4,557 | 3 | 66,803 | 1.25 | (0.26–3.66) |
| Mother | 4,823 | 4 | 71,796 | 1.50 | (0.41–3.84) |
| Sibling | 10,652 | 24 | 200,307 | 2.35 | (1.50–3.49) |
| Sibling's offspring | 14,439 | 25 | 278,142 | 1.86 | (1.21–2.75) |
| Spouse | 9,833 | 6 | 191,816 | 0.60 | (0.22–1.31) |
| Breast cancer4 | |||||
| First degree relatives5 | 27,107 | 83 | 481,064 | 3.67 | (2.92–4.55) |
| Offspring | 9,443 | 32 | 214,269 | 3.61 | (2.47–5.10) |
| Father | 3,943 | 0 | 53,294 | 0.00 | (0.00–314) |
| Mother | 4,228 | 8 | 60,433 | 1.67 | (0.72–3.30) |
| Sibling | 9,493 | 43 | 153,068 | 4.80 | (3.47–6.47) |
| Sibling's offspring | 16,098 | 14 | 315,018 | 1.44 | (0.79–2.42) |
| Spouse | 12,202 | 3 | 196,073 | 0.33 | (0.07–0.97) |
| Colorectal cancer4 | |||||
| First degree relatives5 | 10,117 | 42 | 181,717 | 14 | (9.72‐18) |
| Offspring | 3,227 | 16 | 71,764 | 13 | (7.20–21) |
| Father | 1,520 | 6 | 21,369 | 20 | (7.21–43) |
| Mother | 1,615 | 4 | 22,946 | 14 | (3.72–35) |
| Sibling | 3,755 | 16 | 65,638 | 13 | (7.34–21) |
| Sibling's offspring | 6,228 | 4 | 122,967 | 1.97 | (0.54–5.06) |
| Spouse | 4,364 | 0 | 78,505 | 0.00 | (0.00–2.43) |
| Melanoma4 | |||||
| First degree relatives5 | 15,399 | 14 | 265,724 | 1.93 | (1.05–3.23) |
| Offspring | 4,875 | 6 | 103,170 | 1.98 | (0.73–4.31) |
| Father | 2,520 | 1 | 36,247 | 2.29 | (0.06–13) |
| Mother | 2,666 | 0 | 38,673 | 0.00 | (0.00–6.78) |
| Sibling | 5,338 | 7 | 87,634 | 2.15 | (0.86–4.43) |
| Sibling's offspring | 8,415 | 7 | 163,088 | 1.50 | (0.60–3.10) |
| Spouse | 6,338 | 5 | 107,597 | 1.25 | (0.41–2.92) |
Includes all family members.
Includes early‐onset cancers in family members of the proband.
SIR (95% CI) for concordant cancer in the family member of the proband.
p‐Value for homogeneity of the SIRs between first‐degree relatives combined and spouses was p < 0.001 for cancers of the CNS, breast and colorectum, and p = 0.39 for skin melanoma.
Includes offspring, father, mother and siblings of the proband.
The most elevated cancer risks for family members of the proband were observed for early‐onset colorectal cancer, where the risk of cancers among all different first‐degree relatives was over 10‐fold compared to those expected, based on cancer incidence in the national population.
Regarding CNS cancers, the SIR was elevated for offspring (3.66, 2.32–5.49) and siblings (2.35, 1.50–3.49) of the proband.
Familial risk of early‐onset female breast cancer was elevated for offspring of the proband (SIR 3.61, 2.47–5.10) and siblings (4.80, 3.47–6.47) of the proband, but SIR for the mother of the proband did not reach statistical significance (1.67, 0.72–3.30).
The SIR of skin melanoma was 1.93 (1.05–3.23) in all first‐degree relatives of the proband combined, but none of the SIRs for specific relatives were significantly elevated.
SIR in siblings’ offspring was statistically increased only in CNS cancer (1.86, 1.21–2.75).
All SIRs for probands’ and their siblings’ spouses were at population level, except for breast cancer, where the spouses’ risk was significantly lowered. There was significant heterogeneity (p < 0.001) in SIRs between first‐degree relatives (all combined) and spouses for early‐onset cancers of CNS, breast and colorectum, but not for melanoma of the skin (p = 0.39). There was some heterogeneity in SIRs between different first‐degree relatives (p = 0.05) for breast cancer, but not for other cancers.
Cumulative risks for siblings and offspring
The cumulative sibling and offspring risks by the age of 40 for all cancers studied are plotted in Figure 1. The cumulative risk of colorectal cancer for the offspring of the proband with a concordant cancer was 1.37% (95% CI 0.83–2.25%), and for sibling 0.98% (0.58–1.64%), compared to the population cumulative risk of 0.10%. Cumulative risk of CNS cancer for offspring of CNS probands was 0.75% (0.49–1.17%) and population reference 0.24%. The corresponding cumulative risk for siblings of the CNS proband was 0.53% (0.33–0.85%). Cumulative risk of breast cancer for offspring of the proband with breast cancer was 1.63% (1.15–2.31%) and the population cumulative risk was 0.56%. The corresponding cumulative risk for sibling was 2.05% (1.49–2.81%). Cumulative risk of melanoma for offspring of the proband with melanoma was 0.34% (0.15–0.76%) and accordingly for the sibling of the proband 0.26% (0.12–0.57%), in contrast to population cumulative risk of 0.18%.
Figure 1.
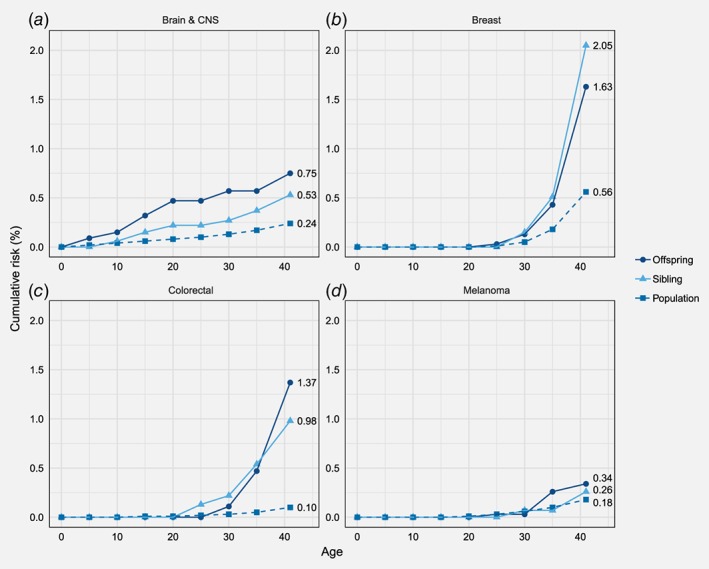
Cumulative risk of cancers of CNS, breast, colorectum and melanoma by age 41 years for offspring and siblings of the early‐onset probands and the population. [Color figure can be viewed at http://wileyonlinelibrary.com]
Familial early‐onset cancer risk by parental cancer status
Table 3 shows the early‐onset familial relative risks (SIRs) of the siblings of the proband by parental cancer status (concordant cancer in at least one parent at any age). The highest SIR was obtained for colorectal cancer in siblings with an affected parent (SIR 90, 43–165). The SIR for colorectal cancer in siblings of the proband without parental cancer was 5.29 (1.94–12). Siblings of the proband with CNS cancer in families with the concordant parental cancer were at considerably higher familial risk of CNS cancer, with a SIR of 29 (11–64), compared to healthy parent's sibling (SIR 1.80, 1.06–2.84). Siblings of the proband with a maternal breast cancer had a SIR of 10 (4.85–19) and siblings without parental breast cancer SIR 4.14 (2.85–5.81).
Table 3.
Numbers of family members of the proband, number of observed cancer cases and standardized incidence ratios (SIR) for early‐onset (≤40 years) concordant cancer in siblings of the proband by concordant parental cancer at any age
| Relatedness to the proband | Number of family members of the proband | Number of cancers | Person‐years | SIR for concordant cancer1 | (95% CI) |
|---|---|---|---|---|---|
| Brain and other CNS cancers2 | |||||
| Sibling with affected parent3 | 194 | 6 | 3,954 | 29 | (11–64) |
| Sibling without affected parent4 | 10,458 | 18 | 196,353 | 1.80 | (1.06–2.84) |
| Breast cancer2 | |||||
| Sibling with affected parent3 | 980 | 10 | 15,226 | 10 | (4.85–19) |
| Sibling without affected parent4 | 8,513 | 33 | 137,842 | 4.14 | (2.85–5.81) |
| Colorectal cancer2 | |||||
| Sibling with affected parent3 | 363 | 10 | 6,405 | 905 | (43–165) |
| Sibling without affected parent4 | 3,392 | 6 | 59,233 | 5.29 | (1.94–12) |
| Melanoma2 | |||||
| Sibling with affected parent3 | 193 | 0 | 3,414 | 0.00 | (0.00–30) |
| Sibling without affected parent4 | 5,145 | 7 | 84,220 | 2.23 | (0.90–4.60) |
SIR (95% CI) for concordant cancer in the family member of the proband.
According to pathological reports retrieved from the Finnish Cancer Registry for siblings with affected parents, all brain and CNS cases were in families with neurofibromatosis in at least one subjects with diagnosed cancer. We found no record of BRCA carriers among breast cancer probands and their siblings or parents. FAP or HNPCC was found in four of the 10 multiplex colorectal cancer families.
At least one sibling diagnosed with the concordant cancer at age ≤40 years and at least either one of the parents diagnosed with the concordant cancer at any age. There were no families with both affected parents.
At least one sibling diagnosed with the concordant cancer at age ≤40 years, no parents with concordant cancer.
SIR for the families without FAP or HNPCC was 56 (21–122).
All CNS cancers in multiplex families with concordant parental cancer were found in families with NF in at least one family member diagnosed with CNS cancer (Table 3). As for multiplex breast cancer families, no BRCA 1/2 gene mutation carriers were reported in the clinical or pathology notifications, but the contents of pathological reports are limited up to 2014. Four of the siblings with early‐onset colorectal cancer and a concordant parental cancer were either in families with FAP or HNPCC. The sibling SIR in the remaining multiplex families with six sibling colorectal cancers was 56 (21‐122).
Early‐onset vs. late‐onset familial cancers
The increase in SIRs was more pronounced in first‐degree relatives of the proband, who were diagnosed at an early age, compared to cases diagnosed at age ≥41 years in all studied cancers, except melanoma of the skin (Supporting Information Table S6). The largest difference in familial SIRs between diagnoses at ≤40 and at ≥41 years was observed for colorectal cancer (p < 0.001 for homogeneity), where the familial SIR for early‐onset cancers in all first‐degree relatives of the proband combined was 14 (9.72–18) and 2.11 (1.83–2.42) for cases diagnosed at 41 years or later. The respective SIRs in CNS cancers were 2.50 (1.88–3.27) and 1.32 (1.08–1.59, p < 0.001 for homogeneity). In breast cancer, the SIR for early‐onset disease in all first‐degree relatives of the proband combined was 3.67 (2.92–4.55) and 1.67 (1.55–1.79) for diagnoses at ≥41 years (p < 0.001 for homogeneity). In skin melanoma, there was no difference between early‐ and late‐onset SIR of the first‐degree relatives (p = 0.25). Regardless of age‐at‐onset, the highest SIRs were observed for siblings of the proband, when also at least either one of the parents was diagnosed with the concordant cancer, except in late‐onset skin melanoma.
Discussion
To the best of our knowledge, this is the first study with a population‐based ascertainment focusing on familial aggregation of early‐onset cancers. We found that even having one other family member in addition to proband with early‐onset cancer is extremely rare. In breast cancer, 98% of the families with an early‐onset proband had no other familial early‐onset breast cancers and the proportion was even larger for all other studied cancers. The cumulative risk of familial cancer by age 41 was highest for breast cancer among offspring (1.63%) and siblings (2.05%) of the proband. This implies that early‐onset breast cancer among offspring and siblings of the proband is very rare event. All studied cancers, except melanoma, showed significant variation in the familial relative risks between first‐degree relatives and spouses. The familial relative risk for early‐onset cancer was the highest among the first‐degree relatives of colorectal cancer probands (SIR of father of the proband 20) and lowest in skin melanoma (none cases among mothers of the proband and some twofold risks in other first‐degree relatives). SIRs of the siblings in the multiplex families were very high for cancers of the colorectum (90) and CNS (29), while in multiplex breast cancer families the SIR was lower (10).
Strengths of the study
Early‐onset cancer is commonly considered an indication of inherited genetic factors or early exposure to carcinogens. Our prospective family‐based cohort design with exclusively early‐onset probands is powerful in identifying novel familial aggregation. Also, owing to broader inclusion of extended family members (spouses and siblings’ offspring), the data are likely to be more powerful than many studies restricted to particular genetic relationships, such as those with twins or selected nuclear families. These reasons could explain our higher familial risks than those found in the Nordic twin studies.18 Completeness and accuracy of cancer information at the FCR have been shown to be high.15 It provides close to complete national cancer data for solid tumors. We have also complete information on cancers of relatives based on registry‐linkage. The SIR‐based method we used is considered reliable, as the statistical analyses adjust for both ascertainment and immortal bias, and the used Poisson excess risk model accounts for censoring in time‐to‐event analysis. The method also adjusts for changes in population cancer risk by calendar time, age and sex. The conventional way of estimating SIR provides as stable estimates as possible for expected numbers of cancers by utilizing cancer incidence rates of the whole target population as the reference. The reported SIR may, to some extent, underestimate the relative risks between the exposed and unexposed, leading to conservative conclusions. As the early‐onset familial cancers are extremely rare events and exposure to early‐onset family history of cancer is rare as well, the potential bias is, however, likely to be small.
Inclusion of probands’ and their siblings’ spouses and siblings’ offspring enables better contrasting of the environmental and genetic influence on cancer risk. Spouses are unrelated to the probands and should reflect population cancer risk if there is no shared environmental effect with the proband. Siblings’ offspring are also informative as they share only 25% of the genetic background with the proband.
Limitations of the study
A shortcoming of our ascertainment scheme is that follow‐up for late‐onset cancers in family members remains rather short and comparison of relative risks between early‐ and late‐onset familial cancers would gain more power with a longer follow‐up. Second, in the current analysis, we have no systematic information on the inherited cancer syndromes or gene mutations influencing cancer risks, such as FAP and HNPCC for colorectal cancer, NF for CNS cancers or BRCA1 or BRCA2 for breast cancer. Overall, FAP is estimated to account for less than 1% and HNPCC for some 3% of all colorectal cancer cases.19, 20 As for breast cancer, Peto et al.21 reported that 6% of the women diagnosed with early‐onset breast cancer were carriers of BRCA1 and BRCA2 gene mutations. It is also well established that the risk of CNS cancer is majorly increased in patients with NF.22
To narrow down the problem of known inherited cancer syndromes or gene mutations in the analyses, we extracted information on FAP, HNPCC, NF and BRCA 1/2 mutations from cancer notifications available at the FCR. This was focused on multiplex families with concordant primary cancers since these would most likely be explained by known inherited mutations. All multiplex CNS families and 40% of the multiplex colorectal cancer families were found to belong to families with either NF or FAP/HNPCC syndrome. We found no information on BRCA 1/2 mutations in multiplex breast cancer families, but it should be noted that data on these mutations at the FCR are incoherent.
Lower fertility among early‐onset cancer subjects may lead to observing more healthy family members and thus underestimating the cancer burden in these families.23 Also, higher mortality of early‐onset cancer patients before reproduction may lead to selection of early‐onset cases.
As our study was registry‐based, we could not adjust the analyses for known environmental risk factors, such as for ultraviolet radiation in the analyses of melanoma risks, or for age at menarche in breast cancer analyses. However, despite some studies,24 there is no convincing evidence of risk factors other than family history and cancer syndromes in early‐onset colorectal cancer and CNS cancers, making the current analyses more valid for these cancers.
Due to the rare nature of early‐onset familial cancers, it would be natural and fruitful to collaborate with other large population‐based genetic‐epidemiological familial studies such as the Swedish Family Cancer Database.9 This would increase the number of familial cancers and the ability to detect novel familial aggregation.
How do the current findings relate to previous knowledge?
To the best of our knowledge, this is the first time, when familial relative risks and cumulative risks for early‐onset cancers are reported from a comprehensive population‐based registry. Majority of the previous studies of early‐onset cancer families are based on clinical or hospital‐based sets of families.
We observed considerably higher sibling risk for early‐onset CNS cancer than studies from the Swedish Family Cancer Database,10, 25 especially when also the parent was diagnosed with the concordant cancer (SIR 29). Dong and Hemminki reported from the Swedish data a SIR of 11 for nervous system cancers when both the parent and the sibling were affected.10 Overall, the analysis of the Utah family data found a relative risk (RR) of 6.4 for astrocytoma and glioblastoma for first‐degree relatives with onset at <20 years of age,26 whereas we obtained a SIR 2.50 for CNS cancer in first‐degree relatives diagnosed at ≤40 years. In our study, all familial cases of CNS cancers in multiplex families were those with NF.
We observed a SIR of 3.67 for early‐onset breast cancer in first‐degree relatives, whereas Frank et al. reported RR 2.15 for first‐degree relatives of a proband diagnosed at <60 years25 and Althuis et al. observed RR of 3.22 for families with family history of breast cancer in adolescents and young adults (<35 years).27 However, our SIR for early‐onset breast cancer of siblings in multiplex families (10) is substantially higher than the RR reported by Frank et al. (2.88).25 The difference can be at least partly due to our families ascertained at a younger age. Our result is, however, in line with the risk ratio of 14 presented by the Collaborative Group on Hormonal Factors in Breast Cancer for women with two first‐degree relatives with a history of breast cancer, with at least one of them diagnosed before the age 40 years.28
Compared to Frank et al.,25 a stronger age‐at‐onset effect of familial colorectal cancer was detected for first‐degree relatives in our study. Our SIR was 2.11 for cases diagnosed at ≥41 years and 14 for diagnoses at ≤40 years, compared to RR 1.80 for familial cases at ≥60 years of age and 3.14 for diagnoses at <60 years reported by Frank et al.25 SIR for colorectal cancer in siblings with a concordant parental cancer in our study was 90, which is much higher than the SIRs observed by Dong and Hemminki for families with affected parent and sibling (28 for colon and 33 for rectum).10 We found only two cases of FAP and two cases of HNPCC out of 10 colorectal cancers among siblings in the multiplex families, based on clinical notifications from the FCR.
Cumulative risk for melanoma of the skin by the age of 41 was modest (0.3%) for proband's siblings and offspring and was at the population level. This is different from that reported by Fallah et al.,12 who reported a lifetime risk of 3% for family members of a melanoma case diagnosed under the age 30. The difference is probably mainly because we only estimated cumulative risk until the age of 41. In melanoma of the skin, familial SIR did not change significantly by age‐at‐onset of the familial case, while Frank et al. observed RR 3.13 for early‐onset familial cancers (diagnosed at <60 years of age) and 2.19 for cases diagnosed at ≥60 years. Notably, there were no early‐onset cases of melanoma in our data among 193 siblings with parental skin melanoma.
The observed familial risks were higher throughout in family members with early‐onset diagnosis, compared to those with late‐onset cancers. Considering that our probands were all ≤40 years of age, the finding supports the dual age effect, where both the probands’ as well as the family members’ age at diagnosis affect the risk of familial cancer. The observation is also supported by Kharazmi et al., who found in their study that familial cancers are likely to be early‐onset mainly in persons, whose family members are affected at early ages and not in those whose family members were affected at older ages.11
Public health point of view
The prospective follow‐up of the family cohort enables estimation of cumulative risks until the age of ≤41 for siblings and offspring of early‐onset cancer patients. From the public health point of view, early‐onset cancers studied here are rare with the highest population cumulative risk in early‐onset breast cancer (0.56%) and cumulative risk of 2.05% for siblings of the proband. It should be noted that in all studied cancers 98% or more of the cases were sporadic, that is, there was no other family member with a concordant early‐onset cancer. The observed relative and cumulative risks should be informed to young (≤40 years) relatives of early‐onset cancer patients in a balanced manner. Despite the large familial relative risks for family members of early‐onset cancer cases, the relatives should be made aware of the modest cumulative risks. However, in case additional information about known familial cancer syndromes or cancer‐predisposing mutations is available, the risk should be assessed taking the carrier status into account.
The generalizability of our findings depends on to what extent etiological factors (genetic or environmental) concerning early‐onset cancers differ between the countries. Finns are often considered genetically different by population history and founder effects, but such an effect would have to be large in order to explain a substantial fraction of early‐onset cancer cases that are not already explained by known inherited mutations or syndromes. Furthermore, we did not find substantially different cancer incidence trends among early‐onset cancers between the Nordic countries, implicating against substantial founder effects. Since little is known about causal risk factors of early‐onset cancers, it is difficult to speculate their role in extrapolation of our findings to other countries.
In summary
The familial relative risks in siblings of the proband differed greatly by the parental cancer status in early‐onset cancers of the colorectum and CNS and less so in breast cancer, implying a major role of inherited factors in the etiologies of colorectal and CNS cancers in multiplex families. Likewise, the increased SIRs in siblings’ offspring in CNS and colorectal cancers provide further evidence for a genetic influence, whereas in breast cancer the respective SIRs were at population level. This combined with smaller observed effects in breast cancer, compared to colorectal and CNS cancers when stratifying by parental cancer status, suggests either an important role of environmental factors or a set of rare genetic factors in causation of early‐onset breast cancer. Considering the notably high dual age effect in familial risk of colorectal cancer in siblings in multiplex families, and as only a small proportion of the effect was explained by FAP or HNPCC, other known or unknown inherited mutations are plausible in these families. Combining our findings with previous reports on the prevalence of BRCA1 and BRCA2 mutations in early‐onset breast cancer (5.9%21) and approximately 10% of other germline predisposing mutations in breast cancer patients at any age,29 it is likely that environmental factors play a large role in early‐onset breast cancer.
In conclusion, early‐onset cancers are mainly sporadic. Our findings support high familial aggregation in early‐onset CNS and colorectal cancers. Familial aggregation in multiplex families with CNS cancers was mainly attributed to NF and in colorectal cancer to FAP and HNPCC. The pattern of familial aggregation of early‐onset breast cancer could be seen to support both very early exposure to environmental factors and/or rare genetic factors.
Supporting information
Appendix S1: Supplementary Information
Acknowledgement
We thank Ms Heidi Ryynänen for data visualizations.
Conflict of interest: The authors report no conflict of interests.
[Correction added on September 24, 2019 after first online publication: copyright statement updated.]
References
- 1. Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981;66:1191–308. [PubMed] [Google Scholar]
- 2. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- 3. Lindor NM, McMaster ML, Lindor CJ, et al. Concise handbook of familial cancer susceptibility syndromes—second edition. J Natl Cancer Inst Monogr 2008;38:3–93. [DOI] [PubMed] [Google Scholar]
- 4. Narod SA, Stiller C, Lenoir GM. An estimate of the heritable fraction of childhood cancer. Br J Cancer 1991;63:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lynch HT, Fusaro RM, Lynch J. Hereditary cancer in adults. Cancer Detect Prev 1995;19:219–33. [PubMed] [Google Scholar]
- 6. National Cancer Institute . The Genetics of Cancer. Bethesda, MD: National Cancer Institute, 2017. Available from: https://www.cancer.gov/about-cancer/causes-prevention/genetics [cited 2018 May 8]. [Google Scholar]
- 7. Thomas DC. Statistical methods in genetic epidemiology. New York, NY: Oxford University Press, 2004. [Google Scholar]
- 8. Goldgar DE, Easton DF, Cannon‐Albright LA, et al. Systematic population‐based assessment of cancer risk in first‐degree relatives of cancer probands. J Natl Cancer Inst 1994;86:1600–8. [DOI] [PubMed] [Google Scholar]
- 9. Hemminki K, Vaittinen P. National database of familial cancer in Sweden. Genet Epidemiol 1998;15:225–36. [DOI] [PubMed] [Google Scholar]
- 10. Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer 2001;92:144–50. [PubMed] [Google Scholar]
- 11. Kharazmi E, Fallah M, Sundquist K, et al. Familial risk of early and late onset cancer: nationwide prospective cohort study. BMJ 2012;345:e8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fallah M, Pukkala E, Sundquist K, et al. Familial melanoma by histology and age: joint data from five Nordic countries. Eur J Cancer 2014;50:1176–83. [DOI] [PubMed] [Google Scholar]
- 13. Madanat‐Harjuoja L‐MS, Malila N, Lähteenmäki P, et al. Risk of cancer among children of cancer patients—a nationwide study in Finland. Int J Cancer 2010;126:1196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Asdahl PH, Winther JF, Bonnesen TG, et al. The adult life after childhood cancer in Scandinavia (ALiCCS) study: design and characteristics. Pediatr Blood Cancer 2015;62:2204–10. [DOI] [PubMed] [Google Scholar]
- 15. Leinonen MK, Miettinen J, Heikkinen S, et al. Quality measures of the population‐based Finnish cancer registry indicate sound data quality for solid malignant tumours. Eur J Cancer 2017;77:31–9. [DOI] [PubMed] [Google Scholar]
- 16. Rothman KJ, Greenland S, Lash TR. Modern epidemiology, 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 17. Fisher RA. The effect of methods of ascertainment upon the estimation of frequencies. Ann Eugen 1934;6:13–25. [Google Scholar]
- 18. Mucci LA, Hjelmborg JB, Harris JR, et al. Familial risk and heritability of cancer among twins in Nordic countries. JAMA 2016;315:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992;33:357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998;338:1481–7. [DOI] [PubMed] [Google Scholar]
- 21. Peto J, Collins N, Barfoot R, et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early‐onset breast cancer. J Natl Cancer Inst 1999;91:943–9. [DOI] [PubMed] [Google Scholar]
- 22. Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive cancer associations in patients with Neurofibromatosis type 1. J Clin Oncol 2016;34:1978–86. [DOI] [PubMed] [Google Scholar]
- 23. Madanat L‐MS, Malila N, Dyba T, et al. Probability of parenthood after early onset cancer: a population‐based study. Int J Cancer 2008;123:2891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosato V, Bosetti C, Levi F, et al. Risk factors for young‐onset colorectal cancer. Cancer Causes Control 2013;24:335–41. [DOI] [PubMed] [Google Scholar]
- 25. Frank C, Fallah M, Sundquist J, et al. Population landscape of familial cancer. Sci Rep 2015;5:12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blumenthal DT, Cannon‐Albright LA. Familiality in brain tumors. Neurology 2008;71:1015–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Althuis MD, Brogan DD, Coates RJ, et al. Breast cancers among very young premenopausal women (United States). Cancer Causes Control 2003;14:151–60. [DOI] [PubMed] [Google Scholar]
- 28. Collaborative Group on Hormonal Factors in Breast Cancer . Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 2001;358:1389–99. [DOI] [PubMed] [Google Scholar]
- 29. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol 2017;3:1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information


