Abstract
Aims
To determine the effects of early sacral neuromodulation (SNM) and pudendal neuromodulation (PNM) on lower urinary tract (LUT) function, minipigs with complete spinal cord injury (cSCI) were analyzed. SNM and PNM have been proposed as therapeutic approaches to improve bladder function, for example after cSCI. However, further evidence on efficacy is required before these methods can become clinical practice.
Methods
Eleven adults, female Göttingen minipigs with cSCI at vertebral level T11‐T12 were included: SNM (n = 4), PNM (n = 4), and SCI control (SCIC: n = 3). Tissue from six healthy minipigs was used for structural comparisons. Stimulation was started 1 week after cSCI. Awake urodynamics was performed on a weekly basis. After 16 weeks follow‐up, samples from the urinary bladder were taken for analyses.
Results
SNM improved bladder function with better capacities and lower detrusor pressures at voiding and avoided the emergence of detrusor sphincter dyssynergia (DSD). PNM and untreated SCI minipigs had less favorable outcomes with either DSD or constant urinary retention. Structural results revealed SCI‐typical fibrotic alterations in all cSCI minipigs. However, SNM showed a better‐balanced distribution of smooth muscle to connective tissue with a trend towards the reduced progression of bladder wall scarring.
Conclusion
Early SNM led to an avoidance of the emergence of DSD showing a more physiological bladder function during a 4 month follow‐up period after cSCI. This study might pave the way for the clinical continuation of early SNM for the treatment of neurogenic LUT dysfunction after SCI.
Keywords: minipigs, pudendal neuromodulation, regeneration, sacral neuromodulation, spinal cord injury, urinary bladder
1. INTRODUCTION
The normal function of the lower urinary tract (LUT) requires fine coordination between the urinary bladder and the urethral sphincter. It depends on the activation of sophisticated neuronal circuits involving peripherally and centrally located neurons. The considerable complexity of regulatory neuronal mechanisms renders LUT function sensitive to a variety of injuries and diseases, particularly those affecting the central nervous system,1 for example, a spinal cord injury (SCI). It leads to severe neurogenic LUT dysfunction (NLUTD). Emerging detrusor sphincter dyssynergia (DSD), simultaneous detrusor contraction and inhibition of sphincter relaxation, results in dangerously high spikes in bladder pressures and leads to renal impairments and upper urinary tract infections on the long term.2, 3 The current urological management of patients with SCI is complex and primarily includes clean intermittent catheterization along with pharmacological management which is symptomatic only. Sacral neuromodulation (SNM) and pudendal neuromodulation (PNM) are already well‐established long‐term therapeutic treatment options in nonneurogenic and some NLUTDs.4 A few clinical trials have evaluated SNM as a treatment for SCI with controversial outcomes.5 One trial highlighted that an early onset of SNM could have a more beneficial outcome on bladder function for patients with SCI2 compared to a late onset of SNM therapy. However, in this study, the patient number was low, so that no definitive conclusion on the success rate of early SNM after complete SCI could be determined. Other studies showed beneficial influences of PNM after SCI in rats and dogs.6, 7 Thus, the application of SNM or PNM for neurogenic bladder management after complete SCI in patients needs further investigation with a carefully selected animal model to predict their therapeutic success.4 Based on its comparable anatomical, physiological and pharmacological characteristics including the LUT, the pig is a good large animal model for the preclinical evaluation of new therapeutic approaches if the field of neurourology.8, 9, 10 Pigs and minipigs have already been successfully used in SCI studies including those with long follow‐up periods.11, 12, 13 The aim of our study was to analyze the effects of early intervention of SNM and PNM in minipigs with complete SCI. We examined functional and structural outcomes of the LUT by urodynamic analyses and molecular biological techniques to determine neuromodulation effects after early stimulation onset.
2. MATERIALS AND METHODS
This study was carried out under the protocols approved by the Ethics and Deontology Committee for Research on Animals, Victor Babes University of Medicine, Timisoara, Romania and obeyed the Association for Assessment of Laboratory Animal Care guidelines for animal use.
Eleven female Göttingen Minipigs, 6 months of age (weight: 21‐26 kg), were included in this study. Upon arrival, all animals were randomly assigned to one of the following groups: SCI control (SCIC: n = 3), SCI‐SNM (SNM: n = 4) and SCI‐PNM (PNM: n = 4). Tissue from the urinary bladder and the spinal cord was obtained from six healthy female, age‐matched minipigs (Ellegaard Minipigs, Dalmose, Denmark) subjected to the group of healthy references (HLY: n = 6). All animals were housed in stable groups of two animals per pen. Animals were subjected to a routine 4‐weeks training period before surgery.
Using computed tomography (CT)‐fluoroscopy, tined leads were inserted bilaterally in the neuroforamina S3 next to the sacral nerve root under visual control, as described in the publication.14 The intraoperative functional test consisted of the observed motor response, due to the required deep anesthesia.
For PNM, a laparoscopic approach15 was chosen to implant plate electrodes (Specify, lead model 3998; Medtronic, Inc, Minneapolis) at the pudendal nerves. For this, a pocket was undermined in the pelvic sidewall muscles in close distance to the pudendal nerve. The modified plate electrode was steeped in the pocket and the pocket sealed with fibrin glue. After both, sacral nerve root S3 or pudendal nerve electrode implantation, electrodes were connected to an extension wire (Extension kit 37082; Medtronic, Inc) and to the implantable pacemaker (SNM: Interstim II; PNM: Prime Advanced, Medtronic, Inc), which was finally subcutaneously implanted in an abdominal fat pouch.
To check the electrode positions, CT scans were performed at weeks 1 and 16 after implantation. The animals obtained standard antibiotic and analgesic care for the first 5 days after surgery. One week after the implantation procedure, the minipigs were subjected to SCI surgery.
Eleven minipigs underwent a CT‐guided complete spinal cord compression, which is described in detail in publication.16 In short, a balloon catheter was inserted to the T11/T12 thoracic vertebra level by means of the Seldinger technique under CT‐guidance. The balloon was air‐filled to a final pressure of 2 atm and left in place for 30 minutes under visual control. Postoperative care contained standard antibiotics and adapted extended analgesic treatment for the first 7 days. Stimulation of SNM and PNM was started 1 week after SCI.
For the SNM group, a clear motoric threshold could be used, which contained contraction of the perianal muscles, tail rotation, and claw rotation. The finally used parameters were slightly under the motoric threshold with 10 Hz at a pulse width of 210 ms and voltage‐dependent on the minipigs functional responses between 0.1 and 1 V. For PNM, the stimulation parameters were identical to the SNM group, only the applied voltage was different and adjusted to the individual minipig responses between 0.5 and 2 V.
All groups were followed up for 16 weeks after SCI. Housing was adapted to the SCI minipigs needs regarding bedding, food, and water supply. Twenty‐four hours of monitoring and care were available during the whole duration. The general health state was monitored three times daily. During the follow‐up period, the urinary bladder was drained regularly at a 4‐hours interval by clean intermittent catheterization. On the basis of a bladder diary, single catheterization volumes, urine strip test results, and observations of atypical odor or color of urine were noted.
A standardized neurological follow‐up was done at 1 and 16 weeks after SCI, comprising measurement of motor function, sensory function, and muscle hypertonia.17, 18
One week after the SCI procedure, a first awake urodynamic analysis (Solar Blue, MMS) was started and continued as a weekly examination until the end of the follow‐up period at 16 weeks. For this purpose, the minipigs were placed in a customized hammock. The urodynamic two‐lumen cystometry catheter with pigtail (8 CHR) was inserted into the bladder, while adhesive electrodes were placed on the perianal skeletal muscle and on a nonresponsive hindlimb skeletal muscle (null electrode). After the short acclimatization of the hammock, the bladder was filled with sterile prewarmed sodium chloride solution at a filling speed of 20 mL/min. At leakage, the filling was stopped and recording continued for further monitoring of bladder activity. If no leakage was seen, the filling was stopped at a maximal infused volume of 400 mL. Recording parameters were the intravesical pressure (in cmH2O) and electromyography activity of the perianal skeletal muscle. Abdominal pressure was not considered due to the minipig's specific anatomical entity. One follow‐up urodynamic unit included two consecutive urodynamics, with complete bladder drainage in between the procedures including a resting period for at least 15 minutes and also drainage at the end. Urodynamic analyses were examined in a blinded manner. Finally, the urodynamic findings of the acute state at 1 month post‐SCI were compared with the final state at 4 months post‐SCI.
Animals were killed at 16 weeks follow‐up time by an overdose intracardial barbiturate injection in deep general anesthesia. Sampling procedure and histological analyses have been described in detail in a previous publication.18 Data are presented as the means ± standard deviations. Due to the investigative character of the study, only a small number of animals were included and thus, data were only descriptively analyzed.
3. RESULTS
3.1. General postsurgical observations
None of the SCI minipigs died during the surgical procedures, nor during the follow‐up period after SCI. No severe infections or injuries were observed. All minipigs showed stable severe neurological deficits (grade 0) and a baseline increase in muscle tone (ie, spastic hypertonia) over the follow‐up time. Spastic tail movements were observed starting 4 weeks after SCI. The front‐limb sensation was normal, while hind‐limb sensation was and remained absent during the whole follow‐up period in all SCI minipigs. Magnetic resonance imaging images of the SCI spine and histological examination of the spinal cord tissue confirmed the completeness of SCI. 16
All SCI minipigs tolerated clean intermittent catheterization well. No urethral lesions, bleedings, or procedural difficulties occurred. Urine strip ruled out urinary tract infections. Events of intermittent isolated leukocyturia were observed occasionally but were not confirmed by repeated tests.
3.2. SNM improves bladder function after SCI
Urodynamics of healthy awake reference minipigs were not conducted due to difficulties of catheterization of awake healthy minipigs. After short sedation and catheterization and fixation in the hammock (propofol or xylazine intramuscular), urodynamic analysis in healthy minipigs gave poor information probably due to the influence of the sedative agents. Chronic implantation of an intravesical catheter (suprapubic) in healthy minipigs was excluded due to the regulatory necessity of minipig group housing during the follow‐up period and the most certain loss of the catheter by biting or movements of the minipigs.
To obtain further information about the healthy minipig bladder function, noninvasive measurements were carried out, like capacity measuring by diaper weight. The average bladder capacity of a healthy minipig was about 360 mL depending on daytime and drinking habits, but not exceeding 400 mL.
Blinded analysis of the urodynamic parameters of the SCI animals (SCI control, SNM, and PNM) revealed three different groups (Figure 1 and Table 1). Three minipigs (2 PNM and 1 SCI control; group 1) developed signs of DSD over the follow‐up period with a reduction in capacity of more than 100 mL, an increase in maximum voiding pressure of more than 10 cmH 2 O on average, moderate to high‐leak point pressures and an increase in leakage episodes (Figure 1 A). Three minipigs (2 PNM and 1 SCI control; group 2) showed constant retention of the bladder. Complete retention was defined with capacities around 300 mL and an inability to void in more than 75% of all urodynamics (Figure 1 B). Five minipigs (4 SNM and 1 SCI control; group 3) showed improvements in bladder function with bladder capacities varying above 150 mL, reduction of maximum voiding pressures and leak point pressures and increasing abilities to void compared with the first 4 weeks after SCI (Figure 1 C).
Figure 1.
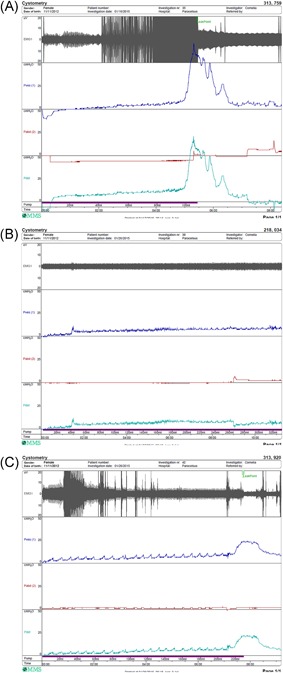
Urodynamic recordings at 4 months of follow‐up after spinal cord injury of one selected representative minipig per group. A, PNM minipig with detrusor sphincter dyssynergia showing high intravesical pressure (blue) and high urethral EMG activity (gray) during prolonged voiding at low bladder filling of only 110 mL. B, SCIC minipigs with permanent urinary bladder retention with continuous low intravesical pressure (blue) and low urethral EMG activity (gray) up to bladder filling of 320 mL. C, SNM minipig with improved voiding and normal bladder capacities (220 mL) with a normal intravesical pressure profile (blue) and regular reduced EMG activity (gray) during voiding. SCIC, SCI control group; EMG, electromyography; PNM, pudendal neuromodulation; SNM, sacral neuromodulation
Table 1.
Urodynamic data from all minipigs of the three distinct groups early (1 mo after SCI) and late (4 mo after SCI)
| Early (1 mo after SCI) | Late (4 mo after SCI) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID (minipig) | Capacity, mL | Compliance, cmH2O/mL | NDO (%) | Retention (%) | Leakage (%) | LPP, cmH2O | Voiding (%) | MVP, cmH2O | Capacity, mL | Compliance, cmH2O/mL | NDO (%) | Retention (%) | Leakage (%) | LPP, cmH20 | Voiding (%) | MVP, cmH2O | |
| SNM | 936 | 244 (±40) | 3 (±3.6) | 33 | 10.0 | 80.0 | 8 (±3.1) | 11% | 40 (±0) | 258 (±62) | 2 (±2.1) | 50.0 | 11 | 38 | 5 (±3.2) | 50.0 | 36 (±2.9) |
| 920 | 189 (±32) | 9 (±5.1) | 29 | 0 | 57 | 18 (±8.2) | 43% | 25 (±3) | 189 (±30) | 7 (±3.0) | 38 | 0 | 0 | 0 | 100 | 25 (±4) | |
| 730 | 212 (±45) | 8 (±4) | 40.0 | 0 | 30.0 | 21 (±6.7) | 50.0% | 34 (±9.3) | 166 (±21) | 9 (±5) | 43 | 0 | 43 | 17 (±5.7) | 57 | 26 (±9.2) | |
| 690 | 158 (±80) | 6 (±6.5) | 50.0 | 0 | 75 | 25 (±18.2) | 25% | 35 (±2.5) | 281 (±39) | 5 (±2.7) | 0 | 75 | 0 | 0 | 75 | 23 (±4) | |
| PNM | 765 | 241 (±90) | 7 (±4.5) | 57 | 28 | 28 | 14 (±2.1) | 43% | 30 (±3.9) | 146 (±95) | 12 (±8.2) | 62 | 0 | 50.0 | 9 (±3.5) | 50.0 | 26 (±4.3) |
| 639 | 329 (±64) | 6 (±4.2) | 0 | 100 | 0 | 0 | 0 | 0 | 299 (±16) | 5 (±1.8) | 25 | 75 | 0 | 0 | 25 | 24 (±2) | |
| 658 | 304 (±9) | 5 (±2.7) | 0 | 100 | 0 | 0 | 0 | 0 | 286 (±13) | 6 (±1) | 20.0 | 60.0 | 20.0 | 0 | 20.0 | 12 (±0) | |
| 759 | 217 (±95) | 14 (±5.1) | 57 | 0 | 29 | 30 (±2.5) | 71% | 41 (±7.5) | 91 (±29) | 24 (±23.8) | 88 | 0 | 25 | 13 (±0) | 75 | 52 (±13.7) | |
| Control | 34 | 268 (±108) | 6 (±4.3) | 14 | 71 | 0 | 29% | 29 (±1.7) | 309 (±14) | 4 (±4.5) | 63 | 100 | 0 | 0 | 0 | 0 | |
| 944 | 241 (±106) | 16 (±9.2) | 50.0 | 17 | 33 | 29 (±8) | 33% | 24 (±1.5) | 112 (±41) | 9 (±9.8) | 38 | 0 | 38 | 6 (±4) | 63 | 25 (±17) | |
| 751 | 306 (±50) | 9 (±6.4) | 57 | 57 | 43 | 14 (±4.3) | 0% | NA | 260 (±257) | 7 (±4) | 40.0 | 20.0 | 0 | 0 | 70.0 | 29 (±8) | |
Abbreviations: ID, minipigs identification number; LPP, leak point pressure; MVP, maximum voiding pressure; NDO, neurogenic detrusor overactivity; PNM, pudendal neuromodulation; SCI, spinal cord injury; SNM, sacral neuromodulation.
3.3. SNM reduces bladder wall scarring
All histological findings were only analyzed by descriptive means only due to the low number of animals per group and the risk of an overestimation of statistical results. It was shown that all SCI minipigs, regardless of treatment, had strong increases in bladder wall thickness (HLY: 0.42 ± 0.08 cm; SCI control: 0.77 ± 0.10 cm; SNM: 0.80 ± 0.18 cm; PNM: 0.81 ± 0.17 cm) compared with the healthy references. Furthermore, the urinary bladder underwent changes in its bladder wall composition in comparison to healthy references. However, the SNM group showed better preserved smooth muscle tissue (Figure 2 ). In detail, when quantifying single bladder wall tissues, the connective tissue amount of all SCI minipigs was slightly increased in comparison to the healthy references (HLY: 44.8% ± 1.2%; SCI control: 62.9% ± 15.7%; SNM: 58.1% ± 13.4%; PNM: 58.2% ± 8.3%;Figure 3 A). From all SCI groups, SNM minipigs showed the lowest increase in connective tissue compared with the PNM and SCI control group. In addition, SNM minipigs showed decreased smooth muscle contents as all SCI groups did, but the remaining amount of smooth muscle tissue was closer to the healthy references compared with the SCI control and PNM groups (HLY: 44.7% ± 1.1%; SCI control: 27.6% ± 7.9%; SNM: 33.8% ± 11.5%; PNM: 30.4% ± 8.6%;Figure 3 B).
Figure 2.
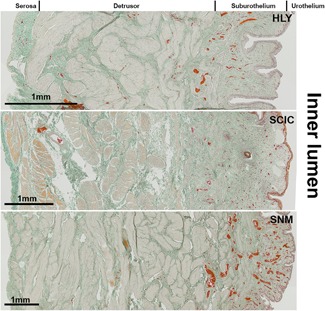
Masson‐Goldner staining of the mid‐region of the urinary bladder wall of healthy reference (HLY) and SCI minipigs. The smooth muscle tissue is shown in rose and the connective tissue in green. Erythrocytes in the lumen of blood vessels are stained in red. Scale bar = 1 mm. SCI, spinal cord injury; SNM, sacral neuromodulation
Figure 3.
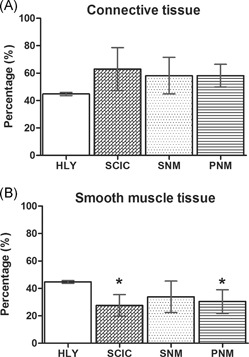
Quantitative results of muscle and connective tissue in the urinary bladder wall. A, Quantity of connective tissue in the urinary bladder wall of healthy reference (HLY) and SCI minipigs. B, Quantity of smooth muscle tissue in the urinary bladder wall of healthy reference (HLY) and SCI minipigs. SCIC: SCI control group; SNM: SCI‐SNM minipig group; PNM: SCI‐PNM minipig group. Values are shown as mean ± standard deviation. Y‐axis: percentage (%). PNM, pudendal neuromodulation; SCI, spinal cord injury; SCIC, spinal cord injury centers; SNM, sacral neuromodulation
When distinguishing between collagenous and noncollagenous connective tissue, the collagenous connective tissue amount did not differ between SCI minipigs and healthy references (HLY: 38.9% ± 4.1%; SCI control: 39.8% ± 6.3%; SNM: 41.9% ± 12.9%; PNM: 40.1% ± 7.7%;Figure 4 A). After SCI, the amount of collagen I was strongly increased in all SCI groups in comparison to the healthy references (HLY: 10.0% ± 2.7%; SCI control: 23.9% ± 3.6%; SNM: 26.3% ± 8.0%; PNM: 21.9% ± 2.0%;Figure 4 B). On the contrary, the amount of collagen III was strongly decreased in all SCI groups in comparison to the healthy references (HLY: 28.6% ± 4.1%; SCI control: 15.9% ± 3.7%; SNM: 15.6% ± 8.6%; PNM: 18.2% ± 6.8%;Figure 4 C). When analyzing the two main collagens, type I and III, a shift from collagen type III towards collagen type I was observed after SCI regardless of SCI treatment.
Figure 4.
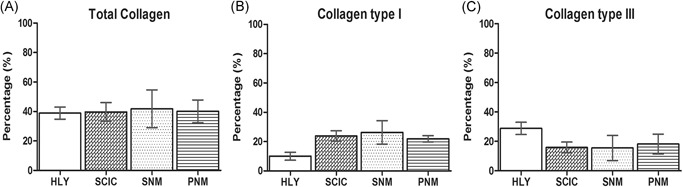
Collagen analyses of the urinary bladder of healthy references (HLY) and SCI minipigs. A, Total collagen amount. B, Collagen I quantity. C, Collagen III quantity. SCIC: SCI control group; SNM: SCI‐SNM minipig group; PNM: SCI‐PNM minipig group. Values are shown as mean ± standard deviation. Y‐axis: percentage (%). PNM, pudendal neuromodulation; SCI, spinal cord injury; SCIC, spinal cord injury centers; SNM, sacral neuromodulation
4. DISCUSSION
SCI is a life‐changing condition for patients with major consequences and a loss of quality of life. The LUT is one of the main regions affected by SCI causing NLUTD. 19 Up to date, the therapeutic modalities for SCI‐associated NLUTD are only symptomatic.
The main finding of our study was the positive effect of early SNM, primarily on LUT function and partially on the urinary bladder structure in minipigs. In the SNM treatment group, no DSD development was observed by urodynamic analysis. The effect of SNM was mainly seen in an amelioration of the intravesical pressures with improved voiding and more physiological bladder capacities. The structural bladder tissue organization of the SNM minipigs partially correlated with the observed functional improvements as these minipigs showed a higher amount of smooth muscle tissue and less connective tissue increase after SCI. Yet, the structural results were less clear than the urodynamic analysis. One clinical study demonstrated that early SNM (onset during the spinal shock phase with prevalent bladder atony) prevented detrusor overactivity and urinary incontinence in patients with complete SCI. The results of our large animal trial support the findings of Sievert et al2 concerning the positive effects of early SNM on bladder function in complete SCI. More recently, another clinical trial reported on SNM success in 94% of patients with NLUTD.5
Minipigs as animal models have proven good translational results with respect to the urinary bladder. The anatomical similarities of the LUT between (mini)pigs and humans have been described in several studies.20, 21 In a previous workup, the pathophysiological changes of the porcine urinary bladder were analyzed after SCI. Furthermore, we developed adjusted care protocols for complete SCI minipigs to enable a safe and reproducible follow‐up.18 In this observation, urinary tract infections were no relevant influencing factor, neither under clinical aspects nor in the dipsticks. Therefore, regular clean intermittent catheterization is an integral element of the SCI minipig care.
After SCI, we observed two patterns of urinary bladder dysfunction: (a) persisting urinary retention and (b) urinary retention followed by the emergence of DSD. The pattern “persisting urinary retention” is described as high bladder capacities at low intravesical pressures, no voiding and no urine leakage without considerable pattern changes during the whole follow‐up. This pattern corresponds well to urinary retention in patients with SCI during the spinal shock phase, which typically lasts between 6 to approximately 12 weeks after SCI.22 The second pattern “urinary retention followed by emergence of DSD” includes the switch from urinary retention towards small capacity bladders with increased maximum detrusor pressures as well as prolonged voiding phases at continuous high detrusor pressures. The DSD pattern usually follows the initial urinary retention phase and worsens during follow‐up. DSD onset and pattern outcomes are comparable between minipigs and humans.22 Regardless of groups, all minipigs showed initial urinary retention, which correlates to the spinal shock phase in humans. Later on, only the SCI control and PNM minipigs showed either persisting urinary retention or the emergence of DSD.
From a structural perspective, a trend towards a fibrotic, smooth muscle reduced urinary bladder wall can be observed after chronic complete SCI in minipigs. To our knowledge, this is the first description of functional and structural changes of the urinary bladder of minipigs with complete SCI.
The minipig sensory and functional responses to SNM correspond to those observed in patients.23 An examination of the electrode location post‐mortem showed electrode encapsulation with varying distances between electrodes and pudendal nerves. Yet, stimuli might have reached the pudendal nerves at varying intensities over time. In our PNM minipig collective, we were not able to detect a modulation effect of PNM on urinary bladder function or structure as described in the literature in humans and dogs.7, 24, 25 PNM nonresponsiveness in our SCI minipigs could have resulted either through (a) difficulties in good electrode fixation techniques, (b) permanent overstimulation with high intensities of the pudendal nerves, (c) encapsulation of the electrodes, or (d) general PNM failure after complete SCI in minipigs. Therefore, the outcomes of early PNM treatment after SCI need to be further investigated.
Limitations of our study were the variations in the functional outcomes between minipigs in the SCI control and PNM group primarily resulting from the low number of animals per group. Furthermore, a longer follow‐up duration would have been ideal to monitor the minipigs with persisting urinary retention to observe, if these animals develop a DSD at a later time point or remain with urinary retention. Discrepancies of the patterns of NLUTD based on varying lesion outcomes (lesion size and level of injury) between animals were excluded based on a thorough examination of cross‐sections of the individual spinal cords based on histological and immunohistochemical analyses (data not shown). However, this is the first known study of urodynamic analysis in SCI minipigs and small collectives were chosen to initially describe the functional and structural changes in the urinary bladder with a moderate follow‐up period. Further studies should include more animals per group with longer follow‐up duration and if possible increase the examined urodynamic parameters to further strengthen the functional outcomes.
In conclusion, this study represents the experimental foundation of an effect of early SNM for NLUTD in SCI minipigs, which might pave the way for future clinical trials with early SNM intervention for patients with complete SCI, which are not considered until today. Furthermore, the minipig has proven its value as an excellent translational animal model to study SCI‐associated NLUTD. On the basis of the course of NLUTD pathophysiology in minipigs, interventions and treatment modalities can be studied in a close‐to human situation narrowing the gap between animal experimentation and clinical application.
ACKNOWLEDGMENTS
The authors wish to thank Professor Sébastien Couillard‐Després (Department of Experimental Neuroregeneration, Spinal Cord Injury and Tissue Regeneration Center Salzburg, Austria) for his valuable advice and the Spinal Cord Injury and Tissue Regeneration Center Salzburg Core Facilities Imaging and Histology for excellent technical support. We also thank Adrian Zeltner from Ellegaard Göttingen Minipigs for his aid. We are grateful to the Pius Branzeu Center for Flap Surgery and Microsurgery and Bogdan Hoinoiu, PhD, for his advice. Finally, we would like to express our appreciation to the team of the experimental unit of the Horia Cernescu Research Unit, established by infrastructure project—SMIS 2669, of the Banat University of Agriculture and Veterinary Sciences, Timisoara, Romania, for their excellent care of the minipigs.
Keller EE, Patras I, Hutu I, et al. Early sacral neuromodulation ameliorates urinary bladder function and structure in complete spinal cord injury minipigs. Neurourology and Urodynamics. 2020;39:586–593. 10.1002/nau.24257
Reinhold Zimmermann & Sophina Bauer: shared last authors.
REFERENCES
- 1. Cruz CD, Cruz F. Spinal cord injury and bladder dysfunction: new ideas about an old problem. ScientificWorldJournal. 2011;11:214‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sievert KD, Amend B, Gakis G, et al. Early sacral neuromodulation prevents urinary incontinence after complete spinal cord injury. Ann Neurol. 2010;67(1):74‐84. [DOI] [PubMed] [Google Scholar]
- 3. Schneider MP, Hughes FM Jr, Engmann AK, et al. A novel urodynamic model for lower urinary tract assessment in awake rats. BJU Int. 2015;115(suppl 6):8‐15. [DOI] [PubMed] [Google Scholar]
- 4. Kessler TM, La Framboise D, Trelle S, et al. Sacral neuromodulation for neurogenic lower urinary tract dysfunction: systematic review and meta‐analysis. Eur Urol. 2010;58(6):865‐874. [DOI] [PubMed] [Google Scholar]
- 5. Wollner J, Krebs J, Pannek J. Sacral neuromodulation in patients with neurogenic lower urinary tract dysfunction. Spinal Cord. 2016;54(2):137‐140. [DOI] [PubMed] [Google Scholar]
- 6. Lin YT, Hsieh TH, Chen SC, et al. Effects of pudendal neuromodulation on bladder function in chronic spinal cord‐injured rats. J Formos Med Assoc. 2015;115:703‐713. [DOI] [PubMed] [Google Scholar]
- 7. Chen G, Liao L, Dong Q, Ju Y. The inhibitory effects of pudendal nerve stimulation on bladder overactivity in spinal cord injury dogs: is early stimulation necessary? Neuromodulation. 2012;15(3):232‐237. [DOI] [PubMed] [Google Scholar]
- 8. Mills IW, Drake MJ, Greenland JE, Noble JG, Brading AF. The contribution of cholinergic detrusor excitation in a pig model of bladder hypocompliance. BJU Int. 2000;86(4):538‐543. [DOI] [PubMed] [Google Scholar]
- 9. Dalmose AL, Bjarkam CR, Djurhuus JC. Stereotactic electrical stimulation of the pontine micturition centre in the pig. BJU Int. 2005;95(6):886‐889. [DOI] [PubMed] [Google Scholar]
- 10. Jensen KN, Deding D, Sorensen JC, Bjarkam CR. Long‐term implantation of deep brain stimulation electrodes in the pontine micturition centre of the Gottingen minipig. Acta Neurochir. 2009;151(7):785‐794. [DOI] [PubMed] [Google Scholar]
- 11. Zurita M, Aguayo C, Bonilla C, et al. The pig model of chronic paraplegia: a challenge for experimental studies in spinal cord injury. Prog Neurobiol. 2012;97(3):288‐303. [DOI] [PubMed] [Google Scholar]
- 12. Jones CF, Lee JH, Kwon BK, Cripton PA. Development of a large‐animal model to measure dynamic cerebrospinal fluid pressure during spinal cord injury: laboratory investigation. J Neurosurg Spine. 2012;16(6):624‐635. [DOI] [PubMed] [Google Scholar]
- 13. Lee JHT, Jones CF, Okon EB, et al. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013;30(3):142‐159. [DOI] [PubMed] [Google Scholar]
- 14. Foditsch EE, Zimmermann R. Development of a CT‐guided standard approach for tined lead implantation at the sacral nerve root S3 in minipigs for chronic neuromodulation. Res Rep Urol. 2016;8:169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foditsch EE, Hoinoiu B, Janetschek G, Zimmermann RP. Laparoscopic placement of a tined lead electrode on the pudendal nerve with urodynamic monitoring of bladder function during electrical stimulation: an acute experimental study in healthy female pigs. SpringerPlus. 2014;3:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foditsch EE, Miclaus G, Patras I, et al. A new technique for minimal invasive complete spinal cord injury in minipigs. Acta Neurochir. 2018;160:459‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navarro R, Juhas S, Keshavarzi S, et al. Chronic spinal compression model in minipigs: a systematic behavioral, qualitative, and quantitative neuropathological study. J Neurotrauma. 2012;29(3):499‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foditsch EE, Roider K, Patras I, et al. Structural changes of the urinary bladder after chronic complete spinal cord injury in minipigs. Int Neurourol J. 2017;21(1):12‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adriaansen JJE, van Asbeck FWA, Tepper M, et al. Bladder‐emptying methods, neurogenic lower urinary tract dysfunction and impact on quality of life in people with long‐term spinal cord injury. J Spinal Cord Med. 2017;40(1):43‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bossowska A, Crayton R, Radziszewski P, Kmiec Z, Majewski M. Distribution and neurochemical characterization of sensory dorsal root ganglia neurons supplying porcine urinary bladder. J Physiol Pharmacol. 2009;60(suppl 4):77‐81. [PubMed] [Google Scholar]
- 21. Dalmose AL, Bjarkam CR, Sorensen JC, Djurhuus JC, Jorgensen TM. Effects of high frequency deep brain stimulation on urine storage and voiding function in conscious minipigs. Neurourol Urodyn. 2004;23(3):265‐272. [DOI] [PubMed] [Google Scholar]
- 22. Goldmark E, Niver B, Ginsberg DA. Neurogenic bladder: from diagnosis to management. Curr Urol Rep. 2014;15(10):448. [DOI] [PubMed] [Google Scholar]
- 23. Spinelli M, Sievert KD. Latest technologic and surgical developments in using InterStim Therapy for sacral neuromodulation: impact on treatment success and safety. Eur Urol. 2008;54(6):1287‐1296. [DOI] [PubMed] [Google Scholar]
- 24. Li P, Liao L, Chen G, Zhang F, Tian Y. Early low‐frequency stimulation of the pudendal nerve can inhibit detrusor overactivity and delay progress of bladder fibrosis in dogs with spinal cord injuries. Spinal Cord. 2013;51(9):668‐672. [DOI] [PubMed] [Google Scholar]
- 25. Kennelly MJ, Bennett ME, Grill WM, Grill JH, Boggs JW. Electrical stimulation of the urethra evokes bladder contractions and emptying in spinal cord injury men: case studies. J Spinal Cord Med. 2011;34(3):315‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]


