Abstract
The 20% prevalence of chronic pain in the general population is a major health concern given the often profound associated impairment of daily activities, employment status, and health-related quality of life in sufferers. Resource utilization associated with chronic pain represents an enormous burden for healthcare systems. Although analgesia based on the World Health Organization’s pain ladder continues to be the mainstay of chronic pain management, aside from chronic cancer pain or end-of-life care, prolonged use of non-steroidal anti-inflammatory drugs or opioids to manage chronic pain is rarely sustainable. As the endocannabinoid system is known to control pain at peripheral, spinal, and supraspinal levels, interest in medical use of cannabis is growing. A proprietary blend of cannabis plant extracts containing delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) as the principal cannabinoids is formulated as an oromucosal spray (USAN name: nabiximols) and standardized to ensure quality, consistency and stability. This review examines evidence for THC:CBD oromucosal spray (nabiximols) in the management of chronic pain conditions. Cumulative evidence from clinical trials and an exploratory analysis of the German Pain e-Registry suggests that add-on THC:CBD oromucosal spray (nabiximols) may have a role in managing chronic neuropathic pain, although further precise clinical trials are required to draw definitive conclusions.
Keywords: THC:CBD oromucosal spray, chronic pain, neuropathic pain, nabiximols
Introduction
Chronic pain is defined as persistent or recurring pain lasting longer than 3 months that is characterized by persistent physical pain, disability, emotional disturbance, and social withdrawal.1 The eleventh International Classification of Diseases (ICD-11) divides chronic pain into seven categories: primary, cancer, post-traumatic and post-surgical, neuropathic, headache and orofacial, visceral, and musculoskeletal.2 The most common conditions associated with chronic pain are back pain, joint pain due to osteoarthrosis, osteoporosis or rheumatic diseases, and cancer.3–5 Neuropathic pain is defined as pain due to a primary lesion or dysfunction in the nervous system.6,7
Although estimates for chronic pain prevalence vary according to its definition, the population analyzed, and the method used to collect data, the burden is sizeable, affecting more than 20% of people globally irrespective of world region.8–14
By interfering with daily activities, employment status and the ability to work effectively, chronic pain can profoundly impair the general well-being of sufferers. Chronic pain is frequently accompanied by depression, anxiety and sleeping difficulties which combine to further reduce health-related quality of life (HR-QoL). The cumulative burden of chronic pain becomes more pronounced with increasing severity.8,15,16 Studies investigating the burden of chronic neuropathic pain report similar findings in terms of high rates of comorbid depression, anxiety, and sleeping difficulties/insomnia, negative impacts on employment status and ability to work, and impaired HR-QoL.17–20
Chronic pain has a substantial economic cost largely due to increased health resource utilization. In 2010 in the United States (US), total annual costs attributable to chronic pain were estimated to be USD$560–635 billion.21 In Europe, the direct and indirect healthcare costs of chronic pain disorders are estimated to be 2–3% of GDP across member states,16 which amounted to about €450 billion in 2016.22
Management of Chronic Pain
Optimal management of chronic pain involves a combination of medical and psychosocial approaches aimed at shifting the focus of treatment from pain relief towards increased function and well-being despite pain.1 In actual practice, however, pharmacological therapies continue to be the mainstay of general management of chronic pain. Analgesics for use in chronic pain include paracetamol, nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids as per the WHO analgesic ladder, supplemented by adjuvant agents (eg muscle relaxants, anxiolytics, or hypnotics).23,24 With the possible exceptions of chronic cancer pain and end-of-life care, however, pharmacological analgesia is rarely a sustainable approach to chronic pain management due to safety limitations and abuse/misuse potential associated with prolonged use of these agents.1
A major contributing factor to the opioid epidemic in the US was increased opioid prescribing as part of a wider strategy to improve pain management, but ultimately leading to a dramatic increase in the number of prescription opioid overdose deaths.25 Epidemiological data suggest that opioid misuse is also a concern in Europe.26–28 In 2017, the six countries with the highest opioid consumption (reported as defined daily doses per million inhabitants per day) were, in order, the US, Germany, Canada, Austria, Belgium, and Switzerland.28
To control the opioid crisis, guidelines currently recommend against the use of opioids to manage chronic pain. In the US, the Centers for Disease Control (CDC) guidelines advocate non-pharmacological and non-opioid pharmacological therapies as the preferred choices to manage chronic pain.25,29 The CDC and American Society of Interventional Pain Physicians guidelines recommend that opioid therapy be considered for chronic pain only if the benefits in terms of pain relief and improved function are likely to outweigh risks to the patient.25,29,30 The European Pain Federation’s expert recommendations about safe and appropriate use of opioids in chronic pain management advise that opioid therapy be initiated only after failure of less potent analgesics and adjuvant therapies and/or rehabilitation to achieve and maintain adequate pain relief.31 In pain types with limited response to opioid medications (eg neuropathic pain), the European Federation of Neurological Societies guidelines recommend a diverse range of agents for first-, second- and third-line treatment (Table 1).32 These recommendations reflect the varying origins and difficult-to-control nature of chronic neuropathic pain, and also highlight the lack of effective new agents to manage neuropathic pain conditions.
Table 1.
European Federation of Neurological Societies Recommended Treatment for Common Neuropathic Pain Conditions
| Condition | First Line | Second or Third Line |
|---|---|---|
| Diabetic neuropathic pain | Duloxetine Gabapentin Pregabalin Tricyclic antidepressants Venlafaxine extended release |
Opioids Tramadol† |
| Post-herpetic neuralgia | Gabapentin Pregabalin Tricyclic antidepressants Lidocaine plasters§ |
Capsaicin Opioids |
| Classical trigeminal neuralgia | Carbamazepine Oxcarbazepine |
Surgery |
| Central pain | Gabapentin Pregabalin Tricyclic antidepressants |
Cannabinoids (multiple sclerosis) Lamotrigine Opioids Tramadol (spinal cord injury) |
Notes: Adapted with permission from Attal N, Cruccu G, Baron R, et al. European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88.32 †Tramadol may be considered first line in patients with acute exacerbations of pain especially in combination with acetaminophen. §Lidocaine is recommended in elderly patients.
Cannabinoids in Chronic Pain Management
Mechanism of Action of Cannabinoids
The discovery of the endocannabinoid system, and its role in pain control and habituation to stress, suggested that cannabinoids may be useful to manage pain conditions.33,34 Delta-9-tetrahydrocannabinol (THC), a partial agonist of cannabinoid type 1 (CB1) and type 2 (CB2) receptors, mimics the effects of endogenous cannabinoids.35–38 CB1 receptors, which mediate many of the psychoactive effects of cannabinoids, are commonly localized on preterminal axonal regions and axons in several brain regions, although they are also present in some peripheral tissues. CB2 receptors are found in a few neurons, but mainly in immune and hematopoietic cells.36,37,39,40 Cannabinoid receptors are members of the G protein-coupled, 7-transmembrane domain receptor superfamily. Endogenous ligands of these receptors, known as endocannabinoids, are the arachidonic acid derivatives N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol.40,41 Endocannabinoids act as neuromodulators,42,43 and are important signaling molecules in neuronal and glial development.44
THC-mediated activation of presynaptic CB1 receptors inhibits neurotransmitter release in excitatory glutamatergic and inhibitory γ-aminobutyric acid (GABA) neurons in pathways including the hippocampus following disruption of synaptic function.45,46 Cannabidiol (CBD) has no agonistic effects on CB1 and CB2 receptors,35 although in experimental conditions (concentrations <1 μM) it has shown non-competitive negative allosteric modulation of CB1 receptors.47 The effects of CBD are likely due to modulation of multiple non-endocannabinoid signaling systems.36,48,49 At micromolar/sub-micromolar concentrations, CBD blocks equilibrative nucleoside transporters that mediate the transport of nucleosides, nucleobases and therapeutic analogs;50 modulates the transient receptor potential of the melastatin type 8 ion channel which is the primary cold sensor in humans;51 and modulates the orphan G-protein-coupled receptor GPR55, a probable lysophosphatidylinositol receptor involved in multiple physiological and pathophysiological pathways.52 In addition, CBD enhances the activity of 5-HT1a receptors which are key mediators of anxiety and depression-like behaviors;53 enhances the activity of α1 and α3 glycine receptors whose inhibitory actions contribute to control of excitability within the central nervous system;54 and modulates the activity of transient receptor potential ankyrin type 1 channels which play a critical role in cortical spreading depression and are a target for pain management.55 CBD has bidirectional effects on intracellular calcium ions, and is a potent antioxidant.36,48,49
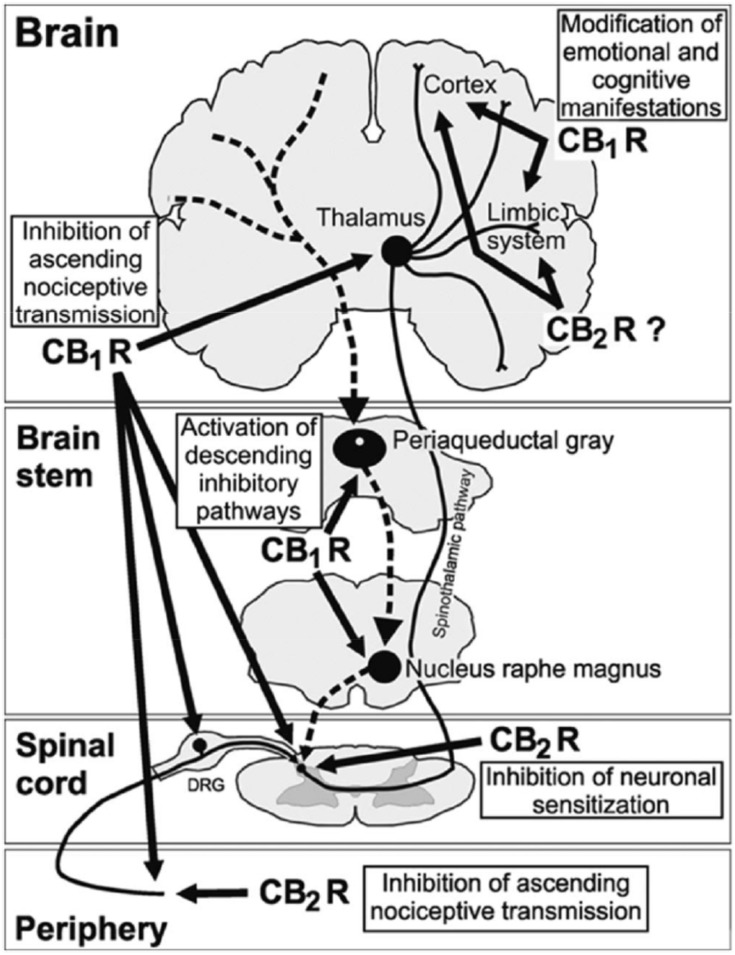
Activation of cannabinoid receptors by endogenous or extraneously administered cannabinoids has multiple analgesia-associated effects mediated by the peripheral and central nervous systems.40 These effects include inhibition of ascending nociceptive transmission, activation of the inhibitory descending pathway, and modification of the emotional component of pain. Activation of CB1 receptors localized in peripheral nociceptive terminals inhibits the activity of nociceptive neurons. At the spinal level, activation of CB1 receptors localized in dorsal root ganglia and in the spinal cord dorsal horn (in nociceptive and non-nociceptive sensitive terminals) inhibits neurotransmitter release and pain transmission. At the supraspinal level, activation of CB1 receptors localized mainly in the thalamus inhibits ascending nociceptive transmission and activates the descending inhibitory pathway by inhibition of GABA release. The emotional and cognitive effects of pain are modified by CB1 receptor activation acting in the limbic system and cortical areas of the brain. In addition, activation of peripheral CB2 receptors localized on immune cells and keratinocytes reduces the release of pronociceptive molecules. At the spinal cord level, CB2 receptor activation modulates immune responses, leading to inhibition of neuronal sensitization during chronic pain (Figure 1).
Figure 1.
Role of the endocannabinoid system in the control of pain at peripheral, spinal, and supraspinal levels. Cannabinoid receptor activity inhibits the ascending nociceptive transmission, activates the inhibitory descending pathway, and modifies the emotional component of pain. CB1R, Cannabinoid type 1 receptor; CB2R, Cannabinoid type 1 receptor. Reproduced with permission from Maldonado R, Baños JE, Cabañero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157(Suppl 1):S23–S32. Available from: https://insights.ovid.com/article/00006396-201602001-00005.40
In preclinical rodent models, activation of cannabinoid receptors reduced neuropathic and chronic inflammatory pain.35,40,48 Recently, in a well-established nerve injury model of neuropathic pain, treatment with CBD for 7 days normalized impaired serotonergic (5-HT) neurotransmission, reduced mechanical allodynia and decreased anxiety-like behavior.56 The findings are important since they show that the effects of CBD relate not only to a reduction in pain intensity and modification of pain quality, but especially to a change in anxiety-related behavior.
Cannabinoids for Pain Management
Use of herbal cannabis (smoked, vaporized, orally ingested), at present at least, is not suitable to manage pain or other medical conditions. Aside from practical issues such as restricted availability as regulated by national or regional health authorities, risks of herbal cannabis include variability in cannabinoid type and potency, potential for misuse/dependency, and possible future mental health-related consequences in young people.57
Numerous studies have investigated cannabis and its derivatives to treat myriad conditions, including chronic pain. As a discussion of the full range of cannabis and cannabis-based products is beyond the scope of this review, interested readers are directed to recent comprehensive reviews on the subject.58,59 Plant-derived (ie phytocannabinoids) and synthetic cannabinoids currently available for medicinal use are described briefly before the focus is turned specifically to THC:CBD oromucosal spray (nabiximols) for management of chronic pain conditions.
Sativex® (USAN name: nabiximols) oromucosal spray is a phytocannabinoid extract containing THC and CBD (1:1 ratio) as its principal components. The methodology for producing THC:CBD spray (nabiximols) is well described.60 The medicine is produced from two chemovars of the C. sativa plant with each clone producing a high level of THC or CBD61 and is standardized to ensure quality, consistency and stability.60 THC and CBD are thought to interact synergistically with other trace cannabinoids to provide activity greater than that of the individual components.62 CBD has a dual mechanism; it potentiates the depressant effects of THC while inhibiting its excitatory and emotional effects.62 The oromucosal route of administration avoids the high THC plasma levels that occur after inhaling herbal cannabis and are responsible for the psychoactivity.63–65 Other negative associations with smoking cannabis such as an increased risk of lung cancer66 are also avoided by oromucosal delivery. THC:CBD oromucosal spray (nabiximols) is approved in several countries as an add-on treatment for moderate to severe resistant multiple sclerosis (MS) spasticity.38
Other phytocannabinoids containing multiple different cannabinoids (eg Bedrocan®) have yet to undergo clinical trial development or receive approval for a first official indication. A plant-derived medicine containing single purified CBD (cannabidiol oral solution, Epidiolex®) was recently approved by the US Food and Drug Administration and European Medicines Agency for treatment of the rare epileptic syndromes, Lennox–Gastaut and Dravet.67,68 Dronabinol (Marinol®, Syndros®) is a synthetic THC indicated for treatment of anorexia associated with weight loss in patients with AIDS, and treatment of severe refractory nausea and vomiting associated with cancer chemotherapy.69,70 Nabilone (Cesamet®, Canemes®) is a THC analog indicated for treatment of severe refractory nausea and vomiting associated with cancer chemotherapy.71
The European Pain Federation has published a position paper about the appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Key points are that: 1) current evidence is insufficient to state whether cannabis-based medicines and medical cannabis differ in their efficacy, tolerability and safety; 2) cannabis-based medicines can be considered as third-line therapy for chronic neuropathic pain; 3) THC:CBD oromucosal spray (nabiximols) can be considered as part of an add-on individual therapeutic trial for individuals with cancer pain who have inadequate pain relief from opioids or other established analgesics,72 although evidence for this latter recommendation is limited.
Search Strategy and Study Identification
To identify clinical trials of THC:CBD oromucosal spray (nabiximols) in chronic pain, searches were conducted of PubMed, Cochrane Library, and ClinicalTrials.gov using the search terms: Sativex, delta-9-tetrahydrocannabinol, cannabidiol, THC:CBD, nabiximols, medical cannabis, chronic pain, pain, and neuropathic pain. There were no language restrictions. In the case of PubMed searches, the “clinical trial” filter was applied. Reference lists of retrieved papers were hand-searched for additional clinical studies. Other references are known to the author or are part of his collection.
THC:CBD Oromucosal Spray (Nabiximols) in Pain Management
Treatment of Multiple Sclerosis Spasticity
In an early randomized controlled trial (RCT) in patients with MS, THC:CBD oromucosal spray (nabiximols) for 6 weeks significantly improved MS spasticity (muscle rigidity with [often painful] spasms) versus placebo as assessed by mean Visual Analogue Scale (VAS) scores.73 In three pivotal RCTs of patients with refractory MS spasticity (treatment duration 6 to 14 weeks), THC:CBD oromucosal spray (nabiximols) significantly improved mean spasticity 0–10 numerical rating scale (NRS) scores and mean pain 0–10 NRS scores compared with placebo.74–76 The post-approval SAVANT (Sativex as Add-on therapy vs further optimized first-line ANTispastics) trial showed that 12 weeks’ add-on treatment with THC:CBD oromucosal spray (nabiximols) produced clinically relevant (≥30% NRS reduction from baseline) improvement of resistant MS spasticity and associated pain than that achieved by adjusting underlying first-line antispasticity medication alone.77
During clinical development of THC:CBD oromucosal spray (nabiximols) for MS spasticity, the most common treatment-related adverse events were mild to moderate transient episodes of dizziness, fatigue or somnolence.38,78 Less commonly, mucosal irritation was reported.38 To date, THC:CBD oromucosal spray (nabiximols) has not been associated with drug tolerance or a withdrawal syndrome79 and there has been no evidence of drug misuse or abuse.38 The estimated total post-marketing exposure of THC:CBD spray (nabiximols) at the end of 2018 was more than 120,000 patient-years.80
European consensus guidelines on pharmacological management of MS spasticity recommend adding THC:CBD oromucosal spray (nabiximols) to current therapy in patients with moderate to severe spasticity who have a suboptimal response or poor tolerance to first-line oral treatments.81
Treatment of Chronic Cancer-Related Pain
Chronic cancer pain is classified pathophysiologically as nociceptive, neuropathic or mixed nociceptive/neuropathic,82 in alignment with the general classification of chronic pain.
The results of placebo-controlled clinical trials of THC:CBD oromucosal spray (nabiximols) in chronic cancer-related pain have been variable. Efficacy was demonstrated in two studies. In a clinical trial involving patients (n = 177) with intractable cancer-related pain, mean pain 0–10 NRS scores were significantly improved after 2 weeks’ treatment with active medication compared with placebo (change from baseline: –1.37 vs –0.69; p = 0.014). About twice as many patients treated with THC:CBD (nabiximols) than placebo (43% vs 21%) achieved a clinically meaningful ≥30% reduction from baseline in pain 0–10 NRS scores. Treatment-related adverse events were mostly mild or moderate and similar to the known safety profile of THC:CBD spray (nabiximols) in MS spasticity, namely somnolence, dizziness and nausea.83 A subsequent open-label extension study involving 39 patients who had received THC:CBD spray in the parent study showed that extended use (median 25 days, range 2–579 days) was generally well tolerated. Patients did not seek to increase their dose of THC:CBD spray (nabiximols) or other pain-relieving medication during extended use. The mean dose during the last 7 days of dosing (5.4 sprays/day) was lower than the mean dose (8.75 sprays/day) in the first weeks of the parent study.84 In another placebo-controlled trial of THC:CBD oromucosal spray (nabiximols) in patients with opioid-refractory cancer pain (n = 360), the primary outcome (30% responder rate) after 5 weeks’ treatment was not met (p = 0.59), although patient-reported analgesia rates were significantly higher with active medication than placebo (p = 0.035). A dose-grading analysis indicated that analgesia rates were significantly higher with low-dose (1–4 sprays/day; p = 0.008) and medium-dose (6–10 sprays/day; p = 0.039) THC:CBD spray (nabiximols) than placebo. Adverse events compared unfavorably with placebo only in the high-dose group.85
In a third large placebo-controlled clinical trial in advanced cancer patients with chronic uncontrolled pain (n = 397), the change from baseline in the mean pain 0–10 NRS score at the end of treatment (2-week titration, 3-week treatment) favored THC:CBD oromucosal spray (nabiximols) but without reaching statistical significance (10.7% vs 4.5%; p = 0.0854). The benefits of THC:CBD spray (nabiximols) on multiple secondary endpoints were observed mainly in US patients, possibly due to lower baseline opioid doses compared with European patients or due to differences in the distribution of cancer pain types between US and Europe patient populations. The safety profile of THC:CBD spray (nabiximols) was consistent with that reported in earlier studies.86
Treatment of Chronic Neuropathic Pain
Randomized clinical trials of THC:CBD oromucosal spray (nabiximols) for the treatment of chronic neuropathic pain conditions are summarized in Table 2.87–90,92-98
Table 2.
Randomized Clinical Trials and Extension Studies of THC:CBD Oromucosal Spray (Nabiximols) in Neuropathic Pain
| Reference | Neuropathic Pain Study | Randomized/Entered Extension Study, n | Completed, n | Treatment Duration | Change in Pain 0–10 NRS for THC:CBD Oromucosal Spray (Nabiximols) vs Placebo | p-value |
|---|---|---|---|---|---|---|
| [87] | Multiple sclerosis | 66 | 64 | 4 weeks | –2.7 vs –1.4 (Δ 1.3) | 0.005 |
| [88] | Multiple sclerosis (open-label extension of87 | 63 | 34 (1 year) 28 (2 years) |
2 years | –2.9† | N/A |
| [89] | Multiple sclerosis | 339 | 297 | 14 weeks | –1.93 vs –1.76 (Δ 0.17) | 0.47 |
| [90] | Multiple sclerosis or other defects of neurological function | 70 | 63 | 3 weeks | –1.3 vs –0.9 (Δ 0.4)§ | 0.33 |
| [92] | Spinal cord injury | 116 | 106 | 3 weeks | –0.74 vs –0.69 (∆ 0.05) | 0.71 |
| [93] | Brachial plexus avulsion | 48 | 45 | 2 weeks | Δ –0.58 vs placebo§ | 0.005 |
| [94] | Allodynia | 125 | 105 | 5 weeks | –1.48 vs –0.52 (Δ 0.97) | 0.004 |
| [95] | Allodynia | 246 | 173 | 14 weeks | Δ –0.34 vs placebo | 0.139 |
| [96] | Diabetes | 297 | 230 | 14 weeks | –1.67 vs –1.55 (∆ 0.12) | 0.63 |
| [97] | Allodynia or diabetes (open-label extension of95,96 | 380 (176 allodynia, 204 diabetes) | 234 (100 allodynia, 134 diabetes) | 38 weeks | –2.7 vs baseline‡ | N/A |
| [98] | Chemotherapy-induced | 18 | 16 | 6 weeks | –1.25 vs –0.44 (Δ 0.81) | 0.29 |
Notes: †Mean pain 0–10 NRS score in patients completing 2 years’ follow-up; §0–10 Box Scale score; ‡Pain 0–10 NRS score decreased over time from a mean of 6.9 points (baseline in the parent studies) to a mean of 5.5 points (end of parent studies), to a mean of 4.2 points (end of open-label treatment) in remaining patients.
Abbreviation: NRS, numerical rating scale.
THC:CBD oromucosal spray (nabiximols) has shown mainly positive results in studies of patients with MS-associated neuropathic pain (Table 2).87–90
A placebo-controlled RCT of patients with central neuropathic pain due to MS reported a near 2-fold higher mean change from baseline on the 0–10 pain NRS (–2.7 vs –1.4; p = 0.005) during 4 weeks’ treatment with THC:CBD oromucosal spray (nabiximols) or placebo as adjunctive analgesia. More patients treated with THC:CBD oromucosal spray (nabiximols) than placebo reported dizziness, dry mouth, and somnolence.87 In a subsequent open-label extension study involving 63 of the 66 original participants, 28 patients (44%) who completed 2 years’ treatment with active medication recorded a mean pain 0–10 NRS score of 2.9, although this evidence is acknowledged to be of lower quality than a RCT. Most patients (92%) experienced one or more adverse events during long-term treatment, mainly dizziness and nausea, which were typically mild to moderate in intensity. There was no evidence of tolerance during up to 2 years’ treatment.88
A larger placebo-controlled trial of add-on THC:CBD oromucosal spray (nabiximols) conducted in 339 patients with refractory central neuropathic pain due to MS failed to meet the primary endpoint due to a high placebo response. After 14 weeks’ treatment, the proportion of patients with ≥30% NRS improvement was 50% and 45% in the THC:CBD spray (nabiximols) and placebo groups, respectively (p = 0.234). Likewise, the change from baseline in pain 0–10 NRS scores did not differ significantly between groups (–1.93 vs –1.76; p = 0.47). Among 58 patients who entered the 4-week randomized withdrawal phase, the primary endpoint of time to treatment failure significantly favored THC:CBD spray (nabiximols), with 24% of patients failing treatment compared with 57% of patients treated with placebo (p = 0.04). THC:CBD spray was generally well tolerated, with most adverse events judged as mild to moderate in severity.89
A RCT available at ClinicalTrials.gov which investigated THC:CBD oromucosal spray (nabiximols) for relief of chronic refractory neuropathic pain due to MS or other defects of neurological function found a numerical but not statistically significant advantage in favor of active medication versus placebo for the change from baseline in the mean pain box scale-11 score (–1.3 vs –0.9; p = 0.33) after 3 weeks’ treatment (ClinicalTrials.gov identifier NCT01606176).90
Lastly, a small independent investigator-initiated non-randomized study of 10 MS patients with neuropathic pain showed that 4 weeks’ treatment with THC:CBD oromucosal spray (nabiximols) significantly reduced the pain rating from baseline (assessed by VAS; p = 0.001) and improved quality of life measures. Interestingly, the clinical effects observed with THC:CBD spray (nabiximols) were paralleled by an increase in fronto-central γ-band oscillation and pain-motor integration strength.91
The results of studies of THC:CBD spray (nabiximols) in patients with neuropathic pain of origins other than MS are mixed (Table 2).92–98
A RCT registered at ClinicalTrials.gov which compared THC:CBD spray (nabiximols) and placebo for relief of intractable neuropathic pain associated with spinal cord injury found no difference between treatments for change from baseline in pain 0–10 NRS scores (–0.74 vs –0.69; p = 0.71) after 3 weeks’ treatment (ClinicalTrials.gov identifier NCT01606202).92
In a study of central neuropathic pain due to brachial plexus avulsion, the difference in the mean pain severity score during the last 7 days of treatment with THC:CBD oromucosal spray (nabiximols) was statistically significant (p = 0.005) compared with placebo, although failed to satisfy the a priori assumed level for clinical significance.93
In 125 patients with neuropathic pain of peripheral origin (eg diabetic neuropathy, post-herpetic neuralgia) characterized by allodynia, 4 weeks’ double-blind treatment with THC:CBD oromucosal spray (nabiximols) was associated with a significant reduction in the mean pain 0–10 NRS score compared with placebo (–1.48 vs –0.52; p = 0.004). Significant improvements with THC:CBD spray (nabiximols) were observed in other endpoints including the Neuropathic Pain Scale composite score (p = 0.007), dynamic allodynia (p = 0.042), punctate allodynia (p = 0.021), Pain Disability Index (p = 0.003) and Patient’s Global Impression of Change (p < 0.001). Adverse events reported by patients on active medication were mainly sedative or gastrointestinal.94 In a subsequent larger trial of THC:CBD spray (nabiximols) for peripheral neuropathic pain associated with allodynia (n = 303), the proportion of treatment-responsive patients (≥30% NRS improvement from baseline) after 14 weeks’ double-blind treatment was significantly higher with THC:CBD spray (nabiximols) than placebo (p = 0.034), but there was no significant difference between treatment groups for change from baseline in the mean pain 0–10 NRS score (p = 0.116). The most common treatment-related events with THC:CBD spray (nabiximols) were dizziness, nausea, fatigue and dysgeusia.95 Likewise, in an aligned ClinicalTrials.gov registered study of THC:CBD spray (nabiximols) in patients with peripheral neuropathic pain associated with diabetes (n = 297), the change from baseline in the mean pain 0–10 NRS score after 14 weeks’ treatment was not significantly different compared with placebo (–1.67 vs –1.55; p = 0.63) (ClinicalTrials.gov identifier NCT00710424).96 Patients who completed either of these two studies (allodynia, n = 176; diabetes, n = 204) were entered into an open-label extension study to receive active medication for a further 38 weeks in addition to their current analgesic therapy. Among 234 patients (62% of the initial sample) who completed the extension phase, the mean pain 0–10 NRS score showed a decrease from 6.9 points at baseline in the parent studies to 4.2 points at the end of open-label follow-up. THC:CBD (nabiximols) spray was well tolerated during follow-on treatment with no evidence of development of tolerance.97
A small pilot trial with a randomized crossover design in patients with chemotherapy-induced neuropathic pain (n = 16) showed no statistically significant effect from treatment with THC:CBD oromucosal spray (nabiximols) compared with placebo for change from baseline in the mean pain 0–10 NRS score, although a responder analysis identified a small number of responders who may derive a clinically meaningful benefit from treatment.98
A placebo-controlled randomized trial of THC:CBD oromucosal spray (nabiximols) in patients with rheumatoid arthritis (n = 58), a disease which causes both nociceptive and neuropathic pain,99 reported improvement after 5 weeks’ treatment in the primary endpoint of morning pain on movement (p = 0.044), and in the secondary endpoints of morning pain at rest (p = 0.018) and “pain at present” component of the Short-Form McGill Pain Questionnaire (p = 0.016).100 Adverse effects were mainly mild or moderate.
Real-World Data for THC:CBD Oromucosal Spray (Nabiximols) in Chronic Pain
A retrospective analysis of anonymized data collected in a large German Pain e-Registry has provided insight into the real-world management of severe chronic pain with THC:CBD oromucosal spray (nabiximols).101 Among 30,228 patients prospectively registered in the German Pain e-Registry within 2017, 800 (2.6%) had received THC:CBD oromucosal spray (nabiximols) as an add-on therapy for pain relief. Prescribing of THC:CBD spray followed a change to German regulations permitting the use of cannabinoid preparations independently of their labels or even in the absence of a label in patients with resistant chronic pain conditions who fail previous management options. The main underlying chronic pain conditions in these patients were lower back pain (234 patients; 29.3%), failed back surgery syndrome (n = 148; 18.5%) and shoulder/neck pain (n = 91; 11.4%). Pain phenotype (nociceptive, mixed, neuropathic) was evaluated using the painDETECT questionnaire (PDQ7).102,103 Most patients were taking analgesic opioids (86.5% strong, 16.1% mild) at baseline.
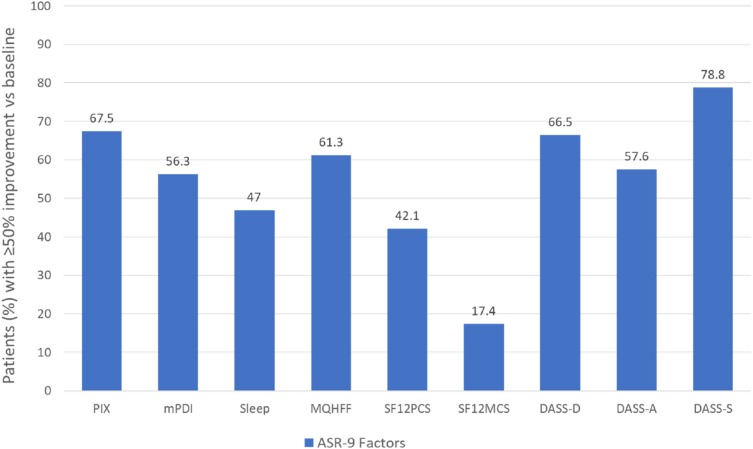
Pain intensity measured on a 0–100 VAS (from 0 = no pain; to 100 = worst pain conceivable) improved by at least 50% in 67.5% of patients after 12 weeks’ treatment with THC:CBD oromucosal spray (nabiximols). Aggregated nine-factor symptom relief (ASR-9), a composite score that summarizes the outputs of nine pain evolution efficacy endpoints measured using validated instruments, was increased by 39% from baseline. Overall, 15.4% of patients (n = 123) showed at least 50% improvement in all nine factors, and 56.0% of patients (n = 488) showed at least 50% improvement in ≥ five ASR-9 factors. Other symptoms with relief rates ≥50% were stress (78.8%), depression (66.5%), anxiety (57.6%) and overall well-being (61.3%) (Figure 2). Outcomes in terms of ≥50% improvement in pain intensity rates and mean ASR-9 symptom relief/improvement scores were significantly greater in the neuropathic pain subgroup (94.8% and 54.9%, respectively; n = 497) versus the mixed pain (24.9% and 18.2%; n = 249) or nociceptive pain (13.6% and 11.9%; n = 54) subgroups (p < 0.001 for all comparisons). Use of concomitant opioids, both as acute (rescue) or continuous pain treatment, was reduced. During the first 12 weeks of use of THC:CBD oromucosal spray (nabiximols), 18.1% of patients discontinued treatment due to inadequate efficacy (14.1%, n = 113) or treatment-emergent adverse events (TEAEs) (4%, n = 32). In all, 206 TEAEs were reported by 159 patients (19.9%), most commonly increased appetite (6.3%) or dysgeusia (2.9%) of mild intensity. This safety profile differs from that observed in other large-scale observational studies of THC:CBD oromucosal spray (nabiximols) in patients with MS spasticity in which the most common adverse events (at mean doses of 6–7 sprays/day) were dizziness, fatigue and drowsiness.104,105
Figure 2.
Proportion of patients with chronic pain (neuropathic 62.1%; mixed 31.1%; nociceptive 6.8%) reporting ≥50% improvement from baseline in Aggregated 9-Factor Symptom Relief (ASR-9) scores after 12 weeks’ treatment with THC:CBD oromucosal spray (nabiximols). Data from Ueberall et al (2019).101
Abbreviations: PIX, pain intensity index; mPDI, modified pain disability index; MQHFF, Marburg Questionnaire on Habitual Health Findings; SF12PCS, Short Form 12-item Health Survey physical component score; SF12MCS, Short Form 12-item Health Survey mental component score; DASS, Depression, Anxiety, Stress Scale.
Conclusions
Chronic pain is a common and frequently debilitating condition that carries a large individual and societal burden. Numerous etiological factors contribute to its development. Discovery of the endocannabinoid system and its role in pain control and habituation to stress suggested that cannabinoids may be useful to manage pain conditions. Activation of cannabinoid receptors has multiple analgesia-associated effects mediated by the peripheral and central nervous systems.
THC:CBD oromucosal spray (nabiximols), a cannabis-derived medicine approved for symptomatic relief of MS-related spasticity, has also been investigated as an add-on treatment for pain. Results of placebo-controlled clinical trials of THC:CBD oromucosal spray (nabiximols) in chronic cancer-related pain were equivocal. The analgesic efficacy of THC:CBD oromucosal spray (nabiximols) was more apparent in placebo-controlled clinical trials of chronic neuropathic pain, particularly MS-associated neuropathic pain, with some patients maintaining long-term (up to 2 years) benefit. A German Pain e-Registry analysis of patients with severe chronic pain treated in daily practice with THC:CBD oromucosal spray (nabiximols) showed best results in the neuropathic pain subgroup versus nociceptive or mixed pain subgroups. Across all reviewed studies in patients with chronic cancer-related or nonmalignant pain, no new safety concerns were identified with THC:CBD oromucosal spray (nabiximols) and there was no evidence of tolerance during extended use.
Anxiety and stress are recognized as major drivers for the development and maintenance of chronic pain. Looking ahead, if the ability of CBD to improve anxiety-related behavior in an animal model of neuropathic pain is corroborated in clinical studies, this may offer patients new perspectives for coping with chronic pain. Interestingly, these preclinical findings correlate with observations from the German Pain Practice e-Registry analysis which indicated that, beyond pain phenomenology, the presence of anxiety and stress are important predictors of response to treatment. Both symptoms improved significantly during treatment with THC:CBD oromucosal spray (nabiximols). This is an intriguing avenue for future research.
The main limitations of studies reviewed herein are the short duration (for a chronic condition), heterogenous patient populations and lack of active comparators. Although the data set for THC:CBD oromucosal spray (nabiximols) in chronic pain includes two extension studies, only limited numbers of patients received treatment long term.
To conclude, as improvement rates appeared to be lower in patients with pain types other than chronic neuropathic pain, proper diagnosis is key towards identifying patients most likely to benefit from THC:CBD oromucosal spray (nabiximols) as an adjunct to other treatment.
Acknowledgments
Writing assistance for this article was provided by Rob Furlong and Kerry Dechant on behalf of Content Ed Net (Madrid, Spain) with funding from Almirall S.A. (Barcelona, Spain).
Disclosure
Michael A Überall has received direct and/or indirect financial support in form of research grants, honorarium for consultancy, scientific advice, and/or lecture activities from Almirall, Aristo Pharma, Berlin Chemie, Bionorica, Esanum, Glaxo Smith Kline, Grünenthal, Hapa Medical, Hexal, Kyowa-Kirin, Lilly, Menarini, Mucos, Mundipharma, Novartis, Pfizer, Pharm Allergan, Servier, SGP Pharma, Shionogi, Teva, and Tilray. The author reports no other conflicts of interest in this work.
References
- 1.Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37:29–42. doi: 10.1007/s00296-016-3481-8 [DOI] [PubMed] [Google Scholar]
- 2.Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156:1003–1007. doi: 10.1097/j.pain.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siebenhuener K, Eschmann E, Kienast A, et al. Chronic pain: how challenging are DDIs in the analgesic treatment of inpatients with multiple chronic conditions?. PLoS One. 2017;12:e0168987. doi: 10.1371/journal.pone.0168987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller-Schwefe GH. European survey of chronic pain patients: results for Germany. Curr Med Res Opin. 2011;27:2099–2106. doi: 10.1185/03007995.2011.621935 [DOI] [PubMed] [Google Scholar]
- 5.Langley PC, Ruiz-Iban MA, Molina JT, De Andres J, Castellón JR. The prevalence, correlates and treatment of pain in Spain. J Med Econ. 2011;14:367–380. doi: 10.3111/13696998.2011.583303 [DOI] [PubMed] [Google Scholar]
- 6.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 7.Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 9.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being. A World Health Organization study in primary care. JAMA. 1998;280:147–151. doi: 10.1001/jama.280.2.147 [DOI] [PubMed] [Google Scholar]
- 10.Bouhassira D, Lantéri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136:380–387. doi: 10.1016/j.pain.2007.08.013 [DOI] [PubMed] [Google Scholar]
- 11.Torrance N, Smith BH, Bennett MI, Lee AJ. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 12.Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6:e010364. doi: 10.1136/bmjopen-2015-010364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatoye F, Gebrye T, Odeyemi I. Real-world incidence and prevalence of low back pain using routinely collected data. Rheumatol Int. 2019;39(4):619–626. doi: 10.1007/s00296-019-04273-0 [DOI] [PubMed] [Google Scholar]
- 14.Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10:918–929. doi: 10.1111/j.1526-4637.2009.00655.x [DOI] [PubMed] [Google Scholar]
- 15.Langley PC. The prevalence, correlates and treatment of pain in the European Union. Curr Med Res Opin. 2011;27:463–480. doi: 10.1185/03007995.2010.542136 [DOI] [PubMed] [Google Scholar]
- 16.Breivik H, Eisenberg E, O’Brien T, OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229. doi: 10.1186/1471-2458-13-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002 [DOI] [PubMed] [Google Scholar]
- 18.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–2843. doi: 10.1016/j.pain.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 19.Smith BH, Torrance N. Epidemiology of neuropathic pain. Pain Manag. 2011;1:87–96. doi: 10.2217/pmt.10.5 [DOI] [PubMed] [Google Scholar]
- 20.Langley PC, Van Litsenburg C, Cappelleri JC, Carroll D. The burden associated with neuropathic pain in Western Europe. J Med Econ. 2013;16:85–95. doi: 10.3111/13696998.2012.729548 [DOI] [PubMed] [Google Scholar]
- 21.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 22.EuroStat. Eurostat news release. Eurostat. [Online]; March30, 2017. Cited September27, 2019 Available from: http://ec.europa.eu/eurostat/documents/2995521/7962764/1-30032017-AP-EN.pdf.
- 23.World Health Organization (WHO). Cancer pain relief; 1986. https://apps.who.int/iris/handle/10665/43944. Accessed January18, 2020.
- 24.Reid C, Davies A. The World Health Organization three-step analgesic ladder comes of age. Palliat Med. 2004;1:175–176. doi: 10.1191/0269216304pm897ed [DOI] [PubMed] [Google Scholar]
- 25.Frieden TR, Houry D. Reducing the risks of relief–the CDC opioid-prescribing guideline. N Engl J Med. 2016;374:1501–1504. doi: 10.1056/NEJMp1515917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Just JM, Schwerbrock F, Bleckwenn M, Schnakenberg R, Weckbecker K. Opioid use disorder in chronic non-cancer pain in Germany: a cross sectional study. BMJ Open. 2019;9:e026871. doi: 10.1136/bmjopen-2018-026871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalkman GA, Kramers C, van Dongen RT, van den Brink W, Schellekens A. Trends in use and misuse of opioids in the Netherlands: a retrospective, multi-source database study. Lancet Publ Health. 2019;4:e498–e505. doi: 10.1016/S2468-2667(19)30128-8 [DOI] [PubMed] [Google Scholar]
- 28.International Narcotics Control Board. Narcotic Drugs. Estimated World Requirements for 2019; 2018. Statistics for 2017 Available from: https://www.drugsandalcohol.ie/30398/1/INCB-Narcotics_Drugs_Technical_Publication_2018.pdf. Accessed January9, 2020..
- 29.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1 [DOI] [PubMed] [Google Scholar]
- 30.Manchikanti L, Kaye AM, Knezevic NN, et al. Responsible, safe, and effective prescription of opioids for chronic non-cancer pain: american Society of Interventional Pain Physicians (ASIPP) guidelines. Pain Physician. 2017;20:S3–S92. doi: 10.36076/ppj.2017.s92 [DOI] [PubMed] [Google Scholar]
- 31.O’Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 2017;21:3–19. doi: 10.1002/ejp.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attal N, Cruccu G, Baron R, et al. European Federation of Neurological Societies. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x [DOI] [PubMed] [Google Scholar]
- 33.Guy GW, Stott CG. The development of Sativex® — a natural cannabis-based medicine In: Mechoulam R editor. Cannabinoids as Therapeutics. Milestones in Drug Therapy MDT. Birkhäuser Basel;2005:231–263. doi: 10.1007/3-7643-7358-X_14 [DOI] [Google Scholar]
- 34.Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70:2409–2438. doi: 10.2165/11585260-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 35.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802. doi: 10.1111/epi.12631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172:737–753. doi: 10.1111/bph.12944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sativex Oromucosal Spray. Summary of product characteristics; April 2019. Available from: https://www.medicines.org.uk/emc/product/602/smpc. Accessed January18, 2020.
- 39.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–14. doi: 10.1111/j.1365-2826.2008.01671.x [DOI] [PubMed] [Google Scholar]
- 40.Maldonado R, Baños JE, Cabañero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157(Suppl 1):S23–S32. doi: 10.1097/j.pain.0000000000000428 [DOI] [PubMed] [Google Scholar]
- 41.Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci. 2015;36:277–296. doi: 10.1016/j.tips.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545 [DOI] [PubMed] [Google Scholar]
- 43.Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369 [DOI] [PubMed] [Google Scholar]
- 44.Maccarrone M, Guzmán M, Mackie K, Doherty P, Harkany T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat Rev Neurosci. 2014;15:786–801. doi: 10.1038/nrn3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffman AF, Lupica CR. Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system. Cold Spring Harb Perspect Med. 2013;3(8):a012237. doi: 10.1101/cshperspect.a012237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci. 2015;16:30–42. doi: 10.1038/nrn3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172:4790–4805. doi: 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699–730. doi: 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhornitsky S, Potvin S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals (Basel). 2012;5:529–552. doi: 10.3390/ph5050529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters - A review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7–30. doi: 10.1080/15257770.2016.1210805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez de Vega MJ, Gómez-Monterrey I, Ferrer-Montiel A, González-Muñiz R. Transient receptor potential melastatin 8 channel (TRPM8) modulation: cool entryway for treating pain and cancer. J Med Chem. 2016;59:10006–10029. doi: 10.1021/acs.jmedchem.6b00305 [DOI] [PubMed] [Google Scholar]
- 52.Leyva-Illades D, Demorrow S. Orphan G protein receptor GPR55 as an emerging target in cancer therapy and management. Cancer Manag Res. 2013;5:147–155. doi: 10.2147/CMAR.S35175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology (Berl). 2014;231:623–636. doi: 10.1007/s00213-013-3389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burgos CF, Yévenes GE, Aguayo LG. Structure and pharmacologic modulation of inhibitory glycine receptors. Mol Pharmacol. 2016;9:318–325. doi: 10.1124/mol.116.105726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang L, Wang Y, Xu Y, Ma D, Wang M. The transient receptor potential ankyrin type 1 plays a critical role in cortical spreading depression. Neuroscience. 2018;382:23–34. doi: 10.1016/j.neuroscience.2018.04.025 [DOI] [PubMed] [Google Scholar]
- 56.De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160:136–150. doi: 10.1097/j.pain.0000000000001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maccarrone M, Maldonado R, Casas M, Henze T, Centonze D. Cannabinoids therapeutic use: what is our current understanding following the introduction of THC, THC:CBD oromucosal spray and others? Expert Rev Clin Pharmacol. 2017;10:443–455. doi: 10.1080/17512433.2017.1292849 [DOI] [PubMed] [Google Scholar]
- 58.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Source. Washington (DC): National Academies Press (US). The National Academies Collection: Reports funded by National Institutes of Health; January 2017. [PubMed]
- 59.Vučković S, Srebro D, Vujović KS, Vučetić Č, Prostran M. Cannabinoids and pain: new insights from old molecules. Front Pharmacol. 2018;9:1259. doi: 10.3389/fphar.2018.01259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potter DJ. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test Anal. 2014;6:31–38. doi: 10.1002/dta.1531 [DOI] [PubMed] [Google Scholar]
- 61.Perez J. Combined cannabinoid therapy via an oromucosal spray. Drugs Today (Barc). 2006;42:495–503. doi: 10.1358/dot.2006.42.8.1021517 [DOI] [PubMed] [Google Scholar]
- 62.Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66(2):234–246. doi: 10.1016/j.mehy.2005.08.026 [DOI] [PubMed] [Google Scholar]
- 63.Potter DJ, Clark P, Brown MB. Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J Forensic Sci. 2008;53:90–94. doi: 10.1111/j.1556-4029.2007.00603.x [DOI] [PubMed] [Google Scholar]
- 64.Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. Cannabis use in patients with multiple sclerosis. Mult Scler. 2006;12:646–651. doi: 10.1177/1352458506070947 [DOI] [PubMed] [Google Scholar]
- 65.Stott CG, Wright S, Guy GW. Comparison of pharmacokinetic profiles of inhaled delta-9-tetrahydrocannabinol (THC) from smoked cannabis with Sativex® oromucosal spray in humans, implications for possible symptomatic treatment in multiple sclerosis. Eur J Neurol. 2008;15:222–390. doi: 10.1111/j.1468-1331.2008.02286.x [DOI] [Google Scholar]
- 66.Aldington S, Harwood M, Cox B, et al. Cannabis use and risk of lung cancer: a case-control study. Eur Respir J. 2008;31:280–286. doi: 10.1183/09031936.00065707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epidiolex prescribing information; June 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf. Accessed January18, 2020.
- 68.European Pharmaceutical Review. Cannabidiol-based treatment receives marketing approval from EMA; July 30, 2019. Available from: https://www.europeanpharmaceuticalreview.com/news/95506/marijuana-based-treatment-approval-ema/. Accessed January18, 2020.
- 69.Marinol prescribing information. August 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf. Accessed January18, 2020.
- 70.Syndros prescribing information. July 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205525s000lbl.pdf. Accessed January18, 2020.
- 71.Cesamet prescribing information. May 2006. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/018677s011lbl.pdf. Accessed January18, 2020.
- 72.Häuser W, Finn DP, Kalso E, et al. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain. 2018;22:1547–1564. doi: 10.1002/ejp.1297 [DOI] [PubMed] [Google Scholar]
- 73.Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–441. doi: 10.1191/1352458504ms1082oa [DOI] [PubMed] [Google Scholar]
- 74.Collin C, Davies P, Mutiboko IK, Ratcliffe S, Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14(3):290–296. doi: 10.1111/j.1468-1331.2006.01639.x [DOI] [PubMed] [Google Scholar]
- 75.Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451–459. doi: 10.1179/016164109X12590518685660 [DOI] [PubMed] [Google Scholar]
- 76.Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex(®)), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18:1122–1131. doi: 10.1111/j.1468-1331.2010.03328.x [DOI] [PubMed] [Google Scholar]
- 77.Markovà J, Essner U, Akmaz B, et al. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: a double-blind, placebo-controlled randomised clinical trial. Int J Neurosci. 2019;129:119–128. doi: 10.1080/00207454.2018.1481066 [DOI] [PubMed] [Google Scholar]
- 78.Vermersch P. Sativex(®) (tetrahydrocannabinol + cannabidiol), an endocannabinoid system modulator: basic features and main clinical data. Expert Rev Neurother. 2011;11:15–19. doi: 10.1586/ern.11.27 [DOI] [PubMed] [Google Scholar]
- 79.Notcutt W, Langford R, Davies P, et al. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler. 2012;18:219–228. doi: 10.1177/1352458511419700 [DOI] [PubMed] [Google Scholar]
- 80.Ziemssen T. Tetrahydrocannabinol:cannabidioloromucosal spray for treating symptoms of multiple sclerosis spasticity: newest evidence. Neurodegener Dis Manag. 2019;9(2s):1–2. doi: 10.2217/nmt-2018-0048 [DOI] [PubMed] [Google Scholar]
- 81.Otero-Romero S, Sastre-Garriga J, Comi G, et al. Pharmacological management of spasticity in multiple sclerosis: systematic review and consensus paper. Mult Scler. 2016;22:1386–1396. doi: 10.1177/1352458516643600 [DOI] [PubMed] [Google Scholar]
- 82.Caraceni A, Shkodra M. Cancer pain assessment and classification. Cancers (Basel). 2019;11(4):E510. doi: 10.3390/cancers11040510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179. doi: 10.1016/j.jpainsymman.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 84.Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46:207–218. doi: 10.1016/j.jpainsymman.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 85.Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain. 2012;13:438–449. doi: 10.1016/j.jpain.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 86.Lichtman AH, Lux EA, McQuade R, et al. Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain. J Pain Symptom Manage. 2018;55:179–188.e1. doi: 10.1016/j.jpainsymman.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 87.Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. doi: 10.1212/01.wnl.0000176753.45410.8b [DOI] [PubMed] [Google Scholar]
- 88.Rog DJ, Nurmikko TJ, Young CA. Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007;29:2068–2079. doi: 10.1016/j.clinthera.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 89.Langford RM, Mares J, Novotna A, et al. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J Neurol. 2013;260:984–997. doi: 10.1007/s00415-012-6739-4 [DOI] [PubMed] [Google Scholar]
- 90.ClinicalTrials.gov. A study to evaluate the effects of cannabis based medicine in patients with pain of neurological origin. Available from: https://clinicaltrials.gov/ct2/show/NCT01606176. Accessed January18, 2020.
- 91.Russo M, Naro A, Leo A, et al. Evaluating Sativex® in neuropathic pain management: a clinical and neurophysiological assessment in multiple sclerosis. Pain Med. 2016;17:1145–1154. doi: 10.1093/pm/pnv080 [DOI] [PubMed] [Google Scholar]
- 92.ClinicalTrials.gov. A study of cannabis based medicine extracts and placebo in patients with pain due to spinal cord injury. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01606202. Accessed January18, 2020.
- 93.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013 [DOI] [PubMed] [Google Scholar]
- 94.Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210–220. doi: 10.1016/j.pain.2007.08.028 [DOI] [PubMed] [Google Scholar]
- 95.Serpell M, Ratcliffe S, Hovorka J, et al. A double-blind, randomized, placebo-controlled, parallel group study of THC/CBD spray in peripheral neuropathic pain treatment. Eur J Pain. 2014;18:999–1012. doi: 10.1002/j.1532-2149.2013.00445.x [DOI] [PubMed] [Google Scholar]
- 96.ClinicalTrials.gov. A double blind, randomized, placebo controlled, parallel group study of Sativex in the treatment of subjects with pain due to diabetic neuropathy. Available from: https://clinicaltrials.gov/ct2/show/NCT00710424. Accessed January18, 2020.
- 97.Hoggart B, Ratcliffe S, Ehler E, et al. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol. 2015;262:27–40. doi: 10.1007/s00415-014-7502-9 [DOI] [PubMed] [Google Scholar]
- 98.Lynch ME, Cesar-Rittenberg P, Hohmann AG. A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain. J Pain Symptom Manage. 2014;47:166–173. doi: 10.1016/j.jpainsymman.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 99.Ito S, Kobayashi D, Murasawa A, et al. An analysis of the neuropathic pain components in rheumatoid arthritis patients. Intern Med. 2018;57:479–485. doi: 10.2169/internalmedicine.9235-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford). 2006;45:50–52. doi: 10.1093/rheumatology/kei183 [DOI] [PubMed] [Google Scholar]
- 101.Ueberall MA, Essner U, Mueller-Schwefe GH. Effectiveness and tolerability of THC:CBD oromucosal spray as add-on measure in patients with severe chronic pain: analysis of 12-week open-label real-world data provided by the German Pain e-Registry. J Pain Res. 2019;12:1577–1604. doi: 10.2147/JPR.S192174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 103.Freynhagen R, Tölle TR, Gockel U, Baron R. The painDETECT project – far more than a screening tool on neuropathic pain. Curr Med Res Opin. 2016;32:1033–1057. doi: 10.1185/03007995.2016.1157460 [DOI] [PubMed] [Google Scholar]
- 104.Flachenecker P, Henze T, Zettl UK. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice–results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur Neurol. 2014;71(5–6):271–279. doi: 10.1159/000357427 [DOI] [PubMed] [Google Scholar]
- 105.Patti F, Messina S, Solaro C, et al. Efficacy and safety of cannabinoid oromucosal spray for multiple sclerosis spasticity. J Neurol Neurosurg Psychiatry. 2016;87:944–951. doi: 10.1136/jnnp-2015-312591 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- EuroStat. Eurostat news release. Eurostat. [Online]; March30, 2017. Cited September27, 2019 Available from: http://ec.europa.eu/eurostat/documents/2995521/7962764/1-30032017-AP-EN.pdf.