Abstract
Autism spectrum disorder (ASD) and other neurodevelopmental disorders (NDs) are behaviorally defined disorders with overlapping clinical features that are often associated with higher‐order cognitive dysfunction, particularly executive dysfunction. Our aim was to determine if the polygenic score (PGS) for ASD is associated with parent‐reported executive dysfunction in everyday life using the Behavior Rating Inventory of Executive Function (BRIEF). Furthermore, we investigated if PGS for general intelligence (INT) and attention deficit/hyperactivity disorder (ADHD) also correlate with BRIEF. We included 176 children, adolescents and young adults aged 5–22 years with full‐scale intelligence quotient (IQ) above 70. All were admitted for clinical assessment of ASD symptoms and 68% obtained an ASD diagnosis. We found a significant difference between low and high ASD PGS groups in the BRIEF behavior regulation index (BRI) (P = 0.015, Cohen's d = 0.69). A linear regression model accounting for age, sex, full‐scale IQ, Social Responsiveness Scale (SRS) total score, ASD, ADHD and INT PGS groups as well as genetic principal components, significantly predicted the BRI score; F(11,130) = 8.142, P < 0.001, R 2 = 0.41 (unadjusted). Only SRS total (P < 0.001), ASD PGS 0.1 group (P = 0.018), and sex (P = 0.022) made a significant contribution to the model. This suggests that the common ASD risk gene variants have a stronger association to behavioral regulation aspects of executive dysfunction than ADHD risk or INT variants in a clinical sample with ASD symptoms. Autism Res 2020, 13: 207–220. © 2019 International Society for Autism Research, Wiley Periodicals, Inc.
Lay Summary
People with autism spectrum disorder (ASD) often have difficulties with higher‐order cognitive processes that regulate thoughts and actions during goal‐directed behavior, also known as executive function (EF). We studied the association between genetics related to ASD and EF and found a relation between high polygenic score (PGS) for ASD and difficulties with behavior regulation aspects of EF in children and adolescents under assessment for ASD. Furthermore, high PGS for general intelligence was related to social problems.
Keywords: autism spectrum disorder, polygenic score, executive function, attention deficit/hyperactivity disorder, behavior rating inventory of executive function
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder (ND) characterized by deficits in social communication and interaction, and restricted and repetitive behaviors and interests [American Psychiatric Association, 2013]. The heritability of ASD is high, with estimates ranging from 70 to 90%, and the disorder is regarded as a complex genetic disorder with multifactorial causes [Lai, Lombardo, & Baron‐Cohen, 2014]. Several rare genetic variants conferring high risk of ASD have been identified, and these rare genetic variants, for example, copy‐number variations (CNVs), are estimated to explain about 5–10% of the genetic risk for ASD [Ramaswami & Geschwind, 2018]. However, most of the heritability of ASD seems to be caused by common genetic variants [Gaugler et al., 2014]. ASD, like attention deficit/hyperactivity disorder (ADHD), schizophrenia, and bipolar disorder, have been shown to be polygenic disorders, in which each single risk variant has a small effect on the disease phenotype [Demontis et al., 2018; Grove et al., 2019; International Schizophrenia Consortium et al., 2009; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014]. Based on genome‐wide association studies (GWASs) summary statistics for a given complex disorder, it is possible to derive measures of the cumulative genetic loads for the disorders inherent to an individual's genotype. Such measures are often called polygenic scores (PGS) [International Schizophrenia Consortium et al., 2009]. Recently, Grove et al. identified five risk loci for ASD based on a genome‐wide association meta‐analysis of 18,381 cases of ASD and 27,969 controls [Grove et al., 2019]. Even though these PGS are getting better at separating ASD cases from controls [Grove et al., 2019], their sensitivity and specificity are not yet high enough for clinical use in diagnostics or treatment planning. However, exploring the potential clinical utility of PGS is central in the pursuit for precision medicine for NDs [Editorial, 2010; Torkamani, Wineinger, & Topol, 2018]. PGS often only explain a few percentiles of variation in disease status, meaning that they have limited predictive power. For many people with “average risk,” quantifying the exact risk might be of limited value. However, identifying those with particularly low or high risk may be of some value. A viable approach is to look at “extreme” PGS, as done by others [Andersson et al., 2018; Khera et al., 2018; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014]. Individuals with high PGS for a given disorder might also be at increased risk for known comorbid traits and disorders. Thus, “extreme” PGS analysis can be a promising tool to investigate the genetic contribution to symptom severity and disease characteristics in NDs.
The key characteristics of ASD, deficits in social skills and abnormal behaviors, strongly affect every day functioning [de Vries & Geurts, 2015], which is often the primary reason for seeking professional help for families with ASD children. Cognitive processes play an important role in the development of social skills, and in moderating behavioral responses [Gardiner & Iarocci, 2018], in particular executive function [Rommelse, Geurts, Franke, Buitelaar, & Hartman, 2011]. These abilities depend on response inhibition, interference control, working memory, and flexibility [Friedman & Miyake, 2017], which enable regulation of thought and goal‐directed behavior [Miyake et al., 2000]. Executive dysfunction is suggested to be involved in the development of key symptoms and behaviors in ASD [Demetriou et al., 2017; Hill, 2004] and other psychiatric disorders characterized by social dysfunctions and abnormal behavior, such as schizophrenia [Amann et al., 2012]. Importantly, social difficulties are associated with executive dysfunctions in everyday life in ASD [Leung, Vogan, Powell, Anagnostou, & Taylor, 2015; Torske, Naerland, Oie, Stenberg, & Andreassen, 2017].
ASD is a spectrum diagnosis with large variation in clinical characteristics and functions. Children and adolescents with subthreshold symptoms who do not receive an ASD diagnosis might still have profound social difficulties and executive dysfunction [American Psychiatric Association, 2013]. Comorbidity is common in ASD, and about 30% also meet the diagnostic criteria for ADHD [Lord, Elsabbagh, Baird, & Veenstra‐Vanderweele, 2018]. Cognitive difficulties are also observed in ADHD [Craig et al., 2016], and particularly executive dysfunctions are present across diagnostic categories like ASD and ADHD as well as many other psychiatric disorders [Dajani, Llabre, Nebel, Mostofsky, & Uddin, 2016; Snyder, Miyake, & Hankin, 2015]. Executive function is a heritable cognitive domain and deficits are found in unaffected ASD family members at higher rates than in the general population [Benca et al., 2017]. Furthermore, in typically developed children, intelligence and executive function correlate [Diamond, 2013].
Still, there are several unclear aspects related to cross‐diagnostic features of NDs, which are behaviorally defined, and their association to cognitive dysfunctions [Gillberg, 2010]. Building on the recent progress in GWAS of different traits and disorders, the PGS has emerged as a tool that enables investigation of the polygenic components of different disorders and to explore the association between genes, symptoms, and functioning. Furthermore, genetic underpinnings of general cognitive ability (intelligence) can be used to identify differences in cognitive factors between NDs [Savage et al., 2018], including executive function. This could provide a novel understanding of the underlying disease mechanisms, as outlined in the Research Domain Criteria initiative [Cuthbert, 2014]. Approaches to dissect social and cognitive traits are also of clinical importance in the ASD field since children and adolescents are admitted to specialist health care due to functional deficits, mainly related to social and/or behavioral impairment.
For people with ASD, structured situations with clear expectations are easier than unstructured everyday situations [Kenworthy, Yerys, Anthony, & Wallace, 2008]. Thus, standardized and structured neuropsychological tests might not capture cognitive deficits important for everyday life functions [Kenworthy et al., 2008]. Actually, questionnaires assessing executive functions have shown to have higher ecological validity than neuropsychological laboratory tests and might provide important information about how executive dysfunctions affect every day functioning. This finding applies to both clinical and nonclinical samples, as well as specifically to children and adults with ASD [Demetriou et al., 2017; Tillmann et al., 2019; Vriezen & Pigott, 2002]. The Behavior Rating Inventory of Executive Function (BRIEF) is one of the most used clinical tools to measure executive functions in everyday life [Gioia, Isquith, Guy, & Kenworthy, 2015]. Furthermore, recent evidence suggests that the Social Responsiveness Scale (SRS) is a valid measure for social impairments in ASD [Constantino & Gruber, 2005]. Because the BRIEF and the SRS both are continuous scales, they can be used to study difficulties beyond diagnostic categories [Geschwind & State, 2015]. Here, we used the BRIEF to measure executive functions and the SRS to measure social skills as they occur in natural social settings.
The primary aim of the present study was to determine if ASD PGS is associated with executive dysfunction in everyday life in a sample of children and adolescents admitted for clinical assessment of ASD. Second, we aimed to disentangle the polygenic components of NDs and cognitive traits in a clinically relevant setting by investigating how the PGS for ASD, ADHD, and general intelligence (INT) are related to executive function. We hypothesized that high level of ASD PGS will be associated with worse BRIEF scores beyond ASD diagnosis in a sample referred for ASD assessment. Furthermore, since the participants in our sample have an ASD diagnosis and/or ASD symptomatology, we expected the ASD PGS to be more strongly associated with BRIEF scores than the PGS for ADHD and INT. We also investigated the association between the PGS and social skills in an everyday setting, and we hypothesized that the PGS for ASD should be positively associated with social difficulties in everyday life.
Method
Participants
The total sample consisted of 176 children, adolescents, and young adults who were referred to a specialized hospital unit for NDs for evaluation of ASD. The current sample was part of the national BUPGEN network and was recruited between 2013 and 2018. For a detailed description of the recruitment procedure see Grove et al. supplementary material [Grove et al., 2019]. Inclusion criteria were ASD‐related difficulties and suspected ASD diagnosis, independent of final ASD diagnosis. The participants were assessed by a team of experienced clinicians (clinical psychologists and child psychiatrist). They assessed the participants based on anamnestic information and descriptions of difficulties in everyday life activities using the gold standard tools, the Autism Diagnostic Observation Schedule (ADOS), and/or the Autism Diagnostic Interview‐Revised (ADI‐R). Exclusion criteria were full scale intelligence quotient (IQ) below 70. No participants had any significant sensory losses (vision and/or hearing) that could interfere with testing. The participants were in the age range 5–22 years and spoke Norwegian fluently. Of the total sample of 176 participants, 120 fulfilled the diagnostic criteria for an ASD diagnosis, and the remaining (n = 56) non‐ASD diagnostic group was named the subthreshold ASD group. The subthreshold ASD group included participants with ASD symptomatology not reaching the criteria of ASD diagnosis, who may also have other diagnoses. Of the 120 children and adolescents with ASD (91 boys and 29 girls), 18 were diagnosed with childhood autism (F84.0), five with atypical autism (F84.1), 56 with Asperger syndrome (F84.5) and 41 with Pervasive developmental disorder‐not otherwise specified (PDD‐NOS) (F84.9). Of the 120 participants with ASD, 42 had a comorbid disorder of ADHD. In the subthreshold ASD group, 26 had an ADHD diagnosis (See Table 1). Of the participants without ASD or ADHD (n = 30), one had chronic motor or vocal tic disorder, four had Tourette syndrome, two had mixed specific developmental disorders, five had specific developmental disorders of speech and language, two had specific developmental disorders of scholastic skills, two had other specified behavioral and emotional disorders, two had disorder of psychological development, one had some other childhood disorder of social functioning and one had obsessive compulsive disorder.
Table 1.
Sample Characteristics (N = 176)
| N (%) | |||
|---|---|---|---|
| Male/boys | 134 (76.1%) | ||
| Female/girls | 42 (23.9%) | ||
| Mean (SD) | Range | ||
| Age (years) | 11.7 (3.7) | 5–22 | |
| Full‐scale IQ | 93.7 (13.5) | 70–133 | |
| n | |||
| ASD | 120 (67.8%) | ||
| ASD with ADHD | 42 | ||
| ADHD without ASD | 26 | ||
| ASD diagnosis n = 120 | n | ||
| Childhood autism (F84.0) | 18 | ||
| Atypical autism (F84.1) | 5 | ||
| Asperger syndrome (F84.5) | 56 | ||
| Pervasive developmental disorder unspecified (F84.9) | 41 |
| Low ASD PGS | High ASD PGS | P‐value | |
|---|---|---|---|
| Boys/girls | n 18/8 | n 22/5 | 0.573 |
| ASD | n 16 | n 20 | 0.618 |
| ADHD | n 8 | n 12 | 0.585 |
| Full‐scale IQ score (SD) | 93.1 (14.6) | 95.9 (10.7) | 0.439 |
ADHD, attention deficit/hyperactivity disorder; ASD, autism spectrum disorder; IQ, intelligence quotient; SD, standard deviation; PGS, polygenic scores; ASD polygenic groups Low and High based on ASD PGS at P‐value <0.1.
In addition, we included 29 typically developed controls (10 girls) with genetic and behavioral data available. The controls were recruited from local schools through invitations/bulletins to all students/parents, and had no history of learning disabilities or psychiatric problems. For a more detailed description of the controls see Hoyland et al. [2019].
We divided the clinical participants (across the ASD and the subthreshold ASD groups) into three groups (Low, Moderate, High) based on each of their PGS for ASD, ADHD and INT. The Low, Moderate and High PGS groups were identified based on the normal distribution curve of the standardized PGS scores: the Moderate group consisted of all participants with PGS within one standard deviation from the mean, and the Low and High groups respectively comprised participants with PGS in the lower tail and the upper tail of the distribution. This subdivision roughly corresponds to the participants with the 15% lowest scores constituting the Low group, those with the 15% highest scores the High group, and everyone in between the Moderate group. In the linear regression, we used the groups Low, Moderate and High PGSs. The discovery samples our PGS are based on consisted of 18,381 ASD cases and 27,969 controls for the ASD PGS [Grove et al., 2019], 20,183 ADHD cases and 35,191 controls for the ADHD PGS [Demontis et al., 2018] and 269,867 cases for the INT PGS [Savage et al., 2018].
Clinical Measures
The participants underwent a thorough clinical evaluation for ASD using the gold standard tools, the ADOS and/or the ADI‐R, administered by an experienced clinician (psychiatrist and/or psychologist) [Lord et al., 2000; Lord, Rutter, & Le Couteur, 1994]. In addition, a comprehensive diagnostic assessment was done based on questionnaires, clinical interviews, naturalistic observations, and formal testing of cognitive function. ADOS and/or ADI‐R were performed in connection with clinical assessment for ASD. IQ was measured using the fourth version of Wechsler's Intelligence Scale for Children [Wechsler, 2003] or another of Wechsler's tests for the appropriate age group [Wechsler, 2002, 2008]. The diagnostic assessments of ADHD were done by a clinician (psychologist and/or psychiatrist) specialized in child and adolescent psychology/psychiatry or a pediatrician, based on all previously mentioned measures and according to formal diagnostic criteria.
Social responsiveness scale
The SRS is a 65 items questionnaire made to identify the presence and severity of social impairment in natural social settings within the autism spectrum. A parent who is familiar with the individual's current behavior and developmental history responds to each question on a Likert scale: (a) not true, (b) sometimes true, (c) often true, or (d) almost always true. The SRS consists of five treatment subscales; Social Awareness, Social Cognition, Social Communication, Social Motivation, and Autistic Mannerisms, which together form the total score [Constantino & Gruber, 2005]. We used the parent rater scale for children/adolescents aged 4–18 years in our study [Constantino & Gruber, 2005]. For the few participants who were over the age of 18, we knew that they lived at home with their parents and that their parents therefore had a good basis for assessing their everyday function. We used continuous t‐scores in our analyses and a higher score means more difficulties related to social function. A total score in the range 60–75 indicates clinically significant deficits in social reciprocal interaction, and a mild to moderate interference in everyday interactions. A t‐score ≥76 represents severe interference in daily social interactions and is strongly associated with a clinical diagnosis of ASD [Constantino & Gruber, 2005]. Internal consistency is found to be high, both in population‐based samples and clinical samples (Cronbach's α = 0.93–0.97) [Constantino & Gruber, 2005].
Behavior rating inventory of executive function
The BRIEF is a 86 items questionnaire designed to assess executive function in everyday life rated by parents, teachers, and/or self‐report [Gioia, Isquith, Guy, & Kenworthy, 2000]. In this study, we used the t‐scores from the parents' report for the age group 5–18 years. For the few participants who were over the age of 18, we knew that they lived at home with their parents and that their parents therefore had a good basis for assessing their everyday function. The parents respond if the described behavior has been a problem for the child/adolescent the past 6 months by circling: (a) never, (b) sometimes, or (c) often. The raw scores were computed into the Software Portfolio (BRIEF SP) that has separate norms based on respondent, age, and gender of the child/adolescent [Gioia et al., 2000]. The BRIEF consists of three indexes and eight nonoverlapping subscales. The Behavior Regulation Index (BRI) incorporates three subscales: inhibit, shift, and emotional control. The Metacognition Index (MI) consists of five subscales: initiate, working memory, plan/organize, organization of materials, and monitor. Together these eight subscales (three from BRI and five from MI) form the Global Executive Composite Index (GEC). The items that make up the BRI index are intended to measure the ability to modulate both behavioral and emotional control, and to move flexibly from one activity to another. The MI index on the other hand is related to active problem solving, and the ability to initiate, organize, and monitor your own actions [Gioia et al., 2000]. A higher score is associated with more problems related to executive function, and t‐scores ≥65 are considered to represent clinically significant levels. In our analysis, we used the continuous t‐scores. The internal consistency is reported to be high (Cronbach's α = 0.80–0.98) [Gioia et al., 2000].
Polygenic Scores
Genotyping
The DNA was extracted with standard methods either from blood samples or saliva collected in the clinic. The genotypes for the study were obtained with Human Omni Express‐24 v.1.1 (Illumina Inc., San Diego, CA) at deCODE Genetics (Reykjavik, Iceland). Quality control was performed using PLINK 1.9 [Purcell et al., 2007]. Briefly, variants were excluded if they had low coverage (<95%), had low MAF (<0.01), deviated from Hardy–Weinberg equilibrium (P < 10−4), or occurred at significantly different frequencies in different genotyping batches (FDR < 0.5). Whole individual genotypes were excluded if they had low coverage (<95%) or high likelihood of contamination (heterozygosity above mean + 5 SD). MaCH software [Li, Willer, Ding, Scheet, & Abecasis, 2010] was used to obtain variant pseudo‐dosages (sums of imputation probabilities for the two haplotypes) for all participants based on reference haplotypes derived from the samples of European ancestry in the 1,000 Genome Project. We had no challenges with kinship in our sample (no relatedness above pi‐hat 0.1 in our sample). All our participants (controls and clinical) were collected in the same time period, genotyped on the same array, and imputed and processed together. Any variants imputed with MAF lower than 0.05 or information score lower than 0.8 were excluded from the subsequent PGS calculations.
Polygenic score
The variants' effects were estimated from an inverse‐variance weighted meta‐analysis of the GWAS summary statistics for all original sub‐studies except any sub‐studies our own participants were drawn from. All summary statistics were quality controlled by removing variants that met any of the following conditions: minor allele frequency <0.05; imputation quality INFO <0.8; not present in more than half of the sub‐studies. The remaining variants were clumped into independent regions on the basis of the linkage disequilibrium structure of the 1,000 Genomes Phase III European population. PLINK v1.9 was used with the following parameters: –clump‐p1 1.0 –clump‐p2 1.0 –clump‐r2 0.2 –clump‐kb 500. The allelic dosage coefficient (or logarithm of the odds ratio) of each of the variants with minimum P‐values from all independent regions were taken as weights in constructing the PGSs. We obtained GWAS summary statistics for ASD from Grove et al. [2019], ADHD from Demontis et al. [2018] and INT from Savage et al. [2018] [Demontis et al., 2018; Grove et al., 2019; Savage et al., 2018]. The summary statistics were used to generate PGS, and test the association with specific phenotypes in the current sample, a procedure referred to as “genetic risk profiling” [Martin, Daly, Robinson, Hyman, & Neale, 2018]. We used a GWAS P‐value threshold for inclusion of 0.1 for all PGS (ASD, ADHD, and INT). This P‐value threshold was chosen because it resulted in the highest explained variance in ASD case/control status in the study our PGS was based on [Grove et al., 2019]. The scores included contributions from about 32,000 variants. The participants in our subsample and Grove et al. [2019], Demontis et al. [2018], and Savage et al. [2018] are all of European ancestry and are therefore genetically relatively homogeneous.
Copy‐number variations
CNVs in molecular studies have been identified as risk factors for ASD [Geschwind, 2011]. Information about the CNVs in our sample was obtained from the chip genotypes, following standard CNV calling methodology [Sonderby et al., 2018].
Statistical Analyses
Data analyses were conducted using the statistical package IBM SPSS Statistics for Windows, version 25.0 (SPSS, Inc., Chicago, IL). Figure 1 was made using jamovi (Version 0.9). We used Pearson's independent t‐tests to investigate the mean differences in all clinical variables (MI, BRI, SRS, full‐scale IQ, and age) between the Low and the High PGS groups. Chi‐square for crosstabs was used to investigate differences in the distribution of sex and ASD and ADHD diagnoses in the Low and the High ASD PGS groups. We also used Pearson's independent t‐tests to investigate the differences in PGS between controls and the clinical group to validate the ASD PGS in our sample. Separate linear regression models were conducted to explain variance in BRIEF scores (BRI, MI, and GEC) across ASD, ADHD, and INT PGS groups. Age, sex, SRS scores, full‐scale IQ, and PCs were entered as covariates. We also used linear regression with total SRS score as the dependent variable and ASD, ADHD and INT PGS groups, age, sex, full‐scale IQ, and BRIEF scores (BRI and MI) as independent variables. To describe the distribution of data, we added scatterplots of age by BRIEF scores and ASD PGS by BRIEF scores (See Supplementary Figs. S1A‐B and S2A‐B) and Q‐Q plots of BRI and MI scores (See Supplementary Figs. S3A‐B). The genetic principal components (PCs) that entered the linear regression model as covariates of no interest were the ones with the highest correlation with ASD, ADHD, and INT PGS (highest and unique correlation PGS ASD = PC02, PGS ADHD = PC01, and PGS INT = PC04) and the dependent variables (BRIEF/GEC = PC06 and SRS = PC08, respectively). We used standard procedures to control for population stratification, and we were unable to identify clear population subgroups in our sample. For scatterplots of the PCs see Supplementary Figures S4A‐D. The coordinates of the study participants in the relevant genetic PCs (PC1, 2, 3, 4, and 6) are inserted in the context of the European reference population from the 1,000 genomes project in Supplementary Figures S5A‐J. We also checked if the outliers had any substantial effect on the analysis and we tried to remove seven of the participants with the highest PC01 (outliers). The main findings were still the same (the difference in BRI score between the high and low ASD groups was still significant) (P = 0.036), and in the regression analysis predicting BRI the same three covariates were significant; sex P = 0.026, SRS P = <0.001, and ASD PGS group P = 0.020; R 2 = 0.390). Furthermore, we also fitted the regression models with continuous PGS instead of PGS groups (See Supplementary Tables S1A‐C). Due to moderate sample size, we did not adjust alpha levels for multiple testing because of the risk of type 2 errors. All tests were considered significant with a two‐sided P‐value lower than 0.05. We report unadjusted P‐values and are cautious in drawing our conclusions.
Figure 1.
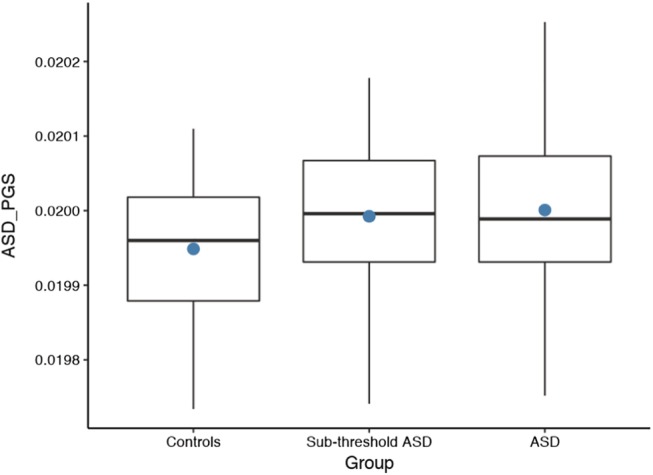
The ASD PGS scores for typically developed controls, the subthreshold ASD group, and the ASD group (ASD PGS at P < 0.1). The dots represent the mean scores, and the bands inside the boxes are the median. ASD, autism spectrum disorder; PGS, polygenic score.
Ethical Considerations
The study was approved by the Regional Ethical Committee and the Norwegian Data Inspectorate (REK #2012/1967), and was conducted in accordance with the Helsinki Declaration of the World Medical Association Assembly. Written informed consent was given from parents or legal guardians for participants under 18 years. Participants over 18 years gave written consent.
Results
Clinical characteristics
In the total sample, there was a significant difference between those who had an ASD diagnosis (n = 120) and the subthreshold ASD group (n = 56) on: total SRS (P < 0.001), total t‐score Global Executive Composite (GEC) from BRIEF (P = 0.003), t‐score BRI from the BRIEF (P < 0.001), and age (P = 0.001). There were no significant differences between the groups on total IQ (P = 0.841) or t‐score MI from BRIEF (P = 0.068). There were no significant differences between those who had an ASD diagnosis and the subthreshold ASD group on any of the PGS; ASD (P = 0.578), ADHD (P = 0.810), and INT (P = 0.842) (all P‐bin 0.1).
Those who had a comorbid diagnosis of ASD and ADHD (n = 42) had a significantly higher score (were more impaired) on both the total t‐score GEC (P = 0.001) and the t‐score MI (P = 0.001) from the BRIEF, than those who only had an ASD diagnosis. There was no significant difference in total SRS, total IQ, or BRI scores between those with an ASD diagnosis only and those with comorbid ASD and ADHD.
In the whole sample (n = 176), there was a small, positive correlation between ASD PGS and full‐scale IQ, r = 0 0.033, but this was not significant (P = 0.673).
Characteristics of the ASD PGS groups
In the total sample (n = 176), there were no significant group differences between the Low and High ASD PGS groups on age (P = 0.059), full‐scale IQ (P = 0.439), SRS total (P = 0.921), or sex distribution (Pearson chi‐square 1.113, P = 0.573). The Low and High ASD PGS groups did not, however, reflect the diagnostic groups, and there was no significance difference between the distribution of ASD or ADHD diagnosis (Pearson chi‐square 0.962, P = 0.618 and Pearson chi‐square 0.107, P = 0.585) in the Low and High PGS groups (See Table 1). We found no significant differences in parental education level, which is highly related to income and socioeconomic status, between the Low and the High ASD PGS groups (fathers education level P = 0.784, mothers education level P = 0.798).
Validation of the PGS in the clinical group compared to typically developed controls
To validate the ASD PGS, we compared the PGS from the ASD group (n = 120), the subthreshold ASD group (n = 56), and the typically developed controls (TDC) (n = 29) using Pearson's independent t‐tests. The PGS for ASD differentiated between the TDC and the clinical group (ASD and subthreshold ASD group, n = 176; t(203) = 2.61, 95% CI [1.2 × 10−5, 8.7 × 10−5], P = 0.010. The PGS for ASD also differentiated between the TDC and both the subthreshold ASD group; t(83) = −2.07, 95% CI [−8.6 × 10−5, −1.7 × 10−6], P = 0.042 and the ASD group t(147) = −2.63, 95% CI [−9.1 × 10−5, −1.3 × 10−5], P = 0.009. The PGS for ASD did not significantly differ between those with ASD and subthreshold ASD group (P = 0.578). There was no significant difference between the TDC and the clinical group (ASD and subthreshold ASD group) on PGS for ADHD (P = 0.205) or INT (P = 0.944). The TDC group had significantly lower scores (less impairment/dysfunction) than the clinical group on both SRS and BRIEF (P < 0.001), and was significantly older than the clinical group (mean control group = 16.3 years, mean clinical group = 11.7 years (ASD and subthreshold ASD), P < 0.001). Altogether the ASD PGS differentiated between the ASD group and TDC, and also between the subthreshold ASD group and the TDC (Fig. 1).
PGS of ASD, ADHD, and INT and their association to executive function (BRIEF)
There was a significant group difference between the Low and the High ASD PGS groups in the BRI scores from the BRIEF; BRI t = 62.9 in the Low PGS group and t = 71.5 in the High PGS group; t(49) = −2.51, 95% CI [−15.45, −1.71], P = 0.015, Cohen's d = 0.69. This means that the group with the highest PGS for ASD had significantly more executive dysfunctions with BRI than the group with lowest PGS for ASD. We did not find a significant difference between the Low and High ASD PGS groups in the MI scores from the BRIEF. We also found no significant group differences between Low and High ADHD or INT PGS groups in either BRI or MI scores from the BRIEF (See Tables 2 and 3).
Table 2.
Independent Sample t‐Test: Differences in BRIEF Scores (BRI and MI) between the Low and the High PGS groups (ASD, ADHD, and INT)
| PGS group | BRI Mean t‐score(SD) | n | df | t | 95% CI | P | MIMean t‐score(SD) | n | df | t | 95% CI | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low ASD PGS |
62.9 (12.6) |
25 |
62.8 (12.2) |
25 | ||||||||
| High ASD PGS |
71.5 (11.9) |
26 | 49 | −2.51 | [−15.45, −1.71] | 0.015* |
65.5 (8.1) |
27 | 41.3a | −0.94 | [−8.57, 3.13] | 0.354 |
| Low ADHD |
67.6 (14.9) |
26 |
63.9 (11.8) |
26 | ||||||||
|
High ADHD PGS |
64.9 (11.2) |
26 | 50 | 0.74 | [−4.64, 10.03] | 0.465 |
67.0 (11.3) |
26 | 50 | −0.97 | [−9.55, 3.32] | 0.336 |
| Low INT PGS |
62.5 (17.1) |
23 |
59.9 (11.9) |
26 | ||||||||
| High INT PGS |
66.2 (12.0) |
26 | 47 | −0.89 | [−12.14, 4.71] | 0.380 |
65.0 (10.6) |
26 | 50 | −1.61 | [−11.31, 1.24] | 0.113 |
BRIEF‐BRI, Behavior Rating Inventory of Executive Functions, Behavior Regulation Index; BRIEF‐MI, Behavior Rating Inventory of Executive Functions, Metacognition Index; CI, confidence interval; df, degrees of freedom.
Low and High ASD PGS: Autism spectrum disorder polygenic score subgroup (Low and High) at P < 0.1.
Low and High ADHD PGS: Attention deficit/hyperactivity disorder polygenic score subgroup (Low and High) at P < 0.1.
Low and High INT PGS: General intelligence polygenic score subgroup (Low and High) at P < 0.1.
*P < 0.05 (two‐tailed); **P < 0.01 (two‐tailed).
Equal variance not assumed; Welch's t‐test.
Table 3.
Independent Sample t‐Test: Differences in BRIEF Scores (GEC) between the Low and the High PGS groups (ASD, ADHD, and INT)
| PGS group | GECMean t‐score (SD) | n | df | t | 95% CI | P |
|---|---|---|---|---|---|---|
| Low ASD PGS |
63.8 (12.7) |
25 | ||||
| High ASD PGS |
69.0 (9.0) |
26 | 49 | −1.68 | [−11.32, 1.00] | 0.099 |
| Low ADHD PGS |
66.5 (13.3) |
26 | ||||
| High ADHD PGS |
67.7 (11.2) |
26 | 50 | −0.36 | [−8.10, 5.64] | 0.721 |
| Low INT PGS |
61.9 (14.3) |
25 | ||||
| High INT PGS |
66.2 (9.1) |
26 | 40.4a | −1.28 | [−11.12, 2.49] | 0.208 |
BRIEF‐GEC, Behavior Rating Inventory of Executive Functions, Global Executive Composite; CI, confidence interval; df, degrees of freedom.
Low and High ASD PGS: Autism spectrum disorder polygenic score subgroup (Low and High) at P < 0.1.
Low and High ADHD PGS: Attention deficit/hyperactivity disorder polygenic score subgroup (Low and High) at P < 0.1.
Low and High INT PGS: General intelligence polygenic score subgroup (Low and High) at P < 0.1.
*P < 0.05 (two‐tailed); **P < 0.01 (two‐tailed).
Equal variance not assumed; Welch's t‐test.
Furthermore, we investigated the presence of CNVs in the Low and the High ASD PGS groups. Three of the participants in the Low ASD PGS group had CNVs, while no CNVs were observed in the High ASD PGS group.
Regression analyses with BRIEF as the dependent variable
A linear regression model accounting for age, sex, full‐scale IQ, SRS total score, ASD, ADHD and INT PGS group as well as genetic PCs explained 41% of the variance in the BRI score; F(11, 130) = 8.142, P < 0.001, R 2 = 0.41(unadjusted). Only SRS total (P = <0.001), PGS ASD 0.1 group (P = 0.018), and sex (P = 0.022) made a significant contribution to the model (See Table 4). This suggests that the ASD PGS is more important than the ADHD PGS and the INT PGS in explaining executive dysfunctions with behavior regulation (BRI) in our sample.
Table 4.
Linear Regression Model Summary: Prediction of Behavior Regulation Index (BRI) (Total sample n = 142a)
| Predictor | B | SE B | 95% confidence interval | t | P | β | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Sex | −5.21 | 2.25 | −9.65 | −0.77 | −2.32 | 0.022* | −0.17 |
| Age | −0.34 | 0.29 | −0.92 | 0.23 | −1.18 | 0.239 | −0.08 |
| Full‐scale IQ | 0.08 | 0.07 | −0.06 | 0.22 | 1.13 | 0.261 | 0.08 |
| SRS_total | 0.52 | 0.06 | 0.39 | 0.65 | 8.10 | <0.001** | 0.59 |
| ASD PGS subgr P < 0.1 | 4.07 | 1.70 | 0.71 | 7.44 | 2.40 | 0.018* | 0.17 |
| ADHD PGS subgr P < 0.1 | −2.05 | 1.61 | −5.23 | 1.13 | −1.27 | 0.205 | −0.09 |
| INT PGS subgr P < 0.1 | −0.61 | 1.79 | −4.16 | 2.93 | −0.34 | 0.732 | −0.02 |
Model's R 2 = 0.408 (unadjusted). Models P‐value = <0.001**.
B, unstandardized regression coefficients; BRIEF‐BRI, Behavior Rating Inventory of Executive Functions, Behavior Regulation Index; BRIEF‐GEC, Behavior Rating Inventory of Executive Functions, Global Executive Composite; BRIEF‐MI, Behavior Rating Inventory of Executive Functions, Metacognition Index; IQ, intelligence quotient; SE , standard error; SRS, Social Responsiveness Scale; β, standardized regression coefficients.
ASD PGS subgroup P < 0.1: ASD polygenic groups low, moderate, and high based on the autism spectrum disorder polygenic score at P < 0.1.
ADHD PGS subgroup P < 0.1: ADHD polygenic groups low, moderate, and high based on the attention deficit/hyperactivity disorder polygenic score at P < 0.1.
INT PGS subgroup P < 0.1: INT polygenic groups low, moderate, and high based on the general intelligence polygenic score at P < 0.1.
*P < 0.05 (two‐tailed); **P < 0.01 (two‐tailed).
n corresponds to participants without any missing variables in outcomes or covariates (in the total sample of the study n = 176, there were 19 missing on SRS total and 11 missing on full‐scale IQ). The covariates are included in the table to show their contribution to the model.
The same model explained 36% of the variance in MI score; F(11, 134) = 6.836, P < 0.001, R 2 = 0.36 (unadjusted). In this case, only SRS total (P < 0.001) made a significant contribution to the model. None of the PGS (ASD, ADHD, or INT) significantly contributed to this model (See Table 5). These results indicate that the PGS did not have a significant role in explaining the metacognitive aspects of executive function in our sample. For the regression model with GEC as dependent variable see Table 6.
Table 5.
Linear Regression Model Summary: Prediction of Metacognition Index (MI) (Total sample n = 146a)
| Predictor | B | SE B | 95% confidence interval | t | P | β | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Sex | −2.45 | 1.87 | −6.15 | 1.25 | −1.31 | 0.192 | −0.10 |
| Age | −0.17 | 0.23 | −0.63 | 0.29 | −0.72 | 0.471 | −0.05 |
| Full‐scale IQ | 0.05 | 0.06 | −0.07 | 0.16 | 0.83 | 0.407 | 0.06 |
| SRS_total | 0.40 | 0.05 | 0.30 | 0.51 | 7.56 | < 0.001** | 0.57 |
| ASD PGS subgr P < 0.1 | 1.36 | 1.41 | −1.43 | 4.16 | 0.97 | 0.336 | 0.07 |
| ADHD PGS subgr P < 0.1 | 0.99 | 1.34 | −1.65 | 3.63 | 0.74 | 0.461 | 0.05 |
| INT PGS subgr P < 0.1 | 1.15 | 1.42 | −1.66 | 3.97 | 0.81 | 0.420 | 0.06 |
Model's R 2 = 0.359 (unadjusted). Models P‐value < 0.001.
ASD PGS subgroup P < 0.1: ASD polygenic groups low, moderate, and high based on the autism spectrum disorder polygenic score at P < 0.1.
ADHD PGS subgroup P < 0.1: ADHD polygenic groups low, moderate, and high based on the attention deficit/hyperactivity disorder polygenic score at P < 0.1.
INT PGS subgroup P < 0.1: INT polygenic groups low, moderate, and high based on the general intelligence polygenic score at P < 0.1.
B, unstandardized regression coefficients; BRIEF‐BRI, Behavior Rating Inventory of Executive Functions, Behavior Regulation Index; BRIEF‐GEC, Behavior Rating Inventory of Executive Functions, Global Executive Composite; BRIEF‐MI, Behavior Rating Inventory of Executive Functions, Metacognition Index; IQ, intelligence quotient; SE, standard error; SRS, Social Responsiveness Scale; β, standardized regression coefficients.
*P < 0.05 (two‐tailed) **P < 0.01 (two‐tailed).
n corresponds to participants without any missing variables in outcomes or covariates (in the total sample of the study n = 176, there were 19 missing on SRS total and 11 missing on full‐scale IQ). The covariates are included in the table to show their contribution to the model.
Table 6.
Linear Regression Model Summary: Prediction of Global Executive Composite (GEC) (Total Sample n = 145a)
| Predictor | B | SE B | 95% confidence interval | t | P | β | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Sex | −3.64 | 1.86 | −7.32 | 0.05 | −1.95 | 0.053 | −0.13 |
| Age | −0.32 | 0.23 | −0.78 | 0.14 | −1.38 | 0.170 | −0.10 |
| Full‐scale_IQ | 0.05 | 0.06 | −0.07 | 0.17 | 0.86 | 0.389 | 0.06 |
| SRS_total | 0.48 | 0.05 | 0.37 | 0.59 | 8.90 | < 0.001** | 0.63 |
| ASD PGS subgr P < 0.1 | 2.68 | 1.44 | −0.18 | 5.53 | 1.85 | 0.066 | 0.13 |
| ADHD PGS subgr P < 0.1 | −0.02 | 1.35 | −2.70 | 2.66 | −0.01 | 0.989 | 0.00 |
| INT PGS subgr P < 0.1 | 0.49 | 1.45 | −2.37 | 3.35 | 0.34 | 0.736 | 0.02 |
R 2 = 0.432 (unadjusted). Models P‐value <0.001.
B, unstandardized regression coefficients; BRIEF‐BRI, Behavior Rating Inventory of Executive Functions, Behavior Regulation Index; BRIEF‐GEC, Behavior Rating Inventory of Executive Functions, Global Executive Composite; BRIEF‐MI, Behavior Rating Inventory of Executive Functions, Metacognition Index; IQ, intelligence quotient; SE, standard error; SRS, Social Responsiveness Scale; β, standardized regression coefficients.
ASD PGS subgroup p0.1: ASD polygenic groups low, moderate, and high based on the autism spectrum disorder polygenic score at P < 0.1.
ADHD PGS subgroup p0.1: ADHD polygenic groups low, moderate, and high based on the attention deficit/hyperactivity disorder polygenic score at P < 0.1.
INT PGS subgroup p0.1: INT polygenic groups low, moderate, and high based on the general intelligence polygenic score at P < 0.1.
*P < 0.05 (two‐tailed); **P < 0.01 (two‐tailed).
n corresponds to participants without any missing variables in outcomes or covariates (in the total sample of the study n = 176, there were 19 missing on SRS total and 11 missing on full‐scale IQ). The covariates are included in the table to show their contribution to the model.
We also fitted regression models with each PGS separately to investigate their relationship to BRI and MI, including sex, age, full‐scale IQ, and total SRS as covariates. The contribution of ASD PGS is mainly the same as when all three PGS were fitted in the same model. Fitting the regression model with BRI as dependent variable results in F(9, 132) = 9.753, P < 0.001, R 2 = 0.40 (unadjusted). SRS total (P < 0.001), sex (P = 0.016), and ASD PGS (P = 0.021) made a significant contribution to the model.
None of the PGSs (ASD, ADHD, or INT) had a significant contribution to the regression models, either with BRI or MI as dependent variables, when we used the continuous PGS (BRI as dependent variable P = 0.234–0.488, MI as dependent variable P = 0.175–0.983) (See Tables S1A–C).
The PGS association to social function (SRS)
We also investigated the association between the PGS and social function in everyday life measured with the SRS. In an independent sample t‐test we found a significant group difference between total SRS scores of the Low and the High INT PGS groups; total t‐score t = 66.0 in the Low PGS group and t = 76.2 in the High PGS group; t(45) = −2.27, 95% CI [−19.11, −1.14], P = 0.028, Cohen's d = 0.66. This means that the group with the highest PGS for INT had significantly more problems related to social function in everyday life than the group with Low PGS for INT. We did not find any significant differences between the Low and High ASD PGS groups or the Low and High ADHD PGS groups on the SRS total score (P = 0.921 and P = 0.865). Furthermore, we performed a regression analysis and controlled for age, sex, full‐scale IQ, MI from the BRIEF, BRI from the BRIEF, ASD, ADHD and INT PGS groups as well as PCs. The linear regression model was significant and explained 48% of the variance in total SRS score; F(12, 128) = 10.010, P < 0.001, R 2 = 0.48 (unadjusted). MI from the BRIEF (P = 0.001), BRI from the BRIEF (P < 0.001), and sex (P = 0.019) had a significant contribution to the model. None of the PGSs made a significant contribution to the total SRS score (P = 0.101–0.742) in the regression model.
Discussion
Our results showed a significant association between ASD PGS, representing the polygenetic components of ASD, and executive function deficits in everyday life in a clinical group seeking specialist health care due to autistic symptomatology. In our study, the BRI from the BRIEF differed significantly between individuals with the highest and the lowest PGS for ASD. The participants in the High PGS group had on average a t‐score of 71.5 on BRI, which is in the clinical range of the scale. In comparison, the participants in the Low PGS group had an average t‐score of 62.9, which is under the clinical cutoff (Cohen's d = 0.69). We did not observe any significant BRI difference between the Low and High PGS groups for ADHD or INT. This finding was in line with our hypothesis that in a sample consisting of participants with an ASD diagnosis and/or ASD symptomatology, the ASD PGS would be more strongly associated with BRIEF scores than the PGS for ADHD and INT. No significant differences in the MI from the BRIEF were detected between the Low and High PGS groups for ASD, ADHD, or INT. Since ASD characteristics are quantitative traits, we included participants under the diagnostic threshold for ASD. Furthermore, because of the large degree of comorbidity in ASD, we included PGS of other diagnostic groups. This enabled us to investigate the polygenic component of core ASD phenomena beyond diagnostic categories. The use of extreme PGSs may already have clinical relevance, for example, for cancer and cardiovascular diseases [Seibert et al., 2018; Torkamani et al., 2018]. In our study, we found a clinically meaningful and significant difference in the BRI scores from the BRIEF where the participants in the High ASD PGS had clinical/pathological t‐scores and the Low ASD PGS group had nonclinical score. Thus the use of extreme scores seems to have a potential for identifying clinically relevant levels of cognitive problems. However, further studies are needed before clinical relevance can be established.
It is possible that polygenic factors in ASD could be related to behavior differences, or other phenotypes not related to executive function. Furthermore, executive function deficits are not related to a specific disorder, but characteristic of several NDs, with few deficits in typically developing controls. This can explain why PGS for ASD in an ASD diagnostic group differs significantly from PGS for ASD in controls, but not significantly from PGS for ASD in a non‐ASD diagnostic group with ASD symptomatology. Thus, in the current clinical sample referred for autism symptomatology it is expected that the PGS for ASD will be higher for the clinical group without ASD than for controls.
The ASD PGS had a stronger relationship to executive function than the PGS for ADHD and INT in our clinical sample under assessment for ASD. This finding might imply that the common genetic variance associated with ASD is of greater importance for executive dysfunction than the genetic variance associated with ADHD or INT. Even though the ADHD PGS and INT PGS were not significant in the regression analysis with BRIEF as dependent variable, they still may have an association to BRIEF, and the findings must be interpreted with caution since the regression analyses are based on null‐hypothesis testing. However, based on the actual t‐scores in the Low and the High groups we found that there is a significant difference between the Low and the High ASD PGS groups in BRI, but not between Low and High ADHD and INT PGS groups. Furthermore, our results indicate that the behavioral regulation aspects of executive function are more strongly related to the polygenetic nature of ASD than the metacognitive aspects. The behavioral regulation index from the BRIEF contains the subscales inhibition, flexibility, and emotional control. The subscale flexibility is the hallmark of ASD, and it is within this area that those with ASD have the most pronounced difficulties, also compared with other clinical groups with executive function deficits [Hovik et al., 2014]. Therefore, executive function deficits most specifically related to ASD may also have the closest link to polygenetic components of ASD.
Our finding that difficulties with the behavior regulatory aspects of executive function and ASD PGS are positively associated contrasts Schork et al.'s finding that ASD PGS is associated with better performance on a cognitive flexibility task [Schork et al., 2018]. This might be explained by differences in sample and method, as Schork et al. investigated typically developing children with neuropsychological tests while we investigated children referred for clinical assessment and measured their executive functioning in everyday life. Furthermore, our finding is in line with studies of clinical populations where executive function deficits are associated with an ASD diagnosis [Demetriou et al., 2017]. The correlations between rating measures like the BRIEF and performance‐based measures of executive function are typically reported to be quite poor. In a review incorporating both clinical and nonclinical samples in addition to both children and adults, the mean correlation between scores on performance‐based measures and behavioral ratings by use of BRIEF was reported to be 0.15 [Toplak, West, & Stanovich, 2013]. Furthermore, it is known that genetic factors can contribute in complex ways to even performance‐based tests intended to measure the same underlying construct like, for example, memory [Kremen et al., 2014]. In a recent meta‐analysis of executive function in ASD, Demetriou et al. found that most measures of executive function did not achieve clinical utility in differentiating between ASD and typical controls [Demetriou et al., 2017]. However, informant‐based measures based on BRIEF achieved absolute clinical marker criteria. They conclude that the BRIEF is based on more representative environmental situations and has therefore a higher ecological validity than many performance‐based measures, and may thus be more appropriate in clinical practice. Furthermore, this is in line with recurrent findings that there is pronounced discrepancy between structured performance‐based measures of general intelligence and adaptive functioning (measures with e.g. Vineland‐II) in ASD [Tillmann et al., 2019]. We therefore think that our finding that a higher ASD risk (higher ASD PGS) is associated with more executive function difficulties is novel and interesting, and is consistent with the clinical representation of more executive function difficulties in the ASD population. Our findings illustrate how the polygenic component of NDs and its association to executive dysfunctions can disentangle psychological constructs and may be used to explore possible underlying biological explanatory models of executive dysfunction.
In a GWAS study, Sun et al. found that ADHD children had genetic variants related to behavioral regulation impairments measured with the BRIEF [Sun et al., 2018]. In children with NDs such as ASD and ADHD, there are indications that BRI from the BRIEF is more strongly associated with genetic factors than the metacognitive aspects of executive function [Sun et al., 2018]. This is in line with our findings of association between the ASD PGS and the BRI subscale, but not the metacognitive scale of BRIEF. Further, in our study we did not find any significant association between the PGS for ADHD and BRI. This seems to suggest a stronger genetic component of executive function in ASD in our sample, as the effect size was bigger for ASD (d = 0.69) than for ADHD (d = 0.20). The lack of significant association between ADHD PGS and BRIEF scores may also be due to a smaller sample of ADHD participants, however the effect sizes for the extreme scores groups (ADHD PGS Low vs. High on BRI: d = 0.20 and MI: d = 0.27) support the notion that ADHD PGS has less influence on BRIEF than the ASD PGS. Another explanation could be that ASD and ADHD involve different genetic mechanisms.
ASD and ADHD are both NDs and often manifest as comorbid conditions, both characterized by executive function deficits. In our study, the PGS for ASD groups did not reproduce the diagnostic categories as both the Low and High PGS group consisted of children/adolescents with ASD and/or ADHD. Dajani et al. [2016] studied a sample of ASD, ADHD, or comorbid ASD and ADHD, and found that executive function performance did not match the diagnostic categories. Sun et al. [2018] argue that is it more important to evaluate executive function than diagnosis for targeting executive function interventions. Taken together, this suggests that the Low and High PGS groups are not only an indirect measurement of ASD, but rather reflect that polygenic disposition for ASD associated with the behavior regulation part of executive function. Furthermore, it is important to be aware of potential factors that can influence the PGSs. In Grove et al.'s ASD sample it is likely that some of the participants might have had comorbid ADHD [Grove et al., 2019], and the ADHD risk score calculated by Demontis et al. might be more specific to ADHD [Demontis et al., 2018]. However, it is likely that there is some overlap between all the different phenotypes, especially at P‐values as high as 0.1.
The INT PGS was significantly related to the amount of social problems in this sample, and we found a moderate effect size in the comparison of Low vs. High INT PGS on the SRS (P = 0.028, d = 0.66). The PGS for ASD or ADHD did not significantly influence the SRS score. This is in line with other studies linking common variants associated with high IQ to social problems [Clarke et al., 2016; Grove et al., 2019]. However, the contribution of INT PGS was not significant in our regression model. SRS measures autistic social impairments in a quantitative score, and can also be viewed as an impairment score. However, we did not find a significant difference in total SRS score between the Low and High ASD PGS groups. We might have too small and heterogeneous sample consisting of different ASD diagnosis to detect a relationship between ASD PGS and SRS. Grove et al. found the Asperger diagnosis to be more strongly correlated to a high ASD PGS than classic autism (childhood autism) [Grove et al., 2019].
We did not find a significant difference in full‐scale IQ between the Low and the High ASD PGS groups in our sample (P = 0.439). In the whole sample, there was a small, positive correlation between ASD PGS and full‐scale IQ, r = 0.033, but this was not significant (P = 0.673). Therefore, our finding is not consistent with the reports showing high risk for ASD associated with higher IQ in the general population [Clarke et al., 2016]. The reason for this is probably the size and heterogeneity of our sample, which groups together different ASD diagnoses. The Asperger diagnosis has previously shown to be the ASD diagnosis with the highest positive correlation with high IQ [Grove et al., 2019].
We found a significant contribution of sex to the behavior regulation of executive function (BRI) in the regression model. This indicates that the relationship between genetic dispositions for ASD and executive function may be different for boys and girls. The low number of girls in our study makes these findings uncertain albeit in line with the “female protective model,” which proposes that more severe genetic mutations are required for a girl to develop ASD than for a boy [Levy et al., 2011]. Thus, common gene variants may also have different vulnerabilities depending on sex.
Strengths and limitations of the study
One of the strengths of the current study is that it includes a clinically relevant sample of participants (n = 176) who were referred for an ASD evaluation and were thoroughly clinically assessed for ASD. Furthermore, the PGSs are based on large discovery samples with large power. Even though we have relatively few participants, we found moderate effect sizes between measures of executive function (BRI) in the Low and High ASD PGS groups. The PGS for ASD is relatively new and based on a smaller sample/cohort than the PGS for ADHD and INT. Yet, only the PGS for ASD is significantly associated with executive function deficits (BRI). However, because of a relatively small sample size, the uncorrected P‐values, and the absence of an effect when using PGS as a continuous variable, the results should be interpreted with caution. Furthermore, the findings are in need of replication in larger samples. We also had information about the presence of possible CNVs in all the participants. We did not find more CNVs in the High vs. the Low ASD PGS group. Therefore, it is not likely that CNVs are the reason for more executive dysfunction in the High ASD PGS group. Another strength of the study is that we had no challenges with kinship or ethnicity as confounding factors.
Even though the participants were thoroughly assessed for ASD, we did not apply specific ADHD measures to compare the degree of ADHD symptoms; we only know whether they have a clinical diagnosis of ADHD. Although we controlled for age in the analyses, nonlinear age effects could bias the results.
Clinical implications
It has been stated that “the end game of PGS […] is personalized medicine” [Zheutling & Ross, 2018]. Children and adolescent with high PGS for ASD may be particularly vulnerable and have difficulties with executive function. Despite the present small effect and predictive power, the findings suggest that PGS may be a clinically useful tool in the future for children with NDs. If children at risk can be identified, it might be of clinical relevance to initiate prevention interventions aimed at the executive difficulties, or stratify the more general ASD treatment by PGS.
Conclusions
We report a significant relationship between PGS for ASD and executive function deficits in terms of behavior regulation in a clinical sample under evaluation for ASD symptomatology. Furthermore, we find that PGS of ASD, and not ADHD nor INT, has a significant contribution to executive function deficits when controlling for confounders. To our knowledge, this is the first study to find an association between the PGS for ASD and executive function in everyday life, and shows how information from PGS can be used in a clinical neurodevelopmental sample.
Supporting information
Supplementary Figure S1A. Scatterplot age by BRI t‐score from the BRIEF.
Supplementary Figure S1B. Scatterplot age by MI t‐score from the BRIEF.
Supplementary Figure S2A. Scatterplot BRI t‐score from the BRIEF by ASD PGS group P < 0.1.
Supplementary Figure S2B. Scatterplot MI t‐score from the BRIEF by ASD PGS group P < 0.1.
Supplementary Figure S3A. Q‐Q plots for BRI t‐scores from the BRIEF.
Shapiro–Wilk test P = 0.089. Kurtosis = −0.469. Skewness = −0.136.
Supplementary Figure S3B. Q‐Q plots for MI t‐scores from the BRIEF.
Shapiro–Wilk test P = 0.208. Kurtosis = −0.246. Skewness = −0.227.
Supplementary Figures S4A–D. Scatterplots principal components.
(PC01 by PC02, PC01 by PC03, PC01 by PC04 and PC01 by PC06).
Supplementary Figures S5A–J. Study participants relevant PCs in the context of the European reference population.
Supplementary Table S1A. Linear regression model summary. Prediction of Behavior Regulation Index (BRI). Total sample n = 1421.
Supplementary Table S1B. Linear regression model summary. Prediction of Metacognition Index (MI). Total sample n = 1461.
Supplementary Table S1C. Linear regression model summary. Prediction of Global Executive Composite (GEC). Total sample n = 1451.
Acknowledgments
We are thankful to the BUPGEN partners and to all the participants. The research was supported by the National Research Council of Norway (Grant #213694), the South‐Eastern Norway Regional Health Authority (Grant #39763), and Vestre Viken Hospital Trust (Grant #6903002).
References
- Amann, B. , Gomar, J. J. , Ortiz‐Gil, J. , McKenna, P. , Sans‐Sansa, B. , Sarro, S. , … Pomarol‐Clotet, E. (2012). Executive dysfunction and memory impairment in schizoaffective disorder: A comparison with bipolar disorder, schizophrenia and healthy controls. Psychological Medicine, 42(10), 2127–2135. 10.1017/S0033291712000104 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5). Washington, DC: American Psychiatric Association. [Google Scholar]
- Andersson, E. , Crowley, J. J. , Lindefors, N. , Ljotsson, B. , Hedman‐Lagerlof, E. , Boberg, J. , … Ruck, C. (2018). Genetics of response to cognitive behavior therapy in adults with major depression: A preliminary report. Molecular Psychiatry, 24, 484–490. 10.1038/s41380-018-0289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca, C. E. , Derringer, J. L. , Corley, R. P. , Young, S. E. , Keller, M. C. , Hewitt, J. K. , & Friedman, N. P. (2017). Predicting cognitive executive functioning with polygenic risk scores for psychiatric disorders. Behavior Genetics, 47(1), 11–24. 10.1007/s10519-016-9814-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, T. K. , Lupton, M. K. , Fernandez‐Pujals, A. M. , Starr, J. , Davies, G. , Cox, S. , … McIntosh, A. M. (2016). Common polygenic risk for autism spectrum disorder (ASD) is associated with cognitive ability in the general population. Molecular Psychiatry, 21(3), 419–425. 10.1038/mp.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. N. , & Gruber, C. P. (2005). Social responsiveness scale (SRS) manual. Los Angeles: Western Psychological Service. [Google Scholar]
- Craig, F. , Margari, F. , Legrottaglie, A. R. , Palumbi, R. , de Giambattista, C. , & Margari, L. (2016). A review of executive function deficits in autism spectrum disorder and attention‐deficit/hyperactivity disorder. Neuropsychiatric Disease and Treatment, 12, 1191–1202. 10.2147/NDT.S104620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert, B. N. (2014). The RDoC framework: Facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry, 13(1), 28–35. 10.1002/wps.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani, D. R. , Llabre, M. M. , Nebel, M. B. , Mostofsky, S. H. , & Uddin, L. Q. (2016). Heterogeneity of executive functions among comorbid neurodevelopmental disorders. Scientific Reports, 6, 36566 10.1038/srep36566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, M. , & Geurts, H. (2015). Influence of autism traits and executive functioning on quality of life in children with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 45, 2734–2743. 10.1007/s10803-015-2438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou, E. A. , Lampit, A. , Quintana, D. S. , Naismith, S. L. , Song, Y. J. C. , Pye, J. E. , … Guastella, A. J. (2017). Autism spectrum disorders: A meta‐analysis of executive function. Molecular Psychiatry, 23, 1198–1204. 10.1038/mp.2017.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis, D. , Walters, R. K. , Martin, J. , Mattheisen, M. , Als, T. D. , Agerbo, E. , … Neale, B. M. (2018). Discovery of the first genome‐wide significant risk loci for attention deficit/hyperactivity disorder. Nature Genetics, 51, 63–75. 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman, N. P. , & Miyake, A. (2017). Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex, 86, 186–204. 10.1016/j.cortex.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner, E. , & Iarocci, G. (2018). Everyday executive function predicts adaptive and internalizing behavior among children with and without autism spectrum disorder. Autism Research, 11(2), 284–295. 10.1002/aur.1877 [DOI] [PubMed] [Google Scholar]
- Gaugler, T. , Klei, L. , Sanders, S. J. , Bodea, C. A. , Goldberg, A. P. , Lee, A. B. , … Buxbaum, J. D. (2014). Most genetic risk for autism resides with common variation. Nature Genetics, 46(8), 881–885. 10.1038/ng.3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind, D. H. (2011). Genetics of autism spectrum disorders. Trends in Cognitive Sciences, 15(9), 409–416. 10.1016/j.tics.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind, D. H. , & State, M. W. (2015). Gene hunting in autism spectrum disorder: On the path to precision medicine. Lancet Neurology, 14(11), 1109–1120. 10.1016/S1474-4422(15)00044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg, C. (2010). The ESSENCE in child psychiatry: Early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Research in Developmental Disabilities, 31(6), 1543–1551. 10.1016/j.ridd.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Gioia, G. A. , Isquith, P. K. , Guy, S. C. , & Kenworthy, L. (2000). Behavior rating rating inventory of executive function (BRIEF). Florida: Psychological Assessment Resources, Inc. [Google Scholar]
- Gioia, G. A. , Isquith, P. K. , Guy, S. C. , & Kenworthy, L. (2015). BRIEF 2: Behavior rating inventory of executive functions (2nd ed). Florida: Psychological Assessment Resources, Inc. [Google Scholar]
- Grove, J. , Ripke, S. , Als, T. D. , Mattheisen, M. , Walters, R. K. , Won, H. , … Borglum, A. D. (2019). Identification of common genetic risk variants for autism spectrum disorder. Nature Genetics, 51(3), 431–444. 10.1038/s41588-019-0344-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, E. L. (2004). Executive dysfunction in autism. Trends in Cognitive Sciences, 8(1), 26–32. [DOI] [PubMed] [Google Scholar]
- Hovik, K. T. , Egeland, J. , Isquith, P. K. , Gioia, G. , Skogli, E. W. , Andersen, P. N. , & Oie, M. (2014). Distinct patterns of everyday executive function problems distinguish children with tourette syndrome from children with ADHD or autism spectrum disorders. Journal of Attention Disorders, 21, 811–823. 10.1177/1087054714550336 [DOI] [PubMed] [Google Scholar]
- Hoyland, A. L. , Naerland, T. , Engstrom, M. , Torske, T. , Lydersen, S. , & Andreassen, O. A. (2019). Atypical event‐related potentials revealed during the passive parts of a Go‐NoGo task in autism spectrum disorder: A case‐control study. Molecular Autism, 10, 10 10.1186/s13229-019-0259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium , Purcell, S. M. , Wray, N. R. , Stone, J. L. , Visscher, P. M. , O'Donovan, M. C. , … Sklar, P. (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature, 460(7256), 748–752. 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy, L. , Yerys, B. E. , Anthony, L. G. , & Wallace, G. L. (2008). Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review, 18(4), 320–338. 10.1007/s11065-008-9077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera, A. V. , Chaffin, M. , Aragam, K. G. , Haas, M. E. , Roselli, C. , Choi, S. H. , … Kathiresan, S. (2018). Genome‐wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nature Genetics, 50(9), 1219–1224. 10.1038/s41588-018-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen, W. S. , Panizzon, M. S. , Franz, C. E. , Spoon, K. M. , Vuoksimaa, E. , Jacobson, K. C. , … Lyons, M. J. (2014). Genetic complexity of episodic memory: A twin approach to studies of aging. Psychology and Aging, 29(2), 404–417. 10.1037/a0035962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M. C. , Lombardo, M. V. , & Baron‐Cohen, S. (2014). Autism. Lancet, 383(9920), 896–910. 10.1016/s0140-6736(13)61539-1 [DOI] [PubMed] [Google Scholar]
- Leung, R. C. , Vogan, V. M. , Powell, T. L. , Anagnostou, E. , & Taylor, M. J. (2015). The role of executive functions in social impairment in Autism Spectrum Disorder. Child Neuropsychology, 22, 1–9. 10.1080/09297049.2015.1005066 [DOI] [PubMed] [Google Scholar]
- Levy, D. , Ronemus, M. , Yamrom, B. , Lee, Y. H. , Leotta, A. , Kendall, J. , … Wigler, M. (2011). Rare de novo and transmitted copy‐number variation in autistic spectrum disorders. Neuron, 70(5), 886–897. 10.1016/j.neuron.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Willer, C. J. , Ding, J. , Scheet, P. , & Abecasis, G. R. (2010). MaCH: Using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genetic Epidemiology, 34(8), 816–834. 10.1002/gepi.20533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Elsabbagh, M. , Baird, G. , & Veenstra‐Vanderweele, J. (2018). Autism spectrum disorder. Lancet, 392(10146), 508–520. 10.1016/S0140-6736(18)31129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord, C. , Risi, S. , Lambrecht, L. , Cook, E. H., Jr. , Leventhal, B. L. , DiLavore, P. C. , … Rutter, M. (2000). The autism diagnostic observation schedule‐generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. [PubMed] [Google Scholar]
- Lord, C. , Rutter, M. , & Le Couteur, A. (1994). Autism Diagnostic Interview‐Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Martin, A. R. , Daly, M. J. , Robinson, E. B. , Hyman, S. E. , & Neale, B. M. (2018). Predicting polygenic risk of psychiatric disorders. Biological Psychiatry, 86, 97–109. 10.1016/j.biopsych.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, A. , Friedman, N. P. , Emerson, M. J. , Witzki, A. H. , Howerter, A. , & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- n.d. (2010). A decade for psychiatric disorders. Nature, 463(7277), 9 10.1038/463009a [DOI] [PubMed] [Google Scholar]
- Purcell, S. , Neale, B. , Todd‐Brown, K. , Thomas, L. , Ferreira, M. A. , Bender, D. , … Sham, P. C. (2007). PLINK: A tool set for whole‐genome association and population‐based linkage analyses. American Journal of Human Genetics, 81(3), 559–575. 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami, G. , & Geschwind, D. H. (2018). Genetics of autism spectrum disorder. Handbook of Clinical Neurology, 147, 321–329. 10.1016/B978-0-444-63233-3.00021-X [DOI] [PubMed] [Google Scholar]
- Rommelse, N. N. , Geurts, H. M. , Franke, B. , Buitelaar, J. K. , & Hartman, C. A. (2011). A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention‐deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neuroscience and Biobehavioral Reviews, 35(6), 1363–1396. 10.1016/j.neubiorev.2011.02.015 [DOI] [PubMed] [Google Scholar]
- Savage, J. E. , Jansen, P. R. , Stringer, S. , Watanabe, K. , Bryois, J. , de Leeuw, C. A. , … Posthuma, D. (2018). Genome‐wide association meta‐analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nature Genetics, 50(7), 912–919. 10.1038/s41588-018-0152-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium . (2014). Biological insights from 108 schizophrenia‐associated genetic loci. Nature, 511(7510), 421–427. 10.1038/nature13595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schork, A. J. , Brown, T. T. , Hagler, D. J. , Thompson, W. K. , Chen, C. H. , Dale, A. M. , … Genetics, S. (2018). Polygenic risk for psychiatric disorders correlates with executive function in typical development. Genes, Brain and Behavior, 18, e12480 10.1111/gbb.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert, T. M. , Fan, C. C. , Wang, Y. , Zuber, V. , Karunamuni, R. , Parsons, J. K. , … Practical Consortium . (2018). Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large scale cohorts. BMJ, 360, j5757 10.1136/bmj.j5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, H. R. , Miyake, A. , & Hankin, B. L. (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6, 328 10.3389/fpsyg.2015.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderby, I. E. , Gustafsson, O. , Doan, N. T. , Hibar, D. P. , Martin‐Brevet, S. , Abdellaoui, A. , … 16p11.2 European Consortium, for the ENIGMA‐CNV working group . (2018). Dose response of the 16p11.2 distal copy number variant on intracranial volume and basal ganglia. Molecular Psychiatry, 1–19. 10.1038/s41380-018-0118-1 [DOI] [Google Scholar]
- Sun, X. , Wu, Z. , Cao, Q. , Qian, Y. , Liu, Y. , Yang, B. , … Wang, Y. (2018). Genetic variant for behavioral regulation factor of executive function and its possible brain mechanism in attention deficit hyperactivity disorder. Scientific Reports, 8(1), 7620 10.1038/s41598-018-26042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann, J. , San Jose Caceres, A. , Chatham, C. H. , Crawley, D. , Holt, R. , Oakley, B. , … EU‐AIMS LEAP Group . (2019). Investigating the factors underlying adaptive functioning in autism in the EU‐AIMS Longitudinal European Autism Project. Autism Research, 12(4), 645–657. 10.1002/aur.2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak, M. E. , West, R. F. , & Stanovich, K. E. (2013). Practitioner review: do performance‐based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry, 54(2), 131–143. 10.1111/jcpp.12001 [DOI] [PubMed] [Google Scholar]
- Torkamani, A. , Wineinger, N. E. , & Topol, E. J. (2018). The personal and clinical utility of polygenic risk scores. Nature Reviews Genetics, 19(9), 581–590. 10.1038/s41576-018-0018-x [DOI] [PubMed] [Google Scholar]
- Torske, T. , Naerland, T. , Oie, M. G. , Stenberg, N. , & Andreassen, O. A. (2017). Metacognitive aspects of executive function are highly associated with social functioning on parent‐rated measures in children with autism spectrum disorder. Frontiers in Behavioral Neuroscience, 11, 258 10.3389/fnbeh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen, E. R. , & Pigott, S. E. (2002). The relationship between parental report on the BRIEF and performance‐based measures of executive function in children with moderate to severe traumatic brain injury. Child Neuropsychology, 8(4), 296–303. 10.1076/chin.8.4.296.13505 [DOI] [PubMed] [Google Scholar]
- Wechsler, D. (2002). Wechsler preschool and primary scale of intelligence ‐ Third edition. San Antonio, TX: Pearson. [Google Scholar]
- Wechsler, D. (2003). Wechler Intelligence Scale for Children ‐ Fourth edition. San Antonio, TX: Pearson. [Google Scholar]
- Wechsler, D. (2008). Wechsler Adult Intelligence Scale–Fourth Edition. San Antonio, TX: Pearson. [Google Scholar]
- Zheutling, A. B. , & Ross, D. (2018). Polygenic risk scores: What are they good for? Biological Psychiatry, 83(11), e51–e53. 10.1016/j.biopsych.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1A. Scatterplot age by BRI t‐score from the BRIEF.
Supplementary Figure S1B. Scatterplot age by MI t‐score from the BRIEF.
Supplementary Figure S2A. Scatterplot BRI t‐score from the BRIEF by ASD PGS group P < 0.1.
Supplementary Figure S2B. Scatterplot MI t‐score from the BRIEF by ASD PGS group P < 0.1.
Supplementary Figure S3A. Q‐Q plots for BRI t‐scores from the BRIEF.
Shapiro–Wilk test P = 0.089. Kurtosis = −0.469. Skewness = −0.136.
Supplementary Figure S3B. Q‐Q plots for MI t‐scores from the BRIEF.
Shapiro–Wilk test P = 0.208. Kurtosis = −0.246. Skewness = −0.227.
Supplementary Figures S4A–D. Scatterplots principal components.
(PC01 by PC02, PC01 by PC03, PC01 by PC04 and PC01 by PC06).
Supplementary Figures S5A–J. Study participants relevant PCs in the context of the European reference population.
Supplementary Table S1A. Linear regression model summary. Prediction of Behavior Regulation Index (BRI). Total sample n = 1421.
Supplementary Table S1B. Linear regression model summary. Prediction of Metacognition Index (MI). Total sample n = 1461.
Supplementary Table S1C. Linear regression model summary. Prediction of Global Executive Composite (GEC). Total sample n = 1451.


