Abstract
Background
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei (PMP) is associated with excessive bleeding and acquired fibrinogen deficiency. Maintaining plasma fibrinogen may support hemostasis.
Objectives
To compare hemostatic efficacy and safety of human fibrinogen concentrate (HFC) vs cryoprecipitate as fibrinogen sources for bleeding patients with acquired fibrinogen deficiency undergoing PMP CRS.
Methods
FORMA‐05 was an off‐label single‐center, prospective, randomized, controlled phase 2 study. Patients undergoing PMP surgery with predicted intraoperative blood loss ≥2 L received human fibrinogen concentrate (HFC; 4 g) or cryoprecipitate (two pools of 5 units, containing approximately 4.0‐4.6 g fibrinogen), repeated as needed. The primary endpoint was a composite of intraoperative and postoperative efficacy, graded using objective 4‐point scales and adjudicated by an independent committee.
Results
One hundred percent of patients receiving HFC (95% confidence interval: 83.9‐100.0, n = 21) or cryoprecipitate (84.6‐100.0, n = 22) achieved hemostatic success. HFC demonstrated noninferior efficacy (P = .0095; post hoc) and arrived in the operating room 46 minutes faster. There were significantly greater mean increases with HFC vs cryoprecipitate in plasma fibrinogen (0.78 vs 0.35 g/L; P < .0001) and FIBTEM A20 (3.33 vs 0.93 mm; P = .003). Factor XIII, factor VIII, and von Willebrand factor activity were maintained throughout surgery. Only red blood cells were transfused intraoperatively (median units: HFC group, 1.0; cryoprecipitate group, 0.5). Thromboembolic events were detected with cryoprecipitate only. Safety was otherwise comparable between groups.
Conclusions
Human fibrinogen concentrate was hemostatically efficacious in patients undergoing major abdominal PMP surgery, with a favorable safety profile. These results are relevant to other surgical settings where bleeding and acquired fibrinogen deficiency occur.
Keywords: cytoreductive surgical procedures, fibrinogen, hemostasis, Pseudomyxoma peritonei, thrombelastography
Essentials.
Cytoreductive surgery for pseudomyxoma peritonei (PMP) is associated with excessive bleeding.
This study compared human fibrinogen concentrate (HFC) vs cryoprecipitate during CRS for PMP.
The primary endpoint of efficacy of HFC was non‐inferior vs cryoprecipitate.
Safety was comparable; however, thromboembolic events were observed with cryoprecipitate only.
1. INTRODUCTION
Pseudomyxoma peritonei (PMP) is a rare malignancy with an estimated incidence of 1 to 3 per million per year.1, 2 It usually arises from a perforated primary appendix tumor with associated mucus production, resulting in the spread of mucus and tumor cells throughout the abdominal cavity.3, 4 Although PMP does not usually metastasize systemically, it is eventually fatal in most cases unless definitively treated by surgery because the space required within the abdomen for nutritional function is replaced by progressive mucinous tumor and mucin accumulation.4
The treatment of peritoneal malignancy has advanced considerably in recent years, and cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is now the established therapeutic modality.5, 6, 7 Also known as the Sugarbaker procedure, this approach uses cytoreductive surgery to remove macroscopic disease, and HIPEC to eradicate microscopic disease and reduce risk of recurrence.8 Cytoreductive surgery usually requires multiple peritonectomy procedures and visceral resections to remove malignant tissue from all involved intra‐abdominal surfaces and organs, followed by intra‐abdominal instillation of HIPEC for 1 hour at 42°C.9, 10 Because of its complexity, the surgery is often prolonged;8 a previous study reported that 84% of such operations required 7 to 12 hours to perform, whereas 9% lasted for more than 12 hours.9 CRS with HIPEC for extensive peritoneal malignancy, such as advanced PMP, may be associated with large‐volume blood loss and substantial use of blood products such as plasma (e.g., 5‐6 units) and red blood cells (RBC; e.g., 4 units).8, 11, 12, 13 Mean blood loss has been estimated to be approximately 1500 mL (ranging up to approximately 5000 mL),11, 13 with as many as three‐quarters of patients requiring transfusions of allogeneic blood products.12, 13 Effective coagulation management is therefore essential to help achieve successful outcomes. The observations of severe bleeding with this type of surgery led to the development of pre‐emptive hemostatic strategies at our center, initially based on fresh frozen plasma (FFP) and more recently based on cryoprecipitate, with the aim of improving hemostatic control during CRS with HIPEC.8
The main coagulopathy observed during this extensive abdominal surgery is the rapid fall in plasma fibrinogen concentration and, consequently, diminished clot quality.14 The fibrinogen concentration decreases through loss of whole blood, hemodilution (from intravenous fluid support), and consumption through the extensive surgical excision, requiring continuous fibrin formation at the wound area. With no endothelial or hepatic reserves of this coagulation factor, this acquired fibrinogen deficiency can only be corrected through administration of exogenous fibrinogen.
Several sources of fibrinogen are available, with cryoprecipitate and human fibrinogen concentrate (HFC) the preferred options in terms of fibrinogen concentration. Both product types have demonstrated ability to increase plasma fibrinogen levels in bleeding patients.15, 16, 17 However, cryoprecipitate has variable fibrinogen content, requires blood type matching, time to thaw, and carries risks of transfusion‐related acute lung injury and pathogen transmission, and has been withdrawn in some European countries.18, 19 In contrast, HFC is a highly purified preparation that contains a defined concentration of fibrinogen, does not require blood type matching, and offers enhanced pathogen safety owing to the virus inactivation steps used in production.20 Robust evidence supporting the use of cryoprecipitate or HFC for correcting acquired fibrinogen deficiency is limited, however, with studies often being retrospective or including only a small number of patients. The objective of the present prospective, randomized, controlled phase 2 study was to evaluate the hemostatic efficacy and safety of a newly developed HFC (Fibryga®; Octapharma AG) compared with cryoprecipitate as sources of fibrinogen for patients with acquired fibrinogen deficiency undergoing cytoreductive surgery with HIPEC for extensive PMP.
2. METHODS
2.1. Study design
This was a prospective, randomized, controlled, open‐label, phase 2 study in patients undergoing cytoreductive surgery with HIPEC for PMP at Basingstoke and North Hampshire Hospital, UK, from March 2017 to July 2018. The study was prospectively registered with the EU Clinical Trials Register on 12 September 2016, under EudraCT Number 2016‐003749‐27. The study was conducted in accordance with Good Clinical Practice (CPMP/ICH/135/95), the Declaration of Helsinki and its amendments, and national law. The study protocol was approved by the National Health Service (NHS) Health Research Authority, South Central ‐ Hampshire A Research Ethics Committee (Ref: 16/SC/0576). All enrolled patients provided voluntary, written informed consent before participating.
2.2. Study population
Patients were enrolled if they were aged ≥18 years, were undergoing cytoreductive surgery with HIPEC for PMP, and had provided written informed consent. Bleeding risk was assessed 60 to 90 minutes after the start of surgery, when excessive blood loss was observed, but before 2 L of blood was lost. When the average intraoperative blood loss in the absence of targeted fibrinogen replacement was predicted to be ≥2 L, the patient was randomized to receive fibrinogen replacement with either HFC (Fibryga®) or cryoprecipitate. A randomization list was prepared by the study statistician and consisted of randomization numbers with treatments associated with each number (1:1 treatment ratio). Randomization envelopes showing only the randomization number were available to the investigator. If the patient was deemed to need fibrinogen replacement based on the bleeding risk assessment, he or she was assigned the next available randomization number by the anesthesiologist; otherwise, the patient was considered a screening failure. Other key exclusion criteria included: a recent history of thromboembolic disease; known presence of chronic infections; undergoing emergency surgery or surgery for other forms of peritoneal malignancy or infection; receipt of fibrinogen‐containing products within 14 days before screening; any known congenital or acquired coagulation disorder; administration of a coagulation‐active drug before the start of treatment; known hypersensitivity to the study medication; and pregnant or lactating women.
2.3. Treatments
A newly developed HFC (Fibryga®) was used in this study, which undergoes two dedicated virus inactivation/removal steps (solvent/detergent treatment and nanofiltration) during its production.20 Lyophilized HFC was delivered by the blood bank to the operating room and then prepared in accordance with the manufacturer's instructions using the dedicated Octajet transfer device and administered immediately after reconstitution. Cryoprecipitate was thawed and prepared for administration by the local blood bank, delivered to the operating room, and administered upon arrival. Based on the prediction of an average intraoperative blood loss ≥2 L, which was made approximately 90 minutes into the surgery when the abdominal cavity was open and the extent of the surgery could be thoroughly assessed, the first dose of HFC (4 g) or cryoprecipitate (two pools, each pool consisting of 5 units/donations, and containing approximately 4.3 [4.0‐4.6 g] fibrinogen) was subsequently administered early intraoperatively via intravenous injection over approximately 20 minutes. Further HFC or cryoprecipitate administration intraoperatively was based on the FIBTEM test of the ROTEM® analysis. A FIBTEM A20 of ≤12 mm triggered the administration of 4 g HFC or 2 pools of cryoprecipitate. Any administration of HFC or cryoprecipitate during the first 24 hours postoperatively was based on clinical judgment of the need for further hemostatic support and guided by FIBTEM analysis, with an A20 of ≤12 mm triggering administration of 2 g HFC or 1 pool of cryoprecipitate. Total amounts of fibrinogen supplemented intraoperatively and postoperatively were calculated according to the actual potency of the HFC batch. For cryoprecipitate, an average of 0.43 g fibrinogen per unit of cryoprecipitate was assumed, which is the midpoint of the mean fibrinogen content of 0.40 to 0.46 g per unit as stated by the NHS Blood and Transplant service.21 The times from patient randomization and surgery start to availability of ready‐to‐administer HFC and cryoprecipitate in the operating room were recorded. For all patients, tranexamic acid (TXA) was permitted according to the clinic's standard practice. RBC concentrates were transfused if hemoglobin levels fell below 8 g/L. Routine thromboprophylaxis was administered to all patients and consisted of low molecular weight heparin together with mechanical means during surgery.
2.4. Evaluation of hemostatic efficacy
The primary endpoint was a composite of intraoperative and postoperative hemostatic efficacy, assessed by the investigators and adjudicated by an Independent Data Monitoring and Endpoint Adjudication Committee (IDMEAC) according to a predefined algorithm (Table S1). Intraoperative efficacy was assessed at the end of surgery by both the surgeon and the anesthesiologist using an objective 4‐point hemostatic efficacy scale, with ratings of excellent, good, moderate, or none (Table S2). Postoperative efficacy was assessed 24 hours after the end of surgery by the hematologist, also using an objective 4‐point hemostatic efficacy scale (Table S3). Overall treatment success was defined as a rating of excellent or good. For all hemostatic efficacy adjudications, the IDMEAC was blinded to the treatment received by each patient.
2.5. Coagulation profiles and clot strength
Plasma fibrinogen levels determined using the Clauss assay, and fibrin clot strength determined by ROTEM analysis (FIBTEM A20), were measured before and after each dose of HFC or cryoprecipitate and also hourly, unless there was overlap between these timings. Other thromboelastometry and coagulation parameters, including factor VIII activity (FVIII:C), von Willebrand factor ristocetin cofactor activity (VWF:RCo) and factor XIII (FXIII), were assessed every 2 hours during surgery. All thromboelastometry and coagulation parameters were also assessed at the preoperative, intraoperative, and end‐of‐surgery assessments, and at 6, 12, 24, and 48 hours and on day 10 after surgery. In addition to FIBTEM A20, thromboelastometry measurements included EXTEM A20 and EXTEM clotting time (CT), which represent the contribution of the extrinsic coagulation pathway to clot strength and time to clot formation, respectively. A separate publication is planned to describe the coagulation profiles in more detail, including results of other coagulation parameters such as VWF antigen, thrombin generation, and platelet count.
2.6. Critical care outcomes
The following critical care outcome data were recorded: duration of surgery; duration of intensive care unit (ICU) stay (including duration of artificial ventilation); duration of hospital stay; intraoperative and 24‐hour, 48‐hour, and 10‐day postoperative requirements for blood products (including FFP, RBC concentrate, and platelets); intraoperative, 24‐hour, and 48‐hour postoperative blood loss; requirement for reoperation or reoperation for bleeding; and 21‐day mortality.
2.7. Safety
All adverse events (AEs) occurring during the study period were recorded (i.e., up to 21 [±7] days after surgery or end of hospitalization, whichever occurred first). Treatment‐emergent AEs were defined as AEs that started or worsened after the start of HFC/cryoprecipitate infusion. For each AE, the severity (mild, moderate, or severe), seriousness (nonserious or serious), and causality (probable, possible, unlikely, or unrelated) were assessed.
2.8. Statistical analysis
A formal sample size calculation was not performed because reliable information on treatment effects with respect to the chosen endpoint was not available. It was considered that this type of surgery was sufficiently standardized to provide a homogenous patient population and to allow for the investigation of the efficacy of fibrinogen supplementation in controlling bleeding.
Fisher's exact test was used to test hypotheses and explore distributions of hemostatic efficacy ratings between treatment groups. Noninferiority of HFC compared with cryoprecipitate for the primary endpoint was analyzed post hoc using the Farrington‐Manning test, with a noninferiority margin of 0.2. Secondary efficacy endpoints were analyzed by presenting sampling statistics by treatment group as well as treatment group differences for means and proportions with 95% confidence intervals (CIs). All statistical analyses were performed using SAS 9.4 software.
The full analysis set (FAS) comprised all randomized patients who received at least one dose of HFC or cryoprecipitate. The per protocol (PP) population excluded patients with major protocol deviations, including substantial deviations from the treatment schedule. This manuscript presents the PP analysis as considered appropriate for a pilot phase 2 study.
3. RESULTS
3.1. Patient characteristics
A total of 48 patients were enrolled in the study (Figure 1). Two patients did not fulfill the eligibility criterion of intraoperative need for fibrinogen replacement, whereas one patient was randomized but did not receive fibrinogen replacement. The FAS therefore comprised 45 patients who received either HFC (n = 22) or cryoprecipitate (n = 23). Of these, two patients (one in the HFC group and one in the cryoprecipitate group) had major protocol deviations (underdosing and overdosing, respectively), and so were excluded from the PP population.
Figure 1.
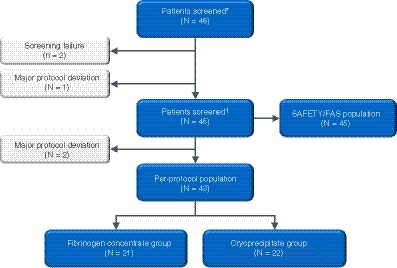
Patient flow. ∗Patients screened were screened and gave consent. †Patients treated received HFC or cryoprecipitate and were included in the study. FAS, full analysis set; HFC, human fibrinogen concentrate; N, number of patients
The median age of the FAS population was 61 years (range: 34‐76) (Table S4). Overall, 55.6% of patients were female and 97.8% were classified as white, with one patient classified as Asian. The mean (±standard deviation [SD]) total peritoneal cancer index score was 27.62 (±7.83). There were no statistically significant differences in the baseline characteristics between the HFC and cryoprecipitate groups. The two groups had similar volumes of disease as reflected by the comparable peritoneal cancer index scores at baseline (Table S4). The coagulation profile was also comparable between groups at the time of randomization (Table S5).
3.2. Hemostatic efficacy
In the analysis of the primary endpoint in the PP population, overall hemostatic efficacy was rated as a success for 100.0% (95% CI: 83.9‐100.0, n = 21) of patients in the HFC and 100% (95% CI: 84.6‐100.0, n = 22) of those in the cryoprecipitate group. A post hoc analysis demonstrated noninferiority of HFC compared with cryoprecipitate for achieving successful overall hemostatic efficacy (P = .0095).
For the HFC group, the surgeon, anesthesiologist, and IDMEAC all rated intraoperative hemostatic efficacy as excellent for 61.9% of patients, good for 33.3%, and moderate for 4.8% (Figure 2). For the cryoprecipitate group, the surgeon and anesthesiologist rated intraoperative efficacy as excellent for 54.6% of patients, good for 27.3%. and moderate for 18.2%, whereas the IDMEAC ratings differed slightly, at 50.0% excellent, 22.7% good, and 27.3% moderate. The hematologist and IDMEAC rated postoperative efficacy as excellent for all patients in both the HFC and cryoprecipitate groups.
Figure 2.
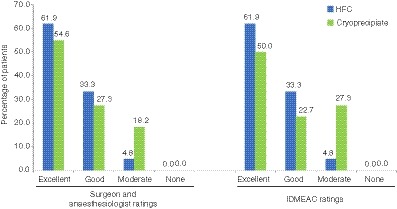
Intraoperative hemostatic efficacy ratings assessed by the surgeon and the anesthesiologist, and the IDMEAC (PP analysis; N = 43). HFC, human fibrinogen concentrate; IDMEAC, Independent Data Monitoring and Endpoint Adjudication Committee; PP, per protocol
The HFC demonstrated a significantly greater capacity to increase fibrinogen levels and fibrin‐based clot strength than cryoprecipitate. In the PP population, the mean (±SD) of the increases in plasma fibrinogen observed after each intraoperative dose of HFC was 0.78 g/L (±0.34); the corresponding mean increase for the cryoprecipitate group was significantly smaller at 0.35 g/L (±0.29; P < .0001 for between‐group comparison). The mean (±SD) of the increases in FIBTEM A20 observed after each intraoperative dose of HFC was 3.33 mm (±1.99). The corresponding mean increase for the cryoprecipitate group was 0.93 mm (±2.91), which was significantly lower than for the HFC group (P = .003).
3.3. Dosing of HFC and cryoprecipitate
In the PP population, the mean (±SD) intraoperative dose of HFC was 6.48 g (±2.96), equating to 89.08 (±38.88) mg/kg body weight, and the mean (±SD) intraoperative dose of cryoprecipitate was 4.09 pools (±2.18). The mean (±SD) total volume of investigational medicinal product infused intraoperatively was 323.8 mL (±148.0) for HFC and 822.7 mL (±431.7) for cryoprecipitate. All patients in the PP population received an initial dose of 4 g HFC or 2 pools of cryoprecipitate. Following this, fewer patients in the HFC group received at least one subsequent intraoperative dose (47.6% vs 63.6% of the cryoprecipitate group) (Table 1). This difference was despite a comparable duration of surgery for the two groups (median 7.5 and 7.9 hours for HFC and cryoprecipitate, respectively). Three patients received further fibrinogen supplementation postoperatively: two in the HFC group and one in the cryoprecipitate group.
Table 1.
Number of doses of HFC or cryoprecipitate administered intraoperatively (PP analysis; N = 43)
| HFC (n = 22) | Cryoprecipitate (n = 23) | |
|---|---|---|
| Initial dose, n% | 21 (100.00) | 22 (100.00) |
| First subsequent intraoperative dose, n (%) | 10 (47.62) | 14 (63.64) |
| Second subsequent intraoperative doses, n (%) | 3 (14.29) | 6 (27.27) |
| Third subsequent intraoperative doses, n (%) | 0 (0.00) | 2 (9.90) |
| Fourth subsequent intraoperative doses, n (%) | 0 (0.00) | 1 (4.55) |
Abbreviations: HFC, human fibrinogen concentrate; PP, per protocol.
Dose of HFC = 4 g; dose of cryoprecipitate = 2 pools of 5 units.
3.4. Time to administration
The time between surgery start and randomization did not differ significantly between the two groups (Table 2). The HFC was delivered to the operating room a mean of 46 minutes earlier than cryoprecipitate. As a result, the mean time between randomization and the start of the initial infusion was 24 minutes shorter for the HFC group (P < .0001).
Table 2.
Time between selected intraoperative time points, mean ± SD, hours (PP analysis; N = 43
| HFC (n = 21) | Cryoprecipitate (n = 22) | Mean difference | P valuea | |
|---|---|---|---|---|
| Surgery start to randomization | 1.13 ± 0.12 | 1.12 ± 0.10 | 0.01 | .7558 |
| Surgery start to availability of IMP in operating room | 1.52 ± 0.13 | 2.30 ± 0.33 | −0.77 | <.0001 |
| Randomization to availability of initial infusion in operating room | 0.39 ± 0.15 | 1.19 ± 0.32 | −0.80 | <.0001 |
| Randomization to initial infusion start | 0.90 ± 0.23 | 1.30 ± 0.33 | −0.40 | <.0001 |
| Surgery start to initial infusion start | 2.02 ± 0.22 | 2.42 ± 0.33 | −0.40 | <.0001 |
Abbreviations: HFC, human fibrinogen concentrate; IMP, Investigational medicinal product; PP, per protocol; SD, standard deviation.
Two‐sided P value was calculated at 5% level of significance by using paired t test for comparison.
Information on infusion duration was available for 33 intraoperative HFC infusions in the PP population. For each infusion, patients received a dose of 4 g (200 mL). The mean (±SD) duration of infusion was 19.00 minutes (±1.32), with a median (range) of 20.00 minutes (16.00‐20.00). The mean (±SD) rate of infusion was 10.58 mL/min (±0.78), with a median (range) of 10.00 mL/min (10.00‐12.50).
3.5. Coagulation parameters and clot strength
In the FAS population, the two groups had comparable plasma fibrinogen levels at baseline and at 1 hour after the start of surgery (Figure 3A). From 2 hours after surgery start, throughout the remaining intraoperative period and at all postoperative time points, fibrinogen levels were numerically higher in the HFC group.
Figure 3.
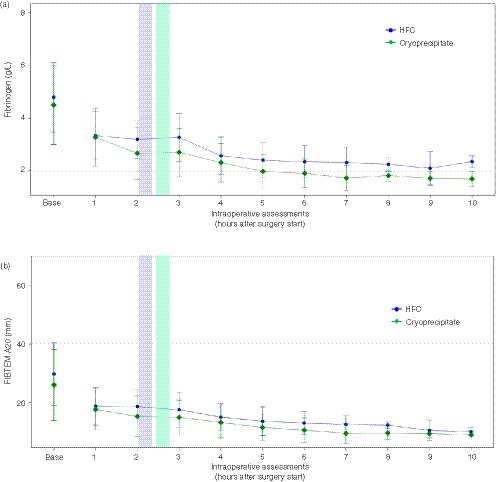
Changes in plasma fibrinogen level and FIBTEM A20 during the intraoperative period (FAS analysis; N = 45) FIBTEM A20, fibrinogen contribution to clot strength at 20 minutes in the ROTEM assay. FAS, full analysis set; HFC, human fibrinogen concentrate. Colored bars represent the period between the mean start time of the initial infusion to the mean end time of the initial infusion; blue, HFC; green, cryoprecipitate
FIBTEM A20 was numerically higher in the HFC group compared with the cryoprecipitate group at baseline, but values were comparable between groups at 1 hour after the start of surgery (Figure 3B). From 2 hours after surgery start, FIBTEM A20 was numerically higher in the HFC group until 8 hours intraoperatively, after which the values were comparable between the groups except for the final measurement, on postoperative day 10, when FIBTEM A20 was numerically higher with cryoprecipitate.
EXTEM A20 values were comparable for the HFC and cryoprecipitate groups at baseline (67.1 mm [±7.9] vs 66.1 mm [±7.2]), then decreased during surgery, with mean values at the end‐of‐surgery assessment being numerically higher for the HFC group (57.8 mm [±6.1] vs 54.6 mm [±9.4]). The mean EXTEM CT was shorter for the HFC group compared with the cryoprecipitate group at baseline (70.4 seconds [±10.7] vs 74.7 seconds [±13.3]), but longer at the end‐of‐surgery assessment (73.2 seconds [±7.6] vs. 67.9 seconds [±6.8]). Differences between the groups diminished during the postoperative period and at the final assessment on day 10 EXTEM A20 values were comparable, although EXTEM CT was shorter in the cryoprecipitate group.
Mean FVIII:C levels remained stable in both groups from baseline up to 4 hours intraoperatively, before decreasing through to the end of surgery (Figure S1A) and then increasing at each postoperative time point (Figure S1B). VWF:RCo showed a slight initial increase early intraoperatively in both groups. VWF:RCo was maintained accordingly in the cryoprecipitate group, whereas in the HFC group it decreased to preoperative levels by the end of surgery (Figure S1C). On the second postoperative day and on the following measurement on the tenth postoperative day, the values were similar in both groups (Figure S1D). Mean FXIII levels decreased sharply in both groups between baseline and 2 hours after surgery start (Figure S1E). They subsequently levelled out, except for a further small decrease up to the end of surgery, and remained stable in the postoperative phase (Figure S1F).
3.6. Critical care outcomes
The median durations of surgery, artificial ventilation in the ICU, ICU stay, and hospitalization were comparable between groups (Table 3). The same was true for actual intraoperative blood loss (Figure S2); following the end of surgery, there was no bleeding in patients in either treatment group through to the assessments at 24 and 48 hours.
Table 3.
Critical care outcomes, median (range) (FAS analysis; N = 45)
|
HFC (n = 22) |
Cryoprecipitate (n = 23) |
Total (N = 45) |
|
|---|---|---|---|
| Duration of surgery, hours | 7.50 (4.9–10.8) | 7.90 (4.4–12.6) | 7.80 (4.4–12.6) |
| Duration of artificial ventilation in the ICU, hours | 24.75 (7.6–29.3)a | 25.75 (6.5–36.2)a | 25.30 (6.5–36.2)a |
| Length of ICU stay, hours | 45.35 (18.2–70.8)a | 48.50 (23.5–521.6)a | 47.50 (18.2–521.6)a |
| Duration of hospitalization, days | 20.90 (12.0–36.1) | 21.50 (14.8–128.8)b | 21.50 (12.0–128.8)a, b |
Abbreviations: FAS, full analysis set; HFC, human fibrinogen concentrate; ICU, intensive care unit.
N = 20 in the HFC group and N = 22 in the cryoprecipitate group as data from two patients and one patient, respectively, were not included because of missing dates/times; N = 42 in total.
N = 22 in the cryoprecipitate group as data from one patient was not included because of missing dates/times; N = 44 in total.
Intraoperatively and postoperatively, RBC concentrate was the only allogeneic blood product transfused in any patient (Table 4). The median (range) number of units of RBCs transfused intraoperatively was 1.0 unit (0‐4) for the HFC group and 0.5 units (0‐5) for the cryoprecipitate group; the mean (±SD) units were 1.3 (±1.4) and 1.3 (±1.6), respectively. In the first 24 hours postoperatively, three patients in each group received an RBC transfusion. Overall, five patients in the HFC group and five in the cryoprecipitate group did not receive an RBC transfusion from the start of surgery until postoperative day 10.
Table 4.
Intraoperative and postoperative transfusion requirements (PP analysis; N = 43)
| HFC (n = 22) | Cryoprecipitate (n = 23) | P value (HFC vs Cryoprecipitate) | |||
|---|---|---|---|---|---|
| Mean ± SD | Median (range) unit | Mean ± SD | Median (range) unit | ||
| Intraoperative | |||||
| RBCs | 1.3 ± 1.4 | 1 (0–4) | 1.3 ± 1.6 | 0.5 (0–5) | .9739 |
| FFP | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| Platelets | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| First 24 hours postoperatively | |||||
| RBCs | 0.2 ± 0.5 | 0 (0–2) | 0.2 ± 0.5 | 0 (0–2) | .9556 |
| FFP | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| Platelets | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| 24 to 48 hours postoperatively | |||||
| RBCs | 0.5 ± 0.7 | 0 (0–2) | 0.2 ± 0.4 | 0 (0–1) | .0882 |
| FFP | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| Platelets | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| 48 hours to 10 days postoperatively | |||||
| RBCs | 0.7 ± 0.9 | 0 (0–2) | 0.5 ± 0.8 | 0 (0–3) | .3233 |
| FFP | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| Platelets | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| Overall | |||||
| RBCs | 2.7 ± 2.0 | 3 (0–6) | 2.1 ± 1.8 | 2 (0–7) | .3292 |
| FFP | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
| Platelets | 0 | 0 (0–0) | 0 | 0 (0–0) | ‐ |
Abbreviations: FFP, fresh frozen plasma; HFC, human fibrinogen concentrate; PP, per protocol; RBC, red blood cell; SD, standard deviation.
4. SAFETY
A total of 225 AEs occurred in 22 patients (100%) in the HFC group and 228 AEs occurred in 23 patients (100%) in the cryoprecipitate group (Table 5). All AEs were treatment‐emergent and none were assessed as related to study drug administration. Twenty‐three AEs in 18 patients were classed as serious AEs (Table S6). Of these, six were in the HFC group and 17 were in the cryoprecipitate group. No thromboembolic events were observed in the HFC group. In the cryoprecipitate group, seven patients (30.4%) experienced seven thromboembolic events (five patients with pulmonary embolism and two patients with deep vein thrombosis) between postoperative days 4 and 11.
Table 5.
Number of patients experiencing any adverse event or serious adverse event (FAS analysis; N = 45)
|
HFC (N = 22) |
Cryoprecipitate (N = 23) |
|
|---|---|---|
| AE, n | 225 | 228 |
| Patients with AE, n (%) | 22 (100) | 23 (100) |
| Severity of AE, n | ||
| Mild | 222 | 214 |
| Moderate | 2 | 12 |
| Severe | 1 | 2 |
| Probably or possibly related AE, n | 0 | 0 |
| SAE, n | 6 | 17 |
Abbreviations: AE, adverse event; FAS, full analysis set; HFC, human fibrinogen concentrate; SAE, serious adverse event.
There was a single death during the study. This death occurred postoperatively in a patient in the cryoprecipitate group who experienced a gastrointestinal anastomotic leak on postoperative day 10. This event was deemed unrelated to study drug administration. The patient underwent reoperation but died on postoperative day 12. No other AEs led to discontinuation of a patient in the study.
5. DISCUSSION
In the surgical setting of cytoreductive surgery for PMP, 100% of patients who received fibrinogen supplementation with either HFC or cryoprecipitate achieved the primary endpoint of overall hemostatic treatment success. Post hoc analysis showed that HFC is non‐inferior to cryoprecipitate for overall hemostatic efficacy. Plasma fibrinogen levels and clot firmness increased after administration of both products, but to a significantly greater extent with HFC. The HFC was available for use earlier after randomization; therefore, the infusion could be initiated sooner than was possible with cryoprecipitate. Critical care outcomes, including duration of surgery, ICU stay, and hospitalization, did not differ significantly between groups. The only allogeneic blood product transfused in either group during the approximately 8 hours of surgery or postoperatively was RBC concentrate in small numbers of units to a few patients. The safety profiles of HFC and cryoprecipitate were comparable; however, thromboembolic events were observed only in the cryoprecipitate group (seven in total).
Owing to the level of blood loss and coagulopathy associated with cytoreductive surgery with HIPEC for PMP, this type of major abdominal surgery could be considered a robust model of acquired fibrinogen deficiency.8 Plasma fibrinogen levels and the contribution of fibrinogen to clot strength, as assessed using FIBTEM, have been shown to decrease significantly during the surgical phase of the procedure.22 The intraoperative course of these two parameters reflected the capability of the new HFC to maintain satisfactory hemostasis.
Administration of hemostatic products for bleeding patients has often consisted of transfusion of fixed ratios of RBC, FFP, and platelet concentrates. Until the end of 2012, FFP was used proactively during CRS in our unit, in a 1:2 ratio with RBCs. However, patients continued to experience significant bleeding, with cryoprecipitate often needed to maintain fibrinogen levels. We therefore instituted a new protocol using cryoprecipitate in place of FFP, plus TXA to reduce fibrinolysis. Prospective analysis subsequently showed that patients receiving upfront TXA and cryoprecipitate (N = 95) had significantly reduced intraoperative transfusion requirements compared with those treated according to the previous standard protocol of early FFP (N = 106): 1.8 vs 4.2 RBC units, 0.2 vs 6.2 FFP units, and 0 vs 0.1 platelet concentrate units, respectively.8
With the increasing use of point‐of‐care coagulation testing, there has been a move toward targeted therapy to correct specific defects.23 In the present study, following the initial dose of HFC or cryoprecipitate, the thromboelastometry FIBTEM A20 parameter was used to guide subsequent intraoperative and 24‐hour postoperative infusions. This ensured that each patient received fibrinogen replacement tailored to their individual fibrin clot profile. RBC concentrate was the only allogeneic blood product transfused in any patient, and at a lower level than previously described by Sargant et al.8
It was surprising to note that coagulation parameters that would be expected to be particularly influenced by the content of cryoprecipitate, such as FVIII, were comparable between the treatment groups. In addition, values for FXIII were also comparable between groups throughout the study. Because FXIII is not an acute phase protein to be promptly delivered to the circulation during surgery, in contrast to FVIII, it can be concluded that satisfactory supplementation with FXIII is also obtained using fibrinogen concentrate.
Although cryoprecipitate has been used for fibrinogen replacement in acquired fibrinogen deficiency for many years, there is close to no efficacy data published on this product.15, 19 There are more studies on use of HFC to correct acquired fibrinogen deficiency, and these have been performed in a variety of clinical settings, including surgery,24, 25, 26, 27, 28, 29 trauma,29, 30, 31, 32 and major obstetric hemorrhage.29, 33 Nevertheless, only one randomized trial, in pediatric cardiac surgery,34 and three observational studies, one in major obstetric hemorrhage,33 one in cardiothoracic surgery,35 and one in acute major hemorrhage requiring coagulation factor replacement or consumptive coagulopathy associated with hypofibrinogenemia,36 have compared fibrinogen concentrates with cryoprecipitate in bleeding patients to date, as identified by a recent systematic review from Jensen et al.37 Current international guidelines recommend use of HFC for treatment of bleeding in surgical patients with hypofibrinogenemia 38 and trauma patients with massive hemorrhage 39. Data from the present study strengthens the recommendations of these guidelines by further adding to the body of evidence that HFC specifically and effectively improves fibrin‐based clot strength and restores hemostasis in bleeding patients, such that transfusion of allogeneic blood products is considerably limited. In addition, these study data support the clinical application of these recommendations because it represents the basis of various regulatory interactions for the approval of the HFC Fibryga in acquired fibrinogen deficiency (e.g., already approved in Switzerland).
In terms of safety, the AEs that occurred in the present study were representative of those expected for surgery of this kind. One potential safety concern related to the use of procoagulants is the occurrence of thromboembolic events. No thromboembolic events were observed in the HFC group, whereas seven were observed in the cryoprecipitate group. On the available evidence, and despite the extended coagulation panel assessed, it is not possible to draw any definitive conclusions as to why thromboembolic events were observed only in the cryoprecipitate group.
This study has demonstrated the efficacy and safety of HFC as a source of fibrinogen for bleeding patients with acquired fibrinogen deficiency undergoing surgery for PMP. Both HFC and cryoprecipitate were efficacious for maintaining hemostasis and increasing plasma fibrinogen levels and FIBTEM A20. HFC appeared to offer additional benefits. The rapid reconstitution of the lyophilized powder in the operating room made it possible to administer the HFC earlier than cryoprecipitate, which required thawing at the blood bank before use. In addition, mean increases in plasma fibrinogen and FIBTEM A20 after each intraoperative dose were significantly higher in the HFC group. Because the mechanisms by which acquired fibrinogen deficiency develops – loss, consumption, and hemodilution – are common to many bleeding situations, these results are not only relevant for patients undergoing cytoreductive surgery for PMP, but also have implications for other types of surgery where significant blood loss is expected.
A limitation of the study was that it could not be conducted in a blinded manner because of inherent differences in aspect and volume between the study drugs. Although the health care professionals carrying out the surgery could not be blinded, the IDMEAC adjudication committee was kept blinded from treatment allocation. A placebo arm was also not possible because delaying fibrinogen supplementation in patients undergoing major abdominal surgery of this nature and experiencing acute bleeding would have been unethical. The noninferiority analysis was carried out as a post hoc as, because of the rarity of the disease, no controlled trials of cryoprecipitate for treatment of acquired hypofibrinogenemia in the setting of abdominal tumor surgery, or indeed generally for major bleeding, could be identified to guide the statistical assumptions on treatment efficacy rates. The low number of patients might also be considered a limitation; however, PMP is a very rare disease and the number of patients in this study (n = 45) is comparable with similar phase 3 studies for congenital disorders of similar prevalence (such as congenital fibrinogen deficiency).
Although this study demonstrated comparable hemostatic efficacy and noninferiority of HFC to cryoprecipitate, a confirmatory multicenter study would further strengthen these results. HFC increased plasma fibrinogen levels and fibrin‐based clotting to a greater extent, but a larger study population would be needed to confirm this. It is noteworthy that thromboembolic events were observed only in the cryoprecipitate group. The study included an extensive panel of coagulation tests at multiple time points, but the results did not identify a potential reason for this observed difference in the incidence of thromboembolic events between the groups. More research is needed to further describe aspects of the coagulation profile to confirm whether there is a difference in the safety profiles of the two products.
6. CONCLUSION
Human fibrinogen concentrate is efficacious for maintaining hemostasis in patients with acquired fibrinogen deficiency undergoing cytoreductive surgery for PMP. HFC is available for use faster than cryoprecipitate and has a comparable safety profile, with the possible exception of thromboembolic events, which were observed only with cryoprecipitate. Owing to the generalizability of the clinical model used, these results have implications for other surgical settings in which patients acquire fibrinogen deficiency and experience acute bleeding. Further studies are needed to explore potential differences between the safety profiles of the two products.
ADDENDUM
A. Roy, F. Mohamed, T. Cecil, S. Nunn, E. Arbuthnot Smith, I. Kruzhkova, C. Solomon, and S. Knaub contributed to the planning of the study and analysis and interpretation of the data. A. Roy, F. Mohamed, S. Stanford, S. Nunn, S. Alves, N. Sargant, S. Rangarajan, J. Bell, S. Dayal, T. Cecil, A. Tzivanakis, and B. Moran were responsible for the conduct of the study and contributed to data acquisition. All authors contributed to reporting of the data and revised the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript for submission to JTH.
CONFLICT OF INTERESTS
This study was sponsored and funded by Octapharma AG. A.R. and F.M. have received investigator fees from Octapharma AG. S.S., S.N., S.A., N.S., S.R., E.A.S., J.B., S.D., T.C., A.T., and B.M. have not received support from any organization for the submitted work. I.K., C.S., and S.K. are employees of Octapharma AG.
Supporting information
ACKNOWLEDGMENTS
The authors thank the study team and patients that participated in this study. This study was sponsored and funded by Octapharma AG. Editorial assistance was provided by Portland Medical Communications Ltd and was funded by Octapharma AG.
Roy A, Stanford S, Nunn S, et al. Efficacy of fibrinogen concentrate in major abdominal surgery – A prospective, randomized, controlled study in cytoreductive surgery for pseudomyxoma peritonei. J Thromb Haemost. 2020;18:352–363. 10.1111/jth.14665
Setting: Study conducted at a single center in the United Kingdom: Basingstoke and North Hampshire Hospital.
Manuscript Handled by: Alan Mast
Final decision: Alan Mast, 22 October 2019
REFERENCES
- 1. 2013/2014 NHS standard contract for pseudomyxoma peritonei service (adult) A08/S(HSS)/b. https://www.england.nhs.uk/wp-content/uploads/2013/06/a08-pseud-peri-ad.pdf. Accessed 17 Jan 2019.
- 2. Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol. 2008;34:196‐201. [DOI] [PubMed] [Google Scholar]
- 3. Hinson FL, Ambrose NS. Pseudomyxoma peritonei. Br J Surg. 1998;85:1332‐1339. [DOI] [PubMed] [Google Scholar]
- 4. Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am. 2003;12:585‐603. [DOI] [PubMed] [Google Scholar]
- 5. National Institute for Health and Clinical Excellence (NICE) .Complete cytoreduction for pseudomyxoma peritonei 2004. Available from: https://www.nice.org.uk/guidance/ipg56 Accessed 17 Jan 2019.
- 6. Sugarbaker PH. Surgical treatment of peritoneal carcinomatosis: 1988 Du Pont lecture. Can J Surg. 1989;32:164‐170. [PubMed] [Google Scholar]
- 7. Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin. Natural history and presentation of a curative approach to treatment. Dis Colon Rectum. 1987;30:772‐779. [DOI] [PubMed] [Google Scholar]
- 8. Sargant N, Roy A, Simpson S, et al. A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus Med. 2016;26:118‐122. [DOI] [PubMed] [Google Scholar]
- 9. Casado‐Adam A, Alderman R, Stuart OA, Chang D, Sugarbaker PH. Gastrointestinal complications in 147 consecutive patients with peritoneal surface malignancy treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Int J Surg Oncol. 2011;2011:468698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315‐325. [DOI] [PubMed] [Google Scholar]
- 12. Preti V, Chang D, Sugarbaker PH. Pulmonary complications following cytoreductive surgery and perioperative chemotherapy in 147 consecutive patients. Gastroenterol Res Pract. 2012;2012:635314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thong SY, Chia CS, Ng O, et al. A review of 111 anaesthetic patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Singapore Med J. 2017;58(8):488‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leversuch K, Needham J, Roy A, Milne A, Knight G. The effect of the Sugarbaker procedure on haemostasis. Haematologica. 2007;92:377. Abstract #1018 at the 12th Congress of the European Hematology Association, Vienna, Austria, 7–10 June 2007. [Google Scholar]
- 15. Lee SH, Lee SM, Kim CS, et al. Fibrinogen recovery and changes in fibrin‐based clot firmness after cryoprecipitate administration in patients undergoing aortic surgery involving deep hypothermic circulatory arrest. Transfusion. 2014;54:1379‐1387. [DOI] [PubMed] [Google Scholar]
- 16. Rahe‐Meyer N, Levy JH, Mazer CD, et al. Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double‐blind phase III study of haemostatic therapy. Br J Anaesth. 2016;117:41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solomon C, Pichlmaier U, Schoechl H, et al. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth. 2010;104:555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nascimento B, Goodnough LT, Levy JH. Cryoprecipitate therapy. Br J Anaesth. 2014;113:922‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sorensen B, Bevan D. A critical evaluation of cryoprecipitate for replacement of fibrinogen. Br J Haematol. 2010;149:834‐843. [DOI] [PubMed] [Google Scholar]
- 20. Schulz PM, Gehringer W, Nohring S, et al. Biochemical characterization, stability, and pathogen safety of a new fibrinogen concentrate (fibryga((R))). Biologicals. 2018;52:72‐77. [DOI] [PubMed] [Google Scholar]
- 21. (NHSBT) NHSBaT . Specification SPN223/8. NHSBT portfolio of blood components and guidance for their clinical use. In: NHSBT, ed. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-hosp/1447/spn223.pdf: NHSBT, 2016, 1‐95.
- 22. Van Poucke S, Huskens D, Van der Speeten K, et al. Thrombin generation and platelet activation in cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy ‐ A prospective cohort study. PLoS One. 2018;13:e0193657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fries D. The early use of fibrinogen, prothrombin complex concentrate, and recombinant‐activated factor VIIa in massive bleeding. Transfusion. 2013;53(Suppl 1):91S‐95S. [DOI] [PubMed] [Google Scholar]
- 24. Rahe‐Meyer N, Solomon C, Hanke A,et al. Effects of fibrinogen concentrate as first‐line therapy during major aortic replacement surgery: a randomized, placebo‐controlled trial. Anesthesiology. 2013;118:40‐50. [DOI] [PubMed] [Google Scholar]
- 25. Ranucci M, Baryshnikova E, Crapelli GB, Rahe‐Meyer N, Menicanti L, Frigiola A. Randomized, double‐blinded, placebo‐controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4:e002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenger‐Eriksen C, Lindberg‐Larsen M, Christensen AQ, Ingerslev J, Sorensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101:769‐773. [DOI] [PubMed] [Google Scholar]
- 27. Rahe‐Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high‐normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102:785‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahe‐Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry‐guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138:694‐702. [DOI] [PubMed] [Google Scholar]
- 29. Negrier C, Ducloy‐Bouthors AS, Piriou V, et al. Postauthorization safety study of Clottafact(R), a triply secured fibrinogen concentrate in acquired fibrinogen deficiency: a prospective observational study. Vox Sang. 2018;113:120‐127. [DOI] [PubMed] [Google Scholar]
- 30. Nardi G, Agostini V, Rondinelli B, et al. Trauma‐induced coagulopathy: impact of the early coagulation support protocol on blood product consumption, mortality and costs. Crit Care. 2015;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schochl H, Nienaber U, Hofer G, et al. Goal‐directed coagulation management of major trauma patients using thromboelastometry (ROTEM)‐guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schochl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry‐guided coagulation factor concentrate‐based therapy versus standard fresh frozen plasma‐based therapy. Crit Care. 2011;15:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ahmed S, Harrity C, Johnson S, et al. The efficacy of fibrinogen concentrate compared with cryoprecipitate in major obstetric haemorrhage–an observational study. Transfus Med. 2012;22:344‐349. [DOI] [PubMed] [Google Scholar]
- 34. Galas FR, de Almeida JP, Fukushima JT, et al. Hemostatic effects of fibrinogen concentrate compared with cryoprecipitate in children after cardiac surgery: a randomized pilot trial. J Thorac Cardiovasc Surg. 2014;148:1647‐1655. [DOI] [PubMed] [Google Scholar]
- 35. Cartwright BL, Kam P, Yang K. Efficacy of fibrinogen concentrate compared with cryoprecipitate for reversal of the antiplatelet effect of clopidogrel in an in vitro model, as assessed by multiple electrode platelet aggregometry, thromboelastometry, and modified thromboelastography. J Cardiothorac Vasc Anesth. 2015;29:694‐702. [DOI] [PubMed] [Google Scholar]
- 36. Theodoulou A, Berryman J, Nathwani A, Scully M. Comparison of cryoprecipitate with fibrinogen concentrate for acquired hypofibrinogenaemia. Transfus Apher Sci. 2012;46:159‐162. [DOI] [PubMed] [Google Scholar]
- 37. Jensen NH, Stensballe J, Afshari A. Comparing efficacy and safety of fibrinogen concentrate to cryoprecipitate in bleeding patients: a systematic review. Acta Anaesthesiol Scand. 2016;60:1033‐1042. [DOI] [PubMed] [Google Scholar]
- 38. Kozek‐Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017;34:332‐395. [DOI] [PubMed] [Google Scholar]
- 39. Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019; 23: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


