Abstract
Background
Even light to moderate alcohol consumption has been shown to increase cancer incidence. However, this association has not been well characterized in Japan.
Methods
Based on a nationwide, hospital‐based data set (2005‐2016), a multicenter case‐control study was conducted (63,232 cancer cases and 63,232 controls matched for sex, age, admission date, and admitting hospital). The total amount of lifetime alcohol consumption (drink‐years) was recalled for each patient by multiplication of the daily amount of standardized alcohol use (drinks per day) and the duration of drinking (years). Odds ratios (ORs) were estimated for overall and specific cancer sites via conditional logistic regression with restricted cubic splines, with adjustments made for smoking, occupational class, and comorbidities. Lifetime abstainers served as the reference group.
Results
Spline curves showed a dose‐response association with overall cancer risk: the minimum risk was at 0 drink‐years, and the OR at 10 drink‐years was 1.05 (95% confidence interval [CI], 1.04‐1.06). In comparison with lifetime abstainers, the OR for >0 to 20 drink‐years was 1.06 (95% CI, 1.01‐1.11). Those who drank 2 drinks or fewer per day had elevated odds for overall cancer risk across all duration‐of‐drinking categories. The same patterns were observed at light to moderate levels of drinking for most gastrointestinal/aerodigestive cancers as well as breast and prostate cancers. Analyses stratified by sex, different drinking/smoking behaviors, and occupational class mostly showed the same patterns for overall cancer incidence associated with light to moderate levels of drinking.
Conclusions
In Japan, even light to moderate alcohol consumption appears to be associated with elevated cancer risks.
Keywords: alcohol, cancer incidence, Japan, lifetime, risk, smoking
Short abstract
Light to moderate levels of lifetime drinking may increase the overall cancer risk with a dose‐response association, and the overall cancer risk appears to be lowest with zero alcohol consumption in Japan. The elevated overall cancer risk appears to be explained by alcohol‐related cancer risk across common sites, including the colorectum, stomach, breast, prostate, and esophagus.
Introduction
Drinking alcohol is a contributor to the overall cancer burden. In Western settings, alcohol‐related cancer risk has been characterized as a J‐shape pattern in some instances (colorectal and kidney cancers), and this suggests potential protective effects of alcohol.1, 2, 3 However, in 2018, the American Society of Clinical Oncology stated that more than 5% of new cancer cases were attributable to alcohol consumption.4 Upper aerodigestive tract (oral, laryngeal, and esophageal), colorectal, and liver cancers represented 60%, 21%, and 13% of alcohol‐related cancer cases in men, respectively, whereas breast, upper aerodigestive tract, liver, and colorectal cancers represented 52%, 25%, 12%, and 6% of alcohol‐related cancer cases in women, respectively.5 On the whole, upper aerodigestive cancers represent approximately 50% of the total cases, and they are followed by colorectal (16%), breast (16%), and liver cancers (13%).5
Recent studies have raised concerns about the risk of even light to moderate levels of alcohol consumption for cancer incidence.6, 7, 8 In Japan as well as East Asian countries, previous studies regarding alcohol‐related cancer risk are widely available for various cancer sites.9, 10, 11, 12 For example, in the Japan Public Health Center (JPHC)–based prospective study, the potential risk of light to moderate levels of alcohol consumption was implied to some extent with the use of trend analysis.9, 10 However, few studies have specifically focused on the cancer risk associated with light to moderate levels of alcohol consumption for overall cancer and specific cancer sites in Japan. Light to moderate levels of alcohol consumption may affect cancer risk through multiple pathways. For example, alcohol use increases circulating sex hormone levels, and this contributes to excess breast cancer risk.13 In addition, acetaldehyde, a metabolite of ethanol classified as a group 1 carcinogen by the International Agency for Research on Cancer, stimulates cell proliferation and induces DNA damage.6, 7, 8, 9, 10, 13, 14 The Japanese have a higher prevalence of polymorphisms in the aldehyde dehydrogenase 2 (ALDH2) enzyme, which makes them slower at metabolizing acetaldehyde.9, 14 Previous studies have indeed suggested an elevated cancer risk from alcohol consumption in the urinary tract and prostate in Japan, which has not been found in Western countries.9, 10, 15, 16, 17, 18, 19 We have hypothesized that there may be an elevated cancer risk at even light to moderate levels of alcohol consumption in Japan due to a higher prevalence of ALDH2 polymorphisms in the Japanese.
Previous studies have elucidated the association between lifetime drinking behaviors and cancer risk by drinking frequency (eg, standard drinks per day)2, 13, 20 and have used the weighted, averaged frequency of drinks over time. This frequency measurement would be relevant for capturing precise drinking behavior. Meanwhile, a less accurate but simple measurement of the lifetime carcinogen burden from drinking—the total amount of lifetime alcohol consumption (called drink‐years hereafter) estimated by multiplication of the average daily amount of standardized alcohol units (drinks per day) and the duration of drinking (years)—has also been used in clinical settings, particularly for upper aerodigestive cancers.21, 22, 23, 24 Yet, the cancer risk associated with light to moderate drink‐year levels has not been well characterized in Japan.
Accordingly, the goal of the current study was to investigate the cancer risk associated with light to moderate levels for the total amount of lifetime alcohol consumption. Using a nationwide, multicenter inpatient data set in Japan that contained clinical, behavioral (smoking and drinking), and occupational information,16, 17, 25, 26, 27, 28 we sought to examine whether light to moderate drink‐year levels were associated with an elevated cancer risk after adjustments for smoking and occupational class disparities. In addition, we sought to determine whether the observed association persisted 1) even after we had fully controlled for alcohol‐related lifestyle comorbidities (eg, hypertension, diabetes, and obesity); 2) within sex strata and with different drinking habits, drinking durations, and occupational classes; and 3) when the analysis was restricted to never smokers.17, 27, 29
Materials and Methods
Study Setting
A nationwide, multicentered, hospital‐based, matched case‐control study was conducted with the Inpatient Clinico‐Occupational Database of Rosai Hospital Group (ICOD‐R), which is administered by the Japan Organization of Occupational Health and Safety. Details of the ICOD‐R have been described elsewhere.16, 17, 25, 26, 27, 28 Briefly, the Rosai Hospital Group consists of 33 general hospitals throughout Japan. The ICOD‐R includes medical chart information confirmed by physicians (eg, basic sociodemographic characteristics, pathological information, clinical history, and diagnosis of current and past diseases), the occupational history (current job and 3 most recent jobs with their duration), and the smoking and alcohol habits (status, daily amount, and duration) of every inpatient. Since 2005, it has also collected self‐reported lifestyle‐related comorbidities diagnosed at annual health check‐ups (eg, hypertension, diabetes, and obesity).17, 27 The clinical diagnosis is coded according to International Classification of Diseases, Ninth Revision (ICD‐9), or International Classification of Diseases, Tenth Revision (ICD‐10), and the profiles of the patients are nationally representative. The ICOD‐R is unique to the Rosai Hospital Group and so differs from medical claims data, which may have less diagnostic accuracy. Written informed consent is obtained, and trained registrars and nurses are responsible for registering the data. The database currently contains details from more than 6 million inpatients. We obtained a deidentified data set under the research agreement, and the local research ethics committees approved the study.
Cases and Controls
The study subjects included 126,464 individuals (63,232 cancer cases and their 63,232 hospital controls) aged 20 years or older who had been admitted to the hospital between 2005 and 2016. The cancer cases included those patients whose main diagnosis was an initial cancer (ICD‐9, 140‐208; ICD‐10, C00‐C97), as confirmed by physicians on discharge. Each cancer case had a diagnosis with a specific cancer site (Table 1). We defined cancer incidence as the first‐time admission among patients who did not have a previous history of cancer, and the validation for the diagnosis has been described elsewhere.16, 17, 25, 26, 27, 28
Table 1.
Odds Ratios Estimated With the Continuous Total Amount of Lifetime Alcohol Consumption With Restricted Cubic Spline Methods
| Primary Site | ICD‐10 | No. of Cases (%) | Age, Mean (SD), y | Women, % | Odds Ratio (95% CI) at 10 Drink‐y Point | |
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | |||||
| All sites | C00‐C97 | 63,232 (100)c | 69 (10) | 34.7 | 1.05 (1.04‐1.06) | 1.05 (1.04‐1.06) |
| Specific sites | ||||||
| Lip, oral cavity, and pharynx | C00‐C14 | 1045 (1.7) | 67 (11) | 25.6 | 1.10 (1.01‐1.19) | 1.09 (1.00‐1.17) |
| Esophagus | C15 | 1408 (2.2) | 69 (9) | 13.1 | 1.45 (1.34‐1.58) | 1.44 (1.33‐1.57) |
| Stomach | C16 | 9355 (14.8) | 70 (10) | 26.2 | 1.06 (1.03‐1.09) | 1.06 (1.04‐1.09) |
| Colon and rectum | C18‐C20 | 9637 (15.2) | 69 (10) | 38.3 | 1.08 (1.05‐1.11) | 1.08 (1.05‐1.11) |
| Liver | C22 | 3604 (5.7) | 70 (9) | 27.8 | 1.03 (0.99‐1.07) | 1.03 (0.99‐1.08) |
| Gallbladder and bile duct | C23, C24 | 1350 (2.1) | 73 (9) | 42.7 | 1.04 (0.97‐1.11) | 1.04 (0.97‐1.11) |
| Pancreas | C25 | 1496 (2.4) | 71 (9) | 42.7 | 1.02 (0.95‐1.09) | 1.03 (0.96‐1.10) |
| Larynx | C32 | 549 (0.9) | 69 (9) | 5.1 | 1.22 (1.08‐1.37) | 1.23 (1.09‐1.38) |
| Lung | C33, C34 | 5972 (9.4) | 71 (9) | 27.1 | 0.97 (0.94‐1.00) | 0.97 (0.93‐1.01) |
| Bone and soft tissue | C40, C41, C46‐C49 | 221 (0.3) | 66 (13) | 46.6 | 1.05 (0.88‐1.27) | 1.10 (0.90‐1.33) |
| Skin | C43, C44 | 1035 (1.6) | 73 (11) | 47.2 | 0.92 (0.86‐0.99) | 0.92 (0.85‐0.99) |
| Breast | C50 | 4452 (7.0) | 63 (13) | 99.1 | 1.08 (1.03‐1.13) | 1.08 (1.03‐1.13) |
| Cervix uteri | C53 | 646 (1.0) | 54 (15) | 100 | 1.12 (1.00‐1.27) | 1.13 (1.00‐1.27) |
| Corpus uteri | C54 | 825 (1.3) | 60 (12) | 100 | 0.99 (0.88‐1.11) | 1.00 (0.89‐1.12) |
| Ovary | C56 | 522 (0.8) | 59 (13) | 100 | 0.98 (0.85‐1.12) | 0.98 (0.85‐1.12) |
| Prostate | C61 | 8371 (13.2) | 71 (7) | 0 | 1.07 (1.05‐1.10) | 1.07 (1.04‐1.09) |
| Kidney | C64 | 1178 (1.9) | 66 (10) | 28.4 | 1.00 (0.94‐1.07) | 1.00 (0.93‐1.07) |
| Renal pelvis and ureter | C65, C66 | 666 (1.1) | 72 (9) | 30.9 | 1.06 (0.96‐1.17) | 1.05 (0.95‐1.16) |
| Bladder | C67 | 3292 (5.2) | 71 (10) | 18.2 | 1.04 (1.00‐1.08) | 1.04 (1.00‐1.08) |
| Brain and nerve system | C70‐C72 | 383 (0.6) | 64 (14) | 37.6 | 0.93 (0.80‐1.07) | 0.93 (0.80‐1.09) |
| Thyroid | C73 | 656 (1.0) | 62 (13) | 74.8 | 0.92 (0.82‐1.03) | 0.92 (0.81‐1.03) |
| Malignant lymphoma | C81‐C85, C96 | 2177 (3.4) | 69 (12) | 43.0 | 1.02 (0.96‐1.08) | 1.02 (0.97‐1.08) |
| Multiple myeloma | C88, C90 | 469 (0.7) | 71 (10) | 48.6 | 0.89 (0.79‐1.01) | 0.88 (0.78‐1.00) |
| Leukemia | C91‐C95 | 616 (1.0) | 69 (12) | 39.4 | 1.01 (0.91‐1.11) | 1.01 (0.91‐1.11) |
Abbreviations: CI, confidence interval; ICD‐10, International Classification of Diseases, Tenth Revision; SD, standard deviation.
Odds ratios and 95% CIs at the 10 drink‐year point were estimated with conditional logistic regression, which was matched for sex, age, admission date, and hospital and adjusted for smoking history and occupational class. A continuous drink‐year variable and restricted cubic spline methods were used.
Additionally adjusted for lifestyle‐related comorbidities (hypertension, hyperlipidemia, diabetes, hyperuricemia, and obesity).
The total number of 63,232 includes the cases from other sites, which are not shown in the specific sites.
According to the methodology used in previous studies, our controls included patients diagnosed with eye and ear disease (360‐389 [ICD‐9] and H00‐H95 [ICD‐10]; 45.4%), genitourinary system disease (580‐629 [ICD‐9] and N00‐N99 [ICD‐10]; 38.3%), infectious and parasitic diseases (1‐136 [ICD‐9] and A00‐B99 [ICD‐10]; 10.6%), or skin diseases (680‐709 [ICD‐9] and L00‐L99 [ICD‐10]; 5.7%).16, 17, 25, 26, 27, 28 To select cases and controls from the same source population, we randomly sampled 1 control for each cancer case who was matched by the basic background characteristics of sex (male or female), age (in the same 1‐year age category), admission date (in the same financial year), and admitting hospital (in the same admitting hospital). Controls were those who were admitted to the hospital for the first time, and those who were later hospitalized for cancer were not eligible to be cases. The matched basic backgrounds were balanced entirely between the cases and controls: the percentage of female patients was 34.7% (21,910 of 63,232), and the mean age was 69 years (standard deviation, 10 years) for both the cases and the controls (Table 2).
Table 2.
Baseline Characteristics of Overall Cancer Cases and Their Matched Controls
| Characteristic | Control (n = 63,232)a | Case (n = 63,232) |
|---|---|---|
| Women, No. (%) | 21,910 (34.7) | 21,910 (34.7) |
| Age, mean (SD), y | 69 (10) | 69 (10) |
| Year, mean (SD) | 2010 (3) | 2010 (3) |
| Drinking history, No. (%)b | ||
| Never | 27,833 (44.0) | 25,353 (40.1) |
| Former | 7144 (11.3) | 8220 (13.0) |
| Current | 28,255 (44.7) | 29,659 (46.9) |
| Average drinks/d, mean (SD)b | 0.8 (1.0) | 0.9 (1.1) |
| Duration of drinking (continuous), mean (SD), yb | 23.5 (23.0) | 25.1 (22.8) |
| Duration of drinking (categorical), No. (%)b | ||
| Never | 27,833 (44.0) | 25,353 (40.1) |
| >0‐19 y | 2331 (3.7) | 2408 (3.8) |
| 20‐39 y | 10,077 (15.9) | 10,905 (17.2) |
| ≥40 y | 22,991 (36.4) | 24,566 (38.9) |
| Total amount of lifetime drinking (continuous), mean (SD), drink‐yb | 33.7 (44.9) | 38.1 (47.4) |
| Total amount of lifetime drinking (categorical), No. (%)b | ||
| Never | 27,833 (44.0) | 25,353 (40.1) |
| >0‐20 drink‐y | 4234 (6.7) | 4143 (6.6) |
| >20‐40 drink‐y | 7972 (12.6) | 7966 (12.6) |
| >40‐60 drink‐y | 10,847 (17.2) | 11,240 (17.8) |
| >60‐90 drink‐y | 5667 (9.0) | 6368 (10.1) |
| >90 drink‐y | 6679 (10.6) | 8162 (12.9) |
| Smoking history, No. (%)b | ||
| Never | 27,849 (44.0) | 24,247 (38.3) |
| Former | 21,641 (34.2) | 22,558 (35.7) |
| Current | 13,742 (21.7) | 16,427 (26.0) |
| Smoking, log(pack‐y), mean (SD)b | 1.8 (1.8) | 2.1 (1.8) |
| High occupational class, No. (%)b | 9167 (14.5) | 8715 (13.8) |
| Hypertension, No. (%) | 23,105 (36.5) | 23,286 (36.8) |
| Hyperlipidemia, No. (%)b | 7695 (12.2) | 7388 (11.7) |
| Diabetes, No. (%)b | 10,324 (16.3) | 9573 (15.1) |
| Hyperuricemia, No. (%)b | 2131 (3.4) | 1942 (3.1) |
| Obesity, No. (%) | 7601 (12.0) | 7596 (12.0) |
Abbreviation: SD, standard deviation.
Controls were matched for sex, age, admission date, and admitting hospital.
P < .05 for t test or chi‐square test.
Total Amount of Lifetime Alcohol Consumption and Other Covariates
According to the methodology used in previous studies that measured the total amount of lifetime alcohol consumption,21, 22, 23, 24 we generated a continuous drink‐year variable for each patient by multiplying the average of the daily amount of standardized alcohol units (drinks per day) and the duration of drinking (years). All study subjects reported their average daily amount of standardized alcohol units and duration of drinking on admission to the hospital (or during their hospital stay due to their acute symptoms). One standardized drink containing 23 g of ethanol was equivalent to one 180‐mL cup (6 ounces) of Japanese sake, one 500‐mL bottle (17 ounces) of beer, one 180‐mL glass (6 ounces) of wine, or one 60‐mL cup (2 ounces) of whiskey.16, 17, 25, 26, 27, 28 The duration of drinking accounted for the years from the age of starting drinking up to the age of quitting drinking or the age on admission if they had not quit drinking. In addition, we categorized patients into 6 categories by their drink‐year levels (0 [lifetime abstainer], >0‐20, >20‐40, >40‐60, >60‐90, and >90 drink‐years). Lifetime abstainers of drinking were defined as those who responded that they had never consumed alcohol.
In addition to basic background characteristics (sex, age, admission date, and admitting hospital), confounding variables included smoking history (never, former, or current) and high occupational class status (defined by the longest held jobs in managerial/professional occupations).25, 26, 27, 28 Other possible mediating variables included lifestyle‐related comorbidities (hypertension, hyperlipidemia, diabetes, hyperuricemia, and obesity) that are potentially linked to alcohol consumption and might explain alcohol‐related cancer risk.17, 27, 29
Statistical Analysis
The odds ratios (ORs) and 95% confidence intervals (CIs) for overall cancer incidence were estimated against continuous drink‐year levels by conditional logistic regression matched for sex, age, admission date, and admitting hospital with a restricted cubic spline method knotted at 0, 23, and 96 drink‐years (corresponding to the 10th, 50th, and 90th percentile points, respectively) on the basis of the distribution of our data.30, 31 Lifetime abstainers with 0 drink‐years served as the referent group for all analyses. To control for potential confounding and mediating variables, we mutually adjusted for smoking history and occupational class (model 1), and we made additional adjustments for comorbidities (model 2). The OR and 95% CI for each drink‐year category (>0‐20, >20‐40, >40‐60, >60‐90, and >90 drink‐years) were also estimated. For specific cancer incidence, we restricted analyses to each cancer site and performed the same analytic procedure.
In sensitivity analyses, we estimated ORs and 95% CIs for men and women, current and former drinkers, and those who drank for <20, 20 to 39, and ≥40 years. In addition, we stratified analyses by occupational class (high vs low) because of occupational class inequalities in cancer risk.25, 26, 27 Furthermore, we restricted analyses to never smokers because of potential synergy effects of smoking and drinking.17, 32 Lifetime abstainers with 0 drink‐years served as the referent group for all analyses. In addition, we used alternative control groups (all available hospital controls diagnosed with benign diseases) as well as alternative drinking categories, which included 7 joint categories for the daily amount and duration of drinking (0 drinks per day [lifetime abstainer], ≤2 drinks per day and <20 years, ≤2 drinks per day and 20‐39 years, ≤2 drinks per day and ≥40 years, >2 drinks per day and <20 years, >2 drinks per day and 20‐39 years, and >2 drinks per day for ≥40 years). In these sensitivity analyses, we analyzed only overall cancer risk because of the limitation of our sample size. α was set at .05, and all P values were 2‐sided. Data were analyzed with STATA/MP13.1 (StataCorp LP, College Station, Texas).
Results
Overall, the cases tended to drink more than the controls (Table 2): the prevalence of ever drinkers among the cases and controls was 59.9% and 56.0%, respectively (P < .001), and the mean drink‐years for the cases and controls were 38.1 and 33.7, respectively (P < .001). In comparison with the controls, smoking behavior was more prevalent, and a high occupational class was less prevalent among the cases (Table 2). Except for nonsignificant associations in hypertension and obesity, comorbidities were slightly less prevalent in the cases versus the controls. As a result, compared with lifetime abstainers, ever drinkers showed increased odds for aerodigestive and gastrointestinal cancers (oral, laryngeal, esophageal, stomach, colorectal, and liver cancers) as well as breast and prostate cancers; this was most pronounced for esophageal cancer (Fig. 1).
Figure 1.
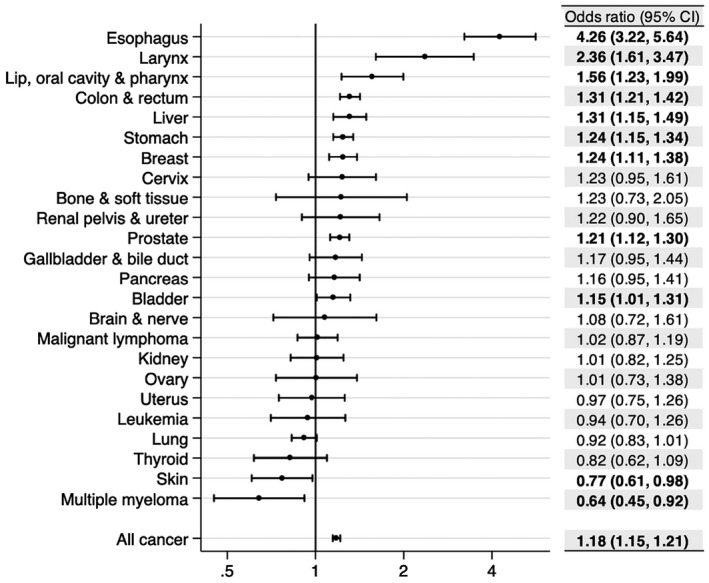
Overall and specific cancer incidence risk associated with ever drinkers. Odds ratios (dots) and 95% CIs (lines) were estimated with 63,232 cases and 63,232 controls by conditional logistic regression matched for sex, age, admission date, and hospital. Smoking history, occupational class, and comorbidities were mutually adjusted. Bolding indicates P < .05. CI indicates confidence interval.
For overall cancer risk, cubic spline curves showed a dose‐response, slightly convex shape (but almost a linear shape up to 20 drink‐years) against light to moderate drink‐year levels, with the minimum risk at 0 (Fig. 2). The observed association persisted even after we had fully controlled for comorbidities (model 2): the OR at 10 drink‐years was 1.05 (95% CI, 1.04‐1.06; Table 1). Compared with lifetime abstainers, the odds were elevated across all levels of categorical drink‐years (Table 3), and the elevated odds persisted even after we had fully controlled for comorbidities (model 2): the OR for >0 to 20 drink‐years was 1.06 (95% CI, 1.01‐1.11). Those who drank 2 drinks or fewer per day had elevated odds for overall cancer risk across all duration‐of‐drinking categories (Table 3).
Figure 2.
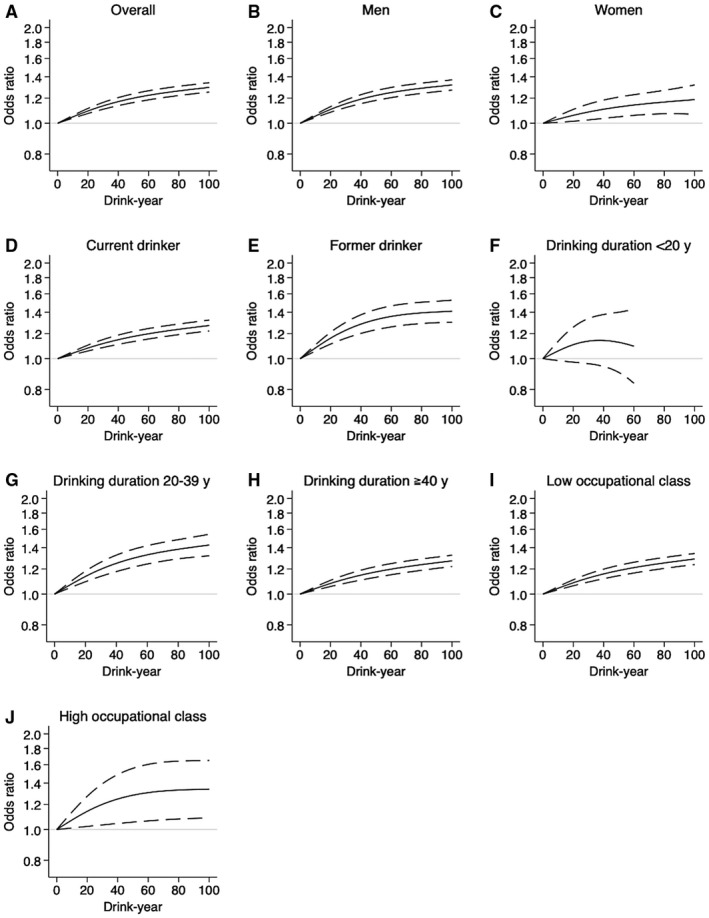
Cubic spline curves for overall cancer risk against the total amount of lifetime alcohol consumption. Odds ratios (solid lines) and 95% confidence intervals (dashed lines) were estimated by conditional logistic regression matched for sex, age, admission date, and hospital. Smoking history and occupational class were mutually adjusted. The numbers of subjects used for the analyses were as follows: (A) 126,464 (overall), (B) 82,644 (men), (C) 43,820 (women), (D) 98,286 (current drinkers), (E) 45,470 (former drinkers), (F) 37,762 (those who drank <20 years), (G) 53,804 (those who drank 20‐39 years), (H) 86,290 (those who drank ≥40 years), (I) 93,826 (those in a low occupational class), and (J) 3126 (those in a high occupational class).
Table 3.
Odds Ratios for Overall and Specific Cancer Incidence Estimated With the Categorical Total Amount of Lifetime Alcohol Consumption
| Characteristic | Odds Ratio (95% CI) | ||
|---|---|---|---|
| Model 1a | Model 2b | ||
| Drink‐y (vs lifetime abstainers) | |||
| Overall | >0‐20 drink‐y | 1.06 (1.01‐1.12) | 1.06 (1.01‐1.11) |
| >20‐40 drink‐y | 1.13 (1.08‐1.17) | 1.12 (1.08‐1.17) | |
| >40‐60 drink‐y | 1.18 (1.13‐1.22) | 1.18 (1.13‐1.22) | |
| >60‐90 drink‐y | 1.26 (1.21‐1.32) | 1.26 (1.21‐1.32) | |
| >90 drink‐y | 1.37 (1.31‐1.43) | 1.37 (1.31‐1.43) | |
| Esophagus | >0‐20 drink‐y | 1.72 (1.06‐2.79) | 1.78 (1.09‐2.90) |
| >20‐40 drink‐y | 2.78 (1.97‐3.93) | 2.74 (1.94‐3.88) | |
| >40‐60 drink‐y | 4.25 (3.08‐5.88) | 4.13 (2.98‐5.71) | |
| >60‐90 drink‐y | 5.31 (3.79‐7.43) | 5.23 (3.72‐7.35) | |
| >90 drink‐y | 7.17 (5.17‐9.96) | 7.03 (5.04‐9.80) | |
| Stomach | >0‐20 drink‐y | 1.09 (0.95‐1.25) | 1.09 (0.95‐1.26) |
| >20‐40 drink‐y | 1.17 (1.05‐1.30) | 1.17 (1.05‐1.30) | |
| >40‐60 drink‐y | 1.21 (1.10‐1.33) | 1.22 (1.10‐1.34) | |
| >60‐90 drink‐y | 1.35 (1.21‐1.52) | 1.36 (1.21‐1.52) | |
| >90 drink‐y | 1.43 (1.28‐1.60) | 1.44 (1.29‐1.61) | |
| Colon and rectum | >0‐20 drink‐y | 1.14 (1.00‐1.30) | 1.14 (1.00‐1.30) |
| >20‐40 drink‐y | 1.12 (1.01‐1.25) | 1.12 (1.01‐1.25) | |
| >40‐60 drink‐y | 1.29 (1.17‐1.43) | 1.29 (1.16‐1.43) | |
| >60‐90 drink‐y | 1.56 (1.39‐1.76) | 1.55 (1.38‐1.75) | |
| >90 drink‐y | 1.69 (1.51‐1.90) | 1.69 (1.50‐1.89) | |
| Liver | >0‐20 drink‐y | 1.13 (0.91‐1.41) | 1.19 (0.95‐1.49) |
| >20‐40 drink‐y | 1.22 (1.02‐1.44) | 1.28 (1.07‐1.53) | |
| >40‐60 drink‐y | 1.10 (0.94‐1.29) | 1.11 (0.94‐1.31) | |
| >60‐90 drink‐y | 1.44 (1.19‐1.76) | 1.46 (1.19‐1.79) | |
| >90 drink‐y | 1.64 (1.38‐1.95) | 1.68 (1.41‐2.01) | |
| Breast | >0‐20 drink‐y | 1.29 (1.12‐1.50) | 1.29 (1.11‐1.49) |
| >20‐40 drink‐y | 1.26 (1.08‐1.47) | 1.25 (1.07‐1.46) | |
| >40‐60 drink‐y | 1.05 (0.83‐1.33) | 1.05 (0.83‐1.33) | |
| >60‐90 drink‐y | 1.42 (1.00‐2.04) | 1.43 (1.00‐2.05) | |
| >90 drink‐y | 1.30 (0.86‐1.96) | 1.27 (0.84‐1.92) | |
| Prostate | >0‐20 drink‐y | 1.17 (1.01‐1.35) | 1.16 (1.00‐1.34) |
| >20‐40 drink‐y | 1.23 (1.11‐1.36) | 1.22 (1.10‐1.35) | |
| >40‐60 drink‐y | 1.27 (1.16‐1.38) | 1.25 (1.14‐1.37) | |
| >60‐90 drink‐y | 1.28 (1.14‐1.43) | 1.25 (1.12‐1.40) | |
| >90 drink‐y | 1.14 (1.03‐1.26) | 1.11 (1.00‐1.24) | |
| Joint category with daily amount and duration of drinking (vs lifetime abstainers) | |||
| Overall | ≤2 drinks/d and <20 y | 1.10 (1.03‐1.17) | 1.10 (1.03‐1.17) |
| ≤2 drinks/d and 20‐39 y | 1.18 (1.13‐1.23) | 1.18 (1.13‐1.23) | |
| ≤2 drinks/d and ≥40 y | 1.16 (1.12‐1.20) | 1.16 (1.12‐1.19) | |
| >2 drinks/d and <20 y | 1.05 (0.86‐1.29) | 1.05 (0.85‐1.29) | |
| >2 drinks/d and 20‐39 y | 1.41 (1.29‐1.53) | 1.41 (1.30‐1.53) | |
| >2 drinks/d and ≥40 y | 1.54 (1.44‐1.64) | 1.54 (1.44‐1.64) | |
Abbreviation: CI, confidence interval.
Conditional logistic regression matched for sex, age, admission date, and hospital and adjusted for smoking history and occupational class.
Additionally adjusted for lifestyle‐related comorbidities (hypertension, hyperlipidemia, diabetes, hyperuricemia, and obesity).
For specific cancer sites, most gastrointestinal and upper aerodigestive cancers (including oral, esophageal, stomach, colorectal, liver, gallbladder, and laryngeal cancers) as well as breast and prostate cancers showed the same pattern (slightly convex or linear shapes) at light to moderate drink‐year levels (Fig. 3 and Tables 2 and 3); this was most pronounced for esophageal cancer (OR at 10 drink‐years, 1.44; 95% CI, 1.33‐1.57; model 2; Table 2). Pancreatic, cervical, renal pelvis and ureter, and bladder cancers as well as bone and soft‐tissue cancers showed a hint of a potential linear association (Fig. 3). No protective association (but a potential linear association) was observed in kidney cancer, whereas light to moderate alcohol consumption was potentially associated with a reduced risk for skin cancer and multiple myeloma (Fig. 3).
Figure 3.
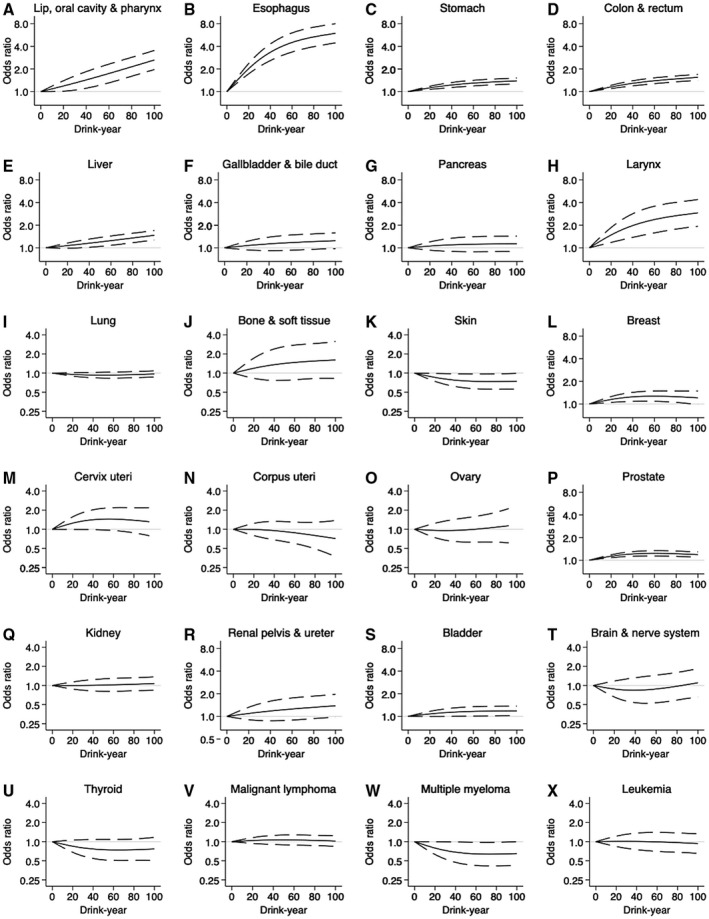
Cubic spline curves for specific cancer risks against continuous total amount of lifetime alcohol consumption. ORs (solid line) and 95% CIs (dashed line) were estimated by conditional logistic regression, mutually adjusted for smoking history, occupational class, and comorbidities. The numbers of subjects used for the analyses were as follows: (A) 2090 (Lip, oral cavity, and pharynx), (B) 2,816 (Esophagus), (C) 18,710 (Stomach), (D) 19,274 (Colon and rectum), (E) 7208 (Liver), (F) 2700 (Gallbladder and bile duct), (G) 2992 (Pancreas), (H) 1098 (Larynx), (I) 11,944 (Lung), (J) 442 (Bone and soft tissue), (K) 2070 (Skin), (L) 8904 (Breast), (M) 1292 (Cervix uteri), (N) 1650 (Corpus uteri), (O) 1044 (Ovary), (P) 16,742 (Prostate), (Q) 2356 (Kidney), (R) 1332 (Renal pelvis and ureter), (S) 6584 (Bladder), (T) 766 (Brain and nerve system), (U) 1312 (Thyroid), (V) 4354 (Malignant lymphoma), (W) 938 (Multiple myeloma), and (X) 1232 (Leukemia).
In sensitivity analyses, the patterns were mostly identical, regardless of sex, drinking habits, drinking durations, or occupational classes (Fig. 2 and Supporting Table 1). The patterns were mostly identical in the analyses with never smokers (Supporting Fig. 1 and Supporting Table 1) and alternative control groups (Supporting Fig. 2).
Discussion
In Japan, overall cancer risk appeared to be the lowest at zero alcohol consumption, with a modest increase in overall cancer risk at light to moderate levels for the total amount of lifetime alcohol consumption. A dose‐response, almost linear association was observed for overall cancer risk and lifetime alcohol consumption without any thresholds, and this suggested that a light level of drinking at the 10‐drink‐year point would increase overall cancer risk by 5%. Although the impact of lifetime alcohol consumption varied across each cancer site, the elevated overall cancer risk appeared to be explained by alcohol‐related cancer risk across relatively common sites, including the colorectum, stomach, breast, prostate, and esophagus.32 Besides, the risk associated with light to moderate levels for the total amount of lifetime alcohol consumption appeared to similarly matter across sexes and different drinking and smoking behaviors or occupational classes in that country.
Our observed patterns of alcohol‐related cancer risk appear to support findings in previous studies.6, 7, 8, 12, 13 For upper aerodigestive and gastrointestinal cancers, our observed patterns would be plausible because of the common genetic vulnerability to acetaldehyde in the Japanese.12 Acetaldehyde is carcinogenic via multiple mechanisms (eg, stimulating cell proliferation and inducing DNA damage) and increases cancer risk even with light levels of lifetime alcohol consumption, regardless of race or region of the world.6, 7, 8, 9, 10, 13, 14 In the current study, even light to moderate levels of lifetime alcohol consumption appeared to increase most of the upper aerodigestive and gastrointestinal cancers.9, 12, 14 In contrast to the patterns observed in Western settings,1, 2, 3 we observed no protective effects of light to moderate lifetime alcohol consumption for colorectal and kidney cancers. For breast and prostate cancer, different pathways such as elevations of circulating sex hormone levels (ie, estrogens and androgens) by alcohol use may explain the alcohol‐related cancer risk at even light to moderate levels of lifetime alcohol consumption.10, 13 In the JPHC study, a dose‐response trend between alcohol consumption and advanced prostate cancer risk (P for trend = .02) was reported.10 As yet, evidence for potential mechanisms that may explain reduced odds for skin cancer and multiple myeloma remains scarce. The potential causal (biologically protective effect) and noncausal explanations (unmeasured confounding) remain unclear for these inverse associations.
The limitations of the current study should be noted. First, the selection of hospital controls may have introduced a selection bias toward the null. Although sensitivity analyses with different drinking behaviors showed almost identical patterns, the lifetime drinking history recalled at the time of hospital admission (ie, not obtained on multiple occasions before the onset of disease) may be subject to recall bias. In addition, our exposure assessment did not inquire about starting/ending dates of drinking habits. Indeed, our observed odds for overall cancer risk (a 5% increase by 10 drink‐years) was roughly equivalent to half of the risk observed in a previous study from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial in the United States (a 10% increase by the lifetime average of 5 drinks per day).8 Therefore, our observed cancer risk associated with light to moderate lifetime alcohol consumption would be underestimated. Second, because of the limitation of our data set, we could not assess alcohol‐related cancer risk by different types of alcoholic beverages (eg, Japanese sake, beer, wine, and whiskey). However, studies suggest that the ethanol (but not the other components of alcoholic beverages) matters primarily for cancer risk, regardless of the types of alcoholic beverages.9 In addition, we could not assess other explanatory variables such as menopausal hormone therapy (for female breast cancer), a family history of cancer, diet (eg, coffee and red meat), physical activities, and ALDH2 genotypes.9, 14, 33 In the JPHC study, alcohol‐related bladder cancer risk was observed in male “flushers” (who are supposed to have polymorphisms in ALDH2 enzyme) but not in male nonflushers.9 In the assessment of how robust our estimate (OR for ever drinkers, 1.18) was to potential unmeasured and uncontrolled confounding, the E‐value was 1.64.34 This means that there would need to be at least a 1.64‐fold association between an unobserved confounder and the exposure/outcome to explain the observed association. Third, although we controlled for smoking in regression analyses, a limited number of cases did not allow us to restrict all analyses within never smokers, and residual smoking effects might have persisted in our results. Despite these limitations, we have demonstrated a comprehensive picture of significant overall cancer risk and risks of various cancers associated with light to moderate levels for the total amount of lifetime alcohol consumption in Japan with a restricted cubic spline method and a clinically useful indicator of drinking intensity. The strengths also include the size of this study, one of the largest multicenter studies for alcohol‐related cancer risk reported in that country,9, 10, 11 and accurate diagnoses directly extracted from medical charts.
Inoue et al32 reported that the population attributable risk for overall cancer incidence by alcohol (9.0% in men and 2.5% in women) was smaller than the risk due to tobacco smoking (29.7% in men and 5.0% in women) and infections such as Helicobacter pylori, hepatitis B virus, and hepatitis C virus (22.8% in men and 17.5% in women), the 2 major prioritized preventable risk factors in Japan. Among alcohol‐related cancer cases, the highest population attributable risk was due to upper digestive cancer,5 which is not one of the most common types in Japan.25, 26 In addition, benefits of adequate, nonheavy alcohol drinking have been reported for overall mortality as well as cardiovascular health.8 However, we observed modest alcohol‐related cancer risk in the most common types (colorectal, stomach, breast, prostate, and liver cancers) even at light to moderate levels of lifetime alcohol consumption in Japan. Thus, given the current burden of overall cancer incidence, we should further encourage promoting public education about alcohol‐related cancer risk.
In summary, we have documented various cancer risks associated with even light to moderate levels for the total amount of lifetime alcohol consumption in Japan, with the minimum risk at zero consumption. The current national cancer control strategy needs to strengthen the emphasis on moderating drinking behavior in the Japanese population to reduce the burden of cancer incidence.
Funding Support
This study was funded by the Ministry of Health, Labour, and Welfare (Industrial Disease Clinical Research Grant 170201‐01) and the Japan Society for the Promotion of Science (KAKENHI JP18K17351).
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Masayoshi Zaitsu: Conception and design; development of methodology; acquisition of data; analysis and interpretation of data; study supervision; writing, review, and/or revision of the manuscript; and administrative, technical, or material support. Takumi Takeuchi: Acquisition of data; writing, review, and/or revision of the manuscript; and administrative, technical, or material support. Yasuki Kobayashi: Study supervision; writing, review, and/or revision of the manuscript; and administrative, technical, or material support. Ichiro Kawachi: Conception and design; development of methodology; study supervision; writing, review, and/or revision of the manuscript; and administrative, technical, or material support.
Supporting information
[Correction added on 20 December 2019, after first online publication: Table 1 and the legend for Figure 3 have been updated.]
Data Availability Statement
The data that support the findings of this study are available from the Japan Organization of Occupational Health and Safety. Restrictions apply to the availability of these data, which were used under license for this study; research data are not shared. If any person wishes to verify the data, they are most welcome to contact the corresponding author.
References
- 1. Antwi SO, Eckel‐Passow JE, Diehl ND, et al. Alcohol consumption, variability in alcohol dehydrogenase genes and risk of renal cell carcinoma. Int J Cancer. 2018;142:747‐756. doi: 10.1002/ijc.31103 [DOI] [PubMed] [Google Scholar]
- 2. Wozniak MB, Brennan P, Brenner DR, et al. Alcohol consumption and the risk of renal cancers in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Int J Cancer. 2015;137:1953‐1966. doi: 10.1002/ijc.29559 [DOI] [PubMed] [Google Scholar]
- 3. Fedirko V, Tramacere I, Bagnardi V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose‐response meta‐analysis of published studies. Ann Oncol. 2011;22:1958‐1972. doi: 10.1093/annonc/mdq653 [DOI] [PubMed] [Google Scholar]
- 4. LoConte NK, Brewster AM, Kaur JS, Merrill JK, Alberg AJ. Alcohol and cancer: a statement of the American Society of Clinical Oncology. J Clin Oncol. 2018;36:83‐93. doi: 10.1200/JCO.2017.76.1155 [DOI] [PubMed] [Google Scholar]
- 5. Praud D, Rota M, Rehm J, et al. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. 2016;138:1380‐1387. doi: 10.1002/ijc.29890 [DOI] [PubMed] [Google Scholar]
- 6. GBD 2016 Alcohol Collaborators . Alcohol use and burden for 195 countries and territories, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015‐1035. doi: 10.1016/S0140-6736(18)31310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. BMJ. 2015;351:h4238. doi: 10.1136/bmj.h4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kunzmann AT, Coleman HG, Huang WY, Berndt SI. The association of lifetime alcohol use with mortality and cancer risk in older adults: a cohort study. PLoS Med. 2018;15:e1002585. doi: 10.1371/journal.pmed.1002585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Masaoka H, Matsuo K, Sawada N, et al. Alcohol consumption and bladder cancer risk with or without the flushing response: the Japan Public Health Center–based prospective study. Int J Cancer. 2017;141:2480‐2488. doi: 10.1002/ijc.31028 [DOI] [PubMed] [Google Scholar]
- 10. Sawada N, Inoue M, Iwasaki M, et al. Alcohol and smoking and subsequent risk of prostate cancer in Japanese men: the Japan Public Health Center–based prospective study. Int J Cancer. 2014;134:971‐978. doi: 10.1002/ijc.28423 [DOI] [PubMed] [Google Scholar]
- 11. Kawai M, Minami Y, Kakizaki M, et al. Alcohol consumption and breast cancer risk in Japanese women: the Miyagi cohort study. Breast Cancer Res Treat. 2011;128:817‐825. doi: 10.1007/s10549-011-1381-x [DOI] [PubMed] [Google Scholar]
- 12. Choi YJ, Lee DH, Han KD, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nationwide population‐based cohort study of South Korea. PLoS One. 2017;12:e0185778. doi: 10.1371/journal.pone.0185778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White AJ, DeRoo LA, Weinberg CR, Sandler DP. Lifetime alcohol intake, binge drinking behaviors, and breast cancer risk. Am J Epidemiol. 2017;186:541‐549. doi: 10.1093/aje/kwx118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol‐related cancers in Asians: a public health perspective. J Biomed Sci. 2017;24:19. doi: 10.1186/s12929-017-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao C, Haque R, Van Den Eeden SK, Caan BJ, Poon KY, Quinn VP. Red wine consumption and risk of prostate cancer: the California Men's Health Study. Int J Cancer. 2010;126:171‐179. doi: 10.1002/ijc.24637 [DOI] [PubMed] [Google Scholar]
- 16. Zaitsu M, Kawachi I, Takeuchi T, Kobayashi Y. Alcohol consumption and risk of upper‐tract urothelial cancer. Cancer Epidemiol. 2017;48:36‐40. doi: 10.1016/j.canep.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 17. Zaitsu M, Nakamura F, Toyokawa S, et al. Risk of alcohol consumption in bladder cancer: case‐control study from a nationwide inpatient database in Japan. Tohoku J Exp Med. 2016;239:9‐15. doi: 10.1620/tjem.239.9 [DOI] [PubMed] [Google Scholar]
- 18. Vartolomei MD, Iwata T, Roth B, et al. Impact of alcohol consumption on the risk of developing bladder cancer: a systematic review and meta‐analysis. World J Urol. Published online June 6, 2019. doi: 10.1007/s00345-019-02825-4 [DOI] [PubMed] [Google Scholar]
- 19. Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74:784‐795. doi: 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 20. Jayasekara H, Juneja S, Hodge AM, et al. Lifetime alcohol intake and risk of non‐Hodgkin lymphoma: findings from the Melbourne Collaborative Cohort Study. Int J Cancer. 2018;142:919‐926. doi: 10.1002/ijc.31123 [DOI] [PubMed] [Google Scholar]
- 21. Hsu WL, Chien YC, Chiang CJ, et al. Lifetime risk of distinct upper aerodigestive tract cancers and consumption of alcohol, betel and cigarette. Int J Cancer. 2014;135:1480‐1486. doi: 10.1002/ijc.28791 [DOI] [PubMed] [Google Scholar]
- 22. Kishikawa H, Sato K, Yamauchi T, et al. Incidence and risk factors for colorectal neoplasia in patients with oral squamous cell carcinoma. Colorectal Dis. 2014;16:888‐895. doi: 10.1111/codi.12717 [DOI] [PubMed] [Google Scholar]
- 23. Morita M, Saeki H, Mori M, Kuwano H, Sugimachi K. Risk factors for esophageal cancer and the multiple occurrence of carcinoma in the upper aerodigestive tract. Surgery. 2002;131(1 suppl):S1‐S6. doi: 10.1067/msy.2002.119287 [DOI] [PubMed] [Google Scholar]
- 24. Maruyama H, Yasui T, Ishikawa‐Fujiwara T, et al. Human papillomavirus and p53 mutations in head and neck squamous cell carcinoma among Japanese population. Cancer Sci. 2014;105:409‐417. doi: 10.1111/cas.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zaitsu M, Kaneko R, Takeuchi T, Sato Y, Kobayashi Y, Kawachi I. Occupational class and male cancer incidence: nationwide, multicenter, hospital‐based case‐control study in Japan. Cancer Med. 2019;8:795‐813. doi: 10.1002/cam4.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zaitsu M, Kaneko R, Takeuchi T, Sato Y, Kobayashi Y, Kawachi I. Occupational inequalities in female cancer incidence in Japan: hospital‐based matched case‐control study with occupational class. SSM Popul Health. 2018;5:129‐137. doi: 10.1016/j.ssmph.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaitsu M, Cuevas AG, Trudel‐Fitzgerald C, Takeuchi T, Kobayashi Y, Kawachi I. Occupational class and risk of renal cell cancer. Health Sci Rep. 2018;1:e49. doi: 10.1002/hsr2.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zaitsu M, Kato S, Kim Y, et al. Occupational class and risk of cardiovascular disease incidence in Japan: nationwide, multicenter, hospital‐based case‐control study. J Am Heart Assoc. 2019;8:e011350. doi: 10.1161/JAHA.118.011350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alkerwi A, Boutsen M, Vaillant M, et al. Alcohol consumption and the prevalence of metabolic syndrome: a meta‐analysis of observational studies. Atherosclerosis. 2009;204:624‐635. doi: 10.1016/j.atherosclerosis.2008.10.036 [DOI] [PubMed] [Google Scholar]
- 30. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 31. Zaitsu M, Yoshihara T, Nakai H, Kubota S. Optimal thermal control with sufficient nutrition may reduce the incidence of neonatal jaundice by preventing body‐weight loss among non‐low birth weight infants not admitted to neonatal intensive care unit. Neonatology. 2018;114:348‐354. doi: 10.1159/000491817 [DOI] [PubMed] [Google Scholar]
- 32. Inoue M, Tsugane S; JPHC Study Group . Impact of alcohol drinking on total cancer risk: data from a large‐scale population‐based cohort study in Japan. Br J Cancer. 2005;92:182‐187. doi: 10.1038/sj.bjc.6602277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nakagawa‐Senda H, Ito H, Hosono S, Oze I, Tanaka H, Matsuo K. Coffee consumption and the risk of colorectal cancer by anatomical subsite in Japan: results from the HERPACC studies. Int J Cancer. 2017;141:298‐308. doi: 10.1002/ijc.30746 [DOI] [PubMed] [Google Scholar]
- 34. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268‐274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Japan Organization of Occupational Health and Safety. Restrictions apply to the availability of these data, which were used under license for this study; research data are not shared. If any person wishes to verify the data, they are most welcome to contact the corresponding author.


