Abstract
Somatic stem/progenitor cells actively proliferate and give rise to different types of mature cells (active state) in embryonic tissues while they are mostly dormant (quiescent state) in many adult tissues. Notch signaling is known to regulate both active and quiescent states of somatic stem cells, but how it regulates these different states is unknown. Recent studies revealed that the Notch effector Hes1 is expressed differently during the active and quiescent states during neurogenesis and myogenesis: high in the quiescent state and oscillatory in the active state. When the Hes1 expression level is high, both Ascl1 and MyoD expression are continuously suppressed. By contrast, when Hes1 expression oscillates, it periodically represses expression of the neurogenic factor Ascl1 and the myogenic factor MyoD, thereby driving Ascl1 and MyoD oscillations. High levels of Hes1 and the resultant Ascl1 suppression promote the quiescent state of neural stem cells, while Hes1 oscillation‐dependent Ascl1 oscillations regulate their active state. Similarly, in satellite cells of muscles, known adult muscle stem cells, high levels of Hes1 and the resultant MyoD suppression seem to promote their quiescent state, while Hes1 oscillation‐dependent MyoD oscillations activate their proliferation and differentiation. Therefore, the expression dynamics of Hes1 is a key regulatory mechanism of generating and maintaining active/quiescent stem cell states.
Keywords: active stem cell, Hes1, Notch signaling, oscillatory expression, quiescent stem cell
Somatic stem/progenitor cells are active in embryonic tissues but quiescent in many adult tissues. Recent studies indicate that the expression dynamics of Hes1 is a key regulatory mechanism of generating and maintaining active/quiescent stem cell states.

1. INTRODUCTION
Somatic stem/progenitor cells have different characteristics in embryonic tissues than they do in adult tissues. In embryonic tissues, they actively divide and give rise to many mature cells, whereas in many adult tissues, they are mostly dormant/quiescent and only occasionally proliferate to give rise to some mature cells. The molecular nature of these differences between the embryonic active state and the adult quiescent state of stem cells is unknown, but recent data suggest that Notch signaling plays an essential role in maintaining both active and quiescent stem cells (Imayoshi, Sakamoto, Yamaguchi, Mori, & Kageyama, 2010). Differentiating cells express Notch ligands such as Delta‐like1 (Dll1), which activate Notch signaling in neighboring cells. Upon activation of Notch signaling, the transmembrane protein Notch is processed in the neighboring cells, releasing the Notch intracellular domain (NICD). NICD then transits to the nuclei, forms a complex with the Notch signaling mediator Rbpj, and induces transcriptional repressors such as Hes1 and Hes5 (Figure 1; Artavanis‐Tsakonas, Rand, & Lake, 1999). Hes1 and Hes5 inhibit cell differentiation by antagonizing cell fate determination factors, such as the neurogenic factors Ascl1 and Neurogenin2 (Neurog2) (Figure 1; Kageyama, Ohtsuka, & Kobayashi, 2007). Thus, differentiating cells inhibit neighboring cells from differentiating by activating Notch signaling, a process called lateral inhibition. However, recent data suggest that Notch signaling molecules exhibit more dynamic expression patterns than previously thought and that such dynamic expression is important for morphogenesis (Kageyama, Ohtsuka, Shimojo, & Imayoshi, 2008). In this review, we discussed the functional significance and mechanism of Notch signaling in regulating active and quiescent stem cells, focusing mainly on neural stem cells.
Figure 1.
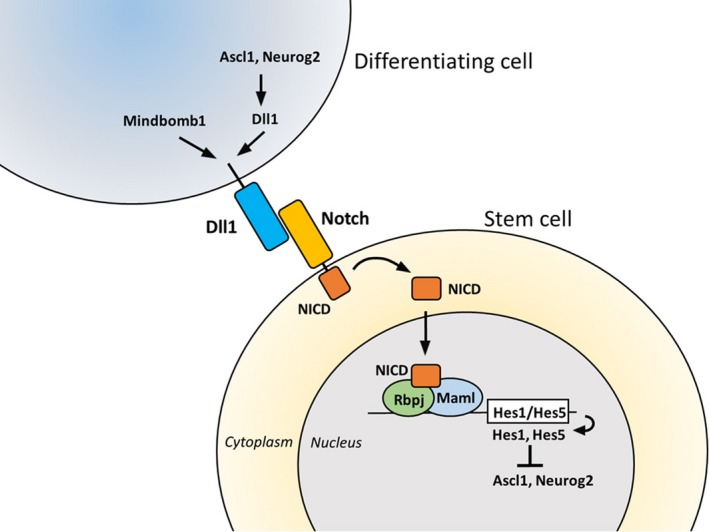
Lateral inhibition of differentiation via Notch signaling. A differentiating cell expresses Notch ligands such as Delta‐like1 (Dll1), which activate Notch signaling in a neighboring cell. The transmembrane protein Notch is then processed in the neighboring cell, releasing the Notch intracellular domain (NICD), which transits to the nucleus, forms a complex with the Notch signaling mediator Rbpj and the co‐activator Mastermind‐like (Maml), and induces transcriptional repressors such as Hes1 and Hes5. Hes1 and Hes5 inhibit cell differentiation and maintain a somatic stem cell by antagonizing cell fate determination factors such as the neurogenic factors Ascl1 and Neurog2
2. NEURAL STEM CELLS IN THE EMBRYONIC AND ADULT BRAIN
In the developing nervous system, neuroepithelial cells, the earliest form of neural stem/progenitor cells, actively proliferate (Figure 2a). Neuroepithelial cells are gradually elongated and transformed into radial glial cells that retain their cell bodies in the innermost neural tube layer, called the ventricular zone (VZ), and extend their processes, called radial fibers, to the external surface of the brain. Radial glial cells undergo asymmetric cell division, each dividing into two distinct cell types: one radial glial cell and one immature neuron or an intermediate progenitor (Figure 2a; Fujita, 2003; Götz & Huttner, 2005). Immature neurons migrate outside of the VZ into outer layers of the brain, where they become mature neurons, while intermediate progenitors migrate into the subventricular zone (SVZ), divide a few times, and give rise to more neurons (Figure 2a). In higher mammals such as primates, some radial glial cells detach from the inner (apical) surface, form a new outer SVZ (OSVZ) layer, and undergo asymmetric cell divisions, producing more neurons (Figure 2a). Neurons are formed in an inside‐out pattern such that inner‐layer neurons are generated first, followed by outer‐layer neurons. Thus, cortical neuron subtypes are sequentially determined by birthdate through progressive lineage restriction of common radial glial cells. After producing neurons, radial glial cells finally differentiate into glial cells (oligodendrocytes and astrocytes), although some of them are maintained as neural stem cells in the postnatal and adult brain. It has been shown that some neural stem cells become slowly dividing or quiescent during embryonic stages and remain as adult neural stem cells (Fuentealba et al., 2015; Furutachi et al., 2015) while others become quiescent postnatally (Berg et al., 2019).
Figure 2.
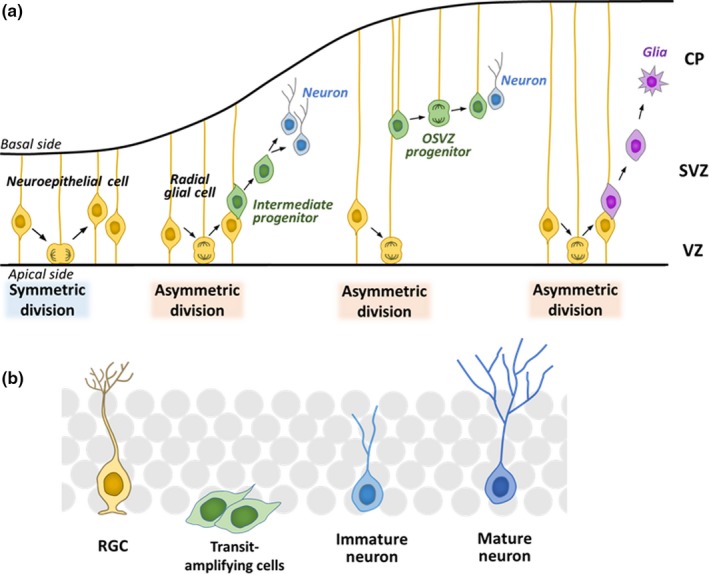
Neurogenesis in the embryonic and adult brain. (a) Differentiation of neural stem/progenitor cells in the embryonic cortex. Neuroepithelial cells initially undergo repeated self‐renewal by symmetric division. As development proceeds, neuroepithelial cells elongate to become radial glial cells, which have cell bodies in the inner region (the ventricular zone, VZ) of the neural tube and long processes (radial fibers) that reach the outer surface. Radial glial cells give rise to neurons and/or intermediate progenitors. Each intermediate progenitor migrates into the subventricular zone (SVZ) and produces neurons. Neurons migrate outward to form the cortical plate (CP). Some radial glial cells detach from the inner (apical) surface, form a new layer called the outer SVZ (OSVZ), and undergo asymmetric cell divisions, producing more neurons. After producing neurons, radial glial cells differentiate into glia (oligodendrocytes and astrocytes). Both neuroepithelial cells and radial glial cells are considered embryonic neural stem/progenitor cells. (b) Neurogenesis in the subgranular zone (SGZ) of the hippocampal dentate gyrus of the adult brain. Neural stem cells with a radial glial cell (RGC) morphology are mostly quiescent/dormant and only occasionally divide to produce transit‐amplifying cells, which divide a few times and differentiate into neurons
In the adult mouse brain, neural stem cells are present in two regions: the subgranular zone (SGZ) of the hippocampal dentate gyrus (Figure 2b) and the SVZ of the lateral ventricles (Doetsch, 2003; Kriegstein & Alvarez‐Buylla, 2009). These adult neural stem cells, which have a radial glial cell morphology, are mostly quiescent/dormant, and only occasionally become activated and divide to produce transit‐amplifying cells (Figure 2b). Transit‐amplifying cells divide a few times and soon differentiate into neurons that integrate into the preexisting neural circuits (Bonaguidi et al., 2011; Encinas et al., 2011; Imayoshi et al., 2008; Lagace et al., 2007; Pilz et al., 2018; Seri, García‐Verdugo, McEwen, & Alvarez‐Buylla, 2001). Thus, the characteristics of neural stem cells are totally different during the embryonic active and adult quiescent states.
3. NOTCH SIGNALING IN ACTIVE AND QUIESCENT NEURAL STEM CELLS
Notch signaling plays an essential role in maintaining active neural stem cells in the developing mouse nervous system (Androutsellis‐Theotokis et al., 2006; Chambers et al., 2001; Imayoshi et al., 2010; Mason et al., 2005; Mizutani, Yoon, Dang, Tokunaga, & Gaiano, 2007). Inactivation of the Notch signaling effector genes Hes1, Hes3, and Hes5 upregulates the expression of proneural genes such as Ascl1 and Neurog2, accelerates neurogenesis, and prematurely depletes neural stem cells in the developing nervous system except for the telencephalon (Hatakeyama et al., 2004; Imayoshi & Kageyama, 2014; Ishibashi et al., 1995; Ohtsuka et al., 1999). In the telencephalon, the Hes1‐related gene Hey1 is highly expressed, suggesting that it compensates for the Hes deficiency. The Hes3;Hes5;Hey1‐null nervous system seems to develop normally, but additional deletion of Hes1 upregulates the expression of Ascl1 and Neurog2, accelerates neurogenesis, and prematurely depletes neural stem cells in the developing telencephalon (Sueda, Imayoshi, Harima, & Kageyama, 2019), indicating that these four Hes/Hey genes cooperatively regulate telencephalic development. Similar defects in the developing nervous system were also observed in the absence of the Notch mediator Rbpj (Imayoshi et al., 2010). Thus, the Notch‐Rbpj‐Hes1/Hes3/Hes5/Hey1 pathway appears to play an essential role in maintaining active neural stem cells in the developing mouse nervous system.
Interestingly, Notch signaling is also important for maintaining quiescent neural stem cells in the adult brain (Ables et al., 2010; Ehm et al., 2010; Imayoshi et al., 2010; Nyfeler et al., 2005; Veeraraghavalu, Choi, Zhang, & Sisoda, 2010). Again, while adult neurogenesis is not significantly affected in the absence of Hes3, Hes5, and Hey1, additional deletion of Hes1 upregulates the expression of Ascl1, accelerates neurogenesis, and prematurely depletes quiescent neural stem cells in the adult brain (Sueda et al., 2019). Furthermore, similar defects in the adult neurogenesis occur in the absence of Rbpj (Imayoshi et al., 2010), indicating that the Notch‐Rbpj‐Hes1/Hes3/Hes5/Hey1 pathway plays an essential role in maintaining quiescent neural stem cells in the adult brain.
Thus, Notch signaling regulates the maintenance of both embryonic active and adult quiescent neural stem cells. But, how does Notch signaling lead to active and quiescent states in the embryonic and adult brains? Our recent data suggest that the dynamics of Hes1 expression are involved in these different states.
4. DYNAMIC EXPRESSION OF NOTCH SIGNALING GENES IN ACTIVE NEURAL STEM CELLS
While Hes genes are required to maintain embryonic neural stem cells, immunostaining and in situ hybridization analyses indicated that their expression levels are variable, exhibiting a granular, salt‐and‐pepper pattern in the VZ. Live‐imaging analyses showed that in embryonic neural stem cells, Hes1 expression oscillates with 2–3‐hr periodicity regulated by negative feedback from Hes1 protein (Figure 3). Notch signaling activates the expression of Hes1, whose protein product represses its own expression by directly binding to its promoter (Hirata et al., 2002; Shimojo, Ohtsuka, & Kageyama, 2008). When the Hes1 promoter is repressed, both Hes1 mRNA and Hes1 protein disappear rapidly because they are extremely unstable, which cancels the negative feedback and initiates the next round of expression. Thus, Hes1 expression oscillates autonomously (Hirata et al., 2002), and a snapshot of Hes1 oscillations in embryonic neural stem cells exhibits a salt‐and‐pepper pattern in the VZ. In these cells, Hes1 oscillations periodically repress the expression of proneural genes and Dll1; therefore, these genes are also expressed in an oscillatory manner and exhibit salt‐and‐pepper patterns in the VZ (Figure 3; Shimojo et al., 2008).
Figure 3.
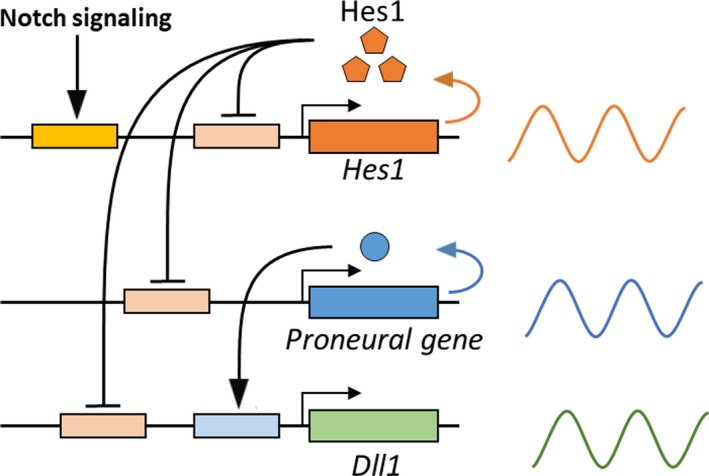
Dynamic expression of Notch signaling genes in active neural stem cells. Notch signaling activates the expression of Hes1, which oscillates with 2–3‐hr periodicity regulated by negative feedback of Hes1 protein. Hes1 oscillations periodically repress the expression of proneural genes and Dll1. Therefore, these genes are also expressed in an oscillatory manner (shown on the right)
It has been shown that the proneural gene Ascl1 has dual, opposing functions: activating the proliferation of neural stem cells and inducing cell cycle exit and subsequent neuronal differentiation (Castro et al., 2011). In neural stem cells, where Notch signaling is active, Hes1 oscillations induce Ascl1 oscillations, while in differentiating neurons, where Notch signaling is inactive, Hes1 expression disappears, enabling Ascl1 to be expressed continuously. Hence, different expression dynamics may be involved in the dual opposing functions of Ascl1. To investigate this possibility, we used an optogenetic method that induces gene expression upon blue‐light illumination (Figure 4a; Wang, Chen, & Yang, 2012). This analysis demonstrated that Ascl1 activates proliferation of neural stem cells when its expression is oscillatory and induces cell cycle exit and neuronal differentiation when its expression is sustained (Figure 4b; Imayoshi et al., 2013). Thus, Ascl1 oscillations, which normally depend on Hes1 oscillations, are important for proliferation of neural stem cells.
Figure 4.
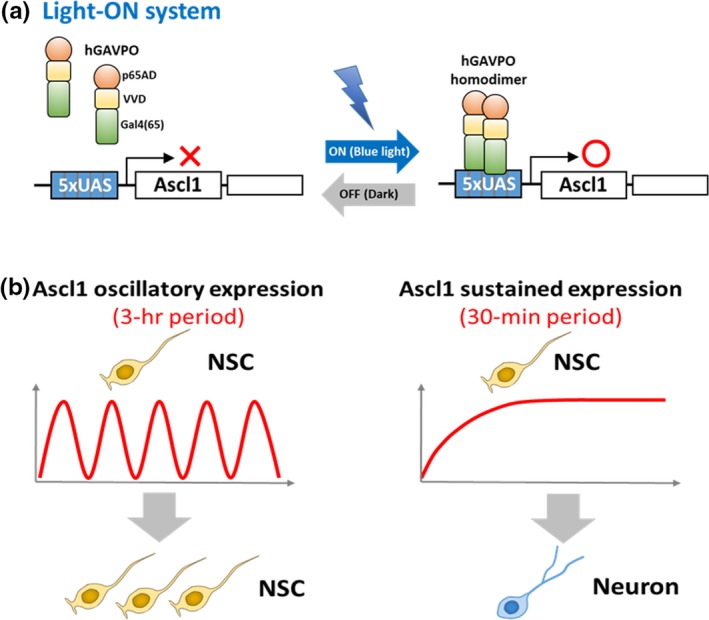
Optogenetic expression of the proneural gene Ascl1. (a) Optogenetic Light‐ON system. Under dark conditions, hGAVPO is an inactive monomer. Upon blue‐light illumination, hGAVPO forms a dimer, binds to the upstream activation sequence (UAS) promoter, and activates the expression of downstream genes. (b) The Light‐ON system can differentially induce oscillatory and sustained Ascl1 expression by changing blue light illumination patterns. Ascl1 activates proliferation of neural stem cells (NSC) when its expression is oscillatory, and induces neuronal differentiation when its expression is sustained
This oscillatory expression is also advantageous to keep a group of cells from differentiating. According to the “lateral inhibition” model of Notch signaling, neurons express Dll1 and activate Notch signaling in neighboring cells, which are inhibited from differentiating into neurons (Figure 1). Therefore, neurons are required to activate Notch signaling and maintain neural stem cells. However, the question arises as to how neural stem cells are maintained before neurons are born. It was found that at an early stage of development, neural stem cells express Hes1, proneural genes, and Dll1 in an oscillatory manner (Shimojo et al., 2008). It is likely that neural stem cells mutually activate Notch signaling at an early stage of development, as follows (Figure 5): When Ascl1/Neurog2 levels are high in one neural stem cell (cell 1), they induce the expression of Dll1, which activates Notch signaling in a neighboring neural stem cell (cell 2). In this neighboring cell, activated Notch signaling induces the expression of Hes1, which represses the expression of Ascl1/Neurog2 and Dll1. However, due to the 2–3‐hr periodicity of the oscillations, Hes1 levels soon become lower, enabling the expression of Ascl1/Neurog2 and Dll1, which activates Notch signaling in the first cell (cell 1). In this way, neighboring cells can mutually activate Notch signaling via Dll1 oscillations (Shimojo et al., 2008). Mathematical modeling suggests that the timing of Dll1 expression is very important for its oscillation and that accelerating or delaying the timing of Dll1 expression dampens its oscillation. Indeed, when the timing of Dll1 expression is accelerated or delayed by shortening or elongating the Dll1 gene, respectively, Hes1 oscillations are severely dampened (Shimojo et al., 2016). Under these conditions, the maintenance and proliferation of neural stem cells are impaired, leading to accelerated neurogenesis and microcephaly (Shimojo et al., 2016). Thus, oscillatory expression is necessary for the maintenance and proliferation of neural stem cells.
Figure 5.
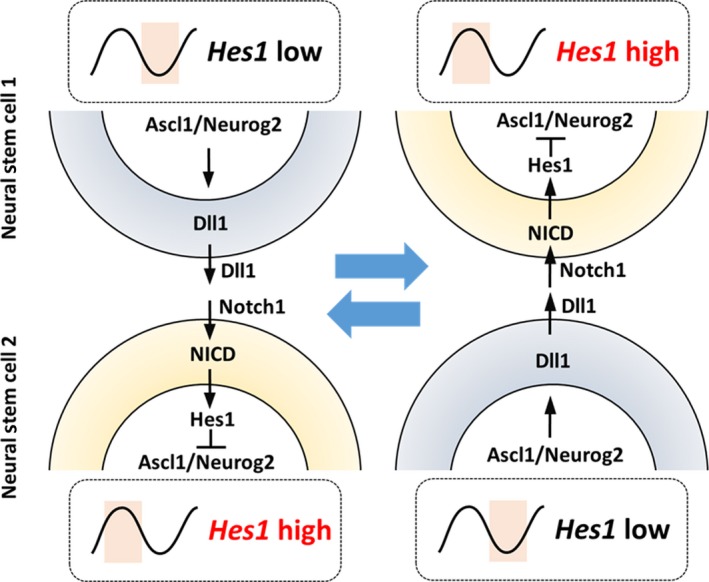
Mutual activation of Notch signaling by oscillations. When Ascl1/Neurog2 are high in a neural stem cell (cell 1), they induce the expression of Dll1, which activates Notch signaling in a neighboring neural stem cell (cell 2). In this neighboring cell, activated Notch signaling induces the expression of Hes1, which represses Ascl1/Neurog2 and Dll1 expression. However, due to the 2–3‐hr periodicity of oscillations, Hes1 levels soon become lower, enabling Ascl1/Neurog2 and Dll1 to be expressed once again, which activates Notch signaling in the first cell (cell 1). In this way, neighboring cells (cell 1 and cell 2) can mutually activate Notch signaling via Dll1 oscillations. NICD, Notch intracellular domain
5. DIFFERENT EXPRESSION DYNAMICS OF NOTCH SIGNALING GENES IN QUIESCENT NEURAL STEM CELLS
Immunohistological analysis indicates that not all cells exhibit the granular, “salt‐and‐pepper” staining patterns of Hes1 in the developing nervous system. Hes1 levels are high and sustained, while proneural proteins are lacking in the boundary regions, such as the roof plate, floor plate, and isthmus (Baek, Hatakeyama, Sakamoto, Ohtsuka, & Kageyama, 2006). In these regions, cells are mostly quiescent, suggesting that patterns of gene expression may be involved in the quiescent versus active state. In agreement with this notion, it was previously shown that induction of high and sustained levels of Hes1 inhibits cell proliferation and that induced inactivation of Ascl1 expression impairs proliferation of neural stem cells (Baek et al., 2006; Castro et al., 2011). These results suggest that high and sustained Hes1 and the resultant suppression of proneural genes may enhance the quiescence of neural stem cells.
How about neural stem cells in the adult brain? The process of activating quiescent neural stem cells in the adult brain has been intensively analyzed. Small fractions of neural stem cells are activated to produce transit‐amplifying cells, which proliferate and soon generate neuroblasts (Figure 2b). Expression of the proneural gene Ascl1 plays a critical role in the activation of quiescent neural stem cells and subsequent formation of neuroblasts in the mouse brain. Ascl1 is expressed at low levels in some activated neural stem cells and at high levels in transit‐amplifying cells (Andersen et al., 2014; Kim, Ables, Dickel, Eisch, & Johnson, 2011; Pastrana, Cheng, & Doetsch, 2009). Furthermore, live‐imaging analysis showed that Ascl1‐expressing neural stem cells exclusively generated neurons in the adult mouse hippocampus (Pilz et al., 2018). By contrast, in the absence of Ascl1, all neural stem cells remained quiescent, indicating that Ascl1 is absolutely required to activate quiescent neural stem cells (Andersen et al., 2014). Recent live‐imaging analysis of the adult mouse brain demonstrated that Hes1 levels were oscillatory, although the peaks and troughs were higher than those in active neural stem cells, causing Ascl1 expression to be continuously suppressed (Figure 6a; Sueda et al., 2019). Inactivation of the Notch pathway upregulates Ascl1 expression, activates neural stem cells, and transiently enhances neurogenesis, but neural stem cells are soon depleted, ending neurogenesis prematurely (Ables et al., 2010; Andersen et al., 2014; Ehm et al., 2010; Imayoshi et al., 2010; Sueda et al., 2019). Conversely, activation of Notch signaling or induction of sustained Hes1 expression represses Ascl1 expression, inhibits neurogenesis, and maintains quiescent neural stem cells in the adult brain (Imayoshi et al., 2010; Mason et al., 2005; Mizutani et al., 2007; Sueda et al., 2019). These results indicate that high levels of Hes1 and the resultant suppression of Ascl1 promote quiescence in neural stem cells in the adult brain (Figure 6a). Indeed, induction of Ascl1 oscillations efficiently activates neural stem cells to produce new neurons in the adult brain (Sueda et al., 2019).
Figure 6.
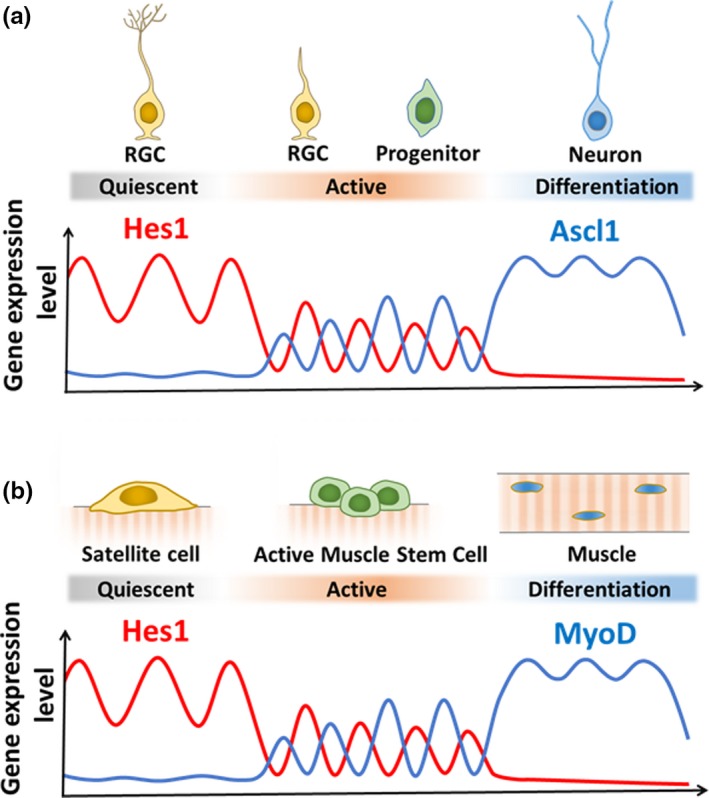
Gene expression dynamics during neurogenesis and myogenesis in adults. (a) Expression dynamics of Hes1 and Ascl1 during neurogenesis. (b) Expression dynamics of Hes1 and MyoD during myogenesis
The detailed mechanism by which high levels of Hes1 are maintained in quiescent neural stem cells remains to be determined. It was shown that bone morphogenetic protein (BMP) signaling is important for maintaining quiescent neural stem cells in the adult brain (Mira et al., 2010). BMP signaling induces the expression of Id1 (Miyazono & Miyazawa, 2002), and Id1 is highly expressed by quiescent neural stem cells in the adult brain (Nam & Benezra, 2009). Interestingly, Id1 inhibits Hes1 activities by forming a complex. Hes1 oscillations are controlled by negative feedback (Hirata et al., 2002), but high levels of Id1 interact with Hes1 and inhibit its negative feedback, thereby upregulating Hes1 expression (Bai et al., 2007; Boareto, Iber, & Taylor, 2017). These results suggest that the BMP‐Id‐Hes axis may be the major pathway for upregulating Hes1, suppressing Ascl1, and maintaining quiescent neural stem cells in the adult brain.
6. ROLES OF HES1 IN ACTIVE AND QUIESCENT MUSCLE STEM CELLS
Similar to neural stem cells, muscle stem cells have different characteristics in embryonic and adult tissues. For example, muscle stem cells derived from the dermomyotome of the somite actively proliferate and generate skeletal muscles in embryos. By contrast, satellite cells in the adult skeletal muscle (known muscle stem cells) are quiescent (Shi & Garry, 2006; Wang & Rudnicki, 2012). When muscles are injured, satellite cells are activated and contribute to muscle regeneration. During this process, the muscle determination gene MyoD plays an important role in muscle formation. It was reported that MyoD not only induces the expression of genes involved in muscle differentiation but also up‐regulates the genes involved in cell proliferation (Cao et al., 2010). Indeed, MyoD directly activates the expression of Id genes (Cao et al., 2010), which inhibit muscle differentiation by antagonizing MyoD activity. Thus, MyoD also appears to have dual activities in muscle development, the initial maintenance of dividing muscle stem cells and the subsequent cell cycle exit and muscle differentiation, similar to that of Ascl1 in neural development. However, it was not clear how MyoD controls these dual activities.
Recent studies showed that in satellite cells of adult muscles, MyoD expression is continuously repressed by the Notch‐Hes1 pathway (Lahmann et al., 2019; Mourikis et al., 2012). When satellite cells are activated, Hes1 expression oscillates with 2–3‐hr periodicity, and Hes1 oscillations periodically repress MyoD expression, thereby driving MyoD oscillations (Lahmann et al., 2019). By contrast, when satellite cells begin muscle differentiation, Hes1 expression disappears, while MyoD expression becomes sustained (Lahman et al., 2019). Furthermore, when Hes1 is inactivated, MyoD expression becomes sustained immediately, and muscle differentiation proceeds without sufficient proliferation of satellite cells. All these data suggest that Hes1 oscillations‐dependent MyoD oscillations regulate proliferation of active muscle stem cells, whereas sustained Hes1 and resultant suppression of MyoD enhance quiescence in muscle stem cells (Figure 6b).
7. CONCLUSIONS
Notch signaling controls the production of active and quiescent somatic stem cells by oscillatory and sustained Hes1 expression, respectively. Oscillating levels of Hes1 drive the oscillatory expression of cell fate determination factors such as Ascl1 and MyoD, which activate proliferation, whereas high, sustained levels of Hes1 suppress cell fate determination factors, leading to quiescence. The oscillatory expression of cell fate determination factors may keep somatic stem cells active, which then proliferate and give rise to mature cells. Thus, the induction of oscillations of cell fate determination factors may be useful to manipulate endogenous stem cells for tissue regeneration. For example, activation of endogenous neural stem cells by Ascl1 oscillations can generate many new neurons, which may ameliorate neurodegenerative diseases such as Alzheimer disease and Huntington disease (Benraiss et al., 2013; Choi et al., 2018). Optogenetic and Hes5‐promoter‐induced oscillations of Ascl1 can activate endogenous neural stem cells in the adult brain, but so far this procedure was not able to maintain active neural stem cells for more than a month (Sueda et al., 2019), probably because Ascl1 expression tends to accumulate and become sustained after a few weeks, which may induce neuronal differentiation. Improvements of this kind of approach should lead to long‐term activation of neural stem cells and may be useful for regenerative medicine in the future.
AUTHOR CONTRIBUTION
RS made figures; RS and RK wrote the manuscript.
ACKNOWLEDGEMENTS
This work was supported by Core Research for Evolutional Science and Technology (CREST) (JPMJCR12W2, R.K.), Grant‐in‐Aid for Scientific Research on Innovative Areas (16H06480, R.K.) from Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, and AMED‐CREST (JP18gm1110002, R.K.) from Japan Agency for Medical Research and Development.
Sueda R, Kageyama R. Regulation of active and quiescent somatic stem cells by Notch signaling. Develop Growth Differ. 2020;62:59–66. 10.1111/dgd.12626
The copyright line for this article was changed on 10 October 2019 after original online publication
REFERENCES
- Ables, J. L. , Decarolis, N. A. , Johnson, M. A. , Rivera, P. D. , Gao, Z. , Cooper, D. C. , … Eisch, A. J. (2010). Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. Journal of Neuroscience, 30, 10484–10492. 10.1523/jneurosci.4721-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, J. , Urbán, N. , Achimastou, A. , Ito, A. , Simic, M. , Ullom, K. , … Guillemot, F. (2014). A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron, 83, 1085–1097. 10.1016/j.neuron.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis‐Theotokis, A. , Leker, R. R. , Soldner, F. , Hoeppner, D. J. , Ravin, R. , Poser, S. W. , … McKay, R. D. (2006). Notch signalling regulates stem cell numbers in vitro and in vivo. Nature, 442, 823–826. 10.1038/nature04940 [DOI] [PubMed] [Google Scholar]
- Artavanis‐Tsakonas, S. , Rand, M. D. , & Lake, R. J. (1999). Notch signaling: Cell fate control and signal integration in development. Science, 284, 770–776. 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- Baek, J. H. , Hatakeyama, J. , Sakamoto, S. , Ohtsuka, T. , & Kageyama, R. (2006). Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development, 133, 2467–2476. 10.1242/dev.02403 [DOI] [PubMed] [Google Scholar]
- Bai, G. , Sheng, N. , Bian, W. , Xie, Z. , Yokota, Y. , Benezra, R. , … Jing, N. (2007). Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Developmental Cell, 13, 283–297. [DOI] [PubMed] [Google Scholar]
- Benraiss, A. , Toner, M. J. , Xu, Q. , Bruel‐Jungerman, E. , Rogers, E. H. , Wang, F. , … Goldman, S. A. (2013). Mobilization of endogenous progenitor cells regenerates functionally‐integrated medium spiny striopallidal projection neurons and delays disease progression in a transgenic model of Huntington's disease. Cell Stem Cell, 12, 787–799. 10.1016/j.stem.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg, D. A. , Su, Y. , Jimenez‐cyrus, D. , Patel, A. , Huang, N. , Morizet, D. , … Bond, A. M. (2019). A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell, 177, 654–668. 10.1016/j.cell.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boareto, M. , Iber, D. , & Taylor, V. (2017). Differential interactions between Notch and ID factors control neurogenesis by modulating Hes factor autoregulation. Development, 144, 3465–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi, M. A. , Wheeler, M. A. , Shapiro, J. S. , Stadel, R. P. , Sun, G. J. , Ming, G. , & Song, H. (2011). In vivo clonal analysis reveals self‐renewing and multipotent adult neural stem cell characteristics. Cell, 145, 1142–1155. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Yao, Z. , Sarkar, D. , Lawrence, M. , Sanchez, G. J. , Parker, M. H. , … Tapscott, S. J. (2010). Genome‐wide MyoD binding in skeletal muscle cells: A potential for broad cellular reprogramming. Developmental Cell, 18, 662–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, D. S. , Martynoga, B. , Parras, C. , Ramesh, V. , Pacary, E. , Johnston, C. , … Guillemot, F. (2011). A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome‐wide characterization of its targets. Genes and Development, 25, 930–945. 10.1101/gad.627811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, C. B. , Peng, Y. , Nguyen, H. , Gaiano, N. , Fishell, G. , & Nye, J. S. (2001). Spatiotemporal selectivity of response to Notch1 signals in mammalian forebrain precursors. Development, 128, 689–702. [DOI] [PubMed] [Google Scholar]
- Choi, S. H. , Bylykbashi, E. , Chatila, Z. K. , Lee, S. W. , Pulli, B. , Clemenson, G. D. , … Tanzi, R. E. (2018). Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science, 361, eaan8821 10.1126/science.aan8821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch, F. (2003). The glial identity of neural stem cells. Nature Neuroscience, 6, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Ehm, O. , Göritz, C. , Covic, M. , Schäffner, I. , Schwarz, T. J. , Karaca, E. , … Lie, D. C. (2010). RBPJκ‐Dependent signaling is essential for long‐term maintenance of neural stem cells in the adult hippocampus. Journal of Neuroscience, 30, 13794–13807. 10.1523/jneurosci.1567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas, J. M. , Michurina, T. V. , Peunova, N. , Park, J.‐H. , Tordo, J. , Peterson, D. A. , … Enikolopov, G. (2011). Division‐coupled astrocytic differentiation and age‐related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell, 8, 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba, L. C. , Rompani, S. B. , Parraguez, J. I. , Obernier, K. , Romero, R. , Cepko, C. L. , & Alvarez‐Buylla, A. (2015). Embryonic origin of postnatal neural stem cells. Cell, 161, 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, S. (2003). The discovery of the matrix cell, the identification of the multipotent neural stem cell and the development of the central nervous system. Cell Structure and Function, 28, 205–228. [DOI] [PubMed] [Google Scholar]
- Furutachi, S. , Miya, H. , Watanabe, T. , Kawai, H. , Yamasaki, N. , Harada, Y. , … Gotoh, Y. (2015). Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nature Neuroscience, 18, 657–665. 10.1038/nn.3989 [DOI] [PubMed] [Google Scholar]
- Götz, M. , & Huttner, W. B. (2005). The cell biology of neurogenesis. Nature Reviews Molecular Cell Biology, 6, 777–788. [DOI] [PubMed] [Google Scholar]
- Hatakeyama, J. , Bessho, Y. , Katoh, K. , Ookawara, S. , Fujioka, M. , Guillemot, F. , & Kageyama, R. (2004). Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development, 131, 5539–5550. 10.1242/dev.01436 [DOI] [PubMed] [Google Scholar]
- Hirata, H. , Yoshiura, S. , Ohtsuka, T. , Bessho, Y. , Harada, T. , Yoshikawa, K. , & Kageyama, R. (2002). Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science, 298, 840–843. [DOI] [PubMed] [Google Scholar]
- Imayoshi, I. , Isomura, A. , Harima, Y. , Kawaguchi, K. , Kori, H. , Miyachi, H. , … Kageyama, R. (2013). Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science, 342, 1203–1208. 10.1126/science.1242366 [DOI] [PubMed] [Google Scholar]
- Imayoshi, I. , & Kageyama, R. (2014). bHLH factors in self‐renewal, multipotency, and fate choice of neural progenitor cells. Neuron, 82, 9–23. 10.1016/j.neuron.2014.03.018 [DOI] [PubMed] [Google Scholar]
- Imayoshi, I. , Sakamoto, M. , Ohtsuka, T. , Takao, K. , Miyakawa, T. , Yamaguchi, M. , … Kageyama, R. (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nature Neuroscience, 11, 1153–1161. [DOI] [PubMed] [Google Scholar]
- Imayoshi, I. , Sakamoto, M. , Yamaguchi, M. , Mori, K. , & Kageyama, R. (2010). Essential roles of Notch signaling in maintenance of neural stem cells in the developing and adult brains. Journal of Neuroscience, 30, 3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi, M. , Ang, S.‐L. , Shiota, K. , Nakanishi, S. , Kageyama, R. , & Guillemot, F. (1995). Targeted disruption of mammalian hairy and Enhancer of split homolog‐1 (HES‐1) leads to up‐regulation of neural helix‐loop‐helix factors, premature neurogenesis and severe neural tube defects. Genes and Development, 9, 3136–3148. 10.1101/gad.9.24.3136 [DOI] [PubMed] [Google Scholar]
- Kageyama, R. , Ohtsuka, T. , & Kobayashi, T. (2007). The Hes gene family: Repressors and oscillators that orchestrate embryogenesis. Development, 134, 1243–1251. 10.1242/dev.000786 [DOI] [PubMed] [Google Scholar]
- Kageyama, R. , Ohtsuka, T. , Shimojo, H. , & Imayoshi, I. (2008). Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nature Neuroscience, 11, 1247–1251. [DOI] [PubMed] [Google Scholar]
- Kim, E. J. , Ables, J. L. , Dickel, L. K. , Eisch, A. J. , & Johnson, J. E. (2011). Ascl1 (Mash1) defines cells with long‐term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One, 6, e18472 10.1371/journal.pone.0018472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein, A. , & Alvarez‐Buylla, A. (2009). The glial nature of embryonic and adult neural stem cells. Annual Review of Neuroscience, 32, 149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace, D. C. , Whitman, M. C. , Noonan, M. A. , Ables, J. L. , DeCarolis, N. A. , Arguello, A. A. , … Eisch, A. J. (2007). Dynamic contribution of nestin‐expressing stem cells to adult neurogenesis. Journal of Neuroscience, 27, 12623–12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahmann, I. , Bröhl, D. , Zyrianova, T. , Isomura, A. , Czajkowski, M.T. , Kapoor, V. , … Birchmeier, C. (2019). Oscillations of Hes1 and MyoD proteins regulate the maintenance of activated muscle stem cells. Genes and Development, 33, 524–535. 10.3410/f.735320311.793559491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, H. A. , Rakowiecki, S. M. , Raftopoulou, M. , Nery, S. , Huang, Y. , Gridley, T. , & Fishell, G. (2005). Notch signaling coordinates the patterning of striatal compartments. Development, 132, 4247–4258. [DOI] [PubMed] [Google Scholar]
- Mira, H. , Andreu, Z. , Suh, H. , Lie, D. C. , Jessberger, S. , Consiglio, A. , … Gage, F. H. (2010). Signaling through BMPR‐IA regulates quiescence and long‐term activity of neural stem cells in the adult hippocampus. Cell Stem Cell, 7, 78–89. [DOI] [PubMed] [Google Scholar]
- Miyazono, K. , & Miyazawa, K. (2002). Id: A target of BMP signaling. Scie STKE, 151, pe40. [DOI] [PubMed]
- Mizutani, K. , Yoon, K. , Dang, L. , Tokunaga, A. , & Gaiano, N. (2007). Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature, 449, 351–355. 10.1038/nature06090 [DOI] [PubMed] [Google Scholar]
- Mourikis, P. , Sambasivan, R. , Castel, D. , Rocheteau, P. , Bizzarro, V. , & Tajbakhsh, S. (2012). A critical requirement for Notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells, 30, 243–252. [DOI] [PubMed] [Google Scholar]
- Nam, H. , & Benezra, R. (2009). High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell, 5, 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyfeler, Y. , Kirch, R. D. , Mantei, N. , Leone, D. P. , Radtke, F. , Suter, U. , & Taylor, V. (2005). Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self‐renewal. EMBO Journal, 24, 3504–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka, T. , Ishibashi, M. , Gradwohl, G. , Nakanishi, S. , Guillemot, F. , & Kageyama, R. (1999). Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. EMBO Journal, 18, 2196–2207. 10.1093/emboj/18.8.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana, E. , Cheng, L.‐C. , & Doetsch, F. (2009). Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proceedings of the National Academy of Sciences of the United States of America, 106, 6387–6392. 10.1073/pnas.0810407106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz, G.‐A. , Bottes, S. , Betizeau, M. , Jörg, D. J. , Carta, S. , Simons, B. D. , … Jessberger, S. (2018). Live imaging of neurogenesis in the adult mouse hippocampus. Science, 359, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri, B. , García‐Verdugo, J. M. , McEwen, B. S. , & Alvarez‐Buylla, A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. Journal of Neuroscience, 21, 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, X. , & Garry, D. J. (2006). Muscle stem cells in development, regeneration, and disease. Genes and Development, 20, 1692–1708. 10.1101/gad.1419406 [DOI] [PubMed] [Google Scholar]
- Shimojo, H. , Isomura, A. , Ohtsuka, T. , Kori, H. , Miyachi, H. , & Kageyama, R. (2016). Oscillatory control of Delta‐like1 in cell interactions regulates dynamic gene expression and tissue morphogenesis. Genes and Development, 30, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo, H. , Ohtsuka, T. , & Kageyama, R. (2008). Oscillations in Notch signaling regulate maintenance of neural progenitors. Neuron, 58, 52–64. [DOI] [PubMed] [Google Scholar]
- Sueda, R. , Imayoshi, I. , Harima, Y. , & Kageyama, R. (2019). High Hes1 expression and resultant Ascl1 suppression regulate quiescent versus active neural stem cells in the adult mouse brain. Genes and Development, 33, 511–523. 10.1101/gad.323196.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavalu, K. , Choi, S. H. , Zhang, X. , & Sisoda, S. S. (2010). Presenilin 1 mutants impair the self‐renewal and differentiation of adult murine subventricular zone‐neuronal progenitors via cell‐autonomous mechanisms involving Notch signaling. Journal of Neuroscience, 30, 6903–6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Chen, X. , & Yang, Y. (2012). Spatiotemporal control of gene expression by a light‐switchable transgene system. Nature Methods, 9, 266–269. [DOI] [PubMed] [Google Scholar]
- Wang, Y. X. , & Rudnicki, M. A. (2012). Satellite cells, the engines of muscle repair. Nature Reviews Molecular Cell Biology, 13, 127–133. 10.1038/nrm3265 [DOI] [PubMed] [Google Scholar]


