Abstract
Objective
Magnetic resonance imaging (MRI) of placental invasion has been part of clinical practice for many years. The possibility of being better able to assess placental vascularization and function using MRI has multiple potential applications. This review summarises up‐to‐date research on placental function using different MRI modalities.
Method
We discuss how combinations of these MRI techniques have much to contribute to fetal conditions amenable for therapy such as singletons at high risk for fetal growth restriction (FGR) and monochorionic twin pregnancies for planning surgery and counselling for selective growth restriction and transfusion conditions.
Results
The whole placenta can easily be visualized on MRI, with a clear boundary against the amniotic fluid, and a less clear placental‐uterine boundary. Contrasts such as diffusion weighted imaging, relaxometry, blood oxygenation level dependent MRI and flow and metabolite measurement by dynamic contrast enhanced MRI, arterial spin labeling, or spectroscopic techniques are contributing to our wider understanding of placental function.
Conclusion
The future of placental MRI is exciting, with the increasing availability of multiple contrasts and new models that will boost the capability of MRI to measure oxygen saturation and placental exchange, enabling examination of placental function in complicated pregnancies.
Short abstract
What is already known about this topic?
Placental function is responsible for significant morbidity and mortality in fetal growth restriction and in monochorionic twin pregnancies complicated by selective growth restriction and transfusion conditions.
Our ability to diagnose placental dysfunction in utero is currently limited, with implications for clinical decision making.
MRI is capable of imaging the whole human placenta at any gestational age and has been shown to demonstrate differences between normally functioning placentas and those with growth restriction.
What does this study add?
This review summarises up‐to‐date research on placental function that has been carried out using different MRI modalities.
We discuss how combinations of these techniques have much to contribute to fetal conditions amenable for therapy such as singletons at high risk for FGR through early recognition, appropriate management, and monitoring response to treatment and monochorionic twin pregnancies for planning surgery and counselling for selective growth restriction and transfusion conditions.
What is already known about this topic?
Placental function is responsible for significant morbidity and mortality in fetal growth restriction and in monochorionic twin pregnancies complicated by selective growth restriction and transfusion conditions.
Our ability to diagnose placental dysfunction in utero is currently limited, with implications for clinical decision making.
MRI is capable of imaging the whole human placenta at any gestational age and has been shown to demonstrate differences between normally functioning placentas and those with growth restriction.
What does this study add?
This review summarises up‐to‐date research on placental function that has been carried out using different MRI modalities.
We discuss how combinations of these techniques have much to contribute to fetal conditions amenable for therapy such as singletons at high risk for FGR through early recognition, appropriate management, and monitoring response to treatment and monochorionic twin pregnancies for planning surgery and counselling for selective growth restriction and transfusion conditions.
1. INTRODUCTION
Magnetic resonance imaging (MRI) of the placenta has been part of clinical practice for many years but is most commonly performed to aid in the diagnosis and management of abnormally adherent placentation. However, there is a growing field investigating imaging of the placenta for other applications (Figure 1). This is down to the technique's ability not only to image structure but also to provide quantitative measures that relate to the tissue properties and function. Several techniques are sensitive to the vascular structure and to properties such as oxygenation and blood flow and thus reveal functional information. Combinations of these techniques have much to contribute to fetal conditions amenable for therapy such as singletons at high risk for fetal growth restriction (FGR) through early recognition, appropriate management, and monitoring response to treatment and monochorionic twin pregnancies for planning surgery and counselling for selective growth restriction and transfusion conditions.
Figure 1.
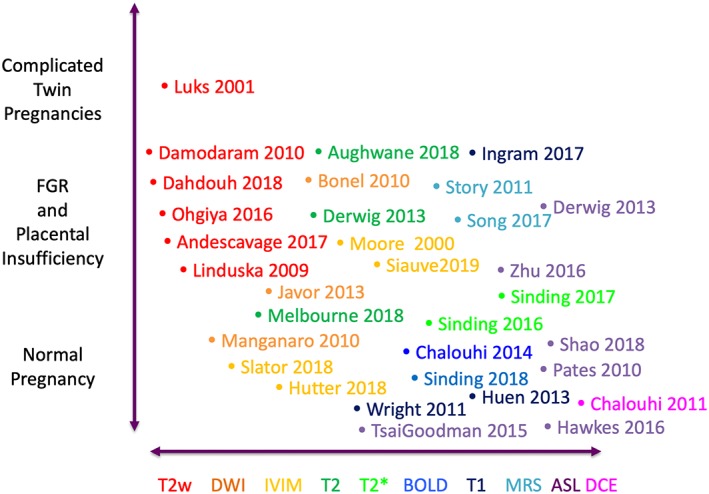
Use of MRI in human placental conditions other than accreta, papers discussed in this review. Abbreviations in text [Colour figure can be viewed at http://wileyonlinelibrary.com]
1.1. Fetal growth restriction
Placental insufficiency leads to FGR, where a fetus fails to reach their genetic growth potential. Poor fetal nutrition and hypoxia result, with increased risk of cognitive impairment, in cerebral palsy and in lifelong metabolic consequences1. The condition is associated with up to two‐thirds of stillbirths in the United Kingdom.2, 3, 4 FGR can be challenging to diagnose as placental function cannot currently be directly measured. Surrogate markers, such as abnormal fetal growth trajectory or abnormal blood flow to the placenta,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 are used with varying success. At present, there is no treatment for FGR, or the associated condition pre‐eclampsia; however, trials are exploring several new therapeutic avenues, including sildenafil,9 esomeprazole,10 metformin,11 pravastatin,12 and vascular endothelial growth factor maternal gene therapy.13, 14 Developing new techniques to assess placental function and response to management is therefore essential.15, 16, 17, 18
FGR is typically divided into early and late‐onset, most frequently defined as diagnosis before or after 32 weeks of gestation.5, 19, 20 These have relatively different clinical phenotypes, with early‐onset FGR being relatively less common, but with a high incidence of placental pathology, and late‐onset being more common, but with a variety of aetiologies. Clinical challenges in these groups also differ. In early‐onset FGR, the difficulty is in balancing in utero mortality and morbidity against the associated complications of iatrogenic preterm birth,21, 22, 23 whereas in late‐onset FGR, the primary issue is detection and delineation from normal small fetuses. Chronic hypoxia is a critical feature of FGR.18, 24 It is possible that measurement of fetal or placental oxygen saturation or oxygen exchange may be useful in differentiating the normal small fetus from one with early or late‐onset FGR and might predict outcome.
Placental insufficiency is generally considered to be as a consequence of inadequate spiral artery remodeling from insufficient trophoblast invasion in early pregancy.25 The most common abnormal histological finding is patchy placental infarcts.19 Lesions relating to hypoxia and therefore suggestive of reduced maternal perfusion are seen more commonly than in normally grown pregnancies. These include syncytiotrophoblast knots, excess cytotrophoblast cells, thickened basement membranes, villous fibrosis, and hypovascular terminal villi, with reduced villous volume, reduced intervillous space, and non‐specific inflammatory lesions.26 Understanding this pathophysiology is key to timely diagnosis and management of FGR. Imaging the placenta is therefore important to our understanding and ability to manage FGR.15, 16, 17, 18
2. COMPLICATED MONOCHORIONIC TWIN PLACENTAS
2.1. Twin‐to‐twin transfusion syndrome
In monochorionic twin pregnancies, the two fetuses are intrinsically linked through connections between their circulatory system within the placenta.27, 28, 29, 30 Twin‐to‐twin transfusion syndrome (TTTS) is caused by haemodynamic unbalance through these vascular connections,31 resulting in one hypovolaemic and one hypervolaemic fetus. If managed conservatively, the overall survival rate for TTTS is around 30%.32 Laser surgery to coagulate the anastomosing vessels along the placental equator has been shown to be the most effective management option for severe TTTS.33 Increasing information on the location of the vascular equator and the flow mismatch between twins may help clinicians in managing these pregnancies and in planning intervention.
There are limited studies of the villous structure and microcirculation, so placental vascular function is poorly understood. Histological studies have found no difference in histomorphometric variables between shared and nonshared lobules of uncomplicated monochorionic pregnancies.34, 35 In TTTS however, the donor has reduced average terminal villous diameter, smaller capillaries, reduced vascularization, and larger feto‐maternal diffusion distance, compared with the recipient twin,34, 35 likely due to the haemodynamic imbalance between the twins.
2.2. Selective FGR
Selective FGR (sFGR) is usually regarded as the combination of one twin less than 10th centile for estimated fetal weight (EFW) and a growth discordance between monochorionic twins of greater than 20% to 25% and occurs in 7% to 11% of monochorionic pregnancies.36, 37, 38 It is an important cause of morbidity and mortality.39, 40 Selective growth restriction provides unique challenges to the obstetrician. Premature delivery comes at the cost of prematurity for the normally grown twin. In some cases, selective reduction of the growth restricted twin is offered in order to optimise the chances for the normally grown fetus. Laser surgery to divide the placentas can also be used, to give both fetuses a chance, whilst protecting the normally grown fetus from harm should the smaller twin die. There is limited information for the clinician on which management option is likely to be the most beneficial for any given situation.
Fetuses with the greater share of the placenta have faster growth velocity than fetuses with the smaller share, unless an arterio‐venous anastomosis is present with net transfusion towards the fetus with the smaller territory which will equalize growth velocities.29 Additionally, the presence of an arterio‐arterial anastomosis has been linked to unequal growth in twins with unequal placental share, and absence of an arterio‐arterial anastomosis breaks the association41 although this is thought to have a protective association for TTTS. Conversely, an increased proportion of arterio‐venous anastomoses, although rare, is linked with twin anemia polycythemia sequence (TAPS).42 Thus, studies suggest a combination of the volume of placental tissue available to each fetus, and the degree and balance of transfusion between them, is responsible for the development of selected growth restriction.43
3. MAGNETIC RESONANCE IMAGING
3.1. Structural Imaging of placenta size and shape
The placenta can easily be visualized on MRI, with a clear boundary against the amniotic fluid, and a less clear placental‐uterine boundary (Figure 2). The entire placenta can be imaged at any gestational age, measuring the anatomical size, shape, and vascular properties across the whole organ. MRI is safe in pregnancy.44 T2 weighted structural imaging shows a homogenous structure with relatively high T2 signal intensity, giving it a light grey appearance. The T2 value falls in placental insufficiency, giving the placenta a darker appearance, with more heterogeneity, possibly due to areas of infarction and fibrosis.45 The placenta is smaller in FGR compared with normally grown controls and has a thickened, globular appearance.46 In twin pregnancies, the two cord locations can be seen, and the larger chorionic vessels identified, allowing identification of the vascular equator. Superresolution reconstruction techniques can be used to combine data from 2D stacks acquired in multiple planes into a single 3D volume.47 This technique has been applied widely to the fetal brain, and extensions of this technique, although made substantially more complicated by non‐rigid motion, are being used for other abdominal organs.48 For placenta size, shape, and thickness estimation, these techniques are likely to represent the best way to acquire data.46, 49, 50, 51, 52, 53 3D reconstruction of structural MRI data has already been shown to have potential in surgical planning for laser division in TTTS,54, 55 and as imaging and reconstruction techniques improve is likely to play an increasingly important role.
Figure 2.
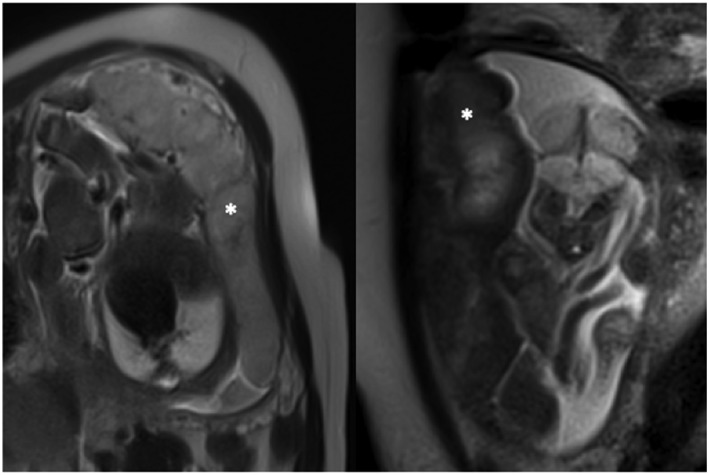
MRI of placenta from a normally grown (left) and FGR (right) fetus. The placenta are marked with white stars. Note the difference in appearance in T2 weighted imaging, with the normal placenta appearing lighter in colour and more homogeneous
3.2. Diffusion weighted imaging
Diffusion weighted imaging (DWI) is widespread in all areas of medical MRI. The sensitisation of the MRI signal to water movement means that the local tissue structure can be measured by changing the parameters of the diffusion pulses. An apparent diffusion coefficient (ADC) value is calculated for each voxel within an image, and this is displayed as a parametric ADC map (Figure 3A). Voxels with higher ADC values represent a greater degree of water diffusion such as within fluid, whereas voxels with low ADC values represent restricted and hindered diffusion, such as within cellular tissue. The ADC therefore depends on the tissue being imaged, and if pathology is present, and thus, the accuracy and the precision of this value depend on the experimental parameters used.56
Figure 3.
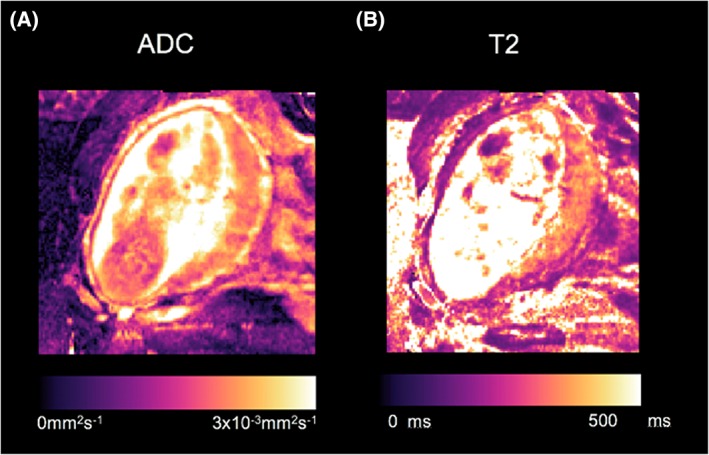
Example of placental single‐compartment ADC and T2 maps generated by linear least‐squares fitting [Colour figure can be viewed at http://wileyonlinelibrary.com]
Several studies have looked at DWI of the growth‐restricted placenta,57, 58 with placental ADC values being found to be significantly lower in the placentas of FGR pregnancies compared with normal controls and in sFGR.59 This suggests the micro‐architectural disturbance in FGR placentas is measurable with MRI.
When DWI is performed in well perfused vascular tissues, the measured signal attenuation at low diffusion sensitisation is due to not only free water diffusion in tissue but also from microcirculation within the capillary network.60, 61 Intra‐voxel incoherent motion (IVIM)62 is the traditional variant of DWI applied to perfused organs. It can be used in the assessment of capillary flow without the need for injecting contrast agents.63 As movement of blood within capillaries has no specific orientation and is dependent on the vascular architecture and velocity of the blood it is termed pseudodiffusion. The IVIM model has two compartments, relating to the solid tissue diffusivity and the tissue perfusion, or pseudodiffusivity. The proportion of each signal is given by the perfusion fraction. Naturally, the product of this perfusion fraction with the pseudodiffusivity is a correlate of blood flow. Although the model fitting is prone to noise, several authors have attempted to make fitting more robust.56, 64, 65
Surgically reduced uterine blood flow in animal models can be observed with IVIM imaging,66 and in humans, the perfusion fraction has been repeatedly shown to be reduced in placental insufficiency compared with normal placentas.67, 68, 69, 70 Caution however should be applied when interpreting quantitative results from single‐contrast MRI which can be confounded by choice of other imaging parameters if not held‐constant; in both the liver and the placenta, quantification of the vascular density is affected by the choice of other image acquisition parameters.71, 72 Specifically, it has been found that the estimated perfusion fraction in IVIM is dependent on the chosen echo time.71 This problem may be overcome using joint models, fitting DWI alongside T2, or T2* relaxation measurements.69, 73
Diffusion measurements of this type can be enhanced by including directional sensitisation,65, 74 and this has been used frequently in other organs to reveal the organisation of the tissue structure, especially the brain.75 In the placenta, the directional sensitivity might reveal information about the structure of the villous tree and how this changes in pathology such as FGR where insufficient spiral artery remodelling is thought to lead to mechanical damage and immaturity in the fetal villous tree which may reduce the measured diffusion of water. In the human haemomonochorial placenta, the technique may be limited by in vivo motion and pulsatility in contrast to the complicated structural exchange interfaces seen in other mammals. The technique is also, in principle, sensitive to water perfusion. There is now some evidence of directionality in flow in the placenta, particularly near to the chorionic plate,65 and this is likely to be associated with net differences in flow properties between chorionic arteries and veins.
Although, to date, most research has been performed investigating singleton growth restriction, in the future, perfusion imaging may be useful to quantify placental perfusion mismatch between twins and the functional volume of placental tissue. This may guide the best location for laser coagulation, ensuring each twin has sufficient functioning tissue to survive, or demonstrate that this is not possible, making selective reduction the safest management option.
3.3. Relaxometry
Relaxometry is the measurement of the signal decay rate in MR by both longitudinal (T1) and transverse (T2/T2*) decay. These contrasts can be explored independently by careful choice of pulse sequence. Theoretically, if not practically, these times correspond to independent physical properties of the tissue.
T2 relaxometry is the quantitative measure of hydrogen proton relaxation following excitation with a radio frequency pulse. The rate of relaxation is different for each tissue; tissue has a short T2 relaxation time, whilst blood has a much longer T2 relaxation time76, 77 (Figure 3B). Tissues with greater all over surface area, whether in the form of cellular membranes or intracellular or extracellular fibrillary macromolecules, tend to have shorter T2 values. In the placenta, T2 relaxation time decreases with increasing gestation,78 possibly because of the proportional increase in villous tissue compared with intervillous space, and increasing fibrin volume density.79 T2 relaxation times are significantly reduced in placentas from pregnancies complicated by FGR compared with those with appropriate growth, possibly due to increases in fibrosis, necrosis, and infarcts within the placental parenchyma80, 81, 82, 83 and reduced fetal oxygen saturation.24, 77
T2 values are dominated by the level of oxygen saturation77, 84, 85; higher oxygen saturation values result in higher T2 values. MRI may provide a useful indirect measurement for feto‐placental oxygenation since oxygen saturation is difficult to measure directly and invasive methods carry a risk of miscarriage. MRI relaxometry provides a non‐invasive way to measure feto‐placental oxygen levels, which has been partially validated in sheep.86, 87 Oxygen saturation in the feto‐placental system is typically quite low when compared with healthy adult measures of oxygen saturation and is found to be significantly lower in growth restricted fetuses.24, 88
Blood oxygenation level dependent (BOLD) MRI is a T2*‐weighted sequence that is able to detect changes in the proportion of deoxyhaemoglobin and hence reflects tissue oxygen saturation. This technique has found much use for mapping brain function where spatial patterns are used to understand functional networks89, 90 but is increasingly finding other applications outside of the brain for its ability, in combination with other flow measurements, to measure oxygen extraction and thus efficiency.91 However, the interpretation of the placental BOLD signal is complex, with signal changes dependent on other factors including blood flow, blood volume fraction, and haematocrit.81, 92, 93
BOLD and T2* measurements are often conflated in the literature. The T2* value cannot be directly related to tissue oxygenation as tissue morphology also affects the T2* value, with a reduction in T2* of the placenta with increasing gestation94 (Figure 4). This gestational relationship may be related to the histological maturation of the placenta and the decrease in placental oxygenation as pregnancy advances.95 During a maternal oxygen‐challenge (hyperoxia), the difference in the absolute T2* value (ΔT2*) signals the change in placental oxygenation independent of baseline conditions, thus demonstrating tissue oxygen saturation. Changes in BOLD signal with controlled hyperoxia and in FGR have been demonstrated in the placenta and other fetal organs.96, 97 However, a difference in ΔT2* has not been demonstrated in cases of placental dysfunction related to FGR to date despite conflicting animal data.81, 98, 99, 100
Figure 4.
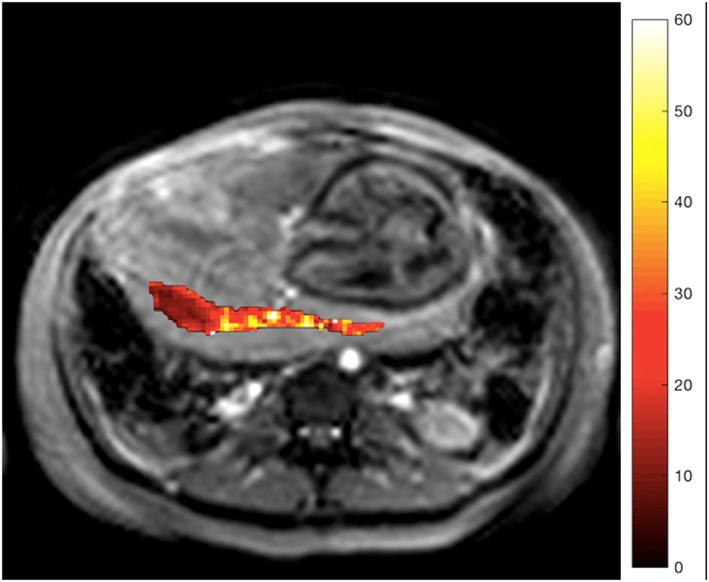
T2 weighted structural image of axial slice through maternal abdomen, demonstrating uterine cavity, fetus, and placenta. Superimposed R2* map of the placental ROI (s−1) [Colour figure can be viewed at http://wileyonlinelibrary.com]
In T1‐weighted oxygen‐enhanced (OE) MRI,98, 101, 102 the signal change related to the maternal oxygen‐challenge reflects changes in tissue pO2, due to the paramagnetic properties of dissolved oxygen. Compared with BOLD, the absolute signal change seen in OE MRI declines with gestational age and is significantly lower in pregnancies with FGR.98, 101 This is thought to support the theory of a relative placental hypoxia in FGR related to placental dysfunction, as more of the dissolved oxygen becomes bound to deoxyhaemoglobin, and hence, less becomes dissolved within the tissue.
The potential to estimate fetal oxygen saturations non‐invasively has obvious potential in the management of singleton and twin growth restriction. It could inform on response to treatment, and also on timing of delivery and might relate to placental function, allowing assessment of each lobule of the placenta. The dependence of T2 on haematocrit may also be useful in assessment of TTTS, and if TAPS is suspected.
3.4. Multicompartment multicontrast models
Conventional T1, T2, and T2* relaxometry are limited having no physiological correlate outside of MRI and an often unknown or intractable dependence on physiological properties of interest such as blood flow, saturation, haematocrit, or cellular composition. Pure tissue regions such as fluid can sometimes be used to infer properties directly,103 but these are more often the exception rather than the rule. Most regions of tissue within an imaging voxel will be mixed, particularly in the heterogenous placenta where fetal blood, maternal blood, and tissue are present within any given voxel. Using joint acquisition protocols, it can be possible to separate the signal contributions from different tissue types.71, 72, 73 This approach does allow physiological properties of the tissue to be inferred, providing the window for potential histological, complementary, or invasive validation methods.
Multicompartment multicontrast models of the type used in DWI can also be generated. The first multicompartment placental specific model is DECIDE,72 which separates the different T2 values of fetal and maternal blood from the background tissue compartment (Figure 5). Doing this results in a mechanism, under certain assumptions, to measure the fetal blood oxygen saturation. This model can also be applied to combined DWI and T2* data. Multicontrast models of this type represent a paradigm shift in the use of MRI for FGR, giving a non‐invasive measurement of placental function.72, 73 Models such as these carry their own assumptions about the physics and physiology of the signal generation process and so researchers should be aware of the limitations of each model for specific pathologies. In general, they carry the same goal of scanner‐independence as for single‐compartment models of T2 or diffusivity, in principle allowing the combination and comparison of data between sites and populations but additionally allowing further validation work because of their physical motivation.
Figure 5.
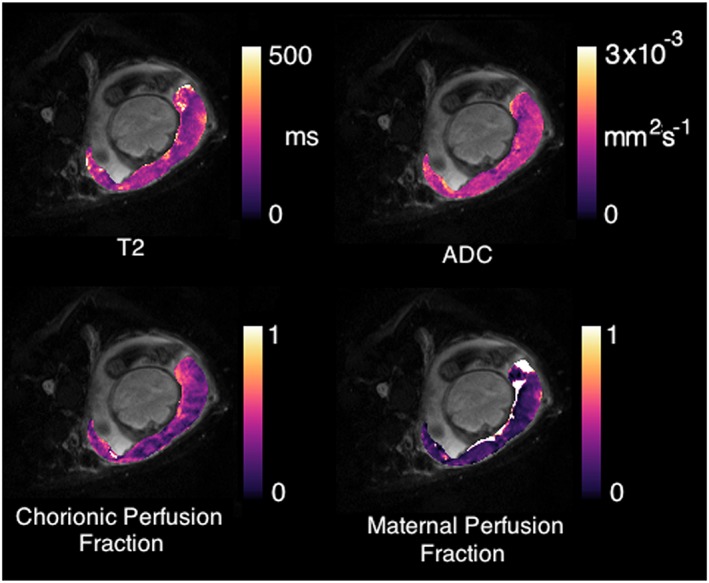
Physiological model‐fitting of the placenta.72 Parametric maps can be produced corresponding to fetal and maternal perfusion fractions (bottom row) simultaneously to conventional ADC and T2 maps (top row) [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.5. MRI flow and metabolic measurement
A key area of MR research is the measurement of the vascular properties of a tissue. The gold‐standard technique for this uses an injected para‐magnetic contrast agent that makes it unsuitable for fetal and maternal clinical MRI except in the most extreme circumstances.104, 105 Dynamic contrast enhanced (DCE) MRI80, 87, 106 does have the capability to reveal the pharmaocokinetics of the placenta including the input of blood to the uterus and placenta and the exchange of contrast agent into the trophoblast and across to the fetus (Figure 6). Common models describe the delivery of contrast to the maternal side of the placenta and the transfer of contrast agent into the fetal blood pool, thus having the potential to improve our understanding of how these processes are affected in different pathologies.105, 107, 108 However, the decision to use contrast to image complex pregnancies is challenging.
Figure 6.
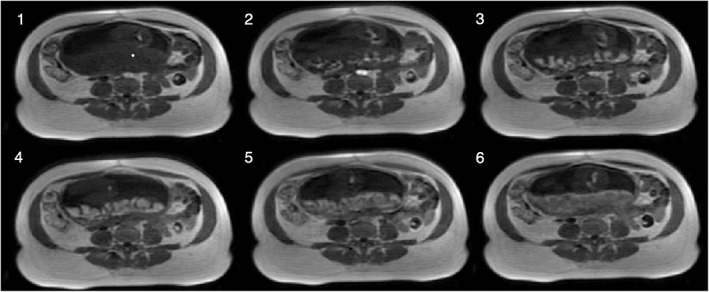
Dynamic enhancement of the placenta with DCE‐MRI. Baseline image (1), arrival and wash‐in (2‐4), wash‐out (5‐6)
Flow can be measured with phase contrast MRI, an imaging technique that encodes the blood flow velocity in large arteries, typically of several millimetres in diameter, directly into the MR imaging data. In combination with knowledge of the vessel area, this gives a quantitative estimate of blood flow.109, 110 Due to the readily available use of Doppler ultrasound, there is little work in this area.111, 112
Arterial spin labeling is a further imaging technique that magnetically labels blood water to visualise larger arterial vessels and blood perfusion.67, 113, 114 Arterial blood water is magnetically labelled just below the region of interest using a radiofrequency inversion pulse. This magnetised tracer flows into the slice of interest, reducing the total tissue magnetisation, and consequently reducing the MR signal and image intensity. The difference between a labelled and unlabelled control image provides a measure of perfusion.115 ASL is exquisitely sensitive to motion and can be relatively time consuming to acquire due to the low average signal. However, its key strength is the ability to acquire multiple different labels with differing postlabel delays or different velocity encodings, thus revealing much about the dynamic perfusion of the placenta. A comparison of IVIM and ASL to assess placental perfusion in the second trimester in normal and FGR pregnancies showed a significant reduction in basal plate ASL signal between normally grown and FGR pregnancies. Basal plate, central placental, and whole placental IVIM vascular density was also different between normally grown and FGR pregnancies.67 As with IVIM, this technique could be useful in monitoring response to treatment in FGR placentas and also perfusion differences in twin pregnancies. The benefit of this technique is that it is a more direct measurement of perfusion; however, it is challenging to apply in practice.
Placenta metabolites can be measured in principle using MRI via proton magnetic resonance spectroscopy which has been investigated in the placenta. However, high acquisition failure rates and difficulty in interpreting the signal mean this is a relatively immature technique within the placenta.116, 117
Lastly, although to the best of our knowledge, it has not yet been tested in humans, hyperpolarised MRI represents a unique way to assess the placental barrier and its metabolic behaviour and permeability.118 The use of different hyperpolarized metabolites could reveal a range of information on different pathways and pathology far beyond that obtained from pharmacokinetic studies of Gadolinium chelates or other heavy contrast molecules.
4. CONCLUSION
The ability of MRI to detect changes in placentas of severely growth restricted fetuses with abnormal Doppler's is well established.70, 78, 80, 94 However, the ability of MRI to measure placental function more broadly has yet to be fully realized or investigated (Table 1). With further development, MRI is likely to increase our understanding of abnormal placental function, improve diagnostic accuracy, and help guide intervention and monitor response. The advances currently being made in the examination of placentas from pregnancies affected by growth restriction will find application in wider conditions such as complicated twin pregnancies, invasive placentation, chorioangioma, caesarean scar pregnancies, and the function of other fetal organs.
Table 1.
Future applications of MRI in placental conditions amenable to therapy
| Technique | MRI Signal Sensitivity | Future Applications |
|---|---|---|
| T2 weighted | Structural features, fluid boundaries, volumetrics | Placental share in complicated twins, cord insertions, chorionic vessel mapping, computer assisted surgical planning |
| DWI | Diffusivity, microarchitecture, fluid not specific to oxygenation/flow. | Microvascular structural differences in FGR/PET/sFGR |
| IVIM | Diffusivity, microvasculature, fluid, perfusion. Chorionic flow. Non‐specific to oxygenation | Functional share in complicated twins. Flow changes in FGR. Post‐intervention redistribution + outcome prediction. |
| T2weighted | Sensitive to oxygenation, tissue compartments | Changes in fetal oxygenation functional redundancy and capacity |
| T2* | Sensitive to oxygenation, tissue compartments | Changes in fetal oxygenation, functional redundancy and capacity |
| BOLD | Sensitive to functional change in oxygenation | Changes in function, and tissue redundancy and capacity over time |
| T1 | Sensitive to oxygenation | Maternal blood flow changes in FGR. Redistribution postlaser TTTS |
| MRS and metabolic | Transfer rates, tissue maturation | Therapeutic changes in transfer and exchange |
| ASL | Sensitive to flow and perfusion | Maternal blood flow changes in FGR. Redistribution postlaser TTTS |
| DCE | Sensitive to flow and transfer rate | Changes in maternal flow and transfer kinetics. |
One of the limitations to the practical use of placental MRI is the relative rarity of some of the conditions being investigated. This can make it difficult to establish studies with sufficient numbers to fully investigate new imaging techniques and hence make recommendations about clinical practice. Enhanced coordination of studies between centres and the sharing of clinical and technical expertise alongside imaging data are essential when investigating these conditions119 and will help to establish the most useful imaging technologies for each pathology. This will speed up the pace of future feto‐placental research for conditions that for the ubiquity of pregnancy remain quite rare but have lifelong impact.
The future of placental MRI is exciting; the use of multiple contrasts and new models to boost the capability of MRI to measure oxygen saturation72 and placental exchange105, 118 will enhance the understanding of placental function in complicated pregnancies.
FUNDING SOURCES
This research was supported by the Wellcome Trust (210182/Z/18/Z and Wellcome Trust/EPSRC NS/A000027/1). The funders had no direction in the study design, data collection, data analysis, manuscript preparation, or publication decision.
CONFLICT OF INTEREST
We have no conflicts of interest to report.
Aughwane R, Ingram E, Johnstone ED, Salomon LJ, David AL, Melbourne A. Placental MRI and its application to fetal intervention. Prenatal Diagnosis. 2020; 40:38–48. 10.1002/pd.5526
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Crispi F, Miranda J, Gratacós E. Long‐term cardiovascular consequences of fetal growth restriction: biology, clinical implications, and opportunities for prevention of adult disease. Am J Obstet Gynecol. 2018;218(2):S869‐S879. 10.1016/j.ajog.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 2. Akolekar R, Bower S, Flack N, Bilardo CM, Nicolaides KH. Prediction of miscarriage and stillbirth at 11‐13 weeks and the contribution of chorionic villus sampling. Prenat Diagn. 2011;31(1):38‐45. 10.1002/pd.2644 [DOI] [PubMed] [Google Scholar]
- 3. Akolekar R, Tokunaka M, Ortega N, Syngelaki A, Nicolaides KH. Prediction of stillbirth from maternal factors, fetal biometry and uterine artery Doppler at 19–24 weeks. Ultrasound Obstet Gynecol. 2016;48(5):624‐630. 10.1002/uog.17295 [DOI] [PubMed] [Google Scholar]
- 4. Lawn JE, Blencowe H, Pattinson R, et al. Stillbirths: Where? When? Why? How to make the data count? Lancet. 2011;377(9775):1448‐1463. 10.1016/S0140-6736(10)62187-3 [DOI] [PubMed] [Google Scholar]
- 5. Figueras F, Caradeux J, Crispi F, Eixarch E, Peguero A, Gratacos E. Diagnosis and surveillance of late‐onset fetal growth restriction. Am J Obstet Gynecol. 2018;218(2):S790‐S802.e1. 10.1016/j.ajog.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 6. DeVore GR. The importance of the cerebroplacental ratio in the evaluation of fetal well‐being in SGA and AGA fetuses. Am J Obstet Gynecol. 2015;213(1):5‐15. 10.1016/j.ajog.2015.05.024 [DOI] [PubMed] [Google Scholar]
- 7. McCowan LM, Figueras F, Anderson NH. Evidence‐based national guidelines for the management of suspected fetal growth restriction: comparison, consensus, and controversy. Am J Obstet Gynecol. 2018;218(2):S855‐S868. [DOI] [PubMed] [Google Scholar]
- 8. Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 2017;14(1):e1002220 10.1371/journal.pmed.1002220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pels A, Kenny LC, Alfirevic Z, et al. STRIDER (Sildenafil TheRapy in dismal prognosis early onset fetal growth restriction): an international consortium of randomised placebo‐controlled trials. BMC Pregnancy Childbirth. 2017;17(1):440 10.1186/s12884-017-1594-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cluver CA, Walker SP, Mol BW, et al. Double blind, randomised, placebo‐controlled trial to evaluate the efficacy of esomeprazole to treat early onset pre‐eclampsia (PIE Trial): a study protocol. BMJ Open. 2015;5(10):e008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brownfoot FC, Hastie R, Hannan NJ, et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms‐like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am J Obstet Gynecol. 2016;214(3):356.e1‐356.e15. [DOI] [PubMed] [Google Scholar]
- 12. Girardi G. Pravastatin to treat and prevent preeclampsia. Preclinical and clinical studies. J Reprod Immunol. 2017;124:15‐20. [DOI] [PubMed] [Google Scholar]
- 13. Spencer R, Ambler G, Brodszki J, et al. EVERREST prospective study: a 6‐year prospective study to define the clinical and biological characteristics of pregnancies affected by severe early onset fetal growth restriction. BMC Pregnancy Childbirth. 2017;17(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carr DJ, Wallace JM, Aitken RP, et al. Uteroplacental adenovirus vascular endothelial growth factor gene therapy increases fetal growth velocity in growth‐restricted sheep pregnancies. Hum Gene Ther. 2014;25(4):375‐384. 10.1089/hum.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol. 2009;587(14):3453‐3458. 10.1113/jphysiol.2009.173252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox H. Pathology of the placenta. Clin Obstet Gynaecol. 1986;17(1):93 10.1097/00004347-199801000-00019 [DOI] [PubMed] [Google Scholar]
- 17. Sebire NJ, Talbert D. The role of intraplacental vascular smooth muscle in the dynamic placenta: a conceptual framework for understanding uteroplacental disease. Med Hypotheses. 2002;58(4):347‐351. 10.1054/mehy.2001.1538 [DOI] [PubMed] [Google Scholar]
- 18. Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92(1):35‐43. 10.1016/S0301-2115(00)00423-1 [DOI] [PubMed] [Google Scholar]
- 19. Mifsud W, Sebire NJ. Placental pathology in early‐onset and late‐onset fetal growth restriction. Fetal Diagn Ther. 2014;36(2):117‐128. [DOI] [PubMed] [Google Scholar]
- 20. Gordijn SJ, Beune IM, Thilaganathan B, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol. 2016;48(3):333‐339. 10.1002/uog.15884 [DOI] [PubMed] [Google Scholar]
- 21. Linsell L, Johnson S, Wolke D, et al. Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: a prospective, population‐based cohort study. Arch Dis Child. 2018;103(4):363‐370. 10.1136/archdischild-2017-313414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ancel PY, Goffinet F, EPIPAGE‐2 Writing Group , et al. Survival and morbidity of preterm children born at 22 through 34 weeks' gestation in France in 2011 results of the EPIPAGE‐2 cohort study. JAMA Pediatr. 2015;169(3):230‐238. 10.1001/jamapediatrics.2014.3351 [DOI] [PubMed] [Google Scholar]
- 23. Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. 2004;191(2):481‐487. [DOI] [PubMed] [Google Scholar]
- 24. Soothill PW, Nicolaides KH, Campbell S. Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J (Clin Res Ed). 1987;294(6579):1051‐1053. 10.1136/bmj.294.6579.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lyall F, Robson SC, Bulmer JN. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction relationship to clinical outcome. Hypertension. 2013;62(6):1046‐1054. 10.1161/HYPERTENSIONAHA.113.01892 [DOI] [PubMed] [Google Scholar]
- 26. Ferrazzi E, Bulfamante G, Mezzopane R, Barbera A, Ghidini A, Pardi G. Uterine Doppler velocimetry and placental hypoxic‐ischemic lesion in pregnancies with fetal intrauterine growth restriction. Placenta. 1999;20(5‐6):389‐394. [DOI] [PubMed] [Google Scholar]
- 27. Bajoria R, Wigglesworth J, Fisk NM. Angioarchitecture of monochorionic placentas in relation to the twin‐twin transfusion syndrome. Am J Obstet Gynecol. 1995;172(3):856‐863. [DOI] [PubMed] [Google Scholar]
- 28. Machin G, Still K, Lalani T. Correlations of placental vascular anatomy and clinical outcomes in 69 monochorionic twin pregnancies. Am J Med Genet. 1996;61(3):229‐236. [DOI] [PubMed] [Google Scholar]
- 29. Denbow ML, Cox P, Taylor M, Hammal DM, Fisk NM. Placental angioarchitecture in monochorionic twin pregnancies: relationship to fetal growth, fetofetal transfusion syndrome, and pregnancy outcome. Am J Obstet Gynecol. 2000;182(2):417‐426. [DOI] [PubMed] [Google Scholar]
- 30. Zhao D, Lipa M, Wielgos M, et al. Comparison between monochorionic and dichorionic placentas with special attention to vascular anastomoses and placental share. Twin Res Hum Genet. 2016;19(3):1‐6. 10.1017/thg.2016.19 [DOI] [PubMed] [Google Scholar]
- 31. Fisk NM, Duncombe GJ, Sullivan MHF. The basic and clinical science of twin‐twin transfusion syndrome. Placenta. 2009;30(5):379‐390. [DOI] [PubMed] [Google Scholar]
- 32. Berghella V, Kaufmann M. Natural history of twin‐twin transfusion syndrome. J Reprod Med. 2001;46(5):480‐484. [PubMed] [Google Scholar]
- 33. Senat M‐V, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin‐to‐twin transfusion syndrome. N Engl J Med. 2004;351(2):136‐144. [DOI] [PubMed] [Google Scholar]
- 34. Fox H, Sebire NJ. Pathology of the Placenta. Saunders Elsevier; 2007. https://www.sciencedirect.com/science/article/pii/S0143400405001396 [Google Scholar]
- 35. Wee LY, Sebire NJ, Bhundia J, Sullivan M, Fisk NM. Histomorphometric characterisation of shared and non‐shared cotyledonary villus territories of monochorionic placentae in relation to pregnancy complications. Placenta. 2006;27(4‐5):475‐482. [DOI] [PubMed] [Google Scholar]
- 36. Sebire NJ, Snijders RJM, Hughes K, Sepulveda W, Nicolaides KH. The hidden mortality of monochorionic twin pregnancies. Br J Obstet Gynaecol. 1997;104(10):1203‐1207. [DOI] [PubMed] [Google Scholar]
- 37. De Paepe ME, Shapiro S, Young L, Luks FI. Placental characteristics of selective birth weight discordance in diamniotic‐monochorionic twin gestations. Placenta. 2010;31(5):380‐386. 10.1016/j.placenta.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 38. Costa‐Castro T, Zhao DP, Lipa M, et al. Velamentous cord insertion in dichorionic and monochorionic twin pregnancies—does it make a difference? Placenta. 2016;42:87‐92. [DOI] [PubMed] [Google Scholar]
- 39. Acosta‐Rojas R, Becker J, Munoz‐Abellana B, et al. Twin chorionicity and the risk of adverse perinatal outcome. Int J Gynecol Obstet. 2007;96(2):98‐102. 10.1016/j.ijgo.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 40. Gratacós E, Carreras E, Becker J, et al. Prevalence of neurological damage in monochorionic twins with selective intrauterine growth restriction and intermittent absent or reversed end‐diastolic umbilical artery flow. Ultrasound Obstet Gynecol. 2004;24(2):159‐163. 10.1002/uog.1105 [DOI] [PubMed] [Google Scholar]
- 41. Hack KEA, Nikkels PGJ, Koopman‐Esseboom C, et al. Placental characteristics of monochorionic diamniotic twin pregnancies in relation to perinatal outcome. Placenta. 2008;29(11):976‐981. [DOI] [PubMed] [Google Scholar]
- 42. Couck I, Lewi L. The placenta in twin‐to‐twin transfusion syndrome and twin anemia polycythemia sequence. Twin Res Hum Genet. 2016;19(3):184‐190. 10.1017/thg.2016.29 [DOI] [PubMed] [Google Scholar]
- 43. Lewi L, Cannie M, Blickstein I, et al. Placental sharing, birthweight discordance, and vascular anastomoses in monochorionic diamniotic twin placentas. Am J Obstet Gynecol. 2007;197(6):587.e1‐587.e8. 10.1016/j.ajog.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 44. Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA ‐ J Am Med Assoc. 2016;316(9):952 10.1001/jama.2016.12126 [DOI] [PubMed] [Google Scholar]
- 45. Gowland P. Placental MRI. Semin Fetal Neonatal Med. 2005;10(5):485‐490. [DOI] [PubMed] [Google Scholar]
- 46. Damodaram M, Story L, Eixarch E, et al. Placental MRI in intrauterine fetal growth restriction. Placenta. 2010;31(6):491‐498. [DOI] [PubMed] [Google Scholar]
- 47. Ebner, M. , Wang G, Li W, Aertsen M, Patel PA, Aughwane R, Melbourne A, Doel T, David AL, Deprest J, Ourselin S. An automated localization, segmentation and reconstruction framework for fetal brain MRI in Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics), 313–320 (2018). Springer: Cham: 10.1007/978-3-030-00928-1_36 [DOI] [Google Scholar]
- 48. Wang G, Zuluaga MA, Pratt R, et al. Slic‐Seg: A minimally interactive segmentation of the placenta from sparse and motion‐corrupted fetal MRI in multiple views. Med Image Anal. 2016;34:137‐147. 10.1016/j.media.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kingdom JC, Audette MC, Hobson SR, Windrim RC, Morgen E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am J Obstet Gynecol. 2018;218(2):S803‐S817. 10.1016/j.ajog.2017.11.575 [DOI] [PubMed] [Google Scholar]
- 50. Dahdouh S, Andescavage N, Yewale S, et al. In vivo placental MRI shape and textural features predict fetal growth restriction and postnatal outcome. J Magn Reson Imaging. 2018;47(2):449‐458. 10.1002/jmri.25806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ohgiya Y, Nobusawa H, Seino N, et al. MR imaging of fetuses to evaluate placental insufficiency. Magn Reson Med Sci. 2016;15(2):212‐219. 10.2463/mrms.mp.2015-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andescavage N, duPlessis A, Metzler M, et al. In vivo assessment of placental and brain volumes in growth‐restricted fetuses with and without fetal Doppler changes using quantitative 3D MRI. J Perinatol. 2017;37(12):1278‐1284. 10.1038/jp.2017.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Linduska N, Dekan S, Messerschmidt A, et al. Placental pathologies in fetal MRI with pathohistological correlation. Placenta. 2009;30(6):555‐559. 10.1016/j.placenta.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 54. Luks FI, Carr SR, Ponte B, Rogg JM, Tracy TF. Preoperative planning with magnetic resonance imaging and computerized volume rendering in twin‐to‐twin transfusion syndrome. Am J Obstet Gynecol. 2001;185(1):216‐219. [DOI] [PubMed] [Google Scholar]
- 55. Pratt R, Deprest J, Vercauteren T, Ourselin S, David AL. Computer‐assisted surgical planning and intraoperative guidance in fetal surgery: a systematic review. Prenat Diagn. 2015;35(12):1159‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Melbourne A, Toussaint N, Owen D, et al. NiftyFit: a software package for multi‐parametric model‐fitting of 4d magnetic resonance imaging data. Neuroinformatics. 2016;14(3):319‐337. 10.1007/s12021-016-9297-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bonel HM, Stolz B, Diedrichsen L, et al. Diffusion‐weighted MR imaging of the placenta in fetuses with placental insufficiency. Radiology. 2010;257(3):810‐819. 10.1148/radiol.10092283 [DOI] [PubMed] [Google Scholar]
- 58. Javor D, Nasel C, Schweim T, Dekan S, Chalubinski K, Prayer D. In vivo assessment of putative functional placental tissue volume in placental intrauterine growth restriction (IUGR) in human fetuses using diffusion tensor magnetic resonance imaging. Placenta. 2013;34(8):676‐680. 10.1016/j.placenta.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 59. Fu L, Zhang J, Xiong S, Sun M. Decreased apparent diffusion coefficient in the placentas of monochorionic twins with selective intrauterine growth restriction. Placenta. 2018;69:26‐31. 10.1016/j.placenta.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 60. Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval‐Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497‐505. 10.1017/CBO9780511659072 [DOI] [PubMed] [Google Scholar]
- 61. Iima M, Le Bihan D. Clinical intravoxel incoherent motion and diffusion MR imaging: past, present, and future. Radiology. 2016;278(1):13‐32. 10.1148/radiol.2015150244 [DOI] [PubMed] [Google Scholar]
- 62. Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval‐Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401‐407. [DOI] [PubMed] [Google Scholar]
- 63. Manganaro L, Fierro F, Tomei A, et al. MRI and DWI: feasibility of DWI and ADC maps in the evaluation of placental changes during gestation. Prenat Diagn. 2010;30(12‐13):1178‐1184. 10.1002/pd.2641 [DOI] [PubMed] [Google Scholar]
- 64. Orton MR, Collins DJ, Koh DM, Leach MO. Improved intravoxel incoherent motion analysis of diffusion weighted imaging by data driven Bayesian modeling. Magn Reson Med. 2014;71(1):411‐420. 10.1002/mrm.24649 [DOI] [PubMed] [Google Scholar]
- 65. Slator PJ, Hutter J, McCabe L, et al. Placenta microstructure and microcirculation imaging with diffusion MRI. Magn Reson Med. 2018;80(2):756‐766. 10.1002/mrm.27036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alison M, Chalouhi GE, Autret G, et al. Use of intravoxel incoherent motion MR imaging to assess placental perfusion in a murine model of placental insufficiency. Invest Radiol. 2013;48(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 67. Derwig I, Lythgoe DJ, Barker GJ, et al. Association of placental perfusion, as assessed by magnetic resonance imaging and uterine artery Doppler ultrasound, and its relationship to pregnancy outcome. Placenta. 2013;34(10):885‐891. 10.1016/j.placenta.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 68. Moore RJ, Strachan BK, Tyler DJ, et al. In utero perfusing fraction maps in normal and growth restricted pregnancy measured using IVIM echo‐planar MRI. Placenta. 2000;21(7):726‐732. [DOI] [PubMed] [Google Scholar]
- 69. Siauve N, Hayot PH, Deloison B, et al. Assessment of human placental perfusion by intravoxel incoherent motion MR imaging. J Matern Neonatal Med. 2019;32(2):293‐300. 10.1080/14767058.2017.1378334 [DOI] [PubMed] [Google Scholar]
- 70. Aughwane, R. , Sokolska M, Bainbridge A, Atkinson D, Kendall G, Deprest J, Vercauteren T, David AL, Ourselin S, Melbourne A. MRI measurement of placental perfusion and fetal blood oxygen saturation in normal pregnancy and placental insufficiency in Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 913–920 (2018). Springer, Cham: 10.1007/978-3-030-00934-2_101 [DOI] [Google Scholar]
- 71. Jerome NP, d'Arcy JA, Feiweier T, et al. Extended T2‐IVIM model for correction of TE dependence of pseudo‐diffusion volume fraction in clinical diffusion‐weighted magnetic resonance imaging. Phys Med Biol. 2016;61(24):N667‐N680. 10.1088/1361-6560/61/24/N667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Melbourne A, Aughwane R, Sokolska M, et al. Separating fetal and maternal placenta circulations using multiparametric MRI. Magn Reson Med. 2018;81(1):350‐361. 10.1002/mrm.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hutter J, Slator PJ, Jackson L, et al. Multi‐modal functional MRI to explore placental function over gestation. Magn Reson Med. 2018;81(2):1191‐1204. 10.1002/mrm.27447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534‐546. 10.1002/jmri.1076 [DOI] [PubMed] [Google Scholar]
- 75. Behrens TEJ, Johansen‐Berg H, Woolrich MW, et al. Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6(7):750‐757. 10.1038/nn1075 [DOI] [PubMed] [Google Scholar]
- 76. de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230(3):652‐659. 10.1148/radiol.2303021331 [DOI] [PubMed] [Google Scholar]
- 77. Portnoy S, Osmond M, Zhu MY, Seed M, Sled JG, Macgowan CK. Relaxation properties of human umbilical cord blood at 1.5 Tesla. Magn Reson Med. 2017;77(4):1678‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Derwig I, Barker GJ, Poon L, et al. Association of placental T2 relaxation times and uterine artery Doppler ultrasound measures of placental blood flow. Placenta. 2013;34(6):474‐479. 10.1016/j.placenta.2013.03.005 [DOI] [PubMed] [Google Scholar]
- 79. Sinding M, Peters DA, Frøkjaer JB, et al. Placental magnetic resonance imaging T2* measurements in normal pregnancies and in those complicated by fetal growth restriction. Ultrasound Obstet Gynecol. 2016;47(6):748‐754. 10.1002/uog.14917 [DOI] [PubMed] [Google Scholar]
- 80. Ingram E, Naish J, Morris D, Myers J, Johnstone ED. MRI measurements of abnormal placental oxygenation in pregnancies complicated by FGR. Am J Obstet Gynecol. 2018;218(1):S40‐S41. [Google Scholar]
- 81. Sinding M, Peters DA, Poulsen SS, et al. Placental baseline conditions modulate the hyperoxic BOLD‐MRI response. Placenta. 2018;61:17‐23. 10.1016/j.placenta.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 82. Schabel MC, Roberts VHJ, Lo JO, et al. Functional imaging of the nonhuman primate Placenta with endogenous blood oxygen level–dependent contrast. Magn Reson Med. 2016;76(5):1551‐1562. 10.1002/mrm.26052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sinding M, Peters DA, Frøkjær JB, et al. Prediction of low birth weight: comparison of placental T2* estimated by MRI and uterine artery pulsatility index. Placenta. 2017;49:48‐54. 10.1016/j.placenta.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 84. Portnoy S, Seed M, Sled JG, Macgowan CK. Non‐invasive evaluation of blood oxygen saturation and hematocrit from T1and T2 relaxation times: in‐vitro validation in fetal blood. Magn Reson Med. 2017;78(6):2352‐2359. 10.1002/mrm.26599 [DOI] [PubMed] [Google Scholar]
- 85. Portnoy S, Milligan N, Seed M, Sled JG, Macgowan CK. Human umbilical cord blood relaxation times and susceptibility at 3 T. Magn Reson Med. 2018;79(6):3194‐3206. 10.1002/mrm.26978 [DOI] [PubMed] [Google Scholar]
- 86. Zhu MY, Milligan N, Keating S, et al. The hemodynamics of late‐onset intrauterine growth restriction by MRI. Am J Obstet Gynecol. 2016;214(3):367.e1‐367.e17. 10.1016/j.ajog.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 87. Schrauben EM, Saini BS, Darby JRT, et al. Fetal hemodynamics and cardiac streaming assessed by 4D flow cardiovascular magnetic resonance in fetal sheep. J Cardiovasc Magn Reson. 2019;21(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Siggaard‐Andersen O, Huch R. The oxygen status of fetal blood. Acta Anaesthesiol Scand. 1995;39:129‐135. 10.1111/j.1399-6576.1995.tb04347.x [DOI] [PubMed] [Google Scholar]
- 89. Kim SG, Ogawa S. Biophysical and physiological origins of blood oxygenation level‐dependent fMRI signals. J Cereb Blood Flow Metab. 2012;32(7):1188‐1206. 10.1038/jcbfm.2012.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676‐682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci. 1999;96(16):9403‐9408. 10.1073/pnas.96.16.9403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ugurbil K, Adriany G, Andersen P, et al. Magnetic resonance studies of brain function and neurochemistry. Annu Rev Biomed Eng. 2000;2(1):633‐660. [DOI] [PubMed] [Google Scholar]
- 93. Chalouhi GE, Salomon LJ. BOLD‐MRI to explore the oxygenation of fetal organs and of the placenta. BJOG Int J Obstet Gynaecol. 2014;121(13):1595 10.1111/1471-0528.12805 [DOI] [PubMed] [Google Scholar]
- 94. Sinding M, Peters DA, Frøkjaer JB, et al. Placental T2* measurements in normal pregnancies and in pregnancies complicated by fetal growth restriction. Ultrasound Obstet Gynecol. 2016;47(6):748‐754. 10.1002/uog.14917 [DOI] [PubMed] [Google Scholar]
- 95. Wright C, Morris DM, Baker PN, et al. Magnetic resonance imaging relaxation time measurements of the placenta at 1.5 T. Placenta. 2011;32(12):1010‐1015. 10.1016/j.placenta.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sørensen A, Sinding M, Peters DA, et al. Placental oxygen transport estimated by the hyperoxic placental BOLD MRI response. Physiol Rep. 2015;3(10):e12582 10.14814/phy2.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sørensen A, Peters D, Fründ E, Lingman G, Christiansen O, Uldbjerg N. Changes in human placental oxygenation during maternal hyperoxia estimated by blood oxygen level‐dependent magnetic resonance imaging (BOLD MRI). Ultrasound Obstet Gynecol. 2013;42(3):310‐314. 10.1002/uog.12395 [DOI] [PubMed] [Google Scholar]
- 98. Ingram E, Morris D, Naish J, Myers J, Johnstone E. MR imaging measurements of altered placental oxygenation in pregnancies complicated by fetal growth restriction. Radiology. 2017;285(3):953‐960. 10.1148/radiol.2017162385 [DOI] [PubMed] [Google Scholar]
- 99. Aimot‐Macron S, Salomon LJ, Deloison B, et al. In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level‐dependent (BOLD) magnetic resonance imaging. Eur Radiol. 2013;23(5):1335‐1342. 10.1007/s00330-012-2712-y [DOI] [PubMed] [Google Scholar]
- 100. Chalouhi GE, Alison M, Deloison B, et al. Fetoplacental oxygenation in an intrauterine growth restriction rat model by using blood oxygen level–dependent MR imaging at 4.7 T. Radiology. 2013;269(1):122‐129. 10.1148/radiol.13121742 [DOI] [PubMed] [Google Scholar]
- 101. Huen I, Morris DM, Wright C, et al. R1 and R2* changes in the human placenta in response to maternal oxygen challenge. Magn Reson Med. 2013;70(5):1427‐1433. 10.1002/mrm.24581 [DOI] [PubMed] [Google Scholar]
- 102. Nye GA, Ingram E, Johnstone ED, et al. Human placental oxygenation in late gestation: experimental and theoretical approaches. J Physiol. 2018;596(23):5523‐5534. 10.1113/JP275633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Duan AQ, Darby JRT, Soo JY, et al. Feasibility of phase‐contrast cine magnetic resonance imaging for measuring blood flow in the sheep fetus. Am J Physiol Integr Comp Physiol. 2017. 10.1152/ajpregu.00273.2017 [DOI] [PubMed] [Google Scholar]
- 104. Chalouhi GE, Deloison B, Siauve N, et al. Dynamic contrast‐enhanced magnetic resonance imaging: definitive imaging of placental function? Semin Fetal Neonatal Med. 2011;16(1):22‐28. 10.1016/j.siny.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 105. Siauve N, Chalouhi GE, Deloison B, et al. Functional imaging of the human placenta with magnetic resonance. Am J Obstet Gynecol. 2015;213(4):S103‐S114. 10.1016/j.ajog.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 106. Frias AE, Schabel MC, Roberts VHJ, et al. Using dynamic contrast‐enhanced MRI to quantitatively characterize maternal vascular organization in the primate placenta. Magn Reson Med. 2015;73(4):1570‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brunelli R, Masselli G, Parasassi T, et al. Intervillous circulation in intra‐uterine growth restriction. Correlation to fetal well being. Placenta. 2010;31(12):1051‐1056. [DOI] [PubMed] [Google Scholar]
- 108. Millischer AE, Deloison B, Silvera S, et al. Dynamic contrast enhanced MRI of the placenta: a tool for prenatal diagnosis of placenta accreta? Placenta. 2017;53:40‐47. 10.1016/j.placenta.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 109. Tsai‐Goodman B, Zhu MY, Al‐Rujaib M, Seed M, Macgowan CK. Foetal blood flow measured using phase contrast cardiovascular magnetic resonance—Preliminary data comparing 1.5 T with 3.0 T. J Cardiovasc Magn Reson. 2015;17(1):30 10.1186/s12968-015-0132-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Jansz MS, Seed M, van Amerom JFP, et al. Metric optimized gating for fetal cardiac MRI. Magn Reson Med. 2010;64(5):1304‐1314. 10.1002/mrm.22542 [DOI] [PubMed] [Google Scholar]
- 111. Pates JA, Hatab MR, McIntire DD, Cunningham FG, Twickler DM. Determining uterine blood flow in pregnancy with magnetic resonance imaging. Magn Reson Imaging. 2010;28(4):507‐510. [DOI] [PubMed] [Google Scholar]
- 112. Hawkes RA, Patterson AJ, Priest AN, et al. Uterine artery pulsatility and resistivity indices in pregnancy: comparison of MRI and Doppler US. Placenta. 2016;43:35‐40. 10.1016/j.placenta.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 113. Shao X, Liu D, Martin T, et al. Measuring human placental blood flow with multidelay 3D GRASE pseudocontinuous arterial spin labeling at 3 T. J Magn Reson Imaging. 2018;47(6):1667‐1676. 10.1002/jmri.25893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wong EC, Cronin M, Wu WC, Inglis B, Frank LR, Liu TT. Velocity‐selective arterial spin labeling. Magn Reson Med. 2006;55(6):1334‐1341. 10.1002/mrm.20906 [DOI] [PubMed] [Google Scholar]
- 115. Alsop DC, Detre JA, Golay X, et al. Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73(1):102‐116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Story L, Damodaram MS, Allsop JM, et al. Brain metabolism in fetal intrauterine growth restriction: a proton magnetic resonance spectroscopy study. Am J Obstet Gynecol. 2011;205(5):483.e1‐483.e8. 10.1016/j.ajog.2011.06.032 [DOI] [PubMed] [Google Scholar]
- 117. Song F, Wu W, Qian Z, Zhang G, Cheng Y. Assessment of the placenta in intrauterine growth restriction by diffusion‐weighted imaging and proton magnetic resonance spectroscopy: a pilot study. Reprod Sci. 2017;24(4):575‐581. 10.1177/1933719116667219 [DOI] [PubMed] [Google Scholar]
- 118. Markovic S, Fages A, Roussel T, et al. Placental physiology monitored by hyperpolarized dynamic 13C magnetic resonance. Proc Natl Acad Sci. 2018;115(10):E2429‐E2436. 10.1073/pnas.1715175115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Slator P, Aughwane R, Cade G, et al. Placenta Imaging Workshop 2018 report: multiscale and multimodal approaches. Placenta. 2018;79:78‐82. 10.1016/j.placenta.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


