Abstract
Background
An implantable cardioverter defibrillator (ICD) is recommended for patients with symptomatic heart failure with ejection fraction ≤35% despite optimal medical therapy. More recently, the benefits of ICDs have been questioned in nonischemic cardiomyopathy (CM).
Aim
To examine the incidence of appropriate therapy, complications, mortality, and cause of death among ICD patients in an unselected validated cohort. In primary prevention, appropriate therapy in ischemic versus nonischemic CM will be evaluated.
Methods
A retrospective observational study of patients in Region Gävleborg, Sweden, who underwent ICD implantation or replacement between 2007 and 2017.
Results
In total, 438 patients (mean age at implant: 65.9 ± 11.2 years, 82.0% males, mean follow‐up: 5.2 ± 4.0 years) were included. There were 108 (24.7%) deaths (49.1% due to heart failure) and 94.9% survived the first year. Cumulative incidence of appropriate therapy at 5‐year was 31.6%. Cumulative incidence of inappropriate shock at 5‐year was 9.1%. A total of 98 complications requiring surgical intervention occurred (annual rate: 4.3%). In total, 236 patients with primary prevention due to ischemic (61.9%) or nonischemic (38.1%) CM were included. During a mean follow‐up of 3.9 ± 2.5 years, for appropriate therapy, there was no significant difference (P = .985) between ischemic (cumulative incidence at 1, 3, and 5 years: 6.4%, 17.1%, and 19.6%) and nonischemic CM (cumulative incidence at 1, 3, and 5 years: 5.6%, 13.6%, and 24.4%).
Conclusion
Ischemic and nonischemic CM confer similar risk of ventricular arrhythmia. This supports current guidelines regarding primary‐prevention ICD. Short‐term survival is excellent but complications remain a problem.
Keywords: arrhythmia, cardiomyopathy, heart failure, implantable cardioverter‐defibrillator, risk stratification, sudden death
1. BACKGROUND
In those deemed at increased risk of ventricular tachyarrhythmia, an implantable cardioverter defibrillator (ICD) is an effective way of preventing sudden cardiac death.1 An ICD treats ventricular tachyarrhythmia by antitachycardia pacing (ATP) or cardioversion, but it also offers bradycardia pacing and can be combined with a cardiac resynchronization therapy system (CRTD).1 In 1996, the Multicenter Automatic Defibrillator Implantation Trial (MADIT) showed a benefit of primary prophylactic ICD with a reduction in all‐cause mortality in patients with heart failure, prior myocardial infarction, and nonsustained ventricular tachycardia.2 The MADIT‐II demonstrated this benefit in patients with prior myocardial infarction and heart failure even without documented nonsustained ventricular tachycardia.3 In the Sudden Cardiac Death in Heart Failure Trial (SCD‐HeFT), an ICD reduced mortality by 23% compared to optimal medical therapy alone in patients with heart failure and reduced left ventricular systolic ejection fraction (EF) either due to ischemic or nonischemic cardiomyopathy (CM); this result was notably driven by the reduction of sudden cardiac death in New York Heart Association (NYHA) functional class II patients.4 In a smaller study, the Defibrillators in Non‐Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) trial, ICDs reduced sudden cardiac death by 80.0% compared to optimal medical therapy alone, but the reduction in all‐cause mortality in this nonischemic CM population was only borderline significant (hazard ratio (HR): 0.65, P = .08).5 Guidelines from the European Society of Cardiology (ESC) recommend primary prophylactic ICD for patients with symptomatic heart failure, NYHA functional class II‐III, EF ≤35% after at least 3 months of optimal medical therapy, and at least 1 year life expectancy.1 This is rated class I (is recommended) for both ischemic and nonischemic CM. The level of evidence is considered stronger for ischemic CM (level A) than for nonischemic CM (level B).1 In 2016, the Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure (DANISH) trial was published; ICD treatment was compared to optimal medical therapy including cardiac resynchronization pacemaker (CRTP) in patients with nonischemic CM.6 Mortality due to sudden cardiac death was reduced in the ICD group (HR: 0.50, P = .005) but there was no significant reduction in all‐cause mortality after 5 years (HR: 0.87, P = .28).6 This has led current guidelines to be put into question. Further studies and real‐life data from unselected patients without tertiary center bias are warranted. Moreover, data are needed regarding the cumulative incidence of appropriate therapy, inappropriate shock, and complications requiring surgery, as well as mortality and cause of death in ICD patients from a modern unselected cohort without tertiary center bias.
2. METHODS
The study was performed as a retrospective observational study of all patients in Region Gävleborg, Sweden who had an ICD implanted or underwent device replacement between 1st January 2007 and 1st January 2017. Eligible patients were identified through Provisio™, a software used for scheduling surgeries, which covered all implants. The study complies with the Declaration of Helsinki and has been approved by the Ethical Review Board in Uppsala (document number 2018/416). Data were retrieved from electronic medical records (Melior™, Cerner Sverige AB, Stockholm) between March 2017 and February 2018 and evaluated according to a predefined protocol and imported from Excel™ 2010 (Microsoft Corporation, Redmond, WA) into SPSS™ version 22 (IBM, Armonk, NY) for statistical analyses and Stata™ (StataCorp (2017), Stata Statistical Software: Release 15, College Station, TX) for figures.
2.1. Definitions
Patients with a first ICD implant due to survival of documented ventricular fibrillation or ventricular tachycardia with hemodynamic compromise were classified as secondary prevention. Primary prevention implies a decision to implant an ICD based on some risk marker, which varies with the underlying disease (eg, hypertrophic CM, long QT‐syndrome, Brugada syndrome). For heart failure with reduced EF, guidelines recommend a primary‐prevention ICD for patients with NYHA functional class II‐III, EF ≤35% after at least 3 months of optimal medical therapy, and at least 1 year life expectancy. Heart failure was classified as either ischemic CM (history of previous myocardial infarction, presence of symptomatic coronary artery disease, or significant coronary artery stenosis on coronary angiography) or nonischemic CM.
2.2. Type of device
ICDs were single chamber (ICD‐VR, one right‐ventricular lead), dual chamber (one right‐atrial and one right‐ventricular lead), or CRTD systems (leads pacing right and left ventricles and right atrium). There were no patients with subcutaneous ICDs.
2.3. Follow‐up
Upgraded or downgraded devices were classified according to the initial ICD type. Patients were followed until loss to follow‐up, ICD explant, downgrade to pacemaker, death, or the end of the study.
2.4. ICD therapy
Appropriate ICD therapies treated ventricular tachycardia/fibrillation with either ATP or cardioversion (shock). An episode of arrhythmia requiring therapy was counted as one event if it happened within 24 h, even if multiple therapies were delivered. Inappropriate therapy was defined as shock in the absence of ventricular tachycardia/fibrillation.
2.5. Statistics
Data are described as frequencies, percentages, and means including standard deviations. The annualized rate was calculated as the proportion of patients experiencing at least one event divided by the follow‐up time calculated as the sum of follow‐up time until first episode or censoring event (loss to follow‐up, death, downgrade to pacemaker, or device explant). A single patient could account for more than one episode (note that several ATP/cardioversion during the same day were counted as one episode) or complication. The cumulative incidence was calculated using time to first event as the censoring event; otherwise, total time of follow‐up for patients without an event was counted as long as they had an active ICD.
t‐test was used for comparisons of continuous variables and χ 2 test for categorical variables. Kaplan‐Meier methods were used to describe time to event and the log‐rank test was used to test for differences. The HR for death was estimated for a risk marker using both univariable and multivariable Cox proportional hazard regression. Two‐sided P‐values <.05 were considered as statistically significant.
3. RESULTS
In total, 438 patients (82.0% males, mean age at implant: 65.9 ± 11.2 years) with ICDs (ICD‐VR: 20.3%, dual lead implantable cardioverter defibrillator [ICD‐DR]: 46.8%) or CRTD (32.9%) were analyzed (Table 1). Patients received β‐blockers (90.0%), angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (83.8%), and mineralcorticoid receptor antagonists (44.5%).
Table 1.
Characteristics of 438 patients with ICD
| All (%) | Primary prevention (%) | Secondary prevention (%) | P‐value | |
|---|---|---|---|---|
| Patients | 438 | 239 | 199 | |
| Mean age | 65.9 ± 11.8 | 65.4 ± 10.8 | 66.4 ± 11.6 | .335 |
| Females | 79 (18.0) | 46 (19.2) | 33 (16.6) | .533 |
| Device type | ||||
| ICD‐VR | 89 (20.3) | 36 (15.1) | 53 (26.6) | .003 |
| ICD‐DR | 205 (46.8) | 89 (37.2) | 116 (58.3) | <.001 |
| CRTD | 144 (32.9) | 114 (47.7) | 30 (15.1) | <.001 |
| Ejection fraction | ||||
| >50% | 63 (14.4) | 1 (0.4) | 62 (31.2) | <.001 |
| 30‐50% | 167 (38.1) | 80 (33.5) | 87 (43.7) | .030 |
| <30% | 208 (47.5) | 158 (66.1) | 50 (25.1) | <.001 |
| Hypertension | 216 (49.3) | 112 (46.9) | 104 (52.3) | .119 |
| Diabetes mellitus | 107 (24.4) | 66 (27.6) | 41 (20.6) | .145 |
| Renal failurea | 69 (15.8) | 44 (18.4) | 25 (12.6) | .286 |
| Atrial fibrillation | 143 (32.6) | 74 (31.0) | 69 (34.7) | .305 |
| β‐blockers | 394 (90.0) | 223 (93.3) | 171 (85.9) | .369 |
| ACE‐i/ARB | 367 (83.8) | 215 (90.0) | 152 (76.4) | .008 |
| MRA | 195 (44.5) | 150 (62.8) | 45 (22.6) | <.001 |
| Amiodarone | 49 (11.2) | 14 (5.9) | 35 (17.6) | <.001 |
Data presented as frequencies (percentage in parenthesis).
ACE‐I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; CM, cardiomyopathy; CRTD, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; ICD‐DR, dual lead implantable cardioverter defibrillator; ICD‐VR, single lead implantable cardioverter defibrillator; MRA, mineralcorticoid receptor antagonists.
aDefined as S‐Creatinine ≥130 µmol/L.
3.1. Appropriate therapy
During a total of 2264 patient‐years (mean: 5.2 ± 4.0 years, n = 438), 28.5% of patients received appropriate therapy and 8.0% of patients received ≥5 episodes of appropriate therapy. The cumulative incidence of appropriate therapy at 1, 3, and 5‐years were 11.5%, 23.4%, and 31.6%, respectively (Figure 1).
Figure 1.
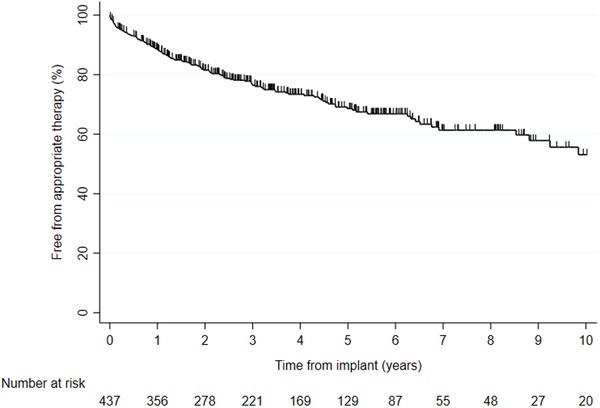
Kaplan‐Meier event‐free appropriate ICD therapy for 438 patients [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Appropriate therapy in primary‐prevention ICD due to ischemic and nonischemic CM
Out of 438 ICD patients, 236 with primary prevention due to ischemic (61.9%) or nonischemic (38.1%) CM were included in the analyses (81.8% males, age at first implant: 65.6 ± 10.6 years), see Table 2. At first implant, device was ICD‐VR (15.3%), ICD‐DR (37.3%), or CRTD (47.5%) and the patients received β‐blockers (93.6%), angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (90.3%), and mineralcorticoid receptor antagonists (62.7%). During 924 patient‐years (mean: 3.9 ± 2.5 years), 38 patients experienced appropriate therapy, 23 (15.8%) patients with ischemic CM, and 15 (16.7%) patients with nonischemic CM. Out of these patients, for ischemic CM 15 out of 23 (65.2%) and for nonischemic CM 10 out of 15 (66.7%) received at least one cardioversion. For appropriate therapy, there was no significant difference (Mantel‐Cox P = .985) between ischemic CM (cumulative incidence at 1, 3, and 5 years: 6.4%, 17.1%, and 19.6%) and nonischemic CM (cumulative incidence at 1, 3, and 5 years: 5.6%, 13.6%, and 24.4%), see Figure 2. A multivariable analysis revealed the following predictors: 10‐year increase of age at implant (HR: 1.10; P = .517), ischemic versus nonischemic etiology (HR: 0.91; P = .786), and male versus female sex (HR: 1.53; P = .596).
Table 2.
Characteristics of 236 patients with primary prevention ICD due to heart failure
| Primary prevention— CM (%) | Primary prevention— ischemic CM (%) | Primary prevention— nonischemic CM (%) | P‐value | |
|---|---|---|---|---|
| Patients | 236 | 146 | 90 | |
| Mean age | 65.6 ± 10.6 | 67.5 ± 8.6 | 62.4 ± 12.7 | <.001 |
| Females | 43 (18.2) | 21 (14.4) | 22 (24.4) | .058 |
| Device type | ||||
| ICD‐VR | 36 (15.3) | 25 (17.1) | 11 (12.2) | .355 |
| ICD‐DR | 88 (37.3) | 64 (43.8) | 24 (26.7) | .009 |
| CRTD | 112 (47.5) | 57 (39.0) | 55 (61.1) | .001 |
| Ejection fraction | ||||
| 30‐50% | 78 (33.1) | 50 (34.2) | 28 (31.1) | .670 |
| <30% | 158 (66.9) | 96 (65.8) | 62 (68.9) | .670 |
| Hypertension | 111 (47.0) | 71 (48.6) | 40 (44.4) | .505 |
| Diabetes mellitus | 65 (27.5) | 48 (32.9) | 17 (18.9) | .024 |
| Renal failurea | 44 (18.6) | 33 (22.6) | 11 (12.2) | .058 |
| Atrial fibrillation | 74 (31.4) | 45 (30.8) | 29 (32.2) | .885 |
| β‐blockers | 221 (93.6) | 137 (93.8) | 84 (93.3) | 1.000 |
| ACE‐i/ARB | 213 (90.3) | 135 (92.5) | 78 (86.7) | .176 |
| MRA | 148 (62.7) | 87 (59.6) | 61 (67.8) | .216 |
| Amiodarone | 14 (5.9) | 9 (6.2) | 5 (5.6) | 1.000 |
Data presented as frequencies (percentage in parenthesis).
ACE‐I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; CM, cardiomyopathy; CRTD, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter defibrillator; ICD‐DR, dual lead implantable cardioverter defibrillator; ICD‐VR, single lead implantable cardioverter defibrillator; MRA, mineralcorticoid receptor antagonists.
aDefined as S‐Creatinine ≥130 µmol/L.
Figure 2.
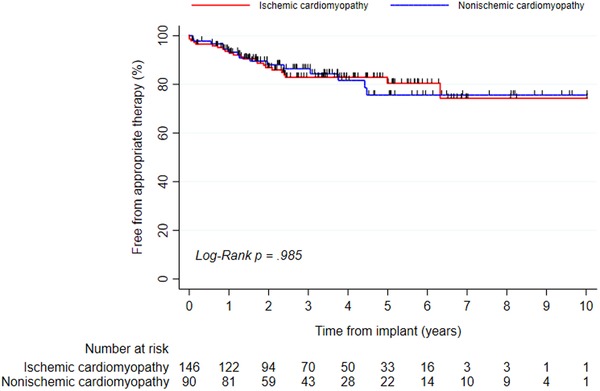
Kaplan‐Meier event‐free appropriate therapy for 236 ICD patients with primary prevention ICD due to ischemic cardiomyopathy or nonischemic cardiomyopathy [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Inappropriate shock and complications requiring surgery
During a total of 2264 patient‐years (mean: 5.2 ± 4.0 years, n = 438), 45 inappropriate shock episodes occurred, for an annual rate 2.0% (8.0% of patients); inappropriate shock was due to atrial arrhythmia (n = 36), lead dysfunction (n = 5), other (n = 2), T‐wave oversensing (n = 1), and external interference (n = 1). The cumulative incidences of inappropriate shocks at 1, 3, and 5 years were 1.9%, 5.2%, and 9.1%, respectively (Figure 3). Increasing age was associated with less risk of inappropriate shock (HR: 0.96; Mantel‐Cox P = .002) while there was no difference with regard to sex (P = .316). A total of 98 complications requiring surgical intervention occurred in 77 patients; these were lead dislodgement (n = 34), lead dysfunction (n = 25), extraction due to infection (n = 16), other (n = 12), connector failure (n = 2), device failure (n = 2), pericardiocentesis (n = 2), Twiddler's syndrome (n = 1), pneumothorax (n = 1), extraction due to multiple leads (n = 1), infection with local revision (n = 1), and upgrade to a high‐voltage ICD (n = 1). The annual rate of complications requiring surgery was 4.3% (males vs females P = .812 and primary vs secondary indication P = .313), see Figure 4.
Figure 3.
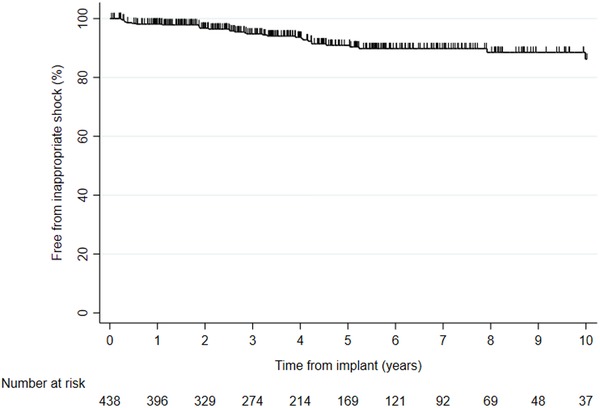
Kaplan‐Meier event‐free inappropriate ICD shock for 438 patients [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
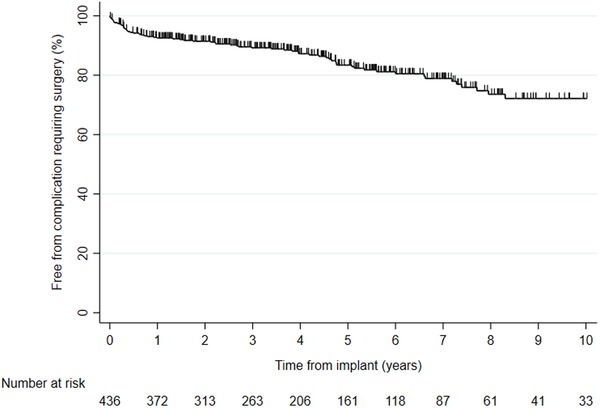
Kaplan‐Meier event‐free complications requiring surgery for 438 ICD patients [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Mortality and cause of death
During a total of 2264 patient‐years (mean: 5.2 ± 4.0 years, n = 438), there were 108 (24.7%) deaths. The main cause of death was heart failure (49.1%) followed by infection (15.7%), sudden cardiac death (9.0 %), malignancy (6.5%), respiratory failure (4.6%), myocardial infarction (2.1%), stroke (1.9%), renal failure (1.9%), suicide (0.9%), nondevice related surgery (0.9%), and unknown causes (7.4%). The cumulative survival at 1, 3, and 5 years was 94.9%, 89.5%, and 82.7%, respectively (Figure 5). There was no significant difference with regard to female versus male sex (16/79 vs 92/359; Mantel‐Cox P = .623). A multivariable analysis revealed the following predictors: 10‐year increase of age at implant (HR: 1.67; P < .001), ischemic vs nonischemic etiology (HR: 2.79; P = .001), secondary versus primary indication (HR: 2.10; P = .002), and male versus female sex (HR: 0.995; P = .989).
Figure 5.
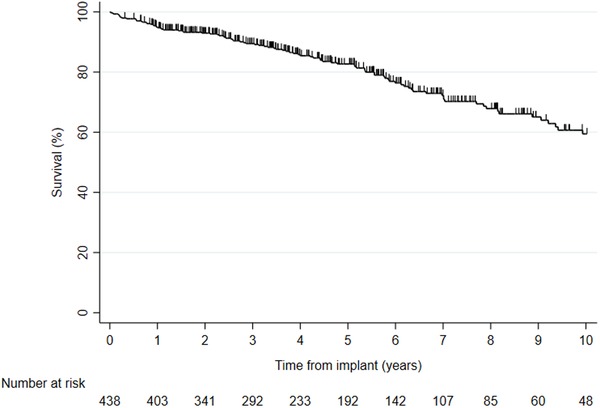
Kaplan‐Meier survival for 438 ICD patients [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
4.1. Appropriate therapy
In our study, the cumulative incidence of appropriate therapy after 5 years was 31.6%. This is comparable to a recently published American study, including patients with both primary and secondary indication, where patients at inclusion had their ICD for on average 4.6 years and 33.1% had experienced appropriate therapy.7
4.2. Appropriate therapy in primary‐prevention ICD due to ischemic and nonischemic CM
In the DEFINITE trial (mean follow‐up: 29 months), the risk of sudden cardiac death was reduced, but the reduction of overall mortality was not statistically significant.5 Recently, the DANISH trial showed a reduction in sudden cardiac death but no significant reduction in overall mortality.6 However, a meta‐analysis of six trials of primary‐prevention ICD in nonischemic CM, including DANISH, showed a significant reduction in all‐cause mortality (HR: 0.76, 95% confidence interval (CI) 0.64 to 0.90, P = .001).8 In a propensity‐score matched analysis of the Swedish Heart Failure Registry, ICD was associated with a 27% reduction in 1‐year all‐cause mortality and this was consistent across subgroups including ischemic CM and nonischemic CM.9
Our results show that in a modern unselected cohort with CRTDs and medical therapy according to guidelines, ischemic and nonischemic CM patients experience appropriate therapy at the same rate.10 Notably, in SCD‐HeFT, ischemic CM was associated with an increased risk of death following appropriate therapy.4, 11 It thus appears that nonischemic CM patients in our study had at least similar benefit from their ICD as ischemic CM patients.
4.3. Inappropriate shock and complications requiring surgery
The proportion of patients who underwent surgery due to complications was 17.6% in this observational study of a regional cohort, without tertiary center bias. In a validation study of the nationwide Danish register of both pacemakers and ICDs, 9.5% of the patients experienced a complication at 6‐month follow‐up but this also included minor complications not requiring surgery.12 Notably, our cohort was followed for a considerably longer time, which increases the number of lead‐related complications because leads are the weakest link of the system.13, 14 The rate of mechanical complications affecting ICD leads after 10 years is approximately 25%.15 According to a systemic review, the pooled complication rate was 9.1% over a mean follow‐up time of 17.9 months.16 The National Cardiovascular Data Registry for ICD data in the United States reported 3.1% complications, but only included complications apparent during the same hospital admission as the primary implant.17 However, registries seem to underestimate the true rate of complications, which emphasizes the importance of validation work.16 The cumulative incidence of an inappropriate shock (not ATP) at 2‐year follow‐up was 3.3% in our study compared to 13% in the MADIT II, but 2.2% over a mean follow‐up of 1.4 years in the subsequent MADIT‐RIT.18, 19 This is likely attributable to differences in programming, pharmaceutical agents, device algorithms, and possibly home monitoring.19, 20, 21 The most common reason for inappropriate shock was atrial arrhythmia, which is consistent with several other studies.19, 22 In our cohort, 90.0% was prescribed β‐blockers which is similar with previous studies.20, 21 In summary, this study confirms the importance of validation to accurately report complications and inappropriate shocks and our findings are consistent with previous studies.
4.4. Mortality and cause of death
An ICD effectively terminates ventricular arrhythmias and improves survival in patients with reduced EF as a primary prevention indication.3, 4, 5 This has been confirmed in extended follow‐up of randomized controlled trials.23 It is standard treatment after life‐threatening ventricular arrhythmias and miscellaneous diseases with specific criteria.1 CRT has beneficial effects on the underlying heart failure and improves survival.24, 25, 26 Furthermore, CRTD as compared with CRTP reduces all‐cause mortality by reducing sudden cardiac death.27 Altogether, this may alter the mode of death. In the current study, the most common cause of death was deteriorated heart failure. Notably, sudden cardiac death occurred in 9% of cases, which is comparable to another retrospective study.28 Death often involves the interplay of several contributing causes and these are not always easily discernable. Nevertheless, our data highlight the importance of following the progression of heart failure and its optimal management. The prediction of death is difficult, but crucial especially in primary prevention. Reduction of sudden cardiac death by implantation of ICDs leads to an increase in other modes of death, in the primary‐prevention ICD population heart failure is the primary cause of death. Interestingly, 95% survived the first year; this indicates selection of patients with acceptable life expectancy. However, this likely also indicates a more restrictive approach to ICD implantation than what is recommended by the ESC. In 2012, the ICD implantation rate in Sweden was 136 per million inhabitants, which is low and likely does not reflect guideline indications.29
5. LIMITATIONS
We assume that appropriate therapy was life‐saving, but this is not necessarily always the case, because ventricular tachycardia may self‐terminate. This is a limitation that we share with other similar studies. Moreover, the programming may affect the occurrence of ICD therapy.30 The ICD implantation rate is lower in Sweden than in many comparable countries, which should be kept in mind when applying these findings to different contexts.31 While offering validated real‐world data, this is a smaller study, which increases the risk of type 2 errors. Risk stratification for sudden cardiac death remains a challenge and more observational studies with long‐term follow‐up are needed to further elucidate this important topic.
6. CONCLUSIONS
Primary‐prevention ICD patients with ischemic and nonischemic CM are at similar risk of life‐threatening ventricular arrhythmias. This supports current guidelines regarding risk stratification for sudden cardiac death. Inappropriate shock is still a non‐negligible problem in ICD patients. A considerable amount of patients undergo surgical intervention due to device‐related complications. Half of patients with ICD/CRTD die from heart failure.
CONFLICT OF INTEREST
Gustav Mattsson: none.
Peter Magnusson has received speaker fees or grants from Abbott, Alnylam, Bayer, AstraZeneca, BMS, Boehringer‐Ingelheim, Internetmedicin, Lilly, Novo Nordisk, Octopus Medical, Pfizer, Vifor Pharma, and Zoll.
AUTHOR CONTRIBUTIONS
Design, data collection and analysis, major writing, and co‐management: Mattsson. Idea, design, data analysis, writing, and project management: Magnusson. Both authors approved the manuscript for submission.
ACKNOWLEDGMENT
The authors acknowledge the contribution of Angelos Pournaras and Rawaz Kader who took part in the data collection. Statistician Krister Ågren provided graphs for the manuscript. This research project was funded by Region Gävleborg. Jo Ann LeQuang of LeQ Medical reviewed the manuscript for American English. Region Gävleborg financed the research project.
Mattsson G, Magnusson P. Long‐term follow‐up of implantable cardioverter defibrillator patients with regard to appropriate therapy, complications, and mortality. Pacing Clin Electrophysiol. 2020;43:245–253. 10.1111/pace.13869
REFERENCES
- 1. Priori S, Blomström‐Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793‐2867. [DOI] [PubMed] [Google Scholar]
- 2. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933‐1940. [DOI] [PubMed] [Google Scholar]
- 3. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877‐883. [DOI] [PubMed] [Google Scholar]
- 4. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225‐237. [DOI] [PubMed] [Google Scholar]
- 5. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151‐2158. [DOI] [PubMed] [Google Scholar]
- 6. Køber L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221‐1230. [DOI] [PubMed] [Google Scholar]
- 7. Witt CM, Waks JW, Mehta RA, et al. Risk of appropriate therapy and death before therapy after implantable cardioverter‐defibrillator generator replacement. Circ Arrhythm Electrophysiol. 2018;11:e006155. [DOI] [PubMed] [Google Scholar]
- 8. Shun‐Shin MJ, Zheng SL, Cole GD, Howard JP, Whinnett ZI, Francis DP. Implantable cardioverter defibrillators for primary prevention of death in left ventricular dysfunction with and without ischaemic heart disease: a meta‐analysis of 8567 patients in the 11 trials. Eur Heart J. 2017;38:1738‐1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schrage B, Uijl A, Benson L, et al. Association between use of primary prevention implantable cardioverter‐defibrillators and mortality in patients with heart failure: a prospective propensity‐score matched analysis from the Swedish heart failure registry. Circulation. 2019;140:1530‐1539. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 11. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkfeldt RE, Johansen JB, Nohr EA, Jørgensen OD, Nielsen JC. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maisel WH. Transvenous implantable cardioverter‐defibrillator leads: the weakest link. Circulation. 2007;115:2461‐2463. [DOI] [PubMed] [Google Scholar]
- 14. Maisel WH, Kramer DB. Implantable cardioverter‐defibrillator lead performance. Circulation. 2008;117:2721‐2723. [DOI] [PubMed] [Google Scholar]
- 15. Koneru JN, Jones PW, Hammill EF, Wold N, Ellenbogen KA. Risk factors and temporal trends of complications associated with transvenous implantable cardiac defibrillator leads. J Am Heart Assoc. 2018;7:e007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ezzat VA, Lee V, Ahsan S, et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real‐world’ data an underestimation? Open Heart 2015;2:e000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Freeman JV, Wang Y, Curtis JP, Heidenreich PA, Hlatky MA. Physician procedure volume and complications of cardioverter‐defibrillator implantation. Circulation 2012;125:57‐64. [DOI] [PubMed] [Google Scholar]
- 18. Daubert JP, Zareba W, Cannom DS, et al. Inappropriate implantable cardioverter‐defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357‐1365. [DOI] [PubMed] [Google Scholar]
- 19. Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275‐2283. [DOI] [PubMed] [Google Scholar]
- 20. Ruwald MH, Abu‐Zeitone A, Jons C, et al. Impact of carvedilol and metoprolol on inappropriate implantable cardioverter‐defibrillator therapy: The MADIT‐CRT trial (Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2013;62:1343‐1350. [DOI] [PubMed] [Google Scholar]
- 21. Brodine WN, Tung RT, Lee JK, et al. Effects of beta‐blockers on implantable cardioverter defibrillator therapy and survival in the patients with ischemic cardiomyopathy (from the Multicenter Automatic Defibrillator Implantation Trial‐II). Am J Cardiol. 2005;96:691‐695. [DOI] [PubMed] [Google Scholar]
- 22. van Rees JB, Borleffs CJ, de Bie MK, et al. Inappropriate implantable cardioverter‐defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556‐562. [DOI] [PubMed] [Google Scholar]
- 23. Goldenberg I, Gillespie J, Moss AJ, et al. Long‐term benefit of primary prevention with an implantable cardioverter‐defibrillator: an extended 8‐year follow‐up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation. 2010;122:1265‐1271. [DOI] [PubMed] [Google Scholar]
- 24. Moss AJ, Hall WJ, Cannom DS, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med. 2009;361:1329‐1338. [DOI] [PubMed] [Google Scholar]
- 25. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539‐1549. [DOI] [PubMed] [Google Scholar]
- 26. Linde C, Abraham WT, Gold MR, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834‐1843. [DOI] [PubMed] [Google Scholar]
- 27. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140‐2150. [DOI] [PubMed] [Google Scholar]
- 28. Thijssen J, van Rees JB, Venlet J, et al. The mode of death in implantable cardioverter‐defibrillator and cardiac resynchronization therapy with defibrillator patients: results from routine clinical practice. Heart Rhythm 2012;9:1605‐1612. [DOI] [PubMed] [Google Scholar]
- 29. Gadler F, Valzania C, Linde C. Current use of implantable electrical devices in Sweden: Data from the Swedish pacemaker and implantable cardioverter‐defibrillator registry. Europace. 2015;17:69‐77. [DOI] [PubMed] [Google Scholar]
- 30. Moss AJ, Schuger C, Beck CA, et al. MADIT‐RIT Trial Investigators. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367:2275‐2283. [DOI] [PubMed] [Google Scholar]
- 31. Torbica A, Banks H, Valzania C, Boriani G, Fattore G. Investigating regional variation of cardiac implantable electrical device implant rates in European healthcare systems: what drives differences? Health Econ. 2017;26:30‐45. [DOI] [PubMed] [Google Scholar]


