In patients with advanced heart failure and reduced ejection fraction (HFrEF), longstanding elevations in left heart filling pressures can result in pulmonary hypertension (PH) that, if severe, may preclude candidacy for heart transplantation. According to the 2016 International Society for Heart and Lung Transplantation listing criteria, PH may be a contraindication if the pulmonary artery (PA) systolic pressure is ≥ 50 mm Hg and either the transpulmonary gradient (TPG) is ≥ 15 or the pulmonary vascular resistance (PVR) is ≥ 3 Wood units.1 Diuresis, vasodilator therapy, and mechanical support (MCS) are often attempted to reverse PH with variable results. We report the novel use of angiotensin receptor-neprilysin inhibitor therapy for refractory PH in end-stage HFrEF patients awaiting heart transplantation. This study was approved by the Cedars-Sinai Medical Center Institutional Review Board, and the subjects gave informed consent for chart review.
Five patients with HFrEF presented with inotrope-dependent heart failure for heart transplantation evaluation (Figure 1; Panel C). All patients had pulmonary artery catheter monitoring while receiving therapy with two or more intravenous inotropic agents titrated to cardiac index > 2.0 L/min/m2. Diuresis was titrated to right atrial pressure <10 mmHg and PA diastolic pressure < 20 mmHg. PA diastolic pressure was used as a surrogate for pulmonary capillary wedge pressure (PCWP) as our institutional policy in patients with indwelling PA catheters is to minimize repeated PA balloon inflation to prevent potential PA injury. At the time of initial PA catheter placement, when PCWP measurement was performed, the PA diastolic pressure was higher than the PCWP by 2–11 mmHg.
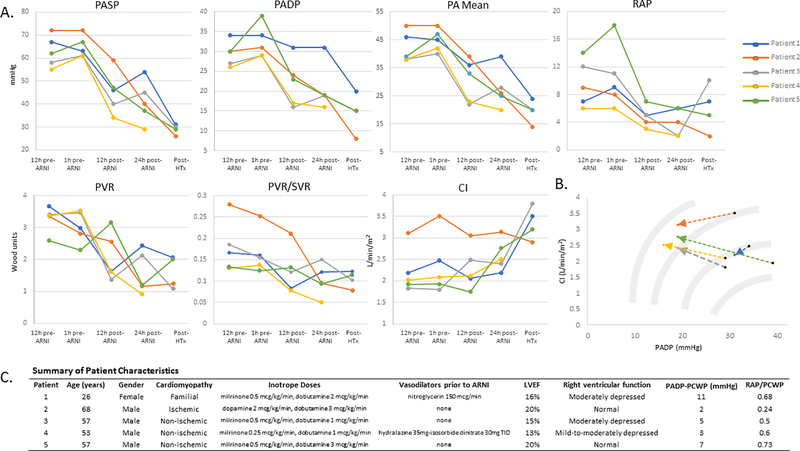
Figure 1.
Hemodynamic changes in response to ARNI treatment in patients with end-stage HFrEF awaiting transplant. A, Pulmonary artery catheter monitoring measures in pre- and post-ARNI and post-heart transplantation. B, Favorable transitions across and along Starling curve schematics. C, Summary of patient characteristics. Echocardiographic parameters were obtained from initial admission echocardiogram, and hemodynamic measurements were obtained on initial placement on pulmonary artery catheter. Abbreviations: PASP, pulmonary artery systolic pressure; PADP, pulmonary artery diastolic pressure; RAP, right atrial pressure; MAP, mean arterial pressure; CI, cardiac index; PVR, pulmonary vascular resistance (calculated using PA diastolic pressure as surrogate for PCWP due to unavailability of regular PCWP measurements, except for post-transplant value); ARNI, angiotensin receptor-neprilysin inhibitor; ICM, ischemic cardiomyopathy; NICM, nonischemic cardiomyopathy; LVEF, left ventricular ejection fraction.
Despite medical optimization, all patients were placed on inactive status on the heart transplant waiting list due to PH with either pulmonary artery systolic pressure (PASP) >60 mmHg (PASP 55–72 mmHg) or PVR >3 Wood units (PVR 2.3–3.7 Wood units). Vasodilator therapies (milrinone, intravenous nitroglycerine, hydralazine-isosorbide dinitrate combination) were administered as blood pressure permitted (Figure 1; Panel C). Intravenous sodium nitroprusside was not prescribed given concerns for potential thiocyanate toxicity with long-term use.
All five patients were evaluated for MCS to resolve PH and restore heart transplant candidacy. However, sacubitril/valsartan, an angiotensin receptor-neprilysin inhibitor (ARNI), was attempted first: 24/26 mg to four patients and a half 24/26 mg tablet to one patient due to hypotension. At the time of ARNI initiation, alternate oral and intravenous vasodilator therapy were discontinued, and dosages of inotropic agents were kept constant.
Sacubitril/valsartan resulted in improvement in filling pressures, PASP, and PVR (Figure 1; Panel A). Within 24 hours of ARNI administration, overall changes in weight (−0.4±1.4 kg), fluid balance (−0.25±1.2 L), and creatinine (−0.1±0.7 mg/dL) were minimal. At 24 hours, systolic and diastolic blood pressure decreased by 9.0 ± 10.8 mmHg and 14.4 ± 6.6 mmHg, respectively. Post ARNI, TPG decreased (−5.6 ± 4.6 mmHg), pulmonary artery pulsatility index (PAPI) increased (+2.0 ± 2.5), and PVR/SVR ratio decreased (−0.06 ± 0.04). Patients were maintained on sacubitril/valsartan with scheduled twice-daily dosing or, if limited by hypotension, as-needed dosing for PASP ≥60 mmHg. All five patients were re-activated on the heart transplant waiting list, and none required MCS to reverse PH.
By using PA diastolic pressure as a surrogate for PCWP in this real-world case series, we may have underestimated the transpulmonary gradient and pulmonary vascular resistance; even with this potential underestimation, the use of sacubitril/valsartan allowed end-stage heart failure patients with previously prohibitive pulmonary hypertension to be successfully listed for heart transplantation. Four patients underwent heart transplantation (one heart-kidney transplant) 5 to 36 days after sacubitril/valsartan initiation with no post-operative PH, right ventricular failure, or post-operative hypotension requiring vasopressor support. Sacubitril/valsartan was not continued post-transplant. The fifth patient is awaiting heart transplantation on home milrinone and sacubitril/valsartan.
These cases illustrate a novel and potentially effective application of angiotensin receptor-neprilysin inhibitor therapy for treatment of advanced HFrEF patients with severe PH awaiting heart transplantation. Sacubitril/valsartan was effective at reversing PH when other vasodilators were not. Although the mechanisms of potential benefit of sacubitril/valsartan on PH remain unknown, the observed decreases in TPG and PVR/SVR ratio suggest the benefit is not explained by systemic vasodilation alone. Mouse models demonstrate favorable effects of neprilysin inhibition on the pulmonary vasculature,2–3 possibly in part due to augmentation of atrial natriuretic peptide levels4 promoting cyclic GMP-mediated vasodilation.5–6 Sacubitril/valsartan may also improve RV-PA coupling, as demonstrated by increased PAPI and decreased PVR, and augment LV function given the observed increase in cardiac index post-ARNI (Figure 1; Panel B). Improvement in PH was achieved in one patient at an unapproved dose of half of the 24–26 mg tablet, highlighting a need for further evaluation of lower-dose sacubitril/valsartan in HFrEF patients.
Further investigation into the effects of the angiotensin receptor-neprilysin inhibitor on the pulmonary vasculature and ventricular function in patients with left heart disease may offer novel therapeutic options, and the use of sacubitril/valsartan may obviate need for mechanical circulatory support in this population of end-stage heart failure patients with PH precluding transplantation.
Supplementary Material
Acknowledgments
Sources of Funding:
This work was supported in part by National Institutes of Health grants R01-HL134168, R01-HL131532, R01-HL143227, the Barbra Streisand Women’s Cardiovascular Research and Education Program, and the Erika Glazer Women’s Heart Health Project.
Footnotes
Disclosures: None
Data Availability: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger-Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EA, Zuckermann A; International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases Council; International Society for Heart Lung Transplantation (ISHLT) Pediatric Transplantation Council; International Society for Heart Lung Transplantation (ISHLT) Heart Failure and Transplantation Council.The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. JHLT. 2016; 35:1–23. [DOI] [PubMed] [Google Scholar]
- 2.Dai ZK, Hsieh CC, Chai CY, Wu JR, Jeng AY, Chou SH, Wu BN, Yeh JL, Chen IJ, Tan MS. Protective effects of a dual endothelin converting enzyme/neutral endopeptidase inhibitor on the development of pulmonary hypertension secondary to cardiac dysfunction in the rat. Pediatric Pulmonology. 2010; 45:1076–85. [DOI] [PubMed] [Google Scholar]
- 3.Clements RT, Vang A, Fernandez-Nicolas A, Kue NR, Mancini TJ, Morrison AR, Mallem K, McCullough DJ, Choudhary G. Treatment of pulmonary hypertension with angiotensin II receptor blocker and neprilysin inhibitor Sacubitril/Valsartan. Circ Heart Fail. 2019; 12:e005819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim N, McCarthy C, Shrestha S, Gaggin HK, Mukai R, Szymonifka J, Apple FS, Burnett JC Jr, Iyer S, Januzzi JL Jr. Effect of neprilysin inhibition on various natriuretic peptide assays. Journal of the American College of Cardiology. 2019; 73:1273–84. [DOI] [PubMed] [Google Scholar]
- 5.Hobbs AJ, Moyes AJ, Baliga RS, Ghedia D, Ochiel R, Sylvestre Y, Dore CJ, Chowdhury K, Maclagan K, Quartly HL, Sofat R, Smit A, Schreiber BE, Coghlan GJ, MacAllister RJ. Neprilysin inhibition for pulmonary arterial hypertension: a randomized, double‐blind, placebo‐controlled, proof‐of‐concept trial. British Journal of Pharmacology. 2019; 176:1251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliga RS, Zhao L, Madhani M, Lopez-Torondel B, Visintin C, Selwood D, Wilkins MR, MacAllister RJ, Hobbs AJ. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2008; 178:861–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.