Abstract
Biomarker measurements can provide unambiguous evidence of environmental exposures as well as the resultant biological responses. Firefighters have a high rate of occupational cancer incidence, which has been proposed to be linked in part to their increased environmental exposure to byproducts of combustion and contaminants produced during fire responses. In this article, the uptake and elimination of targeted volatile organic compounds were investigated by collecting the exhaled breath of firefighters on sorbent tubes before and after controlled structure burns and analyzing samples using automated thermal desorption-gas chromatography (ATD-GC/MS). Volatile organic compounds exposure was assessed by grouping the data according to firefighting job positions as well as visualizing the data at the level of the individual firefighter to determine which individuals had expected exposure responses. When data were assessed at the group level, benzene concentrations were found to be elevated post-exposure in both fire attack, victim search, and outside ventilation firefighting positions. However, the results of the data analysis at the individual level indicate that certain firefighters may be more susceptible to post-exposure volatile organic compounds increases than others, and this should be considered when assessing the effectiveness of firefighting protective gear. Although this work focuses on firefighting activity, the results can be translated to potential human health and ecological effects from building and forest fires.
Keywords: Benzene, environment, occupational exposure, self-contained breathing apparatus (SCBA), thermal desorption (TD), volatile organic compounds (VOCs)
Introduction
Structural firefighting presents a complex environment for which responders must be provided with adequate respiratory and dermal protection to maintain personal safety. A firefighters’ respiratory tract is commonly protected by using self-contained breathing apparatus (SCBA) and the skin is protected by wearing clothing that is specifically designed for the thermal risks of interior structural firefighting.[1] However, despite the use of SCBA and personal protective equipment (PPE) during firefighting, firefighters still have an elevated level of cancer incidence that exceeds that of the general population.[2,3] Previous studies have shown that firefighters may have increased breath and urine concentrations of volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (PAHs) immediately after completing firefighting activities, even when fully outfitted in turnout gear and SCBA.[4–6] However, the benzene and PAH levels detected are similar to those reported for individuals representative of other occupational groups.[4,5]
Breath monitoring is a simple and non-invasive tool for assessing the extent of chemical exposure and provides a way to evaluate the protection afforded by firefighting PPE and identify ways to improve overall chemical protection. Breath measurements reflect the most recent VOC exposures, especially those experienced through inhalation. The lungs have a large surface area, and VOCs are rapidly absorbed across the thin alveoli membrane.[7] In addition to inhalation exposure, transdermal absorption has been postulated as an important route of exposure for VOC and PAHs, as well as other harmful compounds, particularly through the skin around the neck region where the relatively porous hoods that firefighters wear for thermal protection interface with additional components of firefighting PPE.[5,8–10] Transdermal absorption occurs through passive diffusion upon vapor contact with the skin, and is slower than absorption through inhalation.[7] Measuring VOCs and PAHs in breath has been used to assess systemic exposure of firefighters to chemical exposure through both dermal and inhalation routes.[11,12] Although ingestion is also a potential exposure route for VOCs and PAHs, the firefighters participating in this study were not allowed to eat or drink during the controlled structure burns, and therefore this exposure route is not considered to be significant in this study. VOCs and PAHs that are inhaled, ingested, and absorbed through the skin partition into the bloodstream and are distributed throughout the body. VOCs can be exchanged from the bloodstream across the alveoli into the lungs, where the compounds are exhaled in breath.[11] VOCs and PAHs that are not exhaled in the breath are then eliminated from the body through metabolism and excretion in urine and feces.
The general concept of biomarkers research is to establish group-based trends that can ultimately be generalized to larger populations. For example, case and control groups (e.g., cancer vs. not cancer, or firefighter vs. observer) have been compared and the summary statistics of the biomarkers are evaluated for differences.[13–16] Another similar approach is to assess longitudinal (intervention) effects of a group of patients or subjects (e.g., before and after some drug regimen or activity like firefighting) and then evaluate biomarker changes.[4,17] Overall, the statistical trends are interpreted at the group level. This can produce ambiguous results because underlying distributions are skewed,[18] or because the variance of response within the group can outweigh the aggregate differences between the groups.[19,20] One approach for resolving this quandary is to explore biomarkers at the detailed level of the individual and determine if subsets of the results can be explained by trends in meta-data, behavior, or personal history.[21] Herein, VOC concentrations in firefighter breath samples from pre-, post-, and 1-hr post-exposure time points are assessed from both the grouped and individual perspective. The exposure information obtained from the analyses of firefighters who participated in attack, search, and rescue, and outside ventilation positions can be used to identify firefighting positions prone to higher VOC exposure as well as individual firefighters with higher than average VOC exposure. This information can be used in future exercises to rotate firefighters through different positions during live fire responses and to decrease future exposures of individual firefighters by making adjustments and improvements to the use of personal protective gear.
Methods
Study design
The current study of firefighters’ exposures to VOCs was conducted at the University of Illinois Fire Service Institute with Institutional Review Board approval. The Environmental Protection Agency (EPA) and National Institute for Occupational Safety and Health (NIOSH) collaborated on this research effort. Firefighters who participated in the study were between the ages of 18- and 55-years-old, without known cardiovascular disease or tobacco use, and consisted of 37 male and 4 female firefighters (n = 41). Firefighters avoided eating char-grilled and smoked foods for 24 hr prior to the start of the study.
In this study, a total of 12 controlled structure burns with realistic firefighting responses were conducted with only one scenario per day. Groups of 12 firefighters participated in 2 of the controlled structure burns on back-to-back days followed by 2 more backto-back days of controlled burns that were conducted 6–12 days later. Thirty-one of the firefighters participated in four controlled structure burns, nine participated in two controlled structure burns, and one male firefighter withdrew from the study. The controlled structure burns were conducted in a 111 m2 wood framed residential structure with identical home furnishings as fuel, which resulted in a variety of combustion products, including PAHs, VOCs, acid gases, and hydrogen cyanide.[22,23] The 12 firefighters in each controlled structure burn were assigned typical fireground positions, including command and pump operations, attack (fire suppression), search and rescue, outside ventilation, rapid intervention team (RIT)/overhaul, and backup/overhaul, with two firefighters participating in each position per exercise. A total of 12 firefighters participated in the attack position, 12 firefighters participated in the search and rescue position, and 12 firefighters participated in the outside ventilation position for two controlled structure burns each, for a total of 24 person-events per job assignment. Overhaul jobs involved the investigation of fire extension into walls and ceilings as well as controlling smoldering embers. Firefighters participating in attack and search went inside the structures and were required to wear SCBA before entering the building. Firefighters working outside of the structure chose whether to wear SCBA or not (e.g., command/pump and outside ventilation). Firefighters participating in overhaul were allowed to choose whether to wear SCBA or not while outside the structure but were required to don SCBA before entering the structure. Additional details regarding the experimental setup can be found elsewhere.[10,22,23]
Sample collection
Carbograph 2TD/1TD dual bed sorbent tubes were conditioned prior to use according to the manufacturer’s instructions, at 350 C for 2 hr followed by 10 hr at 380 C while purging with approximately 75 mL/min of research grade helium using a TC-20 tube conditioner (Markes International, Gold River, CA). After blank checks, the tubes were subsequently conditioned at 380 C for 2 hr after each desorption. Breath samples were collected from the 12 firefighters who participated in each controlled structure burn immediately before the firefighting scenario (preexposure), directly after (post-exposure), and 1 hr after the scenario (1-hr post-exposure). For two of the firefighting scenarios, samples were collected from only 11 firefighters due to a subject who withdrew from the study. A total of 142 pre-, 142 post-, and 142 1-hr post-exposure breath samples and 20 field blanks from the fireground site were collected for a total n = 446. Firefighters exhaled into BIO-VOC samplers and 129 mL of end-tidal breath was collected onto Carbograph 2TD/1TD dual bed sorbent tubes (catalog no. C2-AXXX-5126, Markes International, Gold River, CA). Samples were shipped to U.S. EPA and stored at 4 C prior to analysis.
ATD-GC/MS analysis
Targeted analysis was performed using a previously developed selected ion monitoring (SIM)/scan method to quantify VOCs in breath samples. Procedures for preparation of VOC standards, instrument calibration and analysis can be found in Wallace et al. 2017.[24] Briefly, benzene, toluene, ethylbenzene, m,p-xylene, styrene, o-xylene, 4-ethyltoluene, and 1,3,5-trimethylbenzene were all targeted in the method using selected target and qualifier ions. A gaseous TO-14A 43 Component Mixture at 1 part-per-million (ppm) in nitrogen was purchased from Linde Electronics & Specialty Gases (Stewartsville, NJ). Calibration levels of 0, 0.5, 1.0, 2.0, 5.0, 10.0, 25.0, and 50.0 parts per billion by volume (ppbv) were achieved by varying the flow rates of the TO-14A calibrant gas and a dilution gas, humidified air, to deliver a constant volume of 200 mL of gas onto each sorbent tube in triplicate using an Easy VOC syringe at 50% relative humidity and 20 C. PAHs were also calibrated for using a stock 16 component 2000 ng/mL Restek Corporation (catalog no. 31011, Bellefonte, PA) calibration mixture at levels of 0, 0.02, 0.05, 0.10, 0.20, 0.50, 1.0, and 2.0 ng/mL in methanol and were loaded onto sorbent tubes in 1 mL volumes. PAHs are not reported herein due to issues with desorption from Carbograph 2TD/1TD sorbent tubes. The VOCs and PAHs were loaded onto the same sorbent tubes for calibration using a dual loading procedure that is described in detail elsewhere.[24] The VOC and PAH standards were paired from low concentration to high during loading (e.g., 0.5 ppbv VOC with 0.02 ng/uL PAH). The samples were analyzed with a series of three calibrations. Instrument drift was monitored by analyzing calibration check standards after every 13 samples, and the instrument was recalibrated when the area counts of the VOCs fell out of a range of þ/- 30% of the calibration value. A detailed description of calibration preparation, standard loading parameters, and method variability can be found in Geer Wallace et al. 2017.[24]
Thermal desorption and analysis were conducted using a TurboMatrix 650 ATD system (PerkinElmer LAS, Shelton, CT) and a 6890N GC coupled to a 5975 inert XL MS (Agilent Technologies, Santa Clara, CA). Thermal desorption was achieved with a purge time of 5 min, desorption flow rate of 20 mL/min, desorption time of 15 min, valve temperature of 270 C, tube temperature of 375 C, and a trap temperature that increased from 10 to 38 C with a 10-min hold. A 30 m Rxi-5Sil MS capillary GC column with a 5 m integra guard column, 0.25 mm ID, and 0.25 lm film (part no. 13623–124) from Restek Corporation (Bellefonte, PA) was used with a 2 mL/min column flow rate. The oven temperature was initially at 35 C for a 2-min hold, then increased at 6 C/min to 190 C, and increased at 28 C/min to 310 C with an 8-min hold. The quadrupole, ion source, and transfer line temperatures were 176, 290, and 290 C, respectively, and ions were monitored from 35–300 m/z. The SIM/scan option was used with a scan rate of 22 and target and qualifier ions for VOCs of interest were monitored in SIM mode. The retention times, target ions, and qualifier ions for VOCs in this method can be found elsewhere.[24]
Data analysis
Chromatographic peaks were integrated using ChemStation software (Version D.02.00, Agilent Corp., Santa Clara, CA). Data for the targeted compounds were quantified using the SIM data and the corresponding external calibration curves, which were fit to second-order polynomial regression equations. Concentrations were corrected for instrument drift by accounting for the weighted average of calibration check standards that were analyzed before and after each sample set and background corrected by subtracting out the average concentration of the analyzed lab blanks. The method detection limit (MDL) of each compound was determined using the method given in the Code of Federal Regulations (40CFR136 Appendix B). Briefly, the lowest analytical calibrator (0.5 ppbv VOC, 0.02 ng PAH) was loaded onto seven sorbent tubes in the first calibration set, the standard deviation of the concentrations obtained for the seven replicates was calculated, and this value was multiplied by 3.14 (the Student’s t-value for a single-tailed t-test with 99% confidence and six degrees of freedom) to obtain the MDLs. Values below the MDL were estimated using quantile-quantile (QQ)-plots and ordered imputations, as previously reported.[18,25] Briefly, the natural log-transformed concentrations in parts per billion by volume (ppbv) were plotted vs. the Z-scores in Excel, and a linear best fit equation was generated. This equation was used to impute the concentrations for the samples below the limit of detection by using the calculated Z-scores.
Two-tailed t-tests (a = 0.05) were performed using log-transformed concentrations in GraphPad Prism version 7 (GraphPad Prism Software, Inc., La Jolla, CA) to determine significant differences in pre- vs. post-exposure breath VOC concentrations for all of the firefighter samples (n = 142). Pre-, post-, and 1- hr post-exposure concentrations of targeted compounds were then assessed by grouping according to firefighting position. The data were combined for attack and search and rescue positions (n = 48), as the firefighters participating in these positions had the presumed highest exposure. The data for outside ventilation firefighters were also investigated (n = 24). The log10 transformed data were visualized in GraphPad Prism (Version 7) using box-and-whisker plots with 5–95% intervals without showing outliers.
As an example, the data for benzene, toluene, and ethylbenzene were analyzed according to expected and unexpected responses of firefighters to exposure conditions in GraphPad Prism (GraphPad Prism Software, Inc.) for comparison with the grouped data response in the box-and-whisker plots. The “expected response” was defined as showing a higher postexposure VOC concentration than pre- or 1-hr post-exposure. Thus, the response would be increased post-exposure concentrations that decrease after one hour of the exercise. The “unexpected response” is firefighters who do not show an increased VOC concentration in post-exposure, or who show a higher 1-hr post-exposure VOC concentration than either pre- or post-exposure. The data were paired in Excel according to individual firefighters who participated in attack and search or outside ventilation positions. All figures were generated using GraphPad Prism.
Results
Identification of targeted compounds in breath samples
VOCs were specifically targeted in this study as these compounds are indicators of combustion and have been shown to have adverse health effects.[26–28] Eight VOCs of interest to firefighting activities were analyzed using ATD-GC/MS, but only six were quantified by external calibration (4-ethyltoluene and 1,3,5-trimethylbenzene were not detected above the MDL in enough samples to be reported). The geometric means and standard deviations for the targeted VOCs are shown in Table 1. Benzene and ethylbenzene were found to have statistically significant increased and decreased post-exposure concentrations, respectively.
Table 1.
Geometric means (geometric standard deviations) of targeted VOCs.
| Compound | Pre-exposure (ppbv)A | Post-exposure (ppbv)A | 1-hr Post-exposure (ppbv)A | Field Blank (ppbv)B |
|---|---|---|---|---|
| BenzeneC | 19.17 (1.97) | 32.36 (1.76) | 19.65 (1.98) | 11.11 (1.76) |
| Toluene | 5.10 (1.82) | 5.39 (1.71) | 4.64 (1.87) | 1.16 (2.53) |
| EthylbenzeneD | 1.11 (2.06) | 0.92 (2.05) | 0.97 (2.33) | 0.53 (2.55) |
| m,p-Xylene | 0.97 (2.40) | 0.79 (2.38) | 0.92 (2.75) | 0.53 (3.00) |
| Styrene | 2.10 (2.01) | 2.33 (2.03) | 1.93 (2.18) | 1.20 (2.09) |
| o-Xylene | 0.98 (2.06) | 0.84 (2.04) | 0.94 (2.28) | 0.53 (2.31) |
n = 142;
n = 20;
statistically significant increase in pre- to post-exposure concentration (p < 0.001);
statistically significant decrease in pre- to post-exposure concentration (p = 0.032)
Assessment of data set distributions: QQ-Plots
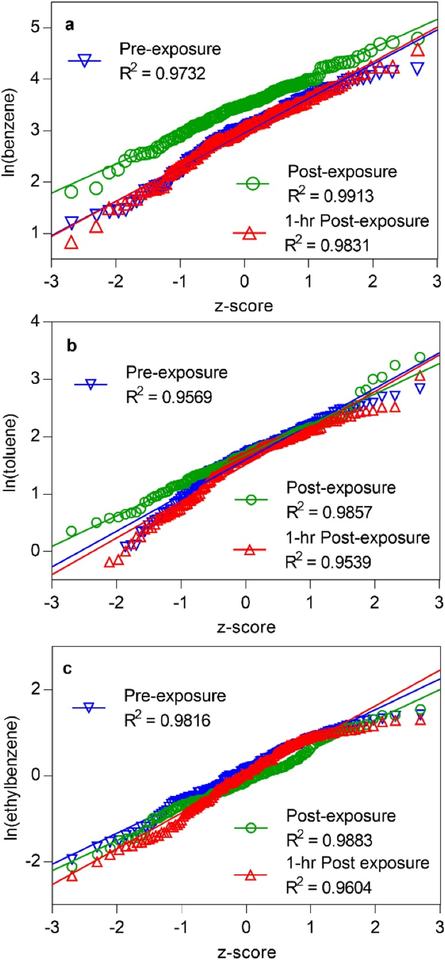
Quantile-quantile (QQ)-plots have been previously shown to be a useful tool for visualizing the spread of data and determining if data sets are normally or lognormally distributed by testing whether the quantiles of the sample approximate the theoretical quantiles.[18, 29] Since many biomonitoring data sets have been previously found to be lognormally distributed,[30] QQplots were constructed using the log-transformed pre-, post-, and 1-hr post-exposure benzene, toluene, and ethylbenzene concentrations for all firefighting positions (Figures 1a–c). These three compounds were selected as examples to show how the data were distributed. Benzene and toluene were identified with the highest concentrations among all of the VOCs monitored, and both benzene and ethylbenzene showed statistically significant differences between pre- and post-exposure concentrations, making these interesting compounds for further investigation. All three of the QQ-plots were linear with R2 values from approximately 0.95–0.99, indicating that the data are lognormally distributed and that log-transformed concentrations should be utilized for calculating statistics.[18,25]
Figure 1.
QQ-plots for (a) benzene, (b) toluene, and (c) ethylbenzene in pre-, post-, and 1-hr post-exposure firefighter breath samples. The R2 values are close to 1, indicating that the data are of uniform lognormal distribution. (1a) Benzene: The increase in the vertical scale for post-exposure shows a distinct difference from the pre- and 1-hr post-exposure groups. Difference in slope between post-exposure and the other two lines indicates that the post-exposure data variance is lower. (1b) Toluene: The tails at the low end of the pre- and 1-hr post-exposure distributions indicate that there a several firefighters with toluene levels that are indistinguishable from zero. (1c) Ethylbenzene: A slight decrease in the scale of post-exposure ethylbenzene was observed at the higher end.
In Figure 1a, the clear separation of the post-exposure benzene data from pre- and 1-hr post-exposure distributions shows a measurable increase in the postexposure concentrations immediately after the firefighting exercise. In contrast to benzene, the toluene QQ-plots for pre-, post-, and 1-hr post-exposure (Figure 1b) overlapped significantly except at the low end. The deviation of the pre- and 1-hr post-exposure concentrations from the trend of the regression lines indicates that many of these low concentrations were basically indistinguishable from zero, showing that some firefighters did not have any measurable toluene in breath until post-exposure. For ethylbenzene, the lower R2 value for the 1-hr post-exposure plot shows that there was slightly more spread in this data set (Figure 1c). The post-exposure ethylbenzene regression line showed only a very slight decrease in intercept compared to pre-exposure. While ethylbenzene showed a statistically significant decrease in postexposure breath concentrations (p = 0.032; see Table 1), there was not as clear of a distinction in the preand post-exposure regression lines as there was for benzene. Therefore, the QQ-plots suggest that toluene and ethylbenzene concentrations as a group are in fact not visually different among the three exposure time points.
Group comparisons of VOC exposure
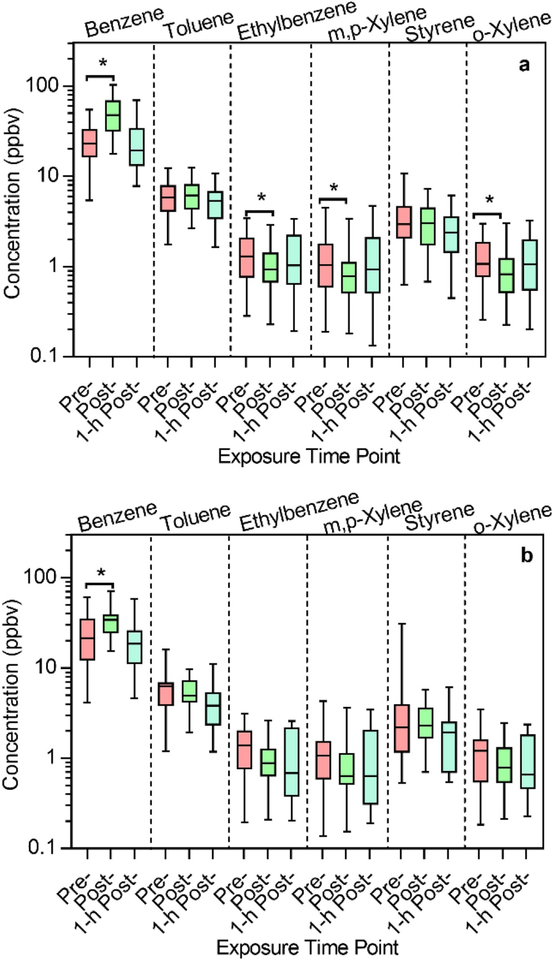
Box-and-whisker plots of the VOC concentrations for the 48 samples collected from firefighters who participated in attack and search and rescue job assignments during the exercises (Figure 2a, n = 48 samples) and the 24 samples collected from firefighters who participated in the outside ventilation job assignment (Figure 2b, n = 24) were constructed to visualize the spread of the data. Attack and search firefighters spent the most time inside the structure during the exercise and were required to wear SCBA inside the structure, while outside ventilation firefighters were in close proximity to the outside of the structure, and only some of these firefighters were on air. As previously seen in the statistical analysis for all 142 samples, benzene showed a significant increase in post-exposure concentrations compared to pre-exposure for the attack and search firefighters (p < 0.001) as well as outside ventilation firefighters (p = 0.0146), and as expected, no significant difference between pre- and 1-hr post-exposure data was detected.
Figure 2.
Exposure levels of targeted compounds measured in firefighters participating in (a) attack and search (n = 48 samples) and (b) outside ventilation (n = 24 samples) positions. *Indicates statistical significance based on two-tailed t-tests. Post-exposure benzene levels were significantly higher than pre-exposure for both Figure 2a and 2b, while post-exposure concentrations were significantly lower than pre-exposure for ethylbenzene, m,p-xylene, and o-xylene in Figure 2a.
As seen previously for all 142 samples combined, ethylbenzene showed a statistically significant decreased post-exposure concentration for attack and search firefighters according to a two-tailed t-test (p = 0.0422) but not for outside ventilation firefighters. Additionally, attack and search firefighters showed statistically significant decreases in post-exposure concentrations of m,pxylene and o-xylene compared to pre-exposure (Figure 2a), although these compounds did not have significant differences for all 142 samples (Table 1).
Evaluation of exposure responses at the individual level
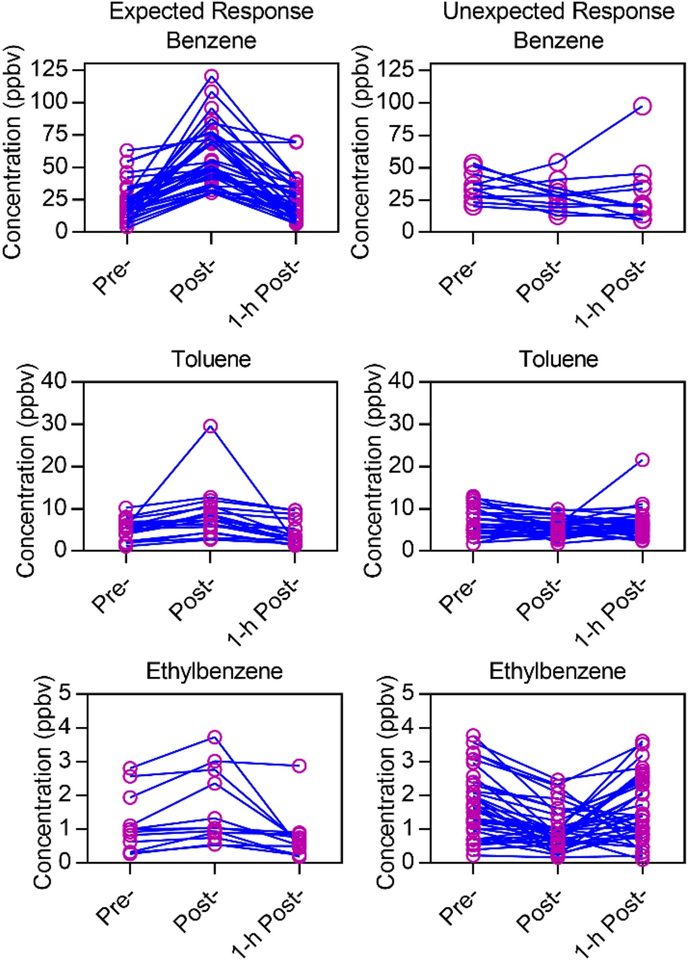
To determine individual variability of the firefighters as a result of VOC exposure, benzene, toluene, and ethylbenzene concentrations were matched across the three timepoints for firefighters who participated in attack and search positions (Figure 3) and outside ventilation positions (Figure 4). The matched VOC concentrations for individual firefighters were split into two categories: those with post-exposure VOC concentrations higher than both pre- and 1-hr postexposure concentrations (expected response) and those with decreased or unchanging post-exposure VOC concentrations (unexpected response)
Figure 3.
Individual responses to VOC exposure during firefighting activity for attack and search positions. Matched data for all individuals were separated into two groups: expected exposure response, and unexpected (random) response, as shown in two columns. Increased exposures were found for 36/48 samples for benzene, 24/48 samples for toluene, and 12/48 samples for ethylbenzene.
Figure 4.
Individual responses to VOC exposure during firefighting activity for outside ventilation positions. Matched data for all individuals were separated into two groups: expected exposure response, and unexpected (random) response, as shown in two columns. Increased exposures were found for 16/24 samples for benzene, 6/24 samples for toluene, and 7/24 samples for ethylbenzene.
Figures 3 and 4 show that not all firefighters experienced responses to firefighting exposure that followed the group trend. While 37 out of 48 attack and search samples showed increased benzene concentrations postexposure that decreased after 1 hr, 11 samples showed decreased post-exposure concentrations, and several samples had higher 1-hr post-exposure concentrations than pre- or post-exposure (Figure 3). For outside ventilation firefighters, only 16 out of 24 samples showed expected increased responses (Figure 4). While these firefighters— analyzed in their groups—had significantly increased post-exposure concentrations according to statistics (pvalue < 0.0001 for attack and search; p = 0.0146 for outside ventilation), these figures show that not all individuals responded to benzene exposure in the same way.
A lower percentage of the participants had the expected response for toluene and ethylbenzene. For attack and search, 18 and 12 samples out of 48 showed the expected increase in post-exposure concentrations of toluene and ethylbenzene, respectively (Figure 3). For outside ventilation, 6 and 7 samples out of 24 showed expected increases in toluene and ethylbenzene, respectively (Figure 4). Some firefighters exhibited unexpected decreased post-exposure concentrations that increased at 1-hr post-exposure. The observed changes in post- and 1-hr post-exposure concentrations may be due to metabolism of the VOCs or exposures experienced after the firefighters removed SCBA. Off-gassing of firefighting equipment could also contribute to the breath levels after firefighting.[31,32]
Discussion
Breath concentrations of VOCs were relatively high during these exercises compared to previous reports of firefighter exposure to VOCs in the literature. For example, in a previous study conducted using a similar experimental setup, geometric mean breath concentrations of benzene ranged from approximately 1.47–1.78 ppbv pre-exposure; 3.28–7.71 ppbv postexposure; and 1.69–4.15 ppbv 6-hr post-exposure.[4] The benzene concentrations observed in the current study were 10 times higher than these previously reported values (Table 1). Concentrations of other VOCs were also high compared to previous studies, although not as high as the observed benzene concentrations (e.g., approximately 2–3 fold higher for m,pxylene).[4] High breath VOC concentrations may be due to high levels of personal air concentrations of VOCs measured in the fire atmosphere in this study.[33]
Interestingly, the field blanks had correspondingly elevated concentrations of VOCs as well. This could be due to residual contamination from the apparatus bay at the training facility where samples were collected, or more likely indicate that the sorbent tube conditioning procedures were not as effective as in previous work. This can be accounted for as the target compounds were generally quantified at levels above those of the field blanks (Table 1). However, 1 field blank out of 20 appeared especially contaminated, showing high concentrations of benzene and toluene (e.g., 25.71 ppbv benzene; 78.99 ppbv toluene). The issue with background contamination is being addressed in current studies by revising the conditioning procedure and processing the sorbent tubes for 15 hr at 380 C. Therefore, the laboratory blanks were utilized for sample collection instead of the field blanks since the contamination was inconsistent. Under the assumption that all sorbent tubes contained approximately the same background contamination, differences between the pre-, post-, and 1-hr postexposure time points were used for data interpretation.
The attack and search firefighters’ exposures were assessed as a group because these firefighters had the presumed highest exposure according to personal air concentrations measured as a part of this study (range 12,000–322,000 ppb for attack and search combined).[33] Due to the requirements of firefighters in these positions to be in close proximity to the active fire during suppression, the exposures of the attack and search firefighters were hypothesized to be similar. Firefighters participating in outside ventilation had much lower personal air concentrations (<9–833 ppb), [33] and thus these firefighters were investigated in this study as a group with lower presumed exposure. Since outside ventilation firefighters spent more time outside of the structure, they likely experienced lower exposure to VOCs.
Additionally, this study showed that firefighters appear to be more prone to benzene uptake compared to toluene or ethylbenzene. This may be indicated by the results of the previous airborne contaminants manuscript from this study, which showed that there were much higher concentrations of benzene in the living room compared to toluene and ethylbenzene.[33] To perform their duties, firefighters working in attack and search positions were required to pass through the living room, which was accessible through the front door of the burn structure, adjacent to the bedrooms, and quickly filled with smoke after ignition.[33]
Some attack and search firefighters were reported to have potential inhalation exposure while working on the fireground prior to entering the structure (i.e., before donning SCBA, in addition to dermal exposure).[31,33] Some of the outside ventilation firefighters chose not to wear SCBA (to reduce the physiological burden imposed by the air packs) and may also have had inhalation exposure. The results presented in Figures 2a and 2b could suggest that brief inhalation exposure to the products of combustion caused a spike in benzene levels in breath (which subsequently returned to background around one hour later). It is also plausible that the firefighters absorbed VOCs through their skin; however, this would most likely happen in the attack and search firefighters who encountered the highest air concentrations while wearing SCBA.
The results in Figure 2a show that the attack and search firefighters experienced significant exposure to benzene during the controlled structure burns. In a previous publication of the room air concentrations of VOCs measured inside the structure, the concentrations of benzene (median = 14 ppm) were substantially higher than other VOCs (next highest was toluene at median = 0.064 ppm) and well above the NIOSH recommended exposure limit of 1 ppm.[33,34] Unlike benzene, air concentrations of ethylbenzene measured in the living room (median = 0.001 ppm) during the fire were extremely low.[33] These results may also suggest that the firefighters’ PPE, including SCBA, hood, and turn-out gear, provided a high enough level of protection against exposure to these VOCs that were present in environmental concentrations at least two orders of magnitude lower than benzene (next highest was toluene at median = 0.064 ppm) such that firefighters did not experience significant systemic exposure. The decreases in VOC concentration post-firefighting may be due to the requirement for the firefighters participating in attack and search positions to wear SCBA throughout the exercise. The clean air provided to the firefighters may have lowered the breath concentrations of these VOCs that may have been present in higher concentrations before the exercise due to residual exposures from other sources (e.g., firehouse, gasoline, engine exhaust, or second-hand tobacco smoke).[35–38]
Additionally, metabolism of benzene and the other VOCs during and immediately after firefighting could change the concentrations of compounds exhaled in breath and the systemic toxicity. Benzene is metabolized by cytochrome P4502E1, and one of the first metabolic products formed is phenol, which is further metabolized to hydroquinone and catechol, and then to p-benzoquinone, o-benzoquinone, and 1,2,4-trihydroxybenzene.[39,40] Urinary metabolites of benzene include phenolic metabolites conjugated with glucuronide or sulfate, mercapturic acids such as phenylmercapturic acid, ring-opened metabolites including trans-trans-Muconic acid as well as DNA adduct residues such as N7 -phenylguanine.[39] Benzene toxicity has been shown to occur through secondary metabolites such as hydroquinone, leading to bone marrow damage.[39,41] Toluene is metabolized to benzyl alcohol by CYP2E1 or CYP2B6 in the liver,[42] and is then oxidized to benzoic acid and conjugated with glycine to form hippuric acid, which is excreted in urine.[43] Another specific urinary metabolite of toluene is ocresol, which is formed at the same time.[7] Toluene can easily cross the blood-brain barrier or cause renal tubular acidosis.[43,44] Previous studies have shown that combined VOC exposures, including those of benzene and toluene, can suppress the metabolism of benzene to phenol and quinol as well as suppress the metabolism of toluene to hippuric acid and o-cresol. The combined exposures of the targeted compounds in this study as well as the non-targeted compounds that are present in the environment likely caused additional effects on VOC metabolism that are outside the scope of this study.[7,45]
Ethylbenzene is predominantly hydroxylated to form 1-phenylethanol by CYP2E1 in the liver, which is either conjugated to glucuronide and excreted in urine or oxidized to form acetophenone. Acetophenone, while minimally excreted in urine, is oxidized to form m-, p, and x-hydroxyacetophenone. In the predominant metabolic pathway, x-hydroxyacetophenone is a precursor for the three major urinary metabolites, mandelic acid, phenylglyoxylic acid, and benzoic acid (which is excreted as the glycine conjugate, hippuric acid).[44,46,47] Ethylbenzene absorption has been shown to be minimal through the skin, and more prevalent through inhalation.[46,48,49] Since many of the firefighters were wearing SCBA (particularly the attack and search firefighters), biological uptake of ethylbenzene which was present at low levels in the environment should have been greatly minimized. While ethylbenzene metabolites in urine were not monitored during this study, it could be that post-exposure concentrations were lower for this compound due to increased metabolism and clearance of this compound by CYP2E1. Monitoring urinary ethylbenzene metabolites, such as mandelic acid and phenylglyoxylic acid, could be of interest in future studies to confirm this hypothesis.
Conclusions
While assessing data in group form is convenient and useful for some evaluations, information about the individual can be lost or overlooked in these types of analyses. Group evaluations were shown to reveal overall post-exposure responses of firefighters, but information regarding the effects of particular firefighting positions and variability among individual firefighters remained hidden in the group statistics. For example, the grouped comparison of the firefighting positions showed that more firefighters who participated in attack and search positions experienced the expected post-exposure VOC increases compared to firefighters in outside ventilation positions. This is likely due to the closer proximity of attack and search firefighters to the active fire during the controlled structure burns, as these firefighters entered the structure while the outside ventilation firefighters remained outside. Thus, firefighters may want to rotate positions in live fire scenarios so that individuals do not consistently participate in positions with high expected exposure.
Additionally, assessment of firefighters’ responses to VOC exposure at the individual level revealed firefighters whose responses did not follow the expected group trend. For example, both attack and search firefighters and outside ventilation firefighters showed statistically significant increased concentrations of benzene in the post-exposure group compared to preexposure, but 12 attack and search samples and 8 outside ventilation samples did not show expected increased post-exposure concentrations of benzene at the individual level. Likewise, although the group statistics showed statistically significant decreased concentrations of ethylbenzene for attack and search firefighters, the assessment of changes for individual firefighters revealed that in 12 out of 48 personevents, the post-exposure concentrations of ethylbenzene increased instead. These deviations from the group trend for particular firefighters can be used to hypothesize why certain individuals may be more or less protected from VOC exposure during controlled structure burns. Factors such as the proximity of the firefighter to the active fire, effectiveness and use of SCBA and PPE, and other environmental exposures of the firefighter before and after the controlled structure burn, including the potential for off-gassing of VOCs from PPE may contribute to the observed differences in individual responses.
Evaluating VOC trends at both the group and individual level was found to be valuable for understanding human exposure to VOCs in controlled structure burns. The results of this study show that differences in the interpretation of exposure at the group and individual levels are important to consider when assessing biomonitoring results and risks of adverse health outcomes in other studies, as certain individuals may be more or less susceptible to exposure than others. Overall, breath levels of VOCs including toluene and ethylbenzene were found to be much lower in firefighters’ breath in this study compared to previous studies, indicating that the firefighter PPE and SCBA were adequately functioning to prevent exposure to these compounds, which were present in the environment at much lower concentrations than benzene. Future work will explore how workplace factors (e.g., tactics and use of SCBA) may affect the observed benzene breath levels among individual firefighters.
Acknowledgments
We acknowledge the volunteer subjects who provided breath samples and were compensated up to $599 to participate. Mention of trade names and commercial products does not constitute endorsement or recommendation for use. This research has been reviewed by EPA and approved for publication. The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official position of NIOSH, Centers for Disease Control and Prevention, or EPA.
Funding
NIOSH and the University of Illinois Institutional Review Boards approved this study, which was funded by the U.S. Department of Homeland Security Assistance to Firefighters Grant Fire Prevention & Safety program (EMW-2013-FP-00766). This study was also conducted through partnership with the CDC Foundation. Dr. Sibel Mentese is grateful for the travel grant received from TUBITAK.
References
- 1.Guidotti TL, and Clough VM: Occupational health concerns of firefighting. Ann. Rev. Publ. Health 13(1):151–171(1992). [DOI] [PubMed] [Google Scholar]
- 2.Tsai RJ, Luckhaupt SE, Schumacher P, Cress RD, Deapen DM, and Calvert GM: Risk of cancer among firefighters in California, 1988–2007. Am. J. Industr. Med 58(7):715–729 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford JO, Winski T, McElvenny D, Graveling R, and Dixon K: Firefighters and cancer: The epidemiological evidence. Instit. Occup. Med TM/17/01(2017). [Google Scholar]
- 4.Pleil JD, Stiegel MA, and Fent KW: Exploratory breath analyses for assessing toxic dermal exposures of firefighters during suppression of structural burns. J. Breath Res 8(3): 037107 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Fent KW, Eisenberg CJ, Evans D, et al. : Evaluation of dermal exposure to polycyclic aromatic hydrocarbons in fire fighters. Health Haz. Eval. Rep (2010–0156): 3196 (2013). [Google Scholar]
- 6.Adetona O, Simpson CD, Li Z, Sjodin A, Calafat AM, and Naeher LP: Hydroxylated polycyclic aromatic hydrocarbons as biomarkers of exposure to wood smoke in wildland firefighters. J. Expos. Sci. Environ. Epidemiol 27(1):78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinrich-Ramm R, Jakubowski M, Heinzow B, Christensen JM, Olsen E, and Hertel O: Biological monitoring for exposure to volatile organic compounds (VOCs)(IUPAC Recommendations 2000). Pure Appl. Chem 72(3):385–436(2000). [Google Scholar]
- 8.Fent KW, Eisenberg J, Snawder J, Stiegel MA et al. : Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann. Occup. Hyg 58(7):830–845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wingfors H, Nyholm JR, Magnusson R, and Wijkmark CH: Impact of fire suit ensembles on firefighter PAH Exposures as assessed by skin deposition and urinary biomarkers. Ann. Work Expos. Health (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fent KW, Alexander B, Roberts J, et al. : Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg 14(10):801–814 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Haick H, Broza YY, Mochalski P, Ruzsanyi V, and Amann A: Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev 43(5):1423–1449 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laitinen J, Mäkelä M, Mikkola J, and Huttu I: Firefighters’ multiple exposure assessments in practice. Toxicol. Lett 213(1):129–133 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Espiridion B, Chen M, Chang JY, et al. : Telomere length in peripheral blood leukocytes and lung cancer risk: a large case–control study in Caucasians. Cancer Res. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotander A, Kärrman A, Toms L-ML, Kay M, Mueller JF, and Gómez Ramos M.a.J.: Novel fluorinated surfactants tentatively identified in firefighters using liquid chromatography quadrupole time-of-flight tandem mass spectrometry and a case-control approach. Environ. Sci. Technol 49(4):2434–2442 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Rozalski R, Gackowski D, Siomek-Gorecka A, et al. : Urinary 5-hydroxymethyluracil and 8-oxo-7,8-dihydroguanine as potential biomarkers in patients with colorectal cancer. Biomarkers 20(5):287–291 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Pleil J, and Giese R: Integrating exhaled breath diagnostics by disease-sniffing dogs with instrumental laboratory analysis. J. Breath Res 11(3):032001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pleil JD, Stiegel MA, and Sobus JR: Breath biomarkers in environmental health science: Exploring patterns in the human exposome. J. Breath Res 5(4):046005 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Pleil JD: QQ-plots for assessing distributions of biomarker measurements and generating defensible summary statistics. J. Breath Res 10(3):035001 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Stiegel MA, Pleil JD, Sobus JR, Morgan MK, and Madden MC: Analysis of inflammatory cytokines in human blood, breath condensate, and urine using a multiplex immunoassay platform. Biomarkers 20(1):35–46 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Pleil JD, Sobus JR, Sheppard PR, Ridenour G, and Witten ML: Strategies for evaluating the environment-public health interaction of long-term latency disease: The quandary of the inconclusive case-control study. Chemico-biol. Interact 196(3):68–78 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Stiegel MA, Pleil JD, Sobus JR, Stevens T, and Madden MC: Linking physiological parameters to perturbations in the human exposome: Environmental exposures modify blood pressure and lung function via inflammatory cytokine pathway. J. Toxicol. Environ. Health, Part A 80(9):485–501 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fent KW, Evans DE, Babik K, et al. : Airborne contaminants during controlled residential fires. J. Occup. Environ. Hyg 15(5):399–412 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Horn GP, Kesler RM, Kerber S, et al. : Thermal response to firefighting activities in residential structure fires: Impact of job assignment and suppression tactic. Ergonomics 61(3):404–419 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Geer Wallace MA, Pleil JD, Mentese S, Oliver KD, Whitaker DA, and Fent KW: Calibration and performance of synchronous SIM/scan mode for simultaneous targeted and discovery (non-targeted) analysis of exhaled breath samples from firefighters. J. Chromatogr, A 1516(Supplement C):114–124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pleil JD: Imputing defensible values for left-censored ‘below level of quantitation’(LoQ) biomarker measurements. J. Breath Res 10(4):045001 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Bolden AL, Kwiatkowski CF, and Colborn T: New look at BTEX: Are ambient levels a problem? Environ. Sci. Technol 49(9):5261–5276 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Bernstein JA, Alexis N, Barnes C, et al. : Health effects of air pollution. J. Allerg. Clin. Immunol 114(5):1116–1123(2004). [DOI] [PubMed] [Google Scholar]
- 28.Stefanidou M, Athanaselis S, and Spiliopoulou C: Health impacts of fire smoke inhalation. Inhal. Toxicol 20(8):761–766 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Das B, and Resnick SI: QQ plots, random sets and data from a heavy tailed distribution. Stoch. Mod 24(1):103–132(2008). [Google Scholar]
- 30.Pleil JD, Sobus JR, Stiegel MA, et al. : Estimating common parameters of lognormally distributed environmental and biomonitoring data: Harmonizing disparate statistics from publications. J. Toxicol. Environ. Health, Part B 17(6):341–368 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Fent KW, Alexander B, Roberts J, et al. : Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg (just-accepted): 00-00 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Fent KW, Evans DE, Booher D, et al. : Volatile organic compounds off-gassing from firefighters’ personal protective equipment ensembles after use. J. Occup. Environ. Hyg 12(6):404–414 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Fent KW, Evans DE, Babik K, Striley C, Bertke S, Kerber S et al. : Airborne contaminants during controlled residential fires. Journal of occupational and environmental hygiene (just-accepted): 01–34 (2018). [DOI] [PubMed] [Google Scholar]
- 34.National Institute for Occupational Safety and Health: Pocket Guide to Chemical Hazards.” [Online] Available at http://www.cdc.gov/niosh/npg/
- 35.Baxter CS, Hoffman JD, Knipp MJ, Reponen T, and Haynes EN: Exposure of firefighters to particulates and polycyclic aromatic hydrocarbons. J. Occup. Environ. Hyg 11(7):D85–D91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira M, Slezakova K, Alves MJ, et al. : Polycyclic aromatic hydrocarbons at fire stations: Firefighters’ exposure monitoring and biomonitoring, and assessment of the contribution to total internal dose. J. Haz. Mater 323:184–194(2017). [DOI] [PubMed] [Google Scholar]
- 37.Jo W-K, and Song K-B: Exposure to volatile organic compounds for individuals with occupations associated with potential exposure to motor vehicle exhaust and/or gasoline vapor emissions. Sci. Total Environ 269(1):25–37 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Hakim M, Broza YY, Barash O, et al. : Volatile organic compounds of lung cancer and possible biochemical pathways. Chem. Rev 112(11):5949–5966 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Snyder R, and Hedli CC: An overview of benzene metabolism. Environ. Health Perspect 104(Suppl 6):1165 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaton MJ, Schlosser PM, Bond JA, and Medinsky MA: Benzene metabolism by human liver microsomes in relation to cytochrome P450 2E1 activity. Carcinogenesis 15(9):1799–1806 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Li J, Jiang S, Chen Y, et al. : Benzene metabolite hydroquinone induces apoptosis of bone marrow mononuclear cells through inhibition of β-catenin signaling. Toxicol. in Vitro 46:361–369 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Kim H, Wang R, Elovaara E, et al. : Cytochrome P450 isozymes responsible for the metabolism of toluene and styrene in human liver microsomes. Xenobiotica 27(7):657–665 (1997). [DOI] [PubMed] [Google Scholar]
- 43.Cruz SL, Rivera-García MT, and Woodward JJ: Review of toluene action: Clinical evidence, animal studies and molecular targets. J. Drug Alcohol Res 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camara-Lemarroy CR, Rodríguez-Gutiérrez R, Monreal-Robles R, and González-González JG: Acute toluene intoxication-clinical presentation, management and prognosis: A prospective observational study. BMC Emerg. Med 15:19 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue O, Seiji K, Watanabe T, et al. : Mutual metabolic suppression between benzene and toluene in man. Int. Arch. Occup. Environ. Health 60(1):15–20 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Sams C, Loizou GD, Cocker J, and Lennard MS: Metabolism of ethylbenzene by human liver microsomes and recombinant human cytochrome P450s (CYP). Toxicol. Lett 147(3):253–260 (2004). [DOI] [PubMed] [Google Scholar]
- 47.IARC: Ethylbenzene. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 77 (2000). [PMC free article] [PubMed] [Google Scholar]
- 48.Gromiec J, and Piotrowski J: Urinary mandelic acid as an exposure test for ethylbenzene. Int. Arch. Occup. Environ. Health 55(1):61–72 (1984). [DOI] [PubMed] [Google Scholar]
- 49.Fishbein L: An overview of environmental and toxicological aspects of aromatic hydrocarbons IV. Ethylbenzene. Sci. Total Environ 44(3):269–287 (1985). [DOI] [PubMed] [Google Scholar]