Abstract
Background:
There are few studies evaluating regional disparities in the care of acute myocardial infarction-cardiogenic shock (AMI-CS).
Methods and Results:
Using the National Inpatient Sample from 2000–2016, we identified adults with a primary diagnosis of AMI and concomitant CS admitted to the United States census regions of Northeast, Midwest, South and West. Inter-hospital transfers were excluded. End-points of interest included in-hospital mortality, use of coronary angiography, percutaneous coronary intervention (PCI), mechanical circulatory support (MCS), hospitalization costs, length of stay and discharge disposition. Multivariable regression was used to adjust for potential confounding. Of the 402,825 AMI-CS admissions, 16.8%, 22.5%, 39.3% and 21.4% were admitted to the Northeast, Midwest, South and West respectively. Higher rates of ST-elevation AMI-CS were noted in the Midwest and West. Admissions to the Northeast were on average characterized by a higher frequency of Whites, Medicare beneficiaries and lower rates of cardiac arrest. Admissions to the Northeast were less likely to receive coronary angiography, PCI and MCS, despite the highest rates of extracorporeal membrane oxygenation use. Compared to the Northeast, in-hospital mortality was lower in the Midwest (adjusted odds ratio [aOR] 0.96 [95% confidence interval (CI) 0.93–0.98]; p<0.001) and West (aOR 0.96 [95% CI 0.94–0.98]; p=0.001), but higher in the South (aOR 1.04 [95% CI 1.01–1.06]; p=0.002). The Midwest (aOR 1.68 [95% CI 1.62–1.74]; p<0.001), South (aOR 1.86 [95% CI 1.80–1.92]; p<0.001) and West (aOR 1.93 [95% CI 1.86–2.00]; p<0.001) had higher discharges to home.
Conclusions:
There remain significant regional disparities in the management and outcomes of AMI-CS.
Keywords: Cardiogenic shock, acute myocardial infarction, health disparities, mechanical circulatory support, percutaneous coronary intervention
INTRODUCTION
In the contemporary era, acute myocardial infarction (AMI) with cardiogenic shock (CS) is associated with high mortality and morbidity.1–14 Despite widespread adoption of quality measures and early revascularization, significant local and regional variations continue to exist in the management and outcomes of AMI.15, 16 Geographical variations in the treatment and outcome of patients with AMI, independent of patient comorbidity and acuity, have been previously reported.17–20 Studies in AMI patients have shown a higher use of medical therapy, lower use of cardiac procedures and lower mortality in the Northeast United States compared to other regions.18, 21–25 Contemporary data has shown higher mortality in the Midwest, South and West regions of the United States compared to the Northeast in patients with ST-elevation myocardial infarction (STEMI) independent of patient and hospital characteristics.18 It is conceivable that similar disparities exist in patients with AMI-CS that encompass the sickest spectrum of AMI; however there are limited data on this subject.26 In light of the recent calls for regionalization of CS care,15, 16 it is crucial to understand if regional disparities exist in AMI-CS care to aid in promoting equitable care to this sick population. Prior studies have evaluated either STEMI or non-ST-elevation myocardial infarction (NSTEMI) etiologies of CS; however in this study we sought to include both STEMI-CS and NSTEMI-CS given the increasing unique comorbidities and acute organ failure.4–9 Using a 17-year nationally-representative database we sought to assess the variation in incidence, temporal trends and outcomes of AMI-CS across the different regions in the United States. We hypothesized that during this 17-year study period there would remain significant disparities in AMI-CS care across regions independent of patient and hospital factors.
MATERIAL AND METHODS
The data are publicly available with the Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project (HCUP) for other researchers to replicate the results of this study.27 Institutional Review Board approval was not sought due to the publicly available nature of the de-identified data. The National (Nationwide) Inpatient Sample (NIS) is the largest all-payer database of hospital inpatient stays in the United States. The NIS contains discharge data from a 20% stratified sample of community hospitals and is a part of the HCUP, sponsored by the Agency for Healthcare Research and Quality.27 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 secondary diagnoses, and procedural diagnoses. The HCUP-NIS does not capture the longitudinal care of individual patients, but captures all information for a given admission / hospitalization.
Using the HCUP-NIS data from 2000–2016, a retrospective cohort study of admissions with AMI-CS were identified. AMI in the primary diagnosis field were identified using codes for STEMI and non-ST-elevation myocardial infarction (NSTEMI) (International Classification of Diseases-9 Clinical Modification [ICD-9CM] 410.x; ICD-10-CM I21.x, I22.2) and a secondary diagnosis of CS (ICD-9CM 785.51; ICD-10-CM R57.0). Admissions transferred from other acute care hospitals were excluded since this might result in misclassification of regional outcomes. The four geographic regions included the Northeast, Midwest, South and West as classified by the HCUP-NIS (Figure 1).18 Coronary angiography, percutaneous coronary intervention (PCI), temporary mechanical circulatory support (MCS) devices (intra-aortic balloon pump [IABP], percutaneous left ventricular assist device [pLVAD] and extra-corporeal membrane oxygenation [ECMO] use were identified for all admissions using previously used methods by our group.1, 2, 4–9, 12–14, 28, 29 The Deyo’s modification of the Charlson Comorbidity Index was used to identify the burden of co-morbid conditions (Supplementary Table 1).30
Figure 1.
United States geographic regions demonstrating states included in census regions as defined by the Healthcare Cost and Utilization Project-National Inpatient Sample
The primary outcome was in-hospital mortality stratified by geographic region. Secondary outcomes included temporal trends in the prevalence and in-hospital mortality of AMI-CS stratified by geographic region, hospital length of stay, hospitalization costs, use of coronary angiography, PCI and MCS stratified by geographic regions.
Statistical Analysis
As recommended by HCUP-NIS, survey procedures using discharge weights provided with HCUP-NIS database were used to generate national estimates. Using the trend weights provided by the HCUP-NIS, samples from 2000–2011 were re-weighted to adjust for the 2012 HCUP-NIS re-design.31 In 2012, the HCUP-NIS was re-designed to sample 20% of the national patient-level sample as compared to 2000–2011 wherein it sampled 100% of the discharges from 20% of the hospitals.31 Using trend weights available on the HCUP-NIS database, samples from 2000–2011 were retroactively re-weighted. The new sampling strategy is expected to result in more precise estimates than the previous HCUP-NIS design by reducing sampling error.27 This methodology has been used by multiple prior studies spanning across year 2012 from the HCUP-NIS.1, 2, 4–9, 12–14, 28 One-way analysis of variance and t-tests were used to compare categorical and continuous variables respectively. The inherent restrictions of the HCUP-NIS database related to research design, data interpretation, and data analysis were reviewed and addressed.31 Pertinent considerations include not assessing individual hospital-level volumes (due to changes to sampling design detailed above), treating each entry as an ‘admission’ as opposed to individual patients, restricting the study details to inpatient factors since the HCUP-NIS does not include outpatient data, and limiting administrative codes to those previously validated and used for similar studies.1, 2, 4–9, 12–14, 28 Adjusted temporal trends for prevalence and in-hospital mortality were calculated using multivariable logistic regression analysis with each year compared to the referent year 2000. Models were adjusted for age sex, race, primary payer, socioeconomic status, hospital location and teaching status, hospital bedsize, comorbidities, cardiac arrest, coronary angiography, PCI, invasive hemodynamic assessment, MCS, invasive ventilation and hemodialysis. In addition, overall odds of all-cause in-hospital mortality by region (Northeast, Midwest, South, and West) were examined using multivariable logistic regression; year of diagnosis was included in the model in addition to all of the variables previously noted. For the multivariable modeling, regression analysis with purposeful selection of statistically (p<0.20) and clinically relevant variables was conducted. Two-tailed p<0.05 was considered statistically significant. A sensitivity analysis was performed to evaluate the clinical outcomes in AMI-CS stratified by the presence of cardiac arrest and by including all AMI-CS admissions (i.e. including those that were transferred). Given the large sample size, all p-values that are statistically significant may not be clinically significant and therefore need careful clinical interpretation. All statistical analyses were performed using SPSS v25.0 (IBM Corp, Armonk NY).
RESULTS
There were 10,866,759 admissions for a primary diagnosis of AMI between January 1, 2000 and December 31, 2016, of which 402,825 (3.7%) constituted non-transferred AMI admissions with concomitant CS. A higher percentage of admissions from the Northeast (27.8%) compared to other regions (Midwest 22.3%, South 20.1% and West 17.2%) (p<0.001) constituted inter-hospital transfers and were excluded. The Northeast, Midwest, South and West had 4348, 4690, 4530 and 4709 admissions with CS per 100,000 AMI admissions during this study period (p<0.001). The Northeast, Midwest, South and West had 16.8%, 22.5%, 39.3% and 21.4% of the total admissions, respectively. The 17-year unadjusted and adjusted (referent year 2000) temporal trends of admissions are presented in Figures 2A and 2B. There was an increase in the proportion of CS admissions in the overall AMI population across all geographic regions over time. The West had a higher percentage of AMI-CS amongst all regions in the unadjusted analysis, but was similar to other regions in the adjusted analysis (Supplementary Table 2). As seen in Table 1, higher rates of STEMI admissions were noted in the Midwest and West. The Midwest and South had a higher proportion of admissions to rural hospitals and the Northeast had a greater proportion of admissions to small-sized hospitals (Table 1).
Figure 2. Unadjusted and adjusted 17-year temporal trends for proportion of acute myocardial infarction admissions with cardiogenic shock by geographic regions.
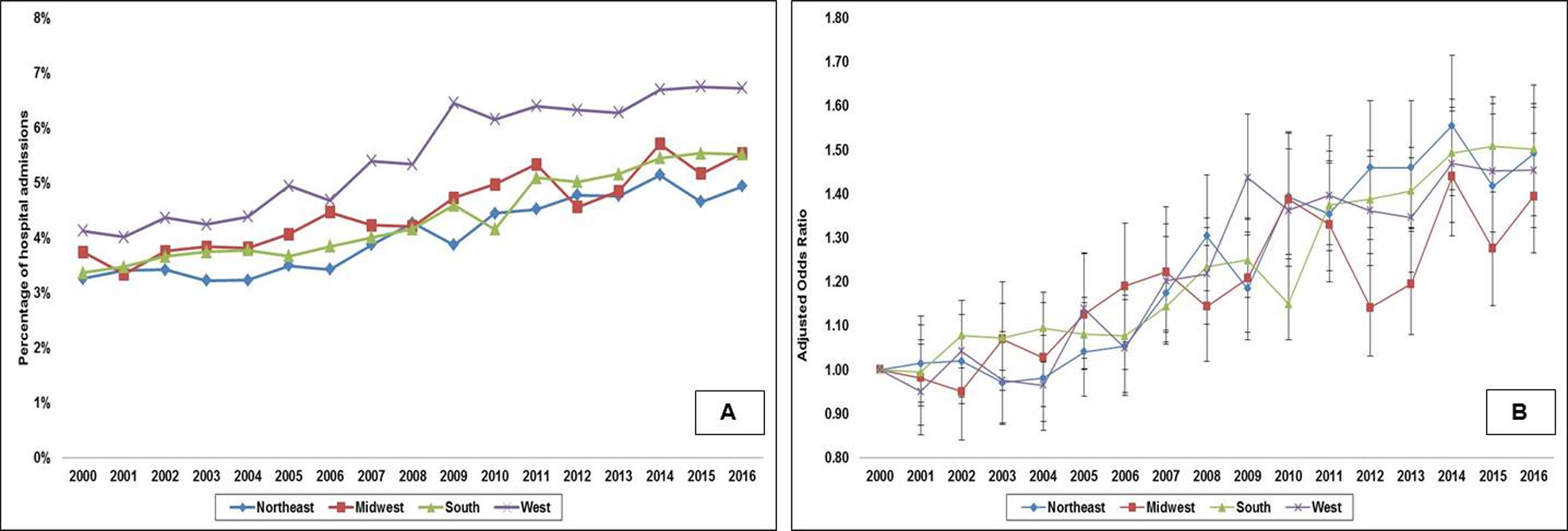
2A: Unadjusted temporal trends of proportion of acute myocardial infarction admissions with cardiogenic shock; all p<0.001 for trend; 2B: Adjusted multivariate logistic regression for temporal trends of proportion of acute myocardial infarction admissions with cardiogenic shock with 2000 as referent year; adjusted for age, sex, race, primary payer, socio-economic status, hospital location/teaching status, hospital bedsize, and comorbidity; all p<0.001 for trend
Table 1.
Baseline characteristics of acute myocardial infarction-cardiogenic shock stratified by geographic region
| Characteristic | Northeast (N=67,638) |
Midwest (N=90,614) |
South (N=158,112) |
West (N=86,461) |
P |
|---|---|---|---|---|---|
| Age (years) | 71.8±13.2 | 69.6±13.3 | 68.6±13.0 | 69.7±12.9 | <0.001 |
| Female sex | 42.6 | 40.4 | 38.8 | 36.6 | <0.001 |
| Race | <0.001 | ||||
| White | 79.0 | 55.6 | 66.0 | 58.6 | |
| Black | 6.0 | 5.3 | 9.2 | 3.1 | |
| Others* | 15.0 | 39.1 | 24.8 | 38.3 | |
| Primary payer | <0.001 | ||||
| Medicare | 67.6 | 63.4 | 61.4 | 58.4 | |
| Medicaid | 6.2 | 5.9 | 5.5 | 9.4 | |
| Private | 21.8 | 24.7 | 22.7 | 24.3 | |
| Uninsured | 2.9 | 4.1 | 6.8 | 4.4 | |
| No charge | 0.3 | 0.1 | 0.7 | 0.2 | |
| Others | 1.2 | 1.8 | 2.9 | 3.2 | |
| Hospital teaching status and location | <0.001 | ||||
| Rural | 6.4 | 10.3 | 9.7 | 4.0 | |
| Urban non-teaching | 29.2 | 34.2 | 44.6 | 58.8 | |
| Urban teaching | 64.4 | 55.6 | 45.7 | 37.2 | |
| Hospital bed-size | <0.001 | ||||
| Small | 12.4 | 10.4 | 7.2 | 6.9 | |
| Medium | 28.3 | 22.0 | 24.1 | 23.3 | |
| Large | 59.3 | 67.7 | 68.7 | 69.7 | |
| Charlson Comorbidity Index | <0.001 | ||||
| 0–3 | 20.6 | 24.2 | 25.6 | 23.2 | |
| 4–6 | 56.4 | 53.5 | 54.1 | 53.0 | |
| ≥ 7 | 23.0 | 22.2 | 20.2 | 23.8 | |
| AMI type | <0.001 | ||||
| ST-segment elevation | 64.4 | 70.3 | 67.2 | 69.0 | |
| Non-ST segment elevation | 35.6 | 29.7 | 32.8 | 31.0 | |
| Out of hospital cardiac arrest | 23.0 | 26.8 | 26.5 | 27.8 | <0.001 |
| Coronary angiography | 61.0 | 75.7 | 73.7 | 73.6 | <0.001 |
| Percutaneous coronary intervention | 40.1 | 53.1 | 49.1 | 50.6 | <0.001 |
| Coronary artery bypass grafting | 11.6 | 15.4 | 16.0 | 15.2 | <0.001 |
| Pulmonary artery / right heart catheterization | 22.2 | 17.0 | 14.8 | 21.2 | <0.001 |
| Mechanical circulatory support | 40.4 | 47.3 | 42.9 | 43.8 | <0.001 |
Represented as percentage or mean ± standard deviation;
Hispanic, Asian, Native American, Others
Admissions with AMI-CS to the Northeast were less likely to receive coronary angiography, PCI and MCS (Table 1). The 17-year temporal trends in the use of coronary angiography, PCI and MCS are presented in Figures 3A–3F. There was an overall increase in the use of coronary angiography and PCI during the study period across all regions. There was a trend towards a decrease in MCS use between 2008 and 2014 that was primarily due to a decrease in IABP use. The Northeast had the highest use of ECMO during 2008–2014, which was significantly higher than other regions; however they had lower rates of overall MCS use. There were significant disparities in the use of invasive hemodynamic monitoring (right heart or pulmonary artery catheterization) between regions in the population that received MCS – Northeast 54.9%, Midwest 35.9%, South 34.5% and West 48.4%.
Figure 3. 17-year temporal trends in cardiac procedures in acute myocardial infarction-cardiogenic shock by geographic regions.
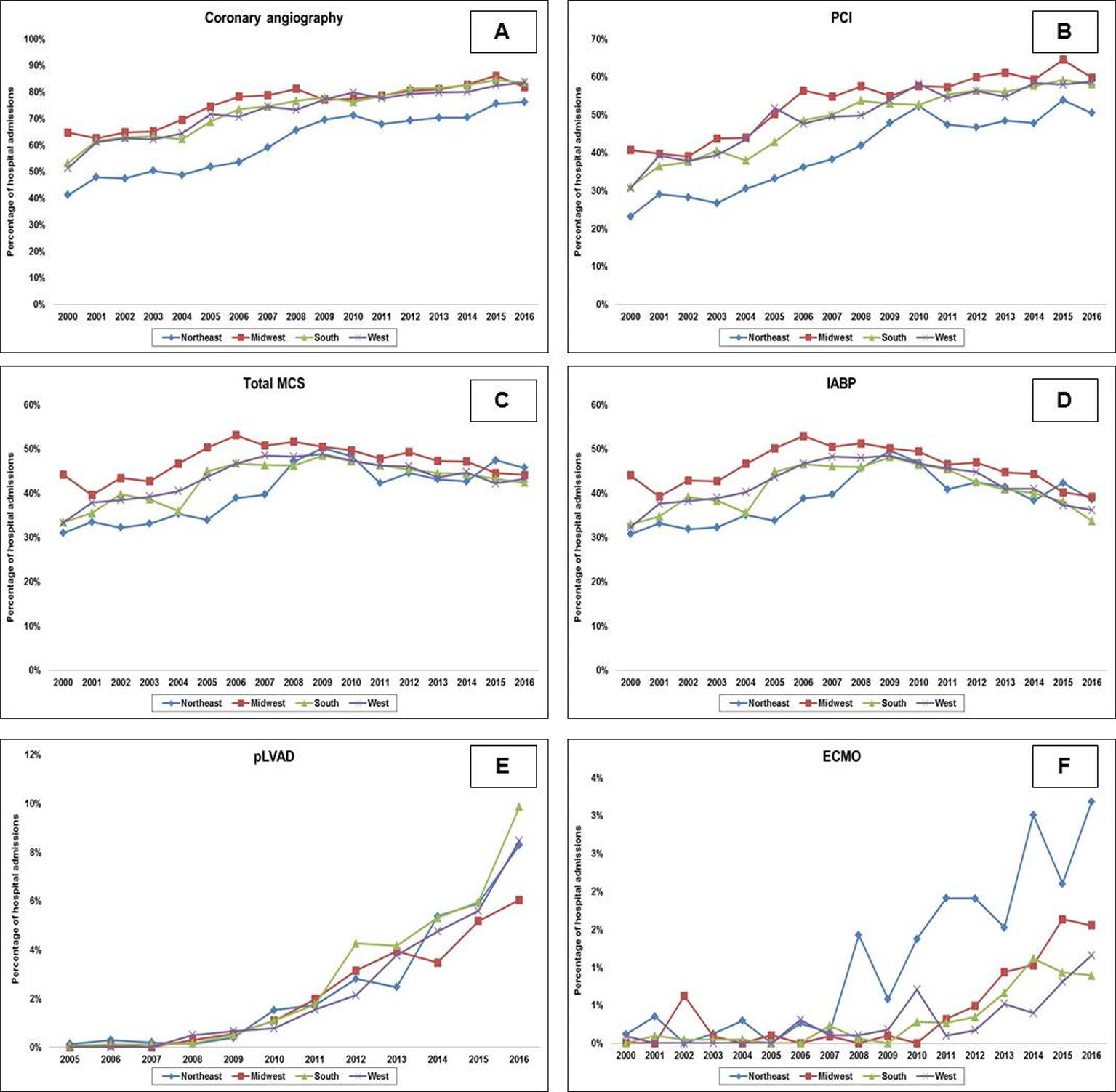
Seventeen-year trends of coronary angiography (3A), PCI (3B), total MCS (3C), IABP (3D), pLVAD* (3E) and ECMO (3F) in acute myocardial infarction-cardiogenic shock stratified by geographic regions; all p<0.001 for trend
*The administrative codes for pLVAD were introduced in 2004, and therefore temporal trends are presented from 2005 onwards
Abbreviations: ECMO: extra-corporeal membrane oxygenation; IABP: intra-aortic balloon pump; MCS: mechanical circulatory support; PCI: percutaneous coronary intervention; pLVAD: percutaneous left ventricular assist device
The in-hospital mortality in the total cohort was 38.9%. As noted in Table 2, unadjusted mortality was higher in the Northeast (42.4%) vs. the other region (38–39%). The 17-year unadjusted and adjusted (referent year 2000) temporal trends of in-hospital mortality stratified by geographic regions are presented in Figure 4. In a multivariable logistic regression analysis for in-hospital mortality with the Northeast region as a reference category, in-hospital mortality was lower in the Midwest (odds ratio [OR] 0.96 [95% confidence interval (CI) 0.93–0.98]; p<0.001) and West (OR 0.96 [95% CI 0.94–0.98]; p=0.001), but higher in the South (OR 1.04 [95% CI 1.01–1.06]; p=0.002) (Table 3). Among the hospital survivors, 28.3% of the admissions in the Northeast were discharged home as compared to 42–47% in the other regions (Table 2). In a multivariable logistic regression analysis incorporating all variables from Table 3, the Midwest (OR 1.68 [95% CI 1.62–1.74]; p<0.001), the South (OR 1.86 [95% CI 1.80–1.92]; p<0.001) and the West (OR 1.93 [95% CI 1.86–2.00]; p<0.001) were all associated with greater discharges to home compared to the Northeast.
Table 2.
Clinical outcomes of acute myocardial infarction-cardiogenic shock stratified by geographic region
| Outcome | Northeast (N=67,638) |
Midwest (N=90,614) |
South (N=158,112) |
West (N=86,461) |
P | |
|---|---|---|---|---|---|---|
| In-hospital mortality | 42.4 | 37.4 | 38.5 | 38.2 | <0.001 | |
| Length of stay (days) | 7 (3–12) | 6 (3–11) | 7 (3–12) | 6 (3–12) | <0.001 | |
| Hospitalization costs (x 1000 United States Dollars) | 76 (32–161) | 77 (42–136) | 88 (43–161) | 126 (64–236) | <0.001 | |
| Discharge disposition | ||||||
| Home | 28.3 | 41.7 | 46.7 | 44.6 | <0.001 | |
| Transferred to other hospitals | 18.9 | 10.5 | 10.4 | 15.0 | ||
| Skilled nursing facility | 33.6 | 30.9 | 27.0 | 25.8 | ||
| Home with home health care | 18.9 | 16.5 | 15.3 | 13.9 | ||
| Against medical advice | 0.2 | 0.3 | 0.5 | 0.7 | ||
Represented as percentage or median (interquartile range)
Figure 4. Unadjusted and adjusted 17-year temporal trends for in-hospital mortality in acute myocardial infarction-cardiogenic shock by geographic regions.
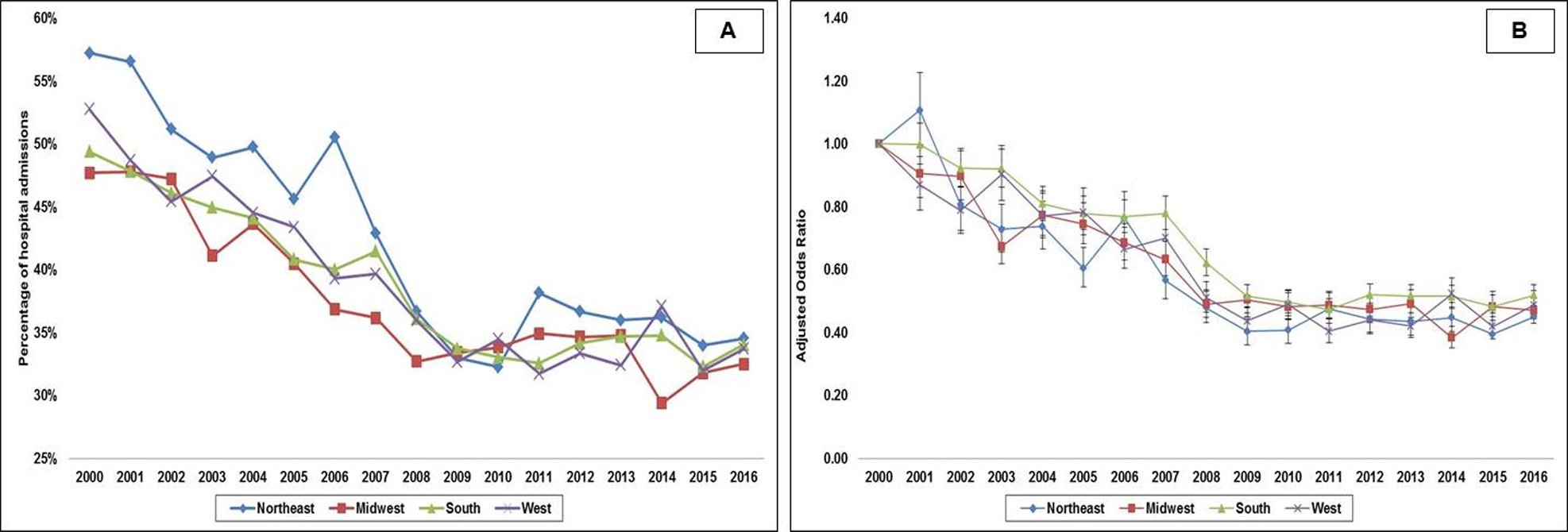
4A: Unadjusted temporal trends of in-hospital mortality in acute myocardial infarction-cardiogenic shock; all p<0.001 for trend; 4B: Adjusted multivariate logistic regression for in-hospital mortality temporal trends in acute myocardial infarction-cardiogenic shock with 2000 as referent year; adjusted for age, sex, race, primary payer, socio-economic status, hospital location/teaching status, hospital bedsize, comorbidity, acute organ failure, cardiac arrest, coronary angiography, percutaneous coronary intervention, invasive hemodynamic monitoring, mechanical circulatory support, invasive mechanical ventilation, hemodialysis; all p<0.001 for trend
Table 3.
Multivariable regression for in-hospital mortality in acute myocardial infarction-cardiogenic shock
| Total cohort (N = 402,290) |
Odds ratio | 95% confidence interval | P | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Hospital region | ||||
| Northeast | Reference category | |||
| Midwest | 0.96 | 0.93 | 0.98 | <0.001 |
| South | 1.04 | 1.01 | 1.06 | 0.002 |
| West | 0.96 | 0.94 | 0.98 | 0.001 |
| Age groups (years) | ||||
| 19–49 | Reference category | |||
| 50–59 | 1.28 | 1.23 | 1.33 | <0.001 |
| 60–69 | 1.87 | 1.79 | 1.94 | <0.001 |
| 70–79 | 3.01 | 2.87 | 3.15 | <0.001 |
| ≥80 | 4.78 | 4.57 | 5.01 | <0.001 |
| Female sex | 1.11 | 1.10 | 1.13 | <0.001 |
| Race | ||||
| White | Reference category | |||
| Black | 1.01 | 0.98 | 1.04 | 0.52 |
| Others | 1.07 | 1.05 | 1.09 | <0.001 |
| Primary payer | ||||
| Medicare | Reference category | |||
| Medicaid | 0.91 | 0.88 | 0.95 | <0.001 |
| Private | 0.76 | 0.74 | 0.78 | <0.001 |
| Uninsured | 1.33 | 1.28 | 1.39 | <0.001 |
| No Charge | 0.83 | 0.73 | 0.94 | 0.004 |
| Others | 0.89 | 0.85 | 0.94 | <0.001 |
| Quartile of median household income for zip code | ||||
| 0–25th | Reference category | |||
| 26th–50th | 0.96 | 0.94 | 0.98 | <0.001 |
| 51st–75th | 0.93 | 0.91 | 0.95 | <0.001 |
| 75th–100th | 0.89 | 0.87 | 0.91 | <0.001 |
| Charlson Comorbidity Index | ||||
| 0–3 | Reference category | |||
| 4–6 | 0.85 | 0.82 | 0.87 | <0.001 |
| ≥ 7 | 0.76 | 0.74 | 0.79 | <0.001 |
| Year | ||||
| 2000 | Reference category | |||
| 2001 | 0.99 | 0.95 | 1.03 | 0.50 |
| 2002 | 0.88 | 0.84 | 0.92 | <0.001 |
| 2003 | 0.80 | 0.76 | 0.83 | <0.001 |
| 2004 | 0.77 | 0.73 | 0.80 | <0.001 |
| 2005 | 0.73 | 0.70 | 0.76 | <0.001 |
| 2006 | 0.72 | 0.69 | 0.75 | <0.001 |
| 2007 | 0.68 | 0.65 | 0.71 | <0.001 |
| 2008 | 0.53 | 0.51 | 0.56 | <0.001 |
| 2009 | 0.47 | 0.45 | 0.49 | <0.001 |
| 2010 | 0.47 | 0.45 | 0.49 | <0.001 |
| 2011 | 0.46 | 0.44 | 0.48 | <0.001 |
| 2012 | 0.48 | 0.46 | 0.50 | <0.001 |
| 2013 | 0.47 | 0.45 | 0.49 | <0.001 |
| 2014 | 0.47 | 0.46 | 0.50 | <0.001 |
| 2015 | 0.46 | 0.44 | 0.48 | <0.001 |
| 2016 | 0.45 | 0.43 | 0.47 | <0.001 |
| Acute organ dysfunction | ||||
| Respiratory | 1.13 | 1.12 | 1.15 | <0.001 |
| Renal | 1.43 | 1.40 | 1.45 | <0.001 |
| Hepatic | 1.42 | 1.38 | 1.46 | <0.001 |
| Hematologic | 0.87 | 0.85 | 0.89 | <0.001 |
| Neurologic | 1.42 | 1.39 | 1.46 | <0.001 |
| Out of hospital cardiac arrest | 2.48 | 2.44 | 2.53 | <0.001 |
| Coronary angiography | 0.33 | 0.32 | 0.34 | <0.001 |
| Percutaneous coronary intervention | 0.86 | 0.84 | 0.88 | <0.001 |
| Pulmonary artery / right heart catheterization | 1.01 | 0.99 | 1.03 | 0.19 |
| Mechanical circulatory support | 1.24 | 1.21 | 1.26 | <0.001 |
| Invasive mechanical ventilation | 1.84 | 1.81 | 1.87 | <0.001 |
| Hemodialysis | 1.24 | 1.19 | 1.29 | <0.001 |
In a sensitivity analysis stratifying for the presence of cardiac arrest, unadjusted in-hospital mortality remained the highest in the Northeast (AMI-CS without cardiac arrest 38.9%; AMI-CS with cardiac arrest 53.9%) as compared to the Midwest (33.0%; 49.3%), South (33.3%; 53.0%) and West (32.7%; 52.6%), respectively (p<0.001). In multivariable logistic regression analysis (Table 3), these regional differences were consistent even after adjusting for cardiac arrest. The in-hospital mortality was consistent with the primary results even if admissions that were transferred were included (Northeast 40.4%, Midwest 36.5%, South 37.7% and West 36.8%).
DISCUSSION
In this large study using a nationally-representative database, we noted that there remains significant geographic heterogeneity in the management and outcomes of AMI-CS across the United States. There was a temporal increase in the prevalence of CS among AMI admissions with a concomitant decrease in overall in-hospital mortality across all geographic regions. Admissions with AMI-CS to the Northeast were less likely to receive coronary angiography, PCI and overall MCS implantation, despite a significant higher use of ECMO in recent years. Adjusted in-hospital mortality was significantly lower in the West and Midwest, but higher in the South compared to the Northeast. Only 28% of the admissions in the Northeast were discharged home as compared to 42–47% in the other regions.
There remain significant disparities across various geographic regions in the management of AMI.18 Consistent with these data, we note important differences in AMI-CS management and outcomes across the four major geographic regions. Prior STEMI-CS studies have noted a lower prevalence of CS in the Northeast without adjusting for potential confounding variables.18 Though the unadjusted analysis in this study did support differences across the geographic regions, we found no such differences after adjusting for differences in care and clinical characteristics. The comparatively lower use of cardiac procedures in the Northeast region we found in our study is consistent with prior studies in patients with AMI.21, 22, 24, 25 Our data are consistent with the real-world literature that reflect reluctance to perform angiography in this sick cohort despite robust guideline recommendations.32 Though Kolte et al. previously demonstrated a ‘regional paradox’ in STEMI patients (i.e. use of angiography was associated with worse outcomes),18 this particular study noted that higher use of angiography and PCI was associated with lower in AMI-CS mortality. In addition to angiography and PCI, this study provides incremental information on the geographic variation in MCS use in AMI-CS. There has been a widespread interest in the use of MCS for AMI-CS in recent years.1–3, 10–12, 33 Given the neutral data on the use of IABP in AMI-CS, there has been a plateau in MCS use that reflects decreased use of IABP in recent years.1, 2, 7 In 2014, the Midwest had the highest use of IABP and lowest use of a pLVAD. There was a notable uptake across all geographic regions for pLVAD and ECMO in AMI-CS with significant differences between regions. These data are consistent with literature from CS in non-AMI patients that demonstrate significant geographic variations in MCS use.1, 2 It is conceivable that local protocols for the management of AMI-CS care may provide impetus for the use of these devices, however these systematic regional differences are worthy of further study.16 Despite the increase in the MCS use in this population, there remain significant regional disparities in the use of invasive hemodynamic monitoring in the admissions receiving MCS suggestive of incomplete management of AMI-CS. Importantly, given the inherent limitations of the HCUP-NIS database, the lower associated in-hospital mortality from the use of angiography and PCI and higher mortality associated with MCS use should be perceived as trends and estimates, given the inability to control for all types of confounding, and need further validation in carefully controlled prospective trials.1, 2
Despite prior studies showing that the Northeast (i.e. the New England region) has lower mortality in AMI patients overall, in this group of AMI admissions with CS, we noted lower mortality in the West and Midwest compared to the Northeast.18 These differences can potentially be explained by, (a) The differences in time frame of the prior studies and the inclusion of a larger and more contemporary cohort in this study; (b) differences in inclusion criteria, i.e. STEMI vs. all AMI patients – prior data have shown that STEMI mortality is lower in the Northeast, however STEMI patients are typically younger and with lower comorbidities that patients with NSTEMI-CS; (c) we excluded inter-hospital transfers that might cross geographic regions for access to a higher level of care; and (d) differences in organization of services and access to complex interventions.16 Additionally, the geographic variations in the outcomes of AMI-CS may be explained by variation in the prevalence of coronary artery disease, a disproportionate increase of cardiovascular risk factors such as obesity and diabetes across certain geographic regions, presence of local multidisciplinary systems of care and the development of geographic hub-and-spoke models to facilitate rapid escalation of care.15, 16, 18 Though there was greater use of cardiac procedures which significantly contribute to total cost of hospitalization in the Midwest and South compared to Northeast, the median hospitalization costs were higher in Northeast compared to those regions. This could be attributed to the increased length of stay and less frequent discharges to home in Northeast compared to other regions which allude to a potentially sicker population. The highest hospitalization costs were in the West, which could be due to the variation in wage index and regional policies in acute care hospitals of the West compared to other regions.34 In addition to the hospital-level and regional disparities in AMI-CS, our multivariable analysis was consistent with prior studies in STEMI population that note insurance-based disparities.35 In our analyses we note admissions with private insurance to have lower mortality than the Medicare population, and these are worthy of further analyses in dedicated studies. Lastly, it is pertinent to note that the most recent societal guidelines on the diagnosis, classification and management of CS were published towards the end of the study period that might partly explain the noted heterogeneity of care.15
In summary, we continued to note geographic disparities in the management of AMI-CS. This study is incremental to prior work that notes a volume-outcome relationship in CS and hospital-level variation in the management of AMI-CS.4, 26 The continued heterogeneity of AMI-CS outcomes may be largely attributed to the differences in system-based practices that include early revascularization, identification and prevention of complications, management of acute organ failure and utilization of multidisciplinary teams.16 Taken in aggregate, our study and similar data from other CS studies, support the need for regionalization of CS care.15, 16 In addition to high costs, these patients typically require multi-disciplinary care and intensive care resources, all of which are cost prohibitive in smaller centers and rural hospitals.16 States such as Arizona and Washington and more recently Georgia have developed acute cardiac care centers analogous to trauma care,16 and it is likely that similar paradigms are required at the state and national levels across the United States.
Limitations
This study has several limitations, some of which are inherent to the analysis of a large administrative database. Coding errors, misrepresentation of procedural volumes and underreporting of comorbidities are potential limitations of using administrative codes. The HCUP-NIS attempts to mitigate potential errors by using internal and external quality control measures. The administrative codes for STEMI, NSTEMI and CS have been previously validated that reduces the inherent errors in the study. This was further strengthened by excluding secondary diagnosis of AMI and inter-hospital transfers. However, it is important to note that the prompt recognition and management of CS remains challenging and may have influenced the results of this study.8, 36 It is important to note that patients who are transferred between hospitals may significantly differ from those that are not.37, 38 Prior work in AMI and critical illness has shown that younger patients with lower comorbidities are transferred more frequently.37, 38 It is possible that the patients who were transferred and thus excluded from our study may have presented to non-PCI capable centers. Therefore, our data are largely applicable to centers with on-site PCI capabilities. Although we adjusted for differences in characteristics using multivariable analysis, it is possible that the observed outcomes could have been influenced, to some extent, by other unidentified confounders because of the inherent limitations of a retrospective study. Given the change in the sampling strategy by HCUP-NIS in 2012, it is conceivable that the procedural volumes may have minor inaccuracies. However since no hospital-level analyses were performed, the intra-regional variation in volumes will likely remain relatively stable in this study. Despite these limitations, this study addresses an important knowledge gap highlighting the national variation in hospital-level outcomes of AMI-CS. The strengths of this analysis include the large sample size, the ability to provide longitudinal data across the 17-year study period and the identification of novel regional variations in AMI-CS care.
CONCLUSIONS
In this large cohort of nationally representative AMI-CS admissions over 17-years, we noted a temporal increase in prevalence and a decrease in in-hospital mortality across all geographic regions. There were significant differences in in-hospital mortality, resource utilization and use of cardiac procedures that were not fully explained by patient or hospital characteristics. This analysis suggests the presence of disparity in healthcare delivery in this high-risk population with significant opportunity for targeted improvement in application of advanced evidence-based therapies.
Supplementary Material
SHORT COMMENTARY.
What is new?
This study notes that admissions to the Northeast were less likely to receive coronary angiography, percutaneous coronary intervention and mechanical circulatory support compared to the Midwest, South and West.
Compared to the Northeast, in-hospital mortality was lower in the Midwest and West, but higher in the South.
What are the clinical implications?
There remain significant regional disparities in the management and outcomes of acute myocardial infarction-cardiogenic shock.
These regional disparities need careful evaluation in prospectively designed studies to shape policies on the regionalization of acute myocardial infarction-cardiogenic shock care.
SOURCES OF FUNDING
Dr. Saraschandra Vallabhajosyula is supported by the Clinical and Translational Science Award (CTSA) Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
ABBREVIATIONS
- AMI
acute myocardial infarction
- CI
confidence interval
- CS
cardiogenic shock
- ECMO
extracorporeal membrane oxygenation
- HCUP
Healthcare Cost and Utilization Project
- IABP
intra-aortic balloon pump
- ICD-9CM
International Classification of Diseases-9 Clinical Modification
- MCS
mechanical circulatory support
- NIS
National/Nationwide Inpatient Sample
- NSTEMI
non-ST-elevation myocardial infarction
- OR
odds ratio
- PCI
percutaneous coronary intervention
- pLVAD
percutaneous left ventricular assist device
- STEMI
ST-elevation myocardial infarction
Footnotes
DISCLOSURES
Dr. Jaffe has been a consultant for Beckman, Abbott, Siemens, Roche, ET Healthcare, Sphingotoec, Quidel, Brava, Blade, and Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Vallabhajosyula S, Arora S, Lahewala S, Kumar V, Shantha GPS, Jentzer JC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A and Deshmukh AJ. Temporary mechanical circulatory support for refractory cardiogenic shock before left ventricular assist device surgery. J Am Heart Assoc. 2018;7:e010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallabhajosyula S, Arora S, Sakhuja A, Lahewala S, Kumar V, Shantha GPS, Egbe AC, Stulak JM, Gersh BJ, Gulati R, Rihal CS, Prasad A and Deshmukh AJ. Trends, predictors, and outcomes of temporary mechanical circulatory support for postcardiac surgery cardiogenic shock. Am J Cardiol. 2019;123:489–497. [DOI] [PubMed] [Google Scholar]
- 3.Vallabhajosyula S, Barsness GW and Vallabhajosyula S. Multidisciplinary teams for cardiogenic shock. Aging (Albany NY). 2019;11:4774–4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallabhajosyula S, Dunlay SM, Barsness GW, Rihal CS, Holmes DR Jr. and Prasad A. Hospital-level disparities in the outcomes of acute myocardial infarction with cardiogenic shock. Am J Cardiol. 2019;124:491–498. [DOI] [PubMed] [Google Scholar]
- 5.Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS and Kashani K. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One. 2019;14:e0222894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallabhajosyula S, Dunlay SM, Kashani K, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS and Barsness GW. Temporal trends and outcomes of prolonged invasive mechanical ventilation and tracheostomy use in acute myocardial infarction with cardiogenic shock in the United States. Int J Cardiol. 2019;285:6–10. [DOI] [PubMed] [Google Scholar]
- 7.Vallabhajosyula S, Dunlay SM, Murphree DH Jr., Barsness GW, Sandhu GS, Lerman A and Prasad A. Cardiogenic shock in Takotsubo cardiomyopathy versus acute myocardial infarction: An 8-year national perspective on clinical characteristics, management, and outcomes. JACC Heart Fail. 2019;7:469–476. [DOI] [PubMed] [Google Scholar]
- 8.Vallabhajosyula S, Dunlay SM, Prasad A, Kashani K, Sakhuja A, Gersh BJ, Jaffe AS, Holmes DR Jr. and Barsness GW. Acute noncardiac organ failure in acute myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2019;73:1781–1791. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhajosyula S, Kashani K, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS and Barsness GW. Acute respiratory failure and mechanical ventilation in cardiogenic shock complicating acute myocardial infarction in the USA, 2000–2014. Ann Intensive Care. 2019;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Dunlay SM, Holmes DR Jr. and Barsness GW. Venoarterial extracorporeal membrane oxygenation with concomitant impella versus venoarterial extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J. 2019; doi: 10.1097/MAT.0000000000001039. [DOI] [PubMed] [Google Scholar]
- 11.Vallabhajosyula S, O’Horo JC, Antharam P, Ananthaneni S, Vallabhajosyula S, Stulak JM, Eleid MF, Dunlay SM, Gersh BJ, Rihal CS and Barsness GW. Concomitant intra-aortic balloon pump use in cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation. Circ Cardiovasc Interv. 2018;11:e006930. [DOI] [PubMed] [Google Scholar]
- 12.Vallabhajosyula S, Prasad A, Dunlay SM, Murphree DH Jr., Ingram C, Mueller PS, Gersh BJ, Holmes DR Jr. and Barsness GW. Utilization of palliative care for cardiogenic shock complicating acute myocardial infarction: A 15-year national perspective on trends, disparities, predictors, and outcomes. J Am Heart Assoc. 2019;8:e011954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallabhajosyula S, Prasad A, Gulati R and Barsness GW. Contemporary prevalence, trends, and outcomes of coronary chronic total occlusions in acute myocardial infarction with cardiogenic shock. Int J Cardiol Heart Vasc. 2019;24:100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallabhajosyula S, Ya’Qoub L, Dunlay SM, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Jaffe AS, Gersh BJ and Kashani K. Sex disparities in acute kidney injury complicating acute myocardial infarction with cardiogenic shock. ESC Heart Fail. 2019;6:874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diepen Sv, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB and Cohen MG. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 16.Rab T, Ratanapo S, Kern KB, Basir MB, McDaniel M, Meraj P, King SB 3rd and O’Neill W. Cardiac shock care centers: JACC review topic of the week. J Am Coll Cardiol. 2018;72:1972–1980. [DOI] [PubMed] [Google Scholar]
- 17.Giugliano RP, Llevadot J, Wilcox RG, Gurfinkel EP, McCabe CH, Charlesworth A, Thompson SL, Antman EM and Braunwald E. Geographic variation in patient and hospital characteristics, management, and clinical outcomes in ST-elevation myocardial infarction treated with fibrinolysis. Results from InTIME-II. Eur Heart J. 2001;22:1702–1715. [DOI] [PubMed] [Google Scholar]
- 18.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Ahmed A, Frishman WH and Fonarow GC. Regional variation across the United States in management and outcomes of ST-elevation myocardial infarction: analysis of the 2003 to 2010 nationwide inpatient sample database. Clin Cardiol. 2014;37:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laskey W, Spence N, Zhao X, Mayo R, Taylor R, Cannon CP, Hernandez AF, Peterson ED and Fonarow GC. Regional differences in quality of care and outcomes for the treatment of acute coronary syndromes: An analysis from the Get With the Guidelines Coronary Artery Disease Program. Crit Pathw Cardiol. 2010;9:1–7. [DOI] [PubMed] [Google Scholar]
- 20.Ko DT, Krumholz HM, Wang Y, Foody JM, Masoudi FA, Havranek EP, You JJ, Alter DA, Stukel TA, Newman AM and Tu JV. Regional differences in process of care and outcomes for older acute myocardial infarction patients in the United States and Ontario, Canada. Circulation. 2007;115:196–203. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor GT, Quinton HB, Traven ND and et al. Geographic variation in the treatment of acute myocardial infarction: The cooperative cardiovascular project. JAMA. 1999;281:627–633. [DOI] [PubMed] [Google Scholar]
- 22.Pilote L, Califf RM, Sapp S, Miller DP, Mark DB, Weaver WD, Gore JM, Armstrong PW, Ohman EM and Topol EJ. Regional variation across the United States in the management of acute myocardial infarction. New Engl J Med. 1995;333:565–572. [DOI] [PubMed] [Google Scholar]
- 23.Krumholz HM, Radford MJ, Wang Y, Chen J, Heiat A and Marciniak TA. National use and effectiveness of β-blockers for the treatment of elderly patients after acute myocardial infarction: National cooperative cardiovascular project. JAMA. 1998;280:623–629. [DOI] [PubMed] [Google Scholar]
- 24.Subramanian U, Weinberger M, Eckert GJ, L’Italien GJ, Lapuerta P and Tierney W. Geographic variation in health care utilization and outcomes in veterans with acute myocardial infarction. J Gen Intern Med. 2002;17:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krumholz HM, Chen J, Rathore SS, Wang Y and Radford MJ. Regional variation in the treatment and outcomes of myocardial infarction: investigating New England’s advantage. Am Heart J. 2003;146:242–249. [DOI] [PubMed] [Google Scholar]
- 26.Shaefi S, O’Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, Mahmood E, Talmor D and Shahul S. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc. 2015;4:e001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Introduction to the HCUP Nationwide Inpatient Sample 2009. http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf. Accessed Jan 18, 2015.
- 28.Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A and Sakhuja A. Tako-tsubo cardiomyopathy in severe sepsis: Nationwide trends, predictors, and outcomes. J Am Heart Assoc. 2018;7:e009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallabhajosyula S, El Hajj SC, Bell MR, Prasad A, Lerman A, Rihal CS, Holmes DR Jr. and Barsness GW. Intravascular ultrasound, optical coherence tomography, and fractional flow reserve use in acute myocardial infarction. Catheter Cardiovasc Interv. 2019; doi: 10.1002/ccd.28543. [DOI] [PubMed] [Google Scholar]
- 30.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE and Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 31.Khera R and Krumholz HM. With great power comes great responsibility: big data research from the National Inpatient Sample. Circ Cardiovasc Qual Outcomes. 2017;10:e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko DT, Wang Y, Alter DA, Curtis JP, Rathore SS, Stukel TA, Masoudi FA, Ross JS, Foody JM and Krumholz HM. Regional variation in cardiac catheterization appropriateness and baseline risk after acute myocardial infarction. J Am Coll Cardiol. 2008;51:716–23. [DOI] [PubMed] [Google Scholar]
- 33.Vallabhajosyula S, Patlolla SH, Sandhyavenu H, Vallabhajosyula S, Barsness GW, Dunlay SM, Greason KL, Holmes DR Jr. and Eleid MF. Periprocedural cardiopulmonary bypass or venoarterial extracorporeal membrane oxygenation during transcatheter aortic valve replacement: A systematic review. J Am Heart Assoc. 2018;7:e009608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medicine Io. Geographic Adjustment in Medicare Payment: Phase I: Improving Accuracy. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 35.Patel N, Gupta A, Doshi R, Kalra R, Bajaj NS, Arora G and Arora P. In-hospital management and outcomes after ST-segment-elevation myocardial infarction in Medicaid beneficiaries compared with privately insured individuals. Circ Cardiovasc Qual Outcomes. 2019;12:e004971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O’Neill W, Ornato JP, Stelling K, Thiele H, van Diepen S and Naidu SS. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 2019;94:29–37. [DOI] [PubMed] [Google Scholar]
- 37.Westfall JM, Kiefe CI, Weissman NW, Goudie A, Centor RM, Williams OD and Allison JJ. Does interhospital transfer improve outcome of acute myocardial infarction? A propensity score analysis from the Cardiovascular Cooperative Project. BMC Cardiovasc Disord. 2008;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nadig NR, Goodwin AJ, Simpson AN, Simpson KN, Richards J and Ford DW. Patient and hospital characteristics associated with interhospital transfer for adults with ventilator-dependent respiratory failure. Ann Am Thorac Soc. 2017;14:730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


