Abstract
Mutations in the isocitrate dehydrogenase (IDH) 1 gene are commonly found in human glioma, with the majority of low-grade gliomas harboring a recurrent point mutation (IDH1 R132H). Mutant IDH reveals an altered enzymatic activity leading to the synthesis of 2-hydroxyglutarate, which has been implicated in epigenetic mechanisms of oncogenesis. Nevertheless, it is unclear exactly how IDH mutations drive glioma initiation and progression, and it is also not clear why tumors with this mutation generally have a better prognosis than IDH wild type tumors. The recognition of the high frequency of IDH mutations in glioma (and also in other malignancies, including acute myeloid leukemia (AML) and cholangiocarcinoma) have led to the development of a number of targeted agents that can inhibit these enzymes. Enasidenib and ivosidenib have both gained regulatory approval for IDH mutant AML. Both agents are still in early clinical phases for glioma therapy, as are a number of additional candidates (including AG 881, BAY1436032, and DS1001). A marked clinical problem in the development of these agents is overcoming the blood-brain-barrier. An alternative approach to target the IDH1 mutation is by the induction of synthetic lethality with compounds that target PARP, glutamine metabolism, and the Bcl-2 family of proteins. We conclude that within the last decade several approaches have been devised to therapeutically target the IDH1 mutation and that potentially both IDH1 inhibitors as well as synthetic lethal approaches might be relevant for future therapies.
1. Epidemiology of IDH mutations in glioma.
Preceding the discovery of mutated isocitrate dehydrogenase (IDH) 1 in gliomas was the earlier observation of mutations in this gene in colon carcinoma [1]. IDH1 was found to be mutated in about 10 % of glioblastomas [3]. Follow up studies demonstrated that a large proportion of roughly 80-90% of low grade gliomas, including astrocytomas and oligodendrogliomas, displayed mutated IDH1 [4, 5]. In gliomas, the most common IDH1 mutation, which comprises roughly 90% of all mutated cases, is located at codon 132, resulting in a switch from arginine to histidine at this position [2]. A relatively small fraction (fewer than 1%) of gliomas harbor IDH2 mutations (leading to IDH2 R172), which is more common in acute myeloid leukemia.
Amongst the low-grade glioma IDH1 mutated group one can distinguish cases harboring the 1p/19q co-deletion and cases that are devoid of this alteration [6]. When no 1p/19q co-deletion is present, the IDH1 mutation is often accompanied by mutations in ATRX and TP53. In glioma pat8ients, the presence of an IDH1 mutation is linked to an improved survival and this holds true even in the setting of high-grade gliomas. However, in AML the IDH1 mutation confers worse patient outcomes [7]. Although gliomas harboring the IDH1 mutation appear to behave more favorably, they nonetheless require therapy. Therefore, targeting IDH1 may be a therapeutic option for low grade gliomas and maybe secondary glioblastomas that arise from lower grade gliomas. A further important notion is the fact that the IDH1 mutation is usually identified in the vast majority of tumor cells, thus when targeted this will affect essentially the entire fraction of tumor cells. Since non-neoplastic cells do not harbor the mutation it is likely that mutation-specific targeting will not affect normal cells, thus providing the potential of low toxicity. Although not part of this review, this has also resulted in the conceptualization of vaccine strategies for IDH1 mutated gliomas.
2. Biology of IDH1 and 2-hydroxyglutarate (2-HG)
2.1. The biological roles of IDH1 enzymes in tumor cells
There are three IDH enzymes that are localized in different cellular compartments. The most well-known IDH enzyme is IDH3, which resides in the mitochondrial matrix and participates in the tricarboxylic acid cycle to produce α-ketoglutarate in a reaction that requires NAD+ that in turn is reduced to NADH2. NADH2 reduces molecular oxygen by transferring it electrons to the respiratory chain. This electron flow generates energy necessary to produce adenosine triphosphate (ATP), the main energy source of cells. While IDH2 resides in the mitochondria as well, IDH1 is found in the cytosol. However, both enzymes produce NADPH2, which is used for redox balance (e.g. the regulation of the ratio between GSH (reduced form of glutathione) and GSSG (oxidized form of glutathione) and biosynthesis, e.g. cholesterol and fatty acid synthesis. [8-11]. Consistently, when IDH1 is mutated, glioma cells tend to produce more reactive oxygen species likely related to the impaired “redox buffering” capacity due to a dysregulated GSH/GSSG ratio. These processes remain critical for the tumors to survive and proliferate.
2.2. Mutant IDH1 produces the oncometabolite 2-hydroxyglutarate (2-HG)
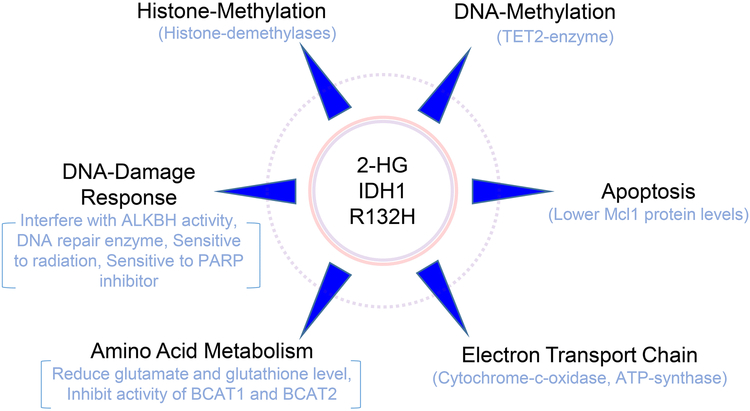
A major property of mutant IDH1 is its ability to produce 2-HG in significant amounts. In tumor tissue, there are reports that suggest that an astonishing level of 30 mM of 2-HG can be found in IDH mutated tumors. To accomplish this, the mutation needs to be present in a heterozygous fashion since for the production of 2-HG both the mutated and wild-type allele need to be present [12]. In turn, mutated IDH1 and IDH2 produce α-ketoglutarate, which gets further converted to 2-HG through depletion of NADPH2 and fixation of CO2 (reductive carboxylation). We have summarized the properties of 2-HG in Figure 1, which includes the impact of 2-HG on the epigenome, the DNA-damage response, apoptosis, amino acid metabolism and the electron transport chain. These aspects will be further reviewed in the following chapters below. Although there are studies related to 2-HG and the immune system, we have not included these recent investigations in the current review article [13, 14] since there is intriguing evidence that 2-HG suppresses the immune system to fight against glioma and hence, vaccine strategies are currently in development for IDH1 R132H mutated gliomas.
Figure 1: Summary of phenotypic changes elicited by 2-HG and/or mutated IDH1.
The scheme summarizes major pathways affected by mutated IDH1 and the oncometabolite, 2-HG.
Another pivotal question about the IDH1 mutation and its related oncometabolite, 2-HG, is the question of “driver vs. passenger” metabolic alteration/mutation since the IDH1 R132H mutation confers a better prognosis and in preclinical model systems there is evidence that IDH1 mutated glioma cells and/or 2-HG exposed tumor cells reveal a reduced proliferation rate. Therefore, several hypotheses emerge with regards to the role of the IDH1 mutation in established gliomas and especially high-grade gliomas. In this context, it is conceivable that the IDH1 mutation may start out as a driver and in the course of tumor development/progression reverts into a passenger mutation [15]. This assumption is coherent with findings that suggest that mutated IDH can block the differentiation of tumor cells although more evidence is necessary in glioma model systems to rigorously validate this point. Recent evidence from elegant transgenic mouse models confirms the earlier notion that the IDH1 mutation is indeed a driver mutation early in tumor development. It is critical to appreciate that IDH1 R132H is insufficient on its own to induce tumor formation. Studies in subventricular zone nestin expressing cells (inducible nestin-cre background) harboring mutated IDH1 showed enhanced proliferation and invasion, but not distinct tumor formation [16]. Nevertheless, IDH1 R132H driven progenitor cell proliferation in the subventricular zone significantly impacted the physiology of the ventricles such that animals developed a pronounced hydrocephalus [16]. However in the presence of PDGFA and loss of Atrx, Pten and Cdkn2a, IDH1 R132H appears to drastically accelerate tumor formation [17]. This model recapitulated the anticipated phenotype with elevated 2-HG, increased DNA-methylation coupled with reduced 5hmC, a proneural signature, and lengthening of telomeres.
2.3. Several major roles of 2-HG in tumor cells
2.3.1. 2-HG suppresses the electron transport chain
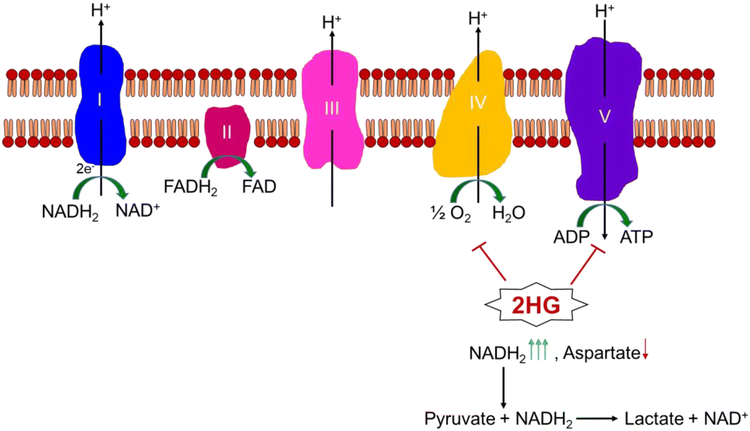
As it was noted above, there is a substantial accumulation of 2-HG in IDH1 mutated neoplasms and the question naturally arises what implications are associated with such a dysregulation. One of the roles appears to be the suppression of oxidative phosphorylation. Several underlying mechanisms have been reported. In the context of glioblastoma and colon carcinoma model systems, in which the IDH1 mutation was ectopically introduced it was demonstrated that 2-HG appears to be capable of binding to complex V of the electron transport chain (ETC). Consisting of five main complexes, the ETC finally generates energy through complex V by utilizing a proton gradient to facilitate the reaction of ADP and phosphate to the energy rich compound ATP (ATP-synthase reaction). Complex I, III and IV are establishing this proton gradient by transporting electrons to molecular oxygen which functions as the final electron acceptor and is in turn reduced to H2O. In this regard, 2-HG elicits its inhibitory function at the same complex as the classical ATP-synthase inhibitor, oligomycin [18]. However, another report suggests a slightly different mechanism that involves complex IV of the respiratory chain [19]. Ultimately, when this complex is not functioning properly a decrease in ATP production will be one of the consequences. In addition, there will be an improper regeneration of NADH and FADH, which will inhibit the proper functionality of the TCA cycle.
The inhibition of the ETC suggests that the ability of aspartate biosynthesis is likely to be impaired in the context of mutated IDH1 and presumably IDH2 as well since recent research has suggested that the pivotal role of the ETC in tumor cells is not primarily the production of ATP, but is the ability of oxygen to serve as an electron acceptor [20]. In this context, it has been known for quite some time that ETC-deficient cells are reliant on pyruvate, which is referred to as auxotrophic for pyruvate. This phenomenon has been described in the 1980s, but the fundamental biochemical principals behind this observation have remained elusive until recently. Pyruvate has multiple roles, but one fundamental aspect is that akin to oxygen it serves as an electron acceptor in metabolism. When oxygen is low or the ETC is impaired, other electron acceptors have to compensate. If no electron acceptor is available, the immediate consequence is a shift in the NAD+/NADH ratio, which would become lower. Therefore, the different isoforms of lactate dehydrogenase (LDH) will aid in rebalancing the ratio by catalyzing the reaction of NADH and pyruvate to lactic acid and NAD+. This point was further supported by the fact that other electron acceptors, such as α-ketobutyrate, are capable of regenerating NADH to NAD+. Following ETC inhibition, α-ketobutyrate rescues from the inhibition of cellular proliferation by rotenone, antimycin, and oligomycin, including the established glioblastoma cells A172 and U87 [20]. They also validate the point that the ETC is pivotal to facilitate the acceptance of electrons by molecular oxygen and that this is crucial for tumor cell proliferation, while ATP production appeared less critical. When the ETC is inhibited, the exogenous α-ketobutyrate helps to restore purine nucleotide levels by elevating TCA cycle metabolites and the non-essential amino acid aspartate, which is critical in nucleotide synthesis [20]. Supporting its critical role in this process, aspartate restored growth in tumor cells when the ETC is inhibited. We have also highlighted the potential roles of 2-HG in the electron transport chain (Figure 2).
Figure 2: Impact of 2-HG on the electron transport chain.
Shown is the electron transport chain with its complexes and electron donors. The final electron acceptor in this system is oxygen that is reduced to water. The flow of the electrons from complex I to complex IV creates energy that is used for synthesis of ATP. In this regard, complex I, III, IV function as proton pumps that create a gradient, which is used by complex V to produce energy (proton-driving force). 2-HG blocks the synthesis of ATP (complex V) and electron transport at the level of complex IV.
2.3.2. The hypermethylated phenotype in IDH1 mutated gliomas is partially mediated by 2-HG
A couple of years ago, an intriguing observation was made in the context of mutated IDH1 and the epigenome [21]. Mutated IDH1 was shown to be associated with the development of a glioma CpG island methylated phenotype (G-CIMP) in intermediate grade gliomas. Notably, human astrocytes transduced with mutated IDH1 R132H resembled the “methylated phenotype” and gene expression observed in low-grade gliomas, strongly suggesting that mutated IDH1 is the driver of this epigenetic phenotype and low-grade gliomas [21]. One of the underlying mechanisms related to the hypermethylated phenotype involved mutant IDH1 mediated inhibition of the TET2 enzyme [21]. TET2 is implicated in the conversion of 5mC to 5hmC (a pivotal step for DNA-demethylation). When 2-HG is present in abundant amounts (such as in the context of mutated IDH1/2) it competes with the cofactor of these enzymes, α-ketoglutarate [22].
3. The quest for better model systems of mutated IDH1 and IDH2
One of the challenges to study the impact of the IDH1 mutations on cancer biology and therapy is presented by the fact that at least for gliomas it is difficult to obtain a stable cell line derived from patients. The IDH mutation is hard to be propagated under in vitro conditions and successfully established IDH1 mutated tumors often end up losing their mutated IDH1 allele [23, 24]. These challenging culture conditions may be related to the fact that IDH1 mutated gliomas have an impaired metabolism with regards to the electron transport chain and oxidative phosphorylation since as mentioned earlier both processes have been shown to be suppressed by 2-HG. Given this implication it may be beneficial to adjust culture conditions in a way that pyruvate and aspartate levels are elevated and possibly also glutamate levels need adjustment since it was shown by another study that IDH1 mutated gliomas have lower glutamate and glutathione levels. To remedy this situation, scientists have started to over-express mutated IDH1 by diverse non-viral and viral constructs in various established glioblastoma cell lines. Thus, many experiments were performed under these artificial conditions. The situation becomes even more challenging when the IDH1 mutation is introduced into a genetic background that does not exist in patients. For instance, it is well accepted that a loss of PTEN or alterations in the EGFR gene are mutually exclusive with the IDH1 mutation. Yet, many experiments in the literature are based on cell culture systems where the IDH1 mutation was introduced in cells that harbor for instance a PTEN loss. Nevertheless, having a knock-in mutation should be the more suitable approach after all since over-expression of the mutation may cause other features that will not be representative of the human disease. An alternative to this scenario represents the utilization of genetic engineered mouse models. For instance, using IDH1-R132H knock-in mice in the background of a TP53 mutation would be a system to be considered to be more representative of the actual scenario in a patient’s tumor. Indeed, there are recent other mouse model systems that might gain traction in the scientific community, such as a mouse model in the context of dual TP53 and ATRX loss [25].
4. Synthetic lethality in IDH1 mutated tumors
4.1. Mutant IDH renders glioma cells susceptible to the standard of care treatment (temozolomide and radiation)
IDH1 mutated tumors have been shown to be susceptible to the inhibition of several targets. This includes the DNA repair machinery, which can be disrupted or challenged through radiation and/or chemotherapy. In this regard, a couple of years ago using model systems of transduced IDH1 R132H mutated established glioblastoma cells it was demonstrated that these cells are more prone to the cytotoxic actions of radiation [26]. Similar effects were seen in the context when the IDH2 R172K mutation was introduced. Mechanistically, it appears that ROS played a crucial role in this process since application of the ROS scavenger, N-acetylcystein, reversed the sensitization effect of the IDH1/IDH2 mutations on radiation. It should be noted also that this study was conducted early following discovery at a time when knock-in models were not available or easily obtainable. The other issue that has arisen over the years is the fact that many studies have used the U87 or other established glioblastoma cell lines that in fact harbor genetic alterations, such as the loss of PTEN that in patients would not co-exist with the IDH1 mutation. Therefore, concerns were generally raised about how representative these established cell line models would be in the context of studying the impact of mutated IDH1. Given the central role of temozolomide for the treatment of brain tumors other groups have interrogated the susceptibility of IDH1 mutated glioma model systems to chemotherapy [27]. They found that the IDH1 mutation appears to enhance the efficacy of temozolomide and a platinum derived chemotherapeutic. Mechanistically, reactive oxygen species and glutathione appeared to play a central role since exogenous glutathione rescued from the reduction in cellular viability by the two compounds.
4.2. Mutant IDH renders glioma cells susceptible to DNA-damage
Several reports have indicated a link between the presence of the IDH1 mutation and impaired repair of the DNA. One group demonstrated that 2-HG is capable of interfering with the DNA-repair enzyme ALKBH [28]. 2-HG competes with α-ketoglutarate for ALKBH. Since ALKBH requires α-ketoglutarate for its functionality, the high abundance of 2-HG produced by the IDH1 mutation interferes with its enzymatic activity. Elegant rescue experiments have provided a strong foundation for this relationship because elimination of the mutated IDH1 allele, which lowers 2-HG, reactivates the enzymatic activity of ALKBH (as noted above, 2-HG will be produced at high levels in the presence of a wild-type and mutated allele of IDH1). By capitalizing on these interesting observations, this group has provided a probable explanation as to why the drug combination of procarbazine, lomustine, and vincristine (PCV) regimen works in a particular class of gliomas. It has been known for many years now that oligodendrogliomas are susceptible to the PCV regimen and molecular studies from the last decade have informed us that oligodendrogliomas commonly harbor the IDH1 mutation.
4.3. Mutant IDH confers sensitivity to inhibition of NAD+ metabolism
As noted above, the IDH1 mutation impacts tumor cell metabolism. While energy metabolism is directly impacted at the level of the electron transport chain by the IDH1 mutation, it was also shown that the electron acceptor NAD+ and its reduced counterpart, NADH2, are affected by the IDH1 mutation. Normally, NAD+ is a complex molecule and requires the synthesis of its adenine portion. This appears to be naturally an energy consuming process. Therefore, salvage pathway enzymes exist to mitigate this issue. These enzymes are called nicotinamide phosphoribosyltransferase (NAMPT) and nicotinate phosphoribosyltransferase (NAPRT1). A couple of years ago it was found that IDH1 mutated gliomas harbor lower levels in the NAPRT1 enzyme [29]. Therefore, it was tempting to speculate whether or not interference with the NAD+ salvage pathway through NAMPT1 inhibitors is synthetically lethal in IDH1 mutated gliomas. Indeed, two inhibitors, FK866 and GMX1778, showed selective reduction of cellular viability in several IDH1 mutated glioma cell lines such as MGG152 and MGG119. Importantly, increasing concentrations of NAD+ rescued from the effects elicited by the NAMPT1 inhibitors, demonstrating that the inhibition of growth/cell death induction indeed relies on NAD+ depletion by these reagents. Given its role as electron acceptor and involvement in energy metabolism, e.g. glycolysis, TCA-cycle, and ETC, it appears conceivable that loss of NAD+ may elicit a state of energy deprivation. Indeed, the authors of this study found a substantial activation of AMPK and lower ATP levels. Cell death associated with energy deprivation may depend on the cellular context and the precipitating event. The NAMPT1 inhibitors were shown to activate cell death with features of autophagy, which was rescued by 3-methyladenine. Regarding translational implications, GMX1778 extended animal survival in a murine orthotopic IDH1 mutated glioblastoma model, suggesting a potential novel treatment option for IDH1 mutated gliomas. In model systems of chondrosarcoma, which harbor IDH1 mutations as well, NAMPT inhibition did not correlate with the IDH1 or IDH2 mutation status. Instead, higher histologic grade and low levels of NAMPT appear to predict the susceptibility to NAMPT inhibitors [29]. More recently, it was found that IDH1 mutated tumors are susceptible to a combination treatment involving temozolomide and NAMPT inhibitors in vitro and in subcutaneous xenograft models. On the biochemical level, temozolomide depletes NAD+ levels through activation of the PARP enzyme, which requires NAD+ for its function to repair DNA damages [30]. Since IDH1 mutated cells are already prone to NAD+ depletion, temozolomide might render these cells particularly sensitive to inhibition of NAMPT.
4.4. Mutant IDH impacts amino acid metabolism
An interesting recent observation was made that IDH1 mutated glioma cells display an impaired ability to synthesize glutamate since 2-HG inhibits the activity of two branched chain amino acid transaminases, BCAT1 and BCAT2, which physiologically require the presence of α-ketoglutarate. Consequently, IDH1 mutated gliomas show suppressed levels of glutamate and in turn are more reliant on glutaminase, which catalyzes the production of glutamate from glutamine [31]. A major compound to be synthesized from glutamate is glutathione, which was reduced in the presence of the IDH1 mutation as well [31]. Another report suggested an increase in enzymes related to glutathione synthesis in IDH1 mutated glioma models, which may serve to support glutathione levels [8]. Therefore, under conditions of oxidative stress IDH1 mutated glioma cells were more sensitive to cell death induction by the clinically validated glutaminase inhibitor, CB-839, which was rescued by exogenous glutathione. Interestingly, when CB-839 was combined with radiation a significant increase in overall survival of animals harboring mutated IDH1 (orthotopic model) was seen [31].
4.5. Mutant IDH impacts DNA-repair and susceptibility to Poly (ADP-Ribose) polymerase (PARP)-inhibitors
Another report showed that DNA double strand break repair is inhibited by 2-HG produced by the IDH1 mutation. In turn, IDH1 mutated solid tumor model systems were shown to be more susceptible to PARP inhibitors in vitro and subcutaneous model systems in vivo. Interestingly, when IDH1 mutated tumors were treated with IDH1 inhibitors, the enhanced sensitivity to these drug compounds was reversed. As anticipated, exogenous 2-HG recapitulates the effect of the IDH1 mutation. Similarly, leukemia cells with mutated IDH1/IDH2 showed higher amounts of DNA damage and were more sensitive to radiation and PARP inhibitors [32].
4.6. Mutant IDH impacts the anti-apoptotic Bcl-2 family of protein, resulting in sensitivity to BH3-mimetics
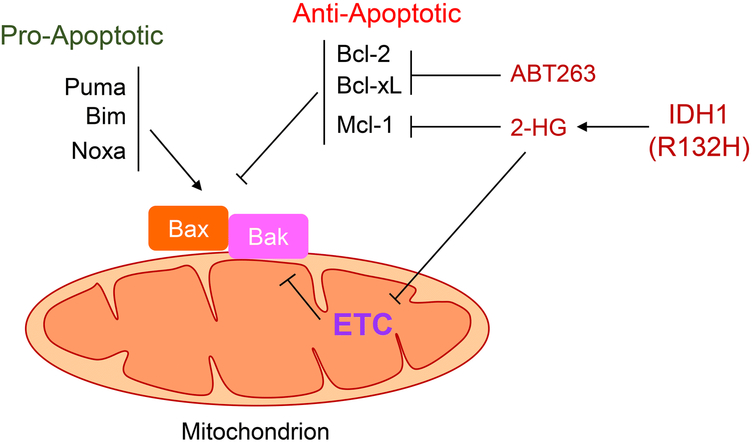
Bcl-2 family proteins are amongst the most important proteins that are known to regulate apoptosis. While Bcl-xL, Bcl-2, and Mcl-1 represent anti-apoptotic members, BIM, BID, Noxa, and PUMA are in fact facilitating programmed cell death. The pro-apoptotic Bcl-2 family members that finally take action to release cytochrome-c from the mitochondria into the cytosol, are BAX and BAK. Cytochrome-c drives activation of the apoptosome followed by cleavage of effector caspases (e.g. caspase-3). The anti-apoptotic Bcl-2 family members bind BAX and BAK to inhibit apoptotic cell death and pro-apoptotic Bcl-2 family members, such as Noxa, can facilitate their release (Figure 3). For most studies related to apoptosis it is usually required to inhibit both molecules to fully rescue from intrinsic apoptosis.
Figure 3: IDH1 R132H impacts the status of the Bcl-2 family of proteins.
The IDH1 mutation confers a neomorphic enzymatic activity, resulting in the production of 2-Hydroxyglutarate (2-HG). 2-HG inhibits the ETC (electron transport chain) through impacting the electron flow to molecular oxygen by interference with cytochrome-c oxidase and in part by inhibition of complex V. This results in lower ATP levels, followed by reduction of protein synthesis and lower levels of protein with a short-half life, such as the anti-apoptotic Bcl-2 family member Mcl-1. Lower levels of Mcl-1 shift the survival dependency towards Bcl-xL and/or Bcl-2, creating a mitochondrial Bcl-2 family related vulnerability, which can be executed by the BH3-mimetic, ABT263, resulting in BAX/BAK activation and permeabilization of the outer mitochondrial membrane. In addition, interference with ETC itself can impact mitochondrial membrane potential prime mitochondrial for BAX/BAK mediated apoptosis. Proapoptotic Bcl-2 family members (Puma, BIM, Noxa) either directly or indirectly activate BAX/BAK to facilitate apoptosis.
With the discovery of Bcl-2 and subsequent successful development of ABT199 (BH3-mimetic drug) [33, 34] several years thereafter these molecules have become “targetable” in patients. Recently, ABT199 (venetoclax) was approved for certain hematological malignancies by the Food and Drug Administration (FDA). In addition, other BH3-mimetics have been designed to target Bcl-xL (WEHI-539) [35] and Mcl-1 (MIK665), respectively [36]. However, it remains elusive which patient population would especially benefit from BH3-mimetics. By performing a high throughput shRNA lentiviral library screen it was demonstrated that IDH mutated myeloid leukemia cells are highly dependent on the expression of Bcl-2 for their survival. In light of the fact that ABT199 was available it was enticing to test the hypothesis that IDH mutated AML cells may be more prone to cell death induction by this BH3-mimetc. Indeed, IDH mutated cells, including patient samples, turned out to be significantly more susceptible to this drug compound [19]. These effects were largely mediated by 2-HG, which was shown to lower the apoptotic threshold by interfering with the activity of complex IV of the respiratory chain. Specifically, 2-HG interacts with a binuclear center established by two heme complexes (heme a3 and CuB), which inhibits the ability of cytochrome-c oxidase to reduce oxygen [37]. Interference with the respiratory chain suffices to render leukemia cells more sensitive to the cytotoxic effects of BH3-mimetics. This observation is especially relevant in light of the fact that in leukemia cells 2-HG and/or the IDH1 mutation did not modulate the levels of anti- or pro-apopotic Bcl-2 family of proteins in a significant manner, suggesting that ETC inhibition is sufficient to enhance the apoptotic effects of ABT199. Although ABT199 was efficient to induce apoptosis in IDH mutated leukemia cells, it was not in solid malignancies. The literature suggests that solid tumors are more often dependent on Bcl-xL for their survival [37]. In line with this observation, it was demonstrated that IDH1 (R132H) mutated glioblastoma cell cultures and xenografts are more susceptible to apoptosis induction by Bcl-xL inhibition. Given that there is a clinically validated Bcl-xL inhibitor available (ABT263) it was obvious to assess its potency in the diverse IDH1 mutated model systems and ABT263 resembled the killing effects observed with genetic silencing of Bcl-xL [38]. This synthetic lethal interaction was not limited to glioblastoma and was validated in a model system of colonic carcinoma, harboring a genetically engineered heterozygous IDH1 mutation. Contrasting the findings in leukemia, the IDH1 mutation was associated with lower Mcl-1 protein levels in IDH1 mutated anaplastic astrocytomas. Similarly, 2HG and mutated IDH1 suppressed Mcl-1 levels in cell cultures. Prior work has unequivocally demonstrated that Mcl-1 drives resistance towards BH3-mimetic (Bcl-2 and Bcl-xL inhibitors) mediated apoptosis and consistently Mcl-1 appeared to be the major driver of resistance towards ABT263 in the model systems tested. The underlying mechanisms as to how 2-HG suppressed Mcl-1 levels was related to tumor cell metabolism since 2-HG suppressed ATP production through inhibition of the ETC in glioblastoma model systems as early as 1h following administration. It is noteworthy that the vulnerability to Bcl-xL inhibition appeared to be selective since IDH1 mutated glioma cells were not sensitive to cell death induction by generic chemotherapeutic drugs or the cytotoxic ligand TRAIL [38].
4.7. Mutant IDH confers sensitivity to SRC inhibitors
IDH1 mutated cholangiocarcinomas have been reported to be more sensitive to SRC kinase inhibition by the drug compound, dasatinib, which was rescued through overexpression of a SRC mutant [39]. Whether this synthetic lethality is observed in IDH mutated gliomas remains elusive, but maybe critical to evaluate in light of the presence of a feasible therapeutic. We provided a brief table about targets and compounds that when inhibited are synthetically lethal in IDH mutated tumors (Table 1). It is acknowledged that these therapies encompass additional tumor entities as well.
Table 1:
Drugs that cause synthetic lethality in IDH1 mutated neoplasms
5. Inhibitors of mutant IDH
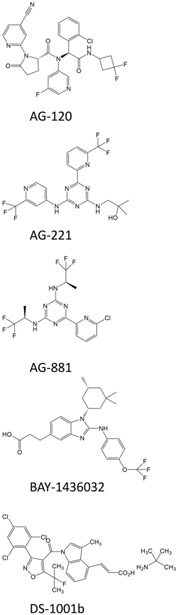
The wide preponderance of IDH mutations in certain types of cancer and a strong biological evidence for these genetic alterations to cause tumor-driving epigenetic changes lead to a rapid success in identifying and developing inhibitors of mutant IDH. While the rationale for targeting mutant IDH in diseases such as AML has been proven to be well founded and clinical proof-of-concept was established [40, 41], the data in glioma are less consistent and substantial clinical benefits following IDH inhibitor treatment remain to be proven. In the following, we will focus on mutant IDH inhibitors AG-120, AG-221, AG-881, BAY1436032, and DS-1001b (Table 2), which are currently evaluated in clinical trials involving patients with gliomas. We have provided the chemical structures of these inhibitors in Figure 4.
Table 2:
Mutant IDH1 inhibitors
Figure 4: Structure of several IDH1 inhibitors as discussed in the present article.
Shown are the chemical structures of AG-120, AG-221, AG-881, BAY1436032, and DS-1001b.
5.1. AG-120 (Ivosidenib, Tibsovo ®)
AG-120 (Agios) is an orally bioavailable approximately 583 Da small-molecule inhibitor. It acts as an allosteric inhibitor and belongs to the phenylglycine class of compounds [41]. AG-120 was shown to have a strong selectivity for mutant IDH1 with an EC50-value of 40nM (R132H) to 50nM (R132C) and an 85-106 fold separation in potency, relative to wild-type IDH1, when tested against purified recombinant protein [42]. The EC50-values for the inhibitory effects of AG-120 on 2D cellular and 3D cellular 2-HG production by U87 R132H cells was reported as 50nM and 108nM, respectively.
AG-120 has been reported to have an acceptable safety profile in a phase 1 clinical trial when applied as a monotherapy for advanced solid cancers (www.clinicaltrials.gov, ). In this study, 35 patients with a non-enhancing glioma were included [43]. Patients in the dose-escalation portion received 100 mg AG-120 BID or 300, 500, 600 or 900 mg QD in continuous 28-day cycles. Patients in the dose-expansion portion received 500 mg QD AG-120 in continuous 28-day cycles. In 91% of the patients AEs were observed. Greater than grade 3 AEs were found in 20% of the patients and among those headache (34%) and diarrhea (26%) represented the most common ones [44]. AG-120 was shown to be readily absorbed and its mean plasma half-life was determined as 40-102 h after a single dose [43]. Notably, in glioma patients, 2-HG baseline concentrations in plasma ranged from 49.7 ng/ml to 97.1 ng/ml and were not significantly altered after treatment with AG-120. With respect to therapeutic efficacy, treatment with AG-120 resulted in a minor response in 6% and stable disease in 83% of patients. Median treatment duration was 16 months and median progression-free survival was reported as 13 months [45]. A phase 1 randomized, controlled, multi-center trial recruiting patients with low grade glioma to determine 2-HG levels in tumor tissue after presurgical treatment with AG-120/AG-881 is ongoing (www.clinicaltrials.gov, ) [45].
5.2. AG-221 (Enasidenib, IDHIFA®)
AG-221 (Celgene/Agios) is an orally bioavailable allosteric inhibitor of mutant IDH2. It has a molecular weight of approximately 473 Da and belongs to the triazine class of compounds [41]. AG-221 has a strong selectivity for mutant IDH2 with an EC50-value of 44 nM (R172Q) when compared to wild-type IDH2 (EC50 > 30 μM) or mutant IDH1 R132H/R132C (EC50 = 15.556 μM) and tested against purified recombinant protein [42].
While AG-221 has gained FDA approval for the treatment of patients with IDH2-mutated acute myeloid leukemia based on encouraging response rates and a favorable safety profile, clinical data in the setting of glioma are sparse [41, 46]. In a phase 1/2 multi-center dose escalation study, patients with IDH2-mutated advanced solid tumors including glioma were enrolled (www.clinicaltrials.gov, ). The results of this study are still awaited. However, considering the facts that in gliomas, IDH2 mutations are much less frequent than IDH1 mutations [47] and a blood-brain barrier crossing pan-mutant IDH inhibitor is in clinical trial it seems currently rather unlikely that selective IDH2 inhibitors will play a predominant role for the treatment of IDH-mutated gliomas.
5.3. AG-881 (Vorasidenib)
AG-881 (Agios) is an orally bioavailable allosteric pan-mutant IDH1/2 inhibitor [48]. It has a molecular weight of approximately 415 Da and belongs to the triazine class of compounds [41]. AG-881 has been described to cross the blood-brain barrier and to be highly potent with IC50-values ranging between less than 1 nM (IDH1-R132H) to 32 nM (IDH2-R140Q) [49]. AG-881 was reported to inhibit tumor growth in an orthotopic mutant IDH1-R132H grade III oligodendroglioma model with a greater than 98% inhibition of intra-tumoral 2-HG levels [50]. Updated data from a phase 1 dose-escalation study of AG-881 in IDH-mutated glioma patients were presented at the 2018 annual SNO meeting [49] (www.clinicaltrials.gov, ). At study entry, of the 52 patients that were enrolled 25 patients had grade II tumors, 22 patients had grade III tumors, 4 had grade IV tumors and 1 patient had a tumor of unknown grade. These patients were treated with daily doses of 10 to 300 mg AG-881. At doses below 100 mg/day, a favorable safety profile was reported. The most common AEs observed were elevated liver enzymes (ALT, n=23; AST, n=21), headache (n=19), and fatigue (n=17). For 10 patients, AEs grade 3 or higher were reported including seizures and elevation of liver enzymes. Five patients experienced dose-limiting toxicities at doses of 100mg and higher. At the time of the data presentation 14 patients were still on trial. Among these patients 13 had non-enhancing lesions. One partial response and 1 minor response were reported. Thirty-six of the patients were reported to have reached a best response of stable disease. Notably, the tumor growth rates were reported to be slowed down based on preliminary volumetric studies when compared to historical controls.
The results of an ongoing perioperative study determining intra-tumoral 2-HG levels after AG-881 treatment are pending (www.clinicaltrials.gov, ) [45].
5.4. BAY1436032
BAY1436032 (Bayer) was identified based on a drug screen searching for compounds with an IC50 lower than 1μM for IDH1-R132H and an IC50 10 fold higher for wild-type IDH1 [51]. Initially, 377 compounds were identified to match those criteria. This process was followed by further selection and chemical modifications leading to BAY1436032. This competitive pan-IDH1 inhibitor was reported to have an IC50-value of 15 nM for both IDH1-R132H and IDH1-R132C. Pre-clinical studies showed that treatment with BAY1436032 lead to reduced 2-HG levels in multiple cell lines bearing different IDH1 mutations and reduced the cellular viability as well as promoted the differentiation of IDH1-R132H mutant NCH551b secondary glioblastoma cells. In a murine orthotopic NCH551b xenograft model, treatment with BAY1436032 resulted in significantly lower intra-tumoral 2-HG levels. In addition, mice subjected to treatment with BAY1436032 at a daily dose of 150 mg/kg showed a slight but statistically significant prolonged survival.
A phase I clinical trial studying the safety profile and maximum tolerated dose of BAY1436032 in patients with IDH1-mutated advanced solid cancers is ongoing (www.clinicaltrials.gov, ).
5.5. DS-1001b
DS-1001b (Daiichi Sankyo Co) represents another inhibitor of mutant IDH1 which is orally bioavailable. In IDH1 mutant chondrosarcoma cells, treatment with DS-1001b resulted in a GI50-value of 289.6 nM (JJ012 IDH1 R132G, after 7 days of treatment) and 160.9 nM (L835 IDH1 R132C, after 21 days of treatment) 1. In contrast, IDH1 wild-type cells displayed GI50-values exceeding 10 μM after 10 days of treatment with DS-1001b. The anti-proliferative effects of DS-1001b in IDH1 mutant chondrosarcoma cells were accompanied by a significant reduction of D-2HG levels, induction of differentiation through up-regulation of SOX9 and a decrease in H3K4me3 and H3K9me3 levels [52].
In glioma, recently the preliminary results from a phase I clinical trial were reported () [53]. In this study, 45 patients with IDH1 mutant gliomas were included and received between 125 and 1400 mg DS-1001b twice a day. The treatment was reported to be well tolerated with no grade 4 or 5 adverse events. In 42.2% of the patients grade 3 adverse events were observed with one patient reaching dose-limiting toxicity at a dose of 1000 mg twice a day. With respect to therapeutic efficacy, among 29 patients with contrast enhancing lesions one patient reached complete response, three patients reached partial response and 10 patients reached stable disease. This trial is ongoing and the final results are awaited.
6. Conclusions:
The discovery of the IDH1 R132H in gliomas remains an important finding in biomedical research. The subsequent observation that mutated IDH1 leads to the production of 2-HG and that this metabolite elicits a significant impact on tumors by regulation of cell death, the epigenome and metabolism, is part of the foundation for targeting the mutated enzyme for therapy. Blocking the neo-enzymatic activity by several IDH1/IDH2 inhibitors has proven to be promising in preclinical model systems. At the moment, the evaluation of efficacy of these molecules (e.g., AG-120, AG-221, AG-881, BAY1436032, and DS-1001b) is still in early clinical development and the results of ongoing and subsequent clinical trials will provide pivotal insight about the efficacy and toxicity of these compounds in patients. An additional concept from preclinical studies is the induction of synthetic lethality since IDH mutations confer unique vulnerabilities that render tumor cells sensitive to inhibition of oncogenic pathways. However, thus far, this concept has not received much attention for clinical trials in the context of IDH1 mutated gliomas. Given the recent emergence of Idh1 R132H mouse models it may be possible to more efficiently identify synthetic lethal interaction in the context of mutated IDH1 in gliomas.
Key points:
IDH1 mutations are common in low grade gliomas and secondary glioblastomas, and IDH1 R132H is the most common IDH1 mutation found in gliomas.
Mutated IDH1 causes a neomorphic enzymatic activity that leads to the production of an oncometabolite, 2-hydroxyglutarate.
Several small molecules have been designed that target mutated IDH enzymes, but the clinical benefit of IDH1 inhibitors in gliomas appears to be limited at the current stage.
A compelling complementary approach to IDH1 inhibitors may be the concept of synthetic lethality whereby mutated IDH1 renders cancer cell dependent on certain pathways that in turn may be targeted therapeutically.
Acknowledgments
Funding
M.D. Siegelin: NIH NINDS R01NS095848, R01NS102366, K08NS083732, Louis V. Gerstner, Jr. Scholars Program (2017-2020) and American Brain Tumor Association Discovery Grant 2017 (DG1700013). Trang Nguyen: American Brain Tumor Association Basic Research Fellowship (BRF1900018).
Footnotes
Conflicts of interest
Georg Karpel-Massler, Trang Thi Thu Nguyen, Enyuan Shang and Markus D. Siegelin declare that they have no conflict of interest that might be relevant to the contents of this manuscript.
References:
- 1.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006. October 13;314(5797):268–74. [DOI] [PubMed] [Google Scholar]
- 2.Miller JJ, Shih HA, Andronesi OC, Cahill DP. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer. 2017. December 1;123(23):4535–46. [DOI] [PubMed] [Google Scholar]
- 3.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008. September 26;321(5897):1807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009. February 19;360(8):765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009. October;118(4):469–74. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research N, Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015. June 25;372(26):2481–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010. August 1;28(22):3636–43. [DOI] [PubMed] [Google Scholar]
- 8.Fack F, Tardito S, Hochart G, Oudin A, Zheng L, Fritah S, et al. Altered metabolic landscape in IDH-mutant gliomas affects phospholipid, energy, and oxidative stress pathways. EMBO Mol Med. 2017. December;9(12):1681–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJF. Wild-type and mutated IDH1/2 enzymes and therapy responses. Oncogene. 2018. April;37(15):1949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horbinski C What do we know about IDH1/2 mutations so far, and how do we use it? Acta Neuropathol. 2013. May;125(5):621–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullarky E, Mattaini KR, Vander Heiden MG, Cantley LC, Locasale JW. PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 2011. December;24(6):1112–5. [DOI] [PubMed] [Google Scholar]
- 12.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010. February 15;207(2):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019. January;565(7738):240–5. [DOI] [PubMed] [Google Scholar]
- 14.Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018. August;24(8):1192–203. [DOI] [PubMed] [Google Scholar]
- 15.Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, et al. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front Oncol. 2019;9:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bardella C, Al-Dalahmah O, Krell D, Brazauskas P, Al-Qahtani K, Tomkova M, et al. Expression of Idh1(R132H) in the Murine Subventricular Zone Stem Cell Niche Recapitulates Features of Early Gliomagenesis. Cancer Cell. 2016. October 10;30(4):578–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philip B, Yu DX, Silvis MR, Shin CH, Robinson JP, Robinson GL, et al. Mutant IDH1 Promotes Glioma Formation In Vivo. Cell Rep. 2018. May 1;23(5):1553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu X, Chin RM, Vergnes L, Hwang H, Deng G, Xing Y, et al. 2-Hydroxyglutarate Inhibits ATP Synthase and mTOR Signaling. Cell Metab. 2015. September 1;22(3):508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015. February;21(2):178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell. 2015. July 30;162(3):552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012. February 15;483(7390):479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011. January 18;19(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luchman HA, Stechishin OD, Dang NH, Blough MD, Chesnelong C, Kelly JJ, et al. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol. 2012. February;14(2):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu F, Cheng G, Yao Y, Kogiso M, Jiang H, Li XN, et al. Inhibition of Mutated Isocitrate Dehydrogenase 1 in Cancer. Med Chem. 2018;14(7):715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunez FJ, Mendez FM, Kadiyala P, Alghamri MS, Savelieff MG, Garcia-Fabiani MB, et al. IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci Transl Med. 2019. February 13;11(479). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li S, Chou AP, Chen W, Chen R, Deng Y, Phillips HS, et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol. 2013. January;15(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L, et al. Decreasing GSH and increasing ROS in chemosensitivity gliomas with IDH1 mutation. Tumour Biol. 2015. February;36(2):655–62. [DOI] [PubMed] [Google Scholar]
- 28.Wang P, Wu J, Ma S, Zhang L, Yao J, Hoadley KA, et al. Oncometabolite D-2-Hydroxyglutarate Inhibits ALKBH DNA Repair Enzymes and Sensitizes IDH Mutant Cells to Alkylating Agents. Cell Rep. 2015. December 22;13(11):2353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N, et al. Extreme Vulnerability of IDH1 Mutant Cancers to NAD+ Depletion. Cancer Cell. 2015. December 14;28(6):773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateishi K, Higuchi F, Miller JJ, Koerner MVA, Lelic N, Shankar GM, et al. The Alkylating Chemotherapeutic Temozolomide Induces Metabolic Stress in IDH1-Mutant Cancers and Potentiates NAD(+) Depletion-Mediated Cytotoxicity. Cancer Res. 2017. August 1;77(15):4102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBrayer SK, Mayers JR, DiNatale GJ, Shi DD, Khanal J, Chakraborty AA, et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell. 2018. September 20;175(1):101–16 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molenaar RJ, Radivoyevitch T, Nagata Y, Khurshed M, Przychodzen B, Makishima H, et al. IDH1/2 Mutations Sensitize Acute Myeloid Leukemia to PARP Inhibition and This Is Reversed by IDH1/2-Mutant Inhibitors. Clin Cancer Res. 2018. April 1;24(7):1705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013. February;19(2):202–8. [DOI] [PubMed] [Google Scholar]
- 34.Davids MS, Letai A. ABT-199: taking dead aim at BCL-2. Cancer Cell. 2013. February 11;23(2):139–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lessene G, Czabotar PE, Sleebs BE, Zobel K, Lowes KN, Adams JM, et al. Structure-guided design of a selective BCL-X(L) inhibitor. Nat Chem Biol. 2013. June;9(6):390–7. [DOI] [PubMed] [Google Scholar]
- 36.Merino D, Kelly GL, Lessene G, Wei AH, Roberts AW, Strasser A. BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines. Cancer Cell. 2018. December 10;34(6):879–91. [DOI] [PubMed] [Google Scholar]
- 37.Karpel-Massler G, Ishida CT, Bianchetti E, Shu C, Perez-Lorenzo R, Horst B, et al. Inhibition of Mitochondrial Matrix Chaperones and Antiapoptotic Bcl-2 Family Proteins Empower Antitumor Therapeutic Responses. Cancer Res. 2017. July 1;77(13):3513–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karpel-Massler G, Ishida CT, Bianchetti E, Zhang Y, Shu C, Tsujiuchi T, et al. Induction of synthetic lethality in IDH1-mutated gliomas through inhibition of Bcl-xL. Nat Commun. 2017. October 20;8(1):1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saha SK, Gordan JD, Kleinstiver BP, Vu P, Najem MS, Yeo JC, et al. Isocitrate Dehydrogenase Mutations Confer Dasatinib Hypersensitivity and SRC Dependence in Intrahepatic Cholangiocarcinoma. Cancer Discov. 2016. July;6(7):727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boddu P, Borthakur G. Therapeutic targeting of isocitrate dehydrogenase mutant AML. Expert Opin Investig Drugs. 2017;26:525–30. [DOI] [PubMed] [Google Scholar]
- 41.Dang L, Su SM. Isocitrate dehydrogenase and (R)-2-hydroxyglutarate: from basic discovery to therapeutics development. Annu Rev Biochem. 2017;86:305–31. [DOI] [PubMed] [Google Scholar]
- 42.Urban DJ, Martinez NJ, Davis MI, Brimacombe KR, Cheff DM, Lee TD, et al. Assessing inhibitors of mutant isocitrate dehydrogenase using a suite of pre-clinical discovery assays. Sci Rep. 2017;7:12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fan B, Mellinghoff IK, Wen PY, Lowery MA, Goyal L, Tap WD, et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Invest New Drugs. 2019:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mellinghoff IK, Touat M, Maher E, De La Fuente M, Cloughesy TF, Holdhoff M, et al. ACTR-46. AG-120, A first-in-class mutant IDH1 inhibitor in patients with recurrent or progressive IDH1 mutant glioma: updated results from the phase 1 non-enhancing glioma population. Neuro Oncol. 2017;19(Suppl 6):vi10–vi1. [Google Scholar]
- 45.Mellinghoff I, Maher E, Wen P, Cloughesy T, Peters K, Choi C, et al. RBTT-03. A phase 1, multi-center, randomized, open-label, perioperative study of AG-120 (Ivosidenib) and AG-881 in patients with recurrent, non-enhancing, IDHI-mutant, low-grade glioma Neuro Oncol. 2018;20(Suppl 6):vi234. [Google Scholar]
- 46.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118:469–74. [DOI] [PubMed] [Google Scholar]
- 48.Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018. July;24(7):1036–46. [DOI] [PubMed] [Google Scholar]
- 49.Mellinghoff IK, Penas-Prado M, Peters KB, Cloughesy TF, Burris III HA, Maher EA, et al. ACTR-31. Phase 1 study of AG-881, an inhibitor of mutant IDH1 and IDH2: results from the recurrent/progressive glioma population. Neuro Oncol. 2018;20(Suppl 6):vi18. [Google Scholar]
- 50.Nicolay B, Narayanaswamy R, Amatangelo MD, Aguado E, Nagaraja R, Murtie J, et al. EXTH-34. Combined use of the pan IDH mutant inhibitor AG-881 with radiation therapy shoes added benefit in an orthotopic IDH1 mutant glioma model in vivo. Neuro Oncol. 2017;19(Suppl 6):vi79. [Google Scholar]
- 51.Pusch S, Krausert S, Fischer V, Balss J, Ott M, Schrimpf D, et al. Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of IDH1 mutant astrocytoma in vivo. Acta Neuropathol. 2017;133:629–44. [DOI] [PubMed] [Google Scholar]
- 52.Nakagawa M, Nakatani F, Matsunaga H, Seki T, Endo M, Ogawara Y, et al. Selective inhibition of mutant IDH1 by DS-1001b ameliorates aberrant histone modifications and impairs tumor activity in chondrosarcoma. Oncogene. 2019. August 12. [DOI] [PubMed] [Google Scholar]
- 53.Natsume A WT, Miyakita Y, Narita Y, Mineharu Y, Arakawa Y, Yamasaki F, Sugiyama K, Hata N, Muragaki Y, Nishikawa R, Shinojima N, Kumabe T, Saito R, Ito K, Tachibana M, Kakurai Y, Nishijima S, Tsubouchi H. Phase I study of a brain penetrant mutant IDH1 inhibitor DS-1001b in patients with recurrent or progressive IDH1 mutant gliomas. Journal of Clinical Oncology. 2019;May 20, 37(no.15_suppl):2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Y, Kwintkiewicz J, Liu Y, Tech K, Frady LN, Su YT, et al. Chemosensitivity of IDH1-Mutated Gliomas Due to an Impairment in PARP1-Mediated DNA Repair. Cancer Res. 2017. April 1;77(7):1709–18. [DOI] [PMC free article] [PubMed] [Google Scholar]