Abstract
Background
Colloids are widely used in the replacement of fluid volume. However, doubts remain as to which colloid is best. Different colloids vary in their molecular weight and therefore in the length of time they remain in the circulatory system. Because of this, and their other characteristics, they may differ in their safety and efficacy.
Objectives
To compare the effects of different colloid solutions in patients thought to need volume replacement.
Search methods
We searched the Cochrane Injuries Specialised Register (searched 1 December 2011), the Cochrane Central Register of Controlled Trials 2011, issue 4 (The Cochrane Library); MEDLINE (Ovid) (1948 to November Week 3 2011); EMBASE (Ovid) (1974 to 2011 Week 47); ISI Web of Science: Science Citation Index Expanded (1970 to 1 December 2011); ISI Web of Science: Conference Proceedings Citation Index‐Science (1990 to 1 December 2011); CINAHL (EBSCO) (1982 to 1 December 2011); National Research Register (2007, Issue 1) and PubMed (searched 1 December 2011). Bibliographies of trials retrieved were searched, and for the initial version of the review drug companies manufacturing colloids were contacted for information (1999).
Selection criteria
Randomised controlled trials comparing colloid solutions in critically ill and surgical patients thought to need volume replacement.
Data collection and analysis
Two review authors independently extracted the data and assessed the quality of the trials. The outcomes sought were death, amount of whole blood transfused, and incidence of adverse reactions.
Main results
Eighty‐six trials, with a total of 5,484 participants, met the inclusion criteria. Quality of allocation concealment was judged to be adequate in 33 trials and poor or uncertain in the rest.
Deaths were reported in 57 trials. For albumin or plasma protein fraction (PPF) versus hydroxyethyl starch (HES) 31 trials (n = 1719) reported mortality. The pooled relative risk (RR) was 1.06 (95% confidence interval (CI) 0.86 to 1.31). When the trials by Boldt were removed from the analysis the pooled RR was 0.90 (95% CI 0.68 to 1.20). For albumin or PPF versus gelatin, nine trials (n = 824) reported mortality. The RR was 0.89 (95% CI 0.65 to 1.21). Removing the study by Boldt from the analysis did not change the RR or CIs. For albumin or PPF versus dextran four trials (n = 360) reported mortality. The RR was 3.75 (95% CI 0.42 to 33.09). For gelatin versus HES 22 trials (n = 1612) reported mortality and the RR was 1.02 (95% CI 0.84 to 1.26). When the trials by Boldt were removed from the analysis the pooled RR was 1.03 (95% CI 0.84 to 1.27). RR was not estimable in the gelatin versus dextran and HES versus dextran groups.
Forty‐one trials recorded the amount of blood transfused; however, quantitative analysis was not possible due to skewness and variable reporting. Twenty‐four trials recorded adverse reactions, with two studies reporting possible adverse reactions to gel and one to HES.
Authors' conclusions
From this review, there is no evidence that one colloid solution is more effective or safe than any other, although the CIs were wide and do not exclude clinically significant differences between colloids. Larger trials of fluid therapy are needed if clinically significant differences in mortality are to be detected or excluded.
Keywords: Humans; Blood Proteins; Blood Proteins/therapeutic use; Colloids; Colloids/therapeutic use; Dextrans; Dextrans/therapeutic use; Fluid Therapy; Fluid Therapy/methods; Fluid Therapy/mortality; Hydroxyethyl Starch Derivatives; Hydroxyethyl Starch Derivatives/therapeutic use; Plasma Substitutes; Plasma Substitutes/therapeutic use; Randomized Controlled Trials as Topic; Rehydration Solutions; Rehydration Solutions/therapeutic use; Resuscitation; Resuscitation/methods; Resuscitation/mortality; Serum Albumin; Serum Albumin/therapeutic use; Serum Albumin, Human; Serum Globulins; Serum Globulins/therapeutic use
Plain language summary
Are particular types of colloid solution safer for replacing blood fluids than others?
When a person is bleeding heavily, the loss of fluid volume in their veins can lead to shock, so they need fluid resuscitation. Colloids and crystalloids are two types of solutions used to replace lost blood fluid (plasma). They include blood and synthetic products. Both colloids and crystalloids appear to be similarly effective at resuscitation. There are different types of colloids and these may have different effects. However, the review of trials found there is not enough evidence to be sure that any particular colloid is safer than any other.
Background
Colloids are used as plasma substitutes for short‐term replacement of fluid volume while the cause of the problem is being addressed (e.g. stopping bleeding). These solutions can be blood products (human albumin solution, plasma protein fraction (PPF)) or synthetic products (modified gelatins, dextrans, etherified starches). Colloid solutions are widely used in fluid resuscitation (Yim 1995) and they have been recommended in a number of resuscitation guidelines and intensive care management algorithms (Armstrong 1994; Vermeulen 1995). Previous systematic reviews have suggested that colloids are no more effective than crystalloids in reducing mortality (Perel 2012; Roberts 2011). Despite this, colloid solutions are still widely used as they are thought to remain in the intravascular space for longer than crystalloids and, therefore, be more effective in maintaining osmotic pressure.
It is plausible that colloids may vary in their safety and effectiveness. Different colloids vary in the length of time they remain in the circulatory system. It may be that some low‐to‐medium molecular weight colloids (e.g. gelatins and albumin) are more likely to leak into the interstitial space (Traylor 1996), whereas some larger molecular weight hydroxyethyl starches (HES) are retained for longer (Boldt 1996). In addition it is thought that some colloids may affect coagulation or cause other adverse effects.
This review examines direct comparisons of the different colloid solutions in randomised trials to complement the earlier reviews on colloids compared to crystalloids (Perel 2012) and human albumin (Roberts 2011).
Objectives
To quantify the relative effects on mortality of different colloid solutions in critically ill and surgical patients requiring volume replacement, by examining direct comparisons of colloid solutions.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Patients clinically assessed as requiring volume replacement or maintenance of colloid osmotic pressure.
Administration of fluid for preoperative haemodilution or volume loading, during plasma exchange, for priming extracorporeal circuits or following paracentesis are excluded.
Types of interventions
The colloid solutions considered are human albumin solutions, PPF, modified gelatins, dextran 70, or etherified starch solutions.
Trials of other blood products not used primarily for volume replacement (e.g. fresh frozen plasma (FFP), pooled serum) were excluded.
The review compares the administration of any regimens of different classes of colloids with each other.
Types of outcome measures
The primary outcome measure was mortality from any cause at the end of the study period.
We also attempted to find data on incidence of adverse reactions, allergies or anaphylactic shock, and the amount of blood (whole blood or red blood cells) transfused in each group. Some of the synthetic colloids may have anticoagulant properties and, therefore, we felt that some measure of blood loss or haemorrhage was important. However, as blood loss is vulnerable to measurement error, we decided to use the amount of blood products transfused as an outcome measure.
Intermediate physiological outcomes were not used for several reasons. These were that they are subject to intra‐ and inter‐observer variation, they have no face value to patients and relatives, and the ones seen as appropriate are not stable over time. Also there would need to exist a strong predictive relationship between the variable and mortality.
Search methods for identification of studies
We did not limit the search for trials by language, date, or publication status.
Electronic searches
We searched the following electronic databases:
Cochrane Injuries Specialised Register (searched 1 Dec 2011);
the Cochrane Central Register of Controlled Trials (2011, issue 4, The Cochrane Library);
MEDLINE (Ovid) (1948 to November Week 3 2011);
EMBASE (Ovid) (1974 to 2011 Week 47);
ISI Web of Science: Science Citation Index Expanded (1970 to 1 December 2011);
ISI Web of Science: Conference Proceedings Citation Index‐Science (1990 to 1 December 2011);
CINAHL (EBSCO) (1982 to 1 December 2011);
PubMed (ncbi.nlm.nih.gov/sites/entrez/) (searched 1 December 2011 limit‐Humans, published in the last 90 days);
National Research Register (issue 1, 2007);
Zetoc (searched 23 March 2007).
Full search strategies are listed in Appendix 1.
Searching other resources
We searched the bibliographies of the retrieved trials and contacted drug companies manufacturing colloids for information. For the original version of the review in 1999 we also identified trials by using the searches undertaken for the pre‐existing review of colloids versus crystalloids (Perel 2012), which included BIDS Index to Scientific and Technical Proceedings, drawing on the handsearching of 29 international journals and the proceedings of several international meetings on fluid resuscitation, and checking the reference lists of the trials found. There were no language restrictions in any of the searches.
To identify unpublished trials we searched the register of the Medical Editors' Trial Amnesty and we contacted the UK Medicines Control Agency.
For the first version of the review (published 1999) we also contacted the medical directors of the following companies, which all manufacture colloids:
Alpha Therapeutic UK Limited (Albutein),
American Critical Care McGraw (Hespan),
Bayer (Plasbumin),
Baxter (Gentran),
Bio Products Laboratory (Zenalb),
Cambridge Laboratories (Rheomacrodex),
Centeon Ltd (Albuminar),
CIS UK Ltd,
CP (Lomodex),
Common Services Agency,
Consolidated (Gelofusine),
DuPont (Hespan),
Fresenius (eloHAES and HAES‐Steril),
Geistlich Sons Ltd (Hespan and Pentaspan),
Hoechst (Haemaccel),
Mallinckrodt Medical GMBH (Infoson),
Nycomed, Oxford Nutrition (Elohes),
Pharmacia and Upjohn Ltd (Rheomacrodex),
Sorin Biomedica Diagnostics Spa.
Data collection and analysis
The Injuries Group Trials Search Co‐ordinator ran the electronic database searches, collated the results, and removed duplicates before sending them to the review authors for screening.
Selection of studies
One review author examined the search results for reports of possibly relevant trials and these reports were then retrieved in full. Two review authors applied the selection criteria independently to the trial reports, resolving disagreements by discussion.
Data extraction and management
Two review authors independently extracted information on the following:
method of allocation concealment,
number of randomised patients,
type of participants,
the interventions,
outcome data (numbers of deaths, volume of blood transfused, and incidence of adverse or allergic reactions).
The review authors were not blinded to the trial authors or journal when doing this, as the value of this has not been established (Berlin 1997). Results were compared and any differences resolved by discussion. Where there was insufficient information in the published report, we attempted to contact the trial authors for clarification.
Assessment of risk of bias in included studies
Since there is evidence that the quality of allocation concealment particularly affects the results of studies (Higgins 2011), two review authors scored this quality on the scale used by Higgins 2011 as shown below, assigning 'high risk of bias' to poorest quality and 'low risk of bias' to best quality:
low risk of bias = trials deemed to have taken adequate measures to conceal allocation (i.e. central randomisation; numbered or coded bottles or containers; drugs prepared by the pharmacy; serially numbered, opaque, sealed envelopes; or other description that contained elements convincing of concealment);
unclear risk of bias = trials in which the authors either did not report an allocation concealment approach at all or reported an approach that did not fall into one of the other categories;
high risk of bias = trials in which concealment was inadequate (such as alternation or reference to case record numbers or to dates of birth).
Where the method used to conceal allocation was not clearly reported, the trial author was contacted, if possible, for clarification. We then compared the scores allocated and resolved differences by discussion.
Data synthesis
The following comparisons were made:
albumin or PPF versus etherified starch,
albumin or PPF versus modified gelatin,
albumin or PPF versus dextran 70,
modified gelatin versus etherified starch,
modified gelatin versus dextran 70,
etherified starch versus dextran 70.
For each trial we calculated the risk ratio (RR) of death and 95% confidence interval (CI), such that a RR of more than 1 indicates a higher risk of death in the first group named.
We examined the groups of trials for statistical evidence of heterogeneity using Chi2 and I2 tests. If there was no obvious heterogeneity on visual inspection or statistical testing, we calculated pooled RRs and 95% CIs using a fixed‐effects model.
We assessed the skewness of continuous data by checking the mean and standard deviation (if available). If the standard deviation is more than twice the mean for data with a finite end point (such as 0 in the case of bleeding), the data are likely to be skewed and it is inappropriate to apply parametric tests (Altman 1996). This is because the mean is unlikely to be a good measure of central tendency. If parametric tests could not be applied, we tabulated the data.
Sensitivity analysis
We examined the effect of excluding trials judged to have inadequate (scoring 'high risk of bias') allocation concealment in a sensitivity analysis.
The editorial group is aware that a clinical trial by Professor Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication pointed out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. All Cochrane Injuries Group reviews that include studies by this author have therefore been edited to show the results with this author's trials included and excluded. Readers can now judge the potential impact of trials by this author on the conclusions of the review.
Results
Description of studies
For more detailed descriptions of individual studies, see 'Characteristics of included studies'.
Eighty‐six studies met the inclusion criteria, with a total of 5488 participants. The earliest trial was from 1980 and the most recent from 2011. From the drug companies that we contacted in 1999, we were sent information by Baxter Healthcare Ltd, CIS UK Ltd, Fresenius Ltd, Hoechst and Pharmacia. No new trials were identified from the information sent to us.
The trials included the following comparisons.
Albumin or PPF versus starch (50 trials with 2458 participants in these groups)
Arellano 2005; Boldt 1986; Boldt 1993a; Boldt 1995; Boldt 1996a; Boldt 1996b; Boldt 1996c; Boldt 1998; Brock 1995; Brutocao 1996; Claes 1992; Diehl 1982; Dolecek 2009; Falk 1988; Friedman 2008; Fulachier 1994; Gahr 1981; Gallagher 1985; Gold 1990; Gondos 2010; Haas 2007; Hausdorfer 1986; Hecht‐Dolnik 2009; Hiippala 1995; Huskisson 1993; Jones 2004; Kirklin 1984; London 1989; Mastroianni 1994; Moggio 1983; Mukhtar 2009; Munoz 1980; Munsch 1988; Niemi 2006; Prien 1990; Rackow 1983; Rackow 1989; Reine 2008; Rosencher 1992; Schramko 2009; Shatney 1983; Standl 2008; Veneman 2004; Verheij 2006; Vogt 1994; Vogt 1996; Vogt 1999; von Sommoggy 1990; Woittiez 1997; Yang 2011.
Albumin or PPF versus dextran (six trials with 410 participants in these groups)
Hedstrand 1987; Hiippala 1995; Jones 2004; Karanko 1987; Lisander 1996; Tollofsrud 1995.
Albumin or PPF versus gelatin (14 trials with 1152 participants in these groups)
Boldt 1986; Du Gres 1989; Evans 2003; Gondos 2010; Haas 2007; Huang 2005; Huskisson 1993; Karanko 1987; Niemi 2006; Stockwell 1992; Stoddart 1996; Tollofsrud 1995; Verheij 2006; Wahba 1996.
Starch versus gelatin (26 trials with 1883 participants in these groups)
Allison 1999; Asfar 2000; Beards 1994; Berard 1995; Beyer 1997; Boldt 1986; Boldt 2000; Boldt 2001; Carli 2000; Dytkowska 1998; Godet 2008; Gondos 2010; Haas 2007; Huskisson 1993; Inal 2010; Jin 2010; Mahmood 2007; Molnar 2004; Niemi 2006, Ooi 2009; Rittoo 2004; Schortgen 2001; Schramko 2010; Van der Linden 2004; Van der Linden 2005; Volta 2007.
Starch versus dextran (one trial with 30 participants in these groups)
Dextran versus gelatin (three trials with 82 participants in these groups)
Gombocz 2007; Karanko 1987; Tollofsrud 1995.
The trials involved patients with hypovolaemia, sepsis, trauma, and patients who had undergone surgery.
The trials tended to report surrogate outcomes such as haemodynamic variables. Data on death were obtainable from 57 trials. Information on the amount of blood or FFP transfused was available in 41 trials. However, the data were reported in a variety of different ways that made combining the data in a meta‐analysis unfeasible.
Inclusion and exclusion criteria varied, but many of the studies excluded patients with previous adverse reactions to colloids, clotting problems, or renal disease.
Risk of bias in included studies
Using the criteria defined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) the quality of allocation concealment was judged to be adequate (at low risk of bias) in 33 trials, unclear in 42 trials, and inadequate (at high risk of bias) in 10 trials. Where the method of allocation concealment was unclear, we attempted to contact all of the trialists and we obtained information from 16 of them. However, due to the lack of reported information on the process of randomisation and allocation concealment, we were unable to assess the quality in many of the trials properly.
Thirteen trials mentioned that some form of blinding was used. In nine, some, or all, of the staff giving treatment were blinded, in six those giving postoperative care were blinded, in two the outcome assessors were blinded, and in one the statisticians performing the analysis were blinded to treatment group.
Effects of interventions
Mortality
Of the 86 trials identified, 41 reported mortality data. Information on death was obtained from a further 16 trials by contact with the trial authors. We, therefore, had data on death from 57 trials.
Albumin or PPF versus HES
Thirty‐one trials (1719 participants) reported mortality data. The pooled RR was 1.06 (95% CI 0.86 to 1.31). When the trials by Boldt (Boldt 1993a; Boldt 1995; Boldt 1996a; Boldt 1996b; Boldt 1996c; Boldt 1998; Boldt 2006a) were removed from the analysis the pooled RR was 0.97 (95% CI 0.70 to 1.35).
Albumin or PPF versus gelatin
Nine trials (824 participants) reported mortality but only three of those trials had any deaths. The RR was 0.89 (95% CI 0.65 to 1.21). The Boldt trial included in this analysis had no events (Boldt 1993a), and therefore contributed no data to the analysis.
Albumin or PPF versus dextran
Four trials (360 participants) reported mortality and were included in the meta‐analysis. Only one of these reported any deaths (Hedstrand 1987). The RR was 3.75 (95% CI 0.42 to 33.09).
Gelatin versus HES
Twenty‐two studies (1612 participants) reported mortality and the pooled RR was 1.02 (95% CI 0.84 to 1.26). The effect was unchanged with removal of the six trials by Boldt (Boldt 1993a; Boldt 2000; Boldt 2001; Haisch 2001c; Haisch 2001c; Huttner 2000a) (RR 1.00; 95% CI 0.80 to 1.25).
Gelatin versus dextran 70
There were three trials (82 participants) that reported mortality. There were no deaths so the RR was not estimable.
HES versus dextran 70
No trials reported mortality.
Amount of blood transfused
Forty‐five trials recorded the amount of blood or FFP transfused. As the data were reported in various ways, often lacking a measure of variation, and was also skewed we did not attempt a quantitative synthesis. These data can be seen in the 'other data' tables.
Adverse events
Twenty‐four trials reported the incidence of adverse or allergic reactions or anaphylactic shock. The majority reported that there were no such incidents. However, one study (Akech 2006) reported a possible adverse reaction to gelatin (Gelufusine) and one (Godet 2008) reported two possible adverse reactions in the HES group and one in the gelatin group.
Sensitivity analysis
The effect of excluding trials judged to have inadequate or unclear allocation concealment was examined in a subgroup analysis. This made no significant difference to the results (albumin or PPF versus HES: pooled RR 1.08; 95% CI 0.86 to 1.36; albumin or PPF versus gelatin pooled RR 0.92; 95% CI 0.47 to 1.81; gelatin versus HES pooled RR 1.10; 95% CI 0.84 to 1.44).
There was also no significant difference when the trials by Boldt were removed from the analysis (albumin or PPF versus HES pooled RR 0.90 (95% CI 0.68 to 1.20), albumin or PPF vs gelatin 0.92 (0.47, 1.81), gelatin versus HES 1.03 (0.84, 1.27).
Removing both the trials with inadequate allocation concealment and the trials by Boldt from the albumin or PPF versus HES analysis gave a pooled effect of RR 0.88 (95% CI 0.63 to 1.24). The RR for gelatin versus HES was 1.12 (95% CI 0.85 to 1.47).
Discussion
Despite finding 90 trials we cannot make any conclusions about the relative effectiveness of different colloid solutions. Previous systematic reviews have suggested that colloids are no more effective than crystalloids in reducing mortality (Perel 2012; Roberts 2011), but there are too few data available to show in direct comparisons whether any of the colloids are safer or more effective than another. The CIs are wide and do not exclude clinically significant differences between colloids.
Mortality was selected as the main outcome measure in this systematic review for several reasons. In the context of critical illness, death or survival is a clinically relevant outcome that is of immediate importance to patients, and data on death are reported in many of the studies. Furthermore, one might expect that mortality data would be less prone to measurement error or biased reporting than would data on pathophysiological outcomes. The use of a pathophysiological end point as a surrogate for an adverse outcome assumes a direct relationship between the two, an assumption that may sometimes be inappropriate. Finally, when trials collect data on a number of physiological end points, there is the potential for bias due to the selective publication of end points showing striking treatment effects.
There was wide variation in the participants, intervention regimens, and the length of follow‐up. The length of follow‐up was not reported in many of the studies. Where it is reported it ranges from a matter of hours to months, which may explain a high proportion of the heterogeneity in overall event rates. The effect of these factors was not examined in a sensitivity analysis, as there was felt to be insufficient data to justify examining subgroups.
Many of the trials were small, and some had been done some time ago. Although older trials will not necessarily be of poorer quality, it may be that treatment protocols have subsequently altered making these trials less relevant to current clinical practice.
Authors' conclusions
Implications for practice.
Previous reviews have not shown a benefit of colloids over crystalloids for volume replacement (Perel 2012; Roberts 2011).
This review does not provide any evidence that one colloid is safer than another, but does not rule out clinically significant differences.
Implications for research.
Trials of fluid therapy need to be larger in order to exclude clinically significant differences between colloids in patient relevant outcomes. However, trials should probably first address the question of whether colloids are any more effective than crystalloid solutions.
Use of surrogate outcomes, such as physiological measurements, should be discouraged unless there is a strong relationship with outcomes of interest to patients and relatives.
What's new
| Date | Event | Description |
|---|---|---|
| 16 October 2012 | Amended | Minor copy edits made to analysis labels |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 12 June 2012 | New citation required but conclusions have not changed | Due to the retraction of four studies (Boldt 2006; Haisch 2001a; Haisch 2001b; Huttner 2000), the review has been amended. The retracted studies, and their associated data, are now excluded from the review. The conclusions of the review have not changed. |
| 1 May 2012 | New citation required but conclusions have not changed | The review has been updated to December 2011. Twenty additional studies have been included (Akech 2006;Dolecek 2009; Friedman 2008; Godet 2008; Gombocz 2007; Gondos 2010; Haas 2007; Hecht‐Dolnik 2009; Inal 2010; Jin 2010; Mahmood 2007; Mittermayr 2007; Mukhtar 2009; Ooi 2009; Reine 2008; Schramko 2009; Schramko 2010; Standl 2008; Volta 2007; Yang 2011). The conclusions of the review have not changed. |
| 30 April 2012 | New search has been performed | The review has been updated to December 2011. |
| 10 February 2011 | New citation required but conclusions have not changed | The editorial group is aware that a clinical trial by Prof. Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. All Cochrane Injuries Group reviews which include studies by this author have therefore been edited to show the results with this author's trials included and excluded. Readers can now judge the potential impact of trials by this author (Boldt 1986, Boldt 1993a, Boldt 1995, Boldt 1996a, Boldt 1996b, Boldt 1996c, Boldt 1998, Boldt 2000, Boldt 2001, Boldt 2006a, Haisch 2001c, Haisch 2001c, Huttner 2000a) on the conclusions of the review. |
| 11 July 2008 | Amended | Converted to new review format. |
| 2 October 2007 | New search has been performed | The search for the review was updated in March 2007 and thirteen new studies were added to the review. |
Notes
The editorial group is aware that a clinical trial by Professor Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. All Cochrane Injuries Group reviews which include studies by this author have therefore been edited to show the results with this author's trials included and excluded. Readers can now judge the potential impact of trials by this author (Boldt 1986; Boldt 1993a;Boldt 1995;Boldt 1996a;Boldt 1996b;Boldt 1996c;Boldt 1998;Boldt 2000;Boldt 2001; Boldt 2006a Haisch 2001c Haisch 2001c Huttner 2000a) on the conclusions of the review.
Emma Sydenham, Managing Editor, performed the sensitivity analysis in 2011. The authors agreed with the changes to the manuscript.
Acknowledgements
We wish to acknowledge the contribution of Phil Alderson, Victoria Hawkins and Syed Ashraf who were authors of earlier versions of this review. In addition, we acknowledge the help of Ralph Bloch, Olivier Duperrex, Andrew Smith, Peter Smith, and Reinhard Wentz, who assisted with translating articles. Also many thanks to the authors who provided us with details of their studies.
We are grateful to the drug companies, Baxter Healthcare Ltd, CIS Ltd, Fresenius Ltd, Hoechst, and Pharmacia who responded to our request for information.
Appendices
Appendix 1. Search strategy
| Cochrane Injuries Specialised Register (searched: 1 December 2011) 1. (colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*) 2. (fluid* or volume or plasma or rehydrat* or blood or oral) and (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*) 3. 1 and 2 |
| Cochrane Central Register of Controlled Trials 2011, issue 4 (The Cochrane Library) #1 MeSH descriptor Colloids explode all trees in MeSH products #2 MeSH descriptor Plasma explode all trees in MeSH products #3 MeSH descriptor Albumins explode all trees in MeSH products #4 (colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*) #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Fluid Therapy explode all trees in MeSH products #7 MeSH descriptor Plasma Volume explode all trees #8 (fluid* or volume or plasma or rehydrat* or blood or oral) near1 (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*) #9 (#6 OR #7 OR #8) #10 (#5 AND #9) #11 (#10), from 2007 to 2011 |
| MEDLINE (Ovid) (1948 to November Week 3 2011) 1. exp Albumins/ 2. exp plasma/ 3. exp colloids/ 4. (colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*).ti,ab. 5. 1 or 2 or 3 or 4 6. Exp Plasma volume/ 7. Exp Fluid Therapy/ 8. ((fluid* or volume or plasma or rehydrat* or blood or oral) adj1 (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*)).ab,ti. 9. 6 or 7 or 8 10. 5 and 9 |
| EMBASE (Ovid) (1974 to 2011 Week 47) 1. exp ALBUMIN/ 2. exp HYDROCOLLOID/ 3. exp PLASMA/ 4. (colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*).ti,ab. 5. 1 or 2 or 3 or 4 6. exp Fluid Therapy/ 7. exp Plasma volume/ 8. ((fluid* or volume or plasma or rehydrat* or blood or oral) adj1 (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*)).ab,ti. 9. 6 or 7 or 8 10. 5 and 9 11. exp Randomized Controlled Trial/ 12. exp controlled clinical trial/ 13. randomi?ed.ab,ti. 14. placebo.ab. 15. *Clinical Trial/ 16. randomly.ab. 17. trial.ti. 18. 11 or 12 or 13 or 14 or 15 or 16 or 17 19. exp animal/ not (exp human/ and exp animal/) 20. 18 not 19 21. 10 and 20 22. (2007* or 2008* or 2009* or 2010* or 2011*).em. 23. 21 and 22 |
| ISI Web of Science: Science Citation Index Expanded (1970 to 1 December 2011), ISI Web of Science: Conference Proceedings Citation Index‐Science (1990 to 1 December 2011) #1 Topic=((colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*)) AND Topic=((fluid* or volume or plasma or rehydrat* or blood or oral) NEAR/1 (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*)) #2 TS=((singl* OR doubl* OR trebl* OR tripl*) NEAR/1 (blind* OR mask*)) OR TS=((clinical OR control* OR placebo OR random*) NEAR/1 (trial* or group* or study or studies or placebo or controlled)) NOT TI=(Animal* or rat or rats or rodent* or mouse or mice or murine or dog or dogs or canine* or cat or cats or feline* or rabbit or rabbits or pig or pigs or porcine or swine or sheep or ovine* or guinea pig*) #3 #1 and #2 |
| CINAHL (EBSCO) (1982 to 2011) S1. (fluid* or volume or plasma or rehydrat* or blood or oral) N3 (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*) S2. colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid* S3. S1 and S2 (limit to Publication Type: Randomized Controlled Trial) |
| PubMed [www.ncbi.nlm.nih.gov/sites/entrez/] (searched 1 December 2011: Limit‐Humans, published in the last 90 days) #1((randomized controlled trial[pt] OR controlled clinical trial[pt]) OR (randomized OR randomised OR randomly OR placebo[tiab]) OR (trial[ti]) OR ("Clinical Trials as Topic"[MeSH Major Topic])) NOT (("Animals"[Mesh]) NOT ("Humans"[Mesh] AND "Animals"[Mesh])) #2 (fluid* or volume or plasma or rehydrat* or blood or oral) and (replac* or therapy or substitut* or restor* or resuscitat* or rehydrat*) #3 (colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*) #4 (("Albumins"[Mesh]) OR "Colloids"[Mesh]) OR "Plasma"[Mesh] #5 #3 or #4 #6 #1 and #2 and #5 |
| NRR up to issue 1, 2007 #1 (colloid* or albumin* or albumen* or plasma* or starch* or dextran* or gelofus* or hemacc* or haemacc* or hydrocolloid*) #2 ((plasma* or fluid* or volum*) and (therap* or restor* or resuscita* or substitut* or replac*)) #3 #1 and #2 |
| ZETOC searched on 23 March, 2007 Colloid* fluid* resusc* |
Data and analyses
Comparison 1. Albumin or PPF versus HES.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 31 | 1719 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.86, 1.31] |
| 2 Blood/red cells transfused (skewed or inadequate data) | Other data | No numeric data |
1.1. Analysis.
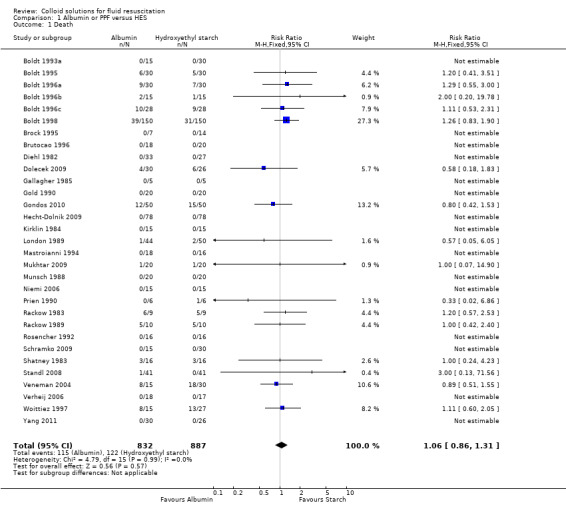
Comparison 1 Albumin or PPF versus HES, Outcome 1 Death.
1.2. Analysis.
Comparison 1 Albumin or PPF versus HES, Outcome 2 Blood/red cells transfused (skewed or inadequate data).
| Blood/red cells transfused (skewed or inadequate data) | ||
|---|---|---|
| Study | Notes | |
| Arellano 2005 | HA group received median of 1 unit each; HES median of 3 units each | |
| Boldt 1998 | Total units of red blood cells transfused given for each group (Hetastarch 356, albumin 371). No means, medians, or measures of variation given | |
| Brock 1995 | The amount of blood derivatives ('blutderivate') was given in millilitres as a mean and standard deviation (SD). In the 10% starch group the mean was 379 (SD 483), in the 6% starch group the mean was 243 (SD 192) and in the 5% albumin group the mean was 171 (SD 236) | |
| Brutocao 1996 | Packed red cell transfusion is given in mL/kg. In the HES group the mean was 0.3, the SD 1.3, and the range of 0 to 6.4. In the albumin group the mean was 1.1, the SD 3.7, and the range 0 to 13.1 | |
| Claes 1992 | Blood transfused was not recorded. Authors state "none of the patients lost an abnormally large quantity of blood or experienced a clinically perceptible coagulation disorder" | |
| Diehl 1982 | 18% (n = 5) of the albumin group and 15% (n = 5) of the HES group received banked blood during their stay. Blood transfused was recorded as mean number of units per person. In the albumin group this was 0.37 units per person and in the HES group this was 0.36 units per person | |
| Falk 1988 | Packed red blood cells transfused at 24 hours was given in millilitres. The albumin group received a mean of 375 with a standard error of the mean (SEM) of 244 and the HES group received a mean of 700 with an SEM 228 | |
| Gallagher 1985 | Amount of blood products transfused postoperatively was given as a mean in millilitres with the SEM. For the albumin group the mean was 560 (SEM 149.2) and for the starch group the mean was 566 (SEM 72.6) | |
| Gold 1990 | Packed red blood cells is given in units. The albumin group received a mean of 2.05 and the HES group received a mean of 2.50 | |
| Hecht‐Dolnik 2009 | Data given as mean number of units (SD) RBC: HES 1.13 (2.52), HA 0.40 (0.89), P = 0.0002 Platelets: HES 0.35 (0.77), HA 0.13 (0.38), P = 0.0001 FFB: HES 0.56 (1.24), HA 0.15 (0.56), P value not significant |
|
| Hiippala 1995 | Amount of red cell concentrates transfused was given as a mean and SD of millilitres per kilogram body weight (mL/kgBW). For albumin the mean was 20 (SD 14), for 4% HES the mean was 20 (SD 14) and for 6% HES the mean was 25 (SD 17) | |
| Jones 2004 | HA group received mean of 0.5 units (range 0 units to 1 unit), HEs group received mean of 1 unit (range of 0 units to 2 units) | |
| Kirklin 1984 | The amount of red cells given up to the first 24 hours postoperatively was recorded. In the HES group the mean was 430 with a standard error of 90, and in the albumin group the mean is 440 with a standard error of 76 | |
| London 1989 | Total postoperative blood transfused is given in millilitres. In the albumin group the figures are given as 838 mL (630 mL) and the HES group 894 mL (600 mL). It does not report what the figures represent (they may be mean and SD). Intraoperatively the blood given in the albumin group was 400 mL (346 mL) and in the HES group 336 mL (400 mL) | |
| Mastroianni 1994 | The mean of packed red cells given was recorded in millilitres. For pentastarch the mean was 167 and for albumin it was 234. Another figure was given 163 for pentastarch and 148 for albumin but it was not clear what this represented | |
| Mukhtar 2009 | Reported as units of PRBCs, mean and range. Intraoperatively HA 4 (0 to 6), HES 4 (0 to 10), postoperatively HA 4 (0 to 8), HES 2 (0 to 8) | |
| Munsch 1988 | The amount of whole blood transfused was given as a median volume. For the albumin group it was 830 mL (range 260 mL to 1800 mL), and for the HES group it was 830 mL (range 50 mL to 1840 mL) | |
| Niemi 2006 | The mean and SD of number of RBC units transfused was given. HA mean 0.2 (SD 0.6), HES mean 0.3 (SD 0.6) | |
| Prien 1990 | The mean and SEM for the amount of packed red cells given was recorded. For the albumin group the mean was 1.2 (SEM 0.7). In the HES group the mean was 1.8 (SEM 0.7) | |
| Rackow 1983 | Total amount of blood transfused was given in millilitres at the end of the maintenance period. For the albumin group the mean was 363.9 (SEM 186) and for the starch group the mean was 757.1 (SEM 201) | |
| Rackow 1989 | No data on units transfused. The authors say "there was no evidence of clinical bleeding" | |
| Shatney 1983 | The amount of red blood cells transfused was given in a graphical form not figures | |
| Standl 2008 | Data given as mean number of units with SD RBC: HES 52.2 (139.2), 53.4 (155.9) FFP: HES 22.4 (117.9), HA 25.2 (90.7) No significant difference between groups |
|
| Vogt 1994 | Amount of EK given was recorded as a mean and SD of the millilitres given. For the albumin group it was 1138 (SD 763.5), and for the HES group it was 944.4 (SD 466.2) | |
| Vogt 1996 | The mean and SD of packed red blood cells transfused was given for the end of surgery and at 6 hours. For the albumin group at the end of surgery the mean was 798 (SD 1147) and at 6 hours it was 1333 (SD 1399). For the HES group at the end of surgery the mean was 763 (SD 923) and at 6 hours the mean was 1538 (SD 1074) | |
| Vogt 1999 | Amount of packed red blood cells was given as mean and SD. In the HES group the mean was 1510 mL (SD 765 mL) and in the albumin group the mean was 1410 mL (SD 946 mL) | |
| von Sommoggy 1990 | The trialists report 'no increased bleeding in the HES group' | |
Comparison 2. Albumin or PPF versus gelatin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 9 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.65, 1.21] |
| 2 Blood/red cells transfused (skewed or inadequate data) | Other data | No numeric data |
2.1. Analysis.
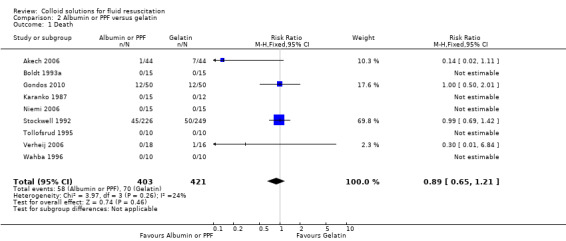
Comparison 2 Albumin or PPF versus gelatin, Outcome 1 Death.
2.2. Analysis.
Comparison 2 Albumin or PPF versus gelatin, Outcome 2 Blood/red cells transfused (skewed or inadequate data).
| Blood/red cells transfused (skewed or inadequate data) | |||||
|---|---|---|---|---|---|
| Study | Notes | ||||
| Evans 2003 | No data on amount of units transfused. Author reports that there was no significant difference in the median total blood loss between the groups (P = 0.5587) | ||||
| Niemi 2006 | The mean and standard deviation (SD) of RBC units transfused was given. HA mean 0.2 (SD 0.6), Gel mean 0.2 (SD 0.4) | ||||
| Stockwell 1992 | The volume of blood products given was recorded as a mean with the range also given. In the albumin group the mean was 1.45 L (range 0‐29) and in the haemacell group the mean was 1.39 L (range 0 L to 66 L) (P = 0.65, Mann‐Whitney U test) | ||||
| Tollofsrud 1995 | The amount of erthrocytes given was recorded as a mean and SD. In the albumin group the mean was 240 (SD 310), and in the polygeline group the mean was 490 (SD 548) | ||||
Comparison 3. Albumin or PPF versus dextran.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 4 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [0.42, 33.09] |
| 2 Blood/red cells transfused (skewed or inadequate data) | Other data | No numeric data |
3.1. Analysis.
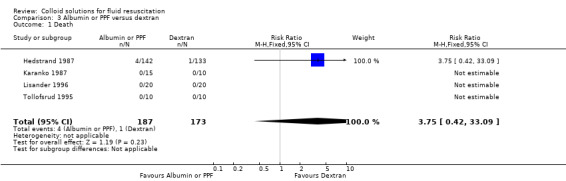
Comparison 3 Albumin or PPF versus dextran, Outcome 1 Death.
3.2. Analysis.
Comparison 3 Albumin or PPF versus dextran, Outcome 2 Blood/red cells transfused (skewed or inadequate data).
| Blood/red cells transfused (skewed or inadequate data) | |||||
|---|---|---|---|---|---|
| Study | Notes | ||||
| Hedstrand 1987 | The perioperative and postoperative amount of red blood cells transfused was reported as a mean and standard deviation (SD) of units given. For the plasma group the mean was 5.2 (SD 4.8) and for the dextran group the mean was 5.8 (SD 4.4) | ||||
| Hiippala 1995 | Amount of red cell concentrates transfused was given as a mean and SD of millilitre per kilo gram body weight (mL/kgBW). For albumin the mean was 20 (SD 14) and for dextran the mean was 19 (SD 12) | ||||
| Jones 2004 | Mean of 0.5 unit HA (range 0 to 1), mean of 1 for DEX (range 0 to 2) | ||||
| Lisander 1996 | Total red blood cells transfused is given. For the albumin group the mean was 2.3 (SD1.6), in the dextran group the mean was 3.8 (SD 2.4). Red cells autotransfused was also given as 312 (SD 184) in the albumin group and 383 (SD 259) in the dextran group | ||||
| Tollofsrud 1995 | Erythrocytes given was recorded as mean and SD. The mean for the albumin group was 240 (SD 310) and the mean for the dextran group was 390 (SD 417) | ||||
Comparison 4. Modified gelatin versus HES.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 22 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.84, 1.26] |
| 2 Blood/red cells transfused (skewed or inadequate data) | Other data | No numeric data |
4.1. Analysis.
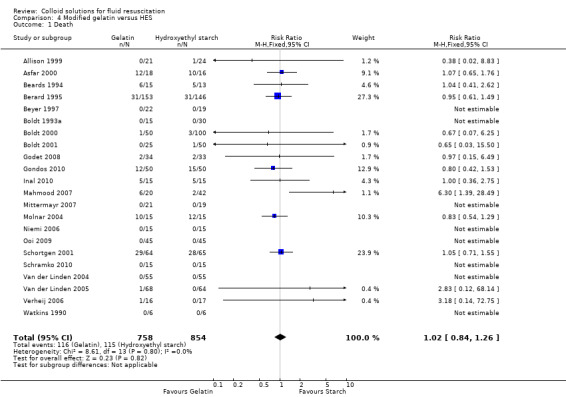
Comparison 4 Modified gelatin versus HES, Outcome 1 Death.
4.2. Analysis.
Comparison 4 Modified gelatin versus HES, Outcome 2 Blood/red cells transfused (skewed or inadequate data).
| Blood/red cells transfused (skewed or inadequate data) | |||||
|---|---|---|---|---|---|
| Study | Notes | ||||
| Allison 1999 | The mean volume of packed red blood cells (PRBC) transfused was given for each day up to and including the 5th day. For the first postoperative day the hydroxyethyl starch (HES) group received a total of 3067 mL of PRBCs and the gelatine group received 2643 mL of PRBCs | ||||
| Berard 1995 | Blood transfused was given in units, 2.6 units for the gel group and 2.5 units for the HES group (presumably this figure is mean) | ||||
| Beyer 1997 | Blood transfused is given in graphical form and not figures | ||||
| Boldt 2000 | The amount of PRBC transfused is given as the total number of units for each group
By the first post operative day the number of units of PRBCs transfused was: HES 70: 38 units, HES 200: 40 units, Gelatin: 44 units |
||||
| Boldt 2001 | The amount of PRBC transfused is given as the total number of units for each group By the first post operative day the number of units of PRBCs transfused was: HES 200: 18 units, HES 130: 16 units, Gelatin 18 units | ||||
| Carli 2000 | The amount of PRBC transfused is given as the total number of units for each group 1 unit of blood was given in the gel group and 0 units of blood were given in the starch group | ||||
| Mahmood 2007 | Amount of red cells and FFP is given as median number of units (range) Red cells: HES 200/0.62 = 7.0 (4.5 to 10), HES 130/0.4 = 6.0 (4.0 to 8.0), gelatin = 7.0 (5.25 to 9.75). P = 0.360 (no statistical difference between groups) FFP: HES 200/0.62 = 4 (0 to 6), HES 130/0.4 = 2 (0 to 5), gelatine = 4 (0 to 7). P = 0.420 (no statistical difference between groups) |
||||
| Mittermayr 2007 | Total red cells units transfused Gelatin n = 13, HES n = 9 Number of patients transfused Gelatin n = 8/21, HES n = 3/19 |
||||
| Niemi 2006 | The mean and SD of red blood cell (RBC) units transfused was given. Gel mean 0.2 (SD 0.4), HES 0.3 (0.6) |
||||
| Ooi 2009 | Data reported as number of patients who received at least 1 unit PRBCs: HES = 40, gelatin = 42. P = 0.46 FFP: HES = 17, gelatin = 24. P = 0.14 No statistical difference between groups |
||||
| Schramko 2009 | Data given as number of units of RBC and FFP transfused RBC: HES 200/0.5 = 11, HES 130/0.4 = 5, HA = 5 FFP: HES 200/0/5 = 1, HES 130/0.4 = 1, HA = 0 No significant difference between groups |
||||
| Schramko 2010 | Data given as number of units of RBC and FFP transfused HES group received 15 units of RBC and 2 units of FFP Gel group received 21 units of RBC and 2 units of FFP No significant difference between groups |
||||
| Van der Linden 2004 | HES group received total of 12 units of PRBC, GEL group received 3 units of PRBC | ||||
| Van der Linden 2005 | No of patients receiving allogenic blood in each group HES group n= 24, GEL n= 21 No of units of PRBC (median and range) HES 0 (range 0‐6), Gel 0 (range 0‐6) |
||||
Comparison 5. Modified gelatin versus dextran.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 3 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Blood/red cells transfused (skewed or inadequate data) | Other data | No numeric data |
5.1. Analysis.
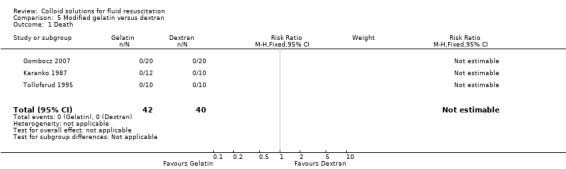
Comparison 5 Modified gelatin versus dextran, Outcome 1 Death.
5.2. Analysis.
Comparison 5 Modified gelatin versus dextran, Outcome 2 Blood/red cells transfused (skewed or inadequate data).
| Blood/red cells transfused (skewed or inadequate data) | |||||
|---|---|---|---|---|---|
| Study | Notes | ||||
| Gombocz 2007 | Units of red blood cells transfused Dextran (group A): mean 1.8 (standard deviation (SD) 1.3) Oxypolygelatin (group B): mean 1.6 (SD 1.2) P = 0.548 |
||||
| Tollofsrud 1995 | Erythrocytes given was recorded as mean and SD Polygeline: mean 490 (SD 548) Dextran: 390 (SD 417) |
||||
Comparison 6. HES versus dextran.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood/red cells transfused (skewed or inadequate data) | Other data | No numeric data |
6.1. Analysis.
Comparison 6 HES versus dextran, Outcome 1 Blood/red cells transfused (skewed or inadequate data).
| Blood/red cells transfused (skewed or inadequate data) | |||||
|---|---|---|---|---|---|
| Study | Notes | ||||
| Hiippala 1995 | Amount of red cell concentrates transfused in millilitres/kilogram body weight (mL/kgBW) was given as a mean and standard deviation Dextran mean 19 (SD 12) 4% Starch mean 20 (SD 14) 6% Starch mean 25 (SD 17) |
||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akech 2006.
| Methods | Randomised design. Fluid interventions allocated sequentially in blocks of 10 ITT analysis |
|
| Participants | 88 children over 3 months of age with severe malaria complicated by metabolic acidosis. Inclusion criteria: severe malaria, metabolic acidosis, and clinical feature of shock. Excluded if had pulmonary oedema, oedematous malnutrition, or papilloedema | |
| Interventions | 1) 4% Modified gelatin (n = 44) 2) 4.5% Albumin (n = 44) |
|
| Outcomes | Death Resolution of shock and acidosis Neurological sequelae at discharge Adverse events |
|
| Notes | Intervention arms not blinded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Authors report that allocation of intervention was not concealed |
Allison 1999.
| Methods | Randomised controlled trial. Randomisation was based on date of admission Analysis not ITT | |
| Participants | 45 patients with blunt trauma who required colloid infusion. Patients were excluded if they were less than 12 years old, did not require admission to the ITU, died within 24 hours, were pregnant or in renal failure 8 gelatin and 6 HES patients excluded after randomisation | |
| Interventions | 1) HES (200/0.45 Pentaspan) (n = 24) 2) Gelatin (Gelofusine) (n = 21) After 24 hours, colloid administration was at the discretion of the clinician | |
| Outcomes | Death Glasgow coma score Volumes of blood and platelets infused Haematological parameters | |
| Notes | Data were collected until the patient left the ITU or for a maximum of 5 days. Main outcome of interest was capillary leak | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Randomisation was based on date of admission (on even dates patients received HES) |
Arellano 2005.
| Methods | Randomised controlled trial. All participants, healthcare workers, and study personnel blinded to allocation | |
| Participants | 50 adults undergoing surgical ablation of oropharyngeal cancer with free flap reconstruction (mean age 55 years). Exclusion criteria ‐ ASA Physical Status Classification 3‐4, cardiac insufficiency, pancreatitis, severe hepatic dysfunction, renal dysfunction, anaemia, coagulation abnormalities, ingestion of NSAID, or ASA within 10 days of surgery and previous major head and neck surgery with free flap reconstruction | |
| Interventions | 1) 5% HA (n = 25) 2) HES 264/0.45 (n = 25) CVP was maintained between 7 mmHg and 10 mmHg | |
| Outcomes | Clinical indices of coagulation Number of units of blood transfused | |
| Notes | Follow‐up 24 hours. 1 patient in each group did not complete the study because planned surgical procedure was abandoned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Study colloids placed in masked container by nurse not involved in other aspects of trial |
Asfar 2000.
| Methods | Randomised controlled trial | |
| Participants | 34 septic, hypovolaemic, ventilated, and haemodynamically controlled patients Inclusion criteria: patients aged over 16 years, systolic arterial pressure higher than 90 mmHg and hypovolaemia defined by PAOP of 12 mmHg or less Patients were excluded if they had an overt haemodynamic, ventilatory, or acid base status instability. Sepsis was identified by either positive bacterial blood cultures, bronchoalveolar lavage, or clinical evidence of infection | |
| Interventions | 1) 6% HES (n = 16) 2) 4% MFG (n = 18) | |
| Outcomes | Death Haemodynamic variables | |
| Notes | Follow‐up 1 hour. 2 patients in the HES group were excluded because they experienced haemodynamic instability. The final analysis was made on remaining 16 patients. Information on allocation concealment obtained from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation using sequentially numbered sealed opaque envelopes |
Beards 1994.
| Methods | Randomised controlled trial | |
| Participants | 28 patients with hypovolaemia, mechanically ventilated for concurrent acute respiratory failure. Patients fulfilled the following inclusion criteria: age >16 years, body weight between 50 kg and 85 kg, MAP < 80 mmHg (or 30 mmHg less than previously recorded); PAOP < 10 mmHg with oliguria (i.e. urine output < 15 mL/hour) | |
| Interventions | 1) Rapid infusion of 500 mL MFG (n = 15) 2) Rapid infusion of 500 mL hetastarch (n = 13) | |
| Outcomes | Death Haemodynamic variables Oxygen variables | |
| Notes | Follow‐up 30 minutes for haemodynamic variables and until discharge for deaths. Information on allocation concealment was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Allocation by alternation |
Berard 1995.
| Methods | Randomised controlled trial. Blinding not mentioned | |
| Participants | 319 patients in a resuscitation service receiving medical (gastrointestinal haemorrhage) and surgical cases. Patients were excluded if they had had a prior allergic reaction | |
| Interventions | 1) Gelatin (n = 153) 2) HES (n = 146) The prescribers chose the quantity of colloid, guided by normal practice | |
| Outcomes | Death Amount of colloid and RBCs given Cost | |
| Notes | 20 patients lost to follow‐up, no explanation given. Follow‐up to discharge. Information on method of randomisation was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. 'A set of 200 tickets (type 1) and another set of 200 tickets (type 2) were mixed in a box. One ticket was drawn at random for each patient' |
Beyer 1997.
| Methods | Randomised controlled trial. No blinding | |
| Participants | 48 patients undergoing major elective hip surgery with an expected blood loss of > 1000 mL. Exclusion criteria were Hb concentration 11 g/dL or less; heart failure and coronary artery disease; MI within the past 6 months; hypertension (> 180 mmHg systolic); impaired renal function; pregnancy; known hypersensitivity to HES or gelatin; patient taking drugs that may specifically affect blood viscosity, diuresis, or clotting | |
| Interventions | 1) 3% MFG (n = 22) 2) 6% HES (n = 19) Both groups also given RL. Fluids administered according to haemodynamic and clinical parameters | |
| Outcomes | Death (information on death was obtained by contact with the study author) Haemodynamic variables Packed cell volume, Hb, clotting times Incidence of allergic reactions | |
| Notes | 7 patients were lost to follow‐up but only 5 were accounted for. Information on method of allocation concealment was obtained by contact with the author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation was by a list of random numbers read by someone not entering patients into the trial (closed list) |
Boldt 1986.
| Methods | Randomised controlled trial, using sealed opaque envelopes Information on allocation concealment was obtained on contact with the study authors Blinding not mentioned Loss to follow‐up not mentioned | |
| Participants | 55 patients undergoing elective aortocoronary bypass surgery Exclusion criteria were ejection fraction < 50% and LVEDP >15 mmHg | |
| Interventions | 1) 500 mL 20% HA (n = 15) 2) 500 mL 3% HES (n = 13) 3) 500 mL 3.5% Gelatin (n = 14) A fourth group received no colloid (n = 13) | |
| Outcomes | Haemodynamic variables Incidence of anaphylactic shock Amount blood transfused | |
| Notes | Follow‐up until discharge from ICU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Boldt 1993a.
| Methods | Randomised controlled trial | |
| Participants | 75 men undergoing elective aortocoronary bypass grafting, who had a PCWP of < 5 mmHg after induction of anaesthesia | |
| Interventions | 1) HA 5% (n = 15) 2) 6% HES, HMW (n = 15) 3) 6% HES, LMW (n = 15) 4) Gelatin 3.5% (n = 15) 5) No additional volume | |
| Outcomes | Death (information obtained on contact with author) Haemodynamic variables | |
| Notes | Follow‐up 1 day. Information on allocation was obtained on contact with study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation by sequentially numbered sealed opaque envelopes |
Boldt 1995.
| Methods | Randomised controlled trial. Blinding of outcome assessors not mentioned | |
| Participants | 30 consecutive trauma patients (injury severity score > 15) and 30 consecutive septic patients who underwent major surgery. Exclusions: patients suffering from renal failure requiring haemofiltration, severe liver dysfunction or coagulation abnormalities in their history were excluded as were patients who were receiving aspirin or other cyclooxygenase inhibitors | |
| Interventions | 1) 10% HES, LMW (15 trauma patients and 15 sepsis patients) 2) 20% HA (15 trauma patients and 15 sepsis patients) Fluid was given to maintain CVP and PCWP between 12 mmHg and 16 mmHg | |
| Outcomes | Death Haemodynamic variables | |
| Notes | Follow‐up at 5 days Deaths were reported within the study period and later (time not specified). Information on allocation concealment was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Boldt 1996a.
| Methods | Randomised controlled trial. Outcome assessors blinded to treatment | |
| Participants | 30 trauma patients and 30 patients with from sepsis secondary to major general surgery. Exclusions were patients with renal impairment, liver insufficiency, disseminated intravascular coagulation, or septic shock | |
| Interventions | 1) 10% HES (n = 30)
2) 20% HA solution (n = 30)
All patients also received RL Volume therapy was given to maintain PCWP between 12 mmHg and 18 mmHg |
|
| Outcomes | Death Haemodynamic variables | |
| Notes | Follow‐up at 5 days and at discharge from ICU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation by sequentially numbered sealed opaque envelopes |
Boldt 1996b.
| Methods | Randomised controlled trial. The doctors giving the fluid were blinded to the solution but blinding of outcome assessors not mentioned. Loss to follow‐up not reported | |
| Participants | 45 consecutive trauma patients transferred to the surgical ICU. Inclusion criteria: injury severity score of > 15 points All patients were haemodynamically stable before being admitted to the study | |
| Interventions | 1) 10% HES (n = 15) 2) 20% HA (n = 15) 3) Unspecified volume therapy regimen (n = 15) The allocated solution was given to maintain CVP and or PAWP between 12 mmHg and 18 mmHg | |
| Outcomes | Death Haemodynamic variables Circulating adhesion molecules | |
| Notes | Deaths were reported within the study period and later (left ITU). Information on allocation concealment was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Boldt 1996c.
| Methods | Randomised controlled trial. Outcome variables were collected by an investigator who was blinded to the treatment. Loss to follow‐up not reported | |
| Participants | 56 patients from the surgical ICU. 28 patients with an injury severity score > 15 and 28 patients with sepsis secondary to major surgery. Patients with renal insufficiency, urine output < 20 mL/hour, severe liver dysfunction, or disseminated intravascular coagulation were excluded | |
| Interventions | 1) 10% HES, LMW (14 trauma patients, 14 sepsis patients) 2) 20% HA (14 trauma patients, 14 sepsis patients) Fluid was infused to maintain PCWP at 10 mmHg to 15 mmHg | |
| Outcomes | Death Haemodynamic variables | |
| Notes | Follow‐up 5 days Deaths were reported within the study period and later (time not specified) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Boldt 1998.
| Methods | Randomised controlled trial. Blinding of outcome assessors not mentioned Loss to follow‐up not mentioned | |
| Participants | 150 traumatised patients (injury severity score >15) and 150 postoperative patients with sepsis. Patients suffering from renal failure, severe liver insufficiency, or with major coagulation abnormalities were not included | |
| Interventions | 1) 10% HES, LMW (n = 150)
2) 20% HA (n = 150) Both for 5 days to maintain the PAWP between 12 Torr and 15 Torr |
|
| Outcomes | Death Haemodynamic variables Organ function Coagulation | |
| Notes | Deaths were reported within the study period and after the study period (time not specified). Information on allocation concealment was obtained on contact with the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Boldt 2000.
| Methods | Randomised controlled trial | |
| Participants | 150 patients undergoing major abdominal surgery | |
| Interventions | 1) 6% HES, LMW (n = 50) 2) 6% HES, MMW (n = 50) 3) 3% MFG (n = 50) To keep MAP > 70 mmHg and CVP between 10 mmHg and 14 mmHg Volume was given perioperatively until the morning of the first postoperative day. For each hour of surgery 500 mL to 800 mL of crystalloids was routinely infused | |
| Outcomes | Death Haemodynamic variables Blood loss Blood transfused Cost | |
| Notes | Follow‐up 1 postoperative day. Deaths recorded after study period. Information on allocation concealment was obtained on contact with the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Boldt 2001.
| Methods | Randomised controlled trial. Volume therapy was done by doctors who did not know the aim of the study | |
| Participants | 75 patients undergoing major abdominal surgery Volume was administered to keep the CVP between 8 mmHg and 12 mmHg | |
| Interventions | 1) 6% HES (n = 25) 2) 6% HES (n = 25) 3) 4% MFG (n = 25) All groups also received 500 mL of RL for each hour of surgery | |
| Outcomes | Death Haemodynamic variables Blood loss Blood units transfused | |
| Notes | There were no deaths in the study period (until first follow‐up on first postoperative day. Deaths until discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. 'Closed envelope system' |
Brock 1995.
| Methods | Randomised controlled trial | |
| Participants | 21 patients who had undergone cardiac surgery | |
| Interventions | 1) 10% HES 200/0.5 in 7.2% saline (n = 7) 2) 5% HA (n = 7) 3) 6% HES in 0.9% saline (n = 7) | |
| Outcomes | Death (data obtained on contact with study author) Haemodynamic variables | |
| Notes | Data on allocation concealment was obtained on contact with the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Allocation by list of random numbers read by someone entering patients into the trial (open list) |
Brutocao 1996.
| Methods | Randomised double‐blind controlled trial with pharmacy‐controlled randomisation | |
| Participants | 38 children aged 1 year or more who were undergoing surgical repair of a congenital heart disease. Exclusion criteria included amrinone therapy, renal disease, coagulopathy, or a known bleeding diathesis | |
| Interventions | 1) 5% Albumin (n = 18) 2) 6% HES (n = 20) Volume expansion was administered as clinically indicated to maintain adequate CVP, perfusion, and urine output. The total amount of colloid therapy was determined by care providers blinded to the randomisation | |
| Outcomes | Death (information on death was obtained on contact with the study authors) Haemodynamic variables Coagulation variables | |
| Notes | Follow‐up until discharge from hospital 9 children excluded post randomisation because they did not require colloid. Information on allocation concealment was obtained on contact with the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Pharmacy‐controlled randomisation |
Carli 2000.
| Methods | Randomised controlled trial. Not ITT analysis | |
| Participants | 164 trauma patients. Patients were included if their SBP was < 100 mmHg, associated with signs of hypoperfusion | |
| Interventions | 1) HES (Hesteril 6%) (n = 85) 2) Gelatin (Plasmion) (n = 79) | |
| Outcomes | Glasgow coma score Haemodynamic variables Units of blood transfused Adverse reaction | |
| Notes | There were 13 deaths from heart failure but these patients were excluded from the final analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. 'Each centre received instructions from the coordinating Institute on the treatment to give the patient' |
Claes 1992.
| Methods | Randomised controlled trial Blinding not mentioned No loss to follow‐up |
|
| Participants | 20 patients undergoing brain tumour surgery and 20 patients undergoing transabdominal hysterectomy. Exclusion criteria: pre‐existing coagulopathies, abnormal preoperative coagulation screening tests, intake of drugs affecting haemostasis within 2 weeks preoperatively, and liver or kidney dysfunction | |
| Interventions | 1000 mL of fluid for volume replacement, as 1) 6% HES (n = 19) 2) 5% HA solution in 0.9% saline (n = 21) | |
| Outcomes | Haemodynamic variables Coagulation variables | |
| Notes | Follow‐up 48 postoperative hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation or allocation |
Diehl 1982.
| Methods | Randomised controlled trial Blinding not mentioned No loss to follow‐up | |
| Participants | 60 patients undergoing coronary artery bypass | |
| Interventions | 1) 6% HES (n = 27) 2) 5% Albumin (n = 33) for volume expansion during the first 24 hours postoperatively. Neither hetastarch nor albumin was used intraoperatively or in the pump prime | |
| Outcomes | Death Coagulation data Haemodynamic variables | |
| Notes | Follow‐up 7 postoperative days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Patients were allocated to groups according to their hospital identification number |
Dolecek 2009.
| Methods | Randomised controlled trial, randomised according to computer‐generated randomisation list | |
| Participants | 56 patients with severe sepsis. Patients were included if they were 18 years or older and developed severe sepsis. Exclusion criteria: severe coagulopathy, pregnant, cardiac failure, acute renal failure, aortal aneurysm, severe aortal regurgitation or dysrhythmia | |
| Interventions | 1) 20% Albumin (n = 30) 2) 6% HES (n = 26) |
|
| Outcomes | Death Haemodynamic variables |
|
| Notes | Follow‐up 28 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered envelopes (information obtained from authors) |
Du Gres 1989.
| Methods | Randomised controlled trial Blinding not mentioned No loss to follow‐up | |
| Participants | 30 patients post cardiac surgery. Patients were included if they were haemodynamically stable, were without serious 'rhythm' problems, had MAP < 90 mmHg, mean pulmonary artery pressure < 20 mmHg and CVP < 10 mmHg. Patients excluded if they needed blood transfusion, had a haematocrit < 28% or Hb < 9 g/100 mL | |
| Interventions | 1) 4% HA (n = 15) 2) Haemaccel (n = 15) | |
| Outcomes | Haemodynamic parameters | |
| Notes | Follow‐up 4 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation |
Dytkowska 1998.
| Methods | Randomised controlled trial | |
| Participants | 40 patients post cardiac surgery. Patients were excluded if they had co‐existing cardiogenic shock, renal failure with creatinine level > 3.0 mg, or severe clotting disorders | |
| Interventions | 1) 200/0 HAES 6% (n = 20) 2) Gelafundin (n = 20) Colloids were administered to patients with diagnosed symptoms of hypovolaemia, during the first 24 hours postoperatively. Infusion rate was adjusted to patients needs but it did not exceed 1000 mL/hour | |
| Outcomes | Haemodynamic parameters Biochemical parameters Adverse reactions | |
| Notes | Follow‐up 2 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation |
Evans 2003.
| Methods | Randomised controlled trial Treatment blinded (fluid set up by independent operator and covered with opaque black bag) | |
| Participants | 55 patients undergoing unilateral cemented hip replacement Exclusion criteria: cardiac insufficiency, renal insufficiency, altered liver function, preoperative anaemia, preoperative coagulation abnormalities, chronic use of corticosteroids and diuretics | |
| Interventions | 1) 4.5% HA (n = 13) 2) 4% Gelosulfine (n = 14) 3) Haemacel (n = 14) 2 L of fluid was infused during the operative period A fourth group received normal saline (n = 14) | |
| Outcomes | Haemodynamic variables Total blood loss | |
| Notes | Follow‐up before surgery, at the end of the surgery, and 2 hours postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ 'sealed envelopes' |
Falk 1988.
| Methods | Randomised controlled trial Blinding not mentioned No loss to follow‐up | |
| Participants | 12 patients with septic shock. Patients were excluded from the study if the pretreatment PAWP > 10 mmHg | |
| Interventions | 1) 250 mL of 5% Albumin (n = 6) 2) 250 mL of 6% HES (n = 6) Given every 15 minutes until the PAWP was increased to 15 mmHg. The test infusion was then continued at 100 mL/hour to maintain PAWP at 15 mmHg for the next 24 hours | |
| Outcomes | Haemodynamic variables Clotting variables | |
| Notes | Follow‐up 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation |
Friedman 2008.
| Methods | Randomised controlled trial | |
| Participants | 34 haemodynamically stable adults with sepsis and suspected hypovolaemia. Exclusion criteria: pregnancy, terminal state, PAOP > 12 mmHg, serum creatinine concentration > 3 mg/dL, severe coagulation abnormalities, history of allergy to any IV fluid | |
| Interventions | 1) 400 mL 10% HES (n=11) 2) 400 mL 6% HES (n=10) 3) 4% HA (n=13) All over 40 minutes |
|
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 160 minutes. No data on mortality or blood transfused | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ sealed, opaque envelope assignment (does not say if sequentially numbered) |
Fries 2004.
| Methods | Randomised controlled trial Treatment not blinded | |
| Participants | 60 patients undergoing primary knee replacement surgery Exclusion criteria: contraindications for regional anaesthesia and puncture of the radial artery, any known allergies, primary and secondary haemostatic disorder | |
| Interventions | 1) 4% Gelofusine (n = 20)
2) 6% HES (n = 20)
A third group received RL Before administrating spinal anaesthesia all patients received 500 mL RL. All patient intraoperatively |
|
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 2 hours postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation or allocation |
Fulachier 1994.
| Methods | Randomised controlled trial Blinding not mentioned No loss to follow‐up | |
| Participants | 16 patients undergoing cardiac surgery (8 were undergoing valve replacement and 8 undergoing coronary bypass). Patients were excluded if they were > 80 years of age, < 18 years of age, had been included in other studies, had received colloids in the month preceding surgery, had coagulation abnormalities, or who were undergoing inotropic treatment | |
| Interventions | 1) 500 mL OF 4% solution of HA in RL (n = 8)
2) 500 mL of HES (n = 8) until starting cardiopulmonary bypass |
|
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 30 minutes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation or allocation |
Gahr 1981.
| Methods | Randomised controlled trial. No information given on method of randomisation No loss to follow‐up | |
| Participants | 20 patients with hypovolaemia following abdominal surgery for malignoma | |
| Interventions | 1) 500 mL HES 450/0.7 (n = 10)
2) 500 mL HA 5% (n = 10) during the first 24 hours after the operation |
|
| Outcomes | Haemodynamic parameters Coagulation data | |
| Notes | Follow‐up 6 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of randomisation or allocation |
Gallagher 1985.
| Methods | Randomised controlled trial | |
| Participants | 10 patients after coronary artery bypass graft surgery Exclusion criteria: patients with significant left main coronary artery stenosis, poor left ventricular function, or poor pulmonary function | |
| Interventions | 1) 5% Albumin (n = 5) 2) 6% HES (n = 5) | |
| Outcomes | Death (data on deaths from study author) Haemodynamic data | |
| Notes | Follow‐up 1 day. Data on allocation obtained on contact with author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Computerised system ‐ patient details were entered before treatment assignment was revealed |
Godet 2008.
| Methods | Randomised controlled trial. Computer‐generated random list with randomisation in balanced blocks | |
| Participants | 65 patients aged 18 years and over with renal dysfunction undergoing abdominal aortic surgery. Exclusion criteria: endovascular aortic surgery, preoperative serum creatinine > 250 µmol/L, history or present diagnosis of severe hepatic insufficiency or coagulation disorders, dialysis, anuria, and post‐transplant surgery | |
| Interventions | 1) 6% HES (n = 32) 2) 3% Gelatin (n = 33) |
|
| Outcomes | Death Haemodynamic variables Renal safety (serum creatinine) Adverse events |
|
| Notes | Follow‐up at 6 days and 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Investigator received a set of envelopes. Envelope only opened when the patient arrived at pre‐induction anaesthesia room |
Gold 1990.
| Methods | Randomised controlled trial Colloid solution was blinded by covering with foil No loss to follow‐up |
|
| Participants | 40 surgical patients undergoing AAA surgery | |
| Interventions | 1) 1 g/kg Albumin 5% solution (n = 20) 2) 1 g/kg Hetastarch 6% solution (n = 20) | |
| Outcomes | Death (data on death was obtained on contact with the author) Haemodynamic and coagulation variables | |
| Notes | Follow‐up not specified. Information on allocation concealment was obtained by contact with the author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Randomisation by alternation |
Gombocz 2007.
| Methods | Randomised double‐blind study (does not specify who was blinded) | |
| Participants | 40 patients undergoing coronary bypass surgery or aortic valve replacement. Exclusion criteria: 'redo' operation, hepatic disease, renal dysfunction, immunological disease, steroid treatment, intake of aspirin or other cyclooxygenase inhibitor within 7 days of surgery, known allergy to volume expanders used in the study | |
| Interventions | 1) 5.5% Gelatin (n = 20) 2) 6% Dextran 70 (n = 20) |
|
| Outcomes | Death Haemodynamic variables Blood transfused |
|
| Notes | Final follow‐up 44 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Gondos 2010.
| Methods | Randomised controlled study | |
| Participants | 200 postoperative haemodynamically stable hypovolaemic patients needing intensive care treatment because of general health status. Exclusion criteria: aged < 18 years, active bleeding or shock, severe pulmonary oedema, known uraemia, anaphylactic reaction to colloid fluids, and life expectancy less than 24 hours | |
| Interventions | 1) 4% Gelatin (n = 50) 2) 6% HES (n = 50) 2) 5% HA (n = 50) A fourth group were given LR (n = 50) |
|
| Outcomes | Death Haemodynamic variables Length of ICU stay |
|
| Notes | Final follow‐up 10th postoperative day. Additional information on allocation obtained from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate ‐ 'randomised by blinded envelope technique ‐ each centre had got 20 closed, opaque envelopes which were sequentially numbered' |
Haas 2007.
| Methods | Randomised controlled trial. Computer‐generated randomisation list | |
| Participants | 42 children undergoing surgery (including craniofacial surgery, tumour resection and abdominal surgery and needing colloid replacement. Exclusion criteria: prematurity; emergency surgery; history of hereditary or acquired coagulopathy including renal, hepatic, and bone marrow disease | |
| Interventions | 1) 4% Modified gelatin (n = 14) 2) 5% Albumin (n = 14) 3) 6% HES (n = 14) |
|
| Outcomes | Haemodynamic variables | |
| Notes | Length of follow‐up not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of allocation concealment |
Hausdorfer 1986.
| Methods | Randomised controlled trial. No information given on method of randomisation | |
| Participants | 30 children undergoing major surgery. During about 3 hours of surgery, the patients lost up to 15% of blood volume | |
| Interventions | 1) HA 5% (n = 15)
2) HES 6% (n = 15) with 14 mL/kg body weight each |
|
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 24 hours postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Hecht‐Dolnik 2009.
| Methods | Randomised controlled trial. Block randomisation with 8 patients in each block. Attending intensivists were blinded to randomisation | |
| Participants | 156 patients undergoing off‐pump coronary artery bypass grafting. Exclusion criteria: history of cardiac surgery, primary bleeding disorders, end‐stage renal disease, and pregnant patients | |
| Interventions | 1) 6% HES (n = 78) 2) 5% HA (n = 78) |
|
| Outcomes | Death PRBC transfused Haemodynamic variables |
|
| Notes | 4 patients excluded after randomisation because they were converted to on‐pump surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. 'Sealed envelopes, attending anaesthetist opened the envelope linked to the patient's study number in the operating room when the procedure was underway' |
Hedstrand 1987.
| Methods | Randomised controlled trial. No information given on method of randomisation Postoperative care staff were blinded No loss to follow‐up | |
| Participants | 275 patients undergoing major surgery. Patients were excluded if they were known to have decreased serum albumin levels or expected to sustain plasma loss, or had pronounced cardiovascular disease | |
| Interventions | 1) PPF (n = 142) 2) Dextran (n = 133) | |
| Outcomes | Volume transfused Complication rates Serum albumin Deaths | |
| Notes | Follow‐up 1 month | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Hiippala 1995.
| Methods | Randomised controlled trial. No information given on method of randomisation Blinding not mentioned 3 patients lost to follow‐up (explanation given) | |
| Participants | 60 patients undergoing major abdominal or urological surgery. Patients who had used platelet‐inhibiting drugs or had a diagnosed haemostatic defect were excluded | |
| Interventions | 1) 3% Dextrose (n = 15) 2) 4% HES (n = 15) 3) 6% HES (n = 15) 4) 5% Albumin (n = 15) | |
| Outcomes | Haemodynamic variables Clotting variables Blood loss | |
| Notes | Follow‐up 3 days postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Huang 2005.
| Methods | Randomised controlled trial No information given on blinding | |
| Participants | 20 patients with burns over 40% of total body surface area admitted 4 to 8 hours after injury | |
| Interventions | 1) PPF (n = 9) 2) Gelofusine (n = 11) In a third control group patients did not receive fluid resuscitation | |
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 48 hours No relevant outcome data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Huskisson 1993.
| Methods | Randomised controlled trial. No information given on method of randomisation | |
| Participants | 27 children returning to the ICU following hypothermic open heart surgery | |
| Interventions | 1) Albumin 2) Gelatin 3) Hetastarch | |
| Outcomes | Haemodynamic variables | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Inal 2010.
| Methods | Randomised controlled trial | |
| Participants | 30 hypovolaemic patients admitted to ICU. Exclusion criteria: pregnancy, haemodynamic instability, heart failure, renal failure, liver failure, known or suspected brain death | |
| Interventions | 1) 3.5% Polygeline (n = 15) 2) 6% HES (n = 15) |
|
| Outcomes | Death Haemodynamic variables Liver function Length of ICU stay |
|
| Notes | Follow‐up 30 minutes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Jin 2010.
| Methods | Randomised controlled trial | |
| Participants | 36 patients undergoing surgery for gastric cancer. Exclusion criteria: cardiac or renal insufficiency, or both; altered liver function; preoperative anaemia or coagulation abnormality, or both; colloid allergy; use of anticoagulants or antiplatelets | |
| Interventions | 1) 6% HES (n = 12) 2) 4% Modified gelatin (n = 12) 3) RL (n = 12) |
|
| Outcomes | Haemodynamic variables Adverse events |
|
| Notes | Follow‐up 4 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐ 'closed envelopes' |
Jones 2004.
| Methods | Randomised controlled trial. Surgeons blinded to the fluid administered although the anaesthetist was aware of the fluid administered to a given patient | |
| Participants | 40 adults scheduled to undergo radical retropubic prostatomy Exclusion criteria: coagulation disorder, platelet count < 100,000/mm3, preoperative Hb < 12 g/dL, if anticoagulant therapy within 10 days of the surgery, aspirin or NSAID use < 10 days before surgery or if they had documented allergy to any of the IV fluids used in the protocol | |
| Interventions | 1) 5% HA (n = 10) 2) 6% Dextran 70 (n = 10) 3) 6% HES (n = 10) A fourth group received RL Haemodilution was done with the target of 9 g/dL All patients underwent moderate haemodilution to a target of Hb 9 g/dL | |
| Outcomes | Haemodynamic variables Blood loss and units transfused | |
| Notes | Follow‐up 3 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Karanko 1987.
| Methods | Randomised controlled trial. Patients were randomised in blocks of 4 Blinding not mentioned No loss to follow‐up | |
| Participants | 48 patients who had undergone coronary bypass surgery 20 hours earlier | |
| Interventions | 1) 4% PPF (n = 15) 2) 6% Dextran 70 (n = 10) 3) 5.5% Oxypolygelatin (n = 12) A fourth group (not randomly selected) acted as a control (n = 11) | |
| Outcomes | Death (data on death was obtained on contact with the author Haemodynamic variables | |
| Notes | Follow‐up 28 hours. Information on allocation was obtained on contact with the author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Paper was put into a hat and taken out by an independent person |
Kirklin 1984.
| Methods | Randomised controlled trial. No information given on method of randomisation Blinding not mentioned No loss to follow‐up | |
| Participants | 30 patients undergoing coronary artery operations. Patients were excluded if they had undergone previous cardiac operations, if they had severe coagulopathies, anaemia, or CRF | |
| Interventions | 1) 6% HES (n = 15) 2) 5% Albumin (n = 15) Both fluids infused over 24 hours to maintain left arterial pressure between 6 mmHg and 12 mmHg and cardiac index > 2.0 L/minute/m2 | |
| Outcomes | Death Haemodynamic and coagulation variables Adverse reactions | |
| Notes | Follow‐up until discharge from ICU 34 patients were originally included in the trial but data from 4 of them was not included in the final analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation concealment |
Lisander 1996.
| Methods | Randomised controlled trial No loss to follow‐up Blinding not mentioned | |
| Participants | 40 patients undergoing revision hip arthroplasty | |
| Interventions | 1) Albumin 40 g/L (n = 20) 2) Dextran 70 60 g/L (n = 20) Patients all received enoxaparin 40 mg/day | |
| Outcomes | Death (data obtained from contact with study author) External blood loss Red cell balance Packed cell volume | |
| Notes | Follow‐up until discharge from hospital. Information on allocation concealment was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
London 1989.
| Methods | Randomised controlled trial. No information given on method of randomisation Blinding not mentioned No loss to follow‐up | |
| Participants | 93 male cardiac surgical patients. Patients were excluded from the study if they had a significant coagulopathy or were anaemic (haematocrit value < 30%) | |
| Interventions | 1) 10% Pentastarch in 0.9% saline (n = 50) 2) 5% HA in 0.9% saline (n = 44) to provide volume expansion during the first 24 hours after cardiac operations | |
| Outcomes | Haemodynamic variables Coagulation variables Death Length of stay | |
| Notes | 1 patient was treated twice with an 8‐month interval. Follow‐up until discharge from hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Mahmood 2007.
| Methods | Randomised controlled trial. Randomisation by blocks of 6 using random number table. The study was not double blind but person analysing data was blind to study group. ITT analysis | |
| Participants | 62 patients undergoing elective infrarenal AAA surgery. Exclusion criteria: preoperative serum creatinine of more than 177 µmol/L and left ventricular ejection fraction < 40%. Also juxtarenal aneurysms and patients who had had a renal transplant | |
| Interventions | 1) HES 200/0.62 (n = 21) 2) HES 130/0.4 (n = 21) 3) Gelatin (n = 20) |
|
| Outcomes | Haemodynamic variables Deaths Red cells infused |
|
| Notes | Follow‐up 5 days, but all‐cause mortality reported for 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate 'sealed envelops allocating the fluid type' were opened on the morning of surgery. Recruitment, randomisation, and concealment were carried out by the trial coordinator |
Mastroianni 1994.
| Methods | Randomised controlled trial. No information given on method of randomisation Blinding not mentioned | |
| Participants | 34 patients undergoing open heart surgery were enrolled | |
| Interventions | 1) 10% Pentastarch. (n = 12) 2) 5% Albumin (n = 17) | |
| Outcomes | Deaths Haemodynamics variables Clotting variables Pulmonary oedema | |
| Notes | Follow‐up 7 days 4 patients in the pentastarch group, and 1 patient in the albumin group were excluded after randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Mittermayr 2007.
| Methods | Randomised controlled trial. Computer‐generated randomisation list | |
| Participants | 66 patients undergoing major orthopaedic surgery (5 excluded from analysis because of pathological baseline measurements of fibrinogen and platelets) | |
| Interventions | 1) Gelatin (n = 21) 2) HES (n = 19) A third group (n = 21) received RL |
|
| Outcomes | Haemodynamic variables RBCs transfused |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Moggio 1983.
| Methods | Randomised controlled trial No loss to follow‐up Blinding not mentioned | |
| Participants | 47 postoperative open heart surgery patients. Operations performed included coronary revascularisation, valve operations, and combined coronary and valve procedures. Patients with pre‐existing hepatic or renal disease were not eligible for the study | |
| Interventions | 1) 5% Albumin in 0.9% saline (n = 23) 2) 6% HES in 0.9% saline (n = 24) | |
| Outcomes | Haemodynamic variables Clotting variables | |
| Notes | Follow‐up not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Randomised according to the last digit of their hospital identification numbers |
Molnar 2004.
| Methods | Randomised controlled trial Blinding unclear | |
| Participants | 30 hypovolaemic patients with ITBVI < 850 in septic shock with ALI Exclusion criteria: CVS failure (NYHA class IV), chronic respiratory failure (chronic hypoxia, hypercapnia) requiring renal replacement therapy, chronic liver failure or those with diabetes mellitus or with known aortic aneurysm | |
| Interventions | 1) 6% HES (n = 15) 2) 4% GEL (n = 15) 250 mL/15‐minute boluses (max 1000 mL) were given until the end point ITBVI > 900 mL/m2 | |
| Outcomes | Death Haemodynamic variables | |
| Notes | Follow‐up 60 minutes after the end point was reached. Follow‐up for deaths was not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
Mukhtar 2009.
| Methods | Randomised controlled trial | |
| Participants | 40 patients undergoing living donor liver transplantation. Exclusion criteria: retransplantation, history of previous upper abdominal surgery, portal vein thrombosis, < 18 years old, primary renal dysfunction | |
| Interventions | 1) 5% HA (n = 20) 2) 6% HES (n = 20) |
|
| Outcomes | Death Haemodynamic variables Renal function |
|
| Notes | Final follow‐up 4 days postoperatively. Mortality given for 2 weeks postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear 'sealed envelope' (does not say if opaque or sequentially numbered) |
Munoz 1980.
| Methods | Randomised controlled trial Blinding not mentioned No mention of loss to follow‐up | |
| Participants | 14 patients with shock due to haemorrhage or sepsis | |
| Interventions | 1) HES (Hespan) 2) 5% Albumin Number in each group not reported | |
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 4 hours post infusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of allocation |
Munsch 1988.
| Methods | Randomised controlled trial. No information given on method of randomisation Blinding not mentioned No loss to follow‐up | |
| Participants | 40 consecutive patients undergoing elective coronary artery bypass graft surgery | |
| Interventions | 1) HES 6% (n = 20) 2) PPF (n = 20) as their postoperative volume expander | |
| Outcomes | Haemodynamic variables Clotting variables Death Adverse reactions | |
| Notes | Follow‐up 7 postoperative days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on method of allocation |
Niemi 2006.
| Methods | Randomised controlled trial Blinding not clear | |
| Participants | 45 patients post cardiac surgery Exclusion criteria: preoperative coagulation disorders; renal or hepatic failure; or taking medication with coumarin anticoagulants, heparin, salicylic acids, or a combination within the previous 5 days | |
| Interventions | 1) 4% HA (n = 15) 2) 4% Gelatine (n = 15) 3) 6% HES (n = 15) | |
| Outcomes | Death (data on death obtained on contact with the author) Clotting variables Blood transfused | |
| Notes | Follow‐up 1 postoperative day 54 patients gave consent but 9 later excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Allocation by closed envelope (not enough information provided to classify as adequate) |
Ooi 2009.
| Methods | Randomised single‐blind controlled study | |
| Participants | 90 patients undergoing coronary artery bypass surgery. Exclusion criteria: repeat coronary artery bypass, congestive heart failure, recent antiplatelet therapy, coagulopathy, renal dysfunction, liver dysfunction, history of pancreatitis, and known hypersensitivity to HES | |
| Interventions | 1) 6% HES (n = 45) 2) 4% Gelatin (n = 45) |
|
| Outcomes | Death PRBCs transfused Postoperative bleeding and renal function |
|
| Notes | Follow‐up 1, 2, and 4 postoperative days. Final follow‐up at 4 weeks. Information on allocation concealment obtained from study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate 'sealed envelopes' ‐ on contact study author confirmed that envelopes opaque and sequentially numbered |
Prien 1990.
| Methods | Randomised controlled trial Blinding not mentioned Loss to follow‐up not mentioned | |
| Participants | 18 patients undergoing modified Whipple's operation (hemipancreato‐duodenectomy). Patients were eligible for the study if there was an absence of major organ dysfunction and serum protein, sodium, glucose, blood urea nitrogen, haematocrit, aPTT and PT times, and platelet times were within normal limits. Specific exclusion criteria included compensated myocardial insufficiency, chronic hypertension, chronic obstructive airways disease, and insulin‐dependent diabetes mellitus | |
| Interventions | 1) 10% HES (n = 6) 2) 20% HA (n = 6) A third group were given RL (n = 6) All given as a volume replacement solution, which was given to maintain CVP at the preoperative level | |
| Outcomes | Death (data on death was obtained on contact with the study author) Haemodynamic variables Clotting variables | |
| Notes | Follow‐up unspecified Study was intraoperative | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information on allocation |
Rackow 1983.
| Methods | Randomised controlled trial Blinding not mentioned | |
| Participants | 18 patients with hypovolaemic and septic shock. Patients were excluded if they were < 18 years of age, considered to be in a terminal state, or had a significant coagulopathy | |
| Interventions | 1) Albumin (n = 9) 2) HES (n = 9) Patients received 250 mL of the treatment fluid every 15 minutes as a fluid challenge. The fluid challenge ended when the WP equalled 15 mmHg. Thereafter the treatment fluid was given in sufficient quantities to maintain the WP at 15 mmHg for the next 24 hours, at which point the study was completed | |
| Outcomes | Death Haemodynamic variables Respiratory variables | |
| Notes | Deaths given for study period and for length of hospital stay. Survival until discharge was used for the mortality data for this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information on allocation |
Rackow 1989.
| Methods | Randomised controlled trial No loss to follow‐up Blinding not mentioned | |
| Participants | 20 patients with severe sepsis and systemic hypoperfusion. Patients were excluded from the study if they were < 21 years of age, pregnant, considered to be terminal, or they manifested spontaneous bleeding | |
| Interventions | 1) 5% Albumin (n = 10) 2) 10% HES (pentastarch) (n = 10) Each group received 250 mL of the treatment fluid every 15 minutes until either the WP was 15 mmHg or less or a maximum volume of 2000 mL of study colloid was infused | |
| Outcomes | Death Haemodynamic variables Clotting variables Allergic reactions | |
| Notes | Follow‐up unspecified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information on allocation |
Reine 2008.
| Methods | Randomised controlled trial. Computerised randomisation | |
| Participants | 38 patients undergoing major orthopaedic, gastrointestinal, or gynaecological surgery | |
| Interventions | 1) 20% HA (n = 19) 2) 6% HES (n = 19) |
|
| Outcomes | Haemodynamic variables Changes in albumin binding capacity |
|
| Notes | Final follow‐up first postoperative day (approximately 22 hours) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate, 'randomisation process was handled by the hospital's office for clinical research' |
Rittoo 2004.
| Methods | Randomised controlled trial Blinding‐not clear | |
| Participants | 40 patients undergoing AAA surgery Exclusion criteria: ejection fraction of < 40% with poor pulmonary function with microalbuminuria and a creatinine concentration of > 150 μmol/L | |
| Interventions | 1) 6% HES (n = 20) 2) 4% Gelosulfine (n = 20) All patients received crystalloid. Colloid infused to maintain stable heart rate, CVP 8 cmH2O to 10 cmH2O and steady MAP and urine output of > 40 mL/hour | |
| Outcomes | Lung function Adverse events | |
| Notes | Follow‐up 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Allocation by sealed envelopes (not enough information provided to classify as adequate) |
Rosencher 1992.
| Methods | Randomised controlled trial No mention of blinding Loss to follow‐up not mentioned | |
| Participants | 32 patients undergoing total hip replacement | |
| Interventions | 1) 4% Albumin (n = 16) 2) LMW HES (n = 16) | |
| Outcomes | Death (data obtained on contact with study author) Bleeding Clotting variables | |
| Notes | Follow‐up 5 postoperative days. Information on allocation concealment was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered, sealed, opaque envelopes |
Schortgen 2001.
| Methods | Randomised controlled trial | |
| Participants | 129 patients with severe sepsis or septic shock over 18 years of age. Patients were excluded if they were pregnant, had a history of allergy to HES or gelatin, had severe acute or chronic renal dysfunction, or previous administration of HES or mannitol | |
| Interventions | 1) 6% HES (n = 65) 2) 3% Fluid‐modified gelatin (n = 64) | |
| Outcomes | Death (data obtained on contact with study author) Length of stay in ICU Acute renal failure | |
| Notes | Follow‐up while in ICU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation was by sealed opaque envelopes serially numbered and used in sequence |
Schramko 2009.
| Methods | Randomised controlled trial | |
| Participants | 45 patients undergoing elective primary cardiac surgery. Exclusion criteria: preoperative coagulation disorder; renal or hepatic failure; received warfarin, heparin, clopidogrel, or acetylsalicylic acid within 5 days before surgery | |
| Interventions | 1) 6% HES 200/0.5 (n = 15) 2) 6% HES 130/0.4 (n = 15) 3) 4% HA (n = 15) |
|
| Outcomes | Haemodynamic variables PRBCs transfused |
|
| Notes | Final follow‐up first postoperative morning. Mortality data obtained from study author (relates to study period only, inhospital mortality not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate 'closed envelopes were prepared before the beginning of the study' |
Schramko 2010.
| Methods | Randomised controlled trial | |
| Participants | 45 patients undergoing elective cardiac surgery. Exclusion criteria: known coagulation disorder; renal or hepatic failure; preoperative left ventricular ejection fraction < 40%; received warfarin, heparin, clopidogrel, or acetylsalicylic acid within previous 5 days | |
| Interventions | 1) 6% HES (n = 15) 2) 4% Gelatin (n = 15) 3) Ringer's acetate (n = 15) |
|
| Outcomes | Haemodynamic variables Units of RBC and FFP transfused |
|
| Notes | Follow‐up 18 hours postoperatively. Mortality data obtained from study author (relates to study period only, inhospital mortality not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate 'closed envelopes were prepared before the beginning of the study by a person who did not take part in the treatment of the study subjects' |
Shatney 1983.
| Methods | Controlled clinical trial. Patients were assigned to groups in an alternating fashion No loss to follow‐up No mention of blinding | |
| Participants | 32 patients with multisystem trauma or haemorrhagic shock, or both. Patients with cardiac arrest on hospital admission or during the first 30 minutes after admission were excluded from the study | |
| Interventions | 1) PPF 5% solution (n = 16) 2) Hetastarch 6% (n = 16) Study patients continued to receive the assigned colloid solution for the first 8 days whenever colloid was thought necessary | |
| Outcomes | Hepatic, pulmonary and renal function Clotting variables Volume of fluids infused Deaths | |
| Notes | Follow‐up 8 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate. Patients assigned by alternation |
Standl 2008.
| Methods | Randomised controlled trial. Randomisation in blocks of 4 using a 1:1 ratio | |
| Participants | 82 children younger than 2 years of age undergoing non‐cardiac surgery. Exclusion criteria: intracranial bleeding within 6 weeks prior to randomisation, ASA risk score > 3, pre‐existing severe organ insufficiencies, coagulation abnormalities and Hb below critical age‐appropriate levels | |
| Interventions | 1) HES 130/0.4 (n = 41) 2) 5% HA (n = 41) |
|
| Outcomes | Death Haemodynamic variables Coagulation variables RBC transfused |
|
| Notes | Final follow‐up first postoperative day | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate 'sealed randomisation envelopes that were opened by the investigator only after final enrolment of the patient' |
Stockwell 1992.
| Methods | Randomised controlled trial. No information given on method of randomisation No loss to follow‐up Blinding not mentioned | |
| Participants | 475 patients admitted to the ICU. Patients were excluded from the study if they were < 18 years or if admitted for cardiac monitoring or cardiac thrombolytic therapy | |
| Interventions | 1) 4.5% Albumin (n = 226) 2) Synthetic colloid polygeline (Haemaccel) (n = 249) for IV volume replacement | |
| Outcomes | Death Length of stay in ICU Incidence of renal failure Pulmonary oedema | |
| Notes | Follow‐up until discharge from ICU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Stoddart 1996.
| Methods | Randomised blinded trial Anaesthetist unaware of intervention No loss to follow‐up | |
| Participants | 30 neonates undergoing major surgery. They were excluded if the body weight < 2 kg or > 5 kg; preoperative Hb < 14 g/dL; they had previously received blood or colloid; or they had suspected major cardiac, renal, metabolic, or chromosomal abnormalities. Neonates were withdrawn from the study if either blood or > 40 mL/kg of colloid was required either during or within the first 24 hour after surgery | |
| Interventions | 1) HA 4.5% (n = 15) 2) Haemaccel (n = 15) | |
| Outcomes | Haemodynamic variables Plasma albumin Hb | |
| Notes | Follow‐up 24 hours postoperatively. Information on allocation concealment was obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Tollofsrud 1995.
| Methods | Randomised controlled trial No loss to follow‐up Blinding not mentioned | |
| Participants | 30 patients undergoing elective coronary artery bypass surgery. Patients with left ventricular ejection fraction < 40%, valvular heart disease, ventricular aneurysm, arrhythmia, diabetes mellitus, renal failure, or lung disease were excluded | |
| Interventions | 1) Polygeline (Haemaccel) (n = 10) 2) Dextran 70 (n = 10) 3) Albumin 40 (n = 10) A fourth group received RL (n = 10) | |
| Outcomes | Death Haemodynamic variables Respiratory data Cost of fluid regimens | |
| Notes | Follow‐up 48 hours during and after surgery. Information on allocation concealment was obtained on contact with the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Sequentially numbered sealed opaque envelopes |
Van der Linden 2004.
| Methods | Randomised controlled trial Blinding not clear | |
| Participants | 110 patients (average age 63 years) undergoing cardiac surgery under cardiopulmonary bypass (elective coronary artery or single valve surgery). Exclusion criteria: undergoing combined cardiac surgery or redo operations, history of allergic reactions to starches or gelatins, significant liver or renal dysfunction | |
| Interventions | 1) 6% HES (n = 55) 2) 3.5% Urea‐lined gelatine (n = 55) If additional colloid required 4.5% HA given | |
| Outcomes | Death Haemodynamic variables Blood transfused | |
| Notes | Follow‐up 18 hours after surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Patients were randomly allocated by opening an envelope (not enough information provided to classify as adequate) |
Van der Linden 2005.
| Methods | Randomised controlled trial Blinding unclear | |
| Participants | 132 patients with a preoperative left ventricular ejection fraction > 35% undergoing elective primary cardiac surgery | |
| Interventions | 1) 6% HES 130/0.4 (48.9 ± 17.2 mL/kg) (n = 64) 2) 3% GEL (48.9 ± 14.6 mL/kg) (n = 68) | |
| Outcomes | Haemodynamic variables
Blood loss Blood transfused |
|
| Notes | Follow‐up until 5 postoperative days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Veneman 2004.
| Methods | Randomised controlled trial | |
| Participants | 61 critically ill hypoalbuminic patients (serum concentration < 20 g/L) | |
| Interventions | 1) Albumin (n = 15) 2) HES 10% 500 mL (n = 15) 3) HES 10% 1000 mL (n = 15) A fourth group received saline | |
| Outcomes | Death Haemodynamic variables Adverse events (from study author) | |
| Notes | Follow‐up 72 hours postoperatively, mortality 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation by sealed envelopes kept outside of hospital |
Verheij 2006.
| Methods | Randomised controlled trial | |
| Participants | 67 patients undergoing either vascular (n = 28) or cardiac surgery (n = 40) Exclusion criteria: age > 79 years and known anaphylactoid reactions to colloids | |
| Interventions | 1) 4% Gelatine (n = 16) 2) 6% HES (n = 18) 3) 5% HA (n = 18) A fourth group received normal saline | |
| Outcomes | Death Haemodynamic variables | |
| Notes | Follow‐up not clear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Hospital pharmacy assigned patients via sealed enveloped method |
Vogt 1994.
| Methods | Randomised controlled trial. No information given on method of randomisation | |
| Participants | 40 patients undergoing major surgery. Exclusion criteria included anaemia and renal, liver, and coagulation disorders | |
| Interventions | 1) 5% HA (n = 20) 2) 6% HES (n = 20) | |
| Outcomes | Haemodynamic variables Coagulation Haematological parameters Blood loss and blood intake | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Vogt 1996.
| Methods | The patients were divided into 2 groups using random numbers Blinding not mentioned No loss to follow‐up | |
| Participants | 41 patients undergoing total hip arthroplasty during the perioperative period. Exclusion criteria: weight < 60 kg, age < 18 years, ASA grade > 3, haematocrit < 34% or > 44%, history of coagulopathies or a Quick's prothrombin test of < 75%, PTT > 45 seconds, platelet count < 100,000/mm3, impaired liver function and renal failure | |
| Interventions | 1) 6% HES (n = 20) 2) 5% HA (n = 21) | |
| Outcomes | Haemodynamic and clotting variables | |
| Notes | Follow‐up 6 hours postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Vogt 1999.
| Methods | Randomised controlled trial. No information given on method of randomisation | |
| Participants | 50 patients undergoing radical prostatectomy or cystectomy with bladder replacement Exclusion criteria: weight < 60 kg; age < 21 years; ASA 1 or 2; Hb < 12 g/dL; history of clotting disorders, liver function disorders, advanced renal insufficiency, or hypoproteinaemia | |
| Interventions | 1) 5% HA 2) 6% HES 200/0.5 | |
| Outcomes | Haemodynamic variables Blood loss | |
| Notes | Follow‐up 3 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Volta 2007.
| Methods | Randomised controlled study. List of random numbers generated by computer. Patients were managed postoperatively by anaesthetists who were masked to the aims of the study | |
| Participants | 36 patients undergoing major abdominal surgery for colon cancer. Exclusion criteria: aged < 18, cardiac insufficiency, kidney dysfunction, altered liver function, preoperative anaemia, preoperative coagulation abnormalities, and long‐term use of corticosteroids or NSAIDs | |
| Interventions | 1) 3.4% Poligeline (n = 12) 2) HES 130/0.4 (n = 12) A third group received RL (n = 12) |
|
| Outcomes | Haemodynamic variables | |
| Notes | Follow‐up 72 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. Data on allocation not provided |
von Sommoggy 1990.
| Methods | Randomised controlled trial. No information given on method of randomisation No loss to follow‐up | |
| Participants | 24 patients undergoing infrarenal aortofemoral bifurcation grafting | |
| Interventions | 1) FFP and 5% HA (n = 13) 2) HES 200 10% and HES 450 6% (n = 11) | |
| Outcomes | Haemodynamic variables Clotting variables Influence on organ function | |
| Notes | Follow‐up 6 hours postoperatively | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Wahba 1996.
| Methods | Randomised controlled trial. Computerised system was used for randomisation Blinding not mentioned Loss to follow‐up not mentioned | |
| Participants | 20 patients who had had coronary artery bypass grafting. Patients with abnormal left‐ventricular function as judged from cine‐angiography were excluded as were patients on anticoagulants < 10 days before the operation | |
| Interventions | 1) 5% Albumin (n = 10) 2) Haemaccel (n = 10) | |
| Outcomes | Death (data on death were obtained on contact with the study author) Haemodynamic variables | |
| Notes | Follow‐up 2 weeks. Data on method of allocation concealment were obtained on contact with the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate |
Watkins 1990.
| Methods | Randomised controlled trial. No information given on method of randomisation | |
| Participants | 12 patients undergoing major surgery | |
| Interventions | 1) LMW polystarch 2) Polygelatine (Haemaccel) for postoperative volume replacement | |
| Outcomes | Death Adverse reactions | |
| Notes | Follow‐up 24 hours after infusion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear. No information given on allocation |
Woittiez 1997.
| Methods | Randomised controlled trial | |
| Participants | 60 patients who had developed hypoalbuminaemia (< 20 g/L) after major surgery 2 patients died after randomisation and before treatment started. These were excluded from the analysis | |
| Interventions | 1) Albumin 20% (300 mL/24 hours) (n = 15) 2) HES 10% (500 mL/24 hours) for 3 days (n = 27) Aim was to restore COP A third group received saline (n = 16) | |
| Outcomes | Death (data on death obtained on contact with the study author) Changes in fluid balance, serum albumin, COP, and clinical signs of oedema were followed daily | |
| Notes | Follow‐up unspecified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Adequate. Allocation by sequentially numbered sealed opaque envelopes |
Yang 2011.
| Methods | Randomised controlled trial. Computer‐generated random numbers | |
| Participants | 90 patients aged 18 to 75 years with hepatocellular carcinoma scheduled for hepatectomy ‐ received fluids postoperatively. Exclusion criteria: renal insufficiency requiring dialysis, cardiac insufficiency, steroid therapy, pre‐existing signs of bacteraemia, and known allergic reactions to starch preparations | |
| Interventions | 1) 20% HA (n = 30) 2) 6% HES (n = 30) 3) LR (n = 30) |
|
| Outcomes | Death Haemodynamic variables Liver function Inflammatory response parameters |
|
| Notes | Follow‐up until hospital discharge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
AAA: abdominal aortic aneurysm; ALI: acute lung injury; aPPT: activated partial thromboplastin time; ASA: American Society of Anesthesiologists; COP: colloid osmotic pressure; CRF: chronic renal failure; CVP: central venous pressure; CVS: cardiovascular system; EVLW: extravascular lung water; FFP: fresh frozen plasma; HA: human albumin; Hb: haemoglobin; HES: hydroxyethyl starch; HMW: high molecular weight; ICU: intensive care unit; ITBVI: intrathoracic blood volume index; ITT: intention to treat; IV: intravenous; LMW: low molecular weight; LVEDP: left ventricular end diastolic pressure; MMW: medium molecular weight; MAP: mean arterial pressure; MFG: modified fluid gelatin; MI: myocardial infarction; NSAID: non‐steroidal anti‐inflammatory drug; NYHA: New York Heart Association; PAWP: pulmonary artery wedge pressure; PAOP: pulmonary artery occlusion pressure; PCWP: pulmonary capillary wedge pressure; PPF: plasma protein fraction; PRBC: packed red blood cell; PT: prothrombin time; RBC: red blood cell; RL: Ringer's lactate; SBP: systolic blood pressure; WP: wedge pressure.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boks 2007 | Pump priming for patients undergoing cardiac surgery |
| Boldt 1993 | Pre‐bypass volume loading |
| Boldt 2000b | Compares 2 starches with each other |
| Boldt 2006 | The paper was retracted by the journal as Institutional Review Board approval could not be verified |
| Boldt 2008 | The paper was retracted by the journal as Institutional Review Board approval could not be verified |
| Brehme 1993 | Haemodilution |
| Bremerich 2000 | Compares 2 different starches (acetyl starch with hydroxyethyl starch) |
| Charlet 1991 | Study compared 2 different gelatins with each other and not with other colloids |
| Christ 1997 | Non‐randomised trial |
| Emery 1992 | Compares 20% and 4.5% albumin with each other and not with other colloids |
| Gan 1999 | Compares Hextend (a plasma volume expander based upon 6% hetastarch) with 6% hetastarch in saline (HES) |
| Green 2010 | Compares HES versus ringers |
| Haisch 2001a | The paper was retracted by the journal as Institutional Review Board approval could not be verified |
| Haisch 2001b | The paper was retracted by the journal as Institutional Review Board approval could not be verified |
| Hankeln 1990 | Haemodilution |
| Harke 1976 | Unable to find out if a randomised controlled trial. Methodology unclear |
| Hiippala 1996 | Patients were expected to have minimal blood loss |
| Hopkins 1994 | Insufficient information to include in review |
| Huet 2000 | Compares 2 starches with each other |
| Huttner 2000 | The paper was retracted by the journal as Institutional Review Board approval could not be verified |
| Jones 2004a | Haemodilution |
| Jovanovic 1997 | Does not mention if study was randomised. Unable to contact author for further information |
| Korttila 1984 | Healthy volunteers and cross‐over trial |
| Kotzampassi 2008 | Not clear how many participants were in each group |
| Langeron 2001 | Compares 2 starches with each other |
| Palumbo 2006 | Authors do not report the number of patients randomised to each group |
| Puri 1983 | There is no mention of a method of randomisation. Just reports "Twenty‐five patients studied in each group were well matched" |
| Rauch 2000 | Compares 2 starches with each other |
| Rehm 2000 | Haemodilution |
| Romero 1999 | Does not mention randomisation |
| Strauss 1985 | Healthy volunteers |
| Vanhoonacker 2009 | Pump priming for cardiac surgery |
| Waxman 1989 | Cross‐over study |
| Yap 2007 | Pump priming cardiac surgery |
Contributions of authors
FB screened citations for eligibility, obtained references, contacted authors, extracted data, entered data and wrote the review. DT screened citations for eligibility and extracted data. PA, VH, and SA contributed to earlier versions of the review.
Sources of support
Internal sources
University of Hertfordshire, UK.
External sources
NHS Research and Development Programme, UK.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Akech 2006 {published data only}
- Akech S, Gwer S, Idro R, Fegan G, Eziefula AC, Newton CRJC, et al. Volume expansion with albumin compared to gelofusine in children with severe malaria: results of a controlled trial. PLoS Hub for Clinical Trials 2006;1(5):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allison 1999 {published data only}
- Allison KP, Gosling P, Jones S, Pallister I, Porter K. Randomized trial of hydroxyethyl starch versus gelatine for trauma resuscitation. Journal of Trauma 1999;47(6):1114‐21. [DOI] [PubMed] [Google Scholar]
Arellano 2005 {published data only}
- Arellano R, Gan BS, Salpeter MJ, Yeo E, McCluskey S, Pinto R, et al. A triple‐blinded randomized trial comparing the hemostatic effects of large‐dose 10% hydroxyethyl starch 264/0.45 versus 5% albumin during major reconstructive surgery. Anesthesia and Analgesia 2005;100:1846‐53. [DOI] [PubMed] [Google Scholar]
Asfar 2000 {published data only}
- Asfar P, Kereni N, Labadie F, Gouello JP, Brenet O, Alquier P. Assessment of hemodynamic and gastric mucosal acidosis with modified fluid versus 6% hydroxyethyl starch: a prospective, randomized study. Intensive Care Medicine 2000;26(9):1282‐7. [DOI] [PubMed] [Google Scholar]
Beards 1994 {published and unpublished data}
- Beards SC, Watt T, Edwards JD, Nightingale P, Farragher EB. Comparison of the hemodynamic and oxygen transport responses to modified fluid gelatin and hetastarch in critically ill patients: a prospective, randomized trial. Critical Care Medicine 1994;22(4):600‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berard 1995 {published data only}
- Berard JP, Curt I, Piech JJ, Ruiz F. Hydroxyethylamidons versus gelatines: impact on the cost of replacement in an emergency (resuscitation) service [Hydroxyethylamidons versus gelatines: impact sur le cout du rempissage dans un service de reanimation]. Annales Francaises d'Anaesthesia et de Reanimation 1995;14:R335. [Google Scholar]
Beyer 1997 {published and unpublished data}
- Beyer R, Harmening U, Rittmeyer O, Zielmann S, Mielck F, Kazmaier S, et al. Use of modified fluid gelatin and hydroxyethyl starch for colloidal volume replacement in major orthopaedic surgery. British Journal of Anaesthesia 1997;78(1):44‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 1986 {published data only}
- Boldt JV, Bormann B, Kling D, Borner U, Mulch J, Hempelmann G. Volume replacement with a new hydroxyethyl starch preparation (3% HES 200/0.5) in heart surgery [Volumenersatz mit einem neuen hydroxyathylstarke ‐ praparat (3% HAS 200/0.5) in der herzchirurgie]. Infusionstherapie und Klinische Ernahrung 1986;13(3):145‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Boldt 1993a {published and unpublished data}
- Boldt J, Knothe C, Zickmann B, Andres P, Dapper F, Hempelmann G. Influence of different intravascular volume therapies on platelet function in patients undergoing cardiopulmonary bypass. Anesthesia and Analgesia 1993;76(6):1185‐90. [DOI] [PubMed] [Google Scholar]
Boldt 1995 {published data only}
- Boldt J, Heesen M, Welters I, Padberg W, Martin K, Hempelmann G. Does the type of volume therapy influence endothelial‐related coagulation in the critically ill?. British Journal of Anaesthesia 1995;75(6):740‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 1996a {published data only}
- Boldt J, Heesen M, Muller M, Pabsdorf M, Hempelmann G. The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesthesia and Analgesia 1996;83(2):254‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 1996b {published data only}
- Boldt J, Heesen M, Padberg W, Martin K, Hempelmann G. The influence of volume therapy and pentoxifylline infusion on circulating adhesion molecules in trauma patients. Anaesthesia 1996;51(6):529‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 1996c {published data only}
- Boldt J, Mueller M, Menges T, Papsdorf M, Hempelmann G. Influence of different volume therapy regimens on regulators of the circulation in the critically ill. British Journal of Anaesthesia 1996;77(4):480‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 1998 {published data only}
- Boldt J, Muller M, Mentges D, Papsdorf M, Hempelmann G. Volume therapy in the critically ill: is there a difference?. Intensive Care Medicine 1998;24(1):28‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 2000 {published data only}
- Boldt J, Suttner S, Kumle B, Huttner I. Cost analysis of different volume replacement strategies in anesthesia. Infusionstherapie und Transfusionsmedizin 2000;27(1):38‐43. [Google Scholar]
Boldt 2001 {published data only}
- Boldt J, Suttner S, Huttner I, Kumle B, Piper S, Krumholz W. Are cost of a crystalloid‐based volume replacement regimen lower than of a colloid‐based volume replacement strategy?. Infusionstherapie und transfusionsmedizin 2001;28(3):144‐9. [Google Scholar]
Brock 1995 {published and unpublished data}
- Brock H, Rapf B, Necek S, Gabriel C, Peterlik C, Polz W. Volume replacement after cardiac surgery. A comparison of small‐volume resuscitation and two different colloid solutions [Vergleichende untersuchungen zur postoperativen volumentherapie]. Anaesthesist 1995;44(7):486‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brutocao 1996 {published and unpublished data}
- Brutocao D, Bratton SL, Thomas JR, Schrader PF, Coles PG, Lynn AM. Comparison of hetastarch with albumin for postoperative volume expansion in children after cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 1996;10(3):348‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carli 2000 {published data only}
- Carli P, Goldstein P, Lejay M, Facon A, Orliaguet G, Petit P. Prehospital care of hypovolemic trauma patients: 6% hydroxyethyl starch versus gelatin [Remplissage vasculaire prehospitalier en traumatologie: Hesteril 6% versus Plasmion]. Journal Europeen des Urgences 2000;13(1‐2):101‐5. [Google Scholar]
Claes 1992 {published data only}
- Claes Y, Hemelrijck J, Gerven M, Arnout J, Vermylen J, Weidler B, et al. Influence of hydroxyethyl starch on coagulation in patients during the perioperative period. Anesthesia and Analgesia 1992;75(1):24‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diehl 1982 {published data only}
- Diehl JT, Lester JL, Cosgrove DM. Clinical comparison of hetastarch and albumin in postoperative cardiac patients. Annals of Thoracic Surgery 1982;34(6):674‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dolecek 2009 {published data only}
- Dolecek M, Svoboda P, Kantorova I, Scheer P, Sas I, Bibrova J, et al. Therapeutic influence of 20% albumin versus 6% hydroxyethylstarch on extravascular lung water in septic patients: a randomized controlled trial. Hepato‐Gastroenterology 2009;56(96):1622‐8. [PubMed] [Google Scholar]
Du Gres 1989 {published data only}
- Du Gres B, Gruner MC, Flamens C. A comparison of the hemodynamic effect of Haemaccel and diluted albumin in the immediate postoperative period after heart surgery [Comparaison des effets hemodynamiques de l'Haemaccel et de l'albumine diluee dans la periode postoperatoire immediate apres chirurgie cardiaque]. Cahiers d'Anesthesiologie 1989;37(5):327‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Dytkowska 1998 {published data only}
- Dytkowska B, Karwacki Z, Suchorzewska J, Wujtewicz M. Comparative assessment of 200/0.5 HAES 6% and Gelafundin in the treatment of hypovolaemia in post‐coronary bypass patients. Medical Science Monitor 1998;4(6):1000‐3. [Google Scholar]
Evans 2003 {published data only}
- Evans PA, Heptinstall S, Crowhurst EC, Davies T, Glenn JR, Madira W, et al. Prospective double‐blind randomized study of the effects of four intravenous fluids on platelet function and hemostasis in elective hip surgery. Journal of Thrombosis and Haemostasis 2003;1:2140‐8. [DOI] [PubMed] [Google Scholar]
Falk 1988 {published data only}
- Falk JL, Rackow EC, Astiz ME, Weil MH. Effects of hetastarch and albumin on coagulation in patients with septic shock. Journal of Clinical Pharmacology 1988;28(5):412‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Friedman 2008 {published data only}
- Friedman G, Jankowski S, Shahla M, Gomez J, Vincent JL. Hemodynamic effects of 6% and 10% hydroxyethyl starch solutions versus 4% albumin solution in septic patients. Journal of Clinical Anesthesia 2008;20(7):528‐33. [DOI] [PubMed] [Google Scholar]
Fries 2004 {published data only}
- Fries D, Streif W, Margreiter J, Klingler A, Kühbacher G, Schobersberger W, et al. The effects of perioperative administered crystalloids and colloids on concentrations of molecular markers of activated coagulation and fibrinolysis. Blood Coagulation and Fibrinolysis 2004;15:213‐9. [DOI] [PubMed] [Google Scholar]
Fulachier 1994 {published data only}
- Fulachier V, Sicard MP, Baille Y, Auffray JP. Effects of fluid expansion using albumin or hydroxyethylstarch on oxygen transport after induction of anesthesia for cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 1994;8(3Supp2):89. [Google Scholar]
Gahr 1981 {published data only}
- Gahr R, Bock PR. Effect of hydroxyethyl starch HES 450/0.7 and 5% human albumin on the colloid osmotic pressure and hemodynamic parameters in hypovolemic patients after major abdominal procedures [Wirkung von hydroxyathylstarke HAS 450/0.7 und humanalbumin 5% auf den kolloidosmotischen druck und hamodynamische parameter bei hypovolamischen patienten nach grosseren abdominalen eingriffen]. Infusionstherapie und Transfusionsmedizin 1981;8(3):147‐52. [MEDLINE: ] [PubMed] [Google Scholar]
Gallagher 1985 {published and unpublished data}
- Gallagher JD, Moore RA, Kerns D, Jose AB, Botros SB, Flicker S, et al. Effects of colloid or crystalloid administration on pulmonary extravascular water in the postoperative period after coronary artery bypass grafting. Anesthesia and Analgesia 1985;64(8):753‐8. [PubMed] [Google Scholar]
Godet 2008 {published data only}
- Godet G, Lehot JJ, Janvier G, Steib A, Castro V, Coriat P. Safety of HES 130/0.4 (Voluven(R)) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel‐group multicentre trial. European Journal of Anaesthesiology 2008;25(12):986‐94. [DOI] [PubMed] [Google Scholar]
Gold 1990 {published and unpublished data}
- Gold MS, Russo J, Tissot M, Weinhouse G, Riles T. Comparison of hetastarch to albumin for perioperative bleeding in patients undergoing abdominal aortic aneurysm surgery. Annals of Surgery 1990;211(4):482‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gombocz 2007 {published data only}
- Gombocz K, Beledi A, Alotti N, Kecskes G, Gabor V, Bogar L, et al. Influence of dextran‐70 on systemic inflammatory response and myocardial ischaemia‐reperfusion following cardiac operations. Critical Care 2007;11(4):R87. [DOI: 10.1186/cc6095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gondos 2010 {published data only}
- Gondos T, Marjanek Z, Ulakcasi Z, Szabo Z, Bogar L, Karolyi M, et al. Short‐term effectiveness of different volume replacement therapies in postoperative hypovolaemic patients. European Journal of Anaesthesiology 2010;27:794‐800. [DOI] [PubMed] [Google Scholar]
- Gondos T, Marjanek Z, Ulakcsai Z, Szabó Z, et al. Evaluation of the effectiveness of different volume replacement therapies in postoperative hypovolemic patients using the PiCCO monitoring system. Critical Care 2009;13(1):Suppl 1: P220. [Google Scholar]
Haas 2007 {published data only}
- Haas T, Preinreich A, Oswald E, Pajk W, Berger J, Kuehbacher G, et al. Effects of albumin 5% and artificial colloids on clot formation in small infants. Anaesthesia 2007;62(10):1000‐7. [DOI] [PubMed] [Google Scholar]
Hausdorfer 1986 {published data only}
- Hausdorfer J, Hagemann H, Heine J. Comparison of volume substitutes human albumin 5% and hydroxyethyl starch 6% in paediatric anaesthesia [Vergleich der volumenersatzmittel humanalbumin 5% und hydroxathylstarke 6% (40.000/0.5) in der kinderanasthesie]. Anasthesie, Intensivtherapie, Notfallmedizin 1986;21(3):137‐42. [MEDLINE: ] [PubMed] [Google Scholar]
Hecht‐Dolnik 2009 {published data only}
- Hecht‐Dolnik M, Barkan H, Taharka A, Loftus J. Hetastarch increases the risk of bleeding complications in patients after off‐pump bypass surgery: a randomized clinical trial. Journal of Thoracic and Cardiovascular Surgery 2009;138:703‐11. [DOI] [PubMed] [Google Scholar]
Hedstrand 1987 {published data only}
- Hedstrand U, Hogman C, Zaren B, Lundkvist B. Postoperative complications after blood replacement with or without plasma. Acta Chirurgica Scandinavica 1987;153(9):501‐5. [PubMed] [Google Scholar]
Hiippala 1995 {published data only}
- Hiippala S, Linko K, Myllyla G, Lalla M, Hekali R, Makelainen A. Replacement of major surgical blood loss by hypo‐oncotic or conventional plasma substitutes. Acta Anaesthesiologia Scandinavica 1995;39(2):228‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2005 {published data only}
- Huang Y, Yan B, Yang Z. Clinical study of a formula for delayed rapid fluid resuscitation for patients with burn shock. Burns 2005;31:617‐22. [DOI] [PubMed] [Google Scholar]
Huskisson 1993 {published data only}
- Huskisson L, Elliott M, Spitz L. Haemodynamic effects of three colloids following pediatric open heart surgery. Clinical Intensive Care 1993;4:302. [Google Scholar]
Inal 2010 {published data only}
- Inal MT, Memi, Karamanlioglu B, Sut N. Effects of polygeline and hydroxyethyl starch solutions on liver functions assessed with LIMON in hypovolemic patients. Journal of Critical Care 2010;25(2):361. [DOI] [PubMed] [Google Scholar]
Jin 2010 {published data only}
- Jin SL, Yu BW. Effects of acute hypervolemic fluid infusion of hydroxyethyl starch and gelatin on hemostasis and possible mechanisms. Clinical and Applied Thrombosis/Hemostasis: Official Journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis 2010;16(1):91‐8. [DOI] [PubMed] [Google Scholar]
Jones 2004 {published data only}
- Jones S, Whitten C, Monk T. Influence of crystalloid and colloid replacement solutions on hemodynamic variables during acute normovolemic hemodilution. Journal of Clinical Anaesthesia 2004;16:11‐7. [DOI] [PubMed] [Google Scholar]
Karanko 1987 {published and unpublished data}
- Karanko MS. Effects of three colloid solutions on plasma volume and hemodynamics after coronary bypass surgery. Critical Care Medicine 1987;15(11):1015‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kirklin 1984 {published data only}
- Kirklin JK, Lell WA, Kouchoukos NT. Hydroxyethyl starch versus albumin for colloid infusion following cardiopulmonary bypass in patients undergoing myocardial revascularization. Annals of Thoracic Surgery 1984;37(1):40‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lisander 1996 {published and unpublished data}
- Lisander B, Jacobsson SA, Ivarsson I, Vegfors M, Engdahl O. Giving both enoxaparin and dextran increases the need for transfusion in revision hip arthroplasty. European Journal of Surgery 1996;162(11):861‐6. [MEDLINE: ] [PubMed] [Google Scholar]
London 1989 {published data only}
- London MJ, Ho JS, Triedman JK, Verrier ED, Levin J, Merrick SH, et al. A randomized clinical trial of 10% pentastarch (low molecular weight hydroxyethyl starch) versus 5% albumin for plasma volume expansion after cardiac operations. Journal of Thoracic and Cardiovascular Surgery 1989;97(5):785‐97. [MEDLINE: ] [PubMed] [Google Scholar]
Mahmood 2007 {published data only}
- Mahmood A, Gosling P, Vohra RK. Randomized clinical trial comparing the effects on renal function of hydroxyethyl starch or gelatine during aortic aneurysm surgery. The British Journal of Surgery 2007;94(4):427‐33. [PUBMED: 17380548] [DOI] [PubMed] [Google Scholar]
Mastroianni 1994 {published data only}
- Mastroianni L, Low HB, Rollman J, Wagle M, Bleske B, Chow MS. A comparison of 10% pentastarch and 5% albumin in patients undergoing open‐heart surgery. Journal of Clinical Pharmacology 1994;34(1):34‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mittermayr 2007 {published data only}
- Mittermayr M, Streif W, Haas T, Fries D, Velik‐Salchner C, Klingler A, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesthesia and Analgesia 2007;105(4):905‐17. [PUBMED: 17898365] [DOI] [PubMed] [Google Scholar]
Moggio 1983 {published data only}
- Moggio RA, Rha CC, Somberg ED, Praeger P, Pooley RW, Reed GE. Hemodynamic comparison of albumin and hydroxyethyl starch in postoperative cardiac surgery patients. Critical Care Medicine 1983;11(12):943‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Molnar 2004 {published data only}
- Molnar Z, Mikor A, Leiner T, Szakmany T. Fluid resuscitation with colloids of different molecular weight in septic shock. Intensive Care Medicine 2004;30:1356‐60. [DOI] [PubMed] [Google Scholar]
Mukhtar 2009 {published data only}
- Mukhtar A, Aboulfetouh F, Obayah G, Salah M, Emam M, Khater Y, et al. The safety of modern hydroxyethyl starch in living donor liver transplantation: a comparison with human albumin. Anesthesia and Analgesia 2009;109:924‐30. [DOI] [PubMed] [Google Scholar]
Munoz 1980 {published data only}
- Munoz E, Raciti A, Dove D, Stahl WM, Guercio L. Effect of hydroxyethyl starch versus albumin on hemodynamic and respiratory function in patients with shock. Critical Care Medicine 1980;8(4):255. [Google Scholar]
Munsch 1988 {published data only}
- Munsch CM, MacIntyre E, Machin SJ, Mackie IJ, Treasure T. Hydroxyethyl starch: an alternative to plasma for postoperative volume expansion after cardiac surgery. British Journal of Surgery 1988;75(7):675‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niemi 2006 {published data only}
- Kuitunen A, Suojaranta‐Ylinen R, Kukkonen S, Niemi T. A comparison of the haemodynamic effects of 4% succinylated gelatin, 6% hydroxyethyl starch (200/0.5) and 4% human albumin after cardiac surgery. Scandinavian Journal of Surgery 2007;96:72‐8. [DOI] [PubMed] [Google Scholar]
- Niemi T, Suojaranta‐Ylinen R, Kukkonen S, Kuitunen A. Gelatin and hydroxyethyl starch, but not albumin, impair hemostasis after cardiac surgery. Anesthesia and Analgesia 2006;102:998‐1006. [DOI] [PubMed] [Google Scholar]
Ooi 2009 {published data only}
- Ooi JS, Ramzisham AR, Zamrin MD. Is 6% hydroxyethyl starch 130/0.4 safe in coronary artery bypass graft surgery?. Asian Cardiovascular & Thoracic Annals 2009;17(4):368‐72. [DOI] [PubMed] [Google Scholar]
Prien 1990 {published and unpublished data}
- Prien T, Backhaus N, Pelster F, Pircher W, Bunte H, Lawin P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. Journal of Clinical Anesthesia 1990;2(5):317‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rackow 1983 {published data only}
- Rackow EC, Falk JL, Fein IA, Siegel JS, Packman MI, Haupt MT, et al. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Critical Care Medicine 1983;11(11):839‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Rackow 1989 {published data only}
- Rackow EC, Mecher C, Astiz ME, Griffel M, Falk JL, Weil MH. Effects of pentastarch and albumin infusion on cardiorespiratory function and coagulation in patients with severe sepsis and systemic hypoperfusion. Critical Care Medicine 1989;17(5):395‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reine 2008 {published data only}
- Reine PA, Kongsgaard UE, Andersen A, Thogersen AK, Olsen H. Infusion of albumin attenuates changes in serum protein binding of drugs in surgical patients compared with volume replacement with HAES. Acta Anaesthesiologica Scandinavica 2008;52(3):406‐12. [DOI] [PubMed] [Google Scholar]
Rittoo 2004 {published data only}
- Rittoo D, Gosling P, Burnley S, Bonnici C, Millns P, Simms MH, et al. Randomized study comparing the effects of hydroxyethyl starch solution with Gelofusine on pulmonary function in patients undergoing abdominal aortic aneurysm surgery. British Journal of Anaesthesia 2004;92:61‐6. [DOI] [PubMed] [Google Scholar]
Rosencher 1992 {published and unpublished data}
- Rosencher N, Vassilieff N, Guigonis V, Toulin P, Conseiller C. Comparison of effects of Elohes and albumin on haemostasis in orthopedic surgery [Comparaison des effets de l'Elohes et de l'albumine sur l'hemostase en chirurgie orthopedique]. Annales francaises d'Anesthesie et de Reanimation 1992;11(5):526‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schortgen 2001 {published data only}
- Schortgen F, Lacherade J, Bruneel F, Cattaneo I, Hemery F, Lemaire F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet 2001;357(9260):911‐6. [DOI] [PubMed] [Google Scholar]
Schramko 2009 {published data only}
- Niemi T, Schramko A, Kukkonen S, Kuitunen A, Suojaranta‐Ylinen R. Haemodynamics and acid‐base equilibrium after cardiac surgery: comparison of rapidly degradable hydroxyethyl starch solutions and albumin. Scandinavian Journal of Surgery 2008;97:259‐65. [DOI] [PubMed] [Google Scholar]
- Schramko A, Suojaranta‐Ylinen R, Kuitunen A, Kukkonen S, Niemi T. Rapidly degradable hydroxyethyl starch solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Anesthesia and Analgesia 2009;108:30‐6. [DOI] [PubMed] [Google Scholar]
Schramko 2010 {published data only}
- Schramko A, Suojaranta‐Ylinen R, Kuitunen A, Raivio P, Kukkonen S, Niemi T. Comparison of the effect of 6% hydroxyethyl starch and gelatine on cardiac and stroke volume index: a randomized, controlled trial after cardiac surgery. Perfusion 2010;25(5):283‐91. [DOI] [PubMed] [Google Scholar]
- Schramko A, Suojaranta‐Ylinen R, Kuitunen A, Raivio P, Kukkonen S, Niemi T. Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. British Journal of Anaesthesia 2010;6:691‐7. [DOI] [PubMed] [Google Scholar]
Shatney 1983 {published data only}
- Shatney CH, Deepika K, Militello PR, Majerus TC, Dawson RB. Efficacy of hetastarch in the resuscitation of patients with multisystem trauma and shock. Archives of Surgery 1983;118(7):804‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Standl 2008 {published data only}
- HES 130/0.4 (Voluven) or human albumin in children younger than 2 yr undergoing non‐cardiac surgery. A prospective, randomized, open label, multicentre trial. European Journal of Anaesthesiology 2008;25(6):437‐45. [DOI] [PubMed] [Google Scholar]
Stockwell 1992 {published data only}
- Stockwell MA, Scott A, Day A, Riley B, Soni N. Colloid solutions in the critically ill. A randomised comparison of albumin and polygeline: 2. Serum albumin concentration and incidences of pulmonary oedema. Anesthesia 1992;47(1):7‐9. [DOI] [PubMed] [Google Scholar]
- Stockwell MA, Soni N, Riley B. Colloid solutions in the critically ill. A randomised comparison of albumin and polygeline: 1. Outcome and duration of stay in the intensive care unit. Anaesthesia 1992;47(1):3‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stoddart 1996 {published data only}
- Stoddart PA, Rich P, Sury MR. A comparison of 4.5% human albumin solution and haemaccel in neonates undergoing major surgery. Paediatric Anaesthesia 1996;6(2):103‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tollofsrud 1995 {published data only}
- Svennevig JL, Tollofsrud S, Kongsgaard U, Noddeland H, Mohr B, Ozer M, et al. Complement activation during and after open‐heart surgery is only marginally affected by the choice of fluid for volume replacement. Perfusion 1996;11(4):326‐32. [DOI] [PubMed] [Google Scholar]
- Tollofsrud S, Svennevig JL, Breivik H, Kongsgaard U, Ozer M, Hysing E, et al. Fluid balance and pulmonary functions during and after coronary artery bypass surgery: Ringer's acetate compared with dextran, polygeline, or albumin. Acta Anaesthesiologica Scandinavica 1995;39(5):671‐7. [DOI] [PubMed] [Google Scholar]
Van der Linden 2004 {published data only}
- Linden PJ, Hert SG, Daper A, Trenchant A, Schmartz D, Defrance P, et al. 3.5% Urea linked gelatine is as effective as 6% HES 200/0.5 for volume management in cardiac surgery patients. Canadian Journal of Anaesthesia 2004;51(3):236‐41. [DOI] [PubMed] [Google Scholar]
Van der Linden 2005 {published data only}
- Linden PJ, Hert SG, Deraedt D, Cromheecke S, Decker K, Paep R, et al. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesthesia and Analgesia 2005;101:629‐34. [DOI] [PubMed] [Google Scholar]
Veneman 2004 {published data only}
- Veneman T, Oude Nijhuis J, Woittiez A. Human albumin and starch administration in critically ill patients: a prospective RCT. Wiener Klinische Wochenschrift 2004;116(9‐10):283‐5. [DOI] [PubMed] [Google Scholar]
Verheij 2006 {published data only}
- Verheij J, Lingen A, Beishuizen A, Christiaans H, Jong J, Girbes A, et al. Cardiac response is greater for colloid than saline fluid loading after cardiac or vascular surgery. Intensive Care Medicine 2006;32:1030‐8. [DOI] [PubMed] [Google Scholar]
- Verheij J, Lingen A, Raijmakers P, Rijnsburgr E, Veerman D, Wisselink W, et al. Effect of fluid loading with saline or colloids on pulmonary permeability, oedema and lung injury score after cardiac and major vascular surgery. British Journal of Anaesthesia 2006;96(1):21‐30. [DOI] [PubMed] [Google Scholar]
Vogt 1994 {published data only}
- Vogt N, Bothner U, Georgieff M. Comparison of 5% human albumin and 6% 200/0.5 HES as exclusive colloid components in large surgical interventions [Vergleich von humanalbumin 5% und 6% HES 200/0.5 als ausschliessliche kolloidkomponente bei grossen chirurgischen eingriffen]. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 1994;29(3):150‐6. [DOI] [PubMed] [Google Scholar]
Vogt 1996 {published data only}
- Vogt NH, Bothner U, Lerch G, Linder KH, Georgieff M. Large‐dose administration of 6% hydroxyethyl starch 200/0.5 for total hip arthroplasty: plasma homeostasis, hemostasis, and renal function compared to use of 5% human albumin. Anesthesia and Analgesia 1996;83(2):262‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vogt 1999 {published data only}
- Vogt N, Bothner U, Brinkmann A, Petriconi R, Georgieff M. Peri‐operative tolerance to large‐dose 6% HES 200/0.5 in major urological procedures compared with 5% human albumin. Anaesthesia 1999;54(2):121‐7. [DOI] [PubMed] [Google Scholar]
- Vogt N, Bothner U, Lerch G, Georgieff M. Pharmacokinetic and oncotic characteristics of high‐dose hydroxyethyl starch compared with human albumin 5% in operative procedures [Pharmakokinetik und onkotisches verhalten von hochdosierter hydroxyathylstarke bei operativen eingriffen im vergleich zu humanalbumin 5%]. Infusion Therapy and Transfusion Medicine 1998;25:212‐21. [Google Scholar]
Volta 2007 {published data only}
- Volta CA, Alvisi V, Campi M, Marangoni E, Alvisi R, Castellazzi M, et al. Influence of different strategies of volume replacement on the activity of matrix metalloproteinases: an in vitro and in vivo study. Anesthesiology 2007;106(1):85‐91. [PUBMED: 17197849] [DOI] [PubMed] [Google Scholar]
von Sommoggy 1990 {published data only}
- Sommoggy S, Fraunhofer J, Jelen‐Esselborn S, Stemberger A. Coagulation changes during aortofemural bifurcation bypass: is volume and plasma substitution possible with hydroxyethyl starch alone? [Gerinnungsveranderungen bei aortofemoralem bifurkationsbypass: ist eine Volumen ‐und Plasmasubstitution mit hydroxyathylstarke allein moglich?]. Anaesthesist 1990;39(7):353‐60. [PubMed] [Google Scholar]
Wahba 1996 {published and unpublished data}
- Wahba A, Sendtner E, Birnbaum DE. Fluid resuscitation with Haemaccel vs. human albumin following coronary artery bypass grafting. The Thoracic and Cardiovascular Surgeon 1996;44(4):178‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watkins 1990 {published data only}
- Watkins J, Wild G, Appleyard TN, Hardy G. Complement activation by polystarch and gelatine volume expanders. Lancet 1990;335(8683):233. [DOI] [PubMed] [Google Scholar]
Woittiez 1997 {published and unpublished data}
- Hondebrink Y, Jeekel L, Oude Nijhuis J, Woittiez AJJ. Restoration of colloid osmotic pressure in hypoalbuminaemic patients. Intensive Care Medicine 1997;23(supp 1):S184. [Google Scholar]
- Timmer B, Hondebrink Y, Oude Nijhuis J, Woittiez AJJ. Restoration of colloid osmotic pressure in hypoalbuminaemic patients. Netherlands Journal of Medicine 1998;52:A42. [Google Scholar]
Yang 2011 {published data only}
- Yang J, Wang WT, Yan LN, Xu MQ, Yang JY. Alternatives to albumin administration in hepatocellular carcinoma patients undergoing hepatectomy: an open, randomized clinical trial of efficacy and safety. Chinese Medical Journal 2011;124(10):1458‐64. [PubMed] [Google Scholar]
References to studies excluded from this review
Boks 2007 {published data only}
- Boks RH, Wijers MJ, Hofland J, Takkenberg JJM, Bogers AJJ. Low molecular starch versus gelatin plasma expander during CPB: does it make a difference?. Perfusion 2007;22(5):333‐8. [DOI: 10.1177/0267659107086656] [DOI] [PubMed] [Google Scholar]
Boldt 1993 {published data only}
- Boldt J, Knothe C, Schindler E, Hammermann H, Dapper F, Hempelmann G. Volume replacement with hydroxyethyl starch solution in children. British Journal of Anaesthesia 1993;70(6):661‐5. [DOI] [PubMed] [Google Scholar]
Boldt 2000b {published data only}
- Boldt J, Lehmann A, Rompert R, Haisch G, Isgro F. Volume therapy with a new hydroxyethyl starch solution in cardiac surgical patients before cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anaesthesia 2000;14(3):264‐8. [DOI] [PubMed] [Google Scholar]
Boldt 2006 {published data only}
- Boldt J, Schollhorn T, Mayer J, Piper S, Suttner S. The value of an albumin‐based intravascular volume replacement strategy in elderly patients undergoing major abdominal surgery. Anesthesia and Analgesia 2006;103:191‐9. [DOI] [PubMed] [Google Scholar]
Boldt 2008 {published data only}
- Boldt J, Brosch C, Röhm K, Papsdorf M, Mengistu A. Comparison of the effects of gelatin and a modern hydroxyethyl starch solution on renal function and inflammatory response in elderly cardiac surgery patients. British Journal of Anaesthesia 2008;100(4):457‐64. [DOI] [PubMed] [Google Scholar]
Brehme 1993 {published data only}
- Brehme S, Keysser G, Turowski A, Schmidt H. Hemorheologic effects of hydroxyethyl starch 200/0.5, dextran 40, oxypolygelatine and full electrolyte sodium over 48 hours [Hamorheologische wirkungen von hydroxyathylstarke 200/0.5, dextran 40, oxypolygelatine und vollelekttrolytlosung uber 48 studen]. Zeitschrift fur die Gesamte Innerve Medizin und ihre Grenzgebiete 1993;48(10):506‐10. [PubMed] [Google Scholar]
Bremerich 2000 {published data only}
- Bremerich DH, Lischke V, Asskali F, Forster H, Behne M. Pharmacodynamics and tolerability of acetyl starch as a new plasma volume expander in patients undergoing elective surgery. International Journal of Clinical Pharmacology and Therapeutics 2000;38(8):408‐14. [DOI] [PubMed] [Google Scholar]
Charlet 1991 {published data only}
- Charlet P, Zerr C, Robert D, Merville C, Renouf P, Khayat MC. Comparative trials of fluid gelatins on hemostasis in heart surgery in adults [Essais comparatifs des gelatines fluides sur l'hemostase dans la chirurgie cardiaque de l'adulte]. Cahiers d'Anesthesiologie 1991;39(4):233‐8. [PubMed] [Google Scholar]
Christ 1997 {published data only}
- Christ F, Niklas M, Kreimeier U, Lauterjung L, Peter K, Messmer K. Hyperosmotic‐hyperoncotic solutions during abdominal aortic aneurysm (AAA) resection. Acta Anaesthesiologica Scandinavica 1997;41(1):62‐70. [DOI] [PubMed] [Google Scholar]
Emery 1992 {published data only}
- Emery EF, Greenough A, Gamsu HR. Randomised controlled trial of colloid infusions in hypotensive preterm infants. Archives of Disease in Childhood, 1992;67(10(S)):1185‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gan 1999 {published data only}
- Gan TJ, Bennett‐Guerrero E, Phillips‐Bute B, Wakeling H, Moskowitz DM, Olufolabi Y, et al. Hextend, a physiologically balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Anesthesia and Analgesia 1999;88(5):992‐8. [DOI] [PubMed] [Google Scholar]
Green 2010 {published data only}
- Green RS, Zed PJ, McIntyre L. Pentastarch Resuscitation in Severe Sepsis and Septic Shock. Canadian Journal of Emergency Medicine 2010;12(1):58‐61. [DOI] [PubMed] [Google Scholar]
Haisch 2001a {published data only}
- Haisch G, Boldt J, Krebs C, Suttner S, Lehmann A, Isgro F. Influence of a new hydroxyethylstarch preparation (HES 130/0.4) on coagulation in cardiac surgical patients. Journal of Cardiothoracic and Vascular Anesthesia 2001;15(3):316‐21. [DOI] [PubMed] [Google Scholar]
Haisch 2001b {published data only}
- Haisch G, Boldt J, Krebs C, Kumle B, Suttner S, Schulz A. The influence of intravascular volume therapy with a new hydroxyethyl starch preparation (6% HES 130/0.4) on coagulation in patients undergoing major abdominal surgery. Anesthesia and Analgesia 2001;92(3):565‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hankeln 1990 {published data only}
- Hankeln K, Senker R, Beez M. Comparative study of the intraoperative effectiveness of 5% human albumin or 10% hydroxyethyl starch (HAES‐steril) on hemodynamics and oxygen transport in 40 patients [Vergleichende Untersuchung zur intraoperativen Wirksamkeit von 5% Humanalbumin oder 10% Hydroxyathylstarke (HAES‐steril) auf Hamodynamik und Sauerstofftransport bei 40 Patienten]. Infusionstherapie 1990;17(3):135‐40. [PubMed] [Google Scholar]
Harke 1976 {published data only}
- Harke H, Thoenies R, Margraf I, Momsen W. The influence of different plasma substitutes on blood clotting and platelet function during and after surgery [Der Einfluss verschiedener Plasmaersatzmittel auf Gerinnungssystem und Thrombocytenfunktion wahrend und nach operativen Eingriffen. Vorlaufige Ergebnisse einer klinischen Studie]. Anaesthesist 1976;25(8):366‐73. [PubMed] [Google Scholar]
Hiippala 1996 {published data only}
- Hiippala S, Teppo AM. Perioperative volume effect of HES 120/0.7 compared with dextran 70 and Ringer acetate. Annales Chirurgiae et Gynaecologiae 1996;85(4):333‐9. [PubMed] [Google Scholar]
Hopkins 1994 {unpublished data only}
- Hopkins PM. 6% Hydroxyethylstarch with 4% gelatine as peri‐operative intravenous volume replacement in surgical patients. National Research Register Version 1/1998.
Huet 2000 {published data only}
- Huet RCGG, Siemons AW, Baus D, Rooyen‐Butijn WT, Haagenaars JAM, Oeveren W, et al. A novel hydroxyethyl starch (Voluven(TM)) for effective perioperative plasma volume substitution in cardiac surgery. Canadian Journal of Anaesthesia 2000;47(12):1207‐15. [DOI] [PubMed] [Google Scholar]
Huttner 2000 {published data only}
- Huttner I, Boldt J, Haisch G, Suttner S, Kumle B, Schulz H. Influence of different colloids on molecular markers of haemostasis and platelet function in patients undergoing major abdominal surgery. British Journal of Anaesthesia 2000;85(3):417‐23. [DOI] [PubMed] [Google Scholar]
Jones 2004a {published data only}
- Jones SB, Whitten CW, Monk TG. Influence of crystalloid and colloid replacement solutions on hemodynamic variables during acute normovolemic hemodilution. Journal of Clinical Anesthesia 2004;16(1):11‐7. [DOI] [PubMed] [Google Scholar]
Jovanovic 1997 {published data only}
- Jovanovic K, Filipovic N, Romic P, Surbatovic M. Hetastarch in replacement of circulation volume compared to haemaccel and dextran 70 in pre‐hospital resuscitation of polytraumatised patients. Intensive Care Medicine 1997;23:S184. [Google Scholar]
Korttila 1984 {published data only}
- Korttila K, Grohn P, Gordin A, Sundberg S, Salo H, Nissinen E, et al. Effect of hydroxyethyl starch and dextran on plasma volume and blood hemostasis and coagulation. Journal of Clinical Pharmacology 1984;24(7):273‐82. [DOI] [PubMed] [Google Scholar]
Kotzampassi 2008 {published data only}
- Kotzampassi K, Grosomanidis V, Andreopoulos K, Skourtis CH, Eleftheriadis E. Albumin versus colloids in colon surgery patients: preliminary results (Abstract no P229). Critical Care 2008;12(Suppl 2):P229. [DOI: 10.1186/cc6450] [DOI] [Google Scholar]
Langeron 2001 {published data only}
- Langeron ODM, Doelberg M, Ang ET, Bonnet F, Capdevila X, Coriat P. Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4) causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesthesia and Analgesia 2001;92(4):855‐62. [DOI] [PubMed] [Google Scholar]
Palumbo 2006 {published data only}
- Palumbo. The effect of HES solution in critically ill patients. Minerva Anesthesiology 2006;72:655‐64. [PubMed] [Google Scholar]
Puri 1983 {published data only}
- Puri VK, Howard M, Paidipaty B, Singh S. Resuscitation in hypovolemia and shock: a prospective study of hydroxyethyl starch and albumin. Critical Care Medicine 1983;11(7):518‐23. [DOI] [PubMed] [Google Scholar]
Rauch 2000 {published data only}
- Rauch S, Sefrin P. Comparison of hydroxyethyl starch solutions derived from potato and corn starch [Vergleich von Hydroxyethylstarkelosungen aus Kartoffel‐ und Maisstarke]. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2000;35(12):750‐5. [DOI] [PubMed] [Google Scholar]
Rehm 2000 {published data only}
- Rehm M, Orth V, Scheingraber S, Kreimeier U, Brechtelsbauer H, Finsterer U. Acid‐base changes caused by 5% albumin versus 6% hydroxyethyl starch solution in patients undergoing acute normovolemic hemodilution: a randomized prospective study. Anesthesiology 2000;93(5):1174‐83. [DOI] [PubMed] [Google Scholar]
Romero 1999 {published data only}
- Romero J, Luna P, Fernandez B, Rojas E, Sarrano X, Alvarez H. The use of HAES‐Steril 6% as a plasma expander after cardiopulmonary bypass in aortocoronary surgery [Uso de HAES esteril 6% como expansor plasmatico despues de la circulacion extracorporea en revascularizacion coronaria]. Revista Mexicana de Anestesiologia 1999;22:160‐7. [Google Scholar]
Strauss 1985 {published data only}
- Strauss RG, Stump DC, Henriksen RA, Saunders R. Effects of hydroxyethyl starch and fibrinogen, fibrin clot formation, and fibrinolysis. Transfusion 1985;25(3):230‐4. [DOI] [PubMed] [Google Scholar]
Vanhoonacker 2009 {published data only}
- Vanhoonacker J, Ongenae M, Vanoverschelde H, Donadoni R. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for cardiopulmonary bypass priming: the effects on postoperative bleeding and volume expansion needs after elective CABG. Acta Anaesthesiologica Belgica 2009;60:91‐7. [PubMed] [Google Scholar]
Waxman 1989 {published data only}
- Waxman K, Holness R, Tominaga G, Chela P, Grimes J. Hemodynamic and oxygen transport effects of pentastarch in burn resuscitation. Annals of Surgery 1989;209(3):341‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yap 2007 {published data only}
- Yap WW, Young D, Pathi V. Effects of gelatine and medium molecular weight starch as priming fluid in cardiopulmonary bypass ‐ a randomised controlled trial. Perfusion 2007;22:57‐61. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armstrong 1994
- Armstrong RF, Bullen C, Cohen SL, Singer M, Webb AR. Critical care algorithms. Vol. Oxford Medical Publications, Oxford University Press, 1994. [Google Scholar]
Berlin 1997
- Berlin JA. Does blinding of readers affect the results of meta‐analyses?. Lancet 1997;350:185‐6. [DOI] [PubMed] [Google Scholar]
Boldt 1996
- Boldt J, Heesen M, Muller M, Pabsdorf M, Hempelmann G. The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesthesia and Analgesia 1996;83:254‐61. [DOI] [PubMed] [Google Scholar]
Boldt 2009
- Boldt J, Suttner S, Brosch C, Lehmann A, Roehm K, Mengitsu A. Cardiopulmonary bypass priming using a high dose of a balanced hydroxyethyl starch versus an albumin‐based priming strategy. Anesthesia and Analgesia 2009;109:1752‐62. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Perel 2012
- Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000567.pub4] [DOI] [PubMed] [Google Scholar]
Reinhart 2011
- Reinhart K, Takala J. Hydroxyethyl starches: what do we still know?. Anesthesia and Analgesia 2011;112:507‐11. [DOI] [PubMed] [Google Scholar]
Roberts 2011
- Roberts I, Blackhall K, Alderson P, Bunn F, Schierhout G. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD001208.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shafer 2011
- Shafer SL. Shadow of doubt. Anesthesia and Analgesia 2011;112:498‐500. [DOI] [PubMed] [Google Scholar]
Traylor 1996
- Traylor RJ, Pearl RG. Crystalloid versus colloid: all colloids are not created equal. Anesthesia and Analgesia 1996;83:209‐12. [DOI] [PubMed] [Google Scholar]
Vermeulen 1995
- Vermeulen LC Jr, Ratko TA, Erstad BL, Brecher ME, Matuszewski KA. A paradigm for consensus. The University Hospital Consortium guidelines for the use of albumin, nonprotein colloid, and crystalloid solutions. Archives of Internal Medicine 1995;155(4):373‐9. [DOI] [PubMed] [Google Scholar]
Yim 1995
- Yim JM, Vermeyken LC, Erstad BL, Matuszewski KA, Burnett DA, Vlasses PH. Albumin and nonprotein colloid solution use in US academic health centers. Archives of Internal Medicine 1995;155(22):2450‐5. [PubMed] [Google Scholar]


