Abstract
Background
Dentine hypersensitivity may be defined as the pain arising from exposed dentine, typically in response to external stimuli, and which cannot be explained by any other form of dental disease. Many treatment regimens have been recommended over the years, and in recent years particular attention has been focused on toothpastes containing various potassium salts.
Objectives
To compare the effectiveness of potassium containing toothpastes with control toothpastes in reducing dentine hypersensitivity.
Search methods
The following databases were searched: Cochrane Oral Health Group Trials Register (searched until August 2005); CENTRAL (until August 2005); EMBASE/MEDLINE, PubMed, Web of Science (until September 2005). Bibliographies of clinical studies and reviews identified in the electronic search were checked for studies published outside the electronically searched journals.
Selection criteria
Randomised controlled trials (RCTs) in which the effect on dentine hypersensitivity of potassium containing toothpastes was tested against non‐potassium containing control toothpastes.
Data collection and analysis
Two of the review authors independently recorded the results of the included trials using a specially designed form. Sensitivity was assessed by using thermal, tactile, air blast, and subjective methods.
Main results
Six studies were included in the meta‐analysis which showed the statistically significant effect of potassium nitrate toothpaste on air blast and tactile sensitivity at the 6 to 8 weeks follow up, e.g. the meta‐analysis of air blast sensitivity showed a standardized mean difference in sensitivity score of ‐1.25 (95% CI: ‐1.65 to ‐0.851) in favour of treatment. The subjective assessment failed to show a significant effect at the 6 to 8 week assessment.
Authors' conclusions
The evidence generated by this review is based on a small number of individuals. Furthermore, the effect varies with the methods applied for assessing the sensitivity. Thus no clear evidence is available for the support of potassium containing toothpastes for dentine hypersensitivity.
Keywords: Adult, Humans, Dentin Sensitivity, Dentin Sensitivity/drug therapy, Potassium Compounds, Potassium Compounds/therapeutic use, Randomized Controlled Trials as Topic, Toothpastes, Toothpastes/chemistry, Toothpastes/therapeutic use
Plain language summary
Potassium containing toothpastes for dentine hypersensitivity
Dentine hypersensitivity is a sharp, sudden pain arising from the teeth when exposed to touch or hot and cold foods. If dental disease is not the cause of the pain, toothpastes containing potassium have been recommended to reduce tooth sensitivity. This review of trials found there was not enough evidence to show that potassium is effective in desensitising teeth. More research is needed.
Background
Dentine hypersensitivity may be defined as the pain arising from exposed dentine, typically in response to external stimuli, and which cannot be explained by any other form of dental disease. The pain most often occurs after exposure of root dentine by removal of cementum and overlying periodontal tissues (Bissada 1994).
The main symptom of dentine hypersensitivity is a sharp sudden pain of short duration in response to thermal stimuli such as intake of cold or hot foods, but may also arise from tactile stimuli e.g. the use of a toothbrush. The proportion of the population experiencing this condition is in the region of 15% (Graf 1977; Flynn 1985).
Dentine hypersensitivity continues to be a problem, although many treatment regimens have been recommended over the years. In recent years particular attention has been focused on potassium containing toothpastes (Schiff 1998). In the same period the consumption of this type of toothpaste has increased significantly in most European countries.
The exact mechanisms by which potassium containing toothpastes desensitises dentine is yet to be elucidated, but the toothpaste companies marketing these products typically claim that the desensitising effect is due to the potassium ion. This statement is based on results from animal studies indicating that by increasing the extracellular potassium ion concentration in very deep dentine cavities it is possible to depolarise nerve fibre membranes and render them unable to repolarise because of the maintained high levels of extracellular potassium ions (Kim 1986). However, the experimental model used in these studies cannot be compared with the situation, where a patient uses a toothpaste twice a day. Daily use of the toothpaste will result in only a slight increase in concentration of potassium ions in saliva and only for a short period of time. Furthermore, during toothbrushing the distance between the desensitising agent and the dental nerves will be large compared to the animal model, where the therapeutic agent is placed in a deep dentine cavity close to the pulp tissue. Clinical trials to investigate active agents in toothpastes for the relief of dentine hypersensitivity are hampered by a number of factors and not least by the very subjective nature of pain assessment (Kaufman 1994; Orchardson 1994; Holland 1997). A great number of clinical trials concerning potassium containing toothpastes have been published since 1974. The literature indicates conflicting results, although most reports state a very positive effect. The aim of this study is to conduct a systematic, up‐to‐date review of the previous randomised controlled trials (RCTs) on this subject.
Objectives
To assess the effectiveness of potassium containing toothpastes in reducing dentine hypersensitivity.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in which potassium containing toothpastes were compared to non‐potassium containing toothpastes.
Types of participants
Healthy human adults (18 years or more) with dentine hypersensitivity from exposed root surfaces.
Types of interventions
Daily home use of potassium containing toothpaste versus control toothpaste. In each study the toothpastes compared will either both contain fluoride or not. The control toothpaste will be exactly the same as the test toothpaste apart from the addition of a potassium salt, and this will be called the 'control' toothpaste throughout the review.
Types of outcome measures
Changes in (1) pain symptoms in response to test procedures including tactile, thermal and airblast stimuli or (2) patients' subjective assessment of pain during every‐day life. Only studies which reported data that allowed them to be included in the meta‐analysis (i.e. mean and standard deviation), are included in the review.
Search methods for identification of studies
The following databases were searched: Cochrane Oral Health Group Trials Register (searched until August 2005); CENTRAL (until August 2005); EMBASE/MEDLINE, PubMed, Web of Science (until September 2005). Bibliographies of clinical studies and reviews identified in the electronic search were checked for studies published outside the electronically searched journals.
The searches attempted to identify all relevant studies irrespective of language. Non‐English articles would be translated. The CENTRAL search strategy (seeAppendix 1) was modified for use in the other databases (seeAppendix 2; Appendix 3; Appendix 4). No handsearching was performed.
From the titles in the electronic search all relevant clinical studies and review articles were identified by review author Sven Poulsen (SP).
Bibliographies of clinical studies and reviews identified in the electronic search were checked for additional studies published outside the electronically searched journals.
All selected articles were screened to identify randomised controlled trials (RCTs), which fulfilled the inclusion criteria (SP).
Named first authors of all included RCTs were contacted (SP) in order to clarify questions relating to the published trials and obtain information on possible unpublished trials or missing data.
Manufacturers were contacted about unpublished studies by the staff at the Cochrane Oral Health Group in Manchester.
Data collection and analysis
Two review authors, Marie Errboe (MAE) and Sven Poulsen (SP) independently selected the papers to be read from the abstracts. Each review author independently selected reports of trials eligible for inclusion in the review. All information and data recording was done independently and any disagreements resolved by discussion.
The quality of all eligible trials was assessed on the basis of the randomisation procedure, allocation concealment, blinding and description of withdrawals.
MAE, Y Lescay Mevil (YLM) and SP independently completed the data extraction on a specially designed form.
The authors of trials were contacted in an attempt to clarify points relating to design and to obtain the mean or standard deviation or both if these parameters were not presented in the article.
As the length of the trials varied, it was decided to use sensitivity measurements after 6 and 8 weeks.
Sensitivity was assessed using the following types of measurements: tactile (pressure with a standardised probe), thermal (heat/cold) stimulation or air blast. Furthermore, patients' subjective assessment of sensitivity was also recorded.
Heterogeneity
The significance of discrepancies in the estimates of the treatment effects from the different trials will be assessed by means of Cochran's test for heterogeneity.
Choice of summary statistic and estimate of overall effect
For each measurement of sensitivity, the mean scores at 6 to 8 weeks were used, although in most of the studies the data were ordinal.
There were insufficient studies with the same outcome to examine publication bias.
Results
Description of studies
See also: Characteristics of included studies table.
Six studies fulfilled the criteria for being included in the review (Nagata 1994; Schiff 1994; Silverman 1996; Schiff 1998; Schiff 2000 (2); Sowinski 2000).
In all six studies the experimental toothpaste contained 5% potassium nitrate whereas the control pastes were without potassium nitrate. Two studies contained sodium monofluoride phosphate in both experimental and control toothpaste in identical concentrations (Schiff 1994; Schiff 1998), two studies contained sodium fluoride in both experimental and control toothpastes in identical concentrations (Schiff 2000 (2); Sowinski 2000), while two studies contained no fluoride (Nagata 1994; Silverman 1996).
All six studies had a parallel group design; four ran for 8 weeks and two for 12. All patients had one or more teeth with dentine hypersensitivity in exposed root surfaces. Teeth with suspected pulpitis, cracked enamel, caries or defective restorations were excluded. The patients in all six trials were instructed to brush twice a day. None of the studies monitored compliance.
Dentinal hypersensitivity was measured using tactile, thermal and airblast tests. Furthermore, in some studies a subjective assessment was also used. In one of the studies (Nagata 1994) tactile sensitivity (method a) was scored using an ordinal scale (increasing sensitivity giving an increasing score) and could thus not be included in a meta‐analysis with the five other studies (Schiff 1994; Silverman 1996; Schiff 1998; Schiff 2000 (2); Sowinski 2000), where tactile sensitivity was assessed as a continuous variable by pressure with a standardized probe (increasing sensitivity giving a decreasing score) (method b). All assessments, except the tactile assessment in these five trials were scored on an ordinal scale (frequently from 0 to 3). None of the studies reported the mean change scores from baseline, all simply reporting the mean scores at the 6 to 8 week assessment.
Risk of bias in included studies
The quality assessment of the studies was done based on randomisation, blinding and descriptions of withdrawals and drop outs (Jadad 1998). All six included studies were described as randomised and double blind but the description of the randomisation procedure and the measures taken to secure double blinding were unclear in all of them, resulting in unclear allocation concealment. Only one of the studies had a description of the withdrawals (Nagata 1994) (Additional Table 1).
1. Quality assessment of studies included in the review.
| Study ID | Randomisation procedure | Allocation concealment | Blinding | Withdrawals described |
| Nagata 1994 | Unclear | Unclear | Double | Not described |
| Schiff 2000 (2) | Unclear | Adequate | Double | Not applicable |
| Schiff 1994 | Unclear | Unclear | Double | Not described |
| Schiff 1998 | Unclear | Unclear | Double | Adequate |
| Silverman 1996 | Unclear | Unclear | Double | Inadequate |
| Sowinsky 2000 | Unclear | Unclear | Double | Not applicable |
Effects of interventions
All six studies measured dentine hypersensitivity by both tactile (methods a or b) and air blast assessments. Three of the studies also included subjective assessments (Nagata 1994; Schiff 1994; Silverman 1996) and one also included thermal assessments (Schiff 1994). In total these six studies were based on 390 participants.
Tactile assessment (method a) (not included in the meta‐analysis)
The mean difference at 6 to 8 weeks for the one study (Nagata 1994) where tactile sensitivity was measured by method a (ordinal scale), was ‐0.65 (95% confidence interval (CI): ‐1.07 to ‐0.23), indicating that the test toothpaste significantly reduced sensitivity. There was evidence of possible baseline imbalance between the groups, with the test toothpaste group having a higher initial mean value, however this would tend to reduce any difference between the groups.
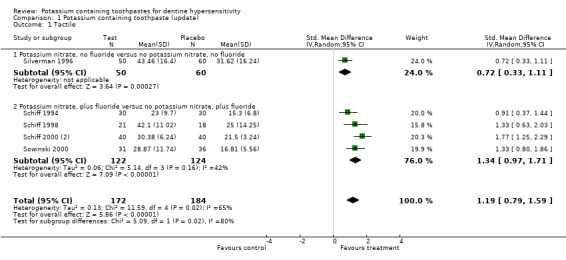
Tactile assessment (method b) (Comparison 1, Outcome 1.1)
There was no evidence of baseline imbalance for any of the five studies that were included in the meta‐analysis for tactile sensitivity measured by method b (continuous variable). The standardised mean difference at 6 to 8 weeks was 1.19 (95% CI: 0.79 to 1.59, Chi2 for heterogeneity = 11.6 (df = 4), P = 0.02) indicating that the test toothpaste reduced sensitivity.
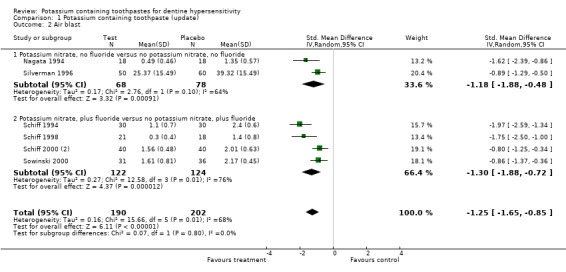
Air blast assessment (Comparison 1, Outcome 1.2)
There was no evidence of baseline imbalance for any of the six studies which were included in the meta‐analysis for air blast. The standardised mean difference at 6 to 8 weeks was ‐1.25 (95% CI: ‐1.65 to ‐0.85, Chi2 for heterogeneity = 15.66 (df = 5), P = 0.008), indicating a reduction in sensitivity in the test toothpaste group.
Thermal assessment (not included in the meta‐analysis)
Only one study (Schiff 1994) measured thermal stimulation for dentinal hypersensitivity and this study had good baseline balance between study groups. The 6 to 8 week mean difference was ‐1.10 (95% CI: ‐1.64 to ‐0.55), indicating a significant difference between the groups favouring the test toothpaste.
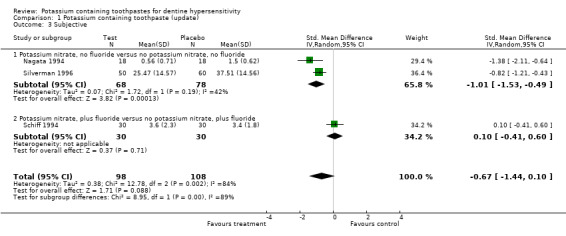
Subjective assessment (Comparison 1, Outcome 1.3)
Three studies included a subjective measurement for dentine hypersensitivity. For two of these studies (Schiff 1994; Silverman 1996) the baseline measurements were balanced between study groups but this information was not available for the third (Nagata 1994). The meta‐analysis for the three studies led to a standardised mean difference of ‐0.67 (95% CI: ‐1.44 to 0.10, Chi2 for heterogeneity = 12.8 (df = 2), P = 0.002), failing to detect a significant difference between the mean subjective assessments.
Discussion
Trials on dentine hypersensitivity are hampered by the problems of measuring a subjective phenomenon such as pain. This is reflected in the fact, that a variety of methods have been employed in existing trials. The degree of standardisation attempted in the different studies varies and it is not known whether some of the methods are more valid than others.
The present review only includes studies that provide sufficient data for meta‐analyses. Whether this might have biased the results is difficult to estimate.
In spite of the fact that the studies measured sensitivity on an ordinal scale, all data were analysed using parametric methods, as no methods of meta‐analysis of ordinal data are available.
The meta‐analysis showed significant differences in mean sensitivity scores assessed by tactile methods, air blast and thermal stimulation, but not for the subjective assessment. However, it must be stressed that the findings are based on only six studies involving a fairly small number of patients (390). In particular, the studies employing subjective assessment involved only 108 patients.
Authors' conclusions
Implications for practice.
Professionals should be aware of the fact that the evidence generated by this review is based on a small number of individuals. Furthermore, the effect varies with methods applied for assessing the sensitivity.
Implications for research.
More large, well designed and well conducted randomised controlled trials (RCTs) are needed.
What's new
| Date | Event | Description |
|---|---|---|
| 6 March 2012 | Amended | Additional table linked to text. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format. |
| 23 May 2006 | New search has been performed | Searches updated. Two new trials have been added to the review. |
| 22 May 2006 | New citation required and conclusions have changed | Substantive amendment. Only studies which reported data that allowed them to be included in the meta‐analysis, are included in this review update. The scope for the present review update has been expanded to include all potassium salts. However, no trials of potassium salts other than potassium nitrate, met the inclusion criteria. Two new trials have been added to the review. The results have been slightly changed. |
Acknowledgements
The review authors want to thank Emma Tavender, Co‐ordinator of the Cochrane Oral Health Group, and Sylvia Bickley, Trials Search Co‐ordinator for their generous help and support. We also want to thank Helen Worthington for her advice on the statistical analysis.
Appendices
Appendix 1. CENTRAL search strategy
(toothpaste* OR tooth paste* OR dentifrice* OR (desensit* AND (agent* OR efficacy OR effect*)) AND ((dentin* OR tooth OR teeth OR root*) AND (hypersensit* OR sensit* OR oversensit* )) Number of hits = 151
Appendix 2. EMBASE/MEDLINE search strategy
(((toothpaste* OR 'tooth paste' OR dentifrice*) OR (desensit* AND (agent* OR efficacy OR effect*))) AND ((hypersensit* OR sensitiv* OR 'oversensit') AND ('dentin' OR ('tooth'/exp OR 'tooth') OR ('teeth'/exp OR 'teeth') OR 'root surface'))) NOT (laser* OR adhesiv* OR endodont* OR bleach* OR whitening OR bond* OR 'caries'/exp) Number of hits = 296
Appendix 3. PubMed search strategy
(((dentifrice OR dentifrices) OR (toothpaste OR toothpastes OR tooth paste*) OR (desensit* AND (agent* OR efficacy OR effect*))) AND ((dentin OR dentine OR tooth OR teeth OR root*) AND (hypersensitivity OR hypersensit* OR sensitivity OR sensitiv* OR over‐sensit*))) NOT (laser* OR adhesiv* OR endodont* OR bleach* OR whitening OR bond* OR caries) Number of hits = 345
Appendix 4. SCI//EXPANDED, Web of Science search strategy
((dentin* OR tooth OR teeth OR root surface*) AND (hypersensit* OR sensitiv* OR (over SAME sensit*))) AND (toothpaste* OR tooth paste* OR dentifrice* OR (desensit* AND (agent* OR efficacy OR effect*))) Science Citation Index Expanded (SCI‐EXPANDED)‐‐1945‐present ((dentin* OR tooth OR teeth OR root surface*) AND (hypersensit* OR sensitiv* OR (over SAME sensit*))) AND (toothpaste* OR tooth paste* OR dentifrice* OR (desensit* AND (agent* OR efficacy OR effect*))) ((dentin* OR tooth OR teeth OR root surface*) AND (hypersensit* OR sensitiv* OR (over SAME sensit*))) AND (toothpaste* OR tooth paste* OR dentifrice* OR (desensit* AND (agent* OR efficacy OR effect*))) Number of hits = 194
Data and analyses
Comparison 1. Potassium containing toothpaste (update).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tactile | 5 | 356 | Std. Mean Difference (IV, Random, 95% CI) | 1.19 [0.79, 1.59] |
| 1.1 Potassium nitrate, no fluoride versus no potassium nitrate, no fluoride | 1 | 110 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.33, 1.11] |
| 1.2 Potassium nitrate, plus fluoride versus no potassium nitrate, plus fluoride | 4 | 246 | Std. Mean Difference (IV, Random, 95% CI) | 1.34 [0.97, 1.71] |
| 2 Air blast | 6 | 392 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐1.65, ‐0.85] |
| 2.1 Potassium nitrate, no fluoride versus no potassium nitrate, no fluoride | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐1.88, ‐0.48] |
| 2.2 Potassium nitrate, plus fluoride versus no potassium nitrate, plus fluoride | 4 | 246 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐1.88, ‐0.72] |
| 3 Subjective | 3 | 206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.44, 0.10] |
| 3.1 Potassium nitrate, no fluoride versus no potassium nitrate, no fluoride | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.53, ‐0.49] |
| 3.2 Potassium nitrate, plus fluoride versus no potassium nitrate, plus fluoride | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.41, 0.60] |
1.1. Analysis.
Comparison 1 Potassium containing toothpaste (update), Outcome 1 Tactile.
1.2. Analysis.
Comparison 1 Potassium containing toothpaste (update), Outcome 2 Air blast.
1.3. Analysis.
Comparison 1 Potassium containing toothpaste (update), Outcome 3 Subjective.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Nagata 1994.
| Methods | 12 weeks, parallel, double blind, randomised | |
| Participants | 36 completing out of 36 | |
| Interventions | 5% potassium nitrate versus 0% potassium nitrate | |
| Outcomes | tactile, air blast and subjective | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schiff 1994.
| Methods | 12 weeks, parallel, double blind, randomised | |
| Participants | 58 completing out of 67 | |
| Interventions | 5% potassium nitrate and 0.243% sodium monofluoride phosphate versus 0% potassium nitrate and 0.243% sodium monofluoride phosphate | |
| Outcomes | thermal, tactile, air blast and subjective sensitivity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schiff 1998.
| Methods | 8 weeks, parallel, double blind, randomised | |
| Participants | 39 completing out of 48 | |
| Interventions | 5% potassium nitrate and 1,500 ppm sodium monofluoride phosphate versus 0% potassium nitrate and 1,500 ppm sodium monofluoride phosphate | |
| Outcomes | tactile and air blast | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schiff 2000 (2).
| Methods | 8 weeks, parallel, double blind, randomised | |
| Participants | 80 completing | |
| Interventions | 5% potassium nitrate and 0.243% sodium fluoride versus 0.243% sodium fluoride | |
| Outcomes | tactile and air blast | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Silverman 1996.
| Methods | 8 weeks, parallel, double blind, randomised | |
| Participants | 110 completing | |
| Interventions | 5% potassium nitrate versus 0% potassium nitrate | |
| Outcomes | tactile, cold air, and subjective | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sowinski 2000.
| Methods | 8 weeks, parallel, double blind, randomised | |
| Participants | 67 completing | |
| Interventions | 5% potassium nitrate and 0.243% sodium fluoride versus 0.243% sodium fluoride | |
| Outcomes | tactile and air blast | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ppm = parts per million
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brook 1994 | Abstract |
| Chesters 1992 | Incomplete data |
| Collins 1984 | Incomplete data |
| Conforti 2000 | Different fluoride concentration in test and comparison toothpastes |
| Cronin 1993 | Abstract |
| Gillam 1996 | Different fluoride concentration in test and comparison toothpastes |
| Gillam 1996, Abstr | Abstract (of Gilham 1996) |
| Hodosh 2001 | No potassium‐free comparison group |
| Hu 2004 | No potassium‐free comparison group |
| Jackson 1987 | Abstract |
| Lecointre 1986 | Tactile, thermal, and air blast sensitivity measures combined into one measurement |
| Manochehr‐Pour 1984 | Incomplete data |
| Perlich 1994 | Abstract |
| Salvato 1989 | Abstract |
| Salvato 1991 | Letter to the Editor |
| Salvato 1992 | Different fluoride concentration in test and comparison toothpastes |
| Schiff 2000 | No potassium‐free comparison group |
| Schwarz 1987 | No information on randomisation |
| Sidi 1991 | Abstract |
| Silverman 1985 | Incomplete data |
| Silverman 1988 | Abstract |
| Silverman 1994 | Incomplete data |
| Sowinski 2000 (2) | No potassium‐free comparison group |
| Sowinski 2001 | Different fluoride concentration in test and comparison toothpastes |
| Tarbet 1980 | Not clear if it was randomised |
| Wara‐aswapati 2005 | No data available at 6 to 8 weeks |
| West 1996 | Abstract |
| West 1997 | Different fluoride concentration and compounds in test and comparison toothpastes |
| Yates 2005 | No potassium‐free comparison group |
| Youssef 1995 | Article not obtainable |
| Zimmer 1998 | Different fluoride concentration and compounds in test and comparison toothpastes |
Contributions of authors
Sven Poulsen (SP) prepared the protocol for the first review; Marie Errboe (MAE) developed the search strategy and literature search; MAE, SP and Y Lescay Mevil (YLM) classified the studies to be included or excluded from the review, and recorded the data from included studies. Anne‐Marie Glenny (AMG) assisted in the statistical analysis. MAE and SP wrote the review.
Sources of support
Internal sources
Royal Dental College, Faculty of Health Sciences, University of Aarhus, Denmark.
External sources
No sources of support supplied
Declarations of interest
One of the review authors, Sven Poulsen (SP), was rewarded a prize from Zendium Inc., Denmark in 1997.
Edited (no change to conclusions)
References
References to studies included in this review
Nagata 1994 {published data only}
Schiff 1994 {published data only}
- Schiff T, Dotson M, Cohen S, Vizio W, McCool J, Volpe A. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: a twelve‐week clinical study. Journal of Clinical Dentistry 1994;5 Spec No:87‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Schiff 1998 {published data only}
- Schiff T, Dos Santos M, Laffi S, Yoshioka M, Baines E, Brasil KD, et al. Efficacy of a dentifrice containing 5% potassium nitrate and 1500 PPM sodium monofluorophosphate in a precipitated calcium carbonate base on dentinal hypersensitivity. Journal of Clinical Dentistry 1998;9(1):22‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Schiff 2000 (2) {published data only}
- Schiff T, Zhang YP, DeVizio W, Stewart B, Chaknis P, Petrone ME, et al. A randomized clinical trial of the desensitizing efficacy of three dentifrices. Compendium of Continuing Education in Dentistry Supplement 2000;(27):4‐10. [PubMed] [Google Scholar]
Silverman 1996 {published data only}
- Silverman G, Berman E, Hanna CB, Salvato A, Fratarcangelo P, Bartizek RD, et al. Assessing the efficacy of three dentifrices in the treatment of dentinal hypersensitivity. Journal of the American Dental Association 1996;127(2):191‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sowinski 2000 {published data only}
- Sowinski JA, Battista GW, Petrone ME, Chaknis P, Zhang YP, DeVizio W, et al. A new desensitizing dentifrice‐‐an 8‐week clinical investigation. Compendium of Continuing Education in Dentistry Supplement 2000;(27):11‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Brook 1994 {published data only}
- Brook A, Francis C, Joshi R, Reekie R, Bransbury D. Efficacy of a potassium nitrate toothpaste in dentinal hypersensitivity. Journal of Dental Research 1994;73(4):955 (Abs 130). [Google Scholar]
Chesters 1992 {published data only}
- Chesters R, Kaufman H, Wolff M, Huntington E, Kleinberg I. The effectiveness of potassium as a desensitizing agent in the presence of SMFP. Journal of Dental Research 1990;69(Spec Issue March):165 (Abs 454). [Google Scholar]
- Chesters R, Kaufman HW, Wolff MS, Huntington E, Kleinberg I. Use of multiple sensitivity measurements and logit statistical analysis to assess the effectiveness of a potassium‐citrate‐containing dentifrice in reducing dentinal hypersensitivity. Journal of Clinical Periodontology 1992;19(4):256‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Collins 1984 {published data only}
- Collins JF, Gingold J, Stanley H, Simring M. Reducing dentinal hypersensitivity with strontium chloride and potassium nitrate. General Dentistry 1984;32(1):40‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Conforti 2000 {published data only}
- Conforti N, Battista GW, Petrone DM, Petrone ME, Chaknis P, Zhang YP, et al. Comparative investigation of the desensitizing efficacy of a new dentifrice: a 14‐day clinical study. Compendium of Continuing Education in Dentistry Supplement 2000;(27):17‐22. [PubMed] [Google Scholar]
Cronin 1993 {published data only}
- Cronin MC, Gordon JM, Reardon RC. Comparison of the desensitizing effectiveness of three dentifrices. Journal of Dental Research 1993;72:249 (Abs 1163). [Google Scholar]
Gillam 1996 {published data only}
- Gillam DG, Bulman JS, Jackson RJ, Newman HN. Comparison of 2 desensitizing dentifrices with a commercially available fluoride dentifrice in alleviating cervical dentine sensitivity. Journal of Periodontology 1996;67(8):737‐42. [DOI] [PubMed] [Google Scholar]
Gillam 1996, Abstr {published data only}
- Gillam DG, Bulman JS, Jackson RJ, Newman HN. Efficacy of desensitizing dentifrices in alleviating dentine hypersensitivity (DH).. Journal of Dental Research 1996;75:428 (Abs 3285). [Google Scholar]
Hodosh 2001 {published data only}
- Hodosh M. Potentiating potassium nitrate's desensitization with dimethyl isosorbide. General Dentistry 2001;49(5):531‐6. [PubMed] [Google Scholar]
Hu 2004 {published data only}
- Hu D, Zhang YP, Chaknis P, Petrone ME, Volpe AR, DeVizio W. Comparative investigation of the desensitizing efficacy of a new dentifrice containing 5.5% potassium citrate: an eight‐week clinical study. Journal of Clinical Dentistry 2004;15(1):6‐10. [PubMed] [Google Scholar]
Jackson 1987 {published data only}
- Jackson RJ, Duke SA. The clinical‐evaluation of dentifrices for the relief of dentin hypersensitivity. Journal of Dental Research 1987;66(4):859 (Abs 219). [Google Scholar]
Lecointre 1986 {published data only}
- Lecointre C, Apiou J, Marty P, Poitou P. Controlled trial of the action of a toothpaste containing nicomethanol hydrofluoride in the treatment of dentine hypersensitivity. Journal of International Medical Research 1986;14(4):217‐22. [DOI] [PubMed] [Google Scholar]
Manochehr‐Pour 1984 {published data only}
- Manochehr‐Pour M, Bhat M, Bissada N. Clinical evaluation of potassium nitrate toothpastes for treatment of dentinal hypersensitivity. Journal of Dental Research 1984;63(Spec Issue):248 (Abs 696). [PubMed] [Google Scholar]
- Manochehr‐Pour M, Bhat M, Bissada N. Clinical evaluation of two potassium nitrate toothpastes for the treatment of dental hypersensitivity. Periodontal Case Reports 1984;6(1):25‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Perlich 1994 {published data only}
- Perlich MA, Bearmann E, Fratarcangelo P, Hanna CB, Salvato A, Silverman G, et al. Desensitizing efficacy of a 5% KNO3/ 0.243% NaF dentifrice. Journal of Dental Research 1994;73:166 (Abs 514). [Google Scholar]
Salvato 1989 {published data only}
- Salvato A, Clark G, Curro, FA. Clinical efficacy of sesodyne‐F as a desensitizing dentifrice. Journal of Dental Research 1989;68:971 (Abs 839). [Google Scholar]
Salvato 1991 {published data only}
- Salvato AR. Potassium chloride dentrifrice as a desensitizing agent. British Dental Journal 1991;171(5):122. [DOI] [PubMed] [Google Scholar]
Salvato 1992 {published data only}
- Salvato AR, Clark GE, Gingold J, Curro FA. Clinical effectiveness of a dentifrice containing potassium chloride as a desensitizing agent. American Journal of Dentistry 1992;5(6):303‐6. [PubMed] [Google Scholar]
Schiff 2000 {published data only}
- Schiff T, Bonta Y, Proskin HM, DeVizio W, Petrone M, Volpe AR. Desensitizing efficacy of a new dentifrice containing 5.0% potassium nitrate and 0.454% stannous fluoride. American Journal of Dentistry 2000;13(3):111‐5. [PubMed] [Google Scholar]
Schwarz 1987 {published data only}
- Schwarz P, Benz C, Sonnabend E. [Study of the effectiveness of a 5% KNO3 toothpaste on dentin hypersensitivity]. Deutsche Zahnarztliche Zeitschrift 1987;42(9):822‐5. [PubMed] [Google Scholar]
Sidi 1991 {published data only}
- Sidi AD, Wilson RF, Ashley FP. Efficacy of a new toothpaste formulation in reducing dentine hypersensitivity. Journal of Dental Research 1991;70:682 (Abs 110). [Google Scholar]
Silverman 1985 {published data only}
- Silverman G. The sensitivity‐reducing effect of brushing with a potassium nitrate‐sodium monofluorophosphate dentifrice. Compendium of Continuing Education in Dentistry 1985;6(2):131‐3, 136. [MEDLINE: ] [PubMed] [Google Scholar]
Silverman 1988 {published data only}
- Silverman G, Gingold J, Clark GE. The effectiveness of KNO3 and NaMFP dentifrices in reducing dentinal hypersensitivity. Journal of Dental Research 1988;67:247 (Abs 1076). [Google Scholar]
Silverman 1994 {published data only}
- Silverman G, Gingold J, Curro FA. Desensitizing effect of a potassium chloride dentifrice. American Journal of Dentistry 1994;7(1):9‐12. [PubMed] [Google Scholar]
Sowinski 2000 (2) {published data only}
- Sowinski JA, Bonta Y, Battista GW, Petrone D, DeVizio W, Petrone M, et al. Desensitizing efficacy of Colgate Sensitive Maximum Strength and Fresh Mint Sensodyne dentifrices. American Journal of Dentistry 2000;13(3):116‐20. [PubMed] [Google Scholar]
Sowinski 2001 {published data only}
- Sowinski J, Ayad F, Petrone M, DeVizio W, Volpe A, Ellwood R, et al. Comparative investigations of the desensitising efficacy of a new dentifrice. Journal of Clinical Periodontology 2001;28(11):1032‐6. [DOI] [PubMed] [Google Scholar]
Tarbet 1980 {published data only}
- Tarbet WJ, Silverman G, Stolman JM, Fratarcangelo PA. Clinical evaluation of a new treatment for dentinal hypersensitivity. Journal of Periodontology 1980;51(9):535‐40. [DOI] [PubMed] [Google Scholar]
Wara‐aswapati 2005 {published data only}
- Wara‐aswapati N, Krongnawakul D, Jiraviboon D, Adulyanon S, Karimbux N, Pitiphat W. The effect of a new toothpaste containing potassium nitrate and triclosan on gingival health, plaque formation and dentine hypersensitivity. Journal of Clinical Periodontology 2005;32(1):53‐8. [DOI] [PubMed] [Google Scholar]
West 1996 {published data only}
- West N, Addy M, Jackson RJ, Ridge BD. Comparison of strontium acetate and potassium nitrate dentifrices on dentine hypersensitivity. Journal of Dental Research 1996;75(5):1150 (Abs 161). [Google Scholar]
West 1997 {published data only}
- West NX, Addy M, Jackson RJ, Ridge DB. Dentine hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. Journal of Clinical Periodontology 1997;24(4):209‐15. [DOI] [PubMed] [Google Scholar]
Yates 2005 {published data only}
- Yates R, Ferro R, Newcombe RG, Addy M. A comparison of a reformulated potassium citrate desensitising toothpaste with the original proprietary product. Journal of Dentistry 2005;33(1):19‐25. [DOI] [PubMed] [Google Scholar]
Youssef 1995 {published data only}
- Youssef HA. An evaluation of four desensitizing agents for treatment of the post preparation tooth pain: silver nitrate, potassium nitrate, strontium chloride and ferric oxalate. Egyptian Dental Journal 1995;41(4):1485‐94. [PubMed] [Google Scholar]
Zimmer 1998 {published data only}
- Zimmer S, Schulte J, Roulet JF. Study of a new toothpaste in the therapy of over‐sensitive necks of teeth. Deutsche Zahnarztliche Zeitschrift 1998;53(8):517‐21. [Google Scholar]
Additional references
Bissada 1994
- Bissada NF. Symptomatology and clinical features of hypersensitive teeth. Archives of Oral Biology 1994;39 Suppl:31S‐2S. [DOI] [PubMed] [Google Scholar]
Flynn 1985
- Flynn J, Galloway R, Orchardson R. The incidence of 'hypersensitive' teeth in the West of Scotland. Journal of Dentistry 1985;13(3):230‐6. [DOI] [PubMed] [Google Scholar]
Graf 1977
- Graf H, Galasse R. Morbidity, prevalence and intraoral distribution of hypersensitive teeth. Journal of Dental Research 1977;56(Spec Issue A):A162. [Google Scholar]
Holland 1997
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. Journal of Clinical Periodontology 1997;24(11):808‐13. [DOI] [PubMed] [Google Scholar]
Jadad 1998
- Jadad A. Randomised controlled trials: a user's guide. BMJ Books, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaufman 1994
- Kaufman HW, Kleinberg I. Design and statistical aspects of the management of clinical trials to assess antihypersensitivity product efficacy. Archives of Oral Biology 1994;39 Suppl:97S‐100S. [DOI] [PubMed] [Google Scholar]
Kim 1986
- Kim S. Hypersensitive teeth: desensitization of pulpal sensory nerves. Journal of Endodontics 1986;12(10):482‐5. [DOI] [PubMed] [Google Scholar]
Orchardson 1994
- Orchardson R, Gangarosa LP Sr, Holland GR, Pashley DH. Towards a standard code of practice for evaluating the effectiveness of treatments for hypersensitive dentine. Archives of Oral Biology 1994;39 Suppl:121S‐4S. [DOI] [PubMed] [Google Scholar]
Schiff 1998
- Schiff T, Dos Santos M, Laffi S, Yoshioka M, Baines E, Brasil KD, et al. Efficacy of a dentifrice containing 5% potassium nitrate and 1500 PPM sodium monofluorophosphate in a precipitated calcium carbonate base on dentinal hypersensitivity. Journal of Clinical Dentistry 1998;9(1):22‐5. [PubMed] [Google Scholar]
References to other published versions of this review
Poulsen 2000
- Poulsen S, Errboe M, Hovgaard O, Worthington HW. Potassium nitrate toothpaste for dentine hypersensitivity. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001476] [DOI] [PubMed] [Google Scholar]


