Abstract
Human in vitro model systems of diabetes are critical to both study disease pathophysiology and offer a platform for drug testing. We have generated a set of tools in the human β-cell line EndoC-βH1 that allows the efficient and inexpensive characterization of β-cell physiology and phenotypes driven by disruption of candidate genes. First, we generated a dual reporter line that expresses a preproinsulin–luciferase fusion protein along with GCaMP6s. This reporter line allows the quantification of insulin secretion by measuring luciferase activity and calcium flux, a critical signaling step required for insulin secretion, via fluorescence microscopy. Using these tools, we demonstrate that the generation of the reporter human β-cell line was highly efficient and validated that luciferase activity could accurately reflect insulin secretion. Second, we used a lentiviral vector carrying the CRISPR-Cas9 system to generate candidate gene disruptions in the reporter line. We also show that we can achieve gene disruption in ~90% of cells using a CRISPR–Cas9 lentiviral system. As a proof of principle, we disrupt the β-cell master regulator, PDX1, and show that mutant EndoC-βH1 cells display impaired calcium responses and fail to secrete insulin when stimulated with high glucose. Furthermore, we show that PDX1 mutant EndoC-βH1 cells exhibit decreased expression of the β-cell-specific genes MAFA and NKX6.1 and increased GCG expression. The system presented here provides a platform to quickly and easily test β-cell functionality in wildtype and cells lacking a gene of interest.
Keywords: diabetes, beta-cell physiology, disease modeling, insulin secretion, calcium imaging, CRISPR/Cas9 gene editing
Diabetes mellitus is rapidly becoming one of the largest and most expensive risks to global public health. Currently, approximately 9% of the world population has a form of diabetes and this prevalence is projected to continue to increase (1). In 2015 alone, global treatment costs related to diabetes were 673 billion dollars (US) (1,2). Unfortunately, diabetes is associated with significant morbidity and mortality, ranking as the 7th leading cause of death in the world and contributing to the mortality of other conditions such as cardiovascular and renal disease (3). Diabetes mellitus results from impaired insulin secretion, action, or both, resulting in high serum glucose levels (4–6). Despite decades of research, our understanding of the underlying pathogenesis of diabetes, especially pancreatic β-cell dysfunction, remains incomplete. Diabetes in all forms has a genetic component that impacts disease penetrance and severity (7,8). Though often multivariable, use of strategies such as genome-wide association studies (GWASs) have helped discover candidate genes that are important in human β-cell physiology and pathophysiology (9–11).
Understanding the precise roles of these candidate genes in β-cell function, however, has been challenging due to lack of good experimental models. These genes can be manipulated in rodent models, but this is time-consuming and expensive, and these models do not always recapitulate human physiology (2). The EndoC-βH1 cell line is a human β-cell line that has been used to model human β-cells using ribonucleic acid interference (RNAi) to inhibit target gene expression (12,13). RNAi, however, does have some limitations that limit its utility in studying β-cell physiology, including off-target effects and failure to completely silence gene expression (14,15). EndoC-βH1 cells can respond to physiologic β-cell stimuli such as glucose and amino acids (13). In β-cell line models, including the EndoC-βH1 line, functionality is generally assessed by quantification of secreted insulin or C-peptide measured using ELISA kits, which are costly and labor-intensive (16). Another method to measure β-cell function is the quantification of calcium flux, but this method is technically challenging and loading cells with calcium-sensitive dyes can impair their normal functionality (17,18).
In this report, we have generated a set of cellular tools in the human EndoC-βH1 line that allows the efficient and inexpensive characterization of candidate genes in β-cell physiology. We found that lentiviral transduction of the EndoC-βH1 cell line is efficient and we were able to generate a dual reporter cell line expressing both an insulin–Gaussia luciferase fusion protein and the genetically encoded protein calcium sensor, GCaMP6s. GCaMP6s was designed to have high baseline brightness, a wide dynamic range and slow kinetics, making it ideal for the study of calcium flux in β-cells (19–21). The use of a genetically encoded fluorescent protein allows the quantification of calcium signaling in live cultures with standard fluorescent microscopy and without the use of dyes. The insulin/luciferase fusion protein is secreted along with insulin as previously reported, allowing simple and inexpensive quantitation of insulin secretion; this approach makes scaling up of high-throughput systems feasible by removing the prohibitive expense of insulin or C-peptide ELISAs (22). The resulting stable cell line is ideal for facilitating the simultaneous quantification of insulin secretion and calcium flux in response to stimuli in both static and perfusion systems. We can then further manipulate this cell line using lentiviral vectors carrying the CRISPR–Cas9 system to generate β-cells carrying candidate gene mutations. The generation of mutant β-cell lines using this system was validated and achieved ~90% efficiency in the deletion of 3 well-described genes critical for β-cell function including PDX1, INS, and HNF1A. Using the insulin–luciferase fusion protein and GCaMP6s, we show that PDX1 mutant β-cells have a poor calcium response and fail to secrete insulin when stimulated with high glucose. Furthermore, we show that PDX1 mutant β-cells exhibit decreased expression of key β-cell genes, including MAFA and NKX6.1, and increased expression of the α-cell gene GCG. These proof of principle studies demonstrate the relevance of this platform for studying human β-cell physiology in the context of normal and diseased states.
Results
Insulin secretion and calcium flux can be quantified in the human β-cell line EndoC-βH1 expressing an insulin–luciferase fusion protein and GCaMP6s
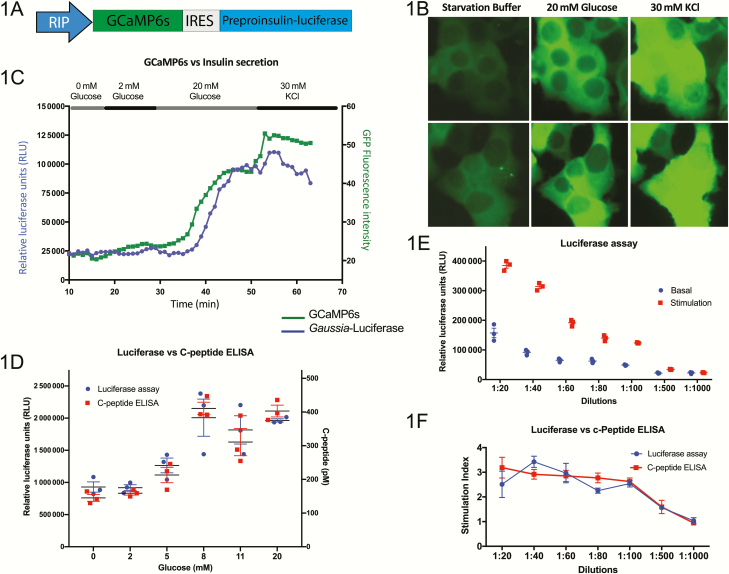
In order to more efficiently measure calcium levels and insulin secretion in the EndoC-βH1 cell line, we cloned the protein calcium sensor GCaMP6s and an insulin–Gaussia luciferase fusion protein into a single lentiviral vector. These transgenes are driven by the rat insulin promoter (RIP), allowing robust expression in these insulin-producing cells (Fig. 1A). The transduction efficiency was >90% (all supplementary material and figures are located in a digital research materials repository (23)). In order to examine the sensitivity of the GCaMP6s calcium reporter, we measured GFP fluorescence intensity in response to different stimuli. Under basal conditions in the absence of glucose, the GFP fluorescence intensity of GCaMP6s is relatively low. This intensity increases 2.5- to 3-fold when cells are incubated in 20 mM glucose. Subsequent exposure to 30 mM KCl to fully depolarize the cells causes green fluorescent protein (GFP) fluorescence intensity to increase ~5-fold over basal levels (Fig. 1B and 1C). We validated the use of both of these reporter tools in a perfusion set-up, allowing for an inexpensive and rapid way to measure calcium levels and insulin secretion in the same cultures and at many time points under varying stimuli (Fig. 1C and (23),). Extensive details of this perfusion set-up have been previously published (24). There is good concordance between calcium flux and insulin secretion with a slight kinetic delay in the latter as would be expected considering that calcium signaling is a mediator of insulin secretion. To show that these changes were calcium-dependent, we performed a perfusion in the absence of Ca2+ and with a Ca2+ chelator, ethyleneglycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, and found that both insulin secretion and calcium flux are markedly decreased in response to KCl in the absence of Ca2+ ((23)). The insulin–luciferase fusion protein has been previously shown to be packaged into the endogenous insulin vesicles and secreted from the cell at a 1:1 ratio with insulin and C-peptide upon stimulation with glucose and KCl (25,26). In order to determine if the insulin–luciferase could be used as a simple and inexpensive surrogate for insulin secretion in our EndoC-βH1 model system, we performed static glucose-stimulated insulin secretion (GSIS) assays and assayed the levels of luciferase and C-peptide secreted into the media after each incubation. Luciferase levels strongly correlate with C-peptide levels over a biologically dynamic range of glucose concentrations (Fig. 1D). When comparing basal (2 mM glucose) and stimulated (20 mM glucose), both C-peptide and luciferase levels increase 2.5- to 3.5-fold under stimulated conditions compared with basal levels (Fig. 1E and 1F). The luciferase assay remains highly sensitive and can be used as a reliable readout for insulin secretion even when samples are diluted 100-fold, similar to the ultrasensitive C-peptide ELISA (Fig. 1E and 1F).
Figure 1.
Generation of a human pancreatic β-cell line with the capability to measure insulin secretion and calcium flux. (A) Schematic representation of the lentiviral construct containing transgenes for GCaMP6s and preproinsulin–Gaussia luciferase, separated by an internal ribosome entry site (IRES) and driven by the rat insulin promoter (RIP). (B) Live-cell imaging of transgenic EndoC-βH1 cells shows an increase in GFP intensity in response to sequential exposure to glucose and KCl (n = 3, representative images from 2 experiments are shown). (C) Measurement of calcium flux by GFP intensity and insulin secretion by luciferase signal of the transgenic EndoC-βH1 line in a perfusion system (n = 3, representative tracking from a single experiment is shown). (D) Luciferase levels show a strong correlation to C-peptide levels measured by ELISA in the transgenic EndoC-βH1 line as a measure of insulin secretion in response to a range of glucose concentrations (n = 3). (E) Serial dilutions measuring luciferase content of the media of transgenic EndoC-βH1 cells under basal (2 mM glucose) and stimulated (20 mM glucose) conditions, showing a sensitivity by limiting dilution (n = 5). (F) Direct comparison of the luciferase assay and human C-Peptide ELISA used to measure the stimulation index (levels at 20 mM glucose over those at 2 mM glucose) of insulin secretion demonstrates the accuracy of measuring luciferase as a surrogate for insulin secretion (n = 5).
CRISPR-Cas9 presents an efficient way to disrupt genes in EndoC-βH1 cells
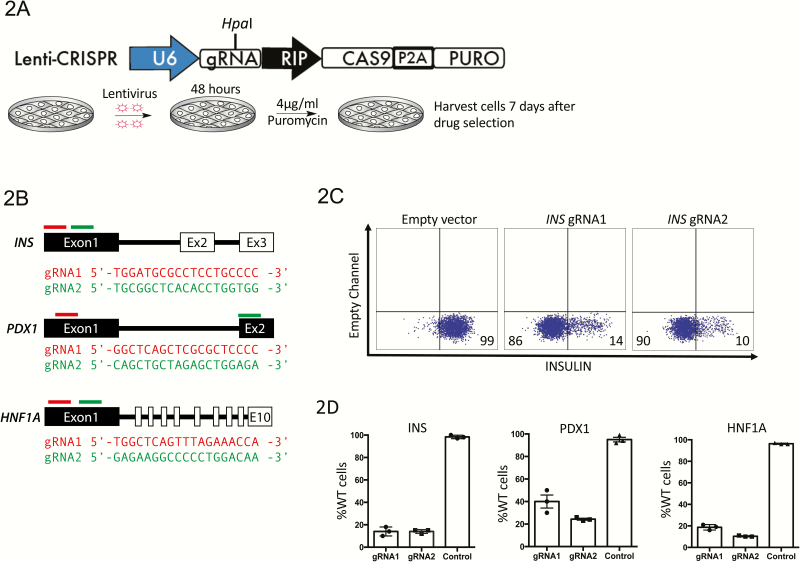
In order for these reporter tools to be effective in the investigation of the roles of gene targets in β-cell function, mutations in gene candidates would have to be easily introduced into the EndoC-βH1 cell line. Unfortunately, EndoC-βH1 cells have a doubling time of approximately 1 week, making the generation of clonal lines difficult. Therefore, we tested the efficiency of the CRISPR–Cas9 system in bulk cultures. We used the lentivirus backbone V2-CRISPR (27), adding a construct using the RIP to drive the expression of Cas9 and puromycin genes. The reporter EndoC-βH1 cells were transduced with this lentivirus and the nontransduced cells were eliminated using puromycin selection for 7 days after viral transduction (Fig. 2A). In order to test the efficiency of this technique, we chose 3 genes of interest: INSULIN (INS), PDX1, and HNF1A. For each gene, we selected 2 guide (g)RNAs and cloned each into the lentiviral backbone (Fig. 2B). After 7 days of puromycin selection, cells were harvested and the knockout efficiency was quantified using flow cytometry (Fig. 2C). Though knockout efficiency varied, robust knockout was achieved in all cases: INS and HNF1A show approximately 90% of cells with a complete absence of protein, while PDX1 have 75% (Fig. 2D and (23)).
Figure 2.
Overview and validation of the strategy to knockout genes in the immortalized EndoC-βH1 pancreatic β-cell line. (A) Schematic representation of the V2 lentiviral construct containing a RIP promoter driving Cas9 expression, a HpaI restriction site allowing for cloning of a desired gRNA and puromycin drug selection to enrich for infected cells. (B) Sequence of gRNAs used to target the INS, PDX1, and HNF1A genes. Red or green bars show the relative position of the gRNAs per gene. (C) Flow cytometry analysis of cells following CRISPR–CAS9 targeting of the INS gene to determine the efficiency of knockout with 2 different gRNAs (n = 3, representative FACS plot from a single experiment is shown). (D) Knock-out efficiency per gRNA in each targeted cell line using flow cytometry (n = 3).
Lack of PDX1 impairs calcium flux and insulin secretion and results in the downregulation of important β-cell genes
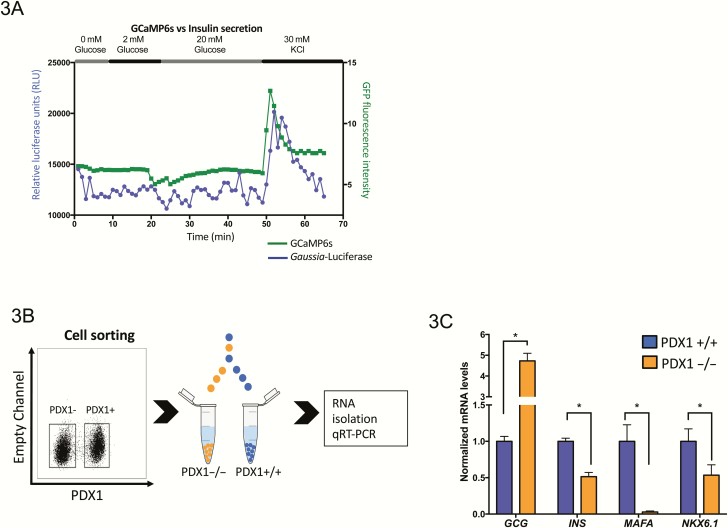
To illustrate the power of combining our dual reporter EndoC-βH1 cell line and the CRISPR-Cas9 system to study the role of candidate genes in β-cell physiology, we further characterized the PDX1 knockout cells. Using the cellular tools in the perfusion system, PDX1 knockout cells show a failure to increase calcium levels and insulin secretion in response to stimulation with 20 mM glucose as measured by fluorescence intensity and luciferase content. However, upon depolarization with KCl, there is an increase in calcium levels and insulin secretion, albeit lower than that observed in the controls (Figs. 1C, 3A, and (23)). To help understand the role of PDX1 in the EndoC-βH1 cell model, we sorted for those cells lacking PDX1 protein after lentiviral infection using intracellular flow cytometry and compared the expression of key genes for β-cell identity and function in sorted cells with persistent PDX1 expression after drug selection (PDX1+) and those without PDX1 expression (PDX1–) (Fig. 3B). Interestingly, loss of PDX1 leads to downregulation of INS, MAFA, and NKX6.1 mRNAs but increased GCG expression (Fig. 3C), consistent with PDX1 known role as a master regulator of β-cell gene expression.
Figure 3.
Loss of PDX1 in EndoC-βH1cells impairs insulin secretion and calcium flux and results in aberrant regulation of β-cell-specific genes. (A) EndoC-βH1 cells PDX1 knockout show impaired insulin secretion and calcium flux as measured by luciferase secretion and GFP intensity of GCaMP6s in a perfusion system compared with wild-type cells in Fig. 1C. (B) EndoC-βH1 cells were transduced with lentivirus carrying CRISPR–CAS9 and gRNA specific for PDX1 gene; EndoC-βH1 transduced cells were then sorted into distinct PDX1+/+ and PDX1–/– populations by intracellular FACS. (C) Quantification of RNA expression by qRT-PCR comparing PDX1+/+ and PDX1–/– cell populations. Dysregulation of β-cell-specific genes is seen including downregulation of INS, MAFA, and NKX6.1 and upregulation of GCG. Unpaired 2-tailed Student’s t-tests were performed for each gene (n = 3, *P < .05).
Discussion
In this report, we have described a set of tools for use in a human β-cell line that allows the detection of insulin secretion using luciferase and calcium flux using GCaMP6s. We have shown that luciferase and insulin levels correlate following stimulation with glucose and KCl. In the same cultures, we can also monitor calcium flux using the genetically encoded protein calcium sensor GCaMP6s by quantifying changes in GFP fluorescence intensity that reflect the amount of calcium present in the cell (19,21,28,29). The generation of this cell line presents an efficient means of quantifying calcium flux and insulin secretion in a human β-cell line. Quantification of insulin secretion is also approximately 10 times less expensive than typical commercial ultrasensitive insulin or c-peptide ELISAs, making this system scalable and amenable to high throughput screening efforts. We demonstrate that the dual reporter cell line can be used to simultaneously track calcium flux and insulin secretion in a perfusion system. In a single culture, we see calcium levels and insulin secretion increase sequentially in response to 20 mM glucose, which recapitulates what has been long described by other groups in perfusion models of rodent and human islets (30–32).
Furthermore, we demonstrate that this dual reporter cell line can be used to interrogate the role of genes critical for β-cell function by using CRISPR–CAS9 to produce mutant lines. Despite the slow growth rate of the EndoC-βH1 cells, which only double every 7 days, and their inability to form clonal colonies, we can knockout the expression of target genes with 70% to 95% efficiency in bulk cultures. Using PDX1 targeted by the CRISPR–Cas9 system as a proof of concept, we were able to investigate the impacts of loss of PDX1 in the EndoC-βH1 cell line. Lack of PDX1 results in the loss of some β-cell-specific gene expression as shown by decreasing INS, NKX6.1, and MAFA expression and increasing GCG expression. Additionally, it also leads to impairment in calcium flux and insulin secretion upon stimulation with 20 mM glucose. These data fit well with the know roles of PDX1 in β-cells (33–36). Together, these findings demonstrate that the use of the dual reporter EndoC-βH1 cell line offers a useful and rapid human model to study candidate genes related to β-cell function and their potential role in diabetes. Furthermore, these tools could be easily adapted and applied in drug screening platforms for subsequent translational applications.
Methods
Cell culture
The EndoC-βH1 cell line was grown according to Ravassard et al. (13). Plates were coated with Matrigel 100 µg/mL and human fibronectin 2 µg/mL (Sigma) in Dulbecco’s modified Eagle’s medium low glucose (Gibco). Cells were cultured in DMEM low glucose supplemented with 2% bovine serum albumin fraction V (Sigma), 50 µM 2-mercaptoethanol, 10 mM nicotinamide (Sigma), 5.5 µg/mL transferrin (Roche Diagnostics), 6.7 ng/mL selenite (Sigma). Cells were split once every 7 days using 0.25% trypsin and replated at a 1:2 ratio.
Cloning and lentivirus
The GCaMP6s plasmid was acquired from Addgene plasmid number 40753 and the Insulin–Gaussia–luciferase construct was a gift from Dr. Pei Wang from UT Health San Antonio. Transgenes were cloned into a lentivirus vector using the In-Fusion cloning kit (Clontech). The expression of the transgenes was driven by the RIP promotor, which was donated by Dr. Christopher Newgard (37). The CRISPR–Cas9 lentivirus vector was purchased from Addgene (Plasmid #52961); using the In-Fusion cloning kit (Clontech), the elongation factor 1alpha binding sequence (EFS) promoter was replaced by the RIP and the BsmBI restriction sites were changed for HpaI to facilitate further cloning of the plasmid. Cloning the gRNAs into the lentivirus vector was performed as described in Mali et al. (38).
Lentiviruses were generated by mixing lentiviral backbone packaging plasmids, vesicular stomatitis virus glycoprotein (VSVG), trans-activator of transcription (TAT), regulator of expression of virion proteins (REV), and high mobility group protein 2 (HMGP2) at a ratio of 0.2:0.2:0.2:0.3. A total of 24.8 μg of the packaging plasmids were mixed with 24.8 μg of the transgene plasmid and transfected into HEK293 cells on a 100-mm dish using 2.5 M CaCl2 and 2× HEPES. The supernatant was collected for 2 days and spun down at 19 500 rpm for 90 minutes at 4°C. The viral pellet was resuspended in 500 μL of HEK293 medium.
Virus infection and drug selection
A total of 5 × 106 cells were plated on a 6-well plate coated with Matrigel and fibronectin. Twenty-four hours after seeding, virus infection was performed by incubating cells with 2 mL of the EndoC-βH1 medium, 40 µL of concentrated lentivirus, and 2 μg/mL of polybrene (Millipore) overnight. The following day, the infected cells were washed twice using IMDM medium and refed with normal EndoC-βH1 medium. Forty-eight hours after infection, 2 µg/mL puromycin was added to the EndoC-βH1 medium for 1 week. After 7 days of drug selection, cells were fed for 2 days with EndoC-βH1 medium without puromycin. All experiments were performed following drug selection.
GSIS perfusion assay and Calcium imaging
In total, 50 000 cells per well were seeded in a 96-well Lumox multiwell plate (SARSTEDT) and each well was transferred to the imaging chamber of a perfusion system (24). Input perfusion solution was supplied using a high-flow peristaltic pump (Instechlabs). Perfusion solution consists of Krebs–Ringer Bicarbonate HEPES Buffer (KRBH) 115 mmol/L NaCl, 24 mmol/L NaHCO3, 5 mmol/L KCl, 1 mmol/L MgCl2, 2.5 mmol/L CaCl2, 10mM HEPES, pH 7.4) prewarmed to 37°C. Cells were incubated for 45 minutes at 37°C and 5% CO2. To perform GSIS and calcium flux imaging, cells were transfer to the perfusion chamber where cells were perfused with a constant flow rate of 350 µL of KRBH for 30 minutes at 37°C; then the input perfusion solution was changed to 2 mM D-glucose KRBH for 15 minutes followed by 30 minutes in 20 mM D-glucose KRBH and finally 10 minutes in 30 mM KCl KRBH. To quantify insulin secretion using the fusion protein preproinsulin–Gaussia luciferase, 50 µL of perfused media per timepoint (per minute) was measured using the Pierce Gaussia luciferase flash assay kit (Life technologies). Collection of output-perfused solution started 20 minutes after KRBH incubation in the chamber and it was collected into a 96-well plate every minute using a Gilson 203B fraction collector. A Leica 8900 series fluorescence microscope was used for live cell imaging. Images were taken every 10 seconds using a GFP channel and processing imaging (GFP fluorescence intensity over time) was performed using the software Leica Application Suite X and ImageJ software (NIH). Results are presented as GFP mean fluorescence intensity of the whole field versus time.
Static GSIS and C-Peptide ELISA
Cells were plated in a 6-well plate (~1.5 × 106 cells per well). Forty-eight hours after adherent cells were used to perform GSIS. Cells were washed twice very gently using KRBH buffer. Before starting GSIS, cells were starved for 60 minutes by leaving cells in 2 mL of KRBH buffer and incubating at 37°C, 5% CO2. After starvation, KRBH buffer was removed and cells were incubated using 1 mL of 2mM D-Glucose KRBH buffer at 37°C, 5% CO2. Supernatant was collected and then cells were finally incubated using 1 mL of 20 mM D-glucose KRBH for 30 minutes at 37°C, 5% CO2, and the supernatant was collected. Supernatant was used to perform C-peptide quantification using the ultrasensitive C-peptide ELISA kit from Mercodia (39). To quantify insulin secretion via Gaussia luciferase, supernatant from GSIS was used to was measured luciferase using the Pierce Gaussia luciferase flash assay kit (Life technologies).
Staining, flow cytometry, and cell sorting
Cells were harvested and fixed in 1.6% paraformaldehyde (PFA) for 30 minutes at 37°C. Intracellular staining was performed using 1× saponin to permeabilize the membrane; cells were incubated in primary antibody for 30 minutes followed by secondary antibody for 30 minutes. Samples were resuspended in FACS buffer and analyzed using a CANTOS BD flow cytometer. The cell lines used as a control expressed GCaMP6s and preproinsulin–Gaussia luciferase and also CAS9 without gRNA. In order to perform cell sorting, cells were fixed using 4% PFA for 30 minutes. Cells were stained the following day, sorted by FACSAria II, and collected for RNA isolation (40). Primary antibodies used were against HNF1A (41), PDX1 (42), C-peptide (43), and insulin (44). Secondary antibodies were purchased from Jackson Immunoresearch (45–47).
RNA isolation and qRT-PCR
In order to extract RNA from fixed cells, we followed the MARIS protocol (40). RNA from sorted samples was isolated using the RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE (Life Technologies/Ambion). cDNA was made using random hexamers and Superscript II Reverse Transcriptase (Invitrogen). Quantification of relative mRNA was performed in triplicate using SYBR-GreenER qPCR Master Mix (Roche). A list of the primers used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) can be found in (23)).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6 software. The results are expressed as the mean ± standard error of the mean. An unpaired 2-tailed Student t-test for groups with equal variance were performed to determine P-values when applicable.
Acknowledgments
The authors thank Dr. Christopher Newgard for supplying the rat insulin promoter construct.
Glossary
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- GSIS
glucose-stimulated insulin secretion
- GWAS
genome-wide association study
- RIP
rat insulin promoter
- RNAi
ribonucleic acid interference
Financial Support: This work was supported by a grant from the National Institutes of Health (NIH) (UC4 DK104196 and R01 DK118155) K.F.L. was supported by NIH, National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) K12 DK94723-8.
Author Contributions. F.L.C., K.F.L., S.K., C.O. designed, conducted, and analyzed data. F.L.C., K.F.L., S.K., D.F., P.G. wrote the manuscript. Y.J.C., B.Z.S., P.W. provided critical reagents. All authors critically reviewed the manuscript text.
Additional Information
Disclosure Summary. The authors declare no conflict of interest.
References
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. [DOI] [PubMed] [Google Scholar]
- 2. King A, Bowe J. Animal models for diabetes: understanding the pathogenesis and finding new treatments. Biochem Pharmacol. 2016;99:1–10. [DOI] [PubMed] [Google Scholar]
- 3. Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Death-final data for 2015. Natl Vital Stat Reports. 2017;66(6):1–4. [PubMed] [Google Scholar]
- 4. Bacos K, Gillberg L, Volkov P, et al. Blood-based biomarkers of age-associated epigenetic changes in human islets associate with insulin secretion and diabetes. Nat Commun. 2016;7:11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121(6):2118–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koster JC, Permutt A Nichols CG. The ATP-sensitive K+ Channel (KATP) connection. J Natl Med Assoc. 2005;54(1):476–478. [Google Scholar]
- 7. Katsarou A, Gudbjörnsdottir S, Rawshani A, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016. [DOI] [PubMed] [Google Scholar]
- 8. DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Prim. 2015;1(July):1–23. [DOI] [PubMed] [Google Scholar]
- 9. Billings LK, Florez JC. The genetics of type 2 diabetes: what have we learned from GWAS? Ann N Y Acad Sci. 2010;1212(1):59–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kong X, Zhang X, Xing X, Zhang B, Hong J, Yang W. The association of type 2 diabetes loci identified in genome-wide association studies with metabolic syndrome and its components in a Chinese population with type 2 diabetes. Plos One. 2015;10(11):e0143607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuchsberger C, Flannick J, Teslovich TM, et al. The genetic architecture of type 2 diabetes. Nature. 2016;536(7614):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsonkova VG, Sand FW, Wolf XA, et al. The EndoC-βH1 cell line is a valid model of human beta cells and applicable for screenings to identify novel drug target candidates. Mol Metab. 2018;8:144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravassard P, Hazhouz Y, Pechberty S, et al. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121(9):3589–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou X, Xu F, Mao H, et al. Nuclear RNAi contributes to the silencing of off-target genes and repetitive sequences in Caenorhabditis elegans. Genetics. 2014;197(1):121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. [DOI] [PubMed] [Google Scholar]
- 16. Nell LJ, Virta VJ, Thomas JW. Application of a rapid enzyme-linked immunosorbent microassay (ELISA) to study human anti-insulin antibody. Diabetes. 1985;34(1):60–66. [DOI] [PubMed] [Google Scholar]
- 17. Gandasi NR, Yin P, Riz M, et al. Ca2+ channel clustering with insulin-containing granules is disturbed in type 2 diabetes. J Clin Invest. 2017;127(6):2353–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rorsman P, Braun M, Zhang Q. Regulation of calcium in pancreatic α- and β-cells in health and disease. Cell Calcium. 2012;51(3–4):300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen TW, Wardill TJ, Sun Y, et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499(7458):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun XR, Badura A, Pacheco DA, et al. Fast GCaMPs for improved tracking of neuronal activity. Nat Commun. 2013;4:2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cai B, Chen X, Liu F, et al. A cell-based functional assay using a green fluorescent protein-based calcium indicator dCys-GCaMP. Assay Drug Dev Technol. 2014;12(6):342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wurdinger T, Badr C, Pike L, et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5(2):171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cardenas-Diaz FL, Leavens K, Kishore S, et al. Cardenas-Diaz supplemental figures, videos and materials. 2020. https://figshare.com/articles/Cardenas_Endo_supplemental_figures_re-upload_docx/11541162. Deposited on January 7, 2020.
- 24. Chen Y, Yamazoe T, Leavens KF, et al. iPreP is a three-dimensional nanofibrillar cellulose hydrogel platform for long-term ex vivo preservation of human islets. J Clin Investig Insight. 2019;4(21):e124644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalwat MA, Wichaidit C, Nava Garcia AY, et al. Insulin promoter-driven Gaussia luciferase-based insulin secretion biosensor assay for discovery of β-cell glucose-sensing pathways. ACS Sens. 2016;1(10):1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burns SM, Vetere A, Walpita D, et al. High-throughput luminescent reporter of insulin secretion for discovering regulators of pancreatic Beta-cell function. Cell Metab. 2015;21(1):126–137. [DOI] [PubMed] [Google Scholar]
- 27. Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11(8):783–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Liu N, He Y, et al. Improved calcium sensor GCaMP-X overcomes the calcium channel perturbations induced by the calmodulin in GCaMP. Nat Commun. 2018;9(1):1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Partridge JG. Utilizing GCaMP transgenic mice to monitor endogenous Gq/11-coupled receptors. Front Pharmacol. 2015;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaminski MT, Lenzen S, Baltrusch S. Real-time analysis of intracellular glucose and calcium in pancreatic beta cells by fluorescence microscopy. Biochim Biophys Acta. 2012;1823(10):1697–1707. [DOI] [PubMed] [Google Scholar]
- 31. Rountree AM, Neal AS, Lisowski M, et al. Control of insulin secretion by cytochrome C and calcium signaling in islets with impaired metabolism. J Biol Chem. 2014;289(27): 19110–19119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sweet IR, Gilbert M. Contribution of calcium influx in mediating glucose-stimulated oxygen consumption in pancreatic islets. Diabetes. 2006;55(12):3509–3519. [DOI] [PubMed] [Google Scholar]
- 33. Gao T, McKenna B, Li C, et al. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014;19(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gutiérrez GD, Bender AS, Cirulli V, et al. Pancreatic β cell identity requires continual repression of non-β cell programs. J Clin Invest. 2017;127(1):244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fujitani Y. Transcriptional regulation of pancreas development and β-cell function [Review]. Endocr J. 2017;64(5):477–486. [DOI] [PubMed] [Google Scholar]
- 36. Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12(12):1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayes HL, Zhang L, Becker TC, et al. A Pdx-1-regulated soluble factor activates rat and human islet cell proliferation. Mol Cell Biol. 2016;36(23):2918–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mali P, Esvelt KM, Church GM. Cas9 as a versatile tool for engineering biology. Nat Methods. 2013;10(10):957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. RRID: AB_2819186. Mercodia Ultrasensitive C-peptide ELISA. https://antibodyregistry.org/AB_2819186. Accessed January 7, 2020. [Google Scholar]
- 40. Hrvatin S, Deng F, O’Donnell CW, Gifford DK, Melton DA. MARIS: method for analyzing RNA following intracellular sorting. Plos One. 2014;9(3):e89459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. RRID:AB_2728751. Cell Signaling HNF1A antibody D7Z2Q. https://antibodyregistry.org/AB_2728751. Accessed January 7, 2020. [Google Scholar]
- 42. RRID:AB_416757. R&D PDX1 antibody BAF2419. https://antibodyregistry.org/AB_416757. Accessed January 7, 2020. [Google Scholar]
- 43. RRID:AB_10691857. Cell Signaling C-peptide antibody 4593S. https://antibodyregistry.org/AB_10691857. Accessed January 7, 2020. [Google Scholar]
- 44. RRID:AB_260137. Sigma Insulin antibody i2018. https://antibodyregistry.org/AB_260137. Accessed January 7, 2020. [Google Scholar]
- 45. RRID:AB_2337985. Goat anti-rabbit IgG-Phycoerythrin. https://antibodyregistry.org/AB_2337985. Accessed January 7, 2020. [Google Scholar]
- 46. RRID:AB_2338620. Goat anti-mouse IgG1-Phycoerythrin. https://antibodyregistry.org/AB_2338620. Accessed January 7, 2020. [Google Scholar]
- 47. RRID:AB_2340625. Donkey anti-rabbit IgG-647. https://antibodyregistry.org/AB_2340625. Accessed January 7, 2020. [Google Scholar]