Abstract
Background
Surgical investigations and interventions account for large health care utilisation and costs, but the scientific evidence for most procedures is still limited.
Objectives
Degenerative conditions affecting the lumbar spine are variously described as lumbar spondylosis or degenerative disc disease (which we regarded as one entity) and may be associated with back pain and associated leg symptoms, instability, spinal stenosis and/or degenerative spondylolisthesis. The objective of this review was to assess current scientific evidence on the effectiveness of surgical interventions for degenerative lumbar spondylosis.
Search methods
We searched CENTRAL, MEDLINE, PubMed, Spine and ISSLS abstracts, with citation tracking from the retrieved articles. We also corresponded with experts. All data found up to 31 March 2005 are included.
Selection criteria
Randomised (RCTs) or quasi‐randomised trials of surgical treatment of lumbar spondylosis.
Data collection and analysis
Two authors assessed trial quality and extracted data from published papers. Additional information was sought from the authors if necessary.
Main results
Thirty‐one published RCTs of all forms of surgical treatment for degenerative lumbar spondylosis were identified. The trials varied in quality: only the more recent trials used appropriate methods of randomization, blinding and independent assessment of outcome. Most of the earlier published results were of technical surgical outcomes with some crude ratings of clinical outcome. More of the recent trials also reported patient‐centered outcomes of pain or disability, but there is still very little information on occupational outcomes. There was a particular lack of long term outcomes beyond two to three years. Seven heterogeneous trials on spondylolisthesis, spinal stenosis and nerve compression permitted limited conclusions. Two new trials on the effectiveness of fusion showed conflicting results. One showed that fusion gave better clinical outcomes than conventional physiotherapy, while the other showed that fusion was no better than a modern exercise and rehabilitation programme. Eight trials showed that instrumented fusion produced a higher fusion rate (though that needs to be qualified by the difficulty of assessing fusion in the presence of metal‐work), but any improvement in clinical outcomes is probably marginal, while there is other evidence that it may be associated with higher complication rates. Three trials with conflicting results did not permit any conclusions about the relative effectiveness of anterior, posterior or circumferential fusion. Preliminary results of two small trials of intra‐discal electrotherapy showed conflicting results. Preliminary data from three trials of disc arthroplasty did not permit any firm conclusions.
Authors' conclusions
Limited evidence is now available to support some aspects of surgical practice. Surgeons should be encouraged to perform further RCTs in this field.
Plain language summary
Surgery for degenerative lumbar spondylosis
Degeneration of the lumbar spine is described as lumbar spondylosis or degenerative disc disease and may lead to spinal stenosis (narrowing of the spinal canal), vertebral instability and/or malalignment, which may be associated with back pain and/or leg symptoms. This review considers the available evidence on the procedures of spinal decompression (widening the spinal canal or laminectomy), nerve root decompression (of one or more individual nerves) and fusion of adjacent vertebrae. There is moderate evidence that instrumentation can increase the fusion rate, but any improvement in clinical outcomes is probably marginal. The effectiveness of intra‐discal electrotherapy (IDET) remains unproven. Only preliminary results are available on disc replacement and it is not possible to draw any conclusions on this subject.
Background
This review includes all forms of surgical treatment of degenerative conditions affecting the lumbar spine. These are variously described as lumbar spondylosis or degenerative disc disease (which we regard as one entity); whether or not they are regarded as the effects of ageing, secondary to trauma or 'wear and tear', or degenerative disease; and whether they involve the inter‐vertebral discs, the vertebrae and/or the associated joints. This includes the associated pathologies or clinical syndromes of instability, spinal stenosis and/or degenerative spondylolisthesis. We have termed the collective conditions 'degenerative lumbar spondylosis'.
Symptoms associated with degenerative lumbar spondylosis vary in severity and have a relatively low correlation with the severity of anatomical or radiographic changes. Only a small proportion of patients come to surgery. Surgical treatment may take the form of either a) fusion with the goal of relieving low back pain (with or without referred leg symptoms, but with the dominant presenting complaint of back pain), and/or b) decompression of nerve root(s) or cauda equina with the goal of relieving neurogenic claudication. Generally, fusion may be considered if there is severe disc degeneration, mal‐alignment, or evidence of spinal instability. Decisions about surgery are usually based not only on the nature of the localized pathology and associated symptoms and disability, but also on other factors such as the patient's occupation, athletic or recreational activity, and socio‐economic situation. The choice of procedure may be influenced by the surgeon's beliefs about the role of surgery in spinal disorders, and the surgical instrumentation and skills available. In the future, it is also likely to be necessary to consider ways of incorporating patient preferences.
Spinal stenosis (narrowing of the spinal canal) was first described as a rare developmental condition, but there is now increasing recognition that stenosis secondary to degenerative lumbar spondylosis may be a cause of low back and leg symptoms, particularly in older patients. Indeed, spinal stenosis is probably now the most common and fastest growing reason for spinal surgery in adults over 65 years of age (Ciol 1996). This fact suggests that surgery is beneficial, but good data relating to the diagnostic criteria and natural history of the condition, the indications for surgery and choice of surgical procedures, and the clinical or patient characteristics associated with a favourable outcome are lacking. Two meta‐analyses provide some information. One suggests that, on average, 64% of patients will obtain a satisfactory outcome from surgery (Turner 1992a). The other suggests that decompression without a fusion will give a 69% satisfactory outcome, whereas with fusion (solid in 86%), this figure would increase to 90% (Mardjetko 1994). However, these two meta‐analyses were based either entirely (Turner 1992a), or mainly (Mardjetko 1994) on largely retrospective case series.
After more than ninety years, there is continued dispute as to whether lumbar fusion is an appropriate and effective method of treating back pain in patients with degenerative lumbar spondylosis. There is heated debate and lack of clear evidence on the nature and role of 'instability', and the clinical indications for surgery are not well defined (Szpalski 1997). There is also wide variation in the surgical techniques used, technical success and rate of fusion. Reported satisfactory clinical outcomes range from 16% to 95% (Turner 1992a).
There is continued interest in, and controversy about, instrumented fusion. Posterior pedicle instrumentation was first used in Europe in the early 1960s (Roy‐Camille 1986). In recent years, there has been an explosion of surgical and commercial interests in a wide variety of methods of instrumented fusion in both Europe and the US. The above noted meta‐analysis of published case series of degenerative spondylolisthesis (Mardjetko 1994) suggested that fusion with pedicle screws produced a higher fusion rate (93% versus 86%) than fusion without instrumentation (which was not statistically significant), but that it did not produce any difference in clinical outcomes (86% versus 90% satisfactory outcomes). There is less available scientific information about other methods of fusion, whether anterior or posterior. In recent years there has been rapidly growing clinical, commercial and public interest in other innovative technologies, such as, intradiscal electrotherapy (IDET) and disc arthroplasty.
In view of these various continued uncertainties, a systematic review of all RCTs of surgical treatment of degenerative lumbar spondylosis remains appropriate.
Objectives
To test the following null hypotheses: (i) Any form of surgical treatment for low back pain and/or associated leg symptoms secondary to degenerative lumbar spondylosis is no more effective than natural history, placebo, conservative treatment, or a rehabilitation program. (ii) Decompression of spinal stenosis secondary to degenerative lumbar spondylosis is no more effective than any of these alternatives. (iii) There is no difference in outcome between different forms of surgical treatment for spinal stenosis. (iv) Fusion for low back pain secondary to degenerative lumbar spondylosis is no more effective than any of these alternatives. (v) There is no difference in outcome between different forms of surgical treatment for low back pain
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials (RCTs) and controlled clinical trials (CCTs) with quasi‐randomised methods (methods of allocating participants to a treatment that are not strictly random e.g. by date of birth, hospital record number or alternation),pertinent to the surgical treatment of degenerative lumbar spondylosis.
Types of participants
Patients over age 18 with degenerative lumbar spondylosis treated by surgery.
Types of interventions
Laminectomy; laminotomy; anterior lumbar intervertebral body (ALIF), postero‐lateral, posterior lumbar intervertebral body (PLIF) fusion, alone or in combination, or other forms of instrumented fusion; intradiscal electrotherapy (IDET), disc arthroplasty; combinations of the preceding interventions.
Types of outcome measures
Outcome measures were designed to cover both patient‐centred clinical outcomes that are of primary interest to patients and surgical outcomes that are often of more interest to surgeons (Deyo 1998).
A) Patient centred outcomes:
1) Proportion of patients with successful outcomes according to self‐assessment 2) Improvement in pain measured on a validated pain scale 3) Improvement in function measured on a disability or quality of life scale 4) Occupational outcomes 5) Economic data as available
B) Surgical outcomes:
1) Proportion of patients with successful outcomes according to clinician's assessment 2) Fusion rate 3) Progression of spondylolisthesis 4) Rate of repeat back surgery 5) Any other technical surgical outcomes 6) Objective clinical measures of physical improvement or impairment, including change in spinal flexion, improvement in straight leg raise, alteration in muscle power and change in neurological signs.
C) Adverse complications:
Note: Small RCTs lack sufficient statistical power to produce any meaningful conclusions about complications of low incidence. A completely different kind of database, that is more representative of routine clinical practice (e.g. Deyo 1992), is necessary to provide sufficient data. However, where mentioned in the primary studies, we extracted information on adverse events.
Search methods for identification of studies
Relevant RCTs in all languages were identified up to March 2005 by: (i) The Cochrane Central Register of Controlled Trials (ii) Computer searching of MEDLINE (Alderson 2003) with specific search terms (see Appendix 1) (iii) PubMed at http://www.ncbi.nlm.nih.gov/. (iv) Hand searching of Spine and ISSLS abstracts from 1975 (v) Communication with members of the Cochrane Back Review Group and other international experts (vi) Personal bibliographies (vii) Citation tracking from all papers identified by the above strategies.
Data collection and analysis
Eligible trials were entered into RevMan 4.2 and sorted on the basis of the inclusion and exclusion criteria. For each included trial, assessment of methodological quality and data extraction were carried out as detailed below.
1. Both authors (JNAG, GW) selected the trials to be included in the review. Disagreement was resolved by discussion, followed, if necessary, by further discussion with an independent colleague.
2. The methodological quality was assessed and internal validity scored by both authors, assessing risk of pre‐allocation disclosure of assignment, intention to treat analysis and blinding of outcome assessors (Schulz 1995). The quality of concealment allocation was rated in three grades: A: Clearly yes ‐ some form of centralized randomisation scheme or assignment system; B: Unclear ‐ assignment envelopes, a "list" or "table", evidence of possible randomisation failure such as markedly unequal control and trial groups, or trials stated to be random but with no description; C: Clearly no ‐ alternation, case numbers, dates of birth, or any other such approach, allocation procedures that were transparent before assignment. Withdrawal, blinding of patients and observers, and intention‐to‐treat analyses were assessed according to standard Cochrane methodology and tabulated in the results tables (van Tulder 2003). The nature, accuracy, precision, observer variation and timing of the outcome measures were also tabulated. Initially, any outcomes specified were noted. The data were then collated and outcome measures collected for later meta‐analysis. In fact, only four categorical outcomes were consistently reported: the patient's and surgeon's ratings of success, the attainment of spinal fusion and the performance of a second surgical procedure. To pool the results, ratings of excellent and good were classified as 'success', while fair and poor were classified as 'failure'. The pooled data are given in the analysis tables.
3. For each study, Odds Ratios (OR) and 95% confidence limits (95% CI) were calculated. Results from clinically comparable trials were pooled using random‐effects models for dichotomous outcomes. It should be noted that in several instances the test for homogeneity was significant, which casts doubt on the statistical validity of the pooling. Nevertheless, there is considerable clinical justification for pooling the trials in this way. In view of the clinical interest, these results are presented as the best available information at present, with the qualification that there may be considerable statistical weaknesses to some of the results. The evidence was rated strong, medium or limited according to the Cochrane Back Review Group levels of evidence (van Tulder 2003).
Results
Description of studies
Thirty‐one RCTs have been included in this review as detailed below. Details of individual trials are presented in the table of Characteristics of Included Studies.
Risk of bias in included studies
Descriptions of randomisation were poor in the earlier trials, but there now appears to be more awareness of the importance of the method of randomisation. In 16 studies there was a clear attempt at concealment of group allocation. In seven trials the method of allocation was not described. Four trials (Herkowitz 1991; Postacchini 1993; Grob 1995; Schofferman 2001) were considered to be quasi‐randomised as the patients were allocated by alternate assignment, according to their date of admission to hospital or by odd and even file numbers. Six trials were clearly 'open' to potential selection bias (Bridwell 1993; Postacchini 1993; Zdeblick 1993; Grob 1995; Schofferman 2001; Kitchel 2002).
Eighteen of the 31 trials had the recommended follow‐up for surgical studies of at least two years. Most had a follow‐up rate of at least 90%. One trial (France 1999) gave different patient outcomes after best and worst case analyses. Blinding is difficult in surgical studies, but three of the recent trials were double blind and several used an independent assessor. Most of the recent trials also provided patient‐oriented, clinical outcomes (Deyo 1998). The majority of the trials gave technical surgical outcomes such as fusion, spondylolisthesis progression or the need for re‐operation. Clinical outcomes were mainly crude ratings on a three to four‐point scale: five trials gave a surgeon's rating and nine gave a patient's rating. Eleven gave direct information on back pain (see Characteristics of Included Trials table) and nine on functional outcome measured on a validated assessment scale. These defects of trial design introduced considerable potential for bias and many of the conclusions of this review are about surgical outcomes rather than patient‐centred clinical outcomes. There is still a lack of long‐term follow‐up beyond two years, which is particularly important in procedures that aim to alter the long‐term natural history or clinical progress of a degenerative condition.
Effects of interventions
Data from thirty‐one RCTs of all forms of surgical treatment for degenerative lumbar spondylosis are included in this updated review. In the first edition of this review nine of the 16 trials identified were found on MEDLINE, four from personal bibliographies and four from abstracts of meeting proceedings. The new trials were mainly collected by the authors from personal literature review or after notification by colleagues of the Cochrane Back Review Group. Three trials originally included have now been deleted from the review (see Characteristics of Excluded Trials table) as originally, they were abstracts of work in progress and no data have been published over the intervening years (Emery 1995; Rogozinski 1995; Zdeblick 1996). Six further trials are included as ongoing studies. The majority of the trials compared two or more surgical techniques. From a surgical perspective, the trials now fall into three broad sections: 1) surgical treatment (decompression with or without fusion) for spinal stenosis and / or nerve root compression 2) surgical treatment (fusion, intra‐discal electrotherapy or disc arthroplasty) for back pain 3) comparison of different techniques of spinal fusion.
In the first section, one trial compared surgical treatment with conservative therapy and one compared different techniques of decompression for spinal stenosis. Three trials compared decompression alone with decompression and some form of fusion. One trial compared outcomes following use of an interspinous spacer with those after a non‐operative regime, including epidural injection. A further two trials of surgery for isthmic spondylolisthesis were included. The second section included two trials of fusion to relieve discogenic back pain compared with different forms of conservative treatment, and preliminary results from three small trials of intra‐discal electrotherapy (IDET) and two trials of disc arthroplasty. In the third section, 15 trials considered the role of instrumentation in fusion and four trials that of electrical stimulation (direct current and pulsed electromagnetic stimulation) in postero‐lateral fusion. Five trials included sub‐groups of participants and are included in more than one section.
Analysis of the included trials is complicated by the inclusion of participants with varied pathology and a lack of consistency in treatment methods. Only five of the trials (Moller 2000; Amundsen 2000; Fritzell 2001; Brox 2003; Brox 2004) had a conservative treatment arm. It was not possible to analyze participants according to duration of their symptoms, type of previous conservative treatment, or indications for surgery, as few of the trials provided these data in usable form. Although many trials provided limited information on selected complications, these were not comparable between trials. Three trials provided comparative information on operating time and blood loss, and three trials provided information on progression of spondylolisthesis. No other adverse effects could be reviewed. A cost analysis was performed in one trial (Fritzell 2001), although the methodological criticisms by Goosens (Goosens 1998) should be noted.
1. Techniques for the decompression of spinal and nerve root stenosis.
The effectiveness of surgical decompression for spinal stenosis has been considered in one new trial (Amundsen 2000). In this trial, 19 patients with severe symptoms were selected for surgical treatment and 50 patients with moderate symptoms for conservative therapy. A further 31 patients were randomised between the two treatments. The overall results were broadly in line with those from meta‐analyses of retrospective case series by Turner (Turner 1992b) and Ciol (Ciol 1996). The results of conservative therapy were better than expected but the authors suggested that, if surgery was deemed necessary, it might be 'good' for up to four‐fifths of severely affected individuals. However, the small, randomised portion of the study showed no statistically significant effect. At ten years, five people of the 11 randomised to decompression had no, or minimal, pain compared with the four of 14 who were initially treated conservatively (six were lost to follow‐up).
Postacchini (Postacchini 1993) considered techniques of decompression for spinal stenosis by comparing laminectomy with multiple laminotomy. This study had several confounding factors. Nine of the 35 patients scheduled for laminotomy actually had a laminectomy for technical reasons and several patients in each group also had an inter‐transverse arthrodesis for degenerative spondylolisthesis. This trial did not demonstrate any difference in clinical outcomes or spondylolisthesis progression between the two treatment methods.
Three trials considered whether some form of postero‐lateral fusion, with or without instrumentation, was a useful adjunct to decompression alone (Herkowitz 1991; Bridwell 1993; Grob 1995). They provided data on a total of 139 participants with 99% follow‐up at two to three years. Pooling of the three trials showed no statistically significant difference in outcomes between decompression plus fusion or decompression alone (random OR 0.44, 95% CI 0.13,1.48), as rated by the surgeon, 18 to 24 months after the procedure, although the precision is too small for definitive conclusions to be drawn. One of these trials (Grob 1995) considered fusion with and without instrumentation in patients with degenerative spinal stenosis with no evidence of instability. In the fusion arm of the trial, patients were allocated to either decompression plus arthrodesis of only the most stenotic segment, or decompression of the whole area. The authors concluded that, in the absence of instability, arthrodesis was not necessary, provided that the posterior elements were preserved during the decompression to maintain spinal stability. The other two trials considered the role of adjunct fusion in spinal stenosis associated with single or two‐level degenerative spondylolisthesis. Herkowitz (Herkowitz 1991) studied non‐instrumented fusion alone, and showed that fusion produced significantly less self‐reported back and leg pain and significantly better surgeon's ratings of outcome. Bridwell (Bridwell 1993) studied both instrumented and non‐instrumented fusion. Those with an instrumented fusion had a significantly higher fusion rate, less spondylolisthesis progression and more improvement in walking ability. Post hoc analysis showed that achieving a solid fusion was associated with subjective improvement. However, there were methodological limitations to this trial: in particular, the control group was too small and there were insufficient data for an intention‐to‐treat analysis to demonstrate any significant effect of performing fusion per se versus decompression alone.
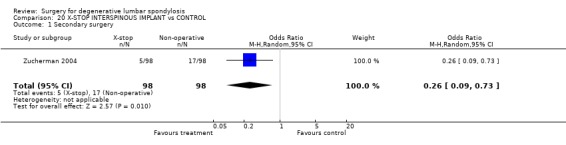
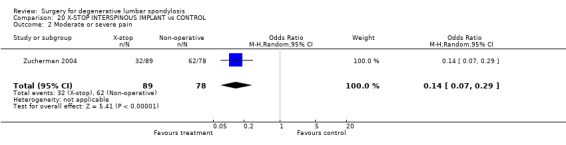
Currently, there are no published RCTs of surgical decompression to relieve isolated nerve root stenosis, but there is one trial examining the effect of an interspinous spacer device (Zucherman 2004) in elderly patients with one or two level central stenosis. Limited results at one year suggest better outcome estimated on the Zurich Claudication Questionnaire and less pain following device use. Trials of intra‐foraminal steroid injection are not included in this surgical review.
There are two trials of surgical treatment for isthmic spondylolisthesis. It may be debated whether this condition falls within our definition of degenerative lumbar spondylosis, but for completeness these trials have been included in this review. Moller (Moller 2000) studied 111 adults with low back pain alone (one third) or with sciatica (two thirds) associated with isthmic spondylolisthesis. The primary aim of the trial was to compare the outcome of posterolateral fusion with conservative treatment in the form of an intensive exercise program. At two years, patients treated surgically had less pain and disability, and better self‐ and observer‐rated outcomes. There was no significant difference in occupational outcomes. However, no separate data were presented for back pain, and it is not clear how much of these successful outcomes was related to relief of sciatica from foraminal stenosis, which is the generally accepted indication for surgery in this condition. Carragee (Carragee 1997) compared the results of fusion alone, or fusion plus laminectomy and decompression for isthmic L5/S1 spondylolisthesis. Again, these patients had both back and leg pain, although without serious neurology. This trial was confounded by the fact that non‐smokers had fusion by bone grafting alone, while smokers had their fusion supplemented by instrumentation. However, in neither group did the addition of decompression to the arthrodesis appear to improve clinical outcome.
2. Surgery for back pain without neurological compromise
At the time of the original Cochrane review of degenerative lumbar spondylosis (1999) there were no published RCTs on the effectiveness of fusion for chronic back pain, compared with natural history, conservative treatment or placebo. There are now two new trials. The Swedish trial of lumbar fusion versus physiotherapy treatment for chronic low back pain (Fritzell 2001) included 294 individuals presenting at 19 spinal centres over a six‐year period. Strict inclusion criteria limited trial entry to those who had low back pain more pronounced than leg pain, lasting longer than two years, and no evidence of nerve root compression. Each patient had to have completed a course of conservative treatment that had failed to produce relief. Nineteen per cent had previous surgery. Individuals were randomised into four treatment groups. Seventy‐two patients had conservative treatment and 222 had one of three different fusion techniques. There was a 98% follow‐up at two years. Twenty‐five subjects did not complete treatment according to random allocation, but these 'group changers' were included in the original 'intention‐to‐treat' analysis. At two years, independent assessors rated 46% of the surgical group as 'excellent' or 'good', compared with 18% of the conservative group (P < 0.0001). More surgical patients rated their results as 'better' or 'much better' (63% versus 29%, P < 0.0001). The surgical patients had significantly greater improvement in pain (visual analogue scale) and disability (Oswestry scale). The "net back to work rate" was significantly in favour of surgical treatment (36% versus 13%, P value 0.002). There were no significant differences in any of these outcomes between the three surgical groups. The Swedish trial also provided one of the few cost‐effective analyses of spinal surgical treatment. The cost differences between the surgical and conservative groups were significant, mainly because more individuals went back to work in the surgical group (Fritzell 2001).
The major question about the Swedish trial was the nature of the conservative treatment used as the control intervention (Mooney 1990). The investigators tried to ensure that each patient understood that "no treatment method, as far as was known, was superior to any other". Nevertheless, the control group essentially received more of the same 'usual non‐surgical treatment' that had already failed, and the failure of which was one of the indications leading to consideration of surgery. In view of the likely negative patient expectations, it is hardly surprising that the results in the control group appear to have been poorer than most epidemiological studies of natural history. Strictly speaking, this trial provided the first substantive evidence that fusion is more effective than continued, standard 1990s, 'usual care'.
The Norwegian trial (Brox 2003; Brox 2004) compared posterolateral fusion with transpedicular screws and post‐operative physiotherapy versus a modern 'rehabilitation' type of programme, consisting of an educational intervention (Indahl 1995) and a three‐week course of intensive exercise sessions, based on cognitive‐behavioural principles. Sixty‐four patients with low back pain lasting longer than one year plus disc degeneration at L4/5, L5/S1 or both (Brox 2003), and a further 60 patients with chronic low back pain more than one year after previous discectomy (Brox 2004) were randomised and reported on separately. There was a 97% follow‐up at one year and intention‐to‐treat analysis. In both series, there were no significant differences in any of the main outcomes of independent observer rating, patient rating, pain, disability or return to work. Radiating leg pain improved significantly more after surgery, whereas fear avoidance beliefs and forward flexion improved significantly more after conservative management. At one‐year follow‐up, the conservative groups had significantly better muscle strength and endurance (Keller 2004). Despite the relatively small size of these trials (though the number randomised to conservative treatment is comparable to the Swedish trial, 57 compared to 72), the consistent results in both first time and previously failed surgical patients and lack of any trends make a Type II error unlikely. In contrast to the Swedish trial, these results suggest that fusion and a modern rehabilitation approach can produce comparable outcomes.
There are now results from three small RCTs of intra‐discal electrotherapy (IDET), each using different protocols. The first (Barendse 2001) randomised 28 patients to either IDET or placebo. At eight weeks, one patient was judged a success in those stimulated (n = 13) and two in the controls (n = 15). No more detailed or longer‐term results have been published. The second (Pauza 2004) reported on a highly selected group of 64 patients (from a potential cohort of 4253) randomised to IDET or placebo. Results (from 56) suggested that IDET resulted in a significantly greater improvement in pain and disability. The final study (Freeman 2003) randomised 57 patients with a 2:1 ratio to IDET or placebo and had 96% follow‐up. No patient in either arm met pre‐defined criteria for clinically significant improvement in the Low Back Outcome Score or SF‐36, or for a successful outcome. These trials are all small so it is not possible to draw any firm conclusions about the effectiveness of IDET. Nevertheless, the extremely poor results of Barendse and Freeman cast serious doubt on the highly selective, positive results reported by Pauza. It is interesting to note that IDET was also found to be ineffective in both arms of a randomised trial published by Ercelen et al (Ercelen 2003). This trial was excluded from the review as it compared two durations of thermocoagulation rather than the intervention versus any form of control therapy.
Three makes of artificial disc ‐ the SB Charite, ProDisc and Maverick ‐ are currently undergoing FDA‐approved multi‐centre RCTs for degenerative lumbar disc disease. McAfee 2003 and Zigler 2003 respectively summarised earlier European experience of these two devices, which did not include any RCTs. McAfee (McAfee 2003) reported on the pilot feasibility study of the US RCT comparing the SB Charite (n = 41) and BAK anterior interbody fusion (n = 19) for single level degenerative disc disease at L4/5 or L5/S1. There was no significant difference in Oswestry Disability scores between the artificial disc and fusion groups at two years. During the period of review of this manuscript, further data from an additional 244 participants (total 304: 205 Charite, 99 BAK) have been published by Geisler et al. 2004 (see sub‐reference McAfee 2003). Oswestry disability scores, VAS scores and device failure rates are provided in the analysis tables. No significant differences were observed. Zigler 2003 (n = 39) and Delamarter 2003 (n = 53) each reported six‐month results from single centres taking part in the US RCT of ProDisc versus circumferential 360 degree fusion for one‐ or two‐level degenerative lumbar disc disease between L3/S1. Zigler 2003 compared 28 patients who received ProDisc and 11 who had fusion. Operative time, blood loss and length of hospital stay were lower with disc replacement. Disc replacement patients had a trend to better Oswestry Disability scores, but at six months there were no significant differences in pain, disability or patient satisfaction. In view of the small numbers, it is not possible to graphically present the results, make multiple statistical comparisons or draw any firm conclusions. Delamarter 2003 compared 35 patients who received the ProDisc and 18 who had fusion. Disc replacement patients had significantly faster improvement in VAS pain and Oswestry Disability scores at six weeks and three months, but by six months there was no significant difference between disc replacement and fusion. Patients with disc replacement at L4/5 preserved significantly better motion.
3) Techniques of fusion
Fifteen trials addressed various questions about the role of instrumentation in fusion. Four of these were sub‐groups from trials already described in sections 1) and 2) (Bridwell 1993; Grob 1995; Moller 2000; Fritzell 2001). This was a very heterogeneous group of studies, in terms of surgical pathology, the technique(s) of instrumentation and the questions addressed. Four trials included patients with back pain associated with mixed pathologies ‐ degenerative disc disease, degenerative spondylolisthesis, isthmic spondylolisthesis, or failed back surgery ‐ and did not present separate results for each condition (Zdeblick 1993; Thomsen 1997; France 1999; Christensen 2002). The Swedish study (Fritzell 2001) focused on people with chronic low back pain due to degenerative disc disease, and excluded stenosis or spondylolisthesis, but 19% of the participants had back pain following previous surgery for disc herniation. Two trials had participants with degenerative spondylolisthesis and stenosis (Bridwell 1993; Fischgrund 1997) and three had participants with isthmic spondylolisthesis (McGuire 1993; Carragee 1997; Moller 2000). Only the recent Norwegian study (Brox 2003) reported separately on participants with chronic low back pain due to degenerative disc disease. There were differences in surgical approach and instrumentation systems in most studies, and only three trials used the same pedicle screw system. There was also lack of uniformity in the outcome measures, with the most common being technical surgical outcomes ‐ fusion rates, progression of spondylolisthesis and re‐operation rates. The results from the trials are summarized in the 'analysis tables'. Note that the test for homogeneity was significant in all the meta‐analyses. Nevertheless, there is strong clinical rationale for pooling this group of trials and, in view of the clinical importance of the issue, the results are presented as the best information available at present, with the qualification that there may be some statistical weakness to their interpretation.
Eight trials directly addressed the question of whether instrumentation improves the outcome of postero‐lateral fusion, with an average 95% patient follow‐up at 16 months to 4.5 years (mean 28 months). These trials provide moderate evidence that instrumentation improves the fusion rate (random OR 0.43, 95% CI 0.21,0.91: favours instrumented). Taken altogether, these trials provide conflicting evidence that instrumentation produces a statistically and clinically significant improvement in clinical outcomes (random OR 0.49, 95% CI 0.28,0.84: favours instrumented). However that is heavily dependent on the suspiciously good results of Bridwell (Bridwell 1993) and Zdeblick (Zdeblick 1996). If only the methodologically stronger trials since 1997 are considered, then any advantage appears to be marginal and non‐significant (74% versus 68%).
Four trials compared various combinations of anterior, posterior or combined fusion. Schofferman (Schofferman 2001) found no difference in clinical outcomes between anterior lumbar interbody fusion (ALIF) plus pedicle screws plus instrumented posterolateral fusion (360°) versus ALIF plus pedicle screws without graft (270°). Health care costs increased with the complexity of surgery. Kitchel (Kitchel 2002) found no difference in outcomes with the addition of a posterior lumbar interbody fusion (PLIF) in degenerative spondylolisthesis (Grade I/II) to a posterolateral instrumented fusion for patients over 60 years of age, but significantly longer surgery time, higher blood loss and complication rate in this group. Christensen (Christensen 2002) found that circumferential fusion using ALIF carbon fiber cages produced a higher fusion rate (90% versus 80%) and lower re‐operation rate (7% versus 22%) than posterolateral fusion with Cotrel‐Dubousset instrumentation. Circumferential fusion produced marginally less back and leg pain (though of borderline significance on multiple comparisons). Finally, Sasso (Sasso 2004) compared fusion rate using a cylindrical threaded titanium cage inserted anteriorly with that obtained after using a femoral ring allograft. Although fusion rate was greater with the cage, disability and neurologic outcome scores were not significantly different. These conflicting results do not permit any conclusions about the relative effectiveness of anterior, posterior or circumferential fusion.
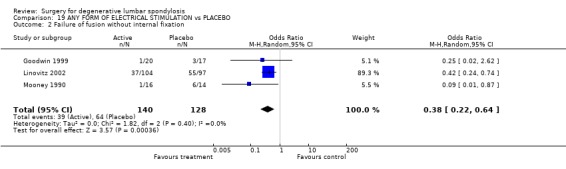
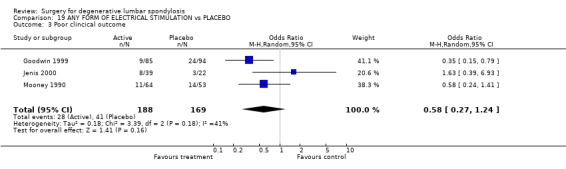
Four trials assessed whether electrical stimulation could enhance fusion, though they all used different methods. Mooney and Linovitz (Mooney 1990; Linovitz 2002) used pulsed electromagnetic stimulation for four hours/day and 30 minutes/day respectively. Goodwin (Goodwin 1999) used capacitively coupled field stimulation 15 to 16 hours/day and Jenis (Jenis 2000) tested both pulsed electromagnetic stimulation and implanted direct current. The anatomical technique of fusion varied. Jenis tested instrumented and Linowitz non‐instrumented fusion, while Mooney and Goodwin tested both instrumented and non‐instrumented fusion. Three trials in non‐instrumented fusion showed a significant effect on the fusion rate [random OR 0.38 95% CI 0.22, 0.64: favoured stimulation]. Two out of three trials in instrumented fusion showed positive results though the third trial had negative results [random OR 0.59 95% CI 0.15, 2.30: not significant]. Although these results suggest that electrical stimulation does have a modest effect on enhancing fusion, it is not possible to assess the relative value of different methods of electrical stimulation. Jenis, Mooney and Goodwin assessed clinical outcomes, but overall there was no significant effect.
Discussion
There is now an increasing scientific database of 31 RCTs on surgical treatments for degenerative lumbar spondylosis. Four RCTs were presented in a single day at the 2003 meeting of the International Society for Study of the Lumbar Spine (ISSLS). Most of the recent trials are of higher quality than those previously reported. However, most still compare different surgical techniques, and few address the more fundamental question of whether these techniques provide effective relief of presenting symptoms. Many trials still report relatively short‐term, technical, surgical outcomes rather than patient‐centred outcomes of pain, disability and capacity for work. The limited evidence on the long‐term effects of either surgical decompression or fusion remains a matter of concern, given the magnitude of the clinical problem and the numbers and costs of surgical procedures being performed.
The trials on spinal stenosis and decompression permit limited conclusions. There is no clear evidence about the most effective technique of decompression for spinal stenosis or the extent of that decompression. There is limited evidence that adjunct fusion to supplement decompression for degenerative spondylolisthesis produces less progressive slip and better clinical outcomes than decompression alone. There is also limited evidence that fusion alone may be as effective as fusion combined with decompression for grade I or II isthmic spondylolisthesis with no significant neurology.
There are now two trials on the effectiveness of fusion compared with conservative treatment. The first (Swedish) trial (Fritzell 2001) appeared to provide strong evidence in favour of fusion, but the more recent (Norwegian) trial (Brox 2003; Brox 2004) refutes this. The difference may lie in the treatment given to the control group. Fusion is more effective than continued, failed, standard 1990s, 'usual care'; it does not appear to be any more effective than a modern rehabilitation programme. Clearly, there are still open questions about the scientific evidence on the clinical effectiveness of fusion. Further evidence is required, which hopefully will be provided by the multi‐centred RCTs of fusion that are presently underway in the US and the UK.
There are now 15 trials of instrumented fusion, but they are clinically and statistically very heterogeneous, and any attempt to combine and interpret the results must be cautious and tentative. These trials dealt with diverse pathological conditions, with different criteria for surgery, and the results were not always presented separately for each sub‐group. Most of the trials used different instrumentation systems. Many of these trials were of low methodological quality with inadequate randomisation, lack of blinding and potential for bias. The published results were mainly surgical outcomes, such as fusion and surgeon's ratings, rather than patient‐centred outcomes. Some of the trials were published in abstract form only. Bearing these limitations in mind, instrumentation of a posterolateral fusion appears to lead to a higher fusion rate, though there are problems assessing fusion in the presence of metalwork, which few of these trials considered (Blumenthal 1993, Kant 1995). Despite enhancing fusion, it appears that any improvement in clinical outcomes is marginal. It is not possible to draw any conclusions from this review about the relative morbidity or complications, except that instrumentation is obviously associated with unique complications. Neither is it possible to draw any conclusions about the possible role of instrumented fusion for any particular pathological condition, or about the relative benefits of any particular instrumentation system.
Bono et al (Bono 2004) recently completed a comprehensive review of a much wider range of randomised and non‐randomised, prospective and retrospective studies of lumbar fusion, which provides a useful check on this more rigorous but more limited Cochrane review. They also concluded that: 1. The surgical literature on lumbar fusion over the past 20 years is 'incomplete, unreliable, haphazard'. They made useful suggestions on how this should be improved in future studies. 2. The use of instrumentation appears to increase the overall fusion rate, but only slightly. 3. The use of instrumentation does not improve overall clinical outcomes (though there is currently insufficient evidence to judge particular sub‐groups of patients). The recent paper (Zucherman 2004) examining the use of an interspinous spacer device for lumbar spinal stenosis provides promising results and further studies are clearly warranted.
There are still only preliminary results available on disc replacement, preventing the drawing of any firm conclusions. It is likely to be another 18 months before the full two‐year outcomes from all the centres of the US RCTs are published.
Only four trials (Thomsen 1997; Fritzell 2001; Brox 2003; Brox 2004) considered occupational status, and it is not possible to draw any conclusions about the efficacy of any of these surgical treatments on capacity for work. There is no good evidence on cost‐effectiveness. There are other data on various aspects of surgical technique that we have not included in this review (e.g. computer assistance on the placement of pedicle screws (Laine 2000)). There is also immense scientific interest in the role of recombinant bone morphogenic protein (Transfeldt 2001; Sandhu 2003) and gene therapy (Cha 2003), but we feel that these topics should be the subject of a separate Cochrane Review.
Authors' conclusions
Implications for practice.
There is now some evidence on various issues of surgical techniques of decompression and fusion for individuals with lumbar spondylosis. There is still insufficient evidence on the effectiveness of surgery on clinical outcomes to draw any firm conclusions. Further studies are needed.
Implications for research.
There is a need for more scientific evidence on the clinical efficacy and cost‐effectiveness of surgical decompression and/or fusion for specific pathological and clinical syndromes associated with degenerative lumbar spondylosis. This will require high quality RCTs, preferably comparing these surgical treatments with natural history, placebo or conservative treatment. Surgeons should seek expert methodological advice when planning trials.
This Cochrane review should be maintained and updated, as further RCTs become available. The authors of this review will be pleased to receive information about any other RCTs of surgical treatment of degenerative lumbar spondylosis.
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 23 August 2005 | New citation required and conclusions have changed | August 2005: re‐analysis of 'Instrumented posterolateral fusion' data changed the level of evidence from "There is moderate evidence that instrumentation can increase the fusion rate, but 'strong evidence that it does not improve clinical outcomes' to 'any improvement in clinical outcomes is probably marginal.' |
| 31 March 2005 | New search has been performed | Issue 2, 2005 update: Identification and inclusion of 17 new trials: Amundsen T et al. Spine 2000;25:1424‐36. Brox I et al. Spine 2003;28:1913‐21. Brox I et al. Spine 2004 (in press). Barendse GAM et al. Spine 2001;26:287‐92. Christensen et al. Spine 2002;27:2674‐83. Delamarter RB et al. Spine 2003; 28(20S): S167. Freeman B et al. Proc of the International Society for the Study of the Lumbar Spine 2003. Fritzell P et al. Spine 2001;26:2521‐34. Jenis LG, An HS, Stein R et al. Journal of Spinal Disorders 2000;13:290‐6. Kitchel SH, Matteri RE. Current Concepts 2002. Linovitz RJ et al. Spine 2002;27:1383‐9. McAfee PC et al. Spine 2003;28(20S):S153‐62. Madan S, Boeree NR. Eur Sp J 2003;12:361‐8. Pauza K et al. The Spine Journal 2004;4:27‐35 Schofferman J et al. Spine 2001;26:E207‐12. Zigler JE et al. Journal of Spinal Disorders 2003;16:352‐61. Zucherman JF et al. Eur Spine J 2004;13:22‐31. Three papers previously included in the review, containing limited abstracted data, were excluded due to lack of a substantive further publication: Emery SE et al. Orthopedic Transactions 1995;19:362. Rogozinski et al. Orthopaedic Transactions 1995;19:362. Zdeblick TA et al. Orthopaedic Transactions 1996;20:10654‐5. |
Acknowledgements
Ms Inga Grant was a co‐author on the original review. The authors would like to thank Professor W.J. Gillespie, Dr Helen Handoll and Mrs Kathryn Quinn of the Department of Orthopaedic Surgery, The University of Edinburgh, for their advice and support in preparation of the original review. We are also grateful to Professor A. Nachemson and Dr M. Szpalski who have provided much assistance in literature searching and retrieval over the years and to the staff of the Cochrane Back Review Group for their editorial work.
Appendices
Appendix 1. MEDLINE search strategy
1. explode SURGERY/ all subheadings 2. explode SPINAL FUSION/ all subheadings 3. explode LAMINECTOMY/ all subheadings 4. (SPINE* or SPINAL) near DECOMPRESS* 5. LAMINOTOMY 6. LAMINOPLASTY 7. PEDICLE near SCREW 8. INTERVERTEBRAL 9. LUMBAR near VERTEBRA* 10.CAUDA‐EQUINA / without‐subheadings, drug‐effects, injuries, surgery 11.FACET near FUSION 12.SPONDYLOLYSIS 13.SPONDYLOSIS 14.explode "SPONDYLOLISTHESIS"/ without‐subheadings, drug‐therapy, economics, mortality, rehabilitation, surgery, therapy 15.LATERAL near MASS 16.ANTERIOR near FUSION 17.POSTERIOR near FUSION 18.explode "INTERVERTEBRAL‐DISK‐DISPLACEMENT"/ without‐subheadings, complications, drug‐therapy, economics, mortality, rehabilitation, surgery, therapy 19.explode BONE‐TRANSPLANTATION/ all subheadings 20.BONE near GRAFT 21.FIXATION near (SPINE* or SPINAL) 22.STABILIS* near (SPINE* or SPINAL) 23.PEDICLE near FUSION 24.explode "BACK‐PAIN"/ without‐subheadings, complications, drug‐therapy, economics, mortality, surgery, therapy 25.explode "LOW‐BACK‐PAIN"/ without‐subheadings, complications, drug‐therapy, economics, mortality, surgery, therapy 26.explode "LUMBAR‐VERTEBRAE"/ without‐subheadings, abnormalities, injuries, surgery, transplantation 27.DEGENERAT* 28.SPINE* or SPINAL or DISC or DISCS or DISK or DISKS 29.explode "SPINAL‐OSTEOPHYTOSIS"/ without‐subheadings, complications, drug‐therapy, economics, mortality, rehabilitation, surgery, therapy 30.#19 and #28 31.#20 and #28 32.#27 near #28 33.SPINAL near STENOSIS 34.FORAMINOTOMY 35.(FORAMEN* or FORAMINA*) near STENOSIS 36.LUMBAR near BODY 37.VERTEBRA* near BODY 38.#28 near BODY 39.#9 near BODY 40.PLIF 41.GRAF 42. LIGAMENTOTAXIS 43. CAGE near FUSION 44. SCREW near FUSION 45. PEDICLE near SCREW 46.#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #21 or #22 or #23 or #24 or #25 or #26 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45
Data and analyses
Comparison 1. DECOMPRESSION vs CONSERVATIVE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary surgery by 4 years | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.89] |
| 2 Bad result at 10 years | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 2.43 [0.09, 67.57] |
1.1. Analysis.
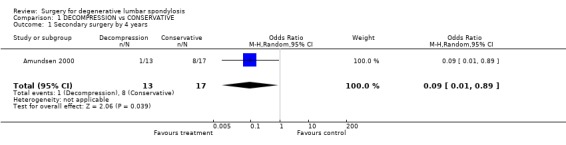
Comparison 1 DECOMPRESSION vs CONSERVATIVE, Outcome 1 Secondary surgery by 4 years.
1.2. Analysis.
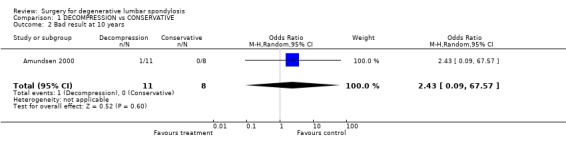
Comparison 1 DECOMPRESSION vs CONSERVATIVE, Outcome 2 Bad result at 10 years.
Comparison 2. MULTIPLE LAMINOTOMY vs LAMINECTOMY.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No success: combined patient / surgeon rating | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.25, 2.88] |
| 2 Spondylolisthesis progression | 1 | 67 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.16, 2.03] |
2.1. Analysis.
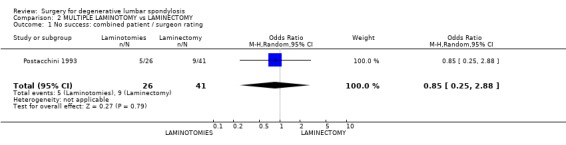
Comparison 2 MULTIPLE LAMINOTOMY vs LAMINECTOMY, Outcome 1 No success: combined patient / surgeon rating.
2.2. Analysis.
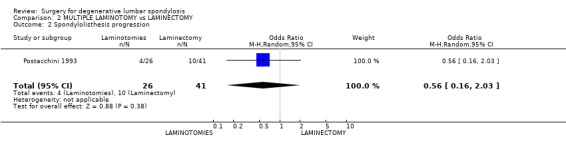
Comparison 2 MULTIPLE LAMINOTOMY vs LAMINECTOMY, Outcome 2 Spondylolisthesis progression.
Comparison 3. LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor result 18‐24 months ‐ Surgeon rating | 3 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.13, 1.48] |
| 2 Re‐operation 2‐4 years | 2 | 64 | Odds Ratio (M‐H, Random, 95% CI) | 4.69 [0.51, 42.83] |
| 3 Spondylolisthesis progression | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.00, 2.07] |
| 4 No improvement in walking distance | 1 | 39 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.06, 2.21] |
| 5 Good result at 18‐24 months | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 4.41 [1.09, 17.76] |
| 6 No spondylolisthesis progression | 2 | 93 | Odds Ratio (M‐H, Random, 95% CI) | 11.53 [0.48, 275.52] |
3.1. Analysis.
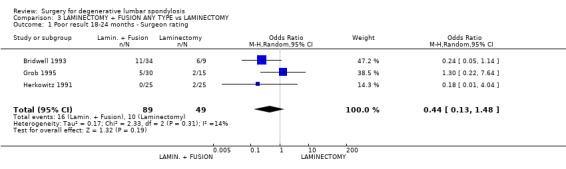
Comparison 3 LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY, Outcome 1 Poor result 18‐24 months ‐ Surgeon rating.
3.2. Analysis.
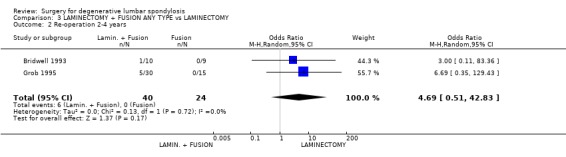
Comparison 3 LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY, Outcome 2 Re‐operation 2‐4 years.
3.3. Analysis.
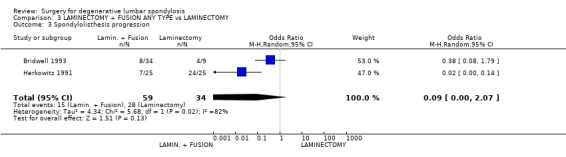
Comparison 3 LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY, Outcome 3 Spondylolisthesis progression.
3.4. Analysis.
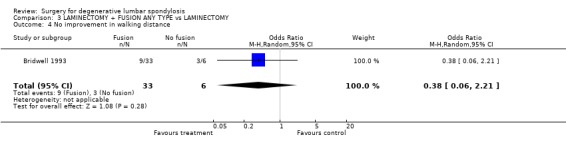
Comparison 3 LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY, Outcome 4 No improvement in walking distance.
3.5. Analysis.
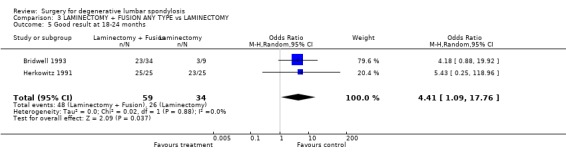
Comparison 3 LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY, Outcome 5 Good result at 18‐24 months.
3.6. Analysis.
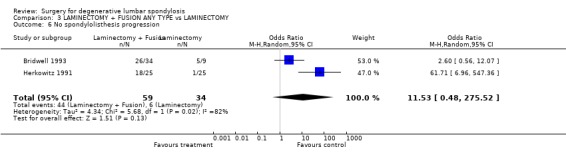
Comparison 3 LAMINECTOMY + FUSION ANY TYPE vs LAMINECTOMY, Outcome 6 No spondylolisthesis progression.
Comparison 4. LAMINECTOMY PLUS MULTI‐LEVEL FUSION vs LAMINECTOMY.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor result as rated by patient ‐ at 2yrs | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 5.74 [0.25, 130.37] |
| 2 Poor result as rated by independent assessor ‐ at 2yrs | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 8.68 [0.41, 184.28] |
| 3 Re‐operation by 28mths | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 3.21 [0.12, 85.20] |
4.1. Analysis.
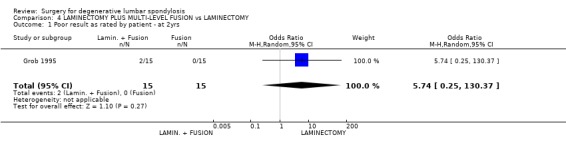
Comparison 4 LAMINECTOMY PLUS MULTI‐LEVEL FUSION vs LAMINECTOMY, Outcome 1 Poor result as rated by patient ‐ at 2yrs.
4.2. Analysis.
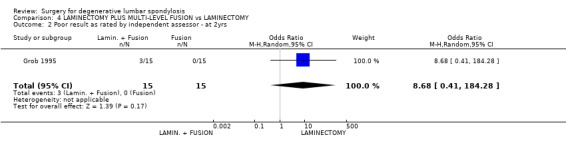
Comparison 4 LAMINECTOMY PLUS MULTI‐LEVEL FUSION vs LAMINECTOMY, Outcome 2 Poor result as rated by independent assessor ‐ at 2yrs.
4.3. Analysis.
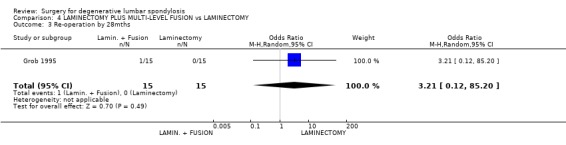
Comparison 4 LAMINECTOMY PLUS MULTI‐LEVEL FUSION vs LAMINECTOMY, Outcome 3 Re‐operation by 28mths.
Comparison 5. LAMINECTOMY vs NO LAMINECTOMY (Isthmic spondylolisthesis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No fusion at 4.5yrs | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 15.21 [0.76, 303.32] |
| 2 No success ‐ Patient rating at 4.5yrs | 1 | 42 | Odds Ratio (M‐H, Random, 95% CI) | 11.5 [1.24, 106.85] |
5.1. Analysis.
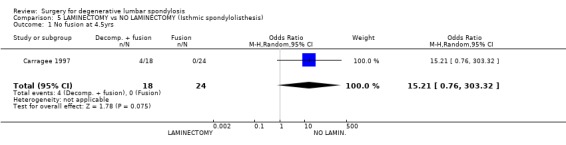
Comparison 5 LAMINECTOMY vs NO LAMINECTOMY (Isthmic spondylolisthesis), Outcome 1 No fusion at 4.5yrs.
5.2. Analysis.
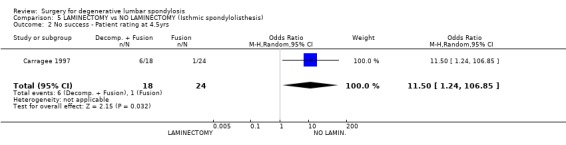
Comparison 5 LAMINECTOMY vs NO LAMINECTOMY (Isthmic spondylolisthesis), Outcome 2 No success ‐ Patient rating at 4.5yrs.
Comparison 6. LAMINECTOMY PLUS ONE LEVEL FUSION (No instrumentation, spinal stenosis + degen spondylolisthesis vs LAMINECT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor result as rated by surgeon ‐ at 36 mths (ave) | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.01, 4.04] |
| 2 Spondylolisthesis progression at 6 months | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 4.67 [0.67, 32.36] |
| 3 Re‐operation required within 4 years | 1 | 19 | Odds Ratio (M‐H, Random, 95% CI) | 3.00 [0.11, 83.36] |
6.1. Analysis.
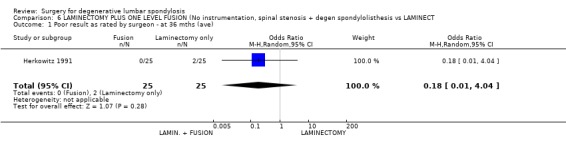
Comparison 6 LAMINECTOMY PLUS ONE LEVEL FUSION (No instrumentation, spinal stenosis + degen spondylolisthesis vs LAMINECT, Outcome 1 Poor result as rated by surgeon ‐ at 36 mths (ave).
6.2. Analysis.
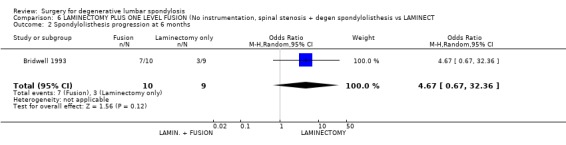
Comparison 6 LAMINECTOMY PLUS ONE LEVEL FUSION (No instrumentation, spinal stenosis + degen spondylolisthesis vs LAMINECT, Outcome 2 Spondylolisthesis progression at 6 months.
6.3. Analysis.
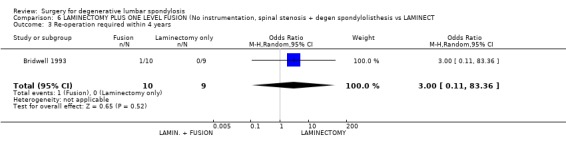
Comparison 6 LAMINECTOMY PLUS ONE LEVEL FUSION (No instrumentation, spinal stenosis + degen spondylolisthesis vs LAMINECT, Outcome 3 Re‐operation required within 4 years.
Comparison 7. LUMBAR FUSION vs CONSERVATIVE (PHYSICAL) THERAPY.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fair or Poor outcome (independent observer rated) | 1 | 262 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.13, 0.52] |
| 2 Not back to work at 2 years | 1 | 208 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.10, 0.64] |
| 3 Unchanged / worse at two years (patient rating) | 1 | 257 | Odds Ratio (M‐H, Random, 95% CI) | 0.28 [0.15, 0.53] |
7.1. Analysis.
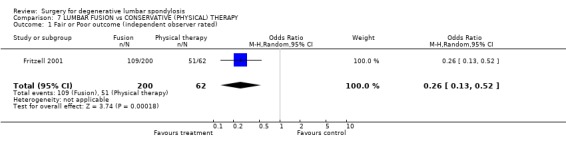
Comparison 7 LUMBAR FUSION vs CONSERVATIVE (PHYSICAL) THERAPY, Outcome 1 Fair or Poor outcome (independent observer rated).
7.2. Analysis.
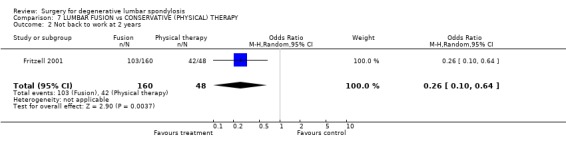
Comparison 7 LUMBAR FUSION vs CONSERVATIVE (PHYSICAL) THERAPY, Outcome 2 Not back to work at 2 years.
7.3. Analysis.
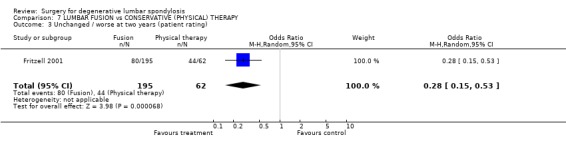
Comparison 7 LUMBAR FUSION vs CONSERVATIVE (PHYSICAL) THERAPY, Outcome 3 Unchanged / worse at two years (patient rating).
Comparison 8. LUMBAR FUSION vs COGNITIVE EXERCISES (Degenerate disc).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure (patient rating) at 1 year | 1 | 61 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.25, 2.25] |
| 2 Failure (independent assessor) at 1 year | 2 | 63 | Odds Ratio (M‐H, Random, 95% CI) | 1.53 [0.48, 4.87] |
8.1. Analysis.
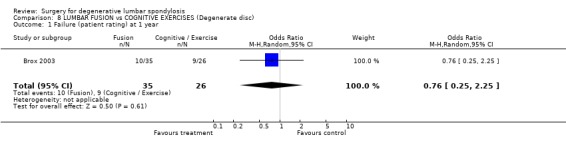
Comparison 8 LUMBAR FUSION vs COGNITIVE EXERCISES (Degenerate disc), Outcome 1 Failure (patient rating) at 1 year.
8.2. Analysis.
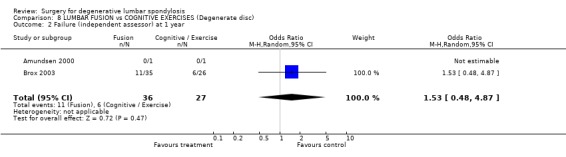
Comparison 8 LUMBAR FUSION vs COGNITIVE EXERCISES (Degenerate disc), Outcome 2 Failure (independent assessor) at 1 year.
Comparison 9. INSTRUMENTED FUSION vs COGNITIVE EXERCISES (Post discectomy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure (patient rating) | 1 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.38, 3.03] |
| 2 Failure (Independent observer rating) | 1 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.49, 4.08] |
9.1. Analysis.
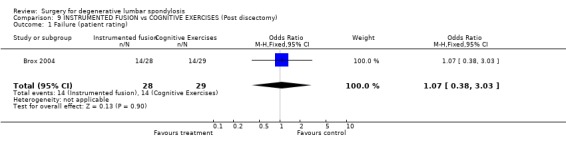
Comparison 9 INSTRUMENTED FUSION vs COGNITIVE EXERCISES (Post discectomy), Outcome 1 Failure (patient rating).
9.2. Analysis.
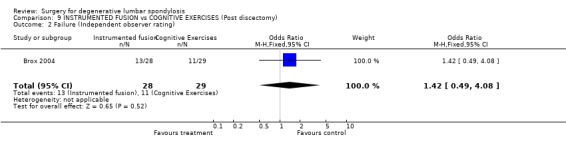
Comparison 9 INSTRUMENTED FUSION vs COGNITIVE EXERCISES (Post discectomy), Outcome 2 Failure (Independent observer rating).
Comparison 10. POSTERO‐LATERAL FUSION +/‐ INSTRUMENTATION vs EXERCISE THERAPY (Isthmic spondylolisthesis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sick leave post treatment | 1 | 106 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.46, 2.46] |
| 2 Failure ‐ patient rating | 1 | 109 | Odds Ratio (M‐H, Random, 95% CI) | 0.23 [0.10, 0.53] |
| 3 Failure ‐ Assessor rating | 1 | 109 | Odds Ratio (M‐H, Random, 95% CI) | 0.09 [0.03, 0.23] |
10.1. Analysis.
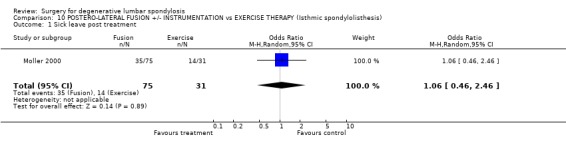
Comparison 10 POSTERO‐LATERAL FUSION +/‐ INSTRUMENTATION vs EXERCISE THERAPY (Isthmic spondylolisthesis), Outcome 1 Sick leave post treatment.
10.2. Analysis.
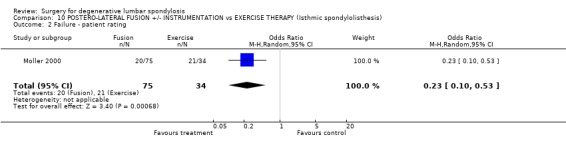
Comparison 10 POSTERO‐LATERAL FUSION +/‐ INSTRUMENTATION vs EXERCISE THERAPY (Isthmic spondylolisthesis), Outcome 2 Failure ‐ patient rating.
10.3. Analysis.
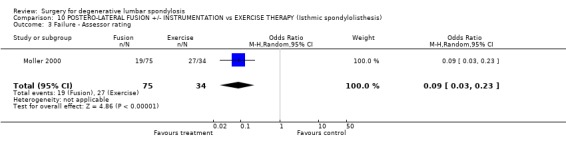
Comparison 10 POSTERO‐LATERAL FUSION +/‐ INSTRUMENTATION vs EXERCISE THERAPY (Isthmic spondylolisthesis), Outcome 3 Failure ‐ Assessor rating.
Comparison 11. INSTRUMENTED FUSION vs LAMINECTOMY (mixed, single/multi‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor result as rated by patient ‐ at >2yrs | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 1.3 [0.22, 7.64] |
| 2 Poor result at 2yrs ‐ surgeon rating | 2 | 113 | Odds Ratio (M‐H, Random, 95% CI) | 1.96 [0.63, 6.16] |
| 3 Re‐operation at 28mths average | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 6.69 [0.35, 129.43] |
| 4 Spondylolisthesis progression | 1 | 33 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.60] |
11.1. Analysis.
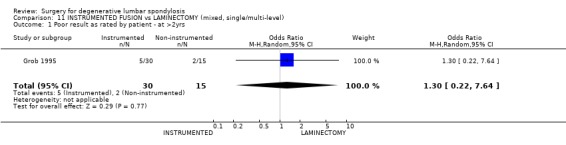
Comparison 11 INSTRUMENTED FUSION vs LAMINECTOMY (mixed, single/multi‐level), Outcome 1 Poor result as rated by patient ‐ at >2yrs.
11.2. Analysis.
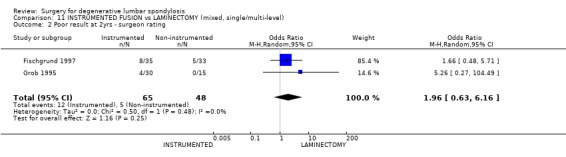
Comparison 11 INSTRUMENTED FUSION vs LAMINECTOMY (mixed, single/multi‐level), Outcome 2 Poor result at 2yrs ‐ surgeon rating.
11.3. Analysis.
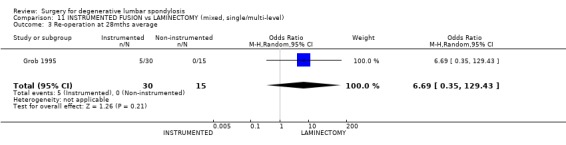
Comparison 11 INSTRUMENTED FUSION vs LAMINECTOMY (mixed, single/multi‐level), Outcome 3 Re‐operation at 28mths average.
11.4. Analysis.
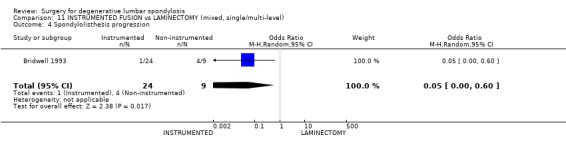
Comparison 11 INSTRUMENTED FUSION vs LAMINECTOMY (mixed, single/multi‐level), Outcome 4 Spondylolisthesis progression.
Comparison 12. INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fair/Poor outcome at 1 ‐ 2yr ‐ Surgeon rating | 3 | 193 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.08, 4.26] |
| 2 2nd procedure by 2yrs | 7 | 494 | Odds Ratio (M‐H, Random, 95% CI) | 1.05 [0.40, 2.73] |
| 3 No fusion at 2 yrs | 8 | 638 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.21, 0.91] |
| 4 Poor clinical outcome | 8 | 653 | Odds Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.84] |
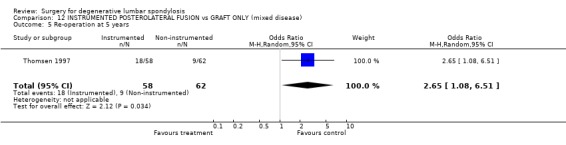
| 5 Re‐operation at 5 years | 1 | 120 | Odds Ratio (M‐H, Random, 95% CI) | 2.65 [1.08, 6.51] |
| 6 Pain score at 5 years | 1 | 109 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.12, 1.18] |
12.1. Analysis.
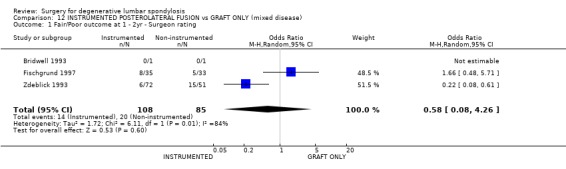
Comparison 12 INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease), Outcome 1 Fair/Poor outcome at 1 ‐ 2yr ‐ Surgeon rating.
12.2. Analysis.
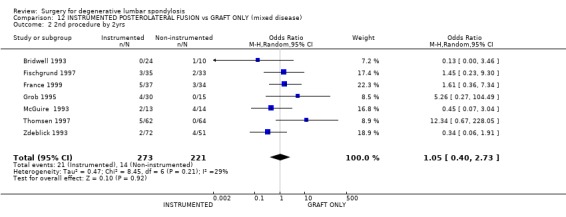
Comparison 12 INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease), Outcome 2 2nd procedure by 2yrs.
12.3. Analysis.
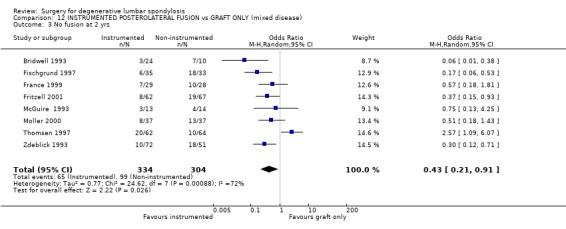
Comparison 12 INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease), Outcome 3 No fusion at 2 yrs.
12.4. Analysis.
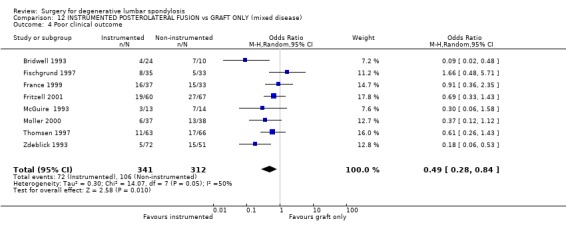
Comparison 12 INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease), Outcome 4 Poor clinical outcome.
12.5. Analysis.
Comparison 12 INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease), Outcome 5 Re‐operation at 5 years.
12.6. Analysis.
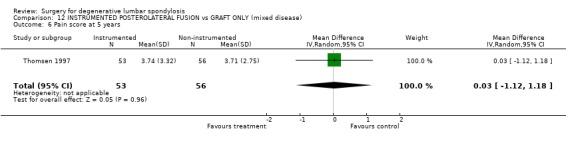
Comparison 12 INSTRUMENTED POSTEROLATERAL FUSION vs GRAFT ONLY (mixed disease), Outcome 6 Pain score at 5 years.
Comparison 13. INSTRUMENTED FUSION vs NON‐INSTRUMENTED FUSION (Isthmic spondylolisthesis).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure ‐ Patient rating at 2 yr | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 0.37 [0.12, 1.12] |
| 2 Failure ‐ Assessor rating | 1 | 75 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.25, 1.92] |
| 3 Failed fusion (definitely not solid) | 1 | 74 | Odds Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.43] |
13.1. Analysis.
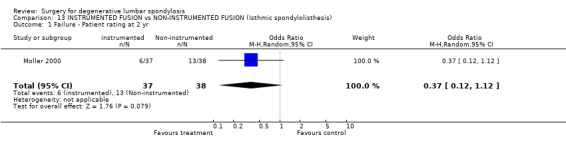
Comparison 13 INSTRUMENTED FUSION vs NON‐INSTRUMENTED FUSION (Isthmic spondylolisthesis), Outcome 1 Failure ‐ Patient rating at 2 yr.
13.2. Analysis.
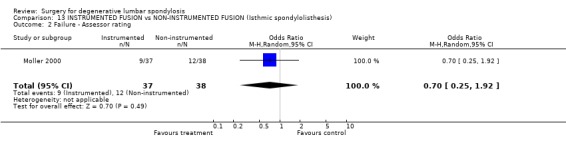
Comparison 13 INSTRUMENTED FUSION vs NON‐INSTRUMENTED FUSION (Isthmic spondylolisthesis), Outcome 2 Failure ‐ Assessor rating.
13.3. Analysis.
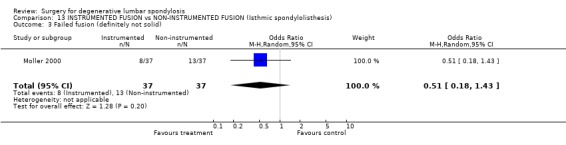
Comparison 13 INSTRUMENTED FUSION vs NON‐INSTRUMENTED FUSION (Isthmic spondylolisthesis), Outcome 3 Failed fusion (definitely not solid).
Comparison 14. INTERBODY FUSION + POSTEROLATERAL FUSION vs POSTERLATERAL FUSION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fusion failure | 2 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.08 [0.51, 2.29] |
| 2 Complications | 2 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.24, 4.17] |
| 3 Not much better | 1 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.59, 2.33] |
| 4 Re‐operation | 1 | 139 | Odds Ratio (M‐H, Random, 95% CI) | 0.25 [0.09, 0.74] |
14.1. Analysis.
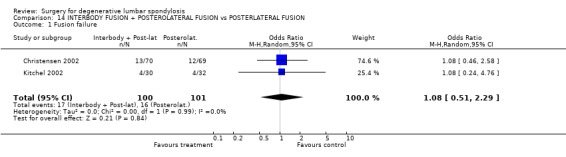
Comparison 14 INTERBODY FUSION + POSTEROLATERAL FUSION vs POSTERLATERAL FUSION, Outcome 1 Fusion failure.
14.2. Analysis.
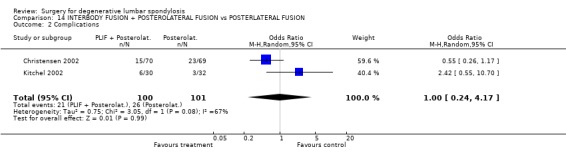
Comparison 14 INTERBODY FUSION + POSTEROLATERAL FUSION vs POSTERLATERAL FUSION, Outcome 2 Complications.
14.3. Analysis.
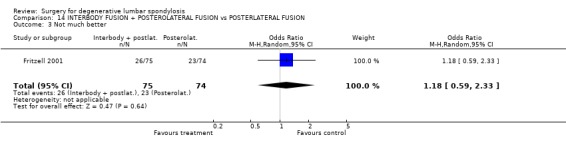
Comparison 14 INTERBODY FUSION + POSTEROLATERAL FUSION vs POSTERLATERAL FUSION, Outcome 3 Not much better.
14.4. Analysis.
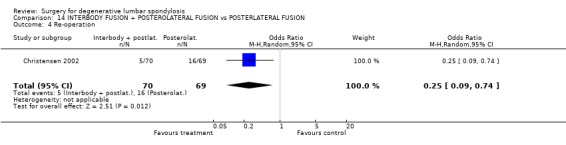
Comparison 14 INTERBODY FUSION + POSTEROLATERAL FUSION vs POSTERLATERAL FUSION, Outcome 4 Re‐operation.
Comparison 15. ALIF PLUS POSTEROLATERAL INSTRUMENTED vs ALIF plus INSTRUMENTED.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fusion failure | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 2.35 [0.40, 13.90] |
| 2 Re‐operation | 1 | 48 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.28, 2.96] |
15.1. Analysis.
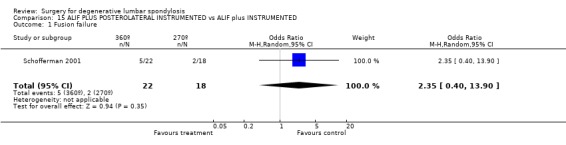
Comparison 15 ALIF PLUS POSTEROLATERAL INSTRUMENTED vs ALIF plus INSTRUMENTED, Outcome 1 Fusion failure.
15.2. Analysis.
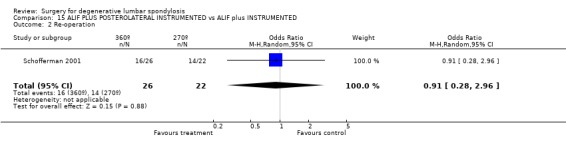
Comparison 15 ALIF PLUS POSTEROLATERAL INSTRUMENTED vs ALIF plus INSTRUMENTED, Outcome 2 Re‐operation.
Comparison 16. GRAF LIGAMENTOPLASTY vs ANTERIOR LUMBAR CAGED FUSION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Re‐operation | 1 | 56 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.24] |
16.1. Analysis.
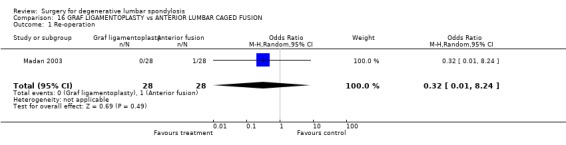
Comparison 16 GRAF LIGAMENTOPLASTY vs ANTERIOR LUMBAR CAGED FUSION, Outcome 1 Re‐operation.
Comparison 17. ANTERIOR THREADED CAGE vs FEMORAL RING FUSION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of fusion | 1 | 118 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.15] |
| 2 Secondary procedure | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.18, 0.76] |
17.1. Analysis.
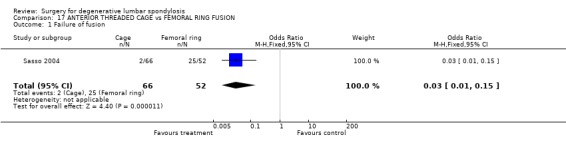
Comparison 17 ANTERIOR THREADED CAGE vs FEMORAL RING FUSION, Outcome 1 Failure of fusion.
17.2. Analysis.
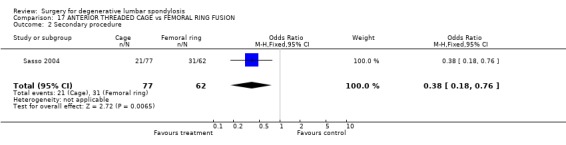
Comparison 17 ANTERIOR THREADED CAGE vs FEMORAL RING FUSION, Outcome 2 Secondary procedure.
Comparison 18. IDET vs SHAM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No success (observer rated) ‐ at 8 weeks | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 1.85 [0.15, 23.07] |
18.1. Analysis.
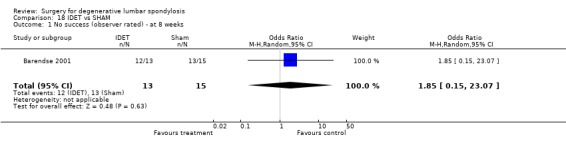
Comparison 18 IDET vs SHAM, Outcome 1 No success (observer rated) ‐ at 8 weeks.
Comparison 19. ANY FORM OF ELECTRICAL STIMULATION vs PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Failure of fusion with internal fixation | 3 | 290 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.30] |
| 2 Failure of fusion without internal fixation | 3 | 268 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.22, 0.64] |
| 3 Poor clincical outcome | 3 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 0.58 [0.27, 1.24] |
19.1. Analysis.
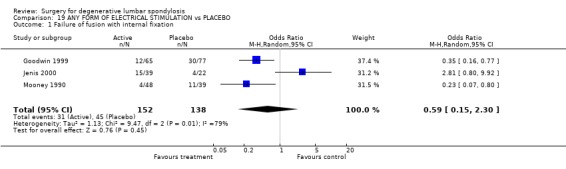
Comparison 19 ANY FORM OF ELECTRICAL STIMULATION vs PLACEBO, Outcome 1 Failure of fusion with internal fixation.
19.2. Analysis.
Comparison 19 ANY FORM OF ELECTRICAL STIMULATION vs PLACEBO, Outcome 2 Failure of fusion without internal fixation.
19.3. Analysis.
Comparison 19 ANY FORM OF ELECTRICAL STIMULATION vs PLACEBO, Outcome 3 Poor clincical outcome.
Comparison 20. X‐STOP INTERSPINOUS IMPLANT vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary surgery | 1 | 196 | Odds Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.73] |
| 2 Moderate or severe pain | 1 | 167 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.29] |
20.1. Analysis.
Comparison 20 X‐STOP INTERSPINOUS IMPLANT vs CONTROL, Outcome 1 Secondary surgery.
20.2. Analysis.
Comparison 20 X‐STOP INTERSPINOUS IMPLANT vs CONTROL, Outcome 2 Moderate or severe pain.
Comparison 21. CHARITE DISC REPLACEMENT vs BAK ANTERIOR INTERBODY FUSION.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oswestry Disability Index at 2 years | 1 | 258 | Mean Difference (IV, Random, 95% CI) | ‐4.30 [‐10.28, 1.68] |
| 2 VAS‐pain | 1 | 258 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐13.71, 2.31] |
| 3 Device failure | 1 | 304 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.32, 2.45] |
21.1. Analysis.
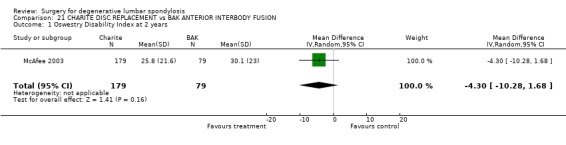
Comparison 21 CHARITE DISC REPLACEMENT vs BAK ANTERIOR INTERBODY FUSION, Outcome 1 Oswestry Disability Index at 2 years.
21.2. Analysis.
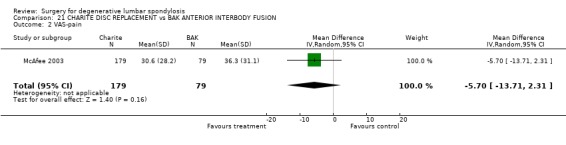
Comparison 21 CHARITE DISC REPLACEMENT vs BAK ANTERIOR INTERBODY FUSION, Outcome 2 VAS‐pain.
21.3. Analysis.
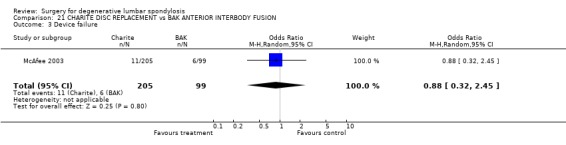
Comparison 21 CHARITE DISC REPLACEMENT vs BAK ANTERIOR INTERBODY FUSION, Outcome 3 Device failure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amundsen 2000.
| Methods | Random number table Allocation concealment: B Lost to follow‐up: 3/31 | |
| Participants | 31 participents; 16 m, 15 f; age 21 to 70+ yrs; Lumbar stenosis Oslo, Norway | |
| Interventions | Exp: Decompression Ctl: Orthosis + "back school" | |
| Outcomes | 2nd procedure Pain degree measured at 10 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Barendse 2001.
| Methods | Randomization by computer programme. Allocation concealment: A Double blind Lost to follow‐up: 0/28 | |
| Participants | 28 participants; 10 m, 18 f; age 30 to 65 yrs; Chronic discogenic pain Maastricht, Netherlands | |
| Interventions | Exp: IDET Ctl: Sham | |
| Outcomes | Observer rating measured at 8 wks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bridwell 1993.
| Methods | Randomization method: not stated Allocation concealment: C Blinding: nil Lost to follow‐up: 1/44 at 2 yrs | |
| Participants | 44 participants; 10 m, 34 f; age 44 to 79 yrs; Spinal claudication St. Louis, Missouri | |
| Interventions | Exp: a) Instrumented posterolateral fusion (Steffee system) b) Posterolateral fusion Ctl: No fusion | |
| Outcomes | Spondylolisthesis progression 2nd procedure required Walking distance measured at 2 yrs | |
| Notes | Non‐randomized allocation of patients with radiological instability | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Brox 2003.
| Methods | Centralized randomization Allocation concealment: A Blinded assessor Lost to follow‐up: 3/60 | |
| Participants | 60 participants; age 25 to 60 yrs; Chronic low back pain Oslo, Norway | |
| Interventions | Exp: Posterolateral instrumented fusion (pedicle systems) Ctl: Cognitive intervention / exercises | |
| Outcomes | Patient rating ODI Back pain rating General function score Hopkins symptom check list Waddell's fear avoidance belief questionnaire Work status Analgesic use measured at 1 yr | |
| Notes | Treatment post laminectomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Brox 2004.
| Methods | Block randomization from computer generated list Allocation concealment: A Lost to follow‐up: 3/60 | |
| Participants | 60 participants age 25 to 60 yrs; Chronic low back pain Oslo, Norway | |
| Interventions | Exp: Posterolateral instrumented fusion (pedicle systems) Ctl: Modern rehabilitation programme | |
| Outcomes | Independent observer rating Patient rating ODI measured at 1 yr Work status | |
| Notes | Treatment post discectomy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Carragee 1997.
| Methods | Randomisation method: sealed envelopes containing random numbers. Concealment: A Blinding: nil Lost to follow‐up: 2 at 4.5 yrs | |
| Participants | 42 participants; 26 m, 16 f; age 19 to 51 yrs; Grade I/II isthmic spondylolisthesis. Stanford, California | |
| Interventions | Exp: a) Smokers with instrumented arthrodesis (Texas SRH system) + decompressive laminectomy b) Non‐smokers with graft alone + decompressive laminectomy Ctl: Same groups without decompressive laminectomy | |
| Outcomes | Back pain rating Fusion Patient rating measured at 3 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Christensen 2002.
| Methods | Randomization by consecutively numbered sealed envelopes Allocation concealment: A Lost to follow‐up: 9/146 | |
| Participants | 148 participants; 88 m, 58 f mean age 45, range 20 to 65 yrs; Heterogeneous conditions Aarhus, Denmark | |
| Interventions | Dubousset system Exp: Circumferential fusion with ALIF Brantigan cage plus posterior instrumentation (CD system or transarticular screws) Ctl: Instrumented posterolateral lumbar fusion (CD system) | |
| Outcomes | Dallas pain questionnaire Low back rating scale Work status measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Delamarter 2003.
| Methods | Central randomization ratio 2:1 Allocation concealment: A Lost to follow‐up: 0/53 at 6 months | |
| Participants | 53 participants; 30 m, 25 f age range 19 to 59 yrs; Chronic disc disease Santa Monica, CA. | |
| Interventions | Exp: ProDisc artificial lumbar disc replacement Ctl: Circumferential fusion (anterior femoral ring allograft plus posterior pedicle screw instrumentation and fusion (pedicle system) | |
| Outcomes | ODI VAS Sagittal motion measured at 6 months | |
| Notes | Interim analysis from one center out of US multi‐center trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fischgrund 1997.
| Methods | Randomization method: closed envelope technique Allocation concealment: A Blinding: assessor Lost to follow‐up: 8/76 at 2.4 yrs. | |
| Participants | 76 participants; 17 m, 59 f; age 52 to 86 yrs; Degenerative spondylolisthesis and spinal stenosis Royal Oak, Michigan | |
| Interventions | Exp: Instrumented posterolateral fusion (Steffee system) Ctl: Postero‐lateral fusion only | |
| Outcomes | Back pain scale Leg pain scale Surgeon rating Fusion Progression of spondylolisthesis measured at 2 yrs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
France 1999.
| Methods | Randomization method: not stated Allocation concealment: B Blinding: nil Lost to follow‐up: 12/83 at 40 months | |
| Participants | 83 participants; 58 m, 25 f; age 19 to 76 yrs; Heterogeneous conditions Multicentre‐U.S. | |
| Interventions | Exp: Instrumented posterolateral fusion (Steffee system) Ctl: Postero‐lateral fusion only | |
| Outcomes | Back pain scale Patient rating Fusion measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Freeman 2003.
| Methods | Randomization method: 2:1 Exp:Ctl Allcocation concealment: B Double blind Lost to follow‐up: 2/57 | |
| Participants | 57 participants Adelaide, Australia | |
| Interventions | Exp: IDET Ctl: Sham therapy | |
| Outcomes | LBOS ODI SF‐36 ZDI Modified somatic perceptions questionnaire measured at 6 mos | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Fritzell 2001.
| Methods | Randomization blindly from computer generated list Allocation concealment: A Independent assessor Lost to follow‐up: 5/294 | |
| Participants | 294 participants; 50% m; age 25 to 64 yrs; Chronic low back pain Multicentre, Sweden | |
| Interventions | Exp: Surgical a) Posterolateral fusion b) Instrumented posterolateral fusion (Steffee system) c) Interbody (ALIF or PLIF (autogenous graft) + b) Ctl: Non‐surgical treatment | |
| Outcomes | Patient rating Observer rating Back to work Back pain (VAS) Oswestry disability index Zung depression scale General function score measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Goodwin 1999.
| Methods | Randomization method: not stated Allocation concealment B Blinding: Assessor Lost to follow‐up 158/337 at 1 yr | |
| Participants | 179 participants at follow‐up; 97 m, 82 f; age 21 to 76 yrs; One or two level fusions ‐ PLIF, ALIF or Postero‐lateral type Multi‐centre, New York | |
| Interventions | Exp: Electrical stimulation Ctl: Placebo stimulation | |
| Outcomes | Surgeon rating Radiographic fusion measured at 1 yr | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Grob 1995.
| Methods | Randomization method: quasi by date of admission to hospital Allocation concealment: C Blinding: nil Lost to follow‐up: 0/30 at 28 months | |
| Participants | 45 participants; 21 m, 24 f; age 48 to 87 yrs; Spinal stenosis History + clinical exam + CT scan. Systemic disease excluded. Stenosis Switzerland | |
| Interventions | Exp: Decompression with arthrodesis (both mono + multi‐segmental) Ctl: Decompression without arthrodesis | |
| Outcomes | Patient rating Surgeon rating 2nd Procedure required measured at 28 months (mean) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Herkowitz 1991.
| Methods | Quasi‐randomized: alternately assigned to treatment Alocation concealment: B Blinding: nil Lost to follow‐up: 0/50 at 3 yrs. | |
| Participants | 50 participants; 14 m, 36 f; age 52 to 84 yrs; Degenerative spondylolisthesis Royal Oak, Michigan | |
| Interventions | Exp: Decompression + fusion Ctl: Decompression | |
| Outcomes | Back pain scale Leg pain scale Surgeon rating Fusion Progression of spondylolisthesis measured at 3 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Jenis 2000.
| Methods | Computer generated randomization Allocation concealment: B Lost to follow‐up: 0/61 | |
| Participants | 61 participants; 32 m, 29 f; age 18 to 75 yrs Boston, Ma; Patients requiring posterolateral fusion | |
| Interventions | Exp: Pulsed electromagnetic field therapy (external coil) Exp. 2: Direct current (implanted electrode) Ctl: Nil | |
| Outcomes | Fusion failure Clinical outcome measured at 1 yr | |
| Notes | Also measurements of fusion mass | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kitchel 2002.
| Methods | Randomization method: not stated Allocation concealment: C | |
| Participants | 62 participants; Degenerative spondylolisthesis | |
| Interventions | Exp: Instrumented posterolateral (pedicle system) and posterior interbody fusin (autogenous graft) Ctl: Instrumented posterolateral fusion | |
| Outcomes | Fusion Surgery time Blood loss Intraoperative complications Oswestry disability index change measured at 2 yrs | |
| Notes | Abstract of data Insufficient for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Linovitz 2002.
| Methods | Randomized from computer generated randomisation code provided by independent third party. Allocation concealment: A Lost to follow‐up: 42/243 | |
| Participants | 243 participants; mean age 57 yrs Heterogeneous group requiring fusion without instrumentation Multicentre, U.S. | |
| Interventions | Exp: Active stimulation 30 min/day for 2 months Ctl: Sham stimulation | |
| Outcomes | Fusion by CT and/or lateral Flex/Ext radiographs measured at 9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Madan 2003.
| Methods | Randomization from chits drawn from box Allocation concealment: A Loss to follow‐up: 0/55 | |
| Participants | 55 participants; 29 m, 26 f; age range 25 to 70 yrs Southampton, UK | |
| Interventions | Exp: Ligamentoplasty (Graf system) Ctl: Anterior lumbar interbody fusion with Hartshill horshoe cage | |
| Outcomes | VAS ODI Re‐operation measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McAfee 2003.
| Methods | Random number generator in ratio 2:1 Allocation concealment: A Lost to follow‐up: 0/60 | |
| Participants | 60 participants, 30 m, 30 f; age range 21 to 56 yrs Baltimore, MD | |
| Interventions | Exp: SB Charite artificial lumbar disc replacement Ctl: BAK anterior interbody fusion | |
| Outcomes | ODI Re‐operation measured at a mean of 2 yrs | |
| Notes | Pilot study only. Further data published by Geisler (see references and analysis tables) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
McGuire 1993.
| Methods | Randomization method: not stated Allocation concealment: B Blinding: nil Lost to follow‐up: 1/27 at 2 yrs | |
| Participants | 27 participants; 23 m, 4 f; age 24 to 42 yrs; Symptomatic grade I to II spondylolisthesis refratory to conservative care. All treated with laminectomy + nerve root decompression Multi‐centre, U.S. | |
| Interventions | Exp: Postero‐lateral fusion (Steffee system) Ctl: Posterolateral fusion only | |
| Outcomes | Fusion, 2nd Procedure required measured at 2 yrs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Moller 2000.
| Methods | Randomization method: blindly selected choice of three. Allocation concealment: A Not blind. Lost to follow‐up 8/114 randomised | |
| Participants | 111 participants; 57 m, 54 f ; 18 to 55 yrs; Isthmic spondylolisthesis of all grades Linkoping, Sweden | |
| Interventions | Exp.1: Instrumented posterolateral fusion (CD system) Exp.2: Posterolateral fusion in situ Ctl: Exercise programme | |
| Outcomes | Disability rating index Pain score Assessor rating Fusion (operated pts.) Patient rating Return to work measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mooney 1990.
| Methods | Randomization method: not stated Allocation concealment: B Blinding: double Lost to follow‐up: 11/206 at 1 yr. | |
| Participants | 195 participants Individuals undergoing initial attempts at interbody spinal fusion (anterior or posterior approach) Irvine, California | |
| Interventions | Exp: Electromagnetic brace ‐ 8 hrs/day Ctl: Placebo brace ‐ 8 hrs/day | |
| Outcomes | Surgeons rating measured at 1 yr | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pauza 2004.
| Methods | Computer generated random numbers Allocation concealment: A Blinding: double Lost to follow‐up: 8/64 | |
| Participants | 64 participants from a potential of 1360 Age 18 to 65 yrs; Chronic low back pain Tyler, Texas | |
| Interventions | Exp: IDET to 90ºC Ctl: Sham generator 16.5 minutes | |
| Outcomes | VAS ODI SF‐36 measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Postacchini 1993.
| Methods | Randomization method: Allocation by pathology and then assigned alternately Allocation concealment: C Blinding: nil Lost to follow up 3/70 at 3.7 yrs. | |
| Participants | 70 participants; 34 m, 36 f; age 43 to 79 yrs; Central lumbar stenosis Rome, Italy | |
| Interventions | Exp: Multiple laminotomy Ctl: Laminectomy | |
| Outcomes | Patient rating Surgeon rating Progression of spondylolisthesis Operating time & Blood loss measured at 3.7 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Sasso 2004.
| Methods | Randomization method not stated. Allocation concealment: B Blinding: nil Lost to follow‐up:22/140 at 2 yrs | |
| Participants | 140 participants; 63 m, 76 f; 18 to 64 yrs; Degenerative disc disease Multicentre, U.S. | |
| Interventions | Exp: Threaded fusion device (Interfix system) Ctl: Femoral ring allograft filled with autogenous iliac crest bone | |
| Outcomes | Radiographic fusion ODI SF‐36 measured at 2 yrs Repeat surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schofferman 2001.
| Methods | Randomization by odd and even file numbers. Allocation concealment: C Lost to follow‐up: 5/53 | |
| Participants | 53 participants; 27 m, 21 f; mean age 42 yrs; Heterogeneous groups requiring fusion Daly City, California | |
| Interventions | Exp: 360º fusion (TSRH system plus allograft ring, plus autogenous posterolateral graft) Ctl: 270º fusion (as above no posterolateral graft) | |
| Outcomes | Pain Oswestry disability index Costs Fusion Re‐operation measured at a mean of 35 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Thomsen 1997.
| Methods | Randomization from consecutively numbered closed envelopes Allocation concealment: A Blinding: assessor Lost to follow‐up: 3/129 at 2 yrs. | |
| Participants | 130 participants; 60 m, 69 f; age 20 to 67 yrs; Chronic low back pain Aarhus, Denmark | |
| Interventions | Exp: Instrumented posterolateral fusion (CD system) Ctl: Postero‐lateral fusion | |
| Outcomes | Functional scale Patient rating Fusion 2nd Procedure required measured at 2 yrs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zdeblick 1993.
| Methods | Random number generator Allocation concealment: C Blinding: nil Lost to follow‐up: 1/124 | |
| Participants | 124participants; age 20 to 80 yrs; Heterogeneous conditions Wisconsin, US | |
| Interventions | Exp: a) Instrumented posterolateral fusion (rigid TSRH system b) Instrumented posterolateral fusion (semi‐rigid Luque II instrumentation Ctl: Posterolateral fusion only | |
| Outcomes | Surgeons rating 2nd procedure required measured at a mean of 16 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zigler 2003.
| Methods | Randomized from central office Allocation concealment: A Blinding of patient and relatives until surgery. Independent assessor blinded Lost to follow‐up: 0/39 | |
| Participants | 39 participants from 1 of 19 participating centers ‐ ratio 28 Exp. to 11Ctl; age 18 to 60 yrs; Degenerative disc disease with primarily back and/or radicular pain Plano, Texas | |
| Interventions | Exp: ProDisc implant Ctl: 360 degree fusion ‐ anterior biconvex‐shaped femoral ring allograft inserted with a 6.5mm retaining cancellous screw to prevent anterior migration plus posterior instrumented fusion (unilateral single level, bilateral two level) | |
| Outcomes | VAS ODI Patient satisfaction on VAS scale Work status (unclear) measured at 6 months | |
| Notes | Results from 1 of 19 centers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zucherman 2004.
| Methods | Block randomization by centre Allocation concealment: A Blinding: nil Lost to follow‐up: 22/200 at 1 yr | |
| Participants | 200 pts; mean age 69 yrs Lumbar spinal stenosis Multi‐centre, U.S. | |
| Interventions | Exp. Interspinous spacer (X‐stop system) Ctl: Epidural injection, NSAIDs, Analgesics, Physical therapy | |
| Outcomes | SF‐36 Zurich claudication questionnaire measured at 1 yr | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Exp = Experimental Ctl = Control m = male f = female yrs = years wks = weeks ODI ‐ Oswestry Disability Index PLIF = posterior lumbar interbody fusion ALIF = anterior lumbar interbody fusion CD system = Cotrel‐Dubousset instrumentation ZCI = Zurich claudication questionnaire
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boden 2002 | Study of human bone morphogenetic protein |
| Christensen 2003 | Study of rehabilitation following surgery |
| Emery 1995 | No subsequent data produced following abstract in 1995 |
| Ercelen 2003 | Comparison of two durations of intradiscal radiofrequency thermocoagulation |
| Gibson S 2002 | Study of method of achieving better fusion (allograft versus autograft) |
| Johnsson 2002 | Study of osteogenic protein‐1 |
| Khot 2004 | Medical treatment for discogenic back pain (steroid vs. saline placebo injection). |
| Korovessis 2003 | Randomly selected group of patients divided according to their surgery |
| Laine 2000 | Study of technique ‐ computer assistance in placement of pedicle screws |
| McAfee 2002 | Study of varying surgical technique |
| Moore 1995 | Initial report of 8 patients published in 1995. No complete data. |
| North 1995 | Preliminary data comparing the effectiveness of an implanted electrode to diminish persistent radicular and back pain after lumbosacral spine surgery with reoperation |
| Rogozinski 1995 | No further data published after abstract in 1995 |
| Sachs 2002 | Study of technique |
| Sanden 2002 | Variation of surgical technique ‐ hydroxyapatite coating of pedicle screws |
| Soegaard 2003 | Study of post spinal fusion rehabilitation programmes |
| Transfeldt 2001 | Study of bone morphogenetic proteins |
| von Strempel 1997 | Study of two different screw types |
| Zdeblick 1996 | No subsequent data produced following abstract in 1996 |
| Zhao 2002 | Study of variation within a given procedure |
Characteristics of ongoing studies [ordered by study ID]
Clarke 2003.
| Trial name or title | A Prospective randomised trial comparing femoral ring allograft versus a titanium cage for circumferential spinal fusion: two year functional and radiological outcome |
| Methods | |
| Participants | 62 participants |
| Interventions | Exp: Titanium interbody cage Ctl: Femoral ring allograft |
| Outcomes | ODI VAS SF‐36 |
| Starting date | 2001 |
| Contact information | Centre for spinal surgery, Queens Medical Centre, Nottingham, UK |
| Notes |
Freeman 2005.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Gibson 2003.
| Trial name or title | Spinal fusion in patients with single level degenerate disc disease and neural compression ‐ a prospective randomised study |
| Methods | |
| Participants | 40 participants; age 39 to 74 yrs |
| Interventions | Exp 1: Transforaminal interbody fusion plus instrumented posterolateral fusion Exp 2: Instrumented posterolateral fusion Ctl: Decompression alone |
| Outcomes | Roland & Morris EuroQol SF‐36 DPQ |
| Starting date | 1999 |
| Contact information | j.n.a.gibson@ed.ac.uk |
| Notes |
Ilharreborde 2005.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Malmivaara 2003.
| Trial name or title | Operative treatment for moderately severe lumbar spinal stenosis: a randomized controlled trial |
| Methods | |
| Participants | 94 participants |
| Interventions | Exp: Segmental decompression and undercutting facetectomy Ctl: Conservative treatment ‐ analgesia & self‐administered exercises guided by a physiotherapist |
| Outcomes | VAS ODI Walking ability on a treadmill |
| Starting date | 2001 |
| Contact information | Finnish Institute of Occupational Health, Helsinki |
| Notes |
Whitaker 2005.
| Trial name or title | |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
Alastair Gibson (JNAG) and Gordon Waddell (GW) initiated the review and wrote the protocol. Miss Inga Grant searched for the trials included in the original review and assembled the database of relevant studies. JNAG and GW then selected the trials to be included in the review and assessed their quality prior to publication of the review in 1999, the 2000/3 updates, and this the 3rd Edition of the Review.
Sources of support
Internal sources
No sources of support supplied
External sources
The Medical Research Council 1998‐9, UK.
Declarations of interest
Nil
Edited (no change to conclusions)
References
References to studies included in this review
Amundsen 2000 {published data only}
- Amundsen T, Weber H, Nordal HJ, Magnaes B, Abdelnoor M, Lilleas F. Lumbar spinal stenosis; conservative or surgical management? A prospective 10‐year study. Spine 2000;11:1424‐36. [DOI] [PubMed] [Google Scholar]
Barendse 2001 {published data only}
- Barendse GAM, Berg SGM, Kessels AHF, Weber WEJ, Kleef M. Randomized controlled trial of percutaneous intradiscal radiofrequency thermocoagulation for chronic discogenic back pain. Spine 2001;26:287‐92. [DOI] [PubMed] [Google Scholar]
Bridwell 1993 {published data only}
- Bridwell KH, Sedgewick TA, O'Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord 1993;6(6):461‐72. [DOI] [PubMed] [Google Scholar]
Brox 2003 {unpublished data only}
- Brox JI, Sorensen R, Friis A, Indahl A, Keller A, Reikeras O, et al. Randomised clinical trial of lumbar instrumented fusion and cognitive intervention and exercises of patients with chronic low back pain and disc degeneration. Proceedings of the International Society for Study of the Lumbar Spine. 2003. [DOI] [PubMed]
- Brox JI, Sorensen R, Friis A, Nygaard O, Indahl A, Keller A, et al. Randomized clinical trial of lumbar instrumented fusion and cognitive intervention and exercises in patients with chronic low back pain and disc degeneration. Spine 2003;28(17):1913‐21. [DOI] [PubMed] [Google Scholar]
Brox 2004 {published data only}
- Keller A, Brox JI, Gunderson R, Holm I, Friis A, Reikeras O. Trunk muscle strength, cross‐sectional area and density in patients with chronic low back pain randomized to lumbar fusion or cognitive intervention and exercises. Spine 2004;29(1):3‐8. [DOI] [PubMed] [Google Scholar]
Carragee 1997 {published data only}
- Carragee EJ. Single‐level posterolateral arthrodesis, with or without posterior decompression, for the treatment of isthmic spondylolisthesis in adults. A prospective, randomised study. J Bone Joint Surg 1997;79‐A:1175‐80. [DOI] [PubMed] [Google Scholar]
Christensen 2002 {published data only}
- Bunger C, Eiskjaer S, Hansen ES, Thomsen K, Hoy K, Helmig P, et al. Controversies in lumbar spine fusion ‐ the role of pedicle screw fixation and 360º fusion ‐ randomized prospective studies. Acta Orthop Scand. 1998; Vol. 69:26.
- Bunger C, Hansen ES, Hoy K, Neumann P, Niedermann B, Lindblad BE, et al. Lumbar spine fusion. A randomized prospective study of circumferential fusion with the ALIF Brantigan cage versus posterolateral fusion with titanium CDI. Proceedings of the British Scoliosis Society. 2001.
- Christensen FB, Hansen ES, Eiskjaer SP, Hoy K, Helmig P, Neumann P, et al. Circumferential lumbar spinal fusion with ALIF Brantigan cage versus posterolateral fusion with titanium CD‐Horizon: a prospective, randomized, clinical study of 146 patients. Spine 2002;27(23):2674‐83. [DOI] [PubMed] [Google Scholar]
Delamarter 2003 {published data only}
- Bae H, Pradhan B, Kanim L, Lam L, Kamrava E, Delamarter R. Clinical results after lumbar total disc replacement: A 2 to 3 year report from the United States clinical trial for the ProDisc‐II prosthesis. Proceedings of the International Society for the Study of the Lumbar Spine. 2005:27.
- Delamarter RB, Fribourg DM, Kanim LE, Bae H. ProDisc artificial total lumbar disc replacement: introduction and early results from the United States clinical trial. Spine 2003;28(20S):S167‐75. [DOI] [PubMed] [Google Scholar]
Fischgrund 1997 {published data only}
- Fischgrund J, McKay M, Herkowitz H, Brower R, Montgomery D, Kurz L. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study, comparing decompression and fusion with and without posterior pedicular instrumentation. Orth Trans 1997;21:158. [Google Scholar]
- Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis: A prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine 1997;22:2807‐12. [DOI] [PubMed] [Google Scholar]
France 1999 {published data only}
- France JC, Yaszemski MJ, Lauerman WC, Cain JE, Glover JM, Lawson KJ, et al. A randomized prospective study of posterolateral lumbar fusion: Outcomes with and without pedicle screw instrumentation. Orthop Trans. 1997; Vol. 21:157. [DOI] [PubMed]
- France JC, Yaszemski MJ, Lauerman WC, Cain JE, Glover JM, Lawson KJ, et al. A randomized prospective study of posterolateral lumbar fusion: Outcomes with and without pedicle screw instrumentation. Spine 1999;24:553‐60. [DOI] [PubMed] [Google Scholar]
Freeman 2003 {published data only}
- Freeman BJC, Fraser RD, Cain CMJ, Hall DJ. A randomised double‐blind controlled efficacy study: intradiscal electrothermal therapy (IDET) versus placebo. J Bone Joint Surg. 2003; Vol. 85‐B, issue Supp III:280.
- Freeman BJC, Fraser RD, Cain CMJ, Hall DJ. A randomized double‐blind controlled efficacy study: Intradiscal electrothermal therapy (IDET) versus placebo. Proceedings of the International Society for Study of the Lumbar Spine. 2003:Abstract 11.
Fritzell 2001 {published data only}
- Fritzell P, Hagg O, Jonsson D, Nordwall A, the Swedish Lumbar Spine Study Group. Cost‐effectiveness of lumbar fusion and nonsurgical treatment for chronic low back pain in the Swedish Lumbar Spine Study: A multicenter, randomized, controlled trial from the Swedish Lumbar Spine Study Group. Spine 2004;29(4):421‐34. [DOI] [PubMed] [Google Scholar]
- Fritzell P, Hagg O, Nordwall A, the Swedish Lumbar Spine Group. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study. A report from the Swedish Lumbar Spine Study Group. Eur Spine J 2003;12(2):178‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzell P, Hagg O, Wessberg P, Nordwall A. 2001 Volvo award winner in clinical studies: Lumbar fusion versus nonsurgical treatment for chronic low back pain: A multicentre randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 2001;26:2521‐34. [DOI] [PubMed] [Google Scholar]
Goodwin 1999 {published data only}
- Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double‐blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine 1999;24(13):1349‐57. [DOI] [PubMed] [Google Scholar]
Grob 1995 {published data only}
- Grob D, Humke T, Dvorak J. Degenerative lumbar spinal stenosis. Decompression with and without arthrodesis. J Bone Joint Surg Am 1995;77(7):1036‐41. [DOI] [PubMed] [Google Scholar]
Herkowitz 1991 {published data only}
- Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am 1991;73‐A(6):802‐8. [PubMed] [Google Scholar]
Jenis 2000 {published data only}
- Jenis LG, An HS, Stein R, Young B. Prospective comparison of the effect of direct current electrical stimulation and pulsed electromagnetic fields on instrumented posterolateral lumbar arthrodesis. Journal of Spinal Disorders 2000;13(4):290‐6. [DOI] [PubMed] [Google Scholar]
Kitchel 2002 {published data only}
- Kitchel SH, Matteri RE. Prospective randomized evaluation of PLIF in degenerative spondylolisthesis patients over 60 years old. The Spine Journal 2002;2(2 Suppl):21S. [Google Scholar]
Linovitz 2002 {published data only}
- Linovitz RJ, Pathria M, Bernhardt M, Green D, Law MD, McGuire RA, et al. Combined magnetic fields accelerate and increase spine fusion. A double‐blind, randomized, placebo controlled study. Spine 2002;27:1383‐9. [DOI] [PubMed] [Google Scholar]
Madan 2003 {published data only}
- Madan S, Boeree NR. Outcome of the Graf ligamentoplasty procedure compared with anterior lumbar interbody fusion with the Hartshill horseshoe cage. Eur Spine J 2003;12:361‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McAfee 2003 {published data only}
- Geisler FH, Blumenthal SL, Guyer RD, McAfee PC, Regan JJ, Johnson JP, et al. Neurological complications of lumbar artificial disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: Results of a multicenter, prospective, randomized investigational device exemption study of Charite intervertebral disc. J Neurosurg Spine 2004;1:143‐54. [DOI] [PubMed] [Google Scholar]
- Holt R, Jajd M, Isaza J, Blumnethal S, McAfee P, Guyer R, et al. Complications of lumbar artificial disc replacement vs fusion: Results from the randomized, multicenter FDA IDE study of the Charite artificial disc. Proceedings of the International Society for the Study of the Lumbar Spine.. 2005:31.
- McAfee PC, Fedder IL, Saiedy S, Shucosky EM, Cunningham B. Experimental design of total disk replacement ‐ experience with a prospective randomized study of the SB Charite. Spine 2003;28(20S):S153‐62. [DOI] [PubMed] [Google Scholar]
- Whitaker C, Hochschuler S, Ohnmeiss D, Zigler J, Guyer R, Sachs B, et al. Proceedings of the International Society for the Study of the Lumbar Spine. 2005:68.
McGuire 1993 {published data only}
- McGuire RA, Amundson GM. The use of primary internal fixation in spondylolisthesis. Spine 1993;18(12):1662‐72. [DOI] [PubMed] [Google Scholar]
Moller 2000 {published data only}
- Moller H, Hedlund R. [Surgery versus conservative treatment in adult isthmic spondylolisthesis. A prospective randomized study]. Thesis: Karolinska Institute. Stockholm: Kongl Carolinska Medico Chirurgiska Institute, 1999. [ISBN 91‐628‐3834‐2] [Google Scholar]
- Moller H, Hedlund R. Surgery versus conservative management in adult isthmic spondylolisthesis. Spine 2000;25(13):1711‐5. [DOI] [PubMed] [Google Scholar]
- Moller H, Hedlund R. Surgery vs. conservative treatment in adult spondylolisthesis ‐ a prospective randomized study. Acta Orthop Scand. 1998; Vol. 69, issue Suppl. 280:13.
- Moller H, Hedlund R. Surgery vs. conservative treatment in adult spondylolisthesis. A prospective randomised study. Orthop Trans. 1996; Vol. 20, issue 2:390.
Mooney 1990 {published data only}
- Mooney V. A randomized double‐blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine 1990;15(7):708‐12. [DOI] [PubMed] [Google Scholar]
Pauza 2004 {published data only}
- Pauza K, Howell S, Dreyfuss P, Peloza J, Park K. A randomized, double‐blind, placebo controlled trial evaluating the efficacy of intradiscal electrothermal anuloplasty (IDET) for the treatment of chronic discogenic low back pain: 6‐month outcomes. Proceedings of the International Spinal Injections Society. 2002.
- Pauza KJ, Howell S, Dreyfuss P, Peloza JH, Dawson K, Bogduk N. A randomized, placebo‐controlled trail of intradiscal electrotermal therapy for the treatment of discogenic low back pain. Spine Journal 2004;4(1):27‐35. [DOI] [PubMed] [Google Scholar]
Postacchini 1993 {published data only}
- Postacchini F, Cinotti G, Perugia D, Gumina S. The surgical treatment of central lumbar stenosis. J Bone Joint Surg 1993;75‐B:386‐92. [DOI] [PubMed] [Google Scholar]
Sasso 2004 {published data only}
- Sasso RC, Kitchel SH, Dawson EG. A prospective, randomized controlled clinical trial of anterior lumbar interbody fusion using a titanium cylindrical threaded fusion device. Spine 2004;29(2):113‐22. [DOI] [PubMed] [Google Scholar]
Schofferman 2001 {published data only}
- Schofferman J, Slosar P, Reynolds J, Goldthwaite N, Koestler M. A prospective randomized comparison of 270º fusions to 360º fusions (circumferential fusions). Spine (electronic) 2001;26(10):E207‐12. [DOI] [PubMed] [Google Scholar]
Thomsen 1997 {published data only}
- Andersen T, Christensen FB, Hansen ES, Bunger C. Pain 5 years after instrumented and non‐instrumented posterolateral lumbar spinal fusion. Eur Spine J 2003;12:393‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen FB, Hansen ES, Laursen M, Thomsen K, Bunger CE. Long‐term functional outcome of pedicle screw instrumentation as a support for posterolateral spinal fusion. Randomized clinical study with a 5‐year follow‐up. Spine 2002;27(12):1269‐77. [DOI] [PubMed] [Google Scholar]
- Korsgaard M, Christensen FB, Thomsen K, Hansen ES, Bonger C. The effect of pedicle screw instrumentation on lordosis in lumbar spinal fusion. J Bone Joint Surg. 1999; Vol. 81‐B, issue Suppl. II:188.
- Thomsen K, Christensen FB, Eiskjaer SP, Hansen ES, Fruensgaard S, Bunger CE. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion. A prospective randomized clinical study. Spine 1997;22:2813‐22. [DOI] [PubMed] [Google Scholar]
- Thomsen K, Eiskjaer S, Hansen ES, Fruensgaard S, Christensen FB, Bunger C. Lumbar posterolateral fusion ‐ the consequences of pedicle screw instrumentation. Acta Orthop Scand. 1996; Vol. 67:48.
Zdeblick 1993 {published data only}
- Zdeblick TA. A prospective, randomized study of lumbar fusion. Preliminary results [see comments]. Spine 1993;18(8):983‐91. [DOI] [PubMed] [Google Scholar]
- Zdeblick TA, Ulschmid S. An outcomes and cost analysis of pedicle screw fusions. Orthop Trans 1996;20:362‐3. [Google Scholar]
Zigler 2003 {published data only}
- Whitaker C, Hochschuler SH, Ohnmeiss DD, Zigler JE, Guyer RD, Sachs BL, et al. Return to work following total disc replacement vs. fusion: A prospective randomized, comparison study. Proceedings of the International Society for Surgery of the Lumbar Spine. 2005:68.
- Zigler JE, Burd TA, Vialle EN, Sachs BL, Rashbaum RF, Ohnmeiss DD. Lumbar spine arthroplasty. Early results using the ProDisc II: A prospective randomized trial of arthroplasty versus fusion. Journal of Spinal Disorders 2003;16(4):352‐61. [DOI] [PubMed] [Google Scholar]
Zucherman 2004 {published data only}
- Zucherman JF, Hsu KY, Hartjen CA, Mehalic TF, Implicito DA, Martin MJ, et al. A prospective randomized multi‐center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1‐year results. Eur Spine J 2004;13:22‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Boden 2002 {published data only}
- Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein‐2 to achieve posterolateral lumbar spine fusion in humans. Spine 2002;27:2662‐73. [DOI] [PubMed] [Google Scholar]
Christensen 2003 {published data only}
- Christensen FB, Laurberg I, Bunger CE. Importance of the back‐cafe concept to rehabilitation after lumbar spinal fusion: A randomized clinical study with a 2‐year follow‐up. Spine 2003;28(23):2561‐9. [DOI] [PubMed] [Google Scholar]
Emery 1995 {published data only}
- Emery SE, Stephens GC, Bolesta MJ. Lumbar fusion with and without instrumentation: A prospective study. [Abstract] Orthop Trans 1995;19(2):362. [Google Scholar]
Ercelen 2003 {published data only}
- Ercelen O, Bulutcu E, Oktenoglu T, Sasani M, Bozkus H, Saryoglu AC, et al. Radiofrequency lesioning using two different time modalities for the treatment of lumbar discogenic pain: A randomized trial. Spine 2003;28(17):1922‐7. [DOI] [PubMed] [Google Scholar]
Gibson S 2002 {published data only}
- Gibson S, McLeod I, Wardlaw D, Urbaniak S. Allograft versus autograft in instrumented posterolateral lumbar spinal fusion: A randomized control trial. Spine 2002;27(15):1599‐1603. [DOI] [PubMed] [Google Scholar]
Johnsson 2002 {published data only}
- Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing osteogenic protein‐1 (BMP‐7) and autograft bone in human noninstrumented posterolateral lumbar fusion. Spine 2002;27:2654‐61. [DOI] [PubMed] [Google Scholar]
Khot 2004 {published data only}
- Khot A, Bowditch M, Powell J, Sharp D. The use of intradiscal steroid therapy for lumbar spinal discogenic pain: a randomized controlled trial. Spine 2004;29:833‐6. [DOI] [PubMed] [Google Scholar]
Korovessis 2003 {published data only}
- Korovessis P, Papazisis Z, Koureas G, Zacharatos S. Rigid vs semirigid and dynamic instrumentation for stabilization the degenerated lumbosacral spine associated with spinal stenosis. Eur Spine J. 2003; Vol. 12, issue Suppl 1:S6‐7.
Laine 2000 {published data only}
- Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D. Accuracy of pedicle screw insertion with and without computer assistance ‐ a randomised controlled clinical study in 100 consecutive patients. ISSLS Proceedings, Adelaide, April 2000. 2000:236. [DOI] [PMC free article] [PubMed]
McAfee 2002 {published data only}
- McAfee PC, Lee GA, Fedder IL, Cunningham BW. Anterior BAK instrumentation and fusion: complete versus partial discectomy. Clin Orthop 2002, (394):55‐63. [DOI] [PubMed] [Google Scholar]
Moore 1995 {published data only}
- Moore KR, Schlegel JD. Early outcome of prospective data for the treatment of degenerative spondylolisthesis comparing in situ fusion versus pedicle screw instrumentation and fusion. Orthop Trans. 1995; Vol. 19:62.
North 1995 {published data only}
- North RB, Kidd DH, Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: A prospective randomized study design. Acta Neurochir 1995;64:106‐8. [DOI] [PubMed] [Google Scholar]
Rogozinski 1995 {published data only}
- Rogozinski A, Rogozinski C. Efficacy of implanted bone growth stimulation in instrumented lumbosacral spinal fusion. Orthop trans 1995;19:362. [DOI] [PubMed] [Google Scholar]
Sachs 2002 {published data only}
- Sachs B, McVoy J, Miller B, Ohnmeiss D. A prospective, randomized comparison of laparoscopic to open anterior lumbar interbody fusion with cages. Proceedings of the Meeting of the Americas II, New York. 2002.
Sanden 2002 {published data only}
- Sanden B, Olerud C, Petren‐Mallmin MP, Larsson S. Hydroxyapatite coating improves fixation of pedicle screws: A clinical study. J Bone Joint Surg 2002;84‐B:387‐91. [DOI] [PubMed] [Google Scholar]
Soegaard 2003 {published data only}
- Soegaard R, Christensen FB, Laurberg I, Bunger CE. Cost‐effectiveness analysis on different rehabilitation strategies after lumbar spinal fusion ‐ A randomized prospective study. Eur Spine J 2003;12(Suppl 1):S10‐11. [Google Scholar]
Transfeldt 2001 {published data only}
- Transfeldt EE, Burkus JK, Kitchel SH, Watkins R, Balderston RA. A prospective and randomized study assessing the clinical and radiographic outcomes of patients treated with rhBMP‐2 and threaded cortical bone dowels in the lumbar spine. Proceedings of the British Scoliosis Society. 2001.
von Strempel 1997 {published data only}
- Strempel AH. Results of dynamic versus rigid instrumentation of the spine. J Bone Joint Surg. 1997; Vol. 79‐B:Supp IV:441.
Zdeblick 1996 {published data only}
- Zdeblick TA, Ulschmid S, Dick JC. The surgical treatment of L5‐S1 degenerative disc disease ‐ A prospective randomized study of laparoscopic fusion. Orthop Transactions 1996;20:75. [Google Scholar]
- Zdeblick TA, Ulschmid S, Dick JC. The surgical treatment of L5‐S1 degenerative disc disease ‐ A prospective randomized study of laparoscopic fusion. Orthopaedic Transactions 1996‐7;20:1064‐5. [Google Scholar]
Zhao 2002 {published data only}
- Zhao J, Wang X, Hou T, He S. One versus two BAK fusion cages in posterior lumbar interbody fusion to L4‐L5 degenerative spondylolisthesis. Spine 2002;27:2753‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fairbank 2005 {published data only}
- Fairbank J, Frost H, Wilson‐MacDonald J, Yu L, Barker K, Collins R and the Spine Stabilisation Group. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ 2005;330:1233‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero‐Arias O, Campbell HE, Gray AM, Fairbank J, Frost H, Wilson‐MacDonald J and the Spine Stabilisation Trial Group. Surgical stabilisation of the spine compared with a programme of intensive rehabilitation for the management of patients with chronic low back pai: cost utility analysis based on a randomised controlled trial. BMJ 2005;330:1239‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2001 {published data only}
- Ma Y, Guo L, Cai X. Posterior interbody fusion or posterolateral fusion for discogenic low back pain. Ahonghua Yi Xue Za Zhi 2001;81(20):1253‐5. [PubMed] [Google Scholar]
References to ongoing studies
Clarke 2003 {published data only}
- A Prospective randomised trial comparing femoral ring allograft versus a titanium cage for circumferential spinal fusion: two year functional and radiological outcome. Ongoing study 2001.
Freeman 2005 {published data only}
- Freeman BJC, McKenna PJ, Mulholland RC, Grevitt MP, Webb JK, Mehdian SH. A prospective randomixed trial: femoral ring allograft versus a titanium cage in circumferential lumbar spinal fusion. Proc. International Society for Study of the Lumbar Spine. 2005:8. [DOI] [PMC free article] [PubMed]
Gibson 2003 {published data only}
- Gibson JNA, Hallett A. Spinal fusion in patients with single level degenerate disc disease and neural compression ‐ A prospective randomised study. Proceedings of the International Society for Study of the Lumbar Spine. 2003.
Ilharreborde 2005 {published data only}
- Ilharreborde B, Olivier E, Rillardon L, Vialle R, Guigui P. Efficiency of total disc replacement arthroplasty in the treatment of chronic low back pain. Proceedings of the International Society for the Study of the Lumbar Spine. 2005:10.
Malmivaara 2003 {published data only}
- Malmivaara A, Slatis P, Heliovaara M, Sainio P, Kinnunen H, Kankare J, et al. Operative treatment for moderately severe lumbar spinal stenosis: a randomized controlled trial. Proceedings of the International Society for Study of the Lumbar Spine. 2003.
Whitaker 2005 {published data only}
- Whitaker C, Hochschuler S, Ohnmeiss D, Zigler J, Guyer R, Sachs B, et al. Return to work following total disc replacement vs. fusion: A prospective, randomized, comparison study. Proceedings of the International Society for the Study of the Lumbar Spine. 2005:68.
Additional references
Alderson 2003
- Alderson P, Green S, Higgins JPT, Editors. The Cochrane Reviewer's Handbook 4.2 [updated December 2003]. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd, 2004, issue 1:Appendix 5c.
Blumenthal 1993
- Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in post‐operative patients? Correlation of routine radiographs with a second surgical look at lumbar fusion. Spine 1993;19:1186‐9. [DOI] [PubMed] [Google Scholar]
Bono 2004
- Bono CM, Lee CK. Critical analysis of trends in fusion for degenerative disc disease over the past 20 years: influence of technique on fusion rate and clinical outcome. Spine 2004;29(4):455‐63. [DOI] [PubMed] [Google Scholar]
Cha 2003
- Cha CW, Boden SD. Gene therapy applications for spine fusion. Spine 2003;28(15):S74‐84. [DOI] [PubMed] [Google Scholar]
Ciol 1996
- Ciol MA, Deyo RA, Howell E, Krief S. An assessment of surgery for spinal stenosis time trends, geographic variations, complications and reoperations. J Amer Geront Soc 1996;44:285‐90. [DOI] [PubMed] [Google Scholar]
Deyo 1992
- Deyo RA, Cherkin DC, Loeser JD, Bigos SJ, Ciol MA. Morbidity and mortality in association with operations on the lumbar spine. The influence of age, diagnosis and procedure. J Bone Joint Surg 1992;74‐A:536‐43. [PubMed] [Google Scholar]
Deyo 1998
- Deyo R, Battie M, Beurskens A, Bombardier C, Croft P, Koes B, et al. Outcome measures for low back pain research: a proposal for standardised use. Spine 1998;23:2003‐13. [DOI] [PubMed] [Google Scholar]
Goosens 1998
- Goosens M, Evers S. Economic evaluation of back pain interventions. In: Nachemson A, Jonsson E editor(s). SBU report on back pain. Stockholm: Swedish Council on Technology Assessment in Health Care, 1998. [Google Scholar]
Indahl 1995
- Indahl A, Velund L, Reikeraas O. Good prognosis for low back pain when left untampered: a randomized clinical trial. Spine 1995;20:473‐7. [DOI] [PubMed] [Google Scholar]
Kant 1995
- Kant AP, Daum WJ, Dean SM, Vehida T. Evaluation of lumbar spine fusion. Plain radiographs versus direct surgical exploration and observation. Spine 1995;20:2313‐7. [PubMed] [Google Scholar]
Keller 2004
- Keller A, Brox JI, Gunderson R, Holm IPT, Friis A, Reikeras O. Trunk muscle strength, cross‐sectional area and density in patients with chronic low back pain randomized to lumbar fusion or cognitive intervention and exercises. Spine 2004;29(1):3‐8. [DOI] [PubMed] [Google Scholar]
Mardjetko 1994
- Mardjetko SM, Connolly PJ, Shott S. Degenerative lumbar spondylosis: a meta‐analysis of the literature 1970‐1993. Spine 1994;19:2256S‐65S. [PubMed] [Google Scholar]
Roy‐Camille 1986
- Roy‐Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop 1986;203:7‐17. [PubMed] [Google Scholar]
Sandhu 2003
- Sandhu HS. Bone morphogenetic proteins and spinal surgery. Spine 2003;28(15):S64‐73. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Szpalski 1997
- Szpalski M, Gunzburg R. Lumbar segmental instability: fact or fiction?. Proceedings of the IVth Brussels International Spine Symposium. Brussels, 1997.
Turner 1992a
- Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis: attempted meta‐analysis of literature 1970‐93. Spine 1992;17:1‐8. [DOI] [PubMed] [Google Scholar]
Turner 1992b
- Turner JA, Ersek M, Herron L, Haselkorn J, Kent D, Ciol MA, et al. Patient outcomes after lumbar spinal fusions. JAMA 1992;268:907‐11. [PubMed] [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L and the Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28:1290‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gibson 1999
- Gibson JNA, Grant IC, Waddell G. The Cochrane review of surgery for lumbar disc prolapse and degenerative lumbar spondylosis. Spine 1999;24(17):1820‐32. [DOI] [PubMed] [Google Scholar]
Gibson 2000
- Gibson JNA, Waddell G, Grant IC. Surgery for degenerative lumbar spondylosis. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD001352] [DOI] [PubMed] [Google Scholar]


