Abstract
Background
The mammalian yolk sac provides nutrients for the growing fetus during critical early developmental processes such as neural tube closure, which precedes the functional maturation of the placenta. In contrast, oviparous species such as the chicken rely solely on the yolk sac for transfer of nutrients from the yolk to the developing embryo. However, the molecular mechanisms that provide the yolk sac with nutrient transfer competence remain poorly understood.
Results
We demonstrate that the chicken endodermal epithelial cells (EEC), which are in close contact with the yolk, gain their nutrient‐transport competence by a paracrine crosstalk with the blood‐vessel forming mesodermal cell layer. Bone morphogenetic proteins (BMP) 4 and 7 produced by ectodermal and mesodermal cell layers likely initiate a differentiation program of EECs during the transition from the area vitellina to the area vasculosa. BMPs, by inducing SMAD signaling, promote the up‐regulation of endocytic receptor expression and thereby provide the EECs with the molecular machinery to produce triglyceride‐rich lipoprotein particles.
Conclusion
This paracrine signaling cascade may constitute the basis for the EEC‐mediated mechanism underlying the efficient uptake, degradation, resynthesis, and transfer of yolk‐derived nutrients into the embryonic circulation, which assures proper energy supply and development of the growing fetus.
Keywords: area vasculosa, feto‐maternal interface, nutrient transfer, paracrine signaling, triglyceride‐rich lipoproteins
Key Findings
Endodermal epithelial cells gain their nutrient transport competence during the transition from the area vitellina to the area vasculosa.
BMPs signal from the ecto/mesodermal cell sheet to the underlying endoderm, thereby initiating endoderm specification.
BMPs thereby provide the endoderm with nutrient transport competence which allows proper nutrition of the growing embryo.
1. INTRODUCTION
In amniotic vertebrates, the yolk sac is the first organ that mediates the transport of nutrients from maternal sources to the growing embryo.1, 2, 3 In mammals, the yolk sac is of major importance before the formation and functional maturation of the chorioallantoic placenta, a highly critical time in embryonic development during which early organogenesis and neural tube closure occur. In contrast, developing embryos of oviparous species such as the chicken depend exclusively on the yolk sac, as it represents the sole organ that transfers maternally derived nutrients stored in the yolk to the growing fetus. Nevertheless, structural and functional features of the yolk sac are highly conserved between species, and comparative RNA sequencing data revealed a significant conservation of the yolk sac transcriptome from human, murine, and chicken embryos.1, 2, 3, 4
In our previous study, we reported that the chicken yolk sac endodermal epithelial cells (EECs), which mediate the efficient uptake, degradation, and resynthesis of yolk nutrients, differentiate into a metabolically highly active type of cells as they become competent for the uptake of nutrients via mesoderm‐derived blood vessels during the transition from the area vitellina to the area vasculosa.5 In addition to a drastic morphological change of EECs during this transition,6 a differentiation process accompanied by the increase in expression of genes involved in nutrient uptake and lipoprotein metabolism, including the LRP2‐Cubilin‐Amnionless receptor tricomplex, Apolipoprotein B (APOB), APOA1, and APOA5 occurs.5
In analogy to the EECs of the chicken yolk sac, the visceral endoderm of murine embryos comprises a single layer of polarized, columnar epithelial cells resting on the extraembryonic mesoderm, which is specialized for the efficient absorption and digestion of maternally derived nutrients.1, 7 Interestingly, most of the genes that we found to be up‐regulated during the vitellina‐to‐vasculosa transition in the chicken have previously been shown to be indispensable for mammalian embryonic development, while being exclusively expressed in the endodermal cells of the mammalian visceral yolk sac. For example, mice deficient in Cubilin (CUBN), Amnionless (AMN), APOB, or MTP die around day 10 of embryonic development (E10) with severe malformations such as neural tube defects and exencephaly.8, 9, 10, 11
Whereas the functions of these proteins in the EECs of the yolk sac are rather well established, the molecular mechanisms that underlie the differentiation and specification of EECs leading to expression of the respective genes are rather poorly understood. From the data obtained in our previous study,5 we concluded that signals from the mesoderm‐derived vasculature most probably induce differentiation of yolk sac EECs responsible for the observed differences in gene expression profiles in the developing chicken yolk sac.12 However, molecular signals dictating the transformation process of the EECs from a resting and lipid‐storing to a metabolically highly active phenotype have not been identified to date in the chicken system.
Bone morphogenetic proteins (BMPs) comprise a subgroup of the large family of transforming growth factor beta (TGFB) gene superfamily, and were originally identified by their capacity to induce cellular processes required for bone formation and differentiation.13 BMPs are secreted proteins and signal through type I and type II TGFB superfamily receptors. Upon ligand binding, the receptors form heterodimers (or heteromultimers) and trigger a downstream phosphorylation cascade of SMAD proteins. In vertebrates, seven type I receptors and five type II receptors are known, but only three type I receptors (BMP type I A receptor, BMP type I B receptor and the activin receptor like kinase, ACVRI or ALK2) and three type II receptors (BMPRII, ActRIIA, and ActRIIB) function as receptors for BMPs. Importantly, activated BMP‐bound type I receptor kinases phosphorylate SMAD1, 5, and 8, which heterodimerize with SMAD4 and translocate to the nucleus to affect gene expression (reviewed in Reference 13).
During murine yolk sac development, BMPs are primarily expressed in mesodermal angioblasts that eventually form the vascular network mediating nutrient transport to the embryo.14, 15 BMP4‐knockout mice die between E6.5 and E9.5, with embryos showing severe developmental retardation, disorganized posterior structures, and a reduction in extraembryonic mesoderm, including yolk sac blood islands.16 Since (a), targeted disruption of the BMP4 receptor ALK2 causes embryonic lethality, abnormal visceral endoderm morphology and disruption of mesoderm formation17 and (b), murine blastocyst‐derived extraembryonic endoderm (XEN) cells acquire features of endodermal cells of the yolk sac upon stimulation with BMP4,18, 19 our major goal was the elucidation of the role of BMP signaling in yolk sac endoderm specification. As the developing chicken yolk sac represents a valuable and amenable tool to study these developmental processes in detail, we set out to shed further light on the potential of BMPs in regulating chicken yolk sac function, and in particular, EEC differentiation.
2. RESULTS
In our previous study,5 we showed that during the vascularization of the EECs, the yolk sac acquires the molecular machinery required for efficient uptake of yolk components, resynthesis of lipoproteins, and their (re)secretion into the embryonic circulation. To gain a more comprehensive understanding of the changes in lipid metabolism during the vascularization of the EECs (i.e., the area vitellina—to vasculosa transition, Figure 1A), we performed qPCR experiments with EECs from the area vasculosa and the area vitellina (Figure 1B). We found that in addition to the LRP2‐Cubilin‐Amnionless receptor tricomplex, which is expressed on the apical aspect of the EECs and mediates efficient uptake of yolk macromolecules,5 genes encoding apolipoproteins associated with triglyceride‐rich lipoproteins (APOB, APOA5, APOA4, and APOC3) as well as with HDL‐like particles (APOA1) are predominantly expressed in the EECs of the area vasculosa (Figure 1B). Additionally, DGAT2, the rate limiting enzyme in triglyceride synthesis and an essential component in the assembly and secretion of TG‐rich lipoproteins,20, 21 shows similar expression patterns as observed for the receptor tricomplex and apolipoproteins (Figure 1B). Whereas mRNA levels for the large subunit of microsomal triglyceride transfer protein (MTP), which mediates the lipidation of APOB essential for VLDL and chylomicron assembly,22 are comparable in the area vasculosa and vitellina (Figure 1B), MTP protein was exclusively detectable in the area vasculosa.5
Figure 1.
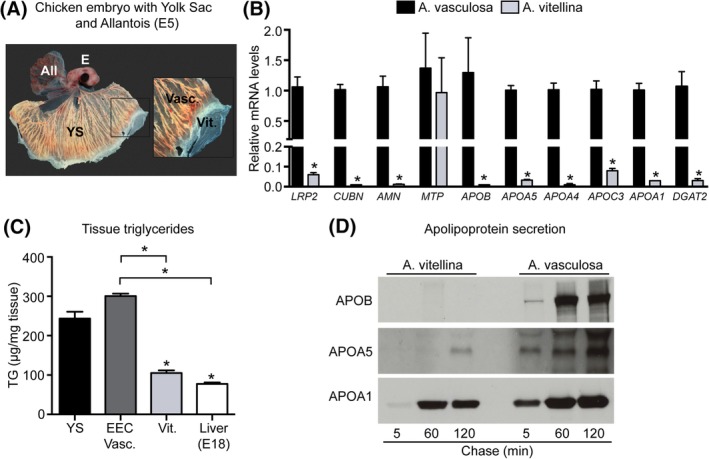
The transition from the area vitellina to the area vasculosa is accompanied by major changes in EEC gene expression, triglyceride content and apolipoprotein secretion. A, Chicken embryo (E) at day 5 of embryonic development (E5) with allantois (All, upper left) and yolk sac (YS) underneath. EECs of the area vitellina are located at the leading edge (Vit) preceding the vascularized, fully functional area vasculosa of the yolk sac (Vasc). B, Transcript levels of the indicated genes were analyzed with qPCR in isolated EECs from the area vasculosa (black bars) and the area vitellina (light grey bars). Relative gene expression levels were normalized to chicken β‐actin C, Quantification of triglyceride levels in tissue samples of total yolk sac from the area vasculosa (YS), isolated EECs from the area vasculosa (EECs Vasc.), EECs from the area vitellina (Vit.) and embryonic liver from E18 chicken embryos. D, Supernatants of radiolabeled EECs from the area vitellina and the area vasculosa were analyzed on their capacity to secrete apolipoproteins by immunoprecipiation. Results in B and C are presented as mean values ± SEM (n = 3 biological replicates) and statistical significance was calculated using Student's t‐test (*P < .05)
Assembly and secretion of TG‐rich lipoproteins such as VLDL or chylomicrons requires not only the protein components, but also considerable amounts of triglycerides, which represent VLDLs' and chylomicrons' most abundant lipid species (reviewed in References 23, 24). Importantly, measurement of TG levels revealed a significant, three‐fold excess of TGs in the area vasculosa compared to the area vitellina EECs (Figure 1C). Accordingly, pulse chase experiments with EEC explant cultures revealed that APOB and APOA5, two apolipoproteins almost exclusively present on TG‐rich lipoproteins, were efficiently secreted from EECs of the area vasculosa, whereas APOA1 (in general a component of triglyceride‐poor lipoproteins) was secreted from EECs of both areas (Figure 1D). Together, these data are compatible with TG‐rich lipoprotein assembly and secretion in EECs of the area vasculosa, initiated by the progressive vascularization of EECs by mesodermally derived blood vessels.
Next, we aimed at the identification of a possible molecular mechanism orchestrating the observed differentiation of EECs. In studies with murine embryos, bone morphogenetic protein 4 (BMP4) has been identified as a candidate inducer of a mesenchymal‐to‐epithelial transition of extraembryonic endodermal cells, eventually resembling extraembryonic visceral endoderm (exVE), a subtype of visceral endoderm forming the definitive yolk sac in mammals.18, 19 To investigate whether BMP family members known to mediate developmental processes could also regulate EEC differentiation in the chicken yolk sac, mRNA levels of BMP4, BMP7, BMP6, and BMP2 were measured using qPCR analysis of embryonic day 5 (E5) yolk sac tissue samples from the area vasculosa (Figure 2A). Interestingly, mRNA transcripts for BMP4 and BMP7 were present in comparable amounts, whereas expression levels of BMP6 and BMP2 were significantly lower (Figure 2A). Robust levels of BMP4 and BMP7 transcripts were readily detectable in the area vasculosa of E3 chicken embryos without time‐dependent regulation up to day 5 of embryonic development (Figure 2B,C). By separating the area vasculosa tissue into EECs and the associated mesodermal/ectodermal cell layers, we could localize BMP4 and BMP7 expression to the ectodermal/mesodermal cell sheet of the area vasculosa, whereas the endodermal cells hardly produced any BMP transcripts (Figure 2D,E). When further comparing BMP transcription levels between ecto/mesodermal cells of the area vasculosa and those of the area vitellina, BMP4 was expressed at significantly higher levels in the area vasculosa (Figure 2F). Although a trend towards higher expression in the area vasculosa was also evident for BMP7, differences were not statistically significant (Figure 2G).
Figure 2.
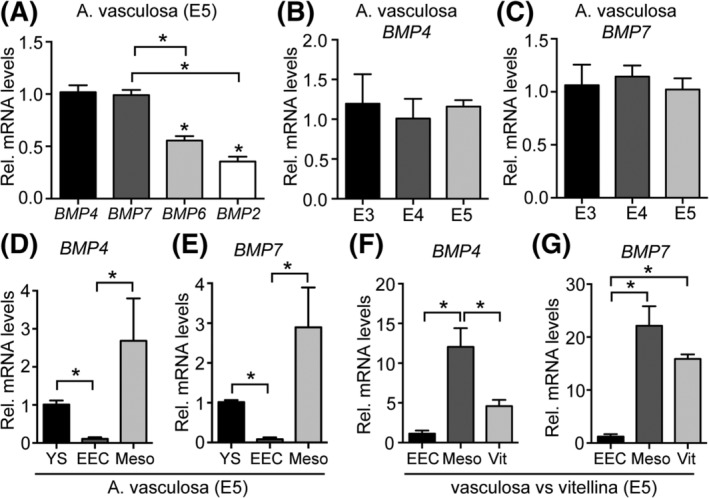
BMP4 and BMP7 are predominantly expressed in the ecto‐ and mesodermal cell layer of the chicken yolk sac's area vasculosa. A, Relative gene expression of chicken BMP4, BMP7, BMP6, and BMP2 was measured with qPCR in E5 yolk sac area vasculosa tissue and normalized to chicken β‐actin. B, C, Relative gene expression of BMP4 and BMP7, respectively, in the yolk sac's area vasculosa of chicken embryos from day 3 (E3) to day 5 (E5) of embryonic development. D, E, Relative gene expression of BMP4 and BMP7 was determined in dissected layers of the yolk sac's area vasculosa, normalized to chicken β‐actin and compared to the expression levels of the genes in the undissected area vasculosa of the yolk sac. F, G, Relative gene expression of BMP4 and BMP7 in EECs and mesodermal cell layer of the vasculosa compared to the expression levels in the area vitellina. Results are presented as mean values ± SEM (n = 3 biological replicates) and statistical significance was calculated using Student's t‐test (*P < .05)
To morphologically gain a more comprehensive understanding of the localization of BMP expression, whole‐mount in‐situ hybridization experiments with E5 yolk sac tissue samples from the area vasculosa and the area vitellina were performed (Figure 3). Clearly, using specific BMP4 and BMP7 antisense oligonucleotide probes, both transcripts could be localized to the ectodermal and mesodermal cell layers that are in direct contact with the basolateral aspect of the EECs in the area vasculosa of the chicken yolk sac (Figure 3A,B). Higher magnifications more precisely illustrate BMP expression in both, ectodermal and mesodermal portions of the yolk sac and also show the tight association of BMP‐positive mesodermal structures with the endodermal absorptive epithelium (Figure 3C,D). Recapitulating our findings from qPCR analysis, the area vitellina ectoderm stained also positive for BMP4 and 7 transcripts, albeit to a lesser extent compared to the area vasculosa (Figure 3E,F). Nonannealing sense RNA probes served as negative control (Figure 3G,H). These findings identified BMP4 and BMP7 as putative paracrine signals secreted from the ecto/mesodermal cell layer that might initiate the cellular differentiation of EECs during the area vitellina‐to‐vasculosa transition.
Figure 3.
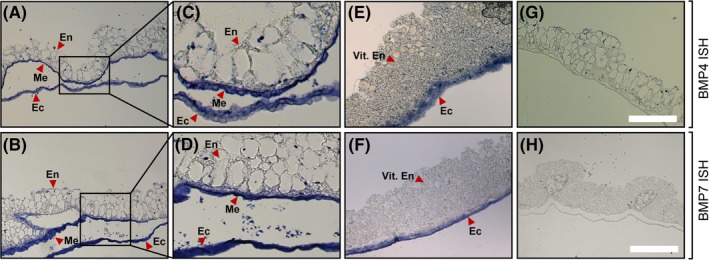
In situ hybridization of BMP4 and BMP7 in the area vasculosa and the area vitellina of yolk sac tissue at day 5 of embryonic development. A, B, Yolk sac area vasculosa tissue samples were subjected to in situ hybridization experiments with digoxigenin‐labeled probes targeting mRNA transcripts of chicken BMP4 and BMP7 (antisense) or G, H, nontargeting control (sense) probes. C, D, Magnifications of the inserts shown in A and B. E, F, Yolk sac area vitellina tissue samples were subjected to in situ hybridization experiments with digoxigenin‐labeled probes targeting mRNA transcripts of chicken BMP4 and BMP7 (antisense). En = Endodermal epithelial cells; Me = Mesodermal cell sheet; Ec = Ectoderm. Samples were embedded in paraffin and sections of 4 μm were prepared. Images were acquired using a Zeiss Axioscope. Magnification in A, B, E, F, G, H: 20×; scale bar, 200 μm
BMPs bind to cell surface receptors of type I and type II serine/threonine kinases, which upon heterotetramerization induce SMAD‐dependent downstream signaling events leading to a diverse repertoire of cellular responses.13, 17 To directly demonstrate the capacity of BMPs to activate signaling pathways in EECs, we established a protocol to culture primary EECs in vitro. Due to the high concentration of intracellular triglycerides, EECs from the area vasculosa did not adhere to the cell culture dish. In contrast, explants of EECs from the area vitellina adhered well, and formed a confluent monolayer rich in intracellular granules and Bodipy‐positive lipid droplets after 2 days of culture (Figure 4A). qPCR analysis revealed that from the three type I serine/threonine kinases known to bind BMPs, type I activin receptor (ALK2) was expressed at highest levels in cultured EECs. In contrast, BMPR1A and BMPR1B showed only basal expression levels (Figure 4B). Importantly, treatment of EECs with either BMP4 or BMP7 induced phosphorylation of SMAD5 (Figure 4C, left panel), one of the central receptor‐regulated R‐SMAD proteins that act as transcription factors for a broad variety of target genes upon BMP‐receptor interaction.18, 19, 25, 26 As a control, treatment of the hepatocellular carcinoma cell line HepG2 resulted in comparable BMP‐mediated SMAD5 phosphorylation, confirming the results obtained with the primary EEC cultures (Figure 4C, right panel).
Figure 4.
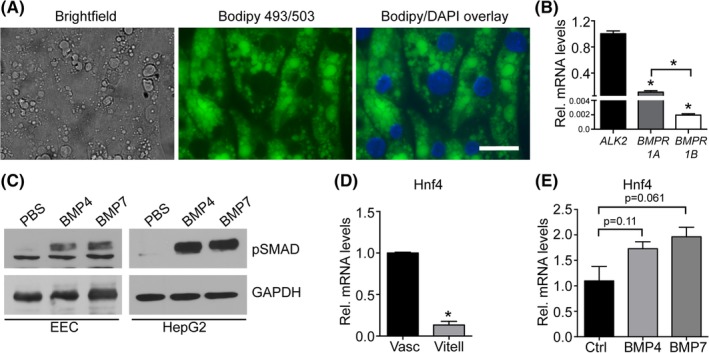
BMP signaling in the chicken yolk sac EECs is mediated through the BMP receptor ALK2 and its downstream mediator SMAD5. A, Light microscopic analysis of cultured EECs from the area vitellina. Granules observed in the brightfield view stain positive for the neutral lipid dye Bodipy 493/503. B, Relative gene expression levels of the potential type I BMP receptors ALK2, BMPR1a, and BMPR1b in EECs of the area vasculosa. C, Cultured EECs from the area vitellina and HepG2 cells were starved for 2 hours and subsequently stimulated with BMP4 or 7 for 40 minutes (200 ng/mL). SMAD5 phosphorylation was analyzed by Western blotting. GAPDH was used as loading control. D, Comparison of HNF4 mRNA levels in EECs of the area vasculosa and area vitellina. E, qPCR analysis of HNF4 mRNA levels in cultured EECs treated with either control PBS or recombinant BMP4 and BMP7. B, D, E, Relative gene expression levels are normalized to chicken β‐actin and represent the average of triplicate measurements. Results in B, D, and E are presented as mean values ± SEM (n = 3 biological replicates) and statistical significance was calculated using Student's t‐test (*P < .05)
Although the secretion of APOB‐containing TG‐rich lipoproteins is mainly regulated via posttranslational mechanisms that control APOB lipidation and intracellular trafficking,23, 24 SMAD transcription factors have been demonstrated to directly bind to enhancer elements located 54 to 63 kb upstream of the APOB promoter region, thereby increasing APOB mRNA levels and protein secretion in intestinal cells.27 Furthermore, the TGFβ‐SMAD signaling axis has been shown to induce APOCIII in synergy with hepatocyte nuclear factor 4 alpha (HNF4α). The transcription factor HNF4α itself is induced upon BMP4 treatment in murine XEN cells18 and was identified as an important regulator of many genes involved in lipid metabolism, including APOB and MTP.18, 19, 28 Here, quantitative mRNA expression analysis revealed a robust increase in HNF4α mRNA levels in EECs of the area vasculosa compared to EECs of the area vitellina (Figure 4D), concurrent with the observed increase in the secretion of APOB (Figure 1D) and of VLDL5 in the area vasculosa of the yolk sac. Notably, BMP4‐ as well as BMP7‐treatment resulted in a trend towards increased mRNA levels of HNF4α in cultured EECs (Figure 4E), pointing to a BMP‐induced induction of HNF4α activity. Together, the responsiveness of EECs towards stimulation with BMPs in terms of SMAD5 phosphorylation as well as the induction of HNF4α expression might represent a synergistic signaling process that drives the induction of TG‐rich lipoprotein metabolism in the EECs of the area vasculosa.
To further strengthen our hypothesis, we treated primary EECs from the area vitellina with either BMP4 or BMP7 and performed expression analysis of LRP2, CUBN, and AMN, encoding the endocytotic receptor tricomplex that transports nutrients from the yolk into the EECs. Likewise, we analyzed the expression of APOB, APOA5, and MTP, essential components of VLDL assembly and secretion. We observed that BMP4 treatment increased expression of LRP2 and CUBN (P = .09, borderline significance), whereas AMN expression levels were unchanged (Figure 5A‐C). Although statistically not significant, BMP4 treatment increased the expression of APOB and MTP (Figure 5D,F). Interestingly, BMP7 treatment resulted in a more robust and significant increase in expression levels of the receptor tricomplex as well as of APOB, APOA5, and MTP (Figure 5A‐F). In contrast, neither BMP4 nor BMP7 had an impact on the expression of PLIN2, an area vitellina‐specific lipid droplet‐associated protein (Figure 5G).5 Finally, to confirm our results at the protein level, BMP4‐stimulated EECs were subjected to Western blot analysis. Unfortunately, it was not possible to detect LRP2, CUBN, APOB, or APOA5 protein in cultured EECs of the area vitellina due to limited amount of cellular material derived from the explant cultures and low expression levels of the respective proteins. However, and in partial contrast to results from qPCR analysis, BMP4 treatment resulted in an increase in AMN and MTP protein levels (Figure 5H). To further support our findings that BMPs induce a differentiation process in the endodermal cell layer, thereby promoting their nutrient transport competence, we performed experiments with explant cultures of endodermal cells from the area vitellina. To mimic BMPs action, we treated vitellina explants with the SMAD5 activator Kartogenin29, 30 and used expression patterns of genes of the receptor tricomplex and critical components of the TG‐rich lipoprotein assembly/secretion machinery as a surrogate marker for the nutrient transport competence of the EECs. Thereby, we found that Kartogenin treatment significantly induced LRP2 expression (Figure 6A) and caused a trend towards increased expression of CUBN and AMN (Figure 6B,C). Importantly, the SMAD5 activator induced expression of APOB and APOA‐IV, two major structural components of TG‐rich lipoproteins such as VLDL and chylomicron particles (Figure 6D,E). In line with the results obtained for MTP in Figure 1B, we did not observe changes in MTP expression in area vitellina EEC explant cultures upon Kartogenin stimulation.
Figure 5.
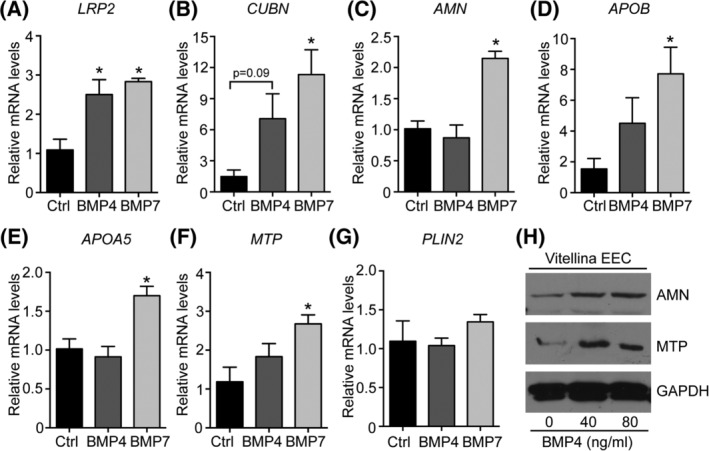
Stimulation of EECs with BMP4 and BMP7 induces an area vasculosa‐like gene expression pattern. A‐G, EECs from the area vitellina were cultured for 48 hours in the presence or absence of 200 ng/mL recombinant mouse BMP4 or BMP7. Relative gene expression levels were normalized to chicken β‐actin and compared to control treated EECs (n = 3, *P < .05, Student's t‐test). H, Cultured EECs from the area vitellina were treated with 0, 40, or 80 ng/mL recombinant mouse BMP4 and analyzed for the expression of AMN and MTP using Western blotting. GAPDH was used as loading control
Figure 6.
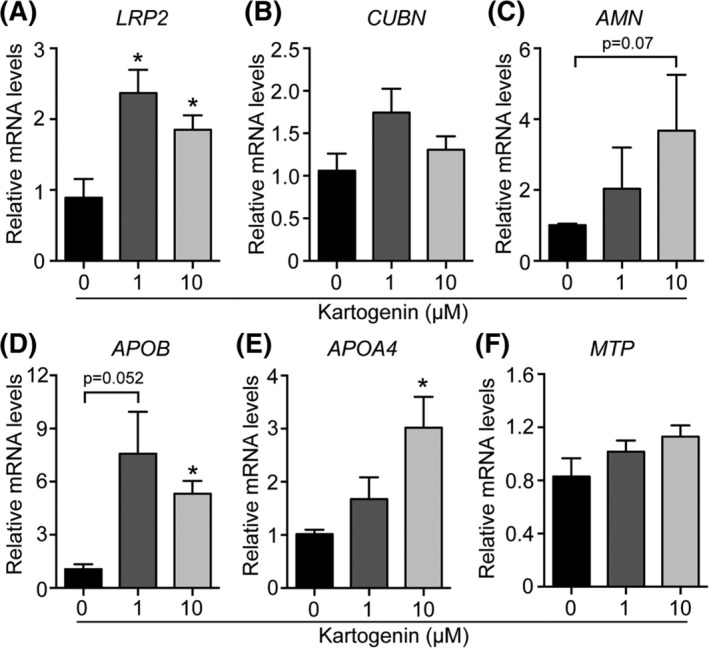
Kartogenin, a small molecule SMAD5 activator partially mimics BMP treatment. A‐F, EECs from the area vitellina were cultured for 48 hours in the presence of 0, 1, or 10 μM of the SMAD5 activator Karotgenin. Relative gene expression levels were normalized to chicken β‐actin. Results are presented as mean values ± SEM (n = 4 biological replicates) and statistical significance was calculated using Student's t‐test (*P < .05)
Altogether, the data appear to support a paracrine, BMP4/7‐mediated differentiation process of EECs that induces TG‐rich lipoprotein synthesis and secretion, providing the basis for efficient yolk‐sac‐mediated nutrient transport to the growing avian embryo.
3. DISCUSSION
The yolk sac endoderm provides the majority of lipid and protein components for the evolving chicken embryo, and supports early developmental processes in embryos of mammalian species.1, 12, 31, 32 As the growing chicken embryo relies exclusively on the yolk sac‐mediated supply with nutrients, the transformation of resting, lipid‐storing EECs in the area vitellina to metabolically highly active EECs in the area vasculosa represents a critical process during yolk sac maturation. Here we describe a control mechanism that coordinates the process of EEC‐differentiation during the progressive supply of EECs with mesodermally derived blood vessels. Together with data published in our previous study,5 we show that in the area vitellina, EECs in the absence of active BMP‐signaling secrete HDL‐like APOA‐I containing particles and contain PLIN2‐covered lipid droplets, but lack the competence to synthesize TG‐rich lipoproteins (Figure 7). During the vitellina‐to‐vasculosa transition, the invading mesodermal cell layer produces significant amounts of BMP4 and BMP7, which act in a paracrine manner on EECs, leading to SMAD5 phosphorylation and the induction of EEC differentiation and remodeling. In turn, the transcriptional profile of EECs changes dramatically so as to afford the efficient uptake of yolk nutrients as well as the synthesis and resecretion of potentially nutritive TG‐rich lipoproteins (Figure 7). Therefore, mesodermally derived BMPs likely represent crucial paracrine signaling components that ensure proper development and function of the yolk sac EECs.
Figure 7.
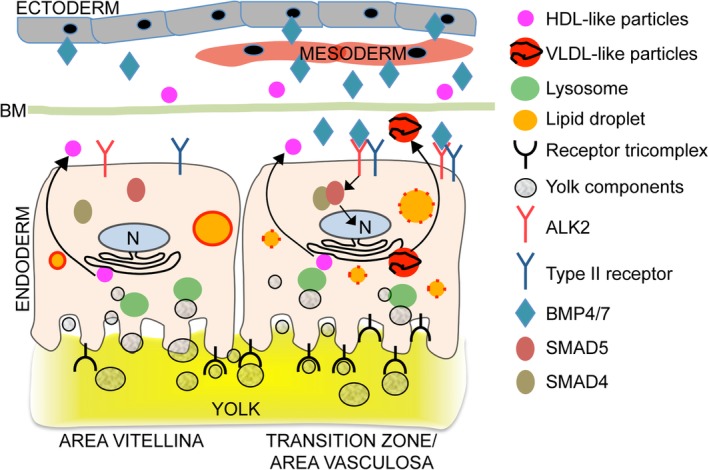
BMP signaling in the chicken yolk sac induces a differentiation process of EECs during the area vitellina to area vasculosa transition. In the absence of BMP signaling, EECs in the area vitellina (left‐hand side) remain in a resting state, characterized by the absence of APOB synthesis and secretion, the absence of the receptor tricomplex, and the expression of PLIN2. In the transition zone/area vasculosa (right‐hand side), BMP4 and BMP7, expressed and secreted from cells of ecto/mesodermal origin, are secreted into the extracellular space where they diffuse and eventually bind the type I receptor ALK2 on the surface of EECs. Activated ALK2 then phosphorylates SMAD5, which in turn translocates to the nucleus and initiates target gene expression and the differentiation process of EECs
Interestingly, extensive research with murine embryos identified signals from the yolk sac's endodermal cells essential for the mesodermal cell layer to develop vascular tubes and mature blood vessels.15 Indian‐ and sonic hedgehog proteins secreted from the endodermal cell layer of the yolk sac signal to the invading, vasculogenesis‐competent mesodermal cells, thereby initiating FOXF1 expression. Of note, FOXF1‐deficient mouse embryos show markedly decreased BMP4 expression in the mesoderm of the yolk sac, an essentially avascular yolk sac phenotype, and early resorption of the fetus. Importantly, the exogenous addition of BMP4 was sufficient to induce vascular tube‐ and blood vessel formation in the yolk sac.33, 34 Thus, BMPs are key to normal mesodermal vasculogenesis that is a result of the crosstalk between the closely associated mesodermal and endodermal cell layers during the formation of the yolk sac.
In contrast, detailed studies on the role of BMPs in the differentiation and specialization program of endodermal yolk sac cells are rather scarce, likely due to the limited amount and poor accessibility to tissues. However, the isolation of stem cell lineages from murine blastocysts, among them the parietal endoderm‐derived extraembryonic endoderm (XEN) cell line, has provided a valuable tool to study this intercellular crosstalk.18, 35 Stimulation of XEN cells with BMP4, expressed in the visceral endoderms' adjacent extraembryonic ectoderm and mesoderm during mouse embryogenesis, established an apical‐basal polarity. In addition, expression profiling of these cells showed that genes normally expressed in the visceral endoderm of the yolk sac, including Lrp2, Cub, and Amn, as well as genes encoding apolipoproteins such as ApoA‐I, ApoA‐2, ApoE, and ApoM were significantly up‐regulated.18, 19 Interestingly, BMP4‐treated XEN cells show increased expression of vascular endothelial growth factor and Indian hedgehog, which previously have been implicated in primitive hematopoiesis and vasculogenesis18 by activating BMP4 expression in mesodermal angioblasts in the yolk sac.33 These findings complement our current data and suggest a paracrine crosstalk that orchestrates the differentiation and specification of the yolk sac's EECs as well as the mesodermally derived vasculature. Our results from qPCR and in situ hybridization experiments revealed that BMPs are readily detectable in area vitellina ectodermal cells in proximity to the sinus terminalis of the area vasculosa (Figures 2F,G and 3E,F). From a functional perspective, the BMP signal in the area vitellina might also be involved in the structural rearrangement of the endodermal cells from a multilayered cell sheet towards a polarized, highly organized epithelium in the area vasculosa.36 Interestingly, Kartogenin, which induced a vasculosa‐like gene expression pattern in EECs from the area vitellina (Figure 6), was previously shown to promote collagen synthesis in human dermal fibroblasts.30 Of note, collagen is a well‐known component of basement membranes and the extracellular matrix, two important factors that regulate epithelial polarization.37
Stimulation of cultured chicken EECs from the area vitellina with either BMP4 or BMP7 resulted in a robust increase in expression of the receptor triad LRP2, CUBN and AMN, which are well known to act as a multiligand receptor complex with high endocytic capacity in the small intestine, the proximal tubules of the kidney, and the endodermal cells of the yolk sac.38, 39, 40 That the receptor tricomplex plays a crucial role in proper nutrient supply during embryonic development has been demonstrated in several studies.11, 39, 40, 41 Cubilin, for example, was identified as the target of teratogenic antibodies when injected into pregnant rats, as they localized almost exclusively to clathrin‐coated pit structures of the visceral endodermal cells of the yolk sac.42 Subsequent studies in mice showed that the lack of either LRP2, CUBN, or AMN leads to similar embryonic‐lethal phenotypes with defects in gastrulation, mesoderm formation, and neural tube closure.9, 11, 41 In humans, neural tube closure usually occurs at very early stages of development, that is, before the establishment of the chorioallantoic placenta.3 In this critical time window, nutrients are absorbed by histotrophic mechanisms involving phagocytosis as well as receptor‐mediated endocytosis. Thereby, despite structural differences, the murine and most likely also the human yolk sac's visceral endodermal cells function as a fetomaternal interface that mediates absorption and transport of nutrients to the growing embryo.3, 4, 39 Additional evidence for an important role of LRP2/CUBN/AMN‐mediated transport of nutrients to the early embryo is provided by studies that analyzed the composition of the coelomic fluid, which is in direct contact with the endodermal cells of the visceral yolk sac and is rich in vitamin A, B12, D, cobalamine, and cholesterol. These macromolecules are ligands for the receptor tricomplex, which binds, internalizes, and thereby provides the accessibility of these factors to the growing embryo.3, 38, 40
Our current data indicate that BMPs activate SMAD signaling and induce the transcription factor HNF4α in EECs of the chicken embryo, apparently resulting in a synergistic target‐gene activation. Notably, ALK2‐deficient mice show reduced expression of the visceral endoderm‐specific transcription factor HNF4α,25, 43 which is highly expressed in the liver, small intestine, and kidney, as well as in the endodermal cells of the yolk sac.28, 44, 45 HNF4α was recently shown to activate LRP2 expression in proximal tubule cells of the kidney by directly binding to an HNF4α‐binding site in the LRP2 promoter.45 The critical importance of HNF4α in embryonic development was first recognized in studies in Drosophila, where maternal HNF4α mRNAs are present in the fertilized egg, deposited by nurse cells. In later stages, HNF4α mRNA is detected in endodermal cells of the midgut primordium, showing some overlapping expression pattern with that found in rodents, in which HNF4α expression is restricted to the cells of the visceral endoderm, starting before gastrulation.44, 46 HNF4α‐deficient mice die early during gestation with severe malformations including gastrulation defects, absence of mesodermal structures, and growth retardation compared to wild‐type and heterozygous littermates.47 However, it is not completely clear whether embryonic lethality is due to decreased absorptive function of the yolk sac.47
The vascularization of the chicken yolk sac's EECs induces a massive turnover of yolk‐derived TGs and the secretion of TG‐rich, APOB containing VLDL‐like particles (Figure 1 and5). Supporting evidence for our findings that the BMP‐SMAD‐HNF4α signaling axis might also regulate APOB expression and VLDL secretion in the developing yolk sac is the identification of binding sites for HNF4α, HNF3β and SMAD transcription factors in the APOB promoter. Of particular interest is the finding that SMADs regulate APOB transcription in a tissue‐specific manner. By binding to an enhancer element 55 kb upstream of the APOB transcription start, SMAD proteins increase APOB transcription in human intestinally derived Caco2 cells, whereas they attenuate transcription in HepG2 cells.27 Of note, the small molecule SMAD5 activator Kartogenin significantly induced mRNA levels for APOB and APOA4 in explant cultures of EECs from the area vitellina (Figure 6D,E). These experiments provide further evidence for an important contribution of SMAD signaling in the process of TG‐rich lipoprotein assembly and secretion in the yolk sac, an absorptive epithelium with unequivocal structural similarities to the small intestine. Finally, liver‐specific ablation of HNF4α in adult mice results in the development of fatty liver and reduction of plasma TG levels by 80%, a consequence of reduced VLDL secretion, whereas overexpression of HNF4α significantly induced mRNA levels of APOB and MTP.28
Besides the differential transcriptional regulation of key components of TG‐rich lipoproteins in the area vasculosa vs. the area vitellina, a block to production of TG‐rich particles might be the presence of the lipid droplet‐stabilizing protein PLIN2 in the EECs of the area vitellina.5 PLIN2 was previously shown to hamper VLDL lipidation by protecting the fatty acid pool stored in lipid droplets from lipolysis, thereby decreasing TG incorporation into VLDL particles.48 Furthermore, PLIN2‐knockout mice showed an increased VLDL secretion rate and a reduction in hepatic TG content.49
In summary, our study (a) provides molecular evidence for a regulatory crosstalk between the cell layers that form and functionally establish the avian yolk sac and (b) sheds light on paracrine signaling mechanisms that dictate the differentiation of EECs. Given the highly conserved structure and the critical importance of the mammalian yolk sac in early gestational periods, that is, neural tube closure and somite formation, the chicken embryo provides an excellent model for the analysis of yolk sac‐dependent developmental defects.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
Sexually mature Derco brown (TETRA‐SL) laying hens were purchased from Diglas Co. (Feuersbrunn, Austria) and maintained as described in.5 Fertilized eggs were incubated in a humidified chamber at 38°C to maintain normal embryonic development.
4.2. Antibodies
The following in‐house raised rabbit polyclonal antisera/antibodies were used: anti‐ggApoA‐I antiserum50; anti‐ggApoA‐V antiserum51; and anti‐ggAPOB antibody.50 Rabbit monoclonal anti‐human SMAD5 (phospho S463 + S465, ab92698) and mouse monoclonal anti‐rabbit GAPDH (G8795) antibodies were purchased from Abcam and Sigma‐Aldrich, respectively.
Preparation and dissection of yolk sac layers was performed as described precisely in Reference 5. Yolk sac tissues used for all experiments in this study were obtained from embryos at day 5 of embryonic development.
4.3. Quantitative PCR
Total RNA was isolated from chicken tissues or from cultured cells using TRI reagent (MRC), and cDNAs were prepared by rev‐transcription of 1 μg of RNA using SuperScriptII rev transcriptase (Invitrogen) and oligo(dT) primers. cDNAs were subjected to quantitative PCR (qPCR) experiments essentially as described in Reference 5. The primers used are listed in Table 1. mRNA levels of genes are expressed as fold change to a respective control and represent the average of triplicate measurements ± SEM. Chicken β‐Actin was used as a housekeeping gene.
Table 1.
Oligonucleotide primers and probes used for qPCR analysis and in situ hybridization, respectively
| qPCR primers | Sequence (5′‐3′) | Amplicon size (bp) | Accession number |
|---|---|---|---|
| ggApoA‐I fwd | GACCGCATTCGGGATATGGT | 197 | NM_205525 |
| ggApoA‐I rev | ATCTCGCGCACCTCCTTGTA | ||
| ggApoA‐IV fwd | ATCACCAAGCAGCTCAACACC | 201 | NM_204938 |
| ggApoA‐IV rev | CACCTCGTCGGCGTAAGGA | ||
| ggApoC‐III fwd | AAAGTGGGAACTCGGGGCAG | 251 | XM_025143168 |
| ggApoC‐III rev | GCCATTTCCTGGCTTGCTGAG | ||
| ggApoA‐V fwd | CGCTGATAAGATCGCCTTCC | 213 | XM_025143219 |
| ggApoA‐V rev | TGGATGTTGCGGTGCAGATC | ||
| ggApoB fwd | CTCCATCAGAGCAGTCGAGT | 216 | NM_001044633 |
| ggApoB rev | ATTAGGCCTCAGGGACAGTG | ||
| ggMtp fwd | GCAGACGCCAGCATCTCT | 256 | NM_001109784 |
| ggMtp rev | TACAACAGCCGGAAGCTTGC | ||
| ggβ‐Actin fwd | AGCTATGAACTCCCTGATGG | 236 | NM_205518 |
| ggβ‐Actin rev | ATCTCCTTCTGCATCCTGTC | ||
| ggLrp2 fwd | GGAGTGTTAGCGGTTGGAGGC | 349 | XM_004942763 |
| ggLrp2 rev | CCACACTACCAGCTCCTGTTA | ||
| ggCubilin fwd | TGAACTCTCTGGATGGCTTC | 264 | XM_025148394 |
| ggCubilin rev | CTCGTTCTGCATCAACACAG | ||
| ggAmn fwd | TCTGGCTCTGGGTTCACAGC | 268 | NM_001277516 |
| ggAmn rev | GAACAGGGATCACTCGCCG | ||
| ggPlin2 fwd | CCCACTGACAAGGTTGTTGCC | 211 | NM_001031420 |
| ggPlin2 rev | GCACCACACGACTTCCCAAG | ||
| ggAbca1 fwd | GCACTGAAGATGATGTAACT | 205 | NM_204145 |
| ggAbca1 rev | AGCTCCTGGAATGTCCTGTTCAC | ||
| ggDgat2 fwd | GGTGAAAACCCACAACCTGC | 218 | XM_419374 |
| ggDgat2 rev | TCACGGGACATATACCCCCA | ||
| ggHnf4α fwd | AAGCAGCCAACCTCAACACC | 248 | XM_015296366 |
| ggHnf4α rev | CTTCATCCCTGCTCGGAAGC | ||
| ggBmp2 fwd | GCAGCTTCCACCACGAAGAAGT | 240 | NM_204358 |
| ggBmp2 rev | TCGTGACAGGGTCCTTGGAG | ||
| ggBmp4 fwd | AGCTTCCACCATGAAGAGCACC | 216 | NM_205237 |
| ggBmp4 rev | CTCCGACAGCGGCTTCATCA | ||
| ggBmp6 fwd | CCAAGTGCTGCAGGAACATCC | 188 | XM_015275997 |
| ggBmp6 rev | TTACACTGAAACCGTCGTGGG | ||
| ggBmp7 fwd | GGAGCACCCAGGAAGGGATT | 226 | XM_417496 |
| ggBmp7 rev | CTGCTTGTTTTGTGGGCCGT | ||
| ggAlk2 fwd | CTATCTGCAGCTCACCACCC | 227 | NM_204560 |
| ggAlk2 rev | CCAACTGGTTCGTGCTTTGG | ||
| ggBmpr1a fwd | ACCAACACTTGCTTCTGGCT | 193 | NM_205357 |
| ggBmpr1a rev | GACTGCCATCCAACGAATGC | ||
| ggBmpr1b fwd | ACTTCAGGTACAAGCGGCAA | 216 | NM_205132 |
| ggBmpr1b rev | AGACTTCCCCATAGCGACCT |
| ISH oligonucleotides | (5′‐3′); underlined: T7 RNA polymerase promoter sequence | Probe length (bp) | Accession number |
|---|---|---|---|
| anitsense ggBMP4 fwd | AGCTTCCACCATGAAGAGCACC | 724 | NM_205237 |
| antisense ggBMP4 rev |
TAATACGACTCACTATAGGGCGGA GTTGACCAACGTCTGC |
||
| sense ggBMP4 fwd |
TAATACGACTCACTATAGGGAGCT TCCACCATGAAGAGCACC |
724 | NM_205237 |
| sense ggBmp4 rev | CGGAGTTGACCAACGTCTGC | ||
| antisense ggBMP7 fwd | GGAGCACCCAGGAAGGGATT | 766 | XM_417496 |
| antisense ggBMP7 rev |
TAATACGACTCACTATAGGGTTCC CCTTCCCCACACATCC |
||
| sense ggBMP7 fwd |
TAATACGACTCACTATAGGGGGA GCACCCAGGAAGGGATT |
766 | XM_417496 |
| sense ggBMP7 rev | TTCCCCTTCCCCACACATC |
4.4. Culture of endodermal epithelial cells from the area vitellina
The area vitellina of yolk sacs was dissected from the area vasculosa as previously described.5 The intact cell sheets of the area vitellina were disrupted by repeated pipetting until a homogenous cell suspension was obtained. Cells were resuspended in fresh DMEM‐F12 medium (Sigma) supplemented with 10% FCS, 2 mM l‐glutamine, 0.1 mg/mL streptomycin, and 100 units/mL penicillin, plated at high density on sterile 6‐well cell culture dishes and grown for 48 hours in complete DMEM‐F12 medium at 37°C and 5% CO2. For the detection of SMAD5 phosphorylation, EECs were washed 2× in PBS, starved for 2 hours in Opti‐MEM reduced serum medium followed by the incubation of the cells with recombinant mouse BMP4 or BMP7 proteins (R&D systems) at the indicated concentrations for 40 minutes. For the analysis of gene expression in BMP‐stimulated EECs, freshly isolated EECs were cultured for 48 hours in complete DMEM‐F12 medium containing 200 ng/mL recombinant BMP4 or BMP7 proteins as indicated in the figure legends (medium and BMPs were replaced after 24 hours).
4.5. Culture of human hepatocellular carcinoma (HepG2) cells
HepG2 cells were obtained from ATCC and cultured in MEM medium (PAA) supplemented with 10% FCS, 2 mM L‐glutamine, 0.1 mg/mL streptomycin, and 100 units/mL penicillin at 37°C and 5% CO2. Downstream experiments were exactly performed as described for EECs.
4.6. Preparation of protein lysates from cultured cells
After washing the cells in PBS, RIPA buffer (50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P‐40, 1% sodium deoxycholate, 0.1% SDS, and complete proteinase and phosphatase inhibitor cocktails) was directly added to the dish, and the cells were detached using a cell scraper. For complete lysis, cells were pipetted up and down several times and sonicated for 10 seconds with constant pulse. After incubation of the lysates for 5 minutes at 4°C, samples were centrifuged for 45 minutes at 10.000 × g and 4°C. The obtained supernatants were immediately used for Western Blotting or stored at −80°C until use.
4.7. Western blotting
Western blotting was performed as described in Reference 5. Briefly, protein samples were analyzed by one‐dimensional SDS‐PAGE and electrophoretically transferred to nitrocellulose membranes (Hybond‐C Extra, AmershamBiosciences) using semidry blotting. Nonspecific binding sites were blocked with TBS (20 mM Tris‐HCl, pH 7.4, 140 mM NaCl) containing 5% (wt/vol) BSA and 0.1% Tween 20 (blocking buffer) for 1 hour at room temperature. Proteins of interest were detected with the indicated specific primary antisera/antibodies, followed by incubation with HRP‐conjugated secondary antibodies and development with the enhanced chemiluminescence protocol (Pierce).
4.8. Pulse‐chase radiolabeling experiments and immunoprecipitation
Five hundred milligrams EEC tissue fragments (either from the area vitellina or the area vasculosa) were washed extensively in PBS and subjected to pulse‐chase radiolabeling experiments as described in detail in Reference 5. Immunoprecipitates were subjected to SDS‐PAGE and subsequent autoradiography.
4.9. Extraction and quantification of triglycerides (TG)
Whole yolk sac tissue, EECs from either the area vasculosa or the area vitellina, or liver tissue from a mature laying hen (60‐80 mg each) were minced and transferred to glass vials containing 3 mL chloroform: methanol (2:1) and incubated for 15 minutes at 55°C followed by an incubation for 16 hours at room temperature. After centrifugation for 30 minutes at 500 × g at room temperature, the lipid fraction from each sample was collected and organic solvents were evaporated in a Bartelt HR‐1 Hetovac vacuum centrifuge. Lipids were resuspended in 1.5 mL chloroform: methanol (2:1), and samples were incubated for 5 minutes at 55°C. After the addition of 0.3 mL diluted H2SO4 (0.05%), the samples were vortexed and centrifuged at 500 × g for 15 minutes. Equal aliquotes (150 μL) of the lower lipid phase were mixed with 300 μL of 1% Triton X‐100 in chloroform and dried in the vacuum centrifuge. Pellets were resuspended in ultrapure H2O (150 μL), and TG concentration was measured using the Wako LabAssay Triglyceride Kit. Total TG concentrations were normalized to tissue wet weight.
4.10. In‐situ hybridization
Digoxigenin‐labeled in situ probes directed against regions of chicken Bmp4 (NM_205237.3) and Bmp7 (XM_417496.4) were generated by in vitro transcription of PCR products containing the T7 RNA polymerase promoter sequence at their 3′ (antisense probes) or 5′ (sense probes) ends.5 In situ hybridization analysis was carried out exactly as described at the GEISHA (Gallus expression in Situ Hybridization Analysis) Web site (“whole‐mount in situ hybridization protocol for mRNA detection”). This protocol represents a slight modification of those published.52, 53 After the staining procedure, tissue pieces were embedded in paraffin and sections of 4 μm were prepared. Slides were mounted with Fluorescence Mounting Medium (Dako) and images were acquired with a Zeiss Axioskope.
4.11. Statistics
Data represent mean ± SEM of representative experiments, unless otherwise stated. To compare the means of two groups, an unpaired, two‐tailed Student's t‐test was used. In all cases statistical significance was assumed when P < .05.
ACKNOWLEDGMENT
We thank Dr. Yasmin Striedner for help with the image acquisition of the chicken embryo shown in Figure 1A.
Bauer R, Tondl P, Schneider WJ. A differentiation program induced by bone morphogenetic proteins 4 and 7 in endodermal epithelial cells provides the molecular basis for efficient nutrient transport by the chicken yolk sac. Developmental Dynamics. 2020;249:222–236. 10.1002/dvdy.129
Funding information Austrian Science Fund, Grant/Award Numbers: J3664‐B19, P 20218
REFERENCES
- 1. Ferner K, Mess A. Evolution and development of fetal membranes and placentation in amniote vertebrates. Respir Physiol Neurobiol. 2011;178:39‐50. [DOI] [PubMed] [Google Scholar]
- 2. Nagai H, Sheng G. Definitive erythropoiesis in chicken yolk sac. Dev Dyn. 2008;237:3332‐3341. [DOI] [PubMed] [Google Scholar]
- 3. Zohn IE, Sarkar AA. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 2010;88:593‐600. [DOI] [PubMed] [Google Scholar]
- 4. Cindrova‐Davies T, Jauniaux E, Elliot MG, Gong S, Burton GJ, Charnock‐Jones DS. RNA‐seq reveals conservation of function among the yolk sacs of human, mouse, and chicken. Proc Natl Acad Sci U S A. 2017;114:E4753‐E4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauer R, Plieschnig JA, Finkes T, Riegler B, Hermann M, Schneider WJ. The developing chicken yolk sac acquires nutrient transport competence by an orchestrated differentiation process of its endodermal epithelial cells. J Biol Chem. 2013;288:1088‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mobbs IG, McMillan DB. Structure of the endodermal epithelium of the chick yolk sac during early stages of development. Am J Anat. 1979;155:287‐309. [DOI] [PubMed] [Google Scholar]
- 7. Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43:183‐205. [PubMed] [Google Scholar]
- 8. Farese RV Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet‐induced hypercholesterolemia in heterozygotes. Proc Natl Acad Sci U S A. 1995;92:1774‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalantry S, Manning S, Haub O, et al. The amnionless gene, essential for mouse gastrulation, encodes a visceral‐endoderm‐specific protein with an extracellular cysteine‐rich domain. Nat Genet. 2001;27:412‐416. [DOI] [PubMed] [Google Scholar]
- 10. Raabe M, Flynn LM, Zlot CH, et al. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci U S A. 1998;95:8686‐8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith BT, Mussell JC, Fleming PA, et al. Targeted disruption of cubilin reveals essential developmental roles in the structure and function of endoderm and in somite formation. BMC Dev Biol. 2006;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakazawa F, Alev C, Jakt LM, Sheng G. Yolk sac endoderm is the major source of serum proteins and lipids and is involved in the regulation of vascular integrity in early chick development. Dev Dyn. 2011;240:2002‐2010. [DOI] [PubMed] [Google Scholar]
- 13. Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265‐278. [DOI] [PubMed] [Google Scholar]
- 14. Byrd N, Becker S, Maye P, et al. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361‐372. [DOI] [PubMed] [Google Scholar]
- 15. Vokes SA, Krieg PA. Endoderm is required for vascular endothelial tube formation, but not for angioblast specification. Development. 2002;129:775‐785. [DOI] [PubMed] [Google Scholar]
- 16. Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein‐4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105‐2116. [DOI] [PubMed] [Google Scholar]
- 17. Gu Z, Reynolds EM, Song J, et al. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551‐2561. [DOI] [PubMed] [Google Scholar]
- 18. Artus J, Douvaras P, Piliszek A, Isern J, Baron MH, Hadjantonakis AK. BMP4 signaling directs primitive endoderm‐derived XEN cells to an extraembryonic visceral endoderm identity. Dev Biol. 2012;361:245‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paca A, Seguin CA, Clements M, et al. BMP signaling induces visceral endoderm differentiation of XEN cells and parietal endoderm. Dev Biol. 2012;361:90‐102. [DOI] [PubMed] [Google Scholar]
- 20. Harris CA, Haas JT, Streeper RS, et al. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 2011;52:657‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Millar JS, Cromley DA, et al. Knockdown of acyl‐CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochim Biophys Acta. 2008;1781:97‐104. [DOI] [PubMed] [Google Scholar]
- 22. Gordon DA, Jamil H. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein‐B lipoprotein assembly. Biochim Biophys Acta. 2000;1486:72‐83. [DOI] [PubMed] [Google Scholar]
- 23. Olofsson SO, Boren J. Apolipoprotein B: a clinically important apolipoprotein which assembles atherogenic lipoproteins and promotes the development of atherosclerosis. J Intern Med. 2005;258:395‐410. [DOI] [PubMed] [Google Scholar]
- 24. Shelness GS, Ingram MF, Huang XF, DeLozier JA. Apolipoprotein B in the rough endoplasmic reticulum: translation, translocation and the initiation of lipoprotein assembly. J Nutr. 1999;129:456S‐462S. [DOI] [PubMed] [Google Scholar]
- 25. de Sousa Lopes SM, Roelen BA, Monteiro RM, et al. BMP signaling mediated by ALK2 in the visceral endoderm is necessary for the generation of primordial germ cells in the mouse embryo. Genes Dev. 2004;18:1838‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hata A, Chen YG. TGF‐beta signaling from receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh K, Batuman OA, Akman HO, Kedees MH, Vakil V, Hussain MM. Differential, tissue‐specific, transcriptional regulation of apolipoprotein B secretion by transforming growth factor beta. J Biol Chem. 2002;277:39515‐39524. [DOI] [PubMed] [Google Scholar]
- 28. Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Decker RS, Koyama E, Enomoto‐Iwamoto M, et al. Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev Biol. 2014;395:255‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang J, Zhou J, Zhang N, Zhang X, Li Q. A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Biophys Res Commun. 2014;450:568‐574. [DOI] [PubMed] [Google Scholar]
- 31. Sheng G. Primitive and definitive erythropoiesis in the yolk sac: a bird's eye view. Int J Dev Biol. 2010;54:1033‐1043. [DOI] [PubMed] [Google Scholar]
- 32. Sheng G, Foley AC. Diversification and conservation of the extraembryonic tissues in mediating nutrient uptake during amniote development. Ann N Y Acad Sci. 2012;1271:97‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Astorga J, Carlsson P. Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development. 2007;134:3753‐3761. [DOI] [PubMed] [Google Scholar]
- 34. Vokes SA, Yatskievych TA, Heimark RL, et al. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371‐4380. [DOI] [PubMed] [Google Scholar]
- 35. Soares ML, Torres‐Padilla ME, Zernicka‐Goetz M. Bone morphogenetic protein 4 signaling regulates development of the anterior visceral endoderm in the mouse embryo. Dev Growth Differ. 2008;50:615‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saitoh M, Shirakihara T, Fukasawa A, et al. Basolateral BMP signaling in polarized epithelial cells. PLoS One. 2013;8:e62659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roignot J, Peng X, Mostov K. Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christensen EI, Verroust PJ. Megalin and cubilin, role in proximal tubule function and during development. Pediatr Nephrol. 2002;17:993‐999. [DOI] [PubMed] [Google Scholar]
- 39. Kozyraki R. Cubilin, a multifunctional epithelial receptor: an overview. J Mol Med (Berl). 2001;79:161‐167. [DOI] [PubMed] [Google Scholar]
- 40. Kozyraki R, Gofflot F. Multiligand endocytosis and congenital defects: roles of cubilin, megalin and amnionless. Curr Pharm Des. 2007;13:3038‐3046. [DOI] [PubMed] [Google Scholar]
- 41. Willnow TE, Hilpert J, Armstrong SA, et al. Defective forebrain development in mice lacking gp330/megalin. Proc Natl Acad Sci U S A. 1996;93:8460‐8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sahali D, Mulliez N, Chatelet F, Dupuis R, Ronco P, Verroust P. Characterization of a 280‐kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med. 1988;167:213‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027‐3037. [DOI] [PubMed] [Google Scholar]
- 44. Duncan SA, Manova K, Chen WS, et al. Expression of transcription factor HNF‐4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF‐4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci U S A. 1994;91:7598‐7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sasaki S, Hara A, Sakaguchi M, Nangaku M, Inoue Y. Hepatocyte nuclear factor 4alpha regulates megalin expression in proximal tubular cells. Biochem Biophys Rep. 2019;17:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhong W, Sladek FM, Darnell JE Jr. The expression pattern of a Drosophila homolog to the mouse transcription factor HNF‐4 suggests a determinative role in gut formation. EMBO J. 1993;12:537‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen WS, Manova K, Weinstein DC, et al. Disruption of the HNF‐4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:2466‐2477. [DOI] [PubMed] [Google Scholar]
- 48. Magnusson B, Asp L, Bostrom P, et al. Adipocyte differentiation‐related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low‐density lipoproteins. Arterioscler Thromb Vasc Biol. 2006;26:1566‐1571. [DOI] [PubMed] [Google Scholar]
- 49. Chang BH, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin‐deficient mice. J Lipid Res. 2010;51:2132‐2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nimpf J, George R, Schneider WJ. Apolipoprotein specificity of the chicken oocyte receptor for low and very low density lipoproteins: lack of recognition of apolipoprotein VLDL‐II. J Lipid Res. 1988;29:657‐667. [PubMed] [Google Scholar]
- 51. Dichlberger A, Cogburn LA, Nimpf J, Schneider WJ. Avian apolipoprotein A‐V binds to LDL receptor gene family members. J Lipid Res. 2007;48:1451‐1456. [DOI] [PubMed] [Google Scholar]
- 52. Acloque H, Wilkinson DG, Nieto MA. In situ hybridization analysis of chick embryos in whole‐mount and tissue sections. Methods Cell Biol. 2008;87:169‐185. [DOI] [PubMed] [Google Scholar]
- 53. Streit A, Stern CD. Combined whole‐mount in situ hybridization and immunohistochemistry in avian embryos. Methods. 2001;23:339‐344. [DOI] [PubMed] [Google Scholar]


