Abstract
In vitro assays presently used for prenatal developmental toxicity (PDT) testing only assess the embryotoxic potential of parent substances and not that of potentially embryotoxic metabolites. Here we combined a biotransformation system, using hamster liver microsomes, with the ES‐D3 cell differentiation assay of the embryonic stem cell test (EST) to compare the in vitro PDT potency of two 5‐ring polycyclic aromatic hydrocarbons (PAHs), benzo[a]pyrene (BaP) and dibenz[a,h]anthracene (DBA), and dimethyl sulfoxide extracts from five PAH‐containing petroleum substances (PS) and a gas‐to‐liquid base oil (GTLb), with and without bioactivation. In the absence of bioactivation, DBA, but not BaP, inhibited the differentiation of ES‐D3 cells into beating cardiomyocytes in a concentration‐dependent manner. Upon bioactivation, BaP induced in vitro PDT, while its major metabolite 3‐hydroxybenzo[a]pyrene was shown to be active in the EST as well. This means BaP needs biotransformation to exert its embryotoxic effects. GTLb extracts tested negative in the EST, with and without bioactivation. The PS‐induced PDT in the EST was not substantially changed following bioactivation, implying that metabolism may not play a crucial role for the PS extracts under study to exert the in vitro PDT effects. Altogether, these results indicate that although some PAH require bioactivation to induce PDT, some do not and this latter appears to hold for the (majority of) the PS constituents responsible for the in vitro PDT of these complex substances.
Keywords: biotransformation, embryonic stem cell test, petroleum substances, polycyclic aromatic hydrocarbons, prenatal developmental toxicity, UVCBs
Short abstract
The present study combines a biotransformation system, using hamster liver microsomes, with the embryonic stem cell test to compare the in vitro prenatal developmental toxicity potency of two 5‐ring polycyclic aromatic hydrocarbons, benzo[a]pyrene and dibenz[a,h]anthracene, and dimethyl sulfoxide extracts from five PAH‐containing petroleum substances and a gas‐to‐liquid base oil, with and without bioactivation.
1. INTRODUCTION
REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) is the first chemical legislation in the world that requires prenatal developmental toxicity (PDT) testing, following the current OECD 414 testing guideline (OECD, 2018), on all chemical substances produced at a volume of ≥100 tonnes/year (ECHA, 2009). Petroleum substances (PS) are substances of Unknown or Variable composition, Complex reaction products or Biological materials (UVCBs) and regulated as such under the REACH legislation. Most PS are produced at a volume far greater than 100 tonnes annually, hence, they need to be assessed for PDT. Testing for PDT is one of the most animal and resource‐intensive regulatory requirements in the field of toxicology (OECD, 2018; van der Jagt et al., 2004). Given the large number of experimental animals and a considerable amount of resources potentially connected to PDT testing of all currently active registered PS (±186 EINECS numbers) (Concawe, 2019), the application of robust and reliable in vitro alternative testing strategies to assess the PDT potency of PS is highly relevant. Such alternative testing strategies may be used to facilitate read‐across from PS for which in vivo PDT data are already available or are being generated under REACH to other PS for which in vivo data are lacking. This will ultimately reduce the required animal tests and resources to study the PDT potency of PS, and support the application of the 3Rs (Reduction, Replacement, and Refinement) principle of animal use in toxicological research.
To date, several in vitro alternative assays have been scientifically validated and are widely used for PDT testing of chemical substances. One of these assays is the mouse embryonic stem cell test (EST) (Genschow et al., 2004, 2002). The EST is generally considered an animal‐free test system, as it uses the permanent embryonic ES‐D3 stem cell line. ES‐D3 cells are able to differentiate spontaneously into any derivatives of all three primary germ layers (endoderm, ectoderm and mesoderm), including cardiomyocytes in the absence of the cytokine, leukemia inhibitory factor (LIF), in the growth medium (Maltsev et al., 1993). In the EST, the ability of test substances to inhibit the differentiation process of ES‐D3 cells is used as the endpoint to study PDT potency of test substances in vitro (Genschow et al., 2004, 2002; Seiler & Spielmann, 2011). Recent studies in our lab showed the ability of the ES‐D3 cell differentiation assay of the EST to assess the PDT potencies of dimethyl sulfoxide (DMSO) extracts of PS (within and across categories with variable polycyclic aromatic hydrocarbon [PAH] content) and GTL products (containing no PAHs) (Kamelia et al., 2017; Kamelia et al., 2019). PDT potencies of different PS extracts, as evaluated in the ES‐D3 cell differentiation assay of the EST, appeared to be in line with their potencies observed in vivo (Kamelia et al., 2019, 2017). GTL extracts tested negative in the EST, while all PAH‐containing PS extracts inhibited the differentiation of ES‐D3 cells into beating cardiomyocytes in a concentration‐dependent manner at non‐cytotoxic concentrations with a potency that was proportional to their three‐ to seven‐ring PAH content (Kamelia et al., 2019, 2017). This result was obtained without including a metabolic system in the ES‐D3 cell differentiation assay, a result that is of interest given that it is generally assumed that PAHs require metabolic activation to exert their toxicity. It has been reported that some PAHs and PS need to be bioactivated to show their carcinogenicity and mutagenicity (Blackburn et al., 1986; Clonfero et al., 1996). However, it is unclear whether bioactivation would also be required for PAH‐containing PS to exert PDT. Initial studies suggest bioactivation may not be required as PS extracts tested positive in the EST, without the inclusion of a mammalian metabolic activation system in this in vitro PDT assay (Kamelia et al., 2019, 2017). In view of this, and in particular to answer the question of whether the PDT potency of PS would be modified upon biotransformation, and to mimic better the in vivo situation, the aim of the present study was to evaluate the consequences of biotransformation for the in vitro PDT of some selected model PAHs and PS extracts by combining a metabolic activation system with the EST. To this purpose, two 5‐ring PAH model compounds: benzo[a]pyrene (BaP) and dibenz[a,h]anthracene (DBA), the DMSO extracts of five PS from different product categories (varying in their PAH content); and of one gas‐to‐liquid base oil (GTLb; totally devoid of PAHs) were tested in the EST, with and without preliminary metabolic activation. The metabolite cocktails of the parent substances were generated using hamster liver microsomes as these have been shown superior for in vitro bioactivation of PAHs and PAH‐containing PS, in comparison with the rat or mouse liver microsomes (Blackburn et al., 1986; Hermann et al., 1980).
2. MATERIALS AND METHODS
2.1. Test substances
BaP (≥ 96% [high‐performance liquid chromatography]; CAS no. 50‐32‐8), DBA (analytical standard grade; CAS no. 53‐70‐3) and 5‐fluorouracil (≥99% [high‐performance liquid chromatography]; CAS no. 51‐21‐8) were purchased from Sigma‐Aldrich, and 3‐hydroxybenzo[a]pyrene (3OH‐BaP; analytical standard grade; CAS no. 13345‐21‐6) was obtained from BIOZOL Diagnostica Vertrieb GmbH. All compounds were of high purity (≥95%) and stock solutions and dilutions were prepared in dimethyl sulfoxide (DMSO; Merck).
Moreover, the DMSO extracts of five PS, varying in the types and levels of PAH, and one gas‐to‐liquid base oil (GTLb; CAS no. 848301‐69‐9) that was totally devoid of PAHs, were also tested in the present study. DMSO extraction and chemical analysis of the PS and GTL products tested was performed essentially as described before by Roy, Johnson, Blackburn and Mackerer (1988), and was carried out at Port Royal Research Laboratory (Hilton Head, SC, USA). An overview of the types and levels of PAHs present in each PS tested, grouped by the number of aromatic rings, is provided in Appendix A (see Supporting Information). Of the five PS, one was a heavy fuel oil (HFO; CAS no. 64741‐62‐4), one was a gas oil (GO; CAS no. 68915‐96‐8), one was a vacuum tower overhead oil (VTO; CAS no. 64741‐49‐7), one was a distillate aromatic extract (DAE; CAS no. 64742‐04‐7) and one was a residual aromatic extract (RAE; CAS no. 91995‐70‐9). The raw material of all PS and GTLb used to generate the aforementioned DMSO extracts were kindly provided by Concawe and Shell International B.V., respectively.
2.2. Microsomal incubations of parent substances for the generation of metabolites
As liver microsomes are toxic for embryonic stem cells (Wobus & Löser, 2011), metabolite cocktails of parent substances were generated by preincubation of the compounds with pooled‐male Golden Syrian Hamster liver microsomes (Xenotech). Incubations were performed in 0.1 m KH2PO4 buffer (pH 7.4) (VWR International), containing 5 mm MgCl2 (VWR International), reduced nicotinamide adenine dinucleotide phosphate (NADPH; final concentration 15 mm) (Roche Diagnostic GmbH), and microsomal protein (final concentration 3 mg/mL), in glass reaction tubes (VWR International). Previous studies suggested that a high concentration of NADPH as a cofactor is needed when generating metabolite mixtures of PAHs and PS extracts in the microsomal incubations (Blackburn et al., 1986; Haugen & Peak, 1983). Considering these findings, the present study applied hamster liver microsomes at a final concentration of 3 mg/mL microsomal protein with the final concentration of NADPH at 15 mm, which is a 1:5 ratio microsomes/NADPH (see Section 2.2). This ratio was obtained by doing an optimization study using BaP as the model compound (data not shown), and the chosen ratio of microsomes and NADPH, in combination with an incubation time of 4 hours at 37°C shows a reasonable compromise between the efficiency of activation and costs, and time of incubation. The total volume of the incubation mixtures was 3 mL and the reaction was initiated by adding the substrate, being the parent substances under study, after the preincubation at 37°C for 1 minute. Incubation mixtures without the cofactor NADPH were used as controls (without bioactivation). In the microsomal incubations (3 mL total volume), BaP and DBA were added at a final concentration of 50 μg/mL (200 μm) and 5.6 μg/mL (20 μm), respectively. All DMSO extracts of PS and GTLb were added at a final concentration of 800 μg raw material/mL, except for sample no. 034‐HFO that was tested at a concentration of 80 μg raw material/mL. It is worth mentioning that all of the aforementioned concentrations of parent substances were chosen based on their highest possible solubility in phosphate buffer used for the microsomal incubations. The microsomal incubation reactions were terminated after 4 hours of incubation at 37°C (tubes were vortexed every 30 minutes) by the addition of 300 μL (10% of the total volume) of ice‐cold 10% v/v perchloric acid (HClO4) (Merck). The incubation mixtures were extracted three times using diisopropyl ether (Acros) and the collected fractions were evaporated to dryness under a stream of nitrogen. Afterward, dried extracts (from incubations with and without bioactivation) were redissolved in 180 μL DMSO (resulting in a final concentration of 16.7 times compared with the final concentration used for the microsomal incubations) and stored at –20°C until further ultra performance liquid chromatography (UPLC) analysis and testing in the EST. Ascorbic acid at a final concentration of 0.5 mg/mL was added to each redissolved sample in DMSO to prevent further oxidation during storage. For testing in the EST, these samples dissolved in DMSO were further diluted and finally tested in 400 times diluted form. This dilution was made to ensure that the final DMSO concentration in the exposure medium is 0.25% v/v, which is the maximum recommended DMSO concentration to be applied in the EST (ECVAM, 2017). By analyzing the metabolite mixtures by UPLC (see Section 2.3, also Figures 2 and 3), it was confirmed that the samples thus tested did contain a substantial amount of metabolites in relatively high amounts. Given the high saturating substrate concentrations applied in the microsomal incubations, it can be expected that all potential metabolites would have been formed to the highest possible level. This set up provides the best testing strategy to allow detection of whether bioactivation could play a role or not in the PDT potency of the test substances under study.
Figure 2.
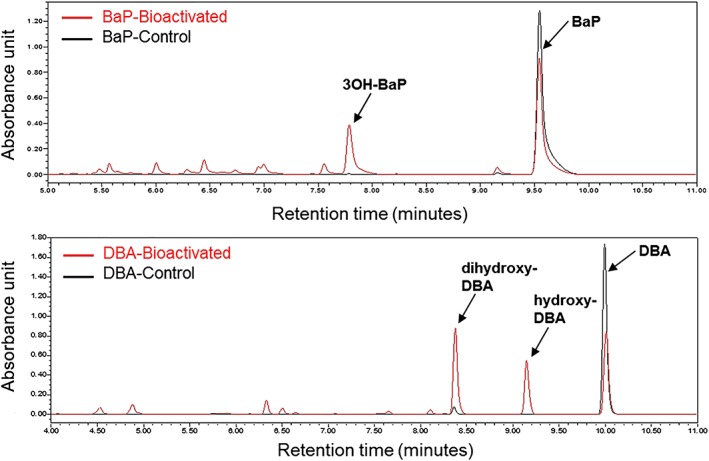
A, BaP. B, DBA. Relevant parts of the chromatograms for ultra performance liquid chromatography of the redissolved extracts from incubations with and without bioactivation of five‐ring polycyclic aromatic hydrocarbon model compounds BaP and DBA. BaP, benzo[a]pyrene. DBA, Dibenz[a,h]anthracene
2.3. Ultra performance liquid chromatography analysis
All redissolved extracts from microsomal incubations of parent substances, with and without bioactivation, were analyzed by UPLC to characterize the level of metabolic conversion. The UPLC system used consisted of a Waters Acquity binary solvent manager, sample manager, and photodiode array detector, equipped with a Waters Acquity UPLC® BEH C18 column (1.7 μm, 2.1 × 50 mm) and Waters Xbridge UPLC® BEH C18 pre‐column (2.5 μm, 2.1 × 5 mm). The temperature of the column was set at 40°C and the autosampler at 10°C during the UPLC analysis. The mobile phase used for the analysis was (A) nanopure water with 0.1% trifluoroacetic acid, and (B) acetonitrile with 0.1% trifluoroacetic acid. The flow rate was set to 0.6 mL/min and the total run time of the UPLC analysis for each sample was 23 minutes. The gradient elution conditions were set as follows: 90:10 (A/B) from 0 to 12 minutes, changing to 10:90 (A/B) from 12 to 20 minutes, then to 90:10 (A/B) from 20 to 23 minutes. Before injection, samples were 100 times diluted in phosphate buffer. The injection volume for each diluted sample was 10 μL and test substances were detected at a wavelength of 270 nm (photodiode array detector; Waters Acquity UPLC®). Calibration curves of PAH model compounds, being BaP, DBA and 3OH‐BaP, were also included in the UPLC analysis for quantification.
2.4. Time‐of‐flight mass spectrometry analysis
The redissolved extracts from microsomal incubations of DBA, with and without bioactivation, were analyzed by time‐of‐flight mass spectrometry (TOF‐MS) to identify, based on the exact mass (m/z), the type of metabolites formed following microsomal incubations of DBA. TOF‐MS data identify whether a metabolite represents a hydroxy‐, dihydroxy‐ or dihydrodiol‐type DBA metabolite. In brief, 10 μL of sample was injected on an Agilent Technologies 1200 U(H)PLC, equipped with a Grace Alltima HP C18 column (3 μm, 50 × 2.1 mm). The mobile phase used for the analysis consisted of a gradient made with solvent A (acetonitrile with 0.1% v/v formic acid) and B (nanopure water with 0.1% v/v formic acid). The flow rate was set to 0.3 mL/min and the total run time for each sample was 60 minutes. The gradient was set as follows: 0:100 (A/B) from 0 to 5 minutes, changing gradually to 100:0 (A/B) from 5 to 45 minutes, followed by a change to 100:0 (A/B) from 45 to 54 minutes and then back to 0:100 (A/B) from 55 to 60 minutes. For MS analysis, a Bruker MicrOTOF‐MS with electrospray ionization interface was used. The instrument was operated in negative ionization mode with full scan mode of mass range between 100 and 1500 m/z.
2.5. Mouse embryonic stem cell test
The ES‐D3 cell viability and differentiation assays of the mouse EST were performed as described in previous published studies (Kamelia et al., 2019, 2017).
2.5.1. ES‐D3 cell culture
The pluripotent mouse embryonic stem cell line ES‐D3 (ES‐D3; CRL‐1934™) was purchased from ATCC (Wesel) and was grown in HyClone AdvanceSTEM Low Osmo Dulbecco's modified Eagle medium (GE Healthcare), supplemented with 15% fetal bovine serum ES cell qualified (ATCC), 2 mm l‐glutamine (Invitrogen, Thermo Fisher Scientific), 50 U/mL penicillin (Invitrogen) and 50 μg/mL streptomycin (Invitrogen). ES‐D3 cells were maintained in 25 cm2 polystyrene cell culture flasks (Greiner Bio‐One), precoated with 0.1% gelatin for at least 1 hour before use. Cells were grown at 37°C, 5% CO2 and routinely subcultured every 2‐3 days using non‐enzymatic cell dissociation solution (Sigma‐Aldrich). To maintain the pluripotency of ES‐D3 cells in the culture, murine LIF (mLIF; Sigma‐Aldrich) at a final concentration of 1000 U/mL was added into the growth medium.
2.5.2. ES‐D3 cell viability assay of the embryonic stem cell test
For the ES‐D3 cell viability assay, cells were seeded in 96‐well plates (100 μL cell suspension/well) at concentrations of 20 × 104 cells/mL (1‐day exposure) or 104 cells/mL (5 days exposure) and incubated at 37°C, 5% CO2 for 1 day to facilitate cell adherence. At 24 hours after cell seeding, cells were exposed to increasing concentrations of test substance in triplicate by the addition of 100 μL exposure medium (containing the test compound at 2× the final concentration) to each well. The exposure media were prepared by mixing 400 times concentrated DMSO stock solutions of the test substances with the growth medium (without mLIF). The final concentration of solvent, DMSO, was kept at 0.25% (v/v). After 1 or 5 days incubation at 37°C, 5% CO2, the WST‐1 assay was performed to assess the effects on ES‐D3 cell viability. For this purpose, WST‐1 reagent (Roche Diagnostics) was added to each well according to the protocol provided by the supplier and cells were incubated for 3 hours at 37°C, 5% CO2. WST‐1, a stable tetrazolium salt, will be cleaved into a soluble formazan by the succinate‐tetrazolium reductase enzyme that is only active in viable cells. Hence, the amount of formed formazan directly correlates with the number of metabolically active cells in the culture. The absorbance of the formed formazan was measured at 440 nm (background at 620 nm) using a SpectraMax M2 (Molecular Devices). The cell viability of each well was expressed as the percentage cell viability compared with that of the solvent control 0.25% DMSO (set at 100%). At least three independent experiments were done for the ES‐D3 cell viability assay of each test substance.
2.5.3. ES‐D3 cell differentiation assay of the embryonic stem cell test
The ES‐D3 cell differentiation assay of the EST lasts for 10 days. On day 0, droplets of 20 μL of a cell suspension (3.75 × 104 cells/mL), with or without test substances, were placed between the well borders on the inner side of the lid of a 96‐well plate to initiate the formation of embryoid bodies (EBs) via hanging drop culture. All wells of the 96‐well plate were filled with 250 μL of phosphate‐buffered saline (Invitrogen) to create an optimal humidity and to prevent evaporation of the hanging drops during 3 days incubation at 37°C, 5% CO2. Sterile caps of Eppendorf tubes were placed in the corner of the 96‐well plates to prevent direct contact of the hanging drops with the plate and the plate was then sealed with Micropore tape (3M). The exposure medium for the ES‐D3 cell differentiation assay was prepared by addition of 400 times concentrated redissolved extracts/samples in DMSO of the respective test substances obtained from the microsomal incubation experiment (see Section 2.2). The bioactivated BaP and DBA were tested in the ES‐D3 cell differentiation assay at the highest final concentration of 2.1 μg/mL and 0.23 μg/mL, respectively. PS and GTLb extracts were tested at the highest final concentration of 33.5 μg raw material/mL, except for sample no. 034‐HFO that was tested at the highest concentration of 3.3 μg raw material/mL, because of limited solubility at higher concentrations. On day 3, the resulting EBs were collected and transferred to bacteriological Petri dishes (60 × 15 mm; Greiner Bio‐One) containing 5 mL exposure medium (with or without test substances). EBs were incubated for another 2 days. On day 5, the EBs were transferred to a 24‐well plate (Greiner Bio‐One) (1 EB/well) containing 1 mL exposure medium/well, and incubated at 37°C, 5% CO2. On day 10, the number of wells (of the 24‐well plate) containing beating cardiomyocytes was scored by visual inspection using a light microscope. The ES‐D3 cell differentiation assay was considered valid if the solvent control had at least 21 of 24 wells that contained beating cardiomyocytes. For each 24‐well plate, the fraction of the 24 wells containing beating cardiomyocytes was determined. The inhibition of differentiation by the test substances was presented as this fraction of the total. Thus, the fraction of 1 indicates that all plated EBs have differentiated into beating cardiomyocytes. 0.25% DMSO (v/v) and 0.065 μg/mL 5‐fluorouracil were used as solvent and positive control of this assay, respectively, and were included in each independent experiment. At least three independent experiments were done for the ES‐D3 cell differentiation assay of each test substance.
2.6. Data analysis
Figures of concentration‐response curves obtained from the EST were made using GraphPad Prism software version 5.0. To this end, results from ES‐D3 cell viability (1 and 5 days exposure) and differentiation assays of the EST were fitted to a sigmoid dose‐response curve with three parameters in the GraphPad Prism 5.0. In parallel, benchmark dose (BMD) software version 2.6.1 from US‐EPA was used to calculate the benchmark concentration (BMC) that corresponds to 50% decrease of ES‐D3 cell viability (BMCv50) or 50% inhibition of ES‐D3 cell differentiation into beating cardiomyocytes (BMCd50). For the BMCv50 determination, concentration‐response curves from the ES‐D3 cell viability assay were fitted to the continuous models available in the BMD software (Hill, exponential, linear, polynomial and power models). For the BMCd50 determination, concentration‐response curves from ES‐D3 cell differentiation assay were fitted to all dichotomous concentration‐response models (gamma, logistic, log‐logistic, probit, log‐probit, Weibull, multistage cancer, multistage and the quantal‐linear models) available in the BMD software. The benchmark response was set at 50%, representing either a 50% decrease in cell viability (BMCv50) or 50% inhibition of cardiomyocyte differentiation (BMCd50). The performance of each fitted model was evaluated based on the scaled residuals, the Akaike's information criterion, the visual inspection of the model fit and the global goodness‐of‐fit, with fitted models showing a goodness‐of‐fit P ≥ .05 being accepted (Gift & Davis, 2017; Haber et al., 2018). The BMC values were selected from the accepted model with the lowest Akaike's information criterion (Haber et al., 2018; Kamelia et al., 2019).
3. RESULTS
3.1. Effects of benzo[a]pyrene and dibenz[a,h]anthracene in the embryonic stem cell test without metabolic activation
Figure 1 presents the effect of the two 5‐ring PAH model compounds, BaP and DBA, in the ES‐D3 cell viability and differentiation assays of the EST. As shown in Figure 1A, BaP did not affect cell viability or inhibit ES‐D3 differentiation up to the highest concentration tested (12.6 μg/mL or 50 μm). Interestingly, DBA, another five‐ring PAHs, inhibited the differentiation of ES‐D3 cells into beating cardiomyocytes in a concentration‐dependent manner (BMCd50: 3 μg/mL equal to 10.8 μm) at non‐cytotoxic concentrations (Figure 1B).
Figure 1.
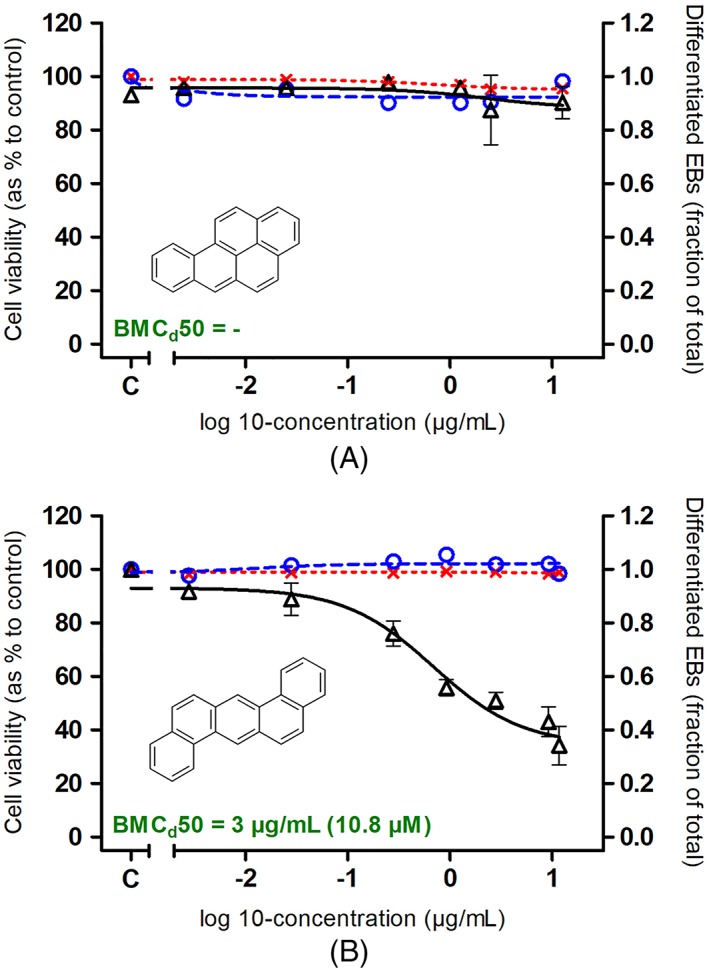
A, Benzo[a]pyrene. B, Dibenz[a,h]anthracene. Concentration‐dependent effects of benzo[a]pyrene and dibenz[a,h]anthracene on ES‐D3 cell viability upon 1 day (red x and red dotted line) and 5 days (blue ○ and blue dashed line) exposure and on ES‐D3 cell differentiation into beating cardiomyocytes (Δ and black‐continuous line). Results represent data from at least three independent experiments and are presented as mean ± SEM. EBs, embryoid bodies
3.2. Microsomal conversion of benzo[a]pyrene and dibenz[a,h]anthracene using hamster liver microsomes
Figure 2 presents the UPLC chromatograms of the redissolved extracts from incubations of BaP and DBA with and without bioactivation. These results reveal that the optimized biotransformation system using hamster liver microsomes has effectively transformed the parent PAH substances into metabolites. For bioactivated BaP, the formation of 3OH‐BaP (retention time: 7.8 minutes) as the major metabolite formed from BaP (retention time: 9.5 minutes) is readily observed (Figure 2A). The concentration of 3OH‐BaP upon bioactivation is ±200 μg/mL (±760 μm). Similarly, DBA has successfully been converted, as reflected by the appearance of metabolite peaks at an earlier retention time (~6‐9.5 minutes) than that of DBA itself (retention time: 9.9 minutes) (Figure 2B). For DBA, the formation of two major metabolites is observed (retention time: 8.4 and 9.1 minutes), which can be tentatively identified as dihydroxy‐DBA and hydroxy‐DBA, based on available literature data on metabolism of DBA (Boyland & Sims, 1964, 1965; Platt et al., 1990). TOF‐MS analysis confirmed that these major metabolites, eluting at 31.8 and 37.6 minutes under the LC conditions used for the TOF measurements are dihydroxy‐DBA, with m/z in negative mode amounting to 309.0921, and hydroxy‐DBA, with m/z in negative mode amounting to 293.0972, respectively (Appendix C; see Supporting Information).
3.3. Microsomal conversion of petroleum substances and gas‐to‐liquid base oil extracts using hamster liver microsomes
Figure 3 presents the UPLC chromatograms obtained for extracts of the microsomal incubations with PS and GTLb, performed using the method shown valid for metabolic conversion of the model PAHs BaP and DBA. Results obtained reveal conversion of various PS constituents into metabolites, which, given the complex nature of these samples were not further identified, but likely reflect cytochrome P450‐mediated formation of metabolites, as they are more polar than the parent compounds, as shown by a higher peak area in the earlier retention time of the UPLC chromatogram as compared with the corresponding control without bioactivation (Figure 3). For the GTLb extract, the differences between the redissolved extracts obtained with and without metabolic activation were limited (bottom right graph of Figure 3).
Figure 3.
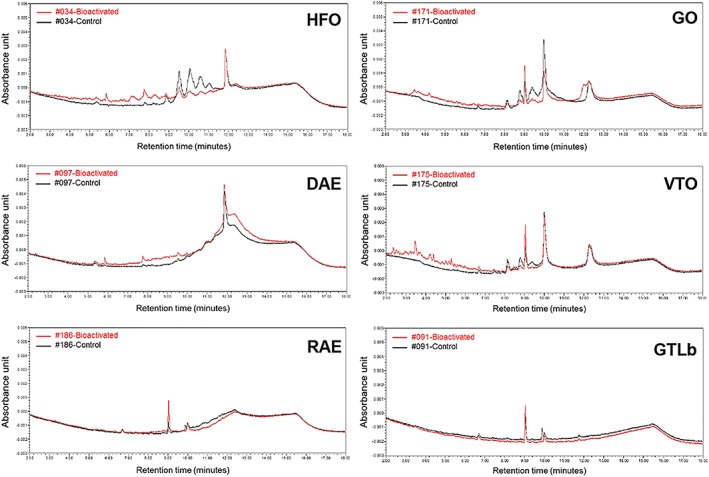
Relevant parts of the chromatograms for ultra performance liquid chromatography of the redissolved extracts from incubations with and without bioactivation of selected petroleum substances and GTLb extracts. Relative increase in the area under the curve of the first part of the chromatogram (retention time: <9.5 min) reflecting formation of polar metabolites, amounted to 15% (sample 034‐HFO), 12% (sample 097‐DAE), 7.6% (sample 171‐GO), 15% (sample 175‐VTO), 2.2% (sample 186‐RAE) and 2.1% (sample 091‐GTLb), respectively, as compared with the area under the curve of the respective control (unmetabolized sample) (Appendix H; see Supporting Information). DAE, distillate aromatic extract; GO, gas oil; GTLb, gas‐to‐liquid base oil; HFO, heavy fuel oil; RAE, residual aromatic extract; VTO, vacuum tower overhead oil
3.4. Effects of benzo[a]pyrene and dibenz[a,h]anthracene extracts with and without preliminary metabolic activation in the embryonic stem cell test
Figure 4 compares the in vitro PDT potency of BaP and DBA with and without metabolic activation as observed in the differentiation assay of the EST. Cytotoxicity at both days 1 and 5 was not observed for any of the samples (data not shown). Figure 4A shows that while BaP tested negative without bioactivation, BaP was able to inhibit the differentiation of ES‐D3 cells after bioactivation using hamster liver microsomes (BMCd50: 1 μg/mL; Appendix D; see Supporting Information). Given the fact that 3OH‐BaP was the major metabolite formed (Figure 2A), this metabolite was also tested in the EST (Figure 5). The concentration of 3OH‐BaP in the bioactivated BaP extract is ±200 μg/mL (±760 μm), which was determined by fitting the area under the curve from the UPLC measurement to the calibration curve of 3OH‐BaP (from DMSO stock solutions). As can be seen in Figure 5, 3OH‐BaP caused a concentration‐dependent inhibition of ES‐D3 cell differentiation at concentrations that do not affect cell viability, giving a BMCd50 value of 1 μg/mL (3.7 μm; Appendix D; see Supporting Information). For DBA, the ability of this five‐ring PAH model compound to inhibit the differentiation of ES‐D3 cell differentiation in the EST was not notably different with and without bioactivation (Figure 4B).
Figure 4.
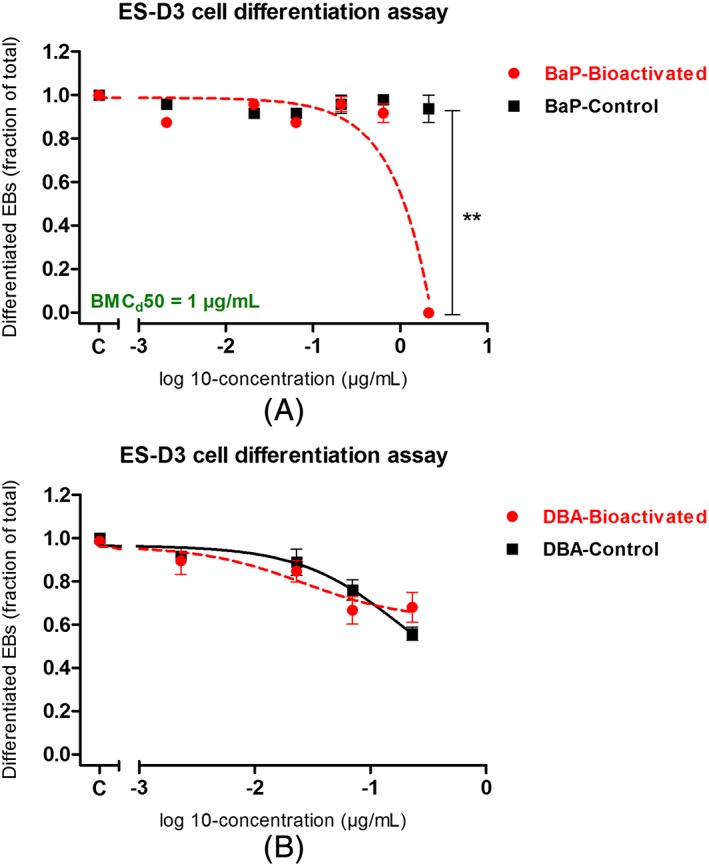
A, BaP. B, DBA. Comparison of effects of BaP and DBA in the ES‐D3 cell differentiation assay of the embryonic stem cell test, with (red ● and red dashed line) and without (■ and black continuous line) bioactivation using hamster liver microsomes. Student t‐test analysis (performed using GraphPad Prism 5.0) showed that no significant difference is obtained when comparing the results of DBA tested in the ES‐D3 cell differentiation assay, with and without bioactivation. In contrast, the bioactivated BaP, in particular at the highest concentration applied, showed more potent activity in inhibiting the ES‐D3 cell differentiation assay as compared with its control (without bioactivation). The concentration ranges tested here are known not to induce any cytotoxicity effects to ES‐D3 cells used for the embryonic stem cell test (data not shown). BaP, benzo[a]pyrene. DBA, Dibenz[a,h]anthracene; EBs, embryoid bodies
Figure 5.
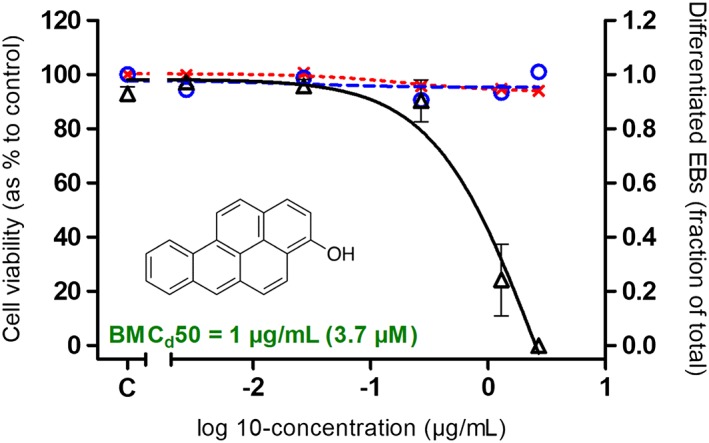
Concentration‐dependent effects of 3‐hydroxybenzo[a]pyrene, identified as the major metabolite formed upon microsomal incubations of benzo[a]pyrene (see Figure 2A), on ES‐D3 cell viability upon 1 day (red x and red dotted line) and 5 days (blue ○ and blue dashed line) exposure and on ES‐D3 cell differentiation into beating cardiomyocytes (Δ and black continuous line). Results represent data from at least three independent experiments and are presented as mean ± SEM. EBs, embryoid bodies
3.5. Effects of petroleum substances and gas‐to‐liquid base oil extracts with and without preliminary metabolic activation in the embryonic stem cell test
Also for the PS and GTLb extracts under study, their potencies in the EST with and without metabolism were compared (Figure 6). The results obtained show that the DMSO extracts of the PS tested (sample 034‐HFO, 097‐DAE, 171‐GO, 175‐VTO, 186‐RAE) were able to inhibit the differentiation of ES‐D3 cells in the EST, as reported before (Kamelia et al., 2019). Interestingly, their PDT potency was not remarkably changed when ES‐D3 cells were exposed to the metabolite mixtures of the corresponding PS extracts (Figure 6A‐E). The GTLb extract was still unable to induce any effect in the EST regardless of the presence or absence of preliminary metabolic activation (Figure 6F).
Figure 6.
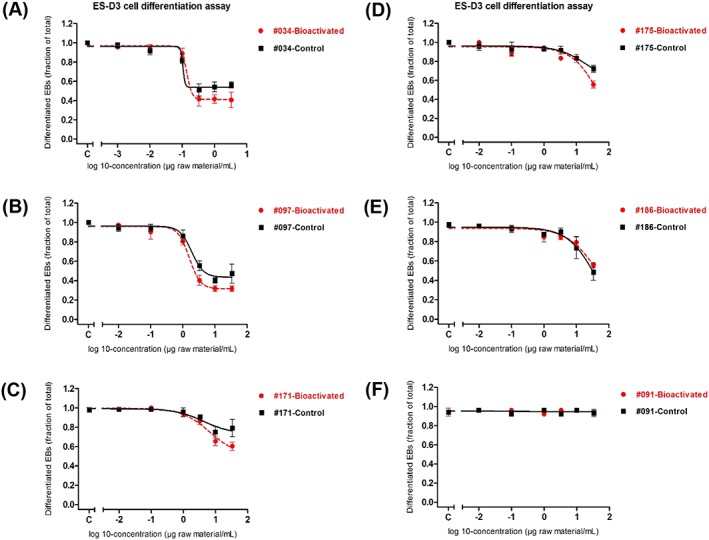
A, 034‐HFO. B, 097‐DAE. C, 171‐GO. D, 175‐VTO. E, 186‐RAE. F, GTLb. Effects of dimethyl sulfoxide extracts of petroleum substances on inhibition of ES‐D3 cell differentiation into beating cardiomyocytes in the embryonic stem cell test, with (red ● and red dashed line) and without (■ and black continuous line) bioactivation using hamster liver microsomes. Student t‐test analysis (performed using GraphPad Prism 5.0) showed that no significant difference is obtained when comparing the results of extracts of petroleum substances tested in the ES‐D3 cell differentiation assay, with and without bioactivation (Appendix F; see Supporting Information). Results represent data from at least three independent experiments and are presented as mean ± SEM. DAE, distillate aromatic extract; EBs, embryoid bodies; GO, gas oil; GTLb, gas‐to‐liquid base oil; HFO, heavy fuel oil; RAE, residual aromatic extract; VTO, vacuum tower overhead oil
4. DISCUSSION
Recently we reported on the use of the EST to study PDT potency of DMSO extracts of PS and GTL products (Kamelia et al., 2019, 2017). A highly significant correlation was found between the EST results and in vivo data (R 2 = 0.97; Kamelia et al., 2019). Additionally, the results obtained in the EST correlated well with the three‐ to seven‐ring PAH content in the tested substances. This is of interest as for several of the adverse effects of PAHs, including particularly their genotoxicity and carcinogenicity, they require bioactivation (Blackburn et al., 1986; Hermann et al., 1980). Whether this also holds for their PDT potency remains to be elucidated. In this respect it is important to note that the ES‐D3 cells used for the EST do not contain substantial xenobiotic metabolizing capacity and will therefore in principle assess the embryotoxicity potential of the parent substances without considering possible bioactivation. The present study aimed to evaluate the consequences of biotransformation for the in vitro PDT of some selected model PAH and DMSO extracts of PS. To this end, the EST was combined with an exogenous biotransformation system. Two 5‐ring PAH model compounds, BaP and DBA, together with DMSO extracts of five PS and one GTLb were tested in the EST, with and without preliminary metabolic activation. Preliminary metabolic activation was achieved by incubating the parent substances with hamster liver microsomes in the presence of NADPH. BaP and DBA were selected as parent PAH model compounds as they represent five‐ring PAH constituents present in the PS extracts under study while both of them are known to induce embryotoxicity in vivo (Bui, Tran, & West, 1986; D'Mello, 2003). The selected PS extracts represented different categories of PS (DAE, GO, HFO, RAE, VTO) with a systematic variation in their PAH content (from 3.3% to 48% wt. PAHs; Appendix A; see Supporting Information) and were shown before to induce concentration‐ and PAH content‐dependent in vitro PDT in the EST (Kamelia et al., 2019).
Results obtained using BaP and DBA as the PAH model compounds show that biotransformation may modify the PDT potency of the test substances, although it appeared not essential for PDT induction by all model substances. BaP tested positive for the ES‐D3 cell differentiation assay of the EST, only upon bioactivation (Figure 4A). These results indicate that bioactivation to 3OH‐BaP likely plays a role in the PDT of BaP (Figure 2A and Figure 5). In contrast, DBA, also a five‐ring PAH, showed a concentration‐dependent inhibition of ES‐D3 cell differentiation by the parent compound, and this potency was not affected upon its microsomal conversion. Furthermore, when ES‐D3 cells were exposed to the extracts of a series of model PS, including nos 034‐HFO, 097‐DAE, 171‐GO, 175‐VTO and 186‐RAE, the PS‐induced PDT in the EST was not remarkably changed following bioactivation (Figure 6). These data suggest that bioactivation may play a minor role in the in vitro PDT of these PS extracts used as model compounds. GTLb extracts remained unable to induce any effect in the EST regardless of the presence or absence of preliminary metabolic activation, which is in line with the absence of PAHs in these substances (Boogaard, Carrillo, Roberts, & Whale, 2017; Kamelia et al., 2019, 2017). To add, the results obtained in the present study for the extracts incubated without microsomes are comparable with our previous published data (Kamelia et al., 2019) on the same PS and GTLb extracts tested in the ES‐D3 cell differentiation assay of the EST as such (see Appendix G; see Supporting Information). Altogether, the results obtained indicate that some PAH constituents may require bioactivation to become able to induce in vitro PDT, but some do not and this latter also appears to hold for the (majority of) the PS constituents responsible for the in vitro PDT of these complex substances in the EST.
This conclusion is corroborated by the observation reported before that the EST, without combining it with a metabolic activation system, appeared to assess, already adequately, the in vitro PDT potency of DMSO extracts of PS UVCBs, as compared with their potencies observed in vivo (Kamelia et al., 2019). The fact that microsomal bioactivation of PAH‐containing PS may not be a prerequisite for their PDT may be linked to the fact that the underlying modes of action may require non‐covalent receptor or enzymes interactions rather than the chemical (DNA) reactivity that is necessary to exert the mutagenic and carcinogenicity potency of these substances (Blackburn et al., 1986; Siddens et al., 2012). Corroborating this notion, our recent observation showed that the in vitro PDT of the PS extracts tested in the present study was at least in part mediated via the aryl hydrocarbon receptor (AhR) (Kamelia et al., 2017). It is generally accepted that three‐ to seven‐ring PAHs are able to activate the AhR (Pieterse et al., 2013; Puga, Tomlinson, & Xia, 2005), and the AhR‐mediated activity of PAH‐containing PS has been linked to the developmental toxicity potency of these groups of substances (Billiard et al., 2006; Goodale et al., 2013; Incardona, Linbo, & Scholz, 2011). The ability of chemical substances to interact with certain nuclear receptors is affected by their molecular size and chemical structure (Balaguer et al., 2017). Comparing the chemical structure of DBA and BaP, the molecular arrangement of the aromatic rings is more angular in DBA as compared with BaP. Regarding their molecular size, BaP (molecular weight: 252.3) has a lower molecular weight compared with the DBA (molecular weight: 278.3). With these differences in molecular size and structure, DBA is able to bind to the AhR with a higher affinity than BaP, as measured in the AhR CALUX assay (Appendix B; see Supporting Information). This may also imply that DBA displays a higher intrinsic potential for interaction with other receptors present in the ES‐D3 cells, including the retinoic acid receptor and the estrogen receptor (ER) that are known to be potentially relevant in PDT (Adam et al., 2019; Dimopoulou et al., 2018; Louisse et al., 2011). Hence, future studies may consider the influence of structural features and size of PAHs, particularly those present in PS extracts, on their ability to activate receptors relevant for their embryotoxicity potency. In the case of 3OH‐BaP, AhR activation may play a relatively smaller contribution to the observed in vitro PDT, as shown by its weak AhR‐mediated activity in the AhR CALUX assay (see Appendix B; see Supporting Information). For ERα‐mediated activity, available data in our lab showed that 3OH‐BaP is a stronger ERα activator than BaP (Appendix B; see Supporting Information). Thus, the role of other receptors than the AhR should be investigated related to the 3OH‐BaP‐induced and PS extracts‐induced in vitro PDT potency.
For bioactivation of the PAHs and PAH‐containing PS, the present study made use of hamster liver microsomes as it has been shown that they are more effective for bioactivation of these groups of substances in comparison with rat or mouse liver microsomes (Blackburn et al., 1986; Haugen & Peak, 1983; Hermann, 1981; Hermann et al., 1980). For instance, Blackburn et al. (1986) modified the standard Ames test, later known as the modified Ames test, for mutagenicity testing of PS by (1) using hamster rather than rat liver S9 mix for generating metabolite mixtures of PS, and (2) using DMSO extracts of PS instead of neat/bulk materials, which improved the interaction of potentially toxic constituents present in PS with the aqueous in vitro test system. Using the above‐mentioned modifications, Blackburn and co‐workers were able to assess correctly the mutagenicity potency of 18 PS samples from different product categories (Blackburn et al., 1986). Furthermore, hamster liver microsomes, and not hamster S9 fractions, were used in the present study because the activity of cytochrome P450 enzymes in hamster liver microsomes (expressed in nmol/mg protein) is three times higher than in the hamster S9 fractions (Appendix E; see Supporting Information), while it was assumed that particularly cytochrome P450‐mediated biotransformation to hydroxylated metabolites would bioactivate the PAHs (Diamante et al., 2017; Geier et al., 2018). The results obtained for 3OH‐BaP support this assumption, while DBA did not need bioactivation. Thus, to what extent bioactivation by cytochrome P450s to hydroxylated metabolites is required for the PDT of other PAH constituents remains to be evaluated, although the data obtained with the PAH‐containing PS seem to indicate that this role may be not essential.
In addition, it is of interest to note that integrating an exogenous biotransformation system with an in vitro test system can be done by either direct addition of the liver S9/microsomes to the cells or via preincubation of parent substances with liver S9/microsomes followed by incubations of cells with the extracted activated parent substances. As it is known that liver S9/microsomes are toxic for embryonic stem cells (Wobus & Löser, 2011), in the present study, preincubation was applied and the extracted bioactivated metabolite mixtures were incubated with the ES‐D3 cells of the EST. This experimental design implies that only relatively stable metabolites will be included in the in vitro PDT testing but it can be foreseen that this matches the in vivo situation where also only relatively stable metabolites may be transported to the developing fetus passing the placenta. The role of 3OH‐BaP in the developmental toxicity potency of BaP corroborates this assumption for a role of relatively stable metabolites. It is important to note that in vivo 3OH‐BaP may be detoxified via Phase II conjugation. Thus, the methodology of the present study enables identification of the hazard of potential PAH metabolites in the developmental toxicity of these compounds, although quantification of their in vivo role, including the potential for their detoxification remains to be established. This could be achieved by so‐called PBK model facilitated reverse dosimetry, at the same time elucidating the internal concentrations and potential role of unconjugated 3OH‐BaP. This approach has been used previously to translate in vitro EST data to in vivo developmental toxicity data taking formation and detoxification of reactive metabolites into account (Louisse et al., 2011; Strikwold et al., 2013).
The results obtained from UPLC analysis following microsomal incubations of PS extracts, with and without bioactivation, do not allow the identification and quantification of parent substances and formed metabolites. However, the presented results in Figure 3 clearly show the difference of the chromatogram pattern between bioactivated PS extracts and their corresponding control (without bioactivation) (Figure 3). Although we could not confirm the peaks in chromatograms in relation to the amount of PAH in the PS extracts tested, the measured UPLC chromatograms (Figure 3) at least represent the ultraviolet‐visual absorbing PAHs with the assumption that all constituents present will also absorb to a similar extent hence the percentage of the total chromatogram would be equal to the percentage/amount of PAHs present in each PS extract tested. In view of this, future research should apply a structured analytical approach tailored to the properties of each PS category to know (at least) the vast majority of constituents present, including PAHs. For example, a combination of several analytical methods, including LC/MS, gas chromatography/MS and nuclear magnetic resonance spectroscopy, may be needed to provide complementary information for characterization of these substances (Concawe, 2012). Such data and information will obviously help to unravel further the role of specific groups of PAH constituents for the observed in vitro PDT induced by these groups of substances.
In conclusion, the present study has successfully combined an exogenous biotransformation system, using hamster liver microsomes, with the EST to compare in vitro PDT potency of two 5‐ring PAHs: BaP and DBA, and the DMSO extracts of PS and GTLb, with and without bioactivation. BaP is able to inhibit the ES‐D3 cell differentiation only upon bioactivation where DBA does not need to be bioactivated to exert its embryotoxicity potency in the EST. Furthermore, PS extract‐induced in vitro PDT in the EST does not substantially change with and without bioactivation, suggesting that metabolism may not play a crucial role in mediating the in vitro embryotoxicity potency. In contrast, GTLb extracts remain unable to induce any effects in the EST regardless of the presence or absence of metabolic activation. By combining a biotransformation system with the EST, the assay may better reflect the in vivo situation. In the future, compounds other than PAHs could be tested to broaden the applicability domain of the presented test system, facilitating the combination of an exogenous biotransformation system and the EST, to evaluate in vitro PDT potency of chemical substances with and without bioactivation. Finally, our findings may serve as a basis for future research to unravel the role of metabolism in the developmental toxicity of PAHs containing PS.
FUNDING
This work was supported by Concawe (Grant number: 201506110) and Operationeel Programma Kansen voor West II (EFRO) (KVW‐00181).
CONFLICT OF INTEREST
LK, LH, BS, BB and IMCMR declare that they have no conflict of interest. Peter J. Boogaard is employed by Shell International, a member company of Concawe, and chairman of the toxicology group of Concawe. Hans Ketelslegers is Science Executive for health at Concawe. Both Prof. Boogaard and Dr. Ketelslegers are totally free (by contract) to freely design and conduct research and express their own scientific opinion without any obligation towards either Shell or Concawe. The current findings are not intended to constitute any product endorsement.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Dr. Jacques Vervoort (Department of Biochemistry, Wageningen University and Research) for his advice and help with the TOF‐MS analysis.
Kamelia L, de Haan L, Spenkelink B, et al. The role of metabolism in the developmental toxicity of polycyclic aromatic hydrocarbon‐containing extracts of petroleum substances. J Appl Toxicol. 2020;40:330–341. 10.1002/jat.3906
REFERENCES
- Adam, A. H. B. , Zhang, M. , de Haan, L. H. J. , van Ravenzwaay, B. , Louisse, J. , & Rietjens, I. M. C. M. (2019). The in vivo developmental toxicity of diethylstilbestrol (DES) in rat evaluated by an alternative testing strategy. Archives of Toxicology, 93(7), 2021–2033. 10.1007/s00204-019-02487-6 [DOI] [PubMed] [Google Scholar]
- Balaguer, P. , Delfosse, V. , Grimaldi, M. , & Bourguet, W. (2017). Structural and functional evidences for the interactions between nuclear hormone receptors and endocrine disruptors at low doses. Comptes Rendus Biologies, 340, 414–420. 10.1016/j.crvi.2017.08.002 [DOI] [PubMed] [Google Scholar]
- Billiard, S. M. , Timme‐Laragy, A. R. , Wassenberg, D. M. , Cockman, C. , & Di Giulio, R. T. (2006). The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicological Sciences, 92, 526–536. 10.1093/toxsci/kfl011 [DOI] [PubMed] [Google Scholar]
- Blackburn, G. R. , Deitch, R. A. , Schreiner, C. A. , & Mackerer, C. R. (1986). Predicting carcinogenicity of petroleum distillation fractions using a modified Salmonella mutagenicity assay. Cell Biology and Toxicology, 2(1), 63–84. 10.1007/BF00117708 [DOI] [PubMed] [Google Scholar]
- Boogaard, P. J. , Carrillo, J. C. , Roberts, L. G. , & Whale, G. F. (2017). Toxicological and ecotoxicological properties of gas‐to‐liquid (GTL) products. 1. Mammalian toxicology. Critical Reviews in Toxicology, 47, 121–144. 10.1080/10408444.2016.1214676 [DOI] [PubMed] [Google Scholar]
- Boyland, E. , & Sims, P. (1964). Metabolism of polycyclic compounds. 24. The metabolism of benz[a]anthracene. Biochemical Journal, 91(3), 493–506. 10.1042/bj0910493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland, E. , & Sims, P. (1965). The metabolism of benz[a]anthracene and dibenz[a,h]anthracene and their 5,6‐epoxy‐5,6‐dihydro derivatives by rat‐liver homogenates. Biochemical Journal, 97(1), 7–16. 10.1042/bj0970007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui, Q. Q. , Tran, M. B. , & West, W. L. (1986). A comparative study of the reproductive effects of methadone and benzo[a]pyrene in the pregnant and pseudopregnant rat. Toxicology, 42, 195–204. 10.1016/0300-483X(86)90009-0 [DOI] [PubMed] [Google Scholar]
- Clonfero, E. , Nardinim, B. , Machioro, M. , Bordin, A. , & Gabbani, G. (1996). Mutagenicity and contents of polycyclic aromatic hydrocarbons in used and recycled motor oils. Mutation Research, 368, 283–291. 10.1016/s0165-1218(96)90070-1 [DOI] [PubMed] [Google Scholar]
- Concawe . (2012). REACH—analytical characterization of petroleum UVCB substances. Retrieved from https://www.concawe.eu/wp-content/uploads/2017/01/rpt_12-7-2012-05443-01-e.pdf.
- Concawe . (2019). REACH roadmap for petroleum substances. Retrieved from https://www.concawe.eu/wp-content/uploads/REACH-Roadmap-for-Petroleum-Substances-2019-update.pdf.
- Diamante, G. , do Amaral E Silva Müller, G. , Menjivar‐Cervantes, N. , Xu, E. G. , Volz, D. C. , Dias Bainy, A. C. , & Schlenk, D. (2017). Developmental toxicity of hydroxylated chrysene metabolites in zebrafish embryos. Aquatic Toxicology, 189, 77–86. 10.1016/j.aquatox.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Dimopoulou, M. , Verhoef, A. , Gomes, C. A. , van Dongen, C. W. , Rietjens, I. M. C. M. , Piersma, A. H. , & van Ravenzwaay, B. (2018). A comparison of the embryonic stem cell test and whole embryo culture assay combined with the BeWo placental passage model for predicting the embryotoxicity of azoles. Toxicology Letters, 286, 10–21. 10.1016/j.toxlet.2018.01.009 [DOI] [PubMed] [Google Scholar]
- D'Mello, J. P. F. (2003). Food safety: Contaminants and toxins. Oxfordshire, UK: CABI Pub; 10.1079/9780851996073.0000 [DOI] [Google Scholar]
- ECHA (European Chemicals Agency) . (2009). Information requirements for repeated dose toxicity and reproductive toxicity—substances over 100 (and 1000) tonnes. Retrieved from https://echa.europa.eu/documents/10162/13644/reach_factsheet_testing_en.pdf.
- European Centre for the Validation of Alternative Methods (ECVAM) . (2017). DB‐ALM protocol no. 113: embryonic stem cell test (EST). Retrieved from https://www.semanticscholar.org/paper/DB-ALM-Protocol-n-%C2%B0-113-%3A-Embryonic-Stem-Cell-Test/ffd673016353d052d0c90b5da26704e13e9e9fc8.
- Geier, M. C. , Chlebowski, A. C. , Truong, L. , Massey Simonich, S. L. , Anderson, K. A. , & Tanguay, R. L. (2018). Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Archives of Toxicology, 92, 571–586. 10.1007/s00204-017-2068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genschow, E. , Spielmann, H. , Scholz, G. , Seiler, A. , Brown, N. , Piersma, A. , … Becker, K. (2002). The ECVAM international validation study on in vitro embryotoxicity tests: Results of the definitive and evaluation of prediction models. ATLA, 30, 151–176. 10.1177/026119290203000204 [DOI] [PubMed] [Google Scholar]
- Genschow, E. , Spielmann, H. , Scholz, G. , Pohl, I. , Seiler, A. , Clemann, N. , … Becker, K. (2004). Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. ATLA, 32, 209–244. 10.1177/026119290403200305 [DOI] [PubMed] [Google Scholar]
- Gift, J. , & Davis, J. A. (2017). Interpreting the results of EPA dose‐response models. Retrieved from https://ntp.niehs.nih.gov/ntp/about_ntp/ntpexpanel/2017/october/presentations/09gift_508.pdf.
- Goodale, B. C. , Tilton, S. C. , Wilson, G. , Corvi, M. M. , Janszen, D. B. , Anderson, K. A. , … Tanguay, R. L. (2013). Structurally distinct polycyclic aromatic hydrocarbons induce differential transcriptional responses in developing zebrafish. Toxicology and Applied Pharmacology, 272, 656–670. 10.1016/j.taap.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber, L. T. , Dourson, M. L. , Allen, B. C. , Hertzberg, R. C. , Parker, A. , Vincent, M. J. , … Boobis, A. R. (2018). Benchmark dose (BMD) modeling: current practice, issues, and challenges. Critical Reviews in Toxicology, 48(5), 387–415. 10.1080/10408444.2018.1430121 [DOI] [PubMed] [Google Scholar]
- Haugen, D. A. , & Peak, M. J. (1983). Mixtures of polycyclic aromatic compounds inhibit mutagenesis in the Salmonella/mammalian microsome mutagenicity test. Mutation Research, 31, 257–269. 10.1016/0165-1218(83)90063-0 [DOI] [PubMed] [Google Scholar]
- Hermann, M. (1981). Synergistic effects of individual polycyclic aromatic hydrocarbons on the mutagenicity of their mixtures. Mutation Research, 90, 339–409. 10.1016/0165-1218(81)90062-8 [DOI] [PubMed] [Google Scholar]
- Hermann, M. , Chaudi, O. , Weill, N. , Bedoulle, H. , & Hofnung, M. (1980). Adaptation of the Salmonella/mammalian microsome test to the determination of the mutagenic properties of mineral oils. Mutation Research, 77, 327–339. 10.1016/0165-1218(80)90004-x [DOI] [PubMed] [Google Scholar]
- Incardona, J. P. , Linbo, T. L. , & Scholz, N. L. (2011). Cardiac toxicity of 5‐ring polycyclic aromatic hydrocarbons is differentially dependent on the aryl hydrocarbon receptor 2 isoform during zebrafish development. Toxicology and Applied Pharmacology, 257, 242–249. 10.1016/j.taap.2011.09.010 [DOI] [PubMed] [Google Scholar]
- Kamelia, L. , Louisse, J. , de Haan, L. , Rietjens, I. M. C. M. , & Boogaard, P. J. (2017). Prenatal developmental toxicity testing of petroleum substances: Application of the mouse embryonic stem cell test (EST) to compare in vitro potencies with potencies observed in vivo. Toxicology In Vitro, 44, 303–312. 10.1016/j.tiv.2017.07.018 [DOI] [PubMed] [Google Scholar]
- Kamelia, L. , de Haan, L. , Ketelslegers, H. B. , Rietjens, I. M. C. M. , & Boogaard, P. J. (2019). In vitro prenatal developmental toxicity induced by some petroleum substances is mediated by their 3‐ to 7‐ring PAH constituent with a potential role for the aryl hydrocarbon receptor (AhR). Toxicology Letters, 315, 64–76. 10.1016/j.toxlet.2019.08.001 [DOI] [PubMed] [Google Scholar]
- Louisse, J. , Gönen, S. , Rietjens, I. M. C. M. , & Verwei, M. (2011). Relative developmental toxicity potencies of retinoids in the embryonic stem cell test compared with their relative potencies in in vivo and two other in vitro assays for developmental toxicity. Toxicology Letters, 203, 1–8. 10.1016/j.toxlet.2011.02.012 [DOI] [PubMed] [Google Scholar]
- Maltsev, V. A. , Rohdewel, J. , Hescheler, J. , & Wobus, A. M. (1993). Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mechanisms of Development, 44(1), 41–50. 10.1016/0925-4773(93)90015-P [DOI] [PubMed] [Google Scholar]
- OECD . (2018). OECD 414 guideline for testing of chemicals: prenatal developmental toxicity study. Retrieved from Organization for Economic Cooperation and Development https://www.oecd-ilibrary.org/docserver/9789264070820-en.pdf?expires=1562837990&id=id&accname=guest&checksum=0895A1195E3968F75ACED092AE4FF01E.
- Pieterse, B. , Felzel, E. , Winter, R. , van der Burg, B. , & Brouwer, A. (2013). PAH‐CALUX, an optimized bioassay for AhR‐mediated hazard identification of polycyclic aromatic hydrocarbons (PAHs) as individual compounds and in complex mixtures. Environmental Science and Technology, 47, 11651–11659. 10.1021/es403810w [DOI] [PubMed] [Google Scholar]
- Platt, K. L. , Schollmeier, M. , Frank, H. , & Oesch, F. (1990). Stereoselective metabolism of dibenz(a,h)anthracene to trans‐dihydrodiols and their activation to bacterial mutagens. Environmental Health Perspectives, 88, 37–41. 10.1289/ehp.908837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga, A. , Tomlinson, C. R. , & Xia, Y. (2005). Ah receptor signals cross‐talk with multiple developmental pathways. Biochemical Pharmacology, 69, 199–207. 10.1016/j.bcp.2004.06.043 [DOI] [PubMed] [Google Scholar]
- Roy, T. A. , Johnson, S. W. , Blackburn, G. R. , & Mackerer, C. R. (1988). Correlation of mutagenic and dermal carcinogenic activities of mineral oils with polycyclic aromatic compound content. Toxicological Sciences, 10, 466–476. 10.1016/0272-0590(88)90293-X [DOI] [PubMed] [Google Scholar]
- Seiler, A. E. , & Spielmann, H. (2011). The validated embryonic stem cell test to predict embryotoxicity in vitro. Nature Protocols, 6, 961–978. 10.1038/nprot.2011.348 [DOI] [PubMed] [Google Scholar]
- Siddens, L. K. , Larkin, A. , Krueger, S. K. , Bradfield, C. A. , Waters, K. M. , Tilton, S. C. , … Baird, W. M. (2012). Polycyclic aromatic hydrocarbons as skin carcinogens: Comparison of benzo[a]pyrene, dibenzo[def,p]chrysene and three environmental mixtures in the FVB/N mouse. Toxicology and Applied Pharmacology, 264(3), 377–386. 10.1016/j.taap.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strikwold, M. , Spenkelink, B. , Woutersen, R. A. , Rietjens, I. M. C. M. , & Punt, A. (2013). Combining in vitro embryotoxicity data with physiologically based kinetic (PBK) modelling to define in vivo dose‐response curves for developmental toxicity of phenol in rat and human. Archives of Toxicology, 87(9), 1709–1723. 10.1007/s00204-013-1107-4 [DOI] [PubMed] [Google Scholar]
- van der Jagt, K. , Munn, S. , Tørsløv, J. , & de Bruijn, J. (2004). Alternative approaches can reduce the use of test animals under REACH. European Commission report EUR 21405EN.
- Wobus, A. M. , & Löser, P. (2011). Present state and future perspectives of using pluripotent stem cells in toxicology research. Archives of Toxicology, 85, 79–117. 10.1007/s00204-010-0641-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


