Summary
Raising crop yield potential is a major goal to ensure food security for the growing global population. Photosynthesis is the primary determinant of crop productivity and any gain in photosynthetic CO2 assimilation per unit of leaf area (A) has the potential to increase yield. Significant intraspecific variation in A is known to exist in various autotrophic organs that represent an unexploited target for crop improvement. However, the large number of factors that influence photosynthetic rates often makes it difficult to measure or estimate A under dynamic field conditions (i.e. fluctuating light intensities or temperatures). This complexity often results in photosynthetic capacity, rather than realized photosynthetic rates being used to assess natural variation in photosynthesis. Here we review the work on natural variation in A, the different factors determining A and their interaction in yield formation. A series of drawbacks and perspectives are presented for the most common analyses generally used to estimate A. The different yield components and their determination based on different photosynthetic organs are discussed with a major focus on potential exploitation of various traits for crop improvement. To conclude, an example of different possibilities to increase yield in wheat through enhancing A is illustrated.
Keywords: photosynthesis, genetic variation, wheat, yield, photosynthetic efficiency
Significance Statement
The MS focuses on natural genetic variation in photosynthesis and other physiological processes as possible targets for exploitation for yield improvements.
Introduction
Photosynthesis is the primary determinant of crop productivity and any gain in photosynthetic efficiency has the potential to result in increases in yield (Flood et al., 2011; Lawson et al., 2012; Gu et al., 2014). Free air CO2 enrichment (FACE) studies have provided substantial evidence that increased photosynthetic rates have translated into greater crop yields, demonstrating the link between photosynthesis and yield (Ainsworth and Long, 2005). The yield potential of a crop can be described by the following equation:
where Q is total solar radiation, εi is the interception efficiency, εc is the efficiency for conversion into biomass and εp is the efficiency of partitioning biomass into harvested organs (Long et al., 2015). In the absence of environmental stress, parameters such as harvest index are already close to the theoretical maximum (Foulkes et al., 2010; Reynolds et al., 2012). Additionally, many canopy traits such as canopy architecture (Long et al., 2006), light interception (Murchie et al., 2009), and photosynthetic duration (Shearman et al., 2005) have been optimized. However, empirical analyses of the yield potential components demonstrate significant inefficiency in εc in C3 crops (Zhu et al., 2008, 2010), in which photosynthetic CO2 assimilation per unit leaf area (A) is the primary process (Kebeish et al., 2007; Maurino and Peterhänsel, 2010; Walker et al., 2016). The maximum potential conversion energy efficiency for C3 plants is 4.6% (Zhu et al., 2010). However, plants including crops attain much less than this and therefore A is far from optimal and a target for further improvements (Parry et al., 2010; Long et al., 2015). Several studies have explored opportunities to increase energy conversion through genetic manipulation, including manipulating Calvin cycle activity (Simkin et al., 2015; Lopez‐Calcagno et al., 2018), RuBisCO properties (Parry et al., 2003) and increasing the kinetics of non‐photochemical quenching for photo‐protection (Kromdijk et al., 2016), all of which have translated into increased A and greater plant biomass. However, restrictions on growing genetically modified crops in many countries especially in Europe means that alternative methods to achieve increases in photosynthesis must be realized. An undervalued and currently unexploited opportunity to increase yield, not mutually exclusive of genetic engineering approaches, is the extensive natural variation in photosynthetic capacity in different C3 crops (Rawson et al., 1983; Blum, 1990; Watanabe et al., 1994; Fischer et al., 1998; Hervé et al., 2001; Pettigrew, 2004; Flood et al., 2011; Gu et al., 2012; Lawson et al., 2012; Driever et al., 2014; Gaju et al., 2016; Carmo‐Silva et al., 2017; Qu et al., 2017; Pater et al., 2017; Faralli et al., 2019b). A number of studies have explored natural variation in photosynthesis in commercial wheat varieties (often relative to the year of release) (Fischer et al., 1981, 1998; Blum, 1990; Watanabe et al., 1994; Reynolds et al., 2000; Xue et al., 2002; Chytyk et al., 2011; Sadras et al., 2012), and demonstrated a correlation between photosynthesis and yield (e.g. Blum, 1990; Fischer et al., 1998), although, such a relationship often depended on growth conditions (Xue et al., 2002), or when measurements were taken during the growing season (Reynolds et al., 2000), while others reported no relationship (e.g. Driever et al., 2014). Variation in photosynthesis has been attributed to differences in radiation use efficiency (Sadras et al., 2012), biochemical differences in RuBisCO activation properties (Carmo‐Silva and Salvucci, 2013), carboxylation efficiency (Driever et al., 2014) and electron transport capacity (Carmo‐Silva et al., 2017). In addition, variations in traits limiting the diffusion of CO2 to the site of carboxylation including mesophyll conductance (gm) (Jahan et al., 2014) and stomatal conductance (gs) (Fischer et al., 1998), which also includes the rapidity of gs responses to changing environmental conditions (Lawson et al., 2010, 2012; Faralli et al., 2019b) have been reported in several crops.
Here we review natural variation in physiological traits with a focus on: (i) photosynthetic capacity, which is determined by plant acclimatory responses and constrained by genetics; and (ii) dynamic short‐term modifications to A (e.g. biochemical factors such as the regulation of enzymes, gs and gm). The most common methods used to estimate A are evaluated and discussed. To conclude, we will focus on natural variation in A, stomatal physiology and the associated photosynthetic limitation in wheat (i.e. source limitation; lack of photo‐assimilates, or sink limitation) with a particular emphasis on the potential exploitation for crop improvement.
Natural variation in photosynthesis
The biochemical processes of photosynthesis in C3 crops are considered essentially identical, (although recent metabolite profiling of C3 species by Arrivault et al. (2019) has reported considerable variation in levels of metabolites), however, significant intraspecific and interspecific variation in photosynthetic rates exists, providing a valuable source of unexploited genetic diversity (Flood et al., 2011) (Table 1a). Furthermore, the physiological or genetic mechanisms underlying these differences in both photosynthetic potential as well as dynamic behaviour may provide valuable information on the performance of different cultivars under specific environments (Driever et al., 2014).
Table 1.
(a) Variation in crop photosynthesis rate per unit leaf area collected at saturating light and current atmospheric [CO2] (A sat). All the data were collected at 400 μmol mol−1 [CO2] while in Blum et al. (1990) and Fischer et al. (1998) measurements were taken at 345 μl L−1 [CO2], in Hervé et al. (2001) and Watanabe et al. (1994) measurements were taken at a [CO2] of 350 μmol mol−1 and in Gu et al. (2012) measurements were taken at a [CO2] of 380 μmol mol−1. (b) Variation in stomatal conductance (gs) and mesophyll conductance (gm) in different studies.
| (a) Reference | Crop | Range of A sat (μmol m−2 sec−1) | Intraspecific variation (μmol m−2 sec−1) | Relation with yield |
|---|---|---|---|---|
| Rawson et al. (1983) | Wheat | 20–45 (mg dm−2 h−1) | 12 mg dm−2 h−1 | A sat and yield not correlated. Cumulative CO2 fixation by flag leaf and yield P < 0.001 r 2 = 0.30 |
| Blum (1990) | Wheat | 9.6–16.6 | 7 | High yielding cultivar showed highest A for the flag leaf |
| Watanabe et al. (1994) | Wheat | 25.5–31.5 | 6 | Yield data not present |
| Fischer et al. (1998) | Wheat | 14.8–25.9 | 11.1 | Asat and mean grain yield r 0.72 and 0.91 (P < 0.01) |
| Reynolds et al. (2000) | Wheat | 20.9–27 at booting, 18–23.6 at anthesis, 23–11.8 at grain filling | 11.2 to 5.6 depending on stage | A sat and grain yield P < 0.01, r = 0.73 |
| Chytyk et al. (2011) | Wheat | 27.5–34.5 | 7 | Yield data not present |
| Sadras et al. (2012) | Wheat | 9.3–19.6 | 10.3 | Data not plotted |
| Driever et al. (2014) | Wheat | 30.5–19.1 | 11.4 | Correlation between grain yield and A not significant (P > 0.05) |
| Carmo‐Silva et al. (2017) | Wheat | 21.2–31.1 (pre‐anthesis), 17.1–23.7 (post‐anthesis) | 9.9–6.6 | P < 0.05 (r = 0.27 pre‐anthesis and r = 0.25 post‐anthesis) |
| Pettigrew (2004) | Cotton | 20.3–37.7 | 17.4 | Yield data not present |
| Pater et al. (2017) | Canola | 5.5–22.5 | 17 | Yield data not present |
| Gu et al. (2012) | Rice | 12.8–25.5 | 12.7 | Yield data not present |
| Hervé et al. (2001) | Sunflower | 17.3 ± 10.2 (mean ± SD) | na | Yield data not present |
| (b) Reference | Crop | gs range (mol m−2 sec−1) | gm range (mol m−2 sec−1) | Note |
|---|---|---|---|---|
| Fischer et al. (1998) | Wheat | 0.34–0.57 | – | Field conditions |
| Jahan et al. (2014) | Wheat | – | 0.51–1.05 | Greenhouse conditions |
| González et al. (1999) | Barley | 0.01–0.06 (cm sec−1) | – | Field conditions |
| Barbour et al. (2010) | Barley | 0.25–0.52 | 0.05–0.50 | Greenhouse conditions |
| Pater et al. (2017) | Canola | 0.12–0.63 | – | Large screening in greenhouse and field conditions |
| Hervé et al. (2001) | Sunflower | 1.01 ± 0.08 (mean ± SD) | – | Greenhouse conditions |
| Ouyang et al. (2017) | Rice | 0.15–0.31 | 0.05–0.21 | Pot experiment |
Intraspecific variation in photosynthetic traits in wheat and the potential effect of selection on photosynthesis was shown initially by Rawson et al. (1983) and then by Blum (1990) where breeding in Mediterranean environments had led to an increase in photosynthetic efficiency at saturating light for the modern cultivars compared with older varieties. Furthermore, Watanabe et al. (1994) followed by Fischer et al. (1998) phenotyped historical Australian and Mexican wheat cultivars for photosynthetic traits and reported a strong correlation between increased rates of CO2 assimilation and yield genetic gain with year of release, demonstrating that breeding has unintentionally selected for higher A. Subsequent research focusing on intraspecific variation in major crops such as cotton (Pettigrew, 2004), canola (Pater et al., 2017), rice (Gu et al., 2012), sunflower (Hervé et al., 2001) and wheat (Reynolds et al., 2000; Sadras et al., 2012; Driever et al., 2014; Carmo‐Silva et al., 2017), highlighted a wide diversity of photosynthetic traits [including A sat and the light and CO2 saturated rate of photosynthesis A max; the maximum carboxylation capacity V cmax as well as the maximum rate of electron transport (J max)]. In addition, at the leaf level, CO2 uptake from the atmosphere to the site of carboxylation is subject to two main restrictions, stomatal and mesophyll, both of which therefore determine the rate photosynthesis. gs (the reciprocal of stomatal resistance) controls CO2 diffusion from the atmosphere into the intercellular air spaces in the gaseous phase (Farquhar and Sharkey, 1982; Sharkey, 1985). Subsequently, gm adds an additional limitation in the liquid phase for the diffusion of CO2 from the intercellular airspaces to the site of carboxylation in chloroplasts (Flexas et al., 2008). Intraspecific variation exists for both gs and gm (Table 1b) in a series of food crops including wheat (Fischer et al., 1998; Jahan et al., 2014), barley (González et al., 1999; Barbour et al., 2010) and rice (Ouyang et al., 2017). Therefore exploiting the existing natural variation in photosynthesis as well as optimizing the components determining A in elite cultivars (Driever et al., 2014), landraces (Gaju et al., 2016) and wild relatives (Prins et al., 2016) could provide novel targets for crop improvement.
However, while Crosbie et al. (1981) showed that leaf photosynthesis of maize can be improved by recurrent selection (i.e. increasing the frequency of favourable alleles for quantitatively inherited traits, in this case for A) five cycles of recurrent phenotypic selection did not produce the expected results in term of productivity, and changes in grain yield were not significant for any of the populations tested (Crosbie and Pearce, 1982). Indeed, correlating photosynthesis with yield is not straight forward, with inconsistencies in the relationship described in the literature, for example positive correlation (Carmo‐Silva et al., 2017), no correlation (Ojima, 1974; Driever et al., 2014), or a correlation but only when photosynthesis was measured at particular phenological stages (Gaju et al., 2016). These inconsistences in the relationship between A and yield emphasize the complexity of yield formation in crops that is based on a series of interrelated subcomponents (Miralles and Slafer, 2007), and that is further complicated by the different methodologies used to estimate A and the influence of fluctuating environmental conditions to which the crop is subjected (Lawson et al., 2012). Individual point measurements of A sat or A max taken either at different times during the crop cycle or on individual leaves within the canopy, often do not correlate with yield (Rawson et al., 1983; Driever et al., 2014). Having said this, in some cases (i.e. Fischer et al., 1998) a significant relationship between A sat and some yield components (i.e. grain number) or the average grain yield (over 5 years) was evident. In addition, when operational A was measured (i.e. single measurements of A at light intensities similar to those experienced by the crop) in the field at the pre‐anthesis and post‐anthesis, a strong correlation with yield was reported (Carmo‐Silva et al., 2017). Although an instantaneous ‘snapshot’ analysis of A sat , carried out by Rawson et al. (1983) did not correlate with yield, a significant (P < 0.001) correlation between cumulative carbon assimilation of the flag leaf (measured as several snapshot A sat measurements over the life cycle) and yield was observed. These studies highlight that the different methods used to measure A, the complexity of the relationship between A, plant growth and yield as well as the influence of the environment on these processes, need to be considered for estimating overall crop photosynthesis.
Factors determining the variation in photosynthetic rate per unit leaf area
Biochemical factors and anatomical features
One of the first studies to examine the underlying biochemical function of interspecific variation in photosynthesis was Wullschleger (1993). Using response curves of A as a function of substomatal CO2 concentration (A/Ci), Wullschleger demonstrated that most of the observed variation in A in the 109 species analyzed was attributed to variation in the underlying biochemistry and photosynthetic capacity with differences in both carboxylation capacity (V cmax) and electron transport capacity for RuBP regeneration (J max). Wullschleger (1993) also reported a positive correlation between V cmax and J max suggesting co‐ordinated regulation by these two processes. A small number of species (23) was reported to be limited by the utilization of triose phosphates, which ranged from 4.9 to 20.1 μmol m−2 sec−1, and reflects the short‐term interaction between A and starch–sucrose production, which ultimately reflects growth. It is clear from the representative A/Ci curves in Wullschleger (1993), that the switch‐over point between limitation by carboxylation capacity and capacity for electron transport differed greatly in the four species illustrated, and that the maximum rates of A achieved were vastly different, which may be due to nitrogen allocation between RuBisCO and light harvesting. Nitrogen (N) concentration is a key determinate of A, as the majority of leaf N is invested in the photosynthetic apparatus, in particular RuBisCO (Hikosaka, 2010). Differences in N‐use efficiency and N concentration in different crops have suggested these as targets for both increased A and optimization of fertilization input (Guarda et al., 2004; Hirel et al., 2007). Although, there is evidence that within species variation in A, can be explained by differences in V cmax and J max (Driever et al, 2014; Carmo‐Silva et al., 2017), Driever et al. (2014) highlighted that the variation in carboxylation capacity was not due to RuBisCO content (or N allocation) but possibly RuBisCO activation, demonstrating further complexity in identifying specific targets for future wheat improvement. Furthermore, the same study also reported that some of the highest V cmax values were found in older species, suggesting that photosynthetic capacity potential has not been fully exploited in past breeding programmes. However, since a major goal of future agriculture is to enhance resource‐use efficiency, it has been hypothesized that increasing RuBisCO carboxylation efficiency while reducing N allocation to RuBisCO might be a successful alternative in crops to improve or sustain A (Reynolds et al., 2012). A reduction in RuBisCO content (up to 20%) led to a 10% lower N requirement in wheat, although reductions in A at high light intensities were also present (Reynolds et al., 2012). More recently, Carmo‐Silva et al. (2017) found significant genotypic variation for RuBisCO carboxylation efficiency and RuBisCO content in wheat, with the cultivar Gatsby combining a high A and a low RuBisCO content, suggesting the potential of this preferable combination for further exploitation. Genetic engineering approaches have shown that increasing protein abundance (e.g. sedoheptulose1,7‐biphosphatase, SBPase) led to a significant increase in A which suggests that although photosynthesis requires a large number of protein–protein interactions, part of the genetic variation can be explained by differences in key protein abundance and activity, that result in improved photosynthetic capacity (Flood et al., 2011; Simkin et al., 2019) and which also might explain variation in metabolite profiles in C3 species (Arrivault et al., 2019). The potential for exploiting natural variation in photosynthetic capacity has been demonstrated by Gu et al. (2014) who used a simulation analyses to assess the contribution that the natural variation in RuBisCO and electron transport rate could make to photosynthesis in rice and showed that exploiting this could increase rice yield by 22–29%, depending on location and year.
Many studies have focused on significant variation in photosynthetic capacity that is determined by acclimation to particular environmental conditions and genetically constrained. However, on a day‐to‐day basis, plants respond dynamically to changes in the surrounding environmental conditions that introduce a further layer of complexity to variation in photosynthesis as there is significant variation in dynamic responses. These dynamic processes include regulation and expression levels of enzymes (Sassenrath‐Cole and Pearcy, 1994; Hikosaka, 2010), dynamic regulation in response to environmental change (Sassenrath‐Cole et al., 1994) including changes in non‐photochemical quenching of excess energy dissipating mechanisms (Külheim et al., 2002; Lawson et al., 2012), and the rapidity of stomatal responses (Lawson et al., 2010, 2012; McAusland et al., 2016) as well as developmental responses to growth environment (Flood et al., 2011; Gilbert et al., 2011).
Other processes, although not directly related to the photosynthetic machinery, also play a role in photosynthetic performance. For instance, sucrose transport from the mesophyll cells to heterotrophic tissues is of pivotal importance to sustain diurnal A, as it is generally accepted that A slowly decreases over the diurnal period due to the accumulation of photosynthates (Ainsworth and Bush, 2011). Recently, Ainsworth and Lemonnier (2018) reported the existence of genetic variation in different phloem loading mechanisms. Apoplastic loading‐unloading strategies are typically common in crop species and optimization cannot only help in sustaining A but also enhance sink strength, therefore these are potential targets to further maximize the diurnal integrated A (Ainsworth and Lemonnier, 2018). Furthermore, morphological factors substantially influenced A with long‐lived evergreen plants showing thicker leaves, with a higher leaf mass per unit leaf area, lower gm and therefore lower A than herbaceous plants (e.g. grasses) (Flood et al., 2011). Therefore, differences in leaf functionality between species are the result of differences in leaf longevity and subsequent optimization of resource investment into photosynthetic organs.
Mesophyll and stomatal limitations of photosynthesis
Mesophyll conductance is considered a key trait for future improvement in A and yield potential, as lower resistance for CO2 diffusion to the chloroplast will allow higher substrate availability for carboxylation. Additionally, an attractive property of increasing gm is the potential to increase A without increasing water loss (Nadal and Flexas, 2018), which is not possible if gs is increased. gm can be dissected into three subcomponents: conductance through intercellular air spaces (g ias), through cell wall (gw) and through the liquid phase inside cells (g liq) (Flexas et al., 2008; Terashima et al., 2011). Variation in gm between species has been associated with alterations in all these components: for instance leaf structure may affect mostly g ias and gw, in particular in thick leaves (Evans and von Caemmerer, 1996). In tobacco and soybean however, the most limiting component to gm appeared to be g liq (Evans and von Caemmerer, 1996). The intraspecific variation in gm in crop species (Table 1) suggests that both morphological and metabolic factors are involved in CO2 diffusion into chloroplasts, with evidence of aquaporin modulation of the gliq component (Gillon and Yakir, 2000; Hanba et al., 2004; Flexas et al., 2006). For instance, overexpression of the aquaporin OsPIP1;2 in rice increased gm by up to 150% compared with the wild type, resulting in greater biomass and yield (Xu et al., 2018) and therefore provided evidence for a major role for aquaporins in the modulation of intracellular CO2 diffusion (Uehlein et al., 2003; Uehlein et al., 2008). Such studies often introduce the question ‘why have such changes not occurred naturally’, however it should be borne in mind that survival to reproduce is the plant’s ultimate goal, while photosynthesis and biomass may or may not be a part of this process, and therefore resource allocation and adaptive capacity will regulate such changes. gm is generally affected by both light and temperature, therefore gm can have a signficant impact on photosynthetic efficiency under fluctuating environments (e.g. Flexas et al., 2008; Kaiser et al., 2018). However, methodologies to quantify gm are time consuming and subject to high levels of uncertainty (see review by Pons et al., 2009 and references therein), severely limiting high‐throughput phenotyping for this trait. In addition, gm is principally dependant on the physical capacity of CO2 to diffuse into the leaf tissue, and therefore dependent on gs and stomatal dynamics.
Increasing CO2 diffusion from the atmosphere to the leaf interior increases A (Lawson et al., 2010) and it has been demonstrated in several studies that manipulating stomatal density (Tanaka et al., 2013) or aperture (Lawson and Blatt, 2014; Duan et al., 2015) increases gs, while recent studies have also suggested that stomatal kinetics and the rapidity of gs responses to the changing environment can increase carbon assimilation (McAusland et al., 2016; Papanatsiou et al., 2019). Increasing gs represents a trait already unintentionally included in breeding for high yielding varieties over many decades (Fischer et al., 1998; Lu et al., 1998; De Vita et al., 2007). The positive effects of higher gs are numerous: in particular, under steady‐state conditions A is co‐related to gs and therefore high gs leads to elevated photosynthetic rates (by limiting the resistance to CO2 diffusion into intracellular airspaces) and, at the same time, increased evaporative cooling maintains optimal leaf temperature for A (Lawson and Blatt, 2014). As in C3 crops, a strong limitation of A is the temperature‐dependent increase in the oxygenation reaction of RuBisCO, the maintenance of optimal leaf temperature through high transpiration rates may be key in limiting photorespiration (Long et al., 2006). In addition, although high gs may lead to early soil water depletion, it has been shown that the extra assimilates gained early in the growing season may enable greater carbon investment in roots (Blum, 2011), facilitating higher water extraction from the deeper soil layers therefore avoiding drought stress (Venuprasad et al., 2011). It is therefore unarguable that gs is a key trait for improving crop yield potential and stability with substantial natural variation known to exist (Faralli et al., 2019a). Stomatal conductance is determined by the number of stomata per unit leaf area and the pore aperture (which is often dependent on the size of stomata) both of which represent breeding targets for altered gs. For example, Arabidopsis lines lacking the epidermal patterning factor (EPF) 1 and 2, exhibited high stomatal density, greater gs and A when compared with the wild type Col‐0 (Franks et al., 2015). Large natural variation in gs has been shown in a number of plants, including crops (i.e. Tichá, 1982; Roche, 2015; Faralli et al., 2019a and Table 1), suggesting gs as a potential target to exploit for increased A, and therefore yield. Stomata open and close in response to changes in environmental cues (i.e. water availability, light, VPD) and depend upon plant hydraulic capacity, which is the plant’s ability to take up and distribute water around the plant (Sack and Scoffoni, 2013; Lawson and Blatt, 2014). In the field and inside a crop canopy, light and VPD can vary within minutes or even seconds. Stomatal responses are an order of magnitude slower than the response of A. For example, gs in wheat can take between 5 and 15 min to reach steady state following a shade or sun fleck (Faralli et al., 2019b) and this lag in behaviour can limit A by up to 15%. Both intraspecific and interspecific variation have been shown to exist for stomatal rapidity (McAusland et al., 2016; Faralli et al., 2019b). In addition significant developmental effects on stomatal responses were shown by Faralli et al. (2019b ), in which a decrease in stomatal rapidity was reported in wheat during the post‐anthesis stage compared with the early booting stage. Therefore, gs and the dynamic response of gs can be potential unexploited targets for future crop improvement.
Measuring photosynthesis
To date, most photosynthetic measurements have been based on two approaches using infrared gas analyzer systems: (i) capacity measurements where photosynthetic CO2 assimilation is measured as a function of substomatal CO2 concentration curves (A/Ci) or as a function of light intensity (A/Q); or (ii) ‘snapshot’ or instantaneous measurements of A at selected times of the day. Additionally, other methods such as carbon isotope discrimination has been successfully used to estimate transpiration efficiency (Rebetzke et al., 2002) and the photosynthetic contribution of different non‐foliar organs to grain yield (Sanchez‐Bragado et al., 2016). In general, A/Ci analysis is a powerful tool from which the biochemical properties under light saturated conditions, a constant leaf temperature and minimal boundary layer resistance can be determined. These conditions, necessary to assess maximum photosynthetic capacity are unlikely to represent those to which a leaf is exposed in the field (Lawson et al., 2012; Driever et al., 2014) (Figure 1). Assessing photosynthesis as a function of light (A/Q analysis) might be considered more representative of field conditions. These measurements can be used to model A rates over the diurnal period if incident light is monitored. However, it should be noted that A/Q curves are usually performed in near optimal environmental conditions, particularly at low vapour pressure deficits and often measured early in the diurnal cycle, both of which promote high gs. Therefore dynamic stomatal behaviour in the field environment could significantly decrease realized A when compared with the ‘theoretical maximum’ (Lawson et al., 2012) (Figure 1c). Indeed, in a study on the effect of dynamic light on Arabidopsis by Vialet‐Chabrand et al. (2017) continuous diurnal gas exchange measurements of A were compared with those determined from A/Q response curve and incident photosynthetic active radiation (PAR), the latter failed to accurately predict the measured photosynthetic rates due to the limitation imposed by stomata (Figure 1c,1) as well as the late‐diurnal negative feedback on A (Vialet‐Chabrand et al., 2017; Matthews et al., 2018). Similar methodological drawbacks are present for simpler (and quicker) analysis of instantaneous or ‘snapshot’ measurements of photosynthesis that are either captured under natural irradiance, or use a light source to mirror in situ irradiance intensities. Stomatal limitation, enzyme activation states and photoinhibition can greatly influence short‐term photosynthesis. Additionally the environmental conditions that the plants have been exposed to before measurements also impact on instantaneous measurements, therefore increasing the complexity for data interpretation (Lawson and Weyers, 1999).
Figure 1.
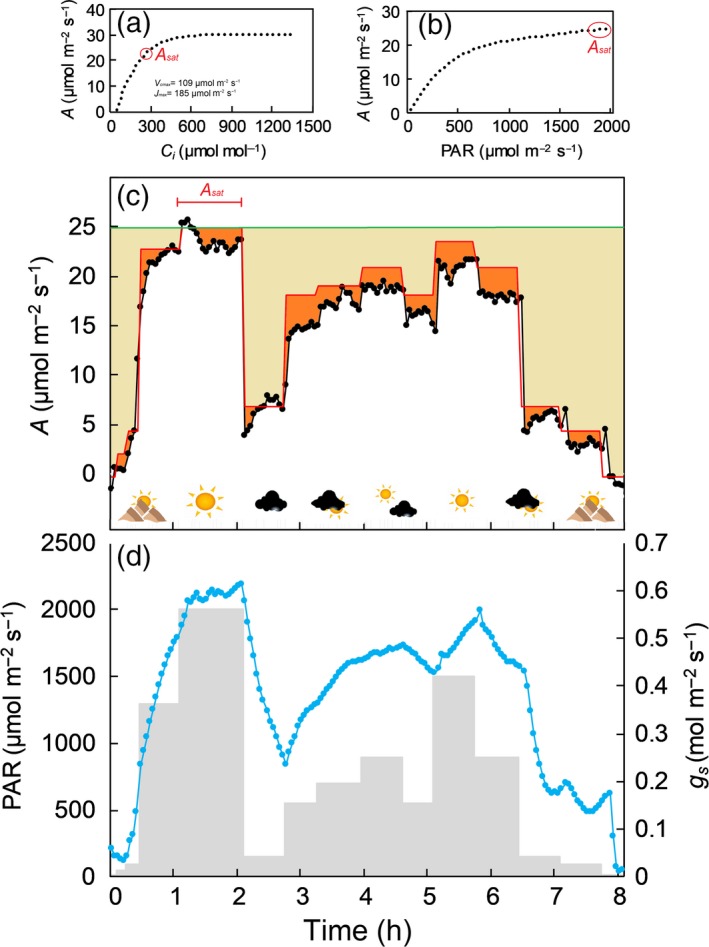
Example of a CO2 assimilation as a function of substomatal CO2 concentration curve (A/Ci) and light (A/Q) (a, b respectively) measured on the flag leaf of wheat (cv. Robigus) at booting stage with an infrared gas analyzer (Li‐Cor 6400, Li‐Cor, USA). The A/Ci was measured at saturating light [1500 µmol m−2 sec−1 photosynthetic active radiation (PAR)] and a leaf temperature of 20°C. RuBisCO carboxylation efficiency (V cmax), the maximum electron transport rate for RuBP regeneration (J max) was estimated following Sharkey et al. (2007) and A sat represents the light saturated A at current ambient [CO2]. (c, d) Diurnal measurement of photosynthetic CO2 assimilation (A) and stomatal conductance (gs) were measured on the same day as the A/Ci and A/Q analysis on an adjacent part of the flag leaf at 20°C leaf temperature following a fluctuating light environment. In (c), black dots represent recorded A values, whereas the red line represents A estimated through the A/Q response. Orange regions represent the discrepancy in A between observed and modelled values from the A/Q. Light brown regions represent the potential overestimation in daily CO2 uptake if A sat is used to assess total daily photosynthesis for plants growing under a natural fluctuating light regime. In (d), grey regions represent the light regimes (therefore the photosynthetically active radiation, PAR) at which the diurnal measurement with the Li‐Cor was carried out (following a simulated sunny‐cloudy pattern shown in (c)), while blue dots represent gs.
Therefore, it is not surprising that A is not always correlated with yield as analyses are often based on either photosynthetic capacity (e.g. A/Ci and A/Q curves) that are not realized in the field or instantaneous measurements that represent a single point measurement of A that fails to characterize the diurnal photosynthetic pattern. In Table 1, the best link between yield and photosynthesis was found when integrated CO2 uptake was determined over the growing season or ‘operational’ photosynthesis was measured in situ (Rawson et al., 1983; Carmo‐Silva et al., 2017 respectively) suggesting that: (i) the different components defining yield are determined over spatial and temporal‐specific phenological stages, and therefore A sat (the most used trait estimated in the literature) may correlate to a particular yield component rather than overall grain yield; (ii) Asat is representative of a steady‐state and optimal condition that crop plants hardly ever experience in the field, and more realistic conditions for the analysis (e.g. subsaturating light intensities) are the most appropriate way to evaluate the realized A in natural dynamic environments; and (iii) although technically challenging, time consuming and subject to a high degrees of errors (e.g. time of senescence initiation), integrated CO2 uptake of the most photosynthetically active leaf (i.e. flag leaf) has the potential to be a representative trait linked to grain yield, at least in wheat (Rawson et al., 1983). Therefore, new instrumentation that would enable diurnal and seasonal measurements of realized photosynthesis to be captured under natural dynamic field conditions and at different layers within the canopy is required (e.g. Salter et al., 2018; Murchie et al., 2018; Vialet‐Chabrand and Lawson, 2019). For example, the development of the ‘OCTOflux’ system by Salter et al. (2018), which is a multiplexed semiportable gas exchange system that enables A max to be measured in eight leaves simultaneously. Furthermore, new tools are needed to facilitate high‐throughput measurements of photosynthetic capacity in situ and on large numbers of plants, such as the recent developments in hyperspectral imaging to rapidly measure V cmax in the field (ca. 10 sec) (Meacham‐Hensold et al., 2019). Although the approaches mentioned above have made significant advancements in measuring photosynthetic capacity, further developments on instrumentation are necessary to enable diel operational or realized photosynthetic rates to be determined, that are subject to the limitations driven by the growth conditions as well as the kinetics of various processes that a plant is subjected to over the dynamic diurnal period.
Exploiting natural variation in photosynthetic capacity and stomatal function for improving crop productivity: a case in wheat
In wheat, several physiological traits have been unintentionally selected for to produce high yielding cultivars with increased grain number m−2 (GN) and hence yield (Fischer et al., 1998). In the last few years, however, yield has stagnated in many countries suggesting the need for greater effort and new targets for increasing productivity (Ray et al., 2012). The critical and source‐limited phase of stem extension determines GN (Slafer et al., 2015). Two not mutually exclusive possibilities have been proposed to increase GN in wheat: (i) lengthening the duration and rate of growth and (ii) increasing resource availability (i.e. photosynthesis) (Miralles and Slafer, 2007). Indeed, increasing sedoheptulose1,7‐biphosphatase activity increased flag leaf photosynthetic capacity and GN per spike in greenhouse‐grown wheat (Driever et al., 2017), suggesting that elevated flag leaf A can increase spike fertility. Most of the work characterizing photosynthesis in wheat has focused on flag leaf A, however, understanding and assessing earlier canopy photosynthetic efficiency (e.g. early stem extension) might be of greater importance to optimize spikelet and floret fertility. Several studies have already reported significant variation in photosynthetic capacity and light saturated rate of photosynthesis in wheat, suggesting the potential exploitation of diversity for selection and/or gene discovery (Driever et al., 2014; Carmo‐Silva et al., 2017). In particular, high‐throughput phenotyping approaches can help detect important genomic regions for leaf and/or canopy photosynthetic traits in wheat and speed up the selection of desirable traits. Either large panels of wheat with unknown ancestry or bi and multiparental populations (for quantitative trait loci analysis) can be used for this approach, as already demonstrated in rice (Teng et al., 2004; Gu et al., 2012) and recently reviewed by van Bezouw et al. (2019). In addition the development of single‐nucleotide polymorphism platforms in wheat (Wilkinson et al., 2012) and the recently annotated genome of bread wheat (Appels et al., 2018) will ensure a more comprehensive understanding of the genetic control of photosynthetic traits or other A‐determining traits such as gs and stomatal dynamics.
As yield generally plateaus at high GN due to the trade‐off with grain weight (GW) (Figure 2, scenarios a and b) (Gambín and Borrás, 2010; Quintero et al., 2018), understanding and potentially optimizing the GW component is of major importance for wheat yield improvement. Recent work reported the presence of a potential source limitation during grain filling (Álvaro et al., 2008; Xie et al., 2015; Quintero et al., 2018). These reports suggest that increased A in post‐anthesis would help facilitate the attainment of the potential maximum individual GW, especially if GN is increased (Figure 2, scenario c). GW can rely on three main sources of assimilates: leaf photosynthesis, spike photosynthesis and the remobilization of the water‐soluble carbohydrates (WSC) from the stem. While efforts have largely focused on selecting and screening for post‐anthesis leaf photosynthetic duration (Blake et al., 2007) and WSC concentration (Rebetzke et al., 2008), spike photosynthesis is an unexplored determining component contributing to GW. When compared with the flag leaf, the spike has shown a higher degree of drought tolerance (Tambussi et al., 2005, 2007) generally explained by a greater intrinsic water‐use efficiency (driven by a low gs per unit area and a high degree of re‐fixed respiratory CO2) and a more pronounced osmotic adjustment (Tambussi et al., 2005, 2007). This situation suggests that spike photosynthesis has an important role in times of water limitation, possibly compensating the flag leaf during grain filling. Furthermore, the assimilates produced in the spike are directly translocated into the grains (Carr and Wardlaw, 1965) leading to a contribution to GW between 10 and 45% depending on environmental conditions and genotype tested (Maydup et al., 2010; Sanchez‐Bragado et al., 2016). Indeed a large variation in gross spike A (calculated as the sum of A and dark respiration (Rd) as a proxy of respiration in the light) has been shown in both durum and bread wheat (Maydup et al., 2010; Molero et al., 2013; Zhou et al., 2016; Sanchez‐Bragado et al., 2016), suggesting the existence of natural genetic diversity for exploitation. For instance, the presence of awns (lemma‐derived organs) has been considered an important source of external CO2 assimilation of the spike (Maydup et al., 2010) although other factors such a spike morphology (e.g. photosynthetic surface area of spikelets) seems to drive the observed variation in spike A (Guo and Schnurbusch, 2016). Earlier evidence proposes that, in the UK, a significant genotypic variation for spike gross A and for the contribution of spike A to GW is present (Faralli et al., 2019c) and confirms the importance of spike photosynthetic CO2 assimilation for grain filling. Additional work is needed to fully understand the underlying mechanism of spike A, as well as the extent of existing natural variation. Further development of high‐throughput phenotyping tools focusing on spike A would take full advantage of this unexploited trait for GW improvement.
Figure 2.
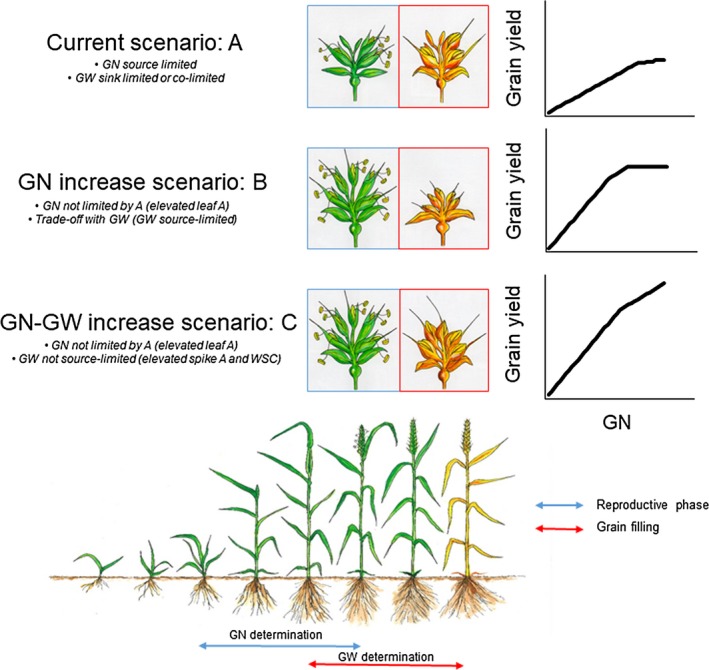
Theoretical scenarios for improving grain yield in wheat. (a) Current scenario with GN source‐limited and GW sink‐limited or both co‐limited. Here, grain yield is limited by GN. (b) Optimization of resources for grain number (GN) leads to a trade‐off with the individual grain weight therefore plateauing grain yield through the progress in GN. (c) Removal of source limitation is required for the reproductive and grain filling stages through optimization of flag leaf photosynthesis, spike photosynthesis and WSC remobilization, leading to a reduced trade‐off with the individual grain weight and therefore increase in grain yield.
Conclusion
Photosynthesis is a key determinant of crop yield. Large natural variation in A and A‐determining traits in different photosynthetic organs exists in a number of crop species that represent a currently unexploited target for crop improvement. Owing to the complexity of the relation between A and yield, improvements in high‐throughput, reliable and relevant methodologies will enable the dissection of useful genetic targets for marker‐assisted selection. In wheat, enhancing leaf canopy photosynthesis will increase GN although greater yield will only be achieved with a parallel increase in GW, which relies primarily on enhanced spike photosynthesis. With this in mind, screening for high photosynthetic capacity in both organs should be considered a prime target for high yielding wheat cultivars. In summary, genetic manipulation and elevated [CO2] experiments have shown a yield advantage when photosynthesis is increased in food crops; therefore exploiting natural genetic variation in photosynthesis will facilitate the development of cultivars with greater yield potential.
Acknowledgements
MF was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) grants awarded to TL (BB/NO16831/1), with IPA co‐funding from BASF. We thank Mike Fryer for the drawing in Figure 2. There are no conflicts of interest.
Data Availability Statement
All data relevant to this review can be found within the manuscript and any supplementary materials if supplied.
References
- Ainsworth, E.A. and Bush, D.R. (2011) Carbohydrate export from the leaf: a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 155(1), 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth, E.A. and Lemonnier, P. (2018) Phloem function: a key to understanding and manipulating plant responses to rising atmospheric [CO 2]? Curr. Opin. Plant Biol. 43, 50–56. [DOI] [PubMed] [Google Scholar]
- Ainsworth, E.A. and Long, S.P. (2005) What have we learned from 15 years of free‐air CO2 enrichment (FACE)? A meta‐analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2 . New Phytol. 165(2), 351–372. [DOI] [PubMed] [Google Scholar]
- Álvaro, F. , Royo, C. , García del Moral, L.F. and Villegas, D. (2008) Grain filling and dry matter translocation responses to source–sink modifications in a historical series of durum wheat. Crop Sci. 48(4), 1523–1531. [Google Scholar]
- Appels, R. , Eversole, K. , Feuillet, C. et al. (2018) Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science, 361(6403), eaar7191. [DOI] [PubMed] [Google Scholar]
- Arrivault, S. , Alexandre Moraes, T. , Obata, T. et al . (2019) Metabolite profiles reveal interspecific variation in operation of the Calvin‐Benson cycle in both C4 and C3 plants. J. Exp. Bot. 70(6), 1843–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour, M.M. , Warren, C.R. , Farquhar, G.D. , Forrester, G.U.Y. and Brown, H. (2010) Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant Cell Environ. 33(7), 1176–1185. [DOI] [PubMed] [Google Scholar]
- van Bezouw, R.F. , Keurentjes, J.J. , Harbinson, J. and Aarts, M.G. (2019) Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant J. 97(1), 112–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake, N.K. , Lanning, S.P. , Martin, J.M. , Sherman, J.D. and Talbert, L.E. (2007) Relationship of flag leaf characteristics to economically important traits in two spring wheat crosses. Crop Sci. 47(2), 491–494. [Google Scholar]
- Blum, A. (1990) Variation among wheat cultivars in the response of leaf gas exchange to light. J. Agric. Sci. 115(3), 305–311. [Google Scholar]
- Blum, A. (2011) Drought resistance–is it really a complex trait? Funct. Plant Biol. 38(10), 753–757. [DOI] [PubMed] [Google Scholar]
- Carmo‐Silva, A.E. and Salvucci, M.E. (2013) The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol. 161(4), 1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo‐Silva, E. , Andralojc, P.J. , Scales, J.C. , Driever, S.M. , Mead, A. , Lawson, T. , Parry, M.A. (2017) Phenotyping of field‐grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 68(13), 3473–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, D.J. and Wardlaw, I.F. (1965) Supply of photosynthetic assimilates to grain from flag leaf and ear of wheat. Aust. J. Biol. Sci. 18(4), 711. [Google Scholar]
- Chytyk, C. , Hucl, P. and Gray, G. (2011) Leaf photosynthetic properties and biomass accumulation of selected western Canadian spring wheat cultivars. Can. J. Plant Sci. 91(2), 305–314. [Google Scholar]
- Crosbie, T.M. and Pearce, R.B. (1982) Effects of recurrent phenotypic selection for high and low photosynthesis on agronomic traits in two maize populations 1. Crop Sci. 22(4), 809–813. [Google Scholar]
- Crosbie, T.M. , Pearce, R.B. and Mock, J.J. (1981) Recurrent phenotypic selection for high and low photosynthesis in two maize populations 1. Crop Sci. 21(5), 736–740. [Google Scholar]
- De Vita, P. , Nicosia, O.L.D. , Nigro, F. , Platani, C. , Riefolo, C. , Di Fonzo, N. and Cattivelli, L. (2007) Breeding progress in morpho‐physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. Eur. J. Agron. 26(1), 39–53. [Google Scholar]
- Driever, S.M. , Lawson, T. , Andralojc, P.J. , Raines, C.A. and Parry, M.A.J. (2014) Natural variation in photosynthetic capacity, growth, and yield in 64 field‐grown wheat genotypes. J. Exp. Bot. 65(17), 4959–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever, S.M. , Simkin, A.J. , Alotaibi, S. , Fisk, S.J. , Madgwick, P.J. , Sparks, C.A. , Raines, C.A. (2017) Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Phil. Trans. R. Soc. B, 372(1730), 20160384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, B. , Ma, Y. , Jiang, M. , Yang, F. , Ni, L. and Lu, W. (2015) Improvement of photosynthesis in rice (Oryza sativa L.) as a result of an increase in stomatal aperture and density by exogenous hydrogen sulfide treatment. Plant Growth Regul. 75(1), 33–44. [Google Scholar]
- Evans, J.R. and Von Caemmerer, S. (1996) Carbon dioxide diffusion inside leaves. Plant Physiol. 110(2), 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli, M. , Matthews, J. and Lawson, T. (2019a) Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 49, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli, M. , Cockram, J. , Ober, E. , Wall, S. , Galle, A. , Van Rie, J. , Lawson, T. (2019b) Genotypic, developmental and environmental effects on the rapidity of gs in wheat: impacts on carbon gain and water‐use efficiency. Frontiers. Plant Sci. 10, 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faralli, M. , Mellers, G. , Chadwick, J.D. et al . (2019c) Spike photosynthetic contribution to grain weight and source‐limitation over the grain filling stage in UK wheat lines with contrasting yield components. Proceedings of the 1st International Wheat Congress, p. 130. Saskatoon, Canada.
- Farquhar, G.D. and Sharkey, T.D. (1982) Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 33(1), 317–345. [Google Scholar]
- Fischer, R.A. , Bidinger, F. , Syme, J.R. and Wall, P.C. (1981) Leaf photosynthesis, leaf permeability, crop growth, and yield of short spring wheat genotypes under irrigation 1. Crop Sci. 21(3), 367–373. [Google Scholar]
- Fischer, R.A. , Rees, D. , Sayre, K.D. , Lu, Z.M. , Condon, A.G. and Saavedra, A.L. (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 38(6), 1467–1475. [Google Scholar]
- Flexas, J. , Ribas‐Carbó, M. , Hanson, D.T. , Bota, J. , Otto, B. , Cifre, J. , Kaldenhoff, R. (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2in vivo. Plant J. 48(3), 427–439. [DOI] [PubMed] [Google Scholar]
- Flexas, J. , Ribas‐Carbo, M. , Diaz‐Espejo, A. , Galmes, J. and Medrano, H. (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ. 31(5), 602–621. [DOI] [PubMed] [Google Scholar]
- Flood, P.J. , Harbinson, J. and Aarts, M.G. (2011) Natural genetic variation in plant photosynthesis. Trends Plant Sci. 16(6), 327–335. [DOI] [PubMed] [Google Scholar]
- Foulkes, M.J. , Slafer, G.A. , Davies, W.J. , Berry, P.M. , Sylvester‐Bradley, R. , Martre, P. , Reynolds, M.P. (2010) Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 62(2), 469–486. [DOI] [PubMed] [Google Scholar]
- Franks, P.J. , W. Doheny‐Adams, T. , Britton‐Harper, Z.J. and Gray, J.E. (2015) Increasing water‐use efficiency directly through genetic manipulation of stomatal density. New Phytol. 207(1), 188–195. [DOI] [PubMed] [Google Scholar]
- Gaju, O. , DeSilva, J. , Carvalho, P. , Hawkesford, M.J. , Griffiths, S. , Greenland, A. and Foulkes, M.J. (2016) Leaf photosynthesis and associations with grain yield, biomass and nitrogen‐use efficiency in landraces, synthetic‐derived lines and cultivars in wheat. Field. Crop. Res. 193, 1–15. [Google Scholar]
- Gambín, B.L. and Borrás, L. (2010) Resource distribution and the trade‐off between seed number and seed weight: a comparison across crop species. Ann. Appl. Biol. 156(1), 91–102. [Google Scholar]
- Gilbert, M.E. , Zwieniecki, M.A. and Holbrook, N.M. (2011) Independent variation in photosynthetic capacity and stomatal conductance leads to differences in intrinsic water use efficiency in 11 soybean genotypes before and during mild drought. J. Exp. Bot. 62(8), 2875–2887. [DOI] [PubMed] [Google Scholar]
- Gillon, J.S. and Yakir, D. (2000) Naturally low carbonic anhydrase activity in C4 and C3 plants limits discrimination against C18OO during photosynthesis. Plant Cell Environ. 23(9), 903–915. [Google Scholar]
- González, A. , Martín, I. and Ayerbe, L. (1999) Barley yield in water‐stress conditions: the influence of precocity, osmotic adjustment and stomatal conductance. Field Crops Res. 62(1), 23–34. [Google Scholar]
- Gu, J. , Yin, X. , Stomph, T.J. , Wang, H. and Struik, P.C. (2012) Physiological basis of genetic variation in leaf photosynthesis among rice (Oryza sativa L.) introgression lines under drought and well‐watered conditions. J. Exp. Bot. 63(14), 5137–5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J. , Yin, X. , Stomph, T.J. and Struik, P.C. (2014) Can exploiting natural genetic variation in leaf photosynthesis contribute to increasing rice productivity? A simulation analysis. Plant Cell Environ. 37(1), 22–34. [DOI] [PubMed] [Google Scholar]
- Guarda, G. , Padovan, S. and Delogu, G. (2004) Grain yield, nitrogen‐use efficiency and baking quality of old and modern Italian bread‐wheat cultivars grown at different nitrogen levels. Eur. J. Agron. 21(2), 181–192. [Google Scholar]
- Guo, Z. and Schnurbusch, T. (2016) Costs and benefits of awns. J. Exp. Bot. 67(9), 2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba, Y.T. , Shibasaka, M. , Hayashi, Y. , Hayakawa, T. , Kasamo, K. , Terashima, I. and Katsuhara, M. (2004) Overexpression of the barley aquaporin HvPIP2; 1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 45(5), 521–529. [DOI] [PubMed] [Google Scholar]
- Hervé, D. , Fabre, F. , Berrios, E.F. , Leroux, N. , Chaarani, G.A. , Planchon, C. , Gentzbittel, L. (2001) QTL analysis of photosynthesis and water status traits in sunflower (Heliantherefore annuus L.) under greenhouse conditions. J. Exp. Bot. 52(362), 1857–1864. [DOI] [PubMed] [Google Scholar]
- Hikosaka, K. (2010) Mechanisms underlying interspecific variation in photosynthetic capacity across wild plant species. Plant Biotechnol. 27(3), 223–229. [Google Scholar]
- Hirel, B. , Le Gouis, J. , Ney, B. and Gallais, A. (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J. Exp. Bot. 58(9), 2369–2387. [DOI] [PubMed] [Google Scholar]
- Jahan, E. , Amthor, J.S. , Farquhar, G.D. , Trethowan, R. and Barbour, M.M. (2014) Variation in mesophyll conductance among Australian wheat genotypes. Funct. Plant Biol. 41(6), 568–580. [DOI] [PubMed] [Google Scholar]
- Kaiser, E. , Morales, A. and Harbinson, J. (2018) Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 176(2), 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebeish, R. , Niessen, M. , Thiruveedhi, K. , Bari, R. , Hirsch, H.J. , Rosenkranz, R. , Peterhänsel, C. (2007) Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 25(5), 593. [DOI] [PubMed] [Google Scholar]
- Kromdijk, J. , Głowacka, K. , Leonelli, L. , Gabilly, S.T. , Iwai, M. , Niyogi, K.K. and Long, S.P. (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science, 354(6314), 857–861. [DOI] [PubMed] [Google Scholar]
- Külheim, C. , Ågren, J. and Jansson, S. (2002) Rapid regulation of light harvesting and plant fitness in the field. Science, 297(5578), 91–93. [DOI] [PubMed] [Google Scholar]
- Lawson, T. and Blatt, M.R. (2014) Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 164(4), 1556–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson, T. and Weyers, J. (1999) Spatial and temporal variation in gas exchange over the lower surface of Phaseolus vulgaris L. primary leaves. J. Exp. Bot. 50(337), 1381–1391. [Google Scholar]
- Lawson, T. , von Caemmerer, S. and Baroli, I. (2010) Photosynthesis and stomatal behaviour In Progress in Botany 72 (Lüttge U., Beyschlag W., Büdel B. and Francis D. eds). Berlin, Heidelberg: Springer, pp. 265–304. [Google Scholar]
- Lawson, T. , Kramer, D.M. and Raines, C.A. (2012) Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr. Opin. Biotechnol. 23(2), 215–220. [DOI] [PubMed] [Google Scholar]
- Long, S.P. , Zhu, X.G. , Naidu, S.L. and Ort, D.R. (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 29(3), 315–330. [DOI] [PubMed] [Google Scholar]
- Long, S.P. , Marshall‐Colon, A. and Zhu, X.G. (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell, 161(1), 56–66. [DOI] [PubMed] [Google Scholar]
- Lopez‐Calcagno, P.E. , Fisk, S. , Brown, K.L. , Bull, S.E. , South, P.F. and Raines, C.A. (2018) Overexpressing the H‐protein of the glycine cleavage system increases biomass yield in glasshouse and field grown transgenic tobacco plants. Plant Biotechnol. J. 17, 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z. , Percy, R.G. , Qualset, C.O. and Zeiger, E. (1998) Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. J. Exp. Bot. 49, 453–460. [Google Scholar]
- Matthews, J.S. , Vialet‐Chabrand, S.R. and Lawson, T. (2018). Acclimation to fluctuating light impacts the rapidity and diurnal rhythm of stomatal conductance. Plant Physiol. 176, 1939–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurino, V.G. and Peterhansel, C. (2010) Photorespiration: current status and approaches for metabolic engineering. Curr. Opin. Plant Biol. 13(3), 248–255. [DOI] [PubMed] [Google Scholar]
- Maydup, M.L. , Antonietta, M. , Guiamet, J.J. , Graciano, C. , López, J.R. and Tambussi, E.A. (2010) The contribution of ear photosynthesis to grain filling in bread wheat (Triticum aestivum L.). Field. Crop. Res. 119(1), 48–58. [Google Scholar]
- McAusland, L. , Vialet‐Chabrand, S. , Davey, P. , Baker, N.R. , Brendel, O. and Lawson, T. (2016) Effects of kinetics of light‐induced stomatal responses on photosynthesis and water‐use efficiency. New Phytol. 211(4), 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham‐Hensold, K. , Montes, C.M. , Wu, J. , Guan, K. , Fu, P. , Ainsworth, E.A. and Bernacchi, C.J. (2019) High‐throughput field phenotyping using hyperspectral reflectance and partial least squares regression (PLSR) reveals genetic modifications to photosynthetic capacity. Remote Sens. Environ. 231, 111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralles, D.J. and Slafer, G.A. (2007). Sink limitations to yield in wheat: how could it be reduced? Journal of Agricultural Sci. 145(2), 139–149. [Google Scholar]
- Molero, G. , Sanchez‐Bragado, R. , Araus, J.L. and Reynolds, M.P. (2013) Phenotypic selection for spike photosynthesis In Proceedings of the 3rd International Workshop of the Wheat Yield Consortium (Reynolds M. and Braun H. eds). Obregon, Sonora, Mexico: CIMMYT, pp. 9–11. [Google Scholar]
- Murchie, E.H. , Pinto, M. and Horton, P. (2009) Agriculture and the new challenges for photosynthesis research. New Phytol. 181(3), 532–552. [DOI] [PubMed] [Google Scholar]
- Murchie, E.H. , Kefauver, S. , Araus, J.L. , Muller, O. , Rascher, U. , Flood, P.J. and Lawson, T. (2018) Measuring the dynamic photosynthome. Ann. Bot. 122(2), 207–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal, M. and Flexas, J. (2018) Mesophyll Conductance to CO2 diffusion: effects of drought and opportunities for improvement In Water Scarcity and Sustainable Agriculture in Semiarid Environment (Tejero I.F.G. and Zuazo V.H.D. eds). Cambridge, MA: Academic Press, pp. 403–438. [Google Scholar]
- Ojima, M. (1974). Improvement of photosynthetic capacity in soybean variety. Jpn. Agric. Res. Q. 8, 6–12. [Google Scholar]
- Ouyang, W. , Struik, P.C. , Yin, X. and Yang, J. (2017) Stomatal conductance, mesophyll conductance, and transpiration efficiency in relation to leaf anatomy in rice and wheat genotypes under drought. J. Exp. Bot. 68(18), 5191–5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanatsiou, M. , Petersen, J. , Henderson, L. , Wang, Y. , Christie, J.M. and Blatt, M.R. (2019) Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science, 363(6434), 1456–1459. [DOI] [PubMed] [Google Scholar]
- Parry, M.A.J. , Andralojc, P.J. , Mitchell, R.A. , Madgwick, P.J. and Keys, A.J. (2003) Manipulation of Rubisco: the amount, activity, function and regulation. J. Exp. Bot. 54(386), 1321–1333. [DOI] [PubMed] [Google Scholar]
- Parry, M.A. , Reynolds, M. , Salvucci, M.E. , Raines, C. , Andralojc, P.J. , Zhu, X.G. , Furbank, R.T. (2010) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 62(2), 453–467. [DOI] [PubMed] [Google Scholar]
- Pater, D. , Mullen, J.L. , McKay, J.K. and Schroeder, J.I. (2017) Screening for natural variation in water use efficiency traits in a diversity set of Brassica napus L. identifies candidate variants in photosynthetic assimilation. Plant Cell Physiol. 58(10), 1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew, W.T. (2004) Cotton genotypic variation in the photosynthetic response to irradiance. Photosynthetica, 42(2), 567–571. [Google Scholar]
- Pons, T.L. , Flexas, J. , Von Caemmerer, S. , Evans, J.R. , Genty, B. , Ribas‐Carbo, M. and Brugnoli, E. (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J. Exp. Bot. 60(8), 2217–2234. [DOI] [PubMed] [Google Scholar]
- Prins, A. , Orr, D.J. , Andralojc, P.J. , Reynolds, M.P. , Carmo‐Silva, E. and Parry, M.A. (2016) Rubisco catalytic properties of wild and domesticated relatives provide scope for improving wheat photosynthesis. J. Exp. Bot. 67(6), 1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, M. , Zheng, G. , Essmine, J. , Hamdani, S. , Song, Q. , Wang, H. , Zhu, X.G. (2017) Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol. 175, 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero, A. , Molero, G. , Reynolds, M.P. and Calderini, D.F. (2018) Trade‐off between grain weight and grain number in wheat depends on GxE interaction: a case study of an elite CIMMYT panel (CIMCOG). Eur. J. Agron. 92, 17–29. [Google Scholar]
- Rawson, H.M. , Hindmarsh, J.H. , Fischer, R.A. and Stockman, Y.M. (1983) Changes in leaf photosynthesis with plant ontogeny and relationships with yield per ear in wheat cultivars and 120 progeny. Funct. Plant Biol. 10(6), 503–514. [Google Scholar]
- Ray, D.K. , Ramankutty, N. , Mueller, N.D. , West, P.C. and Foley, J.A. (2012) Recent patterns of crop yield growth and stagnation. Nat. Commun. 3, 1293. [DOI] [PubMed] [Google Scholar]
- Rebetzke, G.J. , Condon, A.G. , Richards, R.A. and Farquhar, G.D. (2002) Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci. 42(3), 739–745. [Google Scholar]
- Rebetzke, G.J. , Van Herwaarden, A.F. , Jenkins, C. , Weiss, M. , Lewis, D. , Ruuska, S. , Richards, R.A. (2008) Quantitative trait loci for water‐soluble carbohydrates and associations with agronomic traits in wheat. Aust. J. Agric. Res. 59(10), 891–905. [Google Scholar]
- Reynolds, M.P. , Gutiérrez‐Rodríguez, M. and Larqué‐Saavedra, A. (2000) Photosynthesis of wheat in a warm, irrigated environment: I: genetic diversity and crop productivity. Field. Crop. Res. 66(1), 37–50. [Google Scholar]
- Reynolds, M. , Foulkes, J. , Furbank, R. , Griffiths, S. , King, J. , Murchie, E. , Slafer, G. (2012) Achieving yield gains in wheat. Plant Cell Environ. 35(10), 1799–1823. [DOI] [PubMed] [Google Scholar]
- Roche, D. (2015) Stomatal conductance is essential for higher yield potential of C3 crops. Crit. Rev. Plant Sci. 34(4), 429–453. [Google Scholar]
- Sack, L. and Scoffoni, C. (2013) Leaf venation: structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 198(4), 983–1000. [DOI] [PubMed] [Google Scholar]
- Sadras, V.O. , Lawson, C. and Montoro, A. (2012) Photosynthetic traits in Australian wheat varieties released between 1958 and 2007. Field. Crop. Res. 134, 19–29. [Google Scholar]
- Salter, W.T. , Gilbert, M.E. and Buckley, T.N. (2018) A multiplexed gas exchange system for increased throughput of photosynthetic capacity measurements. Plant Methods, 14(1), 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Bragado, R. , Molero, G. , Reynolds, M.P. and Araus, J.L. (2016) Photosynthetic contribution of the ear to grain filling in wheat: a comparison of different methodologies for evaluation. J. Exp. Bot. 67(9), 2787–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenrath‐Cole, G.F. and Pearcy, R.W. (1994) Regulation of photosynthetic induction state by the magnitude and duration of low light exposure. Plant Physiol. 105(4), 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenrath‐Cole, G.F. , Pearcy, R.W. and Steinmaus, S. (1994) The role of enzyme activation state in limiting carbon assimilation under variable light conditions. Photosynth. Res. 41(2), 295–302. [DOI] [PubMed] [Google Scholar]
- Sharkey, T.D. (1985) Photosynthesis in intact leaves of C 3 plants: physics, physiology and rate limitations. The Botanical Review, 51(1), 53–105. [Google Scholar]
- Sharkey, T.D. , Bernacchi, C.J. , Farquhar, G.D. and Singsaas, E.L. (2007) Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 30(9), 1035–1040. [DOI] [PubMed] [Google Scholar]
- Shearman, V.J. , Sylvester‐Bradley, R. , Scott, R.K. and Foulkes, M.J. (2005) Physiological processes associated with wheat yield progress in the UK. Crop Sci. 45(1), 175–185. [Google Scholar]
- Simkin, A.J. , McAusland, L. , Headland, L.R. , Lawson, T. and Raines, C.A. (2015) Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 66(13), 4075–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin, A.J. , López‐Calcagno, P.E. and Raines, C.A. (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 70(4), 1119–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slafer, G.A. , Elia, M. , Savin, R. , García, G.A. , Terrile, I.I. , Ferrante, A. , González, F.G. (2015) Fruiting efficiency: an alternative trait to further rise wheat yield. Food and Energy Security, 4(2), 92–109. [Google Scholar]
- Tambussi, E.A. , Nogués, S. and Araus, J.L. (2005) Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta, 221(3), 446–458. [DOI] [PubMed] [Google Scholar]
- Tambussi, E.A. , Bort, J. , Guiamet, J.J. , Nogués, S. and Araus, J.L. (2007) The photosynthetic role of ears in C3 cereals: metabolism, water use efficiency and contribution to grain yield. Crit. Rev. Plant Sci. 26(1), 1–16. [Google Scholar]
- Tanaka, Y. , Sugano, S.S. , Shimada, T. and Hara‐Nishimura, I. (2013) Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 198(3), 757–764. [DOI] [PubMed] [Google Scholar]
- Teng, S. , Qian, Q. , Zeng, D. , Kunihiro, Y. , Fujimoto, K. , Huang, D. and Zhu, L. (2004) QTL analysis of leaf photosynthetic rate and related physiological traits in rice (Oryza sativa L.). Euphytica, 135(1), 1–7. [Google Scholar]
- Terashima, I. , Hanba, Y.T. , Tholen, D. and Niinemets, Ü. (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol. 155(1), 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichá, I. (1982) Photosynthetic characteristics during ontogenesis of leaves. 7. Stomata density and sizes. Photosynthetica, 16, 375–471. [Google Scholar]
- Uehlein, N. , Lovisolo, C. , Siefritz, F. and Kaldenhoff, R. (2003) The tobacco aquaporin NtAQP1 is a membrane CO 2 pore with physiological functions. Nature, 425(6959), 734. [DOI] [PubMed] [Google Scholar]
- Uehlein, N. , Otto, B. , Hanson, D.T. , Fischer, M. , McDowell, N. and Kaldenhoff, R. (2008) Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell, 20(3), 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuprasad, R. , Impa, S.M. , Gowda, R.V. , Atlin, G.N. and Serraj, R. (2011) Rice near‐isogenic‐lines (NILs) contrasting for grain yield under lowland drought stress. Field. Crop. Res. 123(1), 38–46. [Google Scholar]
- Vialet‐Chabrand, S. and Lawson, T. (2019) Dynamic leaf energy balance: deriving stomatal conductance from thermal imaging in a dynamic environment. J. Exp. Bot. 70(10), 2839–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialet‐Chabrand, S. , Matthews, J.S. , Simkin, A.J. , Raines, C.A. and Lawson, T. (2017) Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 173(4), 2163–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, B.J. , VanLoocke, A. , Bernacchi, C.J. and Ort, D.R. (2016) The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 67, 107–129. [DOI] [PubMed] [Google Scholar]
- Watanabe, N. , Evans, J.R. and Chow, W.S. (1994) Changes in the photosynthetic properties of Australian wheat cultivars over the last century. Funct. Plant Biol. 21(2), 169–183. [Google Scholar]
- Wilkinson, P.A. , Winfield, M.O. , Barker, G.L. , Allen, A.M. , Burridge, A. , Coghill, J.A. and Edwards, K.J. (2012) CerealsDB 2.0: an integrated resource for plant breeders and scientists. BMC Bioinformatics, 13(1), 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger, S.D. (1993) Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 44(5), 907–920. [Google Scholar]
- Xie, Q. , Mayes, S. and Sparkes, D.L. (2015) Carpel size, grain filling, and morphology determine individual grain weight in wheat. J. Exp. Bot. 66(21), 6715–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, F. , Wang, K. , Yuan, W. , Xu, W. , Shuang, L. , Kronzucker, H.J. , Xiao, L. (2018) Overexpression of rice aquaporin OsPIP1; 2 improves yield by enhancing mesophyll CO2 conductance and phloem sucrose transport. J. Exp. Bot. 70(2), 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, Q. , Soundararajan, M. , Weiss, A. , Arkebauer, T.J. and Baenziger, P.S. (2002) Genotypic variation of gas exchange parameters and carbon isotope discrimination in winter wheat. J. Plant Physiol. 159(8), 891–898. [Google Scholar]
- Zhou, B. , Serret, M.D. , Elazab, A. , Bort Pie, J. , Araus, J.L. , Aranjuelo, I. and Sanz‐Sáez, Á. (2016) Wheat ear carbon assimilation and nitrogen remobilization contribute significantly to grain yield. J. Integr. Plant Biol. 58(11), 914–926. [DOI] [PubMed] [Google Scholar]
- Zhu, X.G. , Long, S.P. and Ort, D.R. (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 19(2), 153–159. [DOI] [PubMed] [Google Scholar]
- Zhu, X.G. , Long, S.P. and Ort, D.R. (2010) Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 61, 235–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to this review can be found within the manuscript and any supplementary materials if supplied.


