Abstract
The respiratory system has ideal tissue structure and cell types for efficient gas exchange to intake oxygen and release carbon dioxide. This complex system develops through orchestrated intercellular signaling among various cell types, such as club, ciliated, basal, neuroendocrine, AT1, AT2, endothelial, and smooth muscle cells. Notch signaling is a highly conserved cell–cell signaling pathway ideally suited for very short‐range cellular communication because Notch signals are transmitted by direct contact with an adjacent cell. Enthusiastic efforts by Notch researchers over the last two decades have led to the identification of critical roles of this signaling pathway during development, homeostasis, and regeneration of the respiratory system. The dysregulation of Notch signaling results in a wide range of respiratory diseases such as pulmonary artery hypertension (PAH), chronic obstructive pulmonary disease (COPD), interstitial pulmonary fibrosis (IPF), and lung cancer. Thus, a deep understanding of the biological functions of Notch signaling will help identify novel treatment targets in various respiratory diseases.
Keywords: human disease, lung development, mouse genetics, Notch signaling, organogenesis
Enthusiastic efforts by Notch researchers over the last two decades have led to the identification of critical roles of Notch signaling pathway across the lifespan from development to aging. The dysregulation of Notch signaling results in a wide range of respiratory diseases. We review the roles of Notch signaling in lung development, homeostasis/regeneration, and disease.
1. INTRODUCTION
The respiratory system has ideal tissue structure and cell types for efficient gas exchange to intake oxygen and release carbon dioxide. In mammalians, the respiratory system consists of two structures. Alveoli are distal lung tissues with acinar structures where gas exchange occurs. The airways (or conducting airways), including the trachea, bronchi and bronchiole, are proximal branching tubules that connect the external environment to alveoli and remove various harmful particles, such as viruses and bacteria, to protect alveoli from invasion (Figure 1). This airway epithelium functions as the primary physical barrier against various noxious substances from the environment, but it is easily and frequently damaged. However, cell number, composition, and function are precisely controlled even after severe damage, such as influenza infection. This robust homeostasis is orchestrated by interactions among neighboring cells and by epithelial‐mesenchymal interactions controlling tissue stem cells. Among the pathways that regulate interactions between neighboring cells, the Notch pathway is one of the most well‐studied in lung development and regeneration, in which it plays crucial roles in processes such as proximo‐distal patterning, cell fate choice, cell proliferation, and apoptosis. The present review provides a comprehensive summary of the roles of Notch signaling in lung development, homeostasis, regeneration, and disease.
Figure 1.
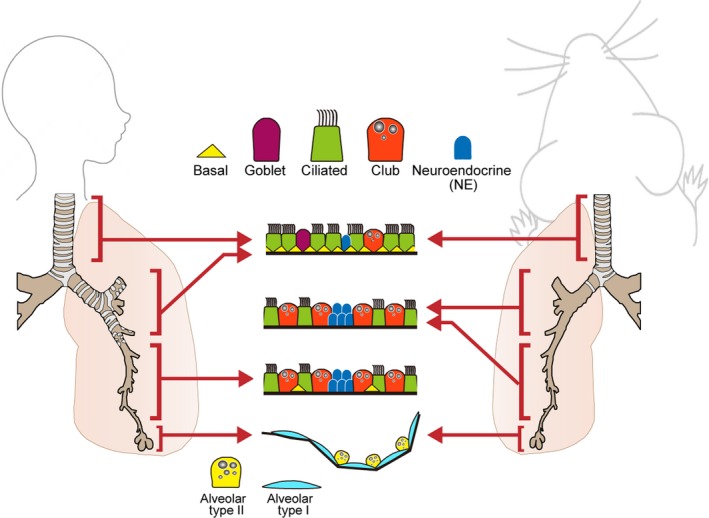
Tissue structure of the respiratory system. A variety of lung epithelial cells exist along the proximal‐distal axis. In proximal regions, there are five major cell populations, including basal cells (yellow triangle), Goblet cells (purple), ciliated cells (green), club cells (orange), and neuroendocrine cells (blue). The cellular composition varies along the proximal‐distal axis. Basal cells reside in only cartilaginous airways in mice, while they also reside in smaller airways, such as bronchioles, in humans. In alveolar regions, there are two types of epithelial cells: flattened alveolar type I cells (pale blue) and cuboidal alveolar type II cells (yellow)
Notch signaling is a highly conserved cell–cell signaling pathway ideally suited for very short‐range cellular communication because it is transmitted to adjacent cells through direct contact. In both humans and mice, this pathway consists of four receptors (Notch 1, 2, 3, and 4) and five canonical ligands (Jagged 1 and 2 and Delta‐like ligand (Dll) 1, 3, and 4) (Figure 2). Through direct binding, the Notch intracellular domain (NICD) is cleaved from the Notch receptor by γ‐secretase and translocates into the nucleus (Kopan and Ilagan Cell 2009; see other reviews in this issue; see also YouTube; https://www.youtube.com/watch?v=IOuuMmvzqQI). This nuclear translocation activates the expression of Notch target genes such as Hes family bHLH transcription factor 1 (Hes1) through interactions with Rbpj and mastermind‐like 1‐3 (Maml1‐3), which are necessary to target specific DNA sites (Kageyama & Ohtsuka, 1999). The functions of Notch signaling can be classified based on three transmission modes (Bray, 2006). The first mode is lateral inhibition, in which ligand‐expressing cells stochastically appear in the uniform progenitor population to activate Notch signaling in adjacent cells and turn off ligand expression, thereby clearly delineating Notch‐active and Notch‐negative cells. Notch‐mediated lateral inhibition plays a critical role in generating a mosaic pattern of differentiation through the exclusive differentiation of ligand‐expressing cells and the coordination of neighboring Notch‐active cell populations. The second mode is induction, in which ligand‐expressing cells and receptor‐expressing cells are derived from different cell lineages and clearly specified in advance. In this mode, Notch signaling is activated in only the receptor‐expressing cell lineage. The last mode is characterized by lineage decisions, which are mainly dependent on the asymmetrical inheritance of Notch regulators such as Numb, a negative regulator of Notch signaling that antagonizes receptors, leading to the downregulation of Notch signaling in Numb‐positive daughter cells. In contrast, Notch signaling is upregulated in Numb‐negative daughter cells. This asymmetric division gives rise to Notch‐active and Notch‐negative daughter cells and contributes to dynamic fate decisions, including the asymmetric division of stem cells and progenitor cells. Hereafter, we will review the roles of Notch signaling in lung development, homeostasis/regeneration, and disease.
Figure 2.
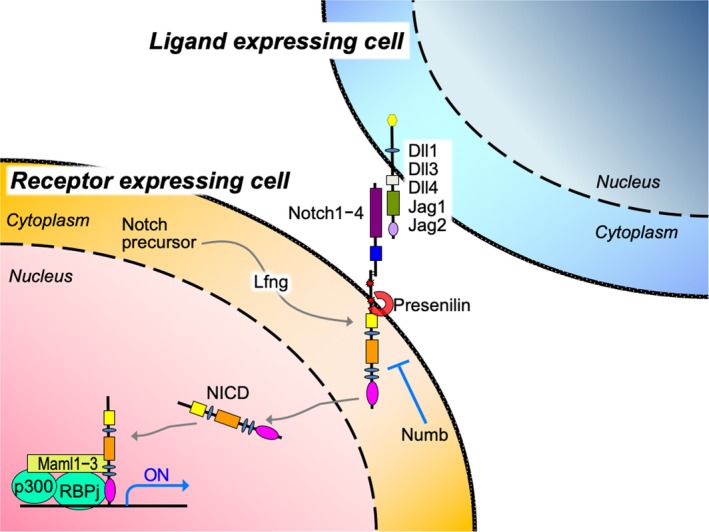
Overview of the Notch signaling pathway. The Notch pathway is regulated by direct interactions between a ligand‐expressing cell (blue) and a receptor‐expressing cell (orange). Gray arrows indicate the transition of precursor Notch protein to the mature and transcriptionally active form. The direct binding of ligands triggers cleavage of the NICD, which translocates into the nucleus. The NICD activates Notch target genes through interactions with Rbpj and Maml1‐3. Numb is a negative regulator of Notch signaling that antagonizes receptors. Lfng is a beta(1‐3)N‐acetylglucosamine transferase that modifies Notch receptors to facilitate their activation
2. NOTCH IN LUNG DEVELOPMENT
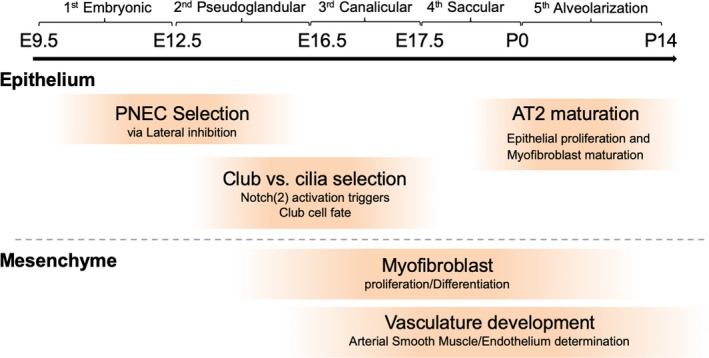
First, prospective respiratory cells appear to express Nkx2.1, a key transcription factor in respiratory epithelial cells, on the ventral side of the anterior foregut endoderm and to form a diverticulum around embryonic day 9.0 (E9.0) in mice and 4 weeks in humans. This primordial tissue develops highly branched and stereotypic airway structures and millions of alveolar sacs in five stages: the embryonic, pseudoglandular, canalicular, saccular, and alveolarization stages (Herriges & Morrisey, 2014) (Figure 3). In the embryonic stage (E9.5–E12.5 in mice), two primary lung buds and a simple trachea tube arise from the Nkx2.1+ diverticulum and separate from the esophagus. During the embryonic and pseudoglandular stages (E12.5–E16.5), the two lung buds start the highly orchestrated branching process to form an airway network with thousands to millions of terminal branches. Following the canalicular stage (E16.5–E17.5) and saccular stage (E17.5‐postnatal day 0 (P0)), these terminal branches begin forming epithelial acinar structures called alveoli, which fully mature during the alveolarization stage (P0–P14). Through these five stages, the respiratory system acquires unique cell types that are precisely regulated in number and space to maximize the efficiency of gas exchange. Tightly regulated cell–cell communication is critical in each stage to achieve this goal. Notably, Notch signaling is known to be a key player in orchestrated cellular interactions. We will highlight several roles of the Notch pathway during development.
Figure 3.
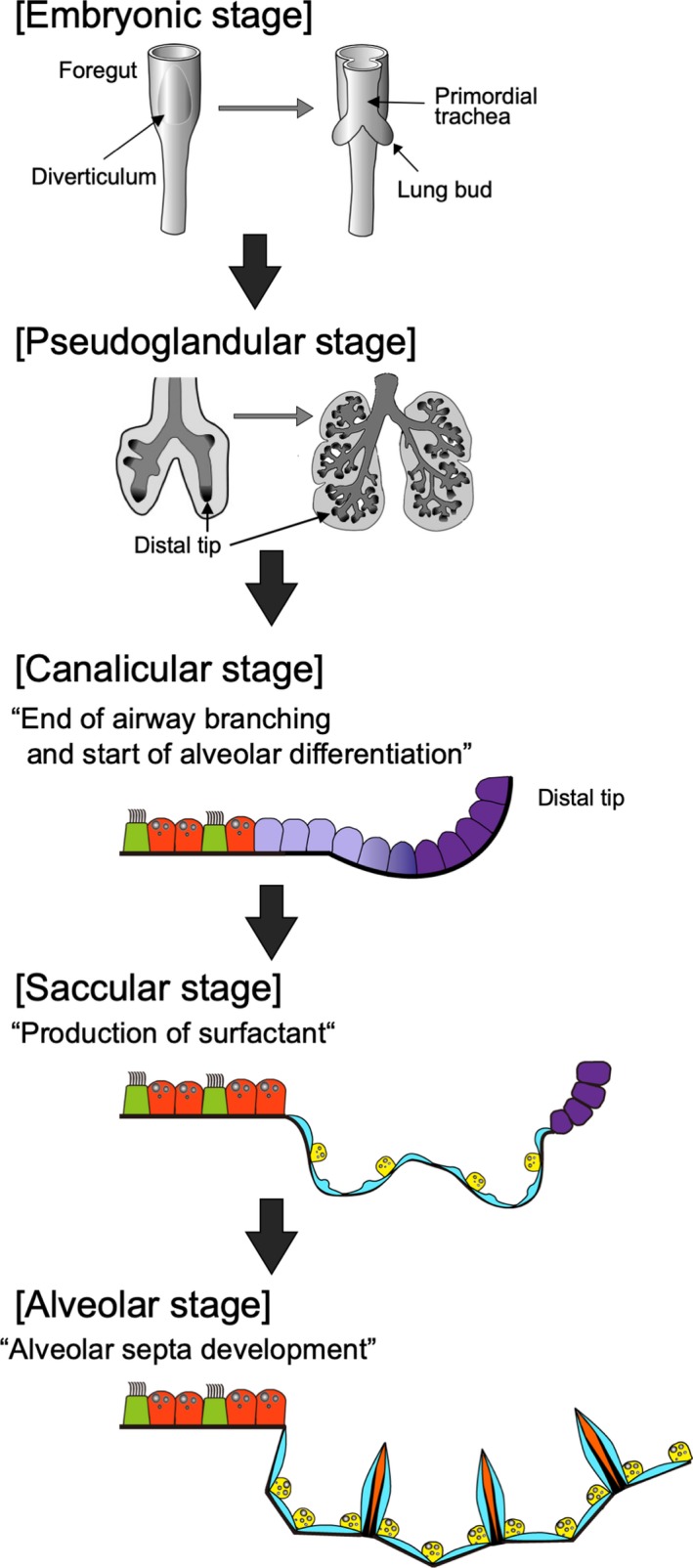
Overview of the stages of lung development. Lung development occurs in five stages: the embryonic (E9.5–E12.5 in mice), pseudoglandular (E12.5–E16.5), canalicular (E16.5–E17.5), saccular (E17.5–P0), and alveolarization stages (P0–P14). In the embryonic stage, two primary lung buds and a simple trachea tube arise from the diverticulum and separate from the esophagus. In the pseudoglandular stage, the highly orchestrated branching process begins to form an airway network. In the canalicular stage, conducting airways stop elongating, and distal tip cells differentiate into alveolar epithelial cells. In the saccular stage, alveolar epithelial type II cells product surfactant to prepare the organism for birth. In the alveolar stage, alveoli become mature with alveolar septum development
2.1. Notch signaling determines multiple cell fates in conducting airways
The role of Notch in mouse development has been studied since 1994 (Swiatek, Lindsell, Del Amo, Weinmaster, & Gridley, 1994), when murine gene manipulation such as gene knockout (KO) became available. In 2000, Brigid Hogan's group first reported the gene expression patterns of Notch receptors and ligands in the developing mouse lung with a knock‐in LacZ reporter and in situ hybridization (Post, Ternet, & Hogan, 2000). This study suggested that Notch signaling might contribute to cell diversification in fetal lungs. This finding was supported by a study by Ito et al. in the same year (Ito et al., 2000). Using Hes1‐ and Mash1‐null mouse embryos, these authors revealed the exclusive differentiation of pulmonary neuroendocrine cells (PNECs) through Notch‐mediated lateral inhibition. Hes1 is a major Notch target gene, and Mash1 regulates Dll1 expression in PNECs. In Hes1‐null lungs, the PNEC population expands alongside the reduction in club cells expressing Notch receptors. At that time, this finding was not conclusive because Hes1 expression during development could potentially be regulated by noncanonical pathways such as the FGF, JAK, and ERK pathways. More detailed information on the various roles of Notch in lung development is now available due to the use of lineage tracing and conditional KO mice since 2009.
Tsao et al. and Morimoto et al. generated endodermal epithelium‐specific deletions of Notch‐related components and found that Notch inhibition resulted in expansion of the PNEC population and a decrease in club cells (Morimoto et al., 2010; Tsao et al., 2009) (Figure 4b–d, Table 1). In addition, the authors revealed the detailed mechanisms of cell fate choice between club cells and ciliated cells in proximal airways. Club cells and ciliated cells reside next to each other in a mosaic pattern in the airway, suggesting that the cell fate choice between club and ciliated cells is balanced by Notch‐mediated lateral inhibition. In fact, the authors showed that epithelium‐specific Rbpj KO disturbed this regulated mosaic pattern and expanded the ciliated population at the expense of club cells. The contribution of Notch1 activation to club cell specification was directly confirmed by detecting the active form of the Notch1 receptor with an antibody against Val1744 of the intercellular domain of Notch1 (NICD1) and lineage tracing with N1IP::Cre mice, in which Cre recombinase translocates into the nucleolus upon Notch1 activation (Morimoto et al., 2010). Zhang S et al. revealed that ciliated cells provide Jagged1 to neighboring club cells to activate Notch (Zhang, Loch, Radtke, Egan, & Xu, 2013). As with the epithelium in Pofut1 or Rbpj KO lungs, Jagged1 deletion within lung epithelial cells also showed ciliated cell expansion with a reduction in club cells. Combinatorial deletion of the Notch 1, 2, and 3 genes further revealed that Notch2 plays a dominant role in the differentiation of club cells, in which Notch1 is activated (Morimoto, Nishinakamura, Saga, & Kopan, 2012). Thus, Jagged1‐Notch2 signaling plays a major role in orchestrating alternative cell fate choices and forming the mosaic pattern of club and ciliated cells, while Notch1 plays an auxiliary role in these processes.
Figure 4.
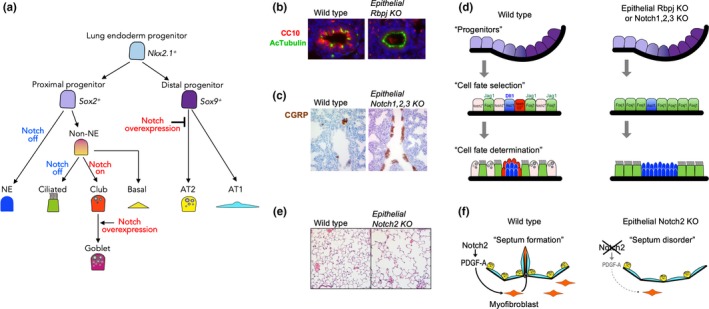
Notch signaling in lung development. (a) Notch‐mediated cell fate choice in epithelial development. In the proximal region, Notch activation is necessary for club and goblet cell differentiation. In the distal area, Notch overexpression inhibits the commitment of distal progenitors into alveolar cells. (b) Immunocytochemistry for Ac‐Tubulin+ ciliated cells (green) and CC10+ club cells (red). Epithelial Rbpj KO expands the ciliated cell population at the expense of CC10+ club cells. (c) Immunohistochemistry for CGRP + neuroendocrine cells (brown). Epithelial triple Notch KO results in the expansion of neuroendocrine cells. (d) Schematic summary of Notch‐mediated cell fate determination in the lung. In wild‐type animals, Foxj1+ ciliated cells (green) and Ascl1+ neuroendocrine (blue) cells express Notch ligands (Jagged1 and Dll1, respectively) to communicate with neighboring cells, which contributes to the specification of club cells, including SPNC cells (red). In contrast, epithelial Notch KO expands the population of Notch‐negative cells, such as ciliated and neuroendocrine cells, at the expense of Notch‐active club and SPNC cells. (e) Hematoxylin‐eosin staining of alveoli in 3‐month‐old mice. Epithelial Notch2 KO mice show an emphysema phenotype. (f) Scheme of alveolar defects in epithelial Notch2 KO mice. Notch activation in epithelial cells induces the secretion of PDGF‐A, an essential inductive signal for the proliferation and differentiation of myofibroblast progenitors. Since myofibroblasts contribute to septa formation, Notch2 KO in epithelial cells results in septum disorders due to impaired proliferation and maturation of myofibroblasts
Table 1.
Phases of developmental events related to Notch signaling
Notch inhibition results in an increase in PNEC number and an expansion of PNEC clusters (neuroendocrine bodies, NEBs). Immunostaining for NICD1 showed that the Notch pathway is activated in epithelial cells adjacent to NEBs expressing the Notch ligand Dll1. These Notch‐active epithelial cells are characterized as SPNC cells or Clara‐like progenitors that express the SSEA‐1 antigen and Upk3 gene (Guha et al., 2012; Morimoto et al., 2012). These cells are absent in the epithelium of Notch1, 2, and 3 triple KO mice, resulting in NEB expansion, similar to the findings in Hes1 KO mice; these results indicate that Notch‐Hes1 signaling is involved in restricting the PNEC fate of epithelial progenitors (Figure 4d, Table 1). To investigate the mechanism of PNEC restriction via Notch‐Hes1, further detailed analyses were performed at early stages, such as E13.5 (Noguchi, Sumiyama, & Morimoto, 2015). High‐resolution live imaging of developing PNECs revealed the stepwise development of NEBs, in which a solitary PNEC appears and then, surprisingly, migrates to the bifurcation point of branching airways to form NEBs as neuroendocrine cell clusters. Notch‐Hes1 signaling is involved in the first step by regulating the number of solitary PNECs by lateral inhibition.
What is the difference between these two neighboring Notch‐active cell types, ciliated cells and PNECs? Notch signaling directly promotes Scgb3a2 gene expression in naïve epithelial cells, which potentially differentiate into club or ciliated cells (Guha et al., 2012). Forced activation of Notch1 and 2 in naïve epithelial cells blocks PNEC differentiation but cannot induce club cell differentiation (Morimoto et al., 2012). In conclusion, the role of Notch signaling in developing proximal airways involves preserving the capacity for bipotential differentiation into club and ciliated cells by avoiding complete commitment to ciliated cells or PNECs.
Notch signaling is also involved in the induction of goblet cells, which secrete mucosa and are related to conditions of chronic inflammation such as asthma. Guseh JS et al. showed that misexpression of NICD1 in epithelial cells expands Muc5ac‐expressing goblet cells and decreases ciliated cells (Guseh et al., 2009) (Figure 4a). In the postnatal lung, Notch2 is involved in cytokine‐induced goblet cell metaplasia both in vitro and in vivo (Danahay et al., 2015), while the Rbpj‐null epithelium shows no induction of goblet cells (Tsao et al., 2009). Cytokines such as IL‐4/IL‐13 bias the airway basal cell fate to goblet cells, leading to goblet cell metaplasia in many airway diseases such as asthma. This goblet cell metaplasia was inhibited by administration of an inhibitory antibody against Jagged1 or Notch2 in vitro and in vivo, which suggests that Jagged1‐Notch2 signaling is involved in goblet cell specification in asthma. This result is consistent with the major role of Notch2, but not of Notch1/3, in club cell specification (Morimoto et al., 2012). Is only Notch2 able to induce a unique genetic program for club cell fate? Do Notch1/3 activate a different gene set than Notch2? To understand the different effects of Notch1 and Notch2 signaling, Liu et al. examined transgenic mice in which the NICDs were swapped (Liu et al., 2015). Surprisingly, chimeric receptors in which the Notch2 extracellular domain was fused to NICD1 (Notch21) showed no obvious phenotype, including no difference in the club/ciliated cell rate. This observation suggested that the phenotypic differences between Notch1 and Notch2 deletion likely reflect differential regulation by tissue‐specific cis enhancers and selectivity in ligand‐receptor interactions; Jagged1 favors Notch2 but is not exclusive.
2.2. Contradictions between phenotypes after chemical inhibition or genetic ablation of Notch‐related factors
Dr. Wellington Cardoso's group proposed that Notch is required to maintain balance in the proximal‐distal axis during the early stage of lung development based on the results of ex vivo embryonic lung cultures (Tsao et al., 2008). In these experiments, Notch activation was globally inhibited by preventing γ‐secretase cleavage of Notch receptors with a γ‐secretase inhibitor (DAPT). Disruption of the Notch pathway in ex vivo cultures of E8.5 or E11.5 lungs resulted in expansion of the distal progenitor population (Sox2 negative) and the increase in ectopic budding in the more proximal region. However, two issues with these experiments must be addressed. First, the authors inhibited Notch signaling globally with pharmacological DAPT treatment, which makes it difficult to elucidate the detailed mechanisms by which Notch signaling affects proximo‐distal axis formation. Second, they used ex vivo lung cultures, which might influence the effects of Notch inhibition in many ways. To address these issues, Cardoso's and Morimoto's group assessed the effects of Notch inhibition on airway epithelial cells using mice with endoderm‐specific conditional KO. Notch‐mediated alternative cell fate selection of club vs. ciliated cells was observed, but impaired proximo‐distal patterning and branching morphogenesis were not observed (Morimoto et al., 2010; Tsao et al., 2009). Collectively, the negative effects of global Notch inhibition on proximo‐distal patterning and branching likely reflect loss of the vascular endothelial network and/or the associated smooth muscle cells (SMCs), which are essential for regulating branching morphogenesis and maintaining distal fates (Morimoto et al., 2010).
2.3. Notch coordinates alveolar development
There is accumulating evidence that Notch plays important roles in alveolar development. The first paper assessing the effects of Notch3 overexpression on distal epithelial cell differentiation during development was published in 2003 (Dang, Eichenberger, Gonzalez, Olson, & Carbone, 2003). In this paper, the Notch3 ICD was overexpressed under control of the surfactant protein C (SPC) promotor/enhancer using transgenic technology; SPC is a marker of alveolar type II (AT2) cells. This transgenic mouse showed altered lung morphology, such as dilated cysts with cuboidal epithelial cells. Examinations with differentiated cell markers and electron microscopy revealed that the majority of the distal epithelial cells were immature, suggesting that Notch3 overexpression blocks alveolar development (Figure 4a). A similar phenotype was observed in mice with NICD1 overexpression (Guseh et al., 2009). These results suggest that NICD overexpression in developing epithelial cells impairs the commitment to alveolar epithelial cells and results in a cystic epithelium. However, epithelial cell‐specific Notch inhibition during prenatal development did not produce an abnormal phenotype in the distal region (Morimoto et al., 2010). These contradictory results between the Notch gain‐ and loss‐of‐function experiments may imply off‐target effects of abnormal Notch activation in the developing epithelium that do not reflect the physiological function of the Notch pathway. In addition, Rbpj conditional KO within the mesenchyme using Dermo1‐Cre did not disrupt prenatal lung development in the distal region, although the recruitment and specification of arterial vascular SMCs (vSMCs) were somewhat impaired. A detailed phenotypic analysis of the loss of Notch signaling in alveologenesis at the postnatal stage was published in 2016 (Tsao et al. 2016). In the alveolarization stage (P0 to P14), a secondary septum forms through the interaction of alveolar epithelial cells with myofibroblasts to dramatically expand the alveolar region for effective gas exchange. In this study, Pofut1 or Notch2 was ablated in fetal lung epithelial cells. Emphysema‐like cystic abnormalities were observed in both mutants after birth (Figure 4e,f, Table 1). Canonical marker and morphological analyses at the postnatal stage revealed that the secondary septation was impaired in the mutants, mainly due to the decreased proliferation and maturation of AT2 cells. In addition, the proliferation and differentiation of myofibroblasts were impaired, in part due to the decrease in PDGF‐AA expression in mutant epithelial cells; PDGF‐AA is an essential signal that induces the proliferation and differentiation of myofibroblast progenitors. Collectively, Notch activation is essential for proper alveolar development after birth, especially for secondary septa formation (Tsao et al., 2016). Similar to these mutants, Jagged1 conditional KO in the AT2 cell lineage using Spc‐rtTA also produced an emphysema‐like phenotype with impaired septation (Zhang et al., 2013). This study proposed that Jagged1 expression before E15.5, rather than in the postnatal stage, is critical for this phenotype. Further study is needed to clarify the exact timing of Notch activation and the cell types required for alveologenesis. A detailed understanding of Notch signaling in alveologenesis is important because it may provide new possibilities for novel treatments for neonatal respiratory diseases such as bronchopulmonary dysfunction.
2.4. Notch controls vascular tissue development in the lung mesenchyme
In general, the Notch pathway has been proven to be one of the key pathways regulating arterial specification, sprouting angiogenesis, and vessel maturation (Benedito & Hellstrom, 2013). Therefore, researchers speculated that Notch signaling is important in the development of the microvascular network in fetal lungs. This hypothesis was proven in 2010 with the Lunatic Fringe (Lfng) KO (Xu et al., 2010). Lfng is a beta(1‐3)N‐acetylglucosamine transferase that modifies Notch receptors to facilitate their activation. The authors found that Lfng‐null lungs showed impaired pulmonary vasculature development and dysregulation of myofibroblast differentiation. Notch activation during pulmonary vascular development was visualized by lineage tracing in N1IP::Cre mice, in which Cre is expressed in cells with activated Notch1 (Morimoto et al., 2010). Notch1‐positive mesenchymal cells in developing lungs were the predominant contributors to lung vasculature, developing into arterial endothelial cells and vSMCs (Table 1). Mesenchyme‐specific Rbpj conditional KO with Dermo1‐Cre impaired selection of the vSMC fate and the migration of vSMCs arising outside the arteries. Notch signaling in vSM progenitor cells activates the expression of PDGFR‐β, which plays an important role in pericyte recruitment and vSMC differentiation during vasculogenesis.
3. NOTCH SIGNALING IN HOMEOSTASIS AND REGENERATION
Homeostasis of the lung epithelium is robustly maintained by the specific functions of resident tissue stem cells, which respond to epithelial damage caused by inhaled harmful particles. The respiratory system harbors various types of epithelial tissue stem cells, from basal cells in the trachea to AT2 cells in alveoli (Hogan et al., 2014; Kotton & Morrisey, 2014) (Figure 1). These cells repopulate mature cell populations after cell loss in a tightly controlled manner under both homeostatic and regeneration conditions, although there are some differences in the mechanisms between maintenance and repair processes. The mechanisms involved in these processes are of primary interest in stem cell biology, and we have just begun to reveal them. It is important to understand the molecular pathways critical for lung development because many of these pathways and processes are used during adult tissue regeneration to promote rapid proliferation and differentiation. Notch is one of the most studied signaling pathways in resident tissue stem cells in conducting airways, while other signaling molecules, such as BMP, Wnt, FGF, and Yap, are also involved in orchestrating these processes (Lee et al., 2014, 2017; Tadokoro, Gao, Hong, Hotten, & Hogan, 2016; Volckaert et al., 2017). In this section, the role of Notch in homeostasis and regeneration processes will be discussed, with a focus on basal cells in the cartilaginous airway and lineage‐negative epithelial progenitors (LNEPs) in the bronchiolar‐alveolar region.
3.1. Homeostasis and regeneration in the proximal airway
Airway basal cells, which reside in the cartilaginous airway (tracheal and proximal bronchi) in mice and in the trachea to bronchioles in humans (Figure 1), contribute to tissue maintenance and regeneration of the airway epithelium in response to various injuries (Hong, Reynolds, Watkins, Fuchs, & Stripp, 2004). Rock JR et al. reported that the Notch pathway is involved in the function of the basal cell population (Rock et al., 2009). Dominant expression of Notch1, Dll1, and Jagged2 in basal cells compared to other cell populations was determined with deep RNA‐seq. The authors examined the role of Notch signaling in basal cells by using mouse genetics and the tracheosphere culture system, a 3D in vitro method for culturing basal cells to assess self‐renewal capacity and multipotency (Rock et al., 2011). Sustained Notch activation in basal cells in mutant mice promoted luminal cell differentiation mainly toward the club cell lineage, while Notch inhibition in tracheosphere culture by dibenzazepine (DBZ), a γ‐secretase inhibitor, resulted in the exclusive expansion of basal cells at the cost of luminal cell differentiation within these spheres. These results suggest that Notch activation is crucial for differentiation into club cells but dispensable for the self‐renewal of basal cells in homeostasis and regeneration, which is consistent with the club cell specification in embryos. Using the tracheal epithelial system as an in vivo model for tissue stem cells, Pardo‐Saganta A et al. proposed a new concept that tissue stem cells function as niches for their daughter cells (Pardo‐Saganta, Tata, et al., 2015). They found that Notch ligands, such as Dll1 and Jagged2, in basal cells maintain club cell homeostasis. Genetic ablation of basal cells by Cre‐mediated expression of diphtheria toxin fragment A (DTA) decreased the club cell population due to the loss of Notch ligands expressed by basal cells as niche factors. Collectively, Notch activation is necessary for club cell specification and maintenance, while the Notch pathway is not needed for basal cell self‐renewal. Once Notch activation is lost in the club cell population, they lose the club cell identity and commit to the ciliated cell fate (Figure 5a). The direction of homeostatic epithelial cell lineage fate (basal cells → club cells → ciliated cells) was confirmed by an elegant clonal analysis performed by Watson JK et al. (Watson et al., 2015). Although respiratory epithelium turnover is very slow under homeostatic conditions, these authors produced a high‐resolution lineage map under such conditions with the combination of lineage tracing, mathematical modeling, and single‐cell qRT‐PCR.
Figure 5.
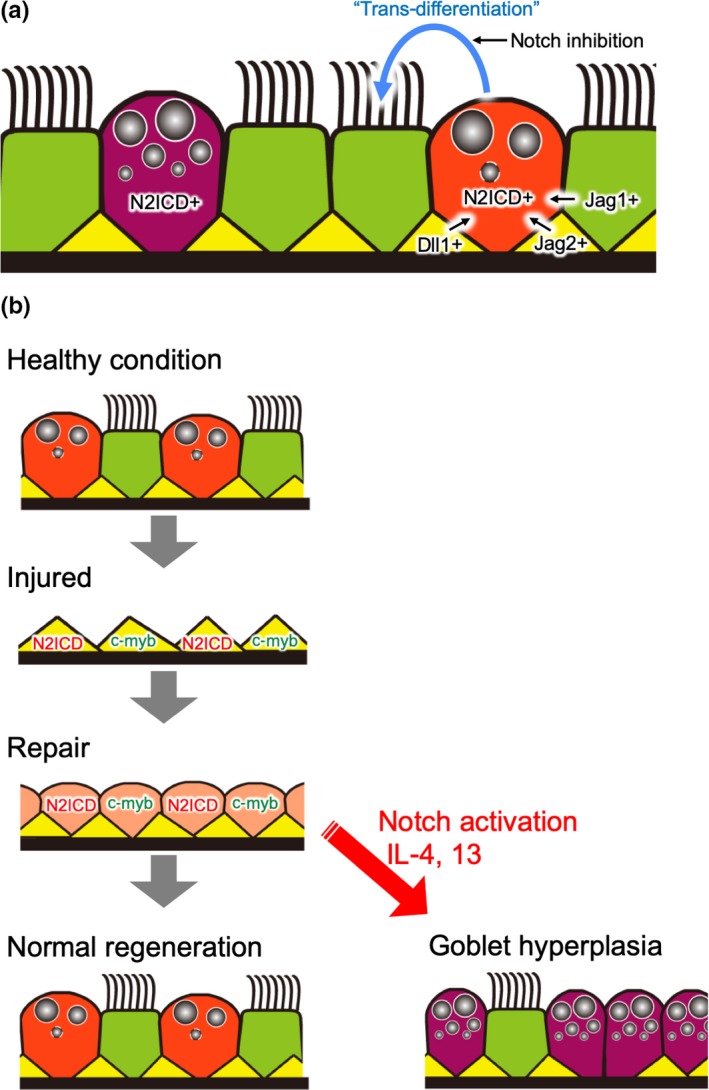
Notch signaling in airways under homeostatic and regenerative conditions. (a) In homeostasis, basal cells (yellow) and ciliated cells (green) express Notch ligands. These cells maintain continuous Notch2 activation in club cells (orange) and goblet cells (purple). If Notch signaling is off, these become ciliated cells. (b) A schematic summary of the regeneration process after naphthalene or SO 2 injury. The luminal cell loss due to injury triggers the expansion of basal cells and the direct segregation into N2ICD + club progenitors and c‐myb+ ciliated progenitors. Abnormal Notch activation in combination with surges of cytokines, such as IL‐4/13, induces goblet hyperplasia
The first paper reporting Notch activation in regenerating airways was published in 2010 (Morimoto et al., 2010). This study used lineage tracing with N1IP::Cre to show that Notch1 activation contributes to the club cell lineage. In 2011, Notch function in airway regeneration was assessed with a sulfur dioxide (SO2) injury model, in which tracheal luminal cells, including club and ciliated cells, are damaged but most basal cells survive. Notch‐active cells were detected 12 h post injury (hpi), with a peak at 36 hpi. The fact that most of the Notch reporter‐positive cells were p63‐ (basal cell marker) and Krt8 + (luminal cell marker) suggests that Notch activation contributes to the recovery from luminal cells after SO2 injury, especially by promoting the differentiation of basal cells into luminal progenitors (Figure 5b). Another study showed that lineage commitment to the club cell fate versus the ciliated cell fate in luminal progenitors after SO2 injury was determined by approximately 6 hpi (Pardo‐Saganta, Law, et al., 2015). The authors found that N2ICD and c‐myb are very early markers of the club and ciliated cell lineages, respectively, after injury, suggesting that the contribution of Notch activation to club cell lineage is shared between homeostatic and regenerative processes (Figure 5b). However, basal cells can directly differentiate into ciliated cells in the repair process, but under homeostatic conditions, they primarily produce club cells that then differentiate into ciliated cells. These different modes of differentiation in homeostasis and regeneration were confirmed by two large‐scale single‐cell RNA‐seq analyses (Montoro et al., 2018; Plasschaert et al., 2018), although the mechanisms remain unclear.
In smaller airways, which do not contain basal cells, club cells are the major tissue stem cell population. In particular, specific club cells called variant club (vClub) cells, which reside next to NEBs, are tissue stem cells resistant to chemical injury, such as naphthalene injury (Hong, Reynolds, Giangreco, Hurley, & Stripp, 2001). In this context, most club cells are damaged and lost, but vClub and neuroendocrine cells function as tissue stem cells to restore the damaged epithelium. Song H et al. performed lineage tracing by using CGRP‐CreER mice, in which the PNEC lineage can be visualized, and revealed that neuroendocrine cells contribute to club cell and ciliated cell populations after naphthalene injury (Song et al., 2012). Because PNECs express Dll1, involvement of Notch signaling was expected. Indeed, epithelium‐specific Notch1 KO using Gata5‐Cre impaired club cell expansion from neuroendocrine cells in smaller airways after naphthalene injury (Xing, Li, Borok, Li, & Minoo, 2012). In addition, NICD overexpression using CGRP‐CreER in conjunction with naphthalene injury led to drastic increases in the proliferation and transdifferentiation of PNECs toward the club cell fate (Yao et al., 2018). Collectively, these results confirm that the Notch pathway is necessary for club cell regeneration, especially for specification from neuroendocrine cells.
3.2. Homeostasis and regeneration in the distal lung
AT2 cells play a central role in both the homeostasis and regeneration of alveoli, which are located in the most distal area of the respiratory system, perform gas exchange, and are robustly maintained by various types of facultative tissue stem cells in response to different types of injuries. Therefore, researchers have focused on the mechanisms regulating the self‐renewal and differentiation of AT2 cells to understand homeostasis and regeneration in the distal lung.
Although there is no direct evidence that Notch is involved in AT2 cell maintenance, Vaughan AE et al. recently revealed that Notch activation is involved in regeneration of the distal lung, including alveoli (Vaughan et al., 2015). LNEP cells, which are a minor stem cell population under homeostatic conditions and mainly reside in small airways, contribute to regeneration in the distal lung region. Severe injury by influenza or bleomycin stimulates quiescent LNEP cells to undergo transdifferentiation into basal‐like cells expressing ΔNp63 (p63 splice variant) and cytokeratin 5. These basal‐like cells expand and migrate toward the wound area to repair the damaged epithelium (Figure 6). Treatment with the γ‐secretase inhibitor DAPT disrupted this repair process in both in vivo and in vitro models. The authors concluded that Notch activation is required for the generation of basal‐like cells from LNEPs but inhibits transdifferentiation to alveolar epithelial cells. This finding is consistent with the result that Notch overexpression during development inhibits the commitment of embryonic progenitors to the alveolar epithelial cell lineage (Dang et al., 2003; Guseh et al., 2009). In a subsequent paper, the authors revealed that Notch activation in LNEPs is initiated by stabilization of HIF1α under conditions of hypoxia (Xi et al., 2017). Epithelium‐specific HIF1α KO blocks Notch and Krt5 expression and promotes the transdifferentiation of LNEPs to AT2 cells, which was phenocopied by constitutive activation of Wnt signaling. As Wnt is known as an important niche factor for AT2 cells (Nabhan, Brownfield, Harbury, Krasnow, & Desai, 2018; Zacharias et al., 2018), the authors concluded that the transdifferentiation of LNEPs to basal‐like cells or AT2 cells depends on the balance between HIF1α‐mediated Notch activation and Wnt activation.
Figure 6.
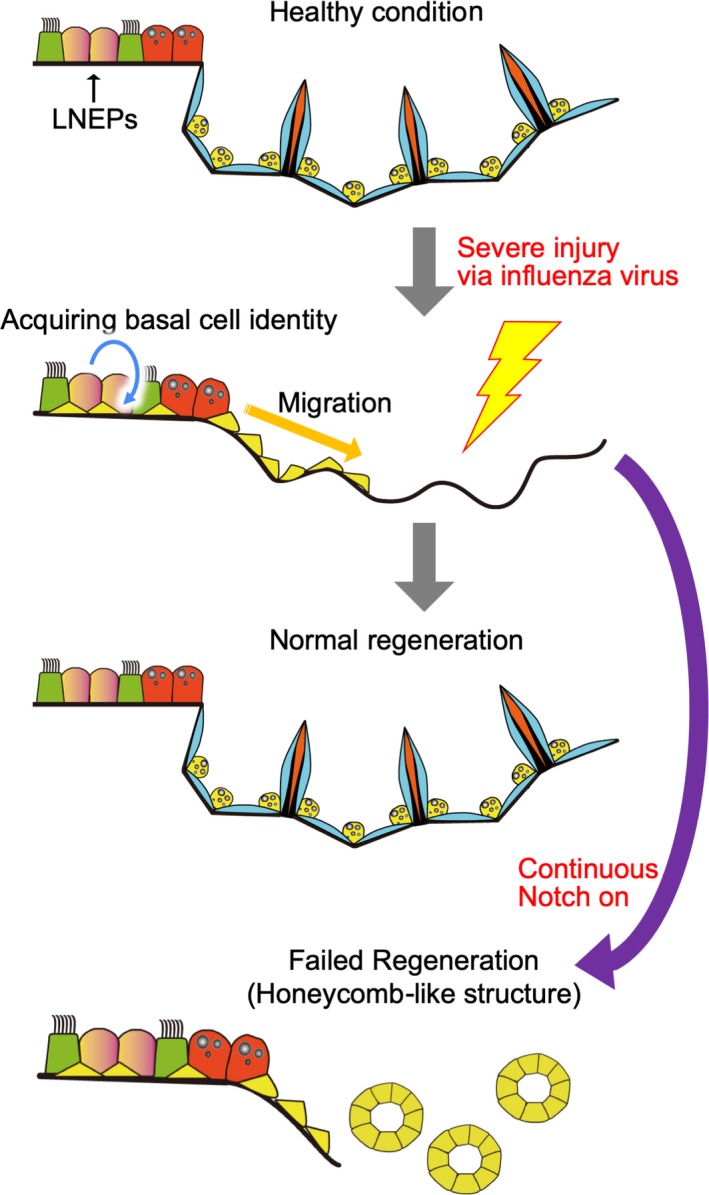
Notch signaling during regeneration in the distal area, including alveoli. In homeostasis, LNEPs mainly reside in small airways. LNEPs contribute to regeneration in the distal lung after severe injury, such as influenza virus infection. LNEPs become basal‐like cells, acquiring ΔNp63/Krt5 expression in a Notch‐dependent manner and migrating to the damaged area. Notch inhibition is necessary for normal repair in the alveoli because Notch blocks the transdifferentiation of basal‐like cells into alveolar cells. Thus, continuous Notch activation results in abnormal regeneration with honeycomb‐like histology, which is often observed in patients with IPF due to the failed transdifferentiation of basal‐like cells to alveolar epithelial cells
4. NOTCH SIGNALING IN RESPIRATORY DISEASES
Given the various important roles of Notch in lung homeostasis and regeneration, the dysregulation of Notch signaling‐related factors is potentially related to a variety of pulmonary diseases (Table 2). Here, we will discuss the current knowledge of Notch signaling in respiratory diseases.
Table 2.
Notch signaling in respiratory diseases
| Related Notch genes | Functions of Notch | |
|---|---|---|
| PAH | ||
| Pulmonary artery hypertension | Notch3 overexpression | Increased proliferation of vascular smooth muscle cells |
| COPD | ||
| Chronic obstructive pulmonary disease | Enhanced NICD1 in mucosal hyperplasic regions | Notch signaling promotes mucosal hyperplasic |
| Decreased Notch signaling as a whole | Notch down regulation induces emphysema via endothelial cell apoptosis | |
| IPF | ||
| Interstitial pulmonary fibrosis | Enhanced NICD1 in highly fibrotic alveolar regions | Profibrotic via inducing endothelial‐mesenchymal transition (EMT) |
| Lung cancer | ||
| Non‐small cell lung cancer (NSCLC) | Notch3 over expression in NSCLC | Highly complex and context‐dependent functions |
| Small cell lung cancer (SCLC) | Decreased Notch signaling via Dlk1/DII3 in SCLC | Tumor promotor in NSCLC |
| Tumor suppressor in SCLC | ||
4.1. Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is characterized by increased blood pressure in the pulmonary artery due to various causes, including progressive remodeling, narrowing, and obliteration of small pulmonary arteries triggered by the proliferation of smooth muscle and endothelial cells (Tuder et al., 2013). In 2009, Li X et al. reported that NOTCH3 is a key factor in the progression of PAH, mainly through controlling the proliferation of vSMCs (Li et al., 2009). The authors found that PAH severity in both humans and mice was significantly correlated with NOTCH3/Notch3 expression in the lungs; NOTCH3 expression was confined to vSMCs and was associated with vSMC proliferation. The causal association between Notch3 activation and vSMC proliferation was shown in Notch3 KO mice and DAPT‐treated PAH model mice. Notch3 KO mice did not develop pulmonary hypertension in response to hypoxic stimulation. In addition, DAPT treatment significantly attenuated the development of PAH. These results suggest that NOTCH inhibition may be a novel treatment option for patients with PAH by antagonizing the NOTCH‐dependent proliferation of vSMCs. Although it was also reported that the administration of soluble Jagged1 inhibited pulmonary hypertension by attenuating Notch signaling (Xiao, Gong, & Wang, 2013), these types of treatments have not been translated to clinical practice. Clinical trials to test whether NOTCH inhibition is a novel treatment for PAH are eagerly anticipated.
4.2. COPD
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease and the world's third leading cause of death; COPD is characterized by chronic airflow limitations and persistent respiratory symptoms, such as dyspnea and cough. Airflow is limited by a mixture of small airway disease and parenchymal destruction known as emphysema, which is mainly triggered by cigarette smoking. The first comprehensive study addressing the effects of cigarette smoking on NOTCH‐related gene expression was published in 2009, reporting the transcriptome analysis of human epithelial cells (Tilley et al., 2009). Microarray analysis of human epithelial cells revealed that NOTCH3 and DLL1 expression levels were significantly downregulated in COPD patients compared to nonsmokers. Reflecting this observation, the HES5 and HEY1/2 genes, which are downstream of Notch signaling, were also downregulated in COPD patients, suggesting that Notch signaling is globally inhibited in the epithelium of COPD patients. A detailed analysis using immunohistochemistry revealed enhanced expression of NICD1/HEY2 + in epithelial cells of COPD patients, especially in regions of mucosal hyperplasia (Boucherat, Chakir, & Jeannotte, 2012). This result suggests that persistent NOTCH activation triggered by cigarette smoking biases the differentiation of basal cells or club cells toward the goblet cell lineage. This phenotype was recapitulated in an in vitro 3D basal cell culture system (Danahay et al., 2015). IL‐13 and IL‐17A biased the basal cell lineage toward the goblet cell fate by blocking the ciliated cell fate, which was rescued by treatment with a Notch2 inhibitor. Therefore, NOTCH inhibitors have potential as therapeutic drugs for moderating mucosal hyperplasia in COPD patients.
The role of NOTCH in the progression of emphysema in COPD patients is not yet clear. A recent paper showed that NOTCH1/2/4 expression in endothelial cells was downregulated in COPD patients compared to healthy subjects (Zong et al., 2018). Given that the mouse Notch pathway plays a critical role in controlling apoptosis in endothelial cells (Limbourg et al., 2005), Notch signaling seems to be a promising key pathway for the progression of emphysema. The apoptosis of endothelial cells exposed to cigarette smoke extract was alleviated by Notch1 overexpression (Zong et al., 2018). These results suggest that the downregulation of NOTCH in endothelial cells increases their apoptosis, resulting in emphysema.
In summary, dysregulation of the Notch pathway is involved in the pathology of COPD, especially in mucosal hyperplasia and endothelial cell apoptosis. However, more in‐depth studies are needed to elucidate the comprehensive role of Notch pathway in COPD progression, especially in the alveolar region.
4.3. IPF
Interstitial pulmonary fibrosis (IPF) is a severe, progressive respiratory disease with poor prognosis characterized by fibroblast proliferation and extracellular matrix (ECM) deposition. The main histopathological feature is fibroblast foci, where mesenchymal cells proliferate and produce aberrant levels of ECM. Fibroblast foci are now believed to form mainly due to the induction of disordered interactions between damaged/aged alveolar epithelial cells and mesenchymal cells by fibrogenic factors, such as TGFβ signaling (Richeldi, Collard, & Jones, 2017). Recently, the Notch pathway was found to be involved in IPF. Aoyagi‐Ikeda K et al. revealed that the expression of NOTCH1 pathway components was significantly enhanced in the highly fibrotic alveolar regions of IPF patients (Aoyagi‐Ikeda et al., 2011). NOTCH1‐expressing cells also express smooth muscle actin (SMA), a marker of myofibroblasts, which form the major population in fibrotic foci in IPF.
Vaughan et al. elegantly showed that persistent Notch activation in LNEPs results in microcystic honeycombing, a hallmark of failed regeneration, phenocopying the lung destruction in IPF patients (Vaughan et al., 2015). Thus, precise control of NOTCH activation to enhance normal epithelial regeneration could be a novel antifibrotic treatment.
4.4. LUNG CANCER
Lung cancer is the leading cause of cancer‐related death worldwide. Deciphering the molecular pathology of lung cancer will provide clues for the development of new molecular targeted drugs for treating cancer. Lung cancer is classified into two main categories based on histology: small cell lung cancer (SCLC) and non‐small cell lung cancer (NSCLC). Genes related to Notch signaling are frequently mutated or aberrantly expressed in SCLC and NSCLC, although the functions of these gene in oncogenesis are context‐dependent (Zou, Zhou, Lai, & Liu, 2018). In 2000, Dang TP et al. reported NOTCH3 overexpression in 40% of NSCLC patients that was related to translocation of 19p (Dang et al., 2000). Westhoff et al. found gain‐of‐function mutations of NOTCH‐1 in 10% of NSCLC patients, while suppression of NUMB protein, a repressor of Notch signaling, was also detected in 30% of the patients in the same cohort (Westhoff et al., 2009). These results indicate that alterations in Notch signaling are relatively frequent events in NSCLC. Loss of NUMB and NOTCH activation could destabilize TP53, the major tumor suppressor in human cancer (Colaluca et al., 2008). In the subgroup of patients without TP53 mutations, a significant correlation was found between Notch activation and poor prognosis, implying that Notch activation is a prognostic indicator in NSCLC patients without TP53 mutations (Westhoff et al., 2009). Another research group conducted a meta‐analysis of 19 studies involving 3663 patients to estimate the role of Notch signaling in NSCLCs (Yuan et al., 2015) and found that higher NOTCH1 expression was related to a greater possibility of lymph node metastasis and a higher TNM stage. Overexpression of NOTCH1 and NOTCH3 was associated with significantly poorer overall survival of NSCLC patients. These results indicate that Notch signaling is a prognostic biomarker of NSCLC patients and may be a treatment target in NSCLC. To verify the clinical role of Notch signaling in NSCLC, however, large, prospective studies are needed, especially those focusing on more reliable markers of Notch activation, such as NICD.
A number of studies have reported that Notch signaling is involved in tumorigenesis, yet its detailed biological roles in NSCLC remain elusive (Zou et al., 2018). The necessity of Notch1 for Kras‐induced lung adenocarcinoma in transgenic mice was demonstrated by the Notch‐mediated suppression of p53‐mediated apoptosis (Licciulli et al., 2013; Xu et al., 2014). In this study, Notch1 deletion in a mouse lung adenocarcinoma model markedly decreased the initiation and burden of lung adenocarcinoma, restoring p53 stability. This study explained the clinical association between NOTCH1 expression and poor prognosis in NSCLC patients at the molecular level (Licciulli et al., 2013). Furthermore, the combination of Kras G12D expression and forced Notch1 activation induced papillary adenocarcinoma in conducting airways (Xu et al., 2014). By contrast, presenilin 2 (PS2) KO mice showed increased incidence of drug‐induced NSCLC (Yun et al., 2014). Given that PS1 and PS2 are the enzymatic subunits of γ‐secretase, this study suggests that impaired Notch cleavage via PS2 KO might have oncogenic effects. PS2 KO mice also developed a mild pulmonary phenotype such as pulmonary fibrosis and hemorrhage in adulthood (Herreman et al., 1999). These studies confirm that PS2 is essential for lung homeostasis. Because PS2 mediates the cleavage of other substrates such as β‐amyloid precursor protein, cadherins, and CD44, epistatic relation between PS2 and Notch or Rbpj genes should be clarified in the future study to determine whether PS/γ‐secretase‐dependent Notch cleavages are responsible for tumorigenesis. In fact, Notch activity showed no significant changes in PS2 KO mice in the study, while iPLA2 activity showed an increase (Yun et al., 2014). More detailed studies are needed to identify the biological mechanisms underlying Notch signaling in NSCLC.
SCLC is a highly progressive, lethal, and metastatic type of lung cancer characterized by neuroendocrine‐like molecular features; most SCLCs express PNEC markers, including ASCL1 (also known as hASH1) (Borges et al., 1997). ASCL1 is a master transcription factor of the neuroendocrine cell program; it positively regulates DLL1 expression (Borromeo et al., 2016), which may activate Notch in neighboring cells (see “Notch in lung development” section). Notch signaling is inactivated in ASCL1‐positive SCLC cells in a cell‐autonomous manner by the expression of NOTCH inhibitors, such as delta‐like noncanonical Notch ligand 1 (DLK1) and DLL3, or by inactivating mutations in Notch pathway genes (George et al., 2015). Given that excessive Notch expression/activation in the developing mouse lung inhibits PNEC differentiation, Notch signaling could function as a tumor suppresser in human SCLC by inhibiting neuroendocrine markers such as ASCL1.
Recently, Lim JS et al. reported that the intratumoral cellular heterogeneity generated by Notch signaling promotes SCLC (Lim et al., 2017). The authors found that non‐neuroendocrine‐type SCLCs are slow growing and have active Notch signaling. Surprisingly, these Notch‐activated SCLC cells are relatively chemoresistant and provide trophic support to neuroendocrine tumor cells in a mouse model of SCLC, which suggests that Notch can play a protumorigenic role in a context‐dependent manner even within the same tumor. Since this complex microenvironment modulated by Notch signaling could be one reason why SCLC becomes chemoresistant after initial therapy, the Notch pathway is a potential novel treatment target.
In summary, Notch signaling fundamentally works as a tumor promotor in NSCLC and as a tumor suppressor in SCLC. However, the molecular pathogenesis is highly complex and context‐dependent.
5. CONCLUSION
Enthusiastic efforts by researchers around the world over the last two decades have led to the identification of various biological roles of Notch signaling in development, homeostasis, and regeneration in the respiratory system, providing useful clues to elucidate the pathological mechanisms underlying challenging respiratory diseases. These discoveries also prompted us to consider the role of Notch signaling in the conserved molecular machineries dictating development, regeneration and disease in the respiratory system. For example, Notch2 signaling is indispensable in club cell development, and Notch is reactivated in the club cell lineage during airway epithelial regeneration in adulthood. The unique contribution of Notch2 in pathology was determined by studying goblet cell hyperplasia in asthma and COPD. Notch signaling plays a fundamental role in secretory cells of the respiratory system throughout life, from embryonic development to the aging process.
Many questions remain to be addressed in future studies. For example, the role of Notch signaling in the development of mesenchymal progenitor cells is unknown. The precise control of Notch signaling in regeneration also remains elusive, and a better understating of this process will enable us to completely regulate it and avoid repair failure. Future studies should address these questions to deepen our understanding of the biological functions of Notch signaling, and these findings may shed light on novel treatment targets in various respiratory diseases.
Kiyokawa H, Morimoto M. Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Develop. Growth Differ. 2020;62:67–79. 10.1111/dgd.12628
REFERENCES
- Aoyagi‐Ikeda, K. , Maeno, T. , Matsui, H. , Ueno, M. , Hara, K. , Aoki, Y. , … Kurabayashi, M. (2011). Notch induces myofibroblast differentiation of alveolar epithelial cells via transforming growth factor‐{beta}‐Smad3 pathway. American Journal of Respiratory Cell and Molecular Biology, 45, 136–144. [DOI] [PubMed] [Google Scholar]
- Benedito, R. , & Hellstrom, M. (2013). Notch as a hub for signaling in angiogenesis. Experimental Cell Research, 319, 1281–1288. 10.1016/j.yexcr.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Borges, M. , Linnoila, R. I. , Van De Velde, H. J. , Chen, N. , Nelkin, B. D. , Mabry, M. , … Ball, D. W. (1997). An achaete‐scute homologue essential for neuroendocrine differentiation in the lung. Nature, 386, 852–855. 10.1038/386852a0 [DOI] [PubMed] [Google Scholar]
- Borromeo, M. D. , Savage, T. K. , Kollipara, R. K. , He, M. , Augustyn, A. , Osbome, J. K. , … Johnson, J. E. (2016). ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Reports, 16, 1259–1272. 10.1016/j.celrep.2016.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat, O. , Chakir, J. , & Jeannotte, L. (2012). The loss of Hoxa5 function promotes Notch‐dependent goblet cell metaplasia in lung airways. Biology Open, 1, 677–691. 10.1242/bio.20121701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray, S. J. (2006). Notch signalling: A simple pathway becomes complex. Nature Reviews Molecular Cell Biology, 7, 678–689. 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- Colaluca, I. N. , Tosoni, D. , Nuciforo, P. , Senic‐Matuglia, F. , Galimberti, V. , Viale, G. , … Di Fiore, P. P. (2008). NUMB controls p53 tumour suppressor activity. Nature, 451, 76–80. 10.1038/nature06412 [DOI] [PubMed] [Google Scholar]
- Danahay, H. , Pessotti, A. D. , Coote, J. , Montgomery, B. E. , Xia, D. , Wilson, A. , … Jaffe, A. B. (2015). Notch2 is required for inflammatory cytokine‐driven goblet cell metaplasia in the lung. Cell Reports, 10, 239–252. 10.1016/j.celrep.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Dang, T. P. , Eichenberger, S. , Gonzalez, A. , Olson, S. , & Carbone, D. P. (2003). Constitutive activation of Notch3 inhibits terminal epithelial differentiation in lungs of transgenic mice. Oncogene, 22, 1988–1997. 10.1038/sj.onc.1206230 [DOI] [PubMed] [Google Scholar]
- Dang, T. P. , Gazdar, A. F. , Virmani, A. K. , Sepetavec, T. , Hande, K. R. , Minna, J. D. , … Carbone, D. P. (2000). Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. Journal of the National Cancer Institute, 92, 1355–1357. 10.1093/jnci/92.16.1355 [DOI] [PubMed] [Google Scholar]
- George, J. , Lim, J. S. , Jang, S. J. , Cun, Y. , Ozretić, L. , Kong, G. , … Thomas, R. K. (2015). Comprehensive genomic profiles of small cell lung cancer. Nature, 524, 47–53. 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha, A. , Vasconcelos, M. , Cai, Y. , Yoneda, M. , Hinds, A. , Qian, J. , … Cardoso, W. V. (2012). Neuroepithelial body microenvironment is a niche for a distinct subset of Clara‐like precursors in the developing airways. Proceedings of the National Academy of Sciences of the United States of America, 109, 12592–12597. 10.1073/pnas.1204710109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseh, J. S. , Bores, S. A. , Stanger, B. Z. , Zhou, Q. , Andersen, W. J. , Melton, D. A. , & Rajagoapl, J. (2009). Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development, 136, 1751–1759. 10.1242/dev.029249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman, A. , Hartmann, D. , Annaert, W. , Satfig, P. , Craessaerts, K. , Serneels, L. , … De Strooper, B. (1999). Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proceedings of the National Academy of Sciences of the United States of America, 96, 11872–11877. 10.1073/pnas.96.21.11872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriges, M. , & Morrisey, E. E. (2014). Lung development: Orchestrating the generation and regeneration of a complex organ. Development, 141, 502–513. 10.1242/dev.098186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan, B. L. , Barkauskas, C. E. , Chapman, H. A. , Epstein, J. A. , Jain, R. , Hsia, C. C. , … Morrisey, E. E. (2014). Repair and regeneration of the respiratory system: Complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell, 15, 123–138. 10.1016/j.stem.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, K. U. , Reynolds, S. D. , Giangreco, A. , Hurley, C. M. , & Stripp, B. R. (2001). Clara cell secretory protein‐expressing cells of the airway neuroepithelial body microenvironment include a label‐retaining subset and are critical for epithelial renewal after progenitor cell depletion. American Journal of Respiratory Cell and Molecular Biology, 24, 671–681. 10.1165/ajrcmb.24.6.4498 [DOI] [PubMed] [Google Scholar]
- Hong, K. U. , Reynolds, S. D. , Watkins, S. , Fuchs, E. , & Stripp, B. R. (2004). Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. American Journal of Pathology, 164, 577–588. 10.1016/s0002-9440(10)63147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T. , Udaka, N. , Yazawa, T. , Okudela, K. , Hayashi, H. , Sudo, T. , … Kitamura, H. (2000). Basic helix‐loop‐helix transcription factors regulate the neuroendocrine differentiation of fetal mouse pulmonary epithelium. Development, 127, 3913–3921. [DOI] [PubMed] [Google Scholar]
- Kageyama, R. , & Ohtsuka, T. (1999). The Notch‐Hes pathway in mammalian neural development. Cell Research, 9, 179–188. 10.1038/sj.cr.7290016 [DOI] [PubMed] [Google Scholar]
- Kotton, D. N. , & Morrisey, E. E. (2014). Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nature Medicine, 20, 822–832. 10.1038/nm.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Bhang, D. H. , Beede, A. , Huang, T. L. , Stripp, B. R. , Bloch, K. D. , … Kim, C. F. (2014). Lung stem cell differentiation in mice directed by endothelial cells via a BMP4‐NFATc1‐thrombospondin‐1 axis. Cell, 156, 440–455. 10.1016/j.cell.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. H. , Tammela, T. , Hofree, M. , Choi, J. , Marjanovic, N. D. , Han, S. , … Kim, C. F. (2017). Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell, 170(1149–1163), e1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Zhang, X. , Leathers, R. , Markino, A. , Huang, C. , Parsa, P. , … Thistlethwaite, P. A. (2009). Notch3 signaling promotes the development of pulmonary arterial hypertension. Nature Medicine, 15, 1289–1297. 10.1038/nm.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciulli, S. , Avila, J. L. , Hanlon, L. , Troutman, S. , Cesaroni, M. , Kota, S. , … Kissil, J. L. (2013). Notch1 is required for Kras‐induced lung adenocarcinoma and controls tumor cell survival via p53. Cancer Research, 73, 5974–5984. 10.1158/0008-5472.can-13-1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, J. S. , Ibaseta, A. , Fischer, M. M. , Cancilla, B. , O'Young, G. , Cristea, S. , … Sage, J. (2017). Intratumoural heterogeneity generated by Notch signalling promotes small‐cell lung cancer. Nature, 545, 360–364. 10.1038/nature22323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg, F. P. , Takeshita, K. , Radtke, F. , Bronson, R. T. , Chin, M. T. , & Liao, J. K. (2005). Essential role of endothelial Notch1 in angiogenesis. Circulation, 111, 1826–1832. 10.1161/01.cir.0000160870.93058.dd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Brunskill, E. , Varnum‐Finney, B. , Zhang, C. , Zhang, A. , Jay, P. Y. , … Kopan, R. (2015). The intracellular domains of Notch1 and Notch2 are functionally equivalent during development and carcinogenesis. Development, 142, 2452–2463. 10.1242/dev.125492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro, D. T. , Haber, A. L. , Biton, M. , Vinarsky, V. , Lin, B. , Birket, S. E. , … Rajagopal, J. (2018). A revised airway epithelial hierarchy includes CFTR‐expressing ionocytes. Nature, 560, 319–324. 10.1038/s41586-018-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, M. , Liu, Z. , Cheng, H. T. , Winters, N. , Bader, D. , & Kopan, R. (2010). Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. Journal of Cell Science, 123, 213–224. 10.1242/jcs.058669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, M. , Nishinakamura, R. , Saga, Y. , & Kopan, R. (2012). Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development, 139, 4365–4373. 10.1242/dev.083840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan, A. N. , Brownfield, D. G. , Harbury, P. B. , Krasnow, M. A. , & Desai, T. J. (2018). Single‐cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science, 359, 1118–1123. 10.1126/science.aam6603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi, M. , Sumiyama, K. , & Morimoto, M. (2015). Directed Migration of Pulmonary Neuroendocrine Cells toward Airway Branches Organizes the Stereotypic Location of Neuroepithelial Bodies. Cell Reports, 13, 2679–2686. 10.1016/j.celrep.2015.11.058 [DOI] [PubMed] [Google Scholar]
- Pardo‐Saganta, A. , Law, B. M. , Tata, P. R. , Villoria, J. , Saez, B. , Mou, H. , … Rajagopal, J. (2015). Injury induces direct lineage segregation of functionally distinct airway basal stem/progenitor cell subpopulations. Cell Stem Cell, 16, 184–197. 10.1016/j.stem.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo‐Saganta, A. , Tata, P. R. , Law, B. M. , Saez, B. , Chow, R. D. , Prabhu, M. , … Rajagopal, J. (2015). Parent stem cells can serve as niches for their daughter cells. Nature, 523, 597–601. 10.1038/nature14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasschaert, L. W. , Zilionis, R. , Choo‐Wing, R. , Savova, V. , Knehr, J. , Roma, G. , … Jaffe, B. (2018). A single‐cell atlas of the airway epithelium reveals the CFTR‐rich pulmonary ionocyte. Nature, 560, 377–381. 10.1038/s41586-018-0394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, L. C. , Ternet, M. , & Hogan, B. L. (2000). Notch/Delta expression in the developing mouse lung. Mechanisms of Development, 98, 95–98. 10.1016/s0925-4773(00)00432-9 [DOI] [PubMed] [Google Scholar]
- Richeldi, L. , Collard, H. R. , & Jones, M. G. (2017). Idiopathic pulmonary fibrosis. Lancet, 389, 1941–1952. 10.1016/s0140-6736(17)30866-8 [DOI] [PubMed] [Google Scholar]
- Rock, J. R. , Gao, X. , Xue, Y. , Randell, S. H. , Kong, Y. Y. , & Hogan, B. L. (2011). Notch‐dependent differentiation of adult airway basal stem cells. Cell Stem Cell, 8, 639–648. 10.1016/j.stem.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock, J. R. , Onaitis, M. W. , Rawlins, E. L. , Lu, Y. , Clark, C. P. , Xue, Y. , … Hogan, B. L. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America, 106, 12771–12775. 10.1073/pnas.0906850106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H. , Yao, E. , Lin, C. , Gacayan, R. , Chen, M. H. , & Chuang, P. T. (2012). Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America, 109, 17531–17536. 10.1073/pnas.1207238109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatek, P. J. , Lindsell, C. E. , Del Amo, F. F. , Weinmaster, G. , & Gridley, T. (1994). Notch1 is essential for postimplantation development in mice. Genes & Development, 8, 707–719. 10.1101/gad.8.6.707 [DOI] [PubMed] [Google Scholar]
- Tadokoro, T. , Gao, X. , Hong, C. C. , Hotten, D. , & Hogan, B. L. (2016). BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors. Development, 143, 764–773. 10.1242/dev.126656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley, A. E. , Harvey, B. G. , Heguy, A. , Hackett, N. R. , Wang, R. , O'Connor, T. P. , & Crystal, R. G. (2009). Down‐regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine, 179, 457–466. 10.1164/rccm.200705-795oc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, P. N. , Chen, F. , Izvolsky, K. I. , Walker, J. , Kukuruzinska, M. A. , Lu, J. , & Cardoso, W. V. (2008). Gamma‐secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. Journal of Biological Chemistry, 283, 29532–29544. 10.1074/jbc.m801565200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, P. N. , Matsuoka, C. , Wei, S. C. , Sato, A. , Hasegawa, K. , Chen, H. K. , … Cardoso, W. V. (2016). Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proceedings of the National Academy of Sciences of the United States of America, 113, 8242–8247. 10.1073/pnas.1511236113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, P. N. , Vasconcelos, M. , Izvolsky, K. I. , Qian, J. , Lu, J. , & Cardoso, W. V. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development, 136, 2297–2307. 10.1242/dev.034884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder, R. M. , Archer, S. L. , Dorfmuller, P. , Erzurum, S. C. , Guignabert, C. , Michelakis, E. , … Morrell, N. W. (2013). Relevant issues in the pathology and pathobiology of pulmonary hypertension. Journal of the American College of Cardiology, 62, D4–D12. 10.1016/j.jacc.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, A. E. , Brumwell, A. N. , Xi, Y. , Gotts, J. E. , Brownfield, D. G. , Treutlein, B. , … Chapman, H. A. (2015). Lineage‐negative progenitors mobilize to regenerate lung epithelium after major injury. Nature, 517, 621–625. 10.1038/nature14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert, T. , Yuan, T. , Chao, C. M. , Bell, H. , Sitaula, A. , Szimmtenings, L. , … De Langhe, S. P. . (2017). Fgf10‐hippo epithelial‐mesenchymal crosstalk maintains and recruits lung basal stem cells. Developmental Cell, 43(48–59), e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, J. K. , Rulands, S. , Wilkinson, A. C. , Wuidart, A. , Ousset, M. , Van Keymeulen, A. , … Rawlins, E. L. (2015). Clonal dynamics reveal two distinct populations of basal cells in slow‐turnover airway epithelium. Cell Reports, 12, 90–101. 10.1016/j.celrep.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff, B. , Colaluca, I. N. , D'ario, G. , Donzelli, M. , Tosoni, D. , Volorio, S. , … Di Fiore, P. P. (2009). Alterations of the Notch pathway in lung cancer. Proceedings of the National Academy of Sciences of the United States of America, 106, 22293–22298. 10.1073/pnas.0907781106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi, Y. , Kim, T. , Brumwell, A. N. , Driver, I. H. , Wei, Y. , Tan, V. , … Vaughan, A. E. (2017). Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nature Cell Biology, 19, 904–914. 10.1038/ncb3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Gong, D. , & Wang, W. (2013). Soluble JAGGED1 inhibits pulmonary hypertension by attenuating notch signaling. Arteriosclerosis, Thrombosis, and Vascular Biology, 33, 2733–2739. 10.1161/atvbaha.113.302062 [DOI] [PubMed] [Google Scholar]
- Xing, Y. , Li, A. , Borok, Z. , Li, C. , & Minoo, P. (2012). NOTCH1 is required for regeneration of Clara cells during repair of airway injury. Stem Cells, 30, 946–955. 10.1002/stem.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Huang, L. , Futtner, C. , Schwab, B. , Ramprasad, R. R. , Lu, Y. , … Onaitis, M. W. (2014). The cell of origin and subtype of K‐Ras‐induced lung tumors are modified by Notch and Sox2. Genes & Development, 28, 1929–1939. 10.1101/gad.243717.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, K. , Nieuwenhuis, E. , Cohen, B. L. , Wang, W. , Canty, A. J. , Danska, J. S. , … Egan, S. E. (2010). Lunatic Fringe‐mediated Notch signaling is required for lung alveogenesis. American Journal of Physiology. Lung Cellular and Molecular Physiology, 298, L45–L56. 10.1152/ajplung.90550.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, E. , Lin, C. , Wu, Q. , Zhang, K. , Song, H. , & Chuang, P. T. (2018). Notch Signaling Controls Transdifferentiation of Pulmonary Neuroendocrine Cells in Response to Lung Injury. Stem Cells, 36, 377–391. 10.1002/stem.2744 [DOI] [PubMed] [Google Scholar]
- Yuan, X. , Wu, H. , Xu, H. , Han, N. , Chu, Q. , Yu, S. , … Wu, K. (2015). Meta‐analysis reveals the correlation of Notch signaling with non‐small cell lung cancer progression and prognosis. Scientific Reports, 5, 10338 10.1038/srep10338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, H. M. , Park, M. H. , Kim, D. H. , Ahn, Y. J. , Park, K. R. , Kim, T. M. , … Han, S. B. (2014). Loss of presenilin 2 is associated with increased iPLA2 activity and lung tumor development. Oncogene, 33, 5193–5200. 10.1038/onc.2014.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias, W. J. , Frank, D. B. , Zepp, J. A. , Morley, M. P. , Alkhaleel, F. A. , Kong, J. , … Morrisey, E. E. (2018). Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature, 555, 251–255. 10.1038/nature25786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Loch, A. J. , Radtke, F. , Egan, S. E. , & Xu, K. (2013). Jagged1 is the major regulator of Notch‐dependent cell fate in proximal airways. Developmental Dynamics, 242, 678–686. 10.1002/dvdy.23965 [DOI] [PubMed] [Google Scholar]
- Zong, D. , Li, J. , Cai, S. , He, S. , Liu, Q. , Jiang, J. , … Ouyang, R. (2018). Notch1 regulates endothelial apoptosis via the ERK pathway in chronic obstructive pulmonary disease. American Journal of Physiology. Cell Physiology, 315, C330–C340. 10.1152/ajpcell.00182.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, B. , Zhou, X. L. , Lai, S. Q. , & Liu, J. C. (2018). Notch signaling and non‐small cell lung cancer. Oncology Letters, 15, 3415–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]


