Abstract
Previous studies demonstrated that delgocitinib ointment, a novel topical Janus kinase inhibitor, rapidly improved clinical signs and symptoms of atopic dermatitis (AD) in Japanese adult patients. We sought to evaluate the long‐term safety and efficacy of delgocitinib 0.5% ointment in a 52‐week study (QBA4‐2). Japanese patients aged 16 years or older with AD received delgocitinib 0.5% ointment b.i.d. for up to 52 weeks. Topical corticosteroids for the treatment of worsening of AD could be used at the investigators’ discretion during the treatment period. Safety end‐points included the incidence and severity of adverse events (AEs). Pooled safety analyses included the data from the other long‐term study (QBA4‐1). Efficacy end‐points included the percentage change from baseline in the modified Eczema Area and Severity Index (mEASI). A total of 506 patients were included in the pooled safety population. Overall, AEs were reported in 69.0% of patients; most AEs were mild and unrelated to delgocitinib ointment. The most common AE was nasopharyngitis, followed by contact dermatitis, acne, and application site folliculitis. No skin atrophy or telangiectasia was found at the application sites of delgocitinib ointment. Application site irritation symptoms were infrequent (<2%) and mild. The incidence of AEs did not increase over time, except for seasonal diseases. The improvement effects on AD as assessed by mEASI were maintained throughout the treatment period. Delgocitinib 0.5% ointment was well tolerated and effective when administrated to Japanese adult patients with AD for up to 52 weeks.
Keywords: atopic dermatitis, delgocitinib, Janus kinase inhibitor, long‐term safety, topical
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disease accompanied by intense pruritus; the signs and symptoms of AD fluctuate with remissions and relapses.1, 2 Because of the chronic course of AD, long‐term treatment is typically required to manage AD. Topical therapies are the mainstay of the management of AD, and topical corticosteroids and calcineurin inhibitors are widely used to reduce skin inflammation.1, 3 These agents, however, are associated with safety concerns. Long‐term use of topical corticosteroids may lead to skin atrophy and telangiectasia.1, 3 Topical calcineurin inhibitors, such as tacrolimus ointment, may cause irritation symptoms including burning and stinging sensations, although these symptoms tend to decrease with AD improvement.1, 3 Crisaborole ointment, a phosphodiesterase 4 inhibitor, has recently been approved for mild to moderate AD in the USA,4 whereas a novel topical agent applicable to more severe AD with minimal safety concerns remains to be developed.
Delgocitinib ointment is a novel topical agent that has inhibitory effects on all types in the Janus kinase (JAK) family (JAK1, JAK2, JAK3 and tyrosine kinase 2).5 The JAK–signal transducer and activator of transcription (STAT) signaling pathway plays an important role in exerting the biological effects of many inflammatory cytokines, including interleukin (IL)‐4, IL‐13 and IL‐31.6, 7 Elevated production of inflammatory cytokines is associated with the pathophysiology of AD.8, 9, 10, 11, 12, 13 Non‐clinical studies showed that topical application of delgocitinib suppresses skin inflammation,14 improves the skin barrier dysfunction15 and reduces pruritus16 through the inhibition of cytokine signaling. In addition, early clinical studies showed the potential effectiveness of delgocitinib ointment in AD.17, 18, 19
Two phase 3 studies of delgocitinib 0.5% ointment, QBA4‐1 and QBA4‐2, were conducted in adult Japanese patients with AD. QBA4‐1 consisted of a 4‐week, randomized, double‐blind, vehicle‐controlled period (part 1) and a 24‐week, open‐label, extension period (part 2) (Fig. S1). In part 1, the percentage change from baseline in the modified Eczema Area and Severity Index (mEASI) score at the end of treatment, the primary efficacy end‐point, was significantly greater in the delgocitinib group than in the vehicle group (−44.3% vs 1.7%, P < 0.001). The improvement in the mEASI score was maintained in part 2. Most adverse events (AEs) were mild and unrelated to delgocitinib across the study periods.19 Here, we report the results of QBA4‐2, a 52‐week open‐label study, to evaluate the long‐term safety and efficacy of delgocitinib ointment, along with pooled safety data from both studies.
Methods
Study design
Study QBA4‐2 was a 52‐week open‐label study (Fig. 1). The sample size (n = 330) was selected to enable adequate evaluation of the long‐term safety of delgocitinib 0.5% ointment.
Figure 1.
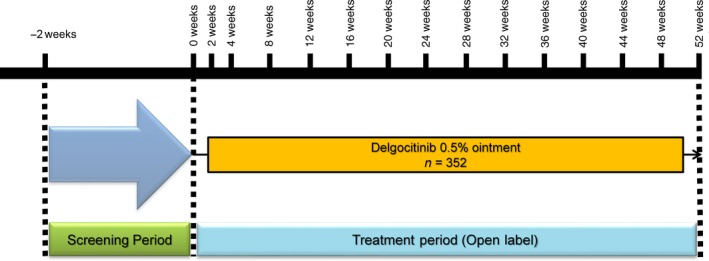
Study design of QBA4‐2.
This study was conducted at 47 medical institutions in Japan in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Study‐related documents, including the study protocol and informed consent forms, were approved by the institutional review boards. Written informed consent was obtained from all patients. The study information is registered with Japan Pharmaceutical Information Center Clinical Trials Information (http://www.clinicaltrials.jp, JapicCTI‐173555).
Patients
Japanese patients aged 16 years or older and diagnosed with AD according to the criteria of the Japanese Dermatological Association1 were enrolled. To be eligible to participate, patients were required to have an Investigator’s Global Assessment (IGA) score between 2 (mild) and 4 (severe), and inflammatory eczema affecting 5–30% of the body surface area.
Patients with any of the following conditions were excluded: active infection at the prospective application site; cutaneous diseases likely to affect the study assessments; and mycobacterium tuberculosis, hepatitis B/C or HIV infection. Patients who had received any of the following therapies before study treatment were excluded: biologics within 24 weeks; systemic corticosteroids, systemic immunosuppressive agents, live vaccines, phototherapy and hyposensitization therapy within 28 days; and strongest or very strong topical corticosteroids1 (with similar potency to class I or II [very high or high potency] corticosteroids in the USA)3 within 14 days. Patients were allowed to continue to use strong or less potent topical corticosteroids1 (with similar potency to class III to VII [medium to lowest] corticosteroids in the USA)3 and tacrolimus hydrate ointment immediately before delgocitinib treatment. All of the therapies above were prohibited during the treatment period, except for topical corticosteroids for the treatment of worsening of AD as described below.
Study treatment
Patients were instructed to apply delgocitinib 0.5% ointment b.i.d. to the areas affected by inflammatory eczema, excluding dry skin areas and the scalp. The maximum dose per application was limited to 5 g. Emollients, oral antihistamines and antiallergenic agents were permitted as concomitant drugs. Topical corticosteroids for the treatment of worsening of AD could be used at the investigators’ discretion; however, concomitant use of delgocitinib ointment and topical corticosteroids to the same area was prohibited.
Study assessments
Patients visited the study sites every 4 weeks, and safety was primarily assessed. Safety assessments were based on symptoms, signs, vital signs and laboratory tests. Plasma concentrations of delgocitinib were measured at selected visits, and the lower limit of quantification was 1.00 ng/mL. Efficacy assessments were based on the mEASI, IGA and pruritus Numeric Rating Scale (NRS) scores. The mEASI score, ranging 0–64.8, was calculated by excluding the head/neck region score from the EASI20 total score, ranging 0–72. This modification was made to assess the treatment effect more precisely in the study where no study treatment could be applied to the scalp. The IGA score was a 6‐point scale ranging from 0 (clear) to 5 (very severe) in reference to the measures of severity by the Research Group granted by the Ministry of Health, Labor and Welfare (Table S1).1 The pruritus NRS score was an 11‐point scale ranging from 0 (no itch) to 10 (the most severe itch), and was obtained through interview at each study visit to assess the average level of pruritus during the past 24 h.
Statistical analyses
Analyses of safety, pharmacokinetics and efficacy were performed on the population comprising patients who underwent the respective study‐specified assessments at least once after the start of delgocitinib treatment. The pooled safety population comprised all patients in this study and patients who received delgocitinib ointment in study QBA4‐1.
Verbatim terms of AEs reported by investigators were coded using the Medical Dictionary for Regulatory Activities, version 19.1. For pooled safety analyses, AEs that occurred during the vehicle treatment in study QBA4‐1 (i.e. AEs that occurred in the patients receiving the vehicle ointment in part 1) were not included.
For efficacy analyses, the mean change or percentage change from baseline was calculated in the mEASI, IGA and pruritus NRS scores. In the mEASI score, the proportions of patients who achieved 50% or more (mEASI‐50) and 75% or more (mEASI‐75) improvements from baseline were also calculated.
Results
Patients
In study QBA4‐2, of the 352 patients who received delgocitinib ointment, 262 (74.4%) completed the study (Table 1). A total of 506 patients were included in the pooled safety population. The most common reason for early study discontinuation was worsening of AD (n = 45 [12.8%] in QBA4‐2, n = 56 [11.1%] in the pooled safety population).
Table 1.
Patient disposition
| QBA4‐2 (n = 352) | Pooled safety population (n = 506) | |
|---|---|---|
| Patients who completed the study | 262 (74.4) | 400 (79.1) |
| Patients who discontinued the study | 90 (25.6) | 106 (20.9) |
| Adverse event | 16 (4.5) | 17 (3.4) |
| Worsening of atopic dermatitis | 45 (12.8) | 56 (11.1) |
| Withdrawal by patient | 23 (6.5) | 26 (5.1) |
| Protocol deviation | 1 (0.3) | 1 (0.2) |
| Lost to follow‐up | 2 (0.6) | 3 (0.6) |
| Physician decision | 3 (0.9) | 3 (0.6) |
Data are displayed as number of patients (%). The pooled safety population includes all patients in QBA4‐2 and patients who received delgocitinib ointment in QBA4‐1.
At baseline, more than half of the patients had moderate AD (n = 215 [61.1%] in QBA4‐2, n = 304 [60.1%] in the pooled safety population; Table 2). The mean duration of exposure to delgocitinib ointment was longer in study QBA4‐2 (286.7 days) than in the pooled safety population (251.3 days), which reflects the difference in the treatment duration between the studies (52 weeks for QBA4‐2 vs up to 28 weeks for QBA4‐1). Because of worsening of AD, topical corticosteroids were used at least once by more than half of the patients (n = 224 [63.6%] in QBA4‐2, n = 288 [56.9%] in the pooled safety population) during the treatment period. In study QBA4‐2, the proportion of patients by baseline IGA score who used topical corticosteroids was the following: 2 (mild), 27.2%; 3 (moderate), 63.8%; and 4 (severe), 8.9%. The proportion of those who did not was the following: 2 (mild), 38.3%; 3 (moderate), 56.3%; and 4 (severe), 5.5%. The patients who used topical corticosteroids appeared to have more severe AD than the patients who did not. In the pooled safety population, however, no apparent difference in baseline IGA score was found (data not shown).
Table 2.
Baseline patient characteristics, extent of exposure to delgocitinib ointment and concomitant use of topical corticosteroids
| QBA4‐2 (n = 352) | Pooled safety population (n = 506) | |
|---|---|---|
| Age (years) | 33.0 (11.1) | 32.6 (10.8) |
| Sex, n (%) | ||
| Men | 223 (63.4) | 318 (62.8) |
| Women | 129 (36.6) | 188 (37.2) |
| Duration of AD (years) | 23.9 (12.2) | 24.2 (11.7) |
| mEASI score | 8.8 (4.9) | 10.5 (5.6) |
| IGA score, n (%) | ||
| 0 (clear), 1 (almost clear) | 0 | 2 (0.4) |
| 2 (mild) | 110 (31.3) | 115 (22.7) |
| 3 (moderate) | 215 (61.1) | 304 (60.1) |
| 4 (severe) | 27 (7.7) | 85 (16.8) |
| Pruritus NRS score | 4.7 (2.0) | 4.8 (2.0) |
| Percentage of BSA affected by AD | 19.6 (6.9) | 21.1 (7.6) |
| Exposure to delgocitinib ointment | ||
| Exposure duration (days) | 286.7 (118.4) | 251.3 (114.5) |
| Amount of drug applied (g) | 1360.8 (869.7) | 1238.6 (786.7) |
| Amount of drug applied per day (g) | 4.8 (2.2) | 5.1 (2.3) |
| Patients who used topical corticosteroids, n (%) | 224 (63.6) | 288 (56.9) |
Data are displayed as mean (SD) unless otherwise indicated. The pooled safety population includes all patients in QBA4‐2 and patients who received delgocitinib ointment in QBA4‐1. AD, atopic dermatitis; BSA, body surface area; IGA, Investigator’s Global Assessment; mEASI, modified Eczema Area and Severity Index; NRS, Numeric Rating Scale.
Safety and tolerability
Overall, AEs were reported in 349 of the 506 patients (69.0%) in the pooled safety population (271/352 [77.0%] in QBA4‐2; Table 3). All AEs were mild or moderate, except one severe AE of rectal cancer, which was considered unrelated to delgocitinib ointment. Most AEs were considered unrelated to delgocitinib ointment, and treatment‐related AEs were reported in 78 patients (15.4%). Serious AEs occurred in seven patients (1.4%), and one serious AE of Kaposi’s varicelliform eruption was considered related to delgocitinib ointment, which developed on day 26. Delgocitinib ointment had not been applied to the area where the event initially developed. Subsequently, the event expanded to the application site of delgocitinib ointment and was resolved on day 38 by withdrawal of delgocitinib ointment and an antiviral therapy. Study discontinuations due to AEs occurred in 17 patients (3.4%), and the most common AEs leading to study discontinuation were contact dermatitis in five patients (1.0%) and application site irritation in three (0.6%).
Table 3.
Summary of adverse events
| QBA4‐2 (n = 352) | Pooled safety population (n = 506) | |
|---|---|---|
| Adverse events | 271 (77.0) | 349 (69.0) |
| Maximum severity | ||
| Mild | 218 (61.9) | 286 (56.5) |
| Moderate | 52 (14.8) | 62 (12.3) |
| Severe | 1 (0.3) | 1 (0.2) |
| Treatment‐related adverse events | 69 (19.6) | 78 (15.4) |
| Serious adverse events | 7 (2.0) | 7 (1.4) |
| Adverse events leading to discontinuation | 16 (4.5) | 17 (3.4) |
Data are displayed as number of patients (%). The pooled safety population includes all patients in QBA4‐2 and patients who received delgocitinib ointment in QBA4‐1.
In the pooled safety population, the most common AE was nasopharyngitis (n = 131 [25.9%]), followed by contact dermatitis (n = 23 [4.5%]), acne (n = 22 [4.3%]), application site folliculitis (n = 18 [3.6%]), influenza (n = 17 [3.4%]), Kaposi’s varicelliform eruption (n = 17 [3.4%]), application site acne (n = 16 [3.2%]) and herpes simplex (n = 15 [3.0%]) (Table 4). The most common treatment‐related AEs were application site events such as application site folliculitis (n = 12 [2.4%]) and application site acne (n = 11 [2.2%]) (Table 5). The incidence of AEs did not increase over time, except for seasonal diseases such as allergic conjunctivitis and seasonal allergy (these two were mostly related to Japanese cedar pollinosis),21 and influenza (Table 6). Application site irritation symptoms (irritation, pruritus, warmth or pain) were reported in less than 2% of the patients, were all mild and occurred mostly in the first 2 weeks of delgocitinib treatment.
Table 4.
Adverse events occurring in 2% or more of patients
| QBA4‐2 (n = 352) | Pooled safety analysis population (n = 506) | |
|---|---|---|
| Eye disorders | ||
| Allergic conjunctivitis | 8 (2.3) | 11 (2.2) |
| Gastrointestinal disorders | ||
| Dental caries | 7 (2.0) | 11 (2.2) |
| General disorders and administration site conditions | ||
| Application site acne | 14 (4.0) | 16 (3.2) |
| Immune system disorders | ||
| Seasonal allergy | 9 (2.6) | 10 (2.0) |
| Infections and infestations | ||
| Nasopharyngitis | 101 (28.7) | 131 (25.9) |
| Application site folliculitis | 15 (4.3) | 18 (3.6) |
| Influenza | 17 (4.8) | 17 (3.4) |
| Kaposi’s varicelliform eruption | 11 (3.1) | 17 (3.4) |
| Herpes simplex | 12 (3.4) | 15 (3.0) |
| Folliculitis | 10 (2.8) | 12 (2.4) |
| Gastroenteritis | 10 (2.8) | 12 (2.4) |
| Paronychia | 7 (2.0) | 11 (2.2) |
| Oral herpes | 10 (2.8) | 10 (2.0) |
| Skin and subcutaneous tissue disorders | ||
| Contact dermatitis | 20 (5.7) | 23 (4.5) |
| Acne | 17 (4.8) | 22 (4.3) |
| Eczema | 9 (2.6) | 10 (2.0) |
Data are displayed as number of patients (%). The pooled safety population includes all patients in QBA4‐2 and patients who received delgocitinib ointment in QBA4‐1.
Table 5.
Treatment‐related adverse events occurring in 1% or more of patients
| QBA4‐2 (n = 352) | Pooled safety population (n = 506) | |
|---|---|---|
| Application site folliculitis | 11 (3.1) | 12 (2.4) |
| Application site acne | 10 (2.8) | 11 (2.2) |
| Application site irritation | 9 (2.6) | 9 (1.8) |
| Kaposi’s varicelliform eruption | 5 (1.4) | 8 (1.6) |
| Application site erythema | 7 (2.0) | 7 (1.4) |
| Contact dermatitis | 6 (1.7) | 6 (1.2) |
Data are displayed as number of patients (%). The pooled safety population includes all patients in QBA4‐2 and patients who received delgocitinib ointment in QBA4‐1.
Table 6.
Adverse events occurring in 2% or more of patients in the pooled safety population by time period
| Onset of adverse events | ||||
|---|---|---|---|---|
| Week 0–12 (n = 506) | Week 12–24 (n = 463) | Week 24–36 (n = 417) | Week 36–52 (n = 279) | |
| Eye disorders | ||||
| Allergic conjunctivitis | 2 (0.4) | 2 (0.4) | 5 (1.2) | 2 (0.7) |
| Gastrointestinal disorders | ||||
| Dental caries | 4 (0.8) | 2 (0.4) | 4 (1.0) | 1 (0.4) |
| General disorders and administration site conditions | ||||
| Application site acne | 10 (2.0) | 3 (0.6) | 2 (0.5) | 4 (1.4) |
| Immune system disorders | ||||
| Seasonal allergy | 0 | 0 | 6 (1.4) | 4 (1.4) |
| Infections and infestations | ||||
| Application site folliculitis | 6 (1.2) | 6 (1.3) | 2 (0.5) | 5 (1.8) |
| Folliculitis | 4 (0.8) | 6 (1.3) | 0 | 2 (0.7) |
| Gastroenteritis | 3 (0.6) | 2 (0.4) | 5 (1.2) | 3 (1.1) |
| Herpes simplex | 9 (1.8) | 4 (0.9) | 1 (0.2) | 6 (2.2) |
| Influenza | 0 | 4 (0.9) | 11 (2.6) | 2 (0.7) |
| Kaposi’s varicelliform eruption | 10 (2.0) | 2 (0.4) | 5 (1.2) | 3 (1.1) |
| Nasopharyngitis | 48 (9.5) | 51 (11.0) | 47 (11.3) | 27 (9.7) |
| Oral herpes | 5 (1.0) | 3 (0.6) | 3 (0.7) | 4 (1.4) |
| Paronychia | 4 (0.8) | 4 (0.9) | 2 (0.5) | 1 (0.4) |
| Skin and subcutaneous tissue disorders | ||||
| Acne | 10 (2.0) | 6 (1.3) | 4 (1.0) | 5 (1.8) |
| Contact dermatitis | 11 (2.2) | 8 (1.7) | 3 (0.7) | 5 (1.8) |
| Eczema | 4 (0.8) | 5 (1.1) | 1 (0.2) | 0 |
Data are displayed as number of patients (%).
Pharmacokinetics
Plasma concentrations of delgocitinib were not detected in more than 80% of patients throughout the treatment period. No apparent difference between study visits was found in the proportion of patients with detectable plasma concentrations of delgocitinib (Table S2).
Efficacy
The proportion of patients with mEASI‐50 and mEASI‐75 was increased after the start of delgocitinib treatment and was maintained throughout the treatment period (Fig. 2). The proportion of patients with mEASI‐50 was 31.5% (104/330) at week 4, 42.3% (121/286) at week 24 and 51.9% (136/262) at week 52. The proportion of patients with mEASI‐75 was 10.9% (36/330) at week 4, 22.7% (65/286) at week 24 and 27.5% (72/262) at week 52. The improvement in the IGA and pruritus NRS scores was also noted at weeks 4, 24 and 52 (Table S3).
Figure 2.
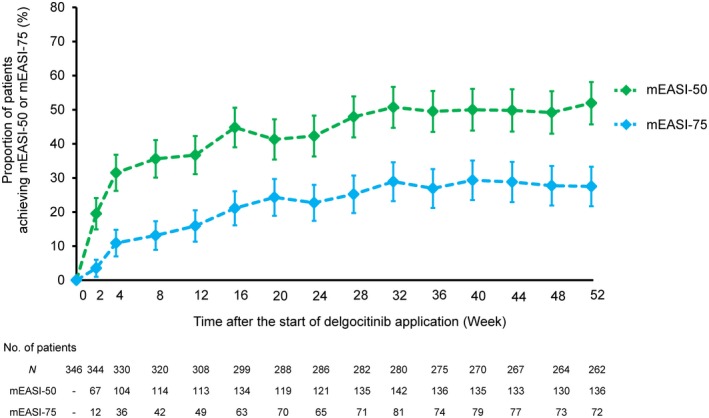
Proportion of patients achieving ≥50% or ≥75% improvement in modified Eczema Area and Severity Index (mEASI) score over time. The error bars represent two‐sided 95% confidence intervals. The data from patients who used topical corticosteroids during the treatment period for the treatment of worsening of atopic dermatitis (AD) were included in the analyses. Six patients were excluded from the analyses because only the face and neck were affected by AD at baseline or the lack of baseline data on mEASI. mEASI‐50, ≥50% improvement from baseline in the mEASI score; mEASI‐75, ≥75% improvement from baseline in the mEASI score.
Discussion
Long‐term treatment with delgocitinib 0.5% ointment, a novel topical JAK inhibitor, was well tolerated in Japanese patients aged 16 years or older with mild to severe AD. Safety results in the long‐term studies were similar to those in the 4‐week phase 2 study.18 No new safety concerns emerged with delgocitinib treatment for up to 52 weeks. Most AEs were mild and unrelated to delgocitinib ointment. The incidence of AEs did not increase over time, except for seasonal diseases.
Long‐term treatment with delgocitinib ointment did not cause skin atrophy or telangiectasia, as seen with topical corticosteroids.1, 3 Application site irritation symptoms, as commonly reported with tacrolimus ointment,1, 3 were infrequent and mild. Most irritation symptoms occurred in the first 2 weeks of delgocitinib treatment, the incidence of which decreased with time. Similarly, local skin infections, including application site folliculitis and Kaposi’s varicelliform eruption, were infrequent; however, appropriate monitoring of local skin infections is mandatory given the immune‐suppressive activity of delgocitinib. As consistent with the previous studies,18, 19 systemic exposure to delgocitinib was low throughout the long‐term treatment, indicating that delgocitinib ointment is unlikely to cause systemic infections due to excessive immunosuppression. Overall, the safety results indicate that delgocitinib ointment has a favorable safety profile as a topical agent for AD.
Previous studies18, 19 demonstrated that delgocitinib ointment rapidly improved clinical signs and symptoms, as assessed with mEASI, IGA and pruritus NRS scores, in Japanese patients aged 16 years or older with moderate to severe AD. In the present study, the improvement effects on AD were maintained with delgocitinib treatment for up to 52 weeks. Because patients were allowed to continue to use strong or less potent topical corticosteroids1 (with similar potency to class III to VII [medium to lowest] corticosteroids in the USA)3 and tacrolimus ointment immediately before delgocitinib treatment initiation, the efficacy results may represent a disease response to switching to delgocitinib ointment from other agents in a real clinical setting.
The results of the present phase 3 studies should not be directly compared with clinical data of other existing topical drugs for AD, such as topical corticosteroids and calcineurin inhibitors, as well as phosphodiesterase 4 inhibitors, because of different study methods and populations. The present study was conducted in an open‐label manner without any control group, which might have led to assessment bias. Additionally, the present study did not include pediatric patients with a higher prevalence of AD. A phase 3 study of delgocitinib ointment is currently being conducted in Japanese pediatric patients with AD.22
In conclusion, delgocitinib 0.5% ointment was well tolerated and effective when administrated to Japanese adult patients with AD for up to 52 weeks. Delgocitinib ointment is expected to be an alternative option with minimal safety concerns for the topical treatment of AD.
Conflict of Interest
H. N. received consulting fees and/or speaker honoraria from AbbVie, Eisai, Eli Lilly Japan, Janssen, Japan Tobacco, Kyowa Hakko Kirin, LEO Pharma, Maruho, Novartis, Torii Pharmaceutical and UCB Japan, and received research grants from AbbVie, Maruho and Tanabe Mitsubishi. O. N. received advisory board honoraria and/or speaker honoraria from Japan Tobacco, Kyowa Hakko Kirin, LEO Pharma and Maruho. A. I. received advisory board honoraria, consulting fees or speaker honoraria from AbbVie, Eli Lilly Japan, Japan Tobacco, Maruho, Novartis, Sanofi and Torii Pharmaceutical, and received research grants from AbbVie, Eli Lilly Japan, Japan Tobacco, Novartis and Sanofi. H. S. received advisory board honoraria and/or speaker honoraria from Japan Tobacco, Kyorin Pharmaceutical, Kyowa Hakko Kirin, Maruho, Sanofi, Taiho Pharma, Tanabe Mitsubishi and Torii Pharmaceutical, and received research grants from AbbVie, Japan Tobacco, LEO Pharma, Maruho, Otsuka Pharmaceutical and Sanofi. R. M., H. K. and T. N. are employees of Japan Tobacco.
Supporting information
Figure S1. Study design of QBA4‐1.
Table S1. Investigator’s Global Assessment score
Table S2. Plasma concentrations of delgocitinib
Table S3. Summary of efficacy results at baseline, week 4, week 24 and week 52
Table S4. List of principal investigators
Acknowledgments
We would like to thank the patients who participated in the study, as well as the investigators and staff at the study sites. The principal investigators and study sites are listed in Table S4. The authors also thank the delgocitinib project team members at Japan Tobacco, especially Shuichi Fukasawa for medical writing and editorial assistance, Kana Yamada for statistical assistance and Manabu Oda for critical review of the manuscript. The study was funded by Japan Tobacco.
References
- 1. Saeki H, Nakahara T, Tanaka A et al. Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016; 43: 1117–1145. [DOI] [PubMed] [Google Scholar]
- 2. Katayama I, Aihara M, Ohya Y et al. Japanese guidelines for atopic dermatitis 2017. Allergol Int 2017; 66: 230–247. [DOI] [PubMed] [Google Scholar]
- 3. Eichenfield LF, Tom WL, Berger TG et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014; 71: 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eucrisa (crisaborol) [prescribing information]. New York, NY: Pfizer Inc; 2018. [Cited 28 November 2019.] Available from URL: http://labeling.pfizer.com/ShowLabeling.aspx?xml:id=5331. [Google Scholar]
- 5. Tanimoto A, Ogawa Y, Oki C et al. Pharmacological properties of JTE‐052: a novel potent JAK inhibitor that suppresses various inflammatory responses in vitro and in vivo. Inflamm Res 2015; 64: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immunemediated disease. Immunity 2012; 36: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bagci IS, Ruzicka T. IL‐31: a new key player in dermatology and beyond. J Allergy Clin Immunol 2018; 141: 858–866. [DOI] [PubMed] [Google Scholar]
- 8. Leung DY, Boguniewicz M, Howell MD, Nomura I, Mamid QA. New insights into atopic dermatitis. J Clin Invest 2004; 113: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res 2012; 52: 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nomura T, Honda T, Kabashima K. Multipolarity of cytokine axes in the pathogenesis of atopic dermatitis in terms of age, race, species, disease stage and biomarkers. Int Immunol 2018; 30: 419–428. [DOI] [PubMed] [Google Scholar]
- 11. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011; 365: 1315–1327. [DOI] [PubMed] [Google Scholar]
- 12. Howell MD, Kim BE, Gao P et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2009; 124: R7–R12. [DOI] [PubMed] [Google Scholar]
- 13. Sonkoly E, Muller A, Lauerma AI et al. IL‐31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006; 117: 411–417. [DOI] [PubMed] [Google Scholar]
- 14. Tanimoto A, Shinozaki Y, Yamamoto Y et al. A novel JAK inhibitor JTE‐052 reduces skin inflammation and ameliorates chronic dermatitis in rodent models: comparison with conventional therapeutic agents. Exp Dermatol 2018; 27: 22–29. [DOI] [PubMed] [Google Scholar]
- 15. Amano W, Nakajima S, Kunugi H et al. The Janus kinase inhibitor JTE‐052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol 2015; 136: 667–677. [DOI] [PubMed] [Google Scholar]
- 16. Yamamoto Y, Otsuka A, Nakashima C et al. The effect of janus kinase inhibitor on pruritus in an atopic dermatitis murine model. Presented at Society of Investigative Dermatology Annual Meeting 2016; Abstract 518. J Invest Dermatol 2016; 136: S92. [Google Scholar]
- 17. Nakagawa H, Nemoto O, Yamada H, Nagata T, Ninomiya N. Phase 1 studies to assess the safety, tolerability and pharmacokinetics of JTE‐052 (a novel Janus kinase inhibitor) ointment in Japanese healthy volunteers and patients with atopic dermatitis. J Dermatol 2018; 45: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakagawa H, Nemoto O, Igarashi A, Nagata T. Efficacy and safety of topical JTE‐052, a Janus kinase inhibitor, in Japanese adult patients with moderate‐to‐severe atopic dermatitis: a phase II, multicentre, randomized, vehicle‐controlled clinical study. Br J Dermatol 2018; 178: 424–432. [DOI] [PubMed] [Google Scholar]
- 19. Clinicaltrials.jp . Phase III clinical study of JTE‐052 Ointment ‐ randomized controlled and long‐term extension study of JTE‐052 Ointment in patients with atopic dermatitis ‐ [JapicCTI‐173554]. [Cited 28 November 2019.] Available from URL: https://www.clinicaltrials.jp/cti-user/trial/Search.jsp (publication in preparation).
- 20. Hanifin JM, Thurston M, Omoto M et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10: 11–18. [DOI] [PubMed] [Google Scholar]
- 21. Yamada T, Saito H, Fujieda S. Present state of Japanese cedar pollinosis: the national affliction. J Allergy Clin Immunol 2014; 133: 632–639. [DOI] [PubMed] [Google Scholar]
- 22. Clinicaltrials.jp . Phase III clinical study of JTE‐052 ointment ‐ randomized controlled and long‐term extension study of JTE‐052 ointment in Japanese Pediatric Patients with Atopic Dermatitis ‐ [JapicCTI‐184064]. [Cited 28 November 2019.] Available from URL: https://www.clinicaltrials.jp/cti-user/trial/Search.jsp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design of QBA4‐1.
Table S1. Investigator’s Global Assessment score
Table S2. Plasma concentrations of delgocitinib
Table S3. Summary of efficacy results at baseline, week 4, week 24 and week 52
Table S4. List of principal investigators


