Summary
Background
Cost‐effective use of biologicals is important. As drug concentrations have been linked to clinical outcomes, monitoring drug concentrations is a valuable tool to guide clinical decision‐making. A concentration–response relationship for ustekinumab at trough is uncertain owing to the contradictory results reported.
Objectives
To investigate the relationship between 4‐week postinjection ustekinumab concentrations and clinical response in patients with psoriasis.
Methods
Forty‐nine patients with moderate‐to‐severe psoriasis treated with 45 mg or 90 mg ustekinumab every 12 weeks for ≥ 16 weeks were included. Ustekinumab serum concentrations and anti‐ustekinumab antibodies were measured at week 4 after injection and disease severity was assessed by Psoriasis Area and Severity Index (PASI).
Results
At week 4 after injection, a significantly negative correlation was observed between ustekinumab concentrations and absolute PASI score up to 5·9 μg mL −1 (ρ = –0·357, P = 0·032). Ustekinumab concentrations were higher in optimal responders (PASI ≤ 2) than in suboptimal responders (PASI > 2) (4·0 vs 2·8 μg mL −1, P = 0·036). The ustekinumab concentration threshold associated with optimal response was determined to be 3·6 μg mL −1 (area under the curve 0·71, sensitivity 86%, specificity 63%). Only one patient (2%) had anti‐ustekinumab antibodies. Psoriatic arthritis was identified as an independent predictor of higher PASI scores and higher ustekinumab concentrations (P = 0·003 and P = 0·048, respectively).
Conclusions
A concentration–response relationship at week 4 after injection was observed for patients with psoriasis treated with ustekinumab. Monitoring 4‐week postinjection ustekinumab concentrations could timely identify underexposed patients who might benefit from treatment optimization.
What's already known about this topic?
Monitoring drug concentrations is a valuable tool that can guide clinical decision‐making when drug concentrations are linked to clinical outcomes.
The presence of a concentration–response relationship for ustekinumab at trough is still debated owing to the contradictory results reported.
What does this study add?
A concentration–response relationship at week 4 after injection for ustekinumab‐treated patients with psoriasis was demonstrated.
Monitoring 4‐week postinjection ustekinumab concentrations could timely identify underexposed patients who might benefit from treatment optimization.
Based on the findings of this study, a treatment algorithm for patients with a suboptimal response is proposed.
Short abstract
Linked Comment: https://doi.org/10.1111/bjd.18709.
https://doi.org/10.1111/bjd.18709 available online
The treatment of psoriasis has dramatically improved with the introduction of biologicals targeting key players in this immune‐mediated inflammatory skin disease, including tumour necrosis factor‐α, interleukin (IL)‐12/23 and IL‐17A. Over the years, more biologicals blocking these crucial cytokines have entered the market and even more are yet to come.1 As physicians have numerous biologicals to choose from, prematurely switching to another drug in case of insufficient response rather than optimizing the current treatment is occurring more frequently, resulting in inefficient use of biologicals. As biologicals constitute a major healthcare expenditure in many countries, cost‐effective use of these drugs is becoming increasingly important.2
Ustekinumab, a monoclonal antibody directed against the common p40 subunit of IL‐12 and IL‐23, has shown efficacy in the treatment of moderate‐to‐severe psoriasis in the pivotal PHOENIX trials.3, 4, 5 Nevertheless, some patients do not respond to ustekinumab treatment or stop responding over time, while others achieve and maintain an optimal response.6, 7 Nowadays, physicians mainly rely on clinical assessment for the management of psoriasis and adhere to standard dosing regimens. However, the one‐size‐fits‐all treatment principle is outdated and the focus is shifting towards a more personalized approach.
Therapeutic drug monitoring – the measurement of drug concentrations – can serve as a tool to guide physicians in clinical decision‐making.8 When a concentration–response relationship is present, monitoring drug concentrations could identify under‐ and overexposed patients who might benefit from treatment optimization. Multiple studies have shown a correlation between serum trough – the drug concentration just before the next drug administration – and clinical response in adalimumab‐treated patients with psoriasis.9, 10 However, for ustekinumab, the presence of a concentration–response relationship at trough is still debated owing to the contradictory results that have been reported.11, 12, 13, 14 The mean ± SD steady‐state trough serum ustekinumab concentration is stated to be 0·69 ± 0·69 μg mL−1 and 0·74 ± 0·78 μg mL−1 for patients with psoriasis receiving 45 and 90 mg, respectively.15 These low values and high variability might hamper the clear distinction between responders and nonresponders in a small cohort. Measuring at 4 weeks postinjection instead of at trough, with the consequently higher ustekinumab concentrations, may be a better time point at which to see a clear concentration–response relationship.
Several patient‐ and treatment‐related factors have been proposed to influence drug concentrations and treatment outcomes. In adalimumab‐ and infliximab‐treated patients with psoriasis, antidrug antibodies have been associated with lower drug concentrations and a decreased treatment response.16, 17, 18 Furthermore, patients with psoriasis who previously received biologicals or who have a high body mass index (BMI) are more likely to have a worse clinical outcome.19, 20 However, which factors exactly influence the drug concentration and clinical response in ustekinumab‐treated patients with psoriasis remain underexplored.
The presence of a concentration–response relationship for ustekinumab at trough is uncertain and the potential of monitoring 4‐week postinjection ustekinumab concentrations in patients with psoriasis has not yet been extensively examined. Therefore, the first aim of this study was to investigate the relationship between ustekinumab concentrations at week 4 after injection and clinical response in a cohort of 49 patients with moderate‐to‐severe psoriasis treated with ustekinumab. The second aim was to identify patient‐ and treatment‐related factors influencing ustekinumab concentration and response.
Patients and methods
Study design and patients
This cross‐sectional study was conducted between December 2014 and April 2015 at Ghent University Hospital, Ghent, Belgium, in accordance with the ethical principles of the Declaration of Helsinki. The local ethics committee approved the study and all patients provided written informed consent (B670201523359). Patients with moderate‐to‐severe plaque psoriasis who were at least 18 years old and treated with ustekinumab for ≥16 weeks were included. Using a weight‐based dosing regimen, patients received ustekinumab subcutaneously at a dose of 45 mg (< 100 kg body weight) or 90 mg (≥ 100 kg body weight) at week 0, week 4 and every 12 weeks from week 4 onwards. Serum samples were prospectively collected 4 weeks [median 28 days, interquartile range (IQR) 27–29, range 23–59] after injection during maintenance and stored at –20 °C.
Outcomes and variables
Disease activity was assessed with absolute Psoriasis Area and Severity Index (PASI) at the time of serum sampling and defined as excellent (PASI ≤1), optimal (PASI ≤ 2) or suboptimal (PASI > 2) response. Clinical improvement was defined as a relative improvement in PASI score with respect to baseline (∆PASI). Absolute PASI ≤ 2 has been shown to correspond to ∆PASI 90.21 Patient characteristics [sex, age, age of psoriasis onset, disease duration, BMI, smoking status, co‐occurrence of psoriatic arthritis (PsA), previous biological use, disease severity at initiation of ustekinumab therapy (PASI baseline)] and treatment characteristics (ustekinumab treatment duration, ustekinumab dosage and concomitant use of methotrexate) were collected at study entry.
Ustekinumab concentration measurements
Ustekinumab serum concentrations were determined using an in‐house developed enzyme‐linked immunosorbent assay. Using a sandwich format, ustekinumab is captured between the anti‐ustekinumab monoclonal antibody MA‐UST56A2D11 and biotinylated MA‐UST56C1H12 as the detection antibody.22 This assay allows quantification of ustekinumab concentrations ranging from 0·25 μg mL−1 to 64 μg mL−1 and has been shown to be comparable with the ustekinumab assay of Janssen R&D in terms of specificity, selectivity, accuracy and precision.23
Anti‐ustekinumab antibody concentrations measurements
When the ustekinumab concentration was < 1 μg mL−1, anti‐ustekinumab antibodies were quantified by means of a drug‐sensitive bridging assay. Briefly, ustekinumab is used as capture and detection antibody and MA‐UST37F12 as calibrator, as described by Verstockt et al.22 All anti‐ustekinumab antibody concentrations were expressed in ng mL−1 MA‐UST37F12 equivalents. When samples were anti‐ustekinumab antibody positive using a drug‐sensitive assay, samples were additionally analysed using a drug‐tolerant assay, as described by Verstockt et al.22
Data analysis
Depending on the research question asked, data analysis was performed on all patients or a subset of patients. Treatment response rates, the concentration–response relationship and a drug concentration cut‐off for clinical response at week 4 after injection were determined in patients who had been on treatment for at least 28 weeks (steady state) and from whom serum samples were collected within 5 days of week 4 after injection during maintenance (n = 38). Evaluation of immunogenicity and identification of confounding factors influencing drug concentration and clinical response was performed in all patients (n = 49).
Statistics
For continuous variables, values are given as median with IQR, and percentages were used for discrete variables. Spearman's rank correlation coefficient (ρ) was used to investigate the relationship between two continuous variables. Unpaired data were analysed using the Mann–Whitney U‐test for continuous variables and the χ2‐test or χ2‐trend test for categorical variables. Receiver–operator characteristic (ROC) curve analysis was performed to identify a cut‐off for optimal response. A cut‐off was chosen based on the performance of the Youden J statistic. Stepwise forward addition‐backward elimination binary linear regression modelling was performed to identify independent predictors of ustekinumab concentration and clinical response. Final model selection was based on the most optimal second‐order Akaike information criterion. A two‐tailed P‐value < 0·05 was considered significant. All statistical analyses were performed with GraphPad Prism 7·0 (GraphPad Software, San Diego, CA, U.S.A.) or R version 3·5·1 (R Development Core Team, Vienna, Austria).
Results
Patient characteristics
Fifty‐five ustekinumab‐treated patients with psoriasis were enrolled, of whom six were excluded from analyses owing to temporary treatment interruption or the absence of PASI score at the time of sampling. The final cohort included 49 patients with psoriasis (73% male) with a median age of 52 years (IQR 41–59) (Table 1). Patients were diagnosed with psoriasis at a median age of 24 years (IQR 16–40) and had a median disease duration of 22 years (IQR 14–28). Most patients were overweight, with a median BMI of 27·4 kg/m², and 27% of patients were smokers. The comorbidity of PsA was diagnosed in 22% of patients. Seventy‐one per cent of patients had previously received at least one biological and 12% received methotrexate during ustekinumab treatment. Median PASI score at baseline was 18·6 and patients had a median ustekinumab treatment duration of 74 weeks (IQR 28–129). Using a weight‐based dosing regimen, 31 patients received 45 mg and 18 patients received 90 mg ustekinumab every 12 weeks.
Table 1.
Baseline demographics and clinical characteristics
| Patient characteristics | n = 49 |
|---|---|
| Male | 36 (73) |
| Median (IQR) age (y) | 52 (41–59) |
| Median (IQR) age of onset of psoriasis (y) | 24 (16–40) |
| Median (IQR) disease duration (y) | 22 (14–28) |
| Median (IQR) BMI (kg m–²) | 27·4 (25·1–30·1) |
| Smoker | 13 (27) |
| Psoriatic arthritis | 11 (22) |
| Previous biological | 35 (71) |
| Concomitant methotrexate | 6 (12) |
| Median (IQR) absolute PASI baseline | 18·6 (14·8–22·5) |
| Median (IQR) treatment duration ustekinumab (wks) | 74 (28–129) |
| Ustekinumab dosage 90 mg | 18 (37) |
Data are n (%) unless otherwise indicated. IQR, interquartile range; y, years; BMI, body mass index; PASI, Psoriasis Area Severity Index.
Treatment response rates
At week 4 after injection, patients had a median absolute PASI score of 1·4 (IQR 0·0–3·0) (Table 2). Twenty‐one (55%) of 38 patients reached a ∆PASI score of 90 and 11 (29%) had a complete clinical improvement. When considering absolute PASI, 24 (63%) patients were optimal responders (PASI ≤ 2), of whom 14 showed an excellent response (PASI ≤ 1).
Table 2.
Treatment response rates at week 4 after injection
| Disease severity and clinical improvement | n = 38 |
|---|---|
| Median (IQR) absolute PASI | 1·4 (0·0–3·0) |
| Absolute PASI ≤ 2 | 24 (63) |
| Absolute PASI ≤ 1 | 14 (37) |
| ∆PASI 90 | 21 (55) |
| ∆PASI 100 | 11 (29) |
Data are n (%) unless otherwise indicated. Only patients treated with ustekinumab for ≥ 28 weeks (steady state) and clinical evaluation at week 4 ± 5 days after injection were included. IQR, interquartile range; PASI, Psoriasis Area Severity Index.
Relationship between 4‐week postinjection ustekinumab concentrations and clinical response
In this cohort, the median ustekinumab concentration at week 4 after injection was 3·3 μg mL−1 (IQR 2·6–4·2). A significantly negative correlation was observed between ustekinumab concentrations and absolute PASI scores up to 5·9 μg mL−1 (ρ = –0·357, P = 0·032) (Fig. 1a). Above this concentration, an increase in ustekinumab concentration no longer correlated with a decrease in absolute PASI score. Additionally, ustekinumab concentrations were positively correlated with ∆PASI (ρ = 0·377, P = 0·024), (Fig. 1b) up to 5·9 μg mL−1, indicating that patients with higher serum ustekinumab concentrations respond better to treatment.
Figure 1.
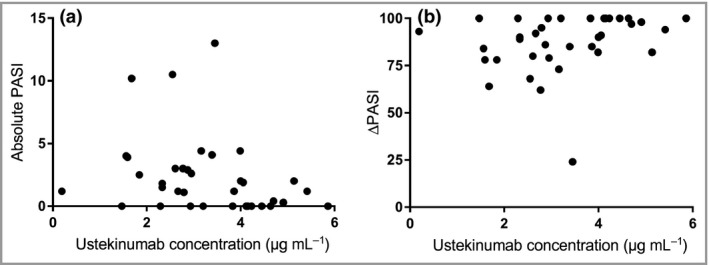
Correlation between ustekinumab serum concentration at week 4 after injection and (a) absolute Psoriasis Area and Severity Index (PASI) score (Spearman's ρ = –0·357, P = 0·032) or (b) ∆PASI (Spearman's ρ = –0·377, P = 0·024). Only patients treated with ustekinumab for ≥ 28 weeks (steady state) and samples collected at week 4 ± 5 days after injection were included (n = 38).
When patients were grouped based on absolute PASI, ustekinumab concentrations were higher in patients with an optimal response (PASI ≤ 2) than in patients with a suboptimal response (PASI > 2) (4·0 μg mL−1 vs 2·8 μg mL−1; P = 0·036) (Fig. 2a). Also, when considering a more stringent PASI cut‐off, patients with PASI ≤ 1 exhibited significantly higher ustekinumab concentrations compared with patients with PASI > 1 (data not shown).
Figure 2.
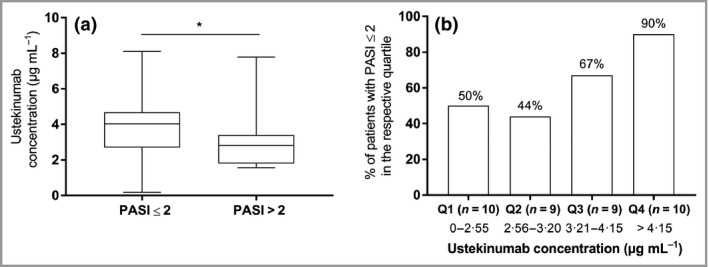
Clinical response based on ustekinumab concentrations at week 4 after injection. (a) Four‐week postinjection ustekinumab serum concentrations in patients with an absolute Psoriasis Area and Severity Index (PASI) score ≤ 2 (4·0 μg mL −1, n = 24) vs patients with an absolute PASI score > 2 (2·8 μg mL −1, n = 14; P = 0·036). (b) Quartile (Q) analysis of 4‐week postinjection ustekinumab concentrations (P = 0·039). Only patients treated with ustekinumab for ≥ 28 weeks (steady state) and samples collected at week 4 ± 5 days after injection were included (n = 38). *P < 0·05.
Quartile analysis of ustekinumab concentrations demonstrated higher percentages of patients achieving an optimal response (PASI ≤ 2) in the higher ustekinumab concentration quartiles than in the lower ustekinumab quartiles (P = 0·039; Fig. 2b), revealing the presence of a concentration–response relationship at 4 weeks postinjection.
Defining an ustekinumab cut‐off for optimal clinical response
In order to identify an ustekinumab concentration cut‐off at week 4 after injection for the optimal clinical response (PASI ≤ 2), ROC curve analysis was performed (Fig. 3a). The best ROC curve (area under the curve 0·71, sensitivity 86%, specificity 63%; P = 0·036) indicated an optimal ustekinumab concentration cut‐off of 3·6 μg mL−1 associated with a positive predictive value and negative predictive value of 88% [95% confidence interval (CI) 67–97] and 57% (95% CI 43–70), respectively. Consequently, when patients are divided based on this threshold, significantly more patients (88%) with an ustekinumab concentration ≥ 3·6 μg mL−1 had an optimal response (PASI ≤ 2) compared with patients with an ustekinumab concentration < 3·6 μg mL−1 (43%; P = 0·004) (Fig. 3b).
Figure 3.
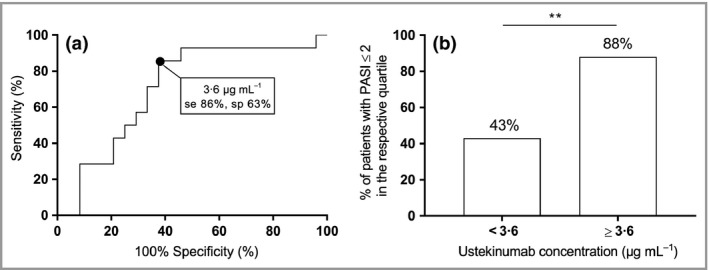
Defining an ustekinumab cut‐off for optimal clinical response. (a) The best receiver–operating characteristic curve [area under the curve 0·71, sensitivity (se) 86%, specificity (sp) 63%; P = 0·036] for optimal clinical response [Psoriasis Area and Severity Index (PASI) ≤ 2] indicated an optimal ustekinumab concentration cut‐off at week 4 after injection of 3·6 μg mL −1. Positive predictive value 88% [95% confidence interval (CI) 67–97], negative predictive value 57% (95% CI 43–70%). (b) Percentage of patients with an optimal response based on the ustekinumab concentration threshold of 3·6 μg mL −1 (P = 0·004). Only patients treated with ustekinumab for ≥ 28 weeks (steady state) and samples collected at week 4 ± 5 days after injection were included (n = 38). **P < 0·01.
Immunogenicity of ustekinumab
Only one patient (2%) had undetectable ustekinumab concentrations and was found to be positive for anti‐ustekinumab antibodies at an intermediate time point using a drug‐sensitive assay. Further analyses revealed that this patient also did not have detectable ustekinumab concentrations before the previous and next ustekinumab injection and was anti‐ustekinumab antibody positive at these time points (Table 3). Additionally, the application of a drug‐tolerant anti‐ustekinumab antibody assay on these samples revealed higher antibody titres. When anti‐ustekinumab antibodies were present, the PASI score increased from 6·3 to 13·4 within 12 weeks, demonstrating the impact of antibodies on treatment response.
Table 3.
Evolution of drug, antidrug antibody concentration and clinical response in one patient
| Time after start of UST therapy | UST concentration (μg mL−1) | AUA concentration: drug‐sensitive assay | AUA concentration: drug‐tolerant assay | PASI |
|---|---|---|---|---|
| Week 0 | NA | NA | NA | 26·2 |
| Week 16 | NA | NA | NA | 2·9 |
| Week 28 | < 0·25 | 138 | 235 | 6·3 |
| Week 37 (= intermediate) | < 0·25 | 52 | 155 | 9·1 |
| Week 40 | < 0·25 | 83 | 190 | 13·4 |
All anti‐ustekinumab antibody concentrations are expressed in ng mL−1 MA‐UST37F12 equivalents. UST, ustekinumab; AUA, anti‐ustekinumab antibody; PASI, Psoriasis Area Severity Index; NA, not available.
Patient‐ and treatment‐related factors influencing ustekinumab concentration or clinical response
Univariate analysis was performed to identify confounding factors influencing ustekinumab concentration or clinical response. No significant effect of age, weight, BMI, prior biological treatment, treatment duration, disease duration, PASI score at baseline, age of psoriasis onset, smoking, concomitant methotrexate use or drug dose was observed on ustekinumab concentration or absolute PASI score.
When evaluating 4‐week postinjection ustekinumab concentrations in patients grouped based on sex, significantly higher values were noted in men compared with women (3·4 μg mL−1 vs 2·3 μg mL−1; P = 0·023) (Fig. 4a). However, men and women did not significantly differ in absolute PASI score (P = 0·298; Fig. 4b). Multivariate analysis revealed that the effect of male sex on ustekinumab concentration disappeared when adjusting for the age of onset of psoriasis and having PsA (Table S1; see Supporting Information).
Figure 4.
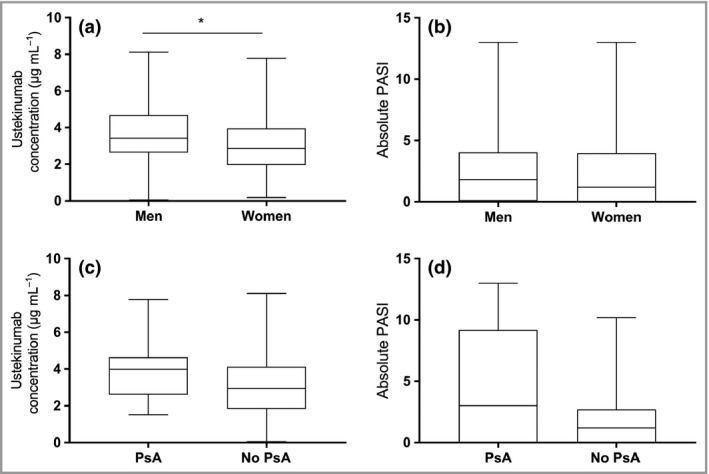
Ustekinumab serum concentrations at week 4 after injection in (a) men (3·4 μg mL −1, n = 36) vs women (2·3 μg mL −1, n = 13; P = 0·023) and (c) patients with psoriatic arthritis (PsA) (4·0 μg mL −1, n = 11) vs patients without PsA (3·4 μg mL −1, n = 38; P = 0·193). Absolute Psoriasis Area and Severity Index (PASI) score in (b) men vs women (P = 0·298) and (d) patients with PsA (3·0, n = 11) vs patients without PsA (1·2, n = 38; P = 0·066). *P < 0·05.
A trend was observed towards higher absolute PASI scores at 4 weeks postinjection, but not at baseline, in patients with PsA vs patients without PsA (3·0 vs 1·2, respectively; P = 0·066) (Fig. 4c). However, in these patients with PsA, 4‐week post injection ustekinumab concentrations were slightly higher, but not significantly so (4·0 μg mL−1 vs 3·4 μg mL−1; P = 0·193 (Fig. 4d). Through multivariate analysis, having PsA was identified as an independent predictor of a higher 4‐week postinjection PASI score and higher ustekinumab concentrations (P = 0·003 and P = 0·048; Table S1).
Discussion
To this day, owing to the contradictory results reported, no consensus has been reached regarding the presence of a concentration–response relationship for ustekinumab and the usefulness of monitoring ustekinumab concentrations. Two independent research groups did not observe a correlation between ustekinumab concentrations at trough and clinical response.11, 12 In contrast, two other studies could distinguish responders from nonresponders based on the ustekinumab trough concentration; one Spanish group made a similar observation at 6 weeks postinjection.12, 13, 14 For therapeutic drug monitoring of ustekinumab to be effective and implemented into clinical practice, physicians should know at which time point to measure ustekinumab concentrations and how to interpret the results.
This study aimed to investigate the relationship between 4‐week postinjection ustekinumab concentrations and clinical response in patients with moderate‐to‐severe psoriasis. A negative correlation was observed between ustekinumab concentrations measured 4 weeks after injection and absolute PASI score, revealing that patients with higher serum ustekinumab concentrations respond better to treatment. Moreover, 4‐week postinjection ustekinumab concentrations were considerably higher in optimal responders than in suboptimal responders and 3·6 μg mL−1 was determined to be the ustekinumab concentration threshold associated with optimal response. In accordance with the findings of Toro‐Montecinos et al., these results reveal the presence of a concentration–response relationship for ustekinumab at 4 weeks postinjection and demonstrate the usefulness of monitoring ustekinumab concentrations.12
Measuring drug concentrations at 4 weeks postinjection has the advantage that drug concentration results are known early enough to allow treatment optimization. Monitoring 4‐week postinjection ustekinumab concentrations can be facilitated by means of dried blood spot (DBS) sampling, in which a blood drop is obtained on a protein saver card after a small finger prick. The patient can perform this at home and send the DBS card by regular mail to the hospital, where the drug concentration can be measured. The feasibility of monitoring biologicals through DBS has already been established for golimumab, adalimumab and ustekinumab.24, 25, 26
In line with immunogenicity rates of ustekinumab seen in clinical trials, only one patient (2%) had anti‐ustekinumab antibodies in this cohort.3, 4 Ustekinumab concentrations were undetectable in this patient and once the anti‐ustekinumab antibodies were present, the PASI score increased dramatically, indicating that although the immunogenicity of ustekinumab is low, anti‐ustekinumab antibodies can have a significant impact on treatment response.
PsA was identified as an independent predictor of higher PASI scores and higher ustekinumab concentrations. Although ustekinumab is approved for both psoriasis and PsA and baseline PASI scores between those groups did not differ in this cohort, patients having both diseases might have a higher disease burden that is not fully captured by PASI score.27, 28 Subsequently, the minimal effective concentration of ustekinumab for patients with psoriasis suffering from PsA might be higher and biologicals targeting other key players in the immune‐mediated disease might be more appropriate to treat two diseases at once. Interestingly, all patients with an ustekinumab concentration higher than the threshold of 3·6 μg mL−1 who did not reach an optimal clinical response were patients with PsA. If these patients are eliminated from the analysis, a positive predictive value of 100% for the cut‐off of 3·6 μg mL−1 was seen.
Biologicals remain to be a major healthcare expenditure in many countries and pose a considerable burden on the healthcare budget.2 Therefore, a rational evidence‐based strategy is needed so that clinicians can optimize treatment in a patient losing response and avoid inefficient use of biologicals. Based on the findings of this study, the following treatment algorithm is proposed (Fig. 5).
Figure 5.
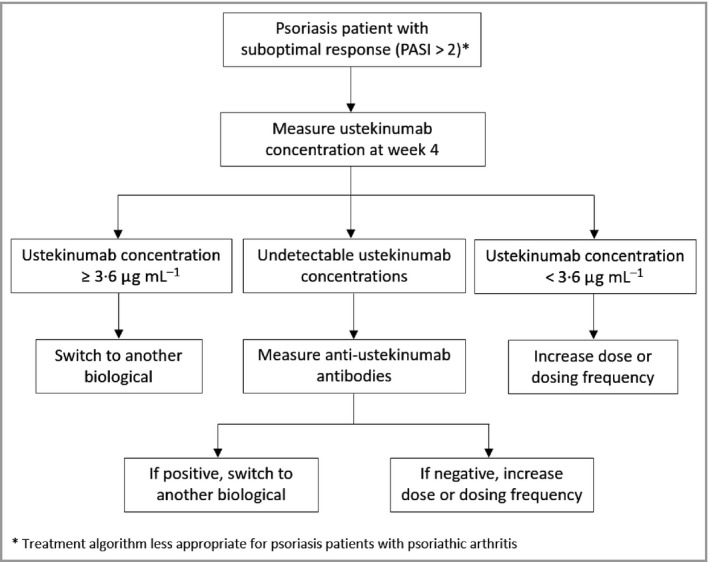
Treatment algorithm for ustekinumab‐treated patients with a suboptimal response based on the study findings. PASI, Psoriasis Area and Severity Index.
This study represents a real‐life clinical practice cohort including a mixture of patients treated with either 45 or 90 mg during maintenance. A major strength is the use of a validated assay to measure ustekinumab concentrations, which has shown to be comparable with the Janssen R&D assay.23 This assay is currently being converted into a CE‐labelled kit (apDia) and will soon be available. Limitations of the study include the small sample size and the cross‐sectional study design. The upper limit of ustekinumab concentrations above which no extra benefit is expected could not be established in this cohort and therefore a treatment algorithm for potentially overtreated patients could not be established. Furthermore, the threshold of 3·6 μg mL−1 that was identified has a negative predictive value of 57%, indicating that there are some patients who have an optimal response despite having drug concentrations below the threshold. Consequently, only reactive therapeutic drug monitoring in which 4‐week postinjection ustekinumab concentrations are measured in patients with a suboptimal response is justified using this drug concentration cut‐off.
To conclude, monitoring 4‐week postinjection ustekinumab concentrations could help in the management of patients with psoriasis by timely identifying patients who are underexposed and could benefit from increased dosing or dosing frequency. For patients with psoriasis suffering from PsA, the therapeutic window of ustekinumab may be higher and alternative biologicals may be more appropriate to treat two diseases at once. As anti‐ustekinumab antibodies are rare, measurement should only be considered in patients with an insufficient clinical response and undetectable drug concentrations. Comparator trials are needed to confirm the clinical and economic value of monitoring 4‐week postinjection ustekinumab concentrations and subsequent treatment optimization.
Supporting information
Table S1 Overview of the predictors of the best model of clinical response and ustekinumab concentration.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.
Funding sources This work was supported, in part, by the TBM Grant T003716N of the Research Foundation – Flanders (FWO), Belgium. N.V.d.B is a SB PhD fellow at FWO.
Conflicts of Interest A.G. has received financial support for research from Pfizer, MSD and Takeda; lecture fees from MSD, Janssen Biologicals, Pfizer, Takeda, Novartis and AbbVie; consultancy fees from Takeda; and advisory board fees from Takeda. KU Leuven holds a license agreement with R‐biopharm, apDia and Merck. J.L. has received financial support for research from Janssen, AbbVie, Novartis, Lilly, Celgene and Pfizer; consultancy fees from Pfizer, Novartis, AbbVie, Janssen Cilag and LEO Pharma; and has carried out clinical trials for Janssen‐Cilag, Merck Serono, Amgen, Pfizer, AbbVie, Celgene and Novartis.
https://doi.org/10.1111/bjd.18709 available online
References
- 1. Ronholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci 2017; 18:E2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs 2015; 7:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371:1665–74. [DOI] [PubMed] [Google Scholar]
- 4. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371:1675–84. [DOI] [PubMed] [Google Scholar]
- 5. Kimball AB, Papp KA, Wasfi Y et al Long‐term efficacy of ustekinumab in patients with moderate‐to‐severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol 2013; 27:1535–45. [DOI] [PubMed] [Google Scholar]
- 6. Zweegers J, Groenewoud JMM, van den Reek J et al Comparison of the 1‐ and 5‐year effectiveness of adalimumab, etanercept and ustekinumab in patients with psoriasis in daily clinical practice: results from the prospective BioCAPTURE registry. Br J Dermatol 2017; 176:1001–9. [DOI] [PubMed] [Google Scholar]
- 7. Langley RG, Lebwohl M, Krueger GG et al Long‐term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 2 study through 5 years of follow‐up. Br J Dermatol 2015; 172:1371–83. [DOI] [PubMed] [Google Scholar]
- 8. Hermans C, Herranz P, Segaert S et al Current practice of therapeutic drug monitoring of biopharmaceuticals in psoriasis patients. Ther Drug Monit 2017; 39:356–9. [DOI] [PubMed] [Google Scholar]
- 9. Menting SP, Coussens E, Pouw MF et al Developing a therapeutic range of adalimumab serum concentrations in management of psoriasis: a step toward personalized treatment. JAMA Dermatol 2015; 151:616–22. [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson N, Tsakok T, Dand N et al Defining the therapeutic range for adalimumab and predicting response in psoriasis: a multicenter prospective observational cohort Study. J Invest Dermatol 2019; 139:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menting SP, van den Reek JM, Baerveldt EM et al The correlation of clinical efficacy, serum trough levels and antidrug antibodies in ustekinumab‐treated patients with psoriasis in a clinical‐practice setting. Br J Dermatol 2015; 173:855–7. [DOI] [PubMed] [Google Scholar]
- 12. Toro‐Montecinos M, Ballesca F, Ferrandiz C et al Usefulness and correlation with clinical response of serum ustekinumab levels measured at 6 weeks versus 12 weeks. J Dermatolog Treat 2019; 30:35–9. [DOI] [PubMed] [Google Scholar]
- 13. Martin‐Gonzalez S, Urigoitia‐Ugalde P, Careaga J et al Optimal concentration range of ustekinumab in patients with plaque‐type psoriasis. J Am Acad Dermatol 2019; 80:1782–4. [DOI] [PubMed] [Google Scholar]
- 14. Chiu HY, Chu TW, Cheng YP et al The association between clinical response to ustekinumab and immunogenicity to ustekinumab and prior adalimumab. PLOS ONE 2015; 10:e0142930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stelara (ustekinumab) (package insert). Janssen Biotech. Available at: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf (last accessed 3 June 2019).
- 16. Adisen E, Aral A, Aybay C et al Anti‐infliximab antibody status and its relation to clinical response in psoriatic patients: a pilot study. J Dermatol 2010; 37:708–13. [DOI] [PubMed] [Google Scholar]
- 17. Lecluse LL, Driessen RJ, Spuls PI et al Extent and clinical consequences of antibody formation against adalimumab in patients with plaque psoriasis. Arch Dermatol 2010; 146:127–32. [DOI] [PubMed] [Google Scholar]
- 18. Bito T, Nishikawa R, Hatakeyama M et al Influence of neutralizing antibodies to adalimumab and infliximab on the treatment of psoriasis. Br J Dermatol 2014; 170:922–9. [DOI] [PubMed] [Google Scholar]
- 19. Notario J, Deza G, Vilarrasa E et al Treatment of patients with plaque psoriasis with secukinumab in a real‐life setting: a 52‐week, multicenter, retrospective study in Spain. J Dermatolog Treat 2018; 30:424–9. [DOI] [PubMed] [Google Scholar]
- 20. Bardazzi F, Balestri R, Baldi E et al Correlation between BMI and PASI in patients affected by moderate to severe psoriasis undergoing biological therapy. Dermatol Ther 2010; 23(Suppl. 1):S14–19. [DOI] [PubMed] [Google Scholar]
- 21. Reich K, Bachhuber T, Melzer N et al From relative to absolute treatment outcomes – correlation of PASI 90 and PASI ≤2 in three clinical trials with secukinumab. J Am Acad Dermatol 2018; 79:AB143. [Google Scholar]
- 22. Verstockt B, Dreesen E, Noman M et al Ustekinumab exposure‐outcome analysis in Crohn's disease only in part explains limited endoscopic remission rates. J Crohns Colitis 2019; 10.1093/ecco-jcc/jjz008. [DOI] [PubMed] [Google Scholar]
- 23. Marini J, Gils A, Shankar G. Comparison of the KU Leuven ustekinumab concentration assay and the antibodies‐to‐ustekinumab assay with assays developed at Janssen R&D and used in clinical studies of IBD patients. Presented at the 13th Congress of ECCO, Vienna, Austria. 14–17 February 2018; abstr. P649.
- 24. Detrez I, Schops G, Lefrere J et al Golimumab Dried Blood Spot Analysis (GOUDA): a prospective trial showing excellent correlation with venepuncture samples and more detailed pharmacokinetic information. AAPS J 2018; 21:10. [DOI] [PubMed] [Google Scholar]
- 25. Kneepkens EL, Pouw MF, Wolbink GJ et al Dried blood spots from finger prick facilitate therapeutic drug monitoring of adalimumab and anti‐adalimumab in patients with inflammatory diseases. Br J Clin Pharmacol 2017; 83:2474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloem K, Schaap T, Boshuizen R et al Capillary blood microsampling to determine serum biopharmaceutical concentration: Mitra((R)) microsampler vs dried blood spot. Bioanalysis 2018; 10:815–23. [DOI] [PubMed] [Google Scholar]
- 27. McInnes IB, Kavanaugh A, Gottlieb AB et al Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double‐blind, placebo‐controlled PSUMMIT 1 trial. Lancet 2013; 382:780–9. [DOI] [PubMed] [Google Scholar]
- 28. Kavanaugh A, Puig L, Gottlieb AB et al Efficacy and safety of ustekinumab in psoriatic arthritis patients with peripheral arthritis and physician‐reported spondylitis: post‐hoc analyses from two phase III, multicentre, double‐blind, placebo‐controlled studies (PSUMMIT‐1/PSUMMIT‐2). Ann Rheum Dis 2016; 75:1984–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Overview of the predictors of the best model of clinical response and ustekinumab concentration.
Powerpoint S1 Journal Club Slide Set.
Video S1 Author video.


