Abstract
Background and purpose
We hypothesized that combining intravenous immunoglobulin (IVIg) and intravenous methylprednisolone (IVMP) leads to more frequent remission compared with IVIg alone while maintaining the fast efficacy of IVIg. In this uncontrolled pilot study, we evaluated remission, rate of improvement and safety in patients with chronic inflammatory demyelinating polyradiculoneuropathy receiving induction treatment with combined IVIg and IVMP.
Methods
Consecutive treatment‐naive patients with chronic inflammatory demyelinating polyradiculoneuropathy were treated with IVIg infusions, consisting of a 2 g/kg loading dose and 1 g/kg maintenance treatment every 3 weeks, combined with 3‐weekly 1‐g IVMP infusions, for a total of 18 weeks. The cumulative steroid dose was 7 g. Primary outcome was remission at 1 year in patients who completed the treatment schedule. Remission was defined as improvement at 18 weeks without the need for further immune treatment between end of the treatment schedule and 1‐year follow‐up. Improvement was defined as a minimal clinically important difference on the Inflammatory Rasch‐Built Overall Disability Scale and/or an increase of ≥8 kPa in grip strength between baseline and week 18.
Results
A total of 20 patients were included; 17 completed the treatment schedule. A total of 13 (76%) of these patients improved at 18 weeks after start of treatment and 10 (59%) patients were in remission at 1 year. Serious adverse events were found in four patients.
Conclusions
Short‐term combined induction treatment with IVIg and IVMP induced remission in almost 60% of patients who completed the treatment schedule. Combined induction therapy was generally well tolerated. A randomized controlled trial is currently running to confirm efficacy and safety of IVMP as add‐on treatment to IVIg.
Keywords: chronic inflammatory demyelinating polyradiculoneuropathy, corticosteroids, intravenous immunoglobulin
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an immune‐mediated neuropathy causing sensory and motor impairment in the arms and legs 1. Induction treatment of CIDP consists of intravenous immunoglobulin (IVIg) or corticosteroids 1, 2, 3. IVIg leads to improvement in most patients within 6 weeks after initial treatment 4. However, time to improve with corticosteroids usually takes several months and patients are frequently switched to IVIg if improvement does not occur quickly enough 3. This relatively long delay to improvement and corticosteroid‐related adverse events are probably the main reasons that corticosteroids are used less often as induction treatment in high‐income countries. However, an important advantage of corticosteroids is that they seem to lead to long‐term remissions 5, 6. We hypothesize that combining intravenous pulsed corticosteroids and IVIg is a safe treatment leading to more remissions in CIDP and to a higher rate of improvement compared with IVIg monotherapy and maintaining the quick clinical response associated with IVIg. Prior to conducting a randomized controlled trial (ISRCTN15893334) to test this hypothesis, we performed an open‐label prospective study to assess the efficacy and safety of combining IVIg and intravenous methylprednisolone (IVMP) as induction treatment in CIDP (OPTIC protocol).
Methods
Inclusion criteria
Adult patients diagnosed with probable or definite CIDP according to the European Federation of Neurological Societies/Peripheral Nerve Society criteria were eligible for inclusion 1. Patients with contraindications for corticosteroids or IVIg were excluded. Patients were included from the Amsterdam UMC, The Netherlands and Maidstone Hospital, UK. The local institutional review board of Amsterdam UMC waived formal review as both treatments and clinical assessments are considered standard practice in CIDP. All patients provided consent to receive treatment.
Treatment schedule
Patients were treated with IVIg and IVMP every 3 weeks for a duration of 18 weeks (Fig. 1). The first treatment consisted of IVIg 2 g/kg in 3–5 days followed by an infusion of 1 g IVMP. This was followed by six maintenance courses of IVIg 1 g/kg over 1 or 2 days and 1 g IVMP every 3 weeks. At 18 weeks, the treating physician was allowed to provide two additional IVIg or IVMP infusions (i.e. at weeks 21 and 24) if further improvement was deemed possible. All patients received osteoporosis prophylaxis consisting of daily calcium and vitamin D and weekly alendronic acid.
Figure 1.
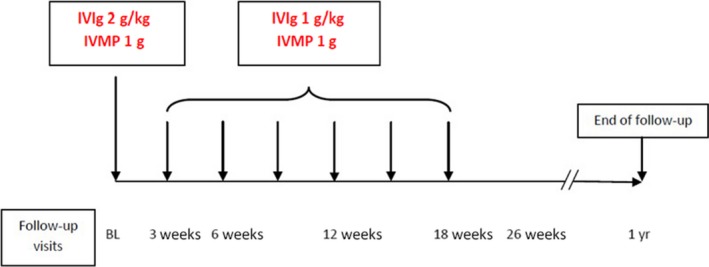
OPTIC treatment protocol. BL, baseline; IVIg, intravenous immunoglobulin; IVMP, intravenous methylprednisolone.
Primary outcome
The primary outcome was the percentage of patients in remission of those who completed the treatment schedule. Remission was defined as improvement in disability or grip strength at 18 weeks or, in the case of two additional treatment cycles, at 24 weeks without the need for further treatment between the end of treatment and 1‐year follow‐up. Improvement was assessed using the Inflammatory Rasch‐Built Overall Disability Scale (iRODS) and grip‐strength measurements. The iRODS is a linearly weighted scale that specifically captures activity and social participation limitations in patients with inflammatory neuropathies 7. Grip strength (in kPa) was measured using a Martin Vigorimeter 8. The best out of three attempts was recorded with each hand. Improvement was defined as an increase of more than the minimal clinically important difference (MCID) on either the iRODS or grip strength. The MCID on the iRODS is related to the individual standard error and was defined as having an MCID standard error ≥1.96 7, 9. The MCID on grip strength was defined as ≥8 kPa in one hand 8. In patients with multifocal CIDP with unilaterally reduced grip strength, an increase of ≥8 kPa in the affected arm was considered as improvement.
Secondary outcomes
Secondary outcomes included percentage of patients with improvement of ≥MCID on the iRODS at 18 weeks, percentage of patients with improvement of ≥8 kPa in grip strength at 18 weeks, and changes between baseline and 18 weeks on the iRODS, Medical Research Council sum score (range 0–60 with higher scores indicating less weakness) and Inflammatory Neuropathy Cause and Treatment sensory sum score (range 0–33 with higher scores indicating more sensory deficits). For the secondary outcomes, we used data from patients who completed the treatment schedule. Adverse events associated with IVIg and IVMP were noted at each follow‐up visit.
Follow‐up
Visits were scheduled at the outpatient clinic at week 3, 6, 12, 18, 26 and 52 after start of treatment (Fig. 1). In case of (possible) deterioration, an extra visit was scheduled. If deterioration occurred after 18 weeks of follow‐up, IVIg treatment was restarted.
Statistical analysis
We used simple descriptive statistics for the primary outcome. Differences between baseline and week 18 were analyzed using the Wilcoxon signed rank test.
Results
A total of 20 patients were included between September 2014 and February 2017. Fourteen patients (70%) had typical CIDP, five patients (25%) had asymmetric CIDP and one patient (5%) had pure motor CIDP. The median duration of symptoms until start of treatment was 14.5 (2–86) months (Table 1). Seventeen patients (85%) completed the treatment schedule (Fig. 2). One patient received two additional IVIg courses after 18 weeks due to slow improvement. Two patients did not complete the treatment schedule because of adverse events (see below), whereas in one patient with typical CIDP, treatment was changed after deterioration following the first treatment course.
Table 1.
Baseline characteristics (n = 20)
| Variable | |
|---|---|
| Age (years) | 59 ± 16 |
| Male | 16 (80%) |
| EFNS/PNS 2010 criteria | |
| Definite | 19 |
| Probable | 1 |
| CIDP subtype | |
| Typical CIDP | 14 (70%) |
| Asymmetric CIDP | 5 (25%) |
| Pure motor CIDP | 1 (5%) |
| Subacute onset | 8 (40%) |
| Duration of symptoms until treatment (months) | 14.5 (2–86) |
Figure 2.
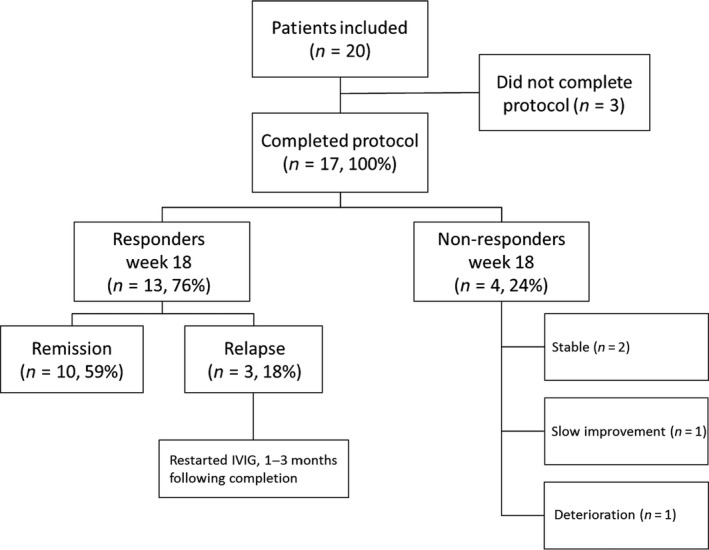
Protocol flowchart and outcome. One patient who did not complete protocol was in remission at 1 year. The one patient who was scored as a non‐responder due to slow improvement was also in remission at 1 year. IVIg, intravenous immunoglobulin.
Remission
In total, 12 patients did not require further treatment at the end of the study (60%), regardless of whether they completed the treatment schedule. Of the 17 patients who completed the treatment schedule, 10 (59%) fulfilled the pre‐defined remission criteria (Fig. 2). Patients with subacute and chronic CIDP showed remission rates of 71% and 50%, respectively. One patient who deteriorated after the first treatment course reached remission after a combination treatment of plasma exchange and pulsed oral dexamethasone. This patient did not have anti‐neurofascin 155/neurofascin186 or contactin antibodies. Another patient did not meet criteria for remission because of slow improvement despite two additional treatment cycles. This patient improved during follow‐up and did not require further treatment at the end of the study.
Treatment responders
A total of 13 of 17 patients (76%) who completed the treatment schedule improved at 18 weeks. Improvement of ≥MCID on the iRODS was seen in 8 (47%) patients. Improvement in grip strength (≥8 kPa) was seen in 12 (71%) patients. Twelve patients reached the MCID on the iRODS and/or grip strength at 6 weeks after first treatment (92%) and one patient at 12 weeks. Time to response did not differ between patients with subacute or chronic CIDP. Secondary outcomes results are summarized in Table 2. Three treatment responders relapsed between 1 and 3 months after last treatment and restarted IVIg, one in combination with IVMP. Figure 3 illustrates change on the iRODS and total grip strength in patients who completed the treatment schedule.
Table 2.
Secondary outcomes for patients who completed the OPTIC protocol
| Parameter | Baseline | Week 18 |
Within‐patient change (baseline to week 18) |
P‐value |
|---|---|---|---|---|
| iRODS score | 55 (46–73) | 80 (57–97) | 15 (−2.0 to 32) | 0.025 |
| Grip strength totala | 96 (69–131) | 170 (110–200) | 41 (−0.5 to 100) | 0.004 |
| MRC sum score | 55 (49–59) | 60 (56–60) | 1.0 (−1.0 to 11) | 0.110 |
| INCAT‐SS | 6.0 (2.5–7.0) | 4.0 (0.5–5.0) | −2.0 (−4.0 to 0.0) | 0.016 |
INCAT‐SS, Inflammatory Neuropathy Cause and Treatment sensory sum score; iRODS, Inflammatory Rasch‐Built Overall Disability Scale, percentile score; MRC, Medical Research Council. Data are given as median (interquartile range).
Grip strength is the sum of both arms.
Figure 3.
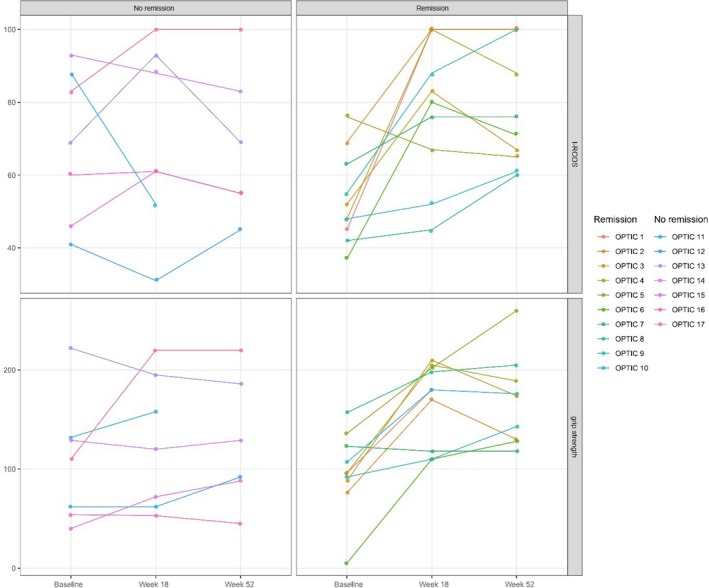
Overview of the Inflammatory Rasch‐Built Overall Disability Scale (iRODS) and total grip strength over time in patients who completed all courses of intravenous methylprednisolone (n = 17). Upper graphs show iRODS scores (centile); lower graphs show (total) grip strength. Patients treated between week 18 and 52 (n = 2) did not classify as remission.
Treatment non‐responders
Four of the 17 patients (24%) who completed the treatment schedule did not meet the criteria for improvement at 18 weeks. Two of these patients improved less than the pre‐defined criteria but no additional treatment was regarded as necessary as patients were considered stable. Of these patients, one experienced a relapse at 11 months after start of treatment. Another patient showed no improvement after completing treatments and was switched to plasmapheresis that led to improvement. As he became plasma exchange dependent, he was treated with rituximab that led to improvement and long‐term treatment‐free remission. Finally, one patient did not meet criteria for improvement at 24 weeks despite two additional courses of IVIg.
Adverse events
Four patients experienced a serious adverse event during treatment. One patient with severe cardiovascular comorbidity suffered a myocardial infarction during the second cycle of IVIg infusions (IVMP had not yet been administered during that course), after which he refused further treatment. One patient developed a diverticulitis with secondary perforation, requiring surgery. IVMP was temporarily stopped to prevent poor wound control after surgery. It was later restarted but stopped again due to nausea and headache after IVMP infusions. Another patient developed a pulmonary embolism 1 week after first treatment. One patient who improved by 18 weeks had a relapse after 2 months and was treated with IVIg and IVMP. This patient died of unknown cause 3 months after last CIDP treatment. In one patient, the last course of IVIg was not administered due to toxicodermia, which was considered a moderate adverse event (Table 3).
Table 3.
Adverse events
| Serious adverse events | No. of events | Treatment action and relation to medication |
|---|---|---|
| Diverticulitis with secondary perforation | 1 | IVMP temporarily stopped. Judged as possibly related to IVMP |
| Myocardial infarction | 1 | Following IVIg infusion, judged as related to IVIg. IVIg discontinued |
| Pulmonary embolism | 1 | After protocol discontinuation (non‐responder) and switch to plasmapheresis. Judged as related to prolonged immobilization and probably related to IVIg and possibly to IVMP |
| Death by unknown cause | 1 | Diseased by an unknown cause 3 months after last treatment |
| Adverse events | Treatment action | |
|---|---|---|
| Skin reactions | 5 | IVIg stopped in one patient |
| Headache | 4 | |
| Flu‐like symptoms | 2 | |
| Insomnia | 2 | |
| Dry mouth after/during infusions | 2 | |
| Mood changes | 1 | |
| Fatigue after infusions | 1 | |
| Metal taste | 1 | |
| Delirium | 1 | |
| Nausea | 1 |
Discussion
The combination of IVIg and IVMP led to improvement and remission at 1 year in 59% of patients who completed the OPTIC treatment schedule. Of all patients, including those who received additional treatments and did not complete or respond to the OPTIC treatment schedule, 60% did not require further treatment at 1 year.
This is the first study on the additional value of IVMP with IVIg as induction treatment in CIDP. The remission rate is similar to, or slightly higher than, two other studies focusing on corticosteroid monotherapy in CIDP. In the IMC trial, a randomized controlled study comparing IVMP with IVIg, 10 of 21 (48%) patients responded to IVMP 2. In the 6‐month treatment‐free follow‐up period, none of these 10 patients required further treatment, corresponding with a 48% remission rate 1 year after start of IVMP treatment. Long‐term follow‐up showed that median remission duration after IVMP was longer (14 months) compared with IVIg (4.5 months) after treatment discontinuation 6. The PREDICT trial, which compared daily oral prednisolone with pulsed dexamethasone, reported a remission rate at 1 year of 36% after treatment with daily oral prednisolone and 44% in patients with pulsed dexamethasone 3. As the diagnosis was changed in some patients during follow‐up of the PREDICT trial, a post hoc analysis showed a 56% remission rate at 1 year in patients with true CIDP who were treated with pulsed dexamethasone 5. This prospective study also showed that about half of patients in remission experienced a relapse in the following years. Both the IMC and PREDICT trial are not completely comparable with the current study. First, we only treated treatment‐naive patients, whereas the IMC trial also included previously treated patients, which might have led to selection bias to patients with a more chronic disease course. Secondly, both trials had a 2–4‐month shorter follow‐up period after stopping treatment compared with our study. In addition, cumulative steroid doses also differed. Patients in the IMC trial were treated with 12 g IVMP over 6 months, whereas patients in the PREDICT trial were treated with an equivalent of 4.8 g IVMP over 6 months. For the OPTIC protocol, we chose a pragmatic schedule of 1 g of IVMP per course, leading to a cumulative steroid dose of 7 g over 18 weeks. Finally, we focused on remission rates at the end of follow‐up in patients who completed the treatment schedule rather than all patients who started on treatment, as we considered this ‘per‐protocol’ analysis more appropriate to investigate our primary hypothesis in this pilot study.
Most IVIg trials focused on short‐term efficacy and therefore there is only limited evidence on the rate of remission after induction treatment with IVIg monotherapy 10. A single dose of IVIg is sufficient in only 14% of patients 11. In the IMC trial, 62% of the IVIg responders remained in remission after 6 months. In the largest IVIg trial in CIDP (ICE trial), patients who responded to IVIg treatment were rerandomized to IVIg or placebo 12. After 6 months, 45% of patients in the placebo group were still in remission. However, this study was not designed to study remission rates of IVIg and a placebo effect might have overestimated the rate of remission in patients who discontinued IVIg. Both the IMC and the ICE trial are difficult to compare with our study as they included known IVIg responders, whereas not all patients improve on IVIg. As improvement was part of our definition of remission, lower rates of remission would be expected if the treatment‐naive patients in our study were treated with IVIg monotherapy.
How to define a treatment responder is still a matter of debate. We chose a combination of a disability scale and grip strength to define improvement as previously reported in the literature 7, 8, 9. In this study, patients who completed treatment showed an improvement of ≥MCID on the iRODS and/or grip strength in 76% of cases. In addition, three patients showed some improvement but failed to reach the pre‐defined criteria for improvement at 18 weeks. Therefore, using a pre‐defined level of improvement in the definition of remission might have led to an underestimation of the remission rate. Alternatively, some patients showed some deterioration not meeting the pre‐defined MCID when comparing 52 weeks with 18 weeks. This probably reflects normal fluctuation in measurement. However, a minimal deterioration and thus active disease cannot be excluded completely.
Most of the patients improved in the first 6 weeks, which is in accordance with the expected fast response 3, 10, 12. Generally, time to improve with corticosteroid monotherapy is longer 3. In addition, some patients do not respond to monotherapy with IVIg or corticosteroids and require a treatment switch from one to the other. Combining both treatment modalities would benefit these patients in particular, at the cost of potential side effects from both treatments.
Intravenous methylprednisolone was well tolerated by most patients, which is in line with previous studies 3, 13, 14, 15. Nevertheless, the main disadvantage of treating patients with the OPTIC protocol rather than a single treatment is the higher risk of adverse effects of both IVIg and corticosteroids. To our knowledge, there is no literature reporting on any synergistic effects of the combination treatment. Two thromboembolic‐related serious adverse events occurred. As one of these patients had severe cardiovascular comorbidity, we would advocate caution or preventive measures when treating patients with increased risk of arterial or venous thrombosis.
The absolute risk of a pulmonary embolism is probably low as a previous trial in Guillain–Barre syndrome did not report any pulmonary embolisms in 112 patients treated with IVMP and IVIg 16. Also, one patient developed a diverticulitis with secondary perforation. It is unclear whether this serious adverse event can be attributed to IVMP. Corticosteroids have been reported to mask early symptoms of diverticulitis, potentially increasing associated mortality and comorbidity, whereas others advocate corticosteroids for treatment of diverticulitis 17.
Limitations
The open and uncontrolled design is the most important limitation of this study as this may have introduced bias. Also, previous studies offer limited guidance on how to combine IVIg and IVMP. Our cumulative IVMP dose and length of the OPTIC protocol was based on the known efficacy of IVIg and corticosteroids, but remains arbitrary. The IVIg dose was based on the ICE trial, which is the largest IVIg trial in CIDP 12. We considered a treatment period of 4 months as the minimum to achieve long‐term remission due to corticosteroids. It is as yet unclear what dose of steroids is needed to achieve remission. As remission rates are comparable with the PREDICT trial, a lower cumulative dose may be sufficient 3. It should be noted that in previous IVIg trials most treatment responders reached their maximum improvement well before 4 months 4, 12. Also in our study, most patients improved in the first 6 weeks 12. In patients who improve after the first few courses of IVIg, continuation with IVMP might have been enough to sustain improvement, which would lead to less IVIg use and would reduce the high healthcare costs associated with IVIg. The open design of this study makes it difficult to attribute treatment effect to IVIg, IVMP or the combination of both.
Conclusion
The combination of IVMP and IVIg led to remission in almost 60% of treated patients and to improvement in almost two‐thirds of patients. The combination of treatments was generally well tolerated although three serious adverse events were possibly attributed to treatment. Based on these results, we recently started the OPTIC trial (ISRCTN15893334), a multi‐center randomized double‐blind placebo‐controlled trial, to confirm efficacy and safety of methylprednisolone treatment as an add‐on treatment to IVIg. Frequent remissions would greatly reduce patients’ burden of frequent infusions and the high healthcare costs associated with long‐term IVIg use.
Disclosure of conflicts of interest
R. Hadden received personal and departmental payments from CSL Behring and Grifols. I.N. van Schaik reports departmental honoraria for serving on scientific advisory boards for CSL Behring and Baxter. He chaired a steering committee for CSL Behring. He is a member of the Scientific Board of the Kreuth III meeting. F. Eftimov reports grants from Prinses Beatrix Spierfonds, Netherlands Organization for Health Research and Development, and a consulting fee from CSL Behring, UCB Pharma and Aserta Pharma that was paid to the Institution, outside the submitted work. The other authors declare no financial or other conflicts of interest.
References
- 1. van den Bergh PY, Hadden RD, Bouche P, et al European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of CIDP: report of a joint task force of the EFNS/PNS – first revision. Eur J Neurol 2010; 17: 356–363. [DOI] [PubMed] [Google Scholar]
- 2. Nobile‐Orazio E, Cocito D, Jann S, et al Intravenous immunoglobulin versus intravenous methylprednisolone for CIDP: a randomised controlled trial. Lancet Neurol 2012; 11: 493–502. [DOI] [PubMed] [Google Scholar]
- 3. van Schaik IN, Eftimov F, van Doorn PA, et al Pulsed high‐dose dexamethasone versus standard prednisolone treatment for CIDP (PREDICT‐study): a double‐blind, randomised, controlled trial. Lancet Neurol 2010; 9: 245–253. [DOI] [PubMed] [Google Scholar]
- 4. Latov N, Deng C, Dalakas MC, et al Timing and course of clinical response to intravenous immunoglobulin in CIDP. Arch Neurol 2010; 67: 802–807. [DOI] [PubMed] [Google Scholar]
- 5. Eftimov F, Vermeulen M, van Doorn PA, Brusse E, van Schaik IN. Long‐term remission of CIDP after pulsed dexamethasone or short‐term prednisolone treatment. Neurology 2012; 78: 1079–1084. [DOI] [PubMed] [Google Scholar]
- 6. Nobile‐Orazio E, Cocito D, Jann S, et al Frequency and time to relapse after discontinuing 6‐month therapy with IVIg or pulsed methylprednisolone in CIDP. J Neurol Neurosurg Psychiatry 2015; 86: 729–734. [DOI] [PubMed] [Google Scholar]
- 7. van Nes SI, Vanhoutte EK, van Doorn PA, et al Rasch‐built Overall Disability Scale (R‐ODS) for immune‐mediated peripheral neuropathies. Neurology 2011; 76: 337–345. [DOI] [PubMed] [Google Scholar]
- 8. Vanhoutte EK, Latov N, Deng C, et al. Vigorimeter grip strength in CIDP: a responsive tool that rapidly measures the effect of IVIG – the ICE‐study. Eur J Neurol 2013; 20: 748–755. [DOI] [PubMed] [Google Scholar]
- 9. Draak TH, Vanhoutte EK, van Nes SI, et al Changing outcome in inflammatory neuropathies: Rasch‐comparative responsiveness. Neurology 2014; 83: 2124–2132. [DOI] [PubMed] [Google Scholar]
- 10. Eftimov F, Winer JB, Vermeulen M, de Haan R, van Schaik IN. Intravenous immunoglobulin for CIDP. Cochrane Database Syst Rev 2013; 12: CD001797. [DOI] [PubMed] [Google Scholar]
- 11. Kuitwaard K, Hahn AF, Vermeulen M, Venance SL, van Doorn PA. Intravenous immunoglobulin response in treatment‐naive CIDP. J Neurol Neurosurg Psychiatry 2015; 86: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 12. Hughes RA, Donofrio P, Bril V, et al Intravenous immune globulin (10% caprylate‐chromatography purified) for the treatment of CIDP (ICE‐study): a randomised placebo‐controlled trial. Lancet Neurol 2008; 7: 136–144. [DOI] [PubMed] [Google Scholar]
- 13. Lopate G, Pestronk A, Al‐Lozi M. Treatment of CIDP with high‐dose intermittent intravenous methylprednisolone. Arch Neurol 2005; 62: 249–254. [DOI] [PubMed] [Google Scholar]
- 14. Molenaar DS, van Doorn PA, Vermeulen M. Pulsed high dose dexamethasone treatment in CIDP: a pilot study. J Neurol Neurosurg Psychiatry 1997; 62: 388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muley SA, Kelkar P, Parry GJ. Treatment of CIDP with pulsed oral steroids. Arch Neurol 2008; 65: 460–1464. [DOI] [PubMed] [Google Scholar]
- 16. van Koningsveld R, Schmitz PI, Meche FG, et al. Effect of methylprednisolone when added to standard treatment with intravenous immunoglobulin for Guillain‐Barre syndrome: randomised trial. Lancet 2004; 363: 192–196. [DOI] [PubMed] [Google Scholar]
- 17. Hwang SS, Cannom RR, Abbas MA, Etzioni D. Diverticulitis in transplant patients and patients on chronic corticosteroid therapy: a systematic review. Dis Colon Rectum 2010; 53: 1699–1707. [DOI] [PubMed] [Google Scholar]


