Abstract
For children under 12 years of age who have chronic hepatitis C virus (HCV) infection, there are currently no approved treatments with direct‐acting antiviral agents. We therefore evaluated the safety and efficacy of ledipasvir‐sofosbuvir in HCV‐infected children aged 3 to <6 years. In an open‐label study, patients 3 to <6 years old chronically infected with HCV genotype 1 (n = 33) or 4 (n = 1) received weight‐based doses of combined ledipasvir‐sofosbuvir as granules (33.75 mg/150 mg for weights <17 kg or 45 mg/200 mg for weights ≥17 kg) for 12 weeks. The primary endpoint was sustained virological response 12 weeks after treatment (SVR12). For the first 14 patients, intensive pharmacokinetic sampling was done on day 10 of treatment. All patients had been infected through perinatal transmission and were treatment naïve. No patients had known cirrhosis. Ten patients (29%) weighed <17 kg. SVR12 was achieved in 97% of patients (33 of 34); the patient who did not achieve SVR12 was 3 years old and discontinued treatment after 5 days because of an adverse event “abnormal drug taste.” The most common adverse events were vomiting (24% of patients), cough (21%), and pyrexia (21%). No patients experienced a serious adverse event. Intensive pharmacokinetic analysis of 13 patients for whom data were evaluable confirmed that the doses selected were appropriate. Conclusion: Ledipasvir‐sofosbuvir was well tolerated and highly effective in children 3 to <6 years old with chronic HCV infection.
Abbreviations
- AUCtau
area under the
curve over the dosing interval
- Cmax
maximum plasma concentration
- BMI
body mass index
- CHC
chronic hepatitis C
- CI
confidence interval
- DAA
direct‐acting antiviral
- HCV
hepatitis C virus
- IFN
interferon
- NI
nucleotide inhibitor
- NS
nonstructural
- RAS
resistance‐associated substitution
- RBV
ribavirin
- SVR
sustained virological response
- SVR12
sustained virological response 12 weeks after treatment
Worldwide, an estimated 71 million people are chronically infected with hepatitis C virus (HCV), of which an estimated 2.1‐5.0 million are children aged ≤15 years.1, 2, 3 In children, the primary route of HCV infection is perinatal transmission.4 The rate of perinatal transmission of HCV infection is approximately 5%, although rates are higher in the presence of inadequately controlled human immunodeficiency virus (HIV) coinfection or high HCV‐RNA viral loads (>6 log IU/mL).4, 5, 6 Chronic hepatitis C (CHC) likely affects at least 1% of pregnant women globally, although prevalence data at the regional level are incomplete and inconsistent.3, 7 High rates exceeding 6% have been reported in certain hyperendemic regions of countries such as Egypt.8 In the past decade, the incidence of acute hepatitis C has increased in young adults in the United States, including women of child‐bearing potential,9, 10 corresponding with a worsening injection opioid epidemic particularly in rural areas and the Appalachian region.10, 11, 12, 13
Following perinatal transmission of HCV, in the absence of treatment, the rate of spontaneous clearance of the infection is reportedly between 11% and 25% and usually occurs within the first 4 years of life.3, 14 In children who become chronically infected, the course of infection is usually slower than in adults; however, liver disease can progress during early life,15 and cases of cirrhosis,16, 17, 18 hepatocellular carcinoma (HCC),19, 20 and end‐stage liver disease (ESLD) requiring liver transplantation (LT) in childhood21, 22 have been reported.
Although sofosbuvir and ledipasvir‐sofosbuvir have been approved to treat HCV in adolescents aged 12 to <18 years, and in certain countries in younger pediatric patients weighing at least 35 kg, the only approved treatment for younger children remains pegylated (Peg‐IFN) interferon (IFN) plus ribavirin (RBV) for up to 48 weeks. It is therefore recommended by international guidelines that children younger than 12 years with chronic HCV defer treatment until IFN‐free regimens become available.2, 23
We previously reported data indicating that once daily ledipasvir–sofosbuvir (45 mg/200 mg) ± RBV is highly effective (sustained virological response [SVR] of 99% [91 of 92]) in children 6 to <12 years old.24 We now report the evaluation of the safety and effectiveness of weight‐based ledipasvir‐sofosbuvir in children 3 to <6 years old. A subset of patients underwent a period of pharmacokinetic evaluation to determine exposures of ledipasvir, sofosbuvir, and GS‐331007, a metabolite of sofosbuvir.
Patients and Methods
Patients
Eligible patients were 3 to <6 years old. Participants were to have chronic infection with HCV genotype 1, 3, 4, 5, or 6 with plasma HCV‐RNA levels ≥104 IU/mL. Patients could either be HCV treatment naïve or experienced. Patients were required to have an absolute neutrophil count ≥1,500/mm3 and a hemoglobin level ≥11 g/dL (≥12 g/dL for males with HCV genotype 3). Liver biopsy was not required for study entry. Confirmed cirrhosis by previous liver biopsy was recorded. Cirrhosis was excluded either by previous liver biopsy or based on the investigator's assessment of a patient's clinical history. Patients whose cirrhosis status was considered indeterminate by the investigator based on clinical history had “unknown” cirrhosis. Patients were excluded from participating in the study if they had any of the following: decompensated liver disease; infection with hepatitis A, hepatitis B, or HIV; alfa‐fetoprotein level >50 ng/mL; serum creatinine >1.5 mg/dL; estimated glomerular filtration rate (eGFR) <90 mL/min/1.73m2 as calculated by the Schwartz Formula; evidence of HCC or other malignancy; significant cardiovascular, pulmonary, or neurological disease; daily use of nonsteroidal anti‐inflammatory drugs; systemic corticosteroid use for more than 2 weeks (pulmonary/nasal administration was permitted); or psychiatric hospitalization, suicide attempt, or disability resulting from psychiatric illness within the past 5 years. Parents or legal guardians provided written informed consent before patients undertook any study–related procedures. Patients who could read and write provided written assent as determined by the local review board or ethics committee, and investigator discretion.
Study Design
This was a phase 2, multicenter, open‐label study. Duration of treatment was 12 weeks for all patients, with the exception of patients with genotype 1 infection with cirrhosis who were to receive 24 weeks of treatment. Patients received weight‐based doses of ledipasvir‐sofosbuvir fixed‐dose combination granules (33.75 mg/150 mg if their weight was <17 kg or 45 mg/200 mg if their weight was ≥17 kg) once‐daily.
Each packet of granules contained 11.25 mg of ledipasvir and 50 mg of sofosbuvir. Patients weighing <17 kg received three packets per dose, and patients weighing ≥17 kg received four packets per dose. The granules could be administered with or without food. If taken with food, the granules were to be sprinkled on a spoonful of nonacidic soft food, such as pudding or ice cream, and then swallowed without chewing. If taken without food, the granules were to be taken first and then washed down with liquid, not mixed into the liquid.
The first 14 patients enrolled underwent an intensive pharmacokinetic evaluation on the 10th day of treatment in order to confirm the appropriateness of the ledipasvir‐sofosbuvir dosages selected. These 14 patients had to be HCV‐treatment naïve and have no documented cirrhosis (“no” or “unknown” cirrhosis), and at least 4 patients of each weight class (≥17 or <17 kg) were to be enrolled. Following the pharmacokinetic lead‐in phase, the 14 patients continued treatment without interruption, while additional patients were enrolled once the dose was confirmed based on the intensive pharmacokinetic analysis.
Study visits occurred at screening, treatment day 1, and weeks 1, 2, 4, 8, and 12. Follow‐up visits were conducted at posttreatment weeks 4, 12, and 24. Instead of the week 1 study visit, patients in the pharmacokinetic lead‐in phase attended two study visits on day 3 and day 10.
The study protocol was approved by the review board or ethics committee of each institution before study initiation. The study was conducted in accord with the International Conference on Harmonization Good Clinical Practice Guidelines and the Declaration of Helsinki.
Assessments
Efficacy
Screening assessments included measurement of the serum HCV‐RNA level and HCV genotype and subtype. HCV‐RNA levels were measured using the Ampliprep/TaqMan HCV Test (v2.0; Roche Molecular Systems, Inc., Branchburg, NJ), which has a lower limit of quantitation (LLOQ) of 15 IU/mL. HCV genotype and subtype were determined using the Siemens VERSANT HCV Genotype INNO‐LiPA2.0 Assay (Siemens Healthcare Diagnostics, Berkeley, CA). Serum HCV‐RNA levels were evaluated on day 1 of treatment; at treatment weeks 1, 2, 4, 8, and 12; and at follow‐up weeks 4, 12, and 24.
Resistance
During treatment and follow‐up, plasma samples for viral sequencing were collected at the same time points as for HCV‐RNA levels. The HCV nonstructural (NS)5A and NS5B coding regions were amplified by DDL Diagnostic Laboratory (Rijswijk, Netherlands), using standard RT‐PCR technology at baseline for all patients. The PCR products from baseline samples were deep sequenced. Sequencing of the HCV NS5B regions was attempted for all enrolled patients at baseline and with virological failure (VF), if applicable. HCV consensus sequences were generated for all successfully deep‐sequenced samples using a 15% cutoff.
Ledipasvir NS5A resistance‐associated substitutions (RASs) were defined as specific amino acid changes in genotype 1a, 1b, or 4 that conferred >2.5‐fold reduced susceptibility to ledipasvir compared to wild type (for genotype 1a: K24G/N/R M28A/G/T Q30E/G/H/L/K/R/T L31I/F/M/V P32L S38F H58D A92K/T Y93C/F/H/N/S; for genotype 1b: L31I/F/M/V P32L P58D A92K Y93C/H/N/S; for genotype 4: K24G/N/R L28A/G/M/T/V L30E/G/H/K/R/S/T M31F/V P32L S38F P58D/L A92K/T Y93C/F/H/N/S). NS5B NI RASs were defined as follows: S96T, N142T, L159F, E237G, S282A/C/D/E/F/G/H/I/K/L/M/N/P/Q/R/S/T/V/W/Y, C289I/L, L320F/I/V, and V321A/I.
Safety
Physical examinations and vital sign assessments were conducted at screening, at every visit during treatment, and at every follow‐up visit. Data regarding reported adverse events, concomitant medication intake, and clinical laboratory assessments were collected at screening, every treatment visit, and at the follow‐up week 4 visit. Clinical laboratory assessments were also collected at the follow‐up week 12 visit.
Palatability
The palatability of the granule formulation was assessed in 17 patients after the first dose of study drug. Patients were asked whether they were able to taste the drug. Patients who reported being able to taste the drug rated the taste using a 5‐point facial hedonic scale with a correlated analog scale numbered 0‐100, with a higher number indicating better perceived taste (0‐20 “very bad,” 20‐40 “bad,” 40‐60 “neither good or bad,” 60‐80 “good,” or 80‐100 “very good”).
Growth and Development
All patients underwent a Tanner pubertal stage assessment at baseline and at follow‐up week 12.25, 26 Z‐scores were calculated for height, weight, and body mass index (BMI) assessed at baseline and at follow‐up week 12.
Pharmacokinetics
An intensive pharmacokinetic lead‐in phase was performed to confirm the appropriateness of the ledipasvir‐sofosbuvir dose selected for this study. Serial blood samples were collected at the day 10 visit from the first 14 patients enrolled to determine the pharmacokinetics of sofosbuvir, its metabolite GS‐331007, and ledipasvir. Blood samples were collected at 0 (≤30 minutes before dosing), after which patients were provided a standardized meal. Within 5 minutes after consuming the meal, patients were dosed with study drug. Blood samples were collected at 0.5, 1, 2, 3, 4, 5, 8, and 12 hours postdose. The predose (0 minute) results also served as t = 24 hours.
Endpoints
The primary efficacy endpoint was the percentage of patients who achieved sustained virological response 12 weeks after treatment (SVR12), defined as HCV RNA <LLOQ (15 IU/mL) 12 weeks after stopping the study drug. The primary safety endpoint was any adverse event leading to the permanent discontinuation of study drug.
Statistical Analyses
Efficacy, safety, and pharmacokinetics were assessed in all patients who received at least one dose of study drug. The SVR12 rate was calculated with a two‐sided 95% exact confidence interval (CI) based on the Clopper‐Pearson method. Missing SVR values were imputed as a success if bracketed by values that were termed successes. Clinical and laboratory adverse events were summarized using the Medical Dictionary for Regulatory Activities (version 20.1). Z‐scores were calculated for height and weight changes over time.
Pharmacokinetic Analyses
The pharmacokinetic parameters of study drug were estimated by noncompartmental analysis using WinNonlin (V7; Certara USA, Inc., Princeton, NJ). Results were summarized descriptively. Appropriateness of the dose was confirmed by comparing the pharmacokinetic parameters of GS‐331007, sofosbuvir, and ledipasvir from this group with the integrated adult data from phase 2 and 3 clinical studies. The 90% CIs were constructed for the ratio of geometric means of pharmacokinetic parameters area under the curve over the dosing interval (AUCtau), maximum plasma concentration (Cmax), and one‐quarter plasma trough concentration (as appropriate). The equivalence boundary was set as 50%‐200%.
Results
Patient Population
From March 21, 2017 to December 11, 2017, 34 patients were screened, enrolled, and treated at 21 study sites in the United States, United Kingdom, and Australia. Among the 34 patients enrolled, the median age of the patients was 5 years (range, 3‐5), and all (34 of 34; 100%) were HCV‐treatment naïve and infected through perinatal transmission (Table 1). Seventy‐one percent of patients were female, 79% were white, and 71% weighed <17 kg. Eighty‐two percent of patients were infected with HCV genotype 1a and 15% with HCV genotype 1b. One patient had HCV genotype 4. No patient was reported to be with cirrhosis.
Table 1.
Patient Demographics and Baseline Characteristics
| Children 3 to <6 Years Old (N = 34) | |
|---|---|
| Median (range) age, years | 5 (3, 5) |
| Female, n (%) | 24 (71) |
| Race, n (%) | |
| White | 27 (79) |
| Black or African American | 1 (3) |
| Asian | 2 (6) |
| Other | 4 (12) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 6 (18) |
| Region, n (%) | |
| United States (US) | 29 (85) |
| Non‐US | 5 (15) |
| Median (range) weight, kg | 19 (11, 34) |
| Weight <17 kg, n (%) | 24 (71) |
| Median weight for age percentile (IQR) | 64.7 (26.8, 78.9) |
| Median (range) height, cm | 107 (89, 120) |
| Median (range) BMI, kg/m2 | 16 (13, 25) |
| Median BMI for age percentile (IQR) | 71.8 (47.0, 95.8) |
| HCV genotype, n (%) | |
| 1a | 28 (82) |
| 1b | 5 (15) |
| 4 | 1 (3) |
| Median (range) HCV RNA, log10 IU/mL | 6.0 (4.8, 7.3) |
| HCV RNA ≥800,000 IU/mL, n (%) | 19 (56) |
| HCV‐treatment naive, n (%) | 34 (100) |
| Cirrhosis, n (%) | |
| No | 14 (41) |
| Yes | 0 |
| Unknown | 20 (59) |
| Median (range) ALT, U/L | 52 (25, 130) |
| Median (range) eGFR,* (mL/min/1.73 m2) | 171 (99, 220) |
| Mode of HCV infection, n (%) | |
| Perinatal transmission | 34 (100) |
Estimated using Schwartz Formula.
Abbreviations: ALT, alanine aminotransferase; IQR, interquartile range.
Of the 34 patients who initiated treatment, 33 completed treatment (Fig. 1).
Figure 1.
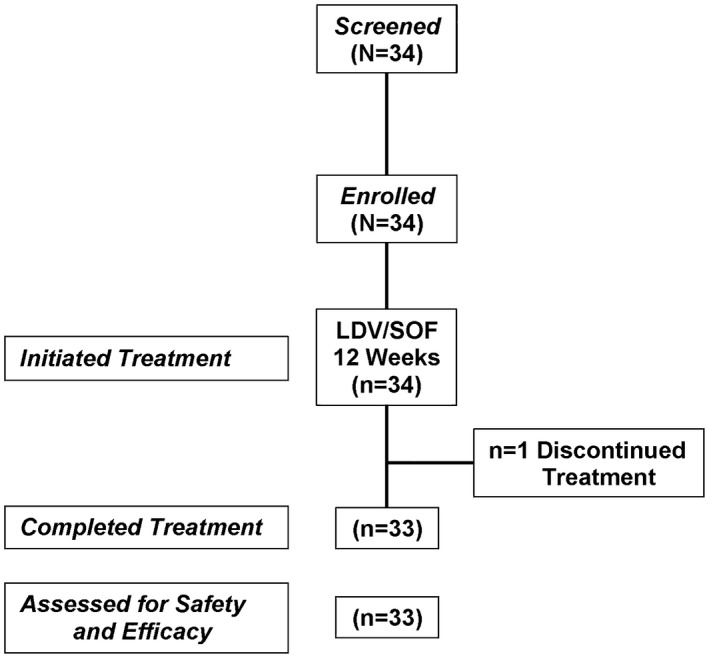
Consort diagram.
Virological Response
Overall, 97% (33 of 34; 95% CI, 85‐100) of patients achieved SVR12 (Table 2). No patients had virological nonresponse or relapse after treatment. One patient discontinued treatment after 5 days of treatment because of an adverse event of “abnormal drug taste.” Subgroup analyses were precluded given that all patients were treatment naïve, none had known cirrhosis, and 97% achieved SVR12.
Table 2.
Treatment Response to Ledipasvir‐Sofosbuvir 12 Weeks
| Children 3 to <6 Years Old (N = 34) | |
|---|---|
| HCV RNA <15 IU/mL, n/n (%) | |
| On treatment | |
| Week 2 | 26/33 (79) |
| Week 4 | 32/33 (97) |
| Week 8 | 33/33 (100) |
| Week 12 | 33/33 (100) |
| After treatment | |
| Week 4 | 33/34 (97) |
| Week 12 (SVR) | 33/34 (97) |
| 95% CI | 85%‐100% |
| VF, n (%) | |
| On treatment | 0 |
| Relapse | 0 |
| Early treatment discontinuation, n (%) | 1 (3) |
Safety
Overall, 25 (74%) patients experienced at least one adverse event. The most commonly reported adverse events were vomiting (24% of patients), cough (21%), and pyrexia (21%; Table 3). No patient experienced a serious adverse event. No patient experienced grade 3 or 4 adverse events.
Table 3.
Adverse Events and Laboratory Abnormalities
| Children 3 to <6 Years Old (N = 34) | |
|---|---|
| No. (%) of patients with any adverse event | 25 (74) |
| No. of grade 3 or 4 adverse events | 0 |
| No. of patients with a serious adverse event | 0 |
| No. of patients with adverse events leading to discontinuation, n (%) | 1 (3) |
| Adverse events in ≥10% patients, n (%) | |
| Vomiting | 8 (24) |
| Cough | 7 (21) |
| Pyrexia | 7 (21) |
| Rhinorrhea | 6 (18) |
| Pharyngitis streptococcal | 4 (12) |
| Grade 3 or 4 laboratory abnormalities, n | 0 |
| Deaths, n | 0 |
Adverse events considered related to study drug by investigators reported in more than 1 patient were abnormal drug taste (n = 3; 9%), fatigue, vomiting, insomnia, and upper abdominal pain (each n = 2; 6%); all were grade 1 and all but one resolved without interruption or discontinuation of study drug. One patient discontinued treatment after 5 days of treatment because of abnormal drug taste. This patient was 3 years old with HCV genotype 1a infection and experienced grade 1 vomiting after administration of study drug on days 2‐4 and grade 1 “abnormal product taste” on days 2‐5. No patient experienced a laboratory abnormality.
Of the 17 patients who assessed the palatability of the oral granule formulation, 12 (71%) rated the taste as palatable (7 did not taste the study drug, and 5 scored the taste at ≥40 points), and 5 patients (29%) rated the taste at <40 points. All 5 patients who rated the taste at <40 points took the granules with food, and all completed treatment.
As assessed by Tanner pubertal staging, study treatment did not affect pubertal development through 12 weeks of posttreatment follow‐up. At baseline, 33 of 34 patients were at Tanner stage 1 for pubic hair and genitalia or breast development. One male patient was at Tanner stage 1 for pubic hair and Tanner stage 2 for genitalia development. At posttreatment week 12, 31 patients with available assessments had no change in Tanner stage. One female patient had an increase from Tanner stage 1 to stage 2 for breast development only.
The median change in Z‐scores for height between baseline and week 12 was –0.01 (range, –0.30, 0.69) and from week 12 to posttreatment week 12 was –0.02 (range, –0.28, 0.62). The median change in Z‐scores for weight between baseline and week 12 was –0.03 (range, –0.64, 0.51) and from week 12 to posttreatment week 12 was –0.1 (range, –0.4, 0.11). The median change in Z‐scores for BMI between baseline and week 12 was –0.09 (range, –1.63, 0.56) and from week 12 to posttreatment week 12 was –0.09 (range, –1.63, 0.56).
Pharmacokinetics
The 14 patients enrolled in the pharmacokinetic lead‐in phase had a median age of 5 years (range, 3‐5), 12 were white (86%), and 9 were female (64%), with a median weight of 20 kg (range, 12‐28). Median BMI was 16 kg/m2 (range, 14‐19) with a corresponding median BMI for age percentile of 73% (range, 2‐98). Of the 14 patients enrolled, 13 were included in the intensive pharmacokinetic analysis. The patient who was not included in the analysis did not take the study drug as required within the 30 minutes of initial sampling. Plasma exposures of sofosbuvir, GS‐331007, and ledipasvir in the 13 patients receiving the weight‐based dosages of 33.75 mg/150 mg or 45 mg/200 mg of ledipasvir‐sofosbuvir were comparable to those observed in adults receiving 90 mg/400 mg of ledipasvir‐sofosbuvir in clinical trials. The 90% CIs for GS‐331007 and ledipasvir AUCtau and Cmax in patients 3 to <6 were within the predefined pharmacokinetic equivalence boundaries of 50%‐200% when compared to adults from the phase 2 and 3 studies (Table 4). Despite the upper bounds of the 90% CI for sofosbuvir AUCtau and Cmax exceeding the upper equivalence boundary, the observed sofosbuvir exposures were within the ranges observed in adult phase 2/3 studies, in which no dose‐response relationships for sofosbuvir exposure and safety metrics were observed. Thus, based on the favorable safety profile of sofosbuvir, the increase in exposure was not deemed clinically significant.
Table 4.
Mean (%CV) Sofosbuvir, GS‐331007, and Ledipasvir Exposures
| Children 3 to <6 Years Old (n = 13) | Adults (n = 2,113)* | Children 3 to <6 Years Old vs. Adults % GMR (90% CI) | |
|---|---|---|---|
| Sofosbuvir† | |||
| AUCtau (ng·h/mL) | 2,500 (16.5)† | 1,380 (34.0) | 188 (143, 246) |
| Cmax (ng/mL) | 1420 (55.5) | 659 (34.0) | 192 (161, 230) |
| GS‐331007 | |||
| AUCtau (ng·h/mL) | 11,700 (29.1) | 12,500 (29.2) | 94 (82, 107) |
| Cmax (ng/mL) | 1,000 (23.3) | 736 (28.2) | 138 (121, 157) |
| Ledipasvir | |||
| AUCtau (ng·h/mL) | 9,320 (35.2) | 8,530 (60.8) | 120 (93, 156) |
| Cmax (ng/mL) | 531 (28.5) | 364 (51.4) | 158 (126, 197) |
Data are presented as mean (% coefficient of variation), unless otherwise specified.
Parameters presented to three significant digits.
Squires and Balistreri27 and n = 1,542 for sofosbuvir parameters in adults.
For sofosbuvir AUCtau in children 3 to <6, n = 3.
Abbreviations: CV, coefficient of variation; GMR, geometric mean ratio.
Resistance Analyses
Deep sequencing of the NS5A and NS5B nucleotide inhibitor (NI) regions were obtained for 33 of 34 patients. The patient who discontinued treatment was not included in the resistance analysis data set. Baseline NS5A RASs were detected in 4 of 33 patients (12%). Each patient had one baseline NS5A RAS: K24R, Q30H, Y93H (each had genotype 1a), and L30R (genotype 4). All 4 patients with baseline NS5A RASs achieved SVR12.
Baseline NS5B NI RASs were detected in 2 of 33 patients (6%), both of whom had genotype 1b infection. Both patients had the NS5B NI baseline RAS L159F, and both achieved SVR12.
Discussion
The global epidemiology of HCV among children 3 to <6 years old is unknown, but the intravenous drug abuse epidemic among adolescents and young adults in the United States raises concern about an increasing number of HCV‐infected newborns at risk of developing chronic infection.27 Although most children chronically infected with HCV are asymptomatic or have mild nonspecific symptoms, 4%‐6% have evidence of advanced fibrosis or cirrhosis and some eventually require LT for ESLD as a consequence of HCV infection.28 Treatment of children with Peg‐IFN plus RBV remains undesirable because of poor tolerability and safety concerns, including potential effects on growth and development.29 However, this regimen is currently the only approved treatment for children under 12 years of age. Therefore, there remains a significant unmet medical need for an all‐oral, IFN‐free, direct‐acting antiviral (DAA) regimen for younger children.
Treatment with direct‐acting antivirals (DAAs) in children aged ≥5 years has been reported on.30 In this study, ledipasvir‐sofosbuvir for 12 weeks was highly effective in treating children 3 to <6 years old with CHC infection, with 97% of participants reaching SVR12 and none experiencing VF. All patients were treatment naïve, without cirrhosis, and all but 1 patient had HCV genotype 1 infection. All patients with baseline NS5A and NS5B NI RASs achieved SVR12. Intensive pharmacokinetic analysis in a subset of patients confirmed that the weight‐based dose selected was appropriate.
Similar to earlier observations in children 6 to <18 years old,24, 31 treatment with ledipasvir‐sofosbuvir was well tolerated in this younger population. The most common adverse events were vomiting, cough, and pyrexia, and no patients experienced serious adverse events or laboratory abnormalities. Although based on a short‐term follow‐up, growth and development, as assessed by Tanner staging and changes in height, weight, and BMI Z‐score, did not appear to be impacted by treatment. The oral granule formulation was considered palatable by most of the patients.
One limitation of this study is that no patient with known cirrhosis was enrolled, which is consistent with a milder evolution of CHC in the pediatric setting compared to adults. Another limitation is that most patients had genotype 1 infection. However the similar exposures, efficacy, and safety observed in the pediatric patients and adult population allows extrapolation to other genotypes and compensated and decompensated cirrhosis.
In summary, ledipasvir‐sofosbuvir was highly effective and safe, and the oral granule formulation was well tolerated in treating children 3 to <6 years with chronic HCV infection. The availability of an all‐oral, DAA regimen for young children with chronic HCV infection would represent a significant advance in the care of patients who currently have limited treatment options.
Acknowledgment
We thank the patients and their families as well as the study‐site personnel. Writing assistance was provided by Jennifer King, Ph.D., of August Editorial, and editorial assistance was provided by Sandra Chen of Gilead Sciences.
Funding for this study was provided by Gilead Sciences, Inc.
EudraCT Number: 2014‐003578‐17.
Potential conflict of interest: Dr. Murray consults for and received grants from Gilead. She received grants from and owns stock in Merck. She received grants from Shire. Dr. Balistreri received grants from Gilead, AbbVie, and Merck. Dr. Rao received grants from Gilead. Dr. Schwarz consults for and received grants from Gilead and Roche/Genentech. She received grants from Bristol‐Myers Squibb. Dr. Rosenthal consults and received grants from Gilead, Retrophin, and Roche. He consults for Audentes and Mirum. He received grants from AbbVie, Bristol‐Myers Squibb, Merck, and Albireo. Dr. Bansal received grants from Gilead and AbbVie. Dr. Narkewicz received grants from and owns stock in AbbVie. He consults for Vertex. He received grants from Gilead. Dr. Brainard is employed by and owns stock in Gilead. Dr. Massetto was employed by and owns stock in Gilead. Dr. Hsueh is employed by and owns stock in Gilead. Dr. Shao is employed by and owns stock in Gilead. Dr. Parhy is employed by and owns stock in Gilead.
References
- 1. The Polaris Observatory HCV Collaborators . Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modelling study. Lancet Gastroenterol Hepatol. 2017;2:161‐176. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461‐511. [DOI] [PubMed] [Google Scholar]
- 3. Nwaohiri A, Schillie S, Bulterys M, Kourtis AP. Hepatitis C virus infection in children: how do we prevent it and how do we treat it? Exp Review Anti Infect Ther 2018;16:689‐694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benova L, Mohamoud YA, Calvert C, Abu‐Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta‐analysis. Clin Infect Dis 2014;59:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother‐to‐infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158:109‐113. [DOI] [PubMed] [Google Scholar]
- 6. Delotte J, Barjoan EM, Berrébi A, Laffont C, Benos P, Pradier C, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern Fetal Neonatal Med 2014;27:664‐670. [DOI] [PubMed] [Google Scholar]
- 7. Spera AM, Eldin TK, Tosone G, Orlando R. Antiviral therapy for hepatitis C: has anything changed for pregnant/lactating women? World J Hepatol 2016;8:557‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khamis HH, Farghaly AG, Shatat HZ, El‐Ghitany EM. Prevalence of hepatitis C virus infection among pregnant women in a rural district in Egypt. Trop Doct 2016;46:21‐27. [DOI] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention . Surveillance for Viral Hepatitis—United States, 2015. Atlanta, GA: Centers for Disease Control and Prevention; 2015. Accessed December 5, 2018. [Google Scholar]
- 10. Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006‐2012. Clin Infect Dis 2014;59:1411‐1419. [DOI] [PubMed] [Google Scholar]
- 11. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C Virus Infection Among Reproductive‐Aged Women and Children in the United States, 2006 to 2014. Ann Intern Med 2017;166:775‐782. [DOI] [PubMed] [Google Scholar]
- 12. Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018;108:175‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koneru A, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011‐2014. MMWR Morb Mortal Wkly Rep 2016;65:705‐710. [DOI] [PubMed] [Google Scholar]
- 14. Dibba P, Cholankeril R, Li AA, Patel M, Fayek M, Dibble C, et al. Hepatitis C in pregnancy. Diseases 2018;6:E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohan P, Barton BA, Narkewicz MR, Molleston JP, Gonzalez-Peralta RP, Rosenthal P, et al. Evaluating progression of liver disease from repeat liver biopsies in children with chronic hepatitis C: a retrospective study. Hepatology 2013;58:1580‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badizadegan K, Jonas MM, Ott MJ, Nelson SP, Perez‐Atayde AR. Histopathology of the liver in children with chronic hepatitis C viral infection. Hepatology 1998;28:1416‐1423. [DOI] [PubMed] [Google Scholar]
- 17. Bortolotti F, Vajro P, Cadrobbi P, Lepore L, Zancan L, Barbera C, et al. Cryptogenic chronic liver disease and hepatitis C virus infection in children. J Hepatol 1992;15:73‐76. [DOI] [PubMed] [Google Scholar]
- 18. Goodman ZD, Makhlouf HR, Liu L, Balistreri W, Gonzalez-Peralta RP, Haber B, et al. Pathology of chronic hepatitis C in children: liver biopsy findings in the Peds‐C Trial. Hepatology 2008;47:836‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez‐Peralta RP, Langham MR Jr., Andres JM, Mohan P, Colombani PM, Alford MK, Schwarz KB. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr 2009;48:630‐635. [DOI] [PubMed] [Google Scholar]
- 20. Strickland DK, Jenkins JJ, Hudson MM. Hepatitis C infection and hepatocellular carcinoma after treatment of childhood cancer. J Pediatr Hematol Oncol 2001;23:527‐529. [DOI] [PubMed] [Google Scholar]
- 21. Barshes NR, Udell IW, Lee TC, O'Mahony CA, Karpen SJ, Carter BA, Goss JA. The natural history of hepatitis C virus in pediatric liver transplant recipients. Liver Transpl 2006;12:1119‐1123. [DOI] [PubMed] [Google Scholar]
- 22. Bortolotti F, Verucchi G, Cammà C, Cabibbo G, Zancan L, Indolfi G, et al. Long‐term course of chronic hepatitis C in children: from viral clearance to end‐stage liver disease. Gastroenterology 2008;134:1900‐1907. [DOI] [PubMed] [Google Scholar]
- 23. AASLD/IDSA HCV Guidance: Recommendations for testing, managing, and treating Hepatitis C. Clinical Liver Disease 2018;12:117-117. [Google Scholar]
- 24. Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez-Peralta RP, et al. Safety and efficacy of ledipasvir‐sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6‐11. Hepatology 2018;68:2158‐2166. [DOI] [PubMed] [Google Scholar]
- 25. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Squires JE, Balistreri WF. Hepatitis C virus infection in children and adolescents. Hepatol Commun 2017;1:87‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu J, Doucette K, Hartling L, Tjosvold L, Robinson J. Treatment of hepatitis C in children: a systematic review. PLoS One 2010;5:e11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jonas MM, Balistreri W, Gonzalez‐Peralta RP, Haber B, Lobritto S, Mohan P, et al. Pegylated interferon for chronic hepatitis C in children affects growth and body composition: results from the pediatric study of hepatitis C (PEDS‐C) trial. Hepatology 2012;56:523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pawlowska M, Sobolewska‐Pilarczyk M, Domagalski K. Hepatitis C virus infection in children in the era of direct‐acting antiviral. World J Gastroenterol 2018;24:2555‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balistreri WF, Murray KF, Rosenthal P, Rosenthal P, Bansal S, Lin CH, Kersey K, et al. The safety and effectiveness of ledipasvir‐sofosbuvir in adolescents 12–17 years old with hepatitis C virus genotype 1 infection. Hepatology 2017;66:371‐378. [DOI] [PubMed] [Google Scholar]


