ABSTRACT
Objective: MicroRNAs (miRNAs) have been demonstrated to engage in the nerve injury, while the effect of microRNA-192-5p (miR-192-5p) on the nerve repair has not yet been well understood. This study is performed to investigate how miR-192-5p affects nerve repair in rats with peripheral nerve injury by regulating X-linked inhibitor of apoptosis protein (XIAP).
Methods: The rat model of left sciatic nerve injury was established, and the expression of miR-192-5p was then detected. A series of experiments were conducted to investigate the role of miR-192-5p on nerve repair in rats with peripheral nerve injury. The expression of apoptosis-related proteins (Caspase-3, Bax and Bcl-2) and nerve repair factors (NGF, BDNF, and GAP-43) was measured. Bioinformatics analysis and dual-luciferase reporter gene assay confirmed the targeting relationship between miR-192-5p and XIAP.
Results: MiR-192-5p inhibition promoted the recovery of sensory function and the recovery and regeneration in rats with sciatic nerve injury. MiR-192-5p inhibition promoted the recovery of muscle atrophy caused by nerve injury. MiR-192-5p inhibition inhibited neuronal apoptosis by affecting the expression of apoptosis-related proteins and promoted the recovery of nerve function by elevating the expression of nerve repair factors induced by peripheral nerve injury. Bioinformatics analysis and dual-luciferase reporter gene assay confirmed that XIAP was a target gene of miR-192-5p.
Conclusion: This study demonstrates that miR-192-5p inhibition can up-regulate the expression of XIAP, decrease the apoptosis of nerve cells, and promote the repair and regeneration of peripheral nerve injury.
KEYWORDS: Microrna-192-5p, nerve repair, peripheral nerve injury
Introduction
Peripheral nerve injury is known as a common global clinical problem, which obviously affects the quality of life of patients and results in enormous financial burden [1]. Patients with severe peripheral nerve injuries often fail to recover their original functional capacity even with the advances in surgical nerve repair [2]. Nerve growth factor (NGF) is the first found member of neurotrophic family, which leads to both the development and phenotype maintenance of the peripheral nervous system (PNS) and ensures the functional integrity of cholinergic neurons in the central nervous system (CNS) [3]. The beneficial functions of NGF on peripheral nerve regeneration have been discussed, while the clinical applications of NGF are still restricted by several factors, including the complexity in NGF delivery and the deleterious side effects of NGF [4]. Schwann cells (SCs) are known to be the principal glial cells in the PNS, which play a significant role in peripheral nerve regeneration due to their interactions with re-growing axons [5]. Based on this premise, accumulating studies have demonstrated that some microRNAs (miRNAs) are able to induce phenotype modulation of SCs in the process of peripheral nerve regeneration [6,7].
In recent years, some studies have indicated the differential expression of several miRNAs following traumatic injury in CNS, such as the brain and spinal cord [8]. Additionally, following spinal nerve ligation (SNL), reduced expression of several sensory organ-specific miRNAs might also be observed in the injured ipsilateral dorsal root ganglion (DRG) [9]. These evidences reveal that miRNAs are found to be essential mediators of plasticity. It is found that an aberrant expression of miRNA was found in an established ischemia (I/R)-reperfusion rat model, and miR-192-5p might be served as vital potential diagnostic markers for I/R-induced kidney injury [10]. A previous study has suggested that X-linked inhibitor of apoptosis protein (XIAP) is a direct target gene for miR-192-5p, and the therapeutic delivery of miR-192-5p by miRNA mimic transfection results in the inhibition of XIAP [11]. Inhibitors of apoptosis (IAPs) are known as a family of proteins that are implicated in many biological functions, such as the regulation of cell proliferation, migration and apoptosis as well as innate immunity and inflammation [12]. Besides, the endogenous XIAP might have a critical function in regulating apoptosis in mammalian cells [13]. Based on the aforementioned evidence, we conducted this present study to investigate the role of miR-192-5p in nerve repair in peripheral nerve injury by targeting XIAP through establishing rar models of left sciatic nerve injury.
Materials and methods
Ethics statement
The experiment was approved by the animal ethics committee of The First Affiliated Hospital of Harbin Medical University. All animal experiments were in line with the Guide for the Care and Use of Laboratory Animal by International Committees.
Experimental animals and grouping
Sixty healthy clean level Sprague-Dawley (SD) rats (weighting 280 ± 20 g, half male and half female) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). With one-week adaptive feeding, the rats were raised in a clean animal room with normal circadian rhythm, a temperature of (22–24)°C, and free access to eating and drinking. All the rats were randomly classified into three groups according to their body weight: sham group, model group (left sciatic nerve injury modeling) and miR-192 inhibitors group (miR-192 inhibitors adenovirus was injected into the protheca of rats after left sciatic nerve injury modeling), with 20 rats in each group. MiR-192 silencing adenovirus was purchased from Shanghai Genechem Co., Ltd. (Shanghai, China).
Establishment of rat model of left sciatic nerve injury
After weighing, the rats in the model group were anesthetized by intraperitoneal injection of 2-% pentobarbital sodium at the dose of 35 mg/kg. The rats were treated with stable breathing, muscle relaxation and no reaction to acupuncture on the plantar, which indicated that the anesthesia was successful. Then, the rats were fixed on the fixed frame, the skin was prepared in the gluteal and femoral junction of the left lower extremity of the rats, and the operation area was disinfected with iodophor. The skin of rats was longitudinally cut with an incision of 1.5 cm to expose the biceps femoris and the sciatic nerve. At 0.5 cm under the femoral nodule, the sciatic nerve trunk was clamped with blood forceps, which was fastened to the third tooth mark. After 10 srelease and 10 s interval (repeated three times), thereby resulting in the width of the 2-mm crush injury. The sciatic nerve was placed in the original place after the measured pressure of 5 kg, and the skin was sutured layer by layer. All clamp operations were performed by one person. The general condition, diet, drinking water, and limb activity were observed with the naked eye.
Pain tolerance threshold and hyperalgesia score (HAS)
The sensory recovery of rats was evaluated by the method of pain tolerance threshold on the 7th and 14th day after operation. The rats were placed in the heat source test box before the test. After the animal adapted to the quiet environment, the radiation light source was placed at the 1/3 back of the foot and plantar, with the start button pressed. When the rats automatically lifted their feet, the timing stopped automatically, and the time on the instrument was recorded as the paw withdrawal latency (PWL). During the whole test, the temperature was 2℃ and the cut off time was set at 21 s to prevent the tissues of rats from burning. Each side was measured 3 times, and the average value was taken. The HAS (the difference value of PWL between the operative side and the control side) was reckoned.
Sciatic nerve functional index (SFI)
The plantar blotting was performed on the 7th and 14th day after operation in each group. The measurement of SFI was based on a reference [14]. SFI was measured using an apparatus as follows. The SFI apparatus was made from wood with 60 × 7 × 20 cm (L, A, H, respectively) dimensions and its floor was covered with white paper. Functional recovery was assessed at 2nd, 4th, 6th, 8th, 10th and 12th weeks after surgery. The lengths of the third toe to its heel (PL), the second toe to the fourth toe (IT) and the first to the fifth toe (TS) were measured on the contralateral normal side (N) and the experimental side (E). SFI was computed by the following modified formula: SFI = – 38.5 (EPL – NPL/NPL) +109.5(ETS – NTS/NTS) +13.3(EIT – NIT/NIT) – 8.8. In this study, SFI oscillates around 0 for normal nerve function, whereas around −100 SFI represents total motor sciatic nerve dysfunction.
Electrophysiological examination
The nerve electrophysiologic examination was performed after plantar blotting on the 7th and 14th day after operation in each group. Six anesthetized rats were randomly selected from each group, and the gross morphology was observed under a microscope, the limbs were properly fixed and the sciatic nerve of the left lower extremity was exposed about the length of 2.0 cm, including end-to-end anastomosis of the nerve. The compound muscle action potential (CMAP) was measured by NHK30-Medelec Synergy V type electrodiagnostic equipment (Medelec Synergy, Oxford Instruments, Surrey, UK). The latency and amplitude were measured and recorded with the stimulation time of 0.1–0.2 ms and the stimulation frequency of 1 Hz. The latent period and amplitude of left sciatic nerve were measured by computer display to calculate the motor nerve conductive velocity (MNCV). MNCV was calculated as the distance between two stimulation electrodes (m)/latency difference of action potential (s).
Wet weight ratio of gastrocnemius muscle
The gastrocnemius muscle was removed completely according to Chen Desong’s method after electrophysiological examination and the determination of photothermal pain tolerance threshold at 7 and 14 days post operation. The wet weight of gastrocnemius muscle was immediately weighed by an electronic balance, and the recovery rate was calculated by using the healthy side as a control.
Sample collection
The sciatic nerve and the corresponding spinal cord were removed 14 days after operation. The anesthetic method was the same with the modeling method. After anesthesia, the rats were fixed in supine position, the abdominal and thoracic incision was performed from the lower jaw to the lower abdomen, the ribs and septum muscles were cut off, and the beating heart was exposed. The left ventricle was punctured from the tip of the left heart with a NO.11 needle. The left ventricle was inserted into the aorta along the left ventricle with a soft vein indwelling catheter, and then the right-atrial appendage was cut open. The blood in tissue was rinsed quickly with 250-mL normal saline, then injected quickly with 100 mL 4% paraformaldehyde solution and injected slowly with 10–150 mL 4% paraformaldehyde solution until the rat’s tail tipped up, the body gradually stiffened to white color. The prone position of the rat was fixed on the operating table immediately after perfusion. The dorsal muscles were exposed and separated from the back to the skeleton along the middle of the spine. The pedicle plate and spinous process were cut open and the lumbar spinal cord was exposed and stripped. The spinal cord of L4-5-S1 segment was removed along the sciatic nerve and the sciatic nerve of the upper and lower 2 cm of the clamp injury was removed. The specimens were partly fixed with 2.5% glutaraldehyde for an electron microscope examination, partly fixed with 4% paraformaldehyde and made into paraffin sections for pathological examination, and some samples were frozen at −80°C for expression detection.
Hematoxylin-eosin (HE) staining
Three or more slices of left sciatic nerve section from each rat of each group were selected. The paraffin sections were dewaxed with xylene and hydrated with gradient ethanol (100%, 95%, 80%, 70%, 50%). Then, the sections were immersed into 0.5-1% eosin solution for 10-min conterstaining, the cytoplasm was dyed to red, and dehydrated twice in 95% ethanol with 1 min each time, and twice in 100% ethanol with 5 min each time. The sections were cleared with xylene twice with 10 min each time and sealed with neutral gum. After HE staining, the regeneration of nerve fibers of rats in each group was observed by light microscope pathological examination and photographed.
Observation of transmission electron microscope (TEM)
The injured sciatic nerve and the corresponding L4-5 segments of spinal cord were taken immediately after the rats were perfused and then prefixed in 2.5% glutaraldehyde solution at 4°C. The specimens were rinsed by phosphate buffer saline (PBS) for 10 min × 3 times, then fixed in 2% osmium for 2 h, then rinsed with PBS for 10 min × 3 times, followed by acetone gradient dehydration, resin Epon618 immersion, embedding, and then preparing 1 mm semi-thin sections. After methylene blue and basic fuchsin staining, the ultrathin sections (Leica, Wetzlar, Germany) were prepared after observed by an optical microscope. The ultrastructural changes of injured sciatic nerve and motor neurons in the corresponding segment of spinal cord were observed under a TEM (Leica, Wetzlar, Germany).
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) staining
Three or more slices of left sciatic nerve sections from each rat of each group were dehydrated with gradient ethanol, rinsed with PBS for 3 min × 3 times. The slices of sciatic nerve tissue were immersed into 0.1 mol/L sodium citrate (200 mL) and heated in a 350 W microwave for 5 min. The slices were taken out immediately and poured into distilled water with a volume of 80 mL. After natural cooling, the slices were rinsed with PBS for 3 times. After the slide was dried, the samples were supplemented with the TUNEL reaction mixture (Roche, Basle, Switzerland), and the sealing membrane was also supplemented to react for 1 h in a dark humidity box at 37°C. Afterward, the samples were supplemented with converter-peroxidase (POD), and the sealing membrane was also supplemented to react for 30 min in a dark humidity box at 37°C. Next, the samples were added with diaminobenzidine (DAB) substrate and then placed at room temperature for 15 min. The samples were re-stained with hematoxylin, treated by flowing water, dehydrated by gradient ethanol, cleared by xylene and sealed by neutral balsam. Those with brown granules in the nucleus were judged to be positive, and the number of positive cells in L5 dorsal root ganglion was counted.
Immunohistochemical staining
Three or more slices of left sciatic nerve sections from each rat of each group were selected, followed by conventional xylene dewaxing, gradient alcohol dehydration, antigenic repair with 100 μL 0.2-mg/mL protein kinase K solution at room temperature for 10 min. After that, 3% peroxidase blocking solution was added and incubated at room temperature for 10 min. Then, 50-μL nonimmune sheep serum was added and incubated at room temperature for 30 min, the serum was dumped. The tissues were added with NGF (1:250), BDNF (5 µg/mL) and GAP-43 (1:500) (all from Abcam, Cambridge, MA, USA) and incubated overnight at 4°C. The negative control (NC) did not add the primary antibody which was replaced by antibody dilution, and then the secondary antibody goat anti-rabbit IgG (1:1000, Abcam, Cambridge, MA, USA) was added and incubated at room temperature for 30 min after 20 min incubation with polymerase adjuvant. After that, the tissues were supplemented with 0.05% diaminobenzidine (DAB) for coloration, counterstained with hematoxylin and sealed. Five fields of vision were randomly selected for each slice and observed. Image-Pro image processing software (Media Cybernetics, Maryland, USA) was used for semi-quantitative analysis of the images. The average optical density (OD) (the percentage of the positive target OD and positive area under each visual field) represented the expression of protein.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
The one-step Trizol method (Invitrogen, Carlsbad, CA, USA) was employed for the extraction of the total RNA nervous tissues. The ultraviolet (UV) analysis and formaldehyde gel electrophoresis were performed to confirm the high-quality RNA. Next, 1-μg RNA was reversed to complementary DNA (cDNA) by avian myeloblastosis virus (AMV) (Takara, Dalian, China). The primers of PCR were designed and synthesized by Invitrogen company (Carlsbad, CA, USA) (Table 1), with U6 and β-actin as internal references. The amplification conditions were as follows: pre-denaturation (94°C) for 5 min, with 40 cycles of denaturation (94°C) for 40 s, annealing (60°C) for 40 s, and extension (72°C) for 1 min, followed by extension (72°C) for 10 min. The products of PCR were verified by agarose gel electrophoresis. The data were analyzed by 2−ΔΔCt, which represented the multiple proportion of the target gene in the experimental group to that of the control group. The formula was as follows: ΔΔCt = (ΔCt target gene – ΔCt reference gene)experimental group – (ΔCt target gene – ΔCt reference gene)control group.
Table 1.
Primer sequence.
| Gene | Sequence |
|---|---|
| miR-192-5p | F: 5ʹ- TGACCAGAGACACCGAACGTCCTTGAGATGG-3’ |
| R: 5ʹ- AACTGCGGCAACTCTTCAGTGTCGTGGAGTG −3’ | |
| U6 | F: 5ʹ- GCTTCGGCAGCACATATACTAAAAT −3’ |
| R: 5ʹ- CGCTTCACGAATTTGCGTGTCAT −3’ | |
| XIAP | F: 5ʹ- GTCTGCCCGCTTATTCTGCACA −3’ |
| R: 5ʹ- CATCGTCTTCCACCTCTTTCA −3’ | |
| β-actin | F: 5ʹ- AGGCCAACCGCGAGAAGATGAC-3’ |
| R: 5ʹ- GTACATGGTGGTGCCGCCAGAC-3’ |
F, forward; R, reverse; miR-192-5p, microRNA-192-5p; XIAP, X-linked inhibitor of apoptosis protein.
Western blot analysis
The protein of nervous tissues was extracted, with the determination of protein concentration conducted according to the bicinchoninic acid (BCA) protein assay kit (Wuhan Boster Biological Technology Co., Ltd., Wuhan, Hubei, China). The extracted protein that supplemented with the uploading buffer was boiled for 10 min at 95°C, with 30 μg for each well. Subsequently, 10% polyacrylamide gel electrophoresis (Wuhan Boster Biological Technology Co., Ltd., Wuhan, Hubei, China) was used to separate proteins. The proteins were then transferred onto polyvinylidene fluoride (PVDF) membranes. Afterward, the membranes were blocked with 5% bovine serum albumin (BSA) at room temperature for 1 h, and then added with the primary antibodies of caspase-3 (1 µg/mL), Bax (1:1000), Bcl-2 (1:1000) and β-actin (1:5000) (all from Abcam, Cambridge, MA, USA) and incubated at 4°C overnight, followed by three washes with Tris-buffered saline with Tween 20 (TBST). Besides, corresponding secondary antibodies (Shanghai Miaotong Biotechnology Co., Ltd., Shanghai, China) were added and incubated for 1 h. Chemiluminescence reagents were used to develop images. β-actin was regarded as an internal reference. The images of the gels were captured in a Bio-Rad Gel Doc EZ Imager (Bio-Rad, Hercules, CA, USA), and the gray values of target protein bands were analyzed by an ImageJ software (National Institutes of Health, Bethesda, Maryland, USA).
Bioinformatics analysis and double luciferase reporter gene assay
Bioinformatics website Starbase http://starbase.sysu.edu.cn/index.php was used to predict the binding site between miR-192-5p and XIAP. The binding relationship between miR-192-5p and XIAP was verified by dual-luciferase reporter gene assay. The wild type (wt) 3ʹ-UTR and mutant (mut) 3ʹ-UTR of XIAP were amplified. The primer sequence was prepared by Shanghai Sangon Bioengineering Co., Ltd. (Shanghai, China), and made endonuclease cleavage with XhoI and NotI and ligated with T4 DNA ligase to the psi-Cpsi-CHECK-2 vector (Promega, USA) so as to construct XIAP-wt and XIAP-mut plasmids. HEK-293T cells (Cell bank of Chinese Academy of Sciences, Shanghai, China) were inoculated in a 24-well plate. The 200 nmol/L miR-192-5p NC or miR-192-5p mimic and 100-ng plasmid (XIAP-wt or XIAP-mut) were co-transfected into 293T cells with reference of Ribo FECTTMCP transfection reagent (Guangzhou Ribo Biotechnology Co., Ltd., Guangzhou, China) and cultured in 24-well plate for 48 h. The cells were lysed to detect the luciferase activity. Three parallel wells were set in each group, and blank control was also set up. According to the instructions of the dual-luciferase reporter gene assay detection kit (Beyotime Biotechnology Institute, Shanghai, China), the cells were washed with PBS and lysed with 200 μL lysate for 15 min. The luminescent value was detected by Tecan Infinite@200 Pro multifunctional microplate reader Tecan Trading Co., Ltd. (Shanghai, China), and the luciferase activity relative light unit (RLU) of the experimental group and the control group was determined by standardizing the luciferase value of renilla luciferase with stable expression.
Statistical analysis
All the data were analyzed by SPSS 21.0 software (IBM Corp, Armonk, NY, USA). The Kolmogorov–Smirnov test verified that the data in this study had a normal distribution. Measurement data were expressed as mean ± standard deviation. The t-test was used for the comparison between the two groups, and one-way analysis of variance (ANOVA) was used for the comparison among multiple groups. The Fisher’s least significant difference t-test (LSD-t) was employed for pairwise comparison after ANOVA. The level of significance was P < 0.05.
Results
miR-192-5p inhibition promotes the recovery of sensory function in rats with sciatic nerve injury
The sensory recovery of rats was evaluated by the method of pain tolerance threshold and HAS. As shown in Table 2, no significant difference was found in pain tolerance threshold and HAS in the sham group on 7 and 14 days after operation (P > 0.05). The pain tolerance threshold and HAS in the model group and the miR-192-5p inhibitors group were higher than those in the sham group. However, the pain tolerance threshold and HAS of the two groups recovered in varying degrees with the prolongation of injury time. On the 7th day after operation, there was no significant difference in pain tolerance threshold and HAS between the model group and the miR-192-5p inhibitors group (P > 0.05). On the 14th day after operation, the pain tolerance threshold and HAS in the miR-192-5p inhibitors group were significantly lower than those in the model group (both P < 0.05). The results suggest that miR-192-5p inhibition can promote the recovery of sensory function in rats with sciatic nerve injury.
Table 2.
The pain tolerance threshold and HAS in rats of each group at different time points.
| PWL |
HAS |
||||
|---|---|---|---|---|---|
| Group | n | At 7th-day after operation | At 14th-day after operation | At 7th-day after operation | At 14th-day after operation |
| Sham group | 5 | 7.69 ± 0.68 | 7.22 ± 0.41 | 2.73 ± 0.51 | 2.34 ± 0.11 |
| Model group | 5 | 10.28 ± 0.40* | 9.56 ± 0.33* | 5.62 ± 0.39* | 4.58 ± 0.12* |
| miR-192-5p inhibitors group | 5 | 9.62 ± 0.62* | 7.17 ± 0.56*# | 4.96 ± 0.96* | 3.16 ± 0.31*# |
HAS, hyperalgesia score; PWL, paw withdrawal latency. * P < 0.05 vs the sham group; # P < 0.05 vs the model group. One-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
miR-192-5p inhibition promotes the recovery and regeneration of sciatic nerve injury in rats
The plantar blotting was performed on the 7th day and 14th day after operation. The SFI was calculated to evaluate the recovery of limb function in rats with sciatic nerve injury according to the aforementioned formula. As shown in Table 3, on the 7th day after operation, the limb movement of rats in the sham group was large and normal, and SFI was almost normal. In the model group and miR-192-5p inhibitors group, the feeding and drinking water were increased. The action response of rats in the miR-192-5p inhibitors group was a little faster than that in the model group, toe flexion improved slightly with stomp drooping, and the foot still walked with the acrotarsium. According to plantar blotting, SFI showed that the miR-192-5p inhibitors group was slightly better than that in the model group except for the sham group, but there was no significant difference between each group (P > 0.05). On the 14th day after operation, the limb movement and SFI were normal in the sham group. Compared with the model group with slower recovery, the activity of rats in the miR-192-5p inhibitors group was significantly increased, the response was relatively sensitive and the speed of movement was faster. From the condition of the rats, the function of the limbs in the miR-192-5p inhibitors group was better than that in the model group, and SFI was also better than that in the model group (P < 0.05). The results suggest that miR-192-5p inhibition can promote the recovery and regeneration of sciatic nerve injury in rats.
Table 3.
The SFI in rats of each group at different time points.
| Group | n | At 7th-day after operation | At 14th-day after operation |
|---|---|---|---|
| Sham group | 5 | −2.10 ± 1.35 | −1.27 ± 0.9 |
| Model group | 5 | −69.16 ± 4.68* | −49.72 ± 5.4* |
| miR-192-5p inhibitors group | 5 | −62.47 ± 6.77* | −26.42 ± 4.35*# |
SFI, sciatic nerve functional index. * P < 0.05 vs the sham group; # P < 0.05 vs the model group. One-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
miR-192-5p inhibition promotes regeneration and repair after sciatic nerve injury in rats
The electromyogram (EMG) was detected at the 7th and 14th day after operation and the change of motor nerve conduction velocity (MNCV) was observed dynamically in each group. As listed in Table 4, the MNCV of rats in the sham group was normal on the 7th and 14th day after operation. The MNCV of rats in the model group and the miR-192-5p inhibitors group decreased significantly but recovered with the prolongation of injury time. On the 7th day after operation, the MNCV of rats in the model group and the miR-192-5p inhibitors group was significantly lower than that in the sham group, but there was no significant difference between the model and the miR-192-5p inhibitors groups (P > 0.05). On the 14th day after operation, the MNCV of rats in the miR-192-5p inhibitors group was significantly higher than that in the model group (P < 0.05). The results suggest that miR-192-5p inhibition can promote the regeneration and repair of the injured sciatic nerve and accelerate the recovery of the injured nerve electrophysiological function.
Table 4.
The MNCV in rats of each group at different time points.
| Group | n | At 7th-day after operation | At 14th-day after operation |
|---|---|---|---|
| Sham group | 5 | 63.84 ± 3.47 | 66.94 ± 4.50 |
| Model group | 5 | 19.03 ± 2.15* | 22.94 ± 4.82* |
| miR-192-5p inhibitors group | 5 | 24.84 ± 2.1* | 57.15 ± 4.64*# |
MNCV, motor nerve conductive velocity. * P < 0.05 vs the sham group; # P < 0.05 vs the model group. One-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
miR-192-5p inhibition promotes the recovery of muscle atrophy caused by nerve injury
The gastrocnemius muscle was removed completely by Chen Desong’s method on the 7th and 14th day after operation, and the weight of gastrocnemius muscle was weighed. The recovery rate of each group was calculated by using the healthy side as the control. The results in Table 5 showed that on the 7th day after operation, except for the sham group, there was no significant difference in the recovery rate of wet weight of gastrocnemius muscle between the model group and the miR-192-5p inhibitors group (P > 0.05), and rats in both groups showed varying degrees of muscle wilting on the injured side. On the 14th day after operation, from the recovery rate of wet weight of gastrocnemius muscle, the miR-192-5p inhibitors group was obviously better than that in the model group (P< 0.05). The results suggest that miR-192-5p inhibition can promote the recovery of muscle atrophy caused by nerve injury.
Table 5.
The wet weight recovery rate of gastrocnemius muscle (%) in rats of each group at different time points.
| Group | n | At 7th-day after operation | At 14th-day after operation |
|---|---|---|---|
| Sham group | 5 | 98.11 ± 1.92 | 99.04 ± 1.84 |
| Model group | 5 | 48.73 ± 3.49* | 52.64 ± 5.6* |
| miR-192-5p inhibitor group | 5 | 54.56 ± 5.24* | 64.15 ± 4.64*# |
* P < 0.05 vs the sham group; # P < 0.05 vs the model group. One-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
miR-192-5p inhibition changes pathological state
The results of HE staining (Figure 1(a)) suggested that on the 14th day after operation, the sciatic nerve fibers in the sham group were arranged neatly and closely, the myelin sheath was clear and the interstitial was evenly stained. In the model group, the nerve fibers were denatured, necrosis was obvious, most of myelin sheath disintegrated with left necrotic contour, axon disappeared completely, and the nerve intima, fascicle and adventitia still existed. The degree of nerve fiber lesion in the miR-192-5p inhibitors group was lighter than that in the model group, axon swelling and degeneration were lighter, myelin sheath was only partially lost, new nerve fibers appeared and Schwann cells had obviously proliferated. The results suggest that miR-192-5p inhibition can reduce the degree of nerve fiber lesion after sciatic nerve injury.
Figure 1.
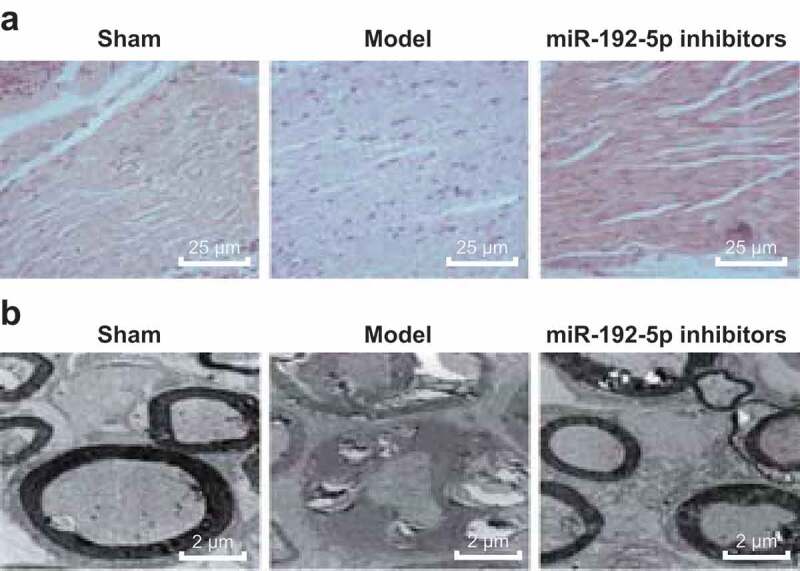
Pathological and ultrastructural changes of nerve tissue in rats in each group. N = 5. (a). The pathological changes of nerve tissue of rats in each group were observed by HE staining. (b). Ultrastructure of nerve tissue of rats observed by a TEM .
The recovery of injured nerve tissue in rats was observed by a TEM (Figure 1(b)). On the 14th day after operation, the nerve fibers in the sham group were closely arranged, axonal microfilaments, microtubules, mitochondria were abundant, and the lamellar structure was clear. In the model group, part of the nuclear membrane was ruptured, nucleoli disappeared, mitochondria swelled, ruptured, cristae disappeared, other organelles degenerated and ruptured seriously. Compared with the model group, the cells in miR-192-5p inhibitors group recovered obviously, the nuclear membrane was slightly disorganized, a few mitochondria were swollen, and the cristae were not decreased significantly. The results suggest that miR-192-5p inhibition can obviously inhibit the degree of degeneration and necrosis of peripheral nerve and promote its regeneration and repair.
miR-192-5p inhibition suppresses neuronal apoptosis of nerve cells
Based on the results of TUNEL staining (Figure 2(a)), compared with the sham group, the number of apoptotic neurons in the model group and the miR-192-5p inhibitors group was significantly increased (P < 0.05). Relative to the model group, the number of neuronal apoptosis in the miR-192-5p inhibitors group was significantly decreased (P< 0.05). The results suggest that miR-192-5p inhibition can inhibit the apoptosis of the corresponding spinal motor neurons induced by peripheral nerve injury and has a protective effect on the neurons.
Figure 2.
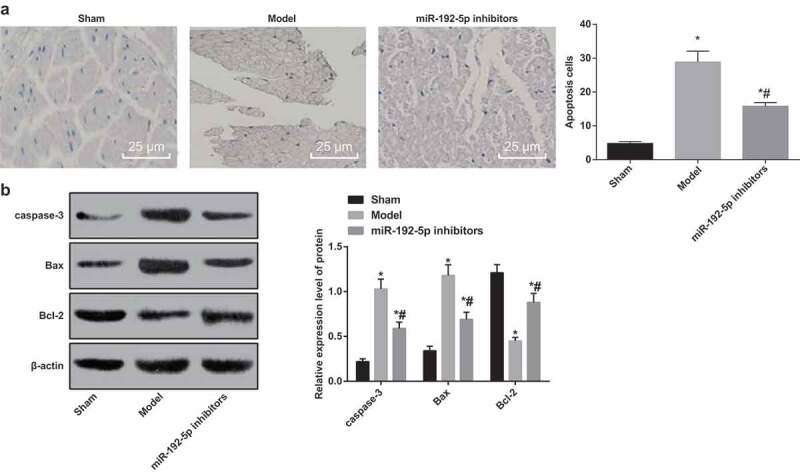
Survival and apoptosis of neurons in rats in each group. N = 5. (a). Detection of neuronal apoptosis by TUNEL staining in rats. (b). Expression of apoptosis-related protein in rat’s nerve tissue detected by western blot analysis. * P < 0.05 vs the sham group; # P < 0.05 vs the model group. One-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
The results of western blot analysis suggested that the expression of caspase-3 and Bax in nerve tissue of rats in the model group and the miR-192-5p inhibitors group was significantly higher than that in the sham group, and the expression of Bcl-2 was significantly down-regulated (all P < 0.05). The expression of caspase-3 and Bax in the nerve tissues of rats in the miR-192-5p inhibitors group was significantly lower than that in the model group, and Bcl-2 was significantly up-regulated (all P < 0.05) (Figure 2(b)). It suggests that miR-192-5p inhibition can inhibit neuronal apoptosis by affecting the expression of apoptosis-related protein.
miR-192-5p inhibition promotes the recovery of nerve function by facilating the expression of nerve repair factors induced by peripheral nerve injury
Immunohistochemical SP method was used to detect the expression of nerve repair factors NGF, BDNF and GAP-43 in rats. In the sham group, there was a little expression in sciatic nerve, the nucleus of positive neurons was blue, and the cytoplasm was stained brown. Compared with the sham group, NGF, BDNF and GAP-43 expression in the cytoplasm of injured sciatic nerve cells raised in the model group (all P < 0.05), indicating that there was a positive significance of NGF, BDNF and GAP-43 on the repair or regeneration of injured sciatic nerve. While in relation to the model group, NGF, BDNF and GAP-43 expression in the cytoplasm of injured sciatic nerve cells further raised in the miR-192-5p inhibitors group (all P < 0.05) (Figure 3). The result suggested that down-regulated miR-192-5p facilitated the repair of injured sciatic nerve may be associated with increased expression of these three factors.
Figure 3.
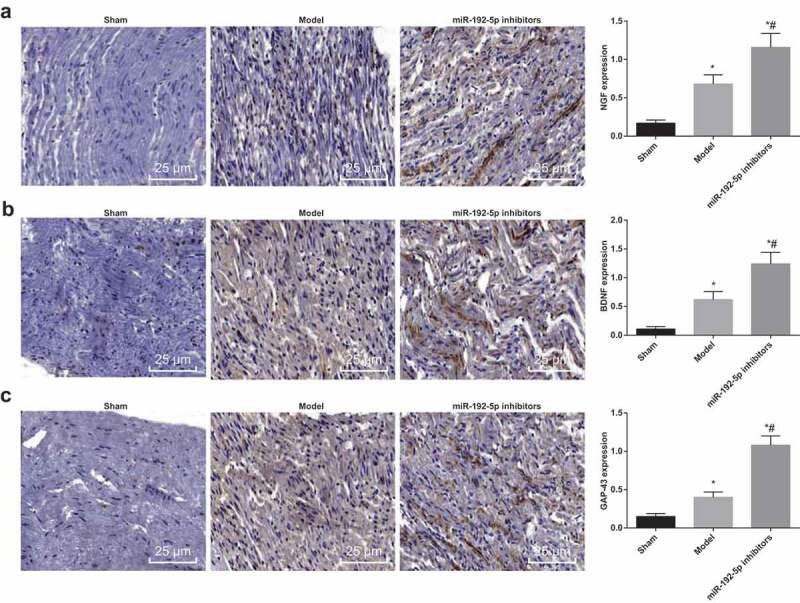
Changes of nerve repair factors in rats in each group. N = 5. (a). Immunohistochemical detection of NGF positive expression in nerve tissue of rats (× 200). (b). Immunohistochemical detection of BDNF positive expression in nerve tissue of rats. (c). Immunohistochemical detection of GAP-43 positive expression in nerve tissue of rats (× 200). * P < 0.05 vs the sham group; # P < 0.05 vs the model group. One-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
XIAP is a target gene of miR-192-5p
The expression of miR-192-5p and XIAP in rats was determined by RT-qPCR and western blot analysis. The results suggested that (Figure 4(a–b)) in contrast to the sham group, the expression of miR-192-5p was up-regulated and the expression of XIAP was down-regulated in the model group (both P < 0.05). Compared with the model group, the expression of XIAP in the nerve tissue of the miR-192-5p inhibitors group was significantly increased. The results suggest that the expression of XIAP was down-regulated by nerve injury and that miR-192-5p inhibition could up-regulate the expression of XIAP.
Figure 4.
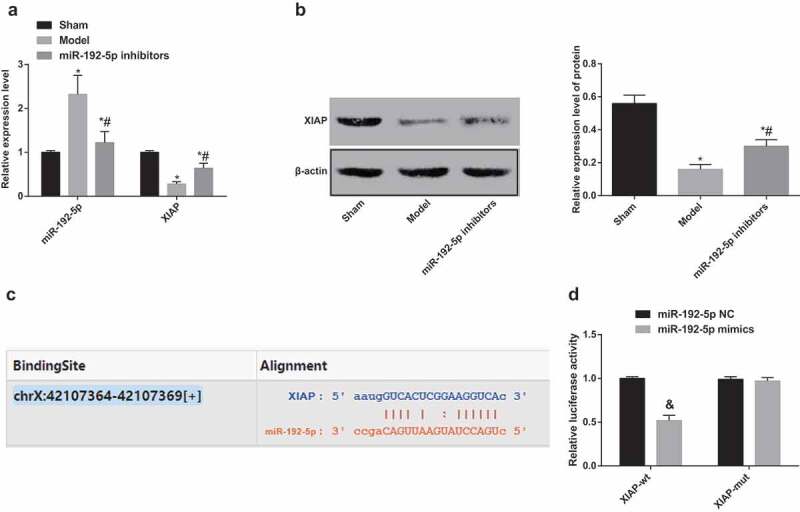
The relationship between miR-192-5p and XIAP. N = 5. (a). Detection of miR-192-5p and XIAP expression in nerve tissue of rats by RT-qPCR. (b). Detection of XIAP protein expression in nerve tissue of rats by western blot analysis. (c). Predicted binding site of miR-192-5p and XIAP by bioinformatics information. (d). Targeting relationship between of miR-192-5p and XIAP by double luciferase reporter gene assay. * P < 0.05 vs the sham group; # P < 0.05 vs the model group; & P < 0.05 vs the NC group. Comparisons between two groups were conducted by t test, one-way ANOVA was used for data analysis, and LSD-t was used for pairwise comparison after ANOVA.
Bioinformatics information predicted that miR-192-5p could directly target sequences of XIAP 3ʹ-UTR from the targetscan.org website (Figure 4(c–d)). Compared with the XIAP-wt and miR-192-5p NC co-transfection group, luciferase activity was significantly decreased in the XIAP-wt and miR-192-5p mimic co-transfection group (P < 0.05). There was no significant difference in the XIAP-mut and miR-192-5p mimic co-transfection group relative to that in the XIAP-mut and miR-192-5p NC co-transfection group (P > 0.05). The results suggest that there was a targeted regulatory relationship between miR-192-5p and 3ʹ-UTR of XIAP.
Discussion
Recently, accumulative evidence has demonstrated that miRNA is a potential biomarker for the detection of various diseases at an early stage [15,16]. However, the expression profiles of these biomarkers differed in different cohorts [17–19]. Nowadays, a large number of therapeutic strategies have been found to ameliorate the functional outcomes of peripheral nerve regeneration. This study is, therefore, focused on the possible regulatory role of miR-192-5p during peripheral nerve injury and regeneration through the regulation of XIAP. Collectively, the results of this study demonstrated that downregulated miR-192-5p can up-regulate the expression of XIAP, decrease the apoptosis of nerve cells, and promote the repair and regeneration of sciatic nerve injury.
One of the most significant findings in this present study was that the expression of miR-192-5p was increased and the expression of XIAP was decreased significantly in sciatic nerve injury rats. In accordance with the results in our study, miR-192 expression was found to be highly expressed in some liver disease and in paints with cancers, and the increased expression was related to tumor metastasis and poor survival [20,21]. In a recent work, it was shown that miRNAs modulated the peripheral nerve system by regulating the migration and proliferation of SCs, and miRNAs expressed in peripheral nerves might able to provide a potential therapeutic target for peripheral nerve injury or repair [22]. Similarly, a study has provided a new insight into the regulation of peripheral nerve regeneration by let-7, which suggested a potential therapy for repair of peripheral nerve injury [23]. XIAP is a member of IAP proteins, which are expressed by neurons in the CNS, and XIAP was absent in dying neurons indicating the protein has a protective role in kainic acid-induced neurodegeneration [24]. It is reported that the downregulation of XIAP inhibited cellular viability, induced apoptosis and increased the anti-leukemia effects of the chemotherapeutic drug doxorubicin in K562 cells [25].
In our study, we also found out that XIAP was a target gene of miR-219-5p. In line with the results in our study, a study has suggested that the XIAP 3ʹ-UTR mRNA contained a miR-219-5p-complementary binding site, and miR-219 might regulate the expression of XIAP through binding to the 3ʹ-UTR of XIAP [26]. Several other miRNAs such as miR-137 is capable of targeting the XIAP gene via a systematic screen, and miR-137 reduced XIAP expression via the special sites in the XIAP 3ʹUTR [27]. Furthermore, in another study, Zeb2, a recently discovered anti-apoptotic target gene of miR-219-5p, is elevated in experimental liver injury and after exposition of Hepal-6 cells to H2O2, suggesting a molecular connection between cell protection and miR-192-5p downregulation [28].
Furthermore, our study suggested that miR-192-5p inhibition can promote the recovery of nerve function by inhibiting the expression of nerve repair factors induced by peripheral nerve injury and inhibit neuronal apoptosis by affecting the expression of apoptosis-related proteins. G Zhang et al. have supported that overexpression of miR-192-5p significantly induced apoptosis in H9c2 cells after hypoxia/reoxygenation (H/R), which was accompanied by a remarked increase in the ratio of Bax/Bcl-2 [29]. In Li et al.’s study, they held the views that miR-192 overexpression promoted apoptosis, which was accompanied by an elevation in caspase-3 activity and Bax/Bcl-2 ratio [30]. DNF promotes motoneuron survival and enhances myelination following injury, increases neurite outgrowth, and acts as a synaptotrophin to promote terminal branching at the neuromuscular junction and synapse remodeling [31]. Evidence has indicated that there is a regulatory negative feedback loop between BDNF and miRNAs; BDNF treatment enables to stimulate neuronal miRNA expression, and miRNAs generally function to suppress expression of BDNF [32]. Also, overexpression of miR-221 promoted neuronal differentiation of PC12 cells without NGF treatment and also induced neuronal differentiation caused by low-dose NGF [33].
In conclusion, this present study provides evidence that in sciatic nerve injury rats, the expression of miR-192-5p was up-regulated and the expression of XIAP was down-regulated. In addition, downregulated miR-192-5p could up-regulate the expression of XIAP, decrease the apoptosis of nerve cells, and promote the repair and regeneration of sciatic nerve injury. This study provides a new insight for the treatment of sciatic nerve injury or even peripheral nerve injury, while the specific mechanisms of miR-192-5p and XIAP in the repair and regeneration of peripheral nerve injury need further confirmation.
Acknowledgments
We would like to acknowledge the reviewers for their helpful comments on this paper.
Authors’ contributions
Guarantor of integrity of the entire study: Xintao Cui
Study design: Guangwei Guan
Experimental studies: Xing Liu, Ying Dong
Manuscript editing: Zhenyu Zhang
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethical statement
This study was approved and supervised by the animal ethics committee of The First Affiliated Hospital of Harbin Medical University. The treatment of animals in all experiments conforms to the ethical standards of experimental animals.
References
- [1].Taylor CA, Braza D, Rice JB, et al. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381–385. [DOI] [PubMed] [Google Scholar]
- [2].Willand MP, Rosa E, Michalski B, et al. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 2016;334:93–104. [DOI] [PubMed] [Google Scholar]
- [3].Aloe L, Rocco M, Bianchi P, et al. Nerve growth factor: from the early discoveries to the potential clinical use. J Transl Med. 2012;10:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manni L, Rocco ML, Bianchi P, et al. Nerve growth factor: basic studies and possible therapeutic applications. Growth Factors. 2013;31(4):115–122. [DOI] [PubMed] [Google Scholar]
- [5].Chen YY, McDonald D, Cheng C, et al. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64(7):613–622. [DOI] [PubMed] [Google Scholar]
- [6].Viader A, Chang L-W, Fahrner T, et al. MicroRNAs modulate Schwann cell response to nerve injury by reinforcing transcriptional silencing of dedifferentiation-related genes. J Neurosci. 2011;31(48):17358–17369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yun B, Anderegg A, Menichella D, et al. MicroRNA-deficient Schwann cells display congenital hypomyelination. J Neurosci. 2010;30(22):7722–7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lei P, Li Y, Chen X, et al. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009;1284:191–201. [DOI] [PubMed] [Google Scholar]
- [9].Aldrich BT, Frakes EP, Kasuya J, et al. Changes in expression of sensory organ-specific microRNAs in rat dorsal root ganglia in association with mechanical hypersensitivity induced by spinal nerve ligation. Neuroscience. 2009;164(2):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zou Y-F, Wen D, Zhao Q, et al. Urinary MicroRNA-30c-5p and MicroRNA-192-5p as potential biomarkers of ischemia-reperfusion-induced kidney injury. Exp Biol Med (Maywood). 2017;242(6):657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ye M, Zhang J, Zhang J, et al. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357(1):196–205. [DOI] [PubMed] [Google Scholar]
- [12].Berthelet J, Dubrez L.. Regulation of Apoptosis by Inhibitors of Apoptosis (IAPs). Cells. 2013;2(1):163–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Potts PR, Singh S, Knezek M, et al. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163(4):789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moattari M, Moattari F, Kaka G, et al. Evaluation of dexamethasone treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. Neurol Res. 2018;40(12):1060–1070. [DOI] [PubMed] [Google Scholar]
- [15].Khalyfa A, Gozal D.. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med. 2014;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mirzaei H, Gholamin S, Shahidsales S, et al. MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. Eur J Cancer. 2016;53:25–32. [DOI] [PubMed] [Google Scholar]
- [17].Weiland M, Gao X-H, Zhou L, et al. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012;9(6):850–859. [DOI] [PubMed] [Google Scholar]
- [18].Molitoris JK, Molitoris BA. Circulating micro-RNAs in acute kidney injury: early observations. Clin J Am Soc Nephrol. 2011;6(7):1517–1519. [DOI] [PubMed] [Google Scholar]
- [19].Ramachandran K, Saikumar J, Bijol V, et al. Human miRNome profiling identifies microRNAs differentially present in the urine after kidney injury. Clin Chem. 2013;59(12):1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang K, Zhang S, Marzolf B, et al. Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci U S A. 2009;106(11):4402–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nagano T, Higashisaka K, Kunieda A, et al. Liver-specific microRNAs as biomarkers of nanomaterial-induced liver damage. Nanotechnology. 2013;24(40):405102. [DOI] [PubMed] [Google Scholar]
- [22].Sohn EJ, Park HT. MicroRNA mediated regulation of schwann cell migration and proliferation in peripheral nerve injury. Biomed Res Int. 2018;2018:8198365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li S, Wang X, Gu Y, et al. Let-7 microRNAs regenerate peripheral nerve regeneration by targeting nerve growth factor. Mol Ther. 2015;23(3):423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Korhonen L. Anti-apoptotic proteins in nerve cell survival and neurodegeneration[J]. 2002. [Google Scholar]
- [25].Lima RT, Martins LM, Guimarães JE, et al. Chemosensitization effects of XIAP downregulation in K562 leukemia cells. J Chemother. 2006;18(1):98–102. [DOI] [PubMed] [Google Scholar]
- [26].Nishioka C, Ikezoe T, Yang J, et al. BCR/ABL increases EZH2 levels which regulates XIAP expression via miRNA-219 in chronic myeloid leukemia cells. Leuk Res. 2016;45:24–32. [DOI] [PubMed] [Google Scholar]
- [27].Li X, Chen W, Zeng W, et al. microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP. Br J Cancer. 2017;116(1):66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim T, Veronese A, Pichiorri F, et al. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208(5):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Y, Huang R, Zhou W, et al. miR-192-5p mediates hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes via targeting of FABP3. J Biochem Mol Toxicol. 2017;31(4):e21873. [DOI] [PubMed] [Google Scholar]
- [30].Li S, Jin Z, Lu X. MicroRNA-192 suppresses cell proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes by downregulating caveolin 1. Mol Cell Biochem. 2017;432(1–2):123–130. [DOI] [PubMed] [Google Scholar]
- [31].Nielsen J, Gotfryd K, Li S, et al. Role of glial cell line-derived neurotrophic factor (GDNF)-neural cell adhesion molecule (NCAM) interactions in induction of neurite outgrowth and identification of a binding site for NCAM in the heel region of GDNF. J Neurosci. 2009;29(36):11360–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Keifer J, Zheng Z, Ambigapathy G. A microRNA-BDNF negative feedback signaling loop in brain: implications for Alzheimer’s Disease. MicroRNA. 2015;4(2):101–108. [DOI] [PubMed] [Google Scholar]
- [33].Hamada N, Fujita Y, Kojima T, et al. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int. 2012;60(8):743–750. [DOI] [PubMed] [Google Scholar]


