Abstract
Background
Gastric cancer (GC) is one of the most common malignancies, and intestinal-type GC is the main histopathologic type of GC in China. We previously reported that casein kinase 2 interacting protein 1 (CKIP-1) acts as a candidate tumor suppressor in intestinal-type GC. CKIP-1 participates in the regulation of multiple signaling pathways, including the Wnt/β-catenin pathway, of which caudal-related homeobox 1 (CDX1) may be a downstream target gene. The purpose of this study was to investigate the relationship between CKIP-1 and CDX1 in intestinal-type GC.
Methods
Sixty-seven gastroscopy biopsy specimens and surgically resected gastric specimens were divided into four groups: gastric mucosa group, intestinal metaplasia (IM) group, dysplasia group, and intestinal-type GC group. The expression levels of CKIP-1 and CDX1 were detected in these groups and GC cell lines, and the correlations between these expression levels were analyzed. SGC7901 and BGC823 cells were divided into CKIP-1 shRNA groups and CKIP-1 over-expression groups, and CDX1 expression was detected. β-Catenin expression was detected in intestinal-type GC tissue samples and CKIP-1 shRNA and CKIP-1 over-expression SGC7901 cells, and its correlation with CKIP-1 expression in intestinal-type GC tissue was analyzed. The Wnt/β-catenin pathway inhibitor DKK-1 and activator LiCl were incubated with SGC7901 cells, BGC823 cells, and CKIP-1 shRNA and CKIP-1 over-expression SGC7901 and BGC823 cells, following which CDX1 and Ki-67 expression were detected.
Results
The expression levels of CKIP-1 and CDX1 were lower in patients with intestinal-type GC than in patients with IM and dysplasia (both P < 0.05). CKIP-1 and CDX1 expression levels were positively correlated in IM, dysplasia, and intestinal-type GC tissue and cell lines (r = 0.771, P < 0.01; r = 0.597, P < 0.01; r = 0.654, P < 0.01; r = 0.811, P < 0.01, respectively). CDX1 expression was decreased in the CKIP-1 shRNA groups and increased in the CKIP-1 over-expression groups of SGC7901 and BGC823 cells compared to that in the corresponding control groups (both P < 0.05). CKIP-1 expression was negatively correlated with β-catenin expression in intestinal-type GC patients (r = −0.458, P < 0.01). Compared to the control group, β-catenin expression was increased in the CKIP-1 shRNA SGC7901 cell group and decreased in the CKIP-1 over-expression SGC7901 cell group (P < 0.05). CDX1 expression was increased in SGC7901 and BGC823 cells treated with DKK-1, DKK-1 increased CDX1 expression and decreased Ki-67 expression in the CKIP-1 shRNA group; the opposite result was observed in SGC7901 and BGC823 cells treated with LiCl, and LiCl decreased CDX1 expression and increased Ki-67 expression in the CKIP-1 over-expression group (both P < 0.05).
Conclusions
Through the Wnt/β-catenin signaling pathway, CKIP-1 may positively regulate CDX1 in intestinal-type GC.
Keywords: Casein kinase 2 interacting protein 1, Caudal-related homeobox 1, Intestinal-type gastric cancer, Intestinal metaplasia
Introduction
Gastric cancer (GC), one of the most common malignancies, is divided into intestinal-type, diffuse-type, mixed-type and uncertain GC by Lauren's Classification, and intestinal-type GC is the main type of GC in China. Studies have demonstrated that intestinal-type GC develops through a sequence of histological changes (Correa's cascade) from diffuse chronic gastritis to intestinal metaplasia (IM), dysplasia, and finally invasive carcinoma.[1] Lesions formed from IM, the trans-differentiation of the gastric epithelium to an intestinal cell type, are crucial to the development of intestinal-type GC. While IM is thought to be a protective response against inflammation, some research suggests that IM increases the risk of intestinal-type GC tumorigenesis.[2] However, the precise mechanism by which IM progresses to intestinal-type GC is not yet fully understood.
Casein kinase 2 interacting protein 1 (CKIP-1), a newly discovered casein kinase 2 (CK2)-interacting protein,[3] is a scaffold protein that mediates multiple protein interactions and plays an important role in bone development, tumorigenesis, tumor development, muscle cell differentiation, and immunomodulatory function.[4–9] In a previous study,[10] we reported that CKIP-1 also acts as a candidate tumor suppressor in intestinal-type GC. IM is mostly induced by Helicobacter pylori infection and the expression of caudal type homeobox transcription factor (CDX). CDX, a mammalian member of the caudal-related homeobox gene family, plays an important role in the differentiation of intestinal cells and maintaining the intestinal phenotype.[11] CDX consists of three homologues, CDX1, CDX2, and CDX4. Among them, CDX1 plays a pivotal role in the development of IM and progression to intestinal-type GC.[12,13] Studies reported that CKIP-1 can participate in the regulation of multiple signaling pathways,[14] including the Wnt/β-catenin signaling pathway,[15] of which CDX1 might be a downstream target gene.[16–18] Therefore, as we speculate that CKIP-1 regulates CDX1 expression through the Wnt/β-catenin signaling pathway to promote the occurrence and development of intestinal-type GC, CKIP-1 was the subject of the present study.
Methods
Ethical approval
The Research Ethics Committee of Guizhou Provincial People's Hospital approved this study (2019 No. 54) and the study design was exempt from the requirement for informed consent. The waiver will not affect the rights and welfare of the subjects.
Patients and samples
Sixty-seven gastroscopy biopsy specimens and surgically resected gastric specimens were obtained from the Department of Pathology, Guizhou Provincial People's Hospital of China from 2014 to 2017. Two senior pathologists reviewed the hematoxylin and eosin-stained sections to confirm the presence of chronic gastritis, IM, dysplasia and intestinal-type GC. Then 67 specimens were divided into four groups: gastric mucosa group, IM group, dysplasia group, and intestinal-type GC group. No patient had received any therapy before biopsy or surgery. The IM and dysplasia grades were determined using the updated Sydney scoring system.[19] The IM samples were categorized as type I, type II, or type III IM[20] by mucin histochemical staining.
Cell lines
Human intestinal GC cell lines (well-differentiated MKN28 cells, moderately differentiated SGC7901 cells, poorly differentiated BGC823 cells, and AGS cells) and the 293T human renal epithelial cell line were obtained from the Shanghai Institutes of Biological Sciences Cell Bank. Cells were cultured in Dulbecco's modified Eagle medium (DMEM) (HyClone, Logan, Ut., USA) containing 10% fetal calf serum in a humidified atmosphere consisting of 5% CO2/95% air at 37°C.
Mucin histochemical staining
Mucin histochemical staining (high iron diamine [HID]/Alcian blue [AB], periodic acid/borohydride [PB]/KOH/periodic acid-Schiff [PAS]) was performed to assess the IM subtype. HID/AB staining was performed as described previously.[21] Briefly, slides were immersed in an HID solution for 20 h at room temperature (RT) and then rinsed with deionized water and stained with AB (pH 2.5) for 20 min. PB/KOH/PAS staining was performed as described previously.[22] Briefly, the slides were immersed in a periodate solution for 30 min at RT, rinsed with deionized water, stained with a boric acid-sodium borohydride solution for 1 h, rinsed with deionized water, and stained with KOH for 30 min and periodic acid for 5 min. After HID/AB staining, type I IM goblet cells were stained blue, and type III IM goblet cells were stained brown. If goblet cells were stained both blue and brown, further PB/KOH/PAS staining was carried out. Type II, IM cells were stained amaranth, while type III IM cells were not stained.
Immunohistochemistry
Immunohistochemical (IHC) staining of gastroscopy biopsy specimens and surgically resected specimens for the CKIP-1, CDX1, and β-catenin proteins was performed as described previously.[23] Briefly, the sections were treated with blocking buffer for 30 min at RT and then incubated with anti-CKIP-1 antibody (1:500 dilution in Tris-NaCl buffer; Abcam, Cambridge, UK), anti-CDX1 antibody (1:200 dilution in Tris-NaCl buffer; Abcam), and anti-β-catenin antibody (1:300 dilution in Tris-NaCl buffer; Abcam) at 4°C overnight. Next, the sections were rinsed in Tris-NaCl buffer and then incubated with biotinylated goat anti-rabbit immunoglobulin G (IgG) or goat anti-mouse IgG (diluted 1:200 in Tris-NaCl buffer; Abcam) for 60 min at RT. The sections were subsequently incubated with an avidin-biotinylated enzyme complex and 3,3’-diaminobenzidine (DAB).
The results of IHC for CKIP-1, CDX1, and β-catenin were independently scored by two observers. Staining was measured in five random fields in each area of interest. CKIP-1 and CDX1 were scored based on the percentage of positive cells and staining intensity. The percentage of positive cells was denoted by four scores as follows: <5% (0), 6% to 25% (1), 26% to 50% (2), 51% to 75% (3), and >75% (4). The staining intensity was scored as follows: negative (0), weak (1), moderate (2), and strong (3). The histologic score (H-score) for each sample was determined by the following formula: H-score = percentage score × intensity score. An overall score of 0 to 12 was calculated and graded as negative (score: 0–1), weak (score: 2–3), moderate (score: 4–8), or strong (score: 9–12). In this study, we defined specimens with negative and weak scores as the CKIP-1 or CDX1 low expression group and those with moderate and strong scores as the CKIP-1 or CDX1 high expression group. Cytoplasmic and/or nuclear staining for β-catenin indicated β-catenin-positive expression (in tumor cells displaying cytoplasmic and/or nuclear immunoreactivity for β-catenin) or β-catenin-negative expression (the absence of cytoplasmic or nuclear staining for β-catenin). The percentage of positive cells was denoted by three scores as follows: 1% to10% (1), 11% to 50% (2), and >50% (3). The staining intensity was scored as follows: weak (1), moderate (2), and strong (3). The H-score was calculated as follows: H-score = percentage score × intensity score. In this study, we defined specimens negative for β-catenin expression and those with an H-score 1 to 4 as the low β-catenin expression group and specimens with an H-score >4 as the high β-catenin expression group.[24]
Plasmids, lentiviral production, and infection
The sequence 5′-CCTGAGTGACTATGAGAAGCTTCTCATAGTCACTCAGG-3′ was designed and cloned into the pLKO.1 vector (Addgene plasmid #1864) to generate a lentiviral plasmid to produce CKIP-1 short hairpin RNA (shRNA). CKIP-1 cDNA was subcloned into the pLenti CMV vector (Addgene plasmid #17482) to obtain a lentiviral over-expression plasmid. Empty plasmid was used as a negative control. Lentiviruses were produced and transfected as previously described.[10] Infectious lentiviruses were produced by co-transfection of the expression vector and packaging plasmids into 293T cells, which were then added to SGC7901 or BGC823 cells in the presence of 8 mg/mL polybrene. At 48 h post-infection, infected cells were selected using 1 μg/mL puromycin for approximately 14 days to generate stable transfectants.
Treatment of GC cells with the Wnt/β-catenin pathway inhibitor DKK-1 and activator LiCl
SGC7901 cells, BGC823 cells, and CKIP-1 shRNA and CKIP-1 over-expression SGC7901 and BGC823 cells were treated with the Wnt/β-catenin pathway inhibitor DKK-1 (200 ng/mL; Peprotech, Rocky Hill, NJ, USA) or the Wnt/β-catenin pathway activator LiCl (10 mmol/L; Sigma-Aldrich Chemical Company, St. Louis, MO, USA) for 24 h.
Western blotting
The cellular protein levels of CKIP-1, CDX1, β-catenin, Ki-67, and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were detected by Western blotting as described previously.[23] Briefly, the total protein was extracted, and proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking with 5% dry milk, the Polyvinylidene difluoride (PVDF) membranes were incubated with anti-CKIP-1 antibody (1:1000 dilution; Abcam), anti-CDX1 antibody (1:2000 dilution; Abcam), anti-β-catenin antibody (1:2000 dilution; Abcam), anti-Ki-67 antibody (1:1000 dilution; Abcam), or anti-GAPDH antibody (1:20,000 dilution; Sigma) for 120 min at RT. After washing, the membranes were incubated with Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG or HRP-conjugated goat anti-mouse IgG for 90 min at RT. Finally, the polyvinylidene difluoride (PVDF) membranes were incubated with Enhanced chemiluminescence (ECL) Plus reagent for 5 min, and signals were visualized by exposure to hyper performance chemiluminescence film. The intensity of each signal on the film was quantitated using a computer-assisted imaging system and normalized to the corresponding signal for GAPDH. Finally, the relative level of each protein was determined and is expressed as a percentage compared to the control group (100%).
Statistical analysis
Statistical analyses were performed using SPSS software version 20.0 (IBM, Armonk, NY, USA). Qualitative variables within groups are expressed as frequencies and percentages. The Chi-squared test was used to analyze qualitative data. Quantitative variables are expressed as the mean ± standard deviation (SD). Quantitative data were compared by the t test or one-way analysis of variance to determine if differences were statistically significant. Correlations were evaluated by Spearman correlation analysis. A P < 0.05 indicated a statistically significant difference.
Results
Expression of CKIP-1 and CDX1 in gastric mucosal epithelium, IM, dysplasia, and intestinal-type GC samples
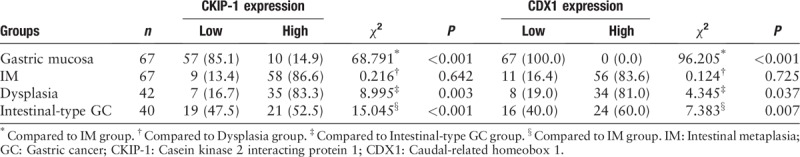
CKIP-1 was mainly expressed in the cytoplasm. CKIP-1 expression in gastric mucosal epithelium cells was negative or weak; however, CKIP-1 expression in IM cells was strikingly high. The proportion of samples showing high CKIP-1 expression was significantly higher in the IM group than in the gastric mucosa group (χ2 = 68.791, P < 0.001). The expression of CKIP-1 was slightly lower in the dysplasia group than in the IM group, but this difference was not significant. Furthermore, compared with the IM and dysplasia groups, CKIP-1 expression was significantly decreased in the intestinal-type GC group (χ2 = 15.045, P < 0.001; χ2 = 8.995, P = 0.003).
All CDX1-positive cases showed CDX1 nuclear staining. Most of the cases did not exhibit CDX1 expression in the gastric mucosal epithelium. Only six of 67 cases exhibited weak CDX1 staining in the gastric mucosal epithelium. However, all IM cases exhibited positive CDX1 expression, and most IM cases showed moderate or strong staining for CDX1. The proportion of samples showing high CDX1 expression was significantly higher in the IM group than in the gastric mucosa group (χ2 = 96.205, P < 0.001). Similar to the expression of CKIP-1, the proportion of samples in the dysplasia group showing high CDX1 expression was slightly lower than that in the IM group, but this difference was not statistically significant. CDX1 expression was lower in intestinal-type GC tissues, and the proportion of samples in the intestinal-type GC group showing high CDX1 expression was significantly lower than that in the IM and dysplasia groups (χ2 = 7.383, P = 0.007; χ2 = 4.345, P = 0.037). The expression levels of CKIP-1 and CDX1 in gastric mucosa, IM, dysplasia, and intestinal-type GC patient tissues are shown in Figure 1 and Table 1.
Figure 1.
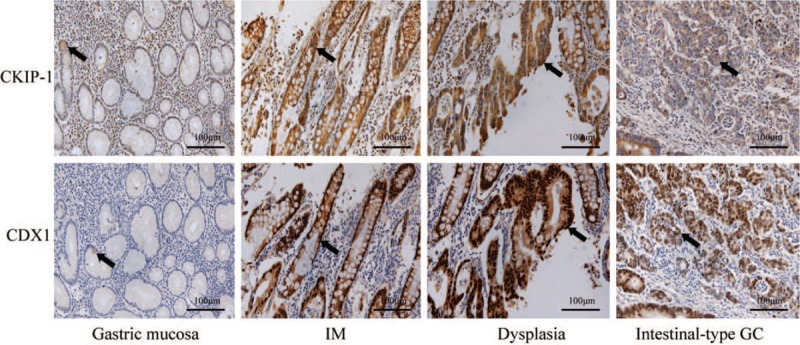
The expression of casein kinase 2 interacting protein 1 (CKIP-1) and caudal-related homeobox 1 (CDX1) in gastric mucosal epithelial cell (black arrow), intestinal metaplasia (IM) cells (black arrow), dysplasia cells (black arrow), and intestinal-type gastric cancer (GC) cells (black arrow) (EnVision, scale bar = 100 μm).
Table 1.
The expression of CKIP-1 and CDX1 in gastric mucosal, IM, dysplasia, and intestinal-type GC patient tissues, n (%).
We also analyzed the relationships between CKIP-1 and CDX1 expression levels and the IM type and grade, dysplasia grade, and differentiation in intestinal-type GC tissues from patients [Table 2]. Both CKIP-1 and CDX1 expression levels were associated with the differentiation of intestinal-type GC (r = −0.463, P = 0.003; r = −0.362, P = 0.022). The less differentiated the intestinal-type GC cells were, the lower the expression of CKIP-1 and CDX1. However, there was no significant correlation between the expression of CKIP-1 and CDX1 and the IM type or grade or the dysplasia grade. Spearman correlation analysis showed that CKIP-1 expression was positively correlated with CDX1 expression in the IM, dysplasia, and intestinal-type GC groups (r = 0.771, P < 0.001; r = 0.597, P < 0.001; r = 0.654, P < 0.001, respectively) [Table 3]. CKIP-1 and CDX1 expression levels in tissues with different types of IM and different levels of intestinal-type GC differentiation are shown in Figures 2 and 3.
Table 2.
The relationship between CKIP-1 and CDX1 expression and the IM type and grade, dysplasia grade, and the differentiation of intestinal-type GC in patient tissues, n (%).
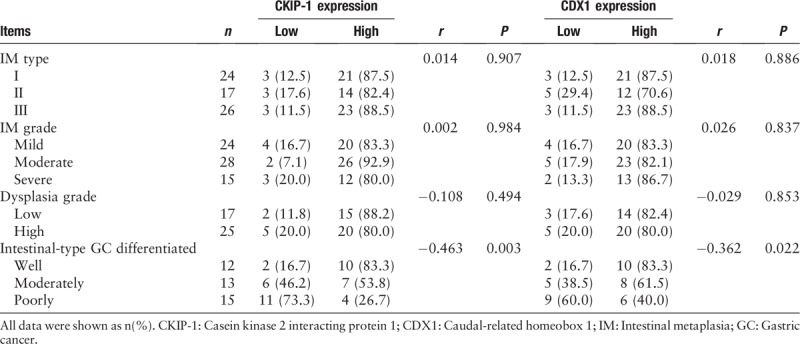
Table 3.
The correlation between CKIP-1 and CDX1 expression levels in different gastric mucosa lesions and intestinal-type GC tissues.
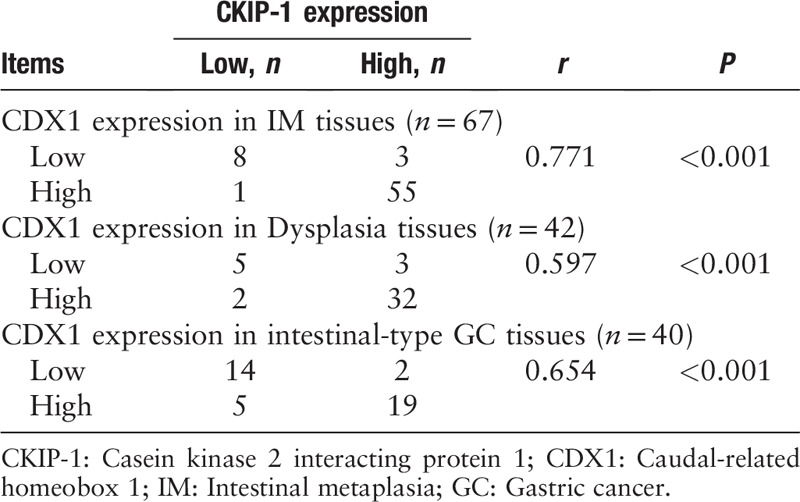
Figure 2.
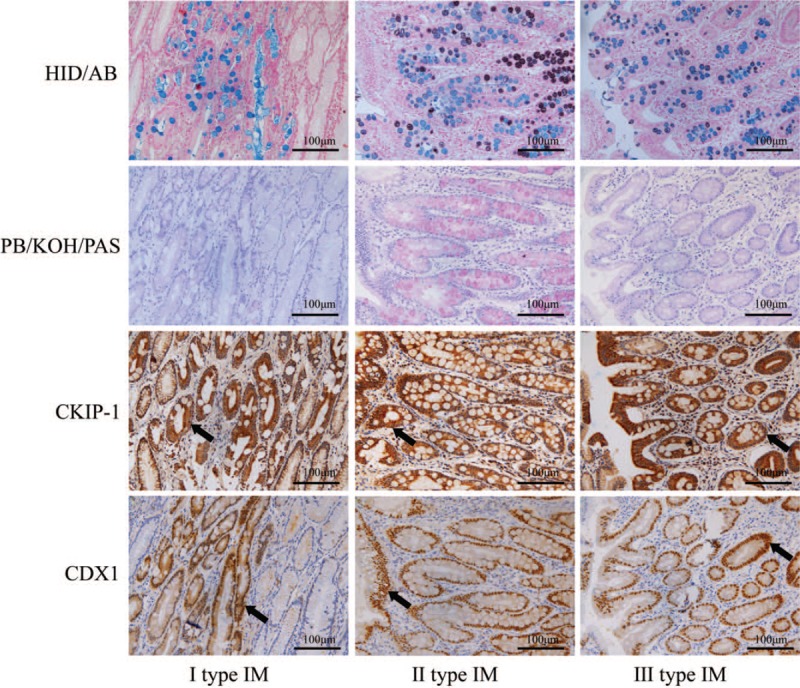
The expression levels of casein kinase 2 interacting protein 1 (CKIP-1) and caudal-related homeobox 1 (CDX1) in patients with different types of intestinal metaplasia (IM) (black arrow shows I, II, and III type IM cells) (high iron diamine [HID]/Alcian blue [AB], periodic acid/borohydride [PB]/KOH/periodic acid-Schiff [PAS]; EnVision, scale bar = 100 μm).
Figure 3.
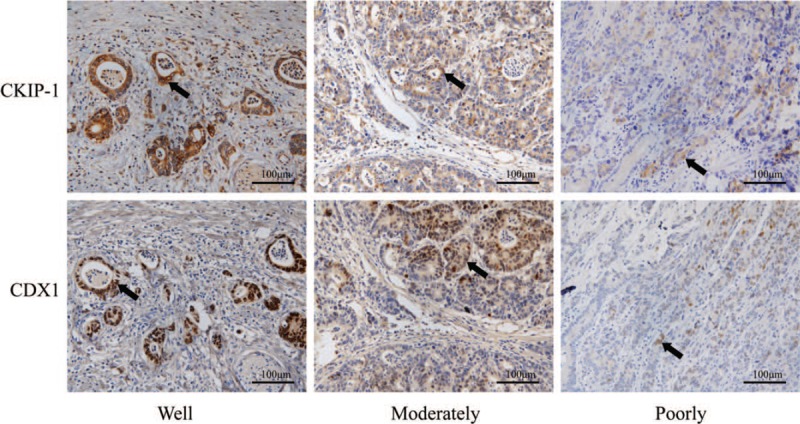
The expression levels of casein kinase 2 interacting protein 1 (CKIP-1) and caudal-related homeobox 1 (CDX1) in differently differentiated intestinal-type gastric cancer (GC) patient tissues (black arrow shows intestinal-type GC cells) (EnVision, scale bar = 100 μm).
Expression of CKIP-1 and CDX1 in intestinal-type GC cell lines
We also detected the expression of CKIP-1 and CDX1 in intestinal-type GC cell lines and observed their relationship [Figure 4]. The CKIP-1 and CDX1 expression differences among the four groups were statistically significant (F = 12.330, P = 0.002; F = 11.614, P = 0.003). Similar to the results observed in tissue samples, the less differentiated the cancer cells were, the lower the CKIP-1 expression (LSD-t = 2.653, P = 0.029; LSD-t = 5.152, P = 0.001; LSD-t = 5.240, P = 0.001; LSD-t = 2.499, P = 0.037; LSD-t = 2.586, P = 0.032, respectively) and CDX1 expression levels (LSD-t = 2.433, P = 0.041; LSD-t = 5.037, P = 0.001; LSD-t = 5.002, P = 0.001; LSD-t = 2.604, P = 0.031; LSD-t = 2.568, P = 0.033, respectively). Spearman correlation analysis showed that CKIP-1 expression was positively correlated with CDX1 expression in intestinal-type GC cell lines (r = 0.811, P = 0.001).
Figure 4.
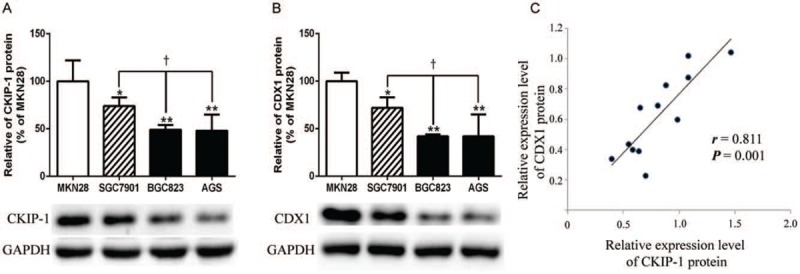
The expression levels of casein kinase 2 interacting protein 1 (CKIP-1) (A), caudal-related homeobox 1 (CDX1) (B), and their relationship (C) in intestinal-type gastric cancer (GC) cell lines. ∗P < 0.05 and ∗∗P < 0.01 compared to MKN28 cells; †P < 0.05 compared to SGC7901 cells.
CDX1 expression in CKIP-1 shRNA and CKIP-1 over-expression SGC7901 and BGC823 cells
After establishing CKIP-1 shRNA transfection and CKIP-1 over-expression SGC7901 and BGC823 cell models, we detected CDX1 expression in these cells. The CDX1 expression differences in SGC7901 and BGC823 cell among the three groups were statistically significant (F = 21.484, P = 0.002; F = 20.125, P = 0.002). As shown in Figure 5, compared with CDX1 expression in the control groups, CDX1 expression was lower in CKIP-1 shRNA SGC7901 cells (LSD-t = 3.611, P = 0.011) and in CKIP-1 shRNA BGC823 cells (LSD-t = 2.918, P = 0.027), and CDX1 expression was higher in CKIP-1 over-expression SGC7901 cells (LSD-t = −2.932, P = 0.026) and CKIP-1 over-expression BGC823 cells (LSD-t = −3.419, P = 0.014).
Figure 5.
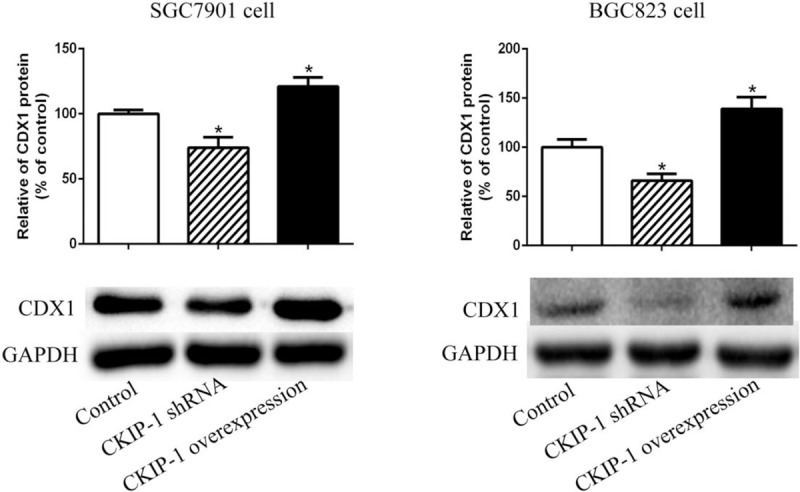
The expression levels of caudal-related homeobox 1 (CDX1) in casein kinase 2 interacting protein 1 (CKIP-1) shRNA and CKIP-1 over-expression SGC7901 and BGC823 cells. ∗P < 0.05 compared to the control group.
β-Catenin expression in intestinal-type GC tissue samples and CKIP-1 shRNA and over-expression SGC7901 cells
Distinct membranous β-catenin staining and rare cytoplasmic or nuclear β-catenin staining were observed in the adjacent normal gastric mucosa epithelium [Figure 6A]. However, reduced or even undetectable membranous and cytoplasmic and/or nuclear staining for β-catenin was commonly observed in intestinal-type GC tissues [Figure 6B–D]. In the CKIP-1 low expression group, the proportion of samples showing high β-catenin expression (15/19, 78.9%) was significantly increased compared with the CKIP-1 high expression group (7/21, 33.3%); correlation analysis showed that CKIP-1 expression was negatively correlated with β-catenin expression in intestinal-type GC patients (r = −0.458, P < 0.001). The β-catenin expression differences among the control group, CKIP-1 shRNA group and CKIP-1 over-expression group were statistically significant (F = 22.060, P = 0.002). The β-catenin expression was increased in the CKIP-1 shRNA group (LSD-t = −2.587, P = 0.041) and decreased in the CKIP-1 over-expression group (LSD-t = 4.005, P = 0.007) compared with the control groups [Figure 6E].
Figure 6.
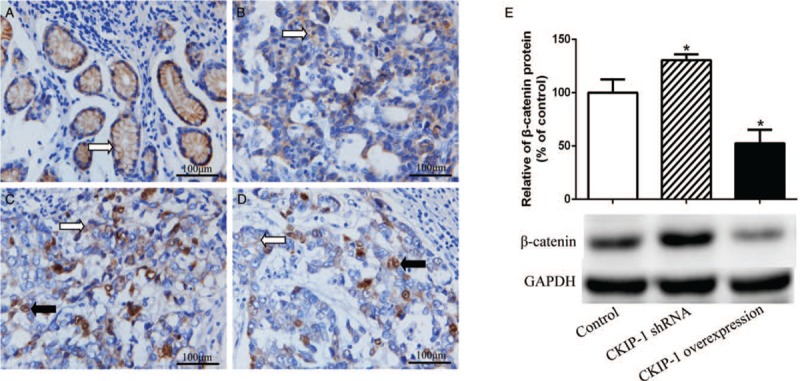
β-Catenin expression in normal gastric mucosa (A, white arrow shows gastric mucosa epithelial β-catenin membranous expression) and intestinal-type gastric cancer (GC) tissue samples (B–D, white arrow shows intestinal-type GC cell β-catenin cytoplasmic expression; black arrow shows intestinal-type GC cell β-catenin nuclear expression) (EnVision, scale bar = 100 μm), β-catenin expression in casein kinase 2 interacting protein 1 (CKIP-1) shRNA and over-expression SGC7901 cells (E). ∗P < 0.05 compared to the control group.
CDX1 expression in GC cells after treatment with the Wnt/β-catenin pathway inhibitor DKK-1 and activator LiCl
CDX1 expression in SGC7901 and BGC823 cells after their treatment with the Wnt/β-catenin pathway inhibitor DKK-1 and activator LiCl were observed. The CDX1 expression differences in SGC7901 and BGC823 cells among the three groups were statistically significant (F = 14.390, P = 0.005; F = 17.706, P = 0.003). As shown in Figure 7A, compared with the control group, CDX1 expression was increased in SGC7901 cell (LSD-t = −2.565, P = 0.043) and BGC823 cells (LSD-t = −2.689, P = 0.036) after treatment with DKK-1, and the opposite effect was observed in SGC7901 cell (LSD-t = 2.798, P = 0.031) and BGC823 cells (LSD-t = 3.253, P = 0.017) after their treatment with LiCl. Then, we observed CDX1 and Ki-67 expression in CKIP-1 shRNA SGC7901 and BGC823 cells after their treatment with DKK-1 and in CKIP-1 over-expression SGC7901 and BGC823 cells after their treatment with LiCl. Compared with the CKIP-1 shRNA group, CDX1 expression was higher (t = −6.760, P = 0.002; t = −3.659, P = 0.022) and Ki-67 expression was lower (t = 4.398, P = 0.012; t = 2.843, P = 0.047) in the CKIP-1 shRNA SGC7901 and BGC823 cells group after DKK-1 treatment. In contrast, CDX1 expression was lower (t = 6.245, P = 0.003; t = 4.925, P = 0.008), and Ki-67 expression was higher (t = −6.272, P = 0.003; t = −2.801, P = 0.049) in the CKIP-1 over-expression SGC7901 and BGC823 cells group after treatment with LiCl [Figure 7B].
Figure 7.
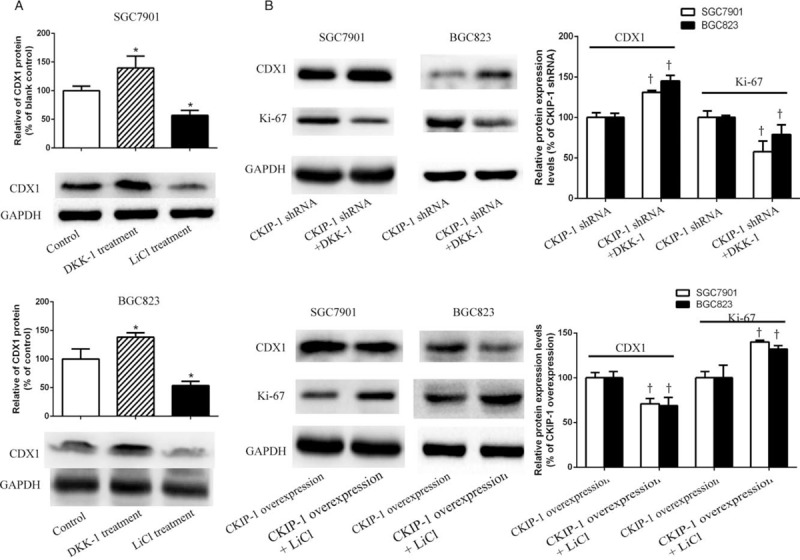
The expression of caudal-related homeobox 1 (CDX1) and Ki-67 in SGC7901 cells, BGC823 cells, and casein kinase 2 interacting protein 1 (CKIP-1) shRNA and CKIP-1 over-expression SGC7901 and BGC823 cells treated with DKK-1 and LiCl. (A) CDX1 expression in SGC7901 and BGC823 cells treated with DKK-1 and LiCl. (B) CDX1 and Ki-67 expression in CKIP-1 shRNA and CKIP-1 over-expression SGC7901 and BGC823 cells treated with DKK-1 and LiCl. ∗P < 0.05 compared to the control group. †P < 0.05 compared to the CKIP-1 shRNA or CKIP-1 over-expression group.
Discussion
Although it is widely accepted that chronic gastritis progresses to IM, dysplasia, and finally GC, the precise molecular alterations underlying the progression of this pathway remain to be delineated.[25] In the current study, we determined the expression levels of CKIP-1 and CDX1 in gastric mucosa, IM, dysplasia, and intestinal-type GC samples from patients by immunohistochemical staining. Our results showed that CKIP-1 was mainly absent or weakly expressed in the gastric mucosal epithelium; however, CKIP-1 expression was strikingly increased in IM lesions. CDX1 was not expressed in the gastric mucosa of most cases but was positively expressed in the nuclei of IM cells in all cases, which is consistent with the results of studies by Silberg et al[26] and Eda et al.[27] This result indicated that CKIP-1 is normally weakly expressed in the gastric mucosal epithelium, but upon IM, the gastric mucosal epithelium obtains an intestinal phenotype and expresses high levels of CKIP-1; and CDX1 may play an important role in the formation of IM. Compared with the IM and dysplasia groups, CKIP-1 and CDX1 expression were lower in the intestinal-type GC group; CKIP-1 and CDX1 expression gradually decreased from the IM group to the dysplasia group to the intestinal-type GC group. These results suggested that CKIP-1 and CDX1 acts as a tumor suppressor in the development of intestinal-type GC. The role of CDX1 in GC remains controversial. Some prior studies reported that the CDX1 expression level in GC tissues was significantly higher than that in control tissues and that CDX1 expression was higher in poorly differentiated GC tissues than in well-differentiated GC tissues.[13,28] In contrast, several studies in surgical specimens reported a reduction in CDX1 in GC compared to IM, indicating that CDX1 plays the role of “tumor suppressor” in tumorigenesis.[26,29] Our previous study showed that CDX1 expression was lower in intestinal-type GC patients with a poorer clinical prognosis; CDX1 over-expression could significantly inhibit the invasion of intestinal-type GC cell lines (data not shown). So we consider that CDX1 might serve as a tumor suppressor gene in intestinal-type GC tumorigenesis. In this study, both CKIP-1 and CDX1 expression levels in the dysplasia group were only slightly lower than those in the IM group, and these differences were not statistically significant. We believe the following reasons explain these differences in CKIP-1 and CDX1 expression levels. Dysplasia can be divided into two types. The first type of dysplasia is dysplasia of the intrinsic epithelium of the gastric mucosa, and the second type of dysplasia is dysplasia of the IM. Diffuse-type GC is believed to develop from dysplasia of the intrinsic epithelium of the gastric mucosa, while intestinal-type GC develops from dysplasia of the IM. The dysplasia cells assessed in this study exhibited dysplasia of the IM. The difference in CKIP-1 and CDX1 expression between the IM and dysplasia groups might be small and insufficient for detection by IHC. In addition, we observed the relationships between CKIP-1 and CDX1 expression and the type and grade of IM, grade of dysplasia and level of intestinal-type GC differentiation. The expression of CKIP-1 and CDX1 tended to decrease from well-differentiated to moderately differentiated to poorly differentiated intestinal-type GC tissues. In intestinal-type GC cell lines, we observed the same results. Poorly differentiated tumor cells are characterized by a high degree of cell atypia; the higher the degree of the malignancy, the more prone cells are to invasion and metastasis, and the more advanced the clinical stage is. These results suggest that both CKIP-1 and CDX1 negatively regulate malignancy in the development of intestinal-type GC. However, our data did not show that CKIP-1 and CDX1 expression was significantly associated with the type and grade of IM or the grade of dysplasia. Follow-up epidemiologic studies showed a faster progression rate to GC in incomplete IM than in complete IM.[30] IM might progress from type I to type III IM,[31] and the risk of developing intestinal-type GC was shown to be significantly higher in types II and III IM than in type I IM.[32,33] There have been only a few studies on CKIP-1 and CDX1 expression in intestinal-type GC. In addition, due to the small number of patient cases in the current study, it will be necessary to increase the number of cases in future studies to clarify the relationship between CKIP-1 or CDX1 and the type and grade of IM or grade of dysplasia.
The Wnt/β-catenin signaling pathway plays a very important role in the tumorigenesis and progression of various tumors. Studies have indicated that the Wnt/β-catenin signaling pathway is abnormally activated in GC cells.[34,35] CKIP-1 is a scaffold protein that may play an important role in regulating a variety of signaling pathways, including the Wnt/β-catenin pathway.[14,15] Zhou et al[15] reported that CKIP-1 silencing significantly promoted the expression of Wnt3a and β-catenin in new bone of distraction osteogenesis animal models. CDX1 was confirmed as a downstream target gene of the Wnt/β-catenin signaling pathway during intestinal morphogenesis or fetal endoderm development.[16,18,36] To explore if CKIP-1 may regulate CDX1 expression through the Wnt/β-catenin signaling pathway in the transformation of IM to intestinal-type GC and the formation of intestinal-type GC, we first investigated the relationship between CKIP-1 and CDX1 expression in patient samples and cell lines. Our data indicated that CKIP-1 expression was positively correlated with CDX1 expression in IM, dysplasia, and intestinal-type GC samples and cell lines. To further explore whether CKIP-1 influences CDX1 expression, we also established CKIP-1 shRNA and CKIP-1 over-expression groups of SGC7901 and BGC823 cells, which showed moderate and lower levels of CKIP-1 expression, respectively, and detected CDX1 expression in these cell models. We found that CDX1 expression was decreased in the CKIP-1 shRNA groups, CDX1 expression was increased in the CKIP-1 over-expression groups compared to the control groups. These data suggested that CKIP-1 positively regulates the expression of CDX1 in intestinal-type GC cells.
β-Catenin acts as a co-activator of transcription factors involved in the canonical Wnt signaling pathway. Dysfunction of this regulatory pathway may result in the accumulation of a hypophosphorylated stable form of β-catenin in the cytoplasm, which is translocated to the nucleus, where it binds the high mobility group domain factors Tcf/LEF and stimulates the transcription of target genes.[37] We also evaluated the expression level and subcellular location of β-catenin in intestinal-type GC tissue samples by IHC staining and analyzed its correlation with CKIP-1 expression to explore whether CKIP-1 regulates Wnt/β-catenin signaling pathway in intestinal-type GC. Our immunohistochemical analysis showed that the normal gastric mucosa epithelium showed membranous β-catenin expression but not cytoplasmic or nuclear β-catenin expression; however, reduced or even undetectable membranous and cytoplasmic and/or nuclear β-catenin staining were commonly observed in GC cells. In addition, we found that in the CKIP-1 low expression group, the proportion of samples showing high β-catenin expression was significantly increased compared with the CKIP-1 high expression group, correlation analysis also showed that CKIP-1 expression was negatively correlated with β-catenin expression in intestinal-type GC patients. In addition, Western blotting results showed that β-catenin expression was increased in CKIP-1 shRNA SGC7901 cells and decreased in CKIP-1 over-expression SGC7901 cells compared with the control groups. All these data imply that the down-regulation of CKIP-1 expression activates the Wnt/β-catenin signaling pathway in GC cells. Only a few data regarding whether the oncogenic activation of this pathway also stimulates CDX1 in tumor cells are available. Interestingly, somewhat conflicting observations on the regulation of CDX1 gene expression by Wnt/β-catenin signaling in human colon cancer were reported.[38,39] In this study, we detected CDX1 expression in GC SGC7901 and BGC823 cells after their treatment with the Wnt/β-catenin signaling pathway inhibitor DKK-1 and activator LiCl. Our data demonstrated that CDX1 expression in GC cells was increased after treatment with DKK-1 and decreased after treatment with LiCl, which suggested that CDX1 may be negatively regulated by the Wnt/β-catenin signaling pathway in GC cells. Next, we investigated CDX1 and Ki-67 expression in CKIP-1 shRNA or over-expression SGC7901 and BGC823 cells after treatment with DKK-1 and LiCl. CDX1 expression was increased, and Ki-67 expression was decreased after treatment with DKK-1. In contrast, CDX1 expression was decreased, and Ki-67 expression was increased after treatment with LiCl. All these data suggest that CKIP-1 down-regulation activates the Wnt/β-catenin signaling pathway to inhibit CDX1 expression and promote the proliferation of GC cells, which might be one of the reasons why CKIP-1 down-regulation causes tumorigenesis and the development of intestinal-type GC.
Based on the above results, we hypothesize that IM is a protective response against inflammation injury. However, the expression of CKIP-1, which serves as a tumor suppressor gene, decreases in the progression from IM to dysplasia to intestinal-type GC. CKIP-1 might positively regulate the Wnt/β-catenin signaling pathway to down-regulate CDX1 expression and promote the transformation to intestinal-type GC. This result is of great significance for understanding the mechanisms by which intestinal-type GC occurs and develops. However, this study is only preliminary, and the details of this regulatory mechanism should be clarified by further research.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81560088) and Guizhou Provincial Science and Technology Foundation (No. [2019]1209).
Conflicts of interest
None.
Footnotes
How to cite this article: Ma L, Cao Y, Hu JJ, Chu ML. Casein kinase 2 interacting protein 1 positively regulates caudal-related homeobox 1 in intestinal-type gastric cancer. Chin Med J 2019;133:154–164. doi: 10.1097/CM9.0000000000000604
References
- 1.Correa P. A human model of gastric carcinogenesis. Cancer Res 1988; 48:3554–3560. [PubMed] [Google Scholar]
- 2.Mesquita P, Raquel A, Nuno L, Reis CA, Silva LF, Serpa J, et al. Metaplasia--a transdifferentiation process that facilitates cancer development: the model of gastric intestinal metaplasia. Crit Rev Oncog 2006; 12:3–26. doi: 10.1615/critrevoncog.v 12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 3.Bosc DG, Graham KC, Saulnier RB, Zhang C, Prober D, Gietz RD, et al. Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J Biol Chem 2000; 275:14295–14306. doi: 10.1074/jbc.275.19.14295. [DOI] [PubMed] [Google Scholar]
- 4.Peng X, Wu X, Zhang J, Zhang G, Li G, Pan X. The role of CKIP-1 in osteoporosis development and treatment. Bone Joint Res 2018; 7:173–178. doi: 10.1302/2046-3758.72.BJR-2017-0172.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han B, Wei SP, Zhang XC, Li H, Li Y, Li RX, et al. Effects of constrained dynamic loading, CKIP-1 gene knockout and combination stimulations on bone loss caused by mechanical unloading. Mol Med Rep 2018; 18:2506–2514. doi: 10.3892/mmr.2018.9222. [DOI] [PubMed] [Google Scholar]
- 6.Tokuda E, Fujita N, Oh-hara T, Sato S, Kurata A, Katayama R, et al. Casein kinase 2-interacting protein-1, a novel Akt pleckstrin homology domain-interacting protein, down-regulates PI3K/Akt signaling and suppresses tumor growth in vivo. Cancer Res 2007; 67:9666–9676. doi: 10.1158/0008-5472.CAN-07-1050. [DOI] [PubMed] [Google Scholar]
- 7.Nie J, Liu L, Xing G, Zhang M, Wei R, Guo M, et al. CKIP-1 acts as a colonic tumor suppressor by repressing oncogenic Smurf1 synthesis and promoting Smurf1 autodegradation. Oncogene 2014; 33:3677–3687. doi: 10.1038/onc.2013.340. [DOI] [PubMed] [Google Scholar]
- 8.Baas D, Caussanel-Boude S, Guiraud A, Calhabeu F, Delaune E, Pilot F, et al. CKIP-1 regulates mammalian and zebrafish myoblast fusion. J Cell Sci 2012; 125:3790–3800. doi: 10.1242/jcs.101048. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Chen JF, Yang YM, Huang XH, Dong XH, Yang HX, et al. CKIP-1 regulates the immunomodulatory function of mesenchymal stem cells. Mol Biol Rep 2019; 46:3991–3999. doi: 10.1007/s11033-019-04844-1. [DOI] [PubMed] [Google Scholar]
- 10.Wang LX, Chu ML, Yin D, Cao Y, Guan ZZ, Ma XB. CKIP-1 serves as a negative regulator and correlates with the degree of differentiation in gastric cancer. Int J Clin Exp Pathol 2017; 10:10674–10680. [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama C, Yamamichi N, Tomida S, Takahashi Y, Kageyama-Yahara N, Sakurai K, et al. Transduced caudal-type homeobox (CDX) 2/CDX1 can induce growth inhibition on CDX-deficient gastric cancer by rapid intestinal differentiation. Cancer Sci 2018; 109:3853–3864. doi: 10.1111/cas.13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology 2000; 119:961–971. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- 13.Kang JM, Lee BH, Kim N, Lee HS, Lee HE, Park JH, et al. CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer. J Korean Med Sci 2011; 26:647–653. doi: 10.3346/jkms.2011.26.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie J, Liu L, He F, Fu X, Han W, Zhang L. CKIP-1: a scaffold protein and potential therapeutic target integrating multiple signaling pathways and physiological functions. Ageing Res Rev 2013; 12:276–281. doi: 10.1016/j.arr.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Zhou ZC, Che L, Kong L, Lei DL, Liu R, Yang XJ. CKIP-1 silencing promotes new bone formation in rat mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol 2017; 123:e1–e9. doi: 10.1016/j.oooo.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Lickert H, Domon C, Huls G, Wehrle C, Duluc I, Clevers H, et al. Wnt/(beta)-catenin signaling regulates the expression of the homeobox gene Cdx1 in embryonic intestine. Development 2000; 127:3805–3813. [DOI] [PubMed] [Google Scholar]
- 17.Prinos P, Joseph S, Oh K, Meyer BI, Gruss P, Lohnes D. Multiple pathways governing Cdx1 expression during murine development. Dev Biol 2001; 239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- 18.Pilon N, Oh K, Sylvestre JR, Savory JG, Lohnes D. Wnt signaling is a key mediator of Cdx1 expression in vivo. Development 2007; 134:2315–2323. doi: 10.1242/dev.001206. [DOI] [PubMed] [Google Scholar]
- 19.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Filipe MI, Potet F, Bogomoletz WV, Dawson PA, Fabiani B, Chauveinc P, et al. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut 1985; 26:1319–1326. doi: 10.1136/gut.26.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Zimaity HM, Ramchatesingh J, Saeed MA, Graham DY. Gastric intestinal metaplasia: subtypes and natural history. J Clin Pathol 2001; 54:679–683. doi: 10.1136/jcp.54.9.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culling CF, Reid PE, Clay MG, Dunn WL. The histochemical demonstration of O-acylated sialic acid in gastrointestinal mucins. Their association with the potassium hydroxide-periodic acid-schiff effect. J Histochem Cytochem 1974; 22:826–831. doi: 10.1177/22.8.826. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, Xiao Y, Ravid R, Guan ZZ. Changed clathrin regulatory proteins in the brains of Alzheimer's disease patients and animal models. J Alzheimers Dis 2010; 22:329–342. doi: 10.3233/JAD-2010-100162. [DOI] [PubMed] [Google Scholar]
- 24.Bachmann IM, Straume O, Puntervoll HE, Kalvenes MB, Akslen LA. Importance of P-cadherin, beta-catenin, and Wnt5a/frizzled for progression of melanocytic tumors and prognosis in cutaneous melanoma. Clin Cancer Res 2005; 11:8606–8614. doi: 10.1158/1078-0432.CCR-05-0011. [DOI] [PubMed] [Google Scholar]
- 25.Correa P, Piazuelo MB. The gastric precancerous cascade. J Dig Dis 2012; 13:2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silberg DG, Furth EE, Taylor JK, Schuck T, Chiou T, Traber PG. CDX1 protein expression in normal, metaplastic, and neoplastic human alimentary tract epithelium. Gastroenterology 1997; 113:478–486. doi: 10.1053/gast.1997.v113.pm9247467. [DOI] [PubMed] [Google Scholar]
- 27.Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, et al. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol 2002; 37:94–100. doi: 10.1007/s005350200002. [DOI] [PubMed] [Google Scholar]
- 28.Feng L, Li JF, Zhang JN, Zhang BG, Hu L, Shao ZF, et al. CDX1 expression in gastric carcinoma and clinical significance (in Chinese). J Surg Concepts Prac 2015; 20:501–504. doi: 10.16139/j.1007-9610.2015.06.011. [Google Scholar]
- 29.Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, et al. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol 2003; 199:36–40. doi: 10.1002/path.1246. [DOI] [PubMed] [Google Scholar]
- 30.González CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer 2013; 133:1023–1032. doi: 10.1002/ijc.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 2010; 105:493–498. doi: 10.1038/ajg.2009.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Zimaity HM, Ota H, Graham DY, Akamatsu T, Katsuyama T. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 2002; 94:1428–1436. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 33.Iida F, Kusama J. Gastric carcinoma and intestinal metaplasia. Significance of types of intestinal metaplasia upon development of gastric carcinoma. Cancer 1982; 50:2854–2858. doi: 10.1002/1097-0142(19821215)50:12 <2854::aid-cncr2820501227>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Yu XW, Xu Q, Xu Y, Gong YH, Yuan Y. Expression of the E-cadherin/β-catenin/tcf-4 pathway in gastric diseases with relation to Helicobacter pylori infection: clinical and pathological implications. Asian Pac J Cancer Prev 2014; 15:215–220. doi: 10.7314/apjcp.2014.15.1.215. [DOI] [PubMed] [Google Scholar]
- 35.Jang BG, Lee BL, Kim WH. Distribution of LGR5+ cells and associated implications during the early stage of gastric tumorigenesis. PLoS One 2013; 8:e82390.doi: 10.1371/journal.pone.0082390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lickert H, Kemler R. Functional analysis of cis-regulatory elements controlling initiation and maintenance of early Cdx1 gene expression in the mouse. Dev Dyn 2002; 225:216–220. doi: 10.1002/dvdy.10149. [DOI] [PubMed] [Google Scholar]
- 37.Jung IM, Chung JK, Kim YA, Kim JE, Heo SC, Ahn YJ, et al. Epstein-Barr virus, beta-catenin, and E-cadherin in gastric carcinomas. J Korean Med Sci 2007; 22:855–861. doi: 10.3346/jkms.2007.22.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong NA, Britton MP, Choi GS, Stanton TK, Bicknell DC, Wilding JL, et al. Loss of CDX1 expression in colorectal carcinoma: promoter methylation, mutation, and loss of heterozygosity analyses of 37 cell lines. Proc Natl Acad Sci U S A 2004; 101:574–579. doi: 10.1073/pnas.0307190101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domon-Dell C, Freund JN. Stimulation of Cdx1 by oncogenic beta-catenin/Tcf4 in colon cancer cells; opposite effect of the CDX2 homeoprotein. FEBS Lett 2002; 518:83–87. doi: 10.1016/s0014-5793(02)02650-9. [DOI] [PubMed] [Google Scholar]


