Abstract
The oxygen reserve index (ORI) is a new technology that provides real-time, non-invasive, and continuous monitoring of patients’ oxygenation status. This review aimed to discuss its clinical utility, prospect and limitations. A systematic literature search of PubMed, MEDLINE, Google Scholar, and ScienceDirect was performed with the keywords of “oxygen reserve index,” “ORI,” “oxygenation,” “pulse oximetry,” “monitoring,” and “hyperoxia.” Original articles, reviews, case reports, and other relevant articles were reviewed. All articles on ORI were selected. ORI can provide an early warning before saturation begins to decrease and expands the ability to monitor the human body's oxygenation status noninvasively and continuously with the combination of pulse oximetry so as to avoid unnecessary hyperoxia or unanticipated hypoxia. Although the technology is so new that it is rarely known and has not been applied to routine practices in hospitals, it shows good prospects for critical care, oxygen therapy, and intraoperative monitoring.
Keywords: Oxygen reserve index (ORI), Oxygenation, Pulse oximetry, Monitoring, Hyperoxia
Introduction
The oxygen status of arterial blood is generally classified into 3 categories: hypoxia (less than normal oxygenation, partial pressure of oxygen [PaO2]: 0–80 mmHg), normoxia (normal oxygenation, PaO2: 81–100 mmHg) and hyperoxia (higher than normal oxygenation, PaO2 > 100 mmHg). Although there are no unified standards, hyperoxia is roughly subdivided into mild hyperoxia (PaO2: 101–200 mmHg) and severe hyperoxia (PaO2 > 200 mmHg).[1] Some researchers divide hyperoxia into three subcategories: mild (PaO2: 100–199 mmHg), moderate (PaO2: 200–299 mmHg), and severe (PaO2 > 300 mmHg).[2] PaO2 of 100 to 200 mmHg is particularly notable in mild hyperoxia. At this range, the patient's oxygenation status is slightly above normoxia, which enriches the body with oxygen but does not pose additional risk or harm from high PaO2 described in many reports; contrarily, it is associated with lower mortality.[3,4] Consequently, an excess PaO2 of approximately 100 mmHg can be regarded as the oxygen reserve in the human body corresponding to the original description of the oxygen reserve index (ORI) by Scheeren et al.[1]
Both hypoxia and normoxia can be assessed noninvasively and continuously using pulse oximetry, the assessment of hyperoxia predominantly relied on the invasive and intermittent arterial blood gas analysis (aBGA) before the introduction of ORI. ORI is a dimensionless index with scores ranging from 0 to 1 at PaO2 from approximately 100 to 200 mmHg, which reflects the oxygenation status of mild hyperoxia.[1,5–7] At present, although the detection of severe hyperoxia still depends on invasive methods of monitoring, ORI can evaluate a part of the hyperoxic range noninvasively.
How is Oxygen Reserve Index Recorded?
In the hypoxia and normoxia range (PaO2 ≤ 100 mmHg), the pulse oxygen saturation (SpO2) can be evaluated using pulse oximetry. When PaO2 exceeds 100 mmHg, arterial oxygen saturation (SaO2) reaches 100% and then remains unchanged, but mixed venous oxygen saturation (SvO2) keeps rising. The change in SvO2 changes the absorption of the incident light, thus changing the signals measured with the increase in PaO2. These signals can be extracted with Masimo Rainbow Signal Extraction Technology. In addition, an increase in PaO2 beyond approximately 200 mmHg allows SvO2 to reach a plateau as well. Consequently, ORI is recorded by optically detecting the changes in SvO2 after SaO2 reaches maximum saturation and before SvO2 reaches maximum saturation. More specifically, ORI is sensitive to the changes in PaO2 with the range of 100 to 200 mmHg.[1]
Correlation Between Oxygen Reserve Index and PaO2
Changes in ORI scores within 0–1 can indirectly reflect the changes in PaO2 within 100 to 200 mmHg. Although ORI and PaO2 do not show one-to-one correspondence, they positively correlate within the aforementioned ranges.
A regression analysis showed a stronger correlation between ORI and PaO2 at PaO2 ≤ 240mmHg (r2 = 0.536) than at PaO2 ≥ 240 mmHg (r2 = 0.0016). Based on this finding, researchers concluded that there is a positive correlation between ORI and PaO2 at PaO2 ≤ 240 mmHg but that there is no correlation between ORI and PaO2 at PaO2 > 240 mmHg. At PaO2 > 100 mmHg, all ORI scores were over 0.24; at PaO2 > 150 mmHg, ORI scores were over 0.55 in 96.6% of the samples. These findings suggest that ORI > 0.24 manifests PaO2 ≥ 100 mmHg. Similarly, an ORI score of 0.55 appears to be the lower threshold for PaO2 > 150 mmHg.[8]
Various Oxygenation Monitoring Methods
Pulse oximetry
Pulse oximetry provides continuous, non-invasive, and real-time monitoring of SpO2. However, the range of detection is limited to 0 to 100 mmHg, and it fails to indicate the downward trend in PaO2 before saturation begins to plummet.[9,10]
Blood gas analysis (BGA)
BGA is the gold standard method to measure oxygenation using PaO2. However, it is invasive, mostly intermittent, and occasionally delayed.[11] Recently, continuous intra-arterial BGA has been introduced.[12–14] However, it is relatively impractical for real-time, point-of-care assessments.
Oxygen reserve index
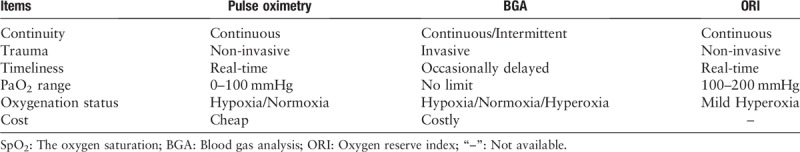
ORI is a novel, continuous, non-invasive, and real-time parameter to assess PaO2 levels in the range of 100 to 200 mmHg. It provides real-time oxygenation monitoring and indicates the downward trend of PaO2, thus alarming clinicians early of the changes in the oxygenation status [Table 1].[1,5–7]
Table 1.
Comparison among SpO2, BGA, and ORI on monitoring oxygenation status.
Clinical Benefits of ORI
Early warning for desaturation
In a pilot study involving 25 children who had undergone general anesthesia after endotracheal intubation, researchers from the University of Texas Southwestern and Children's Medical Center found that ORI assisted clinicians in identifying imminent changes 31.5 s (median; interquartile range, 19.0–34.3 s) before the occurrence of a noticeable drop in SpO2.[6] In another study involving 16 adults who had undergone rapid-sequence induction of general anesthesia before endotracheal intubation, researchers from the Fukushima Medical University School of Medicine also found that ORI could predict a hypoxic state approximately 30 s before the decline in SpO2.[7] Moreover, a validation study involving healthy and conscious volunteers with spontaneous breathing clearly showed that a decline in ORI occurred well before the changes in SpO2.[15] In 2018, a study in Japan found that ORI decreased earlier than SpO2 during one-lung ventilation (OLV),[16] and in 2019, Spanish anesthesiologists declared that ORI predicts hypoxemia during OLV.[17] In addition to these prospective clinical studies, case reports support the role of ORI in early alarm for oxygenation deterioration as well.[18,19] Collectively, the aforementioned studies using different methods and objects demonstrate that ORI predicts desaturation in anesthesia induction and surgery under general anesthesia and awake conditions, in double- and single-lung ventilations, and in adults and children.
In the oxyhemoglobin dissociation curve, the relationship between PaO2 and SaO2 is not linear. In other words, when PaO2 is greater than 80 mmHg, SaO2 may continue to be greater than 95%. Nevertheless, once PaO2 decreases to less than 70 mmHg, SaO2 declines rapidly. This physiological phenomenon is called “slippery slope.”[9] Sudden occurrence of “slippery slope” is undoubtedly a huge challenge for unprepared clinicians. In view of this, pulse oximetry alone is a late detector of hypoxia. Fortunately, “slippery slope,” the enemy that lies in the shadows and can attack the patients at any time, can now be detected 30 s ahead via ORI. Within 30 s, clinicians can make the right decision and respond quickly before rapid desaturation begins so as to avoid hypoxemia-related complications. This is definitely not an overstatement. A case report revealed that doctors accidentally discovered the discontinuation of the oxygen supply through ORI.[19] Therefore, the sharp decline in ORI with an unchanged trend of SpO2 should attract the doctor's attention and vigilance.
Although there are still no reports on the application of ORI in the intensive care unit (ICU), we have every reason to believe that ORI can play an important role in the early warning of desaturation for critically ill patients with acute respiratory failure or other conditions in the ICU. And another entity that deserves to be highlighted is the ORI alarm. However, this alarm activation was predominantly based on the real-time fractional rate of change in ORI rather than on the specific oxygen reserve.
ORI in oxygen therapy
Oxygen supplementation has a widespread and essential application in the perioperative and ICU settings to prevent potential hypoxia-related damage. Hypoxia is easily noticed with the application of pulse oximetry, but its diagnosis depends on BGA, which is invasive and intermittent. Usually, doctors are well-concerned about hypoxia and worry that patients will approach “slippery slope”; therefore, they treat patients with high-flow oxygen. As a result, non-anoxic patients who do not require oxygen therapy are supplemented with excess oxygen, and hypoxic patients are supplemented with inappropriately high concentrations of oxygen. In fact, unnecessary oxygen prescriptions to non-hypoxic patients are a common mistake in respiratory care.[20–23]
Scientific evidence suggests that supplemental oxygen leading to hyperoxia may have potential risks in various clinical situations,[24–27] including deleterious effects on the cardiovascular system, such as systemic vasoconstriction and decrease in the cardiac output and coronary flow, which may result in myocardial infarction,[28–30] nervous system, such as reduction in the cerebral blood flow,[31] and respiratory system, such as atelectasis and increased intrapulmonary shunting, etc.[32] Especially, in critically ill patients, many studies reported a significant correlation between increased mortality and hyperoxia.[2–4,33–36] However, hyperoxia is not always harmful. As mentioned, mild hyperoxia seems to be relatively safe. Recent studies found that the probability of in-hospital deaths Table 1 showed its lowest values at PaO2 values within 110 to 160 mmHg.[3,4]
Attention should be increased on the dangers of prolonged hyperoxia since the harms of severe hyperoxia and hypoxia are well-recognized by all clinicians,[37] and the need for a more precise oxygen therapy is increasing. As we already know, the range for PaO2 monitored using pulse oximeter is limited to 0 to 100 mmHg while that monitored using ORI is limited to 100 to 200 mmHg. Both methods are non-invasive, continuous, and real-time. Therefore, we could infer that ORI in conjunction with pulse oximetry can extend the real-time, continuous, and non-invasive visibility of a patient's oxygenation status up to a PaO2 of 0 to 200 mmHg, which could not be previously monitored. Theoretically, the application of ORI could change the visibility of the patient's oxygenation status [Table 2].[1]
Table 2.
Comparison of visibility among different methods.

Thus, clinicians may assess patients’ oxygenation status more accurately and control the titration of FiO2 more precisely, without BGA to prevent unexpected hypoxia or unintended hyperoxia in the ICU, operating room, or ward. Recently, a case reported in India successfully demonstrated this clinical value of ORI. FiO2 was titrated to 0.4 to 0.5 to maintain an ORI score between 0 and 0.3 and SpO2 above 97% in a neonate undergoing re-exploration of a tracheoesophageal fistula to avoid excessive oxygenation while maintaining a marginal oxygen reserve.[38] On the other hand, Keisuke Yoshida et al[39] validated the important role of ORI to avoid excessive hyperoxia during general anesthesia by adjusting ORI.
ORI in preoxygenation
Preoxygenation is a universal procedure performed before general induction and endotracheal intubation worldwide to increase the patient's oxygen stores and prolong the duration of safe apnea oxygenation.[40–42] Additionally, preoxygenation is a standard technique in general anesthesia but is frequently neglected. With the application of pulse oximetry alone as a monitoring tool, doctors cannot guarantee that the patient's PaO2 would be greater than 100 mmHg or that the intrapulmonary oxygen would keep increasing, as anticipated. Particularly, in obese or critically ill patients, the common methods of 3 min of tidal breathing, four deep breaths within 30 s, or eight deep breaths within 60 s may not achieve high-quality preoxygenation.[43,44] Therefore, ORI may assist us in identifying unsuccessful preoxygenation. As aforementioned, ORI > 0.24 indicates PaO2 ≥ 100 mmHg, and ORI > 0.55 appears to be the lower threshold to indicate PaO2 ≥ 150 mmHg.[5] During preoxygenation, if the ORI scores do not show an upward trend and stay relatively low and even if SaO2 has reached 100%, it is necessary to perform further interventions, such as positive-pressure ventilation, continuous positive airway pressure, positive end-expiratory pressure, bi-level positive airway pressure, and high-flow nasal oxygen.[45,46] However, although ORI could approach the respiratory management individualized in theory, it is regrettable that there has been no relevant clinical study to confirm this point.
Influence of Indigo Carmine on ORI
Indigo Carmine is a blue dye that is generally regarded as safe and biologically inactive. It is rapidly excreted in the urine and routinely administered intravascularly during urologic and gynecologic procedures to localize the ureteral orifices and to identify severed ureters and fistulous communications.[47] The effects of indigo carmine on SpO2, hemoglobin, and regional cerebral saturation have been reported.[48–50]
Recently, a retrospective study demonstrated that the intravenous injection of indigo carmine could greatly influence the ORI score. In all 19 patients evaluated in this retrospective study, ORI decreased rapidly after the intravenous injection of indigo carmine, and in 10 of the 19 patients, it was reduced to 0. The median lowest ORI score was 0 (range: 0–0.16), and the median time to reach the lowest ORI score was 2 min (range: 1–4 min) after indigo-carmine injection. Furthermore, ORI scores returned to the pre-injection level within 20 min in 13 of the 19 patients, and the median time to return to the pre-injection level was 10 min (range: 6–16 min).[51]
Indigo Carmine is a water-soluble dye with a 4 to 5-min half-life after its intravenous injection, and its peak light absorption is at 620 nm.[52] The close numerical value of the wavelength of the ORI recording to the absorbance of indigo carmine may explain the effects of indigo carmine on ORI.[51] In other words, the desaturations are artificial, and the rapid decrease in ORI does not indicate real hypoxia in patients. A transient decrease in ORI scores during urologic or gynecologic surgery is not a cause of panic. We should first verify if indigo carmine was used intravascularly.
In addition, except for indigo carmine, indocyanine green and methylene blue can disturb the measurement recorded with pulse oximeter.[52,53] Moreover, an interference of cerebral near-infrared oximetry by bilirubin has been reported in patients with icterus,[54] and with the increasing incidence of tumors, fluorescence-guided surgery is increasingly being used for tumor resection. Therefore, despite the lack of relevant clinical studies, we should consider the potential interference of various dyes and lights in the ORI measurements.
The greatest limitation of ORI at this stage is that the technology is too new. There seems to be a dearth of evidence for the useful application of ORI. Although ORI has been validated for several clinical utilities, it does not answer all the questions perfectly yet.[55] Naturally, clinicians might think, “Does this stuff really work? It looks like a toy, not a tool.”[56] Indeed, new things are always doubted, as Saugel said, there are no definite answers. Although ORI has a broad application in theory and good application in prospect, its evidence is insufficient. Therefore, we need more high-quality clinical studies on the clinical value of ORI.
Regardless of the original disease, the patient's death had ultimately resulted from lack of oxygen delivery to the brain or heart. Therefore, monitoring and treatment of supplemental oxygen is a race against time. Obviously, ORI is an important tool assisting us to win this race, as it provided clinicians with valuable time of approximately 30 s in advance to correctly respond to the impending hypoxia and to save the patient's life.
In addition, ORI enables clinicians to assess a patient's oxygenation status more accurately without the application of BGA, which avoids unnecessary hyperoxia and unexpected hypoxia to a large extent. However, we must pay close attention to patients who have been intravenously injected with dyes or lights, which would interfere with the ORI measurement. Studies are underway on the application prospect of ORI in various settings. This new technology could provide us with new discoveries in physiology and pathophysiology and open new horizons in the monitoring and treatment of patients.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81873798).
Conflicts of interest
None.
Footnotes
How to cite this article: Chen ST, Min S. Oxygen reserve index, a new method of monitoring oxygenation status: what do we need to know?. Chin Med J 2020;133:229–234. doi: 10.1097/CM9.0000000000000625
References
- 1.Scheeren T, Belda FJ, Perel A. The oxygen reserve index (ORI): a new tool to monitor oxygen therapy. J Clin Monit Comput 2018; 32:379–389. doi: 10.1007/s10877-017-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jakutis G, Norkienė I, Ringaitienė D, Jovaiša T. Severity of hyperoxia as a risk factor in patients undergoing on-pump cardiac surgery. Acta Med Litu 2017; 24:153–158. doi: 10.6001/actamedica.v24i3.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helmerhorst HJ, Arts DL, Schultz MJ, van der Voort PH, Abu-Hanna A, de Jonge E, et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit Care Med 2017; 45:187–195. doi: 10.1097/CCM.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 4.de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care 2008; 12:R156.doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpao AF, Gálvez JA. When seconds count, buy more time: the oxygen reserve index and its promising role in patient monitoring and safety. Anesthesiology 2016; 124:750–751. doi: 10.1097/ALN.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 6.Szmuk P, Steiner JW, Olomu PN, Ploski RP, Sessler DI, Ezri T. Oxygen reserve index: a novel noninvasive measure of oxygen reserve--a pilot study. Anesthesiology 2016; 124:779–784. doi: 10.1097/ALN.0000000000001009. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida K, Isosu T, Noji Y, Hasegawa M, Iseki Y, Oishi R, et al. Usefulness of oxygen reserve index (ORi™), a new parameter of oxygenation reserve potential, for rapid sequence induction of general anesthesia. J Clin Monit Comput 2018; 32:687–691. doi: 10.1007/s10877-017-0068-1. [DOI] [PubMed] [Google Scholar]
- 8.Applegate RL, 2nd, Dorotta IL, Wells B, Juma D, Applegate PM. The relationship between oxygen reserve index and arterial partial pressure of oxygen during surgery. Anesth Analg 2016; 123:626–633. doi: 10.1213/ANE.0000000000001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beasley R, McNaughton A, Robinson G. New look at the oxyhaemoglobin dissociation curve. Lancet 2006; 367:1124–1126. doi: 10.1016/S0140-6736(06)68488-2. [DOI] [PubMed] [Google Scholar]
- 10.Davis DP, Hwang JQ, Dunford JV. Rate of decline in oxygen saturation at various pulse oximetry values with prehospital rapid sequence intubation. Prehosp Emerg Care 2008; 12:46–51. doi: 10.1080/10903120701710470. [DOI] [PubMed] [Google Scholar]
- 11.Ganter MT, Schneider U, Heinzelmann M, Zaugg M, Lucchinetti E, Zollinger A, et al. How often should we perform arterial blood gas analysis during thoracoscopic surgery. J Clin Anesth 2007; 19:569–575. doi: 10.1016/j.jclinane.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Umegaki T, Kikuchi O, Hirota K, Adachi T. Comparison of continuous intraarterial blood gas analysis and transcutaneous monitoring to measure oxygen partial pressure during one-lung ventilation. J Anesth 2007; 21:110–111. doi: 10.1007/s00540-006-0458-x. [DOI] [PubMed] [Google Scholar]
- 13.Nunomiya S, Tsujimoto T, Tanno M, Matsuyama N, Ohtake K, Kubota T. Clinical evaluation of a new continuous intraarterial blood gas monitoring system in the intensive care setting. J Anesth 1996; 10:163–169. doi: 10.1007/BF02471384. [DOI] [PubMed] [Google Scholar]
- 14.Menzel M, Soukup J, Henze D, Engelbrecht K, Senderreck M, Scharf A, et al. Experiences with continuous intra-arterial blood gas monitoring: precision and drift of a pure optode-system. Intensive Care Med 2003; 29:2180–2186. doi: 10.1007/s00134-003-1962-1. [DOI] [PubMed] [Google Scholar]
- 15.Vos JJ, Willems CH, van Amsterdam K, van den Berg JP, Spanjersberg R, Struys M, et al. Oxygen reserve index: validation of a new variable. Anesth Analg 2019; 129:409–415. doi: 10.1213/ANE.0000000000003706. [DOI] [PubMed] [Google Scholar]
- 16.Koishi W, Kumagai M, Ogawa S, Hongo S, Suzuki K. Monitoring the Oxygen Reserve Index can contribute to the early detection of deterioration in blood oxygenation during one-lung ventilation. Minerva Anestesiol 2018; 84:1063–1069. doi: 10.23736/S0375-9393.18.12622-8. [DOI] [PubMed] [Google Scholar]
- 17.Alday E, Nieves JM. Planas A. Oxygen reserve index predicts hypoxemia during one-lung ventilation: an observational diagnostic study. J Cardiothorac Vasc Anesth 2019; doi: 10.1053/j.jvca.2019.06.035. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida K, Isosu T, Imaizumi T, Obara S, Murakawa M. Oxygen Reserve Index (ORiTM) as an alarm for oxygenation deterioration in pediatric tracheostomaplasty: a case report. Paediatr Anaesth 2019; 29:1151–1153. doi: 10.1111/pan.13739. [DOI] [PubMed] [Google Scholar]
- 19.Niwa Y, Shiba J, Fujita H, Oka R, Takeuchi M. Oxygen reserve index (ORi™) contributes to prediction of hypoxemia and patient safety during tracheal stent insertion using rigid bronchoscopy: a case report. J Clin Monit Comput 2019; 33:1011–1014. doi: 10.1007/s10877-018-0232-2. [DOI] [PubMed] [Google Scholar]
- 20.Albin RJ, Criner GJ, Thomas S, Abou-Jaoude S. Pattern of non-ICU inpatient supplemental oxygen utilization in a university hospital. Chest 1992; 102:1672–1675. doi: 10.1378/chest.102.6.1672. [DOI] [PubMed] [Google Scholar]
- 21.Kor AC, Lim TK. Audit of oxygen therapy in acute general medical wards following an educational programme. Ann Acad Med Singapore 2000; 29:177–181. [PubMed] [Google Scholar]
- 22.Skowronski G. The use of oxygen in acute exacerbations of chronic obstructive pulmonary disease: a prospective audit of pre-hospital and hospital emergency management. Clin Med (Lond) 2003; 3:184–185. author reply 185–6. doi: 10.7861/clinmedicine.3-2-183. [PubMed] [Google Scholar]
- 23.de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med 2011; 37:46–51. doi: 10.1007/s00134-010-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care 2003; 48:611–620. [PubMed] [Google Scholar]
- 25.Plant PK, Owen JL, Elliott MW. One year period prevalence study of respiratory acidosis in acute exacerbations of COPD: implications for the provision of non-invasive ventilation and oxygen administration. Thorax 2000; 55:550–554. doi: 10.1136/thorax.55.7.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rønning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke 1999; 30:2033–2037. doi: 10.1161/01.str.30.10.2033. [DOI] [PubMed] [Google Scholar]
- 27.Davis PG, Tan A, O’Donnell CP, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet 2004; 364:1329–1333. doi: 10.1016/S0140-6736(04)17189-4. [DOI] [PubMed] [Google Scholar]
- 28.Asfar P, Singer M, Radermacher P. Understanding the benefits and harms of oxygen therapy. Intensive Care Med 2015; 41:1118–1121. doi: 10.1007/s00134-015-3670-z. [DOI] [PubMed] [Google Scholar]
- 29.Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Weatherall M, Beasley R. Routine use of oxygen in the treatment of myocardial infarction: systematic review. Heart 2009; 95:198–202. doi: 10.1136/hrt.2008.148742. [DOI] [PubMed] [Google Scholar]
- 30.Baron JF, Vicaut E, Hou X, Duvelleroy M. Independent role of arterial O2 tension in local control of coronary blood flow. Am J Physiol 1990; 258:H1388–H1394. doi: 10.1152/ajpheart.1990.258.5.H1388. [DOI] [PubMed] [Google Scholar]
- 31.Niijima S, Shortland DB, Levene MI, Evans DH. Transient hyperoxia and cerebral blood flow velocity in infants born prematurely and at full term. Arch Dis Child 1988; 63:1126–1130. doi: 10.1136/adc.63.10_spec_no.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner PD, Laravuso RB, Uhl RR, West JB. Continuous distributions of ventilation-perfusion ratios in normal subjects breathing air and 100 per cent O2. J Clin Invest 1974; 54:54–68. doi: 10.1172/JCI107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You J, Fan X, Bi X, Xian Y, Xie D, Fan M, et al. Association between arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. J Crit Care 2018; 47:260–268. doi: 10.1016/j.jcrc.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, de Jonge E. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, meta-analysis, and meta-regression of cohort studies. Crit Care Med 2015; 43:1508–1519. doi: 10.1097/CCM.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 35.Damiani E, Adrario E, Girardis M, Romano R, Pelaia P, Singer M, et al. Arterial hyperoxia and mortality in critically ill patients: a systematic review and meta-analysis. Crit Care 2014; 18:711.doi: 10.1186/s13054-014-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raman S, Prince NJ, Hoskote A, Ray S, Peters MJ. Admission PaO2 and mortality in critically Ill children: a cohort study and systematic review. Pediatr Crit Care Med 2016; 17: e444-444e450 doi: 10.1097/PCC.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 37.Martin DS, Grocott MP., III Oxygen therapy in anaesthesia: the yin and yang of O2. Br J Anaesth 2013; 111:867–871. doi: 10.1093/bja/aet291. [DOI] [PubMed] [Google Scholar]
- 38.Ray S, Kulkarni KS, Dave NM, Chincholi I. The utility of the oxygen reserve index™ in a neonate undergoing re-exploration of a tracheoesophageal fistula. Indian J Anaesth 2018; 62:233–234. doi: 10.4103/ija.IJA_778_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshida K, Isosu T, Noji Y, Ebana H, Honda J, Sanbe N, et al. Adjustment of oxygen reserve index (ORi™) to avoid excessive hyperoxia during general anesthesia. J Clin Monit Comput 2019; doi: 10.1007/s10877-019-00341-9. [DOI] [PubMed] [Google Scholar]
- 40.Nimmagadda U, Salem MR, Crystal GJ. Preoxygenation: physiologic basis, benefits, and potential risks. Anesth Analg 2017; 124:507–517. doi: 10.1213/ANE.0000000000001589. [DOI] [PubMed] [Google Scholar]
- 41.Ricard JD. Hazards of intubation in the ICU: role of nasal high flow oxygen therapy for preoxygenation and apneic oxygenation to prevent desaturation. Minerva Anestesiol 2016; 82:1098–1106. [PubMed] [Google Scholar]
- 42.Bouroche G, Bourgain JL. Preoxygenation and general anesthesia: a review. Minerva Anestesiol 2015; 81:910–920. [PubMed] [Google Scholar]
- 43.Shah U, Wong J, Wong DT, Chung F. Preoxygenation and intraoperative ventilation strategies in obese patients: a comprehensive review. Curr Opin Anaesthesiol 2016; 29:109–118. doi: 10.1097/ACO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 44.Pandit JJ, Duncan T, Robbins PA. Total oxygen uptake with two maximal breathing techniques and the tidal volume breathing technique: a physiologic study of preoxygenation. Anesthesiology 2003; 99:841–846. doi: 10.1097/00000542-200310000-00015. [DOI] [PubMed] [Google Scholar]
- 45.Mosier JM, Hypes CD, Sakles JC. Understanding preoxygenation and apneic oxygenation during intubation in the critically ill. Intensive Care Med 2017; 43:226–228. doi: 10.1007/s00134-016-4426-0. [DOI] [PubMed] [Google Scholar]
- 46.McNarry AF, Patel A. The evolution of airway management - new concepts and conflicts with traditional practice. Br J Anaesth 2017; 119:i154–i166. doi: 10.1093/bja/aex385. [DOI] [PubMed] [Google Scholar]
- 47.Lee WJ, Jang HS. Cardiac arrest from intravenous indigo carmine during laparoscopic surgery -A case report. Korean J Anesthesiol 2012; 62:87–90. doi: 10.4097/kjae.2012.62.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isosu T, Obara S, Hakozaki T, Imaizumi T, Iseki Y, Mogami M, et al. Effects of indigo carmine intravenous injection on noninvasive and continuous total hemoglobin measurement with using the Revision L sensor. J Clin Monit Comput 2017; 31:485–486. doi: 10.1007/s10877-016-9850-8. [DOI] [PubMed] [Google Scholar]
- 49.Isosu T, Satoh T, Oishi R, Imaizumi T, Hakozaki T, Obara S, et al. Effects of indigo carmine intravenous injection on noninvasive and continuous total hemoglobin measurement. J Clin Monit Comput 2016; 30:313–316. doi: 10.1007/s10877-015-9719-2. [DOI] [PubMed] [Google Scholar]
- 50.McDonagh DL, McDaniel MR, Monk TG. The effect of intravenous indigo carmine on near-infrared cerebral oximetry. Anesth Analg 2007; 105:704–706. doi: 10.1213/01.ane.0000271917.20429.50. [DOI] [PubMed] [Google Scholar]
- 51.Isosu T, Yoshida K, Oishi R, Imaizumi T, Iseki Y, Sanbe N, et al. Effects of indigo carmine intravenous injection on oxygen reserve index (ORi™) measurement. J Clin Monit Comput 2018; 32:693–697. doi: 10.1007/s10877-017-0064-5. [DOI] [PubMed] [Google Scholar]
- 52.Fujita Y, Yamamoto T, Fuse M, Kobayashi N, Takeda S, Aoyagi T. Pulse dye densitometry using indigo carmine is useful for cardiac output measurement, but not for circulating blood volume measurement. Eur J Anaesthesiol 2004; 21:632–637. doi: 10.1017/s0265021504008087. [DOI] [PubMed] [Google Scholar]
- 53.Sidi A, Paulus DA, Rush W, Gravenstein N, Davis RF. Methylene blue and indocyanine green artifactually lower pulse oximetry readings of oxygen saturation. Studies in dogs. J Clin Monit 1987; 3:249–256. doi: 10.1007/bf03337379. [DOI] [PubMed] [Google Scholar]
- 54.Madsen PL, Skak C, Rasmussen A, Secher NH. Interference of cerebral near-infrared oximetry in patients with icterus. Anesth Analg 2000; 90:489–493. doi: 10.1097/00000539-200002000-00046. [DOI] [PubMed] [Google Scholar]
- 55.Campos JH, Sharma A. Predictors of hypoxemia during one-lung ventilation in thoracic surgery: is oxygen reserve index (ORi) the answer. J Cardiothorac Vasc Anesth 2019; doi: 10.1053/j.jvca.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 56.Saugel B, Belda FJ. The Oxygen Reserve Index in anesthesiology: a superfluous toy or a tool to individualize oxygen therapy. Minerva Anestesiol 2018; 84:1010–1012. doi: 10.23736/S0375-9393.18.13103-8. [DOI] [PubMed] [Google Scholar]


