Abstract
Background:
The effects of keto acid (KA) supplements on Chinese patients receiving maintenance hemodialysis (MHD) are unclear. This study aimed to evaluate the effects of KA supplementation on nutritional status, inflammatory markers, and bioelectric impedance analysis (BIA) parameters in a cohort of Chinese patients with MHD without malnutrition.
Methods:
This was a prospective, randomized, controlled, single-center clinical study conducted in 2011 till 2014. Twenty-nine patients with MHD were randomly assigned to a control (n = 14) or a KA (n = 15) group. The control group maintained a dietary protein intake of 0.9 g/kg/day. The KA group received additional KA supplement (0.1 g/kg/day). BIA was used to determine the lean tissue mass, adipose tissue mass, and body cell mass. The patients’ nutritional status, dialysis adequacy, and biochemical parameters were assessed at the ends of the third and sixth months with t test or Wilcoxon rank-sum test.
Results:
The daily total energy intake for both groups was about 28 kcal/kg/day. After 6 months, the Kt/V (where K is the dialyzer clearance of urea, t is the dialysis time, and V is the volume of the distribution of urea) was 1.33 ± 0.25 in KA group, and 1.34 ± 0.25 in the control group. The median triceps skin-fold thickness in KA group was 12.00 and 9.00 mm in the control group. In addition, the median hand-grip strength in KA group was 21.10 and 25.65 kg in the control group. There were no significant differences between the groups with respect to the anthropometry parameters, dialysis adequacy, serum calcium and phosphorus levels, inflammatory markers, and amino-acid profiles, or in relation to the parameters determined by BIA. Both groups achieved dialysis adequacy and maintained nutritional status during the study.
Conclusions:
In this cohort of Chinese patients with MHD, the patients in the control group whose dietary protein intake was 0.9 g/kg/day and total energy intake was 28 kcal/kg/day, maintained well nutritional status during study period. The KA supplement (0.1 g/kg/day) did not improve the essential amino acid/non-essential amino acid ratio, nor did it change the patients’ mineral metabolism, inflammatory parameters, or body compositions.
Keywords: Chinese, Hemodialysis, Keto acid, Nutrition
Introduction
Diets for patients with chronic kidney disease (CKD) have been recently reviewed regarding how dietary factors could affect the CKD complications treatments.[1] Although the benefits of a low-protein diet are debatable in relation to impeding CKD progression,[2] a very low-protein diet supplemented with keto analogs of essential amino acids is effective at ameliorating the metabolic disturbances associated with advanced CKD and at delaying the initiation of dialysis without causing any deleterious effects on a patient's nutritional status, and it could deliver better outcomes to these patients once dialysis has been initiated.[3,4] Nutritional status is a strong predictor of morbidity and mortality in patients receiving maintenance hemodialysis (MHD).[5] Patients with CKD who are on dialysis are at a very high risk of protein energy malnutrition.[6] It is well documented that malnourished patients have increased frequencies of hospital admissions, longer hospital stays, a lower quality of life, and an increased risk of death.[7]
The clinical practice guidelines for nutrition for patients with MHD,[8] which were established by the National Kidney Foundation's Kidney Disease Outcomes Quality Initiative (KDOQI), recommend a caloric intake of 35 kcal/kg/day and a dietary protein intake (DPI) of 1.2 g/kg/day. However, in practice, many patients fail to comply with these recommendations, because of dietary restrictions, anorexia, and socioeconomic limitations.[9] A comprehensive review of the findings from studies that examined the DPIs in patients with MHD determined that the total energy intakes (TEIs) and DPIs were below those recommended in the current nutrition guidelines.[10] For example, Ohkawa et al[11] investigated the association between the protein equivalent of nitrogen appearance that was normalized according to the ideal body weight (IBW), which is a protein intake index, and the skeletal muscle mass, and suggested that 0.9 to 1.1 g/kg/day of dietary protein was the minimum amount required to maintain adequate protein nutritional statuses in patients with hemodialysis (HD). Similar findings were reported from studies that examined the leucine turnover and nitrogen balances in sedentary healthy subjects (0.89 g/kg/day)[12] and in patients with MHD (0.9–1.0 g/kg/day).[13] During a 1-year study, Jiang et al[14] determined that a diet containing 0.6 to 0.8 g protein/kg/day could be safely administered to their cohort of Chinese peritoneal dialysis patients, and that supplementing the diet with keto acids (KAs), improved the preservation of residual renal function in relatively new peritoneal dialysis patients without significant malnutrition or inflammation.
Chinese patients with MHD have different dietary habits and lifestyles compared with those in western countries.[15] In this prospective, randomized, controlled, single-center clinical study, we aimed to demonstrate the safety of a DPI administered at 0.9 g/kg/day at maintaining the healthy nutritional statuses of Chinese patients with MHD and to investigate the effects of supplementing the diets with KAs (0.1 g/kg/day) on the patients’ anthropometric measurements, biochemical data, and the parameters determined by bioelectric impedance analysis (BIA) over a 6-month period.
Methods
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee at Peking Union Medical College Hospital (No. S 258), and written informed consent was obtained from each patient.
Study sample and design
This was a prospective, randomized, controlled, single-center clinical study conducted in 2011 till 2014. The patients were recruited from the HD center at Peking Union Medical College Hospital. Patients who had undergone HD for at least 3 months with clinically stable nutritional status, older than 18 years old and younger than 75 years old, who had not received supplements comprising essential amino acids or a KA compound were enrolled in the study. The exclusion criteria were patients who required renal transplantation, had severe infectious diseases, had malignant diseases or acquired immunodeficiency syndrome, had advanced organ dysfunction other than that in the kidneys, had enrolled in other clinical studies within the previous 3 months, were pregnant or lactating, had contraindications associated with KA supplementation, could not follow the prescriptions for DPIs or KA supplementation, and had inadequate dialysis.
Twenty-nine patients were enrolled in this study. We undertook pre-study investigations into the patients’ DPIs and TEIs, and found that the average DPI was 0.9 g/kg/day and that the average TEI was about 30 kcal/kg/day. The randomization was undertaken using study numbers in sealed envelopes. The patients were randomized to a control group (DPI: 0.9 g/kg/day) or a KA group [DPI: 0.9 g/kg/day + KA: 0.1 g/kg/day (Ketosteril®; Fresenius Kabi AG, Bad Homburg, Germany)], and the TEIs for both groups were about 30 kcal/kg/day.
Patient follow-up
During a 6-month follow-up period, all of the patients retained their previous medications and were managed in accordance with their daily routines. A dietitian followed up all 29 patients about the goal of the TEI and DPI before the study. A renal exchange list containing groups of food items with the same nutritional content was used during nutritional counseling. In addition, the patients were instructed to complete 3-day diet diaries, of which 1 day was a dialysis day, 1 day was an interdialysis day, and 1 day was a weekend day, every month during the study period. Each patient was assessed once a month by the same dietitian who monitored their clinical condition and nutritional status. The 3-day diet diaries were collected monthly to estimate the DPIs and the TEIs, and to monitor compliance. All of the diet diary data were analyzed using Keto Nutritional Assessment software, version 2.0 (Fresenius Kabi AG).
Parameters assayed during follow-up
An experienced dietitian estimated the DPIs and TEIs from the 3-day diet diaries. The plasma albumin (ALB) and pre-albumin (PA) levels, total lymphocyte counts, hemoglobin (Hgb) levels, and anthropometry parameters were used to assess a patient's nutritional status. The hand-grip strength and triceps skin-fold (TSF) thickness were used as somatometric parameters. BIA parameters, including the post-dialytic lean tissue mass (LTM), the adipose tissue mass (ATM), and the fat tissue mass, were measured trimonthly using a single-frequency body composition monitor (Fresenius Kabi AG). To evaluate dialysis adequacy, the urea kinetic modeling formula, Kt/V, was used, where K is the dialyzer clearance of urea, t is the dialysis time, and V is the volume of the distribution of urea, which is approximately equal to a patient's total body water. The normalized protein catabolic rate was used to monitor compliance with the diet. Since inflammation is another important factor that is associated with the malnutrition of dialysis patients,[16] the high-sensitivity C-reactive protein (CRP) levels were assayed. To assess the effects of KAs on lipid, amino acid, and mineral metabolism, we measured the levels of low-density lipoprotein cholesterol, lipoprotein(a), calcium (Ca), phosphate (P), and parathyroid hormone in the serum and the serum amino acid profiles. All of these markers were tested at baseline, and at the ends of the third and sixth months during the study.
The plasma or serum samples were obtained before dialysis. Routine biochemical assays were used to measure the ALB, PA, and Hgb levels, and the levels of the other biochemical parameters. All of the anthropometric measurements were undertaken after the HD sessions. The levels of 17 amino acids, namely, L-aspartic acid, L-glutamic acid, L-serine, L-histidine, glycine, L-threonine, L-arginine, L-alanine, L-tyrosine, L-cystine, L-valine, L-methionine, L-phenylalanine, L-isoleucine, L-leucine, L-lysine, and L-proline, were measured using high-performance liquid chromatography.
Statistical analyses
The data were presented as mean ± standard deviation or as the median (quartile [Q] 25–Q75) if the data were not normally distributed. The differences between the groups were compared using the Student's t test or Wilcoxon rank-sum test. The statistical analyses were performed using JMP® Pro software, version 13.0.0 (SAS Institute Inc., Cary, NC, USA). A value of P < 0.05 was considered statistically significant.
Results
Patients’ baseline characteristics
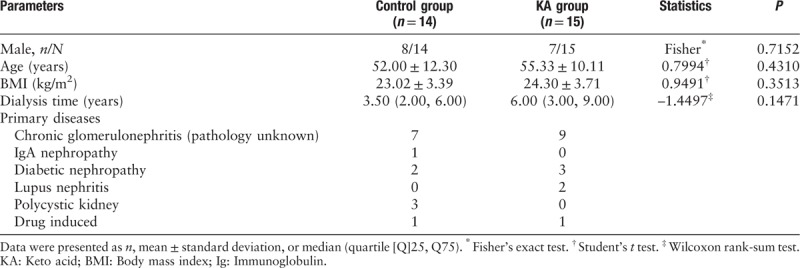
Fifteen male and 14 female patients were enrolled to participate in this prospective, randomized, controlled study. The patients were randomly assigned to the control group (n = 14) or the KA group (n = 15), and all of the patients completed the study. There were no significant differences between the groups with respect to age, gender, the body mass index, and the dialysis duration at baseline [Table 1].
Table 1.
Baseline characteristics of 29 Chinese maintenance hemodialysis patients who were randomized to the control group or the keto acid group.
Dietary compliance and dialysis adequacy
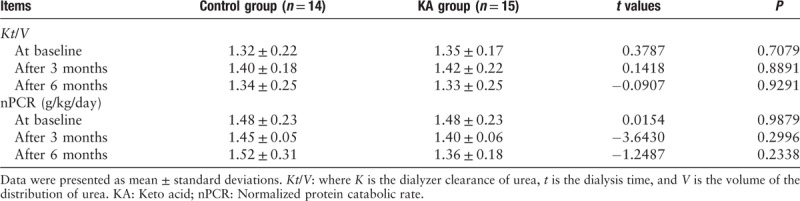
The data from the 3-day diet diaries showed that the patients in the control and KA groups were compliant with the DPI and TEI requirements. There were no significant differences between the control group and the KA group with respect to the mean TEIs (28.04 ± 6.63 vs. 27.68 ± 3.52 kcal/kg IBW/day) and the mean DPIs (0.95 ± 0.26 vs. 0.96 ± 0.30 g/kg IBW/day) during the trial [Figure 1]. The patients in the KA group consumed an additional 0.1 g/kg IBW/day of KAs. There were no significant differences between the control group and the KA group with respect to the normalized protein catabolic rate and the Kt/V, either at baseline or at the end of the sixth month [Table 2].
Figure 1.
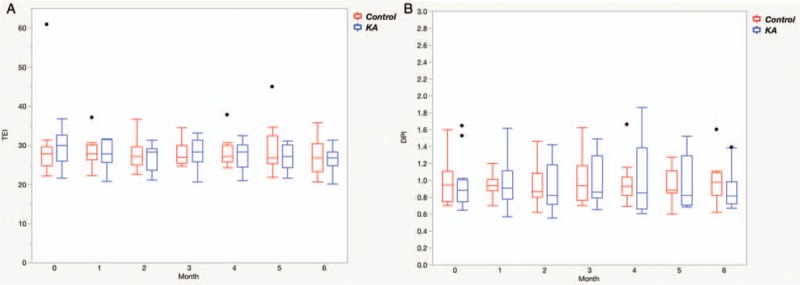
Estimated total energy intake (kcal/kg/day) (A) and dietary protein intake (g/kg/day) (B) in the control group (n = 14) and in the keto acid group (n = 15) during the 6-month observation period. KA: Keto acid; DPI: Dietary protein intake; TEI: Total energy intake. The black dots represent outliers.
Table 2.
The normalized protein catabolic rate and dialysis adequacy in both groups during the study.
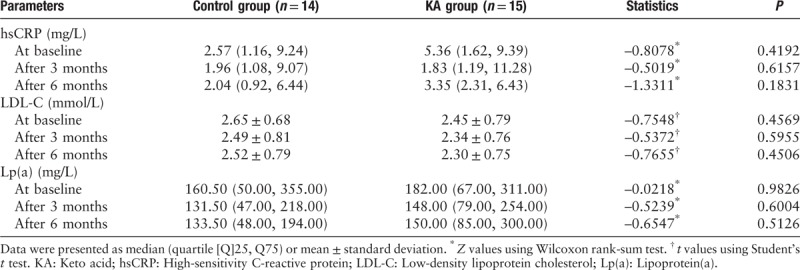
Nutritional assessment and calcium and phosphorus metabolism
Somatometry that involved assessing the TSF thickness and the hand-grip strength was used to estimate the storage of adipose tissue and muscle strength, respectively. The two groups did not differ significantly in relation to the TSF thickness or the hand-grip strength, either at baseline or during the study [Table 3]. There were no obvious changes in the plasma ALB, PA, and Hgb levels or in the total lymphocyte counts in either group during the study [Table 3], and there were no significant differences between the groups in relation to the serum Ca, P, or parathyroid hormone levels [Table 4].
Table 3.
Nutritional parameters determined for both groups during the study.
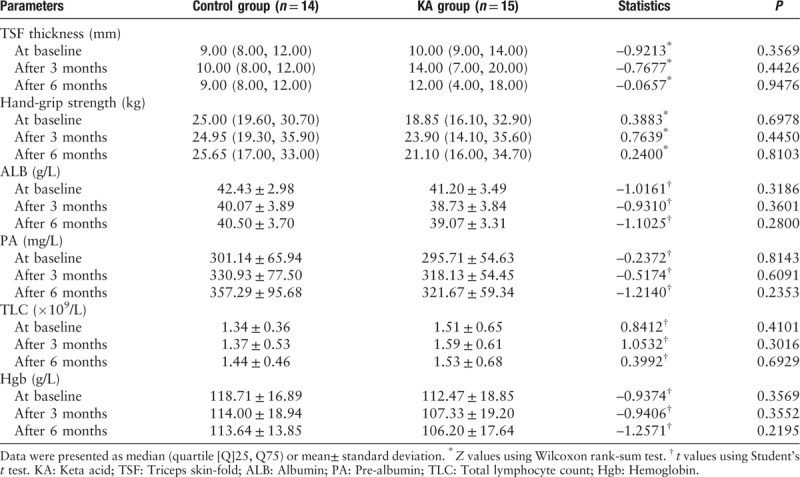
Table 4.
Serum calcium, phosphorus, and parathyroid hormone levels in both groups during the study.
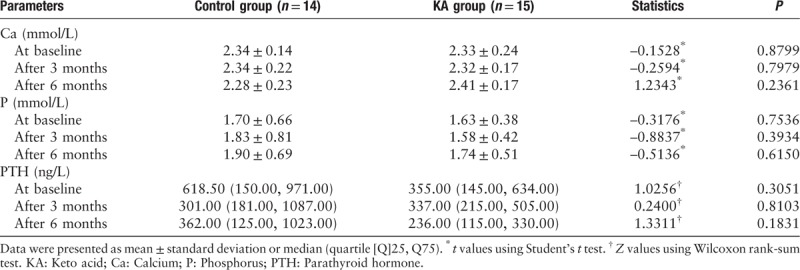
Inflammatory parameters and lipid profiles
There were no significant differences between the groups at baseline or during the study with respect to the serum high-sensitivity CRP, low-density lipoprotein cholesterol, and lipoprotein(a) levels that were monitored every 3 months [Table 5].
Table 5.
Serum high-sensitivity C-reactive protein, low-density lipoprotein cholesterol, and lipoprotein(a) levels in both groups during the study.
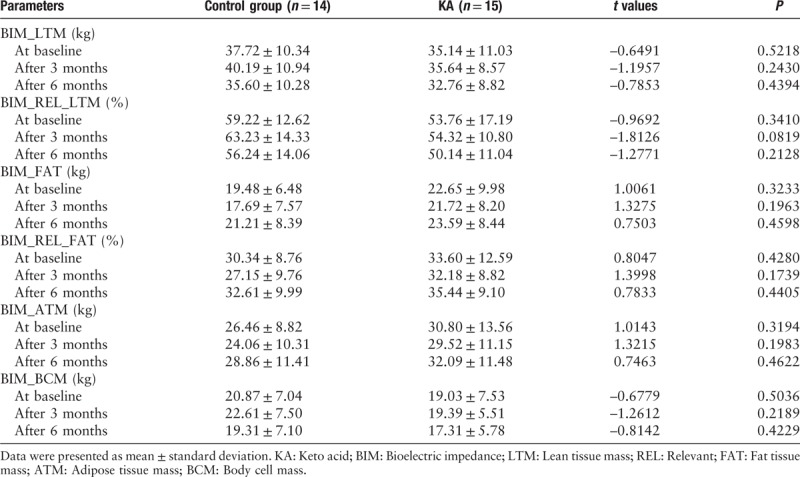
Bioelectrical impedance analysis
At baseline, the LTM in the control group (37.72 ± 10.34 kg) was similar to that in the KA group (35.14 ± 11.03 kg). After 6 months, the LTM in the control group (35.60 ± 10.28 kg) was similar to that in the KA group (32.76 ± 8.82 kg). The fat tissue mass, ATM, and body cell mass were similar in the control group and the KA group at baseline and after 6 months [Table 6].
Table 6.
Bioelectric impedance analysis of both groups during the study.
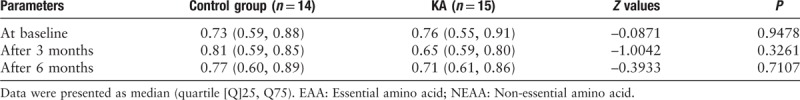
Amino acid profiles
There were no significant differences between the control group and the KA group with respect to any of the individual amino acids investigated in this study at baseline, at the end of the third month, or at the end of the sixth month (data not shown). There were no significant differences between the control group and the KA group in relation to the essential amino acid/non-essential amino acid ratios at baseline, at the end of the third month, or at the end of the sixth month [Table 7].
Table 7.
Serum amino acid profiles (EAA/NEAA) in both groups during the study.
Discussion
The findings from this study demonstrated that Chinese patients with MHD can maintain healthy nutritional statuses when their TEIs are approximately 28 kcal/kg IBW/day and their DPIs are 0.95 g/kg IBW/day. Bossola et al[17] measured the dietary calorie and protein intakes of 128 stable patients on HD at three centers, comprising one in a metropolitan area and two in urban areas of Italy, and they found that the mean dietary calorie and protein intakes of these patients were 22.9 ± 9.1 kcal/kg/day and 0.95 ± 0.76 g protein/kg/day, respectively. They concluded that the daily calorie and protein intakes by patients with MHD in Italy were lower than the recommended values. Our findings once again raise the important issue of the optimal TEIs and DPIs for patients with MHD, particularly those for Asian patients with MHD. The literature used to determine the recommended DPIs and TEIs for patients with MHD in the KDOQI guidelines was limited, and the studies were quite old.[8] Chen[18] proposed that given the advances in dialysis technology and the improvements in the treatment strategies for a variety of metabolic disorders and complications, the target DPI could be reduced.
Careful consideration should be given to determine the best DPI that can maintain a healthy nutritional status in a patient while avoiding an excessive P intake. Hyperphosphatemia is an independent risk factor for mortality in patients with MHD.[19] The findings from an epidemiologic study of 30,075 patients with MHD have shown that hyperphosphatemia is inevitable if the DPI is more than 0.9 g/kg/day.[20] Ichikawa et al[21] studied Japanese patients, and determined that a DPI of approximately 0.9 g/kg IBW/day was sufficient to maintain muscle mass as long as the TEI was greater than 35 kcal/kg/day.
In China, non-calcium-based phosphate binder is not universally available, which probably causes hyperphosphatemia more difficult to control. Li et al[22] studied 40 patients with MHD with uncontrolled hyperphosphatemia who were randomized to receive either a low DPI with a KA-supplement or a normal DPI for 8 weeks. The low-protein diet was individualized and the patients received total caloric intakes of 30 to 35 kcal/kg/day, protein intakes of 0.8 g/kg/day, and phosphate intakes of 500 mg/day. The KA dosage comprised 12 pills per day, which were equivalent to 0.1 g/kg/day if a patient's body weight was 72 kg. They concluded that restricting the DPI for a short period and including a KA supplement could reduce hyperphosphatemia and the level of Ca-P products while retaining stable nutritional statuses in patients with MHD, and that KA supplements benefit some patients with MHD who have hyperphosphatemia.[22]
The amino acid profile did not change in the KA group in our study nor did the KA supplement generate any measurable benefits in relation to the inflammation parameters, lipid profiles, metabolism of calcium and phosphorus, or the patients’ body compositions. These findings indicate that the supplement dose administered was minute relative to the DPI of 0.9 g/kg/day. Other possible explanations for the absence of any measurable effects include the small sample size, differences between the patient populations, the study's short duration, and interventional disparities among the different centers.[23]
Our experiences from this study bring us an impression, that even there would exist any potential positive effect from addition of KAs, such as reduction in morbidity and mortality, which could hardly be demonstrated scientifically given so many confounding factors present during a longer prospective observation of a large-size HD population. However, the evidence that is available demonstrates that nutrition is an indispensable component of the routine care and well-being of patients with MHD.[7] Indeed, as a consequence of this study, the physicians and nurses at our center are now utilizing the parameters used in this study to monitor the patients’ nutritional statuses. Hence, increasing numbers of patients will record their daily dietary intakes in diaries and request counseling from dietitians.
We have also emphasized the importance of routinely including BIA in assessments of the nutritional statuses of patients with MHD.[24] BIA is non-invasive and convenient. Serially recording the changes in the BIA parameters could provide physicians and researchers with greater experience in and more information about the value of BIA for assessing LTM, ATM, and body cell mass in diseases. Ikizler et al[25] used reactance values generated every 3 months by BIA as indirect measurements of the lean body mass in 73 patients with chronic HD over a 15-month period, and they found that the CRP levels and the reactance values were reliable predictors of hospitalization. Recently, Rimsevicius et al[26] determined that of the BIA markers of malnutrition, the most potent marker was the phase angle, which could play a role in predicting malnutrition in patients with MHD.
Clearly, this study has some limitations that are described next. The study's sample size was relatively small, the DPIs assigned to the control and the KA groups were not the same, there was less strong rationale for a comparatively low TEI recommended in patients with HD, and the study's duration was short for observations of malnutrition. Patient motivation is required in clinical studies of the nutritional statuses of patients with HD. Dietary changes and compliance are necessary if patients are to participate in these studies and pursue better clinical outcomes.[27,28] Despite this, we were unable to precisely monitor the DPIs and TEIs, because the study's design involved reviewing 3 days of data from the dietary diaries at monthly intervals only, which is an approach that is commonly used to monitor patient compliance. Another limitation of this study is lacking information of follow-up of residual renal function which will be influenced by nutritional status. Undertaking clinical studies to assess the benefit of nutritional interventions in patients with MHD is tedious, and the integrated network of personnel involved requires physicians, nurses, dietitians, the patients, the patients’ families, and technicians to work harmoniously as a team.
The findings from this study have shown that we should not only use the KDOQI guidelines to establish TEI and DPI goals for our patients. Moreover, these intakes should be individualized based on a patient's lifestyle, dietary preferences, biochemical markers, underlying diseases, cormorbidities, inflammatory status, family support, and compliance.
The scientific purpose of these studies is to deliver the message that nutrition is important for achieving the clinical outcomes for our patients and their practitioners. Meanwhile, we recognize that the more important fundamental requirement is to establish a practical way of educating our patients and to ensure that they are willing and able to follow the clinical path of nutritional care as part of their daily management.
Acknowledgements
The authors thank all of the participants for their cooperation.
Conflicts of interest
None.
Footnotes
How to cite this article: Li HL, Li H, Cao YF, Qi Y, Wang WQ, Liu SQ, Yang CD, Yu XY, Xu T, Zhu Y, Chen W, Tao JL, Li XW. Effects of keto acid supplements on Chinese patients receiving maintenance hemodialysis: a prospective, randomized, controlled, single-center clinical study. Chin Med J 2019;133:9–16. doi: 10.1097/CM9.0000000000000578
References
- 1.Mitch WE, Remuzzi G. Diets for patients with chronic kidney disease, should we reconsider? BMC Nephrol 2016; 17:80.doi: 10.1186/s12882-016-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Moore LW, Tortorici AR, Chou JA, St-Jules DE, Aoun A, et al. North American experience with low protein diet for non-dialysis-dependent chronic kidney disease. BMC Nephrol 2016; 17:90.doi: 10.1186/s12882-016-0304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikizler TA. Dietary protein restriction in CKD: the debate continues. Am J Kidney Dis 2009; 53:189–191.. doi: 10.1053/j.ajkd.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Garneata L, Mircescu G. Effect of low-protein diet supplemented with keto acids on progression of chronic kidney disease. J Ren Nutr 2013; 23:210–213.. doi: 10.1053/j.jrn.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Ruperto M, Sánchez-Muniz FJ, Barril G. A clinical approach to the nutritional care process in protein-energy wasting hemodialysis patients. Nutr Hosp 2014; 29:735–750.. doi: 10.3305/nh.2014.29.4.7222. [DOI] [PubMed] [Google Scholar]
- 6.Ikizler TA. Optimal nutrition in hemodialysis patients. Adv Chronic Kidney Dis 2013; 20:181–189.. doi: 10.1053/j.ackd.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatino A, Regolisti G, Karupaiah T, Sahathevan S, Sadu Singh BK, Khor BH, et al. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr 2017; 36:663–671.. doi: 10.1016/j.clnu.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Clinical practice guidelines for nutrition in chronic renal failure. K/DOQI, National Kidney Foundation. Am J Kidney Dis 2000; 35: Suppl 2: S1–S140.. [DOI] [PubMed] [Google Scholar]
- 9.Kistler BM, Fitschen PJ, Ikizler TA, Wilund KR. Rethinking the restriction on nutrition during hemodialysis treatment. J Ren Nutr 2015; 25:81–87.. doi: 10.1053/j.jrn.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Therrien M, Byham-Gray L, Beto J. A review of dietary intake studies in maintenance dialysis patients. J Ren Nutr 2015; 25:329–338.. doi: 10.1053/j.jrn.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Ohkawa S, Kaizu Y, Odamaki M, Ikegaya N, Hibi I, Miyaji K, et al. Optimum dietary protein requirement in nondiabetic maintenance hemodialysis patients. Am J Kidney Dis 2004; 43:454–463.. doi: 10.1053/j.ajkd.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 12.Tarnopolsky MA, Atkinson SA, MacDougall JD, Chesley A, Phillips S, Schwarcz HP. Evaluation of protein requirements for trained strength athletes. J Appl Physiol 1992; 73:1986–1995.. doi: 10.1152/jappl.1992.73.5.1986. [DOI] [PubMed] [Google Scholar]
- 13.Lim VS, Bier DM, Flanigan MJ, Sum Ping ST. The effect of hemodialysis on protein metabolism. A leucine kinetic study. J Clin Invest 1993; 91:2429–2436.. doi: 10.1172/JCI116477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang N, Qian J, Sun W, Lin A, Cao L, Wang Q, et al. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant 2009; 24:2551–2558.. doi: 10.1093/ndt/gfp085. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Molassiotis A. Dietary and fluid compliance in Chinese hemodialysis patients. Int J Nurs Stud 2002; 39:695–704.. doi: 10.1016/s0020-7489(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 16.Kizil M, Tengilimoglu-Metin MM, Gumus D, Sevim S, Turkoglu I, Mandiroglu F. Dietary inflammatory index is associated with serum C-reactive protein and protein energy wasting in hemodialysis patients: a cross-sectional study. Nutr Res Pract 2016; 10:404–410.. doi: 10.4162/nrp.2016.10.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bossola M, Leo A, Viola A, Carlomagno G, Monteburini T, Cenerelli S, et al. Dietary intake of macronutrients and fiber in Mediterranean patients on chronic hemodialysis. J Nephrol 2013; 26:912–918.. doi: 10.5301/jn.5000222. [DOI] [PubMed] [Google Scholar]
- 18.Chen J. Nutrition, phosphorus, and keto-analogues in hemodialysis patients: a Chinese perspective. J Ren Nutr 2013; 23:214–217.. doi: 10.1053/j.jrn.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Dhillon-Jhattu S, Sprague SM. Should phosphate management be limited to the KDIGO/KDOQI guidelines? Semin Dial 2018; 31:377–381.. doi: 10.1111/sdi.12702. [DOI] [PubMed] [Google Scholar]
- 20.Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 2008; 88:1511–1518.. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichikawa Y, Hiramatsu F, Hamada H, Sakai A, Hara K, Kogirima M, et al. Effect of protein and energy intakes on body composition in non-diabetic maintenance-hemodialysis patients. J Nutr Sci Vitaminol (Tokyo) 2007; 53:410–418.. doi: 10.3177/jnsv.53.410. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Long Q, Shao C, Fan H, Yuan L, Huang BH, et al. Effect of short-term low-protein diet supplemented with keto acids on hyperphosphatemia in maintenance hemodialysis patients. Blood Purif 2011; 31:33–40.. doi: 10.1159/000321376. [DOI] [PubMed] [Google Scholar]
- 23.Esfehani AJ, Dashti S. The impact of education on nutrition on the quality of life in patients on hemodialysis: a comparative study from teaching hospitals. Saudi J Kidney Dis Transpl 2013; 24:130.doi: 10.4103/1319-2442.106308. [DOI] [PubMed] [Google Scholar]
- 24.Raimann JG, Zhu F, Wang J, Thijssen S, Kuhlmann MK, Kotanko P, et al. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int 2014; 85:898–908.. doi: 10.1038/ki.2013.358. [DOI] [PubMed] [Google Scholar]
- 25.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney Int 1999; 55:1945–1951.. doi: 10.1046/j.1523-1755.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 26.Rimsevicius L, Gincaite A, Vicka V, Sukackiene D, Pavinic J, Miglinas M. Malnutrition assessment in hemodialysis patients: role of bioelectrical impedance analysis phase angle. J Ren Nutr 2016; 26:391–395.. doi: 10.1053/j.jrn.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Bellizzi V, Cupisti A, Locatelli F, Bolasco P, Brunori G, Cancarini G, et al. Low-protein diets for chronic kidney disease patients: the Italian experience. BMC Nephrol 2016; 17:77.doi: 10.1186/s12882-016-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuppari L, Nerbass FB, Avesani CM, Kamimura MA. A practical approach to dietary interventions for nondialysis-dependent CKD patients: the experience of a reference nephrology center in Brazil. BMC Nephrol 2016; 17:85.doi: 10.1186/s12882-016-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


