1 |. INTRODUCTION
Endothelial colony-forming cells (ECFCs) are derived from progenitor cells that circulate in peripheral blood. Over the last two decades, human ECFCs have been isolated and studied by multiple laboratories worldwide. Collectively, studies have established a distinct molecular and phenotypic signature for ECFCs that includes expression of endothelial cell markers (e.g., CD31, CD144, CD146, epidermal growth factor–like domain 7 [EGFL-7] and vascular endothelial growth factor receptor 2 [VEGFR2]) and lack of hematopoietic markers (CD45 and CD14). No single identifying marker has been identified yet; however, CD117 (c-kit),1 the side population marker ABCG2,2 CD157,3 PROCR4 and intracellular CD1335 are new, recently identified actors that might help to decipher a clear hierarchy in endothelial stem and progenitor cells. It has become increasingly clear that ECFCs represent a unique population of proliferative cells. ECFCs are distinct from mature circulating endothelial cells (CECs) shed from the vasculature, as they appear in culture after 7–20 days of plating, form highly proliferative colonies, and are present in different numbers in several populations.6,7 To illustrate this, in patients with pulmonary arterial hypertension (PAH), the mean value of ECFC colonies is 1 per 107 mononuclear cells (MNCs). For a normal leukocyte count, this equates to 2.5 ECFC colonies per milliliter of blood (assuming that one progenitor cell gives rise to one colony). This number is negligible in comparison with mature CEC counts in this population (mean value of 80 CEC colonies per milliliter of blood in PAH patients).6,8
Currently, ECFCs are being proposed for a variety of medical applications, including as liquid biopsies to investigate endothelial dysfunction in patients, as cellular vehicles in gene therapy, and as therapeutic cell products.9 In the fields of thrombosis and hemostasis, ECFCs have been instrumental in investigating specific cellular and molecular defects in endothelial cells from patients with von Willebrand disease (VWD) and hereditary hemorrhagic telangiectasia (HHT).10 Despite the broad appeal of ECFCs, several issues remain that prevent their widespread use—most notably, the lack of consensus with regard to the methods used to quantify, culture and evaluate ECFCs. In 2017, the Vascular Biology Standardization Subcommittee (SSC) of the ISTH organized a round table aimed at standardizing methods associated with ECFC isolation and culture. The goals were to revise the procedures used, emphasize existing limitations in published data, and establish guidelines for multicenter studies. To this end, participants from six laboratories responded to a list of questions concerning their experience with ECFCs. The discussion and recommendations that emerged from the round table are summarized in this document.
2 |. NOMENCLATURE FOR ENDOTHELIAL PROGENITOR CELLS IN CULTURE
The panel discussed the most suitable name to be used to refer to this population of progenitor cells, to avoid the confusion caused by differences in nomenclature. Over the last two decades, several terms have been used to name these cells, including endothelial progenitor cells, late outgrowth endothelial cells, blood outgrowth endothelial cells, and ECFCs. The consensus among participant laboratories, and broadly in the literature, appears to be in favor of the last of these terms, first coined by Yoder et al.11
2.1 |. Recommendation 1
The term “ECFC” should preferably be used in all subsequent studies.
3 |. KEY METHODOLOGICAL STEPS IN THE ISOLATION OF ECFCS FROM BLOOD-DERIVED MNCS
Blood is collected in either EDTA-containing, citrated or heparin-containing tubes. Isolation of MNCs is conducted with a 1.077 density gradient step.
The first change of medium occurs between day 2 and day 5 after MNC plating. Also, the time to appearance of the first colony is approximately 7 days for cord blood and 21 days for adult blood. The panel discussed the problem of the definition of colony and of how many cells are required to define a cluster of cells as a colony.
The reported plating density of MNCs varies from 5 × 106 to 5 × 107 per six-well plate; Mauge et al reported that increasing the number of MNCs plated in one well increases the appearance of ECFCs. However, normalized to MNCs, the number of colonies is the same whatever the density plated. Thus, differences in MNC numbers do not influence the number of ECFC colonies observed.12 Various extracellular matrix types have been used to plate MNCs, although fibronectin13 and collagen 114 are more commonly used than gelatin. No differences have been found between these three commonly used matrices, whereas a decreased number of ECFCs was observed with laminin, which was associated with decreased proliferation and vasculogenic effects in a mouse model of hindlimb ischemia.12
Commercially available EGM2 is consistently used as the culture medium. The medium is usually supplemented with 5%−10% fetal bovine serum (FBS), and this range of FBS appears to produce no significant differences in the number of ECFC colonies. A study that compared 5% and 10% FBS on samples from two separate cohorts of patients with idiopathic pulmonary fibrosis (IPF) (IPF-1 and IPF-2 in Figure 1A) found no significant differences in the numbers of isolated ECFCs when different amounts of FBS were used; however, a difference in the number of samples that failed to yield ECFC colonies (“zero colonies”) was observed (Figure 1B). Thus, 10% FBS should be preferred to 5% FBS. Plasma15 and platelet lysate16,17 have been proposed as substitutes for serum, and more ECFC colonies have been reported with platelet lysate.17
FIGURE 1.
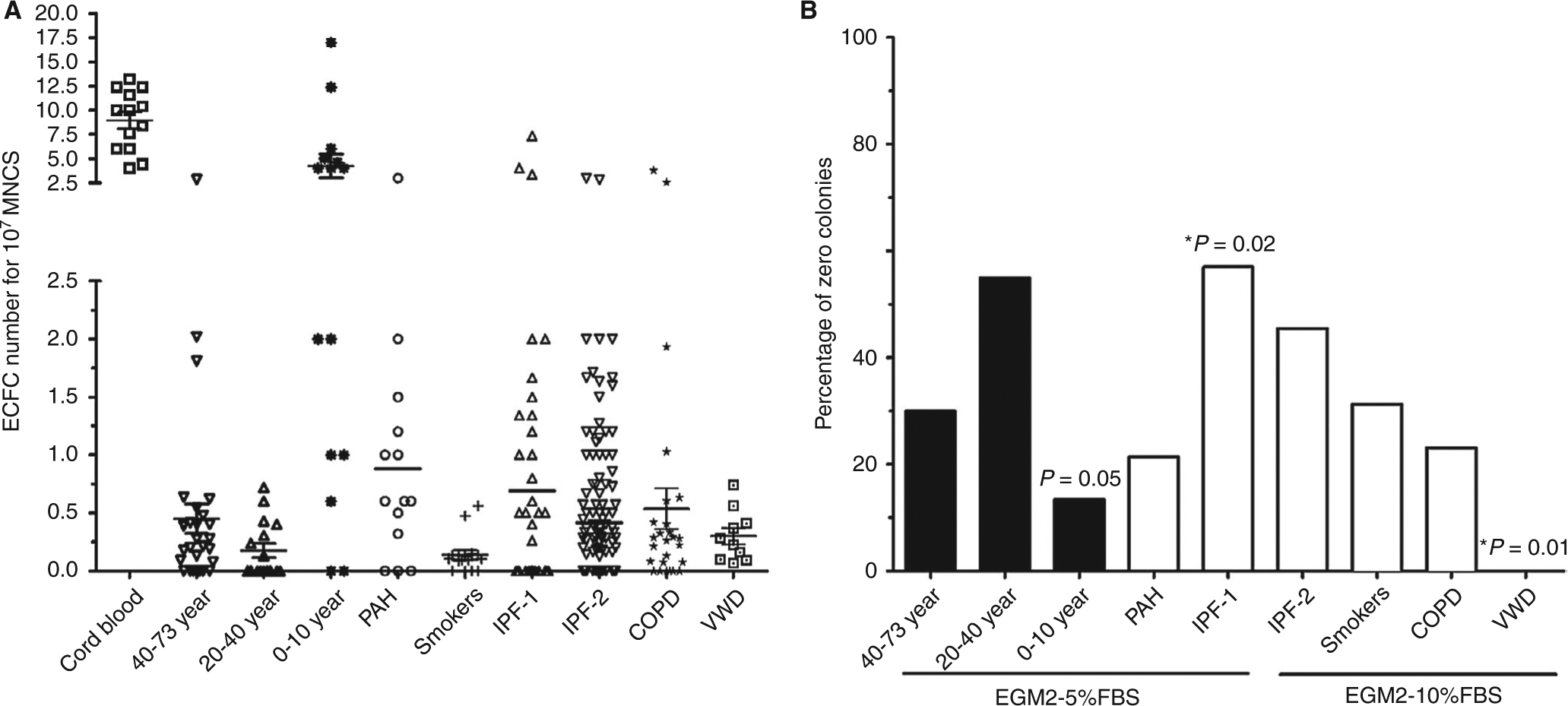
Quantification of endothelial colony-forming cells (ECFCs) in health and disease. A, Umbilical cord blood yields higher numbers of ECFC colonies than adult blood. In children (median age of 2 years), ECFC levels were higher than in adults. The association between age and ECFC levels appears to be lost in older subjects, and no significant differences were observed among subjects aged 20–73 years. ECFC levels have been examined in patients with von Willebrand disease (VWD) and with respiratory diseases, including pulmonary hypertension (PAH), chronic obstructive pulmonary disease (COPD), and idiopathic pulmonary fibrosis (IPF). In these patient groups, ECFC levels (colony numbers) did not appear to be significantly different from those in the healthy controls. For IPF patients, two separate cohorts comparing 5% (IPF-1) and 10% fetal bovine serum (FBS) (IPF-2) were compared and no differences in ECFC numbers were found. B, Levels of zero ECFC colonies were compared with the mean of the whole population (χ2 test or Fisher’s exact test when the number of zero colonies was <5). In healthy volunteers, the levels of zero ECFC colonies were significantly lower in the 0–10–year age group than in older groups (P = 0.05). In other populations, only VWD patients had significantly fewer zero ECFC colonies. For the IPF-1 and IPF-2 populations, we found that IPF-1 patients (with culture performed in EGM2–5% FBS) had more zero ECFC colonies than IPF-2 patients (with culture performed in 10% FBS). MNC, mononuclear cell
3.1 |. Recommendation 2
ECFC colonies should be identified as well-circumscribed colonies with a cobblestone appearance and with more than 50 adherent cells.
ECFC levels should be expressed as the number of colonies per 107 MNCs seeded.
Currently, expert laboratories report a success rate of 70%−75% in isolating ECFCs from peripheral blood of healthy subjects. Lack of success in isolating ECFCs from some subjects is to be expected. Failure to isolate ECFC colonies (defined as “zero colonies”) is a result that needs to be reported.
4 |. HOW TO STANDARDIZE PASSAGE NUMBER AND CELL EXPANSION
Delayed colony appearance is a challenge that affects further subculture and thus the inferred age of the cells. Most publications report ECFC expansion in terms of passage number. However, passaging is not carried out uniformly between different laboratories and studies; moreover, colonies from different individuals and from patients in different disease groups may show different proliferation rates. In some reports, the age of a colony is established according to the first day of MNC culture.18,19 ECFC age is important, because it affects phenotype and function; ECFCs’ display of immature markers declines after 40 days of culture.18,20 Thus, time in culture is a key determinant for the age of the ECFC population.
The most suitable parameter for reporting ECFC proliferation potential is population doubling (PD). Protocols for passaging cells differ widely between laboratories, and PD provides more accurate information than passage number on the state of the cells with regard to their lifespan. However, reporting of PD requires repeated cell counting and some calculations.
Differences in proliferation between ECFCs from peripheral adult blood and those from cord blood have been reported. In adult blood, colonies that appear early in culture after seeding are more proliferative than colonies that appear later. ECFCs from cord blood are both more proliferative and have longer lifespans in culture than ECFCs from adult peripheral blood. Adult blood-derived ECFCs reach their lifespan earlier in culture, so passage number standardization is probably more important than for cord blood-derived ECFCs.
4.1 |. Recommendation 3
The extent of expansion of ECFCs should be characterized not only by passage number, but also by PD time.
ECFC studies should report the number of days in culture from the day of the original MNC isolation (day 0).
5 |. HOW TO STUDY ECFCS IN HEALTH AND DISEASE
In healthy subjects, a very young age appears to affect ECFC levels and properties. Umbilical cord blood yields higher numbers of ECFC colonies than adult blood.21 ECFC levels in children (median age of 2 years) were reported to be higher than those in adults (4.2 colonies per 107 MNCs seeded; n = 15; Figure 1A).6 The association between age and ECFC levels appears to be lost in adult subjects, as no significant differences in ECFC isolation efficiency and function were observed among subjects aged 20–73 years, with the exception of the “zero colonies” category (discussed below). It remains unclear how ECFC levels change from early postnatal life to adulthood.
ECFC levels have been examined in clinical situations, including VWD22,23 and respiratory diseases. In several respiratory diseases, such as PAH,8 chronic obstructive pulmonary disease,10,24 and IPF,7 ECFC levels did not appear to be significantly different from those in healthy controls (Figure 1A).
In some subjects, the isolation procedure yields no colonies—referred to as “zero colonies”. By the use of data from three laboratories (those of Smadja, Sabatier, and Randi), levels of zero ECFC colonies were compared with the mean of the whole population. In healthy volunteers, we found fewer subjects yielding zero colonies in the 0–10–year age group than in older groups. In disease populations, only VWD patients have significantly fewer zero colonies than the whole population. This might be linked to the angiogenic properties described for von Willebrand factor.25 For the IPF-1 and IPF-2 populations, although the median range of ECFCs is similar (Figure 1A), we found than IPF-1 patients (with culture performed in EGM2–5% FBS) have more zero ECFC colonies than IPF-2 patients (with culture performed in 10% FBS). Thus, the amount of FBS could be a major determinant of the appearance of ECFCs in culture. Whether this holds true in other clinical situations, such as diabetes, remains to be determined.26 Often, particularly in vascular genetic disorders, the main changes are not in the levels of ECFCs but in some of their functional properties, including proliferation capacity, vesiculation, and the onset of senescence.27‒29 Alternative measures for regenerative potential need to be explored, including expression of immaturity markers,5,30 the in vivo ability to form stable vessels,31 and long-term proliferation. Importantly, in studies on umbilical cord blood, the health status of the mother needs to be considered. For example, studies have shown that cord blood levels of ECFCs are lower in women with pre-eclampsia than in healthy control pregnancies.32 Also, studies have shown that ECFC levels are higher in women with preterm deliveries than in those with term deliveries, although whether ECFCs are functionally different remains unclear.33 Multiple studies have shown that ECFCs can provide information about endothelial function in vascular diseases, and thus their use as liquid biopsies could be clinically valuable.
The panel discussed the issue of donor reproducibility. Generally, it was agreed that donors who yield ECFCs appear to do so consistently in repeated isolations. Likewise, some donors do not yield ECFCs consistently. Reproducibility is important, considering that the presence of ECFCs has been proposed as a marker of preserved microvascular integrity in patients after acute myocardial infarction.34 The panel agreed on the need to report the ability to isolate ECFCs—i.e., presence or absence of colonies—in clinical studies, which may be as important as the actual number of ECFC colonies obtained (see also Recommendation 2).
5.1 |. Recommendation 4
ECFC levels decrease progressively from birth to adulthood, although the rate at which this variation occurs is unclear. Thus, in adults, age does not seem to be a major determinant of ECFC levels.
ECFCs represent a valuable tool for investigating endothelial function in disease. The functional assessment and number of zero colonies of ECFCs are likely to be more informative than ECFC levels (number of colonies), and thus these cells should be profiled for their molecular signatures and studied with appropriate standardized functional assays.
In summary, the ISTH-SSC workshop identified a number of criteria that are required to increase the reliability of ECFC studies and to reduce interlaboratory discordances. However, some methodological aspects that are difficult to standardize, such as the time between colony appearance and first passage, need to be validated in order to enable comparison of data from multicenter studies. Moreover, preanalytic factors (circadian fluctuations, fasting, exercise, nutrition, etc.) should be considered as areas that could be improved in the future for ECFC standardization. Given the increasing interest in using ECFCs as liquid biopsies, and the prospect of using ECFCs in cell therapy trials for ischemic retinopathies9 and ischemic heart disease,35 standardization is urgently needed.
ACKNOWLEDGEMENTS
We would like to thank Dr Koralia Paschalaki (Randi’s laboratory) and Dr Adeline Blandinieres (Smadja’s laboratory) for methodological support and helpful discussions. We would like to thank Dr Richard Chocron (UMR-S970 and emergency department, Hôpital Européen Georges Pompidou) for statistical support.
Footnotes
CONFLIC T OF INTERESTS
The authors do not have any conflicts of interest to declare.
REFERENCES
- 1.Fang S, Wei J, Pentinmikko N, Leinonen H, Salven P. Generation of functional blood vessels from a single c-kit+ adult vascular endothelial stem cell. PLoS Biol. 2012;10:e1001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naito H, Kidoya H, Sakimoto S, Wakabayashi T, Takakura N. Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels. EMBO J. 2012;31:842–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakabayashi T, Naito H, Suehiro JI, Lin Y, Kawaji H, Iba T, et al. CD157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Cell Stem Cell. 2018;22:384–97 e6. [DOI] [PubMed] [Google Scholar]
- 4.Yu QC, Song W, Wang D, Zeng YA. Identification of blood vascular endothelial stem cells by the expression of protein C receptor. Cell Res. 2016;26:1079–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi E, Poirault-Chassac S, Bieche I, Chocron R, Schnitzler A, Lokajczyk A, et al. Human endothelial colony forming cells express intracellular CD133 that modulates their vasculogenic properties. Stem Cell Rev 2019. In press. https://doi.org/10.1007 [DOI] [PubMed] [Google Scholar]
- 6.Levy M, Bonnet D, Mauge L, Celermajer DS, Gaussem P, Smadja DM. Circulating endothelial cells in refractory pulmonary hypertension in children: markers of treatment efficacy and clinical worsening. PLoS ONE. 2013;8:e65114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smadja DM, Mauge L, Nunes H, d’Audigier C, Juvin K, Borie R, et al. Imbalance of circulating endothelial cells and progenitors in idiopathic pulmonary fibrosis. Angiogenesis. 2013;16:147–57. [DOI] [PubMed] [Google Scholar]
- 8.Smadja DM, Mauge L, Gaussem P, d’Audigier C, Israel-Biet D, Celermajer DS, et al. Treprostinil increases the number and angiogenic potential of endothelial progenitor cells in children with pulmonary hypertension. Angiogenesis. 2011;14:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid E, Guduric-Fuchs J, O’Neill CL, Allen LD, Chambers SEJ, Stitt AW, et al. Preclinical evaluation and optimization of a cell therapy using human cord blood-derived endothelial colony-forming cells for ischemic retinopathies. Stem Cells Transl Med. 2018;7:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paschalaki KE, Randi AM. Recent advances in endothelial colony forming cells toward their use in clinical translation. Front Med (Lausanne) 2018;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat Biotechnol. 2014;32:1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauge L, Sabatier F, Boutouyrie P, D’Audigier C, Peyrard S, Bozec E, et al. Forearm ischemia decreases endothelial colony-forming cell angiogenic potential. Cytotherapy. 2014;16:213–24. [DOI] [PubMed] [Google Scholar]
- 13.Melero-Martin JM, Bischoff J. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol. 2008;445:303–29. [DOI] [PubMed] [Google Scholar]
- 14.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L, Critser PJ, Grimes BR, Yoder MC. Human umbilical cord blood plasma can replace fetal bovine serum for in vitro expansion of functional human endothelial colony-forming cells. Cytotherapy. 2011;13:712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Prasain N, Vemula S, Ferkowicz MJ, Yoshimoto M, Voytik-Harbin SL, et al. Human platelet lysate improves human cord blood derived ECFC survival and vasculogenesis in three dimensional (3D) collagen matrices. Microvasc Res. 2015;101:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasev D, van Wijhe MH, Weijers EM, van Hinsbergh VW, Koolwijk P. Long-term expansion in platelet lysate increases growth of peripheral blood-derived endothelial-colony forming cells and their growth factor-induced sprouting capacity. PLoS ONE. 2015;10:e0129935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smadja DM, Bieche I, Uzan G, Bompais H, Muller L, Boisson-Vidal C, et al. PAR-1 activation on human late endothelial progenitor cells enhances angiogenesis in vitro with upregulation of the SDF-1/CXCR18 system. Arterioscler Thromb Vasc Biol. 2005;25:2321–7. [DOI] [PubMed] [Google Scholar]
- 19.Smadja DM, Bieche I, Helley D, Laurendeau I, Simonin G, Muller L, et al. Increased VEGFR2 expression during human late endothelial progenitor cells expansion enhances in vitro angiogenesis with upregulation of integrin alpha(6). J Cell Mol Med. 2007;11:1149–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bompais H, Chagraoui J, Canron X, Crisan M, Liu XH, Anjo A, et al. Human endothelial cells derived from circulating progenitors display specific functional properties compared with mature vessel wall endothelial cells. Blood. 2004;103:2577–84. [DOI] [PubMed] [Google Scholar]
- 21.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–60. [DOI] [PubMed] [Google Scholar]
- 22.Starke RD, Paschalaki KE, Dyer CE, Harrison-Lavoie KJ, Cutler JA, McKinnon TA, et al. Cellular and molecular basis of von Willebrand disease: studies on blood outgrowth endothelial cells. Blood. 2013;121:2773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randi AM, Laffan MA. Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 2017;15:13–20. [DOI] [PubMed] [Google Scholar]
- 24.Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, et al. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE, et al. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarajapu YP, Hazra S, Segal M, Li Calzi S, Jadhao C, Qian K, et al. Vasoreparative dysfunction of CD34+ cells in diabetic individuals involves hypoxic desensitization and impaired autocrine/paracrine mechanisms. PLoS ONE. 2014;9:e93965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LS, Marchesan D, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:780–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoncini S, Chateau AL, Robert S, Todorova D, Yzydorzick C, Lacroix R, et al. Biogenesis of pro-senescent microparticles by endothelial colony forming cells from premature neonates is driven by SIRT1-dependent epigenetic regulation of MKK6. Sci Rep. 2017;7:8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacha NC, Blandinieres A, Rossi E, Gendron N, Nevo N, Lecourt S, et al. Endothelial microparticles are associated to pathogenesis of idiopathic pulmonary fibrosis. Stem Cell Rev. 2018;14:223–35. [DOI] [PubMed] [Google Scholar]
- 30.Guillevic O, Ferratge S, Pascaud J, Driancourt C, Boyer-Di-Ponio J, Uzan G. A novel molecular and functional stemness signature assessing human cord blood-derived endothelial progenitor cell immaturity. PLoS ONE. 2016;11:e0152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernandez JM, Dominguez-Simeon MJ, et al. Decreased level of cord blood circulating endothelial colony-forming cells in pre-eclampsia. Hypertension. 2014;64:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA. 2014;111:10137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meneveau N, Deschaseaux F, Seronde MF, Chopard R, Schiele F, Jehl J, et al. Presence of endothelial colony-forming cells is associated with reduced microvascular obstruction limiting infarct size and left ventricular remodelling in patients with acute myocardial infarction. Basic Res Cardiol. 2011;106:1397–410. [DOI] [PubMed] [Google Scholar]
- 35.Lee SH, Lee JH, Asahara T, Kim YS, Jeong HC, Ahn Y, et al. Genistein promotes endothelial colony-forming cell (ECFC) bioactivities and cardiac regeneration in myocardial infarction. PLoS ONE. 2014;9:e96155. [DOI] [PMC free article] [PubMed] [Google Scholar]


