Abstract
There is talk of regulatory collaboration worldwide to protect public health and allow patients timely access to medicines. Here, we present the reality of the collaboration between the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA). This takes the form of near daily interactions, which may be less known outside of regulatory agencies. We present a review of what we call clusters, which involve the EMA, the FDA, and many other agencies under the umbrella of confidentiality arrangements. Through a survey of participants, we identified about 30 clusters of variable composition; these allow for the exchange of information and discussion among experts of applying regulatory science to common challenges in global drug development at every phase of its lifecycle and facilitate global medicines development.
In the context of increased globalization of medicinal product (drugs, biologicals, vaccines, and advanced therapies) development, regulators have responded by increasing focus on shared approaches and standards while carefully retaining independence in decision making under their own legal mandates. Standard setting and harmonizing typically occur through multilateral organizations, such as the International Council for Harmonization (ICH) of Technical Requirements for Pharmaceuticals for Human Use.1 Voluntary international forums exist with different objectives: the International Conference of Drug Regulatory Authorities (ICDRA)2 is a forum supported by the World Health Organization (WHO) to discuss priorities and strengthen convergence, in particular, for low‐income and middle‐income countries; and the International Coalition of Medicines Regulatory Authorities (ICMRA)3 was created by heads of agencies to exchange at the executive level on strategic areas and common global challenges.
Strategic partnerships across regions come in other forms as well, and, since 2004, experts from the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) and, subsequently, additional regulatory agencies (or authorities), have been meeting to exchange perspectives and experiences on regulatory science topics in what we now call “clusters.” Information about this type of interaction, its relevance, and opportunities for improvement has not been collected systematically until now. We recently conducted an internal review at the FDA and EMA of the clusters and their activities, which provided an opportunity to take stock of their work. We are providing this review to show, for the first time, how the EMA, the FDA, and other regulators interact daily to protect public health worldwide.
Clusters
Although informal collaboration had existed before, in 2003, the FDA and the EMA signed a memorandum of understanding and a confidentiality agreement that allow commercial confidential information, but not trade secrets, to be shared across agencies.4 More recently, an expanded confidentiality commitment was signed by the European Union and the FDA, allowing for sharing of full inspection reports, including trade secret information.5, 6 The first formal EMA‐FDA cluster was established in 2004 to discuss oncology‐hematology medicines. Over the years, more clusters have been added and these expert groups have grown in participation within the two agencies, then with inclusion of other regulatory agencies with which the EMA and the FDA have confidentiality arrangements.4 Regular interactions are held on topics or issues where scientific collaboration and intensified exchange of information are necessary in the opinion of a participating agency (Figure 1). Each cluster has a slightly different way of operating but always builds on a core team of experts with ad hoc participants brought to the table depending on the topics under discussion. Core participants may be scientific experts, reviewers, regulatory affairs experts, international affairs staff, or managers. Meetings are organized as teleconferences with frequency and duration established by each cluster. Over the years, clusters have been created to cover many therapeutic areas or types of products, including as diverse topics as advance therapies, biosimilars, pediatric medicine development, rare diseases, and patient engagement. They provide a forum to share emerging regulatory science matters, discuss difficult product development, or review issues faced by one or more of the agencies. The discussions are a window to understanding differences in approach and perspectives across regions, even though harmonization is not always the objective. The robust participation, most simply illustrated by the number of clusters operating, affirms that agencies perceive benefit from such exchanges, especially as the complexity of medicine development increases. Not only does information sharing itself create new understandings and value, it provides a form of peer review among fellow regulators, which few other forums can.
Figure 1.
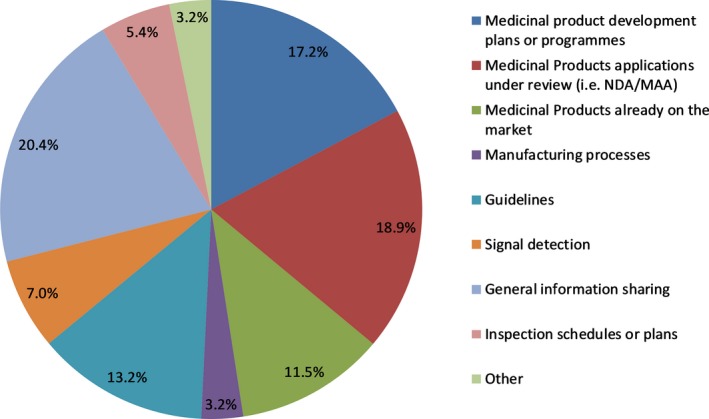
Top topic areas discussed in clusters. Values shown are from aggregate results from a compiled list of the topic areas identified for all clusters. MAA, Marketing Authorisation Application; NDA, New Drug Application.
Initially, the clusters involved the EMA and the FDA only, but today about half of them include at least one other regulatory agency. Shared confidentiality arrangements between agencies are required for participation. The Japanese Ministry of Health, Labour, and Welfare (MHLW) with the Pharmaceuticals and Medical Devices Agency (PMDA), Health Canada (HC), the Australian Therapeutic Goods Administration (TGA), and Swissmedic participate in some clusters, depending largely on the topics, resources, and relevance. The European Directorate for the Quality of Medicines (EDQM) and the WHO have joined several that focus on manufacturing quality and inspection issues. Some regulators initially joined as observers (listening but not adding topics to the agenda) then moved to active participation. For example, in those clusters focusing on a therapeutic area, actively participating regulators have in common programs for early engagement with regulated industry on medicine development, or expedited application assessment. They see benefit in working with other regulators on key aspects of regulatory science. Cluster discussions inform and build trust among regulators facing the same challenges and ultimately may facilitate global development programs as the experts work through similar challenges collectively. Clusters are not limited to discussion of premarket development and may include matters under review in one or more agencies, such as marketing applications, postmarketing safety, or manufacturing inspections, among others, for both human and veterinary medicines. The full list of clusters with participating agencies is in Table 1.
Table 1.
Clusters and participating agencies
| Name | Short description of discussion areas | Meeting frequencya | Participating agencies | Surveyed cluster/comment |
|---|---|---|---|---|
| Advanced therapies/regenerative medicines (ATRM) | Development programs and challenges in regulation of advance therapy medicinal products, such as cell and gene therapies | 5–6 times/year | EMA, FDA, HC, PMDA/MHLW | Yes |
| Anti‐infectives | Development of medicinal products for this therapeutic area | Monthly | EMA, FDA, HC, PMDA | No |
| Antivirals | Development of medicinal products for this therapeutic area | 2–4 times/year | EMA, FDA | No |
| Active Pharmaceutical Ingredients International Inspection Program (API) | Collaboration toward the efficient use of inspection resources and the gain of confidence in each other's inspection outcomes | Monthly | EMA, FDA, PMDA, HC, TGA, EDQM, WHO, MS: France, Denmark, United Kingdom, Italy | Yes |
| Bioequivalence collaboration (BE) | Collaboration toward the efficient use of inspection resources and the gain of confidence in each other's inspection outcomes | 4 times/year | EMA, FDA, MS: Austria, France, Germany (BfArM), Italy, The Netherlands, Spain, United Kingdom | Yes |
| Biomarkers qualification (QBiom) | Activities related to biomarker qualification, parallel Qualification Advice/Opinion procedures | 4 times/year | EMA, FDA | No |
| Biosimilars (Biosim) | Development programs and medicinal products that are biosimilars | 3 times/year | EMA, FDA, PMDA/MHLW, HC, Swissmedic | Yes |
| Biostatistics (Biostats) | Regulatory science and challenges related to biostatistics | 2 times/year | EMA, FDA | No |
| Blood products | Development programs and medicinal products for this therapeutic area | 4 times/year | EMA, FDA, HC | Yes |
| Breakthrough/PRIME | Information sharing on designation decisions for proposals submitted to both agencies (post decision only) | 4 times/year | EMA, FDA | Yes |
| Cardiovascular medicinal products | Development programs and medicinal products for this therapeutic area | 4 times/year | EMA, FDA | Yes |
| Clinical outcome assessment (COA) | Activities related to qualification of novel methodologies in both agencies, parallel Qualification Advice/Opinion procedures | 4 times/year | EMA, FDA | No |
| GCP initiative (GCP) | Collaboration toward the efficient use of inspection resources and the gain of confidence in each other's inspection outcomes | Every 2 months | EMA, FDA, PMDA/MHLW | Yes |
| Mutual Recognition Agreement (MRA) | Collaboration toward implementation of the MRA | Every 2 months | EMA, FDA | No |
| Psychiatry (Psych) | Development programs and medicinal products for this therapeutic area | Every 2 months | EMA, FDA | No |
| Nonclinical Oncology (Pharm Tox) | Nonclinical aspects of oncology product development | Quarterly | EMA, FDA | Yes |
| Oncology‐Hematology medicinal products | Development programs and ongoing assessments of medicinal products for this therapeutic area | Monthly | EMA, FDA, HC, PMDA/MHLW, TGA, Swissmedic | Yes |
| Orphan medicines | Challenges in assessing for orphan designation and product development | 4 times/year | EMA, FDA | Yes |
| Pediatric medicines | Discussion of development programs—pediatric investigation plans—and medicinal products for this patient population | Monthly | EMA, FDA, HC, PMDA/MHLW, TGA | Yes |
| Patient engagement (PE) | Sharing best practices on patient involvement in medicines’ lifecycle | 2 times/year | EMA, FDA, HC | Yes |
| Pharmacogenomics | Challenges and regulatory science related to using pharmacogenomic tools in drug development | 2 times/year | EMA, FDA, PMDA/MHLW | Yes |
| Pharmacometrics (Modeling and Simulation) | Challenges and regulatory science of pharmacometrics and modeling in drug development | 4 times/year | EMA, FDA, PMDA/MHLW, HC | Yes |
| Pharmacovigilance (PhV) | Sharing of information on drug safety issues for human medicinal products and advance notice of regulatory action, public information, and communication | Monthly | EMA, FDA, PMDA/MHLW, HC | Yes |
| Pharmacovigilance Strategy (PhV Strategic call) | Strategic regulatory science topics that are not product specific | 4 times/year | EMA FDA | No |
| Rare diseases | Development programs and medicinal products being studied for rare diseases | Monthly | EMA, FDA | Yes |
| Real‐World Evidence – Big data (RWE) | Platform to foster consistency of approach, address common challenges, leverage data, network and expertise available to facilitate advances in regulatory science | 4 times/year | EMA, FDA |
No Established in 2018 |
| (Medicines) Shortages | Information on drug shortages across regions and shared efforts to mitigate them | 4 times/year | EMA, FDA, HC, TGA | Yes |
| Vaccines (Vacc) | Development programs and medicinal products for this therapeutic area | 4 times/year | EMA, FDA, HC | Yes |
| Veterinary medicines (Vets) | Development programs and challenges related to multiple aspects of veterinary medicinal products | 4 times/year | EMA, FDA | Yes |
| Veterinary Novel therapies (Vets Novel T) | Information exchange on activities related to facilitating development of novel therapies for veterinary use | 4 times/year | EMA, FDA | No |
| Veterinary Pharmacovigilance (Vets PhV) | Sharing of information on drug safety issues for veterinary medicinal products and advance notice of regulatory action, public information, and communication | 2 times/year | EMA, FDA, HC | Yes |
EDQM, European Directorate for the Quality of Medicines; EMA, European Medicines Agency; FDA, US Food and Drug Administration; HC, Health Canada; MHLW, Japanese Ministry of Health, Labour, and Welfare; MS, member state; PMDA, Pharmaceuticals and Medical Devices Agency; TGA, Australian Therapeutic Goods Administration; WHO, World Health Organization.
Meeting frequency is averaged and some have been reduced due to Brexit resource constraints in 2018/19. Frequency of ad hoc calls for emerging topics not shown.
At the end of 2017, the EMA and the FDA international affairs’ teams conducted a review of the clusters to leverage the most successful examples of collaboration and best practices as well as to identify opportunities to increase efficiency.
We surveyed established clusters on, for example, quality of discussions, relevance to their own agency's work, and alignment with agency's priorities. We received responses to the survey from 153 participants from 7 regulatory authorities (the EMA, the FDA, HC, MHLW/PMDA, TGA, Swissmedic, and EDQM). Through this exercise we identified at least 10 more platforms, which at the time were not labeled clusters, but performed similar activities. All were “spin‐off” groups, based on needs, which had arisen from older, more mature human or veterinary medicines clusters but with no overlap of general topic areas.
With regard to operations, each cluster has an administrative and technical lead for each agency. In the case of the EMA and the FDA, these are usually functions that are separate, although when other regions participate the roles tend to be blended. Clusters have terms of reference that outline their objectives and expectations. Most of the clusters seek to address a broad range of scientific and regulatory topics, including product development and review, guidelines and standards, and even plans for workshops and presentations at scientific meetings.
From the survey (with ratings out of 4 or 5 depending on the questions), participants considered the frequency of scheduled meetings sufficient, with the possibility of holding additional ones. Figure 2 shows the results of a series of ratings of the quality of various aspects of cluster function, increasing with maturity of the cluster. Alignment with the agency's priorities in particular was rated at 4.3/5, while assisting the agency with planning and decision making at 3.8/5. As shown in aggregate for all clusters queried, the survey identified the need to better share the value of the clusters’ work by disseminating the outcomes more widely within the respective agencies.
Figure 2.
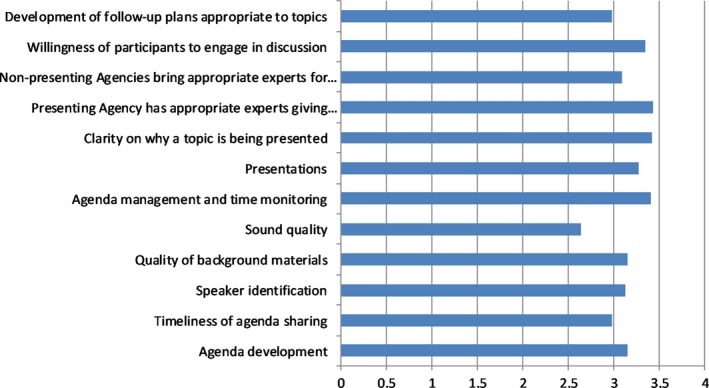
Quality of clusters rating from 1 to 4 (respondents n = 153). Scale: 1 = could be improved; 2 = ok; 3 = good; and 4 = excellent. Values shown are mean scores for the aggregate of all 20 clusters queried.
The most stimulating aspect of cluster discussions cited was that they provide for more informed regulatory decision making and oversight of regulated industry in areas like inspections and data integrity. Participation in cluster discussions was seen as flexible and open‐minded. Some initiatives were born from clusters, such as the FDA‐EMA outputs called pediatric “Common Commentaries.”7 The Patient Engagement cluster exemplifies how sharing best practices boosts innovative ideas for all agencies involved. The FDA and the Clinical Trials Transformation Initiative launched a new work group called the Patient Engagement Collaborative. This was modeled after the EMA's Patients’ and Consumers’ Working Party.8 Safety signal review at the Pharmacovigilance cluster led to coordinated communication, content, and timelines on dolutegravir and neural tube defects. Another outcome has been joint publications, such as multiregional aspects of pharmacogenetics,9 or the FDA and the EMA perspective on drug development in metabolic bone diseases.10 Although cluster interactions may not result in “joint” outputs, they may contribute to harmonization. The nonclinical oncology cluster resolved some divergences ahead of ICH S9 Question and Answer finalization. A protocol was jointly drafted in a rare pediatric disease, Gaucher's disease, allowing for a global drug development program.11 The collaboration can result in presentations or joint workshops, for example, the one on early access tools, PRIME, and Breakthrough Therapies.12 Several participants mentioned that their own learning and professional development benefited from the scientific exchange. A comment summarized the progress made: “We have reached a level of collaboration unthinkable a few years ago!”
The clusters are just one type of platform agencies use to collaborate worldwide. Others, such as the ICMRA,3 the ICDRA,2 or the ICH1 are very active and have specific scopes and formats. Some include industry (ICH) and do not require confidentiality arrangements because they do not address specific products. Other examples include exchange of staff over a short (fellowships) or longer period of time (official liaison based in other agencies).
Each cluster operates according to identified needs and we work closely with the FDA and the EMA to ensure their focus and to increase efficiency, in particular to avoid inefficient overlaps. Many clusters take on activities, such as proposing workshops and using the meetings to develop ideas for things like panel discussions at professional conferences or even shared publications for consideration.
The work of the clusters is robust in its breadth and depth, thus, the information provided here addresses a common misconception or request—heard very often—that the FDA and the EMA “should start talking to each other…or should talk more often.” Figure 3 shows that interactions are, in fact, near daily activities for the EMA and the FDA.
Figure 3.
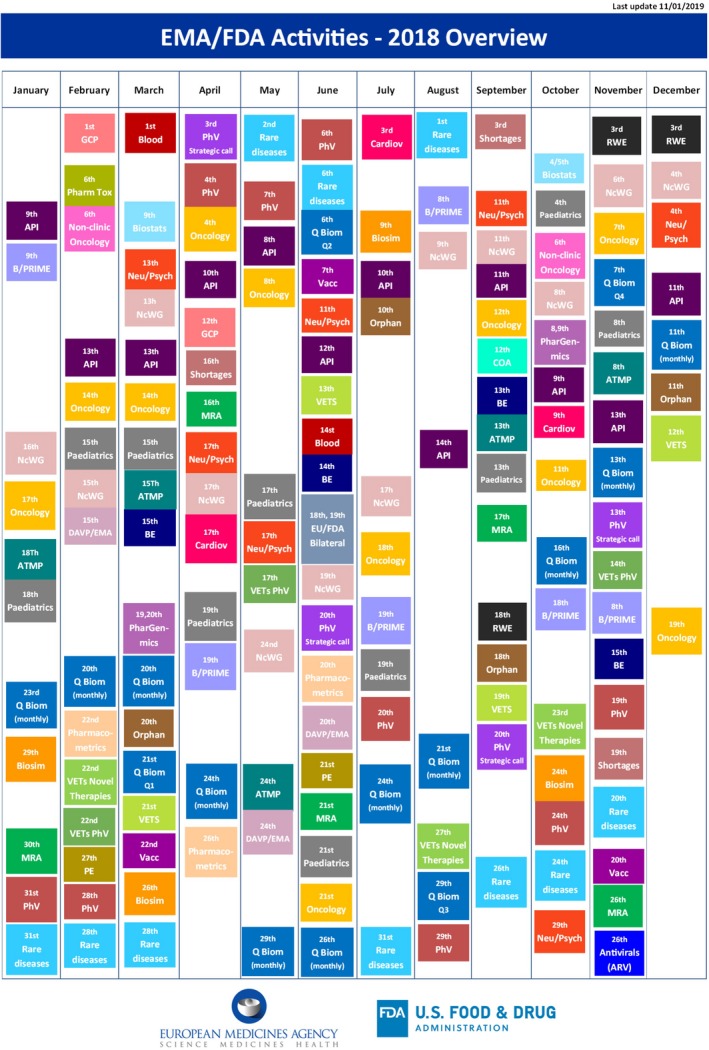
Overview of the European Medicines Agency (EMA)‐US Food and Drug Administration (FDA) cluster activities by month in 2018.
A frequent request, when clusters are mentioned, has been to open them to industry participation, from taking requests for topics to be discussed, or having a company present when one of their products is discussed. Clusters are intended to be meetings between regulators. Regulators must preserve a “safe harbor” time and opportunity to discuss challenges in regulatory science as peers, without pressure to commit to a particular pathway. Additionally, there are significant technical challenges when including multiple participants and having to connect and disconnect them to preserve confidentiality. Meetings with sponsors are opportunities to discuss their developments, applications, or proposals with regulators: They can trigger discussion in clusters, or be informed by those, but various meetings are already offered to serve this purpose. There are, in particular, fora with more than one agency, which allow for industry participation on product‐related development, such as the parallel scientific advice or consultative advice. Globalization of medicines development calls for shared or compatible approaches by regulators. Harmonization is not always possible as regulatory agencies operate under their own legal mandates; however, through clusters the work done toward scientific alignment is underpinning their role in protecting public health.
Conclusion
Dialogue between the EMA and the FDA and other regulatory authorities is an established, near daily activity. Areas of cooperation have been growing and deepening for over a decade and represent substantial effort and engagement by regulators in each agency to ensure robust discussions on sensitive topics and therapeutic areas. These activities, as shown partially through our review, are perceived to be of high added value. Our international collaboration is obviously not limited to clusters only, but extends also to fellowships, joint presentations at international fora, data sharing, collaboration in guidelines, publications, and in trainings. The challenges are to increase continued collaboration among regulators and to add value through scientific alignment in the interest of public health.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Disclaimer
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the EMA, the FDA, or any other agency or organization, or one of their Committees or Working Parties.
Acknowledgments
We thank Nora Lazaro, Monika Hascilo, Mary Jo Salerno, and each cluster lead for their contributions to the collection of data.
References
- 1. International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use. <https://www.ich.org/about/mission.html>. Accessed March 15, 2019.
- 2. International Conference of Drug Regulatory Authorities (ICDRA) . <https://www.who.int/medicines/areas/quality_safety/regulation_legislation/icdra/en/>. Accessed July 8, 2019.
- 3. International Coalition of Medicines Regulatory Authorities (ICMRA) . <http://www.icmra.info/drupal/en>. Accessed July 8, 2019.
- 4. European Medicines Agency . International Partners <https://www.ema.europa.eu/en/partners-networks/international-activities/bilateral-interactions-non-eu-regulators/united-states>. Accessed March 15, 2019.
- 5. European Medicines Agency . Confidentiality arrangements. <https://www.ema.europa.eu/en/documents/other/statement-authority-confidentiality-commitment-european-comissions-directorate-general-health-food_en.pdf>. Accessed July 19, 2019.
- 6. US Food and Drug Administration . Confidentiality commitments <https://www.fda.gov/international-programs/confidentiality-commitments/fda-european-medicines-agency-and-dg-sante-confidentiality-commitment>. Accessed July 19, 2019.
- 7. Common Commentaries . Pediatric cluster <https://www.fda.gov/science-research/pediatrics/international-collaboration-pediatric-cluster>. Accessed July 18, 2019.
- 8. Patient Engagement . <https://www.fda.gov/patients/learn-about-fda-patient-engagement/patient-engagement-collaborative>. Accessed July 18, 2019.
- 9. Maliepaard, M. et al Pharmacogenetics in the evaluation of new drugs: a multiregional regulatory perspective. Nat. Rev. Drug Discov. 12, 103–115 (2013). [DOI] [PubMed] [Google Scholar]
- 10. Kehoe, T. , Blind, E. & Janssen, H. Regulatory aspects of the development of drugs for metabolic bone diseases – FDA and EMA perspective. Br. J. Clin. Pharmacol. 85, 1208–1212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. EMA‐FDA collaborative approach on pediatric Gaucher disease. <https://www.ema.europa.eu/en/gaucher-disease-strategic-collaborative-approach-european-medicines-agency-food-drug-administration>. Accessed July 18, 2019.
- 12. Joint workshop on early development, PRIME, and Breakthrough Therapies of 26/11/2018. <https://www.ema.europa.eu/en/events/stakeholder-workshop-support-quality-development-early-access-approaches-such-prime-breakthrough>. Accessed July 18, 2019.


